-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
Robustness is a property built into biological systems to ensure stereotypical outcomes despite fluctuating inputs from gene dosage, biochemical noise, and the environment. During development, robustness safeguards embryos against structural and functional defects. Yet, our understanding of how robustness is achieved in embryos is limited. While much attention has been paid to the role of gene and signaling networks in promoting robust cell fate determination, little has been done to rigorously assay how mechanical processes like morphogenesis are designed to buffer against variable conditions. Here we show that the cell shape changes that drive morphogenesis can be made robust by mechanisms targeting the actin cytoskeleton. We identified two novel members of the Vinculin/α-Catenin Superfamily that work together to promote robustness during Drosophila cellularization, the dramatic tissue-building event that generates the primary epithelium of the embryo. We find that zygotically-expressed Serendipity-α (Sry-α) and maternally-loaded Spitting Image (Spt) share a redundant, actin-regulating activity during cellularization. Spt alone is sufficient for cellularization at an optimal temperature, but both Spt plus Sry-α are required at high temperature and when actin assembly is compromised by genetic perturbation. Our results offer a clear example of how the maternal and zygotic genomes interact to promote the robustness of early developmental events. Specifically, the Spt and Sry-α collaboration is informative when it comes to genes that show both a maternal and zygotic requirement during a given morphogenetic process. For the cellularization of Drosophilids, Sry-α and its expression profile may represent a genetic adaptive trait with the sole purpose of making this extreme event more reliable. Since all morphogenesis depends on cytoskeletal remodeling, both in embryos and adults, we suggest that robustness-promoting mechanisms aimed at actin could be effective at all life stages.
Published in the journal: . PLoS Genet 9(11): e32767. doi:10.1371/journal.pgen.1003901
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003901Summary
Robustness is a property built into biological systems to ensure stereotypical outcomes despite fluctuating inputs from gene dosage, biochemical noise, and the environment. During development, robustness safeguards embryos against structural and functional defects. Yet, our understanding of how robustness is achieved in embryos is limited. While much attention has been paid to the role of gene and signaling networks in promoting robust cell fate determination, little has been done to rigorously assay how mechanical processes like morphogenesis are designed to buffer against variable conditions. Here we show that the cell shape changes that drive morphogenesis can be made robust by mechanisms targeting the actin cytoskeleton. We identified two novel members of the Vinculin/α-Catenin Superfamily that work together to promote robustness during Drosophila cellularization, the dramatic tissue-building event that generates the primary epithelium of the embryo. We find that zygotically-expressed Serendipity-α (Sry-α) and maternally-loaded Spitting Image (Spt) share a redundant, actin-regulating activity during cellularization. Spt alone is sufficient for cellularization at an optimal temperature, but both Spt plus Sry-α are required at high temperature and when actin assembly is compromised by genetic perturbation. Our results offer a clear example of how the maternal and zygotic genomes interact to promote the robustness of early developmental events. Specifically, the Spt and Sry-α collaboration is informative when it comes to genes that show both a maternal and zygotic requirement during a given morphogenetic process. For the cellularization of Drosophilids, Sry-α and its expression profile may represent a genetic adaptive trait with the sole purpose of making this extreme event more reliable. Since all morphogenesis depends on cytoskeletal remodeling, both in embryos and adults, we suggest that robustness-promoting mechanisms aimed at actin could be effective at all life stages.
Introduction
Every embryo develops under its own unique set of circumstances and challenges. To then ensure a reliable outcome, mechanisms are built into development to buffer against fluctuations in genetic, biochemical, and environmental inputs [1]. This buffering, called “robustness”, can be overwhelmed, ending in miscarriage, shortened gestation, and structural and functional birth defects [2]. Thus, we need to understand how developmental robustness arises in order to define an embryo's susceptibilities to genetic/epigenetic background and environment; and to ultimately promote healthy reproduction.
Many mechanisms are used to buffer biological systems against fluctuating inputs, including redundant protein function [3], [4], secondary or “shadow” enhancers [5], [6], smart network design [7]–[10], and chaperone activity [3], [11]–[14]. Among developmental systems, a rigorous quantitative understanding of these mechanisms has been largely limited to examples where cell fate decisions are made, and robustness is fostered at the level of gene expression [5], [6], [10], [15] or signaling [9], [16], [17]. For morphogenesis, which translates cell fate decisions into embryonic form, the detailed characterization of specific buffering mechanisms has been slower to come. Morphogenesis requires activities that span nuclei, cytoplasm, and whole tissues, and is driven by cell shape change [18], [19]. So robustness could be promoted at many levels (e.g. gene expression, signaling, membrane dynamics, cytoskeletal remodeling, and cell adhesion). But we do not know enough about the molecular and mechanical underpinnings of morphogenesis to predict where its greatest susceptibilities are, or where buffering mechanisms would be most effective. Specifically, we lack a comprehensive understanding of how cell biological steps convert gene expression into reliable tissue-building events. Actin and microtubules seem like good targets for robustness-promoting mechanisms during morphogenesis because they drive cell-shape change and modulate the mechanics of cells and tissues [20], [21]; however, experimental support for this is lacking.
In order to identify the mechanisms that promote robustness, the outcomes of the process in question must be quantifiable [1]. For example, we know of robustness-promoting mechanisms for cell fate decisions in development because the outcomes are binary, and typically happen along well-separated spatial dimensions so that fidelity can be readily tracked and quantified over a range of perturbations [5], [6], [9], [10], [15]–[17]. For morphogenesis, which is spatially complex, challenging to image, and has long been scored by qualitative rather than quantitative methods, fidelity is not easily measured. Consequently, the number of well-tested examples that show how robust morphogenesis is achieved remains low, in the context of both individual cell shape change and whole tissue remodeling [22], [23]. To address this gap in our knowledge, we are using the first tissue-building event in the fly embryo, cellularization, as a simple, quantifiable model to study robustness. Fly embryos first develop as a syncytium, passing through 13 mitoses with no intervening cytokinesis. Then, during cell cycle 14 the embryo undergoes cellularization, during which ∼6000 cortically-anchored nuclei are simultaneously packaged into a sheet of cells that will be the primary epithelium (Figure 1A) [24]. Cellularization takes ∼60 minutes and plasma membrane furrows ingress 35 µm, cutting straight between adjacent nuclei to form mononucleate cells. This simple architecture allows unambiguous quantification of the fidelity of cellularization, where furrow failures or regressions show up as multinucleate cells, and hundreds to thousands of packaging events can be assayed per embryo to generate a ratio of mononucleate cells-to-nuclei (ratio = 1 in wild-type embryos; Figure 1A).
