-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIntermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
Expression of Bacillus subtilis BraB, a branched-chain amino acid permease, is under both negative and positive control by a global transcriptional regulator CodY. The negative control is direct and the positive control is indirect and mediated by another B. subtilis pleiotropic transcriptional regulator, ScoC, which, in turn, is repressed by CodY. Thus, CodY and ScoC form a feed-forward regulatory loop at the braB promoter. In a very unusual manner, the interaction of CodY and ScoC results in high braB expression only at intermediate CodY activities; braB expression remains low both at high and low CodY activities. The novel regulation of braB shows that important, novel regulatory phenomena can be missed by analyzing null mutants in regulatory genes but revealed by using mutants with partial activity.
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005600
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005600Summary
Expression of Bacillus subtilis BraB, a branched-chain amino acid permease, is under both negative and positive control by a global transcriptional regulator CodY. The negative control is direct and the positive control is indirect and mediated by another B. subtilis pleiotropic transcriptional regulator, ScoC, which, in turn, is repressed by CodY. Thus, CodY and ScoC form a feed-forward regulatory loop at the braB promoter. In a very unusual manner, the interaction of CodY and ScoC results in high braB expression only at intermediate CodY activities; braB expression remains low both at high and low CodY activities. The novel regulation of braB shows that important, novel regulatory phenomena can be missed by analyzing null mutants in regulatory genes but revealed by using mutants with partial activity.
Introduction
BraB is one of three permeases demonstrated to be involved in the uptake of branched-chain amino acids (BCAA) in Bacillus subtilis [1]. Given the important role of BCAA in cell metabolism, it is not surprising that the synthesis of the permeases is strictly regulated and coordinated. The most efficient BCAA permease, BcaP, is subject to very strong transcriptional repression by CodY [2], a global regulator in B. subtilis and other Gram-positive bacteria [3, 4]. A second permease, BrnQ, is subject to strong repression by AzlB, a member of the AsnC/Lrp family of transcriptional regulators, in response to an as yet unidentified signal [5]. The regulation of BraB synthesis has not been previously determined.
A fragment containing the regulatory region between the divergently transcribed iscSB (formerly nifZ) and braB genes was found to bind CodY strongly in vivo in a ChIP-to-chip experiment [6]. Moreover, a strong CodY-binding site in the iscSB-braB intergenic region was also detected in vitro during the global characterization of CodY-binding sites by IDAP-Seq [7]. The latter site is well-placed to serve as a potential site of regulation of braB. However, transcription of neither braB nor iscSB was altered >2.0-fold by a null mutation in codY, as detected in DNA microarray or RNA-Seq experiments [6, 8](http://www.genome.jp/kegg/expression/).
CodY controls directly or indirectly the transcription of more than 200 B. subtilis genes [7, 8]. The DNA-binding affinity of CodY from B. subtilis and many other species is increased by interaction with two types of ligands, the BCAA [isoleucine, leucine, and valine (ILV)] [9–11] and GTP [6, 11–14]. Thus, given the presence of CodY-binding sites in the putative braB regulatory region and the influence of BCAA on CodY activity, it was surprising that expression of braB was only minimally affected by a codY null mutation. We describe here a detailed analysis of the mechanisms by which CodY regulates braB.
The braB gene proved to be directly repressed by two proteins, CodY and another pleiotropic regulator, ScoC (formerly known as hpr or catA) [15–18]. Because CodY also represses scoC [19], CodY and ScoC form a feed-forward regulatory loop [20, 21] in which CodY acts an indirect positive regulator of braB. The opposing effects of fully active CodY balance each other; as a result, braB derepression could only be observed at intermediate levels of CodY activity or when both regulators are inactive. These findings emphasize that the phenotypes caused by null mutations in global regulatory protein genes can be misleading.
Results
braB CodY-binding sites
The unexpected absence of an effect of a codY null mutation on expression of a gene with a strong CodY-binding site in its putative regulatory region led us to analyze braB transcription in more detail. A primer extension experiment established that the 5’ end of the braB mRNA is located 72 bp upstream of the initiation codon. The sequences TTGACT and TATAAT, with one and no mismatches to the –35 and –10 regions of σA-dependent promoters, respectively, and a 16-bp spacer region, can be identified upstream of the 5’ end location, suggesting that this position does in fact correspond to the transcription start point (Fig 1A). (Since B. subtilis σA-dependent promoters rarely have a 16-bp spacer, our assignment of the -10 and -35 regions may be off by 1 or 2 bp.) A mutation, T(-29)C, located immediately downstream of the likely -35 region, reduced expression of a braB-lacZ fusion 6-fold (1.97±0.35 Miller units, see below), consistent with our assignment of the promoter.
Fig. 1. The sequence of the braB regulatory region and map of the promoter fragments used. 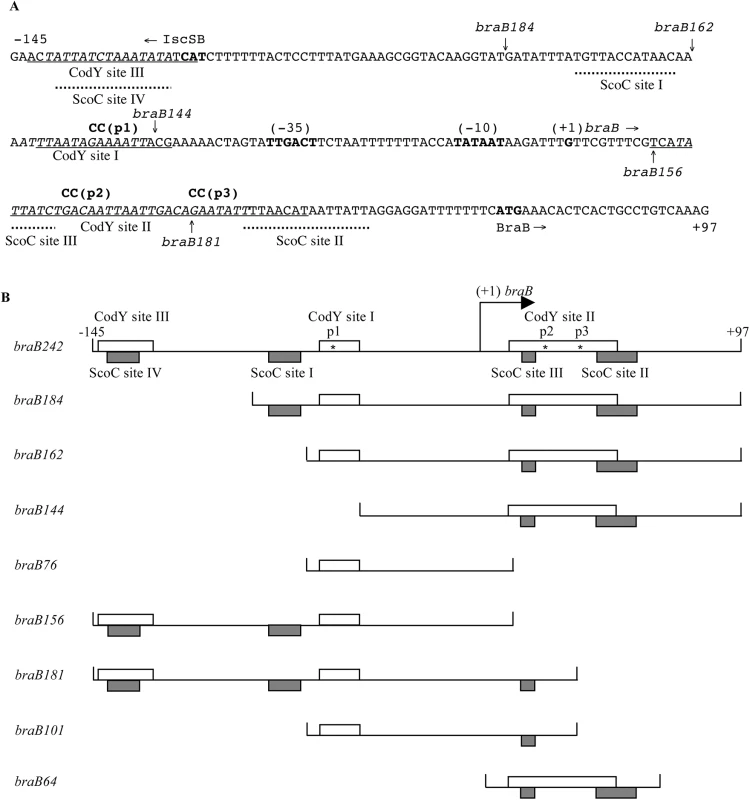
A. The sequence (5’ to 3’) of the coding (non-template) strand of the braB regulatory region within the braB242-lacZ fusion. Coordinates are reported with respect to the transcription start point. The upstream boundary of the braB184, braB162, and braB144 fusions at positions –87, -65, and -47, respectively, are indicated by vertical arrows above the sequence. The vertical arrows below the sequence indicate the junction points, at position +11 and +36, between the braB and lacZ sequences. The likely translation initiation codon, -10 and -35 promoter regions, and apparent transcription start point are in boldface. The directions of transcription and translation are indicated by the horizontal arrows. The sequences on the template strand that were protected by CodY or ScoC in DNase I footprinting experiments are underlined or shown by dotted horizontal lines below the sequence, respectively. The sequences of CodY-binding motifs are italicized and shown in Table 1. The mutated nucleotides are shown in lowercase above the sequence. B. Schematic maps of the braB fragments used to construct lacZ fusions or in DNA-binding experiments. The coordinates indicate the boundaries of different fusions with respect to the braB transcription start point. The location of the apparent transcription start point is indicated by the bent arrow. CodY- and ScoC-binding sites determined in DNase I footprinting experiments are shown as clear or shaded rectangles, respectively. DNase I footprinting experiments showed that CodY protected two sites, I and II, within the 194-bp iscSB-braB intergenic region from positions -62 to -47 and +11 to +50 of the template DNA strand with respect to the braB transcription start point, respectively (Figs 1A, 2B and 2C). Site II is much stronger than site I. Binding to an additional very weak site, III, from positions –143 to –124, which is within the upstream iscSB gene, was observed only at high concentrations of CodY (≥200 nM) (Fig 2B and 2C).
