-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
Peptide hormones are potent signaling molecules that coordinate animal physiology, behavior, and development. A key step in activation of these peptide signals is their proteolytic processing from propeptide precursors by a family of proteases, the subtilisin-like proprotein convertases (PCs). Here, we report the functional dissection of amontillado (amon), which encodes the Drosophila homolog of the mammalian PC2 protein, using cell-type specific inactivation and rescue experiments, and we show that amon is required in the islet-like adipokinetic hormone (AKH)–producing cells that regulate sugar homeostasis. In Drosophila, AKH acts analogously to vertebrate glucagon to increase circulating sugar levels from energy stores, while insulin-like peptides (DILPs) act to decrease sugar levels. amon mutant larvae have significantly reduced hemolymph sugar levels, and thus phenocopy larvae where the AKH–producing cells in the corpora cardiaca have been ablated. Reduction of amon expression in these cells via cell-specific RNA inactivation also results in larvae with reduced sugar levels while expression of amon in AKH cells in an amon mutant background rescues hypoglycemia. Hypoglycemia in larvae resulting from amon RNA inactivation in the AKH cells can be rescued by global expression of the akh gene. Finally, mass spectrometric profiling shows that the production of mature AKH is inhibited in amon mutants. Our data indicate that amon function in the AKH cells is necessary to maintain normal sugar homeostasis, that amon functions upstream of akh, and that loss of mature AKH is correlated with loss of amon activity. These observations indicate that the AKH propeptide is a proteolytic target of the amon proprotein convertase and provide evidence for a conserved role of PC2 in processing metabolic peptide hormones.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000967
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000967Summary
Peptide hormones are potent signaling molecules that coordinate animal physiology, behavior, and development. A key step in activation of these peptide signals is their proteolytic processing from propeptide precursors by a family of proteases, the subtilisin-like proprotein convertases (PCs). Here, we report the functional dissection of amontillado (amon), which encodes the Drosophila homolog of the mammalian PC2 protein, using cell-type specific inactivation and rescue experiments, and we show that amon is required in the islet-like adipokinetic hormone (AKH)–producing cells that regulate sugar homeostasis. In Drosophila, AKH acts analogously to vertebrate glucagon to increase circulating sugar levels from energy stores, while insulin-like peptides (DILPs) act to decrease sugar levels. amon mutant larvae have significantly reduced hemolymph sugar levels, and thus phenocopy larvae where the AKH–producing cells in the corpora cardiaca have been ablated. Reduction of amon expression in these cells via cell-specific RNA inactivation also results in larvae with reduced sugar levels while expression of amon in AKH cells in an amon mutant background rescues hypoglycemia. Hypoglycemia in larvae resulting from amon RNA inactivation in the AKH cells can be rescued by global expression of the akh gene. Finally, mass spectrometric profiling shows that the production of mature AKH is inhibited in amon mutants. Our data indicate that amon function in the AKH cells is necessary to maintain normal sugar homeostasis, that amon functions upstream of akh, and that loss of mature AKH is correlated with loss of amon activity. These observations indicate that the AKH propeptide is a proteolytic target of the amon proprotein convertase and provide evidence for a conserved role of PC2 in processing metabolic peptide hormones.
Introduction
Most peptide hormones and neuropeptides are synthesized as part of larger inactive precursor molecules that must be enzymatically processed by the subtilisin-like proprotein convertases (PCs) to yield bioactive peptide signals. Processing of peptide and neuropeptide hormones is an important regulatory step. Many prohormone precursors encode multiple peptides with distinct functions [1]–[3] and a given precursor may be differentially processed in a cell-specific fashion depending on the PC processing enzyme expressed [4]–[6]. In some cases, the rate and extent of prohormone processing have been shown to be controlled by regulation of PC expression [5], [7], [8]. Modulation of PC expression depending on cell type or upon changing physiological conditions therefore constitutes an important regulatory input for peptide and neuropeptide hormone signaling. Finally, PC activity may be regulated by the action of serpin protease inhibitors [9]–[11], highlighting another control point for peptide hormone production.
Drosophila is a favorable model system for understanding how PCs function at a cellular level to regulate physiology, behavior, and development because of facile genetics, including tools that allow cell-type specific expression and inactivation. In addition, much is already known from Drosophila and other insect systems about the endocrine control of energy metabolism and physiology [12]–[15], neuropeptide control of behavior [16]–[19] and peptide hormone control of developmental progression [20]–[23]. The amontillado (amon) gene (CG6438, Flybase ID FBgn0023179), which encodes a homolog of mammalian PC2 [24], is one of three members of the PC family that have been identified in Drosophila [18]. The remaining two genes, dfurin1 and dfurin2, encode homologs of mammalian furin [25], [26]. amon is expressed in neuroendocrine cells [24] and genetic studies have shown that amon is broadly required throughout the Drosophila life cycle [24], [27], [28]. Hwang et al. [29] have shown that the amon protein is an active protease on a KR containing synthetic peptide when expressed in S2 Drosophila cells. It is nevertheless unclear from these studies whether amon is involved in the processing of any native Drosophila peptide in vivo.
Like humans, Drosophila and other insects employ two antagonistically acting hormones to maintain sugar homeostasis. AKH is the insect analog of vertebrate glucagon and is known to regulate both lipid and sugar mobilization from the fat body during activities such as flight and locomotion [30]–[35] or under conditions of starvation [13], [36], [37]. In insects, trehalose is the major form of sugar found in the hemolymph along with monomeric glucose, and consists of two 1,1-conjugated glucose molecules [38]. AKH also inhibits the synthesis of RNA, fatty acids and proteins in the fat body, the insect equivalent of adipose tissue [39]. In Drosophila, AKH is synthesized in endocrine cells of the corpora cardiaca (CC) as a preprohormone containing a signal peptide, a single AKH of 8–10 amino acids, and a carboxyterminal peptide [40]–[42]. Before AKH is released, the mature AKH peptide is enzymatically cleaved from the carboxyterminal peptide at a dibasic processing site of the kind typically recognized by PCs [40] and then further processed by a carboxypeptidase and amidating enzymes [43]. In contrast to AKH, the Drosophila insulin-like peptides (DILPs) act to lower glucose levels in the hemolymph [15]. The DILPs also possess dibasic cleavage sites and are similar in structure to mammalian insulin [44]. While this suggests that PCs are involved in the processing of metabolic peptide hormones, amon has so far not been linked to a metabolic phenotype in flies.
Here we use cell-type specific inactivation and rescue experiments to show that amon function is required in the AKH cells to maintain normal sugar homeostasis. We find that hypoglycemia resulting from amon RNA inactivation in the AKH cells can be rescued by heat-shock driven expression of the akh gene, indicating that amon acts upstream of akh. In addition, production of mature AKH is inhibited in amon mutants as measured by mass spectrometric profiling. Together, our results are consistent with the model that the AKH propeptide is a proteolytic target of the amon proprotein convertase and suggest a conservation of PC2 function in the processing of peptide hormones regulating sugar homeostasis in insects and vertebrates. Our results also suggest that the amon inactivation and rescue reagents reported here will be generally useful, e.g. in conjunction with cell ablation experiments, to cell-specifically define the functional significance of signals produced by peptidergic cells in Drosophila.
Results
amon mutants have reduced hemolymph sugar levels
amon mutants die early in development, with most arresting as first instar larvae exhibiting molting defects [28]. In order to obtain sufficient volumes of hemolymph for sugar level determination, we provided amon expression via a hs-amon transgene to rescue mutants past the early requirements for amon to the third instar larval stage. While a single heatshock during the first instar larval stage was sufficient to rescue larvae to the second instar larval stage, it was not sufficient to rescue them to the third instar larval stage, suggesting that amon protein turnover occurs in less than 24 h. Thus, total hemolymph sugar levels of amon mutants were determined 24 h after the last heatshock treatment as described in the Materials and Methods section. When hemolymph was collected from amon mutants 3 h after the final heatshock, combined sugar levels (1230 mg/dL, SEM = 51.73) were similar to those seen in wild-type sibling controls (1163 mg/dL, SEM = 76.64, Figure 1A, left bars). Twenty-four hours after the final heatshock, however, amon mutants (1281 mg/dL, SEM = 44.71) were hypoglycemic compared to control larvae (1711 mg/dL, SEM = 51.20, Figure 1A, center bars), indicating that amon mutants failed to properly regulate hemolymph sugar concentrations.