Fig. 1. Sry-α regulates cortical F-actin levels. 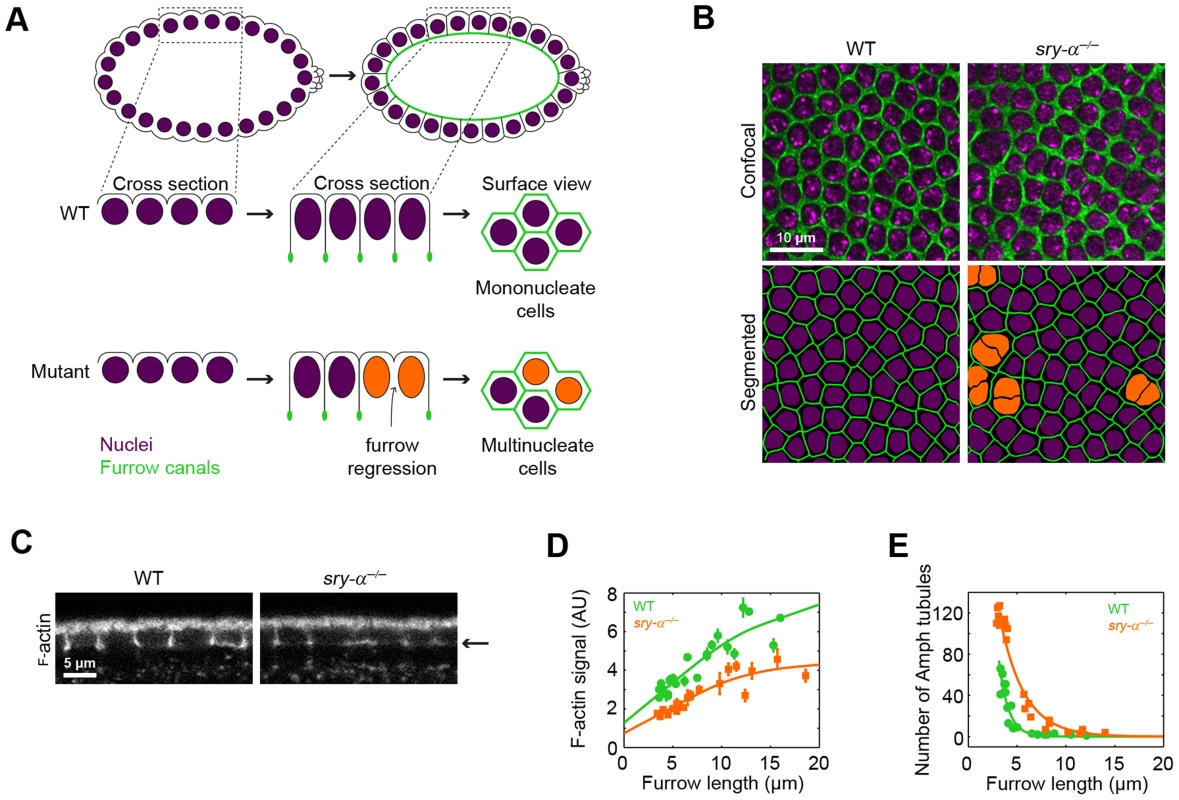
(A) Schematic of cellularization. In wild-type embryos (WT), furrows ingress between nuclei (purple) to form mononucleate cells. In mutants with reduced F-actin, some furrows regress, resulting in multinucleate cells (orange nuclei). (B) Surface views of furrow canals stained for Myosin-2 in embryos of indicated genotypes. Multinucleate cells highlighted (orange nuclei) in corresponding segmented images. (C) Cross sections show F-actin levels, as detected by phalloidin staining. Arrow indicates furrow canal position. (D) Quantification of F-actin levels in furrow canals. Each point represents one embryo with ≥75 furrow canals measured (mean ± s.d.). (E) Quantification of Amphiphysin (Amph) tubules. Each point represents one embryo with ≥100 furrows analyzed. Fly embryos cellularize just after the maternal-to-zygotic transition (MZT) [24], when transcription from the zygotic genome starts to maximally impact the developmental program [25]. Thus, the few zygotic genes that are required for cellularization have long been thought of as “switches” to control this morphogenetic event [26], [27]. However, we now report a new role for one of these long-supposed switches, Sry-α. We identify Sry-α as a zygotic gene product that is expressed at the MZT, not to control cellularization, but rather to make it robust in the face of both environmental and genetic perturbations. We find that Sry-α acts together with its maternally provided paralog Spt to reinforce the actin cytoskeleton and so promote robust cellularization. Our data provides a clear example of how zygotic contributions, made at the MZT, not only instruct development, but also supplement the maternal machinery to ensure the fidelity of specific morphogenetic events. What's more, our data suggests that this robustness is fostered via regulation of the actin cytoskeleton.
Results
Sry-α regulates F-actin levels during cellularization
At the start of cellularization, actin filaments (F-actin) accumulate at incipient furrow tips, which in surface views form furrow “canals” around the nuclei (Figure 1A) [24]. Furrow canals are then maintained throughout cellularization, and are required for stable furrow ingression (Figure 1A) [28]–[30]. We previously showed that mutations or drug treatments that reduce F-actin levels in all furrow canals, precipitate the regression of a fraction of furrows (Figure 1A) [29], [30], consistent with multinucleation phenotypes reported for actin regulators like Rho1 GTPase, RhoGEF2, and the Formin, Diaphanous [28], [31], [32]. In an effort to identify other actin regulators that are required for the fidelity of cellularization, we examined a poorly characterized mutant, serendipity-α (sry-α), that similarly displays a multinucleation phenotype (Figure 1B) [26], [27]. The sry-α gene was previously mapped, and is expressed at the MZT just prior to cellularization [27]. Consequently, sry-α has long been thought of as a developmental cue that provides some new activity to trigger cellularization [26], [27]. We found that all furrow canals in sry-α null mutants (sry-α−/−) have significantly reduced levels of F-actin compared to wild-type, throughout cellularization (Figure 1C, 1D). In addition, incipient furrow canals in sry-α−/− mutants display an increased number of Amphiphysin tubules (Figure 1E), which indicates promiscuous endocytosis upon F-actin reduction [29], [33]. These results show that Sry-α regulates F-actin levels in furrow canals during cellularization.
Sry-α and Spt represent a novel clade of the Vinculin/α-Catenin Superfamily
Based on remote homology searches, including PHYRE [34] and I-TASSER [35], we found that Sry-α is a novel member of the Vinculin/α-Catenin Superfamily (Figure 2A, 2B) [36]. Our analysis also identified a Sry-α paralog in the D. melanogaster genome that we called Spitting Image (Spt, CG8247). Sry-α and Spt align with the middle sequences of Vinculin and α-Catenin, including the Vinculin-Homology 2 domain (VH2; Figure 2A, Figure S1) [37], [38]. Like α-Catulin, Sry-α and Spt represent a distinct clade of the Vinculin/α-Catenin Superfamily (Figure 2B, Figure S2) [39], [40]. Based on a “roll-call” analysis of orthologs in organisms with fully sequenced genomes, sry-α and spt co-exist in all Drosophilids, while spt alone is present in other insects (see Table S1). In higher metazoans, PHYRE analysis also identified other uncharacterized proteins, like Sry-α and Spt, which share remote homology with the middle sequences of Vinculin and α-Catenin (Figure S1, S2). Members of the Vinculin/α-Catenin Superfamily peripherally associate with the plasma membrane and interact with the actin cytoskeleton [36]. Thus, this evolutionary relationship is functionally consistent with a role for Sry-α and Spt at the actin cortex during cellularization.
Fig. 2. Sry-α and Spt are paralogs with redundant F-actin binding activity. 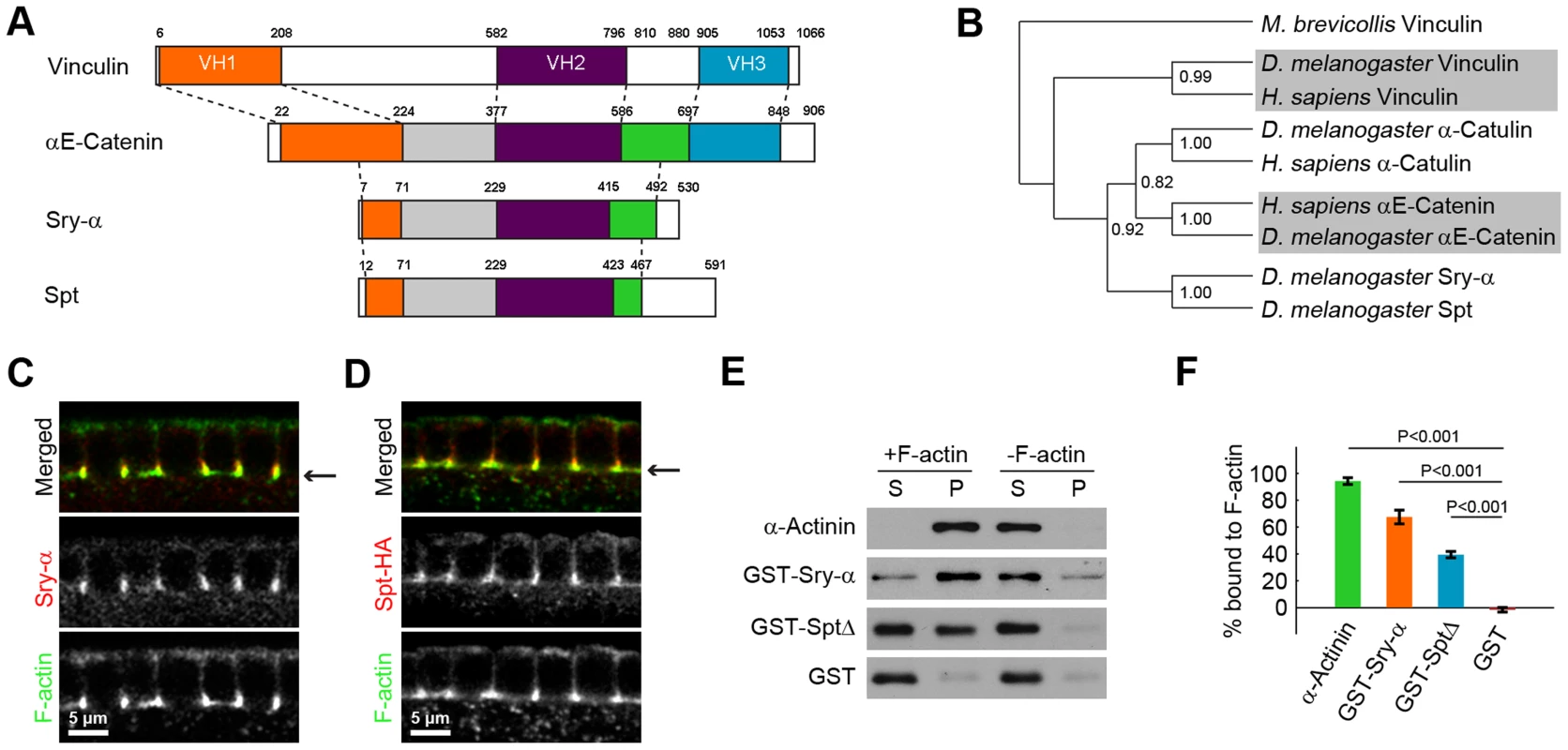
(A) Domain structure of Vinculin/α-Catenin Superfamily members. Vinculin Homology domains 1, 2, and 3 (VH1, VH2, VH3) are orange, purple, and blue, respectively. The green and gray domains indicate sequences shared between α-Catenin, Sry-α, and Spt. White areas indicate unique sequences. Dashed lines are guides to show how the different proteins align with each other. (B) Cladogram of Vinculin/α-Catenin Superfamily. Bootstrap statistics at branch points (1000 iterations). Outgroup is M. brevicollis Vinculin. Shading highlights division of the clades. (C, D) Cross sections show (C) Sry-α and (D) Spt-HA co-localization with F-actin in furrow canals. Arrows indicate furrow canal position. (E) Western blots for an F-actin co-sedimentation assay show Sry-α full-length protein (GST-Sry-α) and Spt truncate (GST-SptΔ; amino acids 250–461) pellet with F-actin. α-Actinin and GST are positive and negative controls, respectively. S, supernatant; P, pellet. (F) Quantification of percent proteins bound to F-actin from three independent co-sedimentation assays (mean ± s.e.m.). Student's t-test was performed to calculate P values as shown. Paralogs Sry-α and Spt have redundant actin-regulating activity
To examine the relationship between Sry-α and Spt, we asked if these paralogs have unique or overlapping functions. Both Sry-α and HA-tagged Spt localize to F-actin rich furrow canals in cellularizing embryos (Figure 2C, 2D). In addition, we used an F-actin co-sedimentation assay to show that both Sry-α and Spt bind F-actin directly. Recombinant Sry-α and Spt proteins were purified from insect cells and mixed with F-actin. Upon centrifugation, F-actin and its interacting proteins pellet (α-Actinin; Figure 2E, 2F), while unbound proteins remain in the supernatant (GST; Figure 2E, 2F). For both Sry-α and Spt, we detected a significant fraction of F-actin bound protein (Figure 2E, 2F). Thus, the co-localization of Sry-α and Spt, as well as their biochemical activity, suggest that they could act redundantly during cellularization.
