-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMosquitoes Reset Malaria Parasites
article has not abstract
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1004987
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1004987Summary
article has not abstract
Serial blood passage of Plasmodium universally increases parasite virulence, which can be reversed by mosquito transmission. How mosquitoes reset Plasmodium virulence has been unknown. We have shown that mosquito transmission modifies expression of Plasmodium subtelomeric multigene families, including those that code for variant surface antigens (VSA), and transforms the systemic immune response to blood-stage infection. In this way, the mosquito regulates malaria disease severity. Here, we present a model in which expression of multigene families is reset by epigenetic reprogramming of Plasmodium within the mosquito. This prepares the malaria parasite for entry into a new unknown host and transforms the early parasite–host interactions that shape disease severity. Studying the molecular mechanisms that operate outside the human host to regulate Plasmodium virulence is therefore a priority.
Historical Perspective
It has been recognised for decades that serial blood passage of Plasmodium through rodents, primates, or humans universally increases parasite virulence. In 1917, induced malaria was first used as pyretic therapy for neurosyphilis, with Plasmodium vivax routinely inoculated to elicit a mild form of disease. Yet passage through the human host elevated parasitaemia and exacerbated disease, increasing the requirement for chemotherapeutic intervention [1]. Blood passage of Plasmodium knowlesi or Plasmodium cynomolgi, whether through human volunteers or nonhuman primates, similarly elevated parasite densities and disease severity [2–4]. And serial blood passage of every rodent malaria parasite species increased parasitaemia and pathogenicity [5–8]. On the other hand, it has been assumed for decades that mosquito transmission resets Plasmodium virulence [8]. At the Horton Mental Hospital, a pioneering centre for malaria therapy, Plasmodium strains were maintained by mosquito transmission to preserve their clinical and parasitological features [9]. Nevertheless, direct evidence that mosquito transmission resets Plasmodium virulence, and a mechanism to explain this phenomenon, have been missing [6,10,11].
Mosquito Transmission Resets Plasmodium Virulence
We have recently shown that mosquito transmission modifies gene expression in blood-stage malaria parasites and in this way resets Plasmodium virulence [12]. Whereas serial blood passage of Plasmodium chabaudi leads to hyperparasitaemia and severe disease in laboratory mice, mosquito transmission of serially blood-passaged parasites leads to a low-grade, chronic, recrudescing infection with minimal pathology. Attenuation of virulence is not parasite clone - or dose-dependent and therefore cannot be explained by bottlenecking during mosquito transmission [13]. Instead, attenuation of the blood-stage parasite is dependent upon host genotype [14] and an intact host immune response [12] and associates with increased expression of Plasmodium subtelomeric multigene families, including those that code for VSA (Box 1). Mosquito transmission therefore modifies expression of parasite virulence genes and transforms host immunity in the pathogenic blood-stage of infection. As such, the mosquito vector both transmits malaria and regulates disease severity.
Box 1. Mosquito Transmission Modifies Expression of Plasmodium Virulence Genes in Human Malaria
Transcriptional profiling [21] and proteomic analysis [22] of cultured Plasmodium falciparum demonstrates that the diversity and magnitude of rifin and var gene expression is increased in sporozoites (isolated from mosquito salivary glands) as compared to merozoites, trophozoites, or gametocytes. Furthermore, 53 of 59 var genes were transcribed in a single human volunteer infected with P. falciparum by mosquito bite just five days after merozoite egress from the liver [23], and diversity of var gene expression has been shown to decrease in human volunteers after blood passage [24]. Collectively, these data support a model in which expression of Plasmodium subtelomeric multigene families is increased as parasites transit through the mosquito and subsequently decreases with time elapsed from the vector.
Epigenetic Reprogramming of Plasmodium
By recognising this key function of the mosquito, new research avenues open that can accelerate our understanding of the pathogenesis of human malaria. It is first important to delineate where, when, and how mosquito transmission modifies expression of Plasmodium virulence genes. This is likely to be a consequence, at least in part, of necessary changes in gene expression for progression through each step of the life cycle in both vector and host [15]. However, epigenetic reprogramming [16] of Plasmodium provides a mechanism by which expression of virulence genes could be reset within the vector. Heritable chromatin modifications control transcription of subtelomeric multigene families in the blood-stage parasite [15] and can thus promote adaptation of malaria parasites to their host [17]. Nevertheless, global erasure of epigenetic marks following gamete fusion in the mosquito could reset expression of multigene families and thus prepare Plasmodium for entry into a new unknown host (Fig 1).
