-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInteraction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
Syngnathia (bony fusion of the upper and lower jaw) is a rare human congenital condition, with fewer than sixty cases reported in the literature. Syngnathia typically presents as part of a complex syndrome comprising widespread oral and maxillofacial anomalies, but it can also occur in isolation. Most cartilage, bone, and connective tissue of the head and face is derived from neural crest cells. Hence, congenital craniofacial anomalies are often attributed to defects in neural crest cell formation, survival, migration, or differentiation. The etiology and pathogenesis of syngnathia however remains unknown. Here, we report that Foxc1 null embryos display bony syngnathia together with defects in maxillary and mandibular structures, and agenesis of the temporomandibular joint (TMJ). In the absence of Foxc1, neural crest cell derived osteogenic patterning is affected, as osteoblasts develop ectopically in the maxillary prominence and fuse with the dentary bone. Furthermore, we observed that the craniofacial musculature is also perturbed in Foxc1 null mice, which highlights the complex tissue interactions required for proper jaw development. We present evidence that Foxc1 and Fgf8 genetically interact and that Fgf8 dosage is associated with variation in the syngnathic phenotype. Together our data demonstrates that Foxc1 – Fgf8 signaling regulates mammalian jaw patterning and provides a mechanistic basis for the pathogenesis of syngnathia. Furthermore, our work provides a framework for understanding jaw patterning and the etiology of other congenital craniofacial anomalies, including temporomandibular joint agenesis.
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1003949
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003949Summary
Syngnathia (bony fusion of the upper and lower jaw) is a rare human congenital condition, with fewer than sixty cases reported in the literature. Syngnathia typically presents as part of a complex syndrome comprising widespread oral and maxillofacial anomalies, but it can also occur in isolation. Most cartilage, bone, and connective tissue of the head and face is derived from neural crest cells. Hence, congenital craniofacial anomalies are often attributed to defects in neural crest cell formation, survival, migration, or differentiation. The etiology and pathogenesis of syngnathia however remains unknown. Here, we report that Foxc1 null embryos display bony syngnathia together with defects in maxillary and mandibular structures, and agenesis of the temporomandibular joint (TMJ). In the absence of Foxc1, neural crest cell derived osteogenic patterning is affected, as osteoblasts develop ectopically in the maxillary prominence and fuse with the dentary bone. Furthermore, we observed that the craniofacial musculature is also perturbed in Foxc1 null mice, which highlights the complex tissue interactions required for proper jaw development. We present evidence that Foxc1 and Fgf8 genetically interact and that Fgf8 dosage is associated with variation in the syngnathic phenotype. Together our data demonstrates that Foxc1 – Fgf8 signaling regulates mammalian jaw patterning and provides a mechanistic basis for the pathogenesis of syngnathia. Furthermore, our work provides a framework for understanding jaw patterning and the etiology of other congenital craniofacial anomalies, including temporomandibular joint agenesis.
Introduction
Jawed vertebrates, or gnathostomes, represent the majority of extant vertebrate species. In fact, more than 99 per cent of the roughly 58,000 living vertebrate species have jaws [1]. A functional, articulating jaw is required for proper nutritional intake, maintenance of oral health, and communication, and its appearance was a turning point in vertebrate evolution. Jaws allowed primitive vertebrates to become effective predators through capturing and processing large, motile prey, and probably account for much of their subsequent success in radiating and adapting to new environments.
The vertebrate jaw consists of separate upper and lower skeletal elements connected by a joint. The mature jaw structures are derived predominantly from the first pharyngeal arch (PA1), an embryonic outgrowth or facial prominence that is composed of (1) a core of mesoderm that will give rise to muscle and vasculature [2]–[4], (2) a population of neural crest cells that will differentiate into bone, cartilage, and connective tissue [5]–[8], (3) an endodermal lining, and (4) a covering of ectoderm both of which provide signals that govern proper survival, patterning, and differentiation of each of these cell populations [9]–[13]. The first pharyngeal arch can be subdivided into discrete upper (maxillary) and lower (mandibular) portions, which contribute to the upper and lower jaw respectively. In addition to these cell populations, the medial (MNP) and lateral (LNP) nasal prominences also make key tissue and signaling contributions to jaw development [14]–[18]. The jaw is constructed from several distinct and separate skeletal elements derived from these prominences including the maxilla, jugal, squamosal, and dentary bones. The temporomandibular joint (TMJ) is the functional jaw joint in mammals and is essential for jaw articulation. The TMJ is a complex synovial joint and consists of the glenoid fossa of the squamosal bone, the condylar head of the dentary, a fibrocartilaginous disc that is located between these two bones, and muscles and tendons that attach to the joint [19].
Craniofacial anomalies constitute approximately one-third of all congenital defects. Given the anatomical complexity associated with jaw development and function, it is not surprising that jaw malformations occur frequently. Syngnathia which is characterized by fusion of the upper and lower jaw, is a rare disorder for which the genetic or environmental etiology and pathogenesis remains unknown.
Foxc1 is a member of the forkhead box winged helix transcription factor family distinguished by its highly conserved forkhead DNA binding domain (for reviews, [20], [21]). In mice, Foxc1 has been reported to play roles in meningeal [22], [23], calvarial [24]–[28], ocular [29]–[31], somitic [32] and renal development [33]. Here we report a novel role for Foxc1 in orofacial development. Foxc1 null mutant mouse embryos display bony syngnathia in addition to defects in maxillary and mandibular structures together with agenesis of the TMJ. We present evidence that Foxc1 interacts genetically with Fgf8 to control patterning of the neural crest cell derived jaw and TMJ and furthermore that the variation in the severity of syngnathia is Fgf8 dosage-dependent. Our results therefore demonstrate that Foxc1 plays a critical role in jaw development and disease; provides a mechanistic basis underpinning the pathogenesis of the syngnathia; and establishes a framework for understanding the etiology of other congenital craniofacial anomalies, including temporomandibular joint agenesis.
Results
Foxc1−/− newborn mice display syngnathia
Foxc1 is initially expressed in the oral ectoderm and cranial mesenchyme in E8.5 embryos (Figure 1A–C). By E9.25, Foxc1 activity has diminished in the oral ectoderm, but continues to be expressed diffusely within the PA1 mesenchyme (Figure 1D, E). At E10.5, Foxc1 is restricted to a discrete caudal-medial domain of the mandibular portion of PA1 (Figure 1F). β-galactosidase expression under the control of the endogenous Foxc1 promoter [26] demarcates a similar spatiotemporal domain of expression in the mandibular mesenchyme (Figure 1G, H). However, the stronger staining intensity may reflect the stability or persistence of lacZ expression over whole mount in situ staining. Nonetheless, this data illustrates the dynamic activity of Foxc1 within the developing PA1 in E8.5 -10.5 embryos.
Fig. 1. Dynamic expression of Foxc1 in PA1. 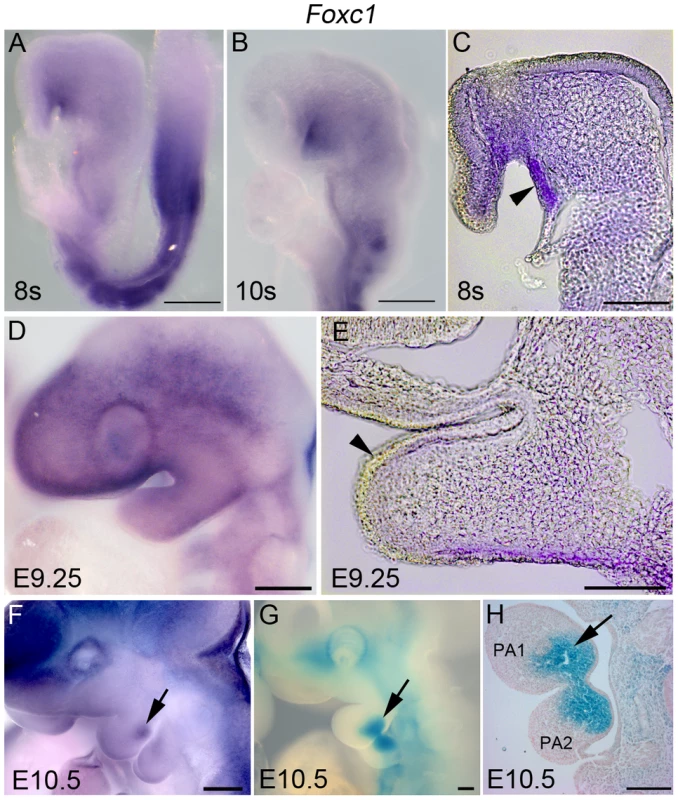
Whole mount in situ hybridization (A–F) and β-galactosidase (β-gal) (G, H) staining showing a timecourse of expression of Foxc1 in the developing first pharyngeal arch. (A–C) At E8.5, Foxc1 is broadly expressed in the cranial mesenchyme, PA1 mesenchyme and strongly expressed in the oral ectoderm. (D, E) By E9.5, expression is diffuse within PA1 mesenchyme, and is no longer detected in the PA1 oral ectoderm. (F–H) By E10.5, a discrete domain of Foxc1 is detected in the mandibular mesenchyme, which is more easily seen in β-gal stained specimens. Scale bars: A, B, D, F–H, 200 µm; C, E, 100 µm. We discovered that Foxc1−/− mutant mice exhibit a bilateral fusion of the upper jaw zygomatic complex to the dentary bone (Figure 2A, B) closely mimicking a condition in humans termed syngnathia. To evaluate the syngnathic phenotype in detail, we dissected the maxillary and mandibular structures from skeletal preparations of Foxc1−/− late gestation to newborn pups (embryonic day 18.5 – postnatal day 0; E18.5-P0 Figure 2). A summary of the skeletal phenotypes is presented in Table 1. Compared to the wild-type controls (Figure 2C), the body of the maxillary bone in Foxc1−/− mutant embryos was reduced in size and the abnormally thickened and shortened zygomatic process of the maxilla was fused to the dentary bone (Figure 2D). Fusion occured either proximal to the molar alveolar ridge (n = 7/13, Figure 2D) or encompassed the entire alveolar region (n = 6/13, Table 1). Although this phenotype has been previously described as an enlarged zygomatic process of the maxilla [26] or as massively ossified zygomatic and dentary bones [27], our more detailed analyses indicate that Foxc1−/− mutant mice represent a unique previously undescribed model for studying the pathogenesis of syngnathia.