To look for overlapping in vivo functions, we first used RNAi [41] and found that spt knockdown (sptRNAi) causes multinucleation during cellularization, which is qualitatively indistinguishable from either the sry-α−/− genetic mutant or sry-α RNAi (sry-αRNAi; Figure 3, Figure S3). Additionally, embryos with double sptRNAi plus sry-αRNAi knockdown display a strongly enhanced multinucleation phenotype (Figure 3B, 3C). Together, the localization, biochemistry, and RNAi phenotypes suggest that Sry-α and Spt share redundant functions during cellularization. To then confirm this, we tested whether Spt overexpression (sptOE) can rescue sry-α−/− multinucleation. We used Sry-α immunostaining to genotype embryos (Figure S4A), and found that 100% of sry-α−/− mutants are rescued by sptOE (Figure 4A, 4B, Figure S4). Rescue of sry-α−/− multinucleation by sptOE is equivalent to that accomplished with a genomic construct encoding sry-α itself (Figure S4B). We also confirmed that sptOE restores F-actin to wild-type levels in sry-α−/− mutants (Figure 4C). Thus, Sry-α and Spt share an overlapping actin regulating function during cellularization.
Fig. 3. sry-α and spt induce the same loss of function phenotype. 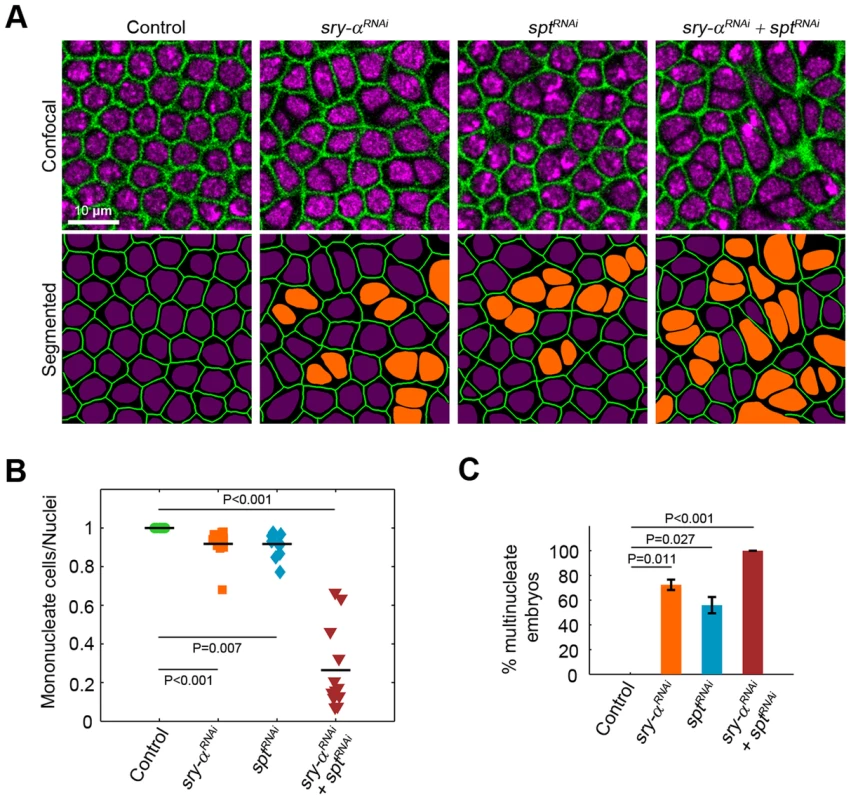
(A) Surface views of furrows and nuclei, detected in living embryos (membrane Gap43-mCherry, green; nuclear Histone-GFP, purple), following the indicated sry-αRNAi or sptRNAi treatment. Multinucleate cells highlighted (orange nuclei) in corresponding segmented images. (B) Quantification of multinucleation phenotypes. Each point represents one embryo with ≥150 nuclei analyzed (n = 12 embryos per condition). (C) Frequency of multinucleation for embryos following the indicated RNAi treatment (n≥6,000 nuclei from 30 embryos per treatment; mean ± s.e.m.). Student's t-test was performed to calculate P values as shown in (B, C). Fig. 4. Sry-α and Spt share redundant function during cellularization. 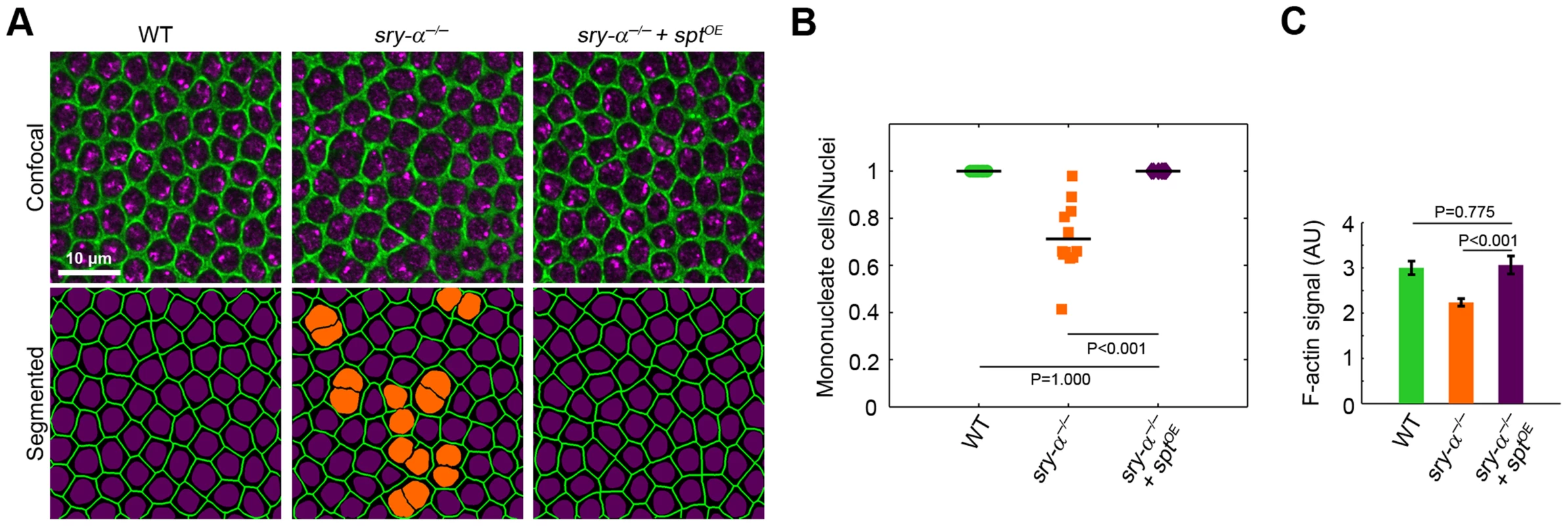
(A) Surface views of furrow canals stained for Myosin-2 in embryos of indicated genotypes. Multinucleate cells highlighted (orange nuclei) in corresponding segmented images. (B) Quantification of multinucleation phenotypes. Each point represents one embryo with ≥150 nuclei analyzed (n = 12 embryos per condition). (C) Quantification of F-actin in furrow canals of furrows of length 3–5.5 µm (n = 9 embryos per genotype, with 15 furrows analyzed per embryo; mean ± s.e.m.). Student's t-test was performed to calculate P values as shown in (B, C). Spt is maternally provided while Sry-α is expressed zygotically
These results challenge a long-standing idea that sry-α is zygotically expressed at the MZT to supply some new activity that instructs cellularization to proceed [26], [27]. In fact, the Sry-α activity may already be available via Spt. We compared the developmental expression profiles of endogenous Sry-α and Spt. As previously shown, sry-α transcript and protein are expressed at the MZT, in a pulse that just coincides with cellularization (Figure 5A, 5B) [27]. Conversely, spt transcript and protein are provided maternally, and Spt levels persist throughout and far beyond cellularization (Figure 5A, 5B, Figure S5). That is, a pulse of zygotically expressed Sry-α adds to a pool of maternally provided Spt during cellularization. (i.e. Sry-α expression only boosts an already existing Spt activity in the embryo.) Given that Spt can completely replace Sry-α during cellularization (Figure 4, Figure S4), Sry-α does not supply some unique activity that triggers the process. Instead, the expression profiles suggest that the level of Spt plus Sry-α is somehow critical for the successful progression of cellularization.
