-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
Approximately a third of the world’s population is burdened with chronic intestinal parasitic helminth infections, causing significant morbidities. Identifying the factors that contribute to the chronicity of infection is therefore essential. Co-infection with other pathogens, which is extremely common in helminth endemic areas, may contribute to the chronicity of helminth infections. In this study, we used a mouse model to test whether the immune responses to an intestinal helminth were impaired following malaria co-infection. These two pathogens induce very different immune responses, which, until recently, were thought to be opposing and non-interchangeable. This study identified that the immune cells required for anti-helminth responses are capable of changing their phenotype and providing protection against malaria. By identifying and blocking the factors that drive this change in phenotype, we can preserve anti-helminth immune responses during co-infection. Our studies provide fresh insight into how immune responses are altered during helminth and malaria co-infection.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1004994
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004994Summary
Approximately a third of the world’s population is burdened with chronic intestinal parasitic helminth infections, causing significant morbidities. Identifying the factors that contribute to the chronicity of infection is therefore essential. Co-infection with other pathogens, which is extremely common in helminth endemic areas, may contribute to the chronicity of helminth infections. In this study, we used a mouse model to test whether the immune responses to an intestinal helminth were impaired following malaria co-infection. These two pathogens induce very different immune responses, which, until recently, were thought to be opposing and non-interchangeable. This study identified that the immune cells required for anti-helminth responses are capable of changing their phenotype and providing protection against malaria. By identifying and blocking the factors that drive this change in phenotype, we can preserve anti-helminth immune responses during co-infection. Our studies provide fresh insight into how immune responses are altered during helminth and malaria co-infection.
Introduction
Infections with Plasmodium and helminths are extremely common, each contributing to substantial morbidity in affected populations [1–3]. Additionally, co-infections with Plasmodium species and intestinal helminths occur frequently in co-endemic areas [4,5]. The impact of co-infection on disease burden, pathogenesis, resistance to infection and immunity is complex and poorly understood. The vast majority of reported co-infection studies have focused on the impact of helminth infection on Plasmodium-associated responses, identifying altered anti-malarial immune responses or malaria-associated pathology during helminth co-infection [6–11]. However, the specific impact of Plasmodium infection on anti-helminth immunity has not been well characterized. Experimental murine models of helminth and Plasmodium co-infections have been established, however these have also mainly focused on how concomitant helminth infection affects Plasmodium immunity and pathology [11–16], with much less focus on how Plasmodium infection impacts helminth-associated type 2 responses.
Murine models of intestinal helminth infections have delineated a clear role for Th2-directed immune responses for proficient immunity. In particular, infection with the natural murine helminth, Heligmosomoides polygyrus, results in a chronic infection with the induction of a polarized type 2 response, characterized by IL-4-producing Th2 cells, alternative activation of macrophages and elevated IgE, closely mimicking human helminthiasis. Following anthelmintic treatment, Th2 cell-dependent immunity protects mice from re-infection (reviewed in [17,18]). In contrast, acute blood-stage infection with the rodent malaria parasite, Plasmodium chabaudi chabaudi (AS), results in polyclonal lymphocyte activation with a strongly polarized Th1 response [19]. Disease is associated with a spectrum of immunopathologies including splenomegaly and anemia [20–22] with peak parasitemia occurring 7–9 days post-infection [23]. These well-studied experimental systems, modeling human disease, provide appropriate tools to dissect the immune responses during co-infection.
There is a large body of literature describing the antagonistic relationship between Th1 and Th2 cell differentiation. In vitro-based studies have clearly established that under Th1 and Th2 polarizing conditions, differentiated cells become more fixed in their phenotype with increasing rounds of cell division, losing their ability to convert to alternative phenotypes [24,25]. Mechanistically, T-bet and GATA-3, transcription factors required to promote Th1 and Th2 differentiation, respectively, inhibit differentiation of the opposing phenotype [26,27]. Despite this clear antagonistic relationship, IL-4+IFNγ+ and T-bet+GATA-3+ Th cells are readily observed in vivo [28,29], and several studies have established that Th subsets retain flexibility in their ability to produce non-lineage-specific cytokines [30–32]. Indeed, recent studies challenging the fate-lineage dogma demonstrated that antigen-restricted TCR transgenic Th2 cells co-produced IFNγ and IL-4 following LCMV infection [33,34].
In light of these new data, it is possible that Th cell conversion occurs during co-infection, altering immunity to one or both pathogens or contributing to the chronicity of helminth infection.
In this study, we observed that Plasmodium and helminth co-infection led to a reduction of helminth-elicited Il4gfp+ Th2 cells and compromised anti-helminth immunity. We hypothesized that helminth-elicited Th2 cells were being converted into IFNγ-secreting Th1 cells during Plasmodium co-infection, as pressure to control both pathogens was placed on the Th cell population. To test this hypothesis, we generated triple cytokine reporter mice to accurately purify and identify Il4gfp, Ifngyfp and Il17aFP635-expressing cells to determine whether Th2 cells had the ability to change their phenotype. We observed that Il4-expressing Th2 cells could readily produce IFNγ following adoptive transfer in Rag–/–recipients, and these cells reduced severe parasitemia during acute P. chabaudi infection. Conversion of Th2 cells was dependent upon IL-12 and IFNγ-signaling, and blockade of these cytokines during co-infection preserved the Th2 response. Overall, this study provides fresh insight into the functional relationship between IFNγ - and IL-4-producing Th cells during co-infection and indicates that limiting acute Th1 responses may preserve Th2-mediated anti-helminth immunity.
Results
Plasmodium infection compromises Th2-dependent anti-helminth immunity
To assess the impact of concomitant Plasmodium infection on the development of Th2 responses, we infected mice with H. polygyrus and 6 days later with 105 P. chabaudi-infected red blood cells (Fig 1A). To accurately identify simultaneous transcription of Th1 (Ifng), Th2 (Il4) and Th17 (Il17a) lineage-defining genes, we generated a triple cytokine reporter mouse (Il4gfpIfngyfpIl17aCreR26FP635) using existing and new fluorescent cytokine reporter mouse strains [35–37] (S1 Fig). Following infection with L3 larvae of the intestinal helminth, H. polygyrus, we observed a significant expansion of Il4gfp+ CD4+ Th2 cells in the mesenteric lymph nodes 14 days post-infection. Co-infected mice had significantly reduced numbers of Il4gfp+ CD4+ Th2 cells in the mesenteric lymph nodes (Fig 1B) as well as a reduction in serum IgE (Fig 1C) and decreased expression of the alternative macrophage activation marker, Retnla (Relmα) in the gut (S2 Fig). These data indicated that helminth-elicited Th2 cells and Th2-driven immune responses were compromised during Plasmodium co-infection. The reduced Il4gfp+ cells in the mesenteric lymph nodes correlated with an increase in Ifngyfp+ cells in the spleen during co-infection.
Fig. 1. H. polygyrus/ P. chabaudi co-infection leads to impaired Th2 responses. 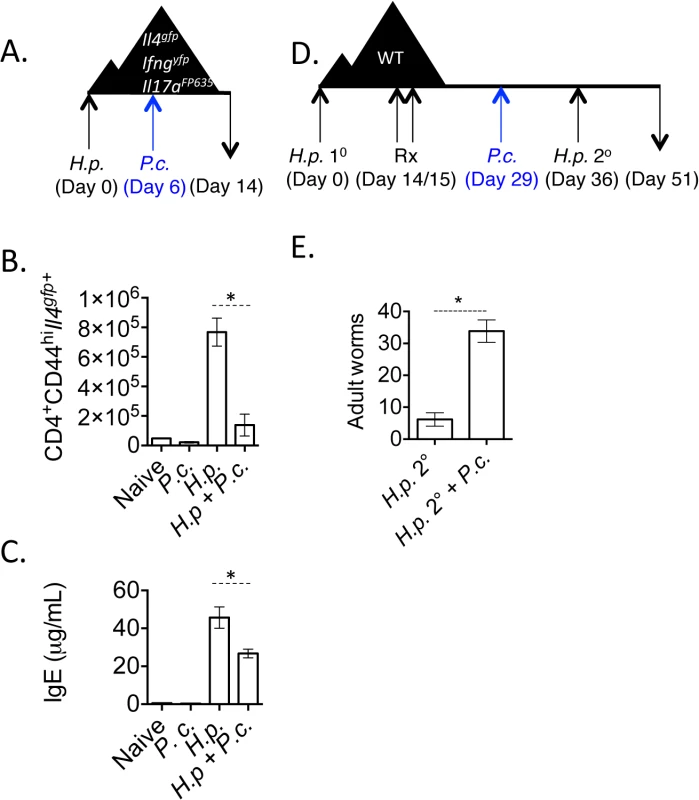
A-C). Triple reporter mice were orally infected with 200 H. polygyrus larvae. 6 days post-infection, mice were infected i.p. with 105 P. chabaudi. At day 8 of P. chabaudi infection (d14 H. polygyrus), mice were harvested. B). Total numbers of CD4+CD44hiIl4gfp+ cells in the mesenteric lymph nodes. Data are representative of 5 independent experiments with 2–4 mice per group. C). IgE measured in the serum by ELISA from 3 pooled experiments. D and E). C57BL/6 mice were infected with 200 H. polygyrus larvae, treated on 2 consecutive days (days 14–15) with pyrantel pamoate (5 mg), infected with 105 P. chabaudi, and re-infected with H. polygyrus. Adult worms in intestine were counted on day 51. Data are representative of 4 independent experiments with 6–7 mice per group. * denotes P<0.05. Very few Il17aFP635+ cells were induced in this model (S2 Fig). Following the resolution of acute malarial parasitemia, Th2 cell numbers in the mesenteric lymph nodes and serum IgE returned to levels observed in mice infected with H. polygyrus only (S2 Fig).
H. polygyrus establishes a chronic infection in wild type C57BL/6 mice. However, treating mice with anthelmintics kills adult parasites and allows a protective memory Th2 response to develop. Upon re-infection, mice expel worms in a CD4+ T cell - and IL-4-dependent manner [38,39]. Following the observation that P. chabaudi infection compromised Th2 cell responses (Fig 1B), we tested whether P. chabaudi infection would impact Th2-dependent anti-helminth immunity. We infected wild type mice with H. polygyrus, treated mice with the anthelmintic, pyrantel pamoate, and then infected mice with P. chabaudi 7-days prior to re-infection with H. polygyrus (Fig 1D). Although H. polygyrus-specific IgG1 levels were comparable between groups of mice (S2 Fig), P. chabaudi-infected mice that had been given a secondary H. polygyrus challenge infection had significantly more adult worms in the intestinal lumen (Fig 1E), indicating that Plasmodium infection compromised proficient anti-helminth immunity.