Fig. 2. Determination of the braB transcription start point and CodY-binding regions. 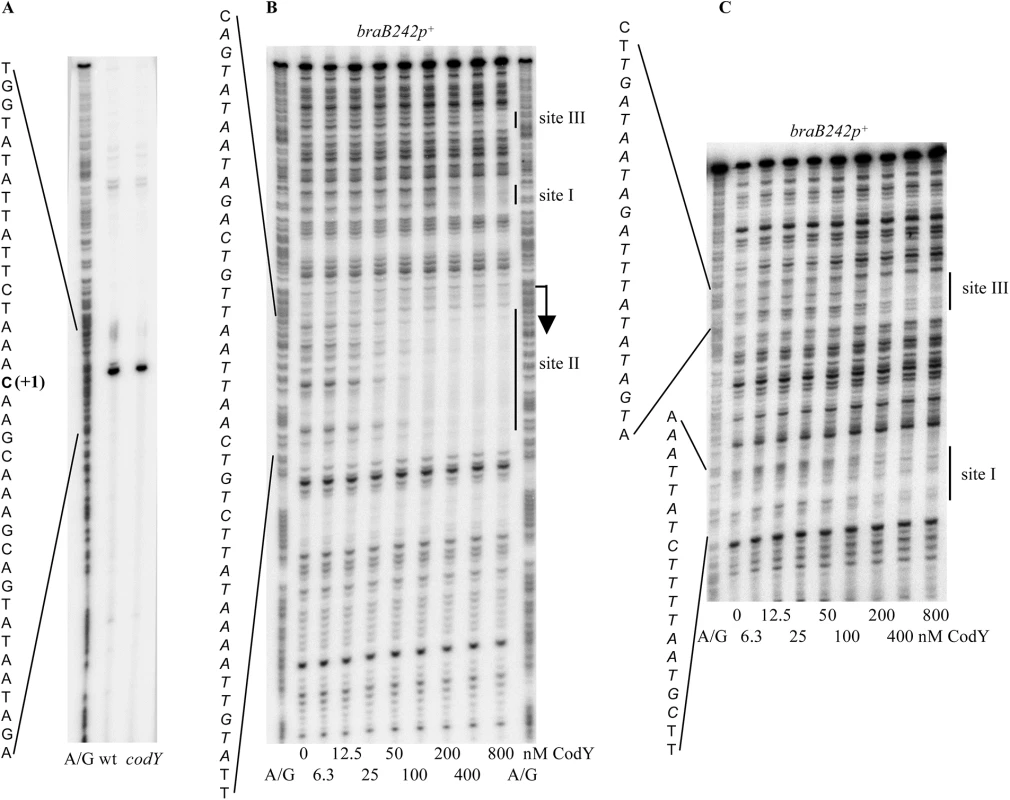
A. Primer extension analysis of the braB mRNA. Primer oBB102 annealing to the lacZ gene of the braB242-lacZ fusion was extended with reverse transcriptase using as the template total RNA from fusion-containing strains BB3076 (wt) and BB3079 (codY) grown in TSS + 16 aa medium. The A+G sequence of the template strand of pBB1593 determined from reactions primed with oBB102 is shown to the left. The apparent transcription start site of the braB gene is in bold and marked by the +1 notation. B. DNase I footprinting analysis of CodY binding to the braB regulatory region. The braB242p+ DNA fragment obtained by PCR with oligonucleotides oBB67 and oBB102 and labelled on the template strand was incubated with increasing amounts of purified CodY in the presence of 10 mM ILV and 2 mM GTP and then with DNase I. The protected areas are indicated by vertical lines and the corresponding sequences are reported; the protected nucleotides are italicized. The apparent transcription start site and direction of transcription are shown by a bent arrow. CodY concentrations used (nM of monomer) are indicated below each lane. The A + G sequencing ladder of the template DNA strand is shown in the flanking lanes. C. Same as B, the gel was run longer to improve the resolution of the upstream CodY-binding sites III and I. The results of the footprinting experiments are consistent with the identification of a strong CodY-binding site in this area by ChIP-to-chip experiments [6]. Moreover, they confirmed and extended the results of the in vitro IDAP-Seq experiments, which identified a strong core binding site from positions +29 to +43, a much weaker core site, which ends at position -45, and an additional, very weak core site, ending at position -116 and detected only at a very high CodY concentration (1 μM) (core sites only include positions that are essential for CodY binding; the beginning positions of the two upstream core sites could not be determined due to limitations of the IDAP-Seq procedure) [7].
The braB regulatory region contains five 15-bp motifs, which resemble the 15-bp CodY-binding consensus sequence, AATTTTCWGAAAATT [22–24] (we use the terms “site” and “motif” to describe an experimentally determined location of CodY binding and a 15-bp sequence that is similar to the consensus motif, respectively). Site I of the braB gene overlaps CodY-binding motif 1, located between positions -64 and -50, that has 4 mismatches with respect to the CodY-binding consensus (Fig 1A and Table 1). The strong site II overlaps two adjoining versions of the 15-bp sequence, motifs 2 and 3, located between positions +14 and +43, each of which has three mismatches with respect to the consensus motif. Another 15-bp sequence, motif 4, with four mismatches is located from positions +40 to +54 and overlaps motif 3 by 4 bp. Site III overlaps CodY-binding motif 5, with 5 mismatches, located from positions -141 to -127 (Fig 1A and Table 1).
Tab. 1. CodY-binding motifs of the braB gene. 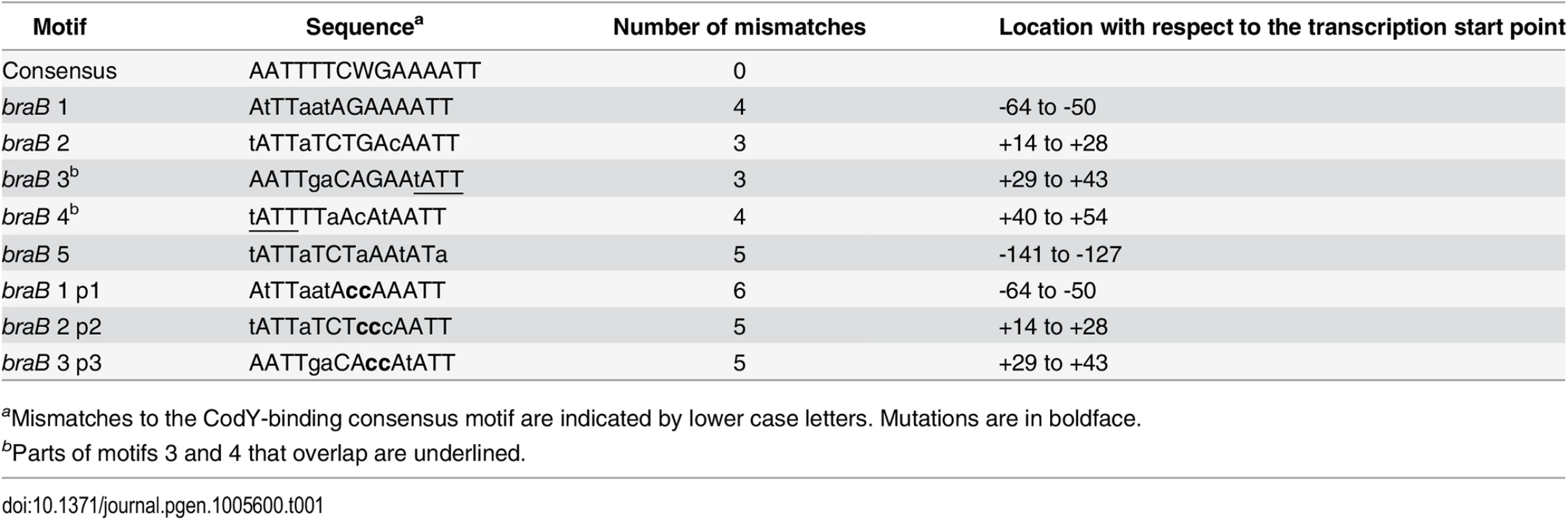
aMismatches to the CodY-binding consensus motif are indicated by lower case letters. Mutations are in boldface. Binding of CodY to upstream braB sites occurred independently of the presence of the downstream site and vice versa (Figs 1 and 3; see below for generation of the truncated fragments), similar to the case for other genes containing multiple CodY-binding sites within their regulatory regions [2, 25, 26].
Fig. 3. Role of braB CodY-binding sites in CodY binding. 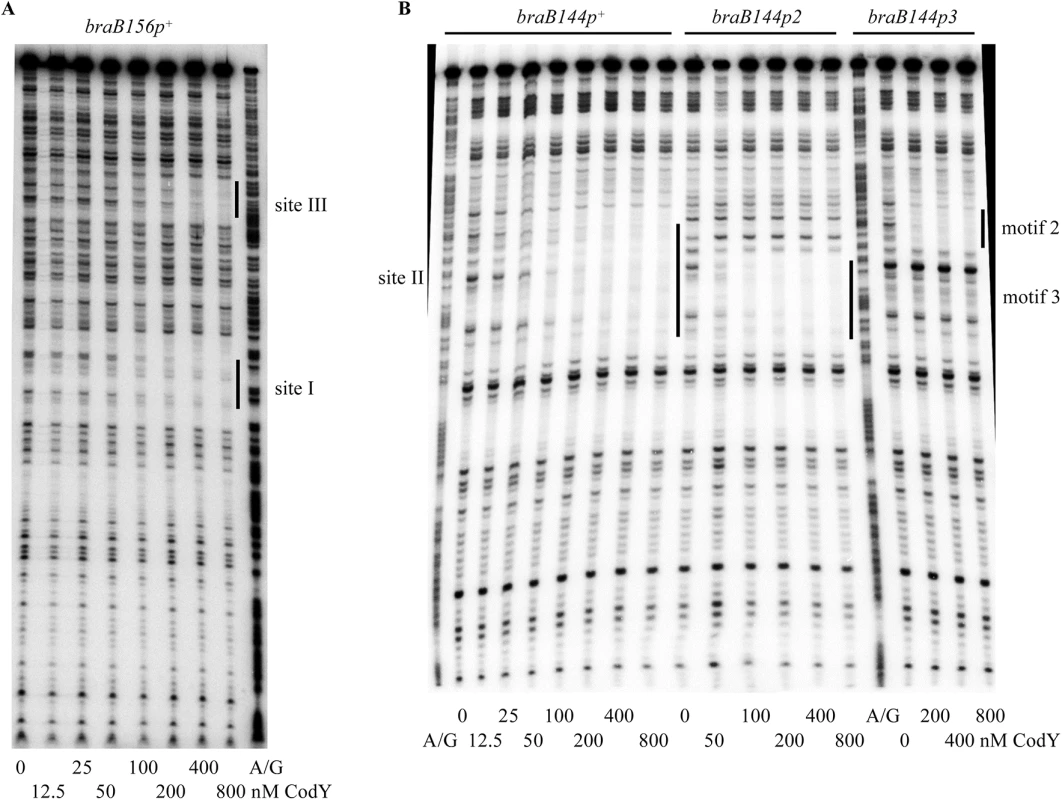
The braB156p+ (A) or braB144p+, braB144p2, and braB144p3 (B) DNA fragments obtained by PCR with oligonucleotides oBB67 and oBB102 and labelled on the template strand were incubated with increasing amounts of purified CodY in the presence of 10 mM ILV and then with DNase I. The protected areas are indicated by vertical lines. CodY concentrations used (nM of monomer) are indicated below each lane. The A + G sequencing ladder of the template DNA strand is shown in the flanking lanes. In gel-shift experiments, CodY bound to DNA fragments containing only sites III and I (braB156) or only site II (braB144) with apparent dissociation constants (KD) of ∼75 nM and ∼4 nM, respectively, compared with ∼3 nM for the full-length fragment, braB242 (Fig 4A, 4B and 4D) (KD reflects the CodY concentration needed to shift 50% of DNA fragments under conditions of vast CodY excess over DNA). Complexes with lower mobility were formed at higher concentrations of CodY for all fragments, indicating apparent changes in stoichiometry of CodY binding (Fig 4).
