-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
Centromeres are specialized chromatin regions marked by the presence of nucleosomes containing the centromere-specific histone H3 variant CENP-A, which is essential for chromosome segregation. Assembly and disassembly of nucleosomes is intimately linked to DNA topology, and DNA topoisomerases have previously been implicated in the dynamics of canonical H3 nucleosomes. Here we show that Schizosaccharomyces pombe Top3 and its partner Rqh1 are involved in controlling the levels of CENP-ACnp1 at centromeres. Both top3 and rqh1 mutants display defects in chromosome segregation. Using chromatin immunoprecipitation and tiling microarrays, we show that Top3, unlike Top1 and Top2, is highly enriched at centromeric central domains, demonstrating that Top3 is the major topoisomerase in this region. Moreover, centromeric Top3 occupancy positively correlates with CENP-ACnp1 occupancy. Intriguingly, both top3 and rqh1 mutants display increased relative enrichment of CENP-ACnp1 at centromeric central domains. Thus, Top3 and Rqh1 normally limit the levels of CENP-ACnp1 in this region. This new role is independent of the established function of Top3 and Rqh1 in homologous recombination downstream of Rad51. Therefore, we hypothesize that the Top3-Rqh1 complex has an important role in controlling centromere DNA topology, which in turn affects the dynamics of CENP-ACnp1 nucleosomes.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003371
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003371Summary
Centromeres are specialized chromatin regions marked by the presence of nucleosomes containing the centromere-specific histone H3 variant CENP-A, which is essential for chromosome segregation. Assembly and disassembly of nucleosomes is intimately linked to DNA topology, and DNA topoisomerases have previously been implicated in the dynamics of canonical H3 nucleosomes. Here we show that Schizosaccharomyces pombe Top3 and its partner Rqh1 are involved in controlling the levels of CENP-ACnp1 at centromeres. Both top3 and rqh1 mutants display defects in chromosome segregation. Using chromatin immunoprecipitation and tiling microarrays, we show that Top3, unlike Top1 and Top2, is highly enriched at centromeric central domains, demonstrating that Top3 is the major topoisomerase in this region. Moreover, centromeric Top3 occupancy positively correlates with CENP-ACnp1 occupancy. Intriguingly, both top3 and rqh1 mutants display increased relative enrichment of CENP-ACnp1 at centromeric central domains. Thus, Top3 and Rqh1 normally limit the levels of CENP-ACnp1 in this region. This new role is independent of the established function of Top3 and Rqh1 in homologous recombination downstream of Rad51. Therefore, we hypothesize that the Top3-Rqh1 complex has an important role in controlling centromere DNA topology, which in turn affects the dynamics of CENP-ACnp1 nucleosomes.
Introduction
Centromeres are unique regions of eukaryotic chromosomes that are essential for chromosome segregation at mitosis and meiosis. The specialized centromeric chromatin directs assembly of kinetochores, which serve as points of attachment for the spindle apparatus. In all eukaryotes, centromeric chromatin is marked by the presence of nucleosomes containing the histone H3 variant Centromere Protein-A (CENP-A), which is a key determinant for centromere identity and essential for centromere function. The specific incorporation and maintenance of CENP-A at centromeres is an epigenetic phenomenon that is not yet fully understood, and new factors involved in CENP-A dynamics are continuing to be discovered. During DNA replication in S-phase pre-existing CENP-A is equally partitioned to sister centromeres and after chromosome segregation newly synthesized CENP-A is incorporated specifically at pre-existing centromeres, possibly involving a feed-forward mechanism between pre-existing CENP-A chromatin and CENP-A assembly factors [1]–[2]. In agreement, it was recently shown that the constitutive centromere-associated network (CCAN) component Centromere Protein C (CENP-C) is required for recruitment of the Mis18 complex [3]. The Mis18 complex is in turn necessary for localization of CENP-A to centromeres and is hypothesized to have a role in centromere priming [4]–[7]. Furthermore, Mis18 is required for centromere targeting of the CENP-A-specific chaperone Holliday Junction Recognition Protein (HJURP), which is both necessary and sufficient for stable recruitment of CENP-A [6], [8]–[9]. It binds specifically to pre-nucleosomal CENP-A and histone H4, and has been shown to facilitate assembly of nucleosomes containing CENP-A in vitro [8]–[14]. The Mis18 complex and/or the CENP-A pre-nucleosomal complex also associates with the chaperone RbAp48, which can mediate assembly of nucleosomes containing CENP-A in vitro [4]–[5], [10]–[11], [15].
Despite continuous advances in the identification of pathways and factors controlling CENP-A nucleosome assembly, the molecular architecture of CENP-A nucleosomes remains unclear. CENP-A, H4, H2A and H2B can be assembled into conventional octameric nucleosomes with left-handed negative wrapping of DNA in vitro [13], [16]–[19]. However, they can also be assembled into tetrameric hemisomes containing only one copy of each histone with right-handed positive wrapping of DNA [20]. Different studies aiming at determining the composition of CENP-A nucleosomes in vivo have found contradicting evidence for both octamers and tetramers [17], [20]–[24]. Interestingly, recent studies imply that there may be cell cycle-dependent transitions in the structure of CENP-A nucleosomes in vivo [25]–[26]. This would reconcile the contradictions between different studies on CENP-A nucleosome structure. A few other models for the structure of CENP-A nucleosomes have also been proposed, but are associated with less experimental evidence.
DNA topoisomerases catalyze changes in DNA topology by cutting, shuffling and re-ligating DNA strands. Nucleosome dynamics are intimately linked to DNA topology [27]. Since DNA is wrapped around the histone core of nucleosomes in a left-handed negative direction, negative supercoiling of DNA favors nucleosome assembly while positive supercoiling of DNA favors nucleosome disassembly. In agreement, DNA topoisomerases have been implicated in the dynamics of canonical H3 nucleosomes in vitro and in vivo [28]–[31]. Eukaryotic topoisomerase III is a type 1A topoisomerase capable of relaxing negatively supercoiled DNA [32]–[33]. Topoisomerase III displays evolutionarily conserved genetic and physical interactions with RecQ helicases and RecQ-mediated genome instability (Rmi) proteins [34]–[39]. RecQ helicases and Rmi1 stimulate relaxation of negative supercoiling by topoisomerase III and together they also have the ability to fully de-catenate and catenate DNA molecules as well as to ‘dissolve’ double Holiday Junctions [40]–[43]. The single RTR complex in Schizosaccharomyces pombe consists of Rqh1, Top3 and Rmi1. These proteins are critical for genome stability and have so far been implicated in homologous recombination (HR) and DNA damage checkpoint activation in vivo [35], [44]–[50]. Both top3 and rmi1 deletion mutants stop dividing after just a few generations with severe defects in nuclear morphology and chromosome segregation [37], [51]–[52]. Thermo-sensitive top3 mutants display growth defects, sensitivity to DNA damaging agents, illegitimate recombination, altered nuclear morphology, and defects in chromosome segregation at restrictive temperatures [46], [53]. The lethality of top3 and rmi1 deletion mutants can be rescued by mutations in rqh1, likely because Rqh1 creates intermediate structures in HR that without Top3 remain unresolved and prevent chromosome segregation [51]–[52]. Mutations in rqh1 result in similar but less severe phenotypes compared to top3 and rmi1 mutations [49], [54]–[55].
In this report, we investigated the genome-wide localization of S. pombe Top3 and discovered that it is preferentially found at intergenic regions (IGRs), sub-telomeres and centromeres. Top3 occupancy at IGRs is similar to that of Top1 and Top2. On the other hand, high relative enrichment of Top3 at centromeric central domains is unique, and is positively correlated with CENP-ACnp1 occupancy. Both top3 and rqh1 mutants display defects in chromosome segregation and increased relative enrichment of CENP-ACnp1 at centromeric central domains. Thus, the Top3-Rqh1 complex normally limits the levels of CENP-ACnp1 in this region. Altered levels of CENP-ACnp1 are accompanied by changes in the levels of the CENP-ACnp1-specific chaperone HJURPScm3 and are independent of HR downstream of Rad51. We therefore suggest that the Top3-Rqh1 complex has an important role in controlling centromere DNA topology and thereby the dynamics CENP-ACnp1 nucleosomes. Specific removal of negative supercoiling by Top3 should inhibit assembly of and destabilize octameric CENP-ACnp1 nucleosomes with left-handed negative wrapping of DNA. In addition, removal of negative supercoiling may limit centromeric transcription, which is hypothesized to promote CENP-ACnp1 nucleosome assembly. In this model, impaired Top3 activity would facilitate assembly of CENP-ACnp1 nucleosomes. Alternatively, the activity of Top3 may create a unique topological state at centromeres that specifically favors formation of tetrameric CENP-ACnp1 hemisomes with right-handed positive wrapping of DNA over octameric CENP-ACnp1 nucleosomes. In this model, impaired Top3 activity would result in a shift from formation of CENP-ACnp1-containing hemisomes towards octameres at centromeres.