Fig. 5. Spt is maternally provided, while Sry-α is zygotically expressed in a pulse for cellularization. 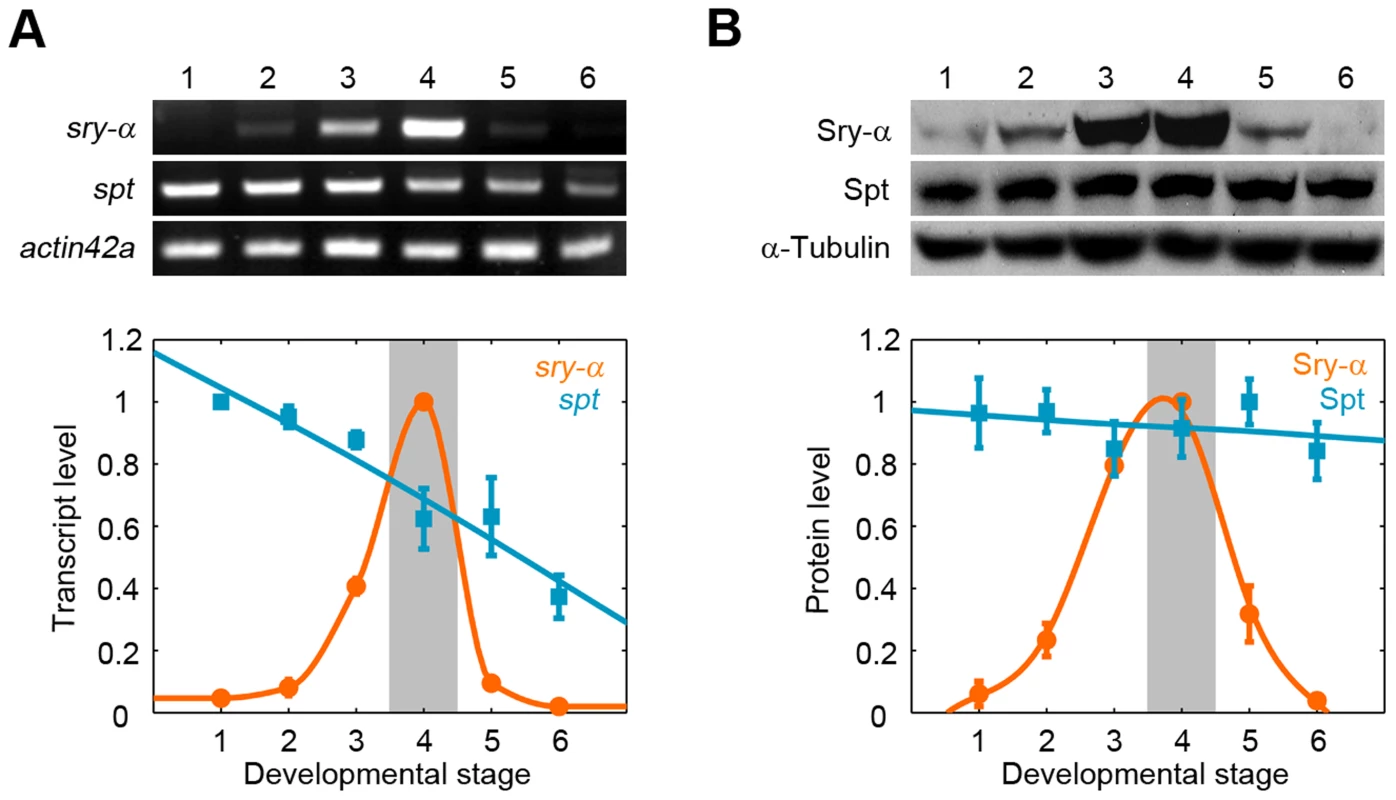
(A) RT-PCR and (B) Western blots show Sry-α and Spt expression profiles. Developmental stages are (1) mitotic cycles 1–9; (2) mitotic cycles 10–11; (3) mitotic cycles 11–13; (4) cellularization (shaded); (5) ventral furrow formation; (6) segment formation. Profiles were obtained from three independent RT-PCR or Western Blotting experiments (mean ± s.e.m.). Spt plus Sry-α work together to promote robust cellularization
It was previously shown that the co-expression of paralogs in worms and yeast promotes biological robustness [3], [4]. Presumably, these “gene duplicates” provide overlapping functions, and so replace or supplement each other in the face of internal and external perturbations [3], [4]. Hence, we hypothesized that there is a threshold level of Sry-α plus Spt activity that is required for cellularization to proceed with high fidelity. This robustness hypothesis predicts that at an optimal condition, Sry-α function is dispensable and Spt alone can support successful cellularization, whereas both are needed at sub-optimal conditions. To test this, we assayed for multinucleation phenotypes in sry-α−/− null mutants at an optimal temperature. We chose 18°C, the lowest temperature at which D. melanogaster thrives. We reasoned that the lower temperature would reduce the demand on the actin cytoskeleton, because F-actin is more stable at lower temperature [42]. As predicted, we found that multinucleation is suppressed in sry-α−/− mutants that are reared at 18°C (Figure 6A, 6B). At this temperature, the ratio of mononucleate cells to nuclei in sry-α−/− mutants is not significantly different than wild-type (Figure 6B). Nor did we detect changes in the dimensions of the cells that formed for sry-α−/− mutants at 18°C (data not shown). Thus, Spt activity is sufficient for cellularization to proceed at an optimal condition. However, multinucleation in sry-α−/− mutants was increasingly severe at higher temperatures (25–32°C; Figure 6A, 6B), showing that Spt is not enough to ensure reliable cellularization when conditions deviate from the optimal.