Fig. 2. Foxc1−/− neonates exhibit syngnathia and TMJ agenesis. 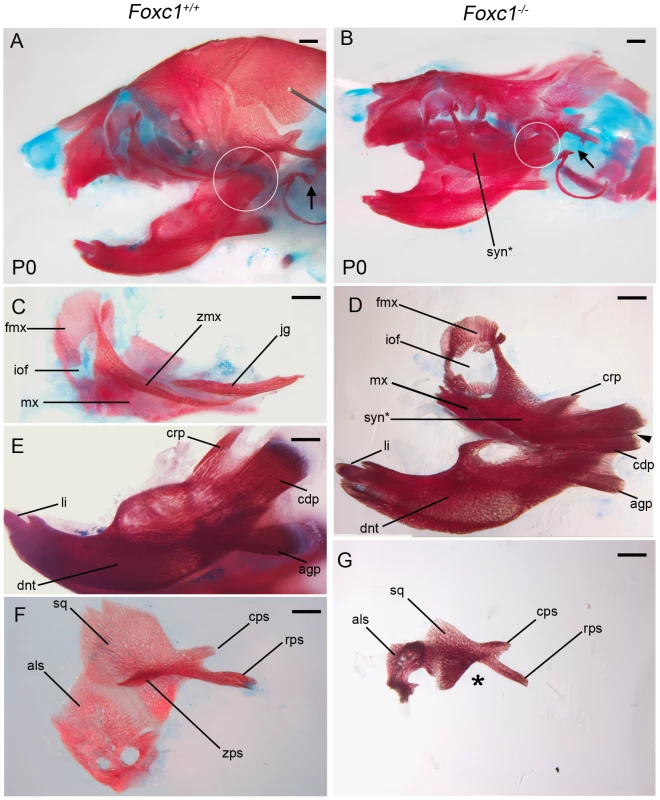
Alizarin red (bone) and alcian blue (cartilage) stained skeletal preparations of Foxc1+/+ (A, C, E, F) and Foxc1−/− (B, D, G) P0 neonates. (A,B) Intact view of cranial skeleton showing relative position of upper and lower jaw elements. Syngnathia (syn*) is evident in Foxc1−/− neonates. Circles highlight the articulating joint. Arrows in A and B highlight lack of ossification of malleus, incus, and stapes in mutant middle ear. (C) Dissected wild-type maxilla (mx) and jugal (jg). (D) The mutant maxilla is fused in the zygomatic region to the dentary (dnt) which displays hypoplastic coronoid (crp), condylar (cdp), and angular (agp) processes compared to controls (E). The Foxc1−/− condyle is bifurcated (arrowhead in D). (F, G) The mutant squamosal (sq) and alisphenoid (als) are hypoplastic, and the squamosal lacks a zygomatic process (asterisk). Scale bars: 500 µm Abbreviations: cps, caudal process of squamosal; fmx, frontal process of maxilla; iof, infraorbital foramen; li, lower incisor; rps, retrotympanic process of squamosal; zmx, zygomatic process of maxilla; zps, zygomatic process of squamosal. Tab. 1. Summary of skeletal phenotypes in jaw related structures. 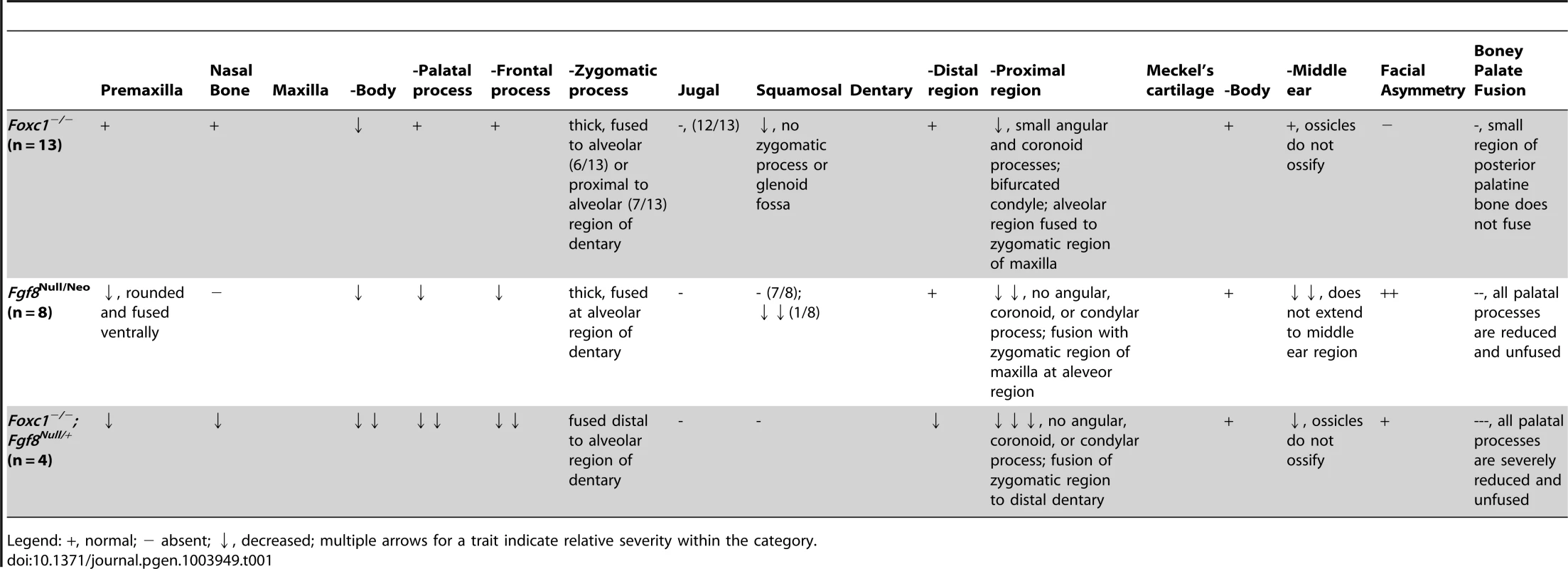
Legend: +, normal; − absent; ↓, decreased; multiple arrows for a trait indicate relative severity within the category. Temporomandibular joint agenesis in Foxc1−/− embryos
The articulation of the mammalian jaw occurs at the TMJ located between the condyle of the dentary and the glenoid fossa of the squamosal bone (circle; Figure 2A, C, E). The condyle in Foxc1−/− mutant embryos was hypoplastic and appeared bifurcated (n = 10/13) (arrowhead; Figure 2B, D). Given the degree of fusion, we cannot however, rule out the possibility that this could also represent a duplicated angular process. Nonetheless, the squamosal body was similarly hypoplastic in Foxc1−/− mutant embryos compared to wild-type controls with no evidence of zygomatic process formation (Figure 2F, G). In E17.5 wild-type embryos, a neural crest cell derived articular disc normally sits between the condyle and glenoid fossa and expresses scleraxis (Scx) [34] (Figure 3A and S1A, B). However, no equivalent domain of Scx positive expression, as evidence of articular disc formation was observed around the hypoplastic condyle in Foxc1−/− embryos (Figure 1C and 3B).
Fig. 3. Molecular analysis of developing TMJ in Foxc1−/− embryos. 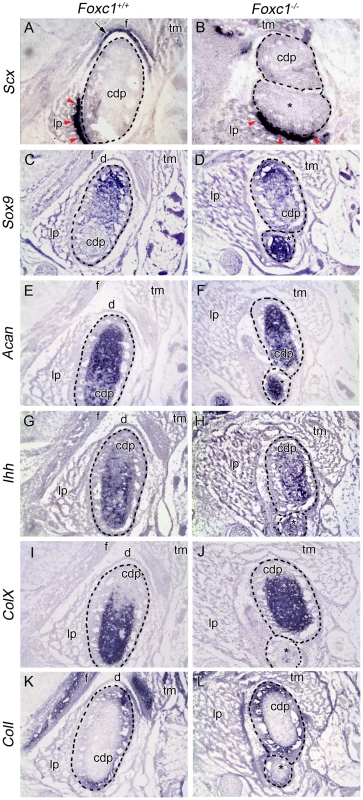
In situ hybridization analysis on serial coronal cryosections in Foxc1+/+ (A, C, E, G, I, K) and Foxc1−/− (B, D, F, H, J, L) mouse heads at E17.5. In (A–L) dashed lines outline the wild-type and bifurcated mutant condyles (cdp and cdp*). (A, B) Scx is expressed in the neural crest-derived disc (arrow) and tendon (red arrowheads) of controls. In mutants, Scx is maintained in the tendon (red arrowheads), but no disc is present. (C–F) Sox9 is localized to the proliferating chondrocytes and Acan is localized to the cartilage of the condyle growth plate in wild-type and Foxc1−/− condylar processes. (G, H) Ihh is localized to the prehypertrophic chondrocytes in wild-type and mutant condyles. (I, J) ColX is expressed in the zone of the hypertrophic chondrocytes of the condylar growth plate. In the absence of Foxc1, the hypertrophic chondrocytes make up a larger proportion of the abnormally bifurcated condyle. (K, L) Coll expression is localized to the osteoblasts of the condyle in control and Foxc1−/− embryos; however, the Coll positive glenoid fossa (f) is only observed in control specimens. Although the structural features of the TMJ are well documented, the genetic, cellular, and molecular mechanisms involved in TMJ morphogenesis remain poorly understood. To explore the mechanistic basis of these jaw joint anomalies, we analyzed the expression of several genes involved in cartilage and bone formation at E17.5. All markers of proliferating and mature chondrocytes were very similar between wild-type and Foxc1−/− condyles (Figure 3C–H). However, the proportion of ColX positive hypertrophic chondrocytes within the Foxc1−/− condyle appeared to be larger relative to controls, suggesting premature ossification (Figure 3I, J). The expression of ColI, a marker of osteoblasts, remained unchanged in the condyle of mutant embryos (Figure 3K, L), but was not seen across from the condyle indicating that no fossa formed. Collectively, our histological and molecular analyses of the TMJ in Foxc1−/− mutant embryos, illustrate the absence of glenoid fossa and articular joint disc, in addition to an abnormal condyle during TMJ development. This indicates that in addition to the syngnathia described above, a functional TMJ does not form in the absence of Foxc1.
Abnormalities of the palate in Foxc1−/− embryos
Skeletal preparations at P0 revealed that in contrast to wild-type littermates, a small region of the posterior palatine bone did not fuse in Foxc1−/− neonates (Figure S2A, C). The soft tissue walls of the buccal cavity were shifted medially in association with the syngnathic jaw, and consequently the palate in Foxc1−/− neonates was slightly more arched than in wild-type (Figure 4C–F). The incisive papilla and rugae were readily identifiable in wild-type and Foxc1−/− palates. Whereas wild-type palates develop 8 rugae in a distinct sequence [35], only 7 rugae form in Foxc1−/− palates. Furthermore, the mutant rugae were not as sharply delineated as in wild-type (Figure S2B, D and S4C, D). Despite abnormalities in the bones of the Foxc1−/− palate, no overt soft tissue clefting was observed in either the primary or secondary palate in Foxc1−/− neonates (Figure S2B, D).
