-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTurning Up the Heat: Inflammasome Activation by Fungal Pathogens
article has not abstract
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1004948
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004948Summary
article has not abstract
The Inflammasome
Since its first description in 2002 [1], the inflammasome has been implicated in the mechanisms underlying a growing number of infectious, autoimmune, and metabolic diseases [2]. Regarding infectious processes, several studies have shown the involvement of this critical component of innate immunity in the outcome of infection with nearly every class of microbe, including fungi [3]. Innate immunity is the frontline of defense against infection and relies on the ability of its main players (phagocytes and epithelial barriers) to detect conserved components of microbes or pathogen-associated molecular patterns (PAMPs). In fungi, the carbohydrate polymers of the cell wall, such as chitin, β-glucan, and mannan are the major PAMPs recognized by the host’s innate immune cells; this recognition occurs via germline-encoded receptors termed pattern recognition receptors (PRRs) [4]. In addition to PAMPs, endogenous molecules associated with damaged host cells, or damage-associated molecular patterns (DAMPs), are released during tissue injury and activate PRRs. This innate detection system includes the Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), and AIM2-like receptors (ALRs). Although the main fungal-recognition PRRs (CLRs and TLRs) are bound to the cytoplasmic membrane of innate immune cells [4], fungal sensing by PRRs located in the cytosol, such as the NLRs and ALRs, is becoming increasingly evident.
A number of NLRs and ALRs can assemble into the inflammasome, a multiprotein complex consisted of PRRs such as NLRP3 (NLR family, pyrin domain-containing 3), NLRC4, or AIM2, adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD), and procaspase-1 [3]. Upon formation of the complex, procaspase-1 is cleaved into an active cysteine protease, which further cleaves the proinflammatory cytokines IL-1β and IL-18 into their mature forms, followed by unconventional secretion. IL-1β and IL-18 mediate several innate antimicrobial responses and are critical to direct adaptive Th17/Th1 cellular responses [5]. In addition, inflammasome activation causes pyroptosis, a lytic inflammatory form of cell death [2,5].
NLRP3 Inflammasome Priming by Fungal Pathogens
Among the inflammasomes, the NLRP3 inflammasome is the main one associated with fungal infection. In contrast to its counterparts, which only respond to a few specific PAMPs, the NLRP3 inflammasome is activated by a diverse array of unrelated triggers that include PAMPs from every class of pathogen, environmental irritants, and DAMPs. Although the precise mechanism of NLRP3 inflammasome activation is unclear, there is evidence suggesting that it is a two-step process [6]. The first, or priming, step is an NF-κB-dependent pathway that triggers expression of pro-IL-1β, pro-IL-18, and optimal NLRP3. In the second, or activation, step, assembly of the inflammasome complex leads to caspase-1 activation to promote cleavage of the immature cytokines. Priming is most frequently achieved via PRR recognition of PAMPs. In this manner, fungal PAMPs are recognized by several CLRs and TLRs that can potentially activate NF-κB [4]. However, dectin-1-dependent signaling is emerging as the key pathway involved in fungus-induced NLRP3 priming (Fig 1) [7]. In addition, this PRR is necessary to activate a caspase-8-dependent inflammasome (see below). Dectin-1, the major β-glucan receptor, uses an immunoreceptor tyrosine-based activation motif to couple itself to Syk kinase for downstream signaling to NF-κB via CARD9-Bcl10-MALT1 (CBM) scaffold, resulting in cytokine production. In addition, phagocytosis and reactive oxygen species (ROS) production result from dectin-1 engagement [8]. The dectin-1 receptor is required for the production of pro-IL-1β in murine and human myeloid cells infected with Candida albicans, Microsporum canis, and Malassezia spp. [9–11]. Consistent with these results, mice deficient in dectin-1 and orally infected with C. albicans presented significantly reduced serum IL-1β levels [12]. Notably, mice lacking dectin-1 or CARD9 and humans with mutations in these proteins are susceptible to candidiasis [12–14]. Although the direct role of dectin-1 has not been evaluated, Syk-dependent NLRP3 priming also occurs in Paracoccidioides brasiliensis-, acapsular Cryptococcus neoformans - and Aspergillus fumigatus-infected cells [15–17]. Interestingly, glucuronoxylomannan, the main capsule component of C. neoformans, inhibits Syk signaling and ultimately NLRP3 inflammasome activation, which may facilitate the intracellular parasitism of this fungus [16]. Considering that the CLRs dectin-2 and mincle share the same downstream pathway as dectin-1, further studies will be necessary to assess the role of these receptors in NLRP3 priming by fungal pathogens.
