-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
Mating in basidiomycetous fungi is often controlled by two unlinked, multiallelic loci encoding homeodomain transcription factors or pheromones/pheromone receptors. In contrast to this tetrapolar organization, Cryptococcus neoformans/Cryptococcus gattii have a bipolar mating system, and a single biallelic locus governs sexual reproduction. The C. neoformans MAT locus is unusually large (>100 kb), contains >20 genes, and enhances virulence. Previous comparative genomic studies provided insights into how this unusual MAT locus might have evolved involving gene acquisitions into two unlinked loci and fusion into one contiguous locus, converting an ancestral tetrapolar system to a bipolar one. Here we tested this model by studying Cryptococcus heveanensis, a sister species to the pathogenic Cryptococcus species complex. An extant sexual cycle was discovered; co-incubating fertile isolates results in the teleomorph (Kwoniella heveanensis) with dikaryotic hyphae, clamp connections, septate basidia, and basidiospores. To characterize the C. heveanensis MAT locus, a fosmid library was screened with C. neoformans/C. gattii MAT genes. Positive fosmids were sequenced and assembled to generate two large probably unlinked MAT gene clusters: one corresponding to the homeodomain locus and the other to the pheromone/receptor locus. Strikingly, two divergent homeodomain genes (SXI1, SXI2) are present, similar to the bE/bW Ustilago maydis paradigm, suggesting one or the other homeodomain gene was recently lost in C. neoformans/C. gattii. Sequencing MAT genes from other C. heveanensis isolates revealed a multiallelic homeodomain locus and at least a biallelic pheromone/receptor locus, similar to known tetrapolar species. Taken together, these studies reveal an extant C. heveanensis sexual cycle, define the structure of its MAT locus consistent with tetrapolar mating, and support the proposed evolutionary model for the bipolar Cryptococcus MAT locus revealing transitions in sexuality concomitant with emergence of a pathogenic clade. These studies provide insight into convergent processes that independently punctuated evolution of sex-determining loci and sex chromosomes in fungi, plants, and animals.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000961
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000961Summary
Mating in basidiomycetous fungi is often controlled by two unlinked, multiallelic loci encoding homeodomain transcription factors or pheromones/pheromone receptors. In contrast to this tetrapolar organization, Cryptococcus neoformans/Cryptococcus gattii have a bipolar mating system, and a single biallelic locus governs sexual reproduction. The C. neoformans MAT locus is unusually large (>100 kb), contains >20 genes, and enhances virulence. Previous comparative genomic studies provided insights into how this unusual MAT locus might have evolved involving gene acquisitions into two unlinked loci and fusion into one contiguous locus, converting an ancestral tetrapolar system to a bipolar one. Here we tested this model by studying Cryptococcus heveanensis, a sister species to the pathogenic Cryptococcus species complex. An extant sexual cycle was discovered; co-incubating fertile isolates results in the teleomorph (Kwoniella heveanensis) with dikaryotic hyphae, clamp connections, septate basidia, and basidiospores. To characterize the C. heveanensis MAT locus, a fosmid library was screened with C. neoformans/C. gattii MAT genes. Positive fosmids were sequenced and assembled to generate two large probably unlinked MAT gene clusters: one corresponding to the homeodomain locus and the other to the pheromone/receptor locus. Strikingly, two divergent homeodomain genes (SXI1, SXI2) are present, similar to the bE/bW Ustilago maydis paradigm, suggesting one or the other homeodomain gene was recently lost in C. neoformans/C. gattii. Sequencing MAT genes from other C. heveanensis isolates revealed a multiallelic homeodomain locus and at least a biallelic pheromone/receptor locus, similar to known tetrapolar species. Taken together, these studies reveal an extant C. heveanensis sexual cycle, define the structure of its MAT locus consistent with tetrapolar mating, and support the proposed evolutionary model for the bipolar Cryptococcus MAT locus revealing transitions in sexuality concomitant with emergence of a pathogenic clade. These studies provide insight into convergent processes that independently punctuated evolution of sex-determining loci and sex chromosomes in fungi, plants, and animals.
Introduction
Although many organisms, in particular microorganisms, can reproduce both asexually and sexually, the vast majority appears to undergo sexual reproduction during their life cycles. The hypotheses advanced as to why sex is so ubiquitous are myriad, but center on several recurrent themes [1], [2]. First, the admixture of genetic material from two genetically distinct individuals that occurs during sexual reproduction may give rise to novel gene combinations that result in offspring better suited to novel or changing environments. Second, sexual reproduction may serve to remove deleterious mutations, such as transposons, that have arisen within the genome. Finally, sex might serve both roles, facilitating combinations of successful alleles and simultaneously purging the genome of deleterious ones.
Until recently it has been difficult to test these models for the possible benefits of sex in experimentally tractable model organisms. However, recent studies with the model yeast S. cerevisiae have provided direct experimental evidence for a benefit of sexual reproduction. Isogenic diploid strains were engineered that are either capable of sporulation with meiotic recombination (sexual) or were able to sporulate but not undergo meiotic recombination (asexual, spo11,13 mutant) [3]. Under a variety of different stressful environments, the sexual strain had a competitive advantage compared to the asexual strain. A series of related studies in both S. cerevisiae and the model alga Chlamydomonas provide additional support for a benefit conferred by sex in response to novel or challenging environments [4]–[6]. In response to constant environments, asexual reproduction is also likely to be of relative benefit by relieving strains from the metabolic and genetic costs associated with sex [7]. Thus, a balance between sexual and asexual reproduction may be struck in response to different environmental conditions.
While the vast majority of sexually reproducing organisms occur as just two sexes or mating types, transitions in sexuality from two to multiple mating types, and vice versa, have occurred in the fungal kingdom. Sexual reproduction is common in fungi, and mating type occurs in two general patterns: bipolar and tetrapolar mating type (reviewed in [8]–[10]. In the bipolar systems, a single genetic locus known as the mating type locus (MAT) occurs in two alternate forms, known as idiomorphs (a or α, a or A, + or −, P or M), and these govern the identity of the cell [11]–[13]. Co-incubation of isolates of opposite mating type under suitable conditions leads to sexual reproduction. Species with bipolar mating systems are found in the ascomycete, basidiomycete, and zygomycete phyla, providing evidence that this is an ancestral organization. In the basidiomycete phylum, many species instead have a more complex sex determining system, known as tetrapolar, in which two unlinked sex determining loci are present [8], [10], [14], [15]. One locus encodes homeodomain transcription factors and the other locus encodes pheromones and pheromone receptors, and both loci must differ for sexual reproduction to occur. In many species, these loci are multiallelic, resulting in literally thousands of different mating types, which promotes outcrossing [16]. Transitions from tetrapolar to bipolar mating type determination have occurred multiple independent times, and in several examples result from fusions of the two unlinked loci to form one contiguous region (reviewed in [17], [18]), potentially illustrating common evolutionary pressures limiting mating-type/sexes to just two.
Although many fungi are currently classified as asexual, genomics has revealed that many such fungi retain the mating type locus and machinery necessary for both mating and meiosis [19]–[24]. In some cases, population genetics studies also reveal an equal distribution of opposite mating types in nature, and evidence for recombination [25]–[28]. In some notable recent examples, such as Aspergillus parasiticus and Aspergillus fumigatus, an extant sexual cycle has been discovered [29], [30]. Thus, there may be few truly asexual fungi, and many fungi, and perhaps most, for which cryptic sexual cycles remain to be discovered [31], [32].
Sexual reproduction in the basidiomycetous Cryptococcus pathogenic species complex involves a well-defined bipolar mating type system with two opposite mating types, a and α [33]–[38]. Cell-cell fusion leads to the production of dikaryotic hyphae with fused clamp connections, and ultimately the hyphal tips differentiate to form basidia in which nuclear fusion and meiosis occur. Budding from the basidial surface then produces long chains of basidiospores, the suspected infectious propagules [39], [40] for reviews see [41]–[43].
Molecular analysis of the Cryptococcus mating type locus reveals it to be unusual, spanning >120 kb and encompassing >20 genes [44]–[49]. With the exception of the homeodomain genes, SXI1α and SXI2a, which govern cell type identity, the a and α mating type alleles are otherwise composed of divergent alleles of a common gene set [50], [51]. A genomic approach comparing the extant, diverged mating type alleles in C. neoformans var. grubii (serotype A), C. neoformans var. neoformans (serotype D), and C. gattii revealed that the MAT locus is composed of four gene strata of distinct evolutionary ages, including an ancient set of ancestral genes, two gene strata of more intermediate evolutionary origin, and a set of apparently recently acquired genes that are species - but not mating type-specific [47]. The finding that the Cryptococcus bipolar MAT locus contains all of the genes normally found in the two unlinked tetrapolar loci suggests that a fusion of the two loci might have been involved in the origins of this unusual gene cluster linked to virulence and differentiation. The hypothesized evolutionary model posits that an ancestral tetrapolar mating system expanded by a series of acquisitions of genes of related function to form two large gene clusters that subsequently underwent fusion via a chromosomal translocation to form an unstable tripolar intermediate stage that subsequently underwent recombination or gene conversion to form the bipolar state. A series of more recent gene conversions and inversions then occurred, giving rise to the extant bipolar mating type alleles and resetting the molecular clock for some genes within distinct strata and even evicting genes from MAT in other examples [47]. The recent discovery that the MAT locus is flanked by and contains recombination hotspots provides evidence that the expansion and fusion of these loci may have been driven by activation of recombination [52]. In a recent study, the homeodomain genes were relocated to an unlinked genomic location, providing direct experimental support that Cryptococcus can be engineered to complete a tetrapolar sexual cycle and that the tripolar intermediate may have conferred an evolutionary disadvantage and therefore have been transitory [53]. A central goal of the studies presented here was to further rigorously examine the proposed MAT evolutionary model by comparative studies with related but divergent fungal species.
In a recent study, a multi-locus gene phylogeny was developed with species closely related to the pathogenic Cryptococcus species complex, which includes C. neoformans var. neoformans, C. neoformans var. grubii, and C. gattii [54]. In this study, the yeasts analyzed defined two clades. One contains the pathogenic species complex as well as Cryptococcus amylolentus, Tsuchiyaea wingfieldii, and Filobasidiella depauperata, and was termed the sensu stricto clade. The other clade encompasses yeasts more distantly related to the pathogenic species complex, and was termed the sensu lato group. C. heveanensis lies in the sensu lato clade, together with Bullera dendrophila, Cryptococcus bestiolae, Cryptococcus dejecticola, and a recently described sexual species, Kwoniella mangroviensis [55]. The fact that C. heveanensis is located in a sister clade to the pathogenic Cryptococcus species complex makes it an excellent candidate to provide insights into the evolution of the MAT locus and sexual reproduction in pathogenic Cryptoccocus species.
The closest yeast with a defined sexual cycle to C. heveanensis is K. mangroviensis, which is also located in the sensu lato clade containing yeasts more distantly related to the pathogenic Cryptococcus species [54]. The basidial structure of K. mangroviensis differs from that in C. neoformans and C. gattii and contains septa (phragmobasidia) whereas the pathogenic Cryptococcus species have nonseptate basidia (holobasidia) [33], [55]. When pairs of compatible K. mangroviensis strains are co-cultured on CMA medium, dikaryotic hyphae with clamp connections and basidia are produced [55]. Of 19 K. mangroviensis strains isolated from the Bahamas, 7 were fertile (37%) and 12 were sterile (63%). Among the seven fertile isolates, six were assigned to the α mating type and one to the A mating type. From these results, the authors proposed that the mating system appeared to be bipolar and biallelic. The nature of the K. mangroviensis mating system will be further clarified by analysis of additional fertile strains, progeny from genetic crosses, and the MAT locus.
In this study, we analyzed the mating behavior and MAT locus structure of C. heveanensis. First, we discovered an extant sexual cycle for C. heveanensis involving dikaryotic hyphae with clamp connections and basidia with cruciate septa and termed this teleomorph Kwoniella heveanensis. Second, we determined the structure of the MAT locus involving two probably unlinked gene clusters corresponding to the homeodomain locus and the pheromone/receptor locus. The presence of two divergently transcribed homeodomain genes in the homeodomain locus suggests this MAT locus arrangement is ancestral to the pathogenic Cryptococcus species, and one or the other homeodomain gene has been lost recently from MAT in C. neoformans and C. gattii. In addition, the presence of numerous other genes around the C. heveanensis MAT locus that correspond to genes located on the same chromosome but far away from MAT in C. neoformans suggests intrachromosomal rearrangements occurred during the evolution of the C. neoformans MAT locus and flanking regions. Characterizing MAT alleles from other C. heveanensis isolates suggests that the homeodomain locus is multiallelic and the pheromone/receptor locus is at least biallelic. Additionally, the MAT-specific region is likely to be restricted to the SXI1 and SXI2 genes in the homeodomain locus and spans at least the STE3, STE12, MFA1/2, CNB00600, and CNG04540 genes in the pheromone/receptor locus, consistent with a tetrapolar mating system. This analysis reveals an evolutionary intermediate with two apparently unlinked MAT loci into which genes have been acquired, lost, and translocated, providing insights into the events that punctuated the evolution of a contiguous sex determining gene cluster in C. neoformans and C. gattii. These studies serve as an evolutionary window both for the evolution of mating type loci in fungi, and also into similar events that have driven the evolution of sex chromosomes in fungi, plants, and animals including insects, fish, and mammals [18], [56]–[58].
Results
Discovery of the sexual cycle of C. heveanensis
To study mating behavior and the MAT locus of C. heveanensis, the isolates (Table 1) were first analyzed to validate their species assignment. When the D1/D2 and ITS regions were sequenced and compared to the type strain CBS 569, all 9 strains were found to have identical D1/D2 sequences (Table 1, Figure S1). Seven isolates also had identical ITS sequences whereas two isolates (BCC 8396, BCC 11754) had one nucleotide difference in the ITS region (Table 1, Figure S1). These findings are in accord with the assignment of these isolates as C. heveanensis. Two other isolates (CBS 9459,CBS 6097) appear to represent related, but distinct species based on a higher level of divergence in the D1/D2 and ITS sequences (see Figure S1). All strains appear to be haploid based on fluorescence-activated cell sorting (FACS) analysis by comparison to C. neoformans haploid and diploid reference isolates (Table 1, Figure S2).
