-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaB1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
The B1 SOX transcription factors SOX1/2/3/19 have been implicated in various processes of early embryogenesis. However, their regulatory functions in stages from the blastula to early neurula remain largely unknown, primarily because loss-of-function studies have not been informative to date. In our present study, we systematically knocked down the B1 sox genes in zebrafish. Only the quadruple knockdown of the four B1 sox genes sox2/3/19a/19b resulted in very severe developmental abnormalities, confirming that the B1 sox genes are functionally redundant. We characterized the sox2/3/19a/19b quadruple knockdown embryos in detail by examining the changes in gene expression through in situ hybridization, RT–PCR, and microarray analyses. Importantly, these phenotypic analyses revealed that the B1 SOX proteins regulate the following distinct processes: (1) early dorsoventral patterning by controlling bmp2b/7; (2) gastrulation movements via the regulation of pcdh18a/18b and wnt11, a non-canonical Wnt ligand gene; (3) neural differentiation by regulating the Hes-class bHLH gene her3 and the proneural-class bHLH genes neurog1 (positively) and ascl1a (negatively), and regional transcription factor genes, e.g., hesx1, zic1, and rx3; and (4) neural patterning by regulating signaling pathway genes, cyp26a1 in RA signaling, oep in Nodal signaling, shh, and mdkb. Chromatin immunoprecipitation analysis of the her3, hesx1, neurog1, pcdh18a, and cyp26a1 genes further suggests a direct regulation of these genes by B1 SOX. We also found an interesting overlap between the early phenotypes of the B1 sox quadruple knockdown embryos and the maternal-zygotic spg embryos that are devoid of pou5f1 activity. These findings indicate that the B1 SOX proteins control a wide range of developmental regulators in the early embryo through partnering in part with Pou5f1 and possibly with other factors, and suggest that the B1 sox functions are central to coordinating cell fate specification with patterning and morphogenetic processes occurring in the early embryo.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000936
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000936Summary
The B1 SOX transcription factors SOX1/2/3/19 have been implicated in various processes of early embryogenesis. However, their regulatory functions in stages from the blastula to early neurula remain largely unknown, primarily because loss-of-function studies have not been informative to date. In our present study, we systematically knocked down the B1 sox genes in zebrafish. Only the quadruple knockdown of the four B1 sox genes sox2/3/19a/19b resulted in very severe developmental abnormalities, confirming that the B1 sox genes are functionally redundant. We characterized the sox2/3/19a/19b quadruple knockdown embryos in detail by examining the changes in gene expression through in situ hybridization, RT–PCR, and microarray analyses. Importantly, these phenotypic analyses revealed that the B1 SOX proteins regulate the following distinct processes: (1) early dorsoventral patterning by controlling bmp2b/7; (2) gastrulation movements via the regulation of pcdh18a/18b and wnt11, a non-canonical Wnt ligand gene; (3) neural differentiation by regulating the Hes-class bHLH gene her3 and the proneural-class bHLH genes neurog1 (positively) and ascl1a (negatively), and regional transcription factor genes, e.g., hesx1, zic1, and rx3; and (4) neural patterning by regulating signaling pathway genes, cyp26a1 in RA signaling, oep in Nodal signaling, shh, and mdkb. Chromatin immunoprecipitation analysis of the her3, hesx1, neurog1, pcdh18a, and cyp26a1 genes further suggests a direct regulation of these genes by B1 SOX. We also found an interesting overlap between the early phenotypes of the B1 sox quadruple knockdown embryos and the maternal-zygotic spg embryos that are devoid of pou5f1 activity. These findings indicate that the B1 SOX proteins control a wide range of developmental regulators in the early embryo through partnering in part with Pou5f1 and possibly with other factors, and suggest that the B1 sox functions are central to coordinating cell fate specification with patterning and morphogenetic processes occurring in the early embryo.
Introduction
The developing embryo must control gene expression to coordinate various embryonic processes such as cell fate specification, embryo patterning and morphogenesis. During the embryonic stages from the blastula to neurula, the coupling of cell lineage specification and gastrulation cell movements is particularly evident. There is also now an increased understanding of the regulatory mechanisms underlying each cell state and each morphogenetic process, but the precise mechanisms that coordinate these events have remained elusive. The group B1 SOX transcription factors are good candidates as coordinators of these embryonic processes. Indeed, they have been implicated in cell fate specification in the early embryo [1]–[7] and also patterning and morphogenetic processes [8]–[10].
B1 Sox comprises sox1a/1b/2/3/19a/19b in zebrafish and Sox1/2/3 in amniotes [11]. The sox19a/19b genes are evolutionary orthologs of mammalian Sox15 (group G), although Sox15 has now been shown to have functionally diversified from the authentic B1 Sox paralogs [11]. Overall, the regulatory functions of B1 sox genes appear to be conserved as a group across vertebrate species, although the paralogs are often differentially employed in a particular process [12]. In zebrafish, sox3/19a/19b are expressed in the blastula [11], whereas the corresponding early expression in mice is covered by Sox2 [1]. Following this stage, the B1 sox genes are thought to be important for specification of the embryonic ectoderm into the neuroectoderm lineage. During this process, their expression becomes confined to the neuroectoderm [11]. As development proceeds to the neurula stage, expression of the B1 sox genes continues in neural precursors, where they function to maintain the neural progenitor states [13]–[15].
The similarities in the characteristics of the B1 SOX proteins as transcriptional regulators [11], [15] suggest redundant functions in tissues where they are coexpressed. In support of this notion, single Sox1 or Sox3 knockout mice display only mild abnormalities in the central nervous system (CNS), presumably because of extensive coexpression of Sox1/2/3 [16]–[18], whereas Sox2-null mouse embryos die around implantation, reflecting its exclusive expression in the ICM [1]. Consistently, a single sox2 or sox3 knockdown (KD) in zebrafish causes only mild developmental abnormalities [19], [20]. Xenopus studies utilizing dominant-negative forms of SOX2 indicate a specific role of Sox2 in neuroectoderm differentiation [2]. To date, however, the overall functions of the B1 sox genes have not been systematically investigated from the blastula to early neurula stages.
An important characteristic of the B1 SOX proteins is that they form a complex with co-DNA-binding partner factors to target specific sequences and this enables them to participate in the regulation of various cell states [21]. The SOX2-Oct3/4 (Pou5f1) complex is a central player in regulatory networks in the ICM and ES cells [1], [22], [23]. Potential target genes of SOX2 and Oct3/4 in ES cells have been identified through genome-wide chromatin immunoprecipitation (ChIP) and microarray expression analyses [22], [24]. The involvement of other B1 SOX-partner combinations in the regulation of specific cell states has also been reported, e.g., B1 SOX-POUIII factors in neural precursors [15] and B1 SOX-Pax6 in lens cells [25]. However, neither B1 SOX-dependent regulatory processes nor B1 SOX target genes in the developing early embryo have been extensively investigated.
In our present study, we performed single to quadruple knockdowns of sox2/3/19a/19b in zebrafish embryos and confirmed that these four genes are functionally redundant in early development. More importantly, phenotypic analyses of the sox2/3/19a/19b quadruple KD embryos uncovered developmental process-specific functions of B1 sox. In the blastula, B1 sox genes regulate the activation of the bmp2b/7 genes, which is critical for dorsoventral (DV) patterning. During gastrulation, B1 sox also regulate the expression of pcdh18a/18b and wnt11, a non-canonical Wnt ligand gene, which together play a role in convergence and extension (C&E) movements. In neural development, the B1 sox genes are essential for the proper regulation of neural bHLH genes of both the her/Hes and proneural classes, and also for the activation of region-specific transcription factor genes such as hesx1, zic1 and rx3. Moreover, the activity of B1 sox is required for the neural expression of various signaling pathway genes: cyp26a1 in RA signaling, oep in Nodal signaling, shh, and also mdkb. ChIP analysis of the her3, hesx1, neurog1, pcdh18a and cyp26a1 genes suggests their direct regulation by B1 SOX. These findings indicate that B1 SOX proteins play a central role in coordinating cell fate specification, embryo patterning and morphogenesis by controlling a wide variety of developmental regulators in the early embryo.
We have also found an interesting overlap between the early phenotypes of the B1 sox quadruple KD embryos and the maternal-zygotic (MZ) spg embryos that are devoid of pou5f1 activity [26]–[29]. This highlights a broad role of the B1 SOX-Pou5f1 complex from the blastoderm to early neural stages of development.
Results
Loss-of-function analysis of the B1 sox genes in the zebrafish embryo
Among the B1 sox genes of zebrafish, sox2/3/19a/19b are expressed at high levels during early development with extensive regional overlaps [11]. sox19b mRNA is maternally supplied. sox3 and sox19a are activated around the 1000-cell stage, and sox2 around the 30% epiboly (30%E) stage [11]. The expression of sox3/19a/19b initially covers the entire blastoderm, but gradually disappears at the embryonic margin after 30%E (Figure S1A). At the shield stage, the expression of sox2/3/19a/19b covers the future ectoderm, but then becomes confined to the presumptive neuroectoderm [11]. Expression of sox1a/1b is initiated only during late gastrulation stages (Figure S1B). These expression patterns suggest that sox2/3/19a/19b are involved in early processes of zebrafish development.
To investigate the function of B1 sox in early stage embryos, we knocked down sox2/3/19a/19b either individually or in combination using morpholino antisense oligonucleotides (MO). Two different MOs were simultaneously used to block translation of each B1 sox gene, which ensures efficient knockdown even when using reduced amounts of MOs [20]. With this double MO strategy, an approximately 90% reduction in translation was achieved using 1.8 ng of a 1∶1 mixture of two MOs, as judged by their effects on luciferase reporters carrying MO-targeting 5′-UTR sequences (sox2 [20]; sox3/19a/19b, Figure S2A). By western blotting, we confirmed the efficient inhibition of the synthesis of endogenous B1 SOX proteins (Figure S2B).
No gross abnormalities were observed in the embryo morphology when any one of sox2/3/19a/19b was knocked down (single KD, Figure 1B), although the development of the CNS may be slightly perturbed and 75% of the sox2 morphants showed an up-turned tail phenotype (Figure 1Ba). When any three of sox2/3/19a/19b were simultaneously knocked down (triple KD), a range of morphological abnormalities was observed depending on the combination of KD targets (Figure 1C). Triple KDs of sox2/19a/19b and sox2/3/19b caused only mild morphological defects (Figure 1Cc and 1Cd), presumably because the remaining sox3 and sox19a genes, respectively, mostly cover the B1 sox expression domains. sox3/19a/19b morphants often showed stronger yet variable defects in their posterior structures (Figure 1Ca and 1Cb), presumably reflecting the weak sox2 expression in the posterior neuroectoderm. sox2/3/19a morphants appeared normal during gastrulation, but later developed morphological abnormalities (Figure 1Ce), likely because sox19b expression decreases in later stages.
