-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDefining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
Hydrolysis of ATP by partition ATPases, although considered a key step in the segregation mechanism that assures stable inheritance of plasmids, is intrinsically very weak. The cognate centromere-binding protein (CBP), together with DNA, stimulates the ATPase to hydrolyse ATP and to undertake the relocation that incites plasmid movement, apparently confirming the need for hydrolysis in partition. However, ATP-binding alone changes ATPase conformation and properties, making it difficult to rigorously distinguish the substrate and cofactor roles of ATP in vivo. We had shown that mutation of arginines R36 and R42 in the F plasmid CBP, SopB, reduces stimulation of SopA-catalyzed ATP hydrolysis without changing SopA-SopB affinity, suggesting the role of hydrolysis could be analyzed using SopA with normal conformational responses to ATP. Here, we report that strongly reducing SopB-mediated stimulation of ATP hydrolysis results in only slight destabilization of mini-F, although the instability, as well as an increase in mini-F clustering, is proportional to the ATPase deficit. Unexpectedly, the reduced stimulation also increased the frequency of SopA relocation over the nucleoid. The increase was due to drastic shortening of the period spent by SopA at nucleoid ends; average speed of migration per se was unchanged. Reduced ATP hydrolysis was also associated with pronounced deviations in positioning of mini-F, though time-averaged positions changed only modestly. Thus, by specifically targeting SopB-stimulated ATP hydrolysis our study reveals that even at levels of ATPase which reduce the efficiency of splitting clusters and the constancy of plasmid positioning, SopB still activates SopA mobility and plasmid positioning, and sustains near wild type levels of plasmid stability.
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1003956
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003956Summary
Hydrolysis of ATP by partition ATPases, although considered a key step in the segregation mechanism that assures stable inheritance of plasmids, is intrinsically very weak. The cognate centromere-binding protein (CBP), together with DNA, stimulates the ATPase to hydrolyse ATP and to undertake the relocation that incites plasmid movement, apparently confirming the need for hydrolysis in partition. However, ATP-binding alone changes ATPase conformation and properties, making it difficult to rigorously distinguish the substrate and cofactor roles of ATP in vivo. We had shown that mutation of arginines R36 and R42 in the F plasmid CBP, SopB, reduces stimulation of SopA-catalyzed ATP hydrolysis without changing SopA-SopB affinity, suggesting the role of hydrolysis could be analyzed using SopA with normal conformational responses to ATP. Here, we report that strongly reducing SopB-mediated stimulation of ATP hydrolysis results in only slight destabilization of mini-F, although the instability, as well as an increase in mini-F clustering, is proportional to the ATPase deficit. Unexpectedly, the reduced stimulation also increased the frequency of SopA relocation over the nucleoid. The increase was due to drastic shortening of the period spent by SopA at nucleoid ends; average speed of migration per se was unchanged. Reduced ATP hydrolysis was also associated with pronounced deviations in positioning of mini-F, though time-averaged positions changed only modestly. Thus, by specifically targeting SopB-stimulated ATP hydrolysis our study reveals that even at levels of ATPase which reduce the efficiency of splitting clusters and the constancy of plasmid positioning, SopB still activates SopA mobility and plasmid positioning, and sustains near wild type levels of plasmid stability.
Introduction
Partition, the elementary form of mitosis that segregates sibling replicons in bacteria, depends critically on replicon-specific NTPases. These proteins, generically termed ParA, contact their replicons by interacting with the cognate ParB protein bound to a set of short sequence repeats (parS) that serves as the centromere. The interaction provokes dynamic behaviour of ParA which somehow directs replicon copies to the new cells-to-be.
By far the most common type of partition NTPase is characterized by a variant ATP-binding Walker A box motif [1], [2] (reviewed by Vecchiarelli et al. [3]). These ParAs are specified by most low copy number plasmids and are the only type encoded by sequenced chromosomes. Mutation of conserved ATP interaction motifs in ParA proteins leads to frequent mis-localization and segregation failure, particularly of low copy-number plasmids [4]–[8], leaving no doubt that ATP is essential to partition.
Just how ATP is involved is harder to define. The ATP hydrolysis activity of Walker-box ParA proteins is intrinsically very weak but is synergistically stimulated in vitro by cognate ParB proteins and by DNA [9]–[11], particularly when the latter includes the centromere [12]. Likewise, the ability of these ParA proteins to migrate en masse from end to end of the nucleoid a few times per cell cycle, presumed to be relevant to plasmid segregation [6], [13], is seen only in the presence of the cognate ParB and parS. These observations, together with the parallel effects of ATPase mutations on partition and ParA dynamics and the correlated movements of ParA and its plasmid [6], [13]–[16], argue for the involvement of ATP hydrolysis in the partition mechanism. On the other hand, binding of ATP without hydrolysis changes the conformation of ParA proteins [7], [17] and alters their properties: it facilitates dimerization of ParA [18]–20 and it enables dimers to bind to non-specific DNA [20]–[23] as well as to each other to form polymeric assemblies [4], [14], [15], [21], [22], [24], [25]. Thus, even in the case of partition-defective mutants that demonstrably bind but fail to hydrolyse ATP, it has not been possible to exclude conformational effects as the key reason for the functional deficiency.
We have recently reported that in the case of the Sop partition system of the Escherichia coli plasmid F, mutation of two arginine residues in the SopB N-terminus, neither of which significantly affects affinity for SopA, reduces stimulation of SopA-catalyzed ATP hydrolysis in vitro [12]. The mutants are interesting for two reasons. One is mechanistic: the characteristics of R36 and R42 mutants are consistent with the former acting as an arginine-finger [26], inserting itself into the ATP-binding pocket of SopA to facilitate processing of the transition state, and the latter serving to optimize positioning of the finger, as previously suggested for analogous mutations in the ParG partition protein of plasmid TP228 [4].
The other reason is the delegation of responsibility for modulating ATP hydrolysis to a physically distinct partner protein. It should be possible to use the SopB arginine-finger mutants to reduce ATP hydrolysis by wild type SopA protein whose conformational responses to ATP binding are normal, and thus to distinguish the substrate and cofactor roles of ATP for any activity in which SopA is involved. We report here that SopB-mediated stimulation of ATP hydrolysis by SopA is needed to restrain oscillatory movement of SopA, to stabilize plasmid positioning and to limit plasmid clustering.
Results
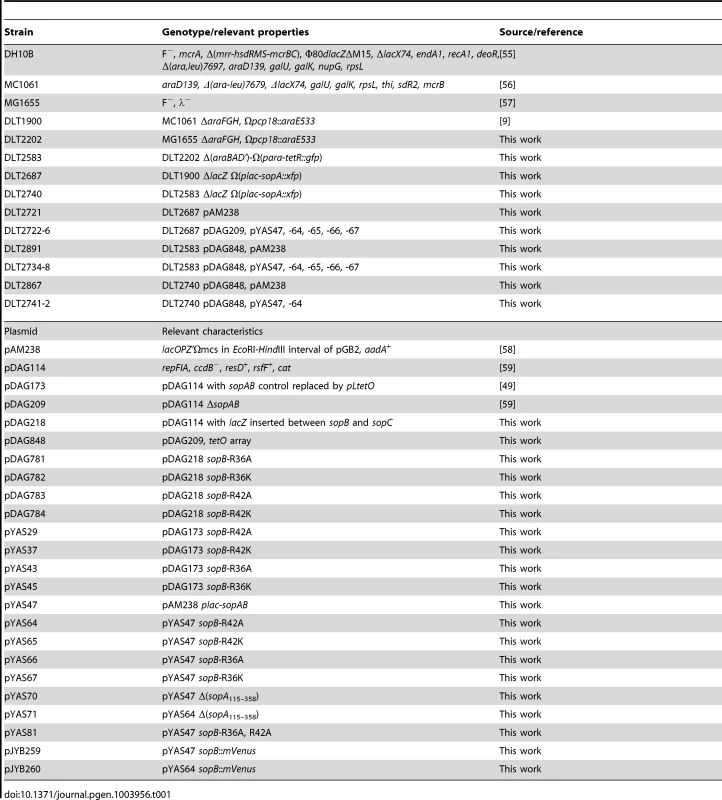
In our previous in vitro study [12], we found hydrolysis of ATP by SopA to be stimulated three-fold by SopB alone and a further 16 - and 45-fold when non-specific and sopC-containing DNA, respectively, were also present. SopB mutation R36A reduced ATPase stimulation by about 20-fold, R36K by seven-fold and R42A by six-fold. Such decreases, if reproduced in vivo, would be expected to provoke frequent loss of mini-F from dividing cells.