Il4gfp+ Th2 cells can functionally adapt, up-regulating IFNγ to control Plasmodium infection
It has become clear in recent years that lineage-committed CD4+ T cells retain a degree of plasticity, with the ability to convert between phenotypes [30]. Plasmodium infection elicits a polyclonal expansion of lymphocytes and IFNγ-secreting T cells [21,22]. We therefore hypothesized that the loss of Il4gfp+ Th2 cells in the mesenteric lymph nodes and the increase in Ifngyfp+ cells in the spleen during H. polygyrus and P. chabaudi co-infection was due to conversion of Th2 cells to an IFNγ-producing Th1-like phenotype. To test whether Th2 cells could produce IFNγ during P. chabaudi infection, we FACS-purified CD4+TCRβ+Il4gfp+Ifngyfp–Il17aFP365– Th2 cells from 2-week in vitro cultures (S1 Fig), adoptively transferred them into Rag1–/–mice and infected the recipient mice with P. chabaudi. Cytokine expression in the transferred cells was analyzed in the spleen at day 8 post-infection (Fig 2A). Transferred Th2 cells (Il4gfp+Ifngyfp–Il17aFP365–) almost completely lost expression of Il4gfp and, comparable to naïve T cells, expanded with approximately 80% of cells expressing Ifngyfp (Fig 2B). Il17aFP635+ cells were barely detectable (<1%) following Plasmodium infection, in line with previous data [21,22,40]. IFNγ protein was also detectable in the serum of mice that received either naive CD4+ T cells or purified Th2 cells, but not in P. chabaudi-infected Rag1–/–mice that received no T cells, indicating that serum IFNγ was T cell-dependent (Fig 2C). Thin blood smears from recipient mice identified that following infection of Rag1–/–mice, very high parasitemia is observed (Fig 2D). The adoptive transfer of naïve T cells to Rag1–/–mice significantly reduced the high parasitemia, confirming an important T cell-dependent role in the control of high parasitemia during acute infection. This system permitted us to test whether Th2 cells, which had converted into IFNγ+ cells, could also control high parasitemia following acute infection. Indeed, adoptive transfer of Th2 cells also significantly reduced parasitemia (Fig 2D), suggesting a functional loss of hemoglobin and severe anemia were also prevented in Rag1–/–mice given Th2 cells (Fig 2E and 2F). Although Th2 cells up-regulated IFNγ in uninfected recipient Rag1–/–mice, significantly greater expansion of these converted cells occurred in P. chabaudi infected recipient mice (S3 Fig). These data demonstrate that purified Il4-expressing Th2 cells were capable of producing IFNγ and could protect mice during acute P. chabaudi infection, similar to naive CD4+ T cells. Finally, to determine whether Th2 cells had the capacity to produce non-lineage cytokines in another model system, we infected Rag1–/–recipient mice with Candida albicans (S4 Fig). At day 6 post C. albicans infection, transferred Il4gfp+ Th2 cells had lost Il4 expression and up-regulated IFNγ, similar to P. chabaudi infection. Interestingly, transferred Th2 cells did not up-regulate IL-17a, unlike naïve controls (S4 Fig).
Fig. 2. In vitro Th2 cells produce IFNγ and protect Rag1–/–mice during Plasmodium infection. 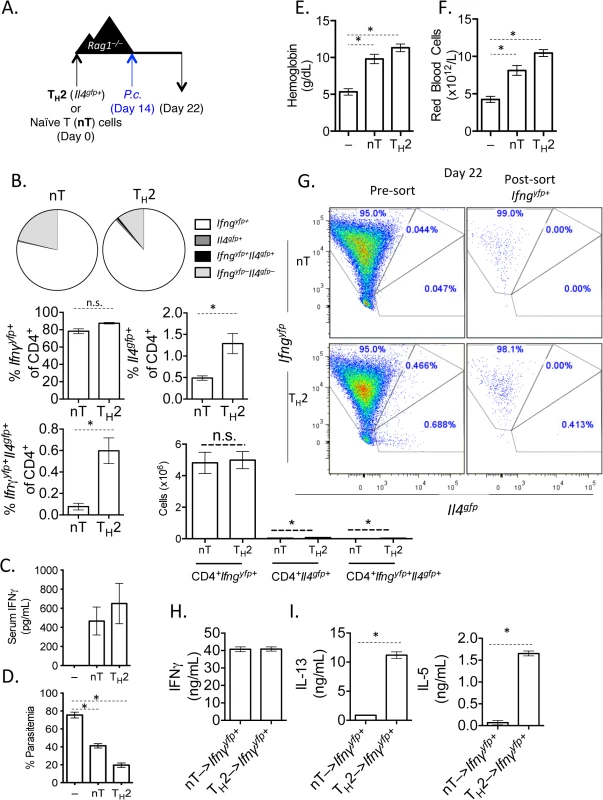
A). Experimental set-up: 2-week in vitro polarized Th2 cells were FACS sorted as CD4+Il4gfp+Ifngyfp–Il17aFP635– and transferred i.v. to Rag1–/–mice. As a control, a group of Rag1–/–mice received naïve CD4+ T cells. A second control group received no T cells. Recipient mice were infected with 105 P. chabaudi i.p. on day 14 post-transfer and harvested at day 8 post-infection. B). Percent and total number of CD4+ Il4gfp+ and Ifngyfp+ cells in the spleen, as determined by FACS. C). Serum IFNγ levels determined by ELISA. D). Percent parasitemia was determined by blinded counting of Giemsa-stained blood smears. E and F). Hemoglobin and eHred blood cell counts were measured in peripheral blood by Vetscan. Data is representative of at least 3 independent experiments, with 3–5 mice per group. G). Converted CD4+TCRβ+Ifngyfp+Il4gfp–Il17aFP635– cells were sorted from pooled spleens of 3 recipient Rag1–/–mice at day 8 post-P. chabaudi infection. H). Sorted Ifngyfp+ cells were cultured in vitro in Th2 conditions for 5 days. ELISAs for IFNγ (H), IL-5 and IL-13 (I) were run on cell supernatants. Error bars represent technical replicates. Data are representative of 3 independent experiments. * denotes P<0.05. Th2 cells that have down-regulated Il4 and up-regulated Ifng undergo significant transcriptional re-wiring yet retain the ability to produce IL-5 and IL-13
We next asked whether Th2 cells that had down-regulated Il4gfp and expressed Ifngyfp retained the ability to re-express Th2-associated cytokines. We transferred Il4gfp+ Th2 cells into Rag1–/–mice and infected recipient mice with P. chabaudi, as in Fig 2A. At day 8 post-infection with P. chabaudi, we sorted CD4+TCRβ+ Ifngyfp+Il4gfp-Il17aFP635– cells from the spleens of recipient mice (Fig 2G). Converted cells were then cultured in vitro with IL-4 and TCR stimulation. As expected, Ifngyfp+ cells that were previously either naïve or Il4gfp+ secreted IFNγ protein (Fig 2H), validating the fidelity of the transcriptional reporter system. However, only Ifngyfp+ cells that were previously Il4gfp+ secreted the Th2-associated cytokines IL-13 and IL-5 (Fig 2I), indicating that converted cells were indeed plastic, retaining the ability to produce Th2 cytokines.
To identify the degree of transcriptional re-wiring of the converted cells in this model, we performed RNA sequencing on Th2 cells (Il4gfp+), converted Th2 cells (Il4gfp+ → Ifngyfp+Il4gfp-), naïve CD4+ T cells, and Th1 cells (naïve → Ifngyfp+Il4gfp–), using the same sorting strategy as in Fig 2G. Comparing the transcriptome of all significantly differentially regulated genes (p<0.05, >2-fold relative to naive T cells) between the populations, we identified that converted cells had adopted a transcriptional profile very similar to Th1 cells (Fig 3A and 3B, S1 Table) with the majority of differentially regulated genes common with Th1 cells, while retaining some transcriptional similarity with their Th2 origin. Converted cells expressed Ifng, Tnf, Il2 and Il10 and largely lost expression of Il4 and Il6, in comparison to the Th2 controls (Fig 3C). Similarly, the transcriptional machinery in converted cells resembled Th1 cells with elevated Tbx21 (Tbet) and Eomes and low expression of Th2-associated transcription factors Gata3 and Nfil3 (Fig 3D). To identify putative mechanistic pathways responsible for Th2 cell conversion, we used an upstream pathways algorithm to predict factors that may contribute to the observed transcriptional profile (Ingenuity Pathways Analysis). This analysis identified canonical Th1 differentiation factors including IL-12, IFNγ and type 1 IFN as potential upstream factors contributing to the observed transcriptional profile in converted cells (Fig 3E). Furthermore, converted cells expressed Il12rb1, Il12rb2, Ifngr1 and Ifnar1 (Fig 3F). In summary, converted Th2 cells had undergone significant re-wiring, closely resembling Th1 cells.