Fig. 4. Gel shift assays of CodY binding to braB fragments. 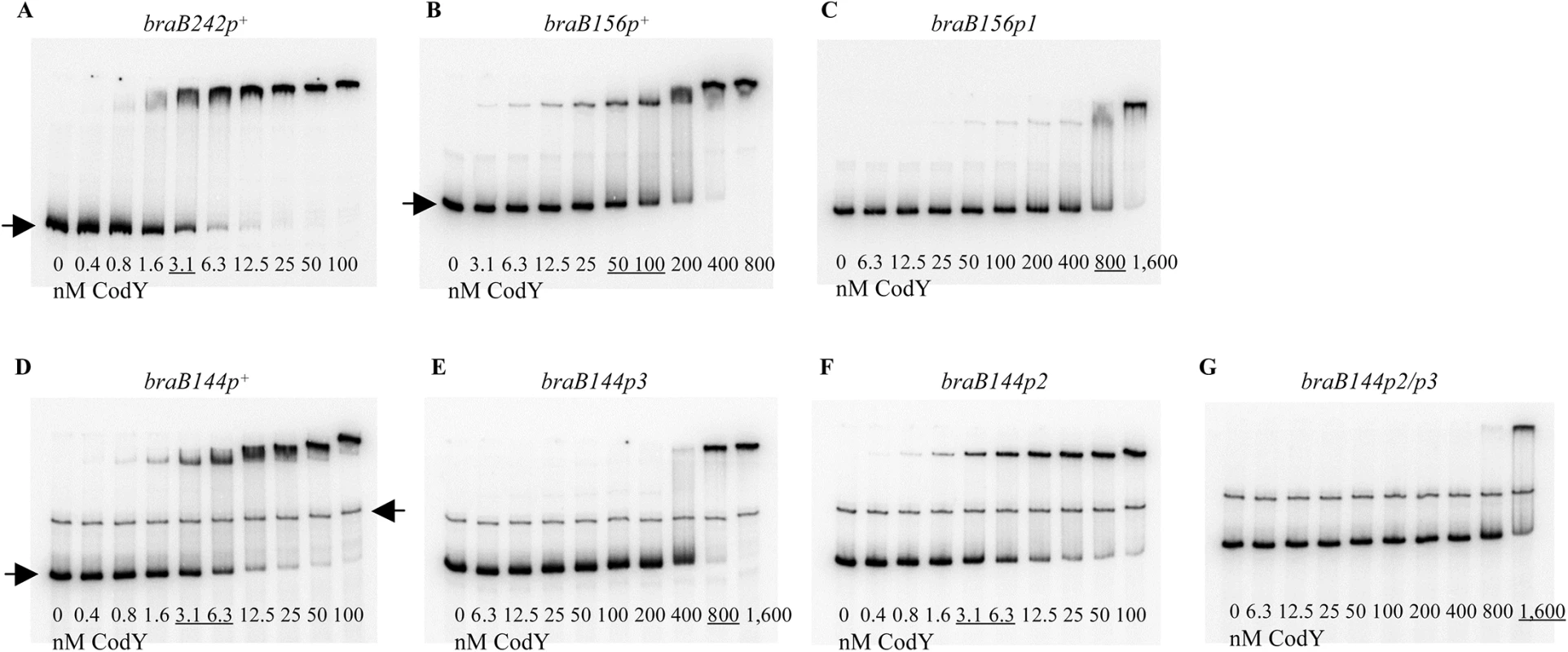
The braB242p+ (A), braB156p+ (B), braB156p1 (C), braB144p+ (D), braB144p3 (E), braB144p2 (F), and braB144p2/3 (G) DNA fragments labeled on the template strand were incubated with increasing amounts of purified CodY in the presence of 10 mM ILV. The fragments were obtained by PCR with oligonucleotides oBB67 and oBB102 (A), oBB358 and oBB102 (B, C), and oBB422 and oBB102 (D-G) and their positions in the gel are indicated by right-pointing arrows. The unspecific DNA fragment, present in some panels, is indicated by a left-pointing arrow. CodY concentrations used (nM of monomer) are reported below each lane; concentrations corresponding to the apparent KD for binding are underlined. Regulation of braB expression
We constructed a transcriptional fusion (braB242-lacZ) containing a 242-bp fragment that includes the entire iscSB-braB intergenic region (Fig 1). Under conditions of maximal CodY activity, in cells grown in TSS glucose–ammonium medium supplemented with ILV and a mixture of 13 other amino acids (referred to here as the 16 aa-containing medium), fusion expression in a codY null mutant strain was very similar (1.3-fold less) to that in the wild-type strain (Table 2, strains BB3076 and BB3079). Consistent with the lacZ fusion results, only very weak, positive regulation (1.6 - to 1.9-fold) in amino acid-containing medium was detected in microarray or RNA-Seq experiments by comparing wild-type and codY null mutant strains [6, 8].
Tab. 2. CodY- and ScoC-mediated regulation of lacZ fusions.a 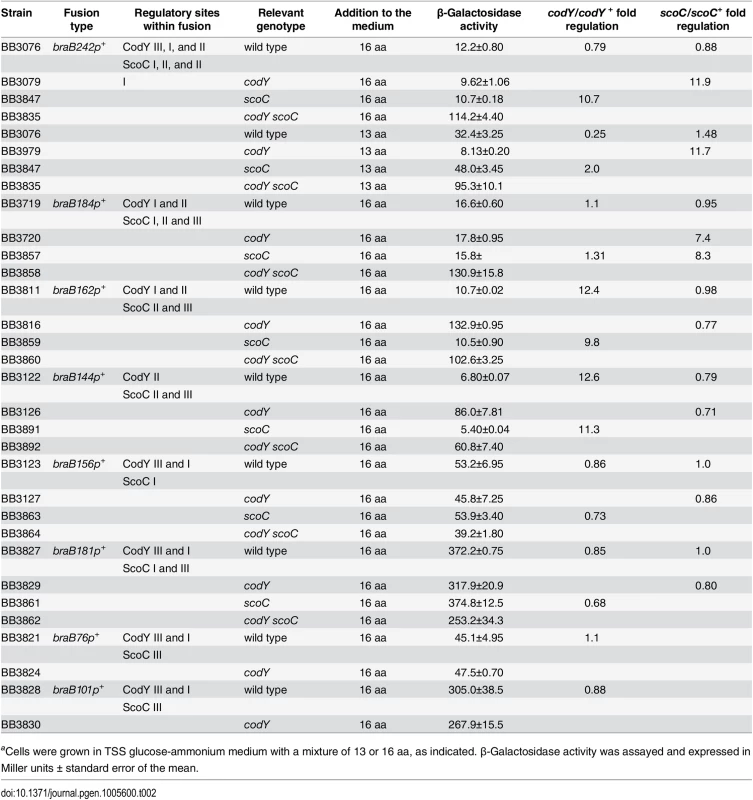
aCells were grown in TSS glucose-ammonium medium with a mixture of 13 or 16 aa, as indicated. β-Galactosidase activity was assayed and expressed in Miller units ± standard error of the mean. braB expression is increased only at intermediate levels of CodY activity
The activity of CodY is reduced to intermediate levels when some amino acids are removed from the medium and decreases strongly in the absence of all amino acid supplements [2, 27]. Expression of the braB242-lacZ fusion in the wild-type strain in the absence of any amino acids or in the presence of ILV only was very similar to that in the presence of 16 aa (11.3 to 14.7 MU versus 12.2 MU). Unexpectedly, almost 3-fold higher activity was found in 13 aa-containing medium (i.e., in the absence of ILV), indicating that CodY, at an intermediate level of activity, may serve as a positive regulator of braB (Table 2).
To test whether expression of the braB gene indeed responds differentially to varying levels of CodY activity in vivo we made use of a previously constructed set of mutant forms of CodY that have different levels of residual responsiveness to ILV. Most of these proteins have alterations in amino acids that form the ILV-binding pocket; they are expressed at wild-type levels and have undiminished activity in effector-independent DNA binding [2, 8, 28]. Since the population of CodY molecules in the cell is in equilibrium between the liganded and unliganded forms of the protein, the unliganded fraction of the population of a mutant protein that has lower affinity for ILV will be greater than for the wild-type protein at a given intracellular ILV concentration. That is, a mutant strain containing a form of CodY that has low affinity for ILV behaves functionally equivalently to the wild-type strain that has a low intracellular pool of ILV.
The analysis of Dataset S1 of Ref. (8) indicates that expression of braB determined by RNA-Seq experiments was up to 4.1-fold higher in three strains containing partially active versions of CodY, F71Y, R61K, or R61H (Fig 5A). The results of the RNA-Seq experiments were confirmed and extended by real-time RT-PCR and by analyzing expression of the braB242-lacZ transcriptional fusion in a larger collection of partial codY mutants (Fig 5B and 5C). The up-and-down expression pattern of the braB fusion, in which maximal activity was seen in mutants with intermediate levels of CodY activity, was in drastic contrast to the plateau-reaching expression pattern of the previously characterized CodY-repressed bcaP283-lacZ fusion (Fig 5F) [2] and all other CodY-regulated genes [2].
Fig. 5. Expression of braB, bcaP, or scoC in mutants containing partially active versions of CodY. 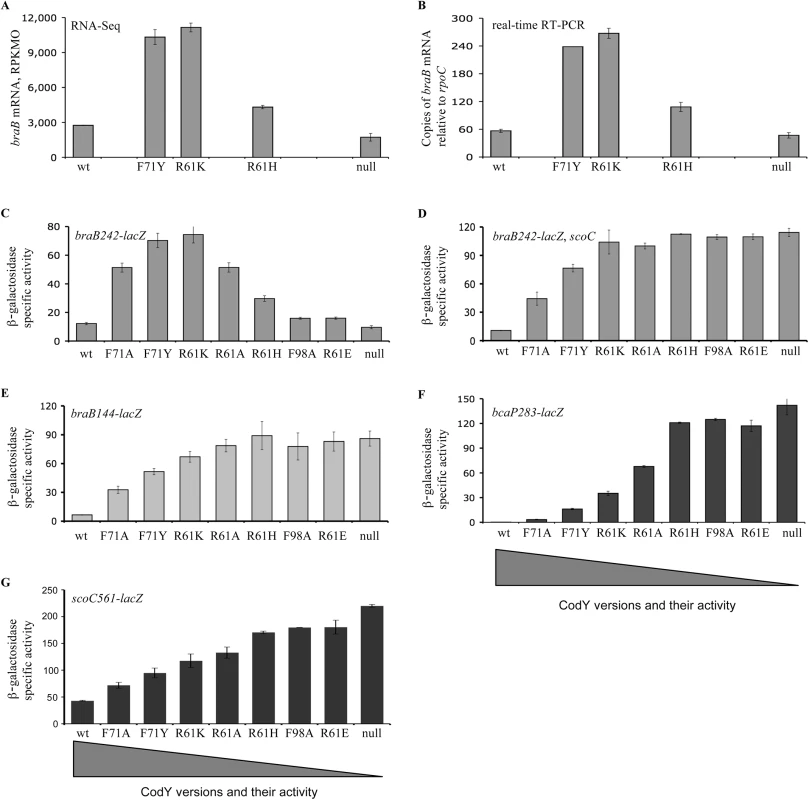
A and B. braB transcript abundance as determined by RNA-Seq (A) or quantitative, real-time RT-PCR (B). Cells were grown in TSS + 16 aa medium. The RNA-Seq values, expressed as reads per kilobase per million ORF (RPKMO) values, were taken from the Dataset S1 of Ref. [8]. The real-time RT-PCR results are expressed as braB transcript abundance relative to rpoC transcript abundance. Point mutant positions along the x-axis are arbitrary and do not imply a linear relationship. Data points are the means of at least two independent experiments, and the error bars show standard errors of the mean. C—G. Expression of braB-lacZ, bcaP-lacZ, and scoC-lacZ fusions in scoC+ or scoC null mutant cells containing partially active versions of CodY. Cells were grown in TSS + 16 aa medium. β-Galactosidase activity was assayed and expressed in Miller units. A braB242-gfp translational fusion was introduced into the wild-type strain, the codY null mutant, and a codY point mutant (R61K) strain with intermediate residual activity. In all cases, the level of braB expression was rather similar across the cell population (Fig 6), eliminating the possibility that a bistable expression pattern could explain our results. As expected, the codY (R61K) mutant strain had elevated expression compared to the wild-type and codY null mutant strains.