Mini-F stability
We substituted the R36 and R42 alleles for wild type sopB in two normally stable mini-F plasmids. In one, pDAG218, a promoterless lacZ gene is inserted between sopB and the sopC centromere, allowing direct monitoring of plasmid loss and of transcription from the natural, autoregulated sop promoter. Assays of plasmid retention during growth in non-selective minimal-glycerol medium (Figure 1A) showed the mutants to be surprisingly stable. The R36A mutation caused about a six-fold increase in mini-F loss, the other mutations almost none, within measurement error. Assays of β-galactosidase showed that only the R36A mutant caused a significant rise in transcription, 1.8-fold (data not shown), a result confirmed by the 1.6-fold increase in SopA protein determined by immunoblot assay of cell extracts (Figure S1A). To determine whether higher sopAB expression accounted for the instability of the R36A mutant relative to the others, we assayed stability of mutant derivatives of the other mini-F, pDAG173, in which sopAB is transcribed constitutively from pL-tetO [27]. With Sop protein levels thus rendered uniform (though 3.4-fold higher than with pDAG218; Figure S1A), the sopB mutant derivatives of pDAG173 showed much the same degree of modest instability as their pDAG218 equivalents (Figure 1A); the increased loss rate of the R36K mutant is offset to some extent by the variability inherent in low loss rate estimates, such that we cannot assess its significance. The essential result is that the R36A, and possibly R36K, mutation results in slight destabilization of mini-F.
Fig. 1. Effect of SopB R36 and R42 mutations on mini-F stability. 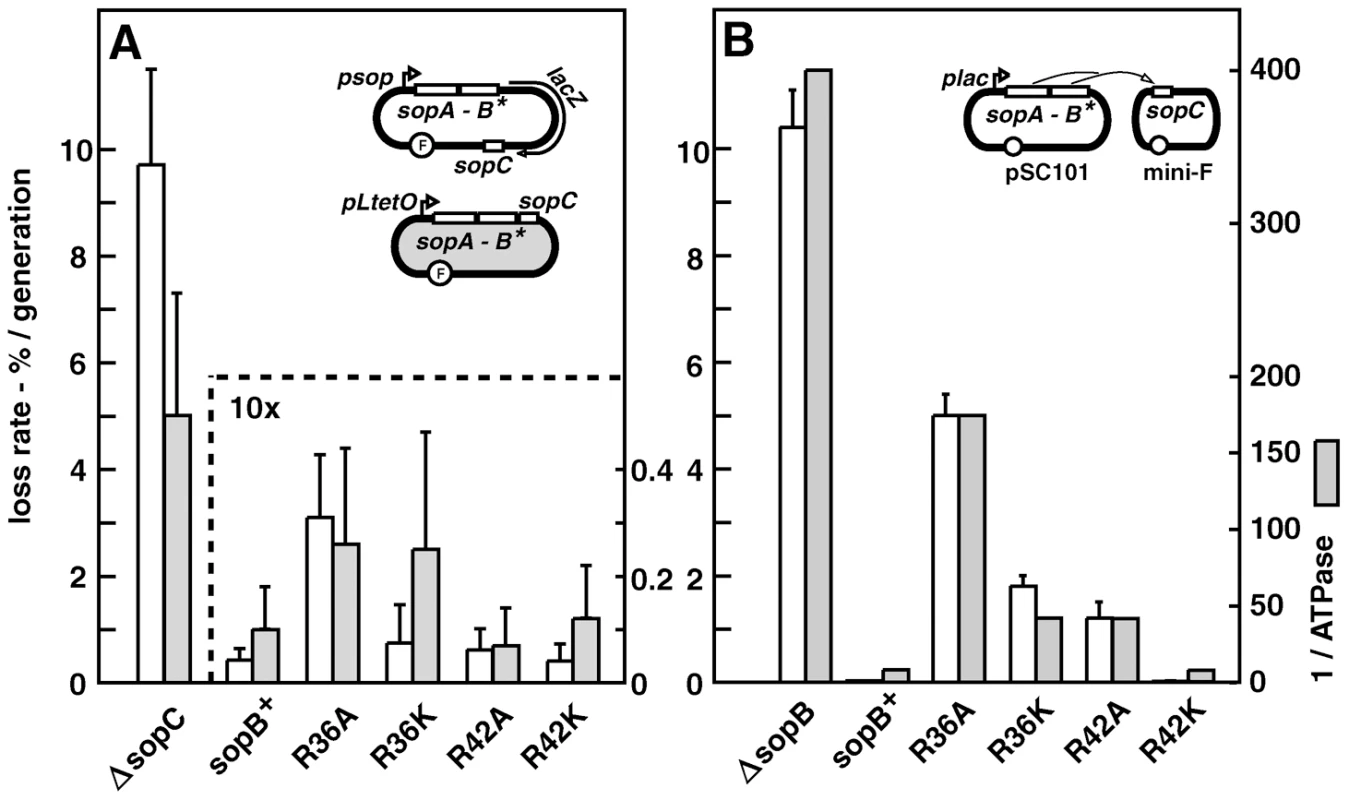
A. sopAB operon and sopC in natural configuration (cis; see sketches): white bars - strain DLT1900 carrying pDAG218 and derivatives, grown at 30°C in minimal-glycerol-leucine medium (t∼160 min); shaded bars - strain DH10B carrying pDAG173 and derivatives, grown in minimal-glycerol-Casamino acids medium (t∼80 min). Error bars are standard deviations of ≥3 loss-rate assays. Note 10-fold scale change for sopC+ plasmids. B. sopAB and sopC on separate plasmids, pAM138 and mini-F respectively (trans), in strain MC1061 grown in minimal-glycerol-leucine medium: white bars – loss rates. Shaded bars show ATPase deficits as the inverse of ATPase stimulation by each SopB in vitro, determined previously [12]. Attempting to increase the sensitivity of the stability assay, we reconfigured it by placing the sopAB operon under plac on an oligocopy (pSC101) vector, in trans to a mini-F ΔsopAB derivative. We considered that SopB protein radiating from the point of its production near the sopAB 3′ end might well be less plentiful at sopC owing to free diffusion and increased competition from non-specific DNA, resulting in formation of smaller, less effective SopB assemblies that amplify the handicap imposed by the mutant SopB defects. While wt sopAB still fully stabilized the mini-F (0.005% loss per generation) in this trans test, the mutations provoked much higher mini-F instability than when in cis (Figure 1B). The R36A mutation had the strongest effect, causing ∼1000-fold destabilization, about half that seen with no active partition, while the R36K and R42A mutations destabilized less, ∼360 - and 240-fold respectively, and the R42K mutation not at all. Whether or not our rationale for applying the trans test is correct, by amplifying loss rates the test serves as an indicator of Sop system performance. All succeeding experiments were carried out using this trans configuration.
Alteration in SopB affinity for DNA, for SopA or for itself is unlikely to explain the partition phenotypes seen. We have shown that the mutant SopB proteins are unchanged with respect to specific and non-specific DNA binding, and exhibit affinity for SopA similar to that of wt SopB [12]. They also behave as wt SopB in destabilizing mini-F when in excess and in silencing transcription from a nearby promoter, two properties which depend on interaction of SopB dimers via their N-terminal domains (Figure S4). More importantly, the deficiency of each SopB in ATPase stimulation is closely correlated with the rate of mini-F loss (Figure 1B). Thus we can be confident that the restricted ability of the SopB R36A, R36K and R42A mutant proteins to stimulate SopA-mediated ATP hydrolysis is the major or exclusive cause of the defective partition seen. Because ATP hydrolysis is thought to be necessary for the segregation event that localizes plasmids in cell-halves, we next sought clues to the role of SopB-stimulated ATP hydrolysis by directly visualizing mini-F molecules.
Mini-F clustering
To observe mini-F, we inserted an array of Tet repressor binding sites into a mini-F equivalent to that used in the trans stability tests, and introduced the resulting plasmid (pDAG848) into a TetR::Gfp-producing strain for visualization by fluorescence microscopy. The concentration of Sop proteins made from the pSC101-based plasmids is only about twice the wt mini-F level (Figure S1B; Table S1), owing to regulation of sopAB expression by Lac repressor in this MG1655 (lacI+)-based strain. Figure 2A shows that cells growing in MGC at 1.2 generations per hour, with an average of 3 copies of pDAG848 per cell, contained 1.2-fold more foci in the presence of wt SopB than of SopB-R36A. The difference was significant (see legend) but not strong, and was not increased by addition of the R42A mutation (Figure 2A). It was accentuated in cells growing in LB at 2.4 generations per hour with an average of 4.6 copies per cell [28], as shown together with foci/cell for the other sopB alleles in Figure 2B. An average of 3 foci per cell were seen in cells with wt SopB, 2.2 with the R42K mutant, 1.5 with R36K or R42A, and 1.2 with R36A. Consistent with this observation, R36A foci were on average brighter than wt foci (Figures 2C, S3A). Copy number per mini-F-carrying cell was not affected by the nature of the SopB protein (Table S2). Hence, whereas in the presence of wt SopB at least half the foci consist of a single plasmid molecule, the number of molecules per focus rises progressively as the ability of SopB to stimulate ATP hydrolysis declines, up to nearly four in the case of R36A. The frequency of cells with no focus also rises, consistent with destabilization by the R36A allele in LB-grown cells (Table S1). SopB-stimulated ATP hydrolysis apparently contributes to splitting mini-F clusters or preventing their formation.
