-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBugs in Transition: The Dynamic World of in Insects
article has not abstract
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1004069
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004069Summary
article has not abstract
The availability of Next Generation Sequencing tools has uncovered an unexpected and highly complex universe of hidden microbial passengers that are transiently or permanently associated with their hosts, hereafter called symbionts. Under natural conditions, these symbionts are often tightly controlled to occur at low densities and/or are restricted to special tissues by intrinsic and extrinsic factors, such as their own replication capacity, nutrition, and stress, as well as host immune competence.
One of the main questions of current symbiosis research is, however, to what extent these microbial passengers affect host phenotypes such as fitness, fecundity, pathogen resistance, or even behavior. In this issue of PLOS Genetics, Luis Teixeira and colleagues [1] have, very elegantly, started to answer some of these essential questions in symbiosis research. The focus of their research is on intracellular bacteria belonging to the genus Wolbachia, one of the most intensively studied symbionts. Wolbachia are found in up to 70% of insect species and in many terrestrial arthropods, and are vertically transmitted with the egg from an infected female to her progeny. In order to enhance their spreading success throughout host populations, Wolbachia can manipulate host reproductive biology by acting as a reproductive parasite, enhancing fitness and fecundity of infected females, enabling them to outcompete uninfected females rapidly in nature [2].
But what happens to the infection as soon as the majority of individuals within a host population are already infected and the spreading of the bacteria comes to a stop? In some cases it has been shown that, in their evolutionary past, Wolbachia have changed their phenotype from reproductive parasitism to obligate mutualism, where the symbiont takes on essential host functions for, e.g., oogenesis, nutrition, or even mate recognition [3]–[5]. Which kind of extrinsic and/or intrinsic factors trigger phenotypic transitions, and what is their genetic basis? In a recent study it was shown that within only 20 years in nature, Wolbachia can transform from a costly reproductive parasite into a mutualist by enhancing fecundity of infected Drosophila simulans females [6].
One of the most puzzling observations, however, is the worldwide replacement during the 20th century of one ancestral Wolbachia strain named wMelCS with the closely related variant wMel in Drosophila melanogaster, the top genetic model system for studying insect host-symbiont interactions [7], [8]. Although neither Wolbachia strain causes any significant level of reproductive parasitism in its respective native host, either in the laboratory or in nature, both persist globally in D. melanogaster at high frequencies worldwide, suggesting they serve some host functions. Moreover, two independent studies have shown that both Wolbachia strains confer significant virus protection [9], [10]. Impressively, D. melanogaster Wolbachia also protects artificially transinfected mosquitos that do not naturally carry Wolbachia from pathogenic RNA viruses, such as Dengue and Chikungunya [11]. A third Wolbachia strain of D. melanogaster that provides even stronger virus resistance is wMelPop, a pathogenic Wolbachia variant that originally emerged after radiation mutagenesis in the laboratory. This virulent Wolbachia strain has recently become a major research focus because it overreplicates massively in somatic host tissues (it is named “popcorn” due to the popcorn-like appearance of infected cells in adult tissues) and hence significantly shortens the lifespan of its host [12]. After transferring wMelPop into the main vector of dengue fever Aedes aegypti, it was shown that the life-shortening effect of wMelPop is also manifest in this medically important insect system [13]. The combination of virus protection, life shortening, and the capacity of maternal spreading render D. melanogaster Wolbachia strains prime candidates for biologically sound insect pest control strategies. Besides their impressive applied capacities for arthropod-borne disease control, however, we currently lack understanding on the following main questions: what are the genotypic and phenotypic differences between the three strains, and why did the wMel strain replace wMelCS globally in the recent past? A better understanding of their genetic basis and their short-term cost–benefit dynamics will be pivotal for further Wolbachia-applied studies in heterologous pest systems.
To this end, Texeira and colleagues studied the molecular and phenotypic basis of short-term Wolbachia dynamics in D. melanogaster. First, they crossed the three Wolbachia variants wMelCS, wMel, and wMelPop into a common genetic fly background in order to exclude any nuclear background effects. Then they carefully assayed for their capacity to suppress viral infections of the Drosophila C virus and Flock house virus in the presence and absence of Wolbachia. They found that the ancestral wMelCS variants proliferate to higher densities and confer greater protection against the two RNA viruses than do flies infected with wMel variants. They also found that flies carrying the ancestral wMelCS infection, although more protected against viral infections, pay a price by living slightly shorter lives than wMel-infected flies (Figure 1). They therefore propose that the ancestral high-cost strain wMelCS (life-shortening due to high titer) has been replaced in nature recently by the less costly Wolbachia variant wMel, which still protects the host significantly from viral infections but replicates at lower densities with lower costs on longevity.