Fig. 1. Larvae lacking functional amon have reduced hemolymph sugar levels. 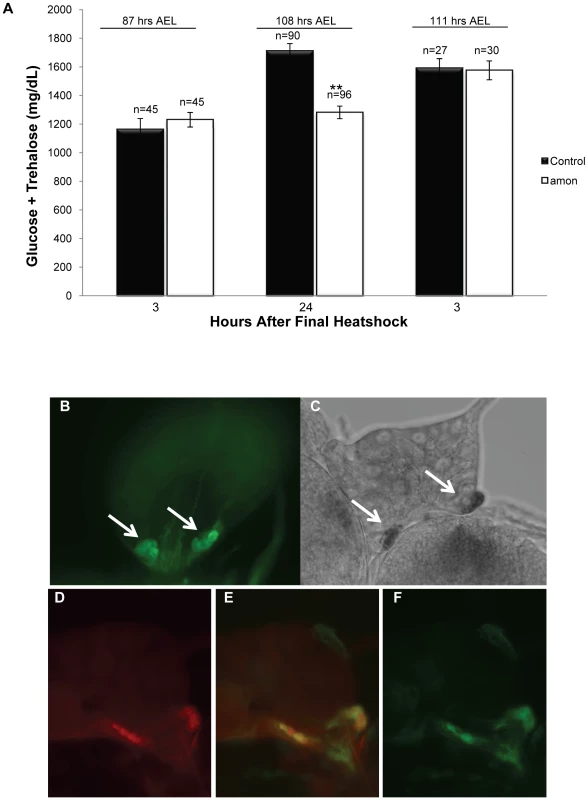
(A) Bars indicate combined glucose and trehalose hemolymph levels in control siblings (black) and amonQ178st mutants (white). Hemolymph carbohydrate levels were measured in control and amon mutant larvae collected 3 h (left bars) and 24 h (center bars) after the last in a series of three heatshocks (at 36, 60, and 84 h AEL) and in control and amon mutant larvae collected 3 h (right bars) after the last of a series of four heatshocks (at 36, 60, 84, and 108 h AEL). (B) amon-gal4 drives expression of uas-CD8-GFP in the corpora cardiaca (CC) cells of the ring gland (white arrows). (C) In situ hybridization of an amon probe to the ring gland. White arrows indicate signal in the ring gland CC cells. (D)AKH cells are visualized using an α-AKH antibody. Signal from amon-gal4 (F) co-localizes to the AKH cells (E). n = number of larvae assayed; larvae were pooled in groups of three. **p<0.0001, Students T-Test. An additional heatshock at 108 h AEL and assay 3 h later was sufficient to restore amon mutant sugar levels (1575 mg/dL, SEM = 65.92) to control levels (1591 mg/dL, SEM = 65.71, Figure 1A, right bars). This observation suggests that maintenance of normal sugar levels is dependent on expression of the amon gene. The hypoglycemia seen in amon mutants is similar to that seen in larvae in which the AKH producing cells have been ablated [36], [37].
To determine if amon is expressed in the AKH producing cells, we created an amon-gal4 transgenic construct in which 424 bp of amon promoter sequence drives expression of the yeast GAL4 protein. Combination of this construct with a uas-cd8-gfp construct promoted expression of GFP in the CNS in a pattern similar to that seen using an amon antibody [27]. Using amon-gal4, we also saw expression of the GFP reporter in the CC portion of the ring gland (Figure 1B) where AKH is produced. Expression of the amon gene in these cells was verified by in situ hybridization using an RNA probe directed against amon (Figure 1C). No hybridization was seen using a control sense probe for amon (data not shown). Finally, we examined the expression patterns of amon and AKH using the amon-gal4 construct and an AKH antibody [45]. Figure 1D–1F show that amon and AKH co-localized to the CC cells of the ring gland.
amon expression in the AKH-producing cells is necessary and sufficient for normal sugar regulation
To ask whether amon expression in the AKH cells is required to maintain normal hemolymph sugar levels, we reduced amon expression in these cells by combining a uas-amon-RNAi transgene with akh-gal4. Figure 2A and 2B shows that ubiquitous expression of the uas-amon-RNAi transgene via a heatshock GAL4 construct reduced amon transcript levels by 90% as measured by quantitative real time PCR (Figure 2A), and that ubiquitous expression of this transgene phenocopied amon mutants. Ninety-one percent of amon knockdown animals (n = 182) died when the uas-amon-RNAi construct was expressed using hs-gal4; expression of the uas-amon-RNAi transgene using an actin-gal4 construct resulted in complete lethality (n = 60). In addition, the phenotypes observed in knockdown animals that arrest during pupal development using the hs-gal4 driver (Figure 2B, bottom) resembled amon mutants (Figure 2B, middle), including a failure to evert the head and a failure of the abdomen to differentiate.
Fig. 2. amon is required in the AKH producing cells for normal sugar regulation. 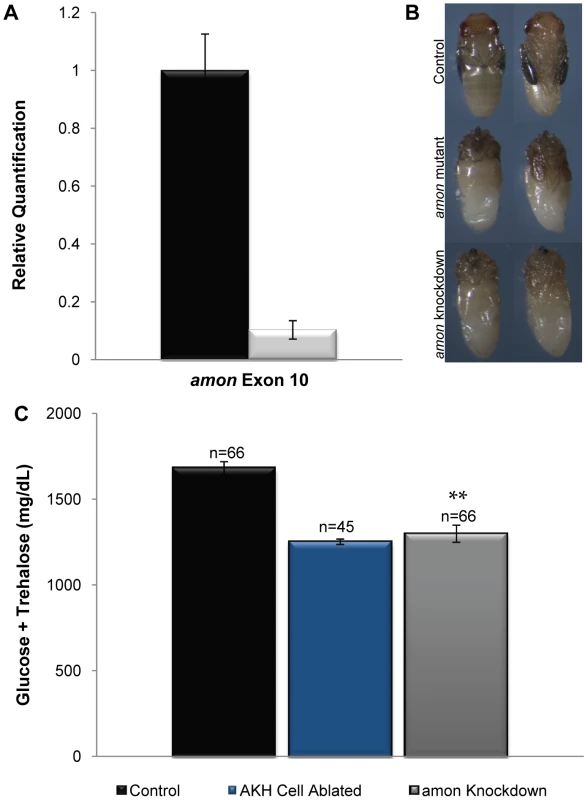
(A) The black bar indicates amon transcript levels in control larvae, while the white bar indicates amon transcript levels when amon-RNAi is ubiquitously expressed. Primers specific to amon exon 10 were used to assess amon transcript levels by quantitative real time PCR. (B) Dorsal and ventral views of a control pupa (top). Middle panels represent amon mutants that are unable to complete metamorphosis, and die with defects in head eversion and abdominal differentiation. amon RNAi knockdown animals also die with phenotypes similar to amon mutants (bottom). (C) Combined glucose and trehalose levels of control larvae are shown in the black bar. The center blue bar shows hemolymph sugar levels in AKH ablated larvae, while the gray bar represents animals in which amon expression has been reduced in the AKH producing cells by RNAi. n = number of larvae assayed; larvae were pooled in groups of three. **p<0.0001, one-way ANOVA. Knockdown of amon activity in the AKH cells using akh-gal4 and the uas-amon-RNAi transgene resulted in a significant decrease in combined glucose and trehalose levels (Figure 2C, gray bar) relative to control larvae (Figure 2C, black bar). In these experiments, this difference was similar to AKH cell ablated larvae produced by combining akh-gal4 and a uas-reaper transgene (Figure 2C, blue bar). Thus, amon activity is necessary in the AKH producing cells to maintain normal sugar homeostasis.