Fig. 3. Converted Th2 cells are transcriptionally similar to Th1 cells. 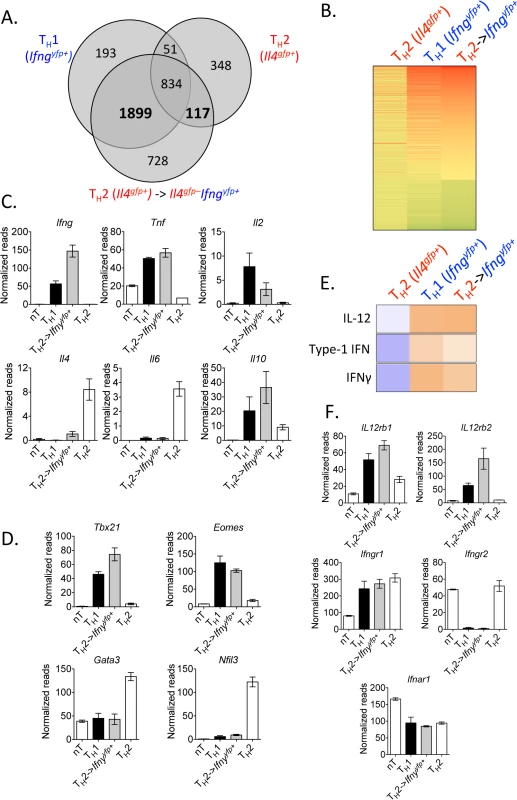
Purified in vitro Th2 cells (CD4+TCRβ+Il4gfp+Ifngyfp-Il17aFP635-) or naive CD4+ T cells were sorted for RNA or transferred to Rag1–/–mice. Recipients were then infected with 105 P. chabaudi, as in Fig 2. CD4+TCRβ+Ifngyfp+Il4gfp-Il17aFP635– cells were then sorted from spleens of recipient mice at day 8 post-infection for RNA. RNA sequencing and IPA analysis was performed on the four cell populations. Data were expressed relative to naïve in A and B. A and B). Venn diagram and heatmap generated from differentially regulated genes (P<0.05, 2-fold relative to naïve T cells) of Th2 cells and Th1 cells, highlighting 1899 genes commonly expressed, which were not changed in Th2 cells. Th2 cells and converted Th2 cells shared 117 differentially regulated genes, which were not expressed in Th1 cells. C, D, and F). Normalized RNA-Seq reads of indicated genes. E). Upstream pathways analysis in Ingenuity Pathways Analysis (IPA) identified IL-12, type 1 IFN, and IFNγ as potential upstream regulators of converted Th2 cells. Samples were generated from 3 biological replicates (each sample representing cells from a single donor mouse). IFNγ production by Th2 cells does not depend on lymphopenia and requires TCR engagement
When T cells undergo expansion in lymphopenic environments a population of rapidly dividing cells up-regulate CD44 and IFNγ [41–43]. To test whether conversion of Th2 cells into IFNγ-expressing cells could occur in a CD4+ T cell replete mouse, we transferred purified Th2 cells or naïve CD4+ T cells into OTII Rag1–/–mice [44], which have CD4+ T cells specific only for OVA peptide. We infected recipient mice with P. chabaudi and analyzed donor and host cells at day 8 post-infection (Fig 4A). Purified Th2 cells transferred into CD4+ OTII Rag1–/–mice, similar to Th2 cells transferred into Rag1–/–mice, produced IFNγ and down-regulated IL-4 (Fig 4B and 4C), contributing to elevated levels of serum IFNγ (Fig 4D). In contrast, host OVA-specific CD4+ T cells did not produce IFNγ following Plasmodium infection (Fig 4C). Thus, Th2 cell conversion was not dependent on lymphopenia.
Fig. 4. IFNγ production by Th2 cells does not depend on lymphopenia. 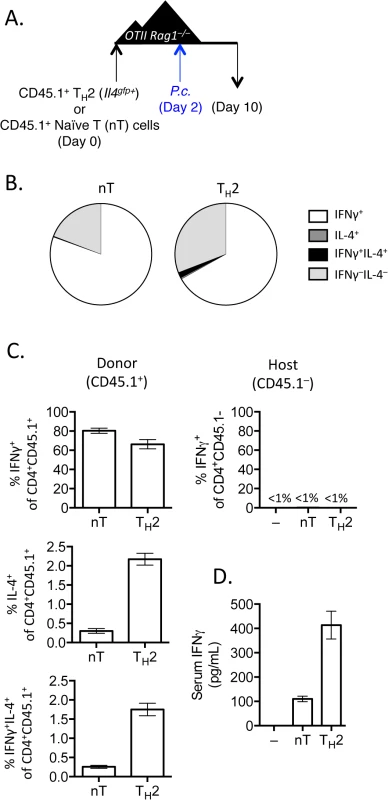
A). Th2 cells generated from Il4gfp mice were polarized in vitro for 2 weeks, sorted as CD4+TCRβ+Il4gfp+, and 2.5x106 were transferred to OTII Rag1–/–recipient mice. Control groups received no T cells or sorted naïve T cells. 2 days post-transfer, mice were infected with 105 P. chabaudi. B and C). Cytokine production in donor CD45.1+ or host cells in spleens, day 8 post-infection with P. Chabaudi, as determined by intracellular cytokine staining. D). IFNγ protein in serum, measured by ELISA. Data are representative of 2 independent experiments with 3–4 mice per group. Given that Th cells require both TCR stimulation and cytokine-mediated signaling for differentiation, it was conceivable that pre-activated Th2 cells in this system would only require a second cytokine receptor-mediated signal to up-regulate IFNγ, without the need for any additional TCR stimulation. We took two independent approaches to test whether TCR engagement was required for Th2 cells to produce IFNγ. First, we generated and FACS-purified TCR-restricted Th2 cells from OTII Rag1–/–mice crossed with Il4gfp reporter mice. We then transferred these OVA-specific Il4gfp+ Th2 cells into Rag1–/–recipients (devoid of OVA) and infected recipient mice with P. chabaudi (Fig 5A). Unlike polyclonal Il4gfp+ Th2 cells that lost expression of Il4gfp and produced IFNγ, antigen-restricted OTII Il4gfp+ Th2 cells retained expression of Il4gfp and failed to produce IFNγ (Fig 5B). Furthermore, IFNγ was not detectable in the serum of mice that received OVA-specific Il4gfp+ Th2 cells (Fig 5C). Functionally, the failure to produce IFNγ correlated with significantly higher parasitemia, comparable to mice that received no T cells (Fig 5D). These data indicate that TCR signaling was required for the functional conversion of Th2 cells into IFNγ-secreting cells. To verify the requirement of TCR-signaling for conversion, we transferred purified Il4gfp+Ifngyfp–Il17aFP365– Th2 cells into Rag1–/–recipient mice which were also deficient in MHC Class II and therefore unable to present antigens to Il4gfp+ Th2 cells. Recipient mice were infected with P. chabaudi, and transferred cells were analyzed at day 8 post-infection (Fig 5E). As before, Il4gfp+ Th2 cells transferred into MHC Class II-sufficient Rag1–/–recipient mice down-regulated Il4gfp and up-regulated Ifngyfp. However, Il4gfp+ Th2 cells transferred to MHC Class II-deficient Rag1–/–recipient mice remained Il4gfp+, did not express Ifngyfp (Fig 5F) and failed to reduce severe parasitemia (Fig 5H). IFNγ was also undetectable in the serum (Fig 5G). Taken together, these two experimental systems demonstrate that conversion of Th2 cells in this model requires TCR engagement.
Fig. 5. TCR stimulation is critical for IFNγ production by Th2 cells. 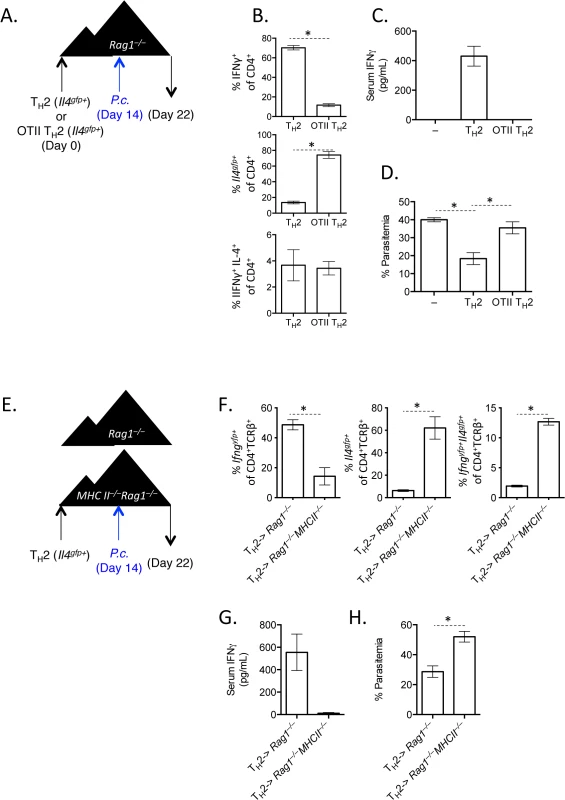
A–D). OTII Rag1–/–Il4gfp or Il4gfp Th2 cells (CD4+TCRβ+Il4gfp+) were transferred to Rag1–/–recipient mice. 14 days later, mice were infected with 105 P. chabaudi, and mice were harvested at d8 post-infection. Representative of 2 independent experiments with 5 mice per group. B). Cytokine expression in transferred cells in the spleen (IFNγ by ICS, Il4gfp reporter expression). C). IFNγ protein in serum, measured by ELISA. D). Percent parasitemia, determined by blinded counting of Giemsa-stained blood smears. E–H). Th2 cells (CD4+TCRβ+Il4gfp+Ifngyfp-Il17aFP635-) were transferred to MHC Class II sufficient or deficient Rag1–/–recipients. Mice were infected with 105 P. chabaudi at day 14, and mice were harvested at day 8 post-infection. Representative of 2 independent experiments with 5 mice per group. F). Cytokine reporter expression in transferred cells in the spleen. G). IFNγ protein in serum, measured by ELISA. H). Percent parasitemia, determined by blinded counting of Giemsa-stained blood smears. * denotes P<0.05. IL-12 and IFNγ, but not type I IFN, promote IFNγ expression by transferred Th2 cells
It has been shown previously that type I IFN signaling was required for IFNγ production from LCMV-specific TCR transgenic Th2 cells [34]. We had also observed that type 1 IFN was a candidate cytokine that could contribute to the transcriptional profile of converted Th2 cells (Fig 3E). We therefore tested the requirement for type 1 IFN signaling by crossing Ifnar–/–mice with Il4gfp reporter mice. FACS purified Il4gfp+Ifnar–/–or Il4gfp+Ifnar+/+ Th2 cells were transferred to Rag1–/–recipient mice, subsequently infected with P. chabaudi and analyzed at day 8 post-infection (Fig 6A). Both type I IFN responsive and unresponsive Th2 cells were capable of up-regulating IFNγ (Fig 6B and 6C), contributing to serum IFNγ levels (Fig 6D). Furthermore, type I IFN responsive and unresponsive Th2 cells afforded similar protection from high parasitemia (Fig 6E), and prevented a loss in hemoglobin and red blood cells (Fig 6F). Thus, type I IFN signaling was dispensable for IFNγ production from ex-Th2 cells and for controlling high parasitemia.