Fig. 1. Single, triple, and quadruple knockdowns of sox2/3/19a/19b. 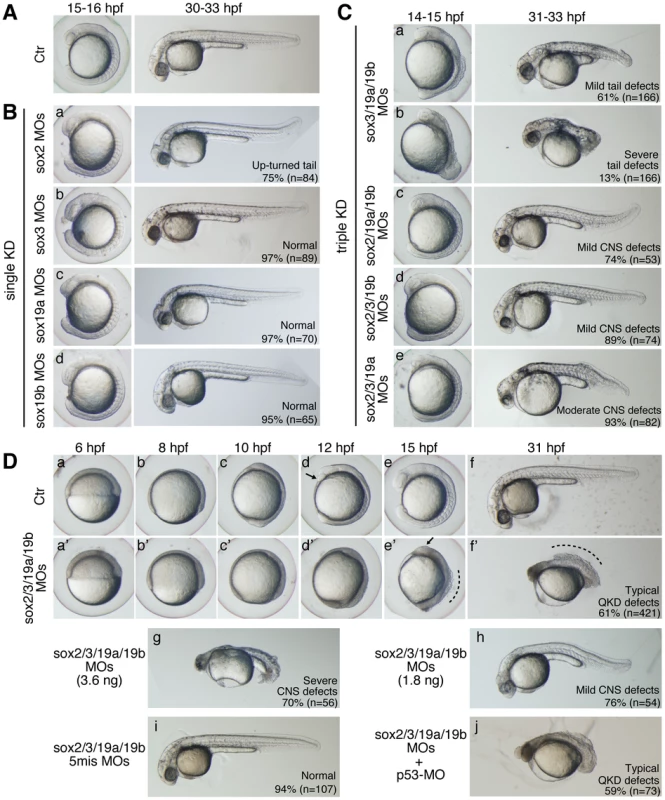
Bright-field images of live embryos observed at the indicated time points. All are lateral views. (A) Uninjected control (Ctr) embryos. (B) Single knockdowns of sox2/3/19a/19b. A 1∶1 mixture of two MOs (0.9 ng each) targeting one of the four B1 sox genes was injected for a single KD. The percentage of embryos in the same morphological class is indicated in each panel. (C) Triple knockdowns of sox2/3/19a/19b. A mixture of indicated combinations of MOs (i.e., a mixture of six MOs, 5.4 ng in total) was used to simultaneously knockdown three out of the four B1 sox genes. The major classes of morphological defects are shown with the percentage of occurrence. The remaining embryos showed either milder or more severe defects. (D) Uninjected control embryos (a–f) and sox2/3/19a/19b quadruple knockdown (QKD) embryos injected with a mixture of MOs targeting the four B1 sox genes (i.e., a mixture of eight MOs, 7.2 ng in total) (a’–f’). The QKD caused very severe developmental abnormalities: a delay in epiboly, a shortened anterior-posterior axis, and impairment of CNS development (61%). The remaining embryos showed either milder defects (7%), more severe defects (24%) or lethality (8%). The aberrant movement of the anterior prechordal plate (arrows in d and e’) suggests a decreased adhesion of ectodermal cells as well as defects in convergence and extension movements in the QKD embryos. The broken lines (e’ and f’) indicate the dorsal trunk regions where cell dissociation was observed. (g, h) Dose-dependent effects of the MOs used for QKD were examined by injecting reduced amounts of the mixture of MOs targeting the four B1 sox genes (3.6 ng in total [g] and 1.8 ng in total [h]). (i) As a negative control, a mixture of 5-base-mismatch control MOs (i.e., a mixture of eight 5mis-MOs, 7.2 ng in total) was injected. (j) The coinjection of a p53-MO (2 ng) had no impact on the neural defects in the QKD embryos. In contrast to the triple KDs, the quadruple knockdown of sox2/3/19a/19b (hereafter called QKD) using a total of 7.2 ng MOs resulted in very severe developmental abnormalities, suggesting essential functions of B1 sox in early embryogenesis (Figure 1Da–1Df). This result also lends support to a model whereby in the triple KD embryos the remaining B1 sox gene compensates for the loss of the other three to a large extent. Taken together, our initial observations indicate that the B1 sox genes are largely functionally redundant in early zebrafish embryos. This is further corroborated by rescue experiments to be described in the next section.
By reducing the amount of MOs used for QKD, hypomorphic phenotypes were produced to different extents depending on the MO levels (Figure 1Dg and 1Dh). Embryos injected with a 50% concentration of MOs for QKD (3.6 ng in total) still showed more severe abnormalities than any of the triple KD embryos. However, injection of only 25% MOs for QKD (1.8 ng in total) resulted in phenotypes similar to the triple KD embryos in terms of severity, further demonstrating the dose-dependent knockdown effects.
The specificity of the MOs we used was confirmed by injection of 5-base mismatch MOs, which caused no developmental abnormalities (Figure 1Di). In addition, the observed QKD phenotypes were not altered by coinjection of p53-MO (Figure 1Dj), which relieves MO-induced non-specific neural cell death [30]. Efficient elimination of B1 SOX activity under this QKD condition was also confirmed by the loss of nestin enhancer activity (Figure S3), which is regulated by B1 SOX and POU [15].
The earliest detectable morphological abnormality of the QKD embryos was a delay in epiboly, notably after the shield stage (Figure 1D). At 10 hour post-fertilization (hpf), when normal embryos reach the tailbud stage, the QKD embryos were still in late epiboly. The thickening of the anterior head region was less prominent in the QKD embryos (Figure 1Dc and 1Dd), suggesting impairment of CNS development. Impaired CNS development was also indicated by the loss of hesx1 expression in the anterior-most neuroectoderm (Figure 2Bd; see also Figure 3Ca) and by the anterolateral displacement of the pax2a expression domains that mark the midbrain-hindbrain boundary (MHB) (Figure 2Bd). An early phase of neurogenesis was also affected in the QKD embryos as indicated by the loss of proneural neurog1 expression (Figure 2Bf). The QKD embryos further displayed a shortened anterior-posterior (AP) axis with a broadened neural plate (marked by hoxb1b) and broadened mesodermal structures including notochord (marked by ntl) (Figure 1D and Figure 2B). Consistently, the gap between the prechordal plate (marked by hgg1) and notochord was reduced in the QKD embryos (Figure 2Be). These abnormalities commonly occur in zebrafish embryos when C&E movements are impaired during gastrulation [31], [32].
Fig. 2. Rescue of the QKD phenotype by exogenous B1 sox mRNAs. 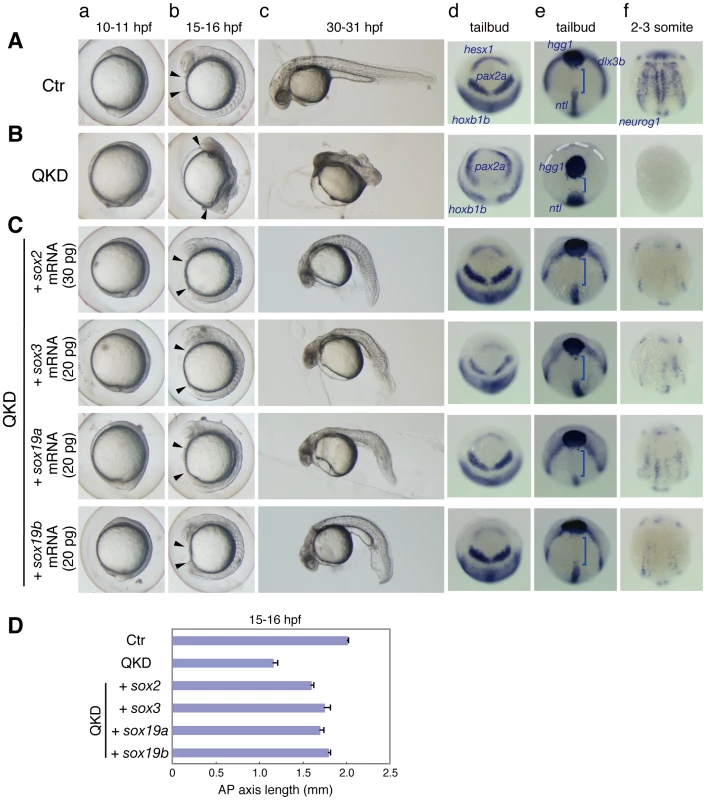
(A, B) Uninjected control (Ctr) embryos (A) and the QKD embryos (B). Live embryos were observed at 10–11 (a), 15–16 (b) and 30–31 (c) hpf. Expression of hesx1, pax2a and hoxb1b (d), dlx3b, hgg1 and ntl (e), and neurog1 (f) was visualized by whole-mount in situ hybridization. Lateral views (a–c); dorsal views with anterior to the top (d–f). (C) The QKD phenotype is similarly rescued by an exogenous supply of any B1 sox mRNA. The MOs for QKD were coinjected with the indicated mRNAs. In the B1 sox mRNA-coinjected embryos, the expression of hesx1, dlx3b and nuerog1 was recovered; patterning of the neural plate marked by pax2a and hoxb1b was normalized; and the expression patterns of hgg1, ntl and dlx3b reflecting C&E movements were also restored. Blue bracket, gap between the hgg1 and ntl expression domains; white dotted line, neural plate border. (D) Recovery of the AP axis elongation in QKD embryos injected with one of the B1 sox mRNAs. The length of the embryos at 15–16 hpf along the AP axis between the arrowheads (Ab, Bb, Cb) was measured for the uninjected control (Ctr) (n = 7), the QKD (n = 9) and QKD with B1 sox mRNA injection (sox2, n = 9; sox3, n = 6; sox19a, n = 7; sox19b, n = 6). The average AP axis lengths with standard errors are shown. Fig. 3. Defects in embryo patterning, early neural development, and C&E movements in the QKD embryos. 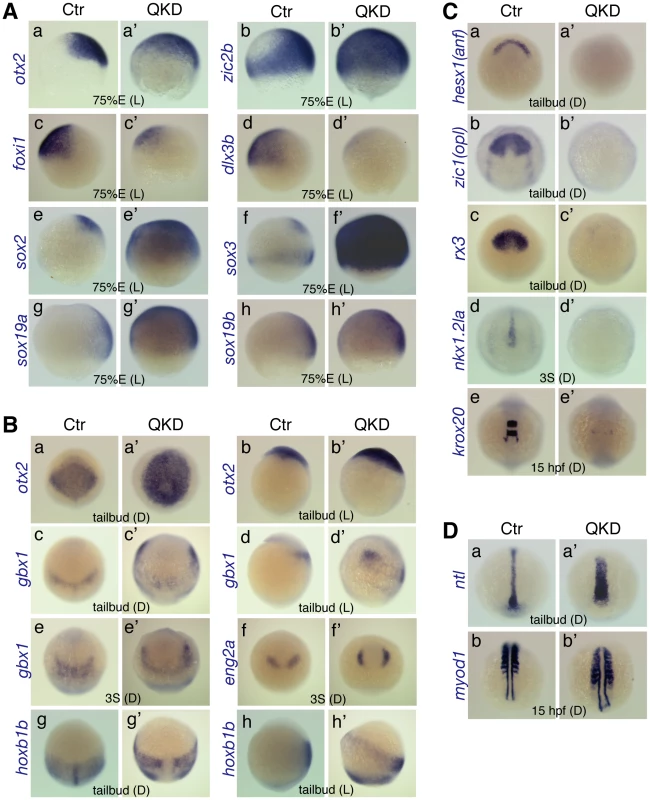
Comparison of the gene expression profiles between uninjected control (Ctr) and QKD embryos. (A) Ventral expansion of early neural gene expression (otx2 [a], zic2b [b] and sox2/3/19a/19b [e–h]), and reduced expression of non-neural ectoderm marker genes (foxi1 [c] and dlx3b [d]) at 75%E. (B) Altered expression patterns of neural genes at the tailbud and 3-somite stages. The expression domains of gbx1 (c–e), eng2a (f) and hoxb1b (g, h) were anterolaterally shifted, which encompass the otx2 expression domain (a, b). (C) Defective neural development revealed by the loss of expression of neural marker genes, hesx1 (a), zic1 (b), rx3 (c), nkx1.2la (d) and krox20 (e). Residual expression of krox20 was observed in r5-derived neural crest cells. (D) Wider mesoderm structures with a shortened axis revealed by mesodermal marker genes, ntl (a) and myod1 (b). The stages and embryo orientations are shown in each panel: E, epiboly stage; S, somite stage; L, lateral view with dorsal to the right; D, dorsal view with anterior to the top. Interchangeable B1 SOX protein functions during early zebrafish development