Fig. 4. Abnormalities of PA1 derived muscle in Foxc1−/−. 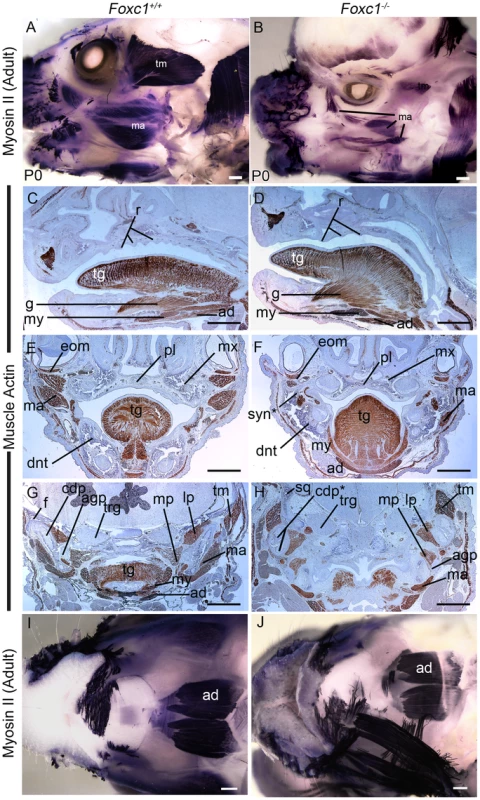
(A,B) Whole mount immunostaining for neonatal myosin II in P0 wild-type (A) and Foxc1−/− (B) heads. The PA1 masseter (ma) and temporalis (tm) muscles are smaller in the mutant than in wild-type. (C–G) Immunostained paraffin sections of E17.5 Foxc1+/+ (C, E, G) and Foxc1−/− (D, F, H) heads showing muscle actin localization (brown). Sagittal (C, D) and frontal (E, F) sections showing organized muscle formation in both wild type and mutant tongues (tg). The palate (pl) is more arched in Foxc1−/− and shows smoother rugae (r) than in Foxc1+/+. (G, H) Frontal sections in the TMJ region. The mutant temporalis muscle (tm) is shifted medially, aberrantly associates with the bifurcated condyle (cdp*), and its fibers are oriented differently than in wild type. The medial (mp) and lateral (lp) pterygoid muscles are appropriately associated with the angular (agp) and condylar processes in Foxc1−/−. The mutant masseter (ma) is reduced to a small component in Foxc1−/− (compare ma regions in F, H to E, G). (I, J) Ventral view of whole mount myosin II immunostained P0 wild type (I) and Foxc1−/− (J) heads. The second heart field derived anterior digastric muscle (ad) is robustly detected in all specimens. Scale bars: 500 µm Abbreviations: dnt, dentary; eom, extraocular muscle; f, glenoid fossa; g, genioglossus muscle; my, mylohyoid muscle; mx, maxilla; trg, trigeminal ganglion. Muscle patterning is abnormal in Foxc1−/− embryos
The pharyngeal arches contribute mesoderm to the tongue and the muscles of mastication – the masseter, temporalis and pterygoid. Given the jaw anomalies evident in Foxc1−/− mutant embryos, we explored whether muscle patterning was also affected. The anterior end of the tongue in Foxc1−/− mutant embryos was abnormally spade shaped and protruded from the oral cavity (Figure S2E, F). Both fungiform and median circumvallate papillae were identified in the Foxc1−/− tongue, and section immunostaining for muscle actin revealed that both intrinsic and extrinsic tongue muscles were well-organized in wild-type and mutant specimens (Figure 4C–F). Thus the tongue muscles form normally in Foxc1−/− mice, although the shape of the tongue may be constricted by the abnormal fusion of bony elements of the jaw.
To examine the patterns of muscle formation further, we performed whole mount immunostaining for neonatal muscle using myosin II in P0 wild-type (n = 8) and Foxc1−/− (n = 4) heads (Figure 4A, B). Compared to wild-type controls the masseter and temporalis muscles were reduced in size in the mutants. In histological sections, the temporalis muscle was shifted medially into the region normally occupied by the squamosal bone (Figure S1D–G) and was abnormally associated with the condyle. The lateral and medial pterygoid muscles, which attach to the condyle and angular process respectively were still associated with the correct processes in Foxc1−/− specimens (Figure 4G, H); however, the orientation of both the lateral and medial pteyrgoid was altered, presumably due to the abnormal position of the bones in the syngnathic Foxc1−/− jaw. The digastric muscle that arises from the second heart field mesoderm was robustly detected in whole mount neonatal muscle myosin II stained heads in both wild-type and mutants (Figure 4I, J). Collectively, these data indicate that abnormal cranial muscle patterning was specific to those derived from PA1, and that these muscles were abnormally shaped, sized, and positioned possibly as a secondary result of altered cranioskeletal patterning.
Ectopic neural crest cell derived osteoblast differentiation in Foxc1−/− embryos
The jaw, TMJ and muscle patterning abnormalities could be due to abnormal development of the neural crest cell derived PA1 mesenchyme. Therefore we examined the formation, migration, proliferation and differentiation of neural crest cells in Foxc1−/− embryos compared to their control littermates. Through Sox10 in situ hybridization (Figure S3A, B) and Wnt1-Cre;Z/EG lineage tracing experiments (Figure S3C–F), we observed that NCC formation, migration, and contribution to PA1, the nasal prominences, and their derivatives between E9.0-12.5 embryos, were all normal in the absence of Foxc1. Similarly, E11.5 Foxc1−/− embryos present with a normal pattern of neurofilament immunostaining, suggesting that neural crest cell contribution to cranial ganglia and peripheral nervous system is not dependent on Foxc1 (Figure S3G, H).
Despite normal neural crest cell colonization, the PA1 in Foxc1−/− mutants was smaller than that of wild type littermates as early as E8.75 (Figure 5A, D). From E9.5-11.5, the mutant maxillary and mandibular prominences remained reduced compared to controls and the invagination of oral ectoderm delineating the maxillary-mandibular constriction was shallower (arrowhead; Figure 5A),
Fig. 5. Early morphologic abnormalities of Foxc1−/− PA1 detected despite no significant alteration of cell cycle dynamics or apoptosis. 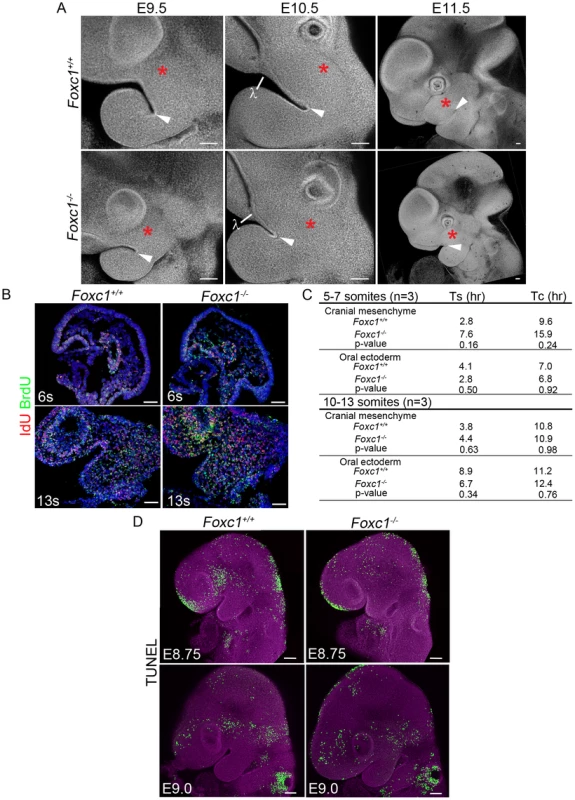
(A) Confocal z-stack projections of DAPI stained, whole mount Foxc1+/+ and Foxc1−/− embryos showing gross PA1 morphology. A red asterisk marks the maxillary prominence in all panels. A white arrowhead indicates the maxilla-mandibular junction (Mx-Md). At E9.0-9.5, the mutant maxillary prominence is smaller than wild-type, and the Mx-Md is shallower than in controls. By E10.5, the lambdoidal junction (λ) and nasal prominence epithelium has formed in Foxc1+/+ and Foxc1−/− embryos. The Foxc1−/− maxillary prominence continues to develop between E10.5-11.5, but remains smaller compared to wild-type embryos. The distance from the λ to Mx-Md junction is reduced in Foxc1−/− embryos. (B) Representative sections of IdU and BrdU immunostained embryos. (C) Average S-phase and cell cycle lengths were determined based upon incorporation of IdU and BrdU. A trend toward longer S-phase and cell cycle length was noted in the cranial mesenchyme at 5–7-s. (D) Projections of confocal Z-stacks showing whole mount TUNEL staining to detect apoptotic cells in Foxc1+/+ and Foxc1−/− embryos at E8.75 and E9.0. Although change in morphology of PA1 is evident, no abnormal level or localization of apoptosis is detected in the mutants. Scale bars: 100 µm. To determine if the smaller PA1 in Foxc1−/− embryos was a result of altered cell proliferation, we used phosphohistone H3 to quantify the mitotic index of E9.0 wild-type and mutant embryos. However, we did not observe any significant difference in the rate of cell division (p = 0.116) (Figure S4). Therefore we turned to combined IdU and BrdU incorporation to determine if there were differences in cell cycle length [36] within the developing PA1 between wild-type and Foxc1−/− embryos (Figure 5B, C). We detected a slightly longer S-phase and consistent with that, a longer cell cycle length in the cranial mesenchyme of Foxc1−/− embryos at the 5–7 somite stage when PA1 size difference is first apparent. Whole mount TUNEL staining revealed no ectopic or abnormal levels of programmed cell death within the pharyngeal arches of Foxc1−/− embryos between E8.75-E9.0 (Figure 5D). The increase in S-phase and cell cycle length in the cranial mesenchyme can account for the smaller appearance of PA1 in Foxc1−/− compared to controls. However, our investigations into neural crest cell migration, cell proliferation, and apoptosis did not reveal any significant disruption in neural crest cell contribution to PA1 growth in Foxc1−/−, embryos. This suggested that perhaps altered neural crest cell differentiation underpinned the pathogenesis of syngnathia and TMJ agenesis.
By E13.5, Foxc1−/− embryos display foreshortened snouts compared to controls (Figure 6A, D). This type of phenotype is typically associated with abnormal cranioskeletal differentiation, therefore we examined cartilage and bone differentiation in wild-type and mutant embryos via alcian blue, and alkaline phosphatase and alizarin red staining respectively. Whole mount alcian blue staining revealed a single Meckel's cartilage in both wild-type and mutant embryos which was indicative of normal cartilage differentiation (Figure 6B, E, G–J). However, early neural crest cell osteogenic differentiation as detected by alkaline phosphatase staining, revealed an expanded maxillary domain that was contiguous and fused at its proximal end with the mandibular domain in E13.5 Foxc1−/− embryos in contrast to the discrete segregated domains observed in wild-type littermate controls (Figure 6C, F). This preceded the manifestation of ossified bony syngnathia which was clearly evident in alizarin red stained E15.0 Foxc1−/− embryos and which progressively worsened through E16.5-18.5 as the craniofacial skeleton matured (Figure 6G–L). In addition to the presence of syngnathia the bony elements of the mandible and maxilla of Foxc1−/− embryos exhibited abnormal morphology and the alisphenoid and squamosal were hypoplastic and malformed compared to wild-type controls. Thus, the pathogenesis of syngnathia in association with maxillomandibular malformation correlates with altered patterns of neural crest cell derived osteogenic differentiation.