Fig. 1. Activation of inflammasomes by fungal pathogens. 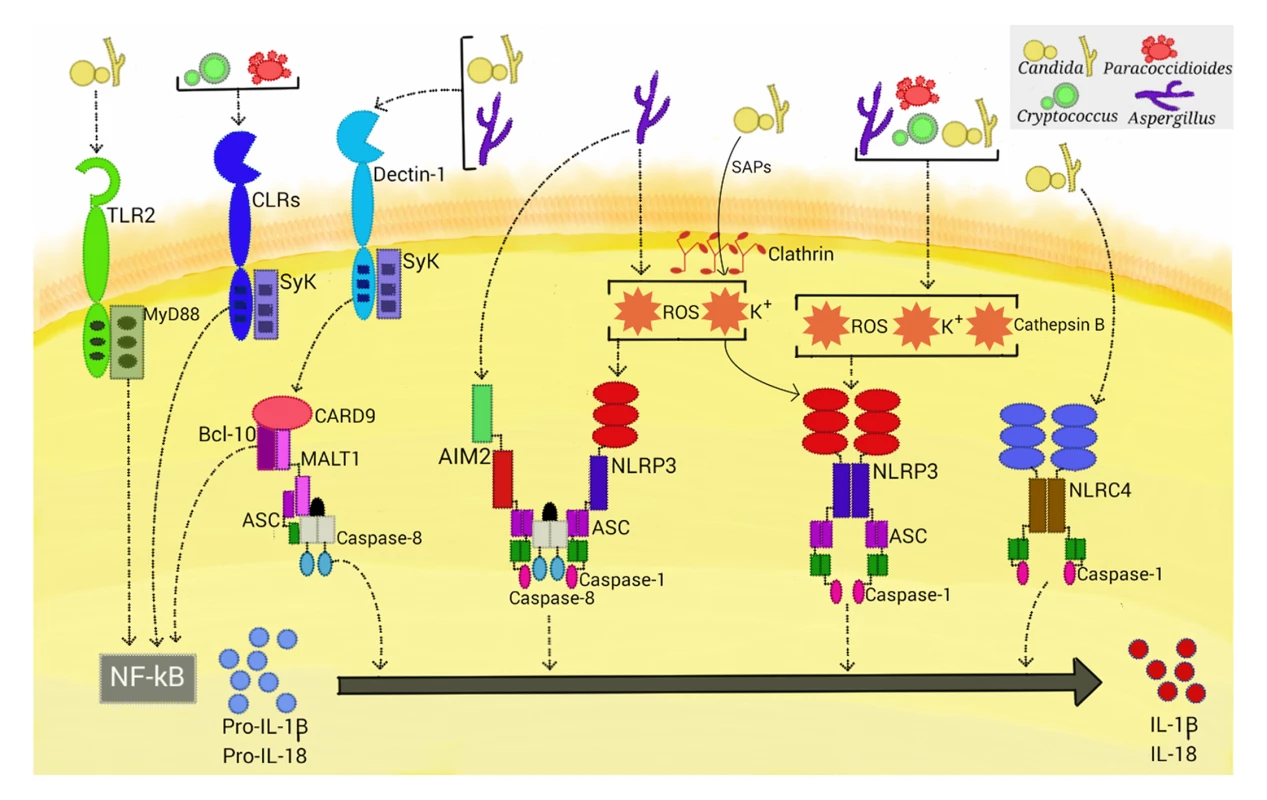
Four inflammasome complexes are activated when engaged by systemic fungal pathogens. Candida, Aspergillus, Cryptococcus, and Paracoccidioides trigger priming-associated receptors (e.g., dectin-1 and TLR2), thereby promoting the first step (priming) of inflammasome activation. All four pathogens activate the canonical caspase-1-dependent NLRP3 inflammasome by inducing ROS, K+ efflux, and cathepsin B release. Candida-secreted aspartic proteases (Saps) are internalized and also activate the NLRP3 inflammasome via induction of K+ efflux and ROS production. Aspergillus activates both caspase-1- and caspase-8-dependent inflammasomes, requiring the simultaneous activation of the NLRP3 and AIM2 receptors. In terms of NLRP3-independent inflammasomes, Candida and Aspergillus activate a caspase-8-dependent inflammasome that requires the engagement of dectin-1, Syk signaling, and ASC recruitment. In addition, Candida triggers the NLRC4 inflammasome. In addition to the dectin-1/Syk pathway, TLR2 and the critical TLR signal adaptor protein MyD88 (Fig 1) are also necessary for the production of pro-IL-1β in murine macrophages infected with C. albicans. In contrast, priming of murine dendritic cells stimulated with this fungus only required the dectin-1/Syk pathway [9,18]. These findings are consistent with those of earlier studies that demonstrate that cooperative signaling between dectin-1 and TLR-2 is necessary for TNF-α production by macrophages stimulated with zymosan (dectin-1 and TLR2 agonist), whereas in dendritic cells, dectin-1 signals directly induce cytokine production [19]. Thus, the simultaneous engagement of CLR and TLR is differentially regulated in macrophages and dendritic cells, suggesting a mechanism by which myeloid cells can be fine-tuned to regulate the inflammatory process.