Results
The top3-105 mutant displays impaired growth and defects in chromosome segregation
In this study we used the previously isolated but uncharacterized thermo-sensitive top3-105 mutant [56]. We sequenced the top3 open reading frame and identified a A762G base pair substitution that results in a Tyr209Cys amino acid change (Figure 1A). This residue is found in a region involved in conformation changes upon DNA binding and is conserved in budding yeast Top3 as well as in metazoan Top3α [57]. Growth of the top3-105 mutant is similar to wild type at 25°C and 30°C, but severely impaired at the restrictive temperature of 36°C (Figure 1B). When cultures grown at 25°C are shifted to 36°C, the top3-105 mutant initially proliferates with kinetics similar to wild type, but after approximately two generations proliferation is severely slowed, with essentially no further increase in cell numbers (Figure 1C). Deletion of rqh1 rescues the lethality of a top3 deletion [51]–[52]. Growth of the top3Δ rqh1Δ double mutant is similar to the rqh1Δ mutant and somewhat slower compared to wild type at all temperatures (Figure 1B and 1C). The lethality of a top3 deletion can also be suppressed by deleting rad51, which is upstream of Top3 and Rqh1 in the ‘dissolution’ pathway in HR [35]. The rad51Δ single mutant displays slow growth, which is further enhanced at 36°C, but not to the same extent as for the top3-105 mutant (Figure 1B and 1C) [58]. The severe growth defect of the top3-105 mutant is not suppressed by deletion of rad51 as the top3-105 rad51Δ double mutant displays similarly impaired growth as the top3-105 mutant at 36°C. Moreover, the top3-105 rad51Δ double mutant displays a small synthetic growth defect at 25°C and 30°C. Growth of the rqh1Δ rad51Δ double mutant is largely similar to the rad51Δ single mutant at all temperatures [59].
Fig. 1. The top3-105 mutant carries an Y209C amino acid substitution and displays impaired growth at 36°C. 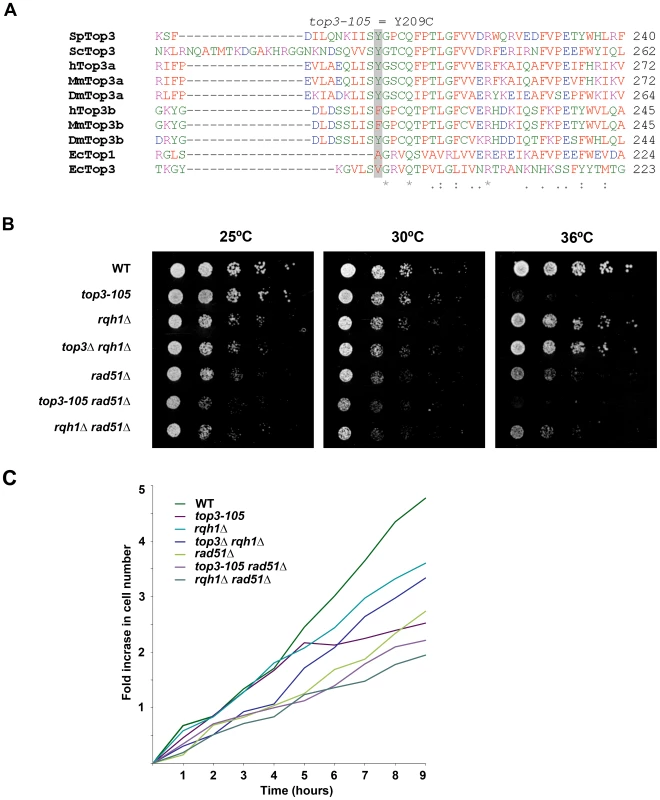
(A) Alignment of Top3 amino acid sequences from different species. The position corresponding to Y209 of S. pombe Top3 is highlighted in grey. The amino acids are colored according to their physiochemical properties. An asterisk (*) indicates a fully conserved residue, a colon (:) indicates conservation between groups of strongly similar properties, and a period (.) indicates conservation between groups of weakly similar properties between all species. (B) Spotting of the indicated strains in 5-fold serial dilutions on plates incubated at 25°C, 30°C and 36°C. (C) Growth kinetics of the indicated strains in liquid media after a shift from 25°C to 36°C at time zero. Subsequently, we looked at nuclear morphology in the mutants using 4′.6-diamidino-2-phenylindole (DAPI) to stain DNA and anti-tubulin immunofluorescence to stain the mitotic spindle, respectively. After 8 hours at 36°C, there is a large increase in the fraction of abnormally long cells (>15 µm) for the top3-105 mutant (28%, n = 500) compared to wild type (0.4%, n = 500), which is indicative of cell cycle delay (Figure 2A). Both the rqh1Δ and top3Δ rqh1Δ mutants display less pronounced increases in the fractions of unusually elongated cells (14% and 17%, respectively, n = 500). The rad51Δ, rad51Δ top3-105 and rad51Δ rqh1Δ mutants all display equally large fractions of elongated cells (53%, 52% and 53%, respectively, n = 500) [58]. Furthermore, the top3-105 mutant display various nuclear and mitotic defects, including amorphous and fragmented nuclei, unequal segregation of DNA, lagging chromosomes and a ‘cut’/‘torn’ phenotype, after 8 hours at 36°C (Figure 2A–2B). The rqh1Δ and top3Δ rqh1Δ mutants display similar but less pronounced defects in chromosome segregation compared to the top3-105 mutant (p = 0.024 and p = 0.00023) (Figure 2A–2B and Table S1). Deletion of rad51 rescues the severe mitotic defects of the top3-105 mutant (p = 0.0046). However, the top3-105 rad51Δ double mutant and the rad51Δ single mutant both still display moderate defects in chromosome segregation. Moreover, deletion of rad51 does not affect the chromosome segregation defects of the rqh1Δ mutant, as the rqh1Δ rad51Δ double mutant displays similar levels of mitotic defects as the rqh1Δ and rad51Δ single mutants (Figure 2A–2B and Table S1). Therefore, it seems that Top3, Rqh1 and Rad51 are all important for normal mitotic chromosome segregation.
Fig. 2. Top3 and Rqh1 are required for chromosome segregation. 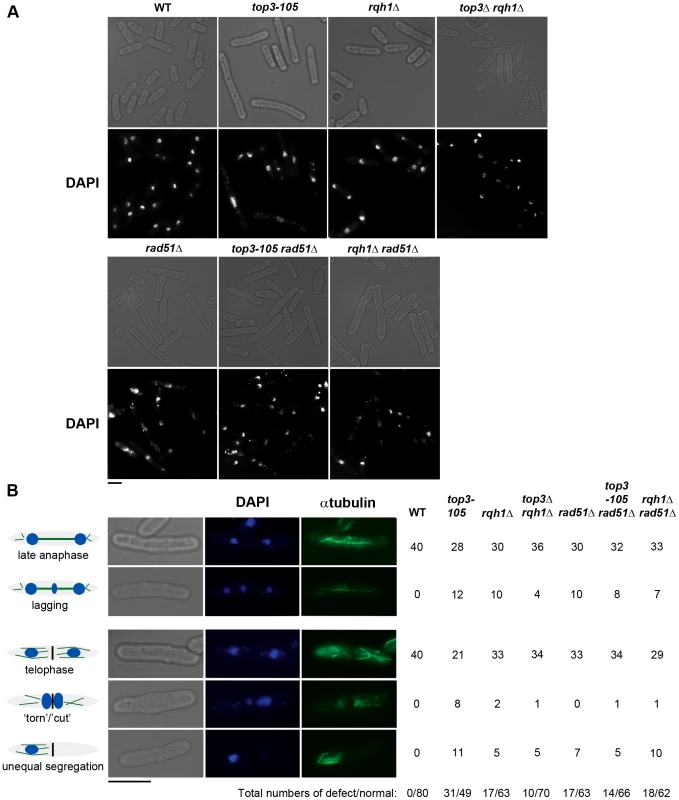
(A) Light and fluorescence microscopy images of the indicated strains with DAPI staining of the DNA after 8 hours at 36°C. (B) Light and fluorescence microscopy images of wild type and top3-105 mutant cells after 8 hours at 36°C. The table shows the numbers of cells displaying normal and defective chromosome segregation among 40 late anaphase cells (with a mitotic spindle and two separate foci of DNA) or 40 telophase cells (with a septum) for the indicated strains after 8 hours at 36°C. The scale bars represents 6.65 µM. Top3 is highly enriched at intergenic regions, sub-telomeres, and centromeres
Next, we investigated the genome-wide relative enrichment of Top3 by chromatin immunoprecipitation (ChIP) of c-Myc epitope tagged Top3 expressed from the endogenous locus and hybridization to high-resolution tiling microarrays (ChIP-chip). Along the euchromatic chromosome arms high relative enrichment of Top3 is preferentially found at intergenic regions (IGRs), while it is generally depleted from open reading frames (ORFs) (Figure 3A). Top3 occupancy is also found at sub-telomeric regions, including the rDNA clusters at the left and right sub-telomeric regions of chromosome III (tel3L and tel3R) (Figure 3A and Figure S1A–S1B). Telomeric repeats are not represented on the array and therefore telomere occupancy could not be investigated. Moreover, we observed a consistently high relative enrichment of Top3 at centromeres (Figure 3A and Figure S1A–S1B). Next, we determined the genome-wide average levels of Top3 when genes are aligned at the transcription start site (TSS) and transcription termination site (TTS), respectively. Top3 display high average relative enrichment at the TTS, but depletion from the TSS and the ORF (Figure 3B). There is also a small average enrichment of Top3 just upstream of the TSS, where gene promoters are generally localized. Overall, this binding pattern is similar to that of Top1 and Top2. Depletion of Top3 from the TSS and ORF as well as enrichment at the TTS is more pronounced for strongly transcribed genes, whereas Top3 occupancy at promoter regions is preferentially found at non-transcribed and weakly transcribed genes (Figure S1C). Looking at individual genes, we identified 233 5′IGRs that display a >1.5-fold and 455 3′IGRs that display a >2-fold average relative enrichment of Top3. These overlap significantly (p<0.001) with 5′ and 3′IGRs that are enriched for Top1 and Top2 (p<0.001 for all pair wise comparisons) (Figure 3C). Thus, similar to Top1 and Top2, Top3 preferentially binds to intergenic regions along the euchromatic chromosome arms. In addition, Top3 is enriched at sub-telomeres and centromeres.