Fig. 6. Time-course of PA1-derived skeletal abnormalities in Foxc1−/− mice. 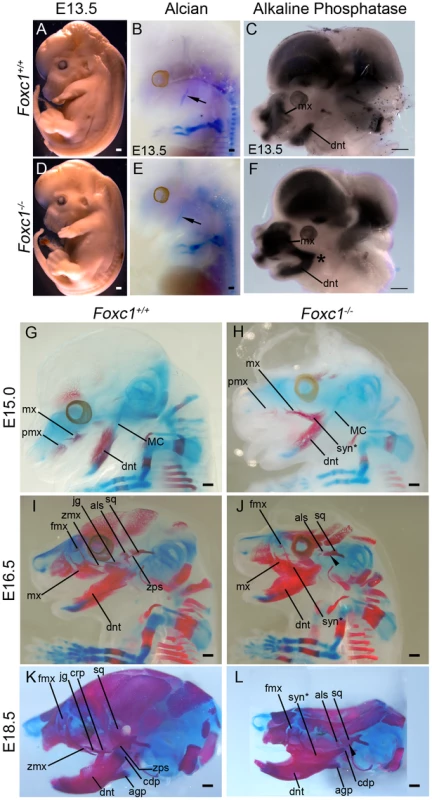
(A, D) Gross view of fixed Foxc1+/+ (A) and Foxc1−/− (D) embryos at E13.5. Cerebral hemispheres are enlarged and the snout is foreshortened in the mutant. (B, E) Whole mount alcian blue staining to detect cartilage differentiation in the wild type (B) and mutant (E) embryos pictured in (A, D). A single, normal Meckel's cartilage (arrows in B, E) is seen in both control and Foxc1−/−. (C, F) Bissected E13.5 heads of Foxc1+/+ (C) and Foxc1−/− (F) embryos stained for endogenous alkaline phosphatase (AP) activity to detect early osteoblast differentiation. In the absence of Foxc1, early osteoblasts of the dentary (dnt) and maxillary (mx) region initially differentiate in a fused, syngnathic pattern (*). (G–L) Whole mount alcian blue (cartilage) and alizarin red (bone) staining of Foxc1+/+ (G, I, K) and Foxc1−/− (H, J, L) embryos. (G, H) At E15.0, ossification of the wild type maxilla (mx) with a wispy frontal process (fmx) and dentary can be seen. In the mutant, wispy projections are seen in the maxillary region similar to controls. However, this ossification is connected to a larger ossified region that is fused (syn*) directly to the dentary. (I–L) In Foxc1+/+ embryos at E16.5 (I) and E18.5 (K), the zygomatic process of the maxilla (zmx), jugal (jg), zygomatic process of the squamosal (zps), and alisphenoid (als) are clearly identified as separately ossifying elements. By contrast, in the Foxc1−/− specimens (J, L), syngnathia is observed in the zygomatic region, rather than the distinct elements found in wild type. The alisphenoid and the squamosal (sq) are hypoplastic compared to controls. The zygomatic process of the squamosal does not form (arrowheads). Scale bars: 500 µm Abbreviations: agp, angular process; cdp, condylar process; crp, coronoid process; mx, premaxilla; tongue, tg. Proximal and distal PA1 markers are normally expressed in absence of Foxc1
After the completion of their migration into the facial prominences, neural crest cells find themselves surrounded by epithelia, and many studies have demonstrated the critical role played by epithelial-mesenchymal interactions in cranioskeletal patterning. Thus patterning of the craniofacial skeleton can be best understood when considered as modules of neural crest progenitors within territories such as the pharyngeal arches, where the signals from the surrounding epithelia interact to generate neural crest cell derived skeletal elements of appropriate size and shape. To explore whether the ectopic osteoblast differentiation and syngnathia observed in Foxc1−/− embryos was a consequence of altered pharyngeal signaling we examined the expression of markers known to play key roles in patterning the proximal-distal axis of PA1 [14], [37]–[39]. In E9.5-10.5 wild-type and Foxc1−/− mutant embryos, the lateral and medial nasal prominences develop such that morphologically the lambdoidal junction is readily identifiable (Figure 5A). Bmp4, which is a key regulator of distal jaw patterning (Figure S5A–D) and its downstream target Msx2 (Figure S5E–H) were expressed normally in the distal mandibular ectoderm, the olfactory epithelium, and at the lambdoidal junction in Foxc1−/− (n = 4) embryos at E10.5.
Endothelin signaling is also required to pattern the distal elements of the jaw but primarily establishes mandibular identity [40]–[43]. Hand2, which is a downstream target of endothelin, was expressed normally in the distal PA1 mesenchyme of Foxc1−/− (n = 4) embryos (Figure S5I–L). Additionally, Gata3 which is required for endothelin-independent expression of Hand2 in the distal mandiblular arch [44], was also properly expressed in both the distal mandibular ectoderm and the lambdoidal junction in E10.5 Foxc1−/− embryos (n = 3) (Figure S5M–P). Thus the normal expression of distal jaw signaling factors such as Bmp4, Msx2, Hand2 and Gata3 is consistent with the normal morphology observed in the distal regions of the dentary and maxilla in Foxc1−/− embryos (Figure 2A–D).
Fgf8 expression in PA1 is altered in the absence of Foxc1
Since patterning cues at the distal ends of PA1 were not altered in Foxc1−/− embryos, we hypothesized that the ectopic osteoblast differentiation and syngnathia reflected altered specification or signaling within the proximal region of PA1. Experimental evidence has implicated Fgf8 as being essential for proximal-distal axis specification and signaling [11], [45]–[49], and it is localized to the oral ectoderm of PA1 near the maxillary-mandibular constriction. Although the oral ectoderm was clearly present, its invagination at the maxillary-mandibular constriction was shallower in E9.0-9.5 Foxc1−/− embryos compared to wild-type (Figure 5A). Therefore we examined Fgf8 expression in wild-type and Foxc1−/− embryos (Figure 7A; n = 4). In Foxc1−/− embryos, Fgf8 expression was expressed at the midbrain-hindbrain boundary and in the frontonasal prominence similar to wild-type controls. However, Fgf8 was reduced in the mandibular oral ectoderm and was absent from the maxillary regions of the oral ectoderm in E8.5-9.5 Foxc1−/− embryos. .
Fig. 7. Foxc1 is required to maintain Fgf8 signaling and genetically interacts with Fgf8. 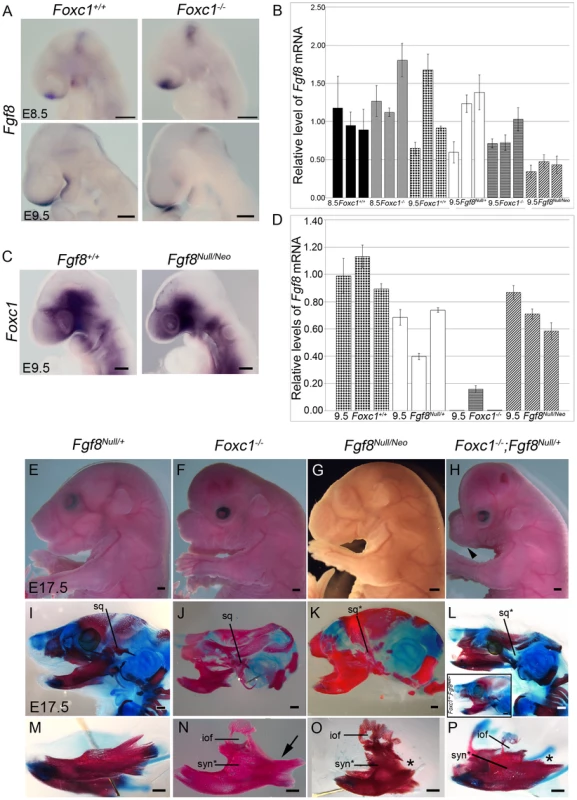
(A) Fgf8 expression is maintained in the frontonasal prominence and midbrain-hindbrain boundary regions of Foxc1−/− embryos, but it is reduced in the PA1 oral ectoderm (red asterisks). (B) Quantification of Fgf8 mRNA in Foxc1, Fgf8Null/+, and Fgf8Null/Neo embryos. (C) Foxc1 is normally localized in Fgf8Null/Neo embryos. (D) Quantification of Foxc1 mRNA in Foxc1, Fgf8Null/+, and Fgf8Null/Neo embryos. Gross appearance (E–H) and skeletal preparations (I–P) of E17.5 embryos comparing Fgf8Null/+ (E, I, M), Foxc1−/− (F, J, N), Fgf8Null/Neo (G, K, O), Foxc1+/−; Fgf8Null/+ (L, inset), and Foxc1−/−;Fgf8Null/+ (H, L, P) phenotypes. In both gross view (E) and skeletal preparations (I, M), Fgf8Null/+ are indistinguishable from wild-type embryos. (F) Foxc1−/− embryos have shortened frontonasal regions, open eyelids, abnormal and shifted external ears, and enlarged, hydrocephalic cerebral hemispheres. (G) Fgf8Null/Neo embryos have a more rounded frontonasal region, small lower jaw, and abnormal, shifted external ears. (H) Compound Foxc1−/−;Fgf8Null/+ embryos resemble Foxc1−/− specimens, but have more severe frontonasal shortening and no externally visible oral opening/lower jaw (black arrowhead). (J, N) Hypoplastic squamosal (sq), syngnathia (syn*), and abnormal condyle formation in the absence of Foxc1. This specimen shows fusion in alveolar region of dentary and absence of the coronoid process (arrow in N). (K, O) Severe hypoplasia and malformation of the squamosal (sq*) was observed in FgfNull/Neo specimens. The frontal process of the maxilla with a characteristic infraorbital foramen (iof) formed, and the maxilla fused to the dentary in the alveolar region, more distally than seen in Foxc1−/−. The proximal processes of the dentary are absent (asterisk in O), but distal incisors form. (L, P) In Foxc1−/−;Fgf8Null/+ embryos, the syngnathic phenotype is further exacerbated. No squamosal formed and a small frontal process of the maxilla is attached to the hypoplastic maxilla. This region is fused to the dentary just proximal to the lower incisors resulting in flattening of the normally curved dentary. The proximal dentary is severely truncated and lacks all processes (asterisk in P). (inset in L) Foxc1+/−;Fgf8Null/+ compound heterozygote (2/12) in which calvaria had developed normally. This specimen also displayed a syngnathic jaw with TMJ abnormalities grossly identical to that of the Foxc1 null. Scale bars: (A,C) 200 µm; (G–H) 1000 µm; (M–P) 500 µm. Therefore we hypothesized that diminished Fgf8 activity correlated with the pathogenesis of syngnathia. To determine if alterations of Fgf8 gene dosage could produce a similar syngnathic phenotype, we examined the PA1 derived upper and lower jaw bones in Fgf8Null/+ and Fgf8Null/Neo embryos (Figure 7E, I, M, G, K, O). Indeed E18.5 Fgf8Null/Neo embryos displayed syngnathia (7/8) in which the maxilla containing a distinctive frontal process was fused to the malformed dentary, which lacked a condylar process. The site of maxilla-dentary fusion was positioned more distally in Fgf8Null/Neo embryos than in Foxc1−/− embryos (Figure 7J, K, N, O). Left-right side differences were often observed in the length of each dentary bone and the location of syngnathia along the proximal-distal axis of Fgf8Null/Neo mutants. As each side of the mutant lower jaw was fused at the mandibular symphysis, this produced a distinct asymmetrical shift of the lower jaw (Figure S6G, H). Additional skeletal phenotype details are provided in Table 1, Figure 5 and Figure S6).
These results indicate that the relative levels of Fgf8 in the developing PA1 are key to the pathogenesis of syngnathia. Therefore we quantified the comparative levels of Fgf8 in each of the mutant Foxc1 and Fgf8 lines by qPCR (Figure 7B) and observed Fgf8 activity in the oral ectoderm of Foxc1−/− embryos to be approximately only 80% of wild-type levels. This was a considerable decrease, well below the levels of Fgf8 maintained by Fgf8Null/+ embryos (100% of wild-type), but not quite as severely reduced as in Fgf8Null/Neo embryos (41% of wild type). Collectively these expression data indicate that Foxc1 may be required to maintain proper levels of Fgf8 in the PA1 oral ectoderm.