NLRP3 Inflammasome Activation by Fungal Pathogens
The mechanisms by which the NLRP3 inflammasome senses its vast array of activators seem to converge on several cellular disturbances that are probably nonexclusive, such as potassium (K+) efflux, calcium influx, ROS production, and the occurrence of cytosolic cathepsins derived from lysosomal disruption [6]. Although there are several controversies surrounding the actual role of each factor in inducing NLRP3 inflammasome assembly, it has been shown that fungi generally induce ROS, K+ efflux, and lysosomal rupture-dependent IL-1β release (Fig 1). ROS, which are a conserved danger signal, and K+ efflux are required by nearly all fungal species found to activate the NLRP3 inflammasome to date. Interestingly, dectin-1/Syk-mediated release of ROS was demonstrated to be critical in inflammasome activation in C. albicans-infected murine and human phagocytes [18]. Thus, Syk-coupled dectin-1 signaling is implicated in both priming and activation of the NLRP3 inflammasome. Recently, secreted aspartic protease (Sap) 2 and Sap6 from Candida were the first fungal proteins demonstrated to provide a caspase-1-dependent NLRP3 inflammasome activation signal (Fig 1) [20]. The inflammasome activation required Sap internalization via a clathrin-dependent mechanism, followed by induction of K+ efflux and ROS production. Additionally, these proteases activate, through the induction of type I IFN production, caspase-11 that cooperates with caspase-1 to maximize IL-1β maturation [21]. In view of the elevated secretion of Saps during vaginal candidiasis, studies are necessary to assess the in vivo relevance of these data.
Although internalization and cytoplasmic leakage of cathepsin B due to lysosomal disruption are common features of particulate activations of the NLRP3 inflammasome, data on the role of cathepsin B in fungal inflammasome activation are conflicting. Pietrella et al. [20] and Joly et al. [22] found this intracellular perturbation to be necessary in macrophages infected with C. albicans, whereas no role was found in dendritic cells. In human monocytes infected with C. neoformans, M. canis, and Trichophyton schoenleinii, cathepsin B release is required for IL-1β maturation [13,23,24]. In this manner, differential requirements for inflammasome activation, as shown for PRR usage in the priming step, may exist in these cells.
Morphogenesis is another important aspect of inflammasome activation by fungal pathogens. It is well established that only A. fumigatus hyphal fragments, and not conidia, activate the NLRP3 inflammasome [17]. A more complex story emerges from Candida studies. Hyphal formation was initially thought to be essential for the activation of the NLRP3 inflammasome [22]. However, studies showing Candida mutants that lack the ability to form hyphae but are able to trigger the inflammasome strongly suggest that filamentation per se is not the only responsible for inflammasome activation [25]. In fact, exposition of PAMPs, such as β-glucan and chitin, is the critical factor for NLRP3 inflammasome activation, even though this process is likely to be coordinately regulated with filamentation [26]. These data support recent studies showing that remodeling of the Candida cell wall, but not hyphal formation, upon internalization by host macrophages is essential for caspase-1/NLRP3-mediated pyroptosis [27,28].