Fig. 6. Expression of the braB242-gfp fusion in individual cells. 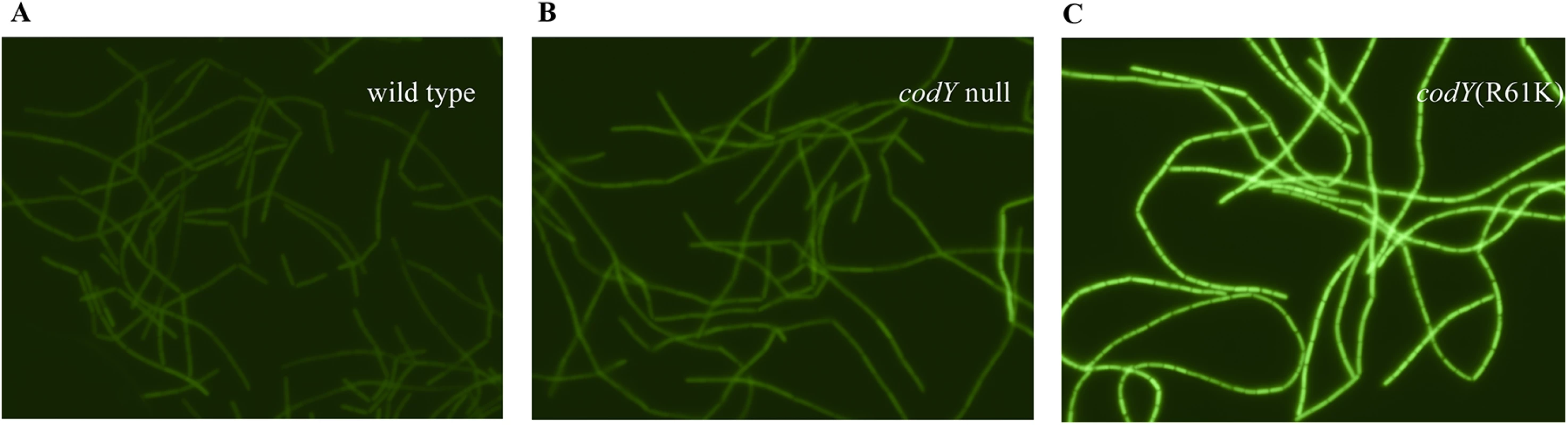
Cells were grown in TSS + 16 aa medium. GFP fluorescence was detected using Zeiss Axio Observer.Z1 microscope. A. Strain BB4082 (wild type). B. Strain BB4083 (codY::spc). C. Strain BB4084 [codY(R61K)]. Deletion analysis of the braB regulatory region
We initially hypothesized that the very unusual pattern of braB regulation observed results from CodY binding independently to negative and positive regulatory sites within the braB regulatory region. If so, the positive and negative effects of fully active CodY might balance each other, but, at intermediate levels of CodY activity, positive regulation might dominate. The CodY-binding sites I and II are located upstream and downstream of the braB promoter in positions appropriate for positive and negative regulation, respectively. To determine their independent effects, we created additional lacZ fusions containing truncated versions of the braB regulatory region lacking the upstream CodY-binding site III (braB184-lacZ and braB162-lacZ) or sites III and I (braB144-lacZ) or the downstream site II (braB156-lacZ) (Fig 1B). (Note that the braB242-, braB184-, braB162- and braB144-lacZ fusions have the identical junction with lacZ; their levels of activity can be directly compared. However, other fusions, such as braB156-lacZ, have different junctions; their activities in wild-type cells can only be compared to the activity of the same fusion in mutant strains or other fusions with a similar junction.)
Surprisingly, deletion of the upstream binding sites III and I did not cause any significant decrease in braB expression in wild-type cells (Table 2; compare strains BB3076, BB3719, BB3811, and BB3122), implying that these are not sites of positive regulation. On the other hand, the braB162-lacZ and braB144-lacZ fusions, but not the braB184-lacZ fusion, were derepressed 12-fold when codY was inactivated (Table 2), suggesting that braB expression is subject to negative regulation by CodY bound to the remaining downstream site II. If so, this regulation must be masked in other fusions by the action of a second repressor that binds to the sequence located between the 5’ ends of braB184-lacZ and braB162-lacZ. Interestingly, no up-and-down expression pattern in mutants with different levels of CodY activity was observed for the braB144-lacZ fusion, which lacks the putative binding site for the predicted second regulator (Fig 5E), suggesting that the latter is responsible for the unusual regulation. As expected from this new model, the braB156-lacZ fusion, which lacks the downstream CodY-binding site II, was not subject to regulation by CodY (Table 2).
Mutations in the braB CodY-binding sites
To confirm that site I is not involved in braB regulation and to quantify more directly the contribution of site II, we changed the very highly conserved G9 and A10 residues of CodY-binding motifs 1, 2, and 3 to CC (the p1, p2, and p3 mutations, respectively) (Fig 1 and Table 1).
The p1 mutation in site I reduced ~10-fold the affinity of CodY for a fragment containing sites III and I, indicating that site I is the major contributor for CodY binding to this fragment (Fig 4B and 4C). However, as expected from our deletion analysis, the p1 mutation did not affect expression of the braB242-lacZ fusion (Table 3, strains BB3731 and BB3076). Thus, as noted previously, many CodY-binding sites have no physiological significance either because they are not positioned appropriately for regulation or because binding is too weak [7].
Tab. 3. Effect of mutations in the CodY-binding sites on braB expression. 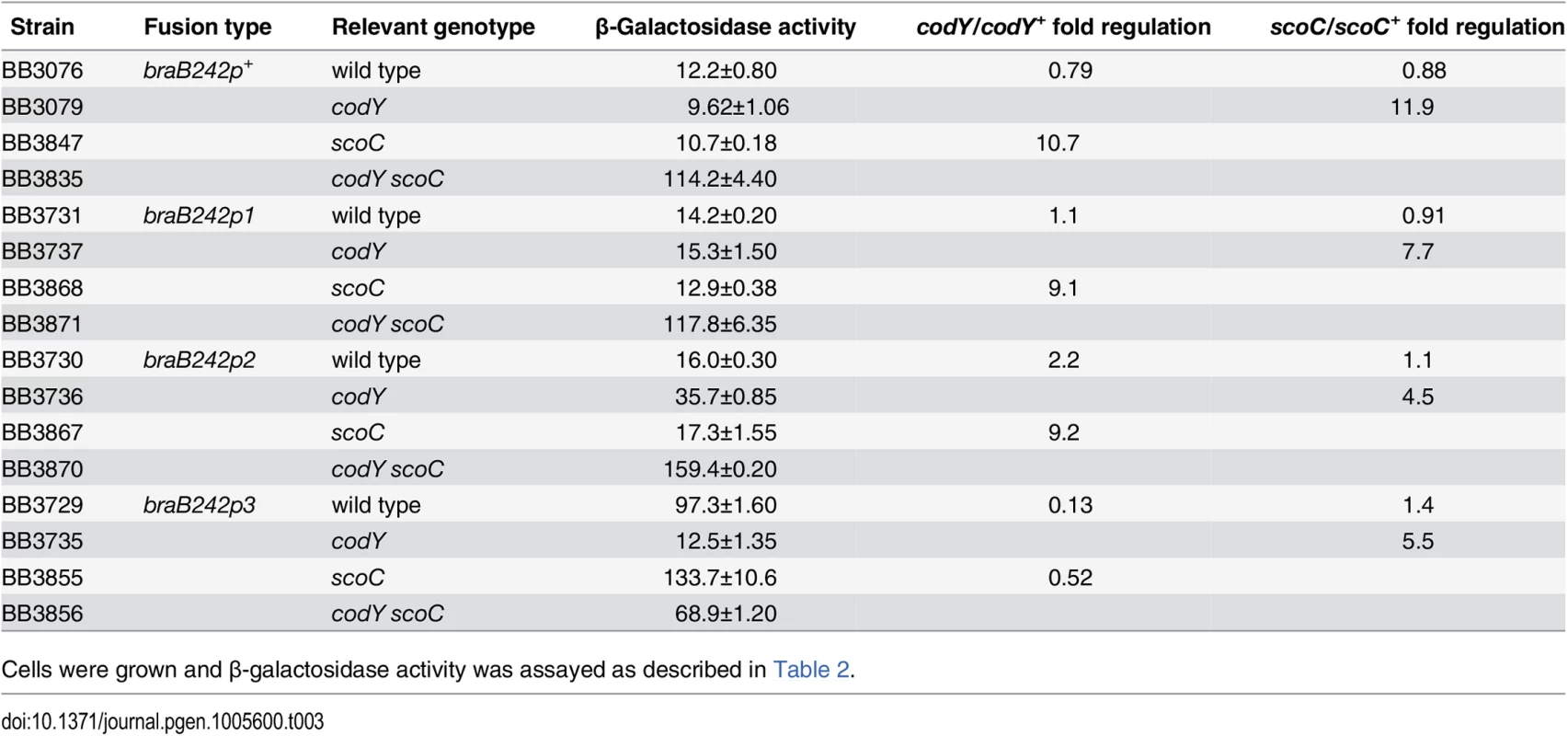
Cells were grown and β-galactosidase activity was assayed as described in Table 2. The p3 mutation reduced the affinity of CodY for site II ≥10-fold (Fig 4E). The p2 mutation did not affect binding of CodY to site II, but further decreased the ability of CodY to interact with this site if it already contained the p3 mutation (Fig 4F and 4G). Footprinting experiments showed that each mutation affected CodY binding to the region of site II, which corresponded to the respective motif (Fig 3B). Taking together the gel-shift and footprinting results, we conclude that interaction of CodY with motif 2 is weaker than with motif 3 and is partly dependent on simultaneous interaction of CodY with motif 3 (see below for the effect of p2 on braB regulation).