Tab. 1. Description of C. heveanensis isolates used in this study. 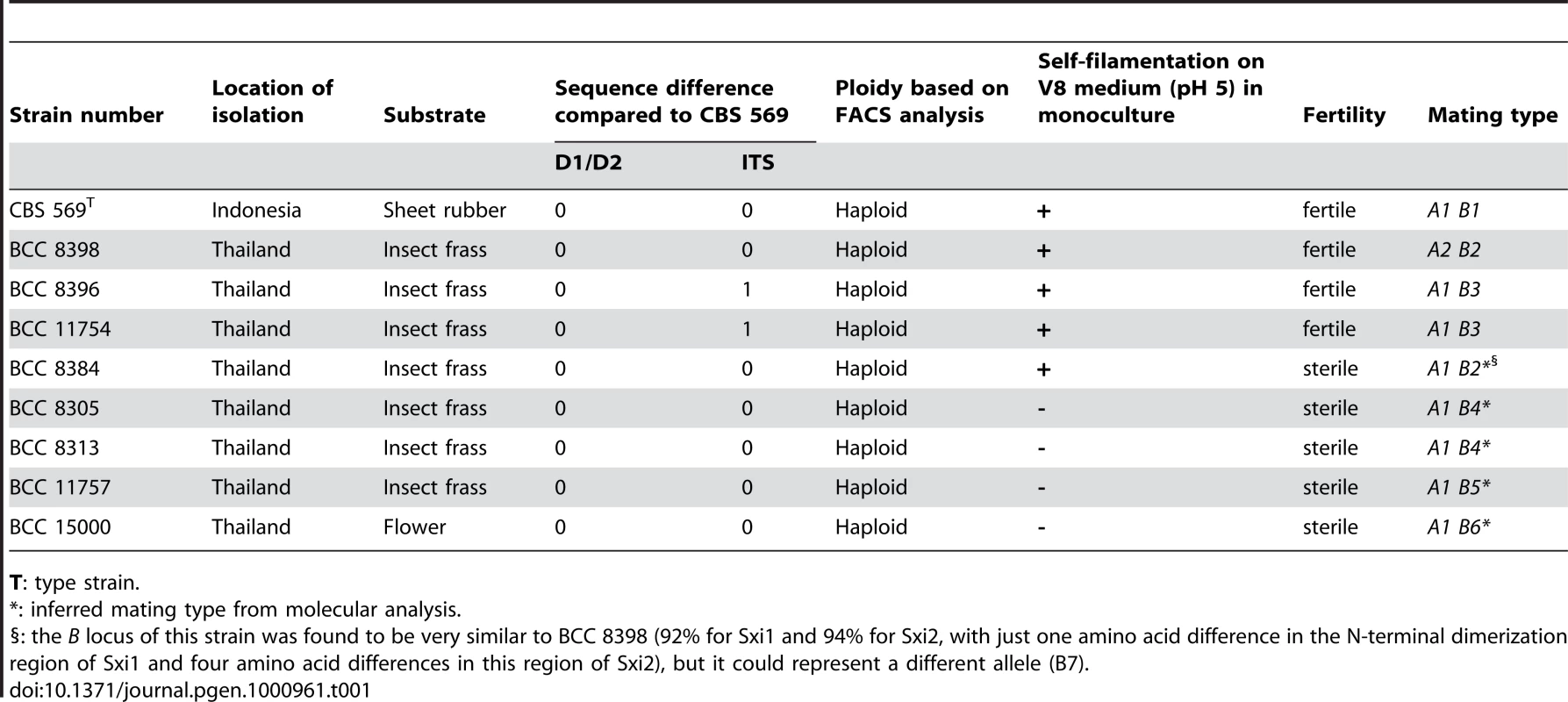
T: type strain. To test if this species has an extant sexual cycle, isolates were co-cultured and examined morphologically for production of mating specific structures. Strains were inoculated on V8 (pH 5 or 7) or MS media alone and in every pair-wise combination and incubated at room temperature in the dark or light. Five strains were self-filamentous when incubated alone while the other strains were not (Table 1). The filaments produced during monoculture were highly branched, irregular in shape, lacked clamp connections, and monokaryotic (nuclei stained with Sytox green) (data not shown). In contrast, dikaryotic hyphae with clamp connections and basidia fruiting bodies indicative of sexual reproduction were produced by three mating combinations (BCC 8398 × CBS 569, BCC 8398 × BCC 8396, and BCC 8398 × BCC 11754). Optimal mating conditions were on V8 medium (pH 5) in the dark. By 10 days incubation, compatible mating pairs had begun to produce dikaryotic filaments with clamp connections (Figure 1A and 1B), and basidia embedded in the agar were first apparent at 2 to 3 weeks of incubation (Figure 1C), and later developed into clusters in a matrix reaching the agar surface and visible by eye (Figure 1D–1G). The basidia are globose with a diameter of 7.5–10.5 µm and cruciate septa, similar to the related species K. mangroviensis and Tremella mesenterica (Figure 1H–1K, [55]). Basidiospores (subglobose, diameter 2.3–3.3 µm) were associated with the basidial clusters (Figure 1J and 1K) and were clearly distinguishable from the larger elliptical budding yeast cells (3.5–4.9 µm×5.0–7.2 µm) (Figure 1L). Fecundity was associated with self-filamentation (4/5 self-filamentous strains were fertile; 4 non-filamentous isolates were sterile (Table 1)). These morphological observations define an extant sexual cycle for this species. The dikaryotic sexual state that formed when compatible pairs of C. heveanensis were co-cultured was named Kwoniella heveanensis because the basidial morphology of this species is quite similar to that of K. mangroviensis (for Standard and Latin descriptions, see Materials and methods). Both species are phylogenetically related in a sister species clade to the pathogenic Cryptococcus species complex [54].
Fig. 1. Sexual reproduction of C. heveanensis. 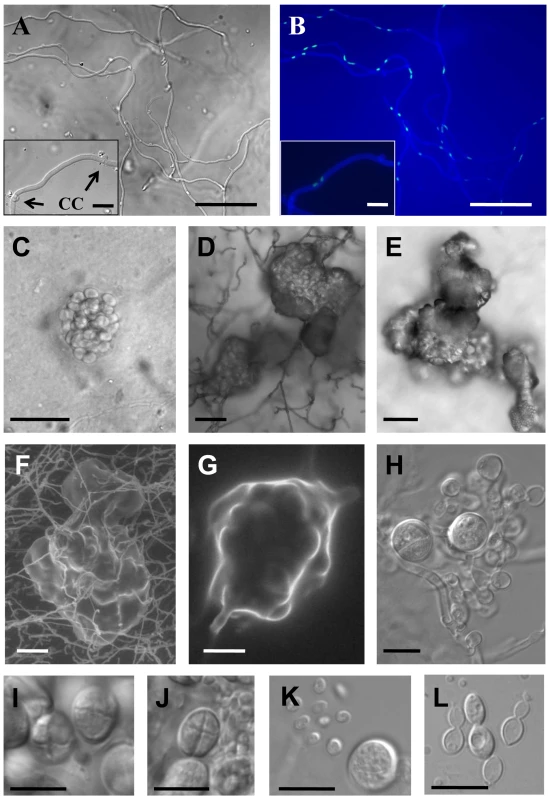
(A,B) Hyphae with clamp connections. Scale bars represent 50 µm. Representative clamp connections (CC) are shown in the enlarged images located in the lower left panels (arrows, scale bars = 10 µm). (A) Differential interference contrast (DIC) image, (B) fluorescence image showing cell walls, septa and nuclei stained with Calcofluor white and Sytox green. (C–E) DIC images of basidial clusters. Scale bars represent 50 µm. (C) A young basidial cluster embedded in the agar, (D,E) mature basidial clusters on the surface of the agar. (F,G) Scanning electron microscopy images of basidial clusters, scale bar in (F) is 50 µm, and in (G) is 10 µm. (H) A developing basidial cluster with two mature and numerous young basidia, scale bar = 10 µm. (I and J) Globose basidia with cruciate septa. Scale bars represent 10 µm. (K) A basidium and basidiospores, scale bar = 10 µm. (L) Vegetative yeast cells, scale bar = 10 µm. Characterization of the C. heveanensis MAT locus
To elucidate the structure of the C. heveanensis MAT locus, probes to MAT-specific genes of C. neoformans and C. gattii were generated by degenerate PCR. These probes (LPD1, RPO41, CAP1, ZNF1, STE20, IKS1, FAO1, and NOG2) were used to screen a fosmid library for the type strain C. heveanensis CBS 569. Sequencing of positive fosmids and assembly of the sequences resulted in two large gene clusters: a region containing the homeodomain locus and flanking regions spanning ∼80 kb and a region containing the pheromone/receptor locus and flanking regions spanning ∼180 kb (Figure 2). The homeodomain locus of C. heveanensis contains two divergent homeodomain genes (SXI1 and SXI2) encoding an HD1 and an HD2 class product, similar to other basidiomycete MAT loci [59]–[61]. Because the MAT locus alleles in C. neoformans and C. gattii contain only SXI1α or SXI2a but not both, this suggests that one or the other homeodomain gene has been lost recently in these pathogenic species.
Fig. 2. C. heveanensis MAT locus structure. 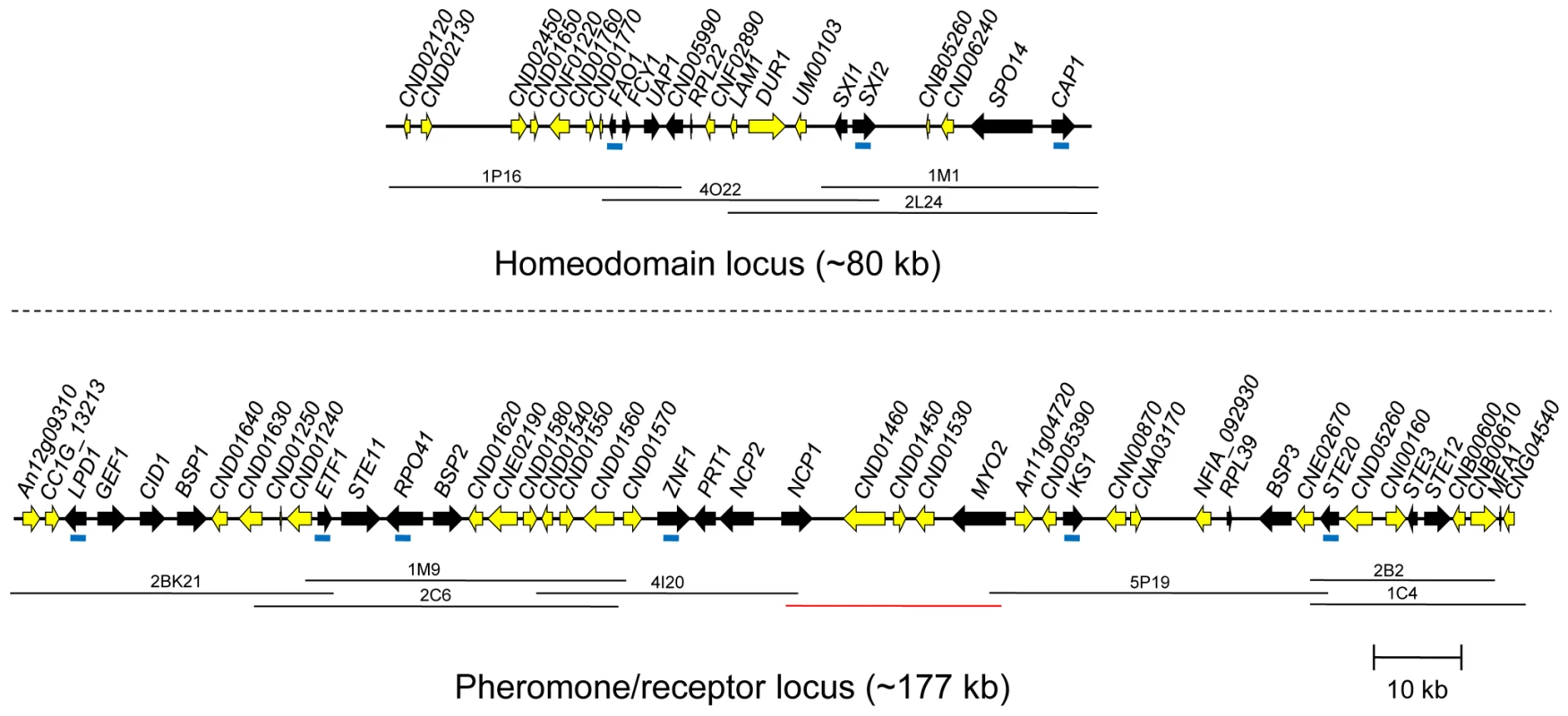
MAT gene probes (represented by blue bars in the figure) were used to screen the fosmid library of C. heveanensis CBS 569. Sequencing the positive fosmids, represented by black lines, and subsequent assembly of the sequences generated two clusters corresponding to the homeodomain locus (∼80 kb) and the pheromone/receptor locus (∼180 kb). The genes that are present in the MAT locus of the pathogenic Cryptococcus species are shown in black, while others are shown in yellow. The red line indicates an ∼20 kb region sequenced by primer walking. The genomic region containing the homeodomain (HD) locus of C. heveanensis also contains the FAO1, FCY1, and UAP1 genes, which form the left boundary of the pathogenic Cryptococcus species MAT locus (Figure 3). Also included are the RPL22, SPO14, and CAP1 genes in addition to the homeodomain genes, which are part of the MAT locus in C. neoformans var. neoformans, C. neoformans var. grubii, and C. gattii [46], [47], [49]. These three genes are also linked to the homeodomain genes of Tremella mesenterica (Figure 3).