The effects of the B1 sox KDs suggested that the B1 SOX proteins act equivalently in transcriptional regulation in the zebrafish embryo. Indeed, SOX1/2/3/19 all activate the nestin and δ-crystallin enhancers in cooperation with Brn2 and Pax6, respectively, in cultured cells [11]. We therefore tested whether injection of a single B1 sox mRNA could rescue the QKD phenotype. Moderate amounts (20–30 pg) of B1 sox mRNAs lacking the MO-target 5′ UTR sequences were individually injected with the MOs for QKD. Coinjection with any one of the sox2/3/19a/19b mRNAs dramatically rescued the QKD phenotype, as judged by the recovery of a normal morphology (Figure 2Ca–2Cc). By measuring the AP axis length of the embryos at 15–16 hpf, we confirmed the recovery of axial elongation in the B1 sox mRNA-injected QKD embryos (Figure 2D). In these embryos also, the expression of hesx1, dlx3b (neural plate border) and neurog1 was recovered and the expression patterns of pax2a and hoxb1b were restored (Figure 2Cd–2Cf). In addition, the C&E movements indicated by the expression patterns of hgg1, ntl and dlx3b [31] were normalized in the B1 sox mRNA-injected embryos (Figure 2Ce). This phenotypic rescue was efficient only to early somitogenesis stages, likely because of the gradual decrease in the exogenously supplied SOX expression [19]. Simultaneous injection of sox2/3/19a/19b mRNAs (5 pg each) had essentially the same rescue effects (data not shown). These observations indicate that the function of the B1 SOX proteins is interchangeable during early zebrafish development.
Phenotypes of B1 sox-deficient embryos
To further explore the functions of B1 sox in early zebrafish embryogenesis, we characterized our QKD embryos by focusing on their defects in neural development and gastrulation movements. In these QKD embryos, expression domains of otx2 (anterior neuroectoderm) and zic2b (entire neuroectoderm) were expanded ventrally at 75%E, whereas expression of both foxi1 and dlx3b (non-neuroectoderm) was reduced (Figure 3Aa–3Ad). Consistently, the neuroectodermal expression of B1 sox was also expanded in the QKD embryo (Figure 3Ae–3Ah). These gene expression changes are reminiscent of the dorsalized phenotype seen in BMP-pathway mutants [33], [34], suggesting impairment of this pathway in the QKD embryos.
At the tailbud stage in the QKD embryos, expression domains of pax2a, gbx1 (hindbrain) and hoxb1b (posterior neuroectoderm) were anterolaterally shifted, which encompass the otx2 expression domain (Figure 2Bd and Figure 3B). A similar change was seen for eng2a expression in the MHB at the 3-somite stage (Figure 3Bf). These observations indicate that some characteristics of the early neural plate can develop even under a severe reduction of B1 SOX activity. However, our initial analyses revealed that expression of many neural genes is abolished in the QKD embryos, including zic1 (forebrain), rx3 (eye field), nkx1.2la (posterior neuroectoderm) and krox20 (rhombomere [r] 3/5) as well as hesx1 and neurog1 (Figure 2B and Figure 3C). Injection of the MOs for QKD into embryos of the nr2f2 enhancer-trap line [35] confirmed the impairment of brain development at later stages (Figure S4A). These findings together suggest that B1 sox activity is critical for neural development, although it is dispensable for the expression of some early neural genes.
The normal expression levels of ntl and myod1 (somite) in the QKD embryos suggest that mesodermal differentiation per se can proceed (Figure 3D). However, the broadened expression domains of these genes indicate defects in convergence movements, which is consistent with the widened expression of neural genes such as gbx1 and hoxb1b. Movement of the anterior prechordal plate (marked by hgg1) was also impaired in the QKD embryos (Figure 2Be), which is characteristic of defective C&E movements. However, hatching gland precursor cells were commonly found to aberrantly move in a dorsal direction and penetrated the ectoderm during mid-somite stages (Figure 1De’). In the severe morphants, these hatching gland cells remained as a single ball-like structure in the head (Figure S4B). This phenotype is unique to the QKD embryo and may reflect a decreased adhesion of ectodermal cells, as also suggested by cell dissociation from the dorsal trunk region (Figure 1De’ and 1Df’).
Interestingly, the QKD embryos show an increase in transcript levels of sox2/3/19a/19b and also sox1b at early developmental stages (Figure 3A and Figure S1B). This implies a negative autoregulation of transcription among the B1 sox members, although a stabilization of these mRNAs by the MOs could not be ruled out. It is also noteworthy that even with these elevated levels of B1 sox transcripts in the QKD embryos, the MO-mediated knockdowns were effective in inhibiting their translation as revealed by western blotting (Figure S2B).
To further characterize the phenotype of the QKD embryos, we examined the changes in gene expression in greater detail by the combined use of in situ hybridization, RT-PCR (summarized in Table S1) and microarray analysis (Figure S5, Table S2 and ). Overall, these analyses indicated that a wide range of developmental processes were affected in the QKD embryos and the major phenotypes can be categorized into: (1) the early dorsoventral patterning defects; (2) defects in gastrulation movements; (3) dysregulation of early neural and neuronal regulatory genes; (4) neural patterning defects associated with the abrogated expression of signaling pathway genes; and (5) early defects resembling those observed for MZspg embryos. Further details of these phenotypes are described below.
Aberrant DV patterning in B1 sox QKD embryos caused by dysregulation of bmp genes
Early DV patterning of the embryo relies on a gradient of Bmp signaling, in which bmp2b/7 play a major role in zebrafish [36], [37]. The phenotypic similarities between the QKD embryos and bmp pathway mutants described above prompted us to examine the genes in this pathway. Expression of bmp2b/7 was found to be reduced in the QKD embryos from the beginning (Figure 4Aa–4Af). bmp4 expression levels were more or less normal initially, but were downregulated at late epiboly stages in the QKD embryos (Figure 4Ag; data not shown). Consistently, expression of the gata2, szl and eve1 genes, which are immediately downstream of Bmp signaling, was also reduced in the QKD embryos (Figure 4Ah–4Aj).
Fig. 4. Defects in DV patterning and gastrulation movements in the QKD embryos. 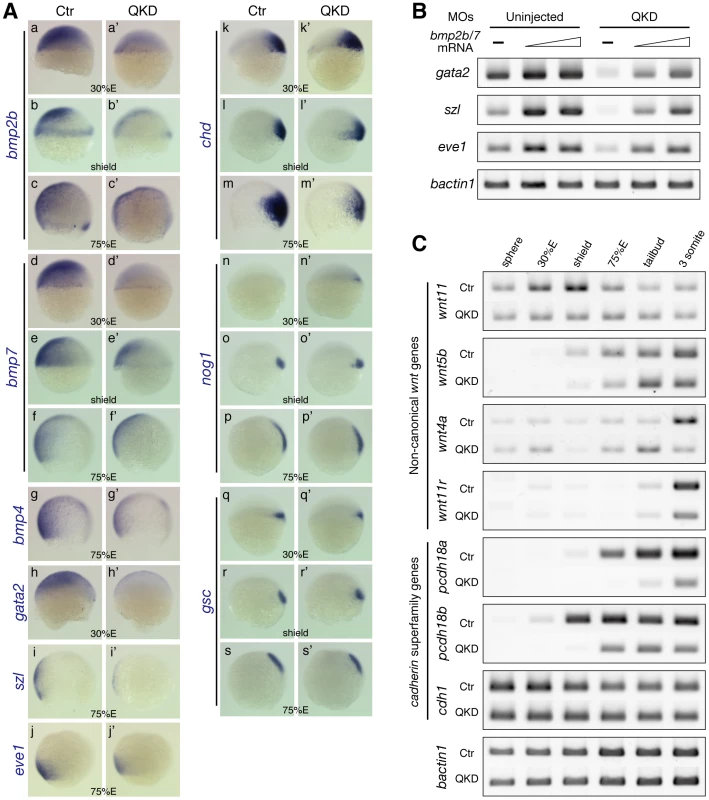
(A) DV patterning defects involving the reduced expression of bmp genes. Expression of bmp2b/7/4 (a–g) and the Bmp downstream genes gata2, szl and eve1 (h–j) is reduced in the QKD embryos. (k–s) Expression of the Bmp antagonist genes, chd and nog1, and the organizer gene gsc. All are lateral views with dorsal to the right. (B) The BMP downstream genes are restored by an exogenous supply of bmp2b/7. Embryos were injected together with the MOs for QKD and a mixture of bmp2b/7 mRNAs (20 or 40 pg each) and subjected to RT-PCR analysis at the shield stage. bactin1 was used as an RT-PCR control. (C) Decreased expression of genes regulating C&E movements in the QKD embryos. Temporal expression profiles of the indicated genes in the uninjected control and QKD embryos from the sphere to 3-somite stages were determined by RT-PCR. Expression of non-canonical wnt genes is reduced in the QKD embryos. Expression of pcdh18a/18b is also reduced in the QKD embryos, whereas cdh1, which is known to be involved in epiboly, is expressed at normal levels. bactin1 was used as an RT-PCR control. The dorsal identity of the zebrafish embryo requires activation of maternal β-catenin, which then activates expression of gsc and the Bmp antagonist genes chd and nog1 at the dorsal side. In the QKD embryos, these genes were initiated normally, although the expression of chd was slightly ventrally expanded at 30%E (Figure 4Ak), which is likely secondary to the reduced expression of bmp2b/7. At later stages in the QKD embryos, however, chd expression was rather decreased (Figure 4Am), contrasting to the bmp pathway mutants [38].
