-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTdp2: A Means to Fixing the Ends
article has not abstract
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003370
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003370Summary
article has not abstract
Topoisomerases carry out the high-wire act of changing DNA structure through transient DNA breaks. Breaks are needed because the topology of DNA can only be changed through cutting the DNA. Topoisomerases are well suited for this tricky enterprise; they hold on to DNA covalently, shielding DNA ends from participation in unwarranted repair signaling or reactions. A downside of this mechanism is that topoisomerases can get trapped on DNA, leading to the new hazard of topoisomerase-mediated DNA damage.
Topoisomerase-mediated damage occurs in at least two important ways. The best appreciated mechanism of trapping topoisomerases on DNA is through the action of anti-cancer drugs such as etoposide [1]. These agents, termed topoisomerase poisons, lead to the accumulation of cleavage complexes, transient intermediates in the enzyme reaction cycle where the DNA is cleaved and the enzyme is covalently bound to DNA via a 5′phosphotyrosyl linkage. DNA metabolic events can also trap topoisomerases: recent work has shown that DNA damage [2], single-strand breaks [3], and mis-insertion of ribonucleotides [4] can trap topoisomerase I, while abasic sites [5] and transcription-related processes [6] may trap topoisomerase II.
The diversity of processes leading to trapping of topoisomerases suggested that cells might have specific mechanisms to repair the protein/DNA covalent complexes. An appealing mechanism is direct cleavage of the tyrosine phosphate ester bond that links topoisomerases to DNA. The first protein to have this activity, tyrosyl DNA phosphodiesterase I (Tdp1), has robust activity against 3′ phosphotyrosyl linkages generated by type 1B topoisomerases [7]. While yeast Tdp1 can also process the 5′ phosphotyrosyl–linked peptides expected to be generated by topoisomerase II or type 1A topoisomerases [8], the activity of the mammalian protein against this type of linkage remains controversial [9], [10]. Subsequent work identified a second tyrosyl DNA phosphodiesterase, Tdp2, with activity against 5′ phosphotyrosyl linkages. In in this week's issue of PLOS Genetics, Gómez-Herreros and colleagues show the importance of Tdp2 in the repair of topoisomerase II covalent complexes [11].
Tdp2 was identified by Cortés-Ledesma and colleagues by a genetic screen using expression of a human cDNA library in yeast followed by selection for camptothecin resistance of a yeast strain lacking tdp1 and rad1 [12]. In addition to Tdp1, they identified a second gene previously identified as TTRAP (TRAF and TNF receptor-associated protein) [13]. They demonstrated that TTRAP had tyrosyl DNA phosphodiesterase activity for both 3′ phosphotyrosyl – and 5′ phosphotyrosyl–linked oligonucleotides, and therefore termed the protein Tdp2. A key finding from the original identification of Tdp2 was that the protein was more active in processing 5′ phosphotyrosyl–linked oligonucleotides, and that siRNA knockdown of Tdp2 in mammalian cells resulted in sensitivity to etoposide, a drug targeting topoisomerase II, but not camptothecin, a drug that targets topoisomerase I. Recent work has greatly enhanced our understanding of the biochemistry and structural biology of Tdp2. Gene knockouts have been described in (avian) DT40 cells [14] and mouse [15], confirming etoposide sensitivity of vertebrate cells lacking Tdp2. In addition, DT40 cells lacking Tdp2 are hypersensitive to camptothecin only if they also lack Tdp1. The structures of Tdp2 from C. elegans, zebrafish, and mouse have been determined, indicating an active site that accommodates adducted single-strand DNA [16], [17]. Structural and biochemical studies indicate that Tdp2 nuclease activity is closely related to the AP endonuclease APE1 [17], [18]. Unlike Tdp1, Tdp2 shows no nuclease or nucleosidase activity.
