-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
Invasive lobular breast cancer (ILC) accounts for 10–15% of invasive breast cancer and is generally ER positive (ER+). To date, none of the genome-wide association studies that have identified loci that predispose to breast cancer in general or to ER+ or ER-negative breast cancer have focused on lobular breast cancer. In this lobular breast cancer study we identified a new variant that appears to be specific to this morphological subtype. We also ascertained which of the known variants predisposes specifically to lobular breast cancer and show for the first time that some of these loci are also associated with lobular carcinoma in situ, a non-obligate precursor of breast cancer and also a risk factor for contralateral breast cancer. Our study shows that the genetic pathways of invasive lobular cancer and ER+ ductal carcinoma mostly overlap, but there are important differences that are likely to provide insights into the biology of lobular breast tumors.
Published in the journal: . PLoS Genet 10(4): e32767. doi:10.1371/journal.pgen.1004285
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004285Summary
Invasive lobular breast cancer (ILC) accounts for 10–15% of invasive breast cancer and is generally ER positive (ER+). To date, none of the genome-wide association studies that have identified loci that predispose to breast cancer in general or to ER+ or ER-negative breast cancer have focused on lobular breast cancer. In this lobular breast cancer study we identified a new variant that appears to be specific to this morphological subtype. We also ascertained which of the known variants predisposes specifically to lobular breast cancer and show for the first time that some of these loci are also associated with lobular carcinoma in situ, a non-obligate precursor of breast cancer and also a risk factor for contralateral breast cancer. Our study shows that the genetic pathways of invasive lobular cancer and ER+ ductal carcinoma mostly overlap, but there are important differences that are likely to provide insights into the biology of lobular breast tumors.
Introduction
Invasive lobular breast cancer (ILC) accounts for 10–15% of all invasive breast carcinomas and it has distinct etiological, clinical and biological characteristics compared with the more common invasive ductal/no special type carcinoma (IDC) [1]. Lobular cancers show stronger associations with the use of hormone replacement therapy (HRT) than IDC, [2] and its incidence follows a similar temporal pattern as the use of combined HRT [3]. ILC is characterized by E-cadherin loss and the malignant cells therefore infiltrate the breast stroma in single files with little associated stromal reaction. This makes it difficult to detect these tumors by palpation or mammography, and they are often larger at presentation than IDCs [4]. ILCs are generally of histological grade 2 and estrogen receptor positive (ER+), with the exception of the pleomorphic subgroup. They typically have a different pattern of metastatic spread to IDCs, tending to infiltrate the peritoneum, ovary and gastrointestinal system. There is some evidence that they are less chemo-sensitive than IDC and that the 10-year survival rate of women with ILC is lower than that of ER+ IDCs [5], [6].
ILC is often associated with lobular carcinoma in situ (LCIS), a form of non-invasive breast cancer that is difficult to detect clinically and typically found incidentally on biopsy. The increased breast biopsy rate associated with screening mammography has led to an increase in the diagnosis of LCIS. LCIS shares many of the same genetic aberrations as ILC, suggesting that it is a precursor lesion in an analogous manner to ductal carcinoma in situ (DCIS) and IDC [7]. Women who have had LCIS are 2.4 times more likely to develop invasive breast cancer compared to the general population, with an excess of ILC (23–80% of cases) [8], [9]. However only 50–70% of invasive cancers associated with LCIS have lobular morphology [10, unpublished data from GLACIER study]. The remaining cancers have a IDC or mixed ductal-lobular appearance, but again are generally ER+ (95% of IDC and mixed ductal-lobular cancers associated with LCIS in the GLACIER study were ER+). Unlike DCIS, LCIS is also a risk factor for developing invasive cancer in the contralateral breast [8].
Genome-wide association studies (GWAS) in breast cancer have identified loci that predispose to invasive breast cancer in general, or specifically to ER+ or ER-negative disease [11]–[25]. However, no previous study has focused specifically on lobular carcinomas. Only one common single nucleotide polymorphism (SNP; rs11249433 at 1p11.2) has been shown to be more strongly associated with lobular than ductal histology [26]. For the remaining SNPs predisposing to ER+ tumors, it is unclear whether the studies have lacked statistical power to identify differential associations by histology, or whether associations tend to be non-differential by morphology after accounting for ER status.
The aim of this study was to identify new breast cancer susceptibility loci specific to lobular carcinoma, and to evaluate the heterogeneity of associations of known loci by morphology. This involved pooling genotyping data from over 6,000 cases of lobular carcinoma (ILC and/or LCIS) and over 34,000 controls genotyped using the iCOGS chip, a custom SNP array that comprises 211,155 SNPs enriched at predisposition loci for breast and other cancers [24].
Results
In a phase I analysis, we evaluated risk associations between SNPs on the iCOGS chip and risk of ILC and LCIS using 1,782 lobular cases (1,470 ILC with or without LCIS, 312 pure LCIS) from GLACIER, a UK study of lobular breast cancer, and 4,755 UK controls from the Breast Cancer Association Consortium, BCAC (Figure 1). There was little evidence for systematic inflation of the test statistics, based on 37,544 uncorrelated SNPs that had not been selected on the basis of breast cancer risk (λ = 1.04; Figure S1). Data were combined by meta-analysis with a further 4,241 cases (4,152 ILC, 89 LCIS) and 29,519 controls of European ancestry, derived from 34 studies in BCAC, and previously typed on the iCOGS chip (Tables S1 and S2). This resulted in a total of 6,023 cases (5,622 ILC, 401 LCIS) and 34,271 controls with data on 199,961 iCOGS SNPs (after quality control exclusions and with minor allele frequency (MAF) >0.01) included in the meta-analysis.