To determine the relationship between the dorsalized phenotype of the QKD embryos and Bmp signaling, we injected a mixture of bmp2b/7 mRNAs (20 or 40 pg each) together with the MOs for QKD. This bmp2b/7 injection rescued the expression of the Bmp downstream genes gata2, szl and eve1 (Figure 4B), indicating that signaling components acting downstream of Bmp2b/7 are not affected in the QKD embryos. Consistently, the mRNA levels of Bmp receptor genes (acvrl1, bmpr1aa, bmpr1ab and bmpr1ba) and smad5 were found to be normal in the QKD embryos by microarray (GEO accession number GSE18830). These observations, together with the normal initiation of the dorsal pathway in the QKD embryos, indicate that the DV patterning defects of the QKD embryos primarily result from the reduction of bmp2b/7 expression.
B1 sox are required for proper gastrulation movements
Components of non-canonical Wnt signaling and cell adhesion molecules are major regulators of gastrulation movements, including epiboly and C&E movements [32], [39]. Since these movements are severely impaired in the QKD embryos, we investigated expression profiles of genes related to these processes. wnt11 and wnt5b are major Wnt ligand genes involved in C&E movements [31], [32]. In the QKD embryos, upregulation of wnt11 that normally occurs during early gastrulation was not observed and expression of wnt5b was slightly reduced at late epiboly stages (Figure 4C). We also observed decreased expression of wnt11r and wnt4a, which are important for convergence movements at later stages [40]. These data suggest that the reduction of non-canonical Wnt ligands contributes to the impairment of C&E movements.
Both classical cadherins and protocadherins are involved in gastrulation movements [39]. We found that expression of pcdh18a/18b was significantly reduced in the QKD embryos (Figure 4C). These genes are expressed in the epiblast at the shield stage and later in the neuroectoderm in an overlapping manner in normal embryos [41], [42]. To examine how reduced activity of Pcdh18a/18b affects embryogenesis, we knocked down these two genes. Although only mild gastrulation defects were observed when these genes were knocked down separately, simultaneous KD caused a delay in epiboly and also C&E defects (Figure S6; for pcdh18a single KD, see also [41]). Delayed epiboly has also been reported for the hypomorphic cdh1 mutants [43], [44], but its expression was not altered in our QKD embryos (Figure 4C). These observations indicate that multiple mechanisms involved in gastrulation movements are simultaneously affected in the QKD embryos.
Dependence of neuronal differentiation programs on B1 sox activity
Several neuronal genes were found to be abnormally upregulated in the QKD embryos. stmn2a is strongly expressed in CNS neurons from mid-somitogenesis stages in wild-type embryos [45] and also weakly expressed throughout the embryo at epiboly stages (Figure 5A and Figure S7A). The latter early stage expression was found to be aberrantly upregulated in the QKD embryos (Figure 5A and Figure S7A). The neuronal tuba1 gene was also upregulated from 75%E (Figure 5A). These observations suggest that a portion of the neuronal differentiation programs is precociously initiated in the QKD embryos.
Fig. 5. Impairment of neural development in the QKD embryos. 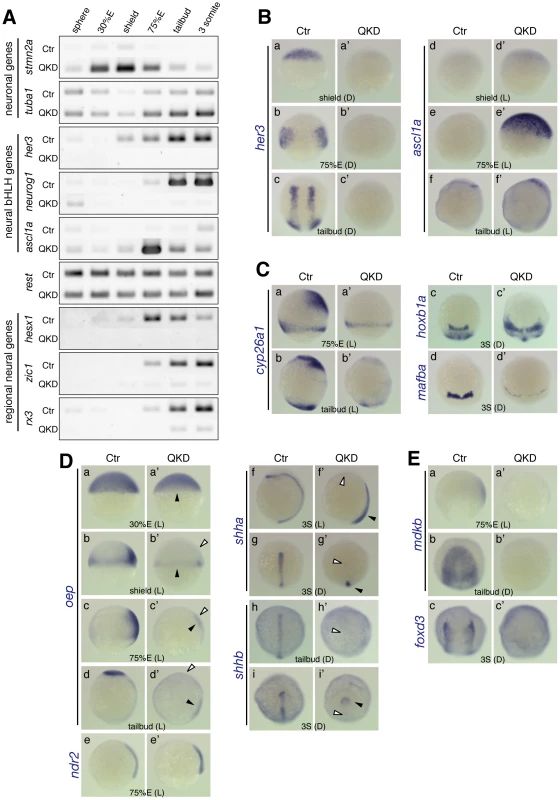
(A) Altered expression of genes involved in neural differentiation. RT-PCR analysis of the indicated genes was performed as described in Figure 4C. (B) Effects of B1 sox QKD on neural bHLH gene expression. The her3 expression is totally lost in the QKD embryos (a–c), whereas ascl1a is transiently upregulated at 75%E in a broad area of the neuroectoderm (d–f). (C) The loss of cyp26a1 expression in the anterior neuroectoderm (a, b) and evidence of hindbrain patterning defects: expansion of hoxb1a expression (c) and a severe reduction of mafba expression (d). (D) The loss of expression of oep and shha/b in the neuroectoderm of the QKD embryos. (a–d) In the QKD embryos, the expression of oep is lost in the ectoderm at the shield stage (b) and the neuroectoderm at the 75%E and tailbud stages (c, d) (marked by open arrowheads in b’–d’), whereas its initial zygotic expression (a) and mesodermal expression (b–d) are maintained (closed arrowheads in a’–d’). (e) ndr2 is also expressed in the QKD mesoderm at normal levels. (f–i) The expression of shha/b in the neuroectoderm is lost (marked by open arrowheads in f’–i’), whereas that in the mesoderm is retained (closed arrowheads in f’–i’). (E) The loss of mdkb expression in the neuroectoderm and severe reduction of foxd3 expression in the neural crest cells of QKD embryos. Neural bHLH transcription factors are key players in the neuronal differentiation programs. Hes/her genes encode repressor-type bHLH proteins, are expressed in undifferentiated neural progenitor cells and maintain their cell state [46]. Among the zebrafish her genes, her3, an ortholog of mammalian Hes3, is initiated in the dorsal region of the epiblast at about 30%E, and its expression continues in bilateral inter-proneuronal domains [47]. This her3 expression is totally lost in the QKD embryos (Figure 5A and 5Ba–5Bc).
Proneural genes encoding activator-type bHLH proteins and participating in neurogenesis are also affected in the QKD embryos. In normal zebrafish embryos, neurog1 expression initially marks primary neurons at the end of gastrulation and then covers the proneuronal domains in a fashion complementary to her3 expression. In the QKD embryos, neurog1 expression is also lost (Figure 2Bf and Figure 5A). However, not all proneural genes behave in this manner as for example ascl1a is transiently upregulated at about 75%E in a broad area of the neuroectoderm in the QKD embryos (Figure 5A and 5B). Proneural genes are known to be repressed by Hes/Her [46], but exogenous injection of her3 mRNA into QKD embryos did not repress aberrant ascl1a expression (data not shown), indicating that the loss of her3 was not causal to this upregulation. The neuronal repressor REST has been implicated in suppression of Ascl1 as well as Stmn2 [48]. However, rest expression was unchanged in the QKD embryos (Figure 5A), although Rest is suggested to be downstream of SOX2 in ES cells [24], indicating that rest is not involved in the aberrant regulation of ascl1a and stmn2a.
Taken together, our results indicate that the proper operation of the neuronal differentiation programs, including regulatory networks involving the neural bHLH genes, is highly dependent on the activity of B1 sox.
Involvement of B1 sox functions in neural patterning
Regional identities of the neural plate are specified through regulatory networks involving various signaling pathways and transcriptional regulators. Genes that are critical for these networks are severely affected in the QKD embryos. As described earlier, in the QKD embryos, expression of the transcription factor genes hesx1, zic1, and rx3, which are required for forebrain and eye development [49]–[51], is lost throughout early embryogenesis (Figure 3C and Figure 5A). The MHB itself was established, as judged from the expression of pax2a and eng2a, but the anterolaterally-shifted expression patterns of these genes suggest an improper formation of the axes of the anterior neural plate (Figure 2Bd and Figure 3Bf). The expression domain of otx2 was expanded and encircled by those of pax2a, eng2a and gbx1 (Figure 2Bd and Figure 3B), which is likely due to the dorsalized phenotype caused by the decreased expression of bmp2b/7, as the bmp2b/7 mutant embryos show similar patterns of gene expression [33], [34]. In addition, the QKD embryos lacked the anterior neuroectoderm expression of cyp26a1 (Figure 5C), which encodes an RA degrading enzyme and thereby plays a role in hindbrain patterning [52]. Hence, the reduction of cyp26a1 expression partly accounts for the hindbrain defects in the QKD embryos, e.g., expansion of hoxb1a in r4 (Figure 5Cc) as observed in the cyp26a1 mutant [53]. However, B1 sox also seem to be more directly involved in gene regulation in hindbrain development as evidenced by severe downregulation of mafba (r5/6) (Figure 5Cd) and the loss of krox20 expression in r3/5 (Figure 3Ce).
Nodal and Sonic hedgehog signaling are crucial for the development of ventral brain structures [54]–[56]. In normal embryos, oep, an ortholog of mouse Cripto, is strongly expressed in the anterior neural plate and is essential as a coreceptor for receiving Nodal signals [54]. Interestingly, oep expression in the ectoderm at the shield stage and the neuroectoderm at later stages is selectively lost in the QKD embryos (Figure 5Db–5Dd), whereas its early zygotic and mesodermal expression was maintained (Figure 5Da–5Dd). Moreover, expression of shha and shhb in the ventral floor of the brain is also lost in the QKD embryos, leaving only shhb expression in the prechordal plate (Figure 5Df–5Di). These findings suggest that the loss of oep and shha/b expression leads to defective ventral brain development in the QKD embryos. It is known that shha expression in the neuroectoderm is regulated by Nodal signals from the mesoderm [57]. However, a more direct link between B1 SOX action and shh regulation is suggested, as the exogenous injection of oep mRNA into the QKD embryos did not restore the shha expression (data not shown), although Nodal-encoding ndr2 is normally expressed (Figure 5De).