Fig. 3. Synteny between MAT locus regions of C. heveanensis, T. mesenterica, and α alleles of C. neoformans var. neoformans, C. neoformans var. grubii, and C. gattii. 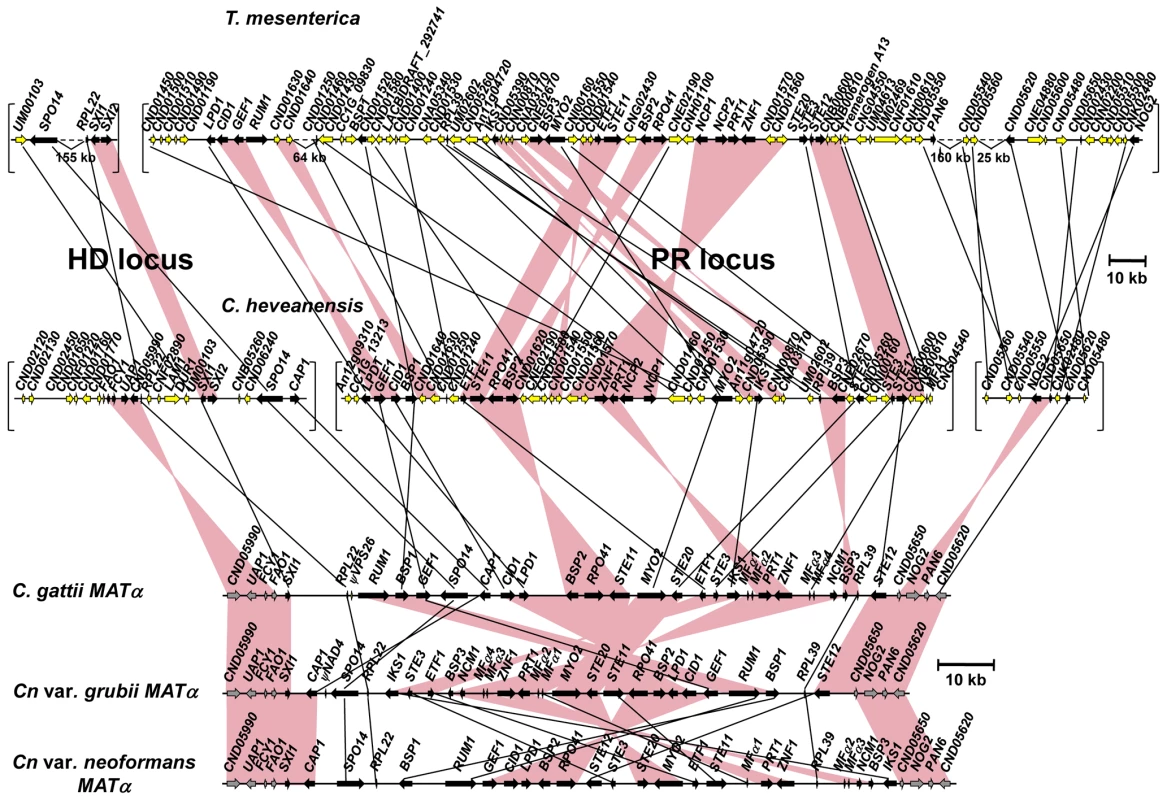
C. heveanensis and T. mesenterica genes that are present in the MAT locus of C. neoformans and C. gattii are shown in black, while others are shown in yellow. Syntenic blocks containing more than one gene are shown as pink bars. The T. mesenterica and C. heveanensis homeodomain locus contains two homeodomain genes homologous with SXI1 and SXI2; whereas, C. neoformans and C. gattii contain a single homeodomain gene, namely SXI1 for α, and SXI2 for the a allele. Upper scale bar refers to T. mesenterica and C. heveanensis, while lower scale bar refers to C. neoformans var. neoformans, C. neoformans var. grubii and C. gattii. JGI annotation IDs for T. mesenterica genes are listed in Table S1. The relative order and the orientations of the C. heveanensis and T. mesenterica contigs are unknown. The remaining genes found in the MAT locus of pathogenic Cryptococcus species are present in the region containing the pheromone/receptor (PR) locus of C. heveanensis (Figure 3). These include the pheromone receptor gene, STE3, the pheromone gene MFA1, the pheromone sensing MAPK cascade components STE11, STE20 and STE12, and other genes whose functions are either unknown, essential, or may not be related to mating (including BSP1, ETF1, ZNF1, PRT1, NCP1, MYO2, IKS1, RPL39, and BSP3). This region also contains the LPD1, GEF1, CID1, RPO41, and BSP2 genes, which were hypothesized to be present at the boundaries of the ancestral sex determining regions in the proposed evolutionary model (RPO41 and BSP2 in the ancestral homeodomain locus and LPD1, GEF1 and CID1 in the ancestral pheromone receptor locus) and to have been entrapped in MAT when the HD and PR loci were brought together via a chromosomal translocation [47]. The presence of these genes in the PR locus region of both C. heveanensis and T. mesenterica implies that these genes were likely to have all been linked to the ancestral PR locus prior to fusion to a contiguous bipolar sex-determinant.
The HD and PR gene clusters of C. heveanensis have numerous other linked genes that are not present in the MAT locus of C. neoformans or C. gattii. Most of these genes (such as CND01630, CND01640, CND05260, CND05390, CNN00870, CNE02670, CNI00160, CND01540, CNG02430, CNE02190, CND01240, CND01560, and CND01570) are also present in the T. mesenterica PR locus region suggesting these genes have recently been lost from the MAT locus and relocated to other genomic locations in C. neoformans and C. gattii (Figure 4). Some of these genes do not have apparent homologs in C. neoformans or C. gattii, suggesting these genes may have been lost during the evolution of the MAT locus. It is also interesting that while a majority of these genes are on the 4th chromosome of C. neoformans strains JEC21/B3501A, where MAT is also located, they correspond to the opposite telomeric region, suggesting intrachromosomal rearrangements may have occurred during MAT locus evolution (Figure 4, [62]).
Fig. 4. C. heveanensis MAT locus region shares synteny with the 4th chromosome of C. neoformans JEC21. 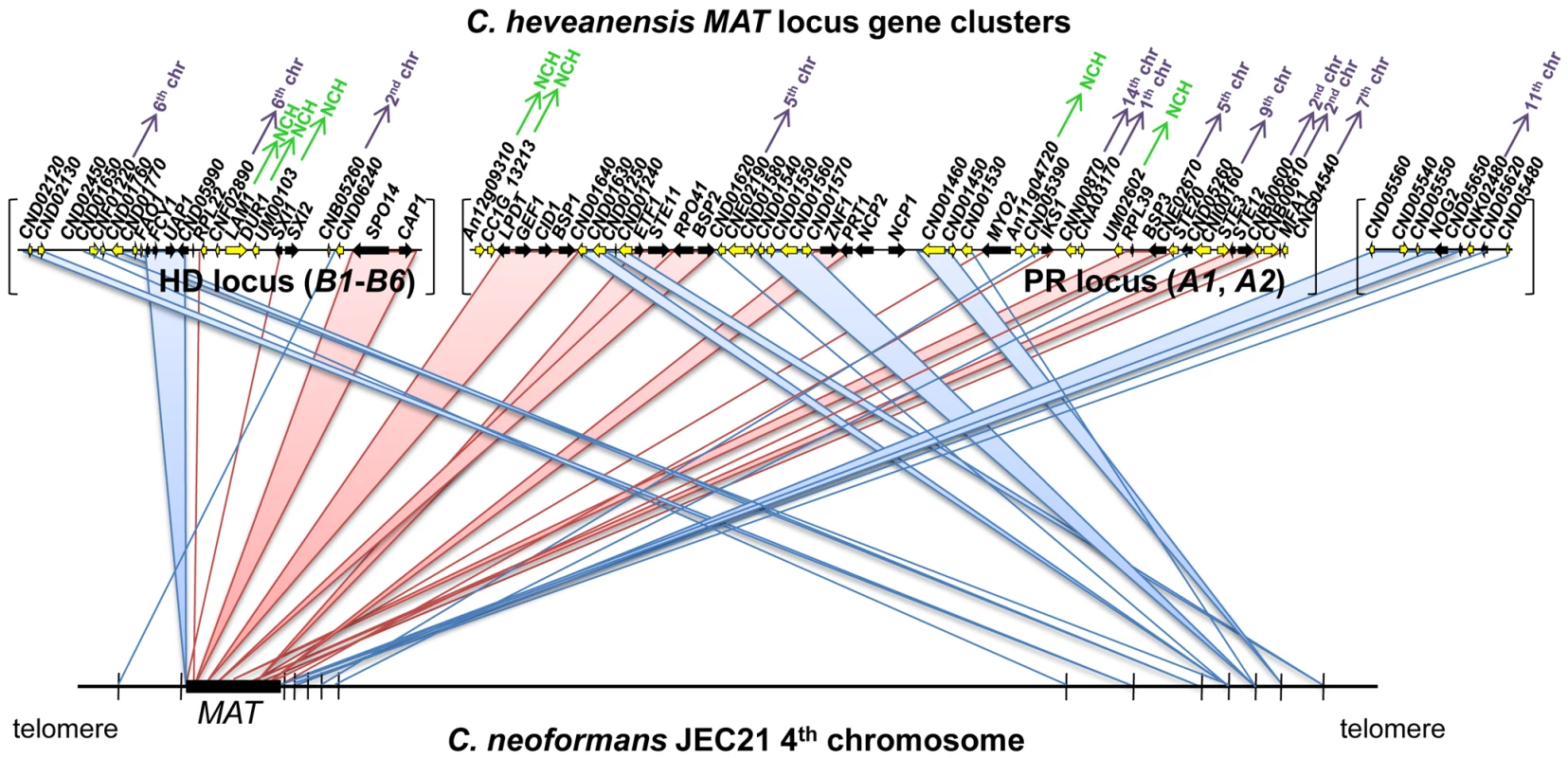
C. heveanensis MAT locus includes numerous genes that are not present in the MAT locus of C. neoformans or C. gattii. Although many of these genes are on the 4th chromosome of C. neoformans strain JEC21 where MAT is located, they correspond to the opposite telomeric region, suggesting intrachromosomal rearragements during the evolution of the MAT locus. Pink lines/bars depict the synteny between genes located within the MAT locus of C. neoformans and the corresponding genes in C. heveanensis. Blue lines/bars show the syntenic relationship of the genes that are linked to the MAT locus of C. heveanensis, but not part of the MAT locus of C. neoformans. The C. heveanensis genes whose C. neoformans homologs are located on different chromosomes are indicated with purple arrows with chromosome numbers. The genes that do not have apparent C. neoformans homologs are indicated by NCH, which stands for “no C. neoformans homolog”. Homeodomain locus of C. heveanensis is multiallelic, and the MAT–specific region is likely to be restricted to the SXI1 and SXI2 genes
Southern blot analysis of the 8 additional C. heveanensis isolates and the type strain with SXI1 and SXI2 gene probes showed a highly divergent profile, suggesting the homeodomain locus structure of C. heveanensis might be multiallelic (Figure 5A). Strains BCC 8305 and BCC 8313 showed the same hybridization profile and sequence analysis also showed that the SXI1, SXI2, LPD1, RPL22, ZNF1, and STE11 genes in these strains are 100% identical for the regions sequenced (data not shown). Strains BCC 8396 and BCC 11754 also appeared identical. Therefore, in these cases, one isolate from each strain pair was selected for further studies. Sequencing of an ∼12 kb region, spanning SXI1 and SXI2, from seven strains provided evidence that the locus is multiallelic with 6 different alleles of SXI1 and SXI2 (Figure S3). Percent sequence identity plots comparing the homeodomain region of CBS 569 with the corresponding region from other isolates showed that there is a highly dissimilar core corresponding to the 5′ ends of the SXI1 and SXI2 genes and spanning a fairly restricted region of ∼2 kb (indicated by a blue box in Figure 5B) followed by a more similar region (∼80%), while the 3′ ends of the genes are almost identical. A representative dot plot comparing the homeodomain region of CBS 569 and BCC 11757 also shows the divergence at the 5′ ends of both genes (Figure 5C). Percent identity plots comparing the homeodomain region from each strain with the others are shown in Figure S4. The homeodomain genes of BCC 8384 and BCC 8398 were found to be very similar (Figure S3, Figure S4). When pairwise comparisons were conducted, percent identity of predicted amino acid sequences between BCC 8384 and BCC 8398 was found to be 92% for Sxi1 and 94% for Sxi2, while percent identity varied between 71–77% for Sxi1, 75–82% for Sxi2 for the other pairwise combinations. Considering the absence of mating between these two isolates, although they are both self-filamentous, these strains may have the same allelic versions of SXI1 and SXI2, preventing mating.