Fig. 6. IFNγ production by Th2 cells does not depend of type I IFN. 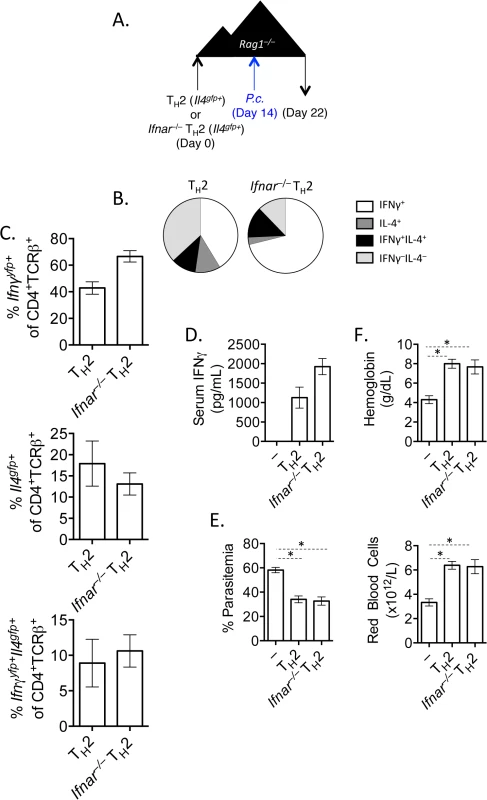
A). CD4+TCRβ+Il4gfp+Ifnar+/+ or Ifnar–/–Th2 cells were transferred to Rag1–/–mice. Recipient mice were infected with 105 P. chabaudi 14 days later and mice were harvested at day 8 post-infection. B and C). Cytokine expression in transferred cells in the spleen (ICS). D). IFNγ protein in serum, measured by ELISA. E). Percent parasitemia, determined by blinded counting of Giemsa-stained blood smears. F). Hemoglobin and red blood cell counts determined by Vetscan. Data are representative of 2 independent experiments with 5–6 mice per group. * denotes P<0.05. From our RNA-Seq analysis we also identified that the canonical Th1 differentiating cytokines, IL-12 and IFNγ, may be responsible for the transcriptional profile observed in our converted cells (Fig 3E). We first tested whether Th2 cells were responsive to IL-12 by measuring the phosphorylation of STAT4 following exposure to IL-12. Supporting previous studies [45–47], neither naïve CD4+ T cells nor sorted Il4gfp+ Th2 cells phosphorylated STAT4 in response to IL-12 (Fig 7A and 7B; Pre - transfer). We then sorted transferred cells from naïve CD4+ T cell or Il4gfp+ Th2 cell recipient Rag1–/–mice 2 weeks post-transfer and found that both populations were responsive to IL-12 (Fig 7A and 7B; Post-transfer). Thus, it was possible that IL-12 was promoting IFNγ expression in Th2 cells following P. chabaudi infection. We tested the role of IL-12 by transferring naïve or Il4gfp+ Th2 cells to Rag1–/–mice and blocking IL-12 prior to and after P. chabaudi infection (Fig 7C). Blocking IL-12 reduced expression of Ifngyfp in naïve T cells (reduced from 78.9% to 52.61%); however, IL-12 blockade did not substantially alter the frequency of Ifngyfp+ cells derived from Th2 cells. Instead, IL-12 blockade maintained expression of Il4gfp+ in the Th2 population, with significantly larger Il4gfp+ and Il4gfp+Ifngyfp+ populations (Fig 7D–7F). These data indicate that in this system IL-12 down-regulated Il4gfp expression, but was not required for IFNγ from Th2 cells. Furthermore, neutralization of IL-12 did not impact parasitemia (Fig 7G).
Fig. 7. Th2 cells become IL-12 responsive following adoptive transfer. 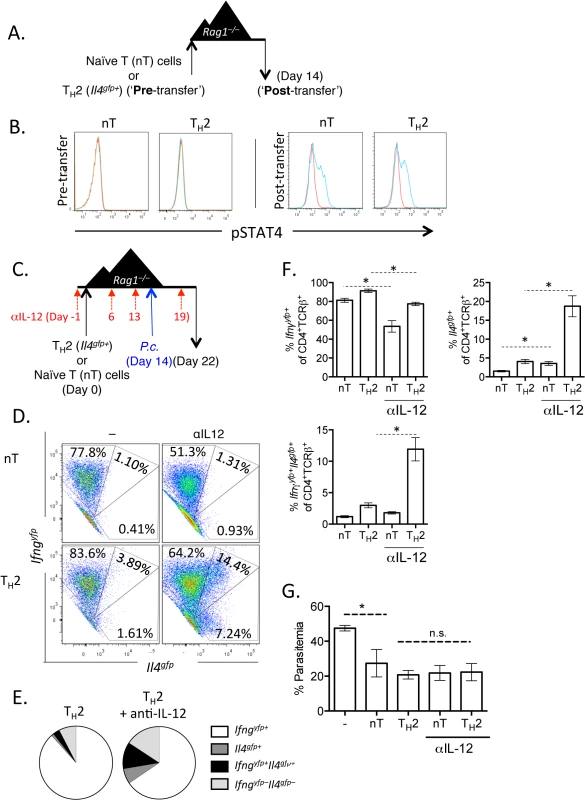
A and B). CD4+Il4gfp+ in vitro Th2 cells or naïve CD4 cells were transferred to Rag1–/–mice for 2 weeks. CD4+TCRβ+ cells were then sorted from spleens of recipient mice and treated with 10ng/mL IL-12 for 15 minutes (blue) (or untreated, red) and then stained for pSTAT4 by FACS. Representative of 2 independent experiments. C—G). Naïve CD4+ T cells or in vitro Th2 cells (CD4+TCRβ+Il4gfp+Ifngyfp-Il17aFP635-) were transferred to Rag1–/–recipient mice for 14 days. Mice were infected with P. chabaudi and harvested at day 8 post-infection. Mice were treated i.p. with 0.5mg of anti-IL12 at days -1, 6, 13, and 19. D–F). Cytokine reporter expression in transferred cells in the spleen, with or without anti-IL-12 treatment. G). Percent parasitemia, determined by blinded counting of Giemsa-stained blood smears. Data are representative of 2 independent experiments with 3–6 mice per group. * denotes P<0.05. We next tested whether IFNγ, which contributes to Th1 differentiation [48], was required for IFNγ expression by Th2 cells. To do this, we blocked IFNγ, IL-12, or both IFNγ and IL-12 throughout the experiment (Fig 8A). Blockade of IFNγ or IL-12 alone did not have a major impact on IFNγ production by Th2 cells (Fig 8B). As above, IL-12 blockade preserved Il4gfp expression in a population of Th2 cells (Fig 8C). However, blockade of both IFNγ and IL-12 led to a >50% reduction in IFNγ-expressing cells deriving from Th2 cells (from 66.7%±1.5% IFNγ+ cells to 31.6%±3.4% IFNγ+ cells, Fig 8B), indicating that both IL-12 and IFNγ were required for optimal conversion of Th2 cells into IFNγ-secreting cells during Plasmodium infection. Despite a 50% reduction in IFNγ-secreting cells following IL-12 and IFNγ blockade, the remaining ~30% of IFNγ+ cells were sufficient to prevent high parasitemia (S5 Fig).
Fig. 8. Blockade of IL-12 and IFNγ prevents optimal IFNγ production by Th2 cells. 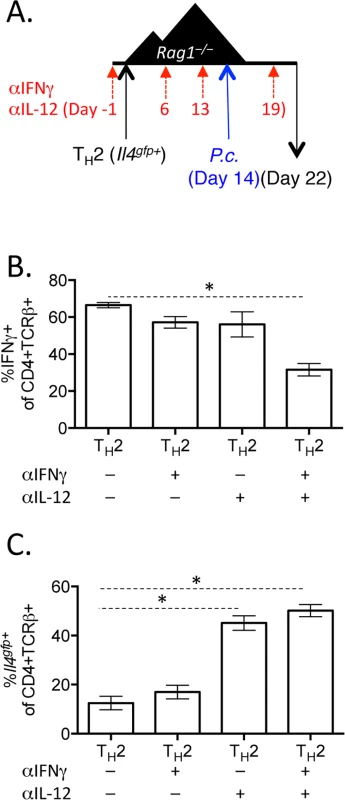
A–C). In vitro Th2 (CD4+TCRβ+Il4gfp+) cells were transferred to Rag1–/–recipient mice for 14 days. Mice were infected with P. chabaudi and harvested at d8 post-infection. Mice were treated i.p. with 0.5mg anti-IL12 and anti-IFNγ at days -1, 6, 13, and 19. B). IFNγ production by transferred Th2 cells in the spleen, as determined by ICS. C). Il4gfp expression in transferred cells in the spleen. Data representative of 2 independent experiments with 5 mice per group. * denotes P<0.05. Blockade of IL-12 and IFNγ during helminth and Plasmodium co-infection preserves Th2 responses
Finally, we translated these new observations back into a co-infection scenario, as presented in Fig 1, and tested whether helminth-induced Th2 cells had the capacity to up-regulate IFNγ in a co-infection scenario. First, we purified ex vivo Il4gfp+Ifngyfp–Il17aFP635– Th2 cells from d14 H. polygyrus-infected mice and transferred them into day 14 H. polygyrus-infected Rag1–/–mice. Recipient mice were then co-infected with P. chabaudi and the transferred cells were analyzed at day 8 post P. chabaudi infection (Fig 9A). Similar to in vitro-derived Th2 cells, H. polygyrus-derived Th2 cells down-regulated Il4gfp and up-regulated Ifngyfp, albeit to a slightly lesser extent than naïve T cells (Fig 9B).