Fig. 2. Effect of R36 and R42 mutations on mini-F clustering. 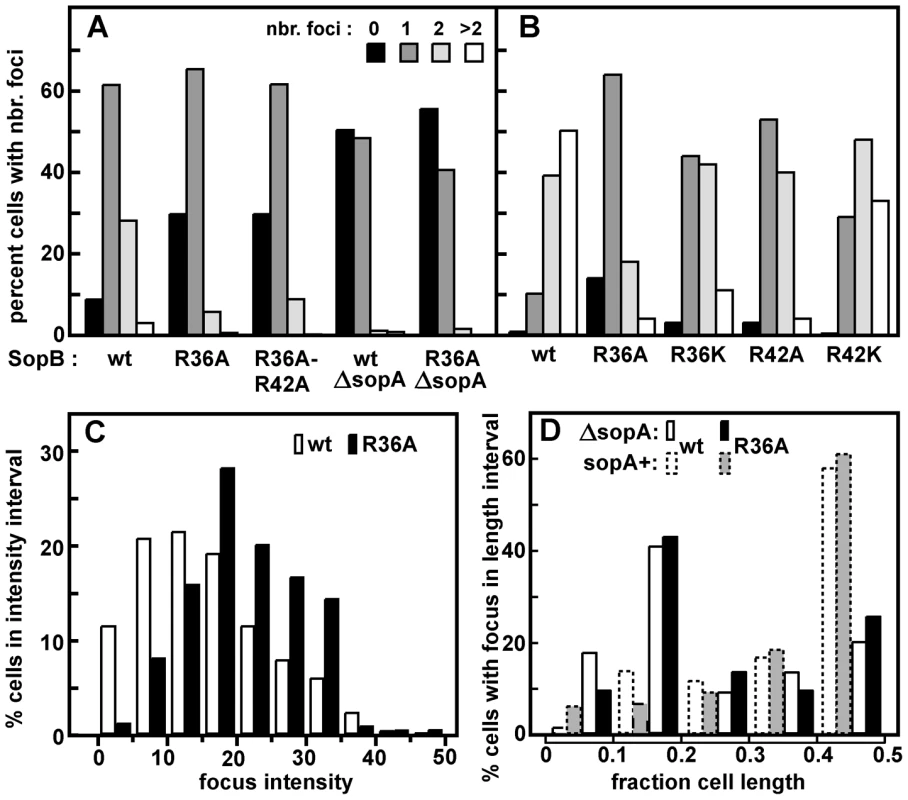
Derivatives of strain DLT2583 (para-tetR::gfp) carrying the tetO-array mini-F, pDAG848, and producing SopA and mutant SopBs in trans were grown in MGC medium (A) or LB broth (B), and the mini-F plasmid foci were visualized as described in Materials and Methods and counted. Total cells scored were: A - 756 (wt), 626 (R36A) 415 (R36A, R42A); 344 (ΔsopA); 368 (ΔsopA, R36A); B - 2118 (wt), 3966 (R36A), 1874 (R36K), 1585 (R42A), 1736 (R42K). The wt and R36A focus number distributions in panel A were shown by the Student t-test to be different at a confidence interval of 95%. Sop protein concentrations relative to wt mini-F were measured by immuno-blot assay to be 1.9- and 2.2-fold (SopA) and 1.7 and 2.4-fold (SopB) higher in cells grown in MGC and LB respectively. C. Brightness of the foci in LB-grown cells, measured using Microbe Tracker. The distributions are different at the 95% confidence level by the Student t-test. Because focus intensity responds non-linearly to plasmid content (as seen elsewhere; e.g. [51]), foci in R36A cells were on average 1.3-fold brighter than in SopB-wt cells while consisting of 2.4 times as many molecules. D. Localization of pDAG848 without SopA. Cells were grown in MGC. The shortest pole-focus distances in single-focus cells were sorted into 1/10 cell-length classes. Dotted-line bars show distributions of single foci in the presence of SopA (Figure 5B). Distributions of focus brightness in ΔsopA cells containing wt and R36A SopB proteins were not significantly different at the 95% confidence level, by the same test as applied to the brightness data of Figure 2C (not shown). To determine whether the R36/42 mutations might influence plasmid clustering independently of SopA we observed pDAG848 supplied with the SopB proteins only. Strong destabilization of mini-F by removal of SopA has been attributed to failure to resolve clusters [29], but inability to direct plasmid movement could also contribute [18], [22]. pDAG848 foci were indeed not seen in half the cells and seldom numbered more than one when present (Figure 2A). In addition, the preferred mid-cell positioning of single mini-F foci gave way to a more polar localization (Figures 2D, S3B). The same reduction in focus number and change in focus distribution occurred whether cells carried the wt or the R36A allele of sopB. Likewise, in the presence of SopA, fluorescent derivatives of wt and R36A SopBs both formed foci distributed over the long cell axis like those of labelled mini-F (Figure S3C). We thus saw no evidence for an effect of the R36/42 residues other than stimulation of ATP hydrolysis by SopA.
SopA relocation
The elements that most effectively stimulate SopA to hydrolyse ATP, SopB and sopC, are also needed to activate the migration of SopA between nucleoid poles, implying that SopB mutants with significant deficiencies in ATPase stimulation, R36A, R36K and R42A, would reduce or eliminate SopA relocation. In order to examine dynamic behaviour of SopA in the presence of wild type and mutant SopB proteins, we introduced into the chromosome of both DLT1900 and DLT2583 a sopA::xfp fusion whose product fully stabilizes mini-F [18]; the construction restored lacI and lacY to DLT1900, putting sopA::xfp expression in this strain under LacI control and enabling the two strains to be used interchangeably. The DLT1900 derivative (DLT2687) was transformed with pYAS47 (pSC101-plac:sopAB) and pDAG209 (mini-F-Δ(sopAB) sopC+), and the movement of SopA::Xfp was followed by fluorescence microscopy. As seen elsewhere [13], fluorescent SopA moved from end to end of the nucleoid 3–5 times per cell cycle provided sopC and wild type SopB were present (data not shown).
We then measured the frequency of SopA relocation after substituting the mutant R36 and R42 codons in sopB. The time-lapse sequences shown in Figure 3A illustrate the surprising result depicted in Figure 3C - a major increase in oscillation frequency for all mutants except R42K, which behaves as wt. The average period of migration to the far nucleoid pole and back was 3.2 minutes for the R36A mutant and 3.6 minutes for R36K and R42A, respectively 2.7 - and 2.4-fold less than the return trip with wt SopB (8.6 minutes). The increase in SopA relocation frequency was only a little higher for the R36A mutant than for R36K and R42A, rather than proportional to ATPase stimulation deficiency as with mini-F in stability and clustering (Figures 1C, 2B); a factor other than reduced ATP hydrolysis may be rate-limiting at this high oscillation frequency. Total SopA levels in these experiments were 3–4 times higher than that in mini-F-carrying cells, but relocation frequencies were comparable at SopA concentrations ∼ten-fold higher still (Figure S2); the high frequencies are thus not a result of SopA excess.
Fig. 3. Effect of R36 and R42 mutations on SopA movement. 