Defects in the anterior neural plate development in the QKD embryos also include the loss of mdkb expression (Figure 5E). Consistent with the proposed role of mdkb in the specification of neural crest cells [58], foxd3 expression in the neural crest was reduced in the QKD embryos (Figure 5Ec).
Regulatory actions of the B1 SOX proteins in early zebrafish embryos
As the B1 SOX proteins primarily function as transcriptional activators [11], [13], [15], [23], [25], the expression of direct target genes is expected to be decreased in response to the QKD. However, the upregulation of several neuronal genes such as stmn2a raised the possibility that B1 SOX might also act as repressors. To test this, we utilized dominant activator and repressor forms of SOX3, SOX3-VP16 and SOX3-EnR (Figure 6A) and compared the effects of these variants under QKD conditions with those of SOX3. As anticipated, genes that were downregulated in the QKD embryos, namely bmp2b/7, pcdh18a/18b, her3, hesx1 and zic1, were efficiently recovered by the exogenous supply of either SOX3 or SOX3-VP16 but not by SOX3-EnR (Figure 6B). These genes are thus likely activation targets of B1 SOX. In addition, the increased expression of stmn2a and ascl1a in the QKD embryos was suppressed in the same way. This suggests an indirect regulation of these genes by B1 SOX through the activation of repressors. However, SOX3-VP16 was less effective than SOX3 in the rescue of some genes such as pcdh18a/18b and ascl1a, suggesting that the activation process may require additional molecular interactions with the intact SOX3 protein.
Fig. 6. Regulatory actions of the B1 SOX proteins. 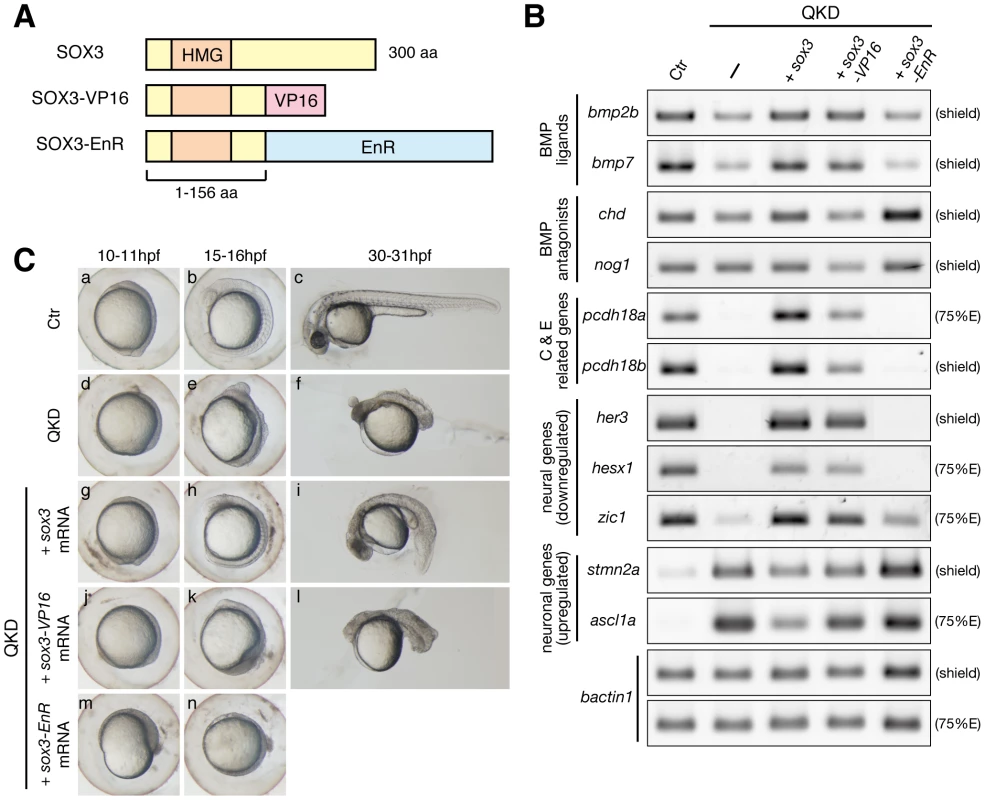
(A) Schematic representation of the protein structures of SOX3, SOX3-VP16 and SOX3-EnR. Dominant activator and repressor forms of SOX3 were constructed by fusing the VP16 activation and Engrailed repression (EnR) domains, respectively, to truncated SOX3(1–156 aa). (B) Gene expression responses to SOX3, SOX3-VP16 or SOX3-EnR under QKD conditions. mRNAs of sox3, sox3-VP16 and sox3-EnR (20 pg) were individually injected with the MOs for QKD and gene expression responses were examined by RT-PCR. The exogenous supply of either SOX3 or SOX3-VP16 but not by SOX3-EnR recovered expression of genes that were downregulated (bmp2b/7, pcdh18a/18b, her3, hesx1 and zic1) in the QKD embryos and also suppressed expression of genes that were upregulated (stmn2a and ascl1a). bactin1 was used as an RT-PCR control. (C) Partial rescue by SOX3-VP16 and strengthening by SOX3-EnR of the morphological phenotypes of the QKD embryos. Live embryo images at 10–11, 15–16, and 30–31 hpf were observed. SOX3-VP16-injected embryos showed a rather ventralized phenotype. Embryos coinjected with SOX3-EnR died during late segmentation stages. All are lateral views. It is noteworthy that the morphological rescue of the QKD embryos by SOX3-VP16 was much less complete when compared to that observed for SOX3, and that the SOX3-VP16-injected embryos showed a rather ventralized phenotype (Figure 6C). In line with this observation, chd and nog1 were unexpectedly reduced in SOX3-VP16-injected embryos, but increased in SOX3-EnR-injected embryos, suggesting that the repressive action of B1 SOX may be required for the proper regulation of dorsally expressed BMP antagonist genes. These findings together indicate that the B1 SOX proteins primarily act as activators in early embryos, whereas a context-dependent repressive action of these factors is also suggested.
Direct regulatory targets of B1 SOX in early zebrafish embryos
To further explore whether the B1 SOX proteins directly regulate the potential downstream genes described above, we searched for possible B1 SOX binding sites (containing the consensus sequence CATTGTT [21], [59] or closely related sequences) in the regulatory regions of these genes. We identified potential SOX-binding sites in the regulatory sequences of her3 [47], hesx1, cyp26a1 [60] and neurog1 [61] and also in the conserved non-coding sequences upstream of pcdh18a (Figure 7Aa). To investigate the direct interaction of the B1 SOX proteins with these genomic sequences in vivo, ChIP experiments were performed using zebrafish embryos at the 70–80%E and tailbud to 2-somite stages. ChIP analysis using anti-SOX2 antibody that weakly cross-reacts with SOX3/19A/19B revealed specific binding of B1 SOX to these regulatory sequences in the zebrafish embryo (Figure 7Ab). Similar results were obtained with anti-SOX3 antibody (data not shown). It is thus likely that these genes are direct downstream targets of B1 SOX.
Fig. 7. Direct regulatory targets of the B1 SOX proteins in the zebrafish embryo. 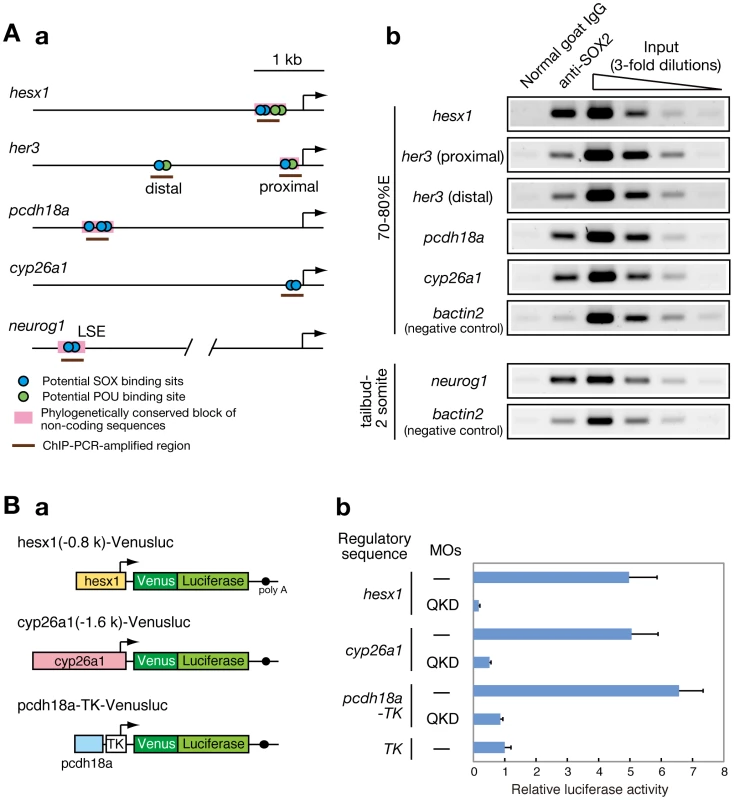
(A) ChIP analysis showing direct association of B1 SOX with regulatory sequences of the downstream genes. (a) Potential binding sites for SOX and POU within the analyzed genomic regions are schematically shown. (b) ChIP-PCR analysis using anti-SOX2 antibody. ChIP experiments were performed using zebrafish embryos at the 70–80%E and tailbud to 2-somite stages. ChIP-PCR analysis using anti-SOX2 antibody that weakly cross-reacts with SOX3/19A/19B revealed specific binding of B1 SOX to the regulatory sequences of the hesx1, her3, pcdh18a, cyp26a1 and neurog1 genes in the zebrafish embryo. bactin2 was used as a negative control. (B) B1 SOX-dependent activities of the regulatory sequences of hesx1, cyp26a1 and pcdh18a. (a) The Venusluc fusion reporter (Venus plus firefly luciferase) constructs containing either of the promoters for hesx1 or cyp26a1 or the upstream conserved sequence of pcdh18a with the HSV TK promoter are schematically shown. (b) The Venusluc reporters were injected into embryos with or without the MOs for QKD together with the reference vector TK-Renilla luciferase. More than 20 injected embryos per sample were collected at the tailbud stage, and luciferase assays were performed. The normalized luciferase activity generated by TK-Venusluc was arbitrarily assigned a value of 1. Data are shown as the average values of four independent injection experiments with standard errors. To further investigate whether the activities of these regulatory sequences are dependent on B1 SOX, we created luciferase reporter vectors containing the promoter sequences for hesx1 and cyp26a1 [60] (Figure 7Ba). The 0.8-kb promoter sequence of the zebrafish hesx1 gene used here corresponds to the chicken Hesx1 promoter that has been shown to have anterior CNS-specific regulatory activity in chicken and also zebrafish embryos [62]. The conserved non-coding sequence upstream of pcdh18a (412 bp) was also cloned into the TK-luciferase reporter vector (Figure 7Ba). When these reporter vectors were injected with or without the MOs for QKD into zebrafish embryos, the promoter activities of hesx1 and cyp26a1 were significantly downregulated upon B1 sox QKD (Figure 7Bb). The 412-bp pcdh18a sequence showed an enhancer activity in normal embryos, whereas this activity was also reduced in the QKD embryos (Figure 7Bb). These data confirm that B1 SOX proteins regulate the hesx1, cyp26a1 and pcdh18a genes through these regulatory elements.
