-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaKeep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
article has not abstract
Published in the journal: . PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004808
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004808Summary
article has not abstract
The study of aging has been dominated by the analysis of degenerative changes in somatic tissues that influence life span, since the decline of life support systems is the cause of age-related death. Reproduction, which is a vital goal of all organisms, also displays an age-related decline. However, this aspect of aging has received much less attention, since the consequence is not death but rather an age-related decline in fertility. Whereas a substantial number of genetic and environmental factors have been demonstrated to influence somatic aging and life span, only a few factors are known to influence reproductive aging. In this issue of PLOS Genetics, Wang et al. use an innovative reverse genetic approach to investigate reproductive aging [1].
Several features of reproductive aging make it an important subject, and the paucity of studies make this a frontier with the promise of new and exciting discoveries. Although reproductive aging is not lethal, it does have important health consequences. In human females, reproductive aging is an important medical issue because the age-related decline in oocyte quality results in increased birth defects and decreased fertility that culminates in reproductive cessation at menopause [2], [3]. Furthermore, reproductive aging is a central issue in understanding the evolution of aging, since it influences progeny number and therefore fitness. The critical questions that are currently being investigated in the area of reproductive aging are (i) what are the genes, pathways, and mechanisms that regulate reproductive aging? (ii) What happens at the molecular, cellular, and tissue level to cause a functioning reproductive organ to degenerate? (iii) What are the relationships between mechanisms that regulate reproductive and somatic aging? Figure 1A shows that the age-related declines of self-fertile and mated reproduction occur well before the age-related declines of neuromuscular activity and survival probability in Caenorhabditis elegans. Are mechanisms controlling these declines distinct, overlapping, or identical?
Fig. 1. Genetic analysis of reproductive aging in C. elegans. 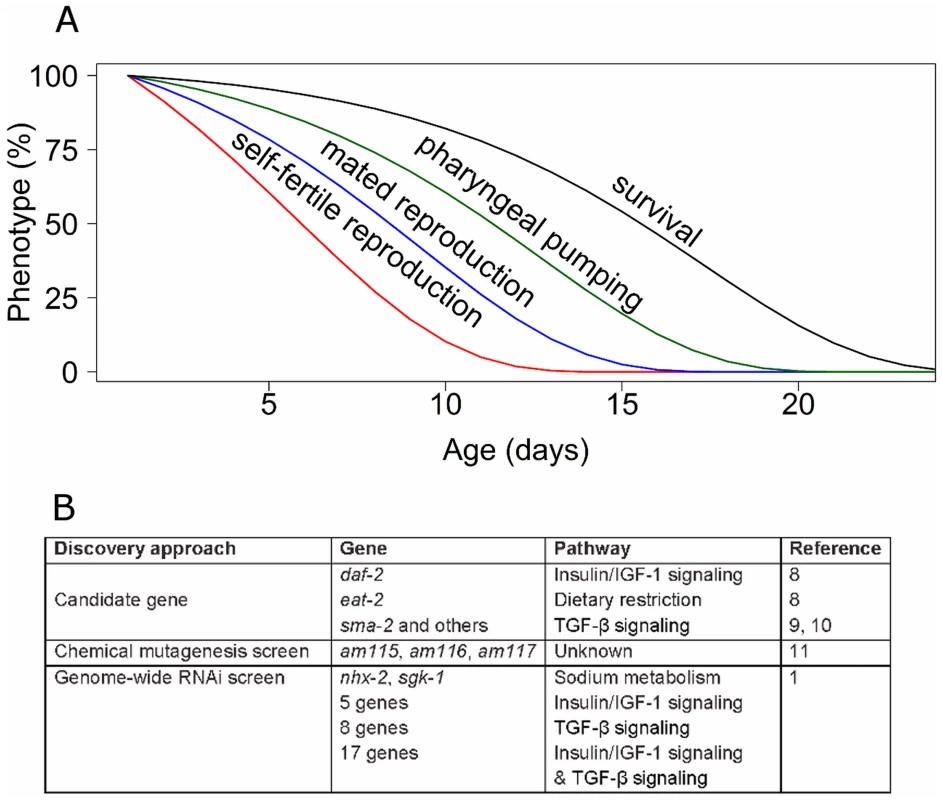
(A) C. elegans displays a series of age-related degenerative changes including loss of self-fertile reproduction (red), mated reproduction (blue), pharyngeal pumping (green), and survival (black). (B) Three major discovery approaches have been used to identify genes that influence reproductive aging. The nematode C. elegans is an important model organism for studies of aging [4], [5]. C. elegans hermaphrodites have a mated reproductive span of approximately ten days and a postreproductive span of approximately six days for a total adult life span of approximately 16 days. A large number of age-related degenerative changes in reproductive and somatic function have been characterized [6], [7]. Sophisticated genetic approaches have resulted in the identification of many genes that influence somatic aging, demonstrating important roles for insulin or insulin-like growth factor (IGF-1) signaling, mitochondrial function, chemosensory function, and dietary intake in modulating adult life span [5].
Two approaches have previously been used to identify genes that modulate reproductive aging in C. elegans: analysis of existing mutations in candidate genes that cause somatic aging delays or other phenotypes, and forward chemical mutagenesis screens (Figure 1B). The candidate gene approach resulted in the discovery that reducing the activity of daf-2, which encodes an insulin or IGF-1 receptor, delays reproductive aging [8]. Dietary restriction extends the life span of many animals, and eat-2 loss-of-function mutations cause dietary restriction by reducing food ingestion. eat-2 mutants display delayed reproductive aging [8]. Mutations of several genes in the TGF-β Sma or Mab signaling pathway, such as sma-2, cause delayed reproductive aging [9], [10]. Thus, high caloric intake and the activity of the insulin or IGF-1 pathway and the TGF-β pathway accelerate reproductive aging. In addition to candidate approaches, the unbiased approach of a forward genetic screen using a chemical mutagen identified three mutants with delayed reproductive aging, although the identity of the affected genes has yet to be reported [11].