Fig. 1. Lobular cancer study design. 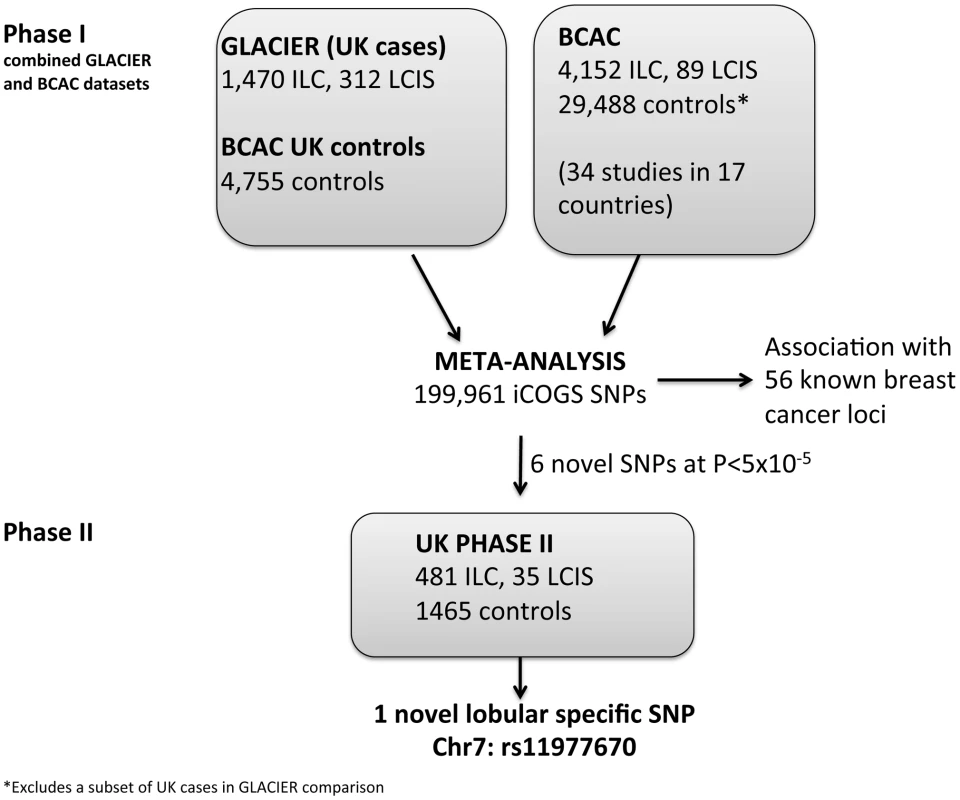
Search for new lobular breast cancer predisposition loci
All SNPs reaching genome-wide significance (P<5×10−8) in the meta-analysis were correlated with one of the known breast cancer predisposition loci. In order to identify new loci that predispose to lobular carcinoma, we selected six uncorrelated SNPs (rs11977670, rs2121783, rs2747652, rs3909680, rs9948182, rs7034265) that were only weakly correlated (r2<0.25) with known loci and that showed the best evidence of association (P between 5×10−8 and 5×10−5) in the overall lobular case-control analysis (ILC and LCIS). These SNPs were genotyped in a Phase II including 516 cases (481 ILC, 35 LCIS) and 1,467 controls, all from white European donors (Figure 1).
One of the six SNPs, rs11977670 at 7q34, reached genome-wide significance in a pooled analysis of phase I and II ILC cases and controls (OR = 1.13, 95%CI = 1.09–1.18, P = 6.0×10−10, Table 1, Figure 2). rs11977670 showed a similar association with LCIS (P-het for ILC vs LCIS = 0.198), and a very weak or no association with IDC (OR = 1.02, 95%CI = 1.00–1.05, P = 0.070; P-het for ILC vs IDC = 1.3×10−5), indicating that this is a lobular specific predisposition locus (Table 2). The risk allele appeared to act in a dominant rather than additive manner: ORAG = 1.21, 95%CI = 1.14–1.30; ORAA = 1.27, 95%CI = 1.17–1.38; P for departure from log-additivity = 0.009; Table S3. rs11977670 was not significantly associated with age at onset of ILC (Ptrend = 0.16) and risk alleles were not significantly over-represented in cases with a positive family history (FH) (P = 0.90, FH+ vs FH−). None of the other 5 SNPs genotyped were associated with lobular breast cancer at a genome-wide significance level, with the strongest association being for rs2121783 at 3p13 (OR = 1.11, 95%CI = 1.07–1.15, P = 4.5×10−7; Table S4).
Fig. 2. Forest plot for rs11977670. 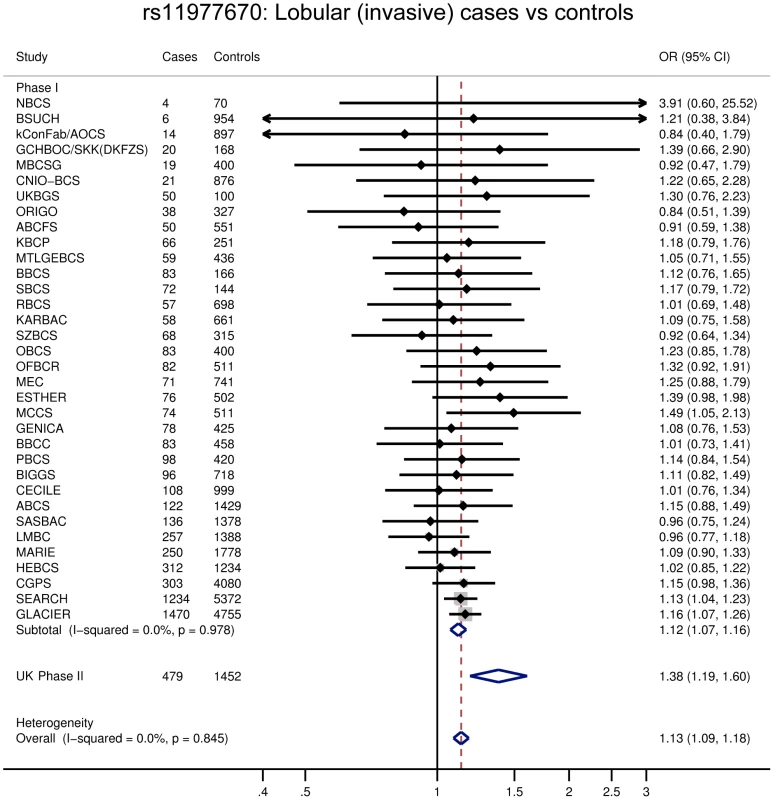
Tab. 1. rs11977670, chromosome 7:139942304 G>A, and association ILC in populations of European ancestry. 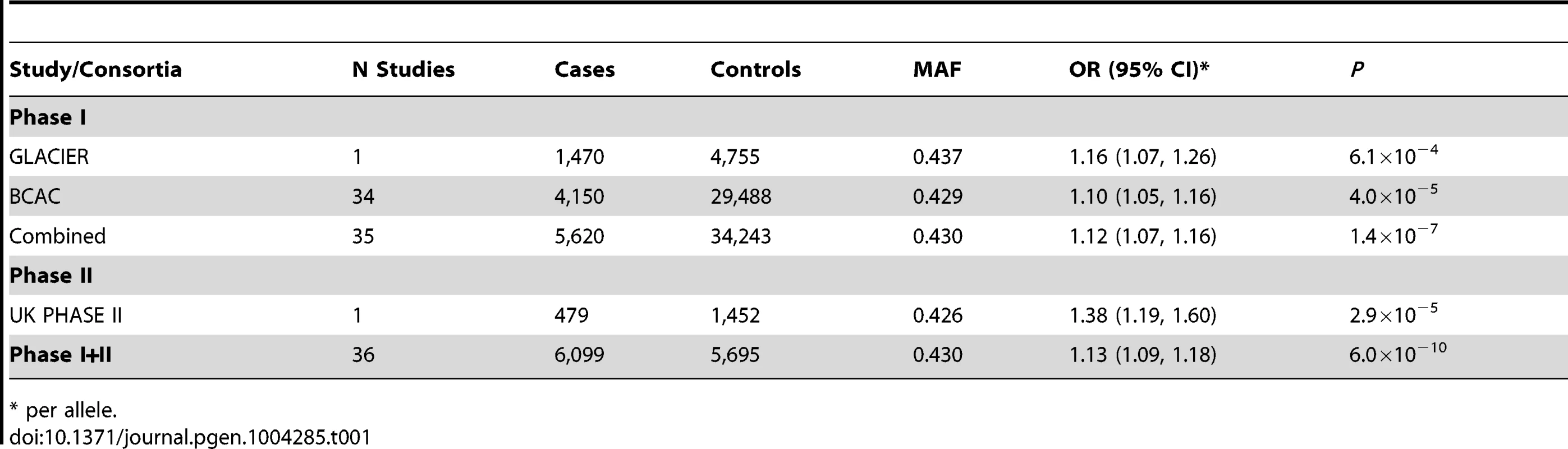
* per allele. Tab. 2. Association with risk of breast cancer for rs11977670 stratified by breast cancer tumour subtypes (Pooled analysis, BCAC, GLACIER, UK PHASE II). 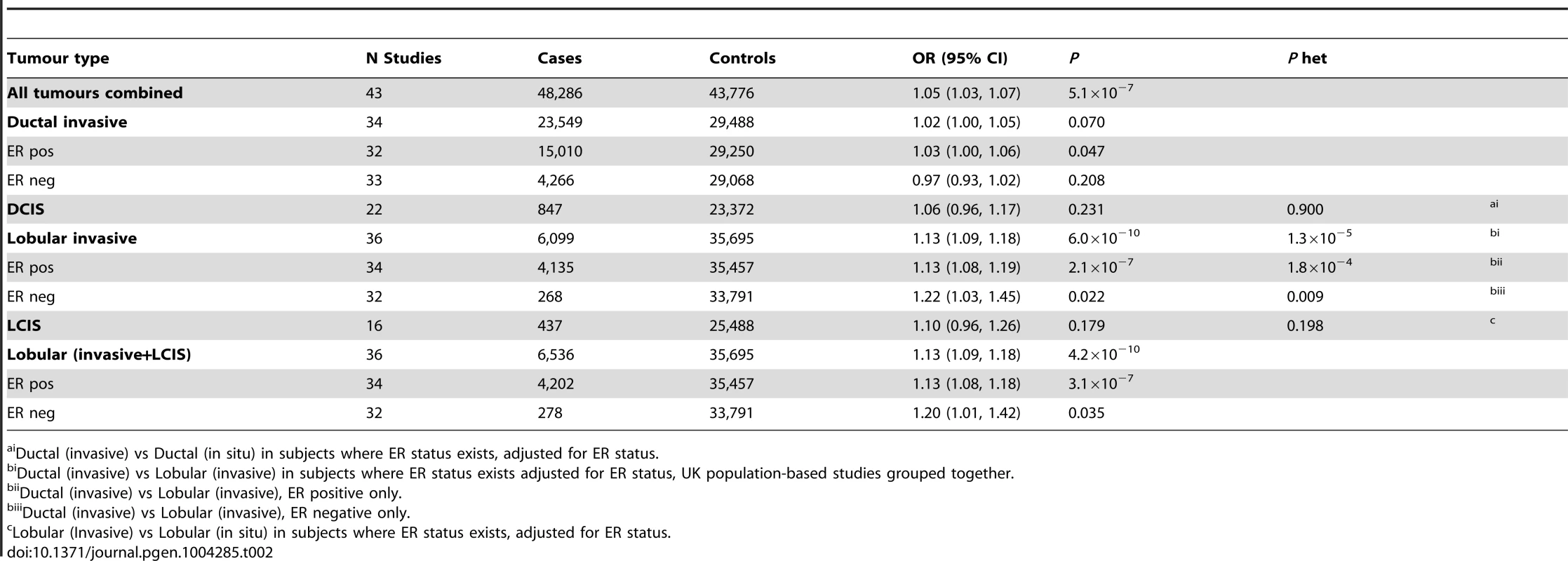
Ductal (invasive) vs Ductal (in situ) in subjects where ER status exists, adjusted for ER status. rs11977670 at 7q34 (position:139942304, GRCh Build 37) is intergenic, 65 kb from the nearest gene, JHDM1D, a histone demethylase and 500 kb from BRAF, a gene frequently mutated in melanoma. It is also in close proximity to a predicted novel U1 spliceosomal RNA that contains two U1 specific promoter motifs (Figure S2). ENCODE data on normal human mammary epithelial cells (HMEC), and breast carcinoma (MCF-7), were used to establish chromatin states in the region and showed that rs11977670 lies in region marked by H3K27 acetylation, Figure S3.
