-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
Computational predictions of the functional impact of genetic variation play a critical role in human genetics research. For nonsynonymous coding variants, most prediction algorithms make use of patterns of amino acid substitutions observed among homologous proteins at a given site. In particular, substitutions observed in orthologous proteins from other species are often assumed to be tolerated in the human protein as well. We examined this assumption by evaluating a panel of nonsynonymous mutants of a prototypical human enzyme, methylenetetrahydrofolate reductase (MTHFR), in a yeast cell-based functional assay. As expected, substitutions in human MTHFR at sites that are well-conserved across distant orthologs result in an impaired enzyme, while substitutions present in recently diverged sequences (including a 9-site mutant that “resurrects” the human-macaque ancestor) result in a functional enzyme. We also interrogated 30 sites with varying degrees of conservation by creating substitutions in the human enzyme that are accepted in at least one ortholog of MTHFR. Quite surprisingly, most of these substitutions were deleterious to the human enzyme. The results suggest that selective constraints vary between phylogenetic lineages such that inclusion of distant orthologs to infer selective pressures on the human enzyme may be misleading. We propose that homologous proteins are best used to reconstruct ancestral sequences and infer amino acid conservation among only direct lineal ancestors of a particular protein. We show that such an “ancestral site preservation” measure outperforms other prediction methods, not only in our selected set for MTHFR, but also in an exhaustive set of E. coli LacI mutants.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000968
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000968Summary
Computational predictions of the functional impact of genetic variation play a critical role in human genetics research. For nonsynonymous coding variants, most prediction algorithms make use of patterns of amino acid substitutions observed among homologous proteins at a given site. In particular, substitutions observed in orthologous proteins from other species are often assumed to be tolerated in the human protein as well. We examined this assumption by evaluating a panel of nonsynonymous mutants of a prototypical human enzyme, methylenetetrahydrofolate reductase (MTHFR), in a yeast cell-based functional assay. As expected, substitutions in human MTHFR at sites that are well-conserved across distant orthologs result in an impaired enzyme, while substitutions present in recently diverged sequences (including a 9-site mutant that “resurrects” the human-macaque ancestor) result in a functional enzyme. We also interrogated 30 sites with varying degrees of conservation by creating substitutions in the human enzyme that are accepted in at least one ortholog of MTHFR. Quite surprisingly, most of these substitutions were deleterious to the human enzyme. The results suggest that selective constraints vary between phylogenetic lineages such that inclusion of distant orthologs to infer selective pressures on the human enzyme may be misleading. We propose that homologous proteins are best used to reconstruct ancestral sequences and infer amino acid conservation among only direct lineal ancestors of a particular protein. We show that such an “ancestral site preservation” measure outperforms other prediction methods, not only in our selected set for MTHFR, but also in an exhaustive set of E. coli LacI mutants.
Introduction
Due to continuing advances in DNA sequencing technologies, our knowledge of human genetic variation is rapidly increasing. It is currently impractical to assay the biological effect of most genetic variants empirically, so computational predictions of their functional impact must play an important role in identifying potential genetic causes underlying human disease. Here, we focus on genetic variation that results in a single amino acid, or nonsynonymous, substitution in an encoded protein. Nonsynonymous changes comprise only a small fraction of known genetic variation, yet account for a disproportionately large fraction of known disease-causing variation in humans [1], [2].
It has long been recognized that amino acid substitutions that impair the function of a protein tend to involve substitution with a chemically very different amino acid [3], [4]. This is not surprising, as relatively few of the amino acids (e.g. active site amino acids of enzymes) in a protein are absolutely required for function. Most positions contribute to stabilizing the requisite structure of the active site and other binding and interaction sites, and such sites tend to be more tolerant of physico-chemically similar amino acid changes. As a result, some algorithms for predicting the functional effect of amino acid substitutions make use of measures of amino acid physico-chemical similarity. Multiple scales of physico-chemical similarity have been developed. The first, developed by Grantham [5], assigns to each possible amino acid substitution a similarity score that summarizes several physico-chemical properties of the two amino acid monomers. Grantham scores are site-independent (i.e. the substitution of a valine for an isoleucine is given the same score, regardless of the protein, or the site within the protein, in which the substitution occurs). However, it has been demonstrated that different sites vary greatly with respect to their tolerance to substitution. Miller and Kumar [4] demonstrated that human disease mutations have a strong statistical tendency to occur at evolutionarily conserved (i.e. slowly evolving) sites. Chasman and Adams [6] showed that mutation tolerance depends on several properties of the local 3D structure of the site, and this information has also been used to make site-specific predictions of the functional effects of substitutions [6], [7]. Such approaches are limited to proteins of known three-dimensional structure, or whose structure can be modeled based on a related protein.
The explosion in comparative genomics data has enabled site-specific predictions that are based on patterns of evolutionary substitution and that do not depend on structural information [8]–[12]. Given enough homologs, there is often considerable information about which substitutions have been “accepted” at a given site across the homologous proteins [13]. If a substitution was accepted (i.e. attained a high frequency in a population), the substitution had no appreciable negative impact on the fitness of the organism that harbored it, and therefore probably did not negatively impact protein function. The implicit assumption in deriving predictions based on this information is that accepted substitutions in one protein would also be accepted in its homologs; i.e. all homologs are assumed to be evolving under the same selective constraints. This assumption has recently been questioned for paralogous genes (genes that diverged by a gene duplication event presumably followed by some degree of functional divergence), and several groups have improved predictions by restricting analysis to orthologs, rather than paralogs [11], [14], [15]. However, it is not known to what extent orthologous genes share selective constraints, particularly after they have diverged substantially. In this paper, we test the utility of orthologs in the prediction of the functional effects of substitutions in the prototypical human enzyme methylenetetrahydrofolate reductase (MTHFR), which catalyzes a critical step necessary for the remethylation of homocysteine to methionine [16], [17].
We selected MTHFR as a model for testing these assumptions for several reasons. From a phenotypic standpoint, even mild defects in MTHFR can lead to metabolic imbalances that increase disease risk and thus would be subject to selective pressures. For example, a common polymorphism (677C→T) that changes an alanine at position 222 to a valine (referred to as A222V hereafter; [18]) impairs enzyme activity by 50% and leads to elevated plasma homocysteine levels in individuals with inadequate folate intakes [16]–[18]. Homocysteine may be a risk factor for several common diseases including cardiovascular disease [19] and neural tube defects [20]. From an evolutionary standpoint, MTHFR was present in the Last Universal Common Ancestor (LUCA) of all extant life, and has been recognizably conserved in nearly every organism sequenced to date. Since there appear to have been few gene duplications, the gene is present in single copy in nearly all animals, making these homologous genes unambiguous orthologs. Finally, a robust yeast complementation assay has been developed, allowing us to experimentally test the function of variants of MTHFR [21].
In addition, from MTHFR resequencing studies in random populations, we and others [21], [22] have identified many novel, low frequency nonsynonymous variants that affect enzyme function. Significantly, like the common A222V variant, many of these are remedial by folate supplementation [21]. As the pace of genetic discovery rapidly increases, there will surely be many novel MTHFR enzyme variants identified. Given the metabolic significance of MTHFR and the ability to nutritionally correct defective alleles, it would be desirable to predict in silico the functional impact of nonsynonymous mutation rather than empirically determine the effect of each variant individually. Furthermore, using MTHFR as a model enzyme may illustrate more general rules for computationally predicting the impact of amino acid substitution on protein function.