Fig. 6. Sry-α is not required for cellularization at an optimal temperature of 18°C, but is required as conditions approach an extreme of 32°C. 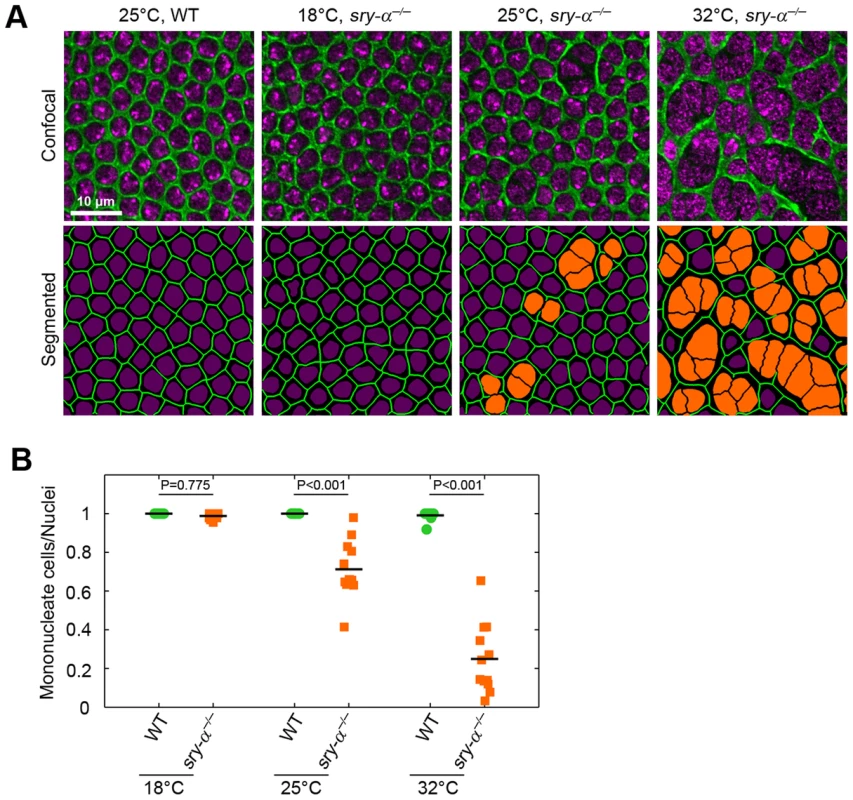
(A) Surface views of furrow canals stained for Myosin-2 in embryos of indicated genotypes. Multinucleate cells highlighted (orange nuclei) in corresponding segmented images. (B) Quantification of multinucleation phenotype for indicated genotype and temperature. Each point represents one embryo with ≥150 nuclei analyzed (n = 12 embryos per condition). Two-way ANOVA analysis was performed to calculate P values as shown. A second prediction of the robustness hypothesis is that reducing sry-α dosage will make cellularization more likely to fail when the embryo is challenged by perturbations [3], [4]. To test this, we reduced the Sry-α level using genetic heterozygosity, and looked for multinucleation at an extreme temperature of 32°C. This temperature marks an upper limit at which D. melanogaster embryos can survive, but developmental events are measurably impaired [5], [6]. We found that the occurrence of multinucleation at 32°C is significantly increased for sry-α heterozygotes (sry-α+/−; Figure 7A, 7B). Genotypes were confirmed by RNA FISH (Figure S6) [43]. So while Spt alone is sufficient for cellularization at an optimal temperature, Spt plus two copies of the sry-α gene are required when environmental conditions are extreme. This suggests that the expression of Sry-α serves as a robustness-promoting mechanism for cellularization.
Fig. 7. Two copies of sry-α are required for reliable cellularization at the extreme temperature of 32°C or when the embryo is challenged by a genetic perturbation. 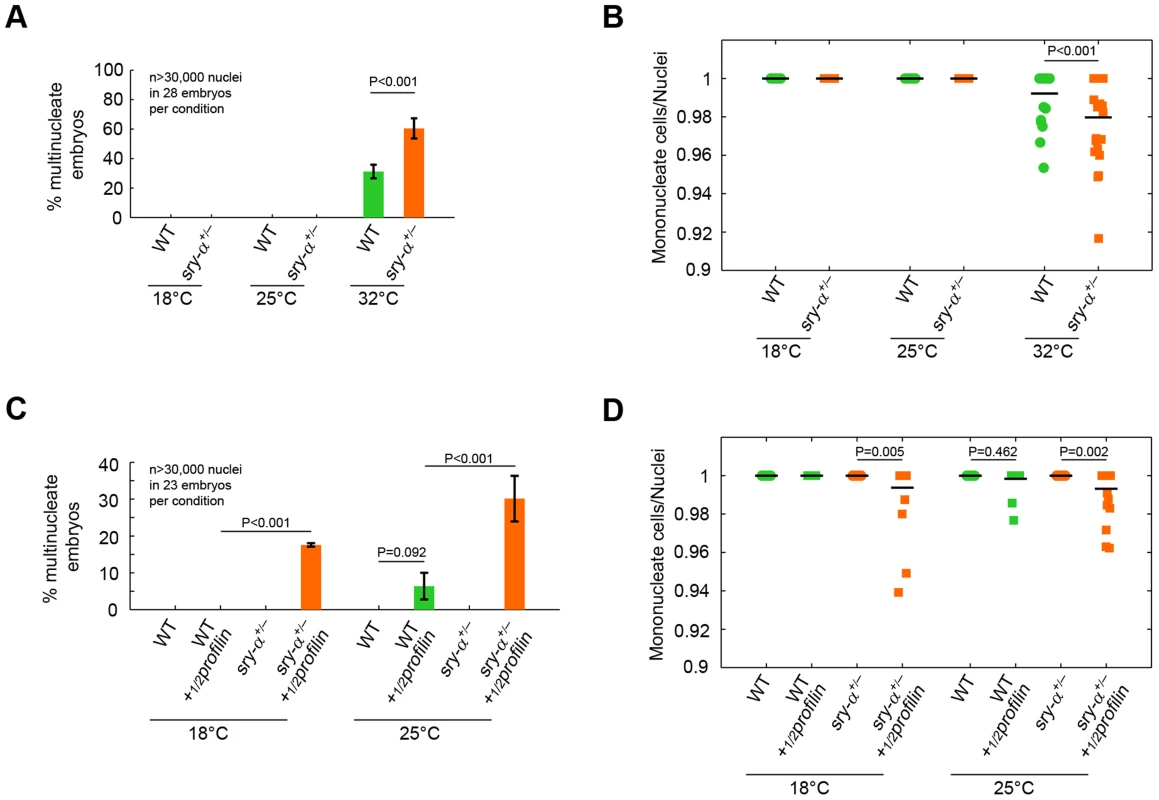
(A, C) Frequency of multinucleation for heterozygous embryos reared (A) at 32°C, or (C) with a reduced dose of maternal Profilin (½profilin; mean ± s.e.m.). (B, D) Quantification of multinucleation phenotype for heterozygous embryos reared (B) at 32°C, or (D) with a reduced dose of maternal Profilin (½profilin). Each point represents one embryo with ≥150 nuclei analyzed (n = 23 embryos per condition). Two-way ANOVA analysis was performed to calculate P values as shown in (A–D). A hallmark of robustness-promoting mechanisms is that they respond equally well to different kinds of perturbations (e.g. genetic, biochemical, or environmental) [44]. For example, shadow enhancers for the fly genes snail and shavenbaby promote robust gene expression at high temperature, and when mutations reduce input from their respective activation pathways [5], [6]. In addition, a systems-level analysis in yeast showed that genes that promote mutational robustness also promote high fitness in a wide range of stressful environments [44]. Thus, we also tested whether the Sry-α level ensures the fidelity of cellularization upon genetic perturbation. We checked for multinucleation in sry-α+/− heterozygotes that carry only a half maternal dose of profilin (½profilin). Profilin is an actin accessory protein that promotes actin polymerization [45]. Strikingly, we saw multinucleation in sry-α+/− heterozygotes in the ½profilin background even at 18°C (Figure 7C, 7D). We conclude that the fidelity of cellularization, in the face of both environmental and genetic perturbations, critically depends on the level of Sry-α.
Discussion
Our data support a model wherein maternal Spt plus zygotic Sry-α work together to promote the robustness of cellularization. Our findings refute the idea that there is a clear passing off of developmental control from the maternal to the zygotic machinery at the MZT [25]–[27], [46]. For example, the maternal genome was previously thought to provide the basic cellular machinery for cellularization, while Sry-α and other zygotic players provided the instructions [26], [27]. More recently zygotic gene products have been shown to actively degrade maternal mRNAs, arguing that there may be a clean break from maternal to zygotic control [47]–[49]. In both cases, the zygotic contribution is largely viewed as being instructive. But our data speaks to a more collaborative interaction between the maternal and zygotic genomes: We show that cellularization can proceed with no input from zygotic Sry-α. Instead of controlling cellularization, we find that Sry-α actually adds to the activity of maternal Spt to make this morphogenetic event more reliable in the face of environmental and genetic perturbation. Sry-α is not taking over for the maternal product because it is only expressed in a pulse during the demanding event of cellularization, while maternal Spt persists far beyond cellularization. So, the relationship of Spt and Sry-α illustrates with exceptional clarity that the maternal and zygotic genomes also work together, with redundant activities, to make development robust. Certainly, this collaboration is likely to be broadly conserved (e.g. maternal plus zygotic contributions of Rac proteins in C. elegans and Cadherins in Xenopus support the progression of specific morphogenetic events [50], [51]).