Fig. 3. Top3 is enriched at IGRs, centromeres, and sub-telomeric regions. 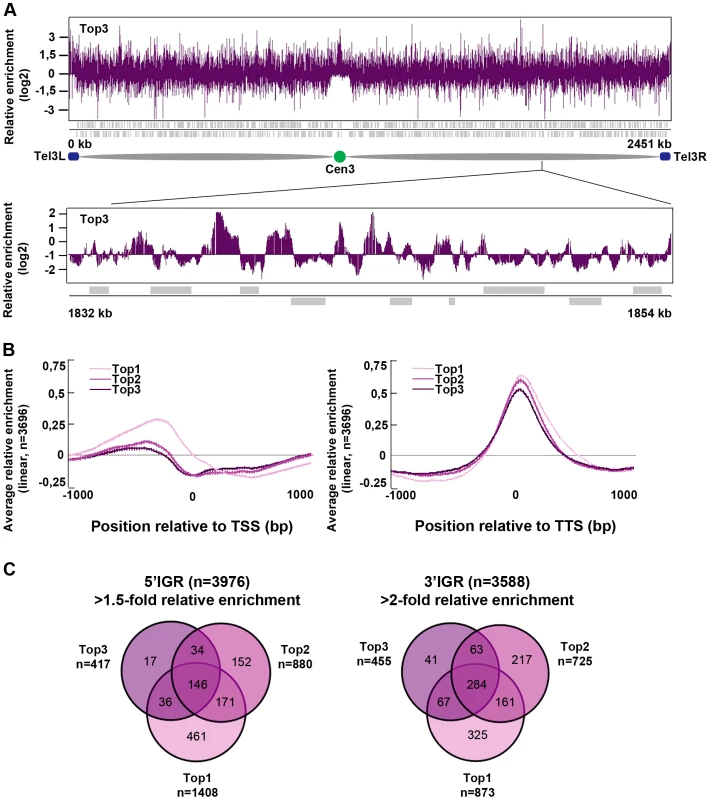
(A) ChIP-chip relative enrichment of Top3-myc along chromosome III at 30°C. The schematic picture shows the approximate position of the centromere and the subtelomeric regions. Telomeric repeats are not represented on the array. (B) Moving average for the relative enrichment of Top3, Top2 and Top1 after alignment of genes at the TSS and TTS, respectively. Error bars represent 99% confidence intervals. The bottom bar illustrates statistical significance by t-tests for the difference between the graphs at each point using a continuous spectrum from black (p = 1) via red to yellow (p = 0). (C) Overlaps between 5′IGRs with an average >1.5-fold and 3′IGRs with an average >2-fold relative enrichment of Top3, Top2 and Top1, respectively. The overlaps are statistically significant (p<0.001) by pair-wise hyper-geometric distribution tests. All data is an average of two independent experiments. Top3 and gene transcription
S. pombe Top1 and Top2 have previously been implicated in various stages of transcription [30]–[31]. We investigated the genome-wide transcription levels in the top3-105 mutant by total RNA extraction, reverse transcription and hybridization to tiling microarrays. After 8 hours at 36°C, there is a small reduction in the genome-wide average RNA levels from ORFs in the top3-105 mutant compared to wild type (Figure 4). However, after filtering out non-transcribed genes (AU<100 in both wild type and the top3-105 mutant) we only identified 118 (106 protein coding) genes that displayed a >1.5-fold decrease of RNA levels in the top3-105 mutant. Moreover, there are also 299 (211 protein coding) genes that display a >1.5-fold increase of RNA levels in the top3-105 mutant. Thus, the top3-105 mutant displays minor changes in RNA levels, associated with both up - and down-regulation of gene transcription.
Fig. 4. Top3 has small effects on gene transcription. 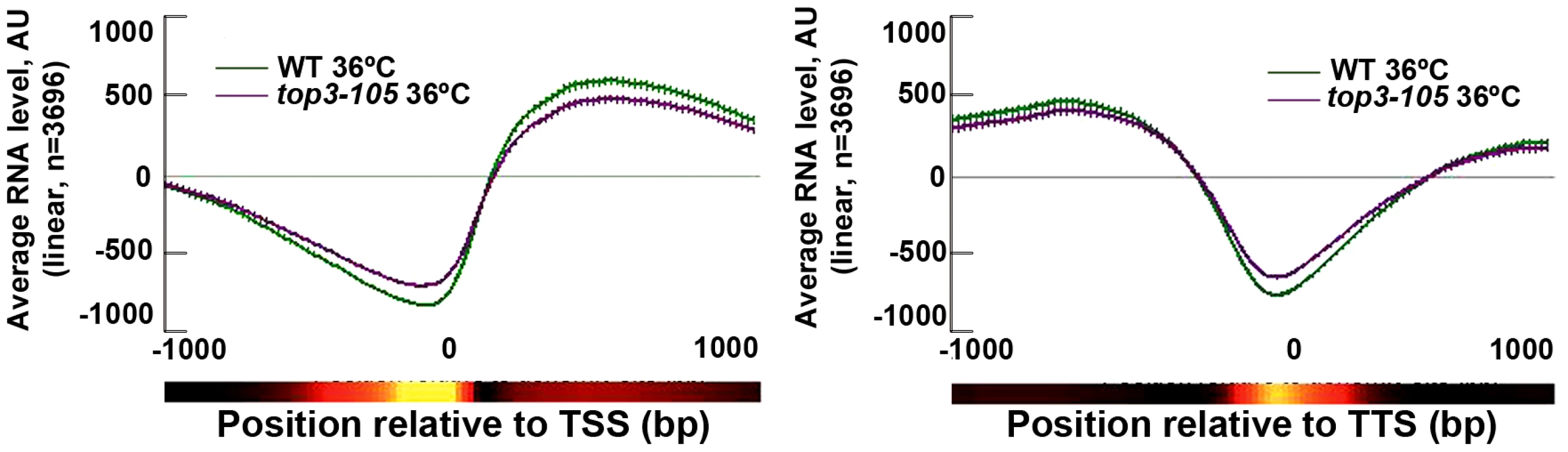
Average RNA levels in wild type and the top3-105 mutant after 8 hours at 36°C when genes are aligned at the TSS and TTS, respectively. Error bars represent 99% confidence intervals. The bottom bar illustrates statistical significance for the difference between the graphs at each point using a continuous spectrum going from black (p = 1) via red to yellow (p = 0). All data is an average of two independent experiments. Top3 and CENP-ACnp1 co-localize at centromeres and 5′IGRs
Top3 occupancy at centromeres is most pronounced at the central domain, consisting of the central core (cnt) and the innermost repeat (imr) regions, where there is 4–8 fold enrichment of Top3 relative to the rest of the genome (Figure 5A and Figure S2). Interestingly, both Top1 and Top2 occupancies are relatively low at this region. The central domain, and particularly the cnt region, is also highly enriched for CENP-ACnp1. Centromeric CENP-ACnp1 occupancy displays a strong positive correlation with Top3 occupancy, but no correlation with Top2 and a weaker positive correlation with Top1 occupancy (Figure 5B). In fact, there is a subset of probes forming a separate cluster in the scatter plot for which there is a unique positive correlation with Top3 enrichment as opposed to Top1 and Top2 enrichment. This cluster corresponds to the majority of the cnt probes, for which the relative enrichment of CENP-ACnp1 is distinctively high. In S. pombe, it has previously been shown that CENP-ACnp1 also localizes to gene promoters [60]. 5′IGRs that display a >3-fold average relative enrichment of CENP-ACnp1 significantly overlap with those that display a >1.5-fold average relative enrichment of Top3 (p<0.001) (Figure 5C). However, as expected from the similar bindings of Top1 and Top2 to promoter regions, there are also significant overlaps with those that display a >1.5-fold average relative enrichment of Top2 and Top1, respectively (p<0.001 for pair-wise comparisons). Moreover, average CENP-ACnp1 occupancies at these 5′IGRs display a weaker positive correlation with Top3 occupancy, as well as with Top2 and Top1 occupancies (Figure 5D). Thus, Top3 co-localizes with CENP-ACnp1 at both centromeres and 5′IGRs, but centromeres are distinctive in that they display a unique correlation between Top3 occupancy and high levels of CENP-ACnp1 occupancy, which is not seen for Top1 and Top2.