Genetic interaction of Foxc1 and Fgf8
Since genetically reduced levels of Fgf8 resulted in syngnathia and Fgf8 levels were reduced in Foxc1 mutants, we hypothesized that Foxc1 and Fgf8 may genetically interact to influence jaw development. Therefore we generated compound heterozygous Foxc1+/−;Fgf8Null/+ and Foxc1−/−;Fgf8Null/+ embryos. Four Foxc1−/−;Fgf8Null/+ embryos were recovered between E18.5 – P0, which closely resembled Foxc1−/− embryos, but the phenotype was much more severe that either Foxc1−/− or Fgf8Null/Neo embryos, lacking an oral opening and a visible lower jaw (arrowhead Figure 7H). In skeletal preparations, Foxc1−/−;Fgf8Null/+ had a more severe phenotype than that of either Foxc1−/− or Fgf8Null/Neo embryos (Figure 7I–P). The premaxilla was smaller than controls and the maxilla consisted of a small frontal process and hypoplastic body of the maxilla. The maxilla was fused to a truncated dentary that lacked all proximal processes. The site of fusion was located quite distally along the length of the dentary, nearing the incisor region, however, incisor formation occurred in each of these mutants. Meckel's cartilage was present, but the proximal portion that gives rise to the malleus was thin and was associated with a small remnant of the incal cartilage (Figure S6A–C). Similar to Foxc1−/− the Foxc1−/−;Fgf8Null/+ mutant middle ear ossicles were still cartilaginous with no evidence of stapes formation at P0, whereas the wild-type malleus and incus were undergoing ossification.
The skeletal phenotypes of the Foxc1−/−;Fgf8Null/+ reflected an exacerbated loss of maxillary and TMJ related structures and a distal shift in the location of fusion along the proximal-distal axis of the dentary. We also observed facial asymmetry in these embryos similar to that observed in Fgf8Null/Neo embryos (Figure S6G–J). Interestingly, we also occasionally observed syngnathia in compound Foxc1+/−;Fgf8Null/+ heterozygotes at E18.5 (n = 2/12) (Figure 7L, inset). Abnormalities of the maxilla, dentary, squamosal, and alisphenoid were identical to those seen in Foxc1−/− embryos. This lends further evidence for the genetic interaction between Foxc1 and Fgf8. Consistent with this idea, we assessed whether Foxc1 expression was altered by decreased levels of Fgf8. While Foxc1 was properly localized to the PA1 mesenchyme, qPCR analysis indicated a slight decrease in Foxc1 mRNA levels in both E9.5 Fgf8Null/+ (59% of wild type) and Fgf8Null/Neo (70% of wild type) embryos (Figure 7C, D). Thus Fgf8 may be required to maintain proper levels of Foxc1 in the PA1 mesenchyme and conversely Foxc1 may be required to maintain proper levels of Fgf8 in the PA1 oral ectoderm forming a feedback loop critical for jaw development and in the pathogenesis of syngnathia and TMJ agenesis.
The proximal Dlx code is disrupted in PA1 of Foxc1−/− embryos
Interestingly Fgf8 is instrumental in activating Dlx genes in neural crest cells thereby triggering the morphogenetic program which specifies regionalized jaw elements [50], [51]. Regionalized patterning within PA1 is elaborated through a nested proximal-distal code of Dlx homeobox transcription factors in the mesenchyme [52]–[57], and the interpretation, pattern, and morphology of the jaw depend on the combinatorial activity of Dlx genes [56]. Therefore we examined the expression of Dlx genes in neural crest derived pharyngeal arch mesenchyme (Figure 8). Dlx2 expression in the maxillary portion of PA1 was reduced or absent in E10.5 Foxc1−/− mutant embryos compared to wild-type controls (n = 4) (Figure 8A–D). Similarly, there was a distinct loss of Dlx5 expression in the mesenchyme at the maxillary-mandibular constriction Foxc1−/− mutant embryos (n = 4) (Figure 8E–H). In contrast, the expression domains of both Dlx3 and Dlx6 appeared to be normal in Foxc1−/− embryos (n = 3) (Figure 8I–P). Therefore, the syngnathia and TMJ agenesis observed in Foxc1−/− embryos likely manifests as a result of perturbed Fgf8 signaling in the proximal region of PA1, together with alterations in distinct subdomains of Dlx2 and Dlx5 which collectively lead to regionalized patterning defects in maxillary and mandibular mesenchyme from which the upper and lower jaw elements are derived.
Fig. 8. Disruption of proximal Dlx code in PA1 of Foxc1−/− embryos. 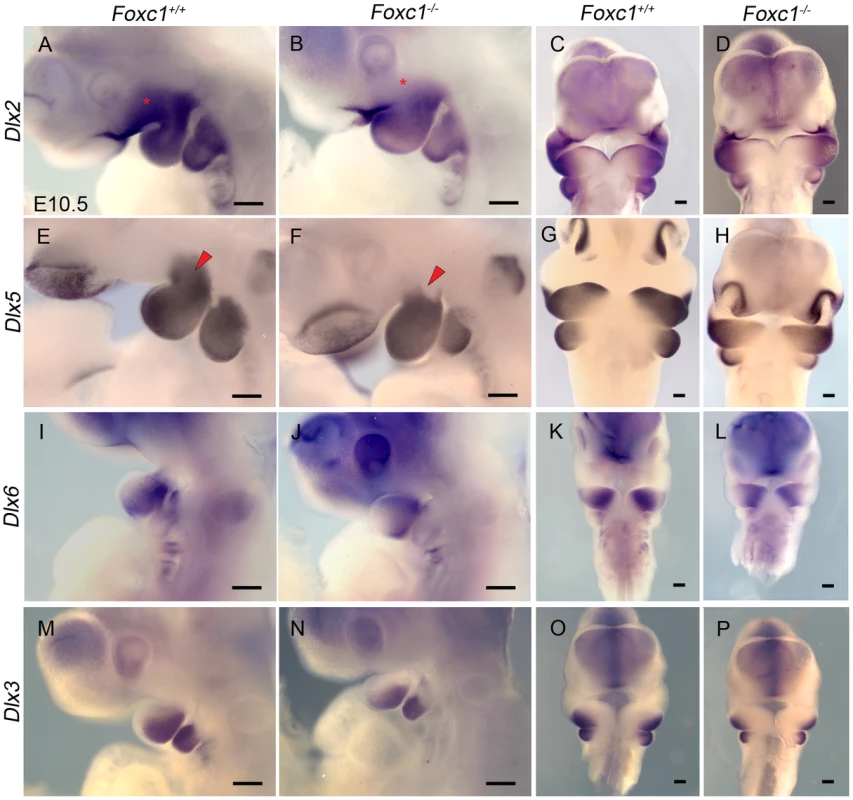
Whole mount in situ hybridization in E10.5 Foxc1+/+ (A, C, E, G, I, K, M, O) and Foxc1−/− (B, D, F, H, J, L, N, P) embryos. Lateral (A, B, E, F, I, J, M, N) and frontal (C, D, G, H, K, L, O, P) views are shown for each probe. (A–D) Dlx2 expression is maintained in the λ-junction and distal mandibular ectoderm and the mandibular mesenchyme of Foxc1−/− embryos. However, expression of Dlx2 is much reduced in the maxillary PA1 mesenchyme in the mutants (red asterisks). (E–H) Dlx5 expression is maintained in nasal prominence epithelia, distal mandibular ectoderm, and most of the mandibular mesenchyme in Foxc1−/− embryos. A discrete domain of mesenchymal Dlx5 expression is lost at the Mx-Md junction (red arrowheads) in the absence of Foxc1. In Foxc1−/− both Dlx6 (I–L) and Dlx3 (M–P) are expressed similarly to controls. Scale bars: 200 µm. Discussion
Syngnathia (fusion of the upper and lower jaw) is a rare human condition with only 56 cases reported to date in the literature (Table S1, and references therein). Syngnathia may involve connection between soft tissues (synechiae) or union between bony elements (synostosis). Bony fusion is rare, requires complex surgical repair, and is often present as part of a broader syndrome of congenital malformation. These cases reveal a high degree of variability in the location and extent of jaw fusion and indicate that bony syngnathia may be isolated or syndromic. In about 18% of cases, syngnathia is associated with known syndromes such as aglossia-adactylia syndrome or hemifacial microsomia. In all the reports of bony syngnathia, there is bony fusion between elements of the upper and lower jaw and no known epigenetic, genetic, or molecular etiology.
Previous reports have speculated that congenital bony syngnathia is caused by various factors, including abnormal development of the stapedial artery [58]; late gestation trauma or pressure defects in utero [59], [60]; and abnormal neural crest cell proliferation and migration [61]. Our review of the case reports suggested that syngnathia arises from defects in pharyngeal arch development and that there was likely a molecular or genetic basis for this disorder. In this study we present the first genetic model, a targeted deletion of Foxc1, with which to further understand the etiology and pathogenesis of human syngnathia.
Foxc1 knockout mice provide a model for understanding human syngnathia
Similar to human bony syngnathia, fusion of the zygomatic complex to the dentary bone in Foxc1−/− mutants occurred distal to the temporomandibular joint region. Furthermore, components of the TMJ were also abnormal in the Foxc1−/− model. Defects were observed in the morphology of the condyle and squamosal, in addition to agenesis of the joint disc and glenoid fossa. Our studies also indicate that jaw associated muscles, including the masseter, temporalis, and pterygoids, were deficient and abnormally positioned in the absence of Foxc1. This reduction in size of the muscles of mastication is very similar to the decrease in muscle volume of the masseter, temporalis, and pterygoid reported in human hemifacial microsomia patients [62]–[65].
Foxc1 plays a role in patterning of maxillary and joint related structures of jaw
Abnormal morphology was observed in Foxc1−/− as early as E8.75 in the form of maxillary and mandibular hypoplasia, with the maxillary prominence more grossly affected. This occcured in the absence of changes in neural crest cell contribution to the arch, or decreases in the mitotic index, or ectopic apoptosis. We did observe a lengthening of the cell cycle in Foxc1−/− cranial mesenchyme, which may contribute to the reduced size of PA1. It is also possible that morphological shape changes contribute to the reduction of the PA1 in Foxc1−/− embryos. Future morphometric analyses may shed further light on the underlying cause of the gross abnormalities of PA1 in Foxc1−/− embryos
Our data indicate that the epithelial signals associated with the distal regions of PA1 remain intact in the absence of Foxc1. We investigated known growth signaling and transcription factors associated with regionalized patterning of PA1 and subsequent jaw development. Reduction in Dlx2 was restricted to the maxillary prominence mesenchyme and loss of Dlx5 was limited to a discrete region of mesenchyme at the maxillo-mandibular constriction. The loss of Foxc1 has the most dysmorphic effect on the maxilla, jugal, and squamosal indicating a requirement for Foxc1 in maxillary patterning and differentiation, particularly structures of the zygomatic complex. The zygomatic complex is also disrupted when Dlx2 is knocked-out in mouse [53]. In addition, targeted disruption of Dlx5 [54] results in abnormal truncation and arrangement of the condylar and angular processes as well as a deviation or split in Meckel's cartilages associated with abnormal ossification. These structures are similarly abnormal in Foxc1−/− mutants. Combinatorial heterozygotic loss of Dlx2 and Dlx5 [56], results in abnormalities of maxillary, palate, and dentary structures as well as severe disruption of both the primary (incal-malleal) and secondary (squamosal-condylar) articulations. Interestingly, loss of Dlx2/5 also results in loss of PA1 derived branchiomeric muscle [66] while Foxc1−/− mutants exhibit significant reduction and abnormality of these muscles. Our data showing discrete changes in the proximal Dlx code and resultant skeletal phenotype in Foxc1−/− mutants is consistent with the previous studies of nested Dlx expression in jaw patterning, and recent studies in mouse indicating that Dlx expression within the arch may be more dynamic than previously appreciated [67]. It appears that the specific levels and domains of Dlx2 and Dlx5 expression that require Foxc1 are critical for patterning elements of the zygomatic complex, maxilla, squamosal and proximal dentary upon which mammalian jaw articulation depends.
Fgf8 dosage affects severity and phenotypic variability in syngnathia
Alteration to the Dlx code in the Foxc1−/− PA1 appears to occur downstream of Fgf8. Interestingly the ectodermal domain of Fgf8 overlies the mesenchymal domain of Dlx2 while Bmp4 overlies the epithelial domain [68]. Moreover, the mesenchymal expression of Dlx2 is positively regulated by Fgf8 signaling while the ectodermal activity of Dlx2 is maintained by Bmp4 signaling. Fgf8 therefore is instrumental in activating Dlx genes in neural crest cells and triggering the morphogenetic program, which specifies different jaw elements [50], [51]. Consistent with this, we demonstrated that simply reducing the overall dosage of Fgf8 could produce bony syngnathia. Fgf8Null/Neo embryos exhibit jaw fusion similar to that observed in Foxc1−/− embryos. Furthermore, genetically reducing one copy of Fgf8 in combination with the Foxc1 null further exacerbated the extent of jaw fusion and the perturbation of maxillary and dentary elements. Together these data indicate that Foxc1 genetically interacts with Fgf8 to influence jaw patterning, and that overall dosage of Fgf8 affects the severity of the syngnathic phenotype.
The Foxc1−/− syngnathic phenotype is similar to, but less severe than the Fgf8Null/Neo jaw phenotype (see Table 1; Figure 7; Figure S6). However, the combinatorial loss of Foxc1 and Fgf8 displayed the most severe phenotype in the spectrum reported here, which may indicate that Foxc1 and Fgf8 function synergistically in their roles in jaw patterning. Interestingly, as Fgf8 dosage decreased in these mouse mutants, we observed facial asymmetry similar to that reported in human syngnathia [69], [70], hemifacial microsomia, and in a high proportion of craniofacial malformations and syndromes [71]. Fgf8 is known to play a key role in establishing left-right asymmetry [72], and conditional loss of Fgf8 in the oral ectoderm of mice [11] and in a hypomorphic zebrafish model [73] result in cranial asymmetry.
Dynamic expression of Foxc1 in PA1
Our data indicate that Foxc1 is initially expressed in the oral ectoderm and cranial mesenchyme contributing to the developing PA1 in E8.5 embryos. At this stage, both Foxc1 and Fgf8 are expressed in overlapping domains within the PA1 oral ectoderm (Figures 1A–C and 7A). In Foxc1−/− embryos at E8.5, the domain and levels of Fgf8 are reduced but not absent in the oral ectoderm (Figure 7C). Foxc1 therefore is not required to induce Fgf8 expression in the oral ectoderm, but may be required to maintain proper levels and localization of Fgf8 (Figure 7). Conversely, we showed that Foxc1 activity was reduced in the Fgf8 allelic series of mutants. Taken together this suggests that Fgf8 and Foxc1 potentially form a feedback loop critical for jaw development and in the pathogenesis of syngnathia and TMJ agenesis.
Given the complex and dynamic expression of Foxc1 in E8.5-10.5 embryos in cranial mesoderm, oral ectoderm and neural crest cell derived PA1 mesenchyme, it will be imperative in the future to delineate the precise requirement for Foxc1 in each of these tissues during craniofacial growth, patterning and morphogenesis using a repertoire of spatially and temporally specific conditional Cre deleter strains. This will shed further light on the spatial and temporal pathogenesis of syngnathia and TMJ agenesis.
Models of jaw patterning and evolution
Given the important role of jaw development in both evolution and disease, many groups have proposed models to address the concept of polarity within the context of pharyngeal arch development and jaw evolution. Two recent models include the dynamic growth zone model (reviewed in [74]) and the hinge and caps model [75], [76]. Briefly, the growth zone model posits that dorso (proximal)-ventral (distal) polarity of the PA relies upon Edn1 signaling to establish a ventral/distal zone within the PA. Once established, distal Edn1 and Bmp4 cues regulate nested Dlx gene expression in the PA to establish a combinatorial, dynamic expression code within the arch. The more distal domains contain undifferentiated cell types, while the cells more intermediately and proximally positioned within the mandible differentiate, resolving into zones. The intermediate zone, which expresses Bapx1, becomes permissive to forming structures of the jaw joint, and the dorsal/proximal zone is established by jag1b expression.
The hinge and caps model [75], [77], [78], places articulation, and subsequently the polarity and modularity, of the upper and lower jaws in the context of cranial neural crest competence to respond to localized epithelial signals. The hinge is defined as the epithelial junction of the maxillary and mandibular prominences of the first arch, also known as the maxillo-mandibular constriction. The caps constitute the lamboidal junction and proximal maxillary region for the upper jaw, and the midline of the mandibular prominences for the lower jaw. Properly patterned placement of the hinge at the sight of articulation and balanced patterning at both caps assures that the elements of the upper and lower jaw will develop in register with one another. Modularity and proximodistal polarity is achieved by the integration of hinge and caps signaling, such that the PA is divided into nested, overlapping developmental fields [75].
The data presented in this study aligns with both the growth zone model and the hinge and caps model. Our data indicate that in the Foxc1−/− mutant embryos, distal Edn1 and BMP4 signaling within PA1 are normal, and that Dlx5 and Dlx3 expression within the recently identified [67] intermediate domain of PA1 is maintained. The loss of a discrete domain of Dlx5 expression near the maxillo-mandibular constriction in Foxc1−/− embryos is similar to the disruption reported in Edn1fl/fl;Foxg1-Cre mutants and provides further evidence of complex control of Dlx expression within specific zones of the developing arch [67]. While it is tempting to speculate that disruption of Foxc1 in our mutants results in a duplication of the condyle or the proximal portion of the dentary rather than a bifurcation, (Figures 3 and 6), we have no molecular data, such as expansion of Dlx5 or Dlx6 into the maxillary region, to support this at this time. Additionally, similar to the reported requirement for ectodermal expression of Edn1 in patterning the intermediate domain, the disruption of Fgf8 expression in the oral ectoderm of Foxc1−/− embryos may similarly disrupt Dlx2 and Dlx5 in the immediately underlying regions of the PA1 mesenchyme.
With respect to the hinge and caps model, the observed defects in Foxc1−/− PA1 morphology and skeletal defects affecting the elements involved in jaw articulation (the squamosal, zygomatic complex, and condyle) indicate disruption may center on the proposed hinge region of PA1. In this manner, the expression of both Foxc1 and Fgf8 in the PA1 oral ectoderm at E8.5-9.0, in combination with the apparent decrease in Fgf8 expression localized to the Foxc1−/− PA1 ectoderm, suggest that Foxc1 may be required to maintain proper hinge associated signals. Localization of Dlx2 and Dlx5 are also primarily altered at the proximal aspect of their expression domains, and the skeletal abnormalities observed are relatively more severe for maxillary jaw elements. Taken together, these may indicate disruption of the symmetric localization of hinge signaling at the maxillo-mandibular constriction in the absence of Foxc1. In agreement with the proposed symmetrical alignment of signals at proximal and distal caps, BMP and Edn1 signals remain intact in the Foxc1−/− embryos and do not display gross evidence of retrognathia (Figure 6), suggesting the skeletal elements at the cap retain their alignment. This is in contrast to the observed retrognathia when Edn1 is conditionally disrupted [67], which could be interpreted as altering the distal caps signaling while proximal caps signals remain intact.
Our data indicate that Foxc1 is required to regulate Fgf8 activity which influences the localization of Dlx2 and Dlx5. In doing so, Foxc1 is a key regulator of jaw patterning cues and the elaboration of proximodistal patterning during jaw development. Consequently our studies provide a mechanistic basis for understanding the etiology and pathogenesis of the rare condition of syngnathia and TMJ agenesis. Future studies are still needed, however, to refine the precise spatiotemporal requirement for Foxc1 in jaw musculoskeletal development. For example, it is unclear whether the abnormal position and size of jaw musclulature in concert with jaw abnormalities reflects a direct requirement for Foxc1 in pharyngeal arch mesoderm development or is an indirect consequence of abnormal patterning of neural crest cell derived mesenchyme. Previous work has shown that cues from neural crest-derived connective tissue can direct the alignment of mesoderm derived myoblasts [79]–[80], and furthermore that Dlx expression in NCCs is required for formation of PA1 derived muscles [66]. This implies that that Foxc1 maybe predominantly required in neural crest cell derived mesenchyme during jaw musculoskeletal depvelopment. However, our data cannot separate the roles of Foxc1 in the early cranial mesenchyme, the oral ectoderm, and in the PA1 mesenchymal domain. The recent generation of a conditional Foxc1 allele [30] and increased understanding of the role of Foxc1 within neural crest-derived populations, as well as the relationship between abnormally patterned skeletal elements and their associated muscles, will enhance our collective knowledge of jaw development and inform treatment strategies for human patients with syngnathia and other related craniofacial malformations.
Materials and Methods
Animal husbandry and genotyping
All mice were housed and all experiments were conducted in compliance with protocols approved by the Institutional Animal Care and Use Committee at the Stowers Institute for Medical Research. Foxc1 mice were obtained from Tsutomu Kume and were maintained on a 129S6/SvEv background. Fgf8Null and Fgf8Neo mice were obtained from Gail Martin and were maintained on a CD1 background. Z/EG (stock number 003920, Tg(CAG-Bgeo/GFP)21Lbe/J) and Wnt1-Cre (stock number 003829, Tg(Wnt1-cre)11Rth Tg(Wnt1-GAL4)11Rth/J) mice were obtained from the Jackson Laboratory and intercrossed with the Foxc1 line to generate both Foxc1;Z/eg and Foxc1; Wnt1-Cre mouse lines. Genotyping of all mouse strains was determined using qPCR with specific probes designed for each strain (Transnetyx, Inc, Cordova, TN, http://www.transnetyx.com). Primer sequences for each assay can be found in Table S2.