Since maternal RNAs and proteins are loaded into oocytes and eggs long before they act in development (up to months) [25], their levels may not be very reliable [46]. Overlapping activities encoded by the zygotic genome could then buffer this variability and ensure successful progression through early embryogenesis. In our case, this relationship was revealed by the distinct expression profiles of paralogs provided from the maternal and zygotic genomes. But the same end is likely accomplished by expressing a single gene both maternally and zygotically. In fact, there is the recurrent observation that some proteins are expressed zygotically, while a significant maternal pool still persists (e.g. Drosophila β-Catenin) [46], [52]. In D. melanogaster, roughly 5% of the genome displays this expression profile, with a maternal contribution supplemented by zygotic expression at the MZT [47].
But why split the contribution between the maternal and zygotic genomes? For cellularization, why is the same level of robustness not achieved by simply expressing more maternal Spt? We can envision at least two possibilities: either Spt and Sry-α have some distinct functions, perhaps at different developmental stages; and/or high levels of Spt/Sry-α protein are harmful.
Sry-α and its expression at the MZT may be an adaptive genetic trait with its function, perhaps its sole function, serving to buffer cellularization against external and internal perturbations. According to our roll-call analysis, both spt and sry-α are present in the genomes of all Drosophilids, while other insects only encode spt (see Table S1). Some aspect of development specific to Drosophila may then stabilize the strategy of maternal Spt plus zygotic Sry-α. For example, cellularization in Drosophila builds tall columnar cells around thousands of nuclei, and so may be more demanding than cellularization in other insects where shorter cuboidal cells form around fewer nuclei [53]–[55]. Alternatively, Drosophilids share a fast rate of embryogenesis in comparison to many other insects [53], [54], [56], which could make their cellularization more difficult. Thus, the pulse of zygotic Sry-α may be advantageous for meeting the challenge of a very extreme cellularization event in Drosophila.
Our data suggests that zygotic Sry-α adds to the activity of maternal Spt to regulate actin, and so ensure the fidelity of cellularization. A significant future challenge will be understanding what specific actin-based activities promote robust morphogenesis. For example, F-actin is a critical determinant of tissue mechanical properties during development because it assembles with Myosin-2 and other crosslinkers, within single cells, to form a cortical network that (1) governs the rigidity and shape of the whole embryo [57]–[59]; and (2) generates the forces for and resistance to the cell shape changes that drive morphogenesis [60]–[64]. Thus, actin could promote robustness by buffering the mechanical properties of cells and tissues. In fact, Spt and Sry-α bind F-actin directly. Also, Spt and Sry-α are related to the F-actin crosslinker Vinculin, and they contain the conserved M-domain at the VH2, which in Vinculin dimerizes to support F-actin crosslinking [38]. So Spt and Sry-α could promote robust cellularization by crosslinking F-actin and modulating tissue mechanics. Alternatively, F-actin also controls the membrane remodeling that accompanies cell shape change and morphogenesis. Specific to Drosophila cellularization, F-actin antagonizes endocytosis to favor plasma membrane growth and furrow ingression [29], [33]. Consequently, F-actin could also promote robustness by controlling the membrane dynamics of morphogenesis. Since all morphogenesis depends on actin remodeling, both in embryos [65] and adults [66], we believe that robustness-promoting mechanisms that target actin are likely to be ubiquitous.
Materials and Methods
Fly stocks and genetics
The sry-α−/− and sry-α+/− embryos were collected from Df(3R)X3F/TM3, Sb [26] crossed to OreR wild-type flies. The nulloX embryos were collected from C(1)DX, ywf [29], [30]. For sry-α−/− plus sptOE, first matαtub-GAL4 (II) was crossed with UASp-Spt-HA (III) (this paper) to make stock matαtub-Gal4; UASp-Spt-HA. Second, these flies were crossed with Df(3R)X3F/TM3, Sb, and finally embryos were collected from matαtub-GAL4/+; UASp-Spt-HA/Df(3R)X3F mothers crossed with their sibling matαtub-GAL4/+; UASp-Spt-HA/Df(3R)X3F fathers. For sry-α−/− plus sry-αrescue, Df(3R)X3F/TM3, Sb was crossed to sry-α genomic rescue stock (II) (gift of E. Wieschaus). For ½profilin, chic221 cn1/CyO; ry506 (Bloomington Stock Center #107932) was crossed with Df(3R)X3F/TM3, Sb; and embryos were collected from chic221 cn1/+; Df(3R)X3F/ry506 mothers crossed with their sibling fathers chic221 cn1/+; Df(3R)X3F/ry506. For RT-PCR and Western Blotting, OreR was used. For RNAi imaging, embryos were injected from stock Gap43-mCherry/CyO; Histone-GFP/TM3, Sb (parental stocks gifts of A. Martin and E. Wieschaus).
Production of Spt transgene and antibody
For UASp-Spt-HA flies, the coding sequence of D. melanogaster spt (CG8247) was fused with a C-terminal hemagglutinin (HA) tag, and cloned into pP(UASP) vector. Transgenesis and mapping followed standard methods (BestGene, Inc.). For anti-Spt antibody, the coding sequence of spt fused with a C-terminal histidine tag was cloned into pET vector, and recombinant protein was purified from E. coli. Antibodies were produced in guinea pigs according to standard methods (Panigen, Inc.). According to the publicly available data on FlyBase (flybase.org), spt is not expressed in adult heads. Thus, adult heads served as the negative control for antibody characterization (Figure S5).
F-actin co-sedimentation assay
Recombinant proteins GST-Sry-α (full length) and GST-SptΔ (amino acids 250–461) were produced in Sf9 cells (Baylor College of Medicine Baculovirus Core/Proteomics Shared Resource). The Spt truncate was used because full-length protein is insoluble in both bacterial and insect expression systems. Proteins were purified with Glutathione Sepharose (GE Healthcare) and dialyzed in F-buffer (20 mM Tris-HCl pH 7.5, 2 mM DTT, 2.5 mM MgCl2, 75 mM KCl and 10 mM NaCl). α-Actinin (Cytoskeleton, Inc.) was used as the positive control, and GST for the negative control was purified from Sf9 cells.
Rabbit muscle monomeric actin (G-actin) was extracted from Rabbit Muscle Acetone powder (Pel-Freez Biologicals). 20 mM G-actin was maintained in G-buffer (2 mM Tris-HCl pH 8.0, 0.2 mM ATP, 0.5 mM DTT, and 0.2 mM CaCl2) until polymerization in F-buffer by adding buffer and salt and incubating at room temperature for 1 hour. For the co-sedimentation assay, purified proteins were pre-cleared by centrifugation at 900,000 rpm for 30 minutes at 4°C to remove any precipitate. Pre-cleared proteins (∼1 µg) were incubated with F-actin for 30 min on ice and then centrifuged at 900,000 rpm for 30 min at 4°C. Supernatant was removed, and replaced by an equal volume of 3X sample buffer to resuspend pellets. Equal volumes of supernatants and pellets were separated on 5–10% SDS-PAGE gels and transferred to nitrocellulose. GST-Sry-α, GST-SptΔ, and GST were detected by 1∶10,000 mouse anti-GST (ab19256, Abcam). α-Actinin was detected by 1∶1000 anti-α-Actinin (A7811, Sigma-Aldrich). The goat secondary antibodies were HRP conjugates used at 1∶10,000 (Jackson ImmunoResearch).
Embryo collection and temperature incubations
Embryo collection cups were set up on apple juice plates according to published protocols [29], [30], [41]. For specific temperature incubations, collection cups were housed in air incubators of 18°C, 25°C, or 32°C within a range of ±1°C for 5, 4, or 3 hours, respectively. Plates were harvested and embryos fixed immediately.
Embryo fixation, staining, and FISH
For immunofluorescence, embryos were fixed in boiling salt buffer; or 4% paraformaldehyde, 0.1 M phosphate buffer (pH 7.4): heptane (1∶1) [29]. Antibody concentrations and fixation methods are listed in Table S2. Due to Myosin-2 antibody incompatibility with FISH, Septin (Peanut) was used as the furrow canal marker for experiments where heterozygosity was scored. For F-actin staining, embryos were fixed in 8% paraformaldehyde, 0.1 M phosphate buffer (pH 7.4): heptane (1∶1) and hand-peeled for staining with 5 U ml−1 Alexa 488-phalloidin (Invitrogen-Molecular Probes). For nuclear staining, Hoescht 33342 was used at either 0.25 µg ml−1 for standard immunofluorescence or 1.0 µg ml−1 for FISH (Invitrogen-Molecular Probes).
For FISH, 44 oligonucleotide probes, covering the sry-α coding sequence were synthesized (Biosearch Technologies), and then labeled with Alexa 488 [43]. Embryos were fixed in 4% paraformaldehyde, 0.1 M phosphate buffer (pH 7.4): heptane (1∶1) for hybridization with 50 µM Alexa 488-sry-α probe followed by immunofluorescence staining.