Fig. 1. Epigenetic reprogramming of Plasmodium within the mosquito. 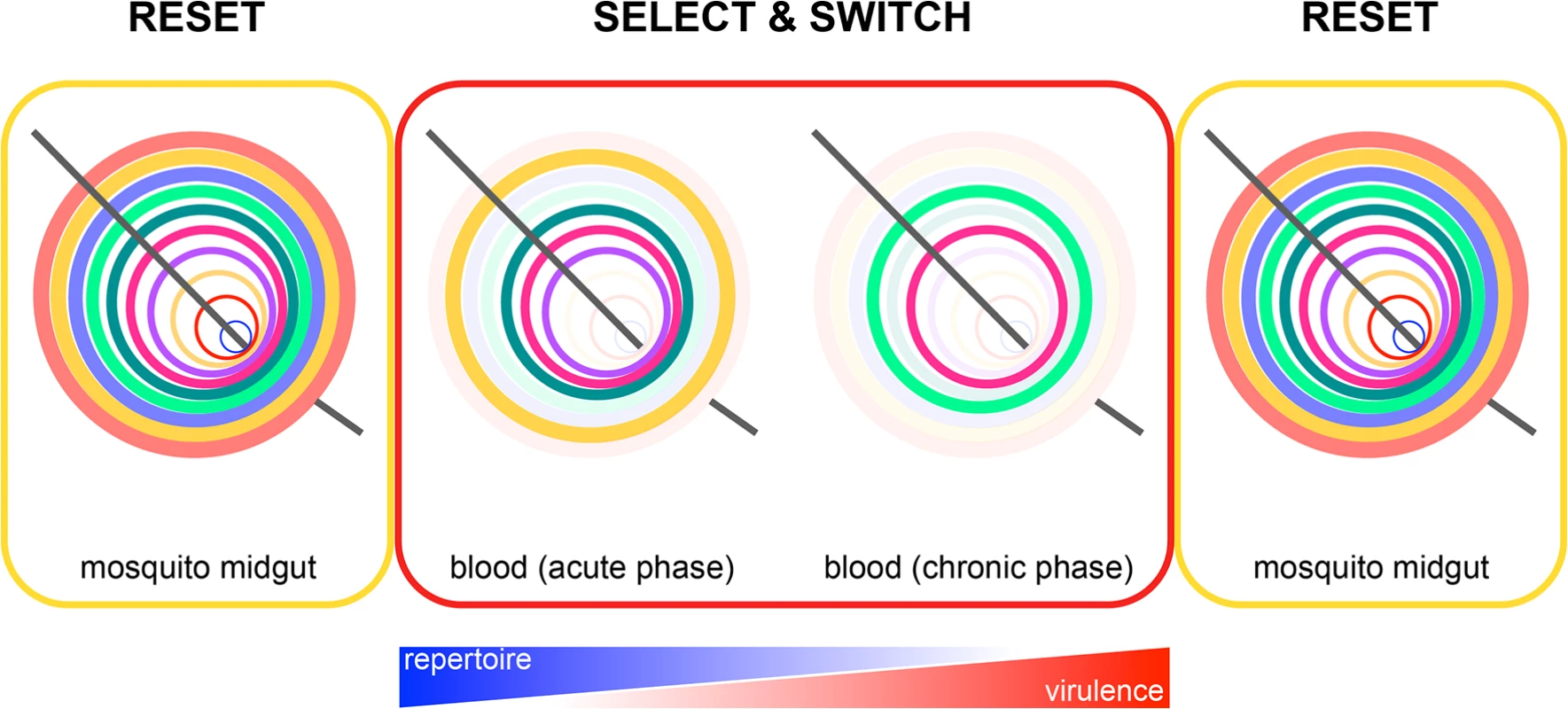
Reset: expression of subtelomeric multigene families is reset in the mosquito by epigenetic reprogramming of the zygote. This ensures that a parasite population will always express all multigene family members from the start of the erythrocytic cycle and gives merozoites the best possible chance of establishing a blood-stage infection every time they emerge from the liver (e.g., it may be beneficial to express all VSA upon liver egress, as a malaria-experienced host will have pre-existing antibodies that recognise a broad repertoire of variant antigens [23]). Select and switch: parasites that express (or switch to) multigene family members offering a survival advantage in their new host are retained, whereas parasites that silence these genes are lost through rounds of selection. In time, this leads to a parasite population expressing a narrow repertoire of multigene family members that promotes survival and chronicity. The further into the chronic phase of infection, the better adapted are parasites to their host. Plasmodium virulence therefore increases, and the need to reset gene expression also increases. Reset: in preparation for entry into the next host, all chromatin marks are again erased following gamete fusion. This model of gene expression provides a general mechanism by which all Plasmodium subtelomeric multigene families could be regulated by the mosquito. Resetting Plasmodium gene expression could be particularly important when transmission is seasonal, and parasites undergo an extended period of host adaptation in a chronically infected individual before their return to the mosquito. In this context, it is important to know whether parasite virulence increases in the chronic phase of infection, as has been observed in human volunteers [3]. Thus, serial blood passage per se may not increase Plasmodium virulence; an alternative explanation is that virulence increases with time elapsed from the mosquito. This will be observed only in a new host and when mosquito transmission is bypassed.