Beyond the NLRP3 Inflammasome in Fungal Infections
In addition to the NLRP3 inflammasome, a NLR-independent caspase-8-dependent inflammasome and other canonical caspase-1-dependent inflammasomes are activated when stimulated with fungi (Fig 1). Gringhuis et al. [29] found that human dendritic cells stimulated with certain strains of C. albicans did not utilize caspase-1 to process IL-1β. This finding led to several experiments demonstrating a NLR-independent dectin-1/Syk-dependent inflammasome activation route, with the assembly of the CBM scaffold and processing of IL-1β mediated by recruitment of MALT-1/caspase-8 and ASC into this complex. This route was also activated by other species of Candida and different A. fumigatus strains. Moreover, the dectin-1-mediated activity, in contrast to NLRP3 inflammasome priming, did not require phagocytosis, suggesting a direct extracellular sensing mechanism. Later, Ganesan et al. [30] demonstrated that caspase-8, dectin-1, and CR3, another receptor implicated in β-glucan sensing, are necessary for IL-1β processing by murine dendritic cells infected with C. albicans. Interestingly, caspase-8 activation in these studies raises questions about Candida-induced programmed cell death pathways, as caspase-8 also has a significant role in initiating apoptosis [31].
Best known for its role in inflammatory responses to flagellated and type 3 secretion system-expressing bacteria, the NLRC4 inflammasome had been found to be dispensable for canonical IL-1β maturation in macrophages and dendritic cells challenged with C. albicans [18]. In contrast, Tomalka et al. [32] showed activation of the NLRC4 inflammasome, along with NLRP3, in a murine model of oral C. albicans infection. One plausible explanation for this discrepancy is that Candida-induced alterations in the mucosal barrier may have allowed bacterial pathobionts to penetrate and infect cells and activate the NLRC4-dependent inflammasome. Interestingly, A. fumigatus induces cooperative and synergistic activation of the NLRP3 and AIM2 inflammasomes in dendritic cells and in a mouse intranasal infection model [33]. Upon ligand binding, these inflammasome receptors trigger the assembly of a single platform, containing ASC, caspase-1, and caspase-8, which are required for IL-1β and IL-18 processing. This study also corroborated the cytoplasmic disturbances (i.e., ROS and K+ efflux) induced by A. fumigatus that are necessary for NLRP3 activation [17] and suggested the availability of fungal dsDNA (the main AIM2 ligand) in the cytosol of infected cells. Considering that bacterial RNA and DNA are sensed by NLRP3 [34], it is possible that NLRP3 and AIM2 both recognize a common nucleic acid composition of A. fumigatus, prompting the formation of a cooperative inflammasome. Altogether, these studies highlight the emerging trend that multiple inflammasome receptors can be activated upon infection with fungal pathogens, as has been shown for several species of bacteria, adding further complexity to the fungus–host relationship.
The Inflammasome and Host Resistance against Fungal Pathogens
The depletion of inflammasome components, inflammasome products, and relevant first-signal receptors has been linked to susceptibility of the host to several bacterial infections. Although the available data are scarce, it seems that this phenomenon extends to fungal infections. In C. albicans, it has been demonstrated that in a disseminated model of infection, loss of NLRP3 leads to increased mortality and an increased fungal burden in several organs, such as the kidney, lung, and liver [18,22]. van de Veerdonk et al. [35] further showed an essential role for ASC and caspase-1 in regulating adaptive antifungal immune responses and host survival during disseminated candidiasis through the induction of Th1 and Th17 development. NLRP3 inflammasome components were also studied in a model of mucosal Candida infection, with NLRP3-, ASC-, or caspase-1-deficient mice being more susceptible to invasive disease, given that they presented a higher fungal burden in the kidneys and the digestive system [9]. Furthermore, loss of the IL-1 receptor (IL-1R) and the priming-associated receptors dectin-1 and TLR-2 aggravated the infection. Using bone marrow chimeras, it was demonstrated that NLRP3 and NLRC4 have a protective but tissue-specific role in oropharyngeal candidiasis [32]. In contrast to NLRP3, whose activation is protective in both the hematopoietic and the stromal compartments, NLRC4 is essential in only the stromal compartment, where its activity is necessary for the induction of neutrophil influx into infected tissues and the avoidance of fungal dissemination, particularly early in infection. Nevertheless, these results should be interpreted with caution since it is also possible that mice lacking NLRC4 possess a diverse microbiota from other controls. In fact, microbiota differences are observed in inflammasome knockout mice [36], resulting in microbiota-driven differences in phenotypes (i.e., microbiota would track with the stromal compartment in bone marrow transplants). Recently, using an invasive pulmonary aspergillosis model, Karki et al. [33] showed that redundant activation of the NLRP3/AIM2 platform is essential for host protection, as it significantly limits dissemination of A. fumigatus hyphae from inflammatory foci. In addition, AIM2 and NLRP3 activity in both the hematopoietic and the stromal compartments is required to protect the host against aspergillosis. It was also shown that loss of key inflammasome-related cytokines (e.g., IL-1β and IL-18) is detrimental for the host. Regarding cryptococcosis, regardless of the route of infection (e.g., intraperitoneal or intranasal), mice lacking NLRP3 or ASC presented poorer survival compared with wildtype mice [23]. In fact, even for intranasal infection with a low virulence acapsular strain of C. neoformans, NLRP3 was required for effective lung leukocyte infiltration and fungal clearance. Finally, our group demonstrated that the presence of the IL-1R-dependent signaling, and NLRP3 is required to control the intracellular growth of P. brasiliensis within macrophages [15].