Interestingly, POU binding sites were found abutting the SOX sites of her3 and hesx1. These genes were found to be commonly downregulated in the QKD embryos (Figure 3C and Figure 5A and 5B) and also MZspg mutants (Table S7; see also [29]). In the QKD embryos, pou5f1 is expressed at normal levels (Figure S7B), indicating that Pou5f1 alone is insufficient to induce her3 or hesx1. These data together suggest that B1 SOX and Pou5f1 proteins synergistically cooperate to activate her3 and hesx1.
Discussion
Previous studies have suggested that the group B1 sox genes are critical for early processes in embryogenesis, particularly during early neural development [1]–[6]. Possibly as a consequence of functional redundancy, however, loss-of-function analyses have not been sufficiently informative to date. In our present study, we successfully depleted the B1 sox activity from early zebrafish embryos by a quadruple knockdown of sox2/3/19a/19b, and present clear evidence that the B1 sox genes are highly redundant and their encoding proteins are functionally interchangeable in early zebrafish embryogenesis. More importantly, we demonstrate that the B1 sox genes are indeed essential for several key processes during early embryogenesis, namely embryonic patterning, gastrulation movements and neural development. The major downstream genes of B1 SOX that function in these processes were found to be developmental transcription factor genes, signaling pathway genes and cell adhesion molecule genes (Figure 8). These data indicate that B1 SOX proteins play a central role in coordinating cell fate specification with embryo patterning and morphogenetic processes by controlling a wide variety of developmental regulators in a process-dependent manner. Among the broad functions of B1 SOX, the transcriptional partnership with Pou5f1 is critical for early embryogenesis from the blastoderm to early neural stages as detailed further below.
Fig. 8. Summary of embryonic stage-dependent target gene regulation by B1 SOX. 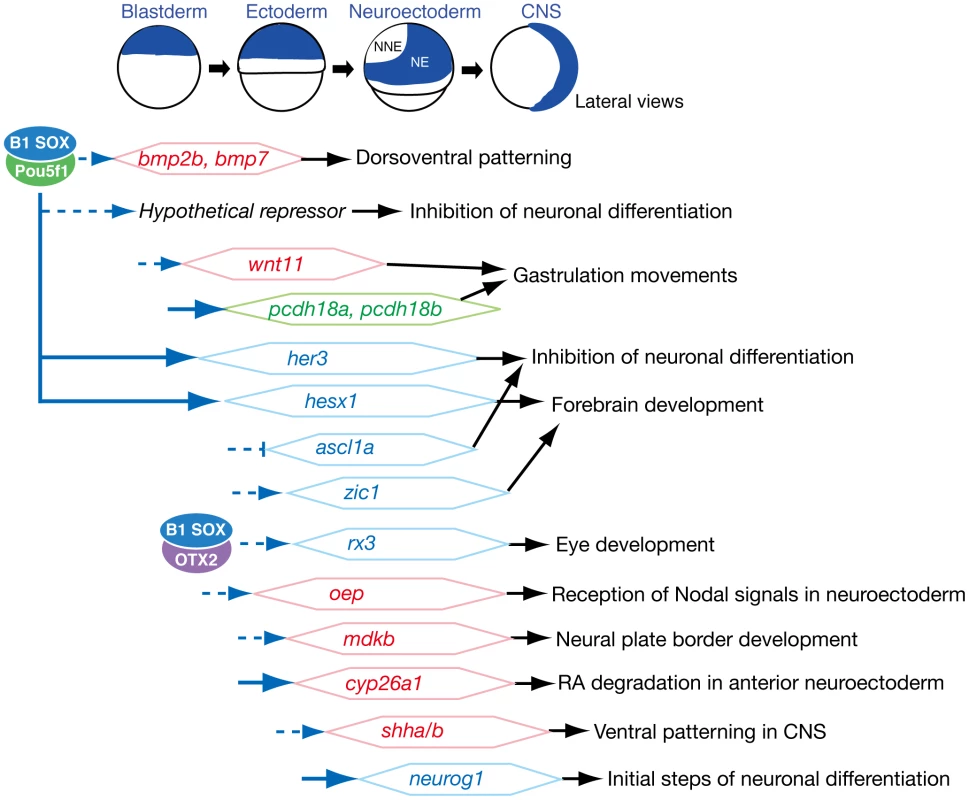
The B1 sox expression domains are schematically illustrated at the top. The B1 SOX-downstream genes that were identified in this study are shown with possible time windows for regulation. The major downstream genes of B1 SOX were found to be developmental transcription factor genes (indicated in blue), signaling pathway genes (red) and cell adhesion molecule genes (green). Our data indicate that, in these regulations, B1 SOX primarily act as activators and appear to indirectly repress several target genes through the activation of hypothetical repressors. A direct regulation of the pcdh18a, her3, hesx1, cyp26a1 and neurog1 genes by B1 SOX is suggested by our ChIP analysis (indicated by blue arrows). The developmental effects of the regulation by B1 SOX are indicated on the right. The partnership of B1 SOX and Pou5f1, a homolog of mammalian Oct3/4, in early embryogenesis
We found similarities in the gene expression profiles between the B1 sox QKD embryos and the MZspg embryos in a wider range of developmental stages from the blastoderm to early neural stages (Figure 8, Table S7 and [29]). This strongly suggests that their cooperation is required not only for the blastoderm stage, which may be similar to the ES cell state, but also for the early neural stage. In contrast to mouse knockouts of Sox2 or Oct3/4, where impairment of the ICM/epiblast lineage development causes early embryonic lethality [1], [63], zebrafish embryos of the B1 sox QKD and MZspg mutants are viable although with severe developmental defects. This enabled us to analyze the functions of B1 sox in later developmental stages as well as the blastoderm stage.
A group of key genes downstream of B1 SOX and Pou5f1 in the blastoderm was found to be bmp2b/7, as the expression of bmp2b/7 is also severely reduced in MZspg embryos [28]. In addition, an overlap of the expression domains of B1 sox, pou5f1 and bmp2b/7 in the blastoderm strongly suggests a direct regulation of bmp2b/7 by B1 SOX and Pou5f1. The dorsalized phenotype of the B1 sox QKD and MZspg embryos can to a large extent be ascribed to the bmp2b/7 defects, as bmp2b/7 mRNA injection into the QKD embryos rescues the expression of Bmp downstream genes (Figure 4B) and bmp2b mRNA injection can also rescue the MZspg embryos [28]. This co-regulation thus appears to be critical for the establishment of the early DV axis, but likely operates only during the initial activation of bmp genes, since during gastrulation the expression domains of B1 sox and bmp2b/7 segregate and eventually become complementary to each other.
her3 and hesx1, which both encode transcriptional repressors, were identified as direct targets of B1 SOX in the early phase of neural development (Figure 5, Figure 6, and Figure 7). The expression of these genes is also lost in the MZspg embryos (Table S7 and [29]). In addition, our ChIP analysis indicated that the proximal and distal SOX-POU elements of the her3 promoter and the hesx1 promoter carrying multiple SOX and POU sites are bound by B1 SOX in vivo (Figure 7A). We further verified, using a luciferase reporter assay, that the activity of hesx1 promoter is dependent upon B1 SOX. These observations together indicate that her3 and hesx1 are regulated under the cooperative action of B1 SOX and Pou5f1. In addition, the B1 SOX and Pou5f1 complex appears to be required for expression of hypothetical repressors that inhibit neuronal differentiation, since the expression of stmn2a is aberrantly upregulated in the B1 sox QKD embryos and also in MZspg embryos (Figure 5A, Figure S7A, and Table S7). These data indicate that in the early phases of neural development B1 SOX proteins cooperate with Pou5f1 and activate the transcriptional repressor genes that inhibit the further differentiation of neural progenitor cells.
The role of the B1 sox genes in coordinating the developmental processes in the gastrulating embryo
In the gastrulating embryo, cell fate specification must be coupled with embryo patterning and gastrulation movements. We found that B1 SOX proteins are involved in all these processes by controlling their respective regulators in the gastrulating embryo.
In the early phase of neural fate specification, the B1 SOX proteins are required for the activation of her3 and the repression of ascl1a, which by themselves are inhibitory to neuronal differentiation (Figure 8). On the other hand, B1 SOX also appear to play a role in an initial phase of neuronal differentiation by directly activating the proneural neurog1 gene. neurog1 is severely downregulated in the QKD embryos (Figure 2 and Figure 5), and in vivo binding of B1 SOX to its regulatory sequence LSE is indicated by our ChIP analysis. In addition, although B1 SOX proteins are known to counteract neurogenesis, this inhibition occurs at late steps of neurogenesis without affecting Neurog1/2 expression [13]. Taken together, these data indicate that B1 SOX are important for the successive generation of neural progenitor cells and immature neuronal cells.
Another important aspect of the functions of the B1 sox genes during neural lineage differentiation is that the initiation of the transcription factor genes zic1 and rx3 depends on their activity (Figure 3 and Figure 5). rx3 may also be directly regulated by B1 SOX, since Xenopus Rx1, a functional homolog of zebrafish rx3, is under the direct regulation of SOX2 and Otx2 in the eye field [64]. Zic1 and Rx3 generally act as a transcriptional activator and are required for forebrain and eye lineage development [50], [51].