To ask whether amon activity in the AKH producing cells is sufficient to maintain hemolymph sugar concentrations, we expressed amon in the AKH cells in an amonC241Y mutant background. Expression of amon in the AKH cells was achieved by combining a uas-amon construct and the akh-gal4 driver in an amon mutant background. Expression of amon in these cells alone was not sufficient to rescue the early developmental requirements for amon. Therefore, in order to obtain larvae large enough for sugar determination, we combined the hs-amon construct with the uas-amon and akh-gal4 constructs in an amon mutant background. amon mutants were rescued by expressing amon via the heatshock promoter once every 24 h until the third instar larval stage. In this background, amon is expressed in the AKH cells by virtue of the uas-amon and akh-gal4 constructs, allowing us to examine requirements for amon in sugar homeostasis. Sugar levels were assayed approximately 24 h after the final heatshock as described earlier. Expression of amon via a uas-amon transgene in the AKH cells of an amon mutant resulted in larvae with wild-type sugar levels (Figure 3, black versus gray bar). This observation indicates that restoring amon activity in the AKH producing cells in an amon mutant background is sufficient to rescue the hypoglycemic defect.
Fig. 3. Expression of amon in the AKH cells of an amonC241Y mutant is sufficient to rescue hypoglycemia. 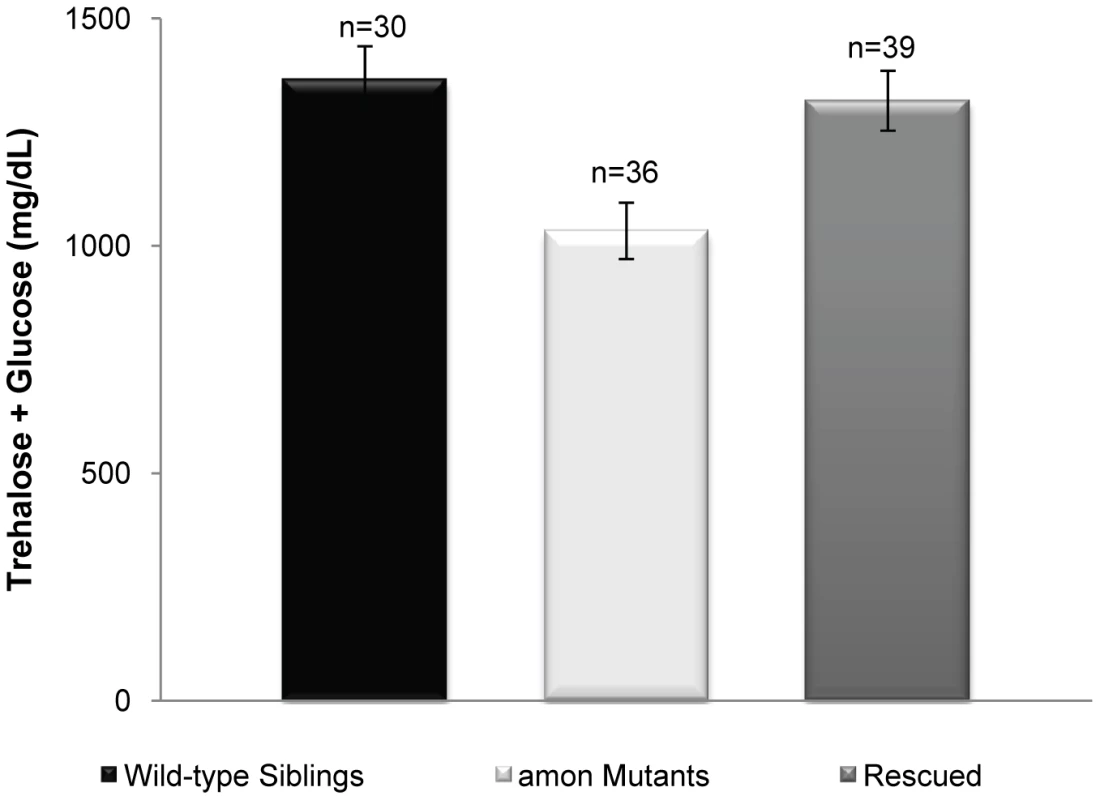
The gray bar represents larvae in which amon expression has been restored in the AKH producing cells (yw; uas-amon/hs-amon; Df(3R) Tl-X e/akh-gal4, amonC241Y) as compared to amon mutants (yw; uas-amon/hs-amon; Df(3R) Tl-X e/amonC241Y white bar) and control siblings (yw; uas-amon/hs-amon; Df(3R) Tl-X e or amonC241Y/TM3 Sb Ser y+ e, black bar). n = number of larvae assayed; larvae were pooled in groups of three. p<0.0015, one way ANOVA. Ubiquitous expression of AKH rescues hypoglycemia induced by amon knockdown in AKH-producing cells
To ask whether the defect in sugar homeostasis observed in larvae in which amon expression has been reduced in the AKH cells via RNAi can be attributed to a lack of mature AKH, we expressed AKH in these larvae using the hs-akh transgene. It has been previously shown that the hypoglycemia induced by ablation of the AKH cells can be rescued through the expression of the akh gene throughout the larva using a hs-akh transgene [13]. Since these animals lack detectable AKH cells, this observation suggests that other cell types possess the proteolytic machinery to produce functional AKH. We found that ubiquitous expression of hs-akh in control larvae (yw; hs-akh/+; akh-gal4/+) had no effect on sugar levels, and that heatshock treatment also did not affect hemolymph sugar levels (Figure 4, black bars). In addition, we recapitulated the rescue of hemolymph sugar levels in AKH cell ablated larvae (yw; hs-akh/uas-reaper; akh-gal4/+, Figure 4, blue bars) demonstrated in earlier studies [13]. Finally, we show that hs-akh expression in larvae with reduced amon function in the AKH cells (yw; hs-akh/uas-amon-RNAi28b; akh-gal4/+) was sufficient to restore glucose and trehalose to control levels (Figure 4, gray bar), indicating that amon functions upstream of AKH to prevent hypoglycemia.
Fig. 4. Ubiquitous expression of AKH rescues the hypoglycemic defect seen in amon knockdown larvae. 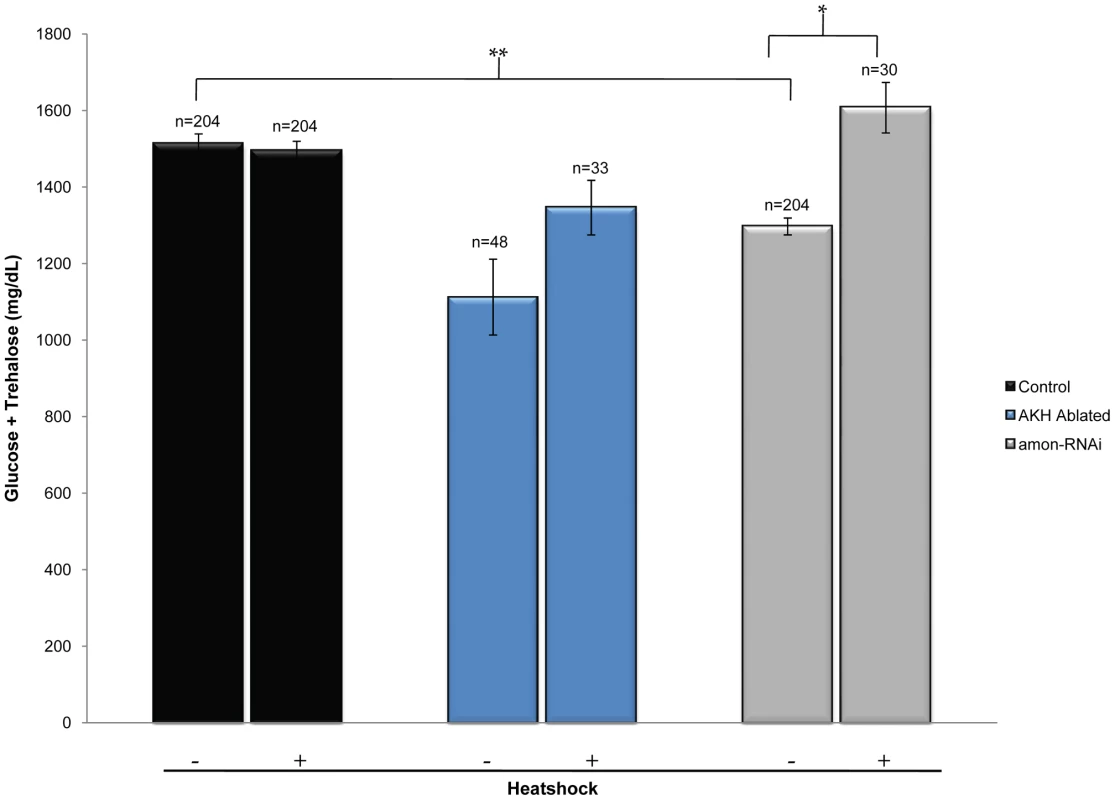
The left black bar represents wild-type levels of combined glucose and trehalose (yw; hs-akh/+; akh-gal4/+). The left blue bar represents combined sugar levels of AKH ablated larvae (yw; hs-akh/uas-reaper; akh-gal4/+) while the left gray bar shows glucose and trehalose levels in which amon has been reduced in the AKH cells by RNAi (yw; hs-akh/uas-amon-RNAi28b; akh-gal4). Bars denoted with a ‘+’ below the graph indicate combined glucose and trehalose levels following heatshock induced expression of akh via a hs-akh transgene. n = number of larvae assayed; larvae were pooled in groups of three. *p = 0.002, **p<0.0001, one-way ANOVA. Direct peptide profiling shows that amon mutants lack mature AKH peptide
The results above suggest that amon is responsible for the proteolytic activation of Drosophila AKH. To determine whether amonC241Y mutants are indeed defective in AKH processing, we directly profiled larval ring glands (a fusion product of the larval CC, corpora allata and prothoracic gland) and adult CC by MALDI-TOF mass spectrometry and compared mass signals for AKH and processing intermediates between wild-type and amon mutant flies. Earlier work has shown that AKH and a processing intermediate with the C-terminal extension GK (AKHGK, Figure 5A) appear as dominant mass signals in direct MALDI-TOF mass spectrometric profiles of single larval ring glands or adult CC [41], [42].