Fig. 5. Homeodomain region of C. heveanensis is multiallelic and likely to be limited to the SXI1 and SXI2 genes. 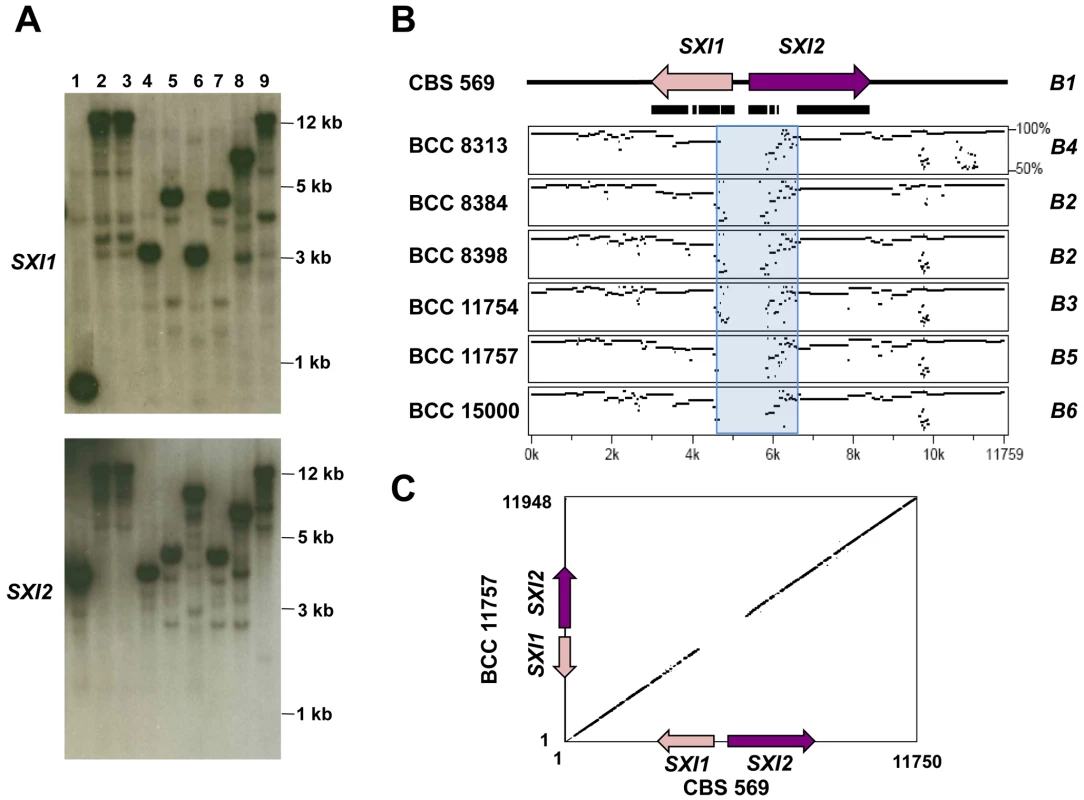
(A) Southern blot analysis showing hybridization patterns of SXI1 and SXI2 gene probes to EcoRV digested genomic DNA from C. heveanensis strains. Lane 1, CBS 569, 2, BCC 8305, 3, BCC 8313, 4, BCC 8384, 5, BCC 8396, 6, BCC 8398, 7, BCC 11754, 8, BCC 11757, 9, BCC 15000. (B) Percent sequence identity plots comparing an ∼12 kb region containing the SXI1 and SXI2 genes from CBS 569 with the corresponding region from other isolates. The blue box shows the highly dissimilar region corresponding to the N-terminal regions of Sxi1 and Sxi2. The black bars under the genes depict the exons. (C) A representative dot plot comparing the homeodomain region of CBS 569 (x-axis) and BCC 11757 (y-axis). Homeodomain proteins have distinct domains for different functions [63]. The homeodomain is the DNA binding region consisting of 3 alpha helices. Fungal homeodomain proteins involved in mating are either HD1 or HD2 type according to the length of this homeodomain region [14]. In HD2 type homeodomain proteins, the distance between the first and second helix is three amino acids shorter than HD1 homeodomain proteins. The C. heveanensis Sxi1 and Sxi2 proteins are HD1 and HD2-type homeodomain proteins, respectively, like their C. neoformans counterparts (Figure 6A, [50], [51]).
Fig. 6. Sxi1 and Sxi2 from C. heveanensis align with HD1 and HD2 homeodomain proteins, respectively. 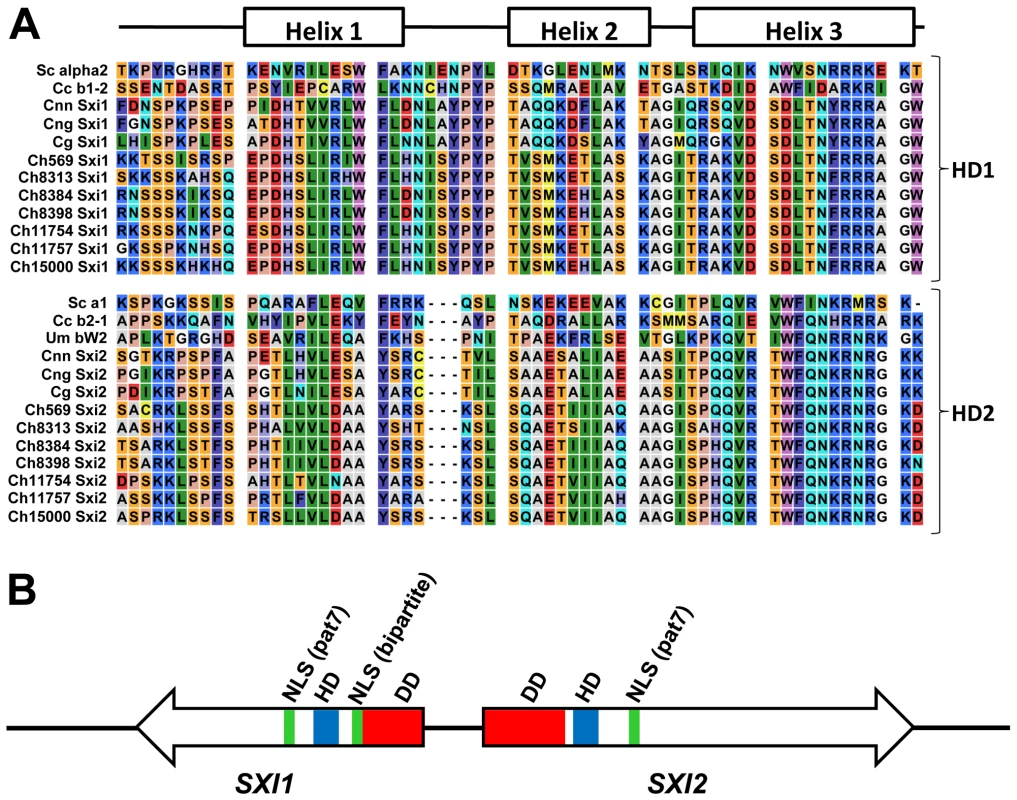
(A) Alignment of the homeodomain regions of S. cerevisiae (Sc), C. cinerea (Cc), C. neoformans var. neoformans (Cnn), C. neoformans var. grubii (Cng), C. gattii (Cg), C. heveanensis (Ch) homeodomain proteins. These proteins are classified as either HD1 or HD2 according to the length of the homeodomain region. There are three extra amino acids between helix 1 and helix 2 of HD1 proteins. C. heveanensis Sxi1 and Sxi2 proteins are of type HD1 and HD2, respectively. (B) Schematic representation of the Sxi1 and Sxi2 domain structures. DD, HD, and NLS denote dimerization domain, homeodomain, and nuclear localization signal, respectively. The types of nuclear localization signal are indicated in parentheses: Bipartite and pat7, pat7 denotes the 7-residue pattern. Studies involving S. cerevisiae a1 and α2, Coprinopsis cinerea HD1 and HD2, and U. maydis bE and bW homeodomain proteins showed that following cell-cell fusion the two homeodomain proteins from compatible partners dimerize via their N-terminal domains [59], [64], [65]. These studies suggest that the interaction is through coiled-coils formed by alpha helices aligned such that the hydrophobic residues are adjacent. Although, we couldn't detect extensive coiled-coil formation in the C. heveanensis homeodomain proteins using the COILS software, there are 3–4 alpha helical regions in the N-terminal domains of both Sxi1 and Sxi2, which might form coiled-coil interactions (Figure S3).
In C. cinerea, two bipartite nuclear localization signals (NLSs) were found in HD1 and this region targeted a reporter protein to the nucleus [66]. In contrast, no NLS was apparent in the C. cinerea HD2 protein and HD2-reporter fusion proteins were not nuclear localized. Similarly in U. maydis, a bipartite NLS in bE (HD1) was predicted [67]. We found one bipartite and one 7-residue pattern (pat7) NLSs in Sxi1 (HD1), while only one pat7 type NLS in Sxi2 (HD2) using the PSORT II software (Figure 6B).
The RPL22 and CAP1 genes are part of the MAT locus in C. neoformans and C. gattii [47], [49]. The RPL22 gene shares 88–90% identity among mating types while the 5′ and 3′ regions of the CAP1 gene show different profiles. For C. neoformans var. neoformans and C. neoformans var. grubii, the identity is 90–92% for the 5′ region of CAP1 and 84–85% for the 3′ region. For C. gattii, the identity is 88% for the 5′ region of CAP1 and 93% for the 3′ region. Sequences from RPL22 and CAP1 were analyzed from C. heveanensis to determine if the HD locus encompasses these genes. When 530 bp of RPL22 gene sequence from all 7 unique strains was analyzed, 97 to 99% sequence identity was observed. Similarly, comparison of 212 bp of CAP1 gene sequence from strains CBS 569, BCC 8313, and BCC 15000 resulted in 99.5–100% identity. Based on this analysis revealing the RPL22 and CAP1 genes are more similar between C. heveanensis isolates than the a and α alleles of C. neoformans, it is likely that the HD locus of C. hevenensis only includes the homeodomain genes, SXI1 and SXI2, although other linked genes should also be examined to further support this conclusion.
These results show that the HD locus of C. heveanensis is multiallelic with at least 6 different alleles determined among 7 strains. Thus, additional extant HD locus alleles likely remain to be discovered. In addition, the MAT-specific region is likely to be limited most prominently to the 5′ end of the SXI1 and SXI2 genes coding for the N-terminal regions of the proteins known to be involved in the specificity of heterodimer formation.
Pheromone/receptor locus of C. heveanensis is at least biallelic
When the pheromone and the pheromone receptor gene sequences were amplified by PCR with CBS 569 specific primers, PCR fragments of the expected sizes were obtained for all strains except BCC 8398. Sequencing of these PCR products showed that the pheromone and receptor gene sequences were identical for all of the strains that yielded PCR products (data not shown). For the atypical strain BCC 8398, degenerate primers were used to amplify the STE3, STE12, CNB00600, and CNG04540 genes and the gaps between these genes were closed by primer walking. This sequence analysis resulted in an ∼9 kb sequence spanning the 5′ end of STE3, STE12, a pheromone gene, which was named MFA2, and the CNG04540 and CNB00600 genes. All of these genes were divergent between BCC 8398 and CBS 569 except CNB00600. Sequence identity values are 54% for STE3, 53% for STE12, 75% for MFA1/2, and 63% for CNG04540. In addition, the gene order also differed as indicated in the comparison and the dot plot (Figure 7A and 7B). Interestingly, the divergent region between BCC 8398 and CBS 569 starts at the 3′ end of the CNB00600 gene. The majority of this gene corresponding to the 5′ portion (1285 bases) is 98% identical, while for a small portion at the 3′ end (139 bases for CBS 569 and 124 bases for BCC 8398), the sequence identity is 53% suggesting this gene may have represented the border of MAT or have been subject to more recent gene conversion.
Fig. 7. Analysis of the pheromone/receptor region of C. heveanensis isolates. 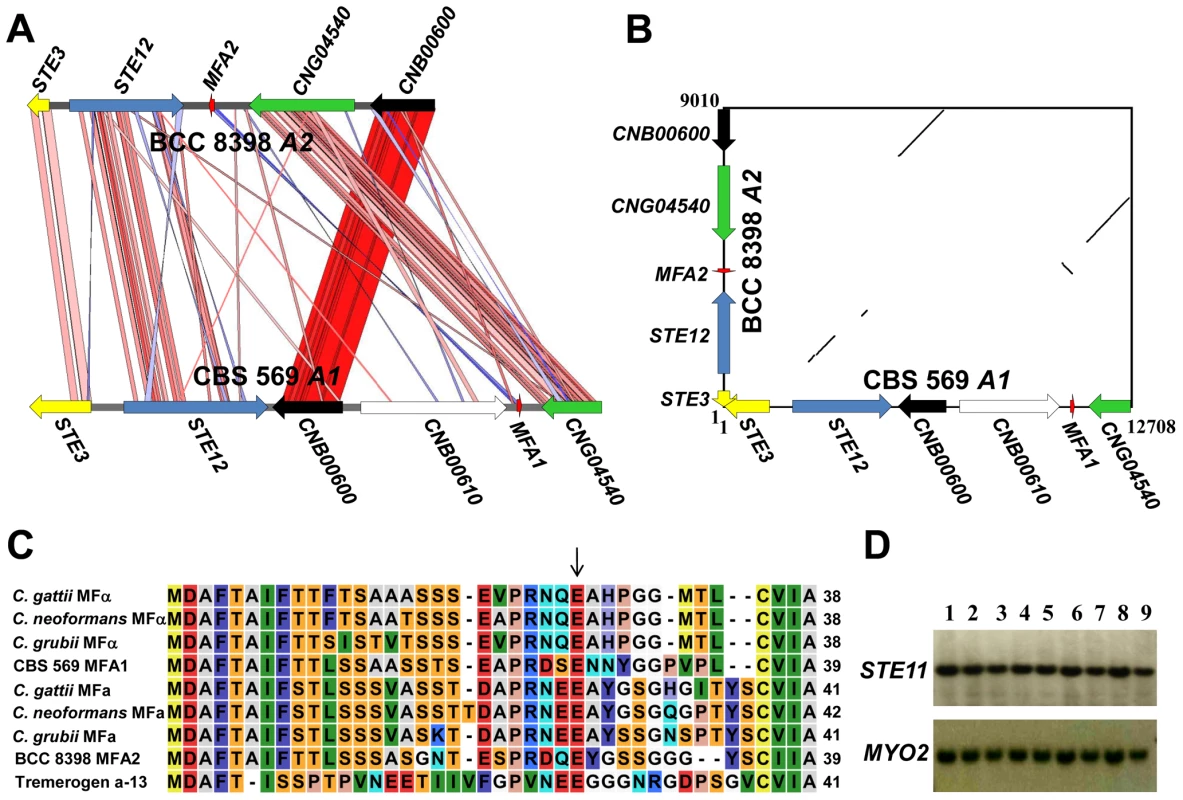
(A) Comparison plot of the PR locus of CBS 569 and BCC 8398. The red and blue bands represent the forward and reverse matches, respectively. The genes are mostly in the same orientation except MFA1/2. The intensity of the color is proportional to the percent identity of the match, where red bands show regions of high sequence identity, while pink bands indicate lower sequence identity. (B) Dot plot analysis of the PR locus of CBS 569 and BCC 8398. (C) Sequence alignment of pheromone sequences from CBS 569, BCC 8398, C. neoformans var. neoformans MFα, C. neoformans var. neoformans MFa, C. neoformans var. grubii MFα, C. neoformans var. grubii MFa, C. gattii MFα, C. gatii MFa and T. mesenterica pheromone tremerogen a-13. The arrow indicates the N-terminal amino acid of tremerogen a-13 after proteolytic cleavage. (D) Southern blot analysis showing hybridization patterns of STE11 and MYO2 gene probes to EcoRV digested genomic DNA from C. heveanensis strains. Lane 1, CBS 569, 2, BCC 8305, 3, BCC 8313, 4, BCC 8384, 5, BCC 8396, 6, BCC 8398, 7, BCC 11754, 8, BCC 11757, 9, BCC 15000. The pheromone genes of C. heveanensis, MFA1 (from CBS 569) and MFA2 (from BCC 8398), encode 39 amino acid-long pheromone precursors (Figure 7C). Both were identified by the characteristic C-terminal motif of lipopeptide pheromones: CAAX, where A is an aliphatic amino acid [68]. While Mfa1 ends with the motif CVIA like pheromone precursors from C. neoformans, C. grubii, C. gattii and T. mesenterica, Mfa2 has CIIA at its C-terminus. Tremerogen a-13, a T. mesenterica pheromone, was isolated and its structure determined [69]. According to this study, after proteolytic cleavage, the mature pheromone starts with a glutamic acid (E) residue indicated with an arrow in Figure 7C. Since this residue is conserved in all Cryptococcus pheromones, the proteolytic cleavage might occur at the same site in other pheromones as well.