Fig. 1. Evolutionary cost–benefit dynamics of Wolbachia in natural populations (below) and laboratory lines (above) of Drosophila melanogaster. 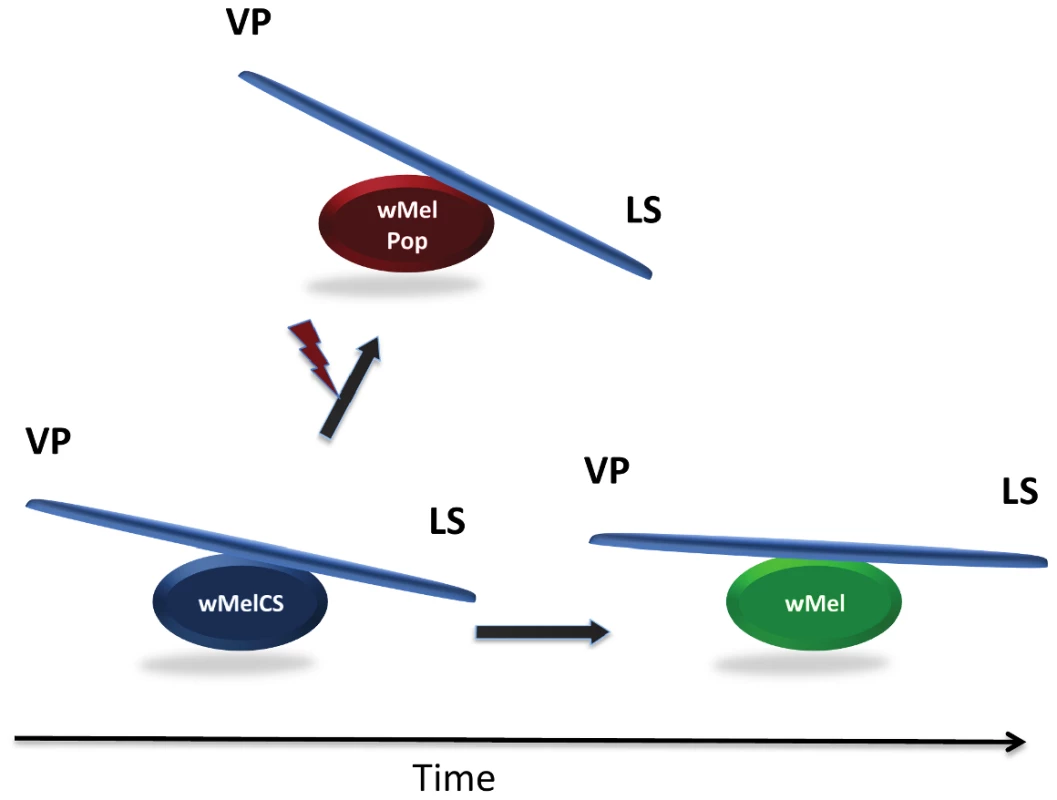
While beneficial Wolbachia phenotypes such as virus protection (VP) enhance host fitness, increased Wolbachia densities are costly by decreasing lifespan (LS). The ancestral Wolbachia variant wMelCS is shown in blue, the laboratory-derived pathogen wMelPop that has been generated by irradiation (red flash) in red, and the recent worldwide infection variant wMel in green. Finally, they trace the genetic basis for the transition from symbiosis to virulence by linking phenotype to genotype in the monophyletic wMelCS Wolbachia group. Through their thoughtful comparative analysis of mutualistic wMelCS-like versus pathogenic wMelPop genomes, they found that these two phenotypically distinct Wolbachia strains form a monophyletic group that mainly differ in only one genomic region: a 7-fold amplification of a 21-kb region in wMelPop encoding eight Wolbachia proteins. These data strongly imply that the selective amplification of one or more genes within this so-called “Octomom region” is quite likely responsible for the expression of the virulent Popcorn phenotype.
This impressive study opens numerous additional questions that will be highly relevant for symbiosis research but also for future applied aspects: Which of the eight candidate genes of the Octomom region of wMelPop are sufficient for triggering Wolbachia pathogenicity? What are the genetic bases for symbiont-directed viral host protection and its strength? And finally, what kind of phenotypic and genetic Wolbachia transitions can be expected to happen in novel host systems such as mosquitos in the near future under natural selection? Stay tuned! It is likely that we are pretty close to monitoring short-term evolutionary dynamics of host–symbiont interactions in real time.
Zdroje
1. ChrostekE, MarialvaMSP, EstevesSS, WeinertLA, MartinezJ, et al. (2013) Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLOS Genet 9: e1003896 doi:10.1371/journal.pgen.1003896
2. WerrenJH, BaldoL, ClarkME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6 : 741–751.
3. DedeineF, VavreF, FleuryF, LoppinB, HochbergME, et al. (2001) Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci U S A 98 : 6247–6252.
4. HosokawaT, KogaR, MengXY, FukatsuT (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A 107 : 769–767.
5. MillerWJ, EhrmanL, SchneiderDI (2010) Infectious speciation revisited: impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLOS Pathog 6: e1001214 doi:10.1371/journal.ppat.1001214
6. WeeksAR, TurelliM, HarcombeWR, ReynoldsKT, HoffmannAA (2007) From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLOS Biol 5: e114 doi:10.1371/journal.pbio.0050114
7. RieglerM, SiduhM, MillerWJ, O'NeillSH (2005) Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol 15 : 1428–1433.
8. RichardsonMF, WeinertLA, WelchJJ, LinheiroRS, MagwireMM, et al. (2012) Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLOS Genet 8: e:1003129 doi:10.1371/journal.pgen.1003129
9. TeixeiraL, FerreiraA, AshburnerM (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLOS Biol 6: e1000002 doi:10.1371/journal.pbio.1000002
10. HedgesLM, BrownlieJC, O'NeillSL, JohnsonKN (2008) Wolbachia and virus protection in insects. Science 322 : 702.
11. MoreiraLA, Iturbe-OrmaetxeI, JefferyJA, LuG, PykeAT, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139 : 1268–1278.
12. MinKT, BenzerS (1997) Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A 94 : 10792–10796.
13. McMenimanCJ, LaneRV, CassBN, FongAW, SidhuM, et al. (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323 : 141–144.
Štítky
Genetika Reprodukční medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