Using expression data from the Cancer Genome Atlas Network (TCGA database) [27], we assessed expression of the nine genes within 0.5 Mb of rs11977670 by breast cancer subtype (ER+ ILC, 40 cases; ER+ IDC, 341 cases; and ER-negative IDC, 108 cases; Figure S4). Three genes showed differential expression in ER+ ILC compared to ER+ IDC (BRAF, P = 0.006; NDUFB2, P = 0.02, SLC37A3, P = 0.05), however none reached statistical significance when correcting for multiple testing. Another two genes, JHDM1D and ADCK2, showed a difference in expression between ER-negative and ER+ cancers, but this was not lobular-specific. To further investigate which genes may be influenced by SNPs tagged by rs11977670, germline genotype data for rs13225058 (A/G), a surrogate for rs11977670 (G/A) (r2 = 0.79) was taken from the TCGA database (SNP6.0 Affymetrix array) and compared to expression of these genes, correcting for copy number variation, in 335 ER+ primary breast cancers where both genotype and expression data was available. A significant difference, after correcting for multiple testing, was found in expression between the AA and GG genotype for two genes JHDM1D (P = 0.0005) and SLC37A3 (P = 0.004), Figure S5a. Confining the analysis to the 36 ILC cases with data in TCGA showed no significant genotype specific expression due small numbers although there was the suggestion of a trend towards overexpression with the GG genotype (2 cases), Figure S5b. 48 of the cases also had expression data on adjacent normal breast tissue, but due to the small numbers no significant genotype specific expression changes were detected, Figure S6. There was no evidence of copy number variation around rs11977670 and no evidence of an excess of somatic mutations in JHDM1D, SLC37A3 or BRAF in ILC.
Assessment of the 75 known breast cancer susceptibility loci for association with ILC and LCIS
Most (56 of 75) known common breast cancer susceptibility loci were associated with ILC at P<0.05 with the effect in the same direction as previously reported (Table S5), and 13 of these reached genome-wide significance (P<5×10−8, Table 3). The strongest associations were with SNPs close to FGFR2 (rs2981579, OR = 1.38, P = 5.1×10−52), TOX3 (rs3803662, OR = 1.33, P = 1.1×10−35), at 1p11.2 (rs11249433, OR = 1.25, P = 2.7×10−25) and 11q13.3 (rs554219, OR = 1.33, P = 1.6×10−22). All 13 loci had previously been shown to be associated with ER+ breast cancer and one locus, rs11249433 (1p11.2), with lobular histology in subgroup analysis. Of the remaining 19 SNPs with P≥0.05, 18 had ORs in the same direction as previously reported for overall breast cancer (Sign test P = 0.0001), suggesting that these SNPs are also likely to predispose to LCIS. Only one of the seven ER-negative specific loci on the iCOGS array showed a significant association with ILC (rs12710696, P = 0.037). In case-only analyses, no SNP showed an association with family history of breast cancer or young age at onset of ILC.