Critical functions of B1 sox in embryo patterning are underscored by our present findings that the neural expression of the signaling pathway genes cyp26a1, oep, shha/b and mdkb is dependent upon B1 sox activity (Figure 5). Signaling pathways involving these genes play a key role in cell fate decisions as well as diverse patterning processes in the developing CNS [52]–[56], [58]. Our ChIP and promoter analyses suggest a direct regulation of cyp26a1 by SOXB1 (Figure 7). Furthermore, the expression of oep, mdkb and shha/b extensively overlaps with that of B1 sox in the neuroectoderm, also implying direct regulation by B1 SOX. Interestingly, the expression of Shh during mouse hippocampal development has recently been shown to be directly regulated by SOX2 [65].
A remarkable defect of the QKD embryos was also found to occur in gastrulation movements. Delayed epiboly and impaired C&E movements are also shared phenotypes with the MZspg embryos [26]–[28], suggesting that these processes may also be co-regulated by B1 SOX and Pou5f1. We speculate that a severe reduction of pcdh18a/18b in combination with a reduced expression of non-canonical wnt genes is largely responsible for the defects in epiboly and C&E movements of the QKD embryos (Figure 4). We have further shown in our present analyses that the conserved sequence block upstream of pcdh18a acts as a B1 SOX-dependent enhancer. The in vivo binding of B1 SOX to this enhancer indicated by our ChIP analysis further supports a direct regulation of pcdh18a by B1 SOX. The knockdown phenotypes of pcdh18a/18b were consistent with their important functions in gastrulation movements (Figure S6 and [41]), but their molecular role in this process is still unclear. Recent studies, however, have reinforced the critical role of cell adhesion molecules in gastrulation movements [39]. Pcdh8 (Papc), structurally similar to Pcdh18, controls C&E movements of the paraxial mesoderm in cooperation with the non-canonical Wnt pathway [66], suggesting analogous roles of Pcdh18a/18b in the ectoderm.
The findings of our current study thus demonstrate that the B1 SOX proteins regulate genes that are critical for a variety of processes in early embryonic development. This suggests that these factors serve as central coordinators of gene regulatory networks in the early developing embryo by coupling cell fate specification with patterning and morphogenetic processes. In transcriptional regulation, B1 SOX proteins likely perform this coordination by partnering with a variety of factors including Pou5f1 [21]. Our microarray analysis also suggests that B1 SOX regulate additional genes and pathways that we did not investigate herein. Future studies of these genes will therefore more fully delineate their multiple functions in coordinating early embryogenesis.
Materials and Methods
MO-mediated knockdowns
MOs were obtained from Gene Tools LLC (OR, USA) and are listed in Table S4. Zebrafish embryos were obtained by natural matings of wild-type TL fish and reared at 28.5°C in 0.03% Red Sea salt solution. Approximately 1 nl of solution containing various combinations of MOs, as indicated in the figures, was injected into 1-cell stage embryos. Unless otherwise noted, a 1∶1 mixture of two MOs (0.9 ng/nl each) was used to knockdown individual B1 sox genes. To knockdown multiple B1 sox genes, the MOs were each mixed at a concentration of 0.9 ng/nl and injected into 1-cell stage embryos.
To estimate the knockdown efficiency of B1 sox MOs, fusion mRNAs of sox3-luc, sox19a-luc and sox19b-luc were prepared by transcription of template vectors, in which the 5′-UTR sequence and a short stretch of the amino-terminal-coding sequence of the respective genes (−131 to +32 of sox3; −151 to +32 of sox19a; −151 to +32 of sox19b) were inserted upstream of the luciferase sequence as previously described [20]. mRNA were microinjected with the relevant MOs into embryos and luciferase activities expressed in the embryos at 10–11 hpf were measured as described [20].
Gene expression analysis
Whole-mount in situ hybridizations of zebrafish embryos were performed as described previously [11]. The genes analyzed are listed in Table S1.
Total RNAs for RT-PCR and microarray analyses were prepared from 40–100 uninjected control embryos and an equivalent number of embryos injected with MOs and/or mRNAs using RiboPure kit (Ambion, TX, USA). 200 ng of total RNA of each sample was reverse-transcribed using oligo-dT primer and Superscript III RT-PCR system (Invitrogen, CA, USA) and a 1/80 fraction of the cDNA was used for PCR templates. PCR was performed using ExTaq polymerase (Takara, Japan) in 25 µl ExTaq buffer containing 5% dimethyl sulfoxide, 0.17 mM cresol red, 10% sucrose and primers listed in Table S5. The PCR temperature profile consisted of 5 min denaturation, 23–30 cycles of a 30 sec denaturation at 94°C, 30 sec annealing at 57–61°C and 15 sec primer extension at 72°C and lastly a 10 min extension at 72°C (see Table S5 for cycle numbers and annealing temperatures). PCR products were separated in a 2% agarose gel (1.5% Methaphor agarose/0.5% agarose) and stained with SYBR green I.
For microarray analysis, cRNA probes were prepared using 4 µg total RNA with a one-cycle cDNA synthesis kit (Affymetrix, CA, USA). Affymetrix Zebrafish Genome arrays were hybridized with 10 µg cRNA probes, and posthybridization staining and washing were performed according to the manufacturer's instructions. RNAs from two independent samples were analyzed for each embryonic stage and the data were processed using the RMA program. Fold changes of the averaged hybridization signals between control and QKD embryo samples were then determined (Figure S5, Table S2 and Table S3). The microarray data have been deposited in the Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo) at the National Center for Biotechnology Information with the accession number GSE18830.
Western blotting
Western blotting with an anti-SOX2 antibody was carried out as described previously [20]. For the detection of SOX3, SOX19A and SOX19B, an anti-SOX3 C-terminal peptide antibody [15] was used. As a loading control, the blotted PVDF membranes were stained with Coomassie Brilliant Blue R-250.
Synthetic mRNAs for embryo injection
The coding sequences of the B1 sox genes and their derivatives were cloned into the pCBA3 vector [20]. The coding sequence of bmp7 was amplified by RT-PCR and cloned into pCBA3. The cDNA clone cb670 (Zebrafish International Resource Center [ZIRC], OR, UAS) was used as a template for bmp2b mRNA. mRNAs were transcribed in vitro from linearized vectors using the mMessage mMachine SP6 kit (B1 sox and bmp7) or mMessage mMachine T7 Ultra kit (bmp2b) (Ambion). For the rescue experiments, each mRNA was mixed with the MOs for QKD and injected into 1-cell stage embryos.
Chromatin immunoprecipitation
ChIP was carried out as described previously [67] with minor modifications. Briefly, zebrafish embryos at the 70–80%E and tailbud to 2-somite stages were enzymatically dechorionated with Pronase and then fixed in 1% formaldehyde in embryo medium for 15 min at room temperature. For each immunoprecipitation experiment, approximately 200 fixed embryos were homogenized in cell lysis buffer and incubated for 15 min on ice. Nuclei were collected by centrifugation, resuspended in 200 µl of nuclei lysis buffer, incubated for 10 min on ice and then sonicated using Bioruptor (Cosmo Bio, Japan) to yield DNA fragments with an average size of 400–500 bases. The supernatant of the sonicated cells was diluted 10-fold with ChIP dilution buffer (50 mM Tris-HCl [pH 8.0], 167 mM NaCl, 1.1% Triton X-100, 0.11% sodium deoxycholate). 950 µl of the diluted lysate was then incubated overnight at 4°C with Protein G Dynabeads (Invitrogen) that had been prebound to 2 µg of anti-SOX2 antibody (AF2018; R&D, MN, USA). The same volume of the lysate was precipitated with normal goat IgG as a negative control. 100 µl of the lysate was used as an input control. Beads were washed four times with RIPA buffer and once with TE buffer containing 50 mM NaCl. Bound complexes were eluted from the beads and cross-links were reversed in 200 µl of elution buffer for six hours at 65°C. Eluted DNA was then purified by treatment with RNase A, followed by proteinase K digestion, phenol∶chloroform∶isoamyl alcohol extraction and ethanol precipitation. Precipitated DNA was resuspended in 30 µl of TE buffer and 1 µl of the DNA suspension was used as a template for ChIP-PCR, which was performed using ExTaq polymerase (Takara) in 20 µl ExTaq buffer containing 0.17 mM cresol red, 10% sucrose and the primers listed in Table S6. PCR products were separated in a 2% agarose gel (1.5% Methaphor agarose/0.5% agarose) and stained with SYBR green I.
Regulatory sequence analysis of the hesx1, cyp26a1, and pcdh18a genes
The fragments of the zebrafish hesx1 promoter (Zv8_NA6682 : 4150–4984, 835 bp), cyp26a1 promoter (Zv8_chr12 : 9333416–9335090, 1.7 kbp) and pcdh18a conserved upstream sequence (Zv7_chr1 : 9017351–9017762, 412 bp) were amplified by PCR from the zebrafish genome using following primers (linker sequences that incorporate restriction sites are indicated by lowercase): hesx1 promoter, gggagatctCGTCAAACTCTCCAAACGTGGAT and ggggtcgacCTCAAGTCCTTTAATTTAACTCCAACTG; cyp26a1 promoter, gggagatctAGTATTCCCCGTCCCATTGC and ggggtcgacGTTGAAGCGCGCAACTGATC; and pcdh18a, gggatgcatAAGGCCCGTCCCAACTGAGGG and gggagatctCTACGTCTCAATCTCCCTGACAGA. The luciferase reporter vectors were constructed using pTK200-Venusluc/ISceI, which was generated by inserting an I-SceI site downstream of the reporter poly(A) sequence of pTK200-Venusluc [35]. The fragments of the hesx1 promoter and cyp26a1 promoter were inserted into the upstream region of the Venusluc sequence by replacing the TK promoter sequence. The pcdh18a-TK-Venusluc vector was constructed by inserting the 412-bp pcdh18a sequence upstream of the TK promoter.
To perform the luciferase assay, 17.5 pg of the Venusluc vectors and 2.5 pg of the reference Renilla luciferase vector phRG-TK (Promega, WI, USA) were co-injected into 1-cell stage zebrafish embryos. Half of the embryos were then subsequently injected with the MOs for B1 sox QKD to assess their effects upon the luciferase expression. Injected embryos were collected at the tail bud stage and luciferase assays were performed as described previously [20].
Transgenic zebrafish lines
The NES30-TK200-nlsVenus/ISceI transgene was constructed by inserting the octamerized NES30 sequence, which is a 30-bp nestin enhancer core sequence composed of SOX and POU binding sites [15], into pTK200-nlsVenus/ISceI. pTK200-nlsVenus/ISceI is identical to pTK200-Venusluc/ISceI except that nlsVenus [35] was used as the reporter. A transgenic line was produced by the I-SceI meganuclease method as previously described [35].