To this end, we constructed 35 MTHFR enzyme variants (34 of which differ from the major allele by a single amino acid and one represents a reconstruction of the human-macaque ancestral allele by a 9-site change) and tested them in a cell-based functional assay based on complementation in the yeast Saccharomyces cerevisiae [21]. Specifically, we targeted sites that have been conserved to varying degrees, and at these sites we created substitutions that have been accepted in at least one known ortholog of human MTHFR. We found, somewhat surprisingly, that most of our selected mutants of MTHFR were not tolerated in the human enzyme despite their presence in orthologous enzymes. These data suggested that the orthologous genes are evolving under different evolutionary constraints than human MTHFR. To remove potentially spurious signals from substitutions accepted in orthologs after their divergence from the human lineage, we subsequently restricted our analysis to only direct lineal ancestors of human MTHFR. Thus, we classified each site by its conservation only in direct ancestors of the human enzyme, which we refer to as “preservation” among ancestors to distinguish it from the common usage of “conservation” among homologs. The results indicated that the extent to which sites are preserved in lineal ancestors was a good predictor for whether that position was tolerable to change, not only for human MTHFR but also in the LacI protein from E. coli.
Methods
Quantitative Yeast Complementation Assay for MTHFR Function
The specific aspects of the assay have been described previously [21]. Briefly, a yeast strain was deleted for both the endogenous MTHFR (MET13, necessary for methionine synthesis) and for a folate biosynthetic enzyme (FOL3, dihydrofolate synthetase). The resulting strain (met13::KanMX fol3::KanMX) requires folate supplementation in the media and expression of a functional human MTHFR for growth in absence of methionine. Under the conditions of the assay, the rate at which the cells grow reflects the cellular activity of the MTHFR variant interrogated [21], [23] for any given level of folate supplementation. Following transformation of the parent strain by each individual variant, growth in the absence of methionine was recorded by optical density (OD595) measured over time in low-volume cultures supplemented with 25 ug/ml folinic acid [21]. To assign a growth-rate metric for quantitative comparisons between alleles, absorbance values were log10-transformed and a maximum slope was calculated which represented the maximal rate of cell growth (see Figure 1). Although this metric was not a cell doubling rate per se, slope calculations were easier to integrate into data handling, and relative differences between variants were exactly the same as they would be for doubling rates. All variants were tested in two or more experiments involving at least 5 replicates per experiment.
Fig. 1. Example growth curves from which rate metrics were calculated. 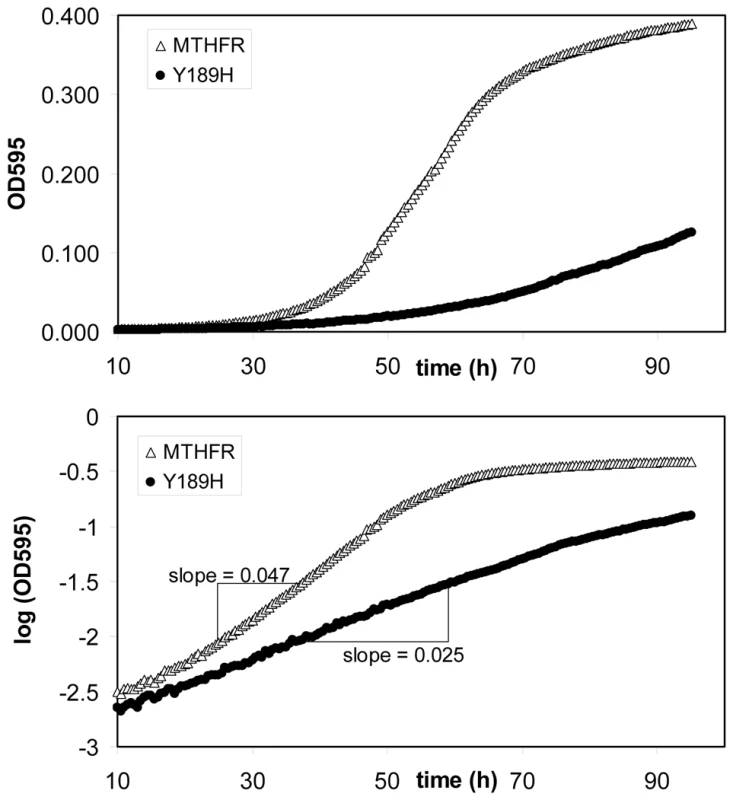
Shown are two examples (major MTHFR allele (open triangle); Y189H substitution variant (closed circle)) where growth in liquid culture was tracked over time, according to Methods. The upper panel shows absorbance (OD595) values and the lower panel shows the log10 transformation of the same absorbance reads. Log10-transformed data were used to calculate maximum slopes that served as growth-rate metrics. Construction of Enzyme Variants
The yeast plasmid driving expression of human MTHFR variants under the inducible GAL1 promoter has been described previously [21], [23]. This plasmid served as the backbone to reconstruct all MTHFR variants by site-directed mutagenesis using QuikChange kits (Stratagene). All variants were verified by sequencing the entire coding region of MTHFR.
Defining Functional Versus Impaired Alleles
Replicate data sets for each substitution variant were compared against a positive control (major MTHFR allele) and a known impaired variant (A222V allele) as a negative control, using two different statistical criteria to evaluate whether variants were significantly different from either control. Significance was determined against each control using both a Student's t-test with a Bonferroni-corrected p-value (p<0.0014 for 35 pairwise comparisons) or Dunnett's test for comparing multiple treatments against a single control (alpha<0.01; [24]). Variants whose activity was not significantly different from the major allele and significantly better than the A222V allele by both statistical measures were classified as functional, whereas variants whose activity was significantly less than the major allele and not significantly better than the negative control were classified as impaired. In the cases where there was not a consensus in the comparisons, these alleles were classified as equivocal. In this way, of the 36 alleles tested in this study (including wild-type human MTHFR), 7 were classified as functional, 25 were classified as impaired and 4 were equivocal (see Figure 2). It should be noted that other statistical determinations of functionality are possible, but they do not appreciably change the results. For example, replacing Dunnett's test with False Discovery Rate analysis (q<0.01; [25]) results in the same classifications for 34 of the 36 alleles.