Tab. 3. SNPs associated with ILC (P<5×10−8) or LCIS (P<0.05) in a pooled lobular analysis (GLACIER and BCAC). 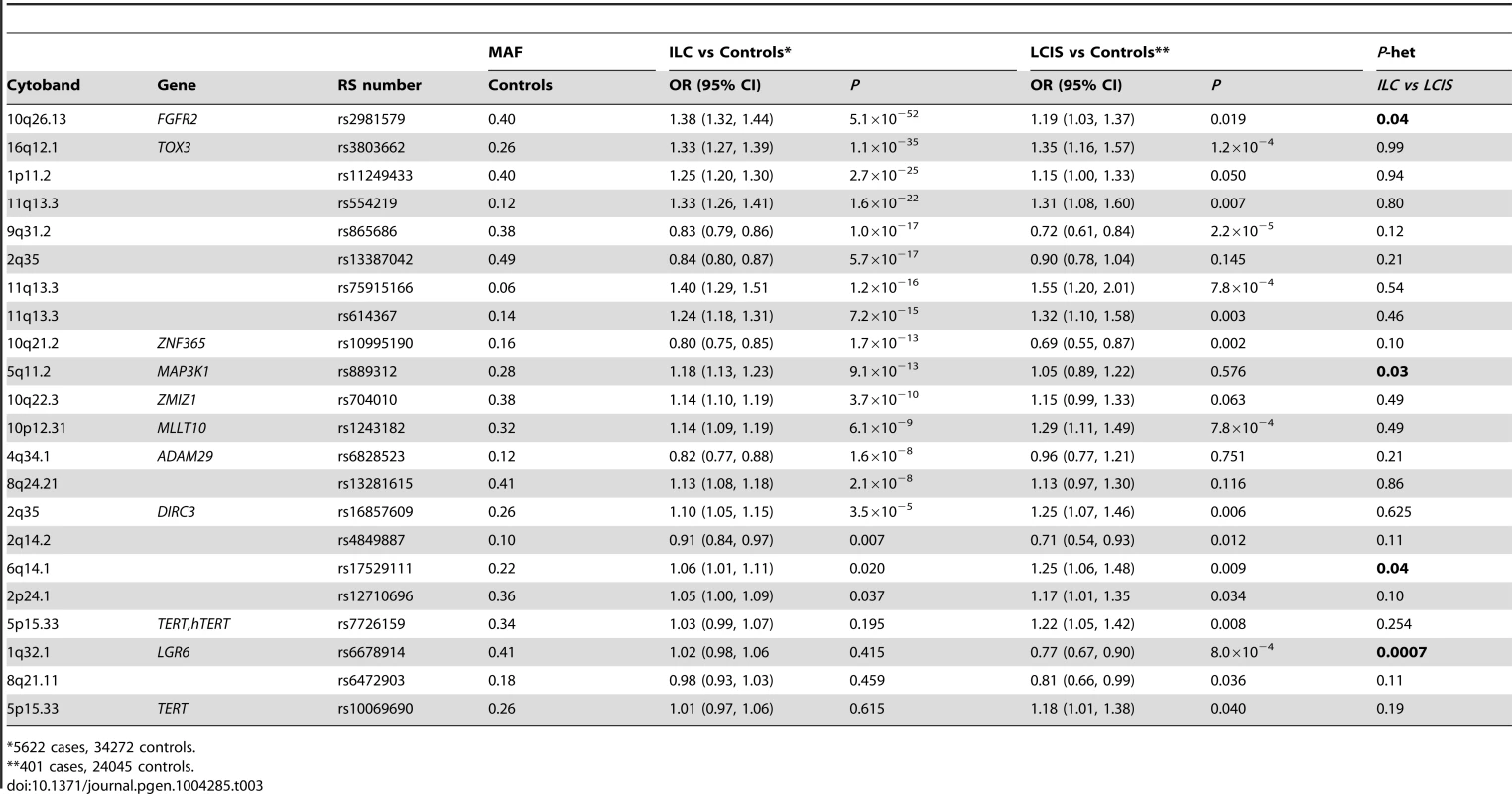
*5622 cases, 34272 controls. For the 75 known breast cancer susceptibility loci, case-control analysis for the 401 cases of pure LCIS (without invasion) and 24,045 controls, revealed 15 out of 75 SNPs associated with LCIS at P<0.05 (Table 3). The strongest associations were for rs865686 (9q31.2, P = 2.2×10−5); rs3803662 (TOX3, P = 1.2×10−4), c11_pos69088342/rs75915166 (11q13.3, P = 7.8×10−4) and rs1243482 (MLLT10, 10p12.31, P = 7.8×10−4) that is partially correlated (r2 = 0.69) with rs7072776, a recently identified ER+ breast cancer predisposition locus that showed a weaker association with LCIS (OR = 1.17, 95%CI = 1.00–1.36, P = 0.05; Table S5). Forty-seven of the remaining 60 SNPs at P>0.05 had ORs in the same direction as for ILC. This is greater than one would expect by chance (Sign Test P = 1.2×10−5) suggesting many of these SNPs predispose to LCIS, but the study did not have enough power to detect these associations with the small sample size.
A global test in case-only analysis (ILC vs LCIS) indicated no significant differences in associations of the 75 SNPs between LCIS and ILC (likelihood ratio test (75 df) = 0.438). However, individual SNP analyses suggested some differences. Two loci showed stronger associations with ILC than pure LCIS: rs2981579, FGFR2 (P-het = 0.02); and rs889312, 5q11.2 (P-het = 0.03). Case-only analysis also suggested that two ER-negative specific SNPs [23], [25] were more strongly associated with LCIS than ILC: rs6678914, 1q32.1 (P-het = 0.0007) and rs17529111, 6q14.1 (P-het = 0.04) Table 3. The remaining SNPs showed no significant heterogeneity between ILC and LCIS.
Assessment of the 75 known susceptibility SNPs for differential effects on ILC and IDC
In order to identify lobular specific SNPs, we performed a case-only analysis of 3,201 ER+ ILC cases and 15,024 ER+ IDC cases from BCAC. Analysis was confined to ER+ cases since 94% of ILC cases were ER+ (compared to 78% of IDC in BCAC). A global test indicated significant differences in SNP associations between ILC and IDC (likelihood ratio test (75 df) P = 5.9×10−6). The SNP showing the largest difference between ILC and IDC was rs11249433 at chr 1p11.2 (P-het = 2.7×10−8; Table 4), a SNP previously associated with lobular histology. At P<0.05, a further two loci were associated more strongly with ILC than IDC: rs2981579, FGFR2 (P-het = 5.3×10−3) and rs10995190, 10q21.2 (P-het = 0.002). This analysis also identified four IDC-specific SNPs at P<0.05: rs10941679, 5p12 (P-het = 1.5×10−4); rs2588809, RAD51L1 (P-het = 0.001); rs6472903, 8q21.11 (P-het = 0.004); rs1550623, CDCA7 (P-het = 0.031) Table S6.