These studies suggest that the activation of inflammasomes is of vital importance for innate immunity to constrain the growth and dissemination of fungal pathogens, conferring fungal resistance upon the host. In support of this notion, patients diagnosed with vulvar vestibulitis syndrome present a length polymorphism in intron 4 of the NLRP3-coding gene, which has been associated with recurrent vulvovaginal candidiasis [37]. It is important, however, to keep in mind that fine-tuning of inflammasome activity is necessary to avoid intense and detrimental inflammatory responses. In fact, in a study utilizing a pulmonary aspergillosis model, Moretti et al. [38] suggested that the activation of the NLRP3 inflammasome may causes pathological sequelae in the face of an unresolved infection.
In summary, significant progress has been made in understanding the role of inflammasomes in fungal diseases. The activation of noncanonical inflammasome and multiple inflammasomes as well as the induction of pyroptosis being the most recent. However, questions remain unanswered. For example, what is the exact mechanism by which NLRP3 is activated and regulated by diverse fungal pathogens? What is the functional relevance of pyroptosis? Also poorly understood are the fungal evasion strategies developed to avoid inflammasome recognition and the inflammasome functionality in nonmyeloid cells during fungal infections. Clearly, it will be exciting to witness the future studies on those topics.
Zdroje
1. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10 : 417–426. 12191486
2. Zhong Y, Kinio A, Saleh M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol. 2013;16 : 4:333. doi: 10.3389/fimmu.2013.00333 24137163
3. Bauernfeind F, Hornung V. Of inflammasomes and pathogens-sensing of microbes by the inflammasome. EMBO Mol Med. 2013;5 : 814–826. doi: 10.1002/emmm.201201771 23666718
4. Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37 : 97–106. doi: 10.1007/s00281-014-0462-4 25420452
5. van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32 : 110–116. doi: 10.1016/j.it.2011.01.003 21333600
6. Vanaja S, Rathinam V, Fitzgerald K. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015;25 : 308–315. doi: 10.1016/j.tcb.2014.12.009 25639489
7. Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 2010;184 : 6335–6342. doi: 10.4049/jimmunol.0903019 20421639
8. Underhill D, Rossnagle E, Lowell C, Simmons R. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106 : 2543–2550. 15956283
9. Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5 : 487–497. doi: 10.1016/j.chom.2009.05.002 19454352
10. Mao L, Zhang L, Li H, Chen W, Wang H, Wu S, et al. Pathogenic fungus Microsporum canis activates the NLRP3 inflammasome. Infect Immun. 2014;82 : 882–892. doi: 10.1128/IAI.01097-13 24478101
11. Kistowska M, Fenini G, Jankovic D, Feldmeyer L, Kerl K, Bosshard P, et al. Malassezia yeasts activate the NLRP3 inflammasome in antigen-presenting cells via Syk-kinase signalling. Exp Dermatol. 2014;23 : 884–889. doi: 10.1111/exd.12552 25267545
12. Taylor P, Tsoni S, Willment J, Dennehy K, Rosas M, Haynes K, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8 : 31–38. 17159984
13. Ferwerda B, Ferwerda G, Plantinga T, Willment J, van Spriel A, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361 : 1760–1767. doi: 10.1056/NEJMoa0901053 19864674
14. Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361 : 1727–1735. doi: 10.1056/NEJMoa0810719 19864672
15. Tavares AH, Magalhães KG, Almeida RD, Correa R, Burgel PH, Bocca AL. NLRP3 inflammasome activation by Paracoccidioides brasiliensis. PLoS Negl Trop Dis. 2013;7: e2595. doi: 10.1371/journal.pntd.0002595 24340123