Fig. 2. Activities of MTHR mutants. 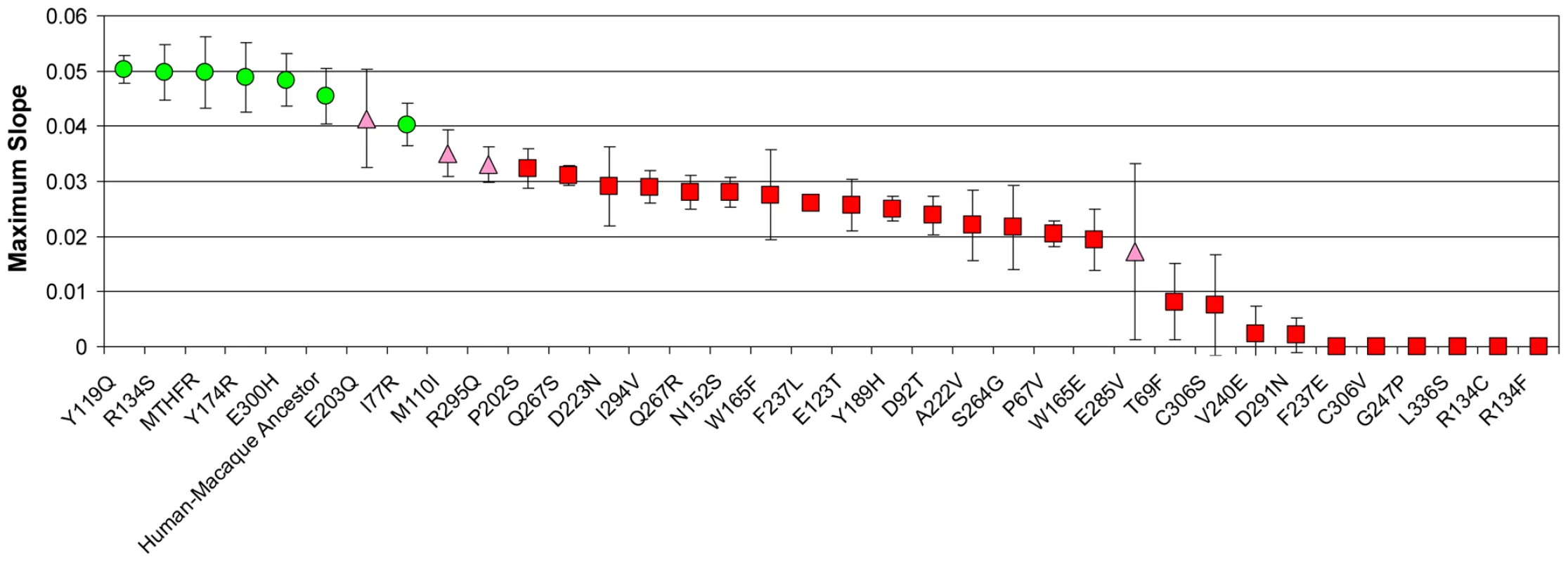
The average maximum slope (growth-rate metric) and standard deviation for each of the 36 MTHFR variants tested as in Methods. Replicate sets (N = 5) were compared against a positive control (major MTHFR allele) and a negative control (A222V allele) using 2 different statistical criteria as described in Methods. Green circles indicate changes not significantly different from the positive control and significantly better than the A222V control and indicate functionality. Red squares indicate changes significantly less active than the positive control and not significantly better than the A222V control and indicate impaired alleles. Pink triangles are classified as equivocal due to disagreement in the statistical methods. The raw replicate data and statistical metrics are in Table S1. Alignment of Orthologs, Phylogenetic Trees, and Ancestral Sequence Determination
Human MTHFR
The alignment and phylogenetic tree were from the PANTHER database [10], in which orthologs of human MTHFR were aligned using MAFFT [26], and a phylogenetic tree was constructed using the GIGA software (Thomas, submitted), which use s known species relationships to help infer the tree topology. Ancestral sequences were reconstructed for human MTHFR using the PAML software package (version 4.2; [27]) with the default parameter file for the aaml program (this uses the WAG empirical model to estimate amino acid substitution probabilities). For calculating ancestral site preservation (see below), we considered a site to have a “known” ancestral amino acid if the reconstruction probability exceeded 90%.
To more accurately reconstruct the human-macaque common ancestral sequence, we used the actual nucleotide coding sequences rather than the protein translation. We identified the predicted coding nucleotide sequences for MTHFR from the following Ensembl genomes: human, gorilla, orangutan, macaque, and mouse lemur. The sequences were aligned using MAFFT (with minor manual adjustment where necessary to maintain codon alignment), and the phylogenetic tree was assumed to match the species tree. Ancestral sequences were reconstructed using the codeml program from PAML with default parameters. This analysis does not use an empirical amino acid substitution model, but rather distinguishes between nucleotide transitions and transversions, and synonymous and nonsynonymous substitutions with a constant value of omega across the tree.
E. coli LacI
Orthologs of E. coli LacI were retrieved by a BLASTP search of the UniProt database, and choosing the top hits that sampled the bacterial clades useful for reconstructing ancestral residues. Included were several clades of enterobacteria, two clades of gamma-proteobacteria, and two actinobacteria as outgroups. A phylogenetic tree (Figure S1) was inferred using the Neighbor-Joining algorithm [28]. Ancestral sequences in the LacI family were determined using the aaml program within PAML as described above for the MTHFR family.
Results
Measuring Enzyme Function by Quantitative Complementation
We have previously shown that human MTHFR enzyme activity can be accurately measured in a simple yeast cell growth assay whereby expression of the human enzyme (or enzyme variant) is asked to complement the methionine biosynthetic defect of yeast MTHFR deletions (met13). Thus, by identifying enzyme variants that are incapable of fully restoring growth in media lacking methionine (relative to wild-type MTHFR), we have identified naturally occurring nonsynonymous polymorphisms that impair enzyme function [21]. To allow quantitative comparisons between variants in this assay, we derived a growth-rate metric by determining maximum slopes from log10 transformed growth curve data (Figure 1). In general, growth rates measured in this manner are directly reflective of the cellular activity of the variant assayed [21].
Intracellular folate levels can remedy defective alleles of MTHFR in humans and this behavior can be recapitulated in the yeast assay [21]. While this is attractive from a therapeutic perspective, this suppression may confound functional assessment of mutant alleles since high folate supplementation can mask enzymatic defects. To avoid this, cultures were supplemented with a level of folate that was mildly rate-limiting for growth (25 ug/ml folinic acid), which allows subtle changes in MTHFR activity to be reflected in the growth readout [21]. Under these conditions, the growth rate driven by the major human allele is approximately 91% of that when folate is not rate-limiting (data not shown). The activities of the complete collection of 36 variants were assayed in this way (Figure 2; raw replicate data for these experiments is in Table S1).
Evolutionary Validation of Cell-Based Functional Determinations: Expected Behaviors of Highly Conserved Sites and a Reconstructed Recent Ancestor
As described in Methods, we used the unchanged major allele of MTHFR (wild type) as a positive control and the impaired A222V allele as a negative control to define growth-rate boundaries for distinguishing functional from impaired variants. Although the A222V variant is not a complete loss-of-function allele, it was chosen as a negative control to effectively set the threshold of activity that distinguishes functional from impaired variants. This change has been well documented to cause a 50% loss in intrinsic activity [16], [22] that, importantly, can lead to metabolic dysfunction and thus would be subject to selective pressures. Even so, this variant has reached a high frequency in the human population (∼30% global frequency; http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=1801133). Thus, selective pressures against this allele must be minimal and this level of activity is likely to be near the borderline of impairment, unless there is some undetected heterozygote advantage.
To establish that the assay and this classification scheme can reliably distinguish functional from impaired variants of MTHFR, and that, at least in straightforward cases, analysis of orthologs accurately predicts whether a given variant will be functional or impaired, we first tested the major human MTHFR allele and a 9-site mutant that “resurrects” the human-macaque ancestral MTHFR (S9G, L53M, Y89F, R132C, I496V, V578I, R594Q, T639A A650E). As expected, we found both human MTHFR and the putative human-macaque ancestral MTHFR to complement the yeast met13 defect with approximately the same, relatively high level of activity (Figure 2). On the other hand, the A222V substitution resulted in significantly reduced activity. We previously demonstrated this allele is defective in this assay [21] and demonstrate here that the quantitative growth defect (rate metric = 0.022 for A222V vs. 0.0497 for the major MTHFR allele) was in good agreement with the reduced level of activity.
We subsequently constructed four single-site mutants of MTHFR that each alter a site that is highly conserved in nearly all orthologs and, thus, were expected to be functionally impaired (see Table S2 for sequence alignments at all sites interrogated in this study). For example, P67V substitutes a chemically very dissimilar amino acid at a site that is conserved among all known eukaryotic (and some prokaryotic) orthologs of human MTHFR and, as expected, was classified as impaired (rate metric = 0.02). D291N targets an aspartic acid residue present in nearly all eukaryotes and despite the chemical similarity of aspartate and asparagine, the D291N variant was severely impaired (rate metric = 0.002). Lastly, we tested two different substitutions of R134, a site at which no single amino acid is highly conserved, but at which only polar amino acids are observed across nearly all MTHFR orthologs (see Figure 3). R134C and R134F each substitute a hydrophobic amino acid and each result in a drastically impaired enzyme (both rate metrics = 0). This is consistent with the prediction that negative selection against hydrophobic amino acids had occurred at this site, even though the identity is not strictly conserved. These examples confirm that our assay is capable of distinguishing between functional and impaired variants, and that, at least in very straightforward cases, evolutionary conservation among orthologs can also make this distinction.