Tab. 4. SNPs showing differential lobular and ductal associations with breast cancer risk in BCAC subjects (ER+ tumours only). 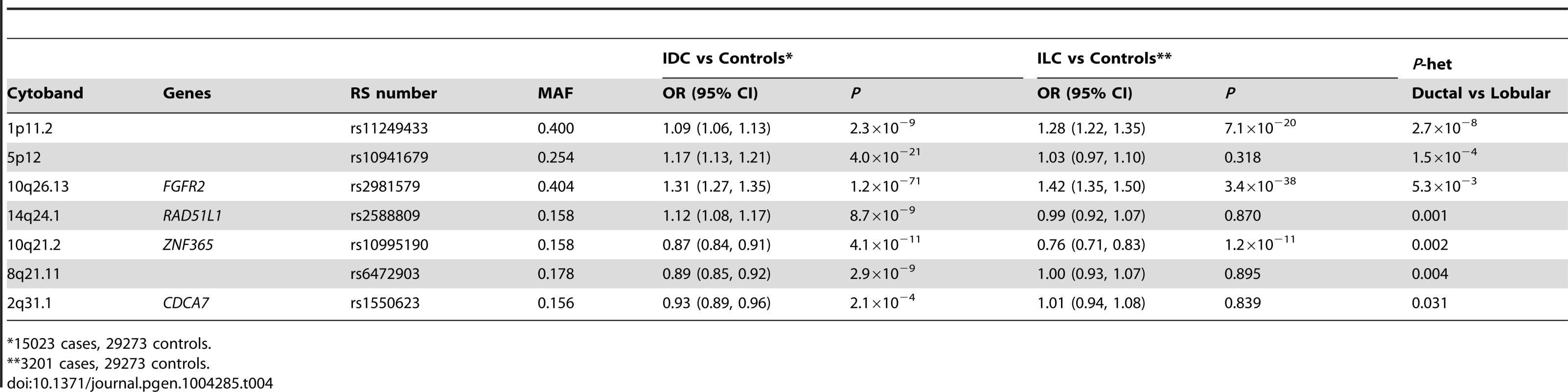
*15023 cases, 29273 controls. Assessment of the 75 known susceptibility SNPs for effects on mixed ILC-IDC cancer predisposition
Case-control analysis of 690 mixed ductal–lobular carcinomas revealed 25 loci that showed an association with these mixed cancers at P<0.05. The top hits were at FGFR2 (rs2981579, OR = 1.37, P = 1.6×10−7), rs941764 (CCDC88C, OR = 1.25, P = 3.6×10−4) and rs10995190 (ZNF365, OR = 0.74, P = 3.9×10−4). The case-only analysis above showed that two of these SNPs are more strongly associated with ILC than IDC (rs2981579, rs10995190). rs941764 showed no association with ILC and only weak association with ER+ IDC, Table S6.
Discussion
Our analyses of a total of 6,539 lobular cancers (including 436 cases of pure LCIS) and 35,710 controls has identified for the first time a lobular-specific SNP, rs11977670 (JHDM1D; OR = 1.13 P = 4.2×10−10, that showed little evidence of association with IDC (P = 0.07) or DCIS (P = 0.23). Identification of the target of this association will require fine mapping of the region, followed by functional assays to determine which gene(s) the key SNPs regulate. The preliminary in silico functional analysis suggests that SNPs in this region may be influencing expression of JHDM1D (a histone demethylase) and SLC37A3 (a sugar-phosphate exchanger). For JHDM1D this appears to be a recessive effect, in contrast to the susceptibility data, which suggests a dominant effect. There are little data on the role of these genes in cancer. There is some evidence that increased expression of JHDM1D can suppress tumor growth by regulating angiogenesis [28] and decreased expression promotes invasiveness, which is contrary to what one would expect from the risk data [29]. This inconsistency does shed some doubt on these results and further analysis of the region is required before any firm conclusion can be made. Studies of syndecan-1-deficient breast cancer cells, which show increased cell motility and invasiveness, demonstrate decreased expression of both JHDM1D and E-cadherin [29], suggesting the two genes may interact. Somatic mutations in CDH1 (E-Cadherin) are frequent in ILC and rare germline frameshift mutations in CDH1 have been described in ILC, particularly in families with hereditary diffuse gastric cancer (HDGC), but also in cases of familial ILC with no HDGC [30], [31]. However, none of the 56 SNPs in CDH1 that were typed on the iCOGS chip showed any association with lobular cancer at P<0.05.
It should also be noted that this study is not a true genome wide association study for lobular breast cancer as the SNPs on the iCOGS chips were chosen on the basis of some prior evidence of association with breast cancer as a whole. Although ILC would have been a small proportion of the samples in the discovery sets for these SNPs it is possible that other lobular specific loci exist that have not been included on the iCOGS chip. This is particularly true for LCIS, which would only have been included in the discovery set as a parallel phenotype when associated with invasive disease.
75 of the known common breast cancer susceptibility loci were assessed for association with ILC and LCIS. As cases of ILC were included in the discovery sets that generated these susceptibility loci and lobular breast cancer is generally ER+ (94% of the ILC cases in this study were ER+) with the majority of ILCs classified as luminal tumors [32], it is not surprising that the majority of SNPs that we found to be associated with ILC were known to also predispose to ER+ breast cancer. However, some loci were only associated with ER+ IDC and not with ILC, particularly rs10941679 at 5p12, previously shown to predispose more strongly to ER-positive, lower-grade cancers [33], P-het = 2.7×10−8. Others showed a much stronger association with ILC than IDC, particularly rs11249433 at 1p11.2, as previously described [26]. These data suggest specific etiological pathways for the development of different histological subtypes of breast cancer, in addition to common pathways that predispose to multiple tumor subtypes.
Despite the small number of pure LCIS cases without invasive disease, our analyses have shown for the first time that many of the SNPs that predispose to ILC also predispose to LCIS. Although only 15 of the known breast cancer SNPs were associated with LCIS risk at P<0.05, 47 of the remaining 60 SNPs at P>0.05 had ORs in the same direction as for ILC (Sign Test P = 1.2×10−5) suggesting that many more SNPs are likely to be associated with pure LCIS but did not reach statistical significance individually because of the relatively few LCIS cases without associated ILC in our sample set. This is not unexpected if LCIS is an intermediate phenotype for ILC. However, a small number of SNPs had differential effects on LCIS or ILC risk. Specifically, rs6678914 at 1q32.1 (LGR6), known to be an ER-negative specific SNP [25], that appeared to be associated with LCIS but not ILC (P-het = 0.0007), and rs17529111 at 6q14 preferentially associated with ER-negative tumors [23] that had a stronger association with LCIS than ILC (P-het = 0.04). We also identified SNPs in FGFR2 and at 5q11.2 (MAP3K1) that appear only to predispose to ILC, but have little effect on LCIS suggesting that SNPs affect different parts of the lobular carcinoma pathway. These findings are surprising and as based on small numbers need confirmation in future studies.
Some of the SNPs associated with both ILC and LCIS showed a stronger effect size in LCIS compared to ILC (for example SNPs at TOX3, 9q31.2, 11q13.3, ZNF365 and MLLT10). It is possible that the SNPs that showed an association with both LCIS and ILC predispose to the development of LCIS rather than ILC, and that the effect size is smaller in ILC as not all cases of LCIS will become invasive cancer. SNPs that predispose strongly to LCIS were also associated with ER+ IDCs but again with stronger effect sizes in LCIS, consistent with the fact that 30–40% of invasive tumors associated with LCIS will not be ILC but will be IDC, mixed ductal-lobular or other morphology.