Gómez-Herreros and colleagues extended the genetic analysis of Tdp2 using the knockouts in DT40 cells and mouse alluded to above. In the accompanying paper [11], the authors characterize the effects of topoisomerase II poisons such as etoposide and doxorubicin in tdp2-deficient cells. They confirm that tdp2-deficient cells are hypersensitive to topoisomerase II poisons, but not other types of DNA damage. Since removal of Top2 will result in a double-strand break (DSB) (see Figure 1), the authors postulated that Tdp2 might function in concert with a specific DSB repair pathway. Indeed, in DT40 cells, they found an epistatic relationship between Tdp2 and Ku70, a component of the non-homologous end-joining (NHEJ) pathway, in the repair of trapped Top2 covalent complexes. In other words, tdp2 ku70 double mutants had the same sensitivity to etoposide as ku70 single mutants. If Tdp2 repaired complexes are preferentially repaired by NHEJ, then loss of Tdp2 should lead to enhanced repair of Top2 complexes by homologous recombination. This was seen, as evidenced by an increase in Rad51 foci in etoposide-treated cells lacking Tdp2 compared to wild type cells, as well as an increase in sister chromatid exchanges. Finally, Gómez-Herreros and colleagues were able to demonstrate the importance of Tdp2 in mice treated with etoposide. Tdp2-deficient mice treated with a relatively low dose of etoposide showed substantial intestinal and lymphoid toxicity compared to mice carrying wild type Tdp2. Taken together, these results clearly demonstrate the importance of Tdp2 in repairing Top2-mediated DNA damage, and suggest that Tdp2 processed damage might be preferentially channeled to NHEJ, perhaps allowing error-free repair of this damage.
Fig. 1. A pathway for repairing topoisomerase II–mediated DNA damage. 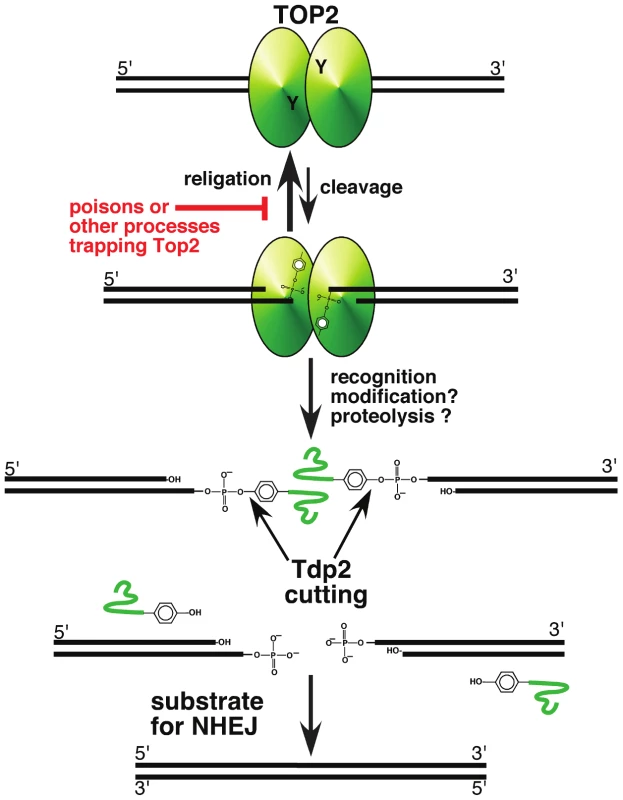
Topoisomerase II, a homodimeric protein, cleaves both strands of DNA, and generates a four base overhang. In the absence of perturbation (such as topoisomerase poisons), religation of the broken strands is kinetically favored, and is likely inhibited by topoisomerase poisons. Recognition of the trapped protein may trigger modification and proteolysis, leaving a short peptide covalently bound to DNA. This peptide can be removed by Tdp2 cleavage of the tyrosyl phosphate bond, leaving DNA with a double-strand break. If the broken DNA is not processed prior to ligation, e.g., by DNA ligase IV in the NHEJ pathway, the result will be error-free repair of the trapped covalent complex. While tyrosyl phosphodiesterases define an important mechanism for removing topoisomerases covalently bound to DNA, there are clearly other important mechanisms at play. Nucleolytic removal of Top2 by the MRN complex (in possible collaboration with Ctip/Sae2) has been suggested by studies in yeast and mammalian cells [19]–[21]. It might be expected that the MRN complex would preferentially channel repair products into homologous recombination pathways.