The Ruvkun lab has been a pioneer in using whole genome RNAi screens to analyze C. elegans biology, and here they describe using this reverse genetic approach to identify genes that influence reproductive aging [1]. The advantage of this approach is that it is relatively comprehensive, in this case a library of 18,413 RNAi clones was analyzed, and the identity of positive genes was known immediately. However, the reduction of gene activity caused by feeding RNAi is partial and variable in different tissues. Wang et al. identified 32 genes that extend the self-fertile reproductive span by at least 20% when RNAi is used to decrease gene activity [1]. To determine how these genes relate to previously described pathways that influence reproductive aging, Wang et al. analyzed interactions with the insulin or IGF-1, TGF-β, and in some cases the eat-2 caloric restriction pathways [1]. Thirty gene inactivations failed to extend self-fertile reproductive span in the background of altered insulin/IGF-1, TGF-β or both, suggesting that these genes may be involved with these signaling networks. Interestingly, the two gene inactivations that caused the largest reproductive span extensions, sgk-1 and nhx-2, did not interact with the previously identified pathways. The molecular identity of these genes suggests a role in sodium metabolism, which has not previously been implicated in reproductive aging (Figure 1B). Manipulations of both sgk-1 and nhx-2 were previously reported to extend life span [12].
To identify the tissue where these genes function, Wang et al. reduced gene activity only in the germline; ten gene inactivations caused extended reproduction, suggesting the site of action is the germline, whereas 22 gene inactivations did not, suggesting the site of action is somatic tissue [1]. Thus, genes that function in both tissues appear to influence reproductive aging. Because self-fertile reproductive span is limited by sperm depletion in C. elegans [8], Wang et al. analyzed mated hermaphrodites that are not sperm limited. Nineteen gene inactivations caused extended reproduction in mated hermaphrodites [1]. Intriguingly, several RNAi clones that did not cause the phenotype in mated hermaphrodites did cause the phenotype when both males and mated hermaphrodites were exposed, and some RNAi clones caused the phenotype when only males were exposed. These findings suggest that male mating, which includes physical contact as well as sperm and seminal fluid transfer, may influence reproductive aging in hermaphrodites.
To address the role of these genes in somatic aging, Wang et al. analyzed life span. Five gene inactivations extended mean life span, indicating these genes play a role in both somatic and reproductive aging [1].
The analysis of reproductive aging is at an early stage, and the identification of new genetic regulators by an RNAi screen is a timely addition to the previous knowledge of genetic regulators identified by candidate approaches and chemical mutagenesis screens. It appears that the relationship between reproductive aging and somatic aging is complex, since genetic manipulations have now been identified that affect both processes, only somatic aging, or only reproductive aging. Further work is necessary to rigorously determine if there are indeed separate pathways that modulate degeneration of somatic and reproductive function, or whether selective effects relate to the specific genetic manipulations that have been analyzed. The most important and challenging issue to be addressed in future studies is the mechanism of action of these genes in influencing reproductive aging. This is a significant challenge because there is likely to be a cascade of effects that proceeds from the immediate activity of the gene product to the phenotype of reproductive degeneration. Solving these puzzles will require detailed analysis of phenotypes at the molecular, cellular, and organ levels. This also remains the major challenge for the genetic analysis of somatic aging, since comprehensive explanations of how gene activities influence degenerative change in somatic tissues remain elusive. By identifying a new group of genes that influence reproductive aging, Wang et al. establish a critical foundation for mechanistic studies that will elucidate how and why gene activities accelerate the decline of reproduction [1].
Zdroje
1. WangMC, OakleyHD, CarrCE, SowaJN, RuvkunG (2014) Gene pathways that delay Caenorhabditis elegans reproductive senescence. PLoS Genet 10: e1004752.
2. Te VeldeER, PearsonPL (2002) The variability of female reproductive ageing. Hum Reprod Update 8 : 141–154.
3. HartgeP (2009) Genetics of reproductive lifespan. Nat Genet 41 : 637–638.
4. GuarenteL, KenyonC (2000) Genetic pathways that regulate ageing in model organisms. Nature 408 : 255–262.
5. KenyonCJ (2010) The genetics of ageing. Nature 464 : 504–512.
6. CollinsJJ, HuangC, HughesS, KornfeldK (2008) The measurement and analysis of age-related changes in Caenorhabditis elegans.. Wormbook 24 : 1–21.
7. PincusZ, SlackFJ (2010) Developmental biomarkers of aging in Caenorhabditis elegans. Dev Dyn 239 : 1306–1314.
8. HughesSE, EvasonK, XiongC, KornfeldK (2007) Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 3: e25.
9. LuoS, ShawWM, AshrafJ, MurphyCT (2009) TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet 5: e1000789.
10. LuoS, KleemannGA, AshrafJM, ShawWM, MurphyCT (2010) TGF-beta and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 143 : 299–312.
11. Hughes SE, Huang C, Kornfeld K. Identification of mutations that delay somatic or reproductive aging of Caenorhabditis elegans. Genetics 189 : 341–356.
12. NehrkeK (2003) A reduction in intestinal cell pHi due to loss of the Caenorhabditis elegans Na+/H+ exchanger NHX-2 increases life span. J Biol Chem 278 : 44657–44666.
Štítky
Genetika Reprodukční medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 12
-
Všechny články tohoto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