One SNP, rs1243182 (MLLT10), that showed a strong association with LCIS (LCIS: P = 7.8×10−4, OR = 1.29; ILC: P = 6.1×10−9,OR = 1.14; ILC+LCIS: P = 3×10−10,OR = 1.15, IDC: P = 1.4×10−5,OR = 1.07, is partially correlated (r2 = 0.69) with rs7072776, a recently identified ER+ breast cancer predisposition locus, which showed no association with LCIS in this study. It is also strongly correlated with rs1243180 (r2 = 0.80), an ovarian cancer predisposition variant [34] and rs11012732 (r2 = 0.57), which predisposes to meningioma [35]. The ovarian SNP, rs1243180, also showed a strong association with lobular cancer (ILC+LCIS: P = 5.54×10−10; OR = 1.13). Conditional analysis confirmed that this was not independent of rs1243182. rs11012732 was not genotyped on the iCOGS chip. The increased risk of ovarian carcinoma after breast cancer is well documented in epidemiological studies [36]. Of note, there are also reports suggesting an association between breast cancer and meningioma [37].
In conclusion, we have identified a novel lobular-specific predisposition SNP at 7q34 close to JHDM1D that does not appear to be associated with IDC. Most known breast cancer predisposition SNPs also predispose to ILC, with some differential effects between ILC and IDC. In addition, many SNPs predisposing to invasive cancer are also likely to increase the risk for LCIS. Overall, our analyses show that genetic predisposition to IDC and lobular lesions (both ILC and LCIS) overlap to a large extent, but there are important differences that are likely to provide insights into the biology of lobular breast tumors.
Methods
Ethics statement
All studies were performed with ethical committee approval, Table S7, and subjects participated in the studies after providing informed consent.
Study populations
Phase I
Cases and controls came from 34 studies forming part of the Breast Cancer Association Consortium (BCAC) included in the COGS Project [13] (Table S1), and GLACIER (A study to investigate the Genetics of LobulAr Carcinoma In situ in EuRope MREC 06/Q1702/64), a UK case-only study of lobular breast cancer. BCAC studies recruited all types of breast cancer. Pathological information in BCAC was collected by the studies individually but combined and checked through standardized data control in a central database. A total of 4,152 ILC and 89 LCIS cases were identified by the central BCAC pathology database (see Table S2 for number of cases by study).
The GLACIER study recruited patients from participating centers throughout the UK with the aim of identifying predisposition genes for LCIS and/or ILC. Any patients aged 60 or less at the time of diagnosis, with a current or past history of LCIS (with or without invasive disease of any histological subtype) were eligible. A total of 2,539 cases were recruited: 2,167 were identified from local pathology reports in 97 UK hospitals, 346 cases were identified through the British Breast Cancer Study (BBCS) using UK Cancer Registry data and 26 cases from the Royal Marsden Breast Tissue Bank. Cryptic relatedness analysis showed no evidence of overlap between these samples and the BCAC samples. All these cases were genotyped with the iCOGS chip and compared to 5,000 UK controls selected from four UK studies participating in BCAC and already typed on the iCOGS chip. Controls were randomly selected prior to analysis so that each of these UK studies, including GLACIER, had a case∶control ratio of at least 1∶2 (Table S8). These controls were excluded from case-control comparisons with BCAC cases from the originating study. This report includes only cases of pure LCIS or ILC with or without LCIS. Cases of LCIS with IDC or mixed lobular and ductal carcinoma in GLACIER were excluded in order to perform meta-analyses with the BCAC studies which do not have information on the presence or absence of LCIS associated with an invasive cancer. After excluding individuals based on genotyping quality (see Genotyping and Analysis) and non-European ancestry, data for the GLACIER study available for analyses included 1,782 cases (1,470 ILC (with or without LCIS), 312 pure LCIS) and 4,755 controls.
Phase II
A further 516 cases (481 ILC, 35 LCIS) and 1,465 controls were analyzed as part of Phase II. Controls were recruited through the GLACIER study, but were not genotyped in Phase I on the iCOGS chip to reduce costs, and were all white West European. Cases came from the following studies: 232 cases from GLACIER, 176 from BBCS, 71 from DietCompLyf [38], 39 from King's Health Partners Cancer Biobank (KHP-CB). All cases were white West European, apart from the 39 samples from the KHP-CB where there were no associated ethnicity data. For studies that had also participated in Phase I, we selected samples so there was no overlap with the samples in Phase I.
Genotyping and analysis
Phase I
After DNA extraction from peripheral blood, GLACIER samples were genotyped on the iCOGS custom Illumina iSelect, which contains 211,155 SNPs, at King's College, London. The remaining cases and controls were genotyped as part of the COGS project described in detail elsewhere [13]. The GLACIER cases were analyzed using the same QC criteria as the COGS project. Briefly, genotypes were called using Illumina's proprietary GenCall algorithm and 10,000 SNPs were manually inspected to verify the algorithm calling. Individuals were excluded if genotypically not female, had overall call rate <95% or were ethnic outliers (248 cases) as identified by multi-dimensional scaling, combining the genotyping data with the three Hapmap2 populations. SNPs with a Gencall rate of <0.25, call rate <95% (call rate <99% if MAF <0.1) and HWE<10−7 or evidence of poor clustering on inspection of cluster plots were excluded. All SNPs with MAF <0.01 were excluded for this analysis. A cryptic relatedness analysis of the whole dataset was performed using 46,918 uncorrelated SNPs and there was no evidence of any duplicates.
For GLACIER cases and controls, principal component analysis (PCA) was carried out on a subset of 46,918 uncorrelated SNPs and used to exclude individuals or groups distinct from the main cluster using the first five principal components (PCs), Figure S7. Following removal of outliers (166 cases and 245 controls), the PCA was repeated and the first five PCs included as covariates in the analysis. The adequacy of the case-control matching was evaluated using quantile-quantile plots of test statistics and the inflation factor (λ) calculated using only uncorrelated SNPs that were not selected by BCAC and were not within one of the four common fine-mapping regions, to minimize selection for SNPs associated with breast cancer, Figure S1. As the majority of the SNPs on the iCOGS array were selected from GWAS of breast, ovarian and prostate cancer the SNPs selected for this analysis were taken from the set of SNPs selected by the prostate consortium, with the assumption that these SNPs were more likely to be representative of common SNPs in terms of population structure in our study than those selected by the breast or ovarian consortia.
For each SNP, we estimated a per-allele log-odds ratio (OR) and standard error by logistic regression, including the 5 PCs as covariates, using PLINK v1.07 (http://pngu.mgh.harvard.edu/purcell/plink/).
Genotyping and analysis of BCAC studies is described in detail elsewhere [24], in brief data were analyzed using the Genotype Library and Utilities (GLU) package to estimate per-allele ORs and standard errors for each SNP using unconditional logistic regression. All analyses were performed in subjects of European ancestry (determined by PC analyses) and adjusted for study and seven principal components.
Case-control odds ratio (OR) for ILC or LCIS cases vs controls from BCAC and GLACIER were combined using inverse variance-weighted fixed-effects meta-analysis, as implemented in METAL [39]. Case-only analyses were also carried out to compare genotype frequencies for ILC vs LCIS (GLACIER and BCAC) and ILC vs IDC (BCAC studies only), and were used as a test for heterogeneity of ORs by tumor subtype. Any study without data on both histological subtypes was dropped from the case-only analysis.
Phase II
SNPs showing the strongest evidence for association with lobular tumors (P<5×10−5) in the meta-analysis (after excluding previously reported loci) were genotyped at LGC Genomics (formerly KBiosciences) in Phase II samples. Duplicate samples genotyped on the iCOGS chip were included to assess the concordance of the two genotyping methods. Cluster plots for rs11977670 are shown in Figure S8.