Fig. 3. Phylogenetic tree and ancestral allele determination from orthologs of human MTHFR. 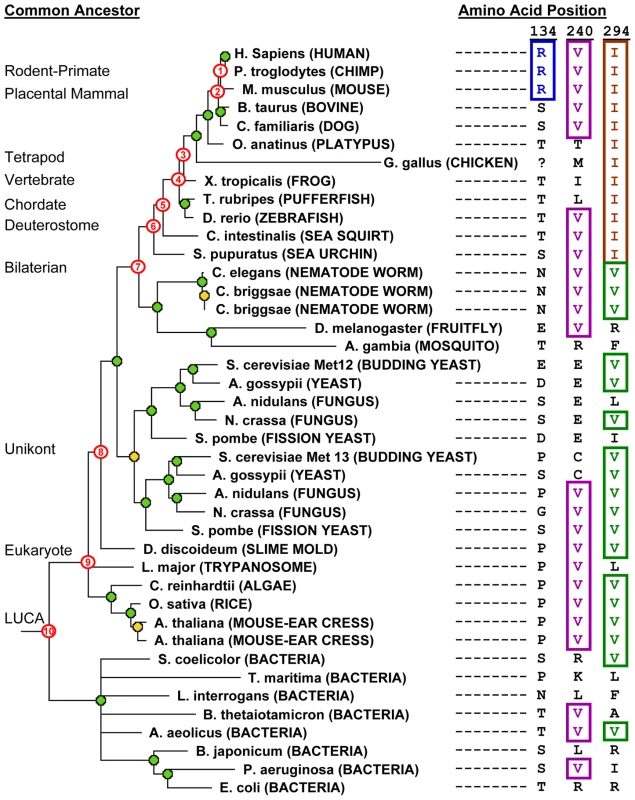
Tree: MTHFR sequences from modern-day species are indicated. Database identifiers for these entries are listed in Table S2. Gene duplication events are shown with orange circles, and speciation events with green circles. Nodes numbered in red correspond to ancestral branch points in the human MTHFR lineage. Longer branch lengths indicate faster evolutionary rate. The chicken sequence was given an arbitrary, long branch length because it is a sequence fragment and the actual branch length could not be accurately determined. Ancestral allele determinations: The right columns show the amino acids found in the modern-day sequences corresponding to positions 134, 240 and 294 in human MTHFR. These are shown to illustrate how ancestral sites are determined and, consequently, how long the identity of the site in the human enzyme has been preserved in the human lineage (see text for details). Testing Single-Site Substitutions that Are Accepted in Orthologs of Human MTHFR
We then selected 30 additional site-specific mutants to interrogate, based on an alignment of orthologs of human MTHFR. Each of the 30 sites was conserved among primates, but diverged to varying degrees in more distant lineages/species. Furthermore, at these sites we introduced substitutions that were accepted in at least one known ortholog (Table 1). We reasoned that these would be relatively difficult cases for prediction methods since this set represents sites that are both partially conserved and partially divergent and little is known how to balance these signals.
Tab. 1. Amino acid substitutions from human MTHFR orthologs tested for functional impact. 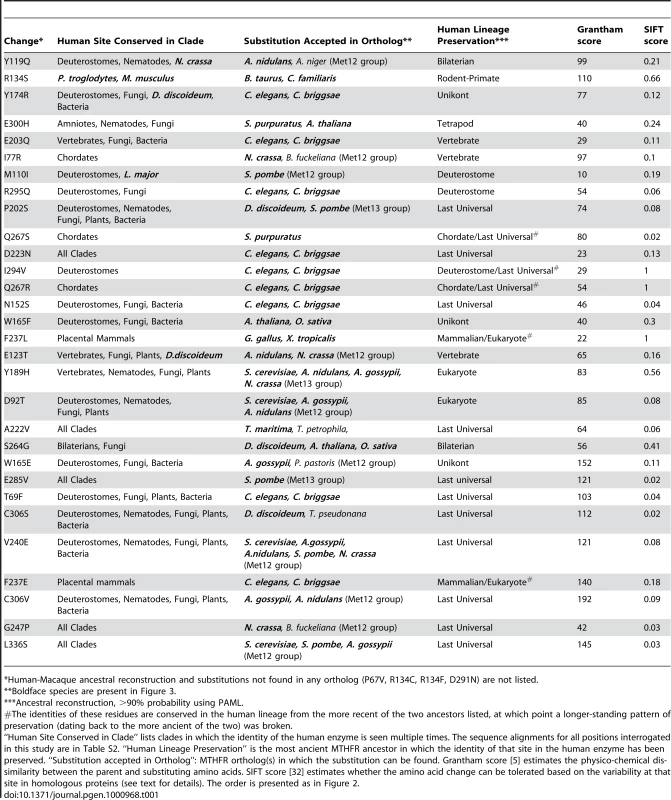
*Human-Macaque ancestral reconstruction and substitutions not found in any ortholog (P67V, R134C, R134F, D291N) are not listed. From a practical standpoint, we limited our changes to the catalytic domain of MTHFR, which comprises the N-terminal half of the 656 amino acid protein (NCBI protein reference NP_005958). This region is more prone to deleterious changes [21], [22] and most mutations that result in severe clinical phenotypes occur in the catalytic domain (http://www.hgmd.cf.ac.uk). In addition, although all genes shown in the phylogenetic tree (Figure 3) are orthologous to human MTHFR by the definition of Fitch [29], we did not choose amino acids accepted in insect orthologs. The insect lineage appears to have an accelerated evolutionary rate and has lost the C-terminal regulatory domain found in other eukaryotes, thus their functional orthology is questionable. Furthermore, we distinguished between the orthologs resulting from the gene duplication event in fungi as belonging to either the Met13 group (defined by S. cerevisiae Met13) or the Met12 group (defined by S. cerevisiae Met12). Evidence from S. cerevisiae and S. pombe suggests that the Met13 group of enzymes may be functionally more similar to human MTHFR than those in the Met12 group, which have diverged to a slightly greater degree [30], [31].
According to our functional definition, 21 mutants in this set of 30 had impaired activity, 5 were functional, and 4 were equivocal (Figure 2). The relatively large number of impaired mutants was unexpected given the results of computational prediction methods. For example, the SIFT (Sorting Intolerant From Tolerant) algorithm [32] predicted that only 7 mutants would be impaired using the recommended 0.05 score threshold (T69F, N152S, G247P, Q267S, E285V, C306S, L336S; Table 1). SIFT calculates a score for each mutant based on the substitutions observed at a given site across a set of homologous proteins. Like most existing methods that incorporate homology as a predictive parameter, SIFT assumes that orthologs are evolving under similar evolutionary constraints. Since all of our substitutions appear in an ortholog of human MTHFR, it is not surprising that SIFT's predictions are strongly biased toward functional substitutions. Concordant with this view, a second commonly used predictive algorithm, Pmut [12], also predicted 80% of these substitutions to be functional (data not shown). Since most changes were accepted in more than one closely related ortholog (Table 1), it is unlikely that the data is skewed by infrequent alleles or spurious mutation in the individual MTHFR genes sequenced.
SIFT displayed only 42% accuracy in discriminating functional from impaired MTHFR alleles when considering only those changes unambiguously classified as functional or impaired in the yeast assay (i.e. ignoring the 4 equivocal calls in this 30 mutation set; Figure 4A). Since all functional alleles had a SIFT score > = 0.09, a threshold empirically set at this point (rather than the 0.05 recommended) would have increased overall accuracy to 62%. However, even this threshold would have still resulted in significant over-prediction of functional alleles (Figure 4A).
Fig. 4. Accuracy of discrimination between functional and impaired variants by different methods. 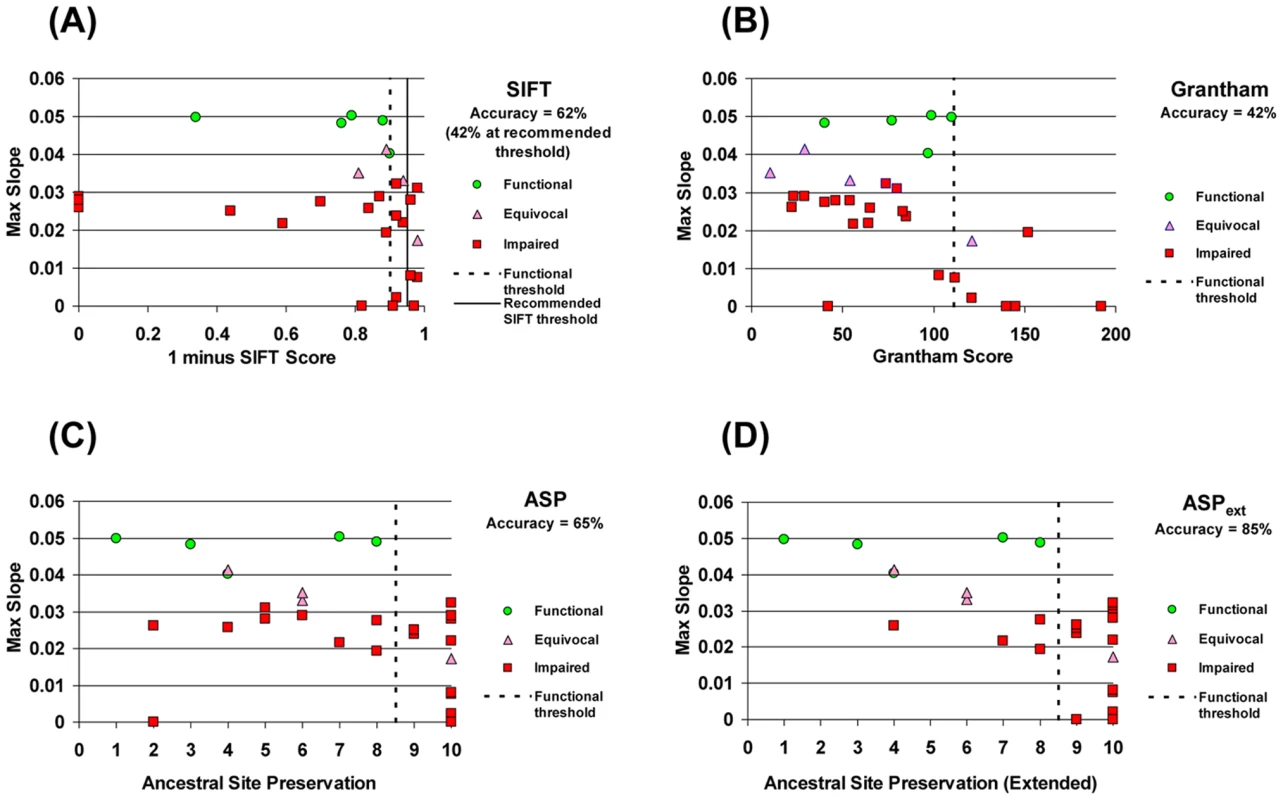
Growth-rate metrics for the 30 variants in Table 1 plotted against scores/classifications from various methods that estimate functional impact. The accuracy of each method was determined by calculating the number of mutations correctly called as functional or impaired divided by the number of mutations unambiguously classified by experimental data. Binning of mutations was determined by using a threshold empirically defined by the functional alleles (dashed vertical line in each panel) to define the functional (left of line) and impaired (right of line) bins. (A) SIFT score; note that the graph plots (1–score) to facilitate comparison with the other methods. All functional variants have a SIFT score >0.09 which, when used as a threshold results in a classification accuracy of 62%. The recommended threshold of 0.05 (solid vertical line) results in a lower classification accuracy (42%). (B) Grantham scale of amino acid dissimilarity between wild-type and substituted amino acid. (C) Ancestral Site Preservation (ASP) measure, using inferred ancestral sequences of human MTHFR. Numbers on the x-axis correspond to increasingly ancient ancestors of human MTHFR as defined by the nodes in Figure 3. (D) Ancestral Site Preservation Extended (ASPext) measure. If a site was preserved in the ancestral lineage for a long period before being substituted by the current-day amino acid, the more ancient ancestor is used to define preservation at this site. The preservation measure for 5 variants is shifted by this criterion (see Table 1). We next assessed a completely different distance metric than evolutionary conservation. The Grantham score provides a measure of the physico-chemical dis-similarity between any pair of amino acids [5]. This measure was evaluated against the observed growth rates for each of these 30 mutations (Figure 4B). Consistent with previous results [4], there was a significant negative correlation (R = −0.48, Pval = 0.007), indicating that, on average, the larger the physico-chemical difference between the wild-type and substituting amino acid, the greater the functional impairment. However, despite this correlation, the Grantham differences were not very useful for discriminating functional from impaired mutations: even a threshold optimized for our dataset yields an accuracy of only 42% (Figure 4B). Similarly to SIFT, such a stratification of the data would have led to an over-prediction of functional alleles. Thus, neither of these tools, which analyze and estimate the impact of amino acid substitution in very different ways, appeared particularly accurate on this dataset.
Distinguishing Functional from Impaired Mutants in MTHFR: the Ancestral Site Preservation (ASP) Measure
Our finding that most of the mutants we tested were impaired, despite the presence of these substitutions in an orthologous protein, suggests that in these cases the site is not under the same selective constraint in human MTHFR as it is in the ortholog. In other words, selective constraints on a given site may differ between lineages in the phylogenetic tree. Therefore it may be more relevant to infer selective constraints by considering only the direct ancestral lineage of a given protein, rather than all lineages (as in methodologies such as SIFT that consider homologous sequences from other organisms). We therefore defined a measure (Ancestral Site Preservation or ASP) that is determined by the most distant ancestor in which a given site is preserved. To determine this, we first constructed a phylogenetic tree for the family and inferred ancestral sequences at specific nodes in the tree (Figure 3). We then traced back among the direct ancestors of the current-day sequences to infer how long a given amino acid had been preserved at a particular site. The ASP score is simply the (relative) number of the most ancient ancestor in which the identity of the site in human MTHFR is still preserved.
To illustrate this ASP score, Figure 3 provides examples of allele determinations in human MTHFR ancestors at three instructive positions in the human enzyme. Site 134 aligns an arginine (R) in human, chimp and mouse MTHFR and, thus, is inferred to be present in the rodent-primate ancestor (node #1 on tree). However, because no other orthologs align R at this position, we cannot infer the ancestral preservation prior to node #1, and the ASP score = 1. The valine (V) at position 240, on the other hand, is extremely well-preserved in ancestors of human MTHFR. V is inferred to be present in the placental mammal ancestor (node #2 on tree) since all descendants align a V at that position. Furthermore, from analyzing increasingly distant outgroups from this node, the presence of a V in vertebrates (zebrafish), deuterostomes (sea urchin, sea squirt), worms and insects strongly suggests the presence of a V back to the bilaterian ancestor (node #7). Analysis of even more distant outgroups shows that V is also aligned at this position in several fungal orthologs, plants, Leishmania, Dictyostelium and several distant bacterial species. The most parsimonious inference, therefore, is that V240 is preserved in all ancestors of human MTHFR back to the LUCA (node #10), giving an ASP score of 10. The third example, position 294, reveals an interesting preservation pattern whereby preservation of isoleucine (I) from a relatively recent ancestor (inferred from the I present in all deuterostome species) broke a longer-standing pattern of ancestral preservation (V is inferred to be present in all more ancient human ancestors: bilaterian through LUCA; nodes 7–10).
For our set of mutants, we found that if the identity of the site was strictly preserved from at least as far back as the Most Recent Common Ancestor (MRCA) of all eukaryotic MTHFRs (node #9, Figure 3), a mutation at that site impaired human MTHFR. On the other hand, all benign changes that resulted in a functional enzyme were at sites whose identity was preserved from no further than the MRCA of all unikont MTHFRs (node #8 in Figure 3). Thus, the unikont MTHFR ancestor empirically defined the functional threshold for the ASP measure (Figure 4C). The ASP measure showed an accuracy of 65% among all unambiguously classified changes and performed as well or better than Grantham scores or SIFT for predicting functional and impaired variants in this dataset.
Furthermore, we observed that five impaired mutants that were mispredicted by ASP (F237L, F237E, Q267R, Q267S, I294V) occurred at 3 positions with a very particular pattern of ancestral preservation in which one amino acid was strictly preserved beginning with the eukaryotic MRCA or LUCA, and substituted only once after that. This is a very interesting pattern in light of our results and one that is not common across MTHFR sites in general. As mentioned above, isoleucine (I) 294 in human MTHFR is an instructive example (Figure 3). Valine is strictly preserved at this site in the human lineage beginning with the LUCA, though it does diverge in some other lineages (e.g. L. major). This strongly suggests the site was under selective constraint in the ancient ancestors of human MTHFR. However, this site was fixed as isoleucine beginning with the MRCA of all deuterostome MTHFRs, suggesting that this ancestral constraint had changed in the deuterostome MTHFR lineage, possibly due to positive selection. Indeed, a reversion to the ancestral amino acid, I294V in human MTHFR, resulted in a significant impairment of function (Figure 2). Likewise at position 267, glutamine (Q) in the human enzyme is preserved since the chordate common ancestor, which broke the long-standing preservation of arginine (R) at this site since the LUCA (Table 1; see Table S2 for alignment). At position 237, phenylalanine (F) is preserved since the placental mammal ancestor, which broke the long-standing preservation of leucine (L) from the eukaryote MRCA. As for I294V, changes reverting to the more ancient amino acid (Q267R and F237L) impaired human MTHFR, even though L237 is accepted in the recently diverged chicken ortholog (Table 1, Figure 2).
The pattern whereby a long-standing ancestral preservation is broken only once by a more recent ancestor in the human lineage indicates that such positions have been under selective constraint for long periods of time and that these constraints changed during evolution of the lineage. If so, these sites are likely to be more sensitive to substitutions even though the site in the human enzyme has been preserved from only a relatively recent ancestor. Thus, to capture the importance of such sites, we further define the Ancestral Site Preservation Extended measure (ASPext). The ASPext is similar to the ASP but traces back to an earlier ancestor if there was a previous long-standing (eukaryotic MRCA or earlier) strict preservation at that site. ASPext significantly improves the ability to predict functional impairment among our set of MTHFR mutants (85% accuracy; Figure 4D).
Generalizing ASP and ASPext to Other Proteins: Predicting Functional and Impaired Mutants of LacI
To determine if the ASP would be a useful measure for predicting functional and impaired variants more generally, we tested its predictive ability against the extensive E. coli lac repressor (LacI) substitution dataset [33], [34]. LacI is a standard test-case for prediction algorithms because it contains nearly comprehensive substitution data: the functional impact of 12–13 nonsynonymous mutants at each of 328 sites in the LacI protein was determined by in vivo assay. Furthermore, this data set, comprising a total of 4041 single-site mutations, was used as a training set for the SIFT algorithm [35], so SIFT has been optimized to perform on these data. We reasoned that if ASP could perform well on LacI predictions, despite not being optimized for this specific data set, this would be good evidence of its more general utility. We would not necessarily expect ASP to perform as well as SIFT, simply because ASP makes relatively crude predictions: a site is predicted to be either completely tolerant (all mutations are predicted to be functional) or completely intolerant (all mutations are predicted to be impaired). On the other hand, SIFT can predict different outcomes for different amino acids at each site.
We were somewhat surprised, then, to find that both ASP and ASPext actually out-performed SIFT on predictions of LacI mutants (Table 2). In this analysis, we constructed a phylogenetic tree for LacI in bacterial species and inferred the ancestral identities of amino acids at divergence points in the tree, as we did for MTHFR (Figure S1). As described above, we empirically defined the common ancestor to unikonts as the “threshold ancestor” for stratifying functional and impaired alleles of MTHFR: all benign changes were at sites preserved for this length of time or less. Interestingly, the prokaryotic threshold ancestor that yielded the highest prediction accuracy was the common ancestor of LacI proteins in E. coli and Enterobacter cancerogenus. LacI proteins in these two species share 52% identity, which is approximately the same overall divergence as between human MTHFR and its D. discoideum ortholog (48%) which both share the unikont ancestor (Figure 3). This suggests a simple method for setting a general threshold across different domains of life.
Tab. 2. Prediction accuracy of different algorithms on the LacI dataset. 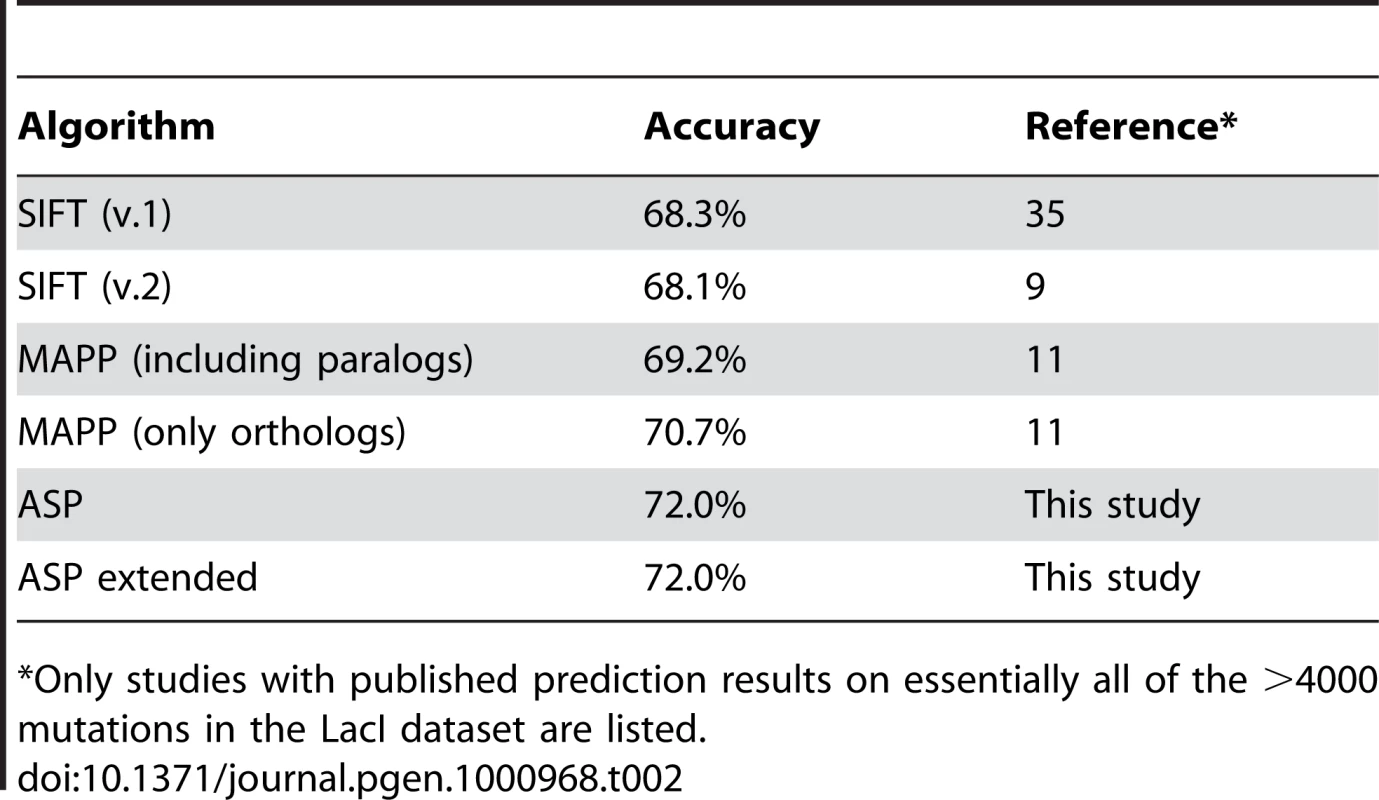
*Only studies with published prediction results on essentially all of the >4000 mutations in the LacI dataset are listed. As shown in Table 2, the accuracy of both ASP and ASPext was 72% when each substitution in LacI is simply classified as functional (wild-type levels) or impaired (less than wild-type levels), as is commonly done [9], [11], [35]. There was no difference in performance between ASP and ASPext as only 4 sites differed between the two methods. The 72% accuracy rate exceeds the best prediction accuracy, to our knowledge, previously reported on the LacI test set (70.7%), obtained by the MAPP method (Multivariate Analysis of Protein Polymorphism; 11) trained on LacI and five of its orthologs. Interestingly, MAPP reportedly performed worse (69.2%) when trained using the full set of 55 LacI homologs, which included numerous putative paralogs. Thus, restricting the training set to a few orthologs minimizes the opportunity for divergence of selective constraints in different lineages, which is consistent with our findings for MTHFR.