The p3 mutation increased expression of the braB242-lacZ fusion 8-fold consistent with relief from CodY-mediated repression (Table 3, strains BB3729 and BB3076). However, expression of the braB242p3-lacZ fusion was substantially reduced in a codY null mutant strain apparently due to repression by the second regulator (Table 3, strain BB3735). This result suggests strongly that the second regulator is active in codY mutant cells, but not in wild-type cells, i.e., its activity or expression is under negative CodY control. Paradoxically, this indicates that our initial hypothesis that braB regulation is subject to simultaneous positive and negative regulation by CodY was likely to be correct, though positive regulation appears to be indirect and mediated through regulation of the second repressor.
Identification of ScoC as a second repressor of braB
CodY is known to regulate the expression of a small number of other regulatory proteins, including ScoC [6–8, 19, 29, 30]. ScoC is a repressor of multiple genes, including those encoding extracellular proteases and oligopeptide permeases, and is also involved in the regulation of sporulation [15–19, 31–33]. Though microarray experiments did not identify braB as a ScoC target [15], we decided to test whether ScoC is the second regulator of braB expression. No effect of a single scoC null mutation on expression of the braB242-lacZ fusion in TSS + 16 aa was detected (Table 2, strain BB3847). However, in a double codY scoC null mutant, expression of the fusion was 11 - to 12-fold higher than in the wild-type strain or in scoC or codY single mutants (Table 2, strain BB3835), indicating that both CodY and ScoC contribute to negative regulation of braB but these effects cannot be dissected if either one of the regulators is active.
Expression of the same fusion in a double null mutant in TSS + 13 aa medium was very similar (Table 2), indicating that our original observation of higher braB expression under these growth conditions in a wild-type strain was indeed due to reduced CodY activity and its effect on ScoC expression.
As expected, in the absence of ScoC, the up-and-down expression pattern of the braB242-lacZ fusion in strains with different CodY activity was replaced by a plateau-reaching pattern, resembling that of the bcaP283-lacZ fusion, which is not subject to ScoC-mediated regulation (Fig 5D and 5F).
Expression of the scoC561-lacZ fusion in strains with different CodY activity also followed a plateau-reaching pattern, characteristic for most genes regulated by CodY, and did not correlate with expression from the braB promoter (Fig 5G).
ScoC binding to the braB regulatory region
In DNase I footprinting experiments, ScoC protected two sites, I and II, within the iscSB-braB intergenic region from positions -79 to -68 and +43 to +57 of the template DNA strand with respect to the braB transcription start point, respectively (Figs 1A and 7). A short, weakly protected region, site III (possibly a part of site II), was also detected from positions +16 to +20. Binding of ScoC to the downstream sites II and III was independent of the presence of the upstream site I on the same DNA fragment (Fig 7).
Fig. 7. Determination of braB ScoC-binding regions. 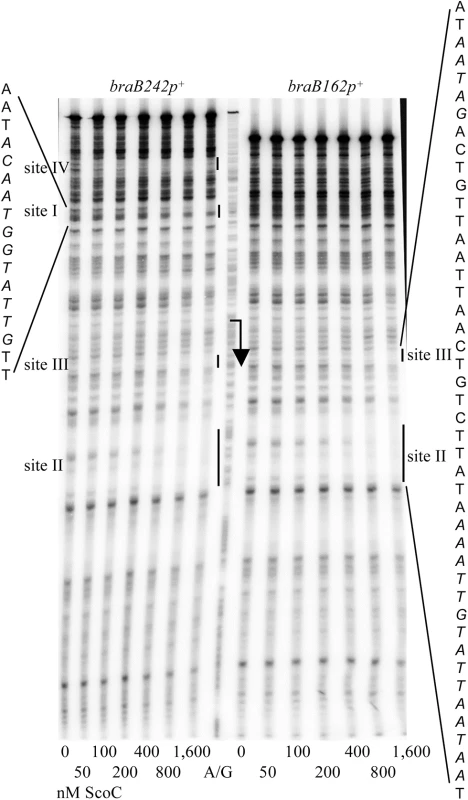
DNase I footprinting analysis of ScoC binding to the braB regulatory region. The braB242p+ or braB162p+ DNA fragment obtained by PCR with oligonucleotides oBB67 and oBB102 and labelled on the template strand was incubated with increasing amounts of purified ScoC and then with DNase I. See the legend to Fig 2B for additional details. The downstream CodY - and ScoC-binding sites partly overlap (Fig 1A). To address the possibility that CodY and ScoC compete for binding at this location, we analyzed interaction of these proteins with a short, 64-bp braB fragment, containing CodY-binding site II and ScoC-binding sites II and III (Fig 1B). In accord with the results described above, ScoC bound this fragment in gel shift experiments less efficiently (KD≈150 nM) than did CodY (KD≈5 nM) (Fig 8A and 8B). Nevertheless, ScoC, in a concentration-dependent manner, was able to replace CodY efficiently in a preformed braB-CodY complex as evidenced by formation of ScoC-specific complexes with higher mobility and the decrease in the amount of braB-CodY complexes with lower mobility (Fig 8C). The CodY-mediated displacement of ScoC from the preformed braB-ScoC complex cannot be recognized confidently because of the low mobility of CodY-specific complexes (complexes containing both proteins would have a similar low mobility). However, by comparing and Fig 8B and 8D, it is clear that CodY bound much less efficiently to preformed braB-ScoC complexes than to free braB DNA, confirming competition between the two proteins for binding. A similar competition between CodY and ScoC was previously detected at the oppA promoter [19].
Fig. 8. Competition between CodY and ScoC for braB binding. 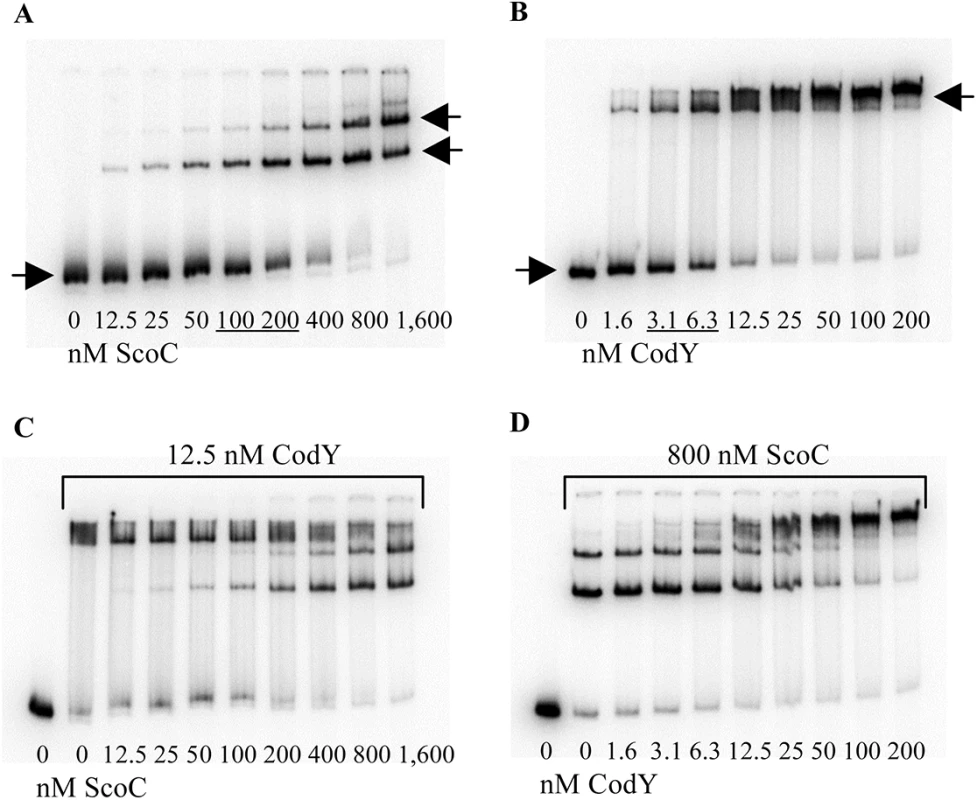
A and B. The braB64 DNA fragment, obtained by PCR with oligonucleotides oBB730 and oBB731 and labelled on the template strand, was incubated for 32 min with increasing amounts of purified ScoC (A) or CodY (B) in the presence of 10 mM ILV. C and D. The braB64 DNA fragment was preincubated for 16 min with 12.5 nM CodY (C) or 800 nM ScoC (D), and then increasing concentrations of either ScoC (C) or CodY (D) were added for an additional 16 min. The positions of the free DNA fragment and protein-DNA complexes are indicated by right-pointing and left-pointing arrows, respectively. Protein concentrations used (nM of monomer) are reported below each lane; concentrations corresponding to the apparent KD for binding are underlined. Another ScoC-binding site, site IV, was detected further upstream within the divergent iscSB gene (Figs 1A and 7). This site was not present in the braB184-lacZ fusion and therefore was not involved in the regulation described. No consensus ScoC-binding motifs, AATAnTATT [18], with ≤2 mismatches were detected within any of the braB binding sites.
Deletion and mutational analysis of the role of ScoC-binding sites in braB expression
The locations of ScoC-binding sites I and II (Figs 1 and 7) correspond well to the binding sites for the predicted second regulator of braB determined by deletion analysis (Table 2). That is, expression of the braB162-lacZ and braB144-lacZ fusions, which lack the upstream ScoC-binding sites, was not affected by a scoC mutation even if the latter was present together with a codY mutation (Table 2). On the other hand, expression of the slightly longer braB184-lacZ fusion, which includes an intact ScoC-binding site I, as well as the downstream site II, was subject to full ScoC repression (as revealed in a double codY scoC mutant) (Table 2).