Image acquisition and analysis
For fixed and living embryos, images were collected on a Zeiss LSM 710 confocal microscope with a 40X/1.2 numerical aperture water-immersion objective (Carl Zeiss, Inc.). Images were collected at a zoom of two, with resolution of 104 nm per pixel.
To segment images, the Image Processing Toolbox in MATLAB (MathWorks) was used. From raw images, the furrow canal network and nuclei masks were generated using a series of morphological operations, as follows. For furrow canal network masks: (1) A Gaussian filter was applied to the raw image, to retain only coarse features of the furrow canals. (2) An intensity threshold was selected manually and applied to generate a preliminary mask. (3) The mask was thinned iteratively until a 1-pixel width network was produced. For nuclei masks: (4) A Gaussian filter was applied to the raw image to smooth out noise, yet retain fine features of the image. A low intensity threshold was selected manually and applied to capture weak furrow canal links present in multinucleated cells. The resulting mask was closed to join disconnected furrow canal links, and thinned iteratively to capture all nuclei separations. Remaining disconnected furrow canal links were removed. (5) The preliminary furrow canal network mask resulting from step 2 above was thinned and added to the mask obtained in step 4. This mask was dilated and inverted to generate a preliminary nuclei mask. Holes inside the mask were filled. (6) Finally, a high threshold was applied to the result of step 1 above; the resulting mask was inverted and then multiplied by the result of step 5 to eliminate boundary artifacts produced by thinning operations.
For quantifications, levels of F-actin in furrow canals and numbers of Amphiphysin tubules were scored as previously reported [29]. The ratio of mononucleate cells to nuclei was generated by manually counting in two quadrants from a raw, single plane, surface view image (quadrant size = 2835 µm2) collected at the furrow canals; and the mean was calculated per embryo. The percent of embryos displaying multinucleation was counted manually using raw, single plane, surface view images collected at the furrow canals, where an entire embryo side was visible (≥1500 nuclei assayed per embryo).
For genotyping by FISH, maximum intensity projections were generated from image stacks, comprising ∼4 µm depth and ≥150 nuclei; and active sry-α transcription sites were manually counted. Embryos with a maximum of 0, 1, or 2 spots were scored as sry-α−/−, sry-α+/−, or sry-α+/+ (wild-type, WT), respectively. For genotyping by immunofluorescence, images were collected at the same microscope settings, and Sry-α signal was scored as either present or absent.
RT-PCR and western blotting
For RT-PCR, embryos were staged in halocarbon oil 27 under a dissecting microscope. Approximately 100 embryos per stage were homogenized in Trizol (Invitrogen Inc.), and total RNA was extracted in phenol: chloroform (1∶1). Total cDNA was made (SuperScript III First-Strand Synthesis System, Invitrogen), and sequences amplified by PCR. For developmental expression profiles, primers were: actin42a-F, actin42a-R, sry-α-F, sry-α-R, spt-F, and spt-R (for sequences see Table S3). Following RNAi, primers were: actin42a-F, actin42a-R, sry-α-F2, sry-α-R2, spt-F2, and spt-R2 (for sequences see Table S3). Samples were loaded on 1% agarose gels.
For Westerns, 1 hour collections of embryos were incubated at room temperature for 0, 1, 2, 3, 4, or 5 hours respectively. Per stage, 50–100 µl embryos were homogenized in 200 µl 0.05 M Tris pH 8.0, 0.15 M KCl, 0.05M EDTA, 0.5% NP-40, 1X protease inhibitor cocktail (Halt Protease Inhibitor Cocktail, Thermo Scientific). For antibody characterization, 20 adult heads were homogenized in the same buffer. Following a 15 minute spin at 15,000 rpm at 4°C, the cytoplasmic fraction was collected and quantified (Pierce BCA Protein Assay Kit, Thermo Scientific). Equal amounts of total protein were separated on 5–10% SDS-PAGE gels and transferred to nitrocellulose. Sry-α, Spt, HA and α-Tubulin were detected by 1∶5 mouse anti-Sry-α (1G10, Developmental Studies Hybridoma Bank), 1∶500 guinea pig anti-Spt (this paper), 1∶100 rat anti-HA (Roche), and 1∶1000 rat anti-α-Tubulin (T9026, Sigma-Aldrich), respectively. The goat secondary antibodies were HRP conjugates used at 1∶10,000 (Jackson ImmunoResearch).
RNAi
Approximately 50 pl of sry-α and spt double-stranded RNA was prepared as previously described [41], with primers: sry-α-RNAi-F, sry-α-RNAi-R, spt-RNAi-F, and spt-RNAi-R (for sequences see Table S3), and injected into freshly laid embryos. Following incubation, mitotic cycle 13 embryos were mounted for imaging. RNAi controls were PBS buffer-injected embryos.
Phylogenetic analysis
Protein sequences were retrieved via UniProt (uniprot.org), and alignments were generated using PROMALS3D (prodata.swmed.edu/promals3d) [37]. Phylogenetic trees were built with PhyML v3.0.1 (pbil.univ-lyon1.fr/software/seaview) [67], and branch supports were tested with the default aLRT SH-like option. In addition, for the small and large trees, bootstrap statistics were determined from 1000 and 500 iterations, respectively. Vinculin from the pre-metazoan M. brevicollis served as the outgroup [36]. For both alignments and trees, the same results were generated using either the entire Sry-α and Spt sequences, or the VH2 domain alone. For the roll call analysis, the D. melanogaster sequences for sry-α and spt were used as input for (1) a PSI-BLAST search of the refseq protein database on NCBI (blast.ncbi.nlm.nih.gov); and (2) a BLAST search of the UniProtKB database on UniProt. For those insects where only one paralog was present, we could not assign them as sry-α or spt based on sequence identity alone because the identity values were the same. Instead, we used the presence or absence of introns as the distinguishing factor: sry-α is intronless, whereas spt has introns in all Drosophilids. Thus, all insect proteins including introns were assigned as spt.
Supporting Information
Zdroje
1. FelixMA, WagnerA (2008) Robustness and evolution: concepts, insights and challenges from a developmental model system. Heredity (Edinb) 100 : 132–140.
2. HamdounA, EpelD (2007) Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci U S A 104 : 1745–1750.
3. BurgaA, CasanuevaMO, LehnerB (2011) Predicting mutation outcome from early stochastic variation in genetic interaction partners. Nature 480 : 250–253.
4. GuZ, SteinmetzLM, GuX, ScharfeC, DavisRW, et al. (2003) Role of duplicate genes in genetic robustness against null mutations. Nature 421 : 63–66.
5. FrankelN, DavisGK, VargasD, WangS, PayreF, et al. (2010) Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466 : 490–493.
6. PerryMW, BoettigerAN, BothmaJP, LevineM (2010) Shadow enhancers foster robustness of Drosophila gastrulation. Curr Biol 20 : 1562–1567.
7. AlonU, SuretteMG, BarkaiN, LeiblerS (1999) Robustness in bacterial chemotaxis. Nature 397 : 168–171.
8. BarkaiN, LeiblerS (1997) Robustness in simple biochemical networks. Nature 387 : 913–917.
9. von DassowG, MeirE, MunroEM, OdellGM (2000) The segment polarity network is a robust developmental module. Nature 406 : 188–192.
10. Manu, SurkovaS, SpirovAV, GurskyVV, JanssensH, et al. (2009) Canalization of gene expression in the Drosophila blastoderm by gap gene cross regulation. PLOS Biol 7: e1000049.
11. RutherfordSL, LindquistS (1998) Hsp90 as a capacitor for morphological evolution. Nature 396 : 336–342.
12. SawarkarR, SieversC, ParoR (2012) Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell 149 : 807–818.
13. SiegalML, RushlowC (2012) Pausing on the path to robustness. Dev Cell 22 : 905–906.
14. SpecchiaV, PiacentiniL, TrittoP, FantiL, D'AlessandroR, et al. (2010) Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature 463 : 662–665.
15. LiX, CassidyJJ, ReinkeCA, FischboeckS, CarthewRW (2009) A microRNA imparts robustness against environmental fluctuation during development. Cell 137 : 273–282.
16. BraendleC, FelixMA (2008) Plasticity and errors of a robust developmental system in different environments. Dev Cell 15 : 714–724.
17. BarkoulasM, van ZonJS, MillozJ, van OudenaardenA, FelixMA (2013) Robustness and epistasis in the C. elegans vulval signaling network revealed by pathway dosage modulation. Dev Cell 24 : 64–75.
18. SawyerJM, HarrellJR, ShemerG, Sullivan-BrownJ, Roh-JohnsonM, et al. (2010) Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol 341 : 5–19.
19. ChungMI, Nascone-YoderNM, GroverSA, DrysdaleTA, WallingfordJB (2010) Direct activation of Shroom3 transcription by Pitx proteins drives epithelial morphogenesis in the developing gut. Development 137 : 1339–1349.