Immune Control of Plasmodium Virulence
By resetting Plasmodium gene expression, the mosquito can also control how blood-stage parasites elicit the systemic immune response in a new host. Mosquito transmission attenuates P. chabaudi virulence because merozoites that emerge from the liver induce an immune response that can rapidly control parasite growth without collateral damage [12]. This contrasts with the host response to serially blood-passaged parasites that causes severe immunopathology. Does increasing expression of Plasmodium VSA explain how the mosquito can transform the elicited host immune response? Or does mosquito transmission change the context in which blood-stage parasites initiate host immunity (e.g., by modifying invasion, cytoadherence, or sequestration)? Furthermore, it remains possible that immune priming and/or regulation during the pre-erythrocytic stages of infection can subsequently modify the systemic immune response to the blood-stage parasite. In all scenarios, the early immune response, elicited in the context of a mosquito bite, can shape malaria disease severity. In turn, the developing immune response is likely to influence expression of Plasmodium virulence genes and could therefore also directly regulate parasite pathogenicity.
Improving Models of Malaria
Mosquitoes reset malaria parasites and can be used to strengthen the relevance of mouse models to human malaria. We should therefore aim to initiate experimental infections by the natural route of transmission wherever possible. We should also strive to study combinations of vector, parasite, and host that exist in nature to validate or improve our current experimental systems. Mouse models are important for interrogating the pathogenesis of malaria because they can answer research questions that cannot be addressed directly in humans. Moreover, relevant mouse models can act as a bridge between human studies. For example, vector regulation of Plasmodium virulence was first observed in human volunteers and subsequently reproduced and delineated in mice; the molecular mechanisms that operate within the mosquito to regulate Plasmodium virulence can now be dissected with human malaria parasites.
To this end, inoculation of human volunteers with Plasmodium is a powerful experimental model [18,19]. In this setting, it is possible to look for evidence of epigenetic reprogramming of P. vivax in laboratory-reared anopheline mosquitoes fed on infected volunteers. Interrogating expression and regulation of subtelomeric multigene families in gametocytes as they circulate, transmit, and then pass through each developmental checkpoint of sporogony is a priority. So, too, is examining how route of transmission influences the systemic host response to blood-stage infection. For this, the immune response to P. falciparum can be compared in peripheral blood obtained from human volunteers infected via mosquito bite versus direct inoculation of blood-stage parasites (isolated just 6–8 days after liver egress [20]). Nevertheless, mice are absolutely required to observe the interactions between parasites and the immune system that shape disease severity because these interactions occur in tissues, such as spleen. We should therefore aim to identify mouse models that share a common immune signature of infection in whole blood with human malaria and use these models to delineate the immune response to Plasmodium in relevant tissues.
Concluding Remarks
A mosquito is not simply a flying syringe. Mosquitoes reset malaria parasites in preparation for entry into a new unknown host and thereby regulate Plasmodium virulence. Furthermore, they are a mixing pot for the generation of new recombinant parasites and can thus transmit previously unseen virulent strains. By studying events within the mosquito, we will accelerate our understanding of malaria disease severity.