Can we infer the relative importance of the Tdp2-dependent pathway compared to other processing pathways? The results of Gómez-Herreros and colleagues clearly show the importance of Tdp2, both in terms of etoposide sensitivity and survival of the organism. However, while they show that tdp2 ku70 double mutant cells had the same sensitivity to etoposide as ku70 single mutants, they also find that tdp2 single mutants were much less sensitive to etoposide than ku70 single mutants. This implies that other pathways are important contributors to processing Top2 complexes besides Tdp2. It should also be noted that their previous work in DT40 cells seems to exclude Tdp1 as an important processing factor [15]. Taken together, these observations suggest other pathways that can process Top2 complexes that are subsequently repaired by NHEJ.
The identification of Tdp2 as a key player in repair of topoisomerase-mediated damage is also important because it provides a necessary tool for working out complete pathways. It has been suggested that an initial step in repairing topoisomerase-mediated damage is covalent modification of the trapped protein by ubiquitylation or other small ubiquitin-like proteins [22]. This recognition of topoisomerases by ubiquitin ligases is likely related to how cells avoid attempting to repair a topoisomerase that is not trapped on DNA but is undergoing a normal reaction cycle, but details of this specificity are not currently understood.
A final intriguing question concerns other functions of Tdp2. Before Tdp2 was identified as a topoisomerase repair protein, it had also been found in other contexts. Tdp2 had been identified as a protein that interacts with CD40, tumor necrosis factor (TNF) receptor-75, and TNF receptor-associated factors (and was named TTRAP) [13], and was separately found as an Ets1-interacting protein (and was named EAPII) [23]. EAPII has recently been suggested to play an important role in lung cancer development, with overexpression leading to activation of the MAPK-ERK pathway [24]. Furthermore, Tdp2 has been studied in zebrafish, where it is an essential modulator of Smad3-dependent Nodal signaling gastrulation [25]. The functions of Tdp2/TTRAP/EAPII have been reviewed recently, highlighting the important biological functions of this protein [26]. Given that there are suggestions from several studies that Tdp2/TTRAP/EAPII may play essential functions [26], the finding by Gómez-Herreros and colleagues that this gene is not essential in mouse will certainly provoke additional investigation.
Zdroje
1. NitissJL (2009) Targeting DNA topoisomerase II in cancer chemotherapy. Nature reviews Cancer 9 : 338–350.
2. NitissJL, NitissKC, RoseA, WaltmanJL (2001) Overexpression of type I topoisomerases sensitizes yeast cells to DNA damage. J Biol Chem 276 : 26708–26714.
3. Nitiss JL (1998) DNA topoisomerases in DNA repair and DNA damage tolerance. In: Nockoloff, JA and Hoekstra, MF, editors. DNA damage and repair: Vol. 2, DNA repair in higher eukaryotes. Totowa: Humana. pp. 517–538.
4. KimN, HuangSN, WilliamsJS, LiYC, ClarkAB, et al. (2011) Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332 : 1561–1564.
5. KingmaPS, OsheroffN (1997) Apurinic sites are position-specific topoisomerase II poisons. J Biol Chem 272 : 1148–1155.
6. JuBG, LunyakVV, PerissiV, Garcia-BassetsI, RoseDW, et al. (2006) A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312 : 1798–1802.
7. PouliotJJ, YaoKC, RobertsonCA, NashHA (1999) Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science 286 : 552–555.
8. NitissKC, MalikM, HeX, WhiteSW, NitissJL (2006) Tyrosyl-DNA phosphodiesterase (Tdp1) participates in the repair of Top2-mediated DNA damage. Proc Natl Acad Sci U S A 103 : 8953–8958.