No or minimal sequence differences were observed for the LPD1 (100% sequence identity in 530 bases), STE11 (99% in 470 bases), ZNF1 (100% in 410 bases) or IKS1 (99% in 1740 bases) genes. Also, Southern blot analysis using STE11 and MYO2 gene fragments as probes showed the same hybridization profile among all strains tested (Figure 7D). This shows that the divergent region is likely to be more restricted than that of C. neoformans and C. gattii and contains at least the receptor gene STE3, the pheromone gene MFA1/2, STE12, and CNG04540.
These results suggest that the PR locus of C. heveanensis is at least biallelic similar to U. maydis and Tremella species, and it contains not only STE3 and MFA1/2, but also other genes including at least STE12, CNB00600, and CNG04540. Sequence analysis of the genomic regions flanking this region from the isolate BCC 8398 will be necessary to establish whether any additional regions of the PR MAT locus remain to be identified.
Mating type genes exhibit three different phylogenetic patterns
Phylogenetic analyses were conducted on selected mating type genes (CID1, GEF1, LPD1, BSP1, SPO14, ETF1, STE20, STE11, STE3 and STE12) from C. heveanensis, T. mesenterica, C. neoformans var. neoformans, C. neoformans var. grubii and C. gattii (Figure S5). These genes were classified into three groups based on their evolutionary history and two representative examples from each group are demonstrated in Figure 8. These analyses show that, STE3 and STE12 exhibit a mating type specific pattern, where C. heveanensis CBS 569 and T. mesenterica show a closer relationship to the α mating type group, while C. heveanensis BCC 8398 is closer to the a mating type group. BSP1, SPO14, ETF1, STE20, and STE11 are mating type-specific in C. neoformans and C. gattii, however, none of these genes from either C. heveanensis or T. mesenterica followed a mating type-specific pattern. The third gene group consists of CID1, GEF1 and LPD1 that do not show a mating type-specific pattern, but demonstrate a species-specific pattern for all species.
Fig. 8. Mating type genes exhibit three phylogenetic patterns. 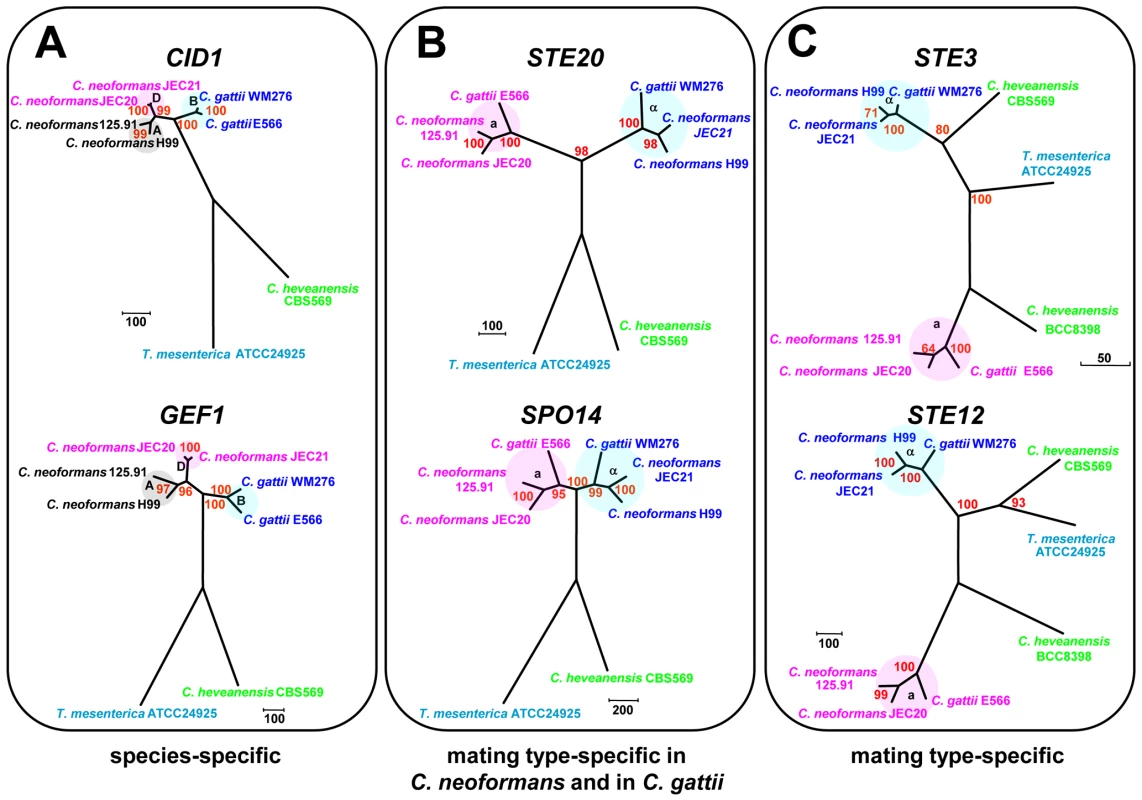
(A) Species-specific profile exhibited by CID1 and GEF1, (B) mating type-specific pattern exhibited by STE20 and SPO14 from C. gattii and C. neoformans, but not from C. heveanensis or T. mesenterica, (C) mating type-specific profile demonstrated by STE3 and STE12. Discussion
We report here two advances with respect to understanding the evolution of sexual reproduction and mating type determination in fungi. First, we discovered and characterized the sexual cycle for C. heveanensis. Second, we cloned and sequenced the mating type locus for this organism. Unlike related species that commonly infect animals, C. heveanensis is not a pathogen and is instead associated with insects and flowers. This species represents an evolutionary window that provides novel insights into understanding transitions that occur between tetrapolar and bipolar mating type systems, and in model and pathogenic species. In particular, this analysis reveals how a tetrapolar ancestral species with two unlinked sex determining regions of the genome gave rise to bipolar extant species in which the sex determinants are linked, providing direct experimental validation of the central tenants of sex chromosome evolution originally proposed by Ohno [56]. These models involve the emergence of sex determinants on autosomes, which are then captured into a sex determining region or chromosome by sequential gene acquisition into strata of different evolutionary ages involving genes of coherent function. The basidiomycete mating type locus represents an exceptional experimental paradigm to test, and validate experimentally, key features of this model. That similar events have transpired repeatedly, and independently, in plants and animals and other fungi, illustrates common underlying principles that are involved in the emergence, evolution, and plasticity of genomic sex determining regions as diverse as fungal mating type loci and sex chromosomes.
Recent multilocus studies support the assignment of C. heveanensis to a clade of species related to but distinct from the pathogenic species C. neoformans and C. gattii [54]. Importantly, this analysis revealed that C. heveanensis occurs in a sensu lato group of species, including K. mangroviensis, which was recently discovered and found to have an extant sexual cycle that was defined as bipolar [55]. A critical aspect in finding the sexual cycle for C. heveanensis was the recent discovery of several new isolates for the species, which were made readily available by their deposition in a public strain collection [70]. Of three isolates previously reported to be C. heveanensis, our D1/D2 and ITS sequence analysis provides evidence that only the type strain CBS 569 is in fact C. heveanensis, whereas two other isolates (CBS 9459 and CBS 6097) appear to represent isolates of closely related cryptic species (Figure S1). Neither of these isolates was fertile with the C. heveanensis type strain. All eight of the recently reported isolates are, based on D1/D2 and ITS sequence analysis, bona fide C. heveanensis isolates. Pairwise incubation of all nine available C. heveanensis isolates under a broad set of conditions and media resulted in mating for three of the 36 possible mating combinations. Thus, at least four of the nine C. heveanensis isolates are fertile. Mating resulted in the production of dikaryotic hyphae with fused clamp connections, basidia with cruciate septa, and basidiospores. Notably, in solo incubations all four of the fertile strains were self-filamentous and produced monokaryotic hyphae, whereas only one of the sterile isolates was self-filamentous. Thus, the formation of monokaryotic hyphae by mating partners appears to be associated with and may precede successful mating, and these may facilitate location of mating partners or fusion of isolates. Based on the similarities of the sexual cycle between C. heveanensis and K. mangroviensis, we have assigned the teleomorphic designation Kwoniella heveanensis. In addition to providing insights into the evolution of mating and MAT, the discovery of the sexual cycle for C. heveanensis will also facilitate further laboratory investigations and strain construction.
How might the self-filamentous phenotype that may be necessary for sexual reproduction be controlled? Five of the nine C. heveanensis isolates are self-filamentous, and four of these are fertile in genetic crosses. The filaments produced may represent something similar to a conjugation tube, however as these occur during mono-culture, they are not induced by pheromones produced by a mating partner of compatible mating type. Possibilities include that elements of the pheromone response pathway have been constitutively activated by mutations, or in response to nutritional cues or limitation alone. Alternatively, it may be that these isolates are self-responsive to their own pheromone as a partial agonist of their Ste3 receptor homolog. In such a model, co-culture leads to more robust pheromone signaling necessary to enable cell-cell fusion and generation of a heterodimeric homeodomain complex that enables completion of sexual reproduction. Future studies to address these and other models will be necessary to test the activity and specificity of the mating pheromones and the Ste3 homologs involved in sensing them, and to isolate mutations that abrogate the self-filamentous phenotype testing if, for example, the pheromone and pheromone receptor genes are involved and whether this is necessary for mating.
Analysis of the mating type locus for C. heveanensis revealed two apparently unlinked genomic regions, one corresponding to the homeodomain gene locus (B) and the second to the pheromone/pheromone receptor gene locus (A) (Figure 9). Notably, the B locus (HD) includes a pair of divergently oriented genes encoding homeodomain proteins related to Sxi1/Sxi2 from C. neoformans and bE/bW from U. maydis. The A locus (PR) includes genes encoding a Ste3 pheromone receptor homolog and a mating pheromone. Both loci are embedded in larger gene clusters, including some previously identified in the C. neoformans MAT locus and novel ones. To establish which genes in the ∼80 and ∼180 kb assemblies are mating type specific, representative regions were isolated by PCR and sequenced from fertile and sterile isolates. This analysis revealed striking diversity in the intergenic region and 5′ coding regions of the SXI1/SXI2 genes, flanked by a less divergent region (∼80% identity) and followed by nearly identical 3′ coding regions (Figure 5). The divergent sequence region spans ∼2 kb. Importantly, this locus appears to be multi-allelic, based on sequence divergence, and at least 6 alleles are present in the 7 isolates examined, suggesting additional alleles may remain to be discovered. This MAT organization is quite similar to the U. maydis b MAT locus [59], [71], [72] corresponding to the diverse N-terminal coiled-coil dimerization domain that dictates self - vs. non-self recognition. The RPL22 and CAP1 genes linked to SXI1/SXI2 in C. heveanensis had very similar sequences from the strains analyzed (97 to 100% identity), suggesting these genes are outside the mating-type specific region. Similar to other basidiomycetes, including U. maydis, S. commune, and C. cinerea, we suggest dimerization occurs between HD1 and an HD2 class homeodomain proteins encoded by different mating type locus alleles [65], [73], [74].