Expression of the braB156-lacZ and braB181-lacZ fusions, which carry the upstream ScoC binding site but lack the downstream site II, was also not affected by a scoC mutation (Table 2). A requirement for interaction with two (or more) binding sites within the same regulatory region appears to be a common theme for ScoC-mediated repression [18, 19, 34–36].
The lack of both ScoC - and CodY-mediated regulation explains why the braB76-lacZ, braB156-lacZ, and braB181-lacZ fusions are expressed at the same level in wild-type cells and in codY null mutant cells (Table 2). On the other hand, the braB242p3-lacZ fusion, which lost direct CodY-mediated regulation, is still subject to repression by increased levels of ScoC accumulated in a codY null mutant strain (Table 3).
Interestingly, the p2 mutation, designed to reduce binding of CodY to motif 2 of site II, in fact may affect ScoC interaction with the braB regulatory region. Indeed, the p2 mutation did not affect expression of the braB242-lacZ fusion in a wild-type strain, in which scoC is repressed, but did so in codY null mutant cells, in which ScoC is expressed (Table 3, strains BB3730 and BB3736); the p2 mutation is located 1 bp downstream of ScoC-binding site III (Fig 1).
The expression levels of different fusions and locations of the ScoC-binding sites confirmed that ScoC is the predicted second repressor of braB. As noted above, deleting of one of the ScoC-binding sites resulted in a plateau-reaching expression pattern of the braB144-lacZ fusion in strains with different CodY activity (Fig 5E).
Discussion
Although previous analysis did not detect any significant regulation of braB by CodY, we now know that braB is subject to complex CodY-mediated regulation by which the protein acts both as a direct repressor and as an indirect positive regulator. The positive effect of CodY is mediated by its repression of the gene encoding a second repressor of braB, ScoC. As a result, braB expression only escapes repression under conditions (e.g., during growth in a medium containing multiple amino acids but lacking ILV) in which CodY activity is limited enough to prevent repression of braB, but high enough to maintain sufficient repression of scoC (Fig 9).
Fig. 9. A model of regulation of the braB promoter by the combined actions of CodY and ScoC. 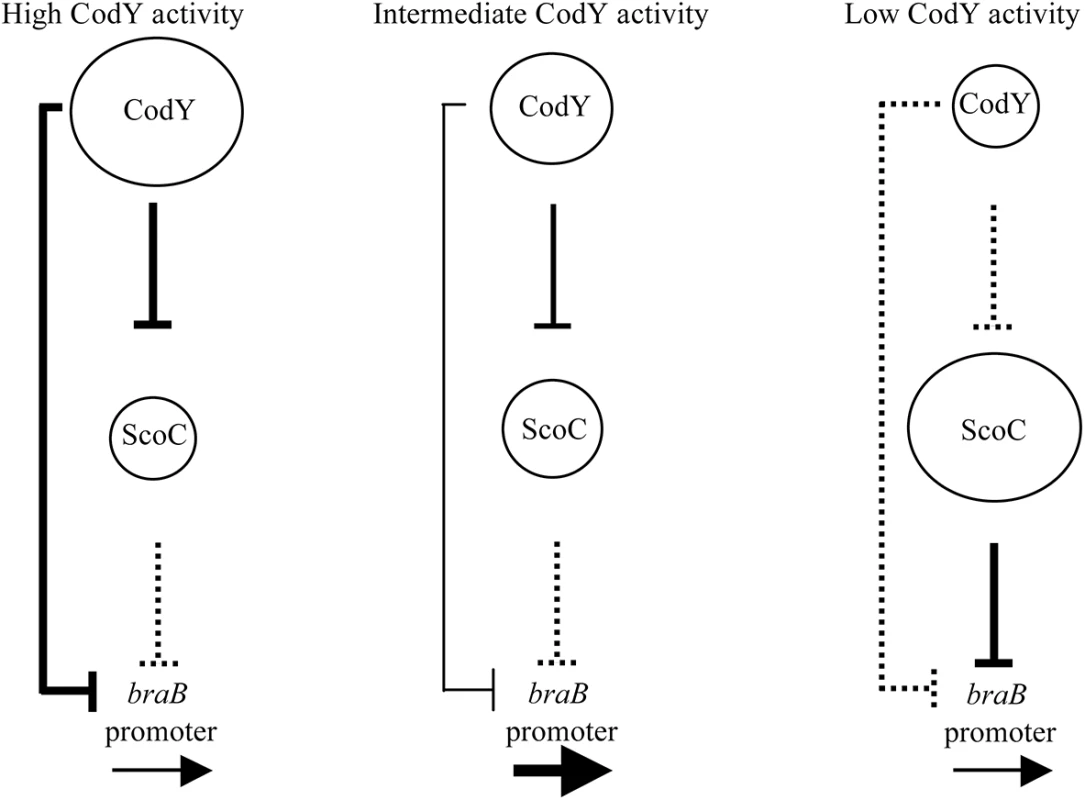
The sizes of the circles reflect the relative amount of the active form of each protein. The solid vertical lines indicate relatively strong effects on transcription. Dotted lines indicate relatively weak effects on transcription. The boldness of the horizontal arrows indicates the relative strength of transcription of the target genes. Our previously described repression of scoC by CodY, coupled with ScoC autorepression [19], keeps the level of ScoC relatively low when cells are growing rapidly. Thus, CodY and ScoC are never fully active or inactive simultaneously. When CodY is inactive, the ScoC level is high enough to repress its target genes, including braB. When CodY is fully active, the ScoC level is insufficient for repression, but CodY is able to repress braB to the same level as fully active ScoC. Because we observe higher expression of braB under conditions of partial CodY activity, we suspect that as CodY activity declines, its binding to the braB regulatory region decreases more rapidly than does its binding to the scoC regulatory region. Alternatively, the affinity of ScoC for its braB binding site might be low enough that ScoC needs to reach a relatively high concentration in order to be effective; by the time this happens, CodY-mediated repression of braB is already very low. In addition, it is possible that the competition between more strongly binding CodY and more weakly binding ScoC for interaction with the same region of the braB regulatory region (at the downstream sites for each protein) may contribute to the differential response of braB expression to varying levels of CodY activity. As a result, even relatively small losses in activity of CodY, such as in CodY(F71A), allow neither efficient direct repression by CodY nor sufficient derepression of ScoC, which would compensate for the loss of CodY-mediated repression.
The novelty of braB regulation reinforces the view that important mechanisms of gene regulation can be missed by using regulatory protein null mutants as the only means of genetic analysis. A null mutant has no activity in any environment and at any stage of its life cycle, but in wild-type cells regulatory proteins are rarely, if ever, totally inactive. What normally varies is the fraction of the population of the regulator that is in the active state. Furthermore, interpreting the phenotype of a null mutant usually assumes that a regulatory protein is either only a positive regulator or only a negative regulator of its target gene(s). Studying the behavior of genes at different levels of a regulator’s activity has the potential to reveal more complex mechanisms in detail.
It should be noted that, although the complex pattern of braB regulation is very interesting, it is not common. Combined repression by CodY and ScoC has also been observed for the B. subtilis opp operon and scoC gene itself. However, in case of opp, ScoC-mediated repression was more efficient than CodY-mediated repression and was detected, although at a reduced level, even in codY+ cells [19]. The opposite was true for expression of the scoC gene, whose regulation by CodY was detected even in scoC+ cells [19]. Among CodY-regulated genes, only the braB gene has shown the described up-and-down pattern, i.e., expression was maximal at the intermediate levels of CodY activity [8]. It remains unknown whether additional regulatory inputs, e.g., through SalA-mediated regulation of scoC expression [37], affect interaction between ScoC and CodY.
We have recently characterized three permeases, BcaP, BraB, and BrnQ, involved in the BCAA uptake in B. subtilis cells [1]. The roles of different BCAA permeases in amino acid uptake under different growth conditions should reflect their levels of expression. The bcaP (yhdG) gene encodes the most efficient permease for isoleucine and valine and is one of the genes most highly repressed by CodY; expression of the bcaP gene is virtually abolished in amino acid-rich media [2, 6]. It is very likely that higher activity of BraB is not needed during strong nutrient limitation when CodY activity is very low, because BcaP is fully derepressed. It is also likely that when bcaP and braB are repressed by highly active CodY, the residual activity of BraB, together with BrnQ, is sufficient for the uptake of high concentrations of BCAA. However, the increase in BraB expression at partial activities of CodY may facilitate the uptake of intermediate concentrations of BCAA.
It is not uncommon for two regulators to control expression of the same gene in such a way that the lack of one regulator is fully compensated for by the increased activity of the other regulator and, as a result, no regulatory effect is observed in single null mutant strains. However, when such regulators act independently and do not form a feed-forward regulatory loop, the full compensatory effect should also be observed at intermediate activities of the regulator. The peculiarity of braB regulation is that the full compensatory effect of ScoC is seen only when CodY has very low or no activity.
The feed-forward regulatory loop formed by CodY and ScoC at the braB promoter, known as a type-2 incoherent loop, is an arrangement in which two regulatory proteins repress the same target gene and one of the regulators represses expression of the other [20, 21]. This regulatory mechanism may have evolved specifically to achieve higher expression of the braB gene at intermediate activities of CodY. Genes that are regulated by a single repressor are also expressed at a higher level when activity of the repressor is reduced. However, expression of such genes reaches its maximum only when the repressor is completely inactive; the regulatory mechanism of braB avoids this scenario.
Materials and Methods
Bacterial strains and culture media
The B. subtilis strains constructed and used in this study were all derivatives of strain SMY [38] and are described in Table 4 or in the text. Escherichia coli strain JM107 [39] was used for isolation of plasmids. Bacterial growth in DS nutrient broth or TSS 0.5% (w/v) glucose-0.2% (w/v) NH4Cl minimal medium was as described [2]. The TSS medium was supplemented as indicated with a mixture of 16 amino acids [40]. This mixture contained all amino acids commonly found in proteins (all concentrations in μg/ml) except for glutamine, asparagine, histidine, and tyrosine: glutamate-Na, 800; aspartate-K, 665; serine, 525; alanine, 445; arginine-HCl, 400; glycine, 375; isoleucine, leucine, and valine, 200 each; methionine, 160; tryptophan, 150; proline, threonine, phenylalanine, and lysine, 100 each; cysteine, 40. In some experiments, ILV were omitted from the amino acid-containing medium.
Tab. 4. B. subtilis strains used. 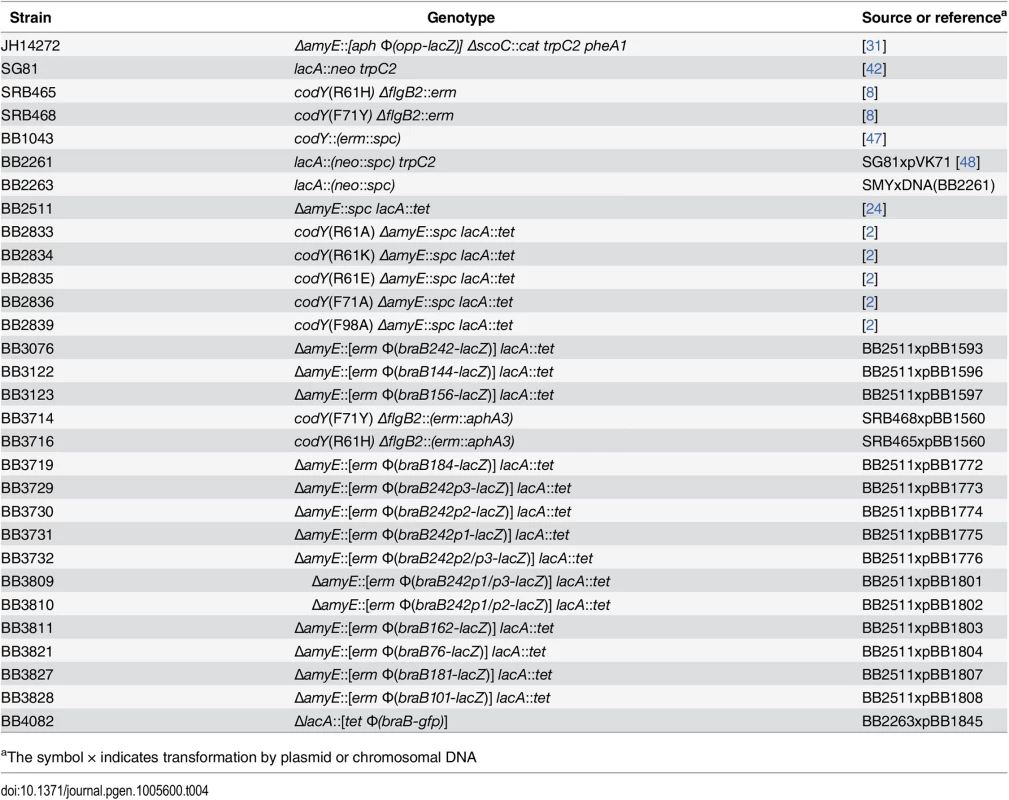
aThe symbol × indicates transformation by plasmid or chromosomal DNA DNA manipulations
Methods for common DNA manipulations, transformation, primer extension, and sequence analysis were as previously described [24, 41]. All oligonucleotides used in this work are described in Table 5. Chromosomal DNA of B. subtilis strain SMY or plasmids constructed in this work were used as templates for PCR. All cloned PCR-generated fragments were verified by sequencing.
Tab. 5. Oligonucleotides used.a 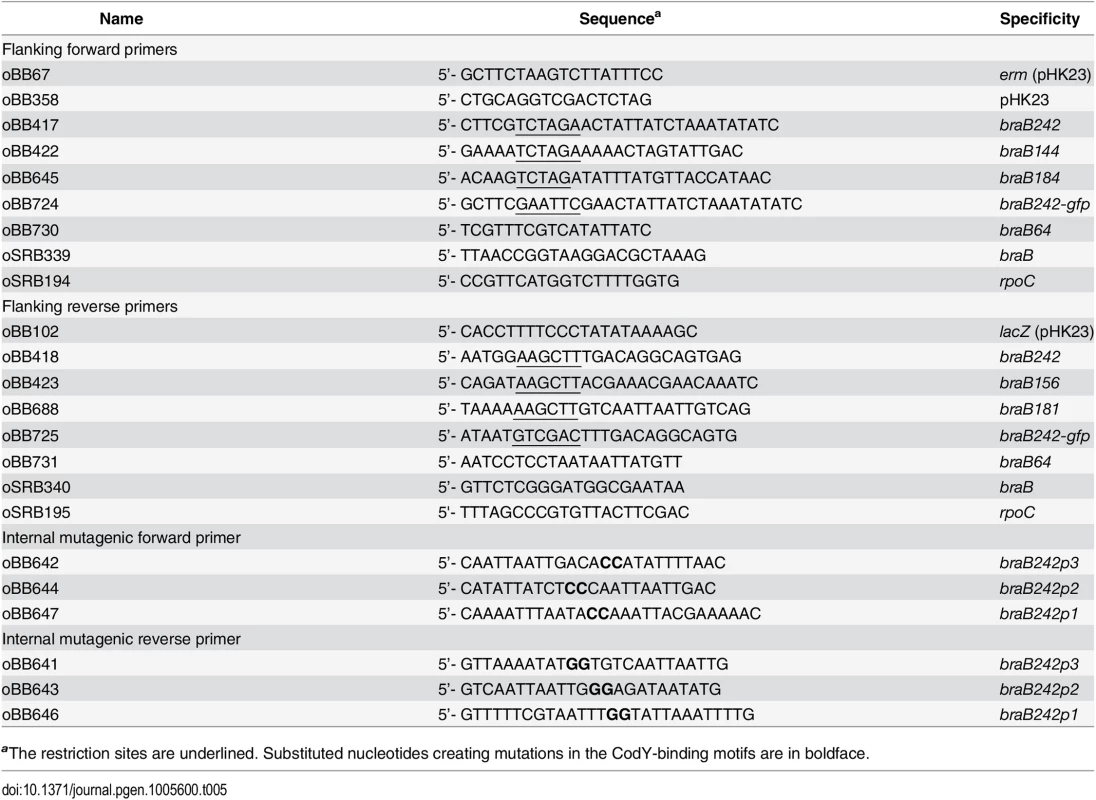
aThe restriction sites are underlined. Substituted nucleotides creating mutations in the CodY-binding motifs are in boldface. Construction of transcriptional braB-lacZ fusions
Plasmid pBB1593 (braB242-lacZ) was created by cloning the XbaI - and HindIII-treated PCR product in an integrative plasmid pHK23 (erm) [24]. The 0.24-kb braB PCR product, containing the entire braB regulatory region, was synthesized with oBB417 and oBB418 as primers. Plasmids pBB1596 (braB144-lacZ) or pBB1772 (braB184-lacZ), containing the braB regulatory region truncated from the 5’ end, were constructed in a similar way using oBB422 or oBB645, respectively, instead of oBB417. Plasmids pBB1597 (braB156-lacZ) and pBB1807 (braB181-lacZ), containing the braB regulatory region truncated from the 3’ end, were created as pBB1593 but using oBB423 or oBB688, respectively, instead of oBB418. Plasmids pBB1803 (braB162-lacZ), pBB1804 (braB76-lacZ), and pBB1808 (braB101-lacZ), in which the braB regulatory region was additionally truncated at the 5’ end, were constructed as pBB1593, pBB1597, and pBB1807, respectively, but using the ApoI and HindIII-digested PCR products that were cloned in pHK23, treated with EcoRI and HindIII.
B. subtilis strains carrying various lacZ fusions at the amyE locus (Table 4) were isolated after transforming strain BB2511 (amyE::spc lacA) with the appropriate plasmids, by selecting for resistance to erythromycin, conferred by the plasmids, and screening for loss of the spectinomycin-resistance marker, which indicated a double crossover, homologous recombination event. Strain BB2511 and all its derivatives have very low endogenous β-galactosidase activity due to a null mutation in the lacA gene [42].
Mutations in the CodY-binding sites
Plasmids pBB1773 (braB242p3-lacZ), pBB1774 (braB242p2-lacZ), and pBB1775 (braB242p1-lacZ), containing 2-bp substitution mutations in CodY-binding motifs, were constructed as described for pBB1593 using fragments generated by two-step overlapping PCR.
In the first step, a product containing the 5’ part of the braB regulatory region was synthesized by using oligonucleotide oBB417 as the forward primer and mutagenic oligonucleotide oBB641, or oBB643, or oBB646 as the reverse primer. A product containing the 3’ part of the braB regulatory region was synthesized by using mutagenic oligonucleotides oBB642, or oBB644, or oBB647 as the forward primer and oligonucleotide oBB418 as the reverse primer. The PCR products were used in a second, splicing step of PCR mutagenesis as overlapping templates to generate a modified fragment containing the entire braB regulatory region; oligonucleotides oBB417 and oBB418 served as the forward and reverse PCR primers, respectively.
Plasmid pBB1776 (braB242p2/p3-lacZ), pBB1801 (braB242p1/p3-lacZ), and pBB1802 (braB242p1/p2-lacZ), containing two mutations, each, were constructed in a similar way, but using a plasmid, containing one of the mutations, pBB1773 or pBB1774, as template for PCR.
Truncated plasmids, containing mutations in the braB regulatory region, were constructed in the same way as plasmids without mutations.
A conversion plasmid for replacing the aphA3 marker for the erm marker, originating from Tn917, was constructed by cloning the 1.5-kb SmaI-StuI fragment of pDG782 [43] into the SnaBI site of pJPM8 [44]. In the resulting plasmid, pBB1560, the aphA3 gene of pDG782, conferring resistance to kanamycin or neomycin, is flanked by 5’ and 3’ parts of the erm cassette of pJPM8; the orientation of the aphA gene coincides with that of erm.
Labeling of DNA fragments
The PCR products containing the regulatory region of the braB gene were synthesized using braB-specific oliginucleotides or vector-specific oligonucleotides oBB67 or oBB358 and oBB102, as the forward and reverse primers, respectively. The reverse primer for each PCR reaction (which would prime synthesis of the template strand of the PCR product) was labeled using T4 polynucleotide kinase and [γ-32P]-ATP. oBB67 and oBB358 start 96 bp or 12 bp upstream of the XbaI site (and 112 bp or 28 bp upstream of the EcoRI site) used for cloning, respectively, and oBB102 starts 36 bp downstream of the HindIII site that serves as a junction between the promoters and the lacZ part of the braB fusion.
The procedures for gel shift and DNase I footprinting experiments were as described [19].