Fig. 5. Top3 and CENP-ACnp1 occupancies are positively correlated. 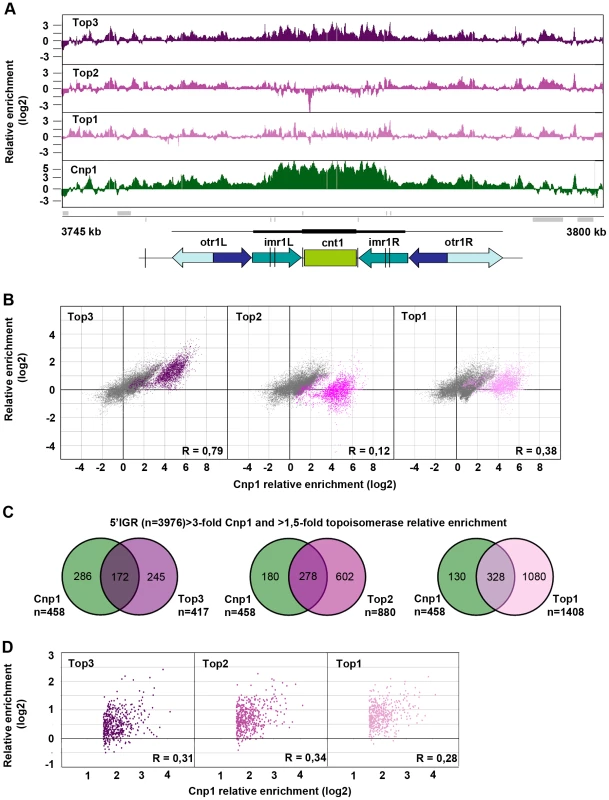
(A) ChIP-chip relative enrichment of Top3-myc, Top2-myc, Top1-myc and CENP-ACnp1 along centromere I at 30°C. Grey boxes represent genes. A schematic representation where arrows represent repeat elements and black lines represent tRNA genes is shown. The vertical arrow indicates the position of primers used in D-F. The scale bar represents 2.5 kb. (B) Density scatter plot for all centromeric probes showing the correlations between the relative enrichment of Cnp1 and Top3, Top2, and Top1, respectively. Probes corresponding to central cores are shown in color. Pearson's correlation coefficients for all centromeric probes (R) are shown. (C) Overlaps between 5′IGRs with an average >3-fold relative enrichment of Cnp1 and >1.5-fold relative enrichment of Top3, Top2 and Top1, respectively. The overlaps are statistically significant (p<0.001) by pair-wise hyper-geometric distribution tests. (D) Scatter plot for 5′IGRs with high (>1.5-fold) relative enrichment of Cnp1 showing the correlations between the average relative enrichment of Cnp1 and Top3, Top2, and Top1, respectively. Pearson's correlation coefficients (R) are shown. All data is an average of two independent experiments. Increased levels and redistribution of non-centromeric CENP-ACnp1 at 5′IGRs in the top3-105 mutant
Next, we used ChIP-chip to investigate the genome-wide relative enrichment of CENP-ACnp1 in the top3-105 mutant after 8 hours at 36°C. There is a small increase in the genome-wide average relative enrichment of CENP-ACnp1 at promoter regions in the top3-105 mutant compared to wild type (Figure 6A). However, there is no difference in the average relative enrichment of CENP-ACnp1 at the 198 5′IGRs that display a high (>3-fold) relative enrichment of CENP-ACnp1 in wild type (Figure 6B). Among these sites, there are 13 that display a >1.5-fold increase and 54 that display a >1.5-fold decrease in the relative enrichment of CENP-ACnp1 in the top3-105 mutant. Moreover, we identified 85 5′IGR that are not enriched for CENP-ACnp1 in wild type, but display a >1.5-fold increase resulting in a rather high (>1.5-fold) relative enrichment of CENP-ACnp1 in top3-105 mutant. Thus, there is both an overall increase in the average levels and a redistribution of CENP-ACnp1 at promoter regions in the top3-105 mutant.
Fig. 6. Top3 and Rqh1 affect CENP-ACnp1 enrichment at 5′IGRs and centromeres. 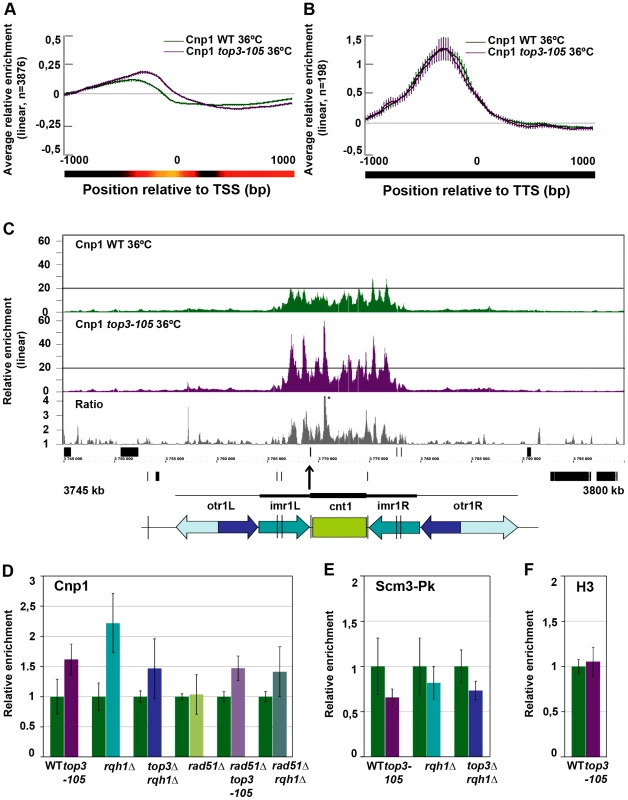
(A) Moving average for the relative enrichment of CENP-ACnp1 in wild type and the top3-105 mutant after alignment of genes at the TSS. Error bars represent 99% confidence intervals. The bottom bar illustrates statistical significance for the difference between the graphs at each point using a continuous spectrum going from black (p = 1) via red to yellow (p = 0). (B) Same as in A for 198 genes with >1.5-fold relative enrichment of CENP-ACnp1 in wild type. (C) ChIP-chip relative enrichment of CENP-ACnp1 in wild type and the top3-105 mutant and the ratio between these along centromere I after 8 hours at 36°C. Grey boxes represent genes. A schematic representation where arrows represent repeat elements and black lines represent tRNA genes is shown. A * indicates that the peak is higher than the maximum value of the axis. (D) ChIP-qPCR relative enrichment of CENP-ACnp1 in wild type and the indicated mutants after 8 hours at 36°C. (E) ChIP-qPCR average relative enrichment of HJURPScm3-Pk/V5 in wild type and the indicated mutants after 8 hours at 36°C. (F) ChIP-qPCR average relative enrichment of H3 in wild type and the top3-105 mutant after 8 hours at 36°C. ChIP-qPCR was performed using triplicate samples in two independent experiments. Relative enrichment at the cnt region of chromosome I was calculated using the ddCt method, normalizing to ChIP input and act1. Samples were also normalized to the average of the wild type samples in each experiment. Error bars represent the standard deviations between six samples. Increased levels of centromeric CENP-ACnp1 and decreased levels of HJURPScm3 in top3-105 and rqh1Δ mutants
At centromeres, where there is a unique overlap between Top3 and CENP-ACnp1 occupancy, there is a consistent increase in CENP-ACnp1 enrichment at the central domains in the top3-105 mutant compared to wild type after 8 hours at 36°C (Figure 6C and Figure S3). This does not seem to be due to indirect effects by changes in transcription, since no genes known to be involved in CENP-ACnp1 assembly or disassembly were found among those that displayed >1.5-fold increase or decrease in RNA levels in the top3-105 mutant (Table S2). We also investigated the total amounts of CENP-ACnp1 protein by acid extraction of histones from chromatin and immunoblotting using strains expressing FLAG epitope tagged CENP-ACnp1 from the endogenous locus. In agreement with the ChIP data, there is an increase in the total levels of CENP-ACnp1 protein associated with chromatin in the top3-105 mutant compared to wild type (Figure S4). The soluble fraction of CENP-ACnp1 is small in both wild type and mutants, but may be slightly higher in the mutant. Using ChIP and qPCR we confirmed that there is an increase in the relative enrichment of CENP-ACnp1 at the cnt region of chromosome I in the top3-105 mutant compared to wild type after 8 hours at 36°C (p = 0.0030) (Figure 6D). This phenotype is found also in the rqh1Δ mutant and the top3Δ rqh1Δ mutant (p = 0.00026 and p = 0.045, respectively). The rad51Δ single mutant on the other hand displays similar levels of CENP-ACnp1 compared to wild type in this region. Moreover, deletion of rad51 does not suppress the increase in CENP-ACnp1 enrichment in the top3-105 and rqh1Δ mutants, as the rad51Δ top3-105 and rad51Δ rqh1Δ double mutants still display increased relative enrichment of CENP-ACnp1 compared to wild type at the cnt region of chromosome I (p = 0.00035 and p = 0.037) (Figure 6D). Thus, Top3 and Rqh1 normally limit the levels of CENP-ACnp1 by a mechanism that is largely independent of their role in HR downstream of Rad51.