A. Time-lapse image series of typical DLT2687 (plac-sopA::xfp)/pDAG848 (mini-F)) cells carrying pYAS47 (sopA+B+) or pYAS64 (sopA+BR36A) grown in MGC-IPTG (0.1 µM) medium and applied to MGC-IPTG agarose slides. Images were acquired with an Olympus microscope at 70-second intervals for 29 minutes using 0.5 sec exposure. B. Kymograph of the SopA::Xfp foci in A. Each point represents the position of the brightest pixel in the image. C. Distribution of oscillation rates in cells producing the wt and the four mutant SopBs. Rate data are binned into 0.5 oscillation/30 min intervals. The levels of SopA::Xfp and SopA were, respectively, about 3-fold and 0.3-fold the wt mini-F levels (Figure S1C); repression of the plasmid-borne plac promoter is stronger than that of the chromosomal plac in the conditions used. The superimposed kymographs of Figure 3B suggested that the more rapid oscillation of SopA in the presence of mutant SopBs arose from virtual elimination of the normal pause between SopA's arrival at one end of the nucleoid and its setting out for the other. Accordingly, we repeated the experiment with shorter image-capture intervals. Because of the shorter viewing period the progressive decline of oscillation rate, owing to decelerating growth or other factors, has less effect, so that the average SopA return trip was shorter in this experiment, 1.8 minutes for R36A; nevertheless, the 3-fold increase relative to wild type was comparable to the 2.7-fold of 30-minute acquisition experiments, as shown in Figure 4. These kymographs confirm the initial indications that the difference in oscillation rates arises mainly from the short time that SopA spends at the nucleoid ends. The average speeds of SopA movement along the nucleoid were similar whether SopB was wt or mutant, whereas the average dwell-time decreased five-fold with the mutant SopB. Indeed the relative intensities of nucleoid-end signal in wt and mutant SopB cells suggest that in the latter, SopA arriving at the ends does not wait for following molecules before setting out again, although we have not ruled out the possibility that in this case much of the SopA is dispersed and relatively immobile. Western blotting showed similar levels of SopA::Xfp protein in both cases (Figure S1C); the difference is thus not due to blockage by piled up or aggregated protein. These observations imply that stimulation of ATP hydrolysis by SopB slows the process by which SopA reinitiates its migration but is not required for the migration itself.
Fig. 4. Effect of SopB-R36A on SopA relocation at high resolution. 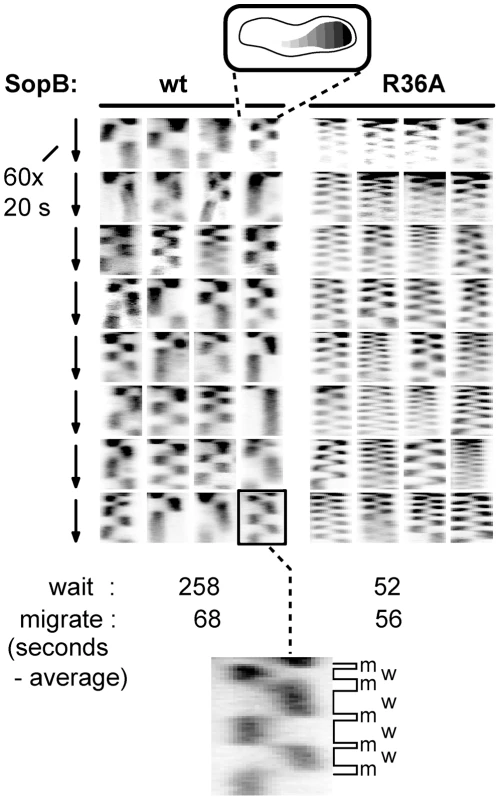
SopA::Xfp movement in DLT2740 (plac-sopA::xfp)/pDAG848 (mini-F)) cells carrying pYAS47 (sopA+B+) or pYAS64 (sopA+BR36A) grown in MGC with 1 µM IPTG was monitored as in Figure 3 but with a Nikon microscope at 20 sec intervals for 20 min using 100 msec exposure. To facilitate comparison, kymograph widths representing cell length have been equalized and brightness has been adjusted. The kymograph at bottom has been expanded to show differentiation of SopA “wait” (w) and “migrate” (m) periods; wait is defined as an exposure in which >80% of the pixels are on one side of the mid-line, migrate as the interval between two waits. Partition ATPase dynamics is generally thought to determine the trajectory and destination of segregating plasmid copies. Accordingly, we next investigated how the R36 and R42 mutations affect mini-F movement.
Mini-F positioning and movement
The average positioning of mini-F plasmids was little altered by substitution of SopB-R36A for wild type. Figure 5A shows the distances from pole to pDAG848 focus in the one - and two-focus classes of cells grown in MGC medium (Figure 2A); although R36A foci predominate at the margins of the distributions, the widening of the spread in R36A cells is slight. Figure 5B summarizes these data as plasmid focus position relative to cell length. Regardless of whether the cells were producing wt SopB or the most defective mutant, R36A, about 60% of single foci were within the central fifth of the cell and about 80% of double foci were grouped around the one-third and two-thirds positions. Similar positioning was seen in cells grown in LB medium (Figure 5B), despite the high degree of plasmid clustering observed in such cells (Figure 2B). Plasmid clusters appear to be as readily positioned as single molecules by the Sop partition apparatus. The most straightforward interpretation of this pattern is that the capacity of SopB to stimulate ATP hydrolysis does not strongly affect the average positions of segregation units once these have been defined by initial separation events. Nevertheless, abnormal positioning was evident in certain R36A cells whose two foci were clearly on the same side of mid-cell (blue symbols in Figure 5A); unless rectified before the next cell division this mis-positioning would result in partition failure.
Fig. 5. Limited effect of R36A mutation on mini-F positioning. 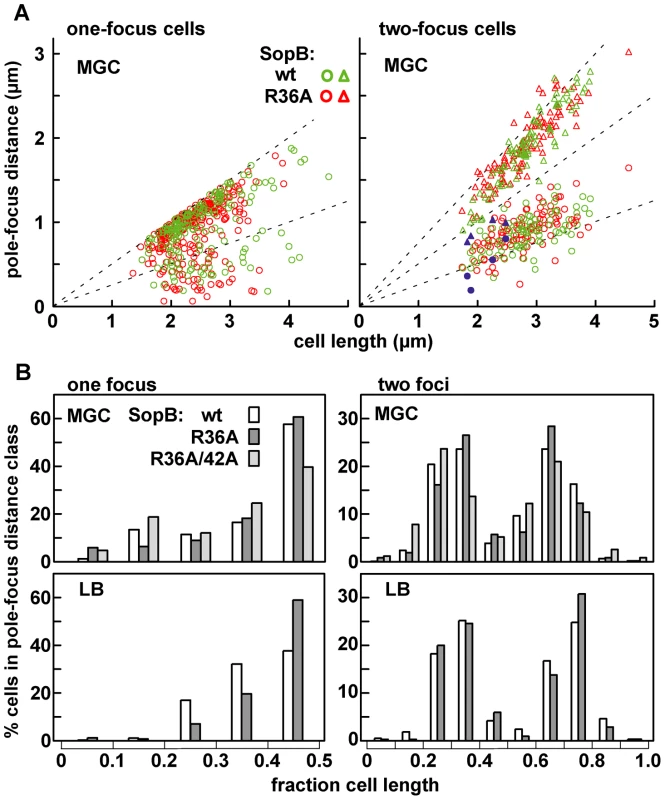
The distance between the pole nearest a focus and the foci in MGC-grown cells of the one- and two-focus classes was measured. A: Pole-focus distances plotted against cell-length. The wt and R36A data-sets are superimposed. Blue symbols denote the four cases (out of 107, all R36A) in which both foci were unambiguously in the same cell-half. B: Pole-focus distances sorted into 1/10-cell intervals. In the right-hand panel distances to foci beyond the mid-point are measured from the same pole as the closer foci. To examine mini-F movement, we observed pDAG848 foci in MGC-grown cells after labelling the plasmid by brief induction of tetR::gfp. Cumulative plasmid trajectories (Figure 6A) show mini-F movement to be less constrained in the presence of the R36A mutant than with wt SopB. By imaging pDAG848 in thirty single-focus cells at 5-second intervals over 5 minutes, we could estimate that on average it moves about 1.5 fold faster (from cumulative distance, Figure 6B). We discerned no consistent relationship between SopA and mini-F movements when these were monitored simultaneously but we could estimate from the oscillation data (above) that in the 5 min viewing time a SopA wave would pass on average once for wt SopB and three times for the mutant. The illustrative kymographs in rows 1 and 2 of Figure 6D reveal that compared to wt SopB, which generally maintains mini-F steady near the centre or quarter positions (row 1 - a–e; h and i show the two exceptions), the R36A mutant provoked a jumpy, exaggerated movement (row 2 - e, f are exceptions) and a frequent approach to the cell poles (c, g, h, i) not seen with wt. The frequencies of detectable direction reversal were similar, 13.6+/−5.5 (s.d.) for wt and 14.6+/−7.1 for R36A.