Fig. 9. H. polygyrus-induced Th2 cells retain capacity to produce IFNγ during co-infection. 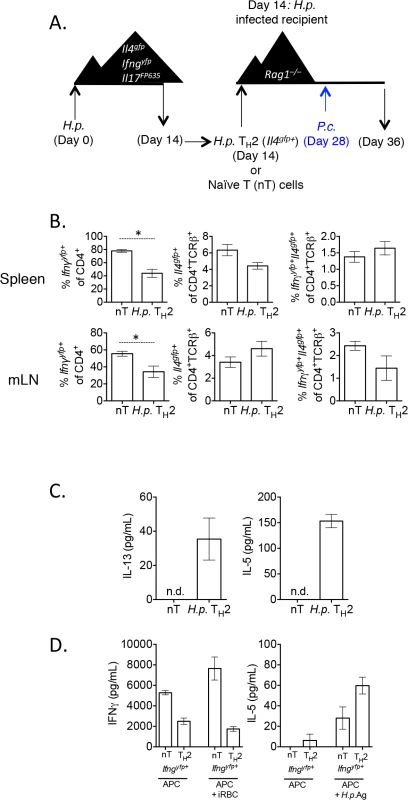
A)–B). CD4+TCRβ+Il4gfp+Ifngyfp-Il17aFP635- ex vivo Th2 cells from d14 H. polygyrus-infected mice or naïve CD4+ T cells were transferred to d14 H. polygyrus-infected Rag1–/–recipient mice. Mice were infected with P. chabaudi and analyzed at day 8 post-infection. B). Cytokine reporter expression in transferred cells from spleens and mesenteric lymph nodes of recipient mice. Data representative of 2 separate experiments, with 4 mice per group. C). Ex vivo Th2 or naïve cells were transferred to recipient Rag1-/- mice, as in 9A. At day 8 post-infection with P. chabaudi, 2x105 mesenteric lymph nodes cells were stimulated with 10 μg/mL H. polygyrus antigen and 10 ng/mL IL-4. ELISAs were performed on cell supernatants after 5 days. Data are representative of 2 separate experiments. Lymph nodes were pooled from 2 recipient mice per group. Error bars represent technical replicates. D). Ex vivo Th2 or naïve cells were transferred to recipient Rag1-/- mice, as in 9A. At day 8 post-infection with P. chabaudi, CD4+TCRβ+Ifngyfp+Il4gfp-Il17aFP635- cells were sorted from pooled spleens of 2 recipient mice per group (following the sorting strategy as in Fig 2G). 9.6x104 purified converted CD4+TCRβ+Ifngyfp+ Il4gfp-Il17aFP635- cells from a naïve or Th2 past were then cultured with 4x105 irradiated CD4+–depleted splenocytes and one of the following: 3x106 red blood cells from a P. chabaudi-infected donor mouse (day 8 post-infection) or 10 μg/mL H. polygyrus antigen. ELISAs were performed on cell supernatants after 5 days. Error bars represent technical replicates. Data are representative of 2 independent experiments. Re-stimulation of lymph node cells with H. polygyrus antigen and IL-4 led to the secretion of IL-5 and IL-13 from mice given H. polygyrus Th2 cells, but not from mice given naïve T cells (Fig 9C). These data suggested that despite a high degree of conversion to IFNγ-secreting cells, cells retained antigen-associated cytokine secretion. To more accurately determine whether converted cells retained the capacity to produce Th2 cytokines in an antigen-specific manner, we sorted Th2 cells, or naïve cells, that had converted into Ifngyfp+ cells from recipient mice and restimulated them in vitro with H. polygyrus antigen or P. chabaudi infected red blood cells (iRBC). Ifngyfp+ cells, which were previously naïve or Il4gfp+ Th2 cells, produced IFNγ when co-cultured with irradiated APCs, supporting the cytokine reporter expression (Fig 9D). iRBCs further stimulated more IFNγ from naive T cells, but not from Th2 cells, suggesting that either ex vivo Th2 cells were not responding to malarial antigens, or that they were already secreting IFNγ at capacity. In addition, ex vivo H. polygyrus elicited Th2 cells which had down-regulated Il4gfp and up-regulated Ifngyfp produced IL-5 in response to H. polygyrus antigen, suggesting that converted cells retained antigen specificity and plasticity in this model (Fig 9D).
Finally, we tested whether the factors promoting IFNγ in the adoptive transfer model, IL-12 and IFNγ (Fig 8B), were responsible for the loss of Th2 cells and type-2 immunity during H. polygyrus and P. chabaudi co-infection. To do this, we infected wild type mice with H. polygyrus and at six days post-infection, mice were co-infected with P. chabaudi with or without blocking antibodies to IL-12 and IFNγ (Fig 10A). Blockade of IL-12 and IFNγ preserved Il4gfp+ Th2 cells in co-infected mice (Fig 10B) and maintained elevated levels of helminth-induced type-2-associated IgE (Fig 10C). However, despite preserving Th2 cells and IgE, proficient anti-helminth immunity was not fully restored in mice given blocking antibodies (S6 Fig). Thus, IL-12 and IFNγ play a major role compromising Th2 responses during helminth/ Plasmodium co-infection, but additional factors also contribute to compromised anti-helminth immunity during co-infection.
Fig. 10. Blockade of IL-12 and IFNγ during co-infection preserves Th2 responses. 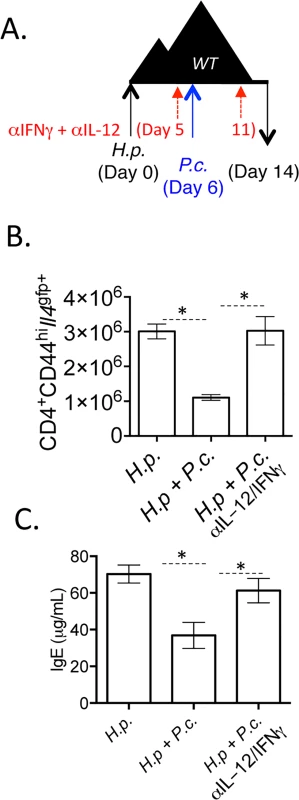
A-C). C57BL/6 mice were orally infected with 200 H. polygyrus larvae. 6 days post-infection, mice were infected with 105 P. chabaudi. At day 8-post infection with P. chabaudi (d14 H. polygyrus), mice were harvested. Mice were treated with 0.5 mg of anti-IL-12 and anti-IFNγ i.p. at days 0, 5, and 11. B). Total numbers of CD4+CD44hiIl4gfp+ cells in the mesenteric lymph nodes. C). IgE measured in the serum by ELISA. Data are representative of 2 independent experiments with 6 mice per group. * denotes P<0.05. Discussion
In this study, we identified that Plasmodium infection significantly reduced CD4+ Th2 cells during co-infection with H. polygyrus and that anti-helminth immunity was compromised during co-infection. Mechanistically, we found that Il4gfp+Ifngyfp–Il17aFP635– Th2 cells, purified from novel triple cytokine reporter mice, converted to IFNγ-secreting cells, contributing significantly to anti-Plasmodium immunity. IFNγ production by Th2 cells was dependent on TCR, IL-12, and IFNγ signaling, all of which contributed to the transcriptional re-programming of Th2 cells. Finally, we found that blockade of IL-12 and IFNγ during Plasmodium and helminth co-infection preserved Th2 responses and IgE production, but was insufficient to fully restore anti-helminth immunity.
There is a large body of literature describing the prevalence of helminth and Plasmodium co-infection in human populations [4,5,8,11,49,50], and mouse models [16,51], with the majority of studies focusing on the impact of helminth infections on anti-Plasmodium responses. Relatively few have focused on how parasite-elicited Th2 responses are affected during Plasmodium co-infection. Our data show that IL-4-expressing Th2 cells, serum IgE, and functional parasite expulsion are reduced during co-infection (Fig 1). This is in line with previous reports, including reduced schistosome-specific IL-4 and IL-5 in Plasmodium and schistosome co-infected individuals [52] and suppressed IL-4 responses during H. polygyrus and Plasmodium yeolii co-infection [53]. Reduced type-2 responses [54] and Th2-mediated immunopathology have also been observed in schistosome and Plasmodium co-infected mice [55], consistent with the notion that anti-helminth associated Th2 responses are compromised during Plasmodium co-infection. However, these studies did not offer mechanistic insight as to how this reduction in type-2 immunity might occur and importantly how type-2 immunity might be preserved during co-infection.
In this study, we focused on the impact of co-infection on CD4+ T cells, which are a critical cell type for immunity to H. polygyrus and contribute significantly to anti-malarial immunity [56]. For our studies, we developed a triple cytokine reporter mouse (Il4gfpIfngyfpIl17aFP635, S1 Fig), which had several important advantages. These mice allowed the determination of T cell phenotype ex vivo without the need for re-stimulation, as well as the ability to obtain highly purified populations of Il4gfp+Ifngyfp–Il17aFP635– Th2 cells, which were not expressing other lineage-associated cytokines[29]. Adoptive transfer of these cells allowed us to accurately determine whether purified Th2 cells changed their phenotype, and finally, simultaneous cytokine reporters allowed us to test whether any conversion was reversible and truly plastic. To this end, we observed that highly-purified Il4gfp+Ifngyfp–Il17aFP635– Th2 cells, either generated in vitro for two weeks (Fig 2) or isolated ex vivo from H. polygyrus-infected mice (Fig 9), were able to produce IFNγ during Plasmodium infection in Rag1–/–mice. This phenomenon is in line with several previous observations 1) identifying that in vitro generated LCMV-specific TCR transgenic Th2 cells could express both IFNγ and IL-4 [34], 2) a ‘bi-functional’ population of Tbet+ GATA3+ cells are generated following H. polygyrus infection [29] and 3) the Tbx21 locus (encoding T-bet) has bivalent epigenetic histone modifications in Th2 cells [57] suggesting Th2 cells retain some flexibility. We observed expression of Ifng, Tbx21, Klrg1, Gzmb, Gzmc in converted Th2 cells, while maintaining low levels of Il4 transcription (Fig 3, S1 Table) and the ability to produce IL-5 and IL-13 (Fig 2). This suggested that converted cells were possibly poly-functional. Whether they are similar to ‘bi-functional’ cells [29] is unclear. Helmby observed exacerbated liver pathology with significantly increased IFNγ and mortality during H. polygyrus and Plasmodium co-infection [58]. Whether Th2 cells converted to IFNγ-secreting cells, contributing to aggravated liver pathology in their study was unclear. Similarly, Th2 cells that up-regulate IL-17 during airway allergen challenge in mice contribute to more severe airway pathology [59], and allergic patients have a greater frequency of IFNγ-secreting cells [60]. Indeed, polyfunctional T cells, which secrete multiple cytokines, correlate with greater protection following vaccination [61], contribute to severe inflammatory syndromes in humans [62] and mice [37] and have greater anti-tumor activity [63]. Thus, understanding the mechanisms of Th cell conversion and the generation of polyfunctional T cells may provide important insight into immunity and immunopathology. Interestingly, in our model of C. albicans, in vitro polarized Th2 cells were unable to produce IL-17a, unlike naïve cells (S4 Fig), suggesting that there is either an important relationship between Th2 and Th1 cells, or that the transcriptional machinery required for IL-17 production is more tightly regulated than for IFNγ.