Next, we investigated whether the altered levels of CENP-ACnp1 is associated with changes in the relative enrichment of the CENP-ACnp1-specific chaperone HJURPScm3 by ChIP-qPCR using strains expressing Pk/V5 epitope tagged HJURPScm3 from the endogenous locus. Surprisingly, the enrichment of HJURPScm3 at the cnt region of chromosome I is reduced in the top3-105, rqh1Δ and top3Δ rqh1Δ mutants compared to wild type after 8 hours at 36°C (p = 0.028, p = 0.24 and p = 0.011, respectively) (Figure 6E). Thus, Top3 and Rqh1 have the opposite effect on the levels of HJURPScm3 compared to the levels of CENP-ACnp1 in this region. Last, we tested if the altered levels of CENP-ACnp1 at centromeres were associated with altered levels of histone H3 in this region. However, there was no significant difference in the relative enrichment of histone H3 at the cnt region of chromosome I in the top3-105 mutant. Thus, Top3 and Rqh1 affect centromeric chromatin in a way that specifically limits the levels of CENP-ACnp1 and promotes association of HJURPScm3 at central domains.
Discussion
Top3 and Rqh1 are required for accurate chromosome segregation
The top3-105 mutant carries an A762G single base pair substitution resulting in a Tyr209Cys amino acid change. This residue is conserved in S. cerevisiae Top3 as well as in metazoan Top3α, and resides in an important region involved in conformational changes upon DNA binding [57]. Similar to other thermo-sensitive top3 mutants, the top3-105 mutant displays impaired growth, altered nuclear morphology and various defects in chromosome segregation soon after a shift to the restrictive temperature [46], [53]. Deletion of top3 is known to cause very severe nuclear and mitotic defects resulting in cell death after just a few generations [51]–[52] Since the lethality can be suppressed by deletion of rqh1 it is hypothesized that these extreme nuclear defects are caused by rapid accumulation of unresolved Rqh1-dependent HR intermediates that prevent chromosome segregation and ultimately causes lethality. However, both the rqh1Δ single mutant and the top3Δ rqh1Δ double mutant still display moderate mitotic defects, indicating that there may be additional roles for the Top3-Rqh1 complex in chromosome segregation. Rad51 has previously been shown to be important for normal mitotic chromosome segregation and the rad51Δ mutant displays moderate defects in chromosome segregation [58]. Deletion of rad51 also suppresses the severe chromosome segregation defects of the top3-105 mutant, but the top3-105 rad51Δ double mutant also still display moderate defects in chromosome segregation. Deletion of rad51 has no effect on the mitotic defects in the rqh1Δ mutant, as the rqh1Δ rad51Δ double mutant display similar levels of chromosome segregation defects as the rqh1Δ and rad51Δ single mutants. Thus, it seems that Top3, Rqh1 and Rad51 are all important for normal mitotic chromosome segregation.
Top3 is the major topoisomerase at centromeric central domains
The genome-wide localization of Top3 reveals high relative enrichment at IGRs, towards subtelomeric regions and at centromeres. The binding pattern for Top3 at IGRs is similar to those of Top1 and Top2, possibly indicating that all tree topoisomerases have overlapping functions at promoters and TTSs, such as maintenance of 5′ and 3′ nucleosome depleted regions (NDRs) important for transcription [30]–[31]. However, we did not find any major effects on transcription for the top3-105 mutant, and Top3 association at promoters is mostly seen at non-transcribed genes. The relative enrichment of Top3 is also high towards subtelomeric regions, including the rDNA clusters at tel3L and tel3R. This is in agreement with the role of S. pombe Top3 and Rqh1 in replication recombination at rDNA and telomere repeats [46]–[47], [61]. Surprisingly, Top3 is also enriched at centromeres and particularly at centromeric central domains, where there is no pronounced enrichment of Top1 and Top2. Thus, Top3 is the major topoisomerase present at centromeric central domains and may have a unique function in this region.
Top3 and Rqh1 affect the relative enrichment of CENP-ACnp1
The chromatin structure found at centromeric central domains is unique in that it contains high levels of the histone H3 variant CENP-ACnp1. Interestingly, Top3 occupancy displays a unique positive correlation with CENP-ACnp1 occupancy in these regions, and especially at the cnt regions where the enrichment of CENP-ACnp1 is particularly high. This led us to investigate if Top3 has an effect on CENP-ACnp1 nucleosomes in this region. Intriguingly, the relative enrichment of CENP-ACnp1 at central domains is increased both in the top3-105 mutant, the rqh1Δ mutant and the top3Δ rqh1Δ double mutant. This suggests that the activity of the Top3-Rqh1 complex normally limits the levels of CENP-ACnp1 in this region. Intriguingly, the altered structure and/or dynamics of centromeric CENP-ACnp1-containing chromatin may contribute to the chromosome segregation defects seen in the top3-105, rqh1Δ and top3-105 rqh1Δ mutants. However, this new role is clearly not the cause of the extremely severe chromosome segregation defects and lethality in the top3Δ mutant, as the top3Δ rqh1Δ mutant is viable while still retaining this phenotype. As previously described, the lethality of the top3Δ mutant likely depends on accumulation of unresolved Rqh1-dependent recombination intermediates, which are probably independent of changes in centromeric chromatin.
Interestingly, CENP-ACnp1 has also been shown to be associated with gene promoters [60]. Like for centromeric CENP-ACnp1, there is an overall increase in the relative enrichment of CENP-ACnp1 at promoter regions in the top3-105 mutant. In addition, there is a partial redistribution of non-centromeric CENP-ACnp1. Thus, it is clear that Top3 also affects the dynamics of CENP-ACnp1 outside of centromeres. However, since Top3, Top2 and Top1 are all enriched at promoter regions, the dynamics of CENP-ACnp1 outside centromeres is likely to depend on all three topoisomerases, making the situation complex.
Top3 may affect CENP-ACnp1 nucleosome dynamics by regulating centromeric DNA topology
The most established role of the Top3-Rqh1 complex is in HR, where they act downstream of Rad51. One possibility is that the effects on CENP-ACnp1 enrichment relates to the role of Top3 and Rqh1 in this pathway. Rad51 is required for accurate chromosome segregation and has previously been shown to suppress chromosomal rearrangements at centromeres [58], [62]. Moreover, it was recently hypothesized that HR could be involved in higher-order organization of S. pombe centromeres [63]. However, increased relative enrichment of CENP-ACnp1 is still seen in the top3-105 rad51Δ and rqh1Δ rad51Δ double mutants, but not in the rad51Δ single mutant. Therefore, the role of the Top3-Rqh1 complex in limiting the levels of CENP-ACnp1 seems largely independent of Rad51-dependent HR. Chromosome segregation defects are on the other hand also seen in the rad51Δ single mutant, indicating that HR may somehow be important for proper chromosome segregation. Thus, chromosome segregation defects in top3 and rqh1 mutants could originate both from defects in HR and from altered levels of CENP-ACnp1 at centromeres. Another possibility is that the Top3-Rqh1 complex has an indirect effect on CENP-ACnp1 dynamics due to altered transcription of genes involved in CENP-ACnp1 nucleosome dynamics. However, we did not find any significant change in transcription for any of the genes currently known to be involved in CENP-ACnp1 dynamics. A third possibility is that Top3 and Rqh1 affects the stability of the CENP-ACnp1 protein. The total amount of CENP-ACnp1 present in cells is increased in the top3-105 mutant compared to wild type. However, this seems to mostly relate to an increase in the total levels of CENP-ACnp1 associated with chromatin, while the amount of soluble CENP-ACnp1 is low in both wild type and mutant. In budding yeast, it has been shown that the amount of soluble CENP-A is tightly controlled by rapid proteolysis, while nucleosome assembly at centromeres stabilizes the protein [64]. In agreement, reduced levels of CENP-ACnp1 at centromeres have previously been associated with a reduction of the total CENP-ACnp1 protein levels found in cells [65]. Thus, increased protein levels of CENP-ACnp1 is in agreement with increased levels of CENP-ACnp1 nucleosomes at centromeres, and it seems less likely that impaired Top3 function would stabilize soluble CENP-ACnp1. Instead, we suggest that Top3 together with Rqh1 affect the assembly and disassembly of CENP-ACnp1 nucleosomes by regulating centromere DNA topology.