A pooled analysis of ILC including Phase I (GLACIER and BCAC) and Phase II data was performed. Data were analyzed using STATA v.12 to estimate per-allele ORs and standard errors for each SNP using unconditional logistic regression. Differences in the strength of the associations with ILC, IDC and LCIS were assessed using case-only analyses. A sign test was used to test whether the number of SNPs showing associations in the same direction in two different subtypes (i.e. LCIS vs ILC, and IDC vs ILC) was significantly grater than expected by chance. A likelihood ratio test was used as a global test of the null hypothesis of no differences between subtypes for any of the ORs of the 75 known loci evaluated. Stratum-specific estimates of per-allele OR by categories of age and family history of disease were obtained from logistic regression models and differences in ORs across strata were tested using an interaction term.
Bioinformatics
In order to establish the SNP's functional role, a window of 10 kb both up and downstream was formed around the marker and pairwise r2 values calculated using 1000 genome CEU population data. Three SNPs were identified as being in LD (r2>0.5) with rs11977670 and were compared to next generation sequence technologies to elucidate the overlap between chromatin states (ENCODE Project). Two cell lines, normal human mammary epithelial (HMEC), and breast carcinoma (MCF-7), were used to establish these chromatin states, i.e. active or engaged enhancers (H3K27ac), nucleosome-depleted regions (DNase I and FAIRE), and RNA polymerase linked regions (Pol II). Expression data from the Cancer Genome Atlas Network for each gene within a 1 Mb window of rs11977670 was analyzed looking for differential expression in each breast cancer subtype (ER+ ILC, 40 cases; ER+ IDC, 341 cases; and ER-negative IDC, 108 cases). Allele data for surrogate SNP rs13225058 was obtained for all ER+ cases from TCGA. These 335 cases were used to produce genotype specific gene expression data in R. Differences in gene expression between the three genotypes were tested for using one-way-anova, verified by t-test and visually by boxplot. Linear regression was performed across all three genotypes using copy number variation as a co-variate. Level 3 copy number variation data (hg19 build) was obtained from the TCGA data portal.
Supporting Information
Zdroje
1. YoderBJ, WilkinsonEJ, MassollNA (2007) Molecular and morphologic distinctions between infiltrating ductal and lobular carcinoma of the breast. Breast J 13 (2) 172–79.
2. ReevesGK, BeralV, GreenJ, GathaniT (2006) BullD (2006) Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol 7 : 910–918.
3. KotsopoulosJ, ChenWY, GatesMA, TworogerSS, HankinsonSE, et al. (2010) Risk factors for ductal and lobular breast cancer: results from the nurses' health study. Breast Cancer Res 12: R106.
4. PestalozziBC, ZahriehD, MallonE, GustersonBA, PriceKN, et al. (2008) Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol 26 (18) 3006–3014.
5. CristofanilliM, Gonzalez-AnguloA, SneigeN, KauSW, BroglioK, et al. (2005) Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol 1;23 (1) 41–48.
6. Tubiana-HulinM, StevensD, LasryS, GuinebretièreJM, BouitaL, et al. (2006) Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas:. Ann Oncol 17 (8) 1228–1233.
7. HwangES, NyanteSJ, Yi ChenY, MooreD, DeVriesS, et al. (2004) Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer 100 (12) 2562–2572.
8. ChubaPJ, HamreMR, YapJ, SeversonRK, LucasD, et al. (2005) Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol 23 (24) 5534–5541.
9. FisherER, LandSR, FisherB, MamounasE, GilarskiL, et al. (2004) Pathologic findings from the NSABBP: twelve-year observations concerning lobular carcinoma in situ. Cancer 15;100 (2) 238–244.
10. SassonAR, FowbleB, HanlonAL, TorosianMH, FreedmanG, et al. (2001) Lobular carcinoma in situ increases the risk of local recurrence in selected patients with stages I and II breast carcinoma treated with conservative surgery and radiation. Cancer 91 : 1862–1869.
11. EastonDF, PooleyKA, DunningAM, PharoahPD, ThompsonD, et al. (2007) Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447 : 1087–1093.
12. HunterDJ, KraftP, JacobsKB, CoxDG, YeagerM, et al. (2007) A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 39 : 870–874.
13. StaceySN, ManolescuA, SulemP, RafnarT, GudmundssonJ, et al. (2007) Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor–positive breast cancer. Nat Genet 39 : 865–869 (2007).
14. StaceySN, ManolescuA, SulemP, ThorlaciusS, GudjonssonSA, et al. (2008) Common variants on chromosome 5p12 confer susceptibility to estrogen receptor–positive breast cancer. Nat Genet 40 : 703–706.
15. AhmedS, ThomasG, GhoussainiM, HealeyCS, HumphreysMK, et al. (2009) Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41 : 585–590.
16. ZhengW, LongJ, GaoYT, LiC, ZhengY, XiangYB, et al. (2009) Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet 41 : 324–328.
17. ThomasG, JacobsKB, KraftP, YeagerM, WacholderS, et al. (2009) A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat Genet 41 : 579–584.
18. TurnbullC, AhmedS, MorrisonJ, PernetD, RenwickA, et al. (2010) Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 42 : 504–507.
19. AntoniouAC, WangX, FredericksenZS, McGuffogL, TarrellR, et al. (2010) A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor–negative breast cancer in the general population. Nat Genet 42 : 885–892.
20. FletcherO, JohnsonN, OrrN, HoskingFJ, GibsonLJ, et al. (2011) Novel breast cancer susceptibility locus at 9q31.2: results of a genome-wide association study. J Natl Cancer Inst 103 : 425–435.
21. HaimanCA, ChenGK, VachonCM, CanzianF, DunningA, et al. (2011) A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor–negative breast cancer. Nat Genet 43 : 1210–1214.
22. GhoussainiM, FletcherO, MichailidouK, TurnbullC, SchmidtMK, et al. (2012) Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet 44 : 312–318.
23. SiddiqA, CouchFJ, ChenGK, LindströmS, EcclesD, et al. (2012) A meta-analysis of genome-wide association studies of breast cancer identifies two novel susceptibility loci at 6q14 and 20q11. Hum Mol Genet 21 : 5373–5384.
24. MichailidouK, HallP, Gonzalez-NeiraA, GhoussainiM, DennisJ, et al. (2013) Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet 45 : 353–361.
25. Garcia-ClosasM, CouchFJ, LindstromS, MichailidouK, SchmidtMK, et al. (2013) Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet 45 (4) 392–398.
26. FigueroaJD, Garcia-ClosasM, HumphreysM, PlatteR, HopperJL, et al. (2011) Associations of common variants at 1p11.2 and 14q24.1 (RAD51L1) with breast cancer risk and heterogeneity by tumor subtype: findings from the Breast Cancer Association Consortium. Hum Mol Genet 20 (23) 4693–706.
27. Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490 (7418) 61–70.
28. OsawaT, MuramatsuM, WangF, TsuchidaR, KodamaT, et al. (2011) Increased expression of histone demethylase JHDM1D under nutrient starvation suppresses tumor growth via down-regulating angiogenesis. PNAS 108 (51) 20725–20729.