Bone and cartilage staining
Early osteoblasts were detected by endogenous alkaline phosphatase activity. At E13.5, heads were bisected, fixed overnight in 4% paraformaldehyde, rinsed in PBS, and then incubated in alkaline phosphatase buffer (100 mM NaCl; 100 mM Tris-HCl, pH 9.5; 50 mM MgCl2; 1% Tween-20). Alkaline phosphatase activity was detected using NBT/BCIP. Whole-mount skeletal preparations were made of embryos and neonates (13.5 dpc-P0) as follows. Embryos E15.0 and older were anesthetized by immersion in cold PBS until no reactive movements were seen, the skin and viscera were removed. All embryos were dehydrated in 95% ethanol, transferred to stain base solution (70% ethanol, 5% acetic acid) for 30 minutes, and then stained in 70% ethanol, 5% acetic acid, 0.02% alcian blue, 0.05% alizarin red for 24–48 hours. Following staining, embryos were rinsed in stain base solution, rinsed in water, incubated in 2.0% potassium hydroxide (KOH) (10 minutes to 6 hours based on embryonic stage), and cleared in a 0.25% KOH-glycerol series.
Histological staining, section and whole-mount in situ hybridization
Unless otherwise noted in text, a minimum of three specimens were examined for each genotype. Heads from E16.5 embryos were fixed in 4% paraformaldehyde (PFA) and embedded in O.C.T. compound. 10 µm sections were stained with hematoxylin and eosin following standard procedures. Section in situ hybridization was performed with digoxigenin-labeled probes as described in [81]. RNA antisense probes for Sox9, Acan, Ihh, ColX, ColI and Scx were generously provided by Dr. Cliff Tabin. Whole mouse embryos (9.0–10.5 dpc) were fixed overnight in 4% PFA at 4°C, then rinsed in phosphate buffered saline (PBS) with 1% Tween-20 followed by step-wise dehydration to 100% methanol. Anti-sense digoxigenin-labeled (dig-UTP, Roche) riboprobes were synthesized for Bmp4, Dlx2, Dlx3, Dlx5, Dlx6, Fgf8, Gata3, Hand2, Msx2, and Sox10. Foxc1 probe was generated by RT-PCR amplification of a 471 bp fragment spanning the 3′end of exon 1 and a portion of the 3′UTR. Primers: 5′GTACCTGAACCAGGCAGGTG3′, and 5′AGGCAAAAATGGAGGAGGTT3′. Whole mount in situ hybridizations were performed according to standard protocols [82], [83] with minor modifications.
Immunohistochemistry and DAPI staining
Newborn (P0) pups were anesthetized by induction of hypothermia followed by decapitation. (Foxc1−/− pups were found dead among littermates on P0.) Isolated heads were placed in PBS for several hours and skin was removed prior to fixation for 48 hours at 4°C in 4% PFA. Heads were rinsed in PBS and dehydrated to 80% methanol, and were stored for at least one week in Dent's fixative at 4°C. Endogenous alkaline phosphatase activity was inactivated by incubation in Dent's bleach prior to rehydration into PBS containing 0.1% Tween-20 (PBST). Heads were incubated overnight in PBST, 10% fetal bovine serum (PBST-FBS) followed by incubation in alkaline phosphatase conjugated anti-Myosin (MY-32, Sigma, A4335), diluted to 10 µg/ml in PBST-FBS for 24 hours at 4°C. Heads were washed in PBST and alkaline phosphatase was detected with NBT/BCIP.
Neurofilament whole mount immunostaining was performed on 4% PFA fixed E11.5 embryos. Endogenous peroxidase activity was blocked by incubation in Dent's bleach overnight at room temperature. Embryos were rehydrated to PBST, blocked in 20% goat serum in PBST, and incubated in 1∶500 dilution of 2H3 antibody (Developmental Studies Hybridoma Bank) overnight at room temperature. Embryos were rinsed in PBST, and then incubated with a 1∶200 dilution of a horseradish peroxidase (HRP) conjugated donkey anti-mouse IgG secondary antibody (Jackson ImmunoResearch, 715-035-150). Signal was detected through diaminobenzidene (DAB) staining (Sigma, D5905). Stained embryos were cleared in 20% glycerol, 0.25% KOH prior to imaging.
For sectional immunohistochemistry to detect muscle actin (HHF35, Dako, M0635), E17.5 embryos were dissected, anesthetized by immersion in cold PBS, and then decapitated. Heads were fixed in 4% PFA, dehydrated to 100% ethanol, and embedded in paraffin. Frontal and sagittal sections were cut (10 µm). Sections were dewaxed and rehydrated to PBS. HHF35 was biotinylated with the MM Biotinylation Kit (Biocare Medical, MMBK G,H) and then applied to sections at a 1∶100 dilution overnight at 4°C. Following streptavidin-HRP incubation, muscle actin reactivity was detected with DAB staining. Sections were counterstained with 10% hematoxylin.
For gross visualization of palate and tongue tissues, upper and lower jaws were separated and incubated overnight in DAPI (2 µg/ml). Tissues were imaged on a Leica MZ FLIII stereoscope using Axiovision software. Similarly, whole embryos between E9.0-11.5 were fixed in 4% PFA, rinsed in PBS, and incubated in DAPI. Embryos were then cleared in 50% glycerol, mounted with Vectashield (Vector Labs, H1000), and scanned using a Zeiss LSM5 Upright Pascal Confocal microscope. Projected Z-stacks were flattened and exported to Adobe Photoshop.
β-galactosidase staining
Whole embryos from E8.5-11.5 were collected and fixed from 30 to 60 minutes in 0.2% glutaraldehyde, 5 µM EGTA, 100 µM MgCl2 on ice. Embryos were rinsed and stained according to manufacturer's protocol (Millipore #BG-6-B, #BG-7-B, #BG-8-C).
Apoptosis and proliferation assays
Embryos (E8.75 - 9.5) were fixed overnight in 4% PFA at 4°C. Embryos were rinsed in PBS and apoptosis was detected in whole embryo samples by TUNEL labeling using the In Situ Cell Death Detection Kit (Roche) for 4 hours at 37°C. Embryos were then counterstained in DAPI, mounted in Vectashield, and scanned using a Zeiss LSM5 Upright Pascal Confocal microscope. Projected Z-stacks were pseudocolored using Zeiss AIM software, flattened, and exported to Adobe Photoshop. Proliferation was assayed by immunohistochemistry for phosphohistone H3 (Upstate, 06-570,1∶500 dilution) on cryosections of E9.0 mouse embryos. Secondary antibody was AlexaFluor 488 conjugated goat anti-rabbit IgG (Invitrogen, A11034, 1∶300 dilution). Sections containing pharyngeal arch one were photographed on a Zeiss Axioplan compound microscope using Axiovision software. Phosphohistone H3 positive cells and DAPI stained nuclei within PA1 were counted using Image J to determine a mitotic index for PA1. An average mitotic index and standard deviation was calculated for both wild-type and mutant embryos. Statistical significance of differences between wild-type and Foxc1−/− counts was assessed using an unpaired, two-tailed Student's t-test with significance taken at p≤0.05.
Determination of cell cycle length
S-phase and cell cycle length were analyzed by incorporation of IdU-BrdU as previously described [36]. Pregnant females were injected intraperitoneally with IdU at 0.1 mg/kg body weight. After 1.5 hours, mice were injected intraperitoneally with 0.1 mg/kg body weight of BrdU. Two hours after IdU injection, mice were euthanized and embryos collected. Cryosections were then prepared and the IdU and BrdU positive cells were detected by immunostaining using mouse anti-BrdU antibody (BD Bioscience, which recognizes both IdU and BrdU) and rat anti-BrdU antibody (Abcam, which recognizes BrdU only). Counts were conducted on 4 non-adjacent sections of the cranial mesenchyme (5–7 s), PA1 mesenchyme (10–13 s), and oral ectoderm for each specimen. Cell cycle length was calculated as described previously [36].
qPCR
The cranial region of E8.5 embryos was isolated by using glass needles to cut transversely just anterior to the heart. For E9.5 embryos, cuts were made posterior and dorsl to PA1 and transversely at the level of the developing eye in order to isolate PA1. For each genotype, 3 pools of total RNA (dissected tissue from 3 embryos per pool) were isolated using the Qiagen RNeasy Mini Kit. Between 250–400 ng of total RNA from each pool was then used to generate random primed single-stranded cDNA (Superscript RTIII First Strand cDNA Synthesis Kit, Invitrogen). Relative levels of mRNA were determined using a qRT-PCR PowerSYBR (Applied Biosystems) assay with the following primers: Fgf8: 5′AATCCAGCCCCAAACTACC3′ and 5′GCTCTGCTCCCTCACATG3′; Foxc1: 5′TTCTTGCGTTCAGAGACTCG3′ and 5′AGGTACTTTCCCGTTCTTTCG3′ and internal control primers for Atp5b, Canx, Gapdh, and Ubc.
Imaging of whole-mount specimens and image processing
Whole mount embryos were photographed using a Leica MZ16 stereoscope, Nikon Digital Sight DS-Ri1 camera, and Nikon NIS Elements BR 3.2 software, unless otherwise noted. A subset of the images (Figure 1A, B, D, F; Figure 2; Figure 5A–D, M–P; Figure 6A, C, E–F, H–J, L–R; Figure S3A–B, I–J, Figure S7A–C, F,G, I, J) was acquired as a manual series of Z-stacks. These images were further processed using Helicon Focus (Helicon Soft, Ltd, http://www.heliconsoft.com) to compile and render the focused regions of the multiple focal planes into a single in focus image.
Supporting Information
Zdroje
1. HirataH, BesshoY, KokubuH, MasamizuY, YamadaS, et al. (2004) Instability of Hes7 protein is crucial for the somite segmentation clock. Nat Genet 36 : 750–754.
2. NodenDM (1982) Patterns and organization of craniofacial skeletogenic and myogenic mesenchyme: a perspective. Prog Clin Biol Res 101 : 167–203.
3. NodenDM (1983) The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat 168 : 257–276.
4. TrainorPA, TanSS, TamPP (1994) Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos. Development 120 : 2397–2408.
5. TrainorPA, TamPP (1995) Cranial paraxial mesoderm and neural crest cells of the mouse embryo: co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development 121 : 2569–2582.
6. Le Douarin N, Kalcheim C (1999) The Neural Crest. Bard J, Barlow P, Kirk D, editors. Cambridge Univesity Press.
7. ChaiY, JiangX, ItoY, BringasPJr, HanJ, et al. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127 : 1671–1679.
8. GrossJB, HankenJ (2008) Review of fate-mapping studies of osteogenic cranial neural crest in vertebrates. Dev Biol 317 : 389–400.
9. CoulyG, CreuzetS, BennaceurS, VincentC, Le DouarinNM (2002) Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 129 : 1061–1073.
10. BrownCB, WenningJM, LuMM, EpsteinDJ, MeyersEN, et al. (2004) Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev Biol 267 : 190–202.
11. TrumppA, DepewMJ, RubensteinJL, BishopJM, MartinGR (1999) Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev 13 : 3136–3148.
12. HaworthKE, HealyC, MorganP, SharpePT (2004) Regionalisation of early head ectoderm is regulated by endoderm and prepatterns the orofacial epithelium. Development 131 : 4797–4806.
13. VeitchE, BegbieJ, SchillingTF, SmithMM, GrahamA (1999) Pharyngeal arch patterning in the absence of neural crest. Curr Biol 9 : 1481–1484.
14. SatokataI, MaL, OhshimaH, BeiM, WooI, et al. (2000) Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nature Genetics 24 : 391–395.