Fig. 6. Effect of R36A mutation on mini-F movement. 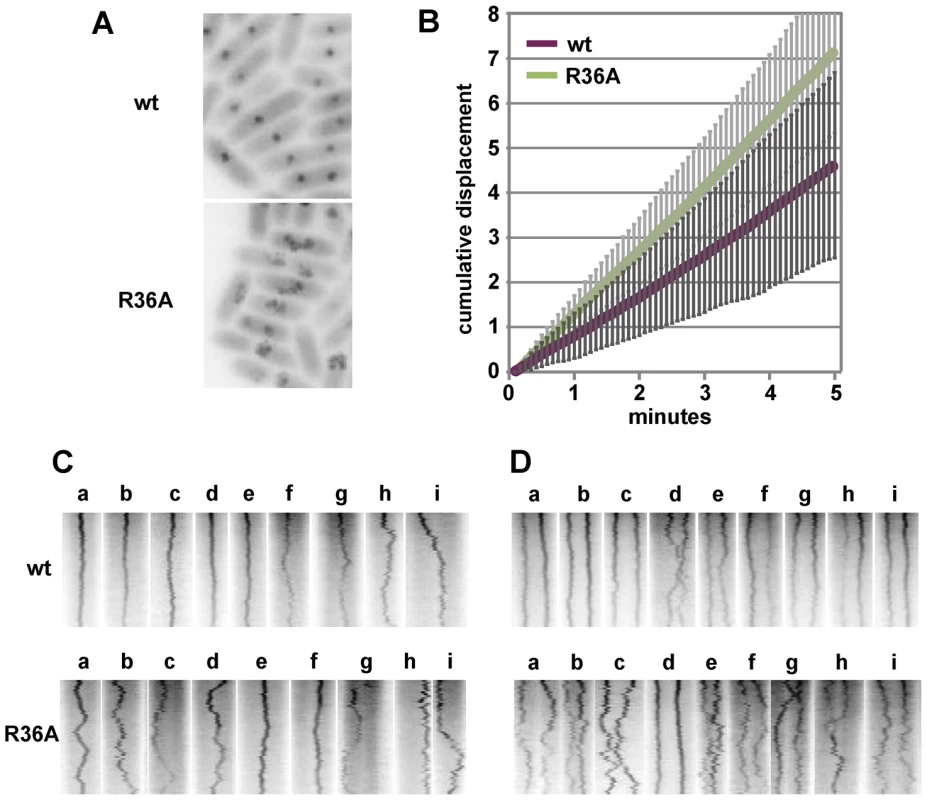
A. Cumulative displacement of mini-F, from images acquired every 5 secs for 5 mins, drawn using the Z project plug-in of Image J. B. Average cumulative displacement in 30 cells as illustrated in A; error bars are standard deviations. C. Illustrative individual kymographs of 1-focus cells; width corresponds to cell length (not equalized). D. As for C, 2-focus cells. Kymographs of 23 two-focus cells (Figure 6E) showed the maintenance of focus position to be, again, fairly constant with wt SopB and erratic with the R36A mutant (d in each row shows exceptions). Nevertheless, the time-averaged locations of the foci corresponded to the quarter positions of the long cell axis, as determined earlier (Figure 5). Correlated deviations in the positions of both foci were infrequent - 3 for wt (e.g. h, i), 4 for R36A (e.g. e, f, i) - but instances of temporary occupation of a single cell-half by both foci were more common for the mutant SopB (7 - e.g. c, e, g, h) than for the wt (1 - d). In R36A mutant cells the two foci often approached each other closely, presumably a simple result of their wider ambit, and in one of the 50 cells examined in this experiment were seen to fuse (row 2 - h). The one close approach seen in 50 wt SopB cells (row 1 - d) did not result in fusion. This frequent and occasionally protracted positioning of both mini-F copies on one side of the eventual division septum in SopB-R36A cells, as seen in the four examples of Figure 5A, is consistent with the higher plasmid loss rate associated with this mutant (Figure 1B).
Discussion
Our approach to defining the role of ATP hydrolysis differs from previous attempts, in that it targets the activator, SopB, rather than the instrument, SopA. The assumption that the behaviour of a mutant ATPase which binds but does not hydrolyse ATP is due to a defect in hydrolysis per se can be misleading; inability to hydrolyze ATP may be a secondary consequence of the mutation or be relevant only at a later step in the pathway. For example, the partition disabilities initially attributed to defective ATP hydrolysis by the K122Q mutant of P1 ParA [30] and the K120R mutant of SopA [7] are now known to be caused by impairment of conformational transitions, seen by changes in tryptophan fluorescence [23] and in CD spectrum (helicity) [7] respectively; these conformational shifts allow the wild type proteins to bind to DNA [23] and to polymerize (our unpublished data), activities which require ATP binding but not hydrolysis. Other observations of reduced ParA dynamics associated with mutations that diminish ATPase activity [6], [13], [15], [16] might require reinterpretation.
We find that when hydrolysis itself is unambiguously targeted, the consequences for plasmid stability are surprisingly modest. Even the SopB-36A mutant, which generates only 5% of normal ATPase activity, allows relocation of SopA along the nucleoid at normal speed, movement of plasmids and a high level of plasmid stability. Indeed we could obtain accurate estimates of partition deficiency only by distancing the sopAB operon from the mini-F test plasmid. Our initial presumption was that reduction of SopB concentration near sopC aggravates the partition deficiencies of the SopB mutants, but other explanations are possible; for example the ten-fold higher average level of Sop proteins in this experiment (Figure S1A) might alter SopA adherence to the nucleoid, as seen in vitro [31]. The possibility that the weak partition phenotype results from a smaller deficit in ATPase stimulation in vivo than that measured in vitro must be put aside provisionally; the lack of a specific in vivo ATPase assay prevents its being tested. The further possibility that the R36 and R42 mutations also affect SopA activity in a way that compensates for loss of ATPase stimulation is not formally excluded but is rendered unlikely by the strong correlation of increased plasmid instability and clustering with in vitro ATPase reduction (Figures 1B, 2). Such instability as there is appears to stem mostly from plasmid clustering or failure to split clusters (Figure 2).
This finding does not sit comfortably with current views of the partition mechanism. The primordial model of Leonard et al [20] and those evolved from it [15], [16], [22], [23], [32], [33] posit hydrolysis of ATP as the primary activator of ParA relocation and hence of plasmid segregation. Binding of ATP induces SopA, like other ParA proteins, to undergo a conformational change that enables it to associate with non-specific DNA [21]–[23] and thereby to coat the nucleoid [13], [14], [18], [34]. It also promotes interaction of SopA with SopB [12]. According to these models, the mini-F partition complex thus encounters SopA-ATP primed by contact with the nucleoid to hydrolyse ATP, and prompts it to do so. After hydrolysis SopA can no longer bind to DNA and departs for the cytoplasm where it reacquires ATP and binds to DNA further along the nucleoid, setting up a wave-like relocation between the nucleoid ends. By acting as a mobile attractor, the SopA wave creates and maintains distance between plasmid copies [35]–[38]. All type I plasmid partition systems studied conform to this general description, regardless of differences in detail.
Thus the main role of ATP hydrolysis is currently thought to be dislodgement of SopA from the nucleoid. But our results (Figures 3, 4) show that SopA relocation, which requires detachment from the nucleoid, is not impeded by severely weakened hydrolysis. The bulk of SopB-stimulated ATP hydrolysis thus appears to be unneeded for SopA detachment from the nucleoid and relocation. On the other hand, basal hydrolysis stimulated two-fold by DNA or residual SopB-mediated stimulation (5% for SopB-R36A) may well suffice to activate or maintain a largely auto-propagating mode of SopA relocation. The observation that pB171 ParA::Gfp continues to retract polewards after disconnection from ParB [16] is in line with this possibility. But if so, a role other than SopA detachment from the nucleoid must be sought for most of the ATP hydrolysis that SopB stimulates. Furthermore, other mechanisms of SopA detachment must be considered. One possibility is competition by the SopB molecules massed in the extended partition complex for the nucleoid surface occupied in meta-stable fashion by SopA-ATP, which could be examined using the approach of Havey et al. [39] for observing behaviour of dynamic complexes of the P1 Par system components in vitro. An alternative is suggested by the recent finding that in an in vitro analogue of the Sop system SopB enhances dissociation of SopA-ATP from a DNA surface [31]. The authors suggested that SopB induces a conformational change in SopA that weakens its affinity for DNA and potentiates subsequent ATP hydrolysis. Specific removal of hydrolysis from this process would generate SopA-ATP unable to immediately rebind the nucleoid but primed to relocate along it. Indeed an even stronger effect of SopB revealed in this study was the inhibition of SopA-ATP association with DNA, implying that once removed from the nucleoid SopA-ATP would be unable to reassociate with it until outside the extended partition complex zone.