Discussion
Most algorithms for the functional prediction of amino acid substitution assume that divergent lineages share the same selective constraints on homologous sites. However, we observed that replacing a single amino acid in the human MTHFR enzyme with one that was accepted in an orthologous protein frequently resulted in functional impairment. Indeed, we observed that even a substitution from a very recently diverged ortholog (F237L, which is fixed in chicken MTHFR) resulted in significant functional impairment. These results suggested that selective constraints can vary in divergent lineages and do not necessarily reflect those directing evolution of the human enzyme.
Our results are in agreement with previous studies that suggest that the selective constraints on homologous sites may differ substantially among orthologous proteins. There are several reasons that selective constraints may differ between lineages, including changes in the strength (from differences in population dynamics that arise from the inability of selection to remove weakly deleterious mutations from a smaller population) or even the direction of selection (e.g. from differences in gene essentiality for different organisms; [36]). However, several studies have concluded that the most likely reason is compensatory mutations, i.e. the change in selective constraints on a given site is actually due to mutations at other sites in the same (or possibly another) protein. Kondrashov et al. [37] estimated that about 10% of all amino acid substitutions producing a pathogenic phenotype in humans are present as the wild-type amino acid in at least one mammalian ortholog. The authors termed these types of substitutions “compensated pathogenic deviations” and suggested that they were tolerated in the orthologous protein because of second-site compensatory mutations. In support of this hypothesis, Gao and Zhang [38] provided evidence that compensatory mutations are the most likely explanation for the majority of human disease mutations fixed in mice. Kulathinal et al. [39] found that debilitating missense mutations in D. melanogaster were fixed in related insect orthologs at a surprisingly high rate, and also speculated that compensatory mutations must be co-evolving in these lineages. Of the substitutions we tested, we found that 70% were functionally impaired, a much greater proportion than reported in the related studies cited above. However, it should be noted that the majority of the substitutions we tested were from more distant orthologs (26 of 30 substitutions were fixed in orthologs whose MRCA with human was over 500 million years ago). The long divergence times have provided more opportunity for compensatory mutations to arise that alter the selective constraints on a given site.
The term “compensatory mutation” may appear to suggest that chronologically a second mutation occurred that “compensates” for a pre-existing deleterious mutation. In fact, the compensatory mutation most likely arises first as a “pre-adaptation” that enables a mutation that would otherwise have been deleterious. Thus, our findings are consistent with the importance of evolutionary trajectory in determining which substitutions are permissable at any given point during evolution. For example, using ancestral protein reconstruction, Zhang and Rosenberg [40] identified two amino acid substitutions that contributed to the evolutionary enhancement of RNase activity of EDN, an eosinophil-associated RNase of primates. Each substitution individually was neutral or perhaps only mildly deleterious to ancestral function, but in combination were complementary in the evolution of a derived, modified function. Likewise, neutral substitutions at some sites in the mineralocorticoid receptor were found to be critical for enabling adaptive substitutions to occur at other sites [41]. In addition, multiple amino acid substitutions in beta lactamase that confer antibiotic resistance seem to arise through only a very small fraction of available trajectories [42]. In other words, the identity of an amino acid at one site can be influenced by the content of other sites and these relationships may be unique to the evolution of specific lineages. These findings highlight the importance of previous substitutions in defining the possibilities of subsequent mutational events.
Given these considerations, we hypothesized that selective constraints on a site might be better estimated from only the evolutionary trajectory of a particular protein (or gene), rather than including substitutions among divergent lineages. This would remove potentially confounding signals from these other lineages, such as differences in population dynamics, adaptation to specific environments and compensatory mutations. To test our hypothesis, we constructed a phylogenetic tree, and inferred ancestral sequences at specific nodes in the tree for the MTHFR family as well as the bacterial LacI family. We could then trace back among the direct ancestors of the current-day sequence, to infer how long a given amino acid had been preserved at a particular site, which we dubbed the Ancestral Site Preservation (ASP) measure. In support of our hypothesis, we found that sites that are preserved for long periods of time in the human lineage (high ASP) will not tolerate substitution, even though such substitutions are tolerated in at least one orthologous protein. In addition, the ASP metric discriminated between functional and impaired variants in both human MTHFR and E. coli LacI (using a comparable threshold in both cases) more accurately than any method reported to date. This was surprising given that the ASP does not consider the nature of the specific amino acid substitution, but rather only the ancestry of a particular site in the protein. This suggests that, to a reasonable approximation, most sites within a protein are either intolerant or tolerant with respect to a wide variety of mutations, and that intolerant and tolerant sites can be discriminated quite well using the evolutionary history of substitution at each site. It is also perhaps somewhat surprising that the ancestral preservation must be for a very long time before it can be considered to be reliably indicative of negative selection against variant amino acids. In MTHFR, all substitutions we tested at sites preserved since at least the eukaryotic common ancestor (approximately 1500 million years ago), undoubtedly due to selection against variant amino acids, resulted in a nonfunctional protein by our definition. However, when preservation has occurred for shorter periods of time, most mutations we tested had no significant functional impact in our assay, suggesting that in MTHFR ancestral preservation even for many hundreds of millions of years may be due in part to random chance rather than selection.
For MTHFR we found that a modified measure, which we dubbed “ASP extended” (ASPext), improved the quality of predictions by accounting for sites that had been preserved for long periods of time during the history of MTHFR, but then changed relatively recently. These are examples of changed evolutionary constraints within the human lineage and, as discussed above for extant orthologous proteins, are most likely due to compensatory mutations. ASPext is capable of highlighting substitutions fixed by recent positive selection in the human lineage (which has been suggested; [36], [43]), if they occurred at previously preserved sites.
Thus, we conclude that as orthologs diverge from their most recent common ancestor, their different evolutionary trajectories lead to the divergence in the selective constraints on homologous sites. While lineage-specific adaptive selection and variable strength of purifying selection contribute to such divergence of constraints, the accumulation of potentially compensatory neutral (or near-neutral) substitutions underlie and enable lineage-specific trajectories. In support of this conclusion, Kondrashov et al. [44] have recently shown that the continued sequence divergence observed for distant orthologs is inconsistent with a model of lineage-independent selective constraints on homologous sites.