Nucleosome assembly and disassembly are intimately linked to DNA topology and DNA topoisomerases have previously been shown to affect the assembly of canonical H3 nucleosomes [27]–[31]. In S. pombe, Top1 and Top2 have been implicated in disassembly of H3 nucleosomes at 5′ and 3′ IGRs [30]–[31]. Here, removal of negative supercoiling by Top1 and Top2 is hypothesized to stimulate nucleosome disassembly mediated by the chromatin remodeler Hrp1. Centromeres are likely to be topologically constrained regions where nucleosome dynamics are highly dependent on DNA topology and topoisomerases. Since Top3 is highly enriched at central domains as compared to the other topoisomerases, nucleosome dynamics in this region likely depends particularly on Top3. Top3 is unique in that it preferentially removes negative supercoiling. Thus, the activity of Top3 should have a negative effect on assembly of CENP-ACnp1 nucleosomes with left-handed wrapping of DNA. This could be a way of controlling and fine-tuning the assembly of CENP-ACnp1 nucleosomes mediated by factors such as HJURPScm3. In the top3-105 mutant, a shift towards a state of more negative supercoiling would result in increased stability and facilitated assembly of CENP-ACnp1 nucleosomes, altering the dynamics of CENP-ACnp1 nucleosomes. In agreement with increased relative enrichment of CENP-ACnp1 also in the rqh1Δ mutant, efficient relaxation of negative supercoils by Top3 has been shown to be dependent on RecQ helicases [41], [66]. HJURPScm3 associates with centromeric chromatin during most of the cell cycle, independently of CENP-ACnp1, but dissociates from centromeres right after assembly of newly synthesized CENP-ACnp1 in the G2 phase of the cell cycle [8]–[9], [67]. Reduced levels of HJURPScm3 at centromeres in the top3-105 and rqh1Δ mutants may thus reflect facilitated loading of CENP-ACnp1 from the pre-nucleosomal complex onto centromeric DNA and a more rapid dissociation of HJURPScm3.
An alternative or additional role in centromeric transcription
In addition to nucleosome dynamics, DNA topology is also known to be important for transcription. Recent studies have shown that transcription is permissive also at the CENP-ACnp1 containing centromeric central domains in fission yeast [60]. Although the exact function is unclear, carefully modulated centromeric transcription has been suggested to play a role in formation of kinetochores as well as assembly of CENP-ACnp1 nucleosomes [68]. In support, factors known to be important for transcription have been implicated in CENP-ACnp1 assembly [65], [69]–[70]. Thus, it is possible that Top3 as the main DNA topoisomerase at centromeric central domains affects transcription-coupled CENP-ACnp1 assembly. However, this is not mutually exclusive with a direct effect on CENP-ACnp1 nucleosome assembly.
Is Top3 a factor that controls the structure of CENP-ACnp1 nucleosomes?
The molecular architecture of CENP-A nucleosomes is a subject of debate. Some studies suggest that CENP-A, H4, H2A and H2B form hemisomes with right-handed wrapping of DNA [20], [22]–[24]. In this case, due to opposite wrapping of the DNA double helix, preferential relaxation of negative supercoils by Top3 should increase the stability of CENP-ACnp1 hemisomes. In this model, impaired Top3 activity in the top3 and rqh1 mutants may result in a shift from assembly of right-handed CENP-ACnp1 hemisomes toward assembly of left-handed octameric CENP-ACnp1 nucleosomes at centromeres, thus giving increased levels of CENP-ACnp1. Such a structural transition has previously been observed upon ectopic incorporation of S. cerevisiae CENP-ACse4 at non-centromeric loci [24]. Recent studies have also suggested that CENP-A nucleosomes may cycle between octameres and tetramers during the cell cycle [25]–[26]. In this case, the Top3-Rqh1 complex may affect one or both species. Interestingly, it was also shown human HJURP and budding yeast Scm3 associates with centromeres specifically during formation of CENP-A hemisomes [25]–[26]. Thus, reduced levels of Scm3 at centromeres in top3 and rqh1 mutants can thus also be reconciled with a structural change in CENP-A chromatin during some part of the cell cycle. Moreover, the fact that CENP-ACnp1 levels are increased, while H3 levels remains the same in the Top3 mutant, would be consistent with a structural change specific for CENP-ACnp1 nucleosomes.
In conclusion, we found that S. pombe Top3 displays a unique enrichment at centromeres where it affects centromeric chromatin in a way that limits the levels of CENP-ACnp1. We suggest that the Top3-Rqh1 complex has an important role in regulating centromeric DNA topology, thereby affecting CENP-ACnp1 nucleosome dynamics and perhaps the structure CENP-ACnp1 nucleosomes. Thus, the Top3-Rqh1 complex may be part of the intricate network of factors and pathways that comes together to carefully regulate CENP-ACnp1 nucleosome dynamics. This function could contribute to the observed chromosome segregation defects in top3 and rqh1 mutants.
Materials and Methods
Fission yeast strains and methods
Standard procedures for genetic manipulation and growth of S. pombe were used [71]. The strain expressing Pk/V5 epitope tagged HJURPScm3 was a gift from Professor M. Yanagida, the thermo-sensitive top3-105 mutant and the rad51Δ mutant were acquired from the Yeast Genetic Resource Center (YGRC). The rqh1Δ mutant was a gift from S-W. Wang. Strains used in this study are listed in Table S3.
Sequencing of the top3-105 mutation
Genomic DNA was isolated as previously described, except that cell walls were digested with 0.4 mg/ml zymolyase 100T (USBiological) and RNA was digested with 10 µg/ml RNase (Roche) for 60 minutes at 37°C [71]. The top3 open reading frame was amplified by PCR and the purified PCR product was sent to Eurofins MWG Operon for custom DNA sequencing. Primers used are listed in Table S4. Protein sequence alignments were generated using ClustalW2 (EMBL-EBI, http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Spotting and analysis of growth kinetics
Cells grown to log-phase at 25°C were spotted in 5-fold serial dilutions and incubated at 25°C, 30°C or 36°C. Cells were grown to early log-phase at 25°C and then shifted to 36°C for 9 hours, while determining cell density hourly using a microscope counting chamber.
Immunofluorescence microscopy
Immunofluorescence microscopy was performed as previously described with the following details and alterations [72]. Cells were grown to mid-log phase first at 25°C and then at 36°C for 8 hours. Cells were fixed in 3.7% formaldehyde and 0.2% glutaraldehyde for 60 minutes before digestion with 0.5 mg/ml zymolyase 100T (US Biological) for 70 minutes at 37°C. Cells were incubated with 1∶80 dilution of TAT1 mouse anti-tubulin serum (a gift from K. Gull) over night and then with 1∶100 dilution of FITC-conjugated goat anti-mouse (F-1010, Sigma) over night. Cells were stained with 0.2 µg/ml 4′.6-diamidino-2-phenylindole (DAPI) and mounted on poly-L-lysine-coated microscopy slides (LabScientific) using Vectashield mounting media (Vector laboratories). Cells were examined by fluorescence microscopy using a Zeiss Axioplan 2 (Carl Zeiss) equipped with a Plan-Neofluar 63X/1.25 PH3 oil objective (Carl Zeiss), an ORCA-100 CCD camera (Hamamatsu Photonix) and Openlab 5.0.2 software (Improvision). P values for comparing chromosome segregation defects were generated using a two-tailed Fishers exact test.