Construction of a translational braB-gfp fusion and fluorescence microscopy
A 0.24-kb braB PCR product, containing the entire braB regulatory region, was synthesized with oBB724 and oBB725 as primers. Plasmid pBB1845 (braB242-gfp) was created by cloning the EcoRI - and SalI-treated PCR product between the EcoRI and XhoI sites of an integrative plasmid pMMB759 (tet), containing a gene encoding a monomeric version (A206K) of GFPmut2 [45]. The braB insert within pBB1845 was identical to the insert in pBB1593 (braB242-lacZ). B. subtilis strain BB4082 carrying the braB242-gfp fusion at the lacA locus was isolated after transforming strain BB2263 (lacA::spc) with pBB1845, by selecting for resistance to tetracycline, conferred by the plasmid, and screening for loss of the spectinomycin-resistance marker, which indicated a double crossover, homologous recombination event.
Cells, containing the braB242-gfp fusion, were grown until mid - to late-exponential phase in TSS + 16 aa medium, centrifuged and resuspended in TSS medium at OD600≈3. The images were collected at a 1,500 ms exposure time using the 100x (1.3 N.A.) objective of the Zeiss Axio Observer.Z1 fluorescent microscope (Zeiss) with the Colibri.2 LED light source, and the ORCA-R2 digital charge-coupled device camera (C10600, Hamamatsu). Zen Pro 2012 software (Zeiss) was used to acquire, view, and analyze the images.
Protein purification
CodY-His5 and His6-ScoC were purified to near homogeneity as described previously [19, 24].
Enzyme assays
β-Galactosidase specific activity was determined as described previously [46].
Real-time RT-PCR
RNA isolation, DNA depletion, and cDNA synthesis were performed as previously described [14]. Quantitative, real-time RT-PCR was used to measure steady state braB transcript abundance during exponential growth using oligonucleotides oSRB339 and oSRB340 as described [14], except that we used B. subtilis strain SMY chromosomal DNA to generate the standard curve. rpoC transcript was used to normalize mRNA abundance.
Zdroje
1. Belitsky BR. Role of branched-chain amino acid transport in Bacillus subtilis CodY activity. J Bacteriol. 2015;197(8):1330–8. doi: 10.1128/JB.02563-14 25645558
2. Belitsky BR, Sonenshein AL. Contributions of multiple binding sites and effector-independent binding to CodY-mediated regulation in Bacillus subtilis. J Bacteriol. 2011;193(2):473–84. doi: 10.1128/JB.01151-10 21097623
3. Sonenshein AL. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol. 2005;8(2):203–7. 15802253
4. Sonenshein AL. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol. 2007;5(12):917–27. 17982469
5. Belitsky BR, Gustafsson MC, Sonenshein AL, Von Wachenfeldt C. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J Bacteriol. 1997;179(17):5448–57. 9287000
6. Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, et al. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J Bacteriol. 2003;185(6):1911–22. 12618455
7. Belitsky BR, Sonenshein AL. Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc Natl Acad Sci U S A. 2013;110(17):7026–31. doi: 10.1073/pnas.1300428110 23569278
8. Brinsmade SR, Alexander EL, Livny J, Stettner AI, Segre D, Rhee KY, et al. Hierarchical expression of genes controlled by the Bacillus subtilis global regulatory protein CodY. Proc Natl Acad Sci USA. 2014;111(22):8227–32. doi: 10.1073/pnas.1321308111 24843172
9. Guedon E, Serror P, Ehrlich SD, Renault P, Delorme C. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol. 2001;40(5):1227–39. 11401725
10. Petranovic D, Guedon E, Sperandio B, Delorme C, Ehrlich D, Renault P. Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol Microbiol. 2004;53(2):613–21. 15228538
11. Shivers RP, Sonenshein AL. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol Microbiol. 2004;53(2):599–611. 15228537
12. Ratnayake-Lecamwasam M, Serror P, Wong KW, Sonenshein AL. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 2001;15(9):1093–103. 11331605
13. Handke LD, Shivers RP, Sonenshein AL. Interaction of Bacillus subtilis CodY with GTP. J Bacteriol. 2008;190(3):798–806. 17993518
14. Brinsmade SR, Sonenshein AL. Dissecting complex metabolic integration provides direct genetic evidence for CodY activation by guanine nucleotides. J Bacteriol. 2011;193(20):5637–48. doi: 10.1128/JB.05510-11 21856856
15. Caldwell R, Sapolsky R, Weyler W, Maile RR, Causey SC, Ferrari E. Correlation between Bacillus subtilis scoC phenotype and gene expression determined using microarrays for transcriptome analysis. J Bacteriol. 2001;183(24):7329–40. 11717292
16. Dod B, Balassa G, Raulet E, Jeannoda V. Spore control (sco) mutations in Bacillus subtilis. II. Sporulation and the production of extracellular protease and α-amylase by scoC mutants. Molec Gen Genet. 1978;163 : 45–56.
17. Higerd TB, Hoch JA, Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972;112(2):1026–8. 4628743
18. Kallio PT, Fagelson JE, Hoch JA, Strauch MA. The transition state regulator Hpr of Bacillus subtilis is a DNA-binding protein. J Biol Chem. 1991;266(20):13411–7. 1906467
19. Belitsky BR, Barbieri G, Albertini AM, Ferrari E, Strauch MA, Sonenshein AL. Interactive Regulation by the Bacillus subtilis Global Regulators CodY and ScoC. Mol Microbiol. 2015;97(4):698–716. doi: 10.1111/mmi.13056 25966844
20. Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci USA. 2003;100(21):11980–5. 14530388
21. Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8(6):450–61. 17510665
22. den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J Biol Chem. 2005;280(40):34332–42. 16040604
23. Guedon E, Sperandio B, Pons N, Ehrlich SD, Renault P. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology. 2005;151(Pt 12):3895–909. 16339935
24. Belitsky BR, Sonenshein AL. Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. J Bacteriol. 2008;190(4):1224–36. 18083814
25. Belitsky BR, Sonenshein AL. Roadblock repression of transcription by Bacillus subtilis CodY. J Mol Biol. 2011;411(4):729–43. doi: 10.1016/j.jmb.2011.06.012 21699902
26. Belitsky BR, Sonenshein AL. CodY-mediated regulation of guanosine uptake in Bacillus subtilis. J Bacteriol. 2011;193(22):6276–87. doi: 10.1128/JB.05899-11 21926227
27. Slack FJ, Mueller JP, Sonenshein AL. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175(15):4605–14. 8335620
28. Villapakkam AC, Handke LD, Belitsky BR, Levdikov VM, Wilkinson AJ, Sonenshein AL. Genetic and biochemical analysis of the interaction of Bacillus subtilis CodY with branched-chain amino acids. J Bacteriol. 2009;191(22):6865–76. doi: 10.1128/JB.00818-09 19749041
29. Serror P, Sonenshein AL. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J Bacteriol. 1996;178(20):5910–5. 8830686
30. Smits WK, Hoa TT, Hamoen LW, Kuipers OP, Dubnau D. Antirepression as a second mechanism of transcriptional activation by a minor groove binding protein. Mol Microbiol. 2007;64(2):368–81. 17493123
31. Koide A, Perego M, Hoch JA. ScoC regulates peptide transport and sporulation initiation in Bacillus subtilis. J Bacteriol. 1999;181(13):4114–7. 10383984
32. Dod B, Balassa G. Spore control (sco) mutations in Bacillus subtilis. III. Regulation of extracellular protease synthesis in the spore control mutations scoC. Molec Gen Genet. 1978;163 : 57–63.
33. Perego M, Hoch JA. Sequence analysis and regulation of the hpr locus, a regulatory gene for protease production and sporulation in Bacillus subtilis. J Bacteriol. 1988;170(6):2560–7. 3131303
34. Henner DJ, Ferrari E, Perego M, Hoch JA. Location of the targets of the hpr-97, sacU32(Hy), and sacQ36(Hy) mutations in upstream regions of the subtilisin promoter. J Bacteriol. 1988;170(1):296–300. 2447063
35. Ogura M, Matsuzawa A, Yoshikawa H, Tanaka T. Bacillus subtilis SalA (YbaL) negatively regulates expression of scoC, which encodes the repressor for the alkaline exoprotease gene, aprE. J Bacteriol. 2004;186(10):3056–64. 15126467
36. Toma S, Del Bue M, Pirola A, Grandi G. nprR1 and nprR2 regulatory regions for neutral protease expression in Bacillus subtilis. J Bacteriol. 1986;167(2):740–3. 3090022
37. Derouiche A, Shi L, Bidnenko V, Ventroux M, Pigonneau N, Franz-Wachtel M, et al. Bacillus subtilis SalA is a phosphorylation-dependent transcription regulator that represses scoC and activates the production of the exoprotease AprE. Mol Microbiol. 2015;97(6):1195–208. doi: 10.1111/mmi.13098 26094643
38. Zeigler DR, Pragai Z, Rodriguez S, Chevreux B, Muffler A, Albert T, et al. The origins of 168, W23, and other Bacillus subtilis legacy strains. J Bacteriol. 2008;190(21):6983–95. doi: 10.1128/JB.00722-08 18723616
39. Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–19. 2985470
40. Atkinson MR, Wray LV Jr., Fisher SH. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J Bacteriol. 1990;172(9):4758–65. 2118500
41. Sambrook J, Fritsch EF, Maniatis TJ. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 1989.
42. Daniel RA, Haiech J, Denizot F, Errington J. Isolation and characterization of the lacA gene encoding beta-galactosidase in Bacillus subtilis and a regulator gene, lacR. J Bacteriol. 1997;179(17):5636–8. 9287030
43. Guerout-Fleury AM, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167(1–2):335–6. 8566804
44. Mueller JP, Bukusoglu G, Sonenshein AL. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992;174(13):4361–73. 1378051
45. Berkmen MB, Lee CA, Loveday EK, Grossman AD. Polar positioning of a conjugation protein from the integrative and conjugative element ICEBs1 of Bacillus subtilis. J Bacteriol. 2010;192(1):38–45. doi: 10.1128/JB.00860-09 19734305
46. Belitsky BR, Sonenshein AL. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J Bacteriol. 1998;180(23):6298–305. 9829940
47. Barbieri G, Voigt B, Albrecht D, Hecker M, Albertini AM, Sonenshein AL, et al. CodY regulates expression of the Bacillus subtilis extracellular proteases Vpr and Mpr. J Bacteriol. 2015;197 : 1423–32. doi: 10.1128/JB.02588-14 25666135
48. Chary VK, Amaya EI, Piggot PJ. Neomycin - and spectinomycin-resistance replacement vectors for Bacillus subtilis. FEMS Microbiol Lett. 1997;153(1):135–9. 9252583
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