In this study we defined preservation strictly in that only the identity of the amino acid at a particular site was considered in determining the ASP. Of course, this definition could be relaxed to include chemically similar amino acids. As we discussed above, there seemed to be a strict requirement for a polar amino acid at position 134 in human MTHFR, although the identity of this site (arginine) is preserved back to only the rodent-primate ancestor. Thus, using polarity as the preservation feature rather than identity would yield a much larger ASP score and may be more informative for changes such as R134F and R134C which are not polar and are quite debilitating. In this case, the R134S change, which was functional, would still be predicted to be functional because it does not break the polarity preservation.
These observations have implications for understanding and reconstructing protein evolution, as well as improving the accuracy of predicting the effects of amino acids substitution on human protein function. The ASP measure appeared to have advantages over other comparative sequence approaches in using phylogeny to predict functionality, though it remains clear that physico-chemical constraints on substitution also play a role. Use of our basic approach should prevent distinct historical constraints from confounding inferences about physico-chemical constraint, and reveal how to better incorporate both ancestral preservation and physico-chemical differences into functional prediction. Perhaps most importantly, by enabling more accurate computational predictions of functional polymorphisms in humans, our results should aid epidemiological efforts in identifying the genetic deficiencies that are etiological for disease.
Supporting Information
Zdroje
1. StensonPD
MortM
BallEV
HowellsK
PhillipsAD
2009 The Human Gene Mutation Database: 2008 update. Genome Med 1 13
2. NgPC
HenikoffS
2006 Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet 7 61 80
3. KrawczakM
BallEV
CooperDN
1998 Neighboring-nucleotide effects on the rates of germ-line single-base-pair substitution in human genes. Am J Hum Genet 63 474 488
4. MillerMP
KumarS
2001 Understanding human disease mutations through the use of interspecific genetic variation. Hum Mol Genet 10 2319 2328
5. GranthamR
1974 Amino acid difference formula to help explain protein evolution. Science 185 862 864
6. ChasmanD
AdamsRM
2001 Predicting the functional consequences of non-synonymous single nucleotide polymorphisms: structure-based assessment of amino acid variation. J Mol Biol 307 683 706
7. WangZ
MoultJ
2001 SNPs, protein structure, and disease. Hum Mutat 17 263 270
8. SunyaevS
RamenskyV
KochI
LatheW3rd
KondrashovAS
2001 Prediction of deleterious human alleles. Hum Mol Genet 10 591 597
9. NgPC
HenikoffS
2002 Accounting for human polymorphisms predicted to affect protein function. Genome Res 12 436 446
10. ThomasPD
CampbellMJ
KejariwalA
MiH
KarlakB
2003 PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13 2129 2141
11. StoneEA
SidowA
2005 Physicochemical constraint violation by missense substitutions mediates impairment of protein function and disease severity. Genome Res 15 978 986
12. Ferrer-CostaC
GelpiJL
ZamakolaL
ParragaI
de la CruzX
2005 PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics 21 3176 3178
13. DayhoffMO
1969 Computer analysis of protein evolution. Sci Am 221 86 95
14. MiyataT
MiyazawaS
YasunagaT
1979 Two types of amino acid substitutions in protein evolution. J Mol Evol 12 219 236
15. ShuY
LeabmanMK
FengB
MangraviteLM
HuangCC
2003 Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci U S A 100 5902 5907
16. RozenR
1997 Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb Haemost 78 523 526
17. SelhubJ
1999 Homocysteine metabolism. Annu Rev Nutr 19 217 246
18. FrosstP
BlomHJ
MilosR
GoyetteP
SheppardCA
1995 A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10 111 113
19. WaldDS
LawM
MorrisJK
2002 Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Bmj 325 1202
20. van der LindenIJ
AfmanLA
HeilSG
BlomHJ
2006 Genetic variation in genes of folate metabolism and neural-tube defect risk. Proc Nutr Soc 65 204 215
21. MariniNJ
GinJ
ZiegleJ
KehoKH
GinzingerD
2008 The prevalence of folate-remedial MTHFR enzyme variants in humans. Proc Natl Acad Sci U S A 105 8055 8060
22. MartinYN
SalavaggioneOE
EckloffBW
WiebenED
SchaidDJ
2006 Human methylenetetrahydrofolate reductase pharmacogenomics: gene resequencing and functional genomics. Pharmacogenet Genomics 16 265 277
23. ShanX
WangL
HoffmasterR
KrugerWD
1999 Functional characterization of human methylenetetrahydrofolate reductase in Saccharomyces cerevisiae. J Biol Chem 274 32613 32618
24. DunnettCW
1964 New tables for Multiple Comparisons with a Control. Biometrics 20 482 491
25. BenjaminiY
HochbergY
1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57 289 300
26. KatohK
TohH
2008 Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9 286 298
27. YangZ
2007 PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24 1586 1591
28. SaitouN
NeiM
1987 The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4 406 425
29. FitchWM
1970 Distinguishing homologous from analogous proteins. Syst Zool 19 99 113
30. RaymondRK
KastanosEK
ApplingDR
1999 Saccharomyces cerevisiae expresses two genes encoding isozymes of methylenetetrahydrofolate reductase. Arch Biochem Biophys 372 300 308
31. NaulaN
WaltherC
BaumannD
SchweingruberME
2002 Two non-complementing genes encoding enzymatically active methylenetetrahydrofolate reductases control methionine requirement in fission yeast Schizosaccharomyces pombe. Yeast 19 841 848
32. NgPC
HenikoffS
2003 SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31 3812 3814
33. MarkiewiczP
KleinaLG
CruzC
EhretS
MillerJH
1994 Genetic studies of the lac repressor. XIV. Analysis of 4000 altered Escherichia coli lac repressors reveals essential and non-essential residues, as well as “spacers” which do not require a specific sequence. J Mol Biol 240 421 433
34. SuckowJ
MarkiewiczP
KleinaLG
MillerJ
Kisters-WoikeB
1996 Genetic studies of the Lac repressor. XV: 4000 single amino acid substitutions and analysis of the resulting phenotypes on the basis of the protein structure. J Mol Biol 261 509 523
35. NgPC
HenikoffS
2001 Predicting deleterious amino acid substitutions. Genome Res 11 863 874
36. LiaoBY
ZhangJ
2008 Null mutations in human and mouse orthologs frequently result in different phenotypes. Proc Natl Acad Sci U S A 105 6987 6992
37. KondrashovAS
SunyaevS
KondrashovFA
2002 Dobzhansky-Muller incompatibilities in protein evolution. Proc Natl Acad Sci U S A 99 14878 14883
38. GaoL
ZhangJ
2003 Why are some human disease-associated mutations fixed in mice? Trends Genet 19 678 681
39. KulathinalRJ
BettencourtBR
HartlDL
2004 Compensated deleterious mutations in insect genomes. Science 306 1553 1554
40. ZhangJ
RosenbergHF
2002 Complementary advantageous substitutions in the evolution of an antiviral RNase of higher primates. Proc Natl Acad Sci U S A 99 5486 5491
41. BridghamJT
CarrollSM
ThorntonJW
2006 Evolution of hormone-receptor complexity by molecular exploitation. Science 312 97 101
42. WeinreichDM
DelaneyNF
DepristoMA
HartlDL
2006 Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312 111 114
43. ClarkAG
GlanowskiS
NielsenR
ThomasPD
KejariwalA
2003 Inferring nonneutral evolution from human-chimp-mouse orthologous gene trios. Science 302 1960 1963
44. KondrashovAS
PovolotskayaIS
IvankovDN
KondrashovFA
Rate of sequence divergence under constant selection. Biol Direct 5 5
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein SignalingČlánek B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish EmbryoČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