Chromatin Immunoprecipitation (ChIP) and preparation for tiling microarrays
ChIP was performed as previously described with the following details and alterations [73]. Cells were grown to mid-log phase at 30°C or first at 25°C and then at 36°C for 8 hours. Cells were lysed using a FastPrep-24 homogenizer (MP Biomedicals) with seven 30 second pulses at 6.5 m/s. Chromatin was fragmented using a Vibra-cell VCX 130 sonicator (Sonics) equipped with a 2 mm stepped microtip, set to 40% amplitude with 10 second pulses and 15 second pauses for two minutes. Chromatin fragments were immunoprecipitated with 1 µg 9E10 mouse anti-Myc (M4439 Sigma), 3 µg rabbit anti-H3 (Ab1791 Abcam), 10 µl rabbit anti-Cnp1 antiserum (a gift from R. Allshire) or 3 µg mouse anti-V5 (MCA1360 Serotec). DNA was recovered using QIAquick PCR Purification with Buffer PB (Qiagen). For analysis on GeneChip S. pombe Tiling 1.0FR Arrays (Affymetrix) 5 mM dUTP was added to the second round of DNA amplification. Fragmentation, labelling and hybridization were performed by the Affymetrix core facility at Karolinska Institiutet (BEA) using standard protocols (http://www.affymetrix.com).
Total RNA extraction and preparation for tiling microarrays
Cells were grown to mid-log phase first at 25°C and then at 36°C for 8 hours. Total RNA was extracted using hot acid-phenol and chloroform. Reverse transcription, labeling, fragmentation and hybridization to GeneChip S. pombe Tiling 1.0FR Arrays (Affymetrix) was performed by BEA.
Microarray data analysis
Raw data (.CEL format) was analyzed using Affymetrix Tiling Analysis Software (TAS) v1.1. One sample analysis and linear scaling was used for RNA samples. Two-sample comparison of immunoprecipitated and input samples, with linear scaling and separate quantile normalization was used for ChIP samples. Probe signals were generated using a bandwidth of 100 and assigned to S. pombe genome coordinates (Sanger 2007 and Sanger 2004 for centromeres). Browser images were generated using Integrated Genome Browser (IGB) (Affymetrix) and PodBat (www.podbat.org). Podbat was used for averaging signals across ORFs and IGRs, for comparing gene lists and for moving averages (bandwidth 100 probes, step size 20 probes) after alignment of genes at the TSS and TTS based on previous annotations [74]. For statistical analysis, a t-test was performed for each bin under the null hypothesis that there is no difference between two sets. 5′IGRs and 3′IGRs were defined as 500 bp upstream or downstream of the ORF or up until the neighbouring gene if shorter. CENP-ACnp1, Top1 and Top2 ChIP-chip and wild type transcription at 30°C raw data (.CEL format) are from previous studies [30], [75]. The microarray data from this publication have been submitted to the GEO database [http://www.ncbi.nlm.nih.gov/geo/] and assigned the accession number GSE44206.
Quantitative PCR
Quantitative real-time PCR (qPCR) was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems) and the associated Sequence Detection Software v.1.3 (Applied Biosystems). ChIP relative enrichment was calculated using the ddCt method, normalizing to ChIP input (dCt) and to a control locus (ddCt) (act1). For each experiment all samples were also normalized to the average of the wild type. Standard deviations were calculated for the total of six samples from two independent experiments. P values were generated using an unpaired two-sample t-test. Primers used are listed in Table S4.
Supporting Information
Zdroje
1. ShelbyRD, MonierK, SullivanKF (2000) Chromatin assembly at kinetochores is uncoupled from DNA replication. J Cell Biol 151 : 1113–1118.
2. JansenLE, BlackBE, FoltzDR, ClevelandDW (2007) Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol 176 : 795–805.
3. MoreeB, MeyerCB, FullerCJ, StraightAF (2011) CENP-C recruits M18BP1 to centromeres to promote CENP-A chromatin assembly. J Cell Biol 194 : 855–871.
4. HayashiT, FujitaY, IwasakiO, AdachiY, TakahashiK, et al. (2004) Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell 118 : 715–729.
5. FujitaY, HayashiT, KiyomitsuT, ToyodaY, KokubuA, et al. (2007) Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev Cell 12 : 17–30.
6. BarnhartMC, KuichPH, StellfoxME, WardJA, BassettEA, et al. (2011) HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol 194 : 229–243.
7. MaddoxPS, HyndmanF, MonenJ, OegemaK, DesaiA (2007) Functional genomics identifies a Myb domain-containing protein family required for assembly of CENP-A chromatin. J Cell Biol 176 : 757–763.
8. PidouxAL, ChoiES, AbbottJK, LiuX, KaganskyA, et al. (2009) Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell 33 : 299–311.
9. WilliamsJS, HayashiT, YanagidaM, RussellP (2009) Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol Cell 33 : 287–298.
10. DunleavyEM, RocheD, TagamiH, LacosteN, Ray-GalletD, et al. (2009) HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell 137 : 485–497.
11. FoltzDR, JansenLE, BaileyAO, YatesJR3rd, BassettEA, et al. (2009) Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell 137 : 472–484.
12. ShuaibM, OuararhniK, DimitrovS, HamicheA (2010) HJURP binds CENP-A via a highly conserved N-terminal domain and mediates its deposition at centromeres. Proc Natl Acad Sci U S A 107 : 1349–1354.
13. DechassaML, WynsK, LiM, HallMA, WangMD, et al. (2011) Structure and Scm3-mediated assembly of budding yeast centromeric nucleosomes. Nat Commun 2 : 313.
14. ShivarajuM, CamahortR, MattinglyM, GertonJL (2011) Scm3 is a centromeric nucleosome assembly factor. J Biol Chem 286 : 12016–12023.
15. FuruyamaT, DalalY, HenikoffS (2006) Chaperone-mediated assembly of centromeric chromatin in vitro. Proc Natl Acad Sci U S A 103 : 6172–6177.
16. SekulicN, BassettEA, RogersDJ, BlackBE (2010) The structure of (CENP-A-H4)(2) reveals physical features that mark centromeres. Nature 467 : 347–351.
17. CamahortR, ShivarajuM, MattinglyM, LiB, NakanishiS, et al. (2009) Cse4 is part of an octameric nucleosome in budding yeast. Mol Cell 35 : 794–805.
18. TachiwanaH, KagawaW, ShigaT, OsakabeA, MiyaY, et al. (2011) Crystal structure of the human centromeric nucleosome containing CENP-A. Nature 476 : 232–235.
19. KingstonIJ, YungJS, SingletonMR (2011) Biophysical characterization of the centromere-specific nucleosome from budding yeast. J Biol Chem 286 : 4021–4026.
20. FuruyamaT, HenikoffS (2009) Centromeric nucleosomes induce positive DNA supercoils. Cell 138 : 104–113.
21. ZhangW, ColmenaresSU, KarpenGH (2012) Assembly of Drosophila centromeric nucleosomes requires CID dimerization. Mol Cell 45 : 263–269.
22. DalalY, WangH, LindsayS, HenikoffS (2007) Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol 5: e218 doi:10.1371/journal.pbio.0050218
23. DimitriadisEK, WeberC, GillRK, DiekmannS, DalalY (2010) Tetrameric organization of vertebrate centromeric nucleosomes. Proc Natl Acad Sci U S A 107 : 20317–20322.
24. KrassovskyK, HenikoffJG, HenikoffS (2011) Tripartite organization of centromeric chromatin in budding yeast. Proc Natl Acad Sci U S A
25. BuiM, DimitriadisEK, HoischenC, AnE, QuenetD, et al. (2012) Cell-cycle-dependent structural transitions in the human CENP-A nucleosome in vivo. Cell 150 : 317–326.
26. ShivarajuM, UnruhJR, SlaughterBD, MattinglyM, BermanJ, et al. (2012) Cell-cycle-coupled structural oscillation of centromeric nucleosomes in yeast. Cell 150 : 304–316.
27. PattertonHG, von HoltC (1993) Negative supercoiling and nucleosome cores. I. The effect of negative supercoiling on the efficiency of nucleosome core formation in vitro. J Mol Biol 229 : 623–636.
28. AlmouzniG, MechaliM (1988) Assembly of spaced chromatin involvement of ATP and DNA topoisomerase activity. Embo J 7 : 4355–4365.
29. GarintherWI, SchultzMC (1997) Topoisomerase function during replication-independent chromatin assembly in yeast. Mol Cell Biol 17 : 3520–3526.
30. Durand-DubiefM, PerssonJ, NormanU, HartsuikerE, EkwallK (2010) Topoisomerase I regulates open chromatin and controls gene expression in vivo. Embo J 29 : 2126–2134.
31. Durand-DubiefM, SvenssonJP, PerssonJ, EkwallK (2011) Topoisomerases, chromatin and transcription termination. Transcription 2 : 66–70.
32. KimRA, WangJC (1992) Identification of the yeast TOP3 gene product as a single strand-specific DNA topoisomerase. J Biol Chem 267 : 17178–17185.