9. InterthalH, ChenHJ, Kehl-FieTE, ZotzmannJ, LeppardJB, et al. (2005) SCAN1 mutant Tdp1 accumulates the enzyme–DNA intermediate and causes camptothecin hypersensitivity. EMBO J 24 : 2224–2233.
10. MuraiJ, HuangSY, DasBB, DexheimerTS, TakedaS, et al. (2012) Tyrosyl-DNA phosphodiesterase 1 (TDP1) repairs DNA damage induced by topoisomerases I and II and base alkylation in vertebrate cells. J Biol Chem 287 : 12848–12857.
11. Gómez-HerrerosF, Romero-GranadosR, ZengZ, Álvarez-QuilónA, QuinteroC, et al. (2013) TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet 9: e1003226 doi:10.1371/journal.pgen.1003226.
12. Cortes-LedesmaF, El KhamisySF, ZumaMC, OsbornK, CaldecottKW (2009) A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461 : 674–678.
13. PypeS, DeclercqW, IbrahimiA, MichielsC, Van RietschotenJG, et al. (2000) TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappa B activation. J Biol Chem 275 : 18586–18593.
14. ZengZ, Cortes-LedesmaF, El KhamisySF, CaldecottKW (2011) TDP2/TTRAP is the major 5′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J Biol Chem 286 : 403–409.
15. ZengZ, SharmaA, JuL, MuraiJ, UmansL, et al. (2012) TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1. Nucleic Acids Res 40 : 8371–8380.
16. ShiK, KurahashiK, GaoR, TsutakawaSE, TainerJA, et al. (2012) Structural basis for recognition of 5′-phosphotyrosine adducts by Tdp2. Nat Struct Mol Biol 19 : 1372–7.
17. SchellenbergMJ, AppelCD, AdhikariS, RobertsonPD, RamsdenDA, et al. (2012) Mechanism of repair of 5′-topoisomerase II-DNA adducts by mammalian tyrosyl-DNA phosphodiesterase 2. Nat Struct Mol Biol 19 : 1363–71.
18. GaoR, HuangSY, MarchandC, PommierY (2012) Biochemical characterization of human tyrosyl-DNA phosphodiesterase 2 (TDP2/TTRAP): a Mg(2+)/Mn(2+)-dependent phosphodiesterase specific for the repair of topoisomerase cleavage complexes. J Biol Chem 287 : 30842–30852.
19. LeeKC, PadgetK, CurtisH, CowellIG, MoianiD, et al. (2012) MRE11 facilitates the removal of human topoisomerase II complexes from genomic DNA. Biol Open 1 : 863–873.
20. HartsuikerE, NealeMJ, CarrAM (2009) Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Molecular Cell 33 : 117–123.
21. HamiltonNK, MaizelsN (2010) MRE11 function in response to topoisomerase poisons is independent of its function in double-strand break repair in Saccharomyces cerevisiae. PLoS ONE 5: e15387 doi:10.1371/journal.pone.0015387.
22. MaoY, DesaiSD, TingCY, HwangJ, LiuLF (2001) 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J Biol Chem 276 : 40652–40658.
23. PeiH, YordyJS, LengQ, ZhaoQ, WatsonDK, et al. (2003) EAPII interacts with ETS1 and modulates its transcriptional function. Oncogene 22 : 2699–2709.
24. LiC, FanS, OwonikokoTK, KhuriFR, SunSY, et al. (2011) Oncogenic role of EAPII in lung cancer development and its activation of the MAPK-ERK pathway. Oncogene 30 : 3802–3812.
25. EsguerraCV, NellesL, VermeireL, IbrahimiA, CrawfordAD, et al. (2007) Ttrap is an essential modulator of Smad3-dependent Nodal signaling during zebrafish gastrulation and left-right axis determination. Development 134 : 4381–4393.
26. LiC, SunSY, KhuriFR, LiR (2011) Pleiotropic functions of EAPII/TTRAP/TDP2: cancer development, chemoresistance and beyond. Cell Cycle 10 : 3274–3283.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