Fig. 9. C. heveanensis is likely to have a tetrapolar mating type system with a multiallelic mating type locus and at least a biallelic pheromone/receptor locus. 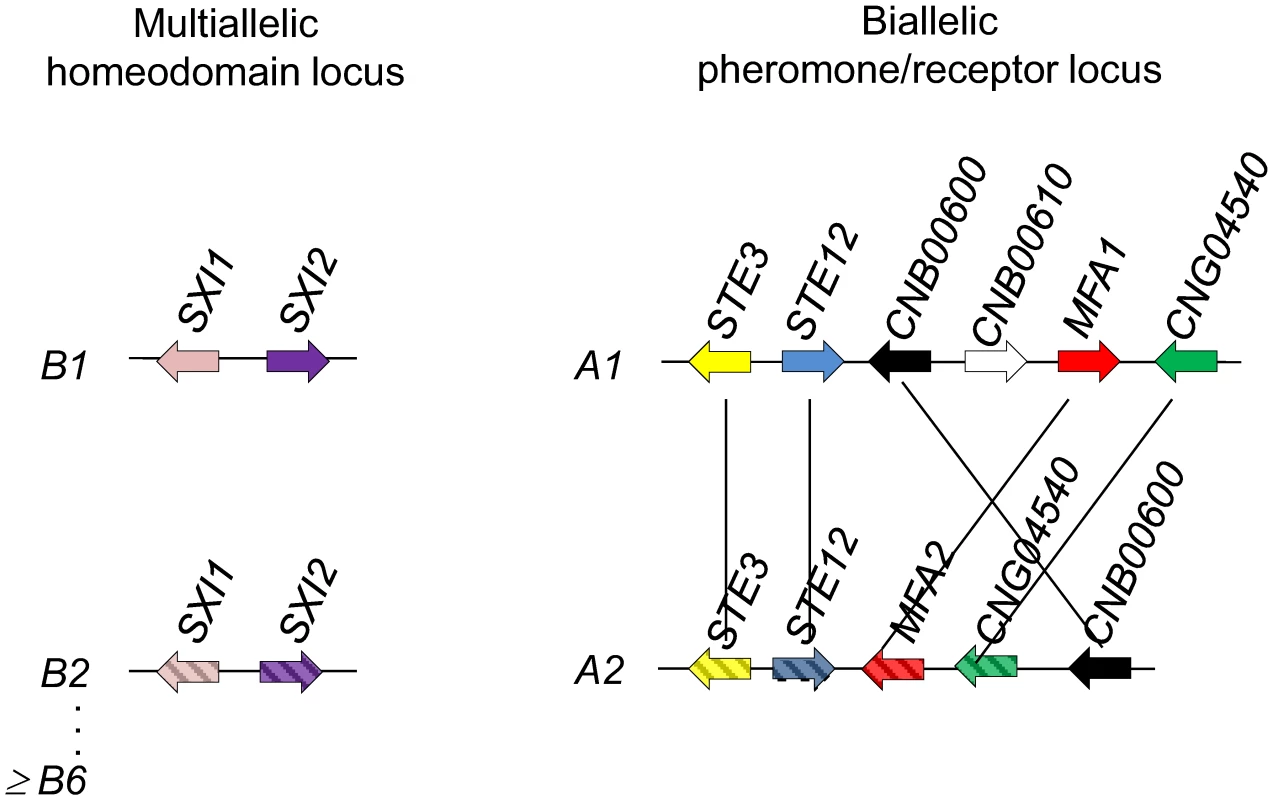
C. heveanensis has a multiallelic HD locus (B locus) including the genes SXI1 and SXI2 with at least 6 alleles among 7 unique strains examined. The PR locus (A locus) of C. heveanensis is at least biallelic and 6 of the strains have the A1 allele, while one of the strains, BCC 8398, has the A2 allele. The PR locus contains at least the STE3, STE12, MFA1/2, CNB00600, and CNG04540 genes. The A locus (PR) of C. heveanensis encodes a Ste3 pheromone receptor homolog, a mating pheromone, and other genes involved in pheromone sensing (Ste11, Ste12, and Ste20) are present or linked. Thus far, our sequence analysis for this large gene cluster spans ∼180 kb for the CBS 569 type strain and representative sequence from other isolates. This analysis allows us to conclude that the gene sequences, and the gene organization, differ substantially between two different alleles present in the population (Figure 7). The conclusion that at least two alleles are present is also supported by consideration of which strain combinations are fertile and which are infertile (Table 1). The finding that the C. heveanensis A MAT locus might be biallelic is similar to the tetrapolar species U. maydis, in which the a mating type locus is also biallelic [75]. The C. heveanensis A locus is distinguished from that of U. maydis in that additional genes are present within the locus, and the linked or flanking genes are also quite distinct. While further analysis of additional genomic regions from other isolates will be required to define the full extent of these MAT locus alleles, it is apparent that highly conserved sequences are embedded within more divergent ones (see Figure 7B). This may illustrate that gene conversion occurs within the locus, as is apparent in the MAT locus of C. neoformans, or that this MAT locus allele represents a transitional form into which related genes have been recruited but have not yet diverged into mating type specific allelic forms. Recent studies of the ectomycorrhizal fungus Laccaria bicolor, a tetrapolar species, have illustrated differences in the evolutionary trajectory for the two mating type loci [76]. Whereas the homeodomain locus appears to be in a region of the genome where syntenic gene organization is conserved, the pheromone and receptor gene locus is more dynamic and subject to different selective pressures, leading to dramatic changes in gene order and organization and insertion of many transposable elements. Similar properties may hold for the two mating type locus alleles of C. heveanensis and may be responsible for some of the unusual features of MAT in this and related species.
The molecular organization of the C. heveanensis MAT locus allows us to propose that this species has a tetrapolar mating type determination system. This conclusion is based on the finding of two apparently unlinked loci that correspond to the A and B mating type locus alleles of other tetrapolar fungal species [16], [77], [78]. Second, a high level of genetic sequence divergence is found for genes within each of the two loci, consistent with a role in mating type determination. Third, the B locus is multiallelic, as is the case of other tetrapolar fungi but not in bipolar species. Fourth, the specific alleles found for the isolates are well correlated with those that are in fact fertile in that all three successful combinations differ at both MAT loci, whereas for at least some of the infertile strain combinations, they share a closely related or identical allele at one or both MAT loci (see Table 1). Fifth, the finding that in the population that the A1 locus allele occurs in isolates with at least six different highly diverged B locus alleles is consistent with two unlinked loci in linkage equilibrium defining mating type. Given the dramatic sequence divergence between the different B alleles, models in which these arose by divergence while linked to the A1 locus seem less plausible. Put another way, if the A and B loci were to be linked, we would expect a higher proportion of the population would consist of A1 linked to, for example, B1, than is observed.
The conclusion that C. heveanensis has a tetrapolar mating type determination system can be further tested by two approaches. First, additional physical evidence that the two MAT loci are unlinked in the genome, based on electrophoretic separation and demonstration by chromoblot that the two lie on different chromosomes, whole genome sequence analysis, or independent segregation following meiosis, would all provide evidence the two are unlinked. Thus far it has not been possible to resolve chromosomes of this species by pulsed field gel electrophoresis, even employing conditions that work effectively for the closely related species C. neoformans, C. gattii, and C. amylolentus. Whole genome analysis may prove to be a more facile approach. The evidence that the two MAT loci are unlinked is that we have been unable, thus far, to find fosmids that physically span both loci or provide evidence the two are linked. It is formally possible that the PR locus could be linked to the right most end of the HD locus, which would place the two ∼20 kb apart. We view this as unlikely as we found no evidence for fosmids in the library that would span such a theoretical region. It is formally possible that the two loci are on the same chromosome but far enough apart to be unlinked genetically, or that the two loci are partially linked. Future studies will be necessary to examine this in further detail.
Second, additional genetic evidence involving the isolation and analysis of meiotic progeny could further establish this species as tetrapolar. The sine qua non of a tetrapolar mating system is the production of four types of meiotic progeny (A1 B1 crossed with A2 B2 yielding four mating types: A1 B1, A2 B2, A1 B2, and A2 B1). Thus far we have not been successful in isolating basidiospores by micromanipulation, and this is a challenge as these are often embedded in a matrix together with basidia. Moreover, in our experience attempts to purify progeny from fungal mating mixtures often leads to a high rate of contamination from the parental isolates, confounding analysis. Future studies could approach this using genetically marked strains to enable selection of meiotic progeny to determine their mating type. A further implication of our findings and conclusions is that the mating type determination system for the closely related species K. mangroviensis, currently classified as a bipolar species with α and A mating types, may also turn out to be tetrapolar with further detailed analysis.
Our findings provide insight and direct experimental support for key features of an evolutionary model that was proposed earlier for the origins of the unusual bipolar mating type locus of the pathogenic Cryptococcus species complex based on a comparative genomic analysis of three closely related species (Figure 10) [47]. The proposed model hypothesized that the ancestral state was a tetrapolar organism with two unlinked loci, one encoding homeodomain genes and the other pheromones and the pheromone receptor that evolved in a stepwise fashion to the large bipolar mating type alleles in C. neoformans and C. gattii. In this model, two unlinked MAT loci first expanded by acquiring additional genes related to sexual reproduction, resulting in a series of gene strata of different evolutionary ages. Second, the two MAT loci fused via a translocation event in one of the two mating types, resulting in an intermediate “tripolar” state. Third, gene conversion between the linked and unlinked sex determinants resulted in fusion of the two loci in the other mating type and collapse to a bipolar state. Finally, more recent gene conversions and rearrangements occurred giving rise to the extant MAT alleles observed today.
Fig. 10. A model depicting the evolution of the bipolar MAT locus in the pathogenic Cryptococcus species from ancestral unlinked homeodomain and pheromone/receptor loci. 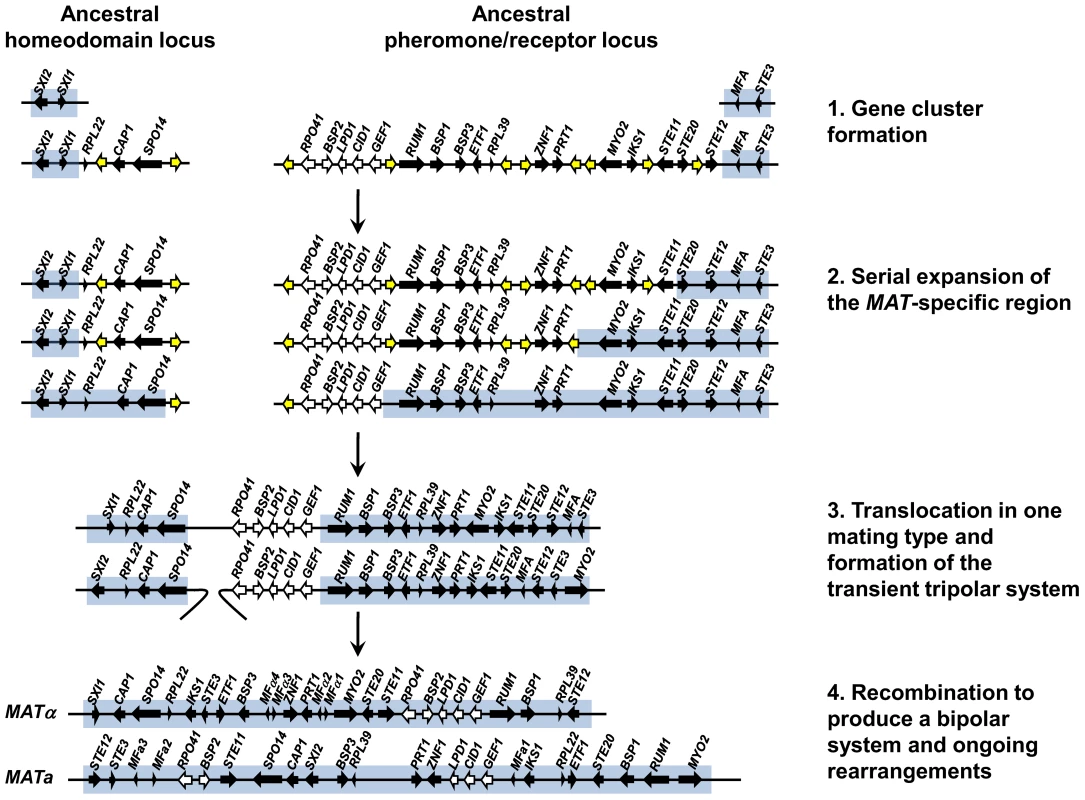
First, mating-related genes together with other genes were acquired into two unlinked loci. Second, the mating type specific region including only the homeodomain genes in the HD locus and the pheromone and the pheromone receptor gene in the PR locus expanded serially by rearrangements or insertions leading to suppressed recombination. During expansion, some of the genes were relocated to different parts of the genome and others were lost. Third, the two loci were combined to a single locus in one mating type by a translocation event forming a transient tripolar system. Fourth, recombination between the mating types occurred to form the bipolar system. Ongoing inversions and rearrangements fashioned the extant C. neoformans/C. gattii MAT locus. Genes shown with black or white arrows are those found in the C. neoformans/C. gattii MAT locus, those in white exhibit a species-specific phylogeny, and those in yellow are not linked to the extant MAT locus in the pathogenic Cryptococcus species complex. Our analysis of the C. heveanensis mating type locus alleles reveals two large gene clusters, one corresponding to the left half of the Cryptococcus mating type locus encoding homeodomain genes and the other to the right half encoding pheromones, pheromone receptors, and signaling components. This organization serves as a molecular window on the organization of the MAT locus in the last shared common ancestor to the two lineages and represents key features of the hypothesized intermediate stages in the MAT evolutionary model (Figure 10). First, we see that the two ancestral MAT loci are probably unlinked and in some cases appear to have acquired additional related flanking genes. Second, the two large gene clusters have been formed, but have not yet undergone fusion to the tripolar or bipolar state. Given that two related large unlinked gene clusters are also present in the related species C. amylolentus (Findley and Heitman, unpublished results), the most parsimonious model is that the organization of MAT in C. heveanensis represents the ancestral state prior to fusion, rather than the result of a recent translocation that separated a contiguous MAT allele. Third, the strata of most recently acquired genes (RPO41, BSP2, GEF1, CID1, and LPD1) are all present in the right gene cluster linked to the pheromone and pheromone receptor genes, and thus were already present prior to linkage of the two regions. This differs in detail from the originally proposed model in which RPO41 and BSP2 had been depicted as linked to the homeodomain gene cluster, and GEF1, CID1, and LPD1 as linked to the pheromone/receptor locus [47]. It also raises the likelihood that these genes are linked because they are functionally related in some way to MAT function, rather than being simply entrapped when the two unlinked MAT loci were fused by translocation. We note that the organization of the two gene clusters in C. heveanensis represents a juxtapositioning of MAT-specific genes (SXI1, SXI2) with genes that are part of the MAT locus in C. neoformans/C. gattii but not yet MAT-specific in C. heveanensis. Similarly, for the pheromone/receptor gene cluster, sequences that are not mating type-specific are embedded between mating type-specific sequences. These gene clusters therefore represent transitional intermediates representing MAT loci to which genes of related function have been recruited, but not yet captured into the mating type-specific regions of the genome or begun to diverge into mating type-specific alleles.
The model presented in Figure 10 represents our model for the events that led to the formation of a large bipolar mating type locus from a tetrapolar ancestral system. The proposed expansion of the ancestral HD and PR loci would likely have involved rearrangements facilitated by repetitive sequences that accumulate in recombinationally suppressed regions of the genome. If these genes have a function in sexual reproduction, as many do, this could have sustained the organization of the locus until the two loci fused. Some features of the gene strata apparent in the extant C. neoformans and C. gattii MAT alleles reflect episodic gene conversion events that occurred after the two ancestral MAT loci fused [44], [47]. Thus, multiple mechanisms operated involving acquisition of linked genes of related function into two expanding gene clusters, as is apparent here for the STE12 gene linked to the PR locus, and gene conversion events between alleles both prior to and following linkage of the HD and PR loci that influenced the evolutionary trajectory and plasticity of this dynamic region of the genome.