15. SatokataI, MaasR (1994) Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 6 : 348–356.
16. QuS, TuckerSC, ZhaoQ, deCrombruggheB, WisdomR (1999) Physical and genetic interactions between Alx4 and Cart1. Development 126 : 359–369.
17. TuckerAS, MatthewsKL, SharpePT (1998) Transformation of tooth type induced by inhibition of BMP signaling. Science 282 : 1136–1138.
18. LeeSH, BedardO, BuchtovaM, FuK, RichmanJM (2004) A new origin for the maxillary jaw. Dev Biol 276 : 207–224.
19. Avery JK (2001) Oral development and histology. Thieme Medical Publishers.
20. CarlssonP, MahlapuuM (2002) Forkhead transcription factors: key players in development and metabolism. Dev Biol 250 : 1–23.
21. HannenhalliS, KaestnerKH (2009) The evolution of Fox genes and their role in development and disease. Nat Rev Genet 10 : 233–240.
22. ZarbalisK, SiegenthalerJA, ChoeY, MaySR, PetersonAS, et al. (2007) Cortical dysplasia and skull defects in mice with a Foxc1 allele reveal the role of meningeal differentiation in regulating cortical development. Proc Natl Acad Sci U S A 104 : 14002–14007.
23. SiegenthalerJA, AshiqueAM, ZarbalisK, PattersonKP, HechtJH, et al. (2009) Retinoic acid from the meninges regulates cortical neuron generation. Cell 139 : 597–609.
24. RiceR, RiceDP, OlsenBR, ThesleffI (2003) Progression of calvarial bone development requires Foxc1 regulation of Msx2 and Alx4. Dev Biol 262 : 75–87.
25. RiceR, RiceDP, ThesleffI (2005) Foxc1 integrates Fgf and Bmp signalling independently of twist or noggin during calvarial bone development. Dev Dyn 233 : 847–852.
26. KumeT, DengKY, WinfreyV, GouldDB, WalterMA, et al. (1998) The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 93 : 985–996.
27. HongHK, LassJH, ChakravartiA (1999) Pleiotropic skeletal and ocular phenotypes of the mouse mutation congenital hydrocephalus (ch/Mf1) arise from a winged helix/forkhead transcriptionfactor gene. Hum Mol Genet 8 : 625–637.
28. VivatbutsiriP, IchinoseS, HytonenM, SainioK, EtoK, et al. (2008) Impaired meningeal development in association with apical expansion of calvarial bone osteogenesis in the Foxc1 mutant. J Anat 212 : 603–611.
29. KidsonSH, KumeT, DengK, WinfreyV, HoganBL (1999) The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev Biol 211 : 306–322.
30. SeoS, SinghHP, LacalPM, SasmanA, FatimaA, et al. (2012) Forkhead box transcription factor FoxC1 preserves corneal transparency by regulating vascular growth. Proc Natl Acad Sci U S A 109 : 2015–2020.
31. SmithRS, ZabaletaA, KumeT, SavinovaOV, KidsonSH, et al. (2000) Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet 9 : 1021–1032.
32. WilmB, JamesRG, SchultheissTM, HoganBL (2004) The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev Biol 271 : 176–189.
33. KumeT, DengK, HoganBL (2000) Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127 : 1387–1395.
34. PurcellP, JheonA, ViveroMP, RahimiH, JooA, et al. (2012) Spry1 and spry2 are essential for development of the temporomandibular joint. J Dent Res 91 : 387–393.
35. EconomouAD, OhazamaA, PorntaveetusT, SharpePT, KondoS, et al. (2012) Periodic stripe formation by a Turing mechanism operating at growth zones in the mammalian palate. Nat Genet 44 : 348–351.
36. QuinnJC, MolinekM, MartynogaBS, ZakiPA, FaedoA, et al. (2007) Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol 302 : 50–65.
37. LiuW, SeleverJ, MuraliD, SunX, BruggerSM, et al. (2005) Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol 283 : 282–293.
38. LiuW, SunX, BrautA, MishinaY, BehringerRR, et al. (2005) Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132 : 1453–1461.
39. BarlowAJ, Francis-WestPH (1997) Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development 124 : 391–398.
40. KuriharaY, KuriharaH, SuzukiH, KodamaT, MaemuraK, et al. (1994) Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368 : 703–710.
41. ClouthierDE, HosodaK, RichardsonJA, WilliamsSC, YanagisawaH, et al. (1998) Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125 : 813–824.
42. YanagisawaH, YanagisawaM, KapurRP, RichardsonJA, WilliamsSC, et al. (1998) Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development 125 : 825–836.
43. SatoT, KuriharaY, AsaiR, KawamuraY, TonamiK, et al. (2008) An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci U S A 105 : 18806–18811.
44. RuestLB, XiangX, LimKC, LeviG, ClouthierDE (2004) Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development 131 : 4413–4423.
45. WilsonJ, TuckerAS (2004) Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Dev Biol 266 : 138–150.
46. TuckerAS, YamadaG, GrigoriouM, PachnisV, SharpePT (1999) Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development 126 : 51–61.
47. MinaM, WangYH, IvanisevicAM, UpholtWB, RodgersB (2002) Region - and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev Dyn 223 : 333–352.
48. Abu-IssaR, SmythG, SmoakI, YamamuraK, MeyersEN (2002) Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 129 : 4613–4625.
49. FrankDU, FotheringhamLK, BrewerJA, MugliaLJ, Tristani-FirouziM, et al. (2002) An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 129 : 4591–4603.
50. ShifleyET, VanhornKM, Perez-BalaguerA, FranklinJD, WeinsteinM, et al. (2008) Oscillatory lunatic fringe activity is crucial for segmentation of the anterior but not posterior skeleton. Development 135 : 899–908.
51. FergusonCA, TuckerAS, SharpePT (2000) Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development 127 : 403–412.
52. QiuM, BulfoneA, GhattasI, MenesesJJ, ChristensenL, et al. (1997) Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol 185 : 165–184.
53. QiuM, BulfoneA, MartinezS, MenesesJJ, ShimamuraK, et al. (1995) Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev 9 : 2523–2538.
54. DepewMJ, LiuJK, LongJE, PresleyR, MenesesJJ, et al. (1999) Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126 : 3831–3846.
55. DepewMJ, LufkinT, RubensteinJL (2002) Specification of jaw subdivisions by Dlx genes. Science 298 : 381–385.
56. DepewMJ, SimpsonCA, MorassoM, RubensteinJL (2005) Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. Journal Of Anatomy 207 : 501–561.
57. JeongJ, LiX, McEvillyRJ, RosenfeldMG, LufkinT, et al. (2008) Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development 135 : 2905–2916.
58. PoswilloD (1973) The pathogenesis of the first and second branchial arch syndrome. Oral Surgery, Oral Medicine, Oral Pathology 35 : 302–328.
59. LasterZ, TemkinD, ZarfinY, KushnirA (2001) Complete bony fusion of the mandible to the zygomatic complex and maxillary tuberosity: case report and review. Int J Oral Maxillofac Surg 30 : 75–79.
60. SnijmanPC, PrinslooJG (1966) Congenital fusion of the gums. Amer J Dis Child 112 : 593–595.
61. Hegtvedt AK (1993) Diagnosis and management of facial asymmetry. Peterson LJ, Indressano AT, Marciani RD, Roser SM, editors. Philadelphia: Lippincott.
62. HeudeE, RivalsI, CoulyG, LeviG (2011) Masticatory muscle defects in hemifacial microsomia: a new embryological concept. Am J Med Genet A 155A: 1991–1995.
63. HirschfelderU, PiechotE, SchulteM, LeherA (2004) Abnormalities of the TMJ and the musculature in the oculo-auriculo-vertebral spectrum (OAV). A CT study. J Orofac Orthop 65 : 204–216.
64. Huisinga-FischerCE, VaandragerJM, Prahl-AndersenB, van GinkelFC (2004) Masticatory muscle right-left differences in controls and hemifacial microsomia patients. J Craniofac Surg 15 : 42–46.
65. Huisinga-FischerCE, ZonneveldFW, VaandragerJM, Prahl-AndersenB (2001) Relationship in hypoplasia between the masticatory muscles and the craniofacial skeleton in hemifacial microsomia, as determined by 3-D CT imaging. J Craniofac Surg 12 : 31–40.
66. HeudeE, BouhaliK, KuriharaY, KuriharaH, CoulyG, et al. (2010) Jaw muscularization requires Dlx expression by cranial neural crest cells. Proc Natl Acad Sci U S A 107 : 11441–11446.
67. TavaresAL, GarciaEL, KuhnK, WoodsCM, WilliamsT, et al. (2012) Ectodermal-derived Endothelin1 is required for patterning the distal and intermediate domains of the mouse mandibular arch. Dev Biol 371 : 47–56.
68. ThomasBL, LiuJK, RubensteinJL, SharpePT (2000) Independent regulation of Dlx2 expression in the epithelium and mesenchyme of the first branchial arch. Development 127 : 217–224.
69. BurketLW (1936) Congenital bony temporomandibular ankylosis and facial hemiatrophy. Review of the literature and report of a case. JAMA 106 : 1719–1722.
70. DawsonKH, GrussJS, MyallRW (1997) Congenital bony syngnathia: a proposed classification. Cleft Palate Craniofac J 34 : 141–146.
71. Gripp K, Escobar LF (2006) Facial Bones. Stevenson RE, Hall JG, editors. New York: Oxford University Press.
72. MeyersEN, MartinGR (1999) Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science 285 : 403–406.
73. AlbertsonRC, YelickPC (2005) Roles for fgf8 signaling in left-right patterning of the visceral organs and craniofacial skeleton. Dev Biol 283 : 310–321.
74. MedeirosDM, CrumpJG (2012) New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev Biol 371 : 121–135.
75. FishJL, VillmoareB, KöbernickK, CompagnucciC, BritanovaO, et al. (2011) Satb2, modularity, and the evolvability of the vertebrate jaw. Evol Dev 13 : 549–564.
76. CompagnucciC, Debiais-ThibaudM, CoolenM, FishJ, GriffinJN, et al. (2013) Pattern and polarity in the development and evolution of the gnathostome jaw: both conservation and heterotopy in the branchial arches of the shark, Scyliorhinus canicula. Dev Biol 377 : 428–448.
77. DepewMJ, CompagnucciC (2008) Tweaking the hinge and caps: testing a model of the organization of jaws. J Exp Zool B Mol Dev Evol 310 : 315–335.
78. DepewMJ, SimpsonCA (2006) 21st century neontology and the comparative development of the vertebrate skull. Developmental Dynamics 235 : 1256–1291.
79. NodenD (1983) The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Devl Biol 96 : 144–165.
80. TokitaM, SchneiderRA (2009) Developmental origins of species-specific muscle pattern. Dev Biol 331 : 311–325.
81. PurcellP, JooBW, HuJK, TranPV, CalicchioML, et al. (2009) Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc Natl Acad Sci U S A 106 : 18297–18302.
82. RiddleRD, JohnsonRL, LauferE, TabinC (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75 : 1401–1416.
83. Nagy A, Gertsenstein M, Vintersten K, Behringer RR (2003) Manipulating the mouse embryo. Cold Spring Harbor: Cold Spring Harbor Laboratory.
Štítky
Genetika Reprodukční medicína
Článek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