29. IbrahimSA, YipGW, StockC, PanJW, NeubauerC, et al. (2012) Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase - and E-cadherin-dependent mechanism. Int J Cancer 131 (6) E884–896.
30. PharoahPD, GuilfordP, CaldasC (2001) International Gastric Cancer Linkage Consortium (2001) Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 121 (6) 1348–53.
31. XieZM, LiLS, LaquetC, Penault-LlorcaF, UhrhammerN, et al. (2011) Germline mutations of the E-cadherin gene in families with inherited invasive lobular breast carcinoma but no diffuse gastric cancer. Cancer 117 (14) 3112–3117.
32. WeigeltB, HorlingsHM, KreikeB, HayesMM, HauptmannM, et al. (2008) Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol 216 (2) 141–150.
33. MilneRL, GoodeEL, García-ClosasM, CouchFJ, SeveriG, et al. (2011) Confirmation of 5p12 as a susceptibility locus for progesterone-receptor-positive, lower grade breast cancer. Cancer Epidemiol Biomarkers Prev 10 : 2222–2231.
34. PharoahPD, TsaiYY, RamusSJ, PhelanCM, GoodeEL, et al. (2013) GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet 45 : 362–370.
35. DobbinsSE, BroderickP, MelinB, FeychtingM, JohansenC, et al. (2011) Common variation at 10p12.31 near MLLT10 influences meningioma risk. Nat Genet 43 (9) 825–7.
36. Curtis RE, Benjamin ER, Hankey BF, Hoover RN. New Malignancies Following Breast Cancer. Seer Cancer Registry. Available: http://www.seer.cancer.gov/publications/
37. CusterBS, KoepsellTD, MuellerBA (2002) The association between breast carcinoma and meningioma in women. Cancer 94 (6) 1626–1635.
38. SwannR, PerkinsKA, VelentzisLS, CiriaC, DuttonSJ, et al. (2013) The DietCompLyf study: A prospective cohort study of breast cancer survival and phytoestrogen consumption. Maturitas 3 : 232–240.
39. WillerCJ, LiY, AbecasisGR (2010) METAL: fast and efficient meta-analysis of genome wide association scans. Bioinformatics 26 : 2190–2191.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 4
-
Všechny články tohoto čísla
- The Challenges of Mitochondrial Replacement
- Concocting Cholinergy
- Genome-Wide Diet-Gene Interaction Analyses for Risk of Colorectal Cancer
- Statistical Power to Detect Genetic (Co)Variance of Complex Traits Using SNP Data in Unrelated Samples
- Mouse Pulmonary Adenoma Susceptibility 1 Locus Is an Expression QTL Modulating -4A
- Transcription-Associated R-Loop Formation across the Human CGG-Repeat Region
- Epigenetic Regulation by Heritable RNA
- Protein Quantitative Trait Loci Identify Novel Candidates Modulating Cellular Response to Chemotherapy
- Genome-Wide Profiling of Yeast DNA:RNA Hybrid Prone Sites with DRIP-Chip
- The Mechanism of Gene Targeting in Human Somatic Cells
- A LINE-1 Insertion in DLX6 Is Responsible for Cleft Palate and Mandibular Abnormalities in a Canine Model of Pierre Robin Sequence
- Interaction between Two Timing MicroRNAs Controls Trichome Distribution in
- DNA Glycosylases Involved in Base Excision Repair May Be Associated with Cancer Risk in and Mutation Carriers
- The Myc-Mondo/Mad Complexes Integrate Diverse Longevity Signals
- Evolutionarily Diverged Regulation of X-chromosomal Genes as a Primal Event in Mouse Reproductive Isolation
- Mutations in Conserved Residues of the microRNA Argonaute ALG-1 Identify Separable Functions in ALG-1 miRISC Loading and Target Repression
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Isl1 Directly Controls a Cholinergic Neuronal Identity in the Developing Forebrain and Spinal Cord by Forming Cell Type-Specific Complexes
- A Synthetic Community Approach Reveals Plant Genotypes Affecting the Phyllosphere Microbiota
- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- Determining the Control Circuitry of Redox Metabolism at the Genome-Scale
- DNA Repair Pathway Selection Caused by Defects in , , and Telomere Addition Generates Specific Chromosomal Rearrangement Signatures
- Methylome Diversification through Changes in DNA Methyltransferase Sequence Specificity
- Folliculin Regulates Ampk-Dependent Autophagy and Metabolic Stress Survival
- Fine Mapping of Dominant -Linked Incompatibility Alleles in Hybrids
- Unexpected Role of the Steroid-Deficiency Protein Ecdysoneless in Pre-mRNA Splicing
- Three Groups of Transposable Elements with Contrasting Copy Number Dynamics and Host Responses in the Maize ( ssp. ) Genome
- Sox5 Functions as a Fate Switch in Medaka Pigment Cell Development
- Synergistic Interactions between the Molecular and Neuronal Circadian Networks Drive Robust Behavioral Circadian Rhythms in
- Chromatin Landscapes of Retroviral and Transposon Integration Profiles
- Widespread Use of Non-productive Alternative Splice Sites in
- Ras GTPase-Like Protein MglA, a Controller of Bacterial Social-Motility in Myxobacteria, Has Evolved to Control Bacterial Predation by
- Cell Type-Specific Functions of Genes Revealed by Novel Adipocyte and Hepatocyte Circadian Clock Models
- Phenotype Ontologies and Cross-Species Analysis for Translational Research
- Embryogenesis Scales Uniformly across Temperature in Developmentally Diverse Species
- In Pursuit of the Gene: An Interview with James Schwartz
- Molecular Mechanisms of Hypoxic Responses via Unique Roles of Ras1, Cdc24 and Ptp3 in a Human Fungal Pathogen
- Analysis of the Genome and Transcriptome of var. Reveals Complex RNA Expression and Microevolution Leading to Virulence Attenuation
- Genotypic and Functional Impact of HIV-1 Adaptation to Its Host Population during the North American Epidemic
- RNA Editome in Rhesus Macaque Shaped by Purifying Selection
- Proper Actin Ring Formation and Septum Constriction Requires Coordinated Regulation of SIN and MOR Pathways through the Germinal Centre Kinase MST-1
- Interplay of the Serine/Threonine-Kinase StkP and the Paralogs DivIVA and GpsB in Pneumococcal Cell Elongation and Division
- A Quality Control Mechanism Coordinates Meiotic Prophase Events to Promote Crossover Assurance
- CNNM2 Mutations Cause Impaired Brain Development and Seizures in Patients with Hypomagnesemia
- The RNA-Binding Protein QKI Suppresses Cancer-Associated Aberrant Splicing
- Uncoupling Transcription from Covalent Histone Modification
- Rad51–Rad52 Mediated Maintenance of Centromeric Chromatin in
- FRA2A Is a CGG Repeat Expansion Associated with Silencing of
- A General Approach for Haplotype Phasing across the Full Spectrum of Relatedness
- A Novel Highly Divergent Protein Family Identified from a Viviparous Insect by RNA-seq Analysis: A Potential Target for Tsetse Fly-Specific Abortifacients
- A Central Role for in Regulation of Islet Function in Man
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- The Mechanism of Gene Targeting in Human Somatic Cells
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Widespread Use of Non-productive Alternative Splice Sites in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