To identify mechanistic pathways contributing to Th2 cell conversion, we employed RNA-Seq analysis of Th1 cells (Ifngyfp+), Th2 cells (Il4gfp+) and Th2 cells that had up-regulated IFNγ (Ifngyfp+Il4gfp–). We identified a high degree of transcriptional similarity between Th1 cells and converted cells, extending significantly beyond cytokine expression. For example, Th1 and converted Ifngyfp+Il4gfp–cells, but not Th2 cells, had similar transcript abundance encoding for several enzymes (Bace2, Cdc25c, Cd38 Chst11, Dusp5, Gzmb, Gzmc and Gzmk, Gstt1, Pdcd1, Ptpn5, Spag5, Troap), chemokine receptors (Cxcr3, Cmklr1, Cx3cr1 and Ccr5), ion channels (Cacna1l and Ttyh2), kinases (Stk32c, Ttbk1, Ttk, Ltk, Cdk1, Pbk, Ccnb1 in addition to many other kinases), nuclear receptors (Nr4a2, Ahr), miRNAs (miR-142, miR-155 and miR-Let7d) and transcriptional regulators (Rai14, E2f7, Gas7, Cdkn2b, E2f8, Klf12, Runx2 and Eomes). Significantly, Th1 cells use a feed-forward regulatory circuit involving Tbx21 (Tbet) and Runx3 for maximal IFNγ production and silencing of Il4 [64]. In our study, both Th1 cells and converted Th2 cells which had lost Il4 and up-regulated Ifng, had elevated Runx3 and Tbet, suggesting that this feed-forward loop was transcriptionally active, supporting optimal IFNγ production in converted cells. Whether the epigenetic landscape of converted cells matched that of their Th1 counterparts is of great interest, as converted Th2 cells retained the capacity to produce Th2-associated IL-5 and IL-13 (Fig 2) in an antigen-specific manner (Fig 9D). Previous studies have indicated that Th1 cells have the capacity to up-regulate Th2-associated features in vivo following helminth infection [65]. In our hands, naïve T cells which had up-regulated IFNγ+ in vivo following Plasmodium infection did not have the capacity to secrete IL-5 or IL-13 when re-stimulated in vitro with anti-CD3/28 and IL-4. Whether there are specific in vivo factors which more readily support T cell plasticity is currently unclear. We would hypothesize that in vitro generated or ex vivo H. polygyrus Th2 cells had bivalent methylation marks in the Il5 and Il13 locus allowing re-expression of these genes following the appropriate activating signal. Supporting this, converted Th2 cells retained some Th2-associated features, including elevated expression of Gfi1, Il4 and Il33r, which may provide the appropriate machinery to re-activate Th2-associated genes, reminiscent of their Th2 past (Fig 3 and S1 Table).
Using an upstream analysis algorithm (Ingenuity Pathways Analysis) with our transcriptional data sets we identified IL-12, IFNγ and to a lesser extent type 1 IFN, as putative factors that could contribute to the observed transcriptional profile of converted cells. This supports a recent study that identified the requirement of Tbet and Stat4 for IFNγ expression in memory Th2 cells [66]. In our study, unlike previous studies, type I IFN signalling in Th2 cells was dispensable for IFNγ production from converted Th2 cells in vivo (Fig 6) [34]. Blocking IL-12 or IFNγ alone did not impact the frequency of converted IFNγ+ cells from transferred Th2 cells (Figs 7 and 8). These data are in agreement with a previous study that found restoring IL-12 responsiveness in Th2 cells, through ectopic expression of IL-12Rβ2, was insufficient to convert Th2 cells into IFNγ-secreting cells [67]. However, in our model, anti-IL-12 treatment alone preserved IL-4 expression in a sub-population of transferred cells (Figs 7 and 8). Blockade of both IFNγ and IL-12 substantially reduced IFNγ+ cells deriving from Th2 cells, suggesting that an IL-12-STAT4 signaling pathway down-regulated IL-4, while an IFNγ / STAT-1 / T-bet pathway was required for optimal IFNγ expression, in accordance with canonical Th1-inducing conditions for naive T cells [68]. While we found that blockade of these cytokines reduced IFNγ+ cells, there was no change in control of parasitemia (S5 Fig). We speculate that this is due to the incomplete loss of conversion, with the remaining IFNγ being sufficient to control levels of parasitemia.
TCR stimulation was essential for in vitro-derived Th2 cells to produce IFNγ (Fig 5) and ex vivo H. polygyrus-elicited Th2 cells required H. polygyrus-infected recipient mice to survive and up-regulate IFNγ. Thus, with sufficient TCR signaling, a change in the local cytokine milieu may be sufficient to re-program Th cells. During helminth and Plasmodium co-infection, either cross-reactive antigens or microflora-derived signals may provide the necessary first TCR signal [69–71]. Alternatively the broad polyclonal activation of non-specific T cells during Plasmodium infection may be sufficient [21,22,72]. Although TCR engagement, IL-12 and IFNγ were required for optimal conversion of Th2 cells into IFNγ-secreting cells, it is possible that other factors also contribute to conversion, including IL-27, which can induce expression of Tbet, and IL-18, which can induce IFNγ production [73,74].
In conclusion, we have shown that IL-12 and IFNγ suppressed Th2 responses during H. polygyrus and P. chabaudi co-infection. Mechanistically, we identified that TCR engagement with IL-12 and IFNγ signaling converted in vitro-generated Th2 cells into IFNγ-producing cells during P. chabaudi infection. Importantly, although blocking IL-12 and IFNγ during co-infection did not retain fulminant anti-helminth immunity, it did preserve Th2 cell numbers and serum IgE, highlighting a novel mechanistic pathway of how Plasmodium infection negatively impacts anti-helminth Th2 responses. Overall, our studies indicate that Plasmodium infection can negatively impact anti-helminth responses, that Th2 cells retain substantial plasticity in the context of Plasmodium infection, and that this plasticity may play a role in the reduced Th2 response during co-infection.
Materials and Methods
Animals
All mice were bred and maintained under specific pathogen-free conditions at the National Institute for Medical Research. Strains used included: C57BL/6, Ifngyfp [36], Il4gfp[35], C57BL/6 Rag1–/–[75], MhcII–/–(B6.129-H-2<dlAb1-Ea)[76] crossed with Rag1–/–at NIMR [77], OTII Rag1–/–(B6.Cg (Tcrαβ)425Cbn/J) [78], OTII Il4gfp Rag1–/–(OTII Rag1–/–crossed with Il4gfp at NIMR), and Ifnar–/–Il4gfp (Ifnar–/–[79] crossed with Il4gfp at NIMR). Triple cytokine reporter mice (Il4gfpIl17CreIfngyfpR26FP635) were established by crossing Il4gfp/gfpIl17Cre/Cre[37] mice with Ifngyfp/+R26FP635/FP635 mice, producing Il4gfp/+Il17Cre/+Ifngyfp/+R26FP635/+. The generation of R26FP635 reporter mice will be presented in detail elsewhere (JB and AP, manuscript in preparation). Briefly, R26FP635 mice were generated by inserting the coding sequences of the red fluorescent protein FP635 [80] into the pROSA26 targeting vector downstream of a loxP-flanked neomycin resistance cassette containing three transcriptional stop signals by homologous recombination. R26FP635 reporter mice in this study were backcrossed to C57BL/6 for more than 8 generations.
Infections
Mice were infected by oral gavage with 200 infective stage 3 (L3) Heligmosomoides polygyrus larvae, diluted in water. The anthelmintic drug pyrantel pamoate (Sigma, 5mg/dose in water) was given orally on two consecutive days. Infections with Plasmodium chabaudi chabaudi (AS) were performed by i.p. injection of 105 parasitized red blood cells. Parasitemia was measured by blinded counting of Giemsa-stained blood smears. Anemia and hemoglobin were measured by diluting blood in Krebs buffered saline with 0.2% glucose and with 100 IU/mL heparin and measured using Vetscan (Abaxis-VetScan HM5 Hematology). Infections with Candida albicans were performed by i.v. injection of 105 yeast forms.
Cell sorting and flow cytometry
Cell sorting was performed using a FACS Aria II (BD Biosciences), MoFlo XDP (Beckman Coulter), or Influx (BD Biosciences) cell sorter. To prepare cells for sorting, CD4+ cells were first positively selected using MACS CD4 beads and magnetic columns (Miltenyi Biotec). Cell suspensions were then stained for 25 minutes with antibodies in PBS with 1% FCS. To prepare for sorting, stained cells were diluted in phenol-red free IMDM (Gibco) (with 1% FCS, 2mM EDTA (Invitrogen), 100 U/mL Penicillin and 100 μg/mL Streptomycin (Gibco), 8 mM L-glutamine (Gibco), and 0.05 mM 2-mercaptoethanol (Gibco)). Propidium iodide (PI) was used to determine cell viability in sorting experiments. Intracellular cytokine staining (ICS) was performed following 6 hours of re-stimulation with 50ng/mL phorbol 12-myristate 13-acetate (PMA, Promega) and 1 μg/mL ionomycin (Sigma) and BD Golgi Stop and BD Golgi Plug (diluted 1 : 1000, BD Biosciences). Following surface stain, cells were incubated with eBioscience Fixation/Permeabilization buffer for 25 minutes followed by 25 minutes in Permeabilization buffer (eBioscience), and incubation with antibodies in Permeabilization buffer for a further 30 minutes. For flow cytometry analysis, cells were analyzed using a BD LSRII (BD Biosciences) and data were analyzed using FlowJo software (Version 7.6.5, Treestar Inc). In all cases using triple cytokine reporter mice, cells from wild type, Ifngyfp or Il4gfp single cytokine reporter mice were used as controls to set gates to differentiate yfp and gfp. Antibodies used include: CD4 (efluor450 and PE-Cy7, RM4-5, eBioscience), CD25 (Fitc, 7D4, BD Pharmingen), CD44 (Fitc, Percpcy5.5, and APC, IM7, eBioscience), CD45.1 (PE-Cy7 and APC, A20, eBioscience), IFNγ (Pacific Blue, XMG1.2, Biolegend), IL4 (PE, 11B11, eBioscience), pSTAT4 (Alexa Fluor 647, BDPhosflow), TCRβ (APC, H57-597, eBioscience) and GFP (Alexafluor647, FM264G, BioLegend). Staining was performed in presence of FcR Blocking Reagent (Miltenyi Biotec). In analysis experiments, viability was determined using the Molecular Probes Live/Dead Fixable Blue Dead Cell Stain Kit (Life Technologies). For phospho-STAT staining, sorted cells were resuspended into serum-free media and incubated at 37 degrees for 20 minutes, followed by incubation with 10 ng/mL IL-12 (R&D) for 15 minutes. Cells were then fixed for 10 minutes at 37 degrees with prewarmed BD Phosflow Lyse/Fix Buffer, washed, permeabilized with BD Phosflow Perm Buffer III for 30 minutes on ice, washed, and stained for 1 hour with antibodies in PBS for FACS analysis.