16. Guo C, Chen M, Fa Z, Lu A, Fang W, Sun B, et al. Acapsular Cryptococcus neoformans activates the NLRP3 inflammasome. Microbes Infect. 2014;16 : 845–854. doi: 10.1016/j.micinf.2014.08.013 25193031
17. Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PloS one. 2010;5: e10008. doi: 10.1371/journal.pone.0010008 20368800
18. Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459 : 433–436. doi: 10.1038/nature07965 19339971
19. Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197 : 1107–1117. 12719479
20. Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, et al. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur J Immunol. 2013;43 : 679–692. doi: 10.1002/eji.201242691 23280543
21. Gabrielli E, Pericolini E, Luciano E, Sabbatini S, Roselletti E, et al. Aspartyl proteinases of Candida albicans induce caspase-11: implication in promoting inflammatory response. Infect Immun. 2015; 83 : 1940–1948. doi: 10.1128/IAI.02895-14 25712931
22. Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183 : 3578–3581. doi: 10.4049/jimmunol.0901323 19684085
23. Lei G, Chen M, Li H, Niu JL, Wu S, Mao L, et al. Biofilm from a clinical strain of Cryptococcus neoformans activates the NLRP3 inflammasome. Cell Res. 2013;23 : 965–968. doi: 10.1038/cr.2013.49 23567555
24. Li H, Wu S, Mao L, Lei G, Zhang L, Lu A, et al. Human pathogenic fungus Trichophyton schoenleinii activates the NLRP3 inflammasome. Protein Cell. 2013;4 : 529–538. doi: 10.1007/s13238-013-2127-9 23686720
25. Wellington M, Koselny K, Krysan D. Candida albicans morphogenesis is not required for macrophage interleukin 1β production. MBio. 2012;4: e00433–12. doi: 10.1128/mBio.00433-12 23269828
26. Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90 : 357–363. doi: 10.1189/jlb.1210702 21531876
27. Wellington M, Koselny K, Sutterwala F, Krysan D. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell. 2014;13 : 329–340. doi: 10.1128/EC.00336-13 24376002
28. O'Meara T, Veri A, Ketela T, Jiang B, Roemer T, Cowen LE, et al. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun. 2015;6 : 6741. doi: 10.1038/ncomms7741 25824284
29. Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13 : 246–254. doi: 10.1038/ni.2222 22267217
30. Ganesan S, Rathinam V, Bossaller L, Army K, Kaiser W, Mocarski ES, et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J Immunol. 2014;193 : 2519–2530. doi: 10.4049/jimmunol.1400276 25063877
31. Stupack D. Caspase-8 as a therapeutic target in cancer. Cancer Lett. 2013;332 : 133–140. doi: 10.1016/j.canlet.2010.07.022 20817393
32. Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Majmudar P, et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7: e1002379. doi: 10.1371/journal.ppat.1002379 22174673
33. Karki R, Man S, Malireddi R, Gurung P, Vogel P, Lamkanfi M, et al. Concerted Activation of the AIM2 and NLRP3 Inflammasomes Orchestrates Host Protection against Aspergillus Infection. Cell Host Microbe. 2015;17 : 357–368. doi: 10.1016/j.chom.2015.01.006 25704009
34. Kailasan Vanaja S, Rathinam V, Atianand M, Kalantari P, Skehan B, Fitzgerald KA et al. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA. 2014;111 : 7765–7770. doi: 10.1073/pnas.1400075111 24828532
35. van de Veerdonk F, Joosten L, Shaw P, Smeekens S, Malireddi R, van der Meer JW, et al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011; 41 : 2260–2268. doi: 10.1002/eji.201041226 21681738
36. Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145 : 745–757. doi: 10.1016/j.cell.2011.04.022 21565393
37. Lev-Sagie A, Prus D, Linhares I, Lavy Y, Ledger. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. J Am J Obstet Gynecol. 2009;200 : 303.e1-6.
38. Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;10: e1004462. doi: 10.1371/journal.ppat.1004462 25375146
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