Figure 7 illustrates the three partition processes in which we propose ATP hydrolysis to play a direct role. Hydrolysis-independent dissociation from DNA, as discussed above, would help account for one of them, the high frequency of SopA::Xfp oscillation seen in response to the SopB R-finger mutants (Figures 3, 4). In the presence of wt SopB, SopA waves were interrupted by a waiting period at the nucleoid poles (as also seen by Hatano et al. [13]), whereas with the R36A mutant this period was very brief, perhaps non-existent at the level of the individual SopA molecule. The difference implies that SopA molecules can arrive at the nucleoid tip in distinct forms that determine their ability to reverse direction. SopA arriving in response to the R36A mutant is already competent to relocate and does so immediately, whereas SopA stimulated by wild type SopB requires adaptation before setting out again, as illustrated in Figure 7B. This conditioning could consist of a time-consuming conformational change in the ATP-bound form of SopA, such as that culminating in ability of P1 ParA to bind DNA [23]. A similar case of apparent reconditioning at nucleoid ends was reported by Autret & Errington [40], who observed that the MinD division topology factor was implicated in relocation of the SopA homologue, Soj, from nucleoid ends in Bacillus subtilis. In a prior study [41] these authors had isolated a mutant of the cognate SopB homologue, Spo0J, (spo0J19) that increased the frequency, but not the speed, of Soj relocation. Modelling of Soj dynamics led Doubrovinski & Howard [36] to propose that Spo0J19 might increase relocation frequency by expelling Soj from the nucleoid in a form that rebinds the nucleoid ends without involving MinD, clearly analogous to bypassing the SopA conditioning that we suggest is a normal prelude to relocation. Our observations suggest that ATP hydrolysis might function as a means of restricting the rate of SopA relocation. Such regulation could be needed to maintain stable positioning of the plasmid.
Fig. 7. Steps in partition at which SopB stimulates SopA to hydrolyse ATP. 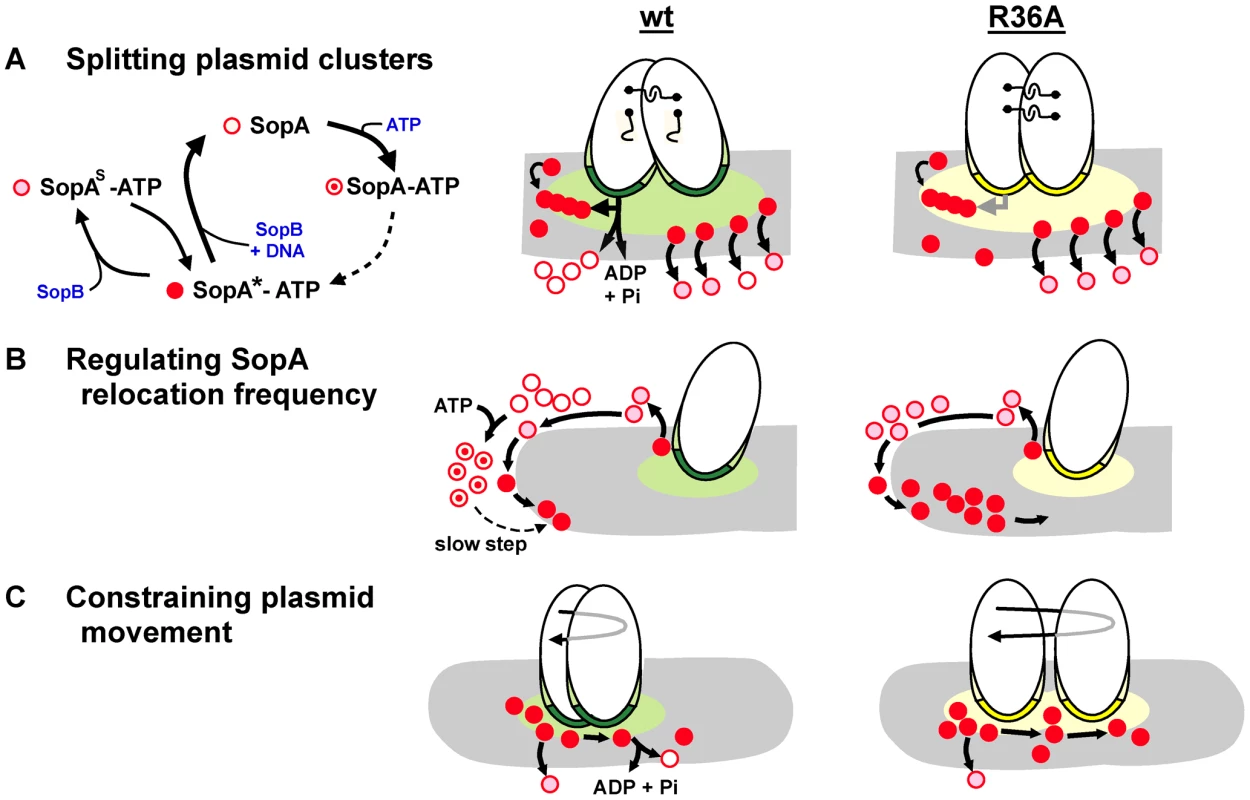
Mini-F plasmids are shown oval with SopB bound to sopC in the partition complex as dark green (wt) or yellow (R36A) and SopB spread in cis or onto the nucleoid (grey) in the corresponding lighter shade. No distinction is made here between direct binding of SopB to the nucleoid and binding via SopA tethers; the latter option is favoured by recent observations of partition complex movement as a function of SopA-ATP disassociation from a DNA carpet [31]. Distinct functional forms of SopA are shown in the key as: SopA*-ATP, able to bind to non-specific DNA (corresponding to ParA*2-ATP2; [23]); SopAS-ATP, modified by SopB to be unable to bind non-specific DNA [31]; SopA, without ATP following hydrolysis; SopA-ATP, associated with ATP but not having undergone transition to the DNA-binding form [23]. A. Model of SopB-stimulated splitting of clusters. SopB spreads from clustered partition complexes, creating a mat which attracts SopA-ATP and shelters it from the nucleoid to allow SopA polymerization [21]. For clarity only one polymer (chain of red dots) and its interactions is shown, at the left of the SopB mat. Polymer contact (horizontal arrow) with SopB-sopC initiates a hydrolysis-driven transfer of each partition complex to successive polymer subunits which generates the force to break inter-plasmid links (shown as hooks). Hydrolysis stimulation by SopB-R36A is too low to drive this process efficiently. Following splitting, the SopB mat shrinks, attenuating polymerization and this mode of plasmid separation. The separation mode that positions plasmid molecules (e.g. diffusion-ratchet; [20], [23]; shown at right of SopB mats) is mostly independent of ATP hydrolysis [31] and is activated by both wt and R36A SopB complexes. B. Model of SopA priming for relocation. Relatively slow conformational transitions [23] must raise the level of SopA-ATP competent to start relocation from the pole. Most of the SopA-ATP dislodged by the R36A partition complex does not hydrolyse its ATP, does not need the transition, and so reverses direction without delay. C. Model of restricted F plasmid movement. The extended partition complex of a segregated plasmid encounters nucleoid-bound or relocating SopA molecules at random and moves as dictated by local SopA dynamics. Wt SopB complexes stimulate ATP hydrolysis, disrupting SopA-SopB contact [35] and curtailing movement, whereas R36A complexes do not, leading to a wider ambit (longer reverse arrow), accounting for the exaggerated position adjustments seen in Figure 6. The effects of the mutant SopBs on mini-F positioning are intuitively easier to relate to plasmid stability. As well as driving segregation of mini-F copies, SopA restricts their subsequent movement [35]. This restriction broke down when the partition complexes contained SopB-R36A instead of wild type. Most of the traces shown in Figure 6 are presumably of positioned mini-F molecules not in the first phase of active segregation. ATPase stimulation is thus necessary for positional stability. If ATP hydrolysis normally repositions plasmids by removing SopA from the nucleoid, we would expect R36A partition complexes to move more sluggishly than wild type, not faster as seen. It is possible that the duration of contact between SopA-ATP and the partition complex is the key determinant of plasmid movement in this context, such that the longer contact expected from weak hydrolysis stimulation by an R36A partition complex might result in the more prolonged pulling of the plasmid by retracting SopA, accounting for the greater displacements seen (Figure 6; depicted in Figure 7C). Regardless of mechanism, the wider ambit of mini-F carrying SopB-R36A could lead to instability by increasing the chance of all plasmid copies finding themselves in the same cell half or in clusters (Figure 6D) at division. There is reason to question, however, whether temporary mis-positioning and cluster formation are the major causes of instability - fluorescent foci of various wild type plasmids have been seen to fuse [16], [42], [43] and fusion/splitting may be part of normal positioning [44]. The greater threat to stability might be failure to break clusters formed immediately after plasmid replication.