Fig. 5. Direct peptide profiling of AKH and AKHGK in control and amonC241Y flies. 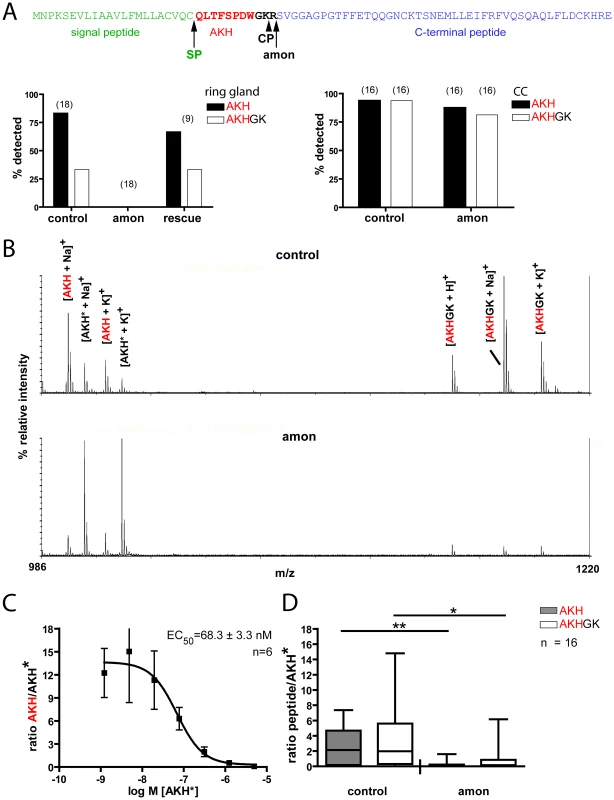
(A) Model of the processing of the AKH prepropeptide (top) and profiling of the larval ring gland (left) and adult corpora cardiaca (right). AKH is processed by a concerted action of a signal peptidase (SP) and amon, likely followed by a two-step carboxypeptidase (CP) action that first removes the C-terminal R yielding the intermediate AKHGK. AKHG is than amidated to bioactive AKH (not shown). While AKH and AKHGK were detected in most preparations from control and rescued (continued heatshock once a day) flies, they were not detectable in amon larvae. (B) Original direct mass profiles from corpora cardiaca of adult control (above) and amon (below) flies. AKH only occurs as the characteristic [M+Na]+ and [M+K]+ adducts, whereas AKHGK also occurs as [M+H]+. In the control fly, both peptides show higher signal intensities as the stable isotope-labelled standard peptide (AKH*). In the amon fly, the signal intensity is clearly higher for AKH* than for the native peptides. As previously reported [41], [42], no other mass peaks occur in the range 990-1220 Da in direct mass spectrometric CC profiles. (C) Standard curve for adult corpora cardiaca obtained with a dilution series of AKH* added to the matrix salt, male OrR wild-type flies. The y axis shows the signal intensity ratio of native AKH/AKH*. Error bars are S.E.M. The relationship of AKH/AKH* is linear for AKH* concentrations of 50–500 nM. (D) Peptide quantification with the labeled AKH* standard at 400 nM. The concentrations of both AKH and AKHGK are significantly reduced in amon flies vs. controls five days after eclosion and final heatshock. *p<0.05, **p<0.01, Mann-Whitney. We detected mature AKH and AKHGK in 89% of third instar larval ring glands from control flies, but not in any amon mutant ring gland (n = 18, Figure 5A, left graph). A continuous heatshock expression of amon rescued larval AKH and AKHGK production to control levels (Figure 5A left), indicating that amon is required for the proteolytic cleavage of AKH. Likewise, a heatshock 1 d before dissection after a 2 d heatshock break rescued AKH production in larvae (100% AKH/80% AKHGK detections, n = 5). To test whether amon similarily affects AKH and AKHGK production in adult flies, we profiled the CC/hypocerebral ganglion complex of 5 d old adult flies heatshocked until eclosion. In both control (94%) and amon mutant (88%, n = 16) adults, mass peaks corresponding to AKH and AKHGK were detected (Figure 5A right), typically with a decreased signal-to-noise ratio for the AKH and AKHGK peaks in amon mutants. While this decreased signal-to-noise ratio indicated a lower amount of AKH and AKHGK in amon mutant CC, it is problematic to use MALDI-TOF signals per se to quantify peptides mainly due to non-homogenous analyte distribution in the co-crystallite and ion suppression effects (see [46], [47]). A solution to minimize these adverse effect is a proper choice of matrix, decomplexing of the sample and the application of chemically similar internal standards (see [46], [47]). We used α-cyano-4-hydroxycinnamic acid as a matrix since it results in relatively homogenous signals and has been found suitable for quantitative analysis of peptides by MALDI-TOF MS [47]. In contrast to homogenized samples, the on-plate extraction during the direct tissue profiling allows only small peptides to permeate in larger amounts through the cell membrane [42], which favourably reduces sample complexity. For quantification, we added a constant amount of heavy isotope-labeled AKH* as chemically identical internal standard with the matrix, and calculated the ratio of the relative signal intensity of native AKH or AKHGK vs. AKH*. A standard curve obtained from CC of 1d old OrR flies showed that the ratio of the relative intensities of native AKH and AKH* was linear when AKH* is present in a concentration of 50–500 nM (Figure 5C). With the matrix, we therefore added 400 nM AKH* as internal standard throughout the quantitative measurements. Control flies 5 days after eclosion and last heat shock showed a significantly higher ratio of AKH/AKH* than amon mutant flies (median: 2.14 vs. 0.13 (n = 15/16), Mann-Whitney test, Figure 5D). They also showed a significantly higher ratio of AKHGK/AKH* (median 1.974 vs. 0.15 (n = 16) Mann-Whitney test, Figure 5D).
The results above imply that the production of AKH is impaired in both larval and adult amon mutants, while the depletion of AKH after stopping heatshock-induced expression of amon happens slower in adult than in larval flies. To test for a slow depletion in adult flies, we profiled the CC of males and females 1 and 10–14 d after eclosion and last heatshock. At 1 d after eclosion and last heatshock, the AKH ratio between control and amon flies (median: males: 0.64 vs. 0.26, females: 0.18 vs. 0.14, ratio±s.e.m. (n = 8–10)) did not differ significantly (p>0,05, Mann-Whitney test,). The ratios became, however, significantly different at d10–14 (median: males: 1.05 vs. 0.03, females: 1.99 vs. 0.04 (n = 6–8), p<0.01, Mann-Whitney test). Based on this ratio data and the assumption of a unity slope of the AKH/AKH* ratio, we calculated the mean amount of AKH present in the CC (Table 1). The AKH levels one day after eclosion and last heat shock ranged between 20–40 fmol/CC and did not significantly differ between control and amon flies. On day 5, AKH levels had increased by more than 4fold in control but not in amon flies. At day 10–14, AKH levels were similar to day 5 in control flies, while amon flies showed reduced levels compared to day 1 and 5. Significant sex-specific differences in AKH levels were not apparent.
Tab. 1. Calculated levels of AKH in the corpora cardiaca of individual flies. 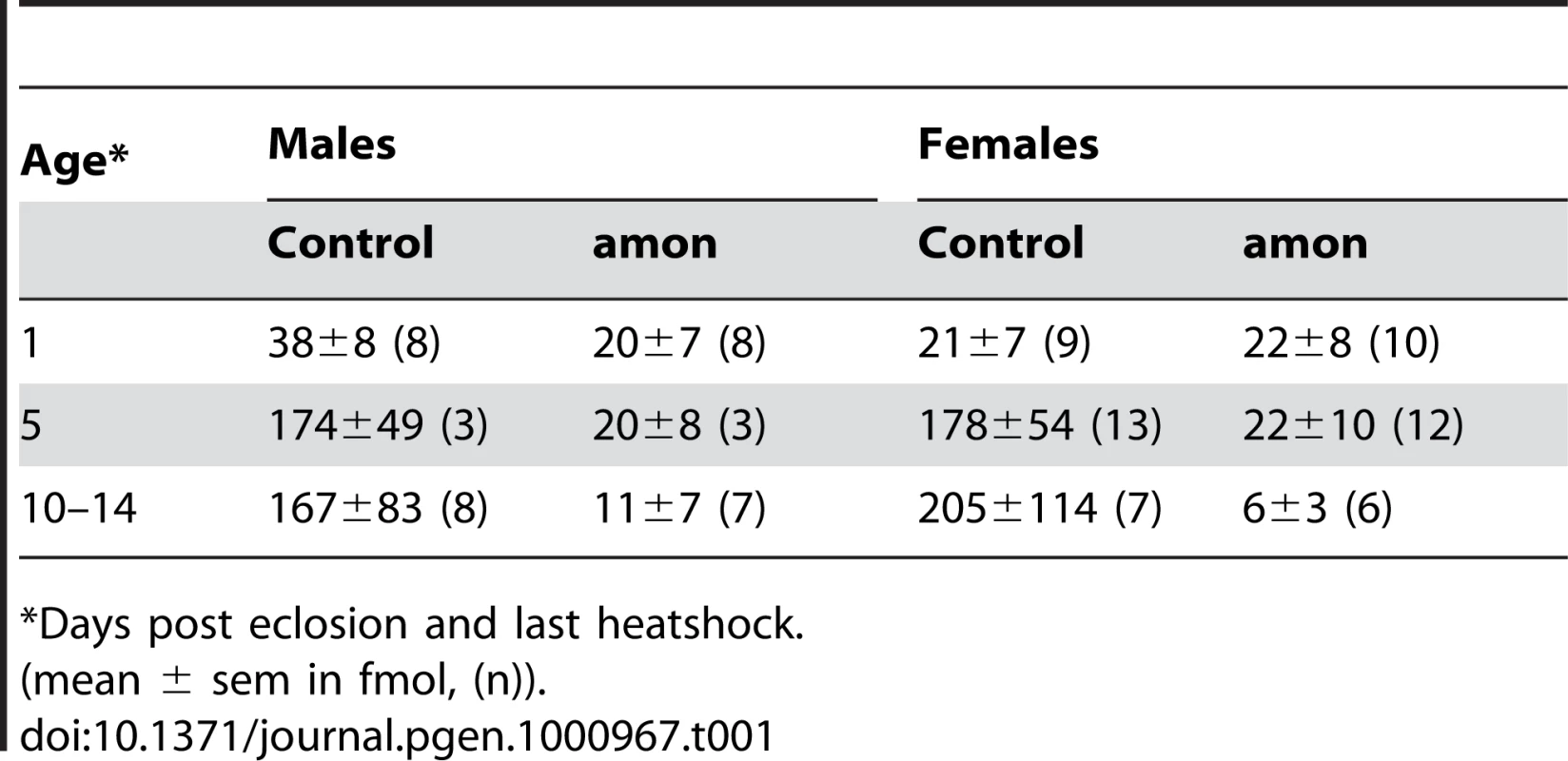
*Days post eclosion and last heatshock. Discussion
The Drosophila proprotein convertase amon is a homolog of mammalian PC2 and is expressed in numerous peptidergic neurons [24], [27]. Phenotypic analysis of animals lacking functional amon have revealed a requirement for this gene during embryonic hatching [24], molting [28], and metamorphosis [27], [28]. In addition, the amon protein is an active protease on a KR containing synthetic protein when expressed in Drosophila S2 cells [29]. While these observations suggest that amon may be required for the proteolytic activation of one or more secretory peptides, it has not been shown that flies lacking amon indeed show a deficiency in peptide production, nor have any of the observed phenotypes of amon-deficient flies been linked to a particular peptide or peptidergic cell. In this study, we identify AKH as a proteolytic target of amon activity, and show that flies lacking amon show a severe deficiency in AKH production. We link this deficiency to a new amon phenotype (hypoglycemia) and show that amon activity is both necessary and sufficient in the AKH cells to maintain sugar homeostasis.
A conserved role of PC2 in metabolic hormone processing
A similar requirement for PC2 activity in the maintenance of sugar homeostasis as described here for Drosophila has also been reported in mice [4], [48]. While in vertebrates PC2 is expressed in the glucagon-producing pancreatic alpha-cells [4], [48], we demonstrate that amon co-localizes with AKH in the endocrine corpora cardiaca cells (Figure 1). These endocrine cells have been suggested to be homologs of the pancreatic alpha-cells [49] and use glucose-sensing and response mechanisms similar to islet cells [13]. We further report that amon mutants fail to produce bioactive AKH, a phenotype similar to PC2 null mice that show defects in the processing of several peptide precursors including proglucagon in the pancreatic alpha-cells [50], [51], prosomatostatin, and proopiomelanocortin [52], [53] (Figure 5). These data not only support our model that AKH production is regulated by amon activity, they also suggest an evolutionarily conserved function of PC2 in the processing of metabolic hormones. This view is consistent with the sequence conservation of amon and vertebrate PC2 and with the small number of PCs expressed in the Drosophila and mammalian endocrine system.
Peptide quantification by direct mass-spectrometric profiling
Direct MALDI-TOF mass spectrometric profiling holds several advantages over liquid chromatography/mass spectrometry (LC/MS) analyses. Most important, it is quick and can be performed on tissues from single animals. Direct MALDI-TOF mass spectrometric peptide quantification has been performed on homogenates of vertebrate and invertebrate endocrine and nervous tissue [54], [55]. We modified this approach by on-plate extraction of isolated CC which is more time-efficient and further minimizes analyte loss, and found a linear signal relationship between analyte and standard around the 100 nM range with the use of an internal stable isotope-labeled standard. This suggests that the method can be used quantitatively within this concentration range. We did, however, not establish calibration curves or precision rates for amon or control flies on the same plate and under the same conditions, which is highly laborious with amon mutants. Our assumption of a unity slope of the ratio curve underlying the calculations of AKH levels may thus not be correct. Nevertheless, our estimates of the AKH levels in the CC of 5 and 10–14 day old adult control flies (around 170–200 fmol/CC) are surprisingly close to the AKH levels previously determined by HPLC from thorax extracts containing the CC (around 100–140 fmol/fly) [32], [40]. In contrast to the results obtained by HPLC, we could not find a significant sex difference in the AKH levels, and observed a lower AKH content in freshly ecdysed flies. While the HPLC-quantification needed extracts from 50 flies, our data originates from single tissue measurements. Our results suggest quantitative direct peptide profiling as a suitable and time-efficient method to (semi)quantify peptide hormones in flies and other small animals which contain small amounts of peptides and are therefore not easily tractable by conventional quantification methods such as enzyme assays or HPLC.
AKH turnover differs between larval and adult flies
In third instar larvae, AKH was undetectable 3 days after the last heat shock, while it was still found in adult flies that had received their last heat shock 5 or more days ago. This suggests that AKH turnover is considerably higher in larvae than in adults under laboratory conditions. Whether this is due to differences in release or production rates, or the size of the AKH storage pool, has yet to be determined. In nature, where longer flights or periods of carbohydrate shortage occur, adult AKH turnover may be much higher. The increase of the AKH level between day 1 and 5 in control flies suggests -similar to the situation in locusts [56] - that AKH production is not coupled to release. Rather, processed AKH seems to be stored in large reserve pools. Interestingly, the biosynthesis of pancreatic glucagon similarly seems not to be coordinated with glucagon release, since e.g. arginine and palmitate induce increased glucagon release, but do not increase glucagon mRNA levels in isolated rat pancreatic islets [50], [57], [58].
Genetic tools to study peptide processing, release, and function in a cell-specific manner
The genetic tools described here allow a reduction of amon activity and the restoration of amon expression in a highly cell-specific fashion in an amon mutant background. These potentially very powerful tools can now be used to identify cell-type specific requirements of amon and to trace the observed phenotypes of amon deficient flies to identified peptidergic signaling networks, revealing their functions. In addition, the apparent evolutionarily conserved mechanism of peptide hormone production by the proprotein convertase family presents the opportunity to use Drosophila and the described genetic tools to study the regulatory mechanisms behind peptide hormone synthesis, processing, and release and to apply the knowledge to mammals.
For example, the hypoglycemic defect of PC2 null mice can be rescued by providing a constant supply of glucagon via an osmotic pump [59]. While this result indicates that supply of glucagon is sufficient to correct the misregulation of glucose levels in PC2 null mice, it does not provide a direct correlation between loss of PC2 activity in the glucagon producing cells with decreases in blood glucose levels. Thus, a more powerful approach would be to reduce PC2 expression only in the alpha-cells of the pancreas and then determine the effect on glucose levels. In Drosophila, such a cell specific reduction of amon can be easily achieved by expression of uas-RNAi constructs using tissue-specific Gal4 expression constructs. Subsequent replacement of hormones can then be accomplished either through direct injection of synthetic hormones, or through broad expression of the cDNA fused to a heatshock promoter. Since peptide processing requires a dedicated enzyme machinery (PCs, carboxypeptidases, amidating enzymes), it is likely that the heatshock-induced expression of AKH or other peptides leads to properly processed and bioactive peptides only in peptidergic neurons and endocrine cells [60].
In a complementary approach, amon can be specifically expressed in target cells in an amon-deficient background. Here, we have validated this approach showing not only that amon is required in the AKH cells to maintain glucose homeostasis in Drosophila (Figure 3), but also that the hypoglycemic defect can be directly attributed to a loss of AKH specifically from these cells (Figure 4).
The fly lines generated here also allow examination of PC requirements in a wide variety of cell types in a relatively short period of time. Furthermore, a combination of the genetic tools and semiquantitative direct peptide profiling as presented here has great potential for the molecular analysis of peptide processing in authentic endocrine cells and peptidergic neurons. Performing such studies in Drosophila is likely to provide valuable insight into the general requirements for PC function in regulating processes including growth, behavior, development, metabolism, and disease.
Materials and Methods
Generation of transgenic fly strains
Injections of transgenic constructs into w1118 embryos were carried out by Duke University Model Systems Genomics. Forward (5′-ATCCAACGCAGCTGAGCAGC-3′) and reverse (5′-CGGAAGGAAAGCACAACAAG-3′) PCR primers were used to amplify an amon fragment extending from position -331 to +133. This fragment was cloned into the BamHI site of pCaSpeR4-Gal4. The homozygous viable amon-gal491D line was used in this study. To create the uas-amon RNAi construct, forward (5′-TGGCGTTGCTTATGACAG-3′) and reverse (5′-ATGTCCCGCCAAGTCAGC3′) primers for the sense insert, and forward (5′-TGGCGTTGCTTATGACAG-3′) and reverse (5′-ATGTCCCGCCAAGTCAGC-3′) primers for the antisense insert were used to amplify exons 7 and 8 of the amon transcript. amon antisense products were cloned into the puasTi vector (Amin Ghabrial, Krasnow Lab, Stanford University, Stanford, Ca) as KpnI/XbaI fragments. amon sense products were cloned into the vector as BglII/XhoI fragments. The homozygous viable uas-amon-RNAi28b line was used in this study. The uas-amon construct was created by digesting the amon cDNA sequence out of the amon #5–8 vector [24] as a EcoRI/EcoRV fragment and subcloning it into pBluescript. pBluescript-amon was then digested with EcoRI and XhoI and cloned into puasT [61]. The homozygous viable uas-amon40L line was used in this study.
Expression of amon-gal4 and in situ hybridization
amon-gal4 was used to drive expression of GFP in cell membranes using the uas-CD8-GFP reporter. Ring glands were dissected from third instar larvae at approximately 108 h AEL, and mounted in glycerol. Tissues were visualized using a Hamamatsu Orca-ER digital camera (model #C4742-80). The generation of DIG-labeled amon probes and in situ hybridization to third instar larval ring glands was performed as described in [62].
Immunohistochemistry
yw; uas-CD8-gfp females were crossed to yw; amon-gal491D males. Immunostaining was carried out essentially as described [27]. For co-localization experiments, tissues were incubated with AKH antiserum (1∶1000, gift from M. Brown, University of Georgia) and anti-Green Fluorescent Protein antibody (1∶1000, Molecular Probes, Invitrogen) overnight at 4°C. Secondary antibodies used were Alexa Fluor 568 goat anti-Rabbit (1∶1000) and Alexa Fluor 488 goat anti-mouse (1∶1000).
Heatshock rescue and trehalose assay of amon mutants
To obtain sufficient volumes of hemolymph, larvae were rescued to third instar larval stages by periodic heatshock driven amon expression. yw; +; amonQ178ST/TM3 Sb Ser y+ e virgin females were crossed to yw; hs-amon; Df(3R)Tl-X e/TM3 Sb Ser y+ e males and placed in an egg collection chamber containing a grape juice agar plate with fresh yeast paste at 25°C. Four hour egg collections were used. Beginning at 36 h AEL, plates were heatshocked at 37°C for 30 min. Subsequent heatshocks were performed at 60 h, 84 h, and in one assay at 108 h AEL. At either 87 h, 108 h, or 111 h AEL, trehalose and sugar measurements were done using pooled hemolymph from groups of three larvae as previously described [15]. Canton S larvae were heat-shocked and assayed in the same manner as the amon mutants described above. To assay combined glucose and trehalose levels of larvae in which amon function was removed in the AKH producing cells, yw; +; akh-gal4 virgin females were crossed to either control males (w1118) or experimental males (w; uas-amon-RNAi28b; +). As an additional control, yw; +; akh-gal4 virgin females were crossed to w; uas-reaper; + males. Flies were raised on standard fly food and incubated at 25°C. At 108 h AEL, glucose and trehalose measurements were performed as described above using feeding third instar larvae. To determine if amon functions upstream of AKH in controlling sugar homeostasis, yw; hs-akh; akh-gal4 virgin females were crossed to either control male flies (w1118) or experimental male flies (yw; uas-amon-RNAi28b; +). As an additional control, yw; hs-akh; akh-gal4 virgin females were crossed to w; uas-reaper; + males. Crosses and egg collections were performed as described above. At 36 h AEL, approximately 100 first instar larvae from control and experimental crosses were transferred to standard fly food. Beginning at 36 h AEL, larvae were heatshocked at 37°C for 45 min every 8 h. At 108 h AEL, glucose and trehalose measurements were performed as described. To determine if amon function in the AKH producing cells is sufficient to maintain sugar homeostasis, yw; uas-amon; Df(3R) Tl-X e/TM3 Sb Ser y+ e virgin females were crossed to yw; hs-amon; akh-gal4, amonC241Y/TM3 Sb Ser y+ e males. As a control cross, yw; uas-amon; Df (3R)Tl-X e/TM3 Sb Ser y+ e virgin females were crossed to approximately yw; hs-amon; amonC241Y/TM3 Sb Ser y+ e males. Crosses and egg collections were performed as described above. At 36 h AEL, plates were heatshocked at 37°C for 30 min. Subsequent heatshocks were performed at 60 h and 84 h AEL. At 108 h AEL trehalose and glucose measurements were done as described previously.
Quantitative real-time PCR
yw; uas-amon-RNAi28b virgin females were crossed to hs-gal4 males in an egg collection chamber containing an apple juice plate with fresh yeast paste, and maintained at 27°C. As a control, w1118 virgin females were crossed to hs-gal4 males in the same manner. After 48 h, a 4 h egg collection was taken. Beginning at 36 h AEL, egg collection plates were heatshocked every 12 h for 1 h at 37°C. Total RNA was isolated from 10 whole third instar larvae at 108 h AEL with TRIzol reagent (Invitrogen), treated with DNaseI, and 5 µg were used for reverse transcription using the Transcriptior First Strand cDNA Synthesis Kit (Roche). The amon transcript was quantified using an Applied Biosystems 7500 Real-Time PCR system according to the manufacturer's instructions. Two sets of primers were used, targeting either exon 10 or exon 11: exon 10 forward (5′-GCCGGCGCCATGGT-3′) and reverse (5′-ATAGCGCGGTGGCACTGA-3′), and exon 11 forward (5′-TTCAACTCGCCCCAAACAC-3′) and reverse (5′-ATGCAGGACCAAGGACCATTC-3′). Ribosomal protein 49 (rp49) was used as an endogenous control.
Lethal phase analysis of amon-RNAi animals
Crosses and egg collections were performed as described in Quantitative Real Time PCR. Beginning at 36 h AEL, both control and experimental animals were heatshocked at 37°C for 1 h every 12 h until approximately 12 days (d) AEL. Death was scored once every 24 h. Pictures were taken with a digital camera (Hamamatsu 3CCD) mounted to a Leitz dissecting scope.
Quantitation of growth defects
yw; uas-amon; Df(3R)Tl-X e/TM3 Sb y+ e virgin females were mated to either control males (yw; +; amonC241Y e/TM3 Sb Ser y+ e) or experimental males (yw; +; akh-gal4, amonC241Y e/TM3 Sb y+ e) and were placed in egg collection chambers at 25°C. Four hour egg collections were taken. Larvae were sorted at 36 h AEL into mutant or control classes using the yellow marker. amon mutant larvae (yw; uas-amon/+; amonC241Y e/Df(3R)Tl-X e), rescued larvae (yw; uas-amon/+; akh-gal4, amonC241Y e/Df(3R)Tl-X e) and control larvae (yw; uas-amon; amonC241Y or Df(3R)Tl-X e/Tm3 Sb Ser y+ e) were washed and killed by microwave as described [15]. Length measurements were made from photographs taken with a digital camera (Hamamatsu 3CCD) mounted to a Leitz dissecting scope.
MALDI-TOF mass spectrometric profiling
Eggs of yw; +; amonC241Y/TM3 Sb Ser y+ e X yw; hs-amon; Df(3R)Tl-X e/TM3 Sb Ser y+ e flies were collected every morning, and heatshocked every 24 h until the larvae had reached the third instar. Three days later, the CNS was dissected free from surrounding tissue in standard Drosophila saline. For adult flies, heatshock was continued until eclosion and then stopped, and the corpora cardiaca (CC)/hypocerebral ganglion were dissected from 5 d old adult males as described [63]. Larval ring glands or the adult CC/hypocerebral ganglion were punched out with pulled glass capillaries, spottet directly onto the MALDI target and left to dry. For the ring gland, matrix (saturated solution of recrystallized α-cyano-4-hydroxycinnamic acid in MeOH/EtOH/Aq.bidest 30/30/40%) was added in small nanoliter volumes with a manual oocyte injector (Drummond Digital, Broomall, PA, USA). For adult CC, 200 nl of matrix was added to each sample with a micropipette. For peptide quantifications, 400 nM heavy isotope-labeled AKH* (pGlu-Leu[13C6, 15N]-Thr-Phe-Ser-Pro-Asp-Trp-amide, Mw = 982.5 Da, Iris Biotech, Marktredwitz, Germany) were added beforehand to the matrix solution. Low protein-binding plasticware was used throughout to minimize peptide loss. MALDI-TOF mass spectra were acquired in positive ion reflectron mode and delayed extraction on an Applied Biosystems Voyager DE RP MALDI-TOF or 4800+ MALDI TOF/TOF mass spectrometer (for quantifications). To suppress matrix ions, the low mass gate was set to 850 Da, with a focus mass of 1100 Da. For quantification, laser power was first adjusted with one sample to provide optimal signal-to-noise ratios, and then kept constant for all samples on the MALDI target. Each spectrum consisted of five subspectra with 50 shots each. For standard curves, adult OrR flies 1 d after eclosion were used; larvae were not sexed. For each amon fly, we measured a control fly taken from the same bottle that had either eclosed on the same day (adults) or originated from the same day of egg laying (larvae) to minimize possible age, food or population density effects. Data were analyzed with Data Explorer 4.3 software (Applied Biosystems). For quantification, mass spectra were base-line corrected and de-isotoped, and the relative peak intensities for the different adducts ([M+H]+,[M+Na]+,[M+K]+) of AKH and AKHGK were summed. Finally, the ratio of the resulting relative peak intensities of AKH/AKH* and AKHGK/AKH* was calculated. Statistics were performed using GraphStat Prism 4.0 (GraphStat Software, San Diego, CA).
Zdroje
1. SossinWS
FisherJM
SchellerRH
1989 Cellular and molecular biology of neuropeptide processing and packaging. Neuron 2 1407 1417
2. StrandFL
1999 New vistas for melanocortins. Finally, an explanation for their pleiotropic functions. Ann N Y Acad Sci 897 1 16
3. ZhouA
WebbG
ZhuXR
SteinerDF
1999 Proteolytic processing in the secretory pathway. Journal of Biological Chemistry 274 20745 20748
4. FurutaM
YanoH
ZhouA
RouilleY
HolstJJ
1997 Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proceedings of the National Academy of Sciences of the United States of America 94 6646 6651
5. NillniEA
2007 Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology 148 4191 4200
6. RouilleY
DuguaySJ
LundK
FurutaM
GongQ
1995 Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol 16 322 361
7. HelwigM
KhorooshiRM
TupsA
BarrettP
ArcherZA
2006 PC1/3 and PC2 gene expression and post-translational endoproteolytic pro-opiomelanocortin processing is regulated by photoperiod in the seasonal Siberian hamster (Phodopus sungorus). J Neuroendocrinol 18 413 425
8. SanchezVC
GoldsteinJ
StuartRC
HovanesianV
HuoL
2004 Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest 114 357 369
9. HookVYH
AzaryanAV
HwangSR
TezapsidisN
1994 Proteases and the emerging role of protease inhibitors in prohormone processing Faseb Journal 8 1269 1278
10. OsterwalderT
KuhnenA
LeisersonWM
KimYS
KeshishianH
2004 Drosophila serpin 4 functions as a neuroserpin-like inhibitor of subtilisin-like proprotein convertases. Journal of Neuroscience 24 5482 5491
11. ReichhartJM
2005 Tip of another iceberg: Drosophila serpins. Trends Cell Biol 15 659 665
12. IkeyaT
GalicM
BelawatP
NairzK
HafenE
2002 Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Current Biology 12 1293 1300
13. KimSK
RulifsonEJ
2004 Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431 316 320
14. ParkJH
SchroederAJ
Helfrich-ForsterC
JacksonFR
EwerJ
2003 Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development 130 2645 2656
15. RulifsonEJ
KimSK
NusseR
2002 Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science 296 1118 1120
16. EwerJ
2005 Behavioral actions of neuropeptides in invertebrates: insights from Drosophila. Horm Behav 48 418 429
17. NasselDR
2002 Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Progress in Neurobiology 68 1 84
18. TaghertPH
VeenstraJA
2003 Drosophila neuropeptide signaling. Advances in Genetics, Vol 49 San Diego Academic Press Inc 1 65
19. ZitnanD
KimYJ
ZitnanovaI
RollerL
AdamsME
2007 Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen Comp Endocrinol 153 88 96
20. DavisMM
O'KeefeSL
PrimroseDA
HodgettsRB
2007 A neuropeptide hormone cascade controls the precise onset of post-eclosion cuticular tanning in Drosophila melanogaster. Development 134 4395 4404
21. HenrichVC
RybczynskiR
GilbertLI
1999 Peptide hormones, steroid hormones, and puffs: mechanisms and models in insect development. Vitam Horm 55 73 125
22. McBrayerZ
OnoH
ShimellM
ParvyJP
BecksteadRB
2007 Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell 13 857 871
23. NijhoutHF
1994 Genes on the wing. Science 265 44 45
24. SiekhausDE
FullerRS
1999 A role for amontillado, the Drosophila homolog of the neuropeptide precursor processing protease PC2, in triggering hatching behavior. Journal of Neuroscience 19 6942 6954
25. RoebroekAJ
CreemersJW
PauliIG
BogaertT
Van de VenWJ
1993 Generation of structural and functional diversity in furin-like proteins in Drosophila melanogaster by alternative splicing of the Dfur1 gene. EMBO J 12 1853 1870
26. RoebroekAJM
AyoubiTAY
CreemersJWM
PauliIGL
VandevenWJM
1995 The dfur2 gene of Drosophila melanogaster - genetic organization, expression during embryogenesis, and pro-protein processing activity of its translational product dfurin2. DNA and Cell Biology 14 223 234
27. RayburnLY
RheaJ
JocoySR
BenderM
2009 The proprotein convertase amontillado (amon) is required during Drosophila pupal development. Dev Biol 333 48 56
28. RayburnLYM
GoodingHC
ChoksiSP
MaloneyD
KiddAR
2003 amontillado, the Drosophila homolog of the prohormone processing protease PC2, is required during embryogenesis and early larval development. Genetics 163 227 237
29. HwangJR
SiekhausDE
FullerRS
TaghertPH
LindbergI
2000 Interaction of Drosophila melanogaster prohormone convertase 2 and 7B2 - Insect cell-specific processing and secretion. Journal of Biological Chemistry 275 17886 17893
30. KodrikD
SochaR
SimekP
ZemekR
GoldsworthyGJ
2000 A new member of the AKH/RPCH family that stimulates locomotory activity in the firebug, Pyrrhocoris apterus (Heteroptera). Insect Biochemistry and Molecular Biology 30 489 498
31. KollischGV
LorenzMW
KellnerR
VerhaertPD
HoffmannKH
2000 Structure elucidation and biological activity of an unusual adipokinetic hormone from corpora cardiaca of the butterfly, Vanessa cardui. European Journal of Biochemistry 267 5502 5508
32. SchafferMH
NoyesBE
SlaughterCA
ThorneGC
GaskellSJ
1990 The fruitfly Drosophila melanogaster contains a novel charged adipokinetic hormone family peptide. Biochemical Journal 269 315 320
33. SiegertKJ
KellnerR
GadeG
2000 A third active AKH is present in the pyrgomorphid grasshoppers Phymateus morbillosus and Dictyophorus spumans. Insect Biochemistry and Molecular Biology 30 1061 1067
34. StoneJV
MordueW
BatleyKE
MorrisHR
1976 Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilization during flight. Nature 263 207 211
35. Van der HorstDJ
2003 Insect adipokinetic hormones: release and integration of flight energy metabolism. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology 136 217 226
36. IsabelG
MartinJR
ChidamiS
VeenstraJA
RosayP
2005 AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 288 R531 R538
37. LeeGH
ParkJH
2004 Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167 311 323
38. WyattGR
1961 Biochemistry of Insect Hemolymph. Annual Review of Entomology 6 75 &
39. GadeG
2004 Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annual Review of Entomology 49 93 113
40. NoyesBE
KatzFN
SchafferMH
1995 Identification and expression of the Drosophila adipokinetic hormone gene. Molecular and Cellular Endocrinology 109 133 141
41. PredelR
WegenerC
RussellWK
TichySE
RussellDH
2004 Peptidomics of CNS-associated neurohemal systems of adult Drosophila melanogaster: a mass spectrometric survey of peptides from individual flies. J Comp Neurol 474 379 392
42. WegenerC
ReinlT
JanschL
PredelR
2006 Direct mass spectrometric peptide profiling and fragmentation of larval peptide hormone release sites in Drosophila melanogaster reveals tagma-specific peptide expression and differential processing. Journal of Neurochemistry 96 1362 1374
43. RayneRC
O'SheaM
1994 Reconstitution of adipokinetic hormone biosynthesis in-vitro indicates steps in prohormone processing. European Journal of Biochemistry 219 781 789
44. BrogioloW
StockerH
IkeyaT
RintelenF
FernandezR
2001 An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Current Biology 11 213 221
45. KaufmannC
BrownMR
2006 Adipokinetic hormones in the African malaria mosquito, Anopheles gambiae: Identification and expression of genes for two peptides and a putative receptor. Insect Biochemistry and Molecular Biology 36 466 481
46. GobomJ
NordhoffE
2002 Quantitative Analysis of neuropeptides by MALDI-TOF MS.
SilberringJ
EckmanR
Mass spectrometry and hyphenated techniques in neuropeptide research New York Wiley and Sons
47. SzajliE
FeherT
MedzihradszkyKF
2008 Investigating the quantitative nature of MALDI-TOF MS. Mol Cell Proteomics 7 2410 2418
48. FurutaM
ZhouA
WebbG
CarrollR
RavazzolaM
2001 Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. Journal of Biological Chemistry 276 27197 27202
49. WangS
TulinaN
CarlinDL
RulifsonEJ
2007 The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proceedings of the National Academy of Sciences of the United States of America 104 19873 19878
50. GromadaJ
FranklinI
WollheimCB
2007 Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28 84 116
51. RouilleY
WestermarkG
MartinSK
SteinerDF
1994 Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proc Natl Acad Sci U S A 91 3242 3246
52. PanH
CheFY
PengB
SteinerDF
PintarJE
2006 The role of prohormone convertase-2 in hypothalamic neuropeptide processing: a quantitative neuropeptidomic study. Journal of Neurochemistry 98 1763 1777
53. AllenRG
PengB
PellegrinoMJ
MillerED
GrandyDK
2001 Altered processing of pro-orphanin FQ/nociceptin and pro-opiomelanocortin-derived peptides in the brains of mice expressing defective prohormone convertase 2. Journal of Neuroscience 21 5864 5870
54. JimenezCR
LiKW
DreisewerdK
MansvelderHD
BrussaardAB
1997 Pattern changes of pituitary peptides in rat after salt-loading as detected by means of direct, semiquantitative mass spectrometric profiling. Proc Natl Acad Sci U S A 94 9481 9486
55. JimenezCR
ter MaatA
PienemanA
BurlingameAL
SmitAB
2004 Spatio-temporal dynamics of the egg-laying-inducing peptides during an egg-laying cycle: a semiquantitative matrix-assisted laser desorption/ionization mass spectrometry approach. J Neurochem 89 865 875
56. DiederenJHB
OudejansR
HarthoornLF
Van der HorstDJ
2002 Cell biology of the adipokinetic hormone-producing neurosecretory cells in the locust corpus cardiacum. Microscopy Research and Technique 56 227 236
57. DumonteilE
MagnanC
Ritz-LaserB
KtorzaA
MedaP
2000 Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology 141 174 180
58. MagnanC
PhilippeJ
KassisN
LauryMC
PenicaudL
1995 In vivo effects of glucose and insulin on secretion and gene expression of glucagon in rats. Endocrinology 136 5370 5376
59. WebbGC
DeyA
WangJ
SteinJ
MilewskiM
2004 Altered proglucagon processing in an alpha-cell line derived from prohormone convertase 2 null mouse islets. Journal of Biological Chemistry 279 31068 31075
60. Helfrich-ForsterC
TauberM
ParkJH
Muhlig-VersenM
SchneuwlyS
2000 Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. J Neurosci 20 3339 3353
61. BrandAH
PerrimonN
1993 Targeted Gene Expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401 415
62. CaiHN
ArnostiDN
LevineM
1996 Long-range repression in the Drosophila embryo. Proc Natl Acad Sci U S A 93 9309 9314
63. WegenerC
NeupertS
PredelR
2010 Direct MALDI-TOF mass spectrometric peptide profiling of neuroendocrine tissue of Drosophila. Methods Mol Biol 615 117 127
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein SignalingČlánek B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish EmbryoČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