A key finding of this comparative analysis is that two divergently oriented homeodomain genes are present in the MAT locus of C. heveanensis, whereas only one or the other homeodomain gene is present in the a or α MAT alleles of C. neoformans and C. gattii. This provides evidence that, like other tetrapolar basidiomycete fungi such as U. maydis, Schizophyllum commune, and C. cinerea, the ancestral Cryptococcal MAT locus contained two paired, divergent homeodomain genes and one or the other was lost recently in the C. neoformans/C. gattii lineage. Examples of homeodomain gene loss are also seen in MAT of pathogenic Candida species [79]–[81]. For example, the alpha2 homeodomain gene has been lost in two haploid emerging yeast pathogenic species, Candida lusitaniae and Candida guillieromondii, both of which have retained complete sexual cycles, and in addition C. guilliermondii has also lost the a1 homeodomain gene. As discussed elsewhere [17], the transition from paired to solo homeodomain genes further serves to restrict outbreeding potential. In species with paired homeodomain genes, there are two opportunities to form functional heterodimeric pairs following cell fusion, whereas species with only a single MAT encoded homeodomain protein have only one chance to form a productive heterodimer required for completion of sexual reproduction. A second key finding is that the C. heveanensis A locus (PR) is at least biallelic; when additional isolates become available it will be possible to test if other extant alleles are present (triallelic or multiallelic). Previous studies of U. maydis, which is tetrapolar with a biallelic pheromone/pheromone receptor locus [75], and the closely related species Sporisorium reilianum [77], which is tetrapolar and triallelic at the pheromone/pheromone receptor locus, provide evidence that transitions have occurred in other fungi from multiallelic/multiallelic to multiallelic/biallelic mating type determination systems. These transitions involving the loss of allelic diversity at one of the two unlinked mating type alleles result in a relative decrease in outcrossing frequency but do not alter the restriction to inbreeding engendered by the tetrapolar state [17]. Thus, a loss of allelic diversity at the A locus encoding pheromone and pheromone receptors may have similarly occurred in the lineages leading to C. heveanensis. The outcome for the species then in both cases is a restriction in outbreeding potential, which may serve to further promote inbreeding and clonality in the population, and this transition in sexuality may have contributed to the emergence of this clade of organisms as successful pathogens of animals, including humans.
Materials and Methods
Strains and media
Strains used in this study are listed in Table 1. C. heveanensis type strain CBS 569, CBS 9459, and CBS 6097 were obtained from the Centraalbureau voor Schimmelcultures (CBS), Netherlands. Other strains of C. heveanensis were from the BIOTEC Culture Collection (BCC), Thailand [70]. Strains were grown in YPD broth or on YPD solid medium. Mating assays were performed on V8 medium (pH 5 and pH 7) [82] and Murashige and Skoog (MS) medium minus sucrose (Sigma-Aldrich, [83]) by pairwise inoculation of strains and incubation at room temperature in the dark on dry upright media without parafilm.
Standard and Latin descriptions of the sexual stage
Standard description: Kwoniella heveanensis Metin, Findley, & Heitman sp. nov.
Etymol.: The epithet is chosen to be identical with that of Cryptococcus heveanensis (Groenewege) Baptist & Kurtzman comb. nov [84].
Heterothallic fungus. Hyphae dikaryotic, clamp connections fused. Basidia clustered, submerged initially, finally on aerial hyphae, globose, 7.5–10.5 µm, cruciately septate, sterigmata not observed. Basidiospores subglobose, 2.3–3.3 µm, germinating by conidia.
Latin description: Kwoniella heveanensis Metin, Findley, & Heitman sp. nov.
Species heterothallica. Hyphae dikaryoticae, fibulae fusae. Basidia aggregata, primum submersa, deinde superficialia, globosa, 7.5–10.5 µm diam, septis cruciatis divisa, sterigmatibus carentia, Basidiosporae subglobosae, 2.3–3.3 µm diam, cito conidia proferentia.
Fluorescence-activated cell-sorting (FACS) analysis
The protocol for the preparation of cells for FACS was based on a previous protocol [85]. Briefly, ∼50 µl of cells scraped from overnight grown cultures of YPD plates were washed with PBS and fixed in 1 ml of 70% ethanol overnight. Then the tubes were centrifuged and the supernatants were discarded. After washing the cells with 1 ml of NS buffer (10 mM Tris-HCl pH 7.2, 0.25 M sucrose, 1 mM EDTA, 1 mM MgCl2, 0.1 mM CaCl2, 0.1 mM ZnCl2), they were resuspended in 180 µl NS buffer. Then, 20 µl RNase A (10 mg/ml, Qiagen) and 5 µl of propidium iodide (0.5 mg/ml, CALBIOCHEM) were added. After incubating at room temperature in the dark with mixing for at least 2 hours, 17.5 µl of the cells were mixed with 700 µl of 50 mM Tris-Cl pH 7.5 and the tubes were sonicated for 45 seconds. Flow cytometry was performed on 10,000 cells and analyzed on the FL1 channel with a Becton-Dickinson FACScan.
Microscopy
For microscopic examination of mating, either the mating mix was inoculated into V8 medium (pH 5) medium plates, and later a block of agar containing the hyphae and basidia was transferred to microscope slides, or the mating mix was directly inoculated onto slides containing a thin layer of mating medium.
For staining, a modified version of a previously published protocol was used [86]. Calcofluor white (0.05% solution, BD) and Sytox green (5 mM, Invitrogen) were used to stain the cell wall and nuclei, respectively. The slide containing the agar piece with hyphae or basidia was first washed with PBS, then, 5–10 µl of Calcofluor white was added and mixed gently with a cover slip. After incubating for 15 min, the excess dye was washed away with PBS. Then fixing was performed by incubating the slide in fixing solution (3.7% formaldehyde, 1% triton-X100 in PBS) for 15 min and the slide was washed with PBS. After that, 5 to 10 µl of 1∶10 diluted Sytox green was spotted on the slide, mixed with a cover slip, and incubated for 15 min. Then the slide was washed with PBS, mounted with a cover slip, and examined under the microscope. Microscopy was performed with an Axioskop 2 plus upright microscope (Zeiss). Images were captured using an AxioCam MRm camera and AxioVision application software (Zeiss). Scanning electron microscopy was performed with an XL30 environmental SEM (FEI).
Fosmid library preparation
For library production, the CopyControl Fosmid Library Production Kit (Epicentre) was used according to the manufacturer's instructions. In each library, a total of ∼16,000 clones were picked into 96 well plates. Purified genomic DNA (∼40 kb fragments) was first sheared, end-repaired, and separated using contour-clamped homogenous electric field (CHEF) on a CHEF DR-II apparatus (Bio-Rad). The following conditions were used: 1 - to 6-sec switch time, 6 V/cm, 14°C for 14 to 15 hrs. The size-fractionated DNA was recovered by gel extraction and precipitated. The insert DNA was then ligated into the CopyControl pCC1FOS cloning-ready vector and incubated overnight at room temperature. The ligated DNA was packaged and plated on E. coli phage-resistant cells overnight at 37°C. Fosmid clones were then picked into 96 well plates and eventually transferred to 384 well plates, which were stored at −80°C.
DNA extraction, degenerate PCR, and library screening
For DNA isolation, strains were inoculated in 50 ml YPD medium, grown for two days at room temperature with shaking and centrifuged. After freezing at −80°C, the pellet was freeze-dried overnight and DNA was isolated as previously described [87].
Degenerate primers were designed for LPD1, RPO41, CAP1, ZNF1, STE20, IKS1, FAO1 and NOG2 based on known gene sequences from C. neoformans var. neoformans, C. neoformans var. grubii, and C. gattii. These primers (Table S2) were used in PCR reactions with C. heveanensis genomic DNA using Ex Taq polymerase (Takara) and the products obtained were gel extracted with QIAquick Gel Extraction Kit (Qiagen). The PCR fragments were cloned into pCR 2.1-TOPO using the TOPO TA Cloning Kit (Invitrogen). From the resulting colonies, plasmid isolation was performed with QIAprep Spin Miniprep Kit (Qiagen) and the plasmids were sequenced with M13F and M13R vector specific primers. After obtaining MAT gene sequences, specific primers were designed using a web-based program, Primer3 (http://frodo.wi.mit.edu/). After PCR with specific primers, the resulting gene fragments were gel-extracted as previously described and used as probes to screen the library.
For library screening, colony lifts were performed on Hybond membranes (Amersham) using standard protocols [88]. The PCR products were labeled with Prime-It II Random Primer Labeling Kit (Stratagene) with dCTP. Hybridization was performed using ULTRAhyb hybridization buffer (Ambion) according to the manufacturer's instructions.
Sequencing and assembly
Positive fosmids were isolated from 200 ml of overnight grown cultures using the Qiafilter plasmid midi kit (Qiagen). The fosmid DNA obtained was sheared using a hydroshear apparatus (Genomic Solutions) to 1.6–3 kb fragments. The gel extracted sheared DNA was then ligated to adapters. To prepare the vector, pUC18 plasmid DNA was cut with EcoRI and HindIII removing the polylinker site, and ligated to single stranded oligos that contain one end complementary to the restriction enzyme site, and the other non-complementary to itself to prevent self-ligation. The sheared and adapter-ligated DNA was then ligated to the pUC18-derived vector and the reaction was used to transform DH5α competent cells (Invitrogen). White colonies were inoculated onto 96 well plates and plasmid isolation was performed with DirectPrep 96 MiniPrep kit (Qiagen). For each positive fosmid, four to five 96 well plates were used.
Sequencing reactions were performed with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and analyzed on PE3700 96-capillary sequencer (Applied Biosystems) in the Sequencing Facility at Duke University. The sequence reads were assembled using Phred, Phrap, and Consed program packages [89]–[91]. The gaps were closed by designing primers from contig ends using Primer3, amplifying across gaps by PCR and sequencing both strands. Short gaps were closed using iProof polymerase (Biorad) and long gaps were closed using LA Taq polymerase (Takara).
Southern blot analysis
For Southern hybridization of C. heveanensis strains, 5 µg of genomic DNA from each strain was digested with EcoRV, electrophoresed on a 0.8% agarose gel, and blotted onto Hybond membranes (Amersham) using standard protocols [88]. The membrane was then hybridized to MAT gene probes generated by PCR as described above.
Annotations and bioinformatic analyses
Annotations of the genes were done manually by comparing the 3-frame translated C. heveanensis sequences with the most similar protein sequences identified by BLASTX. Genbank accession numbers are, GU129941 for CBS 569 homeodomain locus, GU205379 for CBS 569 pheromone/receptor locus, GU129940 for BCC 8398 pheromone/receptor locus, GU129942, GU129943, GU129944, GU129945, GU129946, and GU129947 for homeodomain loci of the strains BCC 8313, BCC 8384, BCC 8398, BCC 11754, BCC 11757, AND BCC 15000, respectively. Genbank accession numbers for D1/D2 and ITS regions are GU585738 and GU585749 for CBS 9459, GU585739 and GU585750 for CBS 6097, GU585740 and GU585751 for BCC 8305, GU585741 and GU585752 for BCC 8313, GU585742 and GU585753 for BCC 8384, GU585743 and GU585754 for BCC 8396, GU585744 and GU585755 for BCC 8398, GU585745 and GU585756 for BCC 11754, GU585746 and GU585757 for BCC 11757, and GU585747 and GU585758 for BCC 15000, respectively. The accession number of the ITS region of CBS 569 is GU585748.
Percent sequence identity plots and dot plots were generated using the MultiPipMaker [92]. Pairwise sequence identity values were calculated using Geneious 4.6.4.
The comparison plot between pheromone/receptor loci of strains CBS 569 and BCC 8398 was produced using Artemis Comparison Tool Release 8 [93]. The input comparison file was generated using WebACT with the tBlastx algorithm.
Homeodomain regions of the proteins Sxi1 and Sxi2 were predicted by comparison to the previously characterized homedomain proteins. Helical content of the N-terminal domains of Sxi1 and Sxi2 were predicted with Jpred 3 [94]. Nuclear localization signals were predicted with PSORT II [95].
Phylogenetic analysis
Phylogenetic analysis was conducted on coding sequences using MEGA4 [96]. The phylogenetic relationship was inferred using the Maximum Parsimony method.