The inter-plasmid links that form clusters are not based on pairing of partition complexes [45], but active partition breaks them [46]. We suggest that segregation of recent replicas requires ATP hydrolysis to play a different role from the triggering of a conformational switch that releases SopA from the nucleoid at later stages of positioning [31], namely provision of energy for the initial segregation event. We proposed previously that following replication SopB spreads from the partition complex onto nucleoid DNA, creating a DNA-free zone that permits SopA to polymerize and thus initiate partition [21]. The recent observation that in an in vitro reconstituted system SopA-ATP does not form filaments on a DNA carpet is consistent with this proposal [31]. Our evidence that clustering is accentuated in proportion to the deficit in ATPase stimulation (Figure 2) encourages a speculation on the role of SopA polymerization - the forced separation of clustered plasmids powered by hydrolysis of ATP (portrayed in Figure 7A), as an initial step that enables partition complexes to then be drawn apart by the retreating SopA wave. While we have no direct evidence for this proposal, it is compatible with the two-phase segregation of F plasmids observed by Gordon et al. [47], in which a very rapid initial separation was followed by gradual progress to the destination. Biochemical and microscopical study focused on this aspect of SopA/B behaviour is now needed to test the proposal
In summary, our results reveal that SopB-mediated stimulation of SopA-catalyzed ATP hydrolysis serves mainly to split F plasmid clusters following replication and to prevent them forming later. SopA relocation and plasmid movement might depend on basal ATP hydrolysis but need little or no further stimulation by SopB. SopB-stimulated hydrolysis appears rather to regulate SopA relocation frequency. The contribution of basal hydrolysis could be approached using SopA mutated at residues directly involved in catalysis whose minimal conformational effects can be demonstrated. This altered perspective should be taken into account in attempts to determine how the partition of other replicons works.
Materials and Methods
Bacterial strains and growth conditions
The strains are derivatives of E.coli K12 and are listed in Table 1. DH10B was used as a transformation recipient for routine plasmid construction. The base strains for mini-F loss rate determinations were MC1061 and DH10B. For microscopic observation, the following mutations were introduced by recombineering, or by P1 transduction from the recombineerants: Δ(araFGH) and Ωpcp18::araE533 into MG1655 as in the construction of DLT1900 [9] to moderate arabinose induction, Ω(para-tetR::egfp) to follow movement of mini-F (pDAG848; see below), and ΔlacZΩ(plac-sopA::xfp) to monitor SopA relocation; details of the two latter constructions are given in Supplementary Information (Text S1). Cultures were grown at 37°C with aeration in Luria-Bertani (LB) broth, or in M9 salts-thiamine (1 µg/ml) supplemented with 0.4% glycerol and either 0.2% Casamino acids (MGC medium) or 20 µg/ml leucine, as noted in the text. When needed for selecting plasmid maintenance, antibiotics (Sigma) were added (µg/ml): kanamycin (30), spectinomycin (30), chloramphenicol (10).
Plasmids
Plasmids are listed in Table 1. Construction outlines are given here, with details available on request. pDAG218: lacZ with its Shine-Dalgarno sequence was incorporated in the sopAB operon by insertion into the 75 bp AccI-ApaI interval between sopB and sopC in pDAG114. pDAG848: deletion of sopAB from pDAG114 and insertion 195 bp downstream from sopC of 2.36 kb of the tetO array of pFX240. pYAS47: insertion of a PCR fragment containing sopAB with its Shine-Dalgarno sequence downstream from the lac promoter in pAM238. sopB alleles encoding the R36A-, R36K-, R42A - and R42K mutant SopBs were introduced into pDAG173, pDAG218 and pYAS47 by restriction fragment replacement to yield the derivatives listed in Table 1. pYAS70,-71: in-phase deletion of sopA codons 115–358 by cleavage with BstXI and SapI, Klenow end-filling and ligation. pYAS81: introduction of R36A (sopB nt106-7 CG>GC) to pYAS66 by quick-change mutagenesis. pJYB259,-260: insertion of a PCR fragment containing a fusion of sopB to mVenus [48], a derivative of Venus rendered monomeric by mutation A207K (this laboratory).
Mini-F stability
Cells grown with selection for mini-F plasmids were diluted into antibiotic-free medium and the resulting cultures sampled at intervals for assay of the plasmid-free fraction as follows. pDAG173 and derivatives, and pDAG209: cells were spread on LB agar and the resulting colonies tested by replica plating to selective medium [49] or by the three-layer method described [50]. pDAG218 and derivatives: cells were spread on MGC agar containing X-gal (40 µg/ml) and white and blue colonies scored after incubation.
Fluorescence microscopy
Cells of strains expressing sopA::xfp from the chromosomal lac promoter, DLT2687 and DLT2740, or tetR::gfp from the tet promoter, DLT2583 and DLT2740, were transformed with the mini-Fs pDAG848 or pDAG209 and pSC101-based vectors expressing sopAB. Transformants grown overnight in MGC medium were diluted 1/400 into MGC (except as noted), supplemented in the case of DLT2740 derivatives with IPTG at 0–10 µM (see Results), and grown at 30°C to OD600 ∼0.25, then sampled for observation; for visualization of pDAG848, tetO::gfp was induced by addition of arabinose to 0.05% 30 min beforehand. Samples were processed for microscopy as described [18]. Slides were maintained at 30°C in a thermostated chamber and examined by phase contrast and fluorescence microscopy using inverted microscopes: (i) Olympus IX81, images captured with a Coolsnap 2 (Roper) camera and processed using Metamorph software; (ii) Nikon Ti-E, acquisition with a EMCCD (Hamamatsu) camera (gain 144), and processing with NIS - element software. Metamorph software was used to generate kymographs, and to measure absolute distances (x,y) of mini-F displacement between images with the “Track Object” tool.
Supporting Information
Zdroje
1. Motallebi-VesharehM, RouchDA, ThomasCM (1990) A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol 4 : 1455–1463.
2. KooninEV (1993) A superfamily of ATPases with diverse functions containing either classical or deviant ATP-binding motif. J Mol Biol 229 : 1165–1174.
3. VecchiarelliAG, MizuuchiK, FunnellBE (2012) Surfing biological surfaces: exploiting the nucleoid for partition and transport in bacteria. Mol Microbiol 86 : 513–523.
4. BarillaD, RosenbergMF, NobbmannU, HayesF (2005) Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF. EMBO J 24 : 1453–1464.
5. DavisMA, RadnedgeL, MartinKA, HayesF, YoungrenB, et al. (1996) The P1 ParA protein and its ATPase activity play a direct role in the segregation of plasmid copies to daughter cells. Mol Microbiol 21 : 1029–1036.
6. EbersbachG, GerdesK (2001) The double par locus of virulence factor pB171: DNA segregation is correlated with oscillation of ParA. Proc Natl Acad Sci U S A 98 : 15078–15083.
7. LibanteV, ThionL, LaneD (2001) Role of the ATP-binding site of SopA protein in partition of the F plasmid. J Mol Biol 314 : 387–399.
8. SiddiqueA, FigurskiDH (2012) Different phenotypes of Walker-like A box mutants of ParA homolog IncC of broad-host-range IncP plasmids. Plasmid 68 : 93–104.
9. BouetJY, BouvierM, LaneD (2006) Concerted action of plasmid maintenance functions: partition complexes create a requirement for dimer resolution. Mol Microbiol 62 : 1447–1459.
10. DavisMA, MartinKA, AustinSJ (1992) Biochemical activities of the parA partition protein of the P1 plasmid. Mol Microbiol 6 : 1141–1147.
11. WatanabeE, WachiM, YamasakiM, NagaiK (1992) ATPase activity of SopA, a protein essential for active partitioning of F plasmid. Mol Gen Genet 234 : 346–352.
12. Ah-SengY, LopezF, PastaF, LaneD, BouetJY (2009) Dual role of DNA in regulating ATP hydrolysis by the SopA partition protein. J Biol Chem 284 : 30067–30075.
13. HatanoT, YamaichiY, NikiH (2007) Oscillating focus of SopA associated with filamentous structure guides partitioning of F plasmid. Mol Microbiol 64 : 1198–1213.
14. LimGE, DermanAI, PoglianoJ (2005) Bacterial DNA segregation by dynamic SopA polymers. Proc Natl Acad Sci U S A 102 : 17658–17663.