Zdroje
1. James SP, Nicol WD, Shute PG. Clinical and Parasitological Observations on Induced Malaria: (Section of Tropical Diseases and Parasitology). Proc R Soc Med. 1936; 29(8): 879–94. 19990731
2. Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am J Trop Med Hyg. 1968; 17(3): 355–8. 4385130
3. Coatney GR, Elder HA, Contacos PG, Getz ME, Greenland R, Rossan RN, et al. Transmission of the M strain of Plasmodium cynomolgi to man. Am J Trop Med Hyg. 1961; 10 : 673–8. 13694174
4. Hartley EG. Increased virulence of Plasmodium cynomolgi bastianellii in the rhesus monkey. Trans R Soc Trop Med Hyg. 1969; 63(3): 411–2.
5. Dearsly AL, Sinden RE, Self IA. Sexual development in malarial parasites: gametocyte production, fertility and infectivity to the mosquito vector. Parasitology. 1990; 100 Pt 3 : 359–68. 2194152
6. Knowles G, Walliker D. Variable expression of virulence in the rodent malaria parasite Plasmodium yoelii yoelii. Parasitology. 1980; 81(1): 211–9. 7422362
7. Mackinnon MJ, Read AF. Selection for high and low virulence in the malaria parasite Plasmodium chabaudi. Proc Biol Sci. 1999; 266(1420): 741–8. 10331293
8. Yoeli M, Hargreaves B, Carter R, Walliker D. Sudden increase in virulence in a strain of Plasmodium berghei yoelii. Ann Trop Med Parasitol. 1975; 69(2): 173–8. 1098585
9. Covell G, Nicol WD. Clinical, chemotherapeutic and immunological studies on induced malaria. Br Med Bull. 1951; 8(1): 51–5. 14944815
10. Alger NE, Branton M, Harant J, Silverman PH. Plasmodium berghei NK65 in the inbred A-J mouse: variations in virulence of P. berghei demes. J Protozool. 1971; 18(4): 598–601. 5133123
11. Mackinnon MJ, Bell A, Read AF. The effects of mosquito transmission and population bottlenecking on virulence, multiplication rate and rosetting in rodent malaria. Int J Parasitol. 2005; 35(2): 145–53. 15710435
12. Spence PJ, Jarra W, Lévy P, Reid AJ, Chappell L, Brugat T, et al. Vector transmission regulates immune control of Plasmodium virulence. Nature. 2013; 498(7453): 228–31. doi: 10.1038/nature12231 23719378
13. Mackinnon MJ. The role of immunity in mosquito-induced attenuation of malaria virulence. Malar J. 2014; 13 : 25. doi: 10.1186/1475-2875-13-25 24443873
14. Spence PJ, Jarra W, Lévy P, Nahrendorf W, Langhorne J. Mosquito transmission of the rodent malaria parasite Plasmodium chabaudi. Malar J. 2012; 11 : 407. doi: 10.1186/1475-2875-11-407 23217144
15. Cortes A, Crowley VM, Vaquero A, Voss TS. A view on the role of epigenetics in the biology of malaria parasites. PLoS Pathog. 2012; 8(12): e1002943. doi: 10.1371/journal.ppat.1002943 23271963
16. Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013; 20(3): 282–9. doi: 10.1038/nsmb.2489 23463313
17. Rovira-Graells N, Gupta AP, Planet E, Crowley VM, Mok S, Ribas de Pouplana L, et al. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res. 2012; 22(5): 925–38. doi: 10.1101/gr.129692.111 22415456
18. McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, Looke D, et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis. 2013; 208(10): 1688–94. doi: 10.1093/infdis/jit394 23908484
19. Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011; 11(1): 57–64. doi: 10.1038/nri2902 21179119
20. Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, et al. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997; 57(4): 495–500. 9347970
21. Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003; 301(5639): 1503–8. 12893887
22. Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002; 419(6906): 520–6. 12368866
23. Wang CW, Hermsen CC, Sauerwein RW, Arnot DE, Theander TG, Lavstsen T. The Plasmodium falciparum var gene transcription strategy at the onset of blood stage infection in a human volunteer. Parasitol Int. 2009; 58(4): 478–80. doi: 10.1016/j.parint.2009.07.004 19616120
24. Peters J, Fowler E, Gatton M, Chen N, Saul A, Cheng Q. High diversity and rapid changeover of expressed var genes during the acute phase of Plasmodium falciparum infections in human volunteers. Proc Natl Acad Sci U S A. 2002; 99(16): 10689–94. 12142467
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