Adoptive cell transfer
Naive CD4+ T cells were sorted from spleens as CD4+TCRβ+CD44–CD25–Il4gfp–PI−(Il4gfp reporter) or CD4+TCRβ+CD44–CD25–Il4gfp-Ifngyfp–Il17aFP635–PI−(triple reporter). Th2 cells were cultured for 2 weeks from splenic CD4+ cells in vitro with 10 ng/mL IL-4 (R&D), 5 ng/mL IL-2 (R&D), 10 μg/mL anti-IFNγ (XMG1.2, BioXcell), and Mouse T-Activator CD3/CD28 Dynabeads (Life Technologies) in IMDM with 10% FCS. Th2 cells were sorted as CD4+TCRβ+Il4gfp+PI−(Il4gfp reporter) or CD4+TCRβ+Il4gfp+Ifngyfp–Il17aFP635–PI−(triple reporter). For each experiment, 0.2x106 to 1x106 cells were adoptively transferred i.v. into recipient C57BL/6 Rag1–/- mice. Blocking antibodies diluted in PBS (anti-IFNγ, XMG1.2, anti-IL12p40 C17.8, BioXcell) were used at 0.4 or 0.5 mg/ dose.
Cell restimulation and ELISA
Sorted cells were cultured in 96 well round bottom plates in various conditions. Where indicated, antigen presenting cells were spleens depleted of CD4+ cells by MACS magnetic separation (Miltenyi Biotec) and irradiated (3000 rads). H. polygyrus antigen was isolated by homogenization of cleaned adult worms in PBS. IFNγ, IL-5, and IL-13 were measured using DuoSet ELISA kits, according to the manufacturer’s instructions (R&D). Total IgE ELISA was performed by coating with Purified Rat Anti-Mouse IgE (R35-72, BD Pharmingen) at 2 μg/mL overnight, followed by overnight incubation with serum and standard (Purified Mouse IgE,k isotype Standard, BD Pharmingen), and detection with Biotin Rat Anti-Mouse IgE at 1 μg/mL (R35-118, BD Pharmingen), Streptavidin HRP at 1 : 000 (BD Pharmingen) and ABTS One Component HRP Microwell Substrate (SurModics). H. polygyrus-specific IgG1 was detected by coating plates with 5 μg/mL H. polygyrus antigen overnight, followed by overnight incubation with serially diluted serum and detection with Biotin Rat Anti-Mouse IgG1 (Invitrogen) and streptavidin and ABTS, as above.
RNA extraction, qRT-PCR, RNA-Seq and IPA analysis
RNA was isolated from cells or tissue using RNeasy Mini Kit according to manufacturer’s instructions (Qiagen). For qRT-PCR of small intestine-derived RNA, 1 cm sections of tissue were harvested and stored in RNAlater (Sigma) before homogenisation and RNA extraction using RNeasy Mini Kit (Qiagen). cDNA was reverse transcribed from RNA using QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions. qRT-PCR analysis was performed using Power SYBR Green PCR master mix (Applied Biosystems) on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Relative quantities of mRNA were determined by the comparative threshold cycle method as described by Applied Biosystems for the ABI Prism 7700/7900HT Sequence Detection Systems using the following primers; Hprt Fwd: 5’-GCCCTTGACTATAATGAGTACTTCAGG-3’ and Rvs: 5’-TTCAACTTGCGCTCATCTTAGG-3’; Retnla Fwd: 5’-CCCTCCACTGTAACGAAGACTC-3’
and Rvs: 5’-CACACCCAGTAGCAGTCATCC-3’; Chil3: Fwd: 5’ - CATGAGCAAGACTTGCGTGAC-3’ and Rvs: 5’-GGTCCAAACTTCCATCCTCCA-3’; Arg1 Fwd: 5’ - GGAAAGCCAATGAAGAGCTG -3’ and Rvs: 5’ - GCTTCCAACTGCCAGACTGT -3’. RNA-seq libraries were constructed using the TruSeq RNA Sample Preparation Kit V2 according to manufacturer’s instructions (Illumina). Libraries were sequenced using the HiSeq 2500 System (Illumina).The raw Illumina reads were analyzed as follows. First, the data quality was analyzed using FastQC (www.bioinformatics.babraham.ac.uk/projects/fastqc). Low quality bases were trimmed using Trimmomatic [81], and the read pairs which passed the trimming quality filters were aligned to mm10 (Ensembl version 75) using Tophat2 [82]. Counts were determined using htseq_count [83]. Normalisation and statistical analysis was performed using edgeR [84]. Statistically significant genes with FDR < 0.05 are reported. Significantly differentially expressed genes were uploaded into Ingenuity Pathways Analysis (IPA) and subjected to upstream analysis to identify factors that could have contributed to the transcriptional profile observed in converted Th2 cells.
Statistical analysis
Data sets were compared by Mann Whitney test using GraphPad Prism (V.5.0). Differences were considered significant at *P ≤ 0.05.
Ethics statement
All animal experiments were carried out following United Kingdom Home Office regulations (project license 80/2506) and were approved by UK National Institute for Medical Research Ethical Review Panel.
Supporting Information
Zdroje
1. Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, et al. (2010) Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med 7: e1000290. doi: 10.1371/journal.pmed.1000290 20563310
2. Pullan RL, Smith JL, Jasrasaria R, Brooker SJ (2014) Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7 : 37. doi: 10.1186/1756-3305-7-37 24447578
3. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, et al. (2008) Helminth infections: the great neglected tropical diseases. J Clin Invest 118 : 1311–1321. doi: 10.1172/JCI34261 18382743
4. Degarege A, Animut A, Legesse M, Medhin G, Erko B (2014) Malaria and helminth co-infection and nutritional status of febrile patients in Southern Ethiopia. J Infect Public Health 7 : 32–37. doi: 10.1016/j.jiph.2013.05.007 23999346
5. Yapi RB, Hurlimann E, Houngbedji CA, Ndri PB, Silue KD, et al. (2014) Infection and Co-infection with Helminths and Plasmodium among School Children in Cote d'Ivoire: Results from a National Cross-Sectional Survey. PLoS Negl Trop Dis 8: e2913. doi: 10.1371/journal.pntd.0002913 24901333
6. Mulu A, Legesse M, Erko B, Belyhun Y, Nugussie D, et al. (2013) Epidemiological and clinical correlates of malaria-helminth co-infections in Southern Ethiopia. Malar J 12 : 227. doi: 10.1186/1475-2875-12-227 23822192
7. Kirwan P, Jackson AL, Asaolu SO, Molloy SF, Abiona TC, et al. (2010) Impact of repeated four-monthly anthelmintic treatment on Plasmodium infection in preschool children: a double-blind placebo-controlled randomized trial. BMC Infect Dis 10 : 277. doi: 10.1186/1471-2334-10-277 20858280
8. Degarege A, Animut A, Legesse M, Erko B (2010) Malaria and helminth co-infections in outpatients of Alaba Kulito Health Center, southern Ethiopia: a cross sectional study. BMC Res Notes 3 : 143. doi: 10.1186/1756-0500-3-143 20500831
9. Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Vannaphan S, et al. (2001) Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am J Trop Med Hyg 65 : 834–836. 11791982
10. Nacher M, Gay F, Singhasivanon P, Krudsood S, Treeprasertsuk S, et al. (2000) Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol 22 : 107–113. 10672191
11. Hartgers FC, Obeng BB, Boakye D, Yazdanbakhsh M (2008) Immune responses during helminth-malaria co-infection: a pilot study in Ghanaian school children. Parasitology 135 : 855–860. doi: 10.1017/S0031182008000401 18474122
12. Laranjeiras RF, Brant LC, Lima AC, Coelho PM, Braga EM (2008) Reduced protective effect of Plasmodium berghei immunization by concurrent Schistosoma mansoni infection. Mem Inst Oswaldo Cruz 103 : 674–677. 19057817
13. Tetsutani K, Ishiwata K, Torii M, Hamano S, Hisaeda H, et al. (2008) Concurrent infection with Heligmosomoides polygyrus modulates murine host response against Plasmodium berghei ANKA infection. Am J Trop Med Hyg 79 : 819–822. 19052285
14. Su Z, Segura M, Stevenson MM (2006) Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection. Infect Immun 74 : 2138–2144. 16552043
15. Tetsutani K, Ishiwata K, Ishida H, Tu L, Torii M, et al. (2009) Concurrent infection with Heligmosomoides polygyrus suppresses anti-Plasmodium yoelii protection partially by induction of CD4(+)CD25(+)Foxp3(+) Treg in mice. European journal of immunology 39 : 2822–2830. doi: 10.1002/eji.200939433 19728313
16. Knowles SC (2011) The effect of helminth co-infection on malaria in mice: a meta-analysis. Int J Parasitol 41 : 1041–1051. doi: 10.1016/j.ijpara.2011.05.009 21777589
17. Reynolds LA, Filbey KJ, Maizels RM (2012) Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin Immunopathol 34 : 829–846. doi: 10.1007/s00281-012-0347-3 23053394
18. Maizels RM, Hewitson JP, Murray J, Harcus YM, Dayer B, et al. (2012) Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol 132 : 76–89. doi: 10.1016/j.exppara.2011.08.011 21875581
19. Langhorne J, Gillard S, Simon B, Slade S, Eichmann K (1989) Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol 1 : 416–424. 2535135
20. Stephens R, Culleton RL, Lamb TJ (2012) The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol 28 : 73–82. doi: 10.1016/j.pt.2011.10.006 22100995
21. Elias RM, Sardinha LR, Bastos KR, Zago CA, da Silva AP, et al. (2005) Role of CD28 in polyclonal and specific T and B cell responses required for protection against blood stage malaria. J Immunol 174 : 790–799. 15634900
22. Muxel SM, Freitas do Rosario AP, Zago CA, Castillo-Mendez SI, Sardinha LR, et al. (2011) The spleen CD4+ T cell response to blood-stage Plasmodium chabaudi malaria develops in two phases characterized by different properties. PLoS One 6: e22434. doi: 10.1371/journal.pone.0022434 21814579
23. Cross CE, Langhorne J (1998) Plasmodium chabaudi chabaudi (AS): inflammatory cytokines and pathology in an erythrocytic-stage infection in mice. Exp Parasitol 90 : 220–229. 9806866
24. Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, et al. (1996) Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med 183 : 901–913. 8642294
25. Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, et al. (2001) Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 14 : 205–215.
26. Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, et al. (2004) Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol 5 : 1157–1165. 15475959
27. Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, et al. (2006) T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med 203 : 755–766. 16520391
28. Krawczyk CM, Shen H, Pearce EJ (2007) Functional plasticity in memory T helper cell responses. J Immunol 178 : 4080–4088. 17371962
29. Peine M, Rausch S, Helmstetter C, Frohlich A, Hegazy AN, et al. (2013) Stable T-bet(+)GATA-3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol 11: e1001633. doi: 10.1371/journal.pbio.1001633 23976880
30. Coomes SM, Pelly VS, Wilson MS (2013) Plasticity within the alphabeta(+)CD4(+) T-cell lineage: when, how and what for? Open Biol 3 : 120157. doi: 10.1098/rsob.120157 23345540
31. Murphy KM, Stockinger B (2010) Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol 11 : 674–680. doi: 10.1038/ni.1899 20644573
32. O'Garra A, Gabrysova L, Spits H (2011) Quantitative events determine the differentiation and function of helper T cells. Nat Immunol 12 : 288–294. doi: 10.1038/ni.2003 21423225
33. Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, et al. (2008) Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med 205 : 53–61. doi: 10.1084/jem.20071855 18195073
34. Hegazy AN, Peine M, Helmstetter C, Panse I, Frohlich A, et al. (2010) Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 32 : 116–128. doi: 10.1016/j.immuni.2009.12.004 20079668
35. Mohrs M, Shinkai K, Mohrs K, Locksley RM (2001) Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15 : 303–311. 11520464
36. Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, et al. (2003) Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med 198 : 1069–1076. 14530376
37. Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, et al. (2011) Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12 : 255–263. doi: 10.1038/ni.1993 21278737
38. Urban JF Jr., Katona IM, Finkelman FD (1991) Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp Parasitol 73 : 500–511. 1683629
39. Urban JF Jr., Katona IM, Paul WE, Finkelman FD (1991) Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A 88 : 5513–5517. 2062833
40. Mastelic B, do Rosario AP, Veldhoen M, Renauld JC, Jarra W, et al. (2012) IL-22 Protects Against Liver Pathology and Lethality of an Experimental Blood-Stage Malaria Infection. Front Immunol 3 : 85. doi: 10.3389/fimmu.2012.00085 22566965
41. Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, et al. (2003) Neonates support lymphopenia-induced proliferation. Immunity 18 : 131–140. 12530982
42. Ernst B, Lee DS, Chang JM, Sprent J, Surh CD (1999) The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11 : 173–181. 10485652
43. Bell EB, Sparshott SM, Drayson MT, Ford WL (1987) The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. J Immunol 139 : 1379–1384. 3305705
44. Bourgeois C, Kassiotis G, Stockinger B (2005) A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells. J Immunol 174 : 5316–5323. 15843528
45. Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM (1995) Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity 2 : 665–675. 7796298
46. Szabo SJ, Dighe AS, Gubler U, Murphy KM (1997) Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med 185 : 817–824. 9120387
47. Usui T, Nishikomori R, Kitani A, Strober W (2003) GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity 18 : 415–428. 12648458
48. Smeltz RB, Chen J, Ehrhardt R, Shevach EM (2002) Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. Journal of immunology 168 : 6165–6172.
49. Remoue F, Diallo TO, Angeli V, Herve M, de Clercq D, et al. (2003) Malaria co-infection in children influences antibody response to schistosome antigens and inflammatory markers associated with morbidity. Trans R Soc Trop Med Hyg 97 : 361–364. 15228260
50. Mwangi TW, Bethony JM, Brooker S (2006) Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol 100 : 551–570. 16989681
51. Salgame P, Yap GS, Gause WC (2013) Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol 14 : 1118–1126. doi: 10.1038/ni.2736 24145791
52. Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, et al. (2008) Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasis and malaria coinfection. Infect Immun 76 : 2212–2218. doi: 10.1128/IAI.01433-07 18285496
53. Noland GS, Urban JF Jr., Fried B, Kumar N (2008) Counter-regulatory anti-parasite cytokine responses during concurrent Plasmodium yoelii and intestinal helminth infections in mice. Exp Parasitol 119 : 272–278. doi: 10.1016/j.exppara.2008.02.009 18396282
54. Helmby H, Kullberg M, Troye-Blomberg M (1998) Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect Immun 66 : 5167–5174. 9784518
55. Abdel-Wahab MF, Powers KG, Mahmoud SS, Good WC (1974) Suppression of schistosome granuloma formation by malaria in mice. Am J Trop Med Hyg 23 : 915–918. 4615597
56. Spence PJ, Langhorne J (2012) T cell control of malaria pathogenesis. Curr Opin Immunol 24 : 444–448. doi: 10.1016/j.coi.2012.05.003 22658628
57. Wei G, Wei L, Zhu J, Zang C, Hu-Li J, et al. (2009) Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30 : 155–167. doi: 10.1016/j.immuni.2008.12.009 19144320
58. Helmby H (2009) Gastrointestinal nematode infection exacerbates malaria-induced liver pathology. Journal of immunology 182 : 5663–5671.
59. Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, et al. (2010) A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med 207 : 2479–2491. doi: 10.1084/jem.20101376 20921287
60. Annunziato F, Cosmi L, Manetti R, Brugnolo F, Parronchi P, et al. (2001) Reversal of human allergen-specific CRTH2+ T(H)2 cells by IL-12 or the PS-DSP30 oligodeoxynucleotide. J Allergy Clin Immunol 108 : 815–821. 11692110
61. Levy Y, Thiebaut R, Montes M, Lacabaratz C, Sloan L, et al. (2014) Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur J Immunol.
62. Mahnke YD, Greenwald JH, DerSimonian R, Roby G, Antonelli LR, et al. (2012) Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood 119 : 3105–3112. doi: 10.1182/blood-2011-09-380840 22219223
63. Ding ZC, Huang L, Blazar BR, Yagita H, Mellor AL, et al. (2012) Polyfunctional CD4(+) T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood 120 : 2229–2239. doi: 10.1182/blood-2011-12-398321 22859605
64. Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, et al. (2007) Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol 8 : 145–153. 17195845
65. Panzer M, Sitte S, Wirth S, Drexler I, Sparwasser T, et al. (2012) Rapid in vivo conversion of effector T cells into Th2 cells during helminth infection. J Immunol 188 : 615–623. doi: 10.4049/jimmunol.1101164 22156341
66. Williams CL, Schilling MM, Cho SH, Lee K, Wei M, et al. (2013) STAT4 and T-bet are required for the plasticity of IFN-gamma expression across Th2 ontogeny and influence changes in Ifng promoter DNA methylation. J Immunol 191 : 678–687. doi: 10.4049/jimmunol.1203360 23761633
67. Heath VL, Showe L, Crain C, Barrat FJ, Trinchieri G, et al. (2000) Cutting edge: ectopic expression of the IL-12 receptor-beta 2 in developing and committed Th2 cells does not affect the production of IL-4 or induce the production of IFN-gamma. J Immunol 164 : 2861–2865. 10706670
68. Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, et al. (2002) T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol 3 : 549–557. 12006974
69. Fairlie-Clarke KJ, Lamb TJ, Langhorne J, Graham AL, Allen JE (2010) Antibody isotype analysis of malaria-nematode co-infection: problems and solutions associated with cross-reactivity. BMC Immunol 11 : 6. doi: 10.1186/1471-2172-11-6 20163714
70. Naus CW, Jones FM, Satti MZ, Joseph S, Riley EM, et al. (2003) Serological responses among individuals in areas where both schistosomiasis and malaria are endemic: cross-reactivity between Schistosoma mansoni and Plasmodium falciparum. J Infect Dis 187 : 1272–1282. 12696007
71. Pierrot C, Wilson S, Lallet H, Lafitte S, Jones FM, et al. (2006) Identification of a novel antigen of Schistosoma mansoni shared with Plasmodium falciparum and evaluation of different cross-reactive antibody subclasses induced by human schistosomiasis and malaria. Infect Immun 74 : 3347–3354. 16714563
72. Sardinha LR, D'Imperio Lima MR, Alvarez JM (2002) Influence of the polyclonal activation induced by Plasmodium chabaudi on ongoing OVA-specific B - and T-cell responses. Scand J Immunol 56 : 408–416. 12234262
73. Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, et al. (1995) Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378 : 88–91. 7477296
74. Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, et al. (1997) IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol 158 : 1541–1550. 9029088
75. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, et al. (1992) RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68 : 869–877. 1547488
76. Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, et al. (1999) Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A 96 : 10338–10343. 10468609
77. Sinclair C, Bains I, Yates AJ, Seddon B (2013) Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proc Natl Acad Sci U S A 110: E2905–2914. doi: 10.1073/pnas.1304859110 23858460
78. Barnden MJ, Allison J, Heath WR, Carbone FR (1998) Defective TCR expression in transgenic mice constructed using cDNA-based alpha - and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76 : 34–40. 9553774
79. Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, et al. (1994) Functional role of type I and type II interferons in antiviral defense. Science 264 : 1918–1921. 8009221
80. Shcherbo D, Murphy CS, Ermakova GV, Solovieva EA, Chepurnykh TV, et al. (2009) Far-red fluorescent tags for protein imaging in living tissues. The Biochemical journal 418 : 567–574. doi: 10.1042/BJ20081949 19143658
81. Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 : 2114–2120. doi: 10.1093/bioinformatics/btu170 24695404
82. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36. doi: 10.1186/gb-2013-14-4-r36 23618408
83. Simon Anders PTP, Wolfgang Huber (2014) HTSeq—A Python framework to work with high-throughput sequencing data. bioRxiv preprint.
84. Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 : 139–140. doi: 10.1093/bioinformatics/btp616 19910308
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