33. GoulaouicH, RoulonT, FlamandO, GrondardL, LavelleF, et al. (1999) Purification and characterization of human DNA topoisomerase IIIalpha. Nucleic Acids Res 27 : 2443–2450.
34. GangloffS, McDonaldJP, BendixenC, ArthurL, RothsteinR (1994) The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol Cell Biol 14 : 8391–8398.
35. LaursenLV, AmpatzidouE, AndersenAH, MurrayJM (2003) Role for the fission yeast RecQ helicase in DNA repair in G2. Mol Cell Biol 23 : 3692–3705.
36. WuL, DaviesSL, NorthPS, GoulaouicH, RiouJF, et al. (2000) The Bloom's syndrome gene product interacts with topoisomerase III. J Biol Chem 275 : 9636–9644.
37. ChangM, BellaouiM, ZhangC, DesaiR, MorozovP, et al. (2005) RMI1/NCE4, a suppressor of genome instability, encodes a member of the RecQ helicase/Topo III complex. Embo J 24 : 2024–2033.
38. MullenJR, NallasethFS, LanYQ, SlagleCE, BrillSJ (2005) Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex. Mol Cell Biol 25 : 4476–4487.
39. YinJ, SobeckA, XuC, MeeteiAR, HoatlinM, et al. (2005) BLAP75, an essential component of Bloom's syndrome protein complexes that maintain genome integrity. Embo J 24 : 1465–1476.
40. HarmonFG, DiGateRJ, KowalczykowskiSC (1999) RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: a conserved mechanism for control of DNA recombination. Mol Cell 3 : 611–620.
41. WuL, HicksonID (2002) The Bloom's syndrome helicase stimulates the activity of human topoisomerase IIIalpha. Nucleic Acids Res 30 : 4823–4829.
42. WuL, HicksonID (2003) The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426 : 870–874.
43. CejkaP, PlankJL, BachratiCZ, HicksonID, KowalczykowskiSC (2010) Rmi1 stimulates decatenation of double Holliday junctions during dissolution by Sgs1-Top3. Nat Struct Mol Biol 17 : 1377–1382.
44. OakleyTJ, GoodwinA, ChakravertyRK, HicksonID (2002) Inactivation of homologous recombination suppresses defects in topoisomerase III-deficient mutants. DNA Repair (Amst) 1 : 463–482.
45. CromieGA, HyppaRW, SmithGR (2008) The fission yeast BLM homolog Rqh1 promotes meiotic recombination. Genetics 179 : 1157–1167.
46. WinTZ, GoodwinA, HicksonID, NorburyCJ, WangSW (2004) Requirement for Schizosaccharomyces pombe Top3 in the maintenance of chromosome integrity. J Cell Sci 117 : 4769–4778.
47. WinTZ, MankouriHW, HicksonID, WangSW (2005) A role for the fission yeast Rqh1 helicase in chromosome segregation. J Cell Sci 118 : 5777–5784.
48. HopeJC, CruzataLD, DuvshaniA, MitsumotoJ, MaftahiM, et al. (2007) Mus81-Eme1-dependent and -independent crossovers form in mitotic cells during double-strand break repair in Schizosaccharomyces pombe. Mol Cell Biol 27 : 3828–3838.
49. DaveyS, HanCS, RamerSA, KlassenJC, JacobsonA, et al. (1998) Fission yeast rad12+ regulates cell cycle checkpoint control and is homologous to the Bloom's syndrome disease gene. Mol Cell Biol 18 : 2721–2728.
50. DoeCL, DixonJ, OsmanF, WhitbyMC (2000) Partial suppression of the fission yeast rqh1(−) phenotype by expression of a bacterial Holliday junction resolvase. Embo J 19 : 2751–2762.
51. GoodwinA, WangSW, TodaT, NorburyC, HicksonID (1999) Topoisomerase III is essential for accurate nuclear division in Schizosaccharomyces pombe. Nucleic Acids Res 27 : 4050–4058.
52. MaftahiM, HanCS, LangstonLD, HopeJC, ZigourasN, et al. (1999) The top3(+) gene is essential in Schizosaccharomyces pombe and the lethality associated with its loss is caused by Rad12 helicase activity. Nucleic Acids Res 27 : 4715–4724.
53. OhM, ChoiIS, ParkSD (2002) Topoisomerase III is required for accurate DNA replication and chromosome segregation in Schizosaccharomyces pombe. Nucleic Acids Res 30 : 4022–4031.
54. MurrayJM, LindsayHD, MundayCA, CarrAM (1997) Role of Schizosaccharomyces pombe RecQ homolog, recombination, and checkpoint genes in UV damage tolerance. Mol Cell Biol 17 : 6868–6875.
55. StewartE, ChapmanCR, Al-KhodairyF, CarrAM, EnochT (1997) rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. Embo J 16 : 2682–2692.
56. YuasaT, HayashiT, IkaiN, KatayamaT, AokiK, et al. (2004) An interactive gene network for securin-separase, condensin, cohesin, Dis1/Mtc1 and histones constructed by mass transformation. Genes Cells 9 : 1069–1082.
57. ChangelaA, DiGateRJ, MondragonA (2001) Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule. Nature 411 : 1077–1081.
58. JangYK, JinYH, ShimYS, KimMJ, YooEJ, et al. (1995) Evidences for possible involvement of Rhp51 protein in mitotic events including chromosome segregation. Biochem Mol Biol Int 37 : 329–337.
59. MiyabeI, MorishitaT, HishidaT, YoneiS, ShinagawaH (2006) Rhp51-dependent recombination intermediates that do not generate checkpoint signal are accumulated in Schizosaccharomyces pombe rad60 and smc5/6 mutants after release from replication arrest. Mol Cell Biol 26 : 343–353.
60. ChoiES, StralforsA, CastilloAG, Durand-DubiefM, EkwallK, et al. (2011) Identification of noncoding transcripts from within CENP-A chromatin at fission yeast centromeres. J Biol Chem 286 : 23600–23607.
61. RogO, MillerKM, FerreiraMG, CooperJP (2009) Sumoylation of RecQ helicase controls the fate of dysfunctional telomeres. Mol Cell 33 : 559–569.
62. NakamuraK, OkamotoA, KatouY, YadaniC, ShitandaT, et al. (2008) Rad51 suppresses gross chromosomal rearrangement at centromere in Schizosaccharomyces pombe. Embo J 27 : 3036–3046.
63. McFarlaneRJ, HumphreyTC (2010) A role for recombination in centromere function. Trends Genet 26 : 209–213.
64. CollinsKA, FuruyamaS, BigginsS (2004) Proteolysis contributes to the exclusive centromere localization of the yeast Cse4/CENP-A histone H3 variant. Curr Biol 14 : 1968–1972.
65. CarlstenJO, SzilagyiZ, LiuB, LopezMD, SzasziE, et al. (2012) Mediator Promotes CENP-A Incorporation at Fission Yeast Centromeres. Mol Cell Biol 32 : 4035–4043.
66. HarmonFG, BrockmanJP, KowalczykowskiSC (2003) RecQ helicase stimulates both DNA catenation and changes in DNA topology by topoisomerase III. J Biol Chem 278 : 42668–42678.
67. LandoD, EndesfelderU, BergerH, SubramanianL, DunnePD, et al. (2012) Quantitative single-molecule microscopy reveals that CENP-A(Cnp1) deposition occurs during G2 in fission yeast. Open Biol 2 : 120078.
68. ChanFL, WongLH (2012) Transcription in the maintenance of centromere chromatin identity. Nucleic Acids Res 40 (22) 11178–88 doi:10.1093/nar/gks921
69. ChenES, SaitohS, YanagidaM, TakahashiK (2003) A cell cycle-regulated GATA factor promotes centromeric localization of CENP-A in fission yeast. Mol Cell 11 : 175–187.
70. WalfridssonJ, BjerlingP, ThalenM, YooEJ, ParkSD, et al. (2005) The CHD remodeling factor Hrp1 stimulates CENP-A loading to centromeres. Nucleic Acids Res 33 : 2868–2879.
71. MorenoS, KlarA, NurseP (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194 : 795–823.
72. HaganIM, HyamsJS (1988) The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci 89 (Pt 3) 343–357.
73. Durand-DubiefM, EkwallK (2009) Chromatin immunoprecipitation using microarrays. Methods Mol Biol 529 : 279–295.
74. LantermannAB, StraubT, StralforsA, YuanGC, EkwallK, et al. (2010) Schizosaccharomyces pombe genome-wide nucleosome mapping reveals positioning mechanisms distinct from those of Saccharomyces cerevisiae. Nat Struct Mol Biol 17 : 251–257.
75. StralforsA, WalfridssonJ, BhuiyanH, EkwallK (2011) The FUN30 chromatin remodeler, Fft3, protects centromeric and subtelomeric domains from euchromatin formation. PLoS Genet 7: e1001334 doi:10.1371/journal.pgen.1001334
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