15. PrattoF, CicekA, WeihofenWA, LurzR, SaengerW, et al. (2008) Streptococcus pyogenes pSM19035 requires dynamic assembly of ATP-bound ParA and ParB on parS DNA during plasmid segregation. Nucleic Acids Res 36 : 3676–3689.
16. RinggaardS, van ZonJ, HowardM, GerdesK (2009) Movement and equipositioning of plasmids by ParA filament disassembly. Proc Natl Acad Sci U S A 106 : 19369–19374.
17. DaveyMJ, FunnellBE (1997) Modulation of the P1 plasmid partition protein ParA by ATP, ADP, and P1 ParB. J Biol Chem 272 : 15286–15292.
18. CastaingJP, BouetJY, LaneD (2008) F plasmid partition depends on interaction of SopA with non-specific DNA. Mol Microbiol 70 : 1000–1011.
19. DaveyMJ, FunnellBE (1994) The P1 plasmid partition protein ParA. A role for ATP in site-specific DNA binding. J Biol Chem 269 : 29908–29913.
20. LeonardTA, ButlerPJ, LoweJ (2005) Bacterial chromosome segregation: structure and DNA binding of the Soj dimer–a conserved biological switch. Embo J 24 : 270–282.
21. BouetJY, Ah-SengY, BenmeradiN, LaneD (2007) Polymerization of SopA partition ATPase: regulation by DNA binding and SopB. Mol Microbiol 63 : 468–481.
22. HesterCM, LutkenhausJ (2007) Soj (ParA) DNA binding is mediated by conserved arginines and is essential for plasmid segregation. Proc Natl Acad Sci U S A 104 : 20326–20331.
23. VecchiarelliAG, HanYW, TanX, MizuuchiM, GhirlandoR, et al. (2010) ATP control of dynamic P1 ParA-DNA interactions: a key role for the nucleoid in plasmid partition. Mol Microbiol 78 : 78–91.
24. BattSM, BingleLE, DaffornTR, ThomasCM (2009) Bacterial genome partitioning: N-terminal domain of IncC protein encoded by broad-host-range plasmid RK2 modulates oligomerisation and DNA binding. J Mol Biol 385 : 1361–1374.
25. EbersbachG, RinggaardS, Moller-JensenJ, WangQ, SherrattDJ, et al. (2006) Regular cellular distribution of plasmids by oscillating and filament-forming ParA ATPase of plasmid pB171. Mol Microbiol 61 : 1428–1442.
26. ScheffzekK, AhmadianMR, WittinghoferA (1998) GTPase-activating proteins: helping hands to complement an active site. Trends Biochem Sci 23 : 257–262.
27. LutzR, BujardH (1997) Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res 25 : 1203–1210.
28. BouetJY, RechJ, EgloffS, BiekDP, LaneD (2005) Probing plasmid partition with centromere-based incompatibility. Mol Microbiol 55 : 511–525.
29. RavinNV, RechJ, LaneD (2003) Mapping of functional domains in F plasmid partition proteins reveals a bipartite SopB-recognition domain in SopA. J Mol Biol 329 : 875–889.
30. FungE, BouetJY, FunnellBE (2001) Probing the ATP-binding site of P1 ParA: partition and repression have different requirements for ATP binding and hydrolysis. Embo J 20 : 4901–4911.
31. VecchiarelliAG, HwangLC, MizuuchiK (2013) Cell-free study of F plasmid partition provides evidence for cargo transport by a diffusion-ratchet mechanism. Proc Natl Acad Sci U S A 110 : 1390–1397.
32. FogelMA, WaldorMK (2006) A dynamic, mitotic-like mechanism for bacterial chromosome segregation. Genes Dev 20 : 3269–3282.
33. PtacinJL, LeeSF, GarnerEC, ToroE, EckartM, et al. (2010) A spindle-like apparatus guides bacterial chromosome segregation. Nat Cell Biol 12 : 791–798.
34. HiranoM, MoriH, OnogiT, YamazoeM, NikiH, et al. (1998) Autoregulation of the partition genes of the mini-F plasmid and the intracellular localization of their products in Escherichia coli. Molecular & General Genetics 257 : 392–403.
35. DermanAI, Lim-FongG, PoglianoJ (2008) Intracellular mobility of plasmid DNA is limited by the ParA family of partitioning systems. Mol Microbiol 67 : 935–946.
36. DoubrovinskiK, HowardM (2005) Stochastic model for Soj relocation dynamics in Bacillus subtilis. Proc Natl Acad Sci U S A 102 : 9808–9813.
37. IvanovV, MizuuchiK (2010) Multiple modes of interconverting dynamic pattern formation by bacterial cell division proteins. Proc Natl Acad Sci U S A 107 : 8071–8078.
38. MeinhardtH, de BoerPA (2001) Pattern formation in Escherichia coli: a model for the pole-to-pole oscillations of Min proteins and the localization of the division site. Proc Natl Acad Sci U S A 98 : 14202–14207.
39. HaveyJC, VecchiarelliAG, FunnellBE (2012) ATP-regulated interactions between P1 ParA, ParB and non-specific DNA that are stabilized by the plasmid partition site, parS. Nucleic Acids Res 40 : 801–812.
40. AutretS, ErringtonJ (2003) A role for division-site-selection protein MinD in regulation of internucleoid jumping of Soj (ParA) protein in Bacillus subtilis. Mol Microbiol 47 : 159–169.
41. AutretS, NairR, ErringtonJ (2001) Genetic analysis of the chromosome segregation protein Spo0J of Bacillus subtilis: evidence for separate domains involved in DNA binding and interactions with Soj protein. Mol Microbiol 41 : 743–755.
42. CampbellCS, MullinsRD (2007) In vivo visualization of type II plasmid segregation: bacterial actin filaments pushing plasmids. J Cell Biol 179 : 1059–1066.
43. LiY, AustinS (2002) The P1 plasmid in action: time-lapse photomicroscopy reveals some unexpected aspects of plasmid partition. Plasmid 48 : 174–178.
44. SenguptaM, NielsenHJ, YoungrenB, AustinS (2010) P1 plasmid segregation: accurate redistribution by dynamic plasmid pairing and separation. J Bacteriol 192 : 1175–1183.
45. WeitaoT, DasguptaS, NordstromK (2000) Plasmid R1 is present as clusters in the cells of Escherichia coli. Plasmid 43 : 200–204.
46. DorokhovB, RavinN, LaneD (2010) On the role of centromere dispersion in stability of linear bacterial plasmids. Plasmid 64 : 51–59.
47. GordonS, RechJ, LaneD, WrightA (2004) Kinetics of plasmid segregation in Escherichia coli. Mol Microbiol 51 : 461–469.
48. SanchezA, RechJ, GascC, BouetJY (2013) Insight into centromere-binding properties of ParB proteins: a secondary binding motif is essential for bacterial genome maintenance. Nucleic Acids Res 41 : 3094–3103.
49. LemonnierM, BouetJY, LibanteV, LaneD (2000) Disruption of the F plasmid partition complex in vivo by partition protein SopA. Mol Microbiol 38 : 493–505.
50. DubarryN, PastaF, LaneD (2006) ParABS systems of the four replicons of Burkholderia cenocepacia: new chromosome centromeres confer partition specificity. J Bacteriol 188 : 1489–1496.
51. BurtonBM, MarquisKA, SullivanNL, RapoportTA, RudnerDZ (2007) The ATPase SpoIIIE transports DNA across fused septal membranes during sporulation in Bacillus subtilis. Cell 131 : 1301–1312.
52. LaemmliUK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 : 680–685.
53. JohnstonC, MartinB, GranadelC, PolardP, ClaverysJP (2013) Programmed protection of foreign DNA from restriction allows pathogenicity island exchange during pneumococcal transformation. PLoS Pathog 9: e1003178.
54. SurteesJA, FunnellBE (2001) The DNA binding domains of P1 ParB and the architecture of the P1 plasmid partition complex. J Biol Chem 276 : 12385–12394.
55. GrantSG, JesseeJ, BloomFR, HanahanD (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A 87 : 4645–4649.
56. CasadabanMJ, CohenSN (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol 138 : 179–207.
57. BlattnerFR, PlunkettG3rd, BlochCA, PernaNT, BurlandV, et al. (1997) The complete genome sequence of Escherichia coli K-12. Science 277 : 1453–1462.
58. Gil D (1990) Elaboration et caractérisation d'un nouveau type de vecteur de clonage à nombre de copies régulable. [PhD thesis]. Toulouse: Université de Paul Sabatier.
59. RavinN, LaneD (1999) Partition of the linear plasmid N15: interactions of N15 partition functions with the sop locus of the F plasmid. J Bacteriol 181 : 6898–6906.
Štítky
Genetika Reprodukční medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání