-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDNA Repair Pathway Selection Caused by Defects in , , and Telomere Addition Generates Specific Chromosomal Rearrangement Signatures
Recent advances in the sequencing of human cancer genomes have revealed that some types of genome rearrangements are more common in specific types of cancers. Thus, these cancers may share defects in DNA repair mechanisms, which may play roles in initiation or progression of the disease and may be useful therapeutically. Linking a common rearrangement signature to a specific genetic or epigenetic alteration is currently challenging, because we do not know which rearrangement signatures are linked to which DNA repair defects. Here we used a genetic assay in the model organism Saccharomyces cerevisiae to specifically link two classes of chromosomal rearrangements, interstitial deletions and inverted duplications, to specific genetic defects. These results begin to map out the links between observed chromosomal rearrangements and specific DNA repair defects and in the present case, may provide insights into the chromosomal rearrangements frequently observed in metastatic pancreatic cancer.
Published in the journal: . PLoS Genet 10(4): e32767. doi:10.1371/journal.pgen.1004277
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004277Summary
Recent advances in the sequencing of human cancer genomes have revealed that some types of genome rearrangements are more common in specific types of cancers. Thus, these cancers may share defects in DNA repair mechanisms, which may play roles in initiation or progression of the disease and may be useful therapeutically. Linking a common rearrangement signature to a specific genetic or epigenetic alteration is currently challenging, because we do not know which rearrangement signatures are linked to which DNA repair defects. Here we used a genetic assay in the model organism Saccharomyces cerevisiae to specifically link two classes of chromosomal rearrangements, interstitial deletions and inverted duplications, to specific genetic defects. These results begin to map out the links between observed chromosomal rearrangements and specific DNA repair defects and in the present case, may provide insights into the chromosomal rearrangements frequently observed in metastatic pancreatic cancer.
Introduction
Large numbers of complex chromosomal rearrangements (called gross chromosomal rearrangements or GCRs) are seen in many cancers, potentially due to ongoing genome instability. Much of our present knowledge on the genome rearrangements seen in cancer is from cytogenetic observations of large-scale genome rearrangements and processes associated with their formation. Some examples include cytogenetically observable genome rearrangements that appear to be triggered by dicentric chromosomes undergoing cycles of bridge-fusion-breakage [1]–[3] or breakage of chromosomes by anaphase bridges that have been observed in early stages of carcinogenesis [4] and in cells containing defects in cancer susceptibility genes like BLM [5]. The advent of genomics methods including whole-genome next generation sequencing of the genomes from tumors and paired normal tissue has greatly expanded the information available about the kinds of somatic GCRs present in cancers. Interestingly, some types of GCRs may be specifically enhanced in subsets of cancer, including retrotransposition events in colorectal cancers [6], inversions in pancreatic cancer [7], tandem duplications in ovarian and triple-negative breast cancer [8], [9], and focal copy number changes in ovarian cancer [10]. The presence of these rearrangements in a subset of cancers of a specific type suggests that the genetic background in different cancers may influence the mechanisms of GCRs formation. The limited understanding of the types of genetic defects that affect GCR formation and the enormous genetic variation seen in many cancers pose challenges to understanding the influence of genetic background on the types of GCRs seen and their rates of formation.
Quantitative measurements of the accumulation of GCRs in the yeast Saccharomyces cerevisiae have been useful for identifying pathways that normally suppress the formation of GCRs. These measurements have typically measured the loss of genetic markers present on a non-essential terminal region of chromosomes in haploid strains [11]–[15]. This feature of these assays allows the formation of different types of GCRs by a diversity of mechanisms depending on the assay and the genotype of the strain used. The types of GCRs observed include terminal deletions healed by de novo telomere addition, simple monocentric translocations including the formation of circular chromosomes, and complex GCRs that are initiated by the formation of dicentric translocations and end-to-end chromosome fusions followed by multi-step rearrangements that resolve the initial dicentric translocations to monocentric GCRs [11], [14], [16]–[20].
During the analysis of GCRs formed in assays utilizing a CAN1/URA3 cassette placed at various locations along the left arm of chromosome V (chrV L; [14], [21]), we noticed that a high proportion of GCRs in some mutants, including tel1Δ, sae2Δ, mrc1Δ tof1Δ and rad53Δ sml1Δ, retained a hygromycin resistance marker (hph) present on the assay chromosome telomeric to the CAN1/URA3 cassette. We initially characterized the GCRs formed in the tel1Δ mutant, which lacks the gene encoding a DNA damage checkpoint protein kinase that is important for telomere maintenance [16], [22]–[25], and determined that hph-retention was due to the formation of interstitial deletions by non-homologous end-joining (NHEJ) or by formation of inverted duplications that were then resolved by homologous recombination (HR) between the ura3-52 allele (a Ty element insertion at the URA3 locus in the duplicated region) and URA3 in the CAN1/URA3 cassette. Both types of hph+ products were observed in wild-type strains, but at much lower frequencies than in the tel1Δ mutant. Importantly, the hph − GCRs formed in the tel1Δ mutant were also associated with increased frequencies of inverted duplications that differed from the hph+ GCRs only with respect to the homologies used for telomere capture. Deletion of SAE2 also caused an increase in hph retention. However, unlike the tel1Δ mutation, this increase was solely due to increased levels of inverted duplications. Detailed analysis of the interactions between tel1 and sae2 single mutations and mutations affecting the Mre11-Rad50-Xrs2 (MRX) complex, which functions in the resection of DNA at double-stranded breaks (DSBs), or the DNA damage checkpoint revealed that complex interactions between repair pathways promote the formation of specific rearrangements. Furthermore, genetic defects that suppressed de novo telomere addition increased hph retention, whereas genetic defects that increased de novo telomere addition decreased hph retention. Together, these results suggest a mechanism by which Tel1, Sae2, and de novo telomere addition play a role in suppressing inverted duplications and, in some cases, interstitial deletions, and further demonstrate that defects in these pathways/genes result in GCRs with a specific structural signature.
Results
Retention of telomeric DNA in GCRs is assay - and genotype-specific
Two GCR assays on chrV that incorporated a telomeric hygromycin resistance marker (hph) (Figure 1A and B) [14] were used to characterize the GCR rate and the frequency of GCRs retaining hph in over 95 mutant strains [14], [21]. The yel072w::CAN1/URA3 GCR (dGCR) assay primarily mediates GCRs by duplication-mediated rearrangements with chromosomes IV, X, and XIV; the GCRs derived using this assay frequently lost the telomeric portion of chrV that includes the hph marker [14], [21]. Consistent with this, 0 of 62 GCRs (0%) derived in the wild-type dGCR assay strain and 15 of 2435 GCRs (0.6%) formed in all tested dGCR assay strains retained hph. In contrast, the frequency of hph retention was higher in the GCRs formed in the yel068c::CAN1/URA3 GCR (uGCR) assay, which mediates GCRs by single copy or “unique” genomic sequences. In the wild-type uGCR assay strain, 2 of the 27 GCR-containing isolates (7%) retained hph, and 367 of 2670 GCRs (14%) formed in all tested uGCR assay strains retained the hph marker. Specific mutations significantly increased the frequency of hph+ GCRs relative to wild type (Figure 1C). These mutations included tel1Δ (58% hph+; p = 3×10−13, G-test), sae2Δ (50% hph+; p = 2×10−9, G-test), rad53Δ sml1Δ (31% hph+; p = 7×10−6, G-test), and mrc1Δ tof1Δ (64% hph+; p = 6×10−8, G-test).
Fig. 1. Biased distribution of GCRs retaining hph. 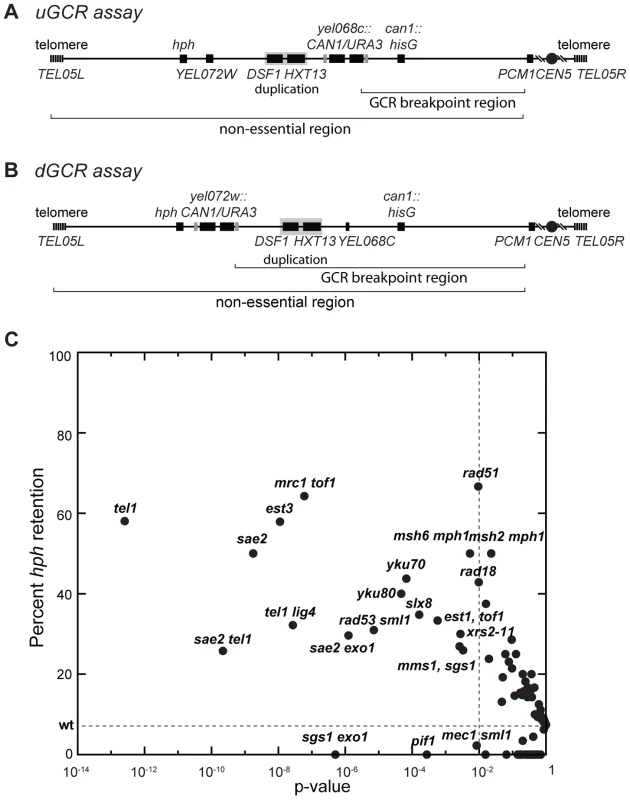
(A and B) Schematic showing the positions of the CAN1/URA3 cassette in the uGCR and dGCR assays relative to the 4.2 kb HXT13-DSF1 segmental duplication on chrV. The GCR breakpoint region (horizontal bracket) is the region in which rearrangements must occur to lose CAN1/URA3 cassette but not the essential gene PCM1. (C) Plot of the percent retention of hph in the uGCR assay in various mutant backgrounds against the respective p-value for retention (G-test) using the wild-type distribution (2 of 27) as the expected distribution. These data include strains generated and analyzed in this study. Points to the left of the vertical dashed line correspond to mutations with p-values<0.01. The horizontal dashed line is the frequency of hph retention in the wild-type uGCR assay strain. hph+ uGCRs from the tel1Δ strain are either interstitial deletions or inverted duplications
We characterized 18 hph+ GCRs isolated from the tel1Δ uGCR assay strain by pulsed-field gel electrophoresis (PFGE) and Southern blotting. Probes complementary to hph and to MCM3, which is an essential gene on chrV, hybridized to the same band in lanes with undigested chromosomes (Figures 2 and S1), indicating that hph was retained on chrV. The size of chrV was similar to wild-type in 8 isolates and was larger than wild-type in 10 isolates. Digestion of chrV by AscI generates three fragments from the starting chromosome: the left telomeric fragment contains hph sequence, the internal fragment contains both hph and MCM3 sequence, and the right telomeric fragment has neither hph nor MCM3 sequence (Figure 2A). In all cases with a larger than wild-type chrV, the change in size appeared to be due to changes in the internal AscI fragment (Figure 2B and C; Figure S1).
Fig. 2. GCRs retaining hph belong to two size classes. 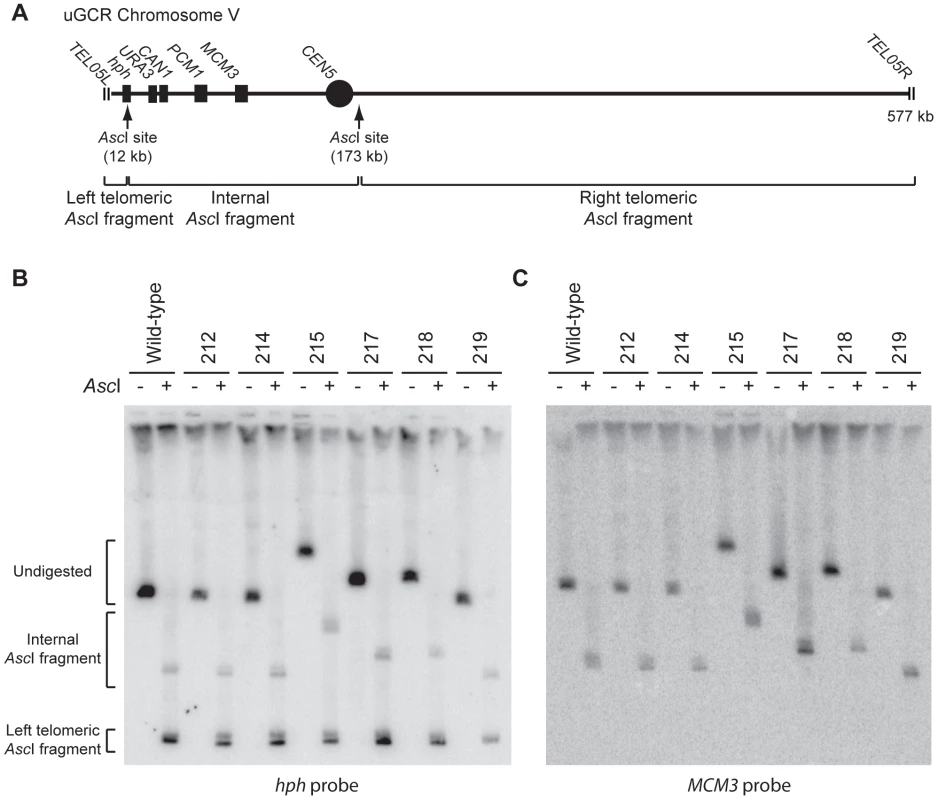
(A) Digestion of the uGCR chrV divides the uGCR chrV into left telomeric, internal, and right telomeric fragments. Vertical arrows indicate the AscI cleavage sites and relevant chromosomal features are labeled. (B) Southern blot using an hph probe of a pulsed-field gel (PFG) with DNA from the wild-type strain (RDKY6677) and 6 GCR-containing isolates (212, 214, 215, 217, 218, and 219) with and without AscI digestion. The hph probe hybridizes to the intact chromosome and the internal and left telomeric fragments. (C) Southern blot of a second PFG with the same samples as in panel B using an MCM3 probe. The MCM3 probe hybridizes to the intact chromosome and the internal fragment. Analysis of the 8 hph+ GCRs with a wild-type-sized chrV revealed that they all contained interstitial deletions. We used PCR to map and amplify the rearrangement breakpoints. Sanger sequencing of the PCR products revealed the presence of interstitial deletions that spanned the CAN1/URA3 cassette (Figure 3A and B) and had short sequence identities at the breakpoint junctions (0–5 basepairs in length; Figure S2), consistent with previous observations [18]. In addition, isolate 214 contained an insertion of a ∼4 kb fragment of a Ty retrotransposon at the breakpoint. Lack of copy number changes other than the interstitial deletion was verified by array comparative genomic hybridization (aCGH) of isolate 3118 (Figure 3C). Paired-end whole genome sequencing (WGS) of isolate 3118 (Table S1 and S2) confirmed the interstitial deletion by the identification of 572 read pairs (‘junction-defining’ read pairs) that had mapped inter-read distances of ∼5.4 kb as compared to the median mapped inter-read distance of 417 bp for all 11,333,616 uniquely mapping read pairs (Figure S3). Additionally, alignment of 114 unmapped reads, which were paired with a read that mapped adjacent to the junction-defining read pairs (‘junction-sequencing’ reads), identified the same junction sequence observed by PCR amplification and Sanger sequencing (Figure S3).
Fig. 3. hph+ GCRs associated with wild-type-sized chrV are interstitial deletions. 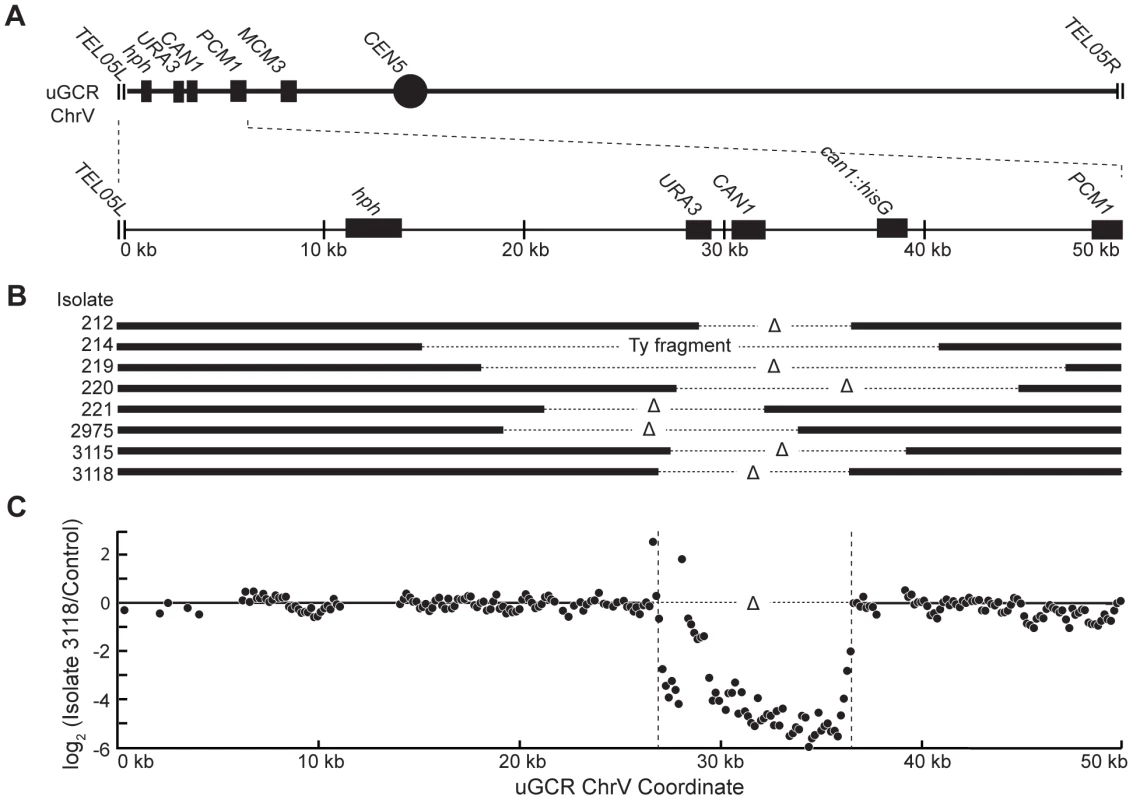
(A) Diagram of the uGCR chrV and the features on the first 50 kb containing hph, the CAN1/URA3 cassette and the GCR breakpoint region. (B) Map of the retained (solid bar) and deleted (dotted line) regions for the 8 hph+ GCR isolates with wild-type-sized chrV. Interstitial deletions on chrV entirely (isolates 214, 219, 220, 2975, 3115, and 3118) or partially (isolates 212 and 221) spanned the CAN1/URA3 cassette. All of the isolates are simple deletions, indicated by a Δ symbol, other than 214, which is fused to a fragment of a Ty element. (C) The log base 2 ratio of the aCGH hybridization intensity for a portion of chrV L from isolate 3118 illustrating the agreement between aCGH and sequenced junctions. The coordinates are mapped to the “uGCR Chromosome V” of RDKY6677, which differs somewhat from the database S288c sequence due to modifications introduced onto chrV during strain construction. No data are present for the hph and can1::hisG insertions because these regions were not probed by the aCGH array. The 10 hph+ GCRs with a large chrV were inverted duplications associated with a second homology-mediated rearrangement. aCGH analysis revealed that in the strains containing these GCRs all of the copy number changes detected were restricted to chrV: the changes associated with these GCRs included a ∼4–19 kb chrV L deletion spanning the CAN1/URA3 cassette, and a ∼80–100 kb chrV L duplication extending from the GCR breakpoint region, which is bounded by the CAN1/URA3 cassette and PCM1 (Figure 1A), to a centromeric repetitive element, which was most frequently the Ty-containing ura3-52 (Figure 4A). In each case, the aCGH data also indicated that the GCRs retained the hph-containing region of chrV from TEL05L to the telomeric half of YEL068C, consistent with HR-mediated fusion between ura3-52 and URA3 in the CAN1/URA3 cassette. We verified the ura3-52/URA3 fusion by PCR amplification and Sanger sequencing (Figure S4). WGS of 8 isolates (Table S1) identified and sequenced an inversion junction at the telomeric end of the chrV L duplication (Figure S5; Table S2). If these junctions were formed by folding back and priming of a single strand (Figure 4B), then the homologies for priming were 3–9 bases and the unpaired single-stranded hairpin ranged from 25 to 44 bases (Figure S5). Palindromes are typically difficult to amplify, which may explain the reduced number of junction-defining read pairs recovered for the inversion junctions relative to other rearrangement junctions introduced during strain construction (Table S2). The analyses of these inverted duplication GCRs were consistent with the changes observed by PFGE (Figure 2B and C; Figure S1), because the duplicated regions lacked AscI sites, and the rearranged chromosomes were capped by the AscI-containing left telomeric fragment.
Fig. 4. hph+ GCRs associated with chrV larger than wild-type contain duplicated chrV sequences. 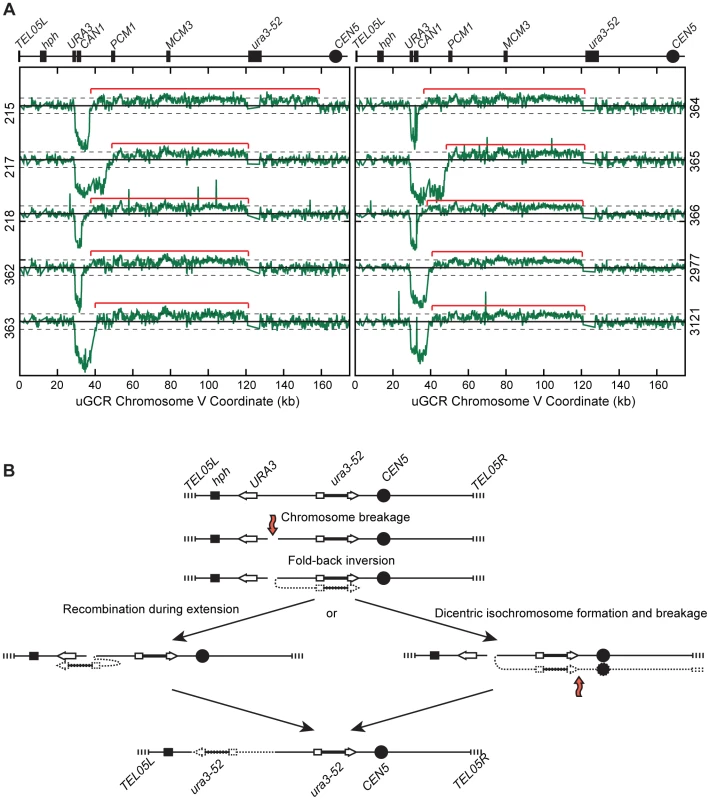
(A) The log base 2 ratio of the aCGH hybridization intensity for chrV L of hph+ isolates with larger than wild-type-sized chrV. The solid horizontal bar is at 0 and dashed lines are at −1 and 1 (2-fold decreased and increased, respectively). Probes were mapped onto the “uGCR Chromosome V” coordinate system. Chromosomal features such as hph, the CAN1/URA3 cassette, the ura3-52 mutation, and the centromere (CEN5) are indicated at top. Red brackets indicate duplicated chromosomal regions that span from the GCR breakpoint region (between the CAN1/URA3 cassette and PCM1) to a Ty-related element, most frequently ura3-52. (B) Proposed mechanism for rearrangement formation (see Discussion). Orange arrows indicate DSBs. Some hph − rearrangements isolated using the tel1Δ uGCR assay are inverted duplications
PFGE analysis of the 13 hph − GCR-containing isolates from the tel1Δ uGCR assay strain revealed that 9 contained a wild-type-sized chrV and 4 contained a large chrV (Figure S6A). PCR mapping [26] revealed that the 9 isolates with wild-type-sized chrV had deletions that included the CAN1/URA3 cassette; sequencing the breakpoints of 4 of these GCRs confirmed that one was a translocation and 3 were de novo telomere additions (Figure S6B). In contrast, aCGH analysis of the 4 isolates with a larger than wild-type chrV (Figure 5A–C) was consistent with a chrV inverted duplication combined with rearrangements targeting homologies unrelated to URA3: these GCRs contained a chrV L deletion from the telomere to the GCR breakpoint region (Figure 5A), a chrV L duplication from the GCR breakpoint region to a Ty-related repetitive element (Figure 5A), and an additional duplication of at least one other additional genomic region bounded by Ty-related elements and telomeres (Figure 5B and C). Isolate 3125 had two duplicated regions (between the inverted Ty pairs YDRWTy2-2/YDRCTy1-2 and YDRWTy2-3/YDRCTy1-3 and between YDRWTy1-5 and TEL04R), which was consistent with a mechanism involving more than one round of HR-mediated rearrangements similar to GCRs obtained using other GCR assays [15], [20]. The inversion junctions were identified and sequenced by analysis of WGS data from isolates 3124 and 3125 (Figure S5; Table S1 and S2). Thus, the hph − inverted duplications differed from the hph+ inverted duplications only with regard to the homologies involved in the resolution of the initial inversion chromosome (Figure 5D).
Fig. 5. hph− GCRs associated with chrV larger than wild-type contain duplicated chrV sequences. 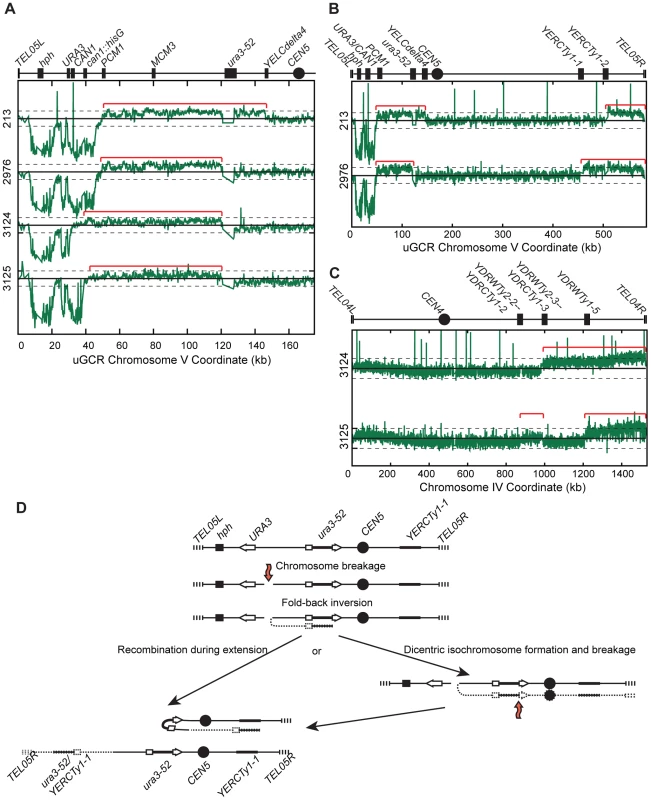
(A) The log base 2 ratio of the aCGH hybridization intensity for chrV L for hph− isolates with chrV larger than wild-type. The solid horizontal bar is at 0 and dashed lines are at −1 and 1 (2-fold decreased and increased, respectively). Probes were mapped onto the “uGCR Chromosome V” coordinate system. Chromosomal features such as hph, the CAN1/URA3 cassette, the ura3-52 mutation, and the centromere (CEN5) are indicated at top. Red brackets indicate duplicated chromosomal regions that span from the GCR breakpoint region (between the CAN1/URA3 cassette and PCM1) to a Ty-related element, most frequently ura3-52. (B) The log base 2 ratio of aCGH hybridization intensity for all of chrV for isolates 213 and 2976. Red brackets indicate duplicated chromosomal regions. (C) The log base 2 ratio of aCGH hybridization intensity for all of chrIV for isolates 3124 and 3125. Red brackets indicate duplicated chromosomal regions. (D) Proposed mechanism for rearrangement formation (see Discussion). Orange arrows indicate DSBs. Detection of chrV L duplications by a multiplex ligation-mediated probe amplification (MLPA) assay
Because inverted duplications could form with or without hph retention, we developed an MLPA probe set [27], [28] to identify chrV L duplications. MLPA results were validated by comparison with aCGH data for isolates 213, 217, 362, and 3178 (Figure S7). Using MLPA we verified that the 9 hph − GCRs with a wild-type sized chrV from the tel1Δ uGCR assay strain lacked a chrV L duplication. The aggregate data indicated that 14 of 31 GCRs isolated in the tel1Δ uGCR assay strain contained chrV L duplications consistent with inverted duplications (Table 1), whereas the remaining 17 GCRs lacked chrV L duplications and were consistent with interstitial deletions, de novo telomere additions, or translocations.
Tab. 1. Count of GCR events with and without duplication of the left arm of chromosome V from the uGCR assay. 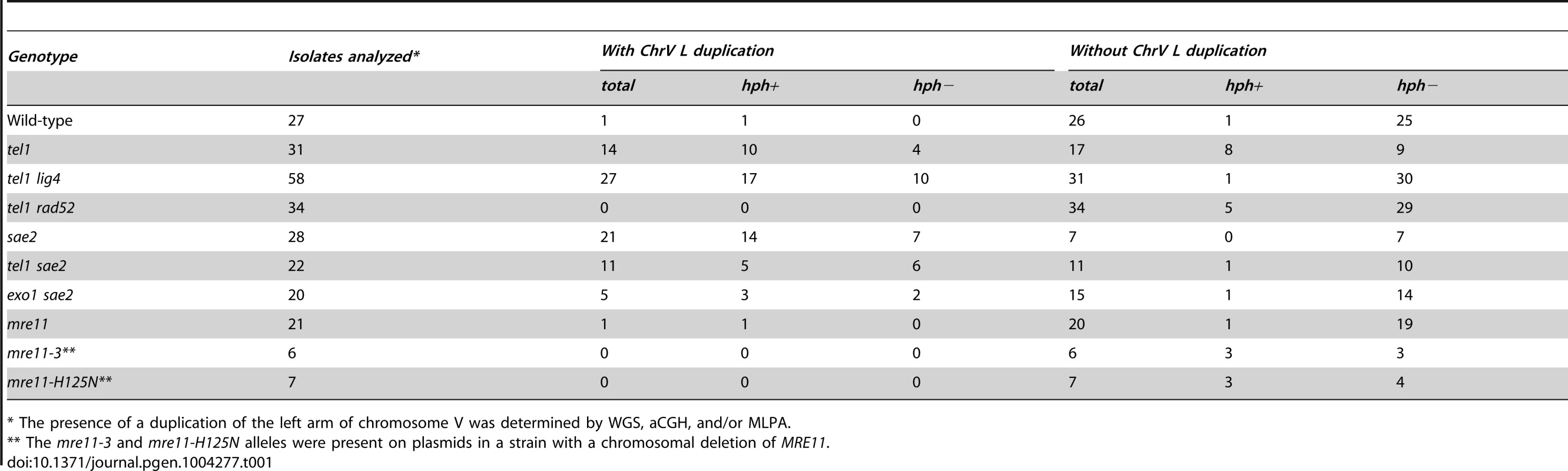
* The presence of a duplication of the left arm of chromosome V was determined by WGS, aCGH, and/or MLPA. NHEJ is required for efficient formation of hph+ interstitial deletions
To investigate the mechanisms of GCR formation, we first tested the effect of a lig4Δ mutation, which causes an NHEJ defect [29]. In the dGCR assay, lig4Δ caused a modest increase in GCR rate (Table 2), and the tel1Δ lig4Δ double mutation caused a higher rate in the dGCR assay relative to each single mutation (p = 0.0003 and p = 0.0016, respectively, Mann-Whitney test). The lig4Δ mutation did not affect the GCR rate or hph+ retention in the uGCR assay, but the tel1Δ lig4Δ double mutation modestly decreased the GCR rate and increased the frequency of hph retention relative to the wild-type strain (p = 2×10−8, G-test). The most striking change in the uGCR product spectrum of the tel1Δ lig4Δ strain relative to the tel1Δ strain was the lack of GCRs that did not contain a chrV L duplication that retained hph (Table 1). Eighteen of 19 hph+ GCRs from the tel1Δ lig4Δ uGCR assay strain belonged to the inverted duplication class of GCRs: these GCRs had a chrV that was larger than wild-type, fusion of ura3-52 with URA3 (data not shown), and a chrV L duplication (Table 1). Thus, the major mechanism forming the interstitial deletion class of hph+ GCRs is NHEJ, which is consistent with the short homologies found at the interstitial deletion breakpoints (Figure S2).
Tab. 2. GCR rates and percent hph retention in tel1, sae2, and related mutants. 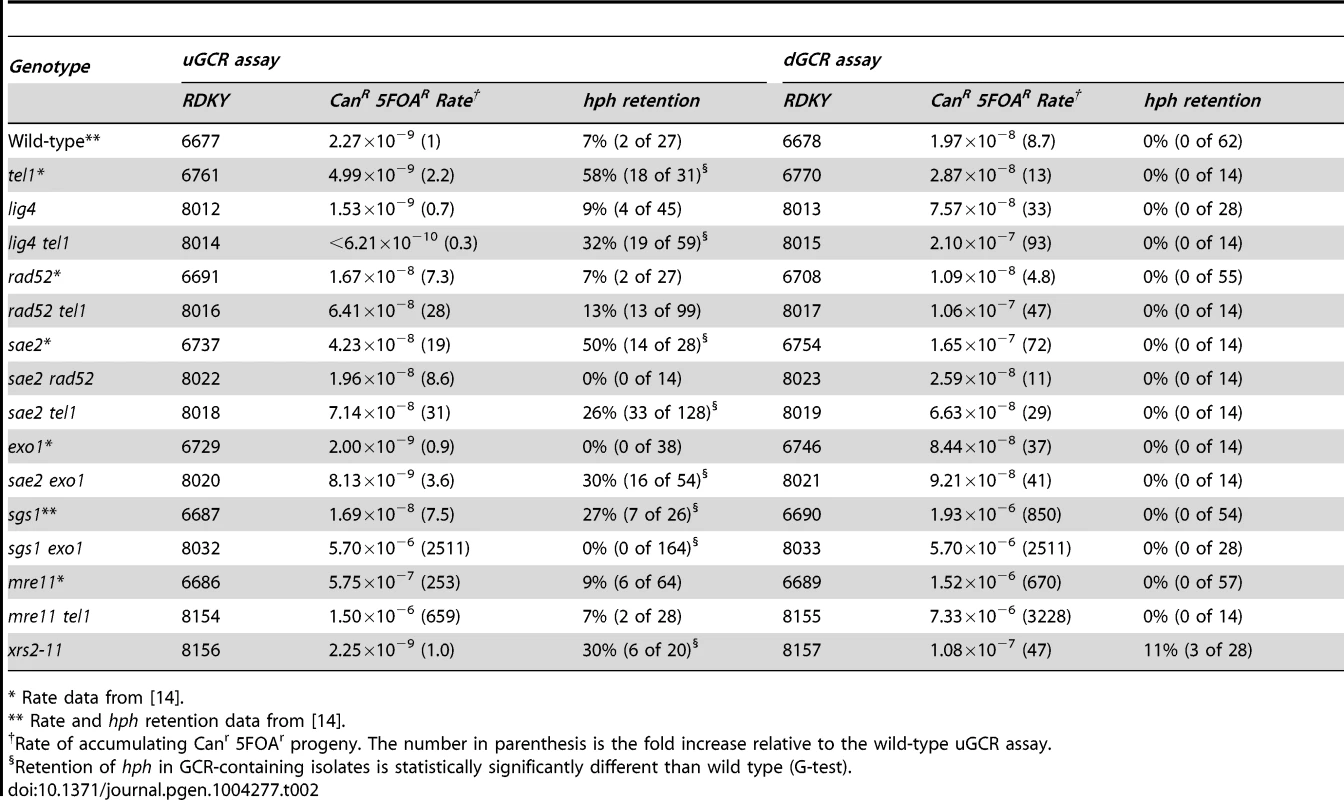
* Rate data from [14]. HR is required for efficient formation of hph+ inverted duplications
Because the inverted duplication class of GCRs involved homology-mediated rearrangements, we tested the effect of a rad52Δ mutation that eliminates HR. In the dGCR assay, a rad52Δ mutation suppressed the GCR rates [14], and the tel1Δ rad52Δ double mutant had modestly increased GCR rates relative to both single mutants (Table 2). The rad52Δ and the tel1Δ rad52Δ mutants had higher GCR rates in the uGCR assay, but had no significant increase in the frequency of hph retention relative to the wild-type strain (p = 1.0 and 0.5, respectively, G-test; Table 1). Analyses of the 13 hph+ GCRs from the rad52Δ tel1Δ uGCR assay strain was consistent with these GCRs belonging to the interstitial deletion class of GCRs: these GCRs had wild-type-sized chrV, no ura3-52/URA3 fusions (data not shown), and none of the 5 hph+ isolates tested by MLPA had a chrV L duplication (Table 1). These data suggest that HR mediates a key step in the formation of the inverted duplication class of GCRs, likely by the formation of stable monocentric chromosomes (Figure 4B and 5D).
Interstitial deletions and inverted duplications are formed in the wild-type uGCR assay strain
Two of 27 GCRs formed in the wild-type uGCR assay strain retained hph+ (isolates 3178 and 3255). The GCR in isolate 3255 was an interstitial deletion: chrV was of wild-type size with no chrV L duplication, and WGS identified an interstitial deletion (Table 1; Figure S8A, B, and D; Table S1 and S2). The GCR in isolate 3178 was an inverted duplication: chrV was larger than wild-type due to a change in the size of the central AscI fragment; it contained a chrV L duplication extending from the GCR breakpoint region to ura3-52; a ura3-52/URA3 breakpoint junction was present that could be amplified by PCR; and an inversion junction was present that was identified by WGS (Table 1, S1, and S2; Figure S4, S5G, and S8A–C). The remaining 25 hph − GCRs formed in the wild-type uGCR assay strain were hph − GCRs that lacked chrV L duplications (Table 1). Thus, both the interstitial deletion and inverted duplication classes of hph+ GCRs were observed with the wild-type uGCR assay strain, suggesting that deletion of TEL1 changes the efficiency rather than the pathways by which these GCRs are formed.
DNA hairpins are likely intermediates in the formation of inverted duplications
The structures of the inverted duplication GCRs were consistent with the formation of single-stranded hairpins (Figure S5), but do not rule out interchromosomal Break-Induced Replication (BIR) events occurring after DNA replication [30], [31]. Because hairpin-capped duplexes are substrates for Sae2-promoted cleavage [32]–[34], we determined the effect of deleting SAE2 on hph retention in the uGCR assay. Half of the GCRs from the sae2Δ uGCR assay strain were hph+ (14 of 28 isolates; p = 2×10−9, G-test). All 14 hph+ isolates and 7 of 14 hph − isolates contained GCRs that were consistent with the inverted duplication class of GCRs: all had a chrV that was larger than wild-type and had chrV L duplications as measured by MLPA (Table 1). Additionally, 13 of the 14 hph+ isolates had a URA3/ura3-52 fusion (data not shown). These results support the hypothesis that the formation of the inverted duplication class of GCRs involves a DNA hairpin intermediate. Deletion of RAD52 eliminated the sae2Δ-mediated increase in the frequency of hph+ GCRs in the uGCR assay (Table 2), which is consistent with the importance of RAD52 for the HR-dependent event that occurs after inversion formation and stabilizes the inverted duplication GCRs formed in the tel1Δ uGCR assay strain.
TEL1 promotes the formation of the hph+ inverted duplication class of GCRs in sae2Δ mutants
Mec1 and Tel1 promote Sae2 activity by phosphorylation [35]–[37]. To test if TEL1 and SAE2 function in the same pathway in the formation of hph+ GCRs, we generated tel1Δ sae2Δ double mutant strains. In the dGCR assay, the double mutant had a 2.5-fold lower GCR rate relative to the sae2Δ single mutant (p = 0.0001, Mann-Whitney test) and a 2.3-fold higher GCR rate relative to the tel1Δ single mutant (p = 0.006; Table 2). In the uGCR assay, the double mutant had a 1.7-fold higher GCR rate relative to the sae2Δ single mutant, and the frequency of hph retention was lower than seen in both the tel1Δ and sae2Δ single mutant strains (p = 1×10−13 and p = 2×10−8, respectively, G-test), but still was higher than wild-type (p = 2×10−10; Table 2). MLPA analysis of hph+ and hph − GCRs from the tel1Δ sae2Δ uGCR assay strain revealed that 50% (11 of 22) contained chrV L duplications, and the frequency of hph+ GCRs without chrV L duplications (probable interstitial deletions), like the case of the sae2Δ strain, was much lower than seen with the tel1Δ strain (Table 1). These data suggest that TEL1 is not required for the formation of chrV L inverted duplications in the sae2Δ uGCR assay strain, but does promote the formation of chrV L inverted duplications associated with hph retention.
Mutations affecting Sae2 phosphorylation sites do not cause increased hph retention
We then tested the ability of different plasmid-borne phosphorylation-defective alleles of SAE2 to complement the sae2Δ mutation (Table 3). In the dGCR assay, the sae21–9 and sae22,4,5,8,9 alleles, which eliminated multiple Mec1 and Tel1 phosphorylation sites [35], either did not or partially suppressed the increased GCR rate caused by deleting SAE2. In the uGCR assay, these sae2 alleles partially complemented the increased GCR rate and decreased the hph retention observed in the sae2Δ single mutant. Sae2 is also phosphorylated by the Cdc28 cyclin-dependent kinase at Ser267, and the sae2-S267A mutation is phenotypically similar to a sae2Δ single mutant [38]. The sae2-S267A allele did not suppress the higher GCR rate of the sae2Δ mutation in either GCR assay; however, the sae2-S267A allele did not cause increased hph retention in the uGCR assay. The lack of hph retention in strains containing these sae2 phosphorylation-defective alleles suggests that these alleles are not simply null mutations but additionally disrupt hph retention potentially by affecting Tel1 signaling or by affecting the capture of the acentric hph-containing fragment.
Tab. 3. GCR formation in plasmid-complemented strains. 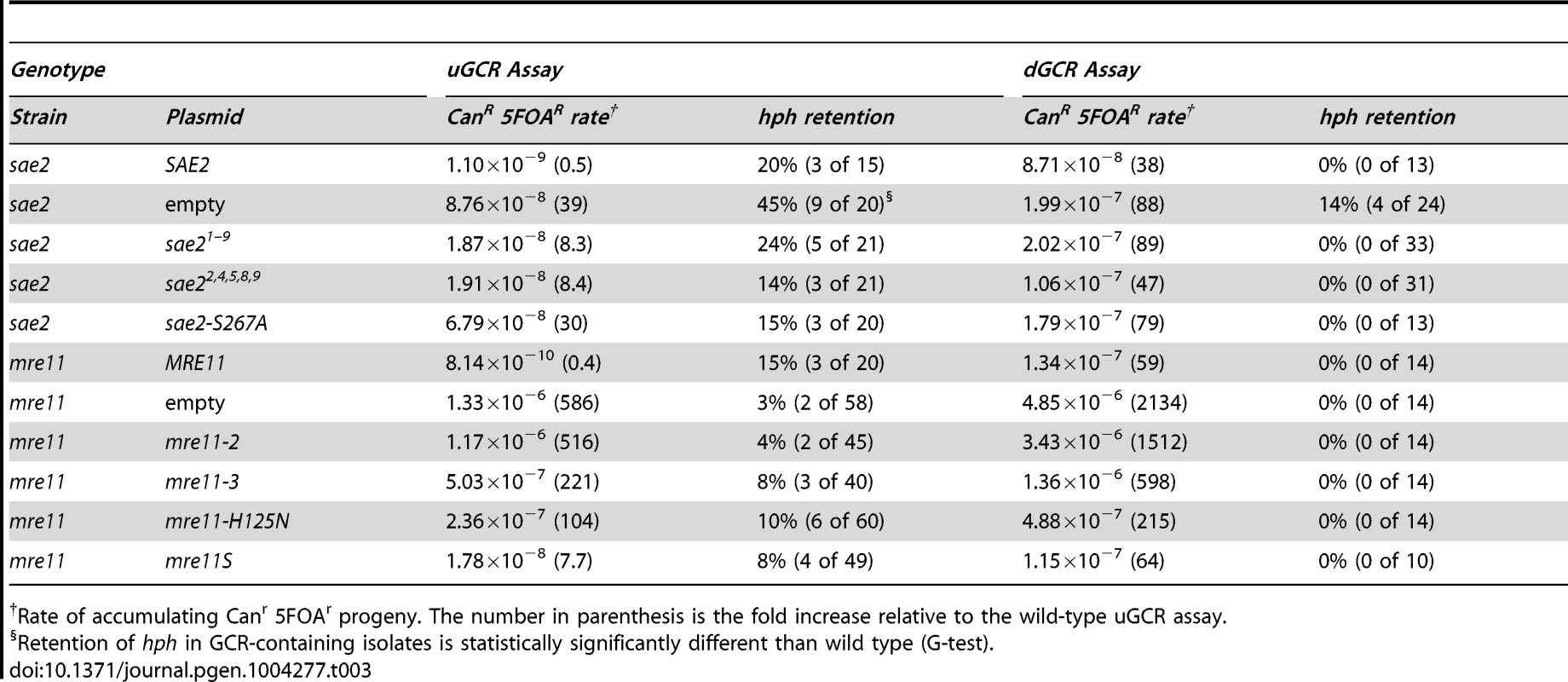
Rate of accumulating Canr 5FOAr progeny. The number in parenthesis is the fold increase relative to the wild-type uGCR assay. Disruption of the Tel1 interaction with Mre11-Xrs2-Rad50, but not other Mre11 defects, causes increased hph retention
Tel1 and Sae2 interact functionally with the Mre11-Rad50-Xrs2 (MRX) complex [39], [40]. However, the mre11Δ single mutation caused increased GCR rates in both GCR assays without affecting the frequency of hph-retention in the uGCR assay (Table 2). In addition, the mre11Δ uGCR strain did not have an increased frequency of hph − GCRs associated with chrV L duplications (Table 1). Consistent with the differences in rate and types of GCRs formed, the tel1Δ and mre11Δ mutations were not epistatic; the tel1Δ mre11Δ double mutant had higher GCR rates relative to both single mutants in both GCR assays and did not have increased hph retention in the uGCR assay, similar to the mre11Δ single mutant (Table 2). These results suggest that Mre11 plays roles in maintaining genome stability that are independent of Tel1 and Sae2.
The stability of the MRX complex is more important than the Mre11 nuclease function in maintaining genome stability [41], [42]; however, the nuclease-defective mre11-D56N and mre11-H125N alleles are similar to the sae2Δ mutation in causing persistent Mre11 foci and in reducing recombination at inverted repeats [33], [43]. We therefore investigated if nuclease-defective mre11 alleles might increase hph retention in the uGCR assay like the sae2Δ mutation. Plasmid-borne wild-type MRE11 and the meiotic-processing defective mre11S allele [44] complemented or largely complemented the mre11Δ defect, respectively, in both GCR assays (Table 3). In contrast, the mre11-2 allele, which causes defects in MRX complex formation [45], caused defects similar to those caused by the mre11Δ mutation, and the nuclease-defective mre11-3 and mre11-H125N alleles [45], [46] caused partial defects. None of the tested mre11 alleles tested significantly changed the frequency of hph-retaining GCRs in the uGCR assay relative to the wild-type or mre11Δ mutant strains (Table 3), and all of the GCRs analyzed from the nuclease-defective mre11 alleles lacked inverted duplications (Table 1). Thus, the GCRs accumulating in strains with a sae2Δ mutation differ from GCRs in the mre11Δ and nuclease-defective mre11 mutations, despite the similarity of these mutations in assays for inverted repeat-mediated recombination likely caused by defects in hairpin cleavage [33]. The differences in types and rates of GCRs formed in strains with mre11 mutations indicate that defects in MRE11 cause additional defects relative to defects in SAE2 and that the GCRs that are initially formed in these mre11 strains are not mediated by hairpin-mediated formation of inverted duplications.
Tel1 is recruited to DSBs through interaction with the C-terminus of Xrs2, and consequently the xrs2-11 allele, which encodes a truncated Xrs2 protein lacking the C-terminal 162 residues that does not interact with Tel1, is similar to a tel1Δ mutation in some assays [39]. The xrs2-11 mutant had an increased GCR rate in the dGCR assay that was 4 - to 5-fold higher than that of the wild-type and tel1Δ strains (Table 2). In contrast, the GCR rate in the uGCR assay in the xrs2-11 mutant was not distinguishable from that of the wild type or tel1Δ strains, whereas the frequency of hph retention in the xrs2-11 uGCR assay strain was increased relative to wild-type (p = 0.003; G-test) but not significantly different from that caused by the tel1Δ mutation. These data suggest that Tel1 recruitment to the MRX complex is required to suppress the formation of hph-retaining GCRs, despite the fact that other MRX defects cause higher GCR rates without increasing hph retention.
End resection promotes GCRs associated with chrV L duplications and hph retention
End resection during double strand break repair (DSB) repair in S. cerevisiae is proposed to involve two steps [47]–[49]: the initial removal of a short oligonucleotide by the MRX complex in conjunction with Sae2 followed by extensive resection by either Exo1 alone or by Sgs1 in combination with Dna2. Deletion of both SAE2 and SGS1 causes synthetic lethality, which can be suppressed by deleting YKU70 [50], but the sae2Δ mutation is not lethal in combination with an exo1Δ mutation. In the uGCR assay, the sae2Δ exo1Δ double mutant had a level of GCRs retaining hph and having a chrV L duplication that was intermediate between that of the wild-type and sae2Δ strains (Tables 1 and 2). Additionally, the double mutant had modestly reduced GCR rates relative to the sae2Δ single mutant in both assays (p = 0.002 uGCR assay, p = 0.06 dGCR assay; Mann-Whitney) (Table 2). Elimination of both resection pathways in the sgs1Δ exo1Δ double mutant caused a substantial increase in GCR rate relative to the single mutants in both GCR assays (Table 2). The fact that the sgs1Δ exo1Δ double mutant had the same GCR rate in both GCR assays is consistent with the observation that the sgs1Δ exo1Δ double mutant repairs DSBs primarily through the formation of de novo telomeres [51] and with the significantly reduced frequency of hph retention in the uGCR assay (Table 2; p = 5×10−7, G-test). Together these results suggest that at least Exo1 contributes to the formation of hph-retaining chrV L inverted duplications in sae2Δ mutants, potentially by mediating resection to initiate a DNA hairpin structure.
Retention of hph in GCRs formed in checkpoint-defective mutant strains
Because TEL1 is involved in DNA damage checkpoint signaling [52]–[54], we analyzed other mutations affecting the DNA damage and replication checkpoints (Table 4). Most of the mutations tested did not cause increased frequency of hph retention in the uGCR assay, except for rad53Δ sml1Δ, tof1Δ, and mrc1Δ tof1Δ. The hph+ GCRs obtained from the rad53Δ sml1Δ strain were primarily inverted duplications (11 of 13) and the hph+ GCRs from the mrc1Δ tof1Δ double mutant were primarily interstitial deletions (8 of 10) on the basis of the size of the rearranged chrV and the presence of a URA3/ura3-52 fusion junction detected by PCR (data not shown). Thus, the defects in tel1Δ mutants appear to be distinct from defects causing increased hph retention in the mrc1Δ tof1Δ or rad53Δ sml1Δ mutants.
Tab. 4. GCR rates and percent hph retention in checkpoint defective mutants. 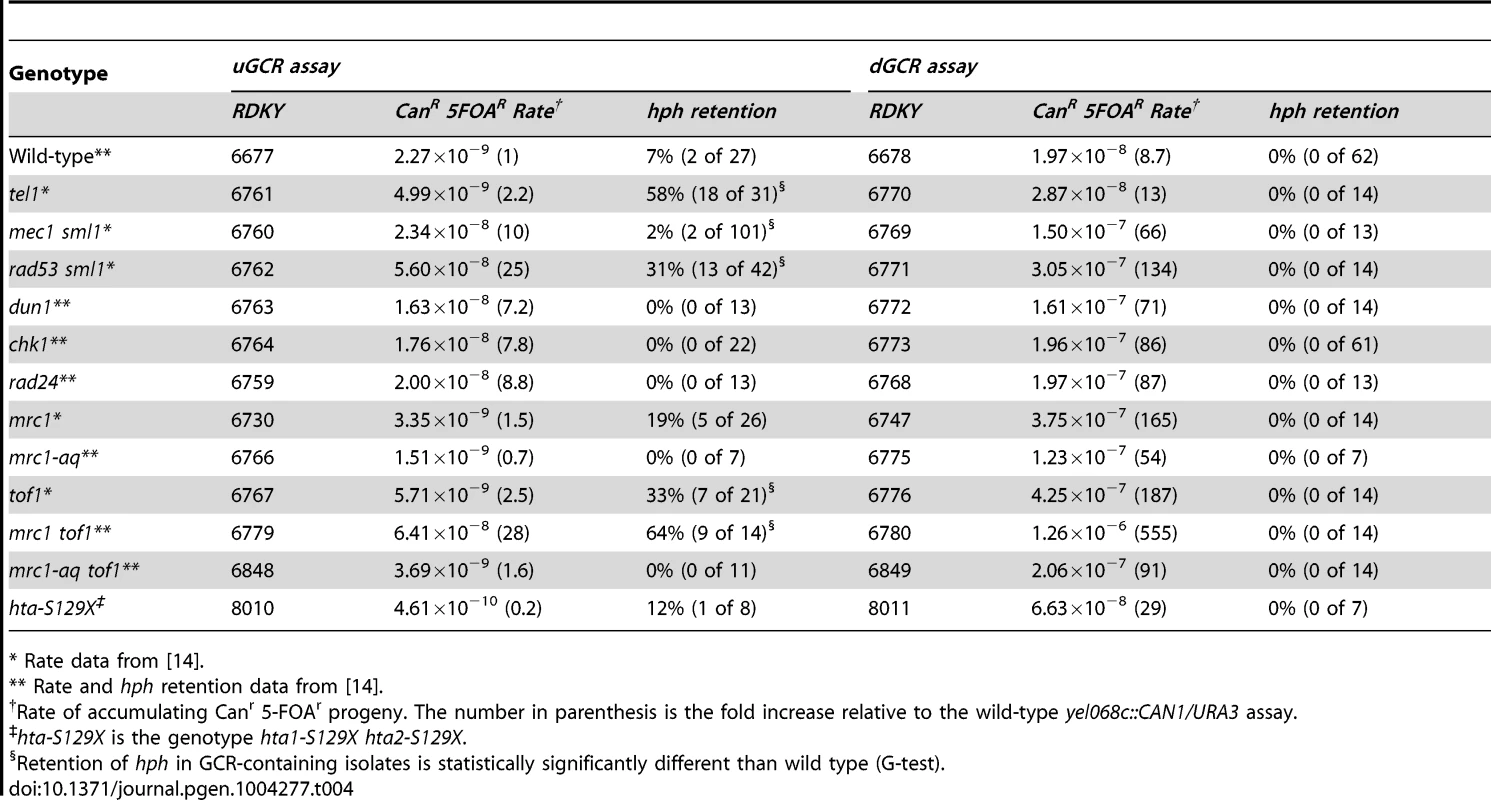
* Rate data from [14]. Retention of hph in GCRs formed in strains containing mutations affecting de novo telomere addition
Strains with tel1 mutations have short telomeres and can form de novo telomere additions, even if the efficiency appears to be decreased in some cases [16], [17], [22], [55]. Because efficient de novo telomere addition might be predicted to prevent the formation of both interstitial deletion and inverted duplication GCRs, we investigated strains with mutations affecting de novo telomere addition (Table 5). To test mutations affecting telomerase, we generated post-senescent type II survivor est1Δ and est3Δ strains after sporulating heterozygous est1Δ/EST1 or est3Δ/EST3 diploids. These strains had an increased frequency of hph retention in the uGCR assay (p = 0.0006 and p = 2×10−9, respectively, G-test; Table 5). Similarly, deletion of YKU70 and YKU80, which are required for de novo telomere addition and NHEJ but not telomere maintenance [29], [56], increased the frequency of hph retention to 44% and 40%, respectively (p = 7×10−5 and p = 5×10−5, respectively, G-test; Table 5). In contrast, deletion of LIG4, which is required for NHEJ but not de novo telomere addition [29], [56], did not increase the frequency of hph retention (Table 2).
Tab. 5. GCR rates and percent hph retention in mutants affecting de novo telomere addition. 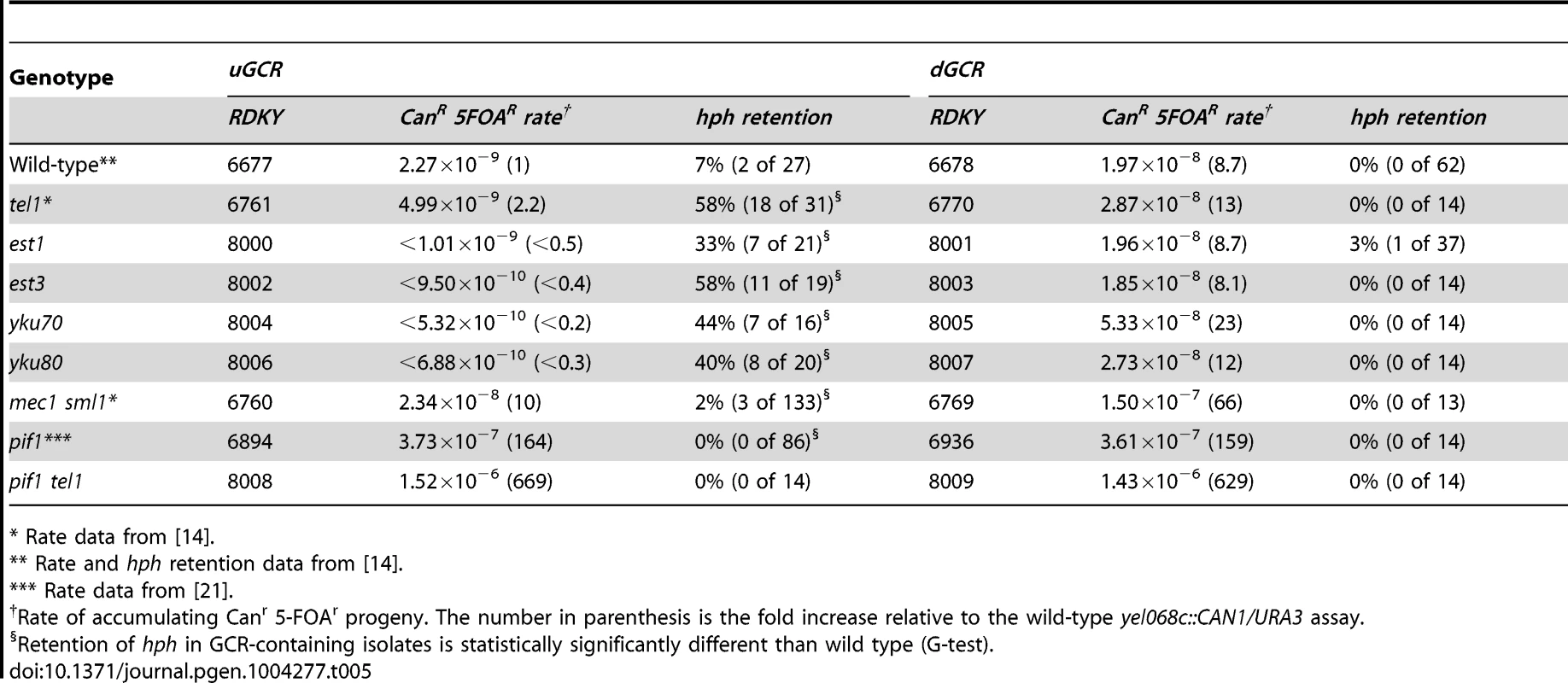
* Rate data from [14]. Consistent with the effects of mutations eliminating de novo telomere addition, mutations that increase the rate of de novo telomere addition caused a reduced frequency of hph retention. Deletion of MEC1, which causes increased rates of GCRs that are mediated primarily by de novo telomere addition due to loss of inhibition of CDC13 [22], [57], simultaneous deletion of SGS1 and EXO1, which results in high rates of healing of DSBs by de novo telomere addition [51], and deletion of PIF1, which causes increased rates of GCRs mediated primarily by de novo telomere addition due to loss of inhibition of telomerase at sites of de novo telomere addition [16], [58]–[60], caused a significantly reduced frequencies of retention of hph relative to that of the wild-type strain in the uGCR assay (mec1Δ sml1Δ p = 0.008; sgs1Δ exo1Δ p = 5×10−7; pif1Δ p = 0.0002; Table 2 and 5). In addition, hph+ GCRs were not observed in the pif1Δ tel1Δ double mutant, reminiscent of the inability of tel1Δ to suppress the increased GCR rate caused by de novo telomere addition in the pif1-m2 mutant [16]. Consistent with the hypothesis that de novo telomere additions were the primary type of GCR formed in the pif1Δ tel1Δ double mutant strains, the rates in the uGCR and dGCR assays were essentially the same, like that seen in the pif1Δ single mutant and the sgs1Δ exo1Δ double mutant (Table 2 and 5). Together, these results suggest that de novo telomere addition suppresses hph retention, potentially by competing for broken ends that could otherwise undergo either NHEJ or resection leading to interstitial deletions or inverted duplications.
Discussion
The observed genotype-specific increase in retention of the telomeric hph marker in the uGCR assay was due to the formation of NHEJ-dependent interstitial deletions spanning the CAN1/URA3 cassette or inverted duplications that recaptured the telomeric end of chrV using the homology between URA3 of the CAN1/URA3 cassette and ura3-52 located on chrV L. Previous studies identified inverted duplication GCRs involving dicentric isoduplication intermediates; however, these occurred at low rates [18] and were difficult to identify, because their identification required sequencing of their rearrangement breakpoints. The ability of the uGCR assay to capture these events combined with more facile product analysis provided a convenient genetic assay for use in studying the structural features and genetic requirements underlying these types of GCRs. The tel1Δ uGCR assay strain had increased frequencies of forming both types of hph+ GCRs, whereas other mutant backgrounds with increased hph retention yielded primarily interstitial deletions (mrc1Δ tof1Δ) or inverted duplications (sae2Δ and rad53Δ sml1Δ). Remarkably, both types of rearrangements were observed in GCRs formed in the wild-type uGCR assay strain. These hph-retaining GCRs were suppressed by de novo telomere addition; mutations promoting de novo telomere addition (mec1Δ, pif1Δ, and sgs1Δ exo1Δ) suppressed hph retention, whereas mutations inhibiting de novo telomere addition (est1Δ, est3Δ, yku70Δ, and yku80Δ) enhanced hph retention. In contrast, extensive competition between other implicated pathways likely precludes simple extrapolation of the conclusions based on the phenotypes caused by individual mutations that result in increased frequencies of hph-retaining GCRs to the predicted effects of other mutations affecting the same or related pathways. Examples include the differences between tel1Δ and mec1Δ, between tel1Δ and rad53Δ, between tel1Δ and mre11Δ, between sae2Δ and mre11Δ, as well as the differences between mutations affecting different features of Mre11-Rad50-Xrs2. Despite this, our analysis allowed us to link an unusual signature of GCRs to specific genetic defects.
Current and previous results suggest that several mechanisms contribute to the hph+ GCRs observed. The GCRs could be initiated by one or more DSBs between hph and PCM1, the most telomeric essential gene, although a DSB-independent mechanism for generating similar products has been proposed for forks stalling in the context of large inverted repeats [61], [62]. Interstitial deletions then appear to be formed by NHEJ-mediated rejoining of the two ends associated with potential processing of the ends at the DSB in some cases. Inverted duplications appear to be initiated by 5′ resection of a DSB followed by fold-back invasion of the 3′ single stranded end (Figure S5 and S9A). Subsequently, one of three mechanisms operate (Figure S9B): 1) intramolecular BIR occurs up to the position of ura3-52 followed by HR-mediated template switching to the telomeric URA3 and continuation of BIR to the end of chrV; 2) intermolecular BIR extends the entire length of chrV yielding an isoduplication chromosome that then breaks during cell division and is resolved by secondary rearrangements to yield a stable monocentric chromosome [63]; and 3) the fold-back hairpin is covalently closed followed by replication to yield an isoduplication chromosome, which is further processed as described above in mechanism 2. Notably, hph+ inverted duplications were much more prevalent than hph − inverted duplications resolved by HR between a chrV Ty element and any of the other 254 Ty related elements in the genome. Strand switching during BIR (mechanism 1; [64], [65]) combined with the possibility that the telomeric hph+ fragment is recombinogenic because it contains a DSB could explain this bias. However, if an isoduplication chromosome is formed first (mechanisms 2 and 3), it must subsequently break during cell division before undergoing a secondary rearrangement(s) to capture a new telomere. Consequently, the telomeric hph-containing fragment might be diluted out by loss or segregation into the wrong progeny during cell division, thereby reducing the formation of hph+ recombinants; this would allowing other Ty-related sequences to serve as substrates for HR with the broken isoduplication chromosome at higher relative efficiencies relative to the hph-containing fragment. Together, these models predict that genetic alterations that either directly or indirectly facilitate hairpin formation, protect hairpins that have formed, promote the use of the hph-containing fragment as a template, facilitate NHEJ, or suppress pathways that compete with these events will increase the formation of the types of hph+ GCRs seen in the present study.
The sae2Δ and tel1Δ uGCR assay strains accumulated high frequencies of hph+ inverted duplications that required HR for their formation. However, these results are not consistent with a simple model in which hph+ inverted duplications are suppressed primarily by Tel1-activated Sae2, which is phosphorylated by Tel1 and Mec1 [35], because sae2 phosphosite mutations did not caused increased levels of hph+ GCRs in the uGCR assay, the tel1Δ mutant uGCR assay strain differed from the sae2Δ strain by accumulating interstitial deletion GCRs in addition to inverted duplication GCRs, and the tel1Δ sae2Δ double mutant uGCR assay strain had suppressed levels of hph+ GCRs relative to both single mutants. The lack of interstitial deletion GCRs in the sae2Δ uGCR assay strain would be consistent with Sae2 primarily promoting cleavage of DNA hairpins [33], [34], [66] and with Tel1 affecting multiple pathways, potentially including promotion of de novo telomere additions, suppression of NHEJ, and/or suppression of hairpin cleavage by Sae2-MRX. In addition, GCRs formed in the tel1Δ sae2Δ uGCR strain do have higher levels of hph− GCRs, indicating that the retention or preferential use of the acentric hph-containing telomeric chrV fragment during BIR is dependent on Tel1 in the absence of Sae2. Consistent with this model, TEL1 can more readily compensate for the deletion of MEC1 in strains with sae2Δ mutations [54], presumably because of increased Tel1 signaling from DSBs that are not resected due to the uncleaved terminal hairpins that accumulate in sae2Δ mutants. Thus, our data would suggest that the sae2 phosphosite mutations, unlike a sae2Δ mutation, may disrupt functions of Tel1 that promote use of the hph-containing telomeric chrV fragment during BIR. Our data, however, do not rule out a scenario in which moderate overexpression of the mutant sae2 alleles from low copy number ARS CEN plasmids might be sufficient to overcome the effect of these phosphosite mutants. The fact that TEL1 and SAE2 jointly suppress inverted duplications suggests an alternative explanation for the apparent requirement of TEL1 and SAE2 in microhomology-mediated end joining (MMEJ; [67]): TEL1 and SAE2 may suppress pathways that compete with MMEJ for substrates rather than directly functioning in MMEJ.
Consistent with the differences in the effects of defects in TEL1 and SAE2 on the rate and types of GCRs formed, genetic analyses revealed that the effects of mutations affecting related pathways are difficult to predict. For example, the increased hph retention seen in the rad53Δ sml1Δ strain, which is primarily due to inverted duplications and not interstitial deletions, argues that Tel1 has additional repair-related functions that can suppress the formation of hph+ GCRs. However, extrapolation of this result to other DNA damage checkpoint defective mutations is problematic. For example, the mec1Δ sml1Δ double mutant uGCR assay strain had a significantly increased accumulation of hph − GCRs relative to the wild-type strain likely due to a failure to suppress de novo telomere additions [22], [57]. Similarly, both Tel1 and Sae2 function in conjunction with the MRX complex in vivo, and disruption of the Tel1-Xrs2 interaction caused increased formation of hph+ GCRs similar to that caused by the tel1Δ mutation. Thus, the recruitment of Tel1 to DSBs by MRX is likely required for suppressing hph retention. Yet, the mre11Δ mutation and mre11 point mutations that disrupt complex formation and nuclease activity result in much higher GCR rates than the tel1Δ and sae2Δ single mutations but did not result in increased accumulation of hph+ GCRs in the uGCR assay, suggesting that these mre11 mutants cause defects in addition to defects in hairpin cleavage. In sum, our results indicate that GCR signatures observed here often reflect the properties caused by individual genetic defects rather than inactivation of entire pathways in which genes of interest function, except in the case of mutations that directly affect de novo telomere addition; this likely limits our ability to predict the exact GCR signature caused by individual pathway defects.
Our results support the hypothesis that extensive competition between different DNA repair mechanisms determines the spectrum of genome rearrangements that accumulate in cells and this spectrum can be altered by subtle changes in the efficiencies of different pathways. This competition likely underlies the fact that rearrangement spectra caused by mutations in related genes tend to differ. Therefore, the spectrum of genome rearrangements that accumulate can provide insights into the underlying genetic defects in DNA repair pathways. For example, in a recent analysis of GCRs in human metastatic pancreatic cancers, 1 out of every 6 GCRs was a copy number change mediated by an inverted duplication that showed an association with hallmarks of telomere dysfunction and a dysregulated G1-to-S-phase transition in conjunction with an intact G2/M checkpoint [7]. These phenotypes are highly reminiscent of the phenotypes caused by defects in TEL1 as described here and in previous studies [16], [22]–[25]. Together, these results are consistent with the notion that defects in signaling by the ATM pathway, which involves the human homolog of TEL1, may play important roles in the formation of the GCRs seen in a fraction of metastatic pancreatic cancer. Similarly, other defects such as defects in RBBP8, which encodes the human Sae2 homolog CtIP, might also play a role in the formation of the inverted duplications seen in metastatic pancreatic cancer. However, additional experimentation will be required to determine if the genetic insights into the origin of genome instability signatures in S. cerevisiae can be used to predict genetic changes with functional consequences in human cancer.
Materials and Methods
Construction and propagation of strains and plasmids
GCR assays were performed using derivatives of RDKY6677 (yel068c::CAN1/URA3) or RDKY6678 (yel072w::CAN1/URA3) that in addition have the genotype MATa leu2Δ1 his3Δ200 trp1Δ63 lys2ΔBgl hom3-10 ade2Δ1 ade8 ura3-52 can1::hisG iYEL072::hph as previously described [14]. Mutant derivatives of these strains (Table S3) were constructed using standard PCR-based gene disruption methods or mating to strains containing mutations as described [11]. The xrs2-11 allele [39] was generated by integrating a HIS3 marker at the 3′ end of XRS2 to introduce a stop codon and delete the codons encoding residues 693–854 of Xrs2.
Post-senescent est1Δ and est3Δ survivors were generated by deleting one copy of either EST1 or EST3 in diploid versions of RDKY6677 and RDKY6678, sporulating the heterozygous diploids, and performing multiple sequential re-streaks of individual spore clones on YPD agar media until growth of the mutants was equivalent to a wild-type control as described [68]. Type I and type II survivors were distinguished on the basis of Southern blotting of XhoI digested genomic DNA as described [68] and by the fact that chromosomes of type I survivors do not properly enter PFGE gels [69].
Alleles of sae2 were introduced into the sae2::TRP1 deletion strains using pRS313-based ARS CEN plasmids containing the HIS3 marker [70]. Integration plasmids bearing the sae21–9 and sae22,4,5,8,9 alleles, pML468.6 and pML488.15, were kind gifts of Maria Pia Longhese (Università degli Studi di Milano-Bicocca). The SAE2-bearing fragments from pML468.6 and pML488.15 were subcloned into the EcoRI site of pRS313 and verified by sequencing to generate pRDK1698 and pRDK1699. The wild-type SAE2-bearing plasmid, pRDK1700, was generated by PCR amplification of SAE2 from wild-type genomic DNA with primers 5′-TGC AAT AGA GTC GTG AAT TCG TCT GAG TTA GCG TCT GAT TTT GAC TCT TTC TTC TTC TTT TTC GTC TT-3′ and TGC AAT AGA GTC GTG AAT TCC CTG GTA GTT AGG TGT CAT TTG TTT AAC GTC CGT TAA CTT CCC CTT TCT-3′ to generate an insert spanning the same genomic region as pRDK1698 and pRDK1699. The sae2-S267A plasmid, pRDK1701, was generated by site-directed mutagenesis of pRDK1700 and verified by sequencing. For GCR rate determination, the transformed query strains were grown in –HIS liquid media, viable cell determination was performed by plating on –HIS media, and GCR-containing progeny were selected on Can/5-FOA media lacking histidine.
Alleles of mre11 were introduced into the mre11::HIS3 deletion strains using pRS314 ARS CEN plasmids containing the TRP1 marker. The wild-type MRE11 plasmid was generated by PCR amplifying MRE11 from wild-type genomic DNA with the primers 5′-CTG AGG AAT TCG ATT TGG CTA AAC TAG GCT GAG GTA GGC TCG-3′ and 5′-CTG AGC TCG AGG GTA TTG TTT CCC ACA AGG GGA CGG TTA ATG-3′ and cloning the PCR product into pRS314 cut with EcoRI and XhoI. The resulting plasmid, pRDK1702, was verified by sequencing. The mre11-H125N and mre11S (mre11-P84S,T188I) plasmids, pRDK1703 and pRDK1704, were generated by site-directed mutagenesis and verified by sequencing. The mre11-2 and mre11-3 plasmids, pRS314-mre11-2 and pRS314-mre11-3, were kind gifts of John Petrini (Sloan-Kettering Institute). For GCR rate determination, the transformed query strains were grown in –TRP liquid media, viable cell determination was performed by plating on –TRP media, and GCR-containing progeny were selected on GCR media lacking tryptophan.
Determination of GCR rates and hph retention
GCR rates were determined using multiple independent biological isolates as previously described [71]. The frequency of hph retention was determined by testing a single GCR-containing isolate from each of a number of individual independent cultures for growth on YPD media supplemented with 200 µg/mL hygromycin B (Invitrogen).
Statistical analysis
The significance of the deviation of hph-retention for each genotype was measured using the maximum likelihood statistical significance G-test [72] as implemented for R by P. Hurd (http://www.psych.ualberta.ca/~phurd/cruft/). Probabilities for the null model that the observed distributions were generated by the same underlying rate were calculated using the two-tailed Mann-Whitney U-test (http://faculty.vassar.edu/~lowry/utest.html). A significant differences was inferred when the probability of the null model was 0.01 or less.
PFGE gel and Southern blotting
DNA plugs for PFGE were prepared as described [73]. Asc I-digested plugs were prepared by treating plugs with 50 units of Asc I (New England Biolabs) overnight at 37°C. Electrophoresis was performed using a Bio-Rad CHEF-DRII apparatus at 7 V/cm, with a 60 to 120 s switch time for 24 h. The gels were stained with ethidium bromide and imaged. The DNA in the gel was transferred to Hybond-XL membranes by neutral capillary blotting. The DNA was crosslinked to the membrane by UV irradiation in a Stratalinker™ (Stratagene) apparatus at maximum output for 60 seconds. Probes were generated by random primer labeling of MCM3 and hph fragments with the Prime-It II kit (Stratagene). Probe hybridization was performed at 68°C for 2–4 hr. The membrane was then washed extensively and imaged with a PhosphoImager (Molecular Dynamics, Inc.).
Multiplex ligation-mediated probe amplification analysis
Primers targeted to the left arm of chromosome V (Figure S7A) were designed according to the recommendations on the MRC-Holland website (http://www.mrc-holland.com) with the length of each amplification product differing by 6 basepairs (Table S4). The reagents were purchased from MRC-Holland, and the amplification, fragment separation, and fragment detection steps were performed essentially as described [28]. Data were collected on an ABI 3730XL sequencer using the POP7 polymer and GS500-LIZ sizing standard (Life Technologies). The raw data for each run were integrated using GeneMapper software (Life Technologies) and analyzed using a custom Python script that uses gnuplot (http://www.gnuplot.info) to plot the integrated area for each peak in wild-type controls against the respective peak in experimental samples (Figure S7B and C). Amplification detected by MLPA was verified by comparison with aCGH data for isolates 213, 217, 362, and 3178 (Figure S7D).
Array comparative genomic hybridization
One µg of genomic DNA was prepared from GCR-containing isolates and the wild-type strain RDKY6677 using the Purgene kit (Qiagen) and concentrated to >100 ng µL−1. The DNA from GCR-containing isolates was amplified and labeled with Cy5, and wild-type control DNA was amplified and labeled with Cy3. Subsequently, four mixtures containing GCR isolate/wild-type pairs were hybridized to a NimbleGen 4-plex chip. Data were analyzed using the SignalMap software (NimbleGen) and remapped from the chrV sequence of the reference genome to the coordinates of chrV in RDKY6677. Microarray data have been deposited at ArrayExpress (http://www.ebi.ac.uk/arrayexpress) with under the accession E-MTAB-2377.
Whole genome paired-end sequencing
Multiplexed paired-end libraries were constructed from 5 µg of genomic DNA purified using the Purgene kit (Qiagen). The genomic DNA was sheared by sonication and end-repaired using the End-it DNA End-repair kit (Epicentre Technologies). Common adaptors from the Multiplexing Sample Preparation Oligo Kit (Illumina) were then ligated to the genomic DNA fragments, and the fragments were then subjected to 18 cycles of amplification using the Library Amplification Readymix (KAPA Biosystems). The amplified products were fractionated on an agarose gel to select 600 bp fragments, which were subsequently sequenced on an Illumina HiSeq 2000 using the Illumina GAII sequencing procedure for paired-end short read sequencing. Reads from each read pair were mapped separately by bowtie version 0.12.7 [74] to a reference sequence that contained revision 64 of the S. cerevisiae S288c genome (http://www.yeastgenome.org), hisG from Samonella enterica, and the hphMX4 marker (Table S1). Sequencing data have been deposited at NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra) under the accession SRP039033.
Rearrangement and copy number analysis of paired-end sequencing data
Chromosomal rearrangements were identified after bowtie mapping by version 0.5 of the Pyrus suite (http://www.sourceforge.net/p/pyrus-seq). Briefly, after PCR removal of PCR duplicates, read pairs with 2 uniquely mapping reads were used to generate 2 distributions. The number of times each base pair was read (the ‘nread’ distribution) was determined for identifying the sequence variants observed a significant number of times, and the number of times each base pair was spanned by a pair of reads (the ‘nspan’ distribution) was determined to identify the candidate chromosomal rearrangements that were supported by a significant number of read pairs. The data were then analyzed for junction-defining read pairs that indicated the presence of structural rearrangements relative to the reference genome, such as the tel1::HIS3 deletion or GCR-related fold-back inversions. The junction-sequencing reads were identified from read pairs in which one read could not be mapped and the other read mapped next to the junction-defining read pairs. Sequences of the junctions were generated by de novo alignment of the junction-sequencing reads associated with rearrangements defined by statistically significant junction-defining read pairs (Figure S3). The identified rearrangements included all known rearrangements in the strains that could be defined based on the average distance between the read pairs in the library (Table S2).
Supporting Information
Zdroje
1. SaundersWS, ShusterM, HuangX, GharaibehB, EnyenihiAH, et al. (2000) Chromosomal instability and cytoskeletal defects in oral cancer cells. Proc Natl Acad Sci U S A 97 : 303–308.
2. GisselssonD, PetterssonL, HoglundM, HeidenbladM, GorunovaL, et al. (2000) Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc Natl Acad Sci U S A 97 : 5357–5362.
3. FouladiB, SabatierL, MillerD, PottierG, MurnaneJP (2000) The relationship between spontaneous telomere loss and chromosome instability in a human tumor cell line. Neoplasia 2 : 540–554.
4. GisselssonD, HoglundM (2005) Connecting mitotic instability and chromosome aberrations in cancer–can telomeres bridge the gap? Semin Cancer Biol 15 : 13–23.
5. ChanKL, NorthPS, HicksonID (2007) BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J 26 : 3397–3409.
6. LeeE, IskowR, YangL, GokcumenO, HaseleyP, et al. (2012) Landscape of somatic retrotransposition in human cancers. Science 337 : 967–971.
7. CampbellPJ, YachidaS, MudieLJ, StephensPJ, PleasanceED, et al. (2010) The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467 : 1109–1113.
8. McBrideDJ, EtemadmoghadamD, CookeSL, AlsopK, GeorgeJ, et al. (2012) Tandem duplication of chromosomal segments is common in ovarian and breast cancer genomes. J Pathol 227 : 446–455.
9. StephensPJ, McBrideDJ, LinML, VarelaI, PleasanceED, et al. (2009) Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 462 : 1005–1010.
10. The Cancer Genome Atlas (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474 : 609–615.
11. ChenC, KolodnerRD (1999) Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat Genet 23 : 81–85.
12. HackettJA, FeldserDM, GreiderCW (2001) Telomere dysfunction increases mutation rate and genomic instability. Cell 106 : 275–286.
13. KanellisP, GagliardiM, BanathJP, SzilardRK, NakadaS, et al. (2007) A screen for suppressors of gross chromosomal rearrangements identifies a conserved role for PLP in preventing DNA lesions. PLoS Genet 3: e134.
14. PutnamCD, HayesTK, KolodnerRD (2009) Specific pathways prevent duplication-mediated genome rearrangements. Nature 460 : 984–989.
15. ChanJE, KolodnerRD (2011) A genetic and structural study of genome rearrangements mediated by high copy repeat Ty1 elements. PLoS Genet 7: e1002089.
16. MyungK, ChenC, KolodnerRD (2001) Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411 : 1073–1076.
17. PutnamCD, PennaneachV, KolodnerRD (2004) Chromosome healing through terminal deletions generated by de novo telomere additions in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 101 : 13262–13267.
18. PutnamCD, PennaneachV, KolodnerRD (2005) Saccharomyces cerevisiae as a model system to define the chromosomal instability phenotype. Mol Cell Biol 25 : 7226–7238.
19. PennaneachV, KolodnerRD (2004) Recombination and the Tel1 and Mec1 checkpoints differentially effect genome rearrangements driven by telomere dysfunction in yeast. Nat Genet 36 : 612–617.
20. PennaneachV, KolodnerRD (2009) Stabilization of dicentric translocations through secondary rearrangements mediated by multiple mechanisms in S. cerevisiae. PLoS One 4: e6389.
21. PutnamCD, HayesTK, KolodnerRD (2010) Post-replication repair suppresses duplication-mediated genome instability. PLoS Genet 6: e1000933.
22. MyungK, DattaA, KolodnerRD (2001) Suppression of spontaneous chromosomal rearrangements by S phase checkpoint functions in Saccharomyces cerevisiae. Cell 104 : 397–408.
23. ChanSW, BlackburnEH (2003) Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining. Mol Cell 11 : 1379–1387.
24. DuBoisML, HaimbergerZW, McIntoshMW, GottschlingDE (2002) A quantitative assay for telomere protection in Saccharomyces cerevisiae. Genetics 161 : 995–1013.
25. RitchieKB, MalloryJC, PetesTD (1999) Interactions of TLC1 (which encodes the RNA subunit of telomerase), TEL1, and MEC1 in regulating telomere length in the yeast Saccharomyces cerevisiae. Mol Cell Biol 19 : 6065–6075.
26. SchmidtKH, PennaneachV, PutnamCD, KolodnerRD (2006) Analysis of gross-chromosomal rearrangements in Saccharomyces cerevisiae. Methods Enzymol 409 : 462–476.
27. SchoutenJP, McElgunnCJ, WaaijerR, ZwijnenburgD, DiepvensF, et al. (2002) Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 30: e57.
28. ChanJE, KolodnerRD (2012) Rapid analysis of Saccharomyces cerevisiae genome rearrangements by multiplex ligation-dependent probe amplification. PLoS Genet 8: e1002539.
29. DudasovaZ, DudasA, ChovanecM (2004) Non-homologous end-joining factors of Saccharomyces cerevisiae. FEMS Microbiol Rev 28 : 581–601.
30. McEachernMJ, HaberJE (2006) Break-induced replication and recombinational telomere elongation in yeast. Annu Rev Biochem 75 : 111–135.
31. Flores-RozasH, KolodnerRD (2000) Links between replication, recombination and genome instability in eukaryotes. Trends Biochem Sci 25 : 196–200.
32. LengsfeldBM, RattrayAJ, BhaskaraV, GhirlandoR, PaullTT (2007) Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol Cell 28 : 638–651.
33. LobachevKS, GordeninDA, ResnickMA (2002) The Mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell 108 : 183–193.
34. RattrayAJ, ShaferBK, NeelamB, StrathernJN (2005) A mechanism of palindromic gene amplification in Saccharomyces cerevisiae. Genes Dev 19 : 1390–1399.
35. BaroniE, ViscardiV, Cartagena-LirolaH, LucchiniG, LongheseMP (2004) The functions of budding yeast Sae2 in the DNA damage response require Mec1 - and Tel1-dependent phosphorylation. Mol Cell Biol 24 : 4151–4165.
36. ClericiM, MantieroD, LucchiniG, LongheseMP (2006) The Saccharomyces cerevisiae Sae2 protein negatively regulates DNA damage checkpoint signalling. EMBO Rep 7 : 212–218.
37. Cartagena-LirolaH, GueriniI, ViscardiV, LucchiniG, LongheseMP (2006) Budding Yeast Sae2 is an In Vivo Target of the Mec1 and Tel1 Checkpoint Kinases During Meiosis. Cell Cycle 5 : 1549–1559.
38. HuertasP, Cortes-LedesmaF, SartoriAA, AguileraA, JacksonSP (2008) CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature 455 : 689–692.
39. NakadaD, MatsumotoK, SugimotoK (2003) ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev 17 : 1957–1962.
40. SymingtonLS, GautierJ (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45 : 247–271.
41. SmithS, GuptaA, KolodnerRD, MyungK (2005) Suppression of gross chromosomal rearrangements by the multiple functions of the Mre11-Rad50-Xrs2 complex in Saccharomyces cerevisiae. DNA Repair (Amst) 4 : 606–617.
42. Tittel-ElmerM, AlabertC, PaseroP, CobbJA (2009) The MRX complex stabilizes the replisome independently of the S phase checkpoint during replication stress. EMBO J 28 : 1142–1156.
43. LisbyM, BarlowJH, BurgessRC, RothsteinR (2004) Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118 : 699–713.
44. NairzK, KleinF (1997) mre11S–a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes Dev 11 : 2272–2290.
45. BressanDA, OlivaresHA, NelmsBE, PetriniJH (1998) Alteration of N-terminal phosphoesterase signature motifs inactivates Saccharomyces cerevisiae Mre11. Genetics 150 : 591–600.
46. MoreauS, FergusonJR, SymingtonLS (1999) The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol 19 : 556–566.
47. GravelS, ChapmanJR, MagillC, JacksonSP (2008) DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev 22 : 2767–2772.
48. MimitouEP, SymingtonLS (2008) Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455 : 770–774.
49. ZhuZ, ChungWH, ShimEY, LeeSE, IraG (2008) Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134 : 981–994.
50. MimitouEP, SymingtonLS (2010) Ku prevents Exo1 and Sgs1-dependent resection of DNA ends in the absence of a functional MRX complex or Sae2. EMBO J 29 : 3358–3369.
51. LydeardJR, Lipkin-MooreZ, JainS, EapenVV, HaberJE (2010) Sgs1 and exo1 redundantly inhibit break-induced replication and de novo telomere addition at broken chromosome ends. PLoS Genet 6: e1000973.
52. MorrowDM, TagleDA, ShilohY, CollinsFS, HieterP (1995) TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell 82 : 831–840.
53. SanchezY, DesanyBA, JonesWJ, LiuQ, WangB, et al. (1996) Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271 : 357–360.
54. UsuiT, OgawaH, PetriniJH (2001) A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol Cell 7 : 1255–1266.
55. LustigAJ, PetesTD (1986) Identification of yeast mutants with altered telomere structure. Proc Natl Acad Sci U S A 83 : 1398–1402.
56. PennaneachV, PutnamCD, KolodnerRD (2006) Chromosome healing by de novo telomere addition in Saccharomyces cerevisiae. Mol Microbiol 59 : 1357–1368.
57. ZhangW, DurocherD (2010) De novo telomere formation is suppressed by the Mec1-dependent inhibition of Cdc13 accumulation at DNA breaks. Genes Dev 24 : 502–515.
58. SchulzVP, ZakianVA (1994) The saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76 : 145–155.
59. BouleJB, VegaLR, ZakianVA (2005) The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature 438 : 57–61.
60. ZhouJ, MonsonEK, TengSC, SchulzVP, ZakianVA (2000) Pif1p helicase, a catalytic inhibitor of telomerase in yeast. Science 289 : 771–774.
61. MizunoK, LambertS, BaldacciG, MurrayJM, CarrAM (2009) Nearby inverted repeats fuse to generate acentric and dicentric palindromic chromosomes by a replication template exchange mechanism. Genes Dev 23 : 2876–2886.
62. PaekAL, KaocharS, JonesH, ElezabyA, ShanksL, et al. (2009) Fusion of nearby inverted repeats by a replication-based mechanism leads to formation of dicentric and acentric chromosomes that cause genome instability in budding yeast. Genes Dev 23 : 2861–2875.
63. McClintockB (1941) The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics 26 : 234–282.
64. SchmidtKH, WuJ, KolodnerRD (2006) Control of translocations between highly diverged genes by Sgs1, the Saccharomyces cerevisiae homolog of the Bloom's syndrome protein. Mol Cell Biol 26 : 5406–5420.
65. SmithCE, LlorenteB, SymingtonLS (2007) Template switching during break-induced replication. Nature 447 : 102–105.
66. RattrayAJ, McGillCB, ShaferBK, StrathernJN (2001) Fidelity of mitotic double-strand-break repair in Saccharomyces cerevisiae: a role for SAE2/COM1. Genetics 158 : 109–122.
67. LeeK, LeeSE (2007) Saccharomyces cerevisiae Sae2 - and Tel1-dependent single-strand DNA formation at DNA break promotes microhomology-mediated end joining. Genetics 176 : 2003–2014.
68. ChenQ, IjpmaA, GreiderCW (2001) Two survivor pathways that allow growth in the absence of telomerase are generated by distinct telomere recombination events. Mol Cell Biol 21 : 1819–1827.
69. WellingerRJ, ZakianVA (2012) Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: beginning to end. Genetics 191 : 1073–1105.
70. SikorskiRS, HieterP (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 : 19–27.
71. Putnam CD, Kolodner RD (2010) Determination of gross chromosomal rearrangement rates. Cold Spring Harb Protoc 2010: pdb prot5492.
72. Sokal RR, Rohlf FJ (1994) Biometry: the principles and practice of statistics in biological research. New York: Freeman.
73. GerringSL, ConnellyC, HieterP (1991) Positional mapping of genes by chromosome blotting and chromosome fragmentation. Methods Enzymol 194 : 57–77.
74. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25.
75. ChenH, LisbyM, SymingtonLS (2013) RPA coordinates DNA end resection and prevents formation of DNA hairpins. Mol Cell 50 : 589–600.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 4
-
Všechny články tohoto čísla
- The Challenges of Mitochondrial Replacement
- Concocting Cholinergy
- Genome-Wide Diet-Gene Interaction Analyses for Risk of Colorectal Cancer
- Statistical Power to Detect Genetic (Co)Variance of Complex Traits Using SNP Data in Unrelated Samples
- Mouse Pulmonary Adenoma Susceptibility 1 Locus Is an Expression QTL Modulating -4A
- Transcription-Associated R-Loop Formation across the Human CGG-Repeat Region
- Epigenetic Regulation by Heritable RNA
- Protein Quantitative Trait Loci Identify Novel Candidates Modulating Cellular Response to Chemotherapy
- Genome-Wide Profiling of Yeast DNA:RNA Hybrid Prone Sites with DRIP-Chip
- The Mechanism of Gene Targeting in Human Somatic Cells
- A LINE-1 Insertion in DLX6 Is Responsible for Cleft Palate and Mandibular Abnormalities in a Canine Model of Pierre Robin Sequence
- Interaction between Two Timing MicroRNAs Controls Trichome Distribution in
- DNA Glycosylases Involved in Base Excision Repair May Be Associated with Cancer Risk in and Mutation Carriers
- The Myc-Mondo/Mad Complexes Integrate Diverse Longevity Signals
- Evolutionarily Diverged Regulation of X-chromosomal Genes as a Primal Event in Mouse Reproductive Isolation
- Mutations in Conserved Residues of the microRNA Argonaute ALG-1 Identify Separable Functions in ALG-1 miRISC Loading and Target Repression
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Isl1 Directly Controls a Cholinergic Neuronal Identity in the Developing Forebrain and Spinal Cord by Forming Cell Type-Specific Complexes
- A Synthetic Community Approach Reveals Plant Genotypes Affecting the Phyllosphere Microbiota
- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- Determining the Control Circuitry of Redox Metabolism at the Genome-Scale
- DNA Repair Pathway Selection Caused by Defects in , , and Telomere Addition Generates Specific Chromosomal Rearrangement Signatures
- Methylome Diversification through Changes in DNA Methyltransferase Sequence Specificity
- Folliculin Regulates Ampk-Dependent Autophagy and Metabolic Stress Survival
- Fine Mapping of Dominant -Linked Incompatibility Alleles in Hybrids
- Unexpected Role of the Steroid-Deficiency Protein Ecdysoneless in Pre-mRNA Splicing
- Three Groups of Transposable Elements with Contrasting Copy Number Dynamics and Host Responses in the Maize ( ssp. ) Genome
- Sox5 Functions as a Fate Switch in Medaka Pigment Cell Development
- Synergistic Interactions between the Molecular and Neuronal Circadian Networks Drive Robust Behavioral Circadian Rhythms in
- Chromatin Landscapes of Retroviral and Transposon Integration Profiles
- Widespread Use of Non-productive Alternative Splice Sites in
- Ras GTPase-Like Protein MglA, a Controller of Bacterial Social-Motility in Myxobacteria, Has Evolved to Control Bacterial Predation by
- Cell Type-Specific Functions of Genes Revealed by Novel Adipocyte and Hepatocyte Circadian Clock Models
- Phenotype Ontologies and Cross-Species Analysis for Translational Research
- Embryogenesis Scales Uniformly across Temperature in Developmentally Diverse Species
- In Pursuit of the Gene: An Interview with James Schwartz
- Molecular Mechanisms of Hypoxic Responses via Unique Roles of Ras1, Cdc24 and Ptp3 in a Human Fungal Pathogen
- Analysis of the Genome and Transcriptome of var. Reveals Complex RNA Expression and Microevolution Leading to Virulence Attenuation
- Genotypic and Functional Impact of HIV-1 Adaptation to Its Host Population during the North American Epidemic
- RNA Editome in Rhesus Macaque Shaped by Purifying Selection
- Proper Actin Ring Formation and Septum Constriction Requires Coordinated Regulation of SIN and MOR Pathways through the Germinal Centre Kinase MST-1
- Interplay of the Serine/Threonine-Kinase StkP and the Paralogs DivIVA and GpsB in Pneumococcal Cell Elongation and Division
- A Quality Control Mechanism Coordinates Meiotic Prophase Events to Promote Crossover Assurance
- CNNM2 Mutations Cause Impaired Brain Development and Seizures in Patients with Hypomagnesemia
- The RNA-Binding Protein QKI Suppresses Cancer-Associated Aberrant Splicing
- Uncoupling Transcription from Covalent Histone Modification
- Rad51–Rad52 Mediated Maintenance of Centromeric Chromatin in
- FRA2A Is a CGG Repeat Expansion Associated with Silencing of
- A General Approach for Haplotype Phasing across the Full Spectrum of Relatedness
- A Novel Highly Divergent Protein Family Identified from a Viviparous Insect by RNA-seq Analysis: A Potential Target for Tsetse Fly-Specific Abortifacients
- A Central Role for in Regulation of Islet Function in Man
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- The Mechanism of Gene Targeting in Human Somatic Cells
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Widespread Use of Non-productive Alternative Splice Sites in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