Supporting Information
Zdroje
1. AvilionAA
NicolisSK
PevnyLH
PerezL
VivianN
2003 Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 17 126 140
2. KishiM
MizusekiK
SasaiN
YamazakiH
ShiotaK
2000 Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development 127 791 800
3. MizusekiK
KishiM
MatsuiM
NakanishiS
SasaiY
1998 Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125 579 587
4. PapanayotouC
MeyA
BirotAM
SakaY
BoastS
2008 A mechanism regulating the onset of Sox2 expression in the embryonic neural plate. PLoS Biol 6 e2 doi:10.1371/journal.pbio.0060002
5. TakemotoT
UchikawaM
KamachiY
KondohH
2006 Convergence of Wnt and FGF signals in the genesis of posterior neural plate through activation of the Sox2 enhancer N-1. Development 133 297 306
6. UchikawaM
IshidaY
TakemotoT
KamachiY
KondohH
2003 Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev Cell 4 509 519
7. ZornAM
BarishGD
WilliamsBO
LavenderP
KlymkowskyMW
1999 Regulation of Wnt signaling by Sox proteins: XSox17α/β and XSox3 physically interact with β-catenin. Mol Cell 4 487 498
8. GontanC
de MunckA
VermeijM
GrosveldF
TibboelD
2008 Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol 317 296 309
9. MatsumataM
UchikawaM
KamachiY
KondohH
2005 Multiple N-cadherin enhancers identified by systematic functional screening indicate its Group B1 SOX-dependent regulation in neural and placodal development. Dev Biol 286 601 617
10. QueJ
OkuboT
GoldenringJR
NamKT
KurotaniR
2007 Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134 2521 2531
11. OkudaY
YodaH
UchikawaM
Furutani-SeikiM
TakedaH
2006 Comparative genomic and expression analysis of group B1 sox genes in zebrafish indicates their diversification during vertebrate evolution. Dev Dyn 235 811 825
12. KamachiY
IwafuchiM
OkudaY
TakemotoT
UchikawaM
2009 Evolution of non-coding regulatory sequences involved in the developmental process: reflection of differential employment of paralogous genes as highlighted by Sox2 and group B1 Sox genes. Proc Jpn Acad Ser B Phys Biol Sci 85 55 68
13. BylundM
AnderssonE
NovitchBG
MuhrJ
2003 Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci 6 1162 1168
14. GrahamV
KhudyakovJ
EllisP
PevnyL
2003 SOX2 functions to maintain neural progenitor identity. Neuron 39 749 765
15. TanakaS
KamachiY
TanouchiA
HamadaH
JingN
2004 Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol 24 8834 8846
16. NishiguchiS
WoodH
KondohH
Lovell-BadgeR
EpiskopouV
1998 Sox1 directly regulates the γ-crystallin genes and is essential for lens development in mice. Genes Dev 12 776 781
17. RizzotiK
BrunelliS
CarmignacD
ThomasPQ
RobinsonIC
2004 SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet 36 247 255
18. WeissJ
MeeksJJ
HurleyL
RaverotG
FrassettoA
2003 Sox3 is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol 23 8084 8091
19. DeeCT
HirstCS
ShihYH
TripathiVB
PatientRK
2008 Sox3 regulates both neural fate and differentiation in the zebrafish ectoderm. Dev Biol 320 289 301
20. KamachiY
OkudaY
KondohH
2008 Quantitative assessment of the knockdown efficiency of morpholino antisense oligonucleotides in zebrafish embryos using a luciferase assay. Genesis 46 1 7
21. KondohH
KamachiY
2010 SOX-partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. Int J Biochem Cell Biol 42 391 399
22. MasuiS
NakatakeY
ToyookaY
ShimosatoD
YagiR
2007 Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 9 625 635
23. YuanH
CorbiN
BasilicoC
DaileyL
1995 Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev 9 2635 2645
24. BoyerLA
LeeTI
ColeMF
JohnstoneSE
LevineSS
2005 Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122 947 956
25. KamachiY
UchikawaM
TanouchiA
SekidoR
KondohH
2001 Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev 15 1272 1286
26. LachnitM
KurE
DrieverW
2008 Alterations of the cytoskeleton in all three embryonic lineages contribute to the epiboly defect of Pou5f1/Oct4 deficient MZspg zebrafish embryos. Dev Biol 315 1 17
27. LundeK
BeltingHG
DrieverW
2004 Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Curr Biol 14 48 55
28. ReimG
BrandM
2006 Maternal control of vertebrate dorsoventral axis formation and epiboly by the POU domain protein Spg/Pou2/Oct4. Development 133 2757 2770
29. OnichtchoukD
GeierF
PolokB
MesserschmidtD
MössnerR
2010 Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Bio 6 354
30. RobuME
LarsonJD
NaseviciusA
BeiraghiS
BrennerC
2007 p53 activation by knockdown technologies. PLoS Genet 3 e78 doi:10.1371/journal.pgen.0030078
31. HeisenbergCP
TadaM
RauchGJ
SaudeL
ConchaML
2000 Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405 76 81
32. MyersDC
SepichDS
Solnica-KrezelL
2002 Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet 18 447 455
33. NguyenVH
SchmidB
TroutJ
ConnorsSA
EkkerM
1998 Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol 199 93 110
34. NguyenVH
TroutJ
ConnorsSA
AndermannP
WeinbergE
2000 Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development 127 1209 1220
35. OguraE
OkudaY
KondohH
KamachiY
2009 Adaptation of GAL4 activators for GAL4 enhancer trapping in zebrafish. Dev Dyn 238 641 655
36. LittleSC
MullinsMC
2009 Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11 637 643
37. SchmidB
FurthauerM
ConnorsSA
TroutJ
ThisseB
2000 Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127 957 967
38. Miller-BertoglioVE
FisherS
SanchezA
MullinsMC
HalpernME
1997 Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol 192 537 550
39. HammerschmidtM
WedlichD
2008 Regulated adhesion as a driving force of gastrulation movements. Development 135 3625 3641
40. MatsuiT
RayaA
KawakamiY
Callol-MassotC
CapdevilaJ
2005 Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev 19 164 175
41. AamarE
DawidIB
2008 Protocadherin-18a has a role in cell adhesion, behavior and migration in zebrafish development. Dev Biol 318 335 346
42. KubotaF
MurakamiT
TajikaY
YorifujiH
2008 Expression of protocadherin 18 in the CNS and pharyngeal arches of zebrafish embryos. Int J Dev Biol 52 397 405
43. KaneDA
McFarlandKN
WargaRM
2005 Mutations in half baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development 132 1105 1116
44. ShimizuT
YabeT
MuraokaO
YonemuraS
AramakiS
2005 E-cadherin is required for gastrulation cell movements in zebrafish. Mech Dev 122 747 763
45. BurzynskiGM
DelalandeJM
ShepherdI
2009 Characterization of spatial and temporal expression pattern of SCG10 during zebrafish development. Gene Expr Patterns 9 231 237
46. KageyamaR
OhtsukaT
KobayashiT
2008 Roles of Hes genes in neural development. Dev Growth Differ 50 Suppl 1 S97 103
47. HansS
ScheerN
RiedlI
v WeizsackerE
BladerP
2004 her3, a zebrafish member of the hairy-E(spl) family, is repressed by Notch signalling. Development 131 2957 2969
48. BallasN
GrunseichC
LuDD
SpehJC
MandelG
2005 REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121 645 657
49. AndoniadouCL
SignoreM
SajediE
Gaston-MassuetC
KelbermanD
2007 Lack of the murine homeobox gene Hesx1 leads to a posterior transformation of the anterior forebrain. Development 134 1499 1508
50. LoosliF
StaubW
Finger-BaierKC
OberEA
VerkadeH
2003 Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep 4 894 899
51. MaurusD
HarrisWA
2009 Zic-associated holoprosencephaly: zebrafish Zic1 controls midline formation and forebrain patterning by regulating Nodal, Hedgehog, and retinoic acid signaling. Genes Dev 23 1461 1473
52. WhiteRJ
SchillingTF
2008 How degrading: Cyp26s in hindbrain development. Dev Dyn 237 2775 2790
53. HernandezRE
PutzkeAP
MyersJP
MargarethaL
MoensCB
2007 Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development 134 177 187
54. GritsmanK
ZhangJ
ChengS
HeckscherE
TalbotWS
1999 The EGF-CFC protein one-eyed pinhead is essential for Nodal signaling. Cell 97 121 132
55. MathieuJ
BarthA
RosaFM
WilsonSW
PeyrierasN
2002 Distinct and cooperative roles for Nodal and Hedgehog signals during hypothalamic development. Development 129 3055 3065
56. RohrKB
BarthKA
VargaZM
WilsonSW
2001 The Nodal pathway acts upstream of Hedgehog signaling to specify ventral telencephalic identity. Neuron 29 341 351
57. MüllerF
AlbertS
BladerP
FischerN
HallonetM
2000 Direct action of the Nodal-related signal Cyclops in induction of sonic hedgehog in the ventral midline of the CNS. Development 127 3889 3897
58. LiedtkeD
WinklerC
2008 Midkine-b regulates cell specification at the neural plate border in zebrafish. Dev Dyn 237 62 74
59. ChakravarthyH
BoerB
DeslerM
MallannaSK
McKeithanTW
2008 Identification of DPPA4 and other genes as putative Sox2:Oct-3/4 target genes using a combination of in silico analysis and transcription-based assays. J Cell Physiol 216 651 662
60. HuP
TianM
BaoJ
XingG
GuX
2008 Retinoid regulation of the zebrafish cyp26a1 promoter. Dev Dyn 237 3798 3808
61. BladerP
PlessyC
SträhleU
2003 Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech Dev 120 211 218
62. SpielerD
BaumerN
SteblerJ
KoprunnerM
Reichman-FriedM
2004 Involvement of Pax6 and Otx2 in the forebrain-specific regulation of the vertebrate homeobox gene ANF/Hesx1. Dev Biol 269 567 579
63. NicholsJ
ZevnikB
AnastassiadisK
NiwaH
Klewe-NebeniusD
1998 Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95 379 391
64. DannoH
MichiueT
HitachiK
YukitaA
IshiuraS
2008 Molecular links among the causative genes for ocular malformation: Otx2 and Sox2 coregulate Rax expression. Proc Natl Acad Sci U S A 105 5408 5413
65. FavaroR
ValottaM
FerriAL
LatorreE
MarianiJ
2009 Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci 12 1248 1256
66. UnterseherF
HefeleJA
GiehlK
De RobertisEM
WedlichD
2004 Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. EMBO J 23 3259 3269
67. WardleFC
OdomDT
BellGW
YuanB
DanfordTW
2006 Zebrafish promoter microarrays identify actively transcribed embryonic genes. Genome Biol 7 R71
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein SignalingČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