Supporting Information
Zdroje
1. BellG
1982 The Masterpiece of Nature: the Evolution and Genetics of Sexuality. Berkeley University of California Press
2. SmithJM
1978 The Evolution of Sex. Cambridge, UK Cambridge University Press
3. GoddardMR
GodfrayHC
BurtA
2005 Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434 636 640
4. BirdsellJ
WillsC
1996 Significant competitive advantage conferred by meiosis and syngamy in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 93 908 912
5. KaltzO
BellG
2002 The ecology and genetics of fitness in Chlamydomonas. XII. repeated sexual episodes increase rates of adaptation to novel environments. Evolution 56 1743 1753
6. GrimbergB
ZeylC
2005 The effects of sex and mutation rate on adaptation in test tubes and to mouse hosts by Saccharomyces cerevisiae. Evolution 59 431 438
7. XuJ
2005 Cost of interacting with sexual partners in a facultative sexual microbe. Genetics 171 1597 1604
8. KronstadJW
StabenC
1997 Mating type in filamentous fungi. Annu Rev Genet 31 245 276
9. CasseltonLA
OlesnickyNS
1998 Molecular genetics of mating recognition in basidiomycete fungi. Microbiol Mol Biol Rev 62 55 70
10. FraserJA
HsuehY-P
FindleyK
HeitmanJ
2007 Evolution of the mating-type locus - the basidiomycetes.
HeitmanJ
KronstadJW
TaylorJW
CasseltonLA
Sex in Fungi: molecular determination and evolutionary implications Washington, DC ASM Press 19 34
11. GlassNL
NelsonMA
1994 Mating-type genes in mycelial ascomycetes.
EsserK
LemkePA
The Mycota I Growth, Differentiation and Sexualtiy Berlin Heidelberg Springer-Verlag 295 306
12. DebuchyR
TurgeonBG
2006 Mating-Type structure, evolution, and function in euascomycetes.
EsserK
The Mycota I - Growth, Differentiation and Sexuality Berlin Heidelberg Springer-Verlag 293 324
13. ButlerG
2007 The evolution of MAT: the ascomycetes.
HeitmanJ
KronstadJW
TaylorJW
CasseltonLA
Sex in Fungi: molecular determination and evolutionary implications Washington, DC ASM Press 3 18
14. CasseltonLA
KuesU
1994 Mating-type genes in Homobasidiomycetes.
EsserK
LemkePA
The Mycota I -Growth, Differentiation and Sexuality Berlin Heidelberg Springer-Verlag 307 321
15. CasseltonLA
ChallenMP
2006 The mating type genes of the Basidiomycetes.
EsserK
The Mycota I -Growth, Differentiation and Sexuality Berlin Heidelberg Springer-Verlag 356 374
16. KotheE
1996 Tetrapolar fungal mating types: sexes by the thousands. FEMS Microbiol Rev 18 65 87
17. HsuehY-P
HeitmanJ
2008 Orchestration of sexual reproduction and virulence by the fungal mating-type locus. Curr Opin Microbiol 11 517 524
18. FraserJA
HeitmanJ
2005 Chromosomal sex-determining regions in animals, plants and fungi. Curr Opin Genet Dev 15 645 651
19. NiermanWC
PainA
AndersonMJ
WortmanJR
KimHS
2005 Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438 1151 1156
20. GalaganJE
CalvoSE
CuomoC
MaLJ
WortmanJR
2005 Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438 1105 1115
21. FraserJA
StajichJE
TarchaEJ
ColeGT
InglisDO
2007 Evolution of the mating type locus: insights gained from the dimorphic primary fungal pathogens Histoplasma capsulatum, Coccidioides immitis, and Coccidioides posadasii. Eukaryot Cell 6 622 629
22. BubnickM
SmulianAG
2007 The MAT1 locus of Histoplasma capsulatum is responsive in a mating type-specific manner. Eukaryot Cell 6 616 621
23. MandelMA
BarkerBM
KrokenS
RounsleySD
OrbachMJ
2007 Genomic and population analyses of the mating type loci in Coccidioides species reveal evidence for sexual reproduction and gene acquisition. Eukaryot Cell 6 1189 1199
24. PöggelerS
HoffB
KückU
2008 Asexual cephalosporin C producer Acremonium chrysogenum carries a functional mating type locus. Appl Environ Microbiol 74 6006 6016
25. BurtA
CarterDA
KoenigGL
WhiteTJ
TaylorJW
1996 Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc Natl Acad Sci U S A 93 770 773
26. PaolettiM
RydholmC
SchwierEU
AndersonMJ
SzakacsG
2005 Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr Biol 15 1242 1248
27. PringleA
BakerDM
PlattJL
WaresJP
LatgeJP
2005 Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59 1886 1899
28. RydholmC
SzakacsG
LutzoniF
2006 Low genetic variation and no detectable population structure in Aspergillus fumigatus compared to closely related Neosartorya species. Eukaryot Cell 5 650 657
29. HornBW
Ramirez-PradoJH
CarboneI
2009 Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet Biol 46
30. O'GormanCM
FullerHT
DyerPS
2009 Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457 471 474
31. GrosseV
KrappmannS
2008 The asexual pathogen Aspergillus fumigatus expresses functional determinants of Aspergillus nidulans sexual development. Eukaryot Cell 7 1724 1732
32. PyrzakW
MillerKY
MillerBL
2008 Mating type protein Mat1-2 from asexual Aspergillus fumigatus drives sexual reproduction in fertile Aspergillus nidulans. Eukaryot Cell 7 1029 1040
33. Kwon-ChungKJ
1975 A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67 1197 1200
34. Kwon-ChungKJ
1976a Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68 821 833
35. Kwon-ChungKJ
1976b A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia 68 943 946
36. FraserJA
SubaranRL
NicholsCB
HeitmanJ
2003 Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans variety gattii: implications for an outbreak on Vancouver Island. Eukaryot Cell 2 1036 1045
37. KellerSM
VivianiMA
EspostoMC
CogliatiM
WickesBL
2003 Molecular and genetic characterization of a serotype A MATa Cryptococcus neoformans isolate. Microbiology 149 131 142
38. NielsenK
CoxGM
WangP
ToffalettiDL
PerfectJR
2003 Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α isolates. Infect Immun 71 4831 4841
39. GilesSS
DagenaisTR
BottsMR
KellerNP
HullCM
2009 Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun: Epub ahead of print, May 18
40. BottsMR
GilesSS
GatesMA
KozelTR
HullCM
2009 Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryot Cell 8 595 605
41. HullCM
HeitmanJ
2002 Genetics of Cryptococcus neoformans. Annu Rev Genet 36 557 615
42. WickesBL
2002 The role of mating type and morphology in Cryptococcus neoformans pathogenesis. Int J Med Microbiol 292 313 329
43. McClellandCM
ChangYC
VarmaA
Kwon-ChungKJ
2004 Uniqueness of the mating system in Cryptococcus neoformans. Trends Microbiol 12 208 212
44. LengelerKB
WangP
CoxGM
PerfectJR
HeitmanJ
2000 Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc Natl Acad Sci USA 97 14555 14460
45. KarosM
ChangYC
McClellandCM
ClarkeDL
FuJ
2000 Mapping of the Cryptococcus neoformans MATα locus: presence of mating type-specific mitogen-activated protein kinase cascade homologs. J Bacteriol 182 6222 6227
46. LengelerKB
FoxDS
FraserJA
AllenA
ForresterK
2002 Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot Cell 1 704 718
47. FraserJA
DiezmannS
SubaranRL
AllenA
LengelerKB
2004 Convergent evolution of chromosomal sex-determining regions in the animal and fungal kingdoms. PLoS Biol 2 e384 doi:10.1371/journal.pbio.0020384
48. RenP
RoncagliaP
SpringerDJ
FanJ
ChaturvediV
2005 Genomic organization and expression of 23 new genes from MATα locus of Cryptococcus neoformans var. gattii. Biochem Biophys Res Commun 326 233 241
49. FraserJA
GilesSS
WeninkEC
Geunes-BoyerSG
WrightJR
2005 Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437 1360 1364
50. HullCM
DavidsonRC
HeitmanJ
2002 Cell identity and sexual development in Cryptococcus neoformans are controlled by the mating-type-specific homeodomain protein Sxi1α. Genes Dev 16 3046 3060
51. HullCM
BoilyMJ
HeitmanJ
2005 Sex-specific homeodomain proteins Sxi1α and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot Cell 4 526 535
52. HsuehYP
IdnurmA
HeitmanJ
2006 Recombination hotspots flank the Cryptococcus mating-type locus: implications for the evolution of a fungal sex chromosome. PLoS Genet 2 e184 doi:10.1371/journal.pgen.0020184
53. HsuehYP
FraserJA
HeitmanJ
2008 Transitions in sexuality: recapitulation of an ancestral tri - and tetrapolar mating system in Cryptococcus neoformans. Eukaryot Cell 7 1847 1855
54. FindleyK
Rodriguez-CarresM
MetinB
KroissJ
FonsecaA
2009 Phylogeny and phenotypic characterization of pathogenic Cryptococcus species and closely related saprobic taxa in the Tremellales. Eukaryot Cell 8 353 361
55. Statzell-TallmanA
BellochC
FellJW
2008 Kwoniella mangroviensis gen. nov., sp.nov. (Tremellales, Basidiomycota), a teleomorphic yeast from mangrove habitats in the Florida Everglades and Bahamas. FEMS Yeast Res 8 103 113
56. OhnoS
1967 Sex chromosomes and sex-linked genes;
A LabhartTM
SamuesLT
ZanderJ
Berlin, Heidelberg, and New York Springer-Verlag
57. FraserJA
HeitmanJ
2004 Evolution of fungal sex chromosomes. Mol Microbiol 51 299 306
58. Marshall GravesJA
2008 Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annu Rev Genet 42 565 586
59. KamperJ
ReichmannM
RomeisT
BolkerM
KahmannR
1995 Multiallelic recognition: nonself-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell 81 73 83
60. SchulzB
BanuettF
DahlM
SchlesingerR
SchaferW
1990 The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60 295 306
61. JamesTY
LiouSR
VilgalysR
2004 The genetic structure and diversity of the A and B mating-type genes from the tropical oyster mushroom, Pleurotus djamor. Fungal Genet Biol 41 813 825
62. LoftusBJ
FungE
RoncagliaP
RowleyD
AmedeoP
2005 The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307 1321 1324
63. Asante-OwusuRN
BanhamAH
BohnertHU
MellorEJ
CasseltonLA
1996 Heterodimerization between two classes of homeodomain proteins in the mushroom Coprinus cinereus brings together potential DNA-binding and activation domains. Gene 172 25 31
64. HoCY
AdamsonJG
HodgesRS
SmithM
1994 Heterodimerization of the yeast MATa1 and MATα2 proteins is mediated by two leucine zipper-like coiled-coil motifs. EMBO J 13 1403 1413
65. BanhamAH
Asante-OwusuRN
GottgensB
ThompsonS
KingsnorthCS
1995 An N-terminal dimerization domain permits homeodomain proteins to choose compatible partners and initiate sexual development in the mushroom Coprinus cinereus. Plant Cell 7 773 783
66. SpitA
HylandRH
MellorEJ
CasseltonLA
1998 A role for heterodimerization in nuclear localization of a homeodomain protein. Proc Natl Acad Sci U S A 95 6228 6233
67. KronstadJW
LeongSA
1990 The b mating-type locus of Ustilago maydis contains variable and constant regions. Genes Dev 4 1384 1395
68. CaldwellGA
NaiderF
BeckerJM
1995 Fungal lipopeptide mating pheromones: a model system for the study of protein prenylation. Microbiol Rev 59 406 422
69. SakagamiY
YoshidaM
IsogaiA
SuzukiA
1981 Peptidal sex hormones inducing conjugation tube formation in compatible mating-tye cells of Tremella mesenterica. Science 212 1525 1527
70. NakaseT
JindamorakotS
Am-InS
PotacharoenW
TanticharoenM
2006 Yeast biodiversity in tropical forests of Asia.
PeterG
RosaC
The Yeast Handbook: Biodiversity and Ecophysiology of Yeasts: Springer Berlin Heidelberg 441 460
71. KahmannR
BolkerM
1996 Self/nonself recognition in fungi: old mysteries and simple solutions. Cell 85 145 148
72. FeldbruggeM
KamperJ
SteinbergG
KahmannR
2004 Regulation of mating and pathogenic development in Ustilago maydis. Curr Opin Microbiol 7 666 672
73. KüesU
2000 Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol Mol Biol Revl 64 316 353
74. BrownAJ
CasseltonLA
2001 Mating in mushrooms: increasing the chances but prolonging the affair. Trends Genet 17 393 400
75. BölkerM
UrbanM
KahmannR
1992 The a mating type locus of U. maydis specifies cell signaling components. Cell 68 441 450
76. Niculita-HirzelH
LabbeJ
KohlerA
le TaconF
MartinF
2008 Gene organization of the mating type regions in the ectomycorrhizal fungus Laccaria bicolor reveals distinct evolution between the two mating type loci. New Phytol 180 329 342
77. SchirawskiJ
HeinzeB
WagenknechtM
KahmannR
2005 Mating type loci of Sporisorium reilianum: novel pattern with three a and multiple b specificities. Eukaryot Cell 4 1317 1327
78. BakkerenG
KamperJ
SchirawskiJ
2008 Sex in smut fungi: Structure, function and evolution of mating-type complexes. Fungal Genet Biol 45 Suppl 1 S15 21
79. ButlerG
RasmussenMD
LinMF
SantosMA
SakthikumarS
2009 Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459 657 662
80. ReedyJL
FloydAM
HeitmanJ
2009 Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr Biol 19 891 899
81. SollDR
PujolC
SrikanthaT
2009 Sex: deviant mating in yeast. Curr Biol 19 R509 511
82. Kwon-ChungKJ
BennettJE
RhodesJC
1982 Taxonomic studies on Filobasidiella species and their anamorphs. Antonie van Leeuwenhoek 48 25 38
83. XueC
TadaY
DongX
HeitmanJ
2007 The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 1 263 273
84. BaptistJN
KurtzmanCP
1976 Comparative enzyme patterns in Cryptococcus laurentii and its taxonomic varieties. Mycologia 68 1195 1203
85. TanakaR
TaguchiH
TakeoK
MiyajiM
NishimuraK
1996 Determination of ploidy in Cryptococcus neoformans by flow cytometry. J Med Vet Mycology 34 299 301
86. WickesBL
MayorgaME
EdmanU
EdmanJC
1996 Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc Natl Acad Sci USA 93 7327 7331
87. PitkinJW
PanaccioneDG
WaltonJD
1996 A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142 1557 1565
88. SambrookJ
FritschEF
ManiatisT
1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY Cold Spring Harbor Laboratory Press
89. EwingB
HillierL
WendlMC
GreenP
2008 Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res 8 175 185
90. EwingB
GreenP
1998 Base-calling of automated sequencer traces using Phred. II. Error probabilities. Genome Res 8 186 194
91. GordonD
AbajianC
GreenP
1998 Consed: A graphical tool for sequence finishing. Genome Res 8 195 202
92. SchwartzS
ZhangZ
FrazerKA
SmitA
RiemerC
2000 PipMaker–a web server for aligning two genomic DNA sequences. Genome Res 10 577 586
93. CarverTJ
RutherfordKM
BerrimanM
RajandreamMA
BarrellBG
2005 ACT: the Artemis Comparison Tool. Bioinformatics 21 3422 3423
94. ColeC
BarberJD
BartonGJ
2008 The Jpred 3 secondary structure prediction server. Nucleic Acids Res 36 W197 201
95. NakaiK
HortonP
1999 PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 24 34 36
96. TamuraK
DudleyJ
NeiM
KumarS
2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein SignalingČlánek B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish EmbryoČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