20. von DassowM, DavidsonLA (2007) Variation and robustness of the mechanics of gastrulation: the role of tissue mechanical properties during morphogenesis. Birth Defects Res C Embryo Today 81 : 253–269.
21. von DassowM, DavidsonLA (2011) Physics and the canalization of morphogenesis: a grand challenge in organismal biology. Phys Biol 8 : 045002.
22. SaundersTE, PanKZ, AngelA, GuanY, ShahJV, et al. (2012) Noise reduction in the intracellular pom1p gradient by a dynamic clustering mechanism. Dev Cell 22 : 558–572.
23. HowellAS, JinM, WuCF, ZylaTR, ElstonTC, et al. (2012) Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell 149 : 322–333.
24. SchejterED, WieschausE (1993) Functional elements of the cytoskeleton in the early Drosophila embryo. Annu Rev Cell Biol 9 : 67–99.
25. TadrosW, LipshitzHD (2009) The maternal-to-zygotic transition: a play in two acts. Development 136 : 3033–3042.
26. MerrillPT, SweetonD, WieschausE (1988) Requirements for autosomal gene activity during precellular stages of Drosophila melanogaster. Development 104 : 495–509.
27. SchweisguthF, LepesantJA, VincentA (1990) The serendipity alpha gene encodes a membrane-associated protein required for the cellularization of the Drosophila embryo. Genes Dev 4 : 922–931.
28. CaoJ, AlbertsonR, RiggsB, FieldCM, SullivanW (2008) Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J Cell Biol 182 : 301–313.
29. SokacAM, WieschausE (2008) Local actin-dependent endocytosis is zygotically controlled to initiate Drosophila cellularization. Dev Cell 14 : 775–786.
30. SokacAM, WieschausE (2008) Zygotically controlled F-actin establishes cortical compartments to stabilize furrows during Drosophila cellularization. J Cell Sci 121 : 1815–1824.
31. GrosshansJ, WenzlC, HerzHM, BartoszewskiS, SchnorrerF, et al. (2005) RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development 132 : 1009–1020.
32. Padash BarmchiM, RogersS, HackerU (2005) DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J Cell Biol 168 : 575–585.
33. YanS, LvZ, WinterhoffM, WenzlC, ZobelT, et al. (2013) The F-BAR protein Cip4/Toca-1 antagonizes the formin Diaphanous in membrane stabilization and compartmentalization. J Cell Sci 126 : 1796–1805.
34. KelleyLA, SternbergMJ (2009) Protein structure prediction on the Web: a case study using the PHYRE server. Nat Protoc 4 : 363–371.
35. ZhangY (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9 : 40.
36. ZhaoZM, ReynoldsAB, GaucherEA (2011) The evolutionary history of the catenin gene family during metazoan evolution. BMC Evol Biol 11 : 198.
37. PeiJ, KimBH, GrishinNV (2008) PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res 36 : 2295–2300.
38. YangJ, DokurnoP, TonksNK, BarfordD (2001) Crystal structure of the M-fragment of alpha-catenin: implications for modulation of cell adhesion. EMBO J 20 : 3645–3656.
39. GuindonS, DufayardJF, LefortV, AnisimovaM, HordijkW, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59 : 307–321.
40. JanssensB, StaesK, van RoyF (1999) Human alpha-catulin, a novel alpha-catenin-like molecule with conserved genomic structure, but deviating alternative splicing. Biochim Biophys Acta 1447 : 341–347.
41. MartinAC, KaschubeM, WieschausEF (2009) Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457 : 495–499.
42. ZimmerleCT, FriedenC (1986) Effect of temperature on the mechanism of actin polymerization. Biochemistry 25 : 6432–6438.
43. RajA, van den BogaardP, RifkinSA, van OudenaardenA, TyagiS (2008) Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods 5 : 877–879.
44. LehnerB (2010) Genes confer similar robustness to environmental, stochastic, and genetic perturbations in yeast. PLOS One 5: e9035.
45. CampelloneKG, WelchMD (2010) A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11 : 237–251.
46. WieschausE (1996) Embryonic transcription and the control of developmental pathways. Genetics 142 : 5–10.
47. De RenzisS, ElementoO, TavazoieS, WieschausEF (2007) Unmasking activation of the zygotic genome using chromosomal deletions in the Drosophila embryo. PLOS Biol 5: e117.
48. BushatiN, StarkA, BrenneckeJ, CohenSM (2008) Temporal reciprocity of miRNAs and their targets during the maternal-to-zygotic transition in Drosophila. Curr Biol 18 : 501–506.
49. GiraldezAJ, MishimaY, RihelJ, GrocockRJ, Van DongenS, et al. (2006) Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312 : 75–79.
50. LundquistEA, ReddienPW, HartwiegE, HorvitzHR, BargmannCI (2001) Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development 128 : 4475–4488.
51. NandadasaS, TaoQ, MenonNR, HeasmanJ, WylieC (2009) N - and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development 136 : 1327–1338.
52. StrongTC, ThomasJH (2011) Maternal and zygotic requirements for src64 during Drosophila cellularization. Genesis 49 : 912–918.
53. HandelK, GrunfelderCG, RothS, SanderK (2000) Tribolium embryogenesis: a SEM study of cell shapes and movements from blastoderm to serosal closure. Dev Genes Evol 210 : 167–179.
54. MiuraT, BraendleC, ShingletonA, SiskG, KambhampatiS, et al. (2003) A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J Exp Zool B Mol Dev Evol 295 : 59–81.
55. KanayamaM, Akiyama-OdaY, OdaH (2010) Early embryonic development in the spider Achaearanea tepidariorum: Microinjection verifies that cellularization is complete before the blastoderm stage. Arthropod Struct Dev 39 : 436–445.
56. HavelkaJ, LandaV, LandaV (2007) Embryogenesis of Aphidoletes aphidimyza (Diptera: Cecidomyiidae): Morphological markers for staging of living embryos. Eur J Entomol 104 : 81–87.
57. KofronM, SpagnuoloA, KlymkowskyM, WylieC, HeasmanJ (1997) The roles of maternal alpha-catenin and plakoglobin in the early Xenopus embryo. Development 124 : 1553–1560.
58. KofronM, HeasmanJ, LangSA, WylieCC (2002) Plakoglobin is required for maintenance of the cortical actin skeleton in early Xenopus embryos and for cdc42-mediated wound healing. J Cell Biol 158 : 695–708.
59. TaoQ, LloydB, LangS, HoustonD, ZornA, et al. (2005) A novel G protein-coupled receptor, related to GPR4, is required for assembly of the cortical actin skeleton in early Xenopus embryos. Development 132 : 2825–2836.
60. MartinAC, GelbartM, Fernandez-GonzalezR, KaschubeM, WieschausEF (2010) Integration of contractile forces during tissue invagination. J Cell Biol 188 : 735–749.
61. SkoglundP, RoloA, ChenX, GumbinerBM, KellerR (2008) Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135 : 2435–2444.
62. RoloA, SkoglundP, KellerR (2009) Morphogenetic movements driving neural tube closure in Xenopus require myosin IIB. Dev Biol 327 : 327–338.
63. ZhouJ, KimHY, WangJH, DavidsonLA (2010) Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development 137 : 2785–2794.
64. ZhouJ, KimHY, DavidsonLA (2009) Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 136 : 677–688.
65. GorfinkielN, SchambergS, BlanchardGB (2011) Integrative approaches to morphogenesis: lessons from dorsal closure. Genesis 49 : 522–533.
66. SimpsonCL, PatelDM, GreenKJ (2011) Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol 12 : 565–580.
67. GouyM, GuindonS, GascuelO (2010) SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27 : 221–224.
Štítky
Genetika Reprodukční medicína
Článek Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis inČlánek Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 11
-
Všechny články tohoto čísla
- Molecular Recognition by a Polymorphic Cell Surface Receptor Governs Cooperative Behaviors in Bacteria
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis in
- Retrotransposon Silencing During Embryogenesis: Cuts in LINE
- Roles of XRCC2, RAD51B and RAD51D in RAD51-Independent SSA Recombination
- Parallel Evolution of Chordate Regulatory Code for Development
- A Genetic Approach to the Recruitment of PRC2 at the Locus
- Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
- Elevated Mutagenesis Does Not Explain the Increased Frequency of Antibiotic Resistant Mutants in Starved Aging Colonies
- Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
- Interplay between Active Chromatin Marks and RNA-Directed DNA Methylation in
- Recombinogenic Conditions Influence Partner Choice in Spontaneous Mitotic Recombination
- Crosstalk between NSL Histone Acetyltransferase and MLL/SET Complexes: NSL Complex Functions in Promoting Histone H3K4 Di-Methylation Activity by MLL/SET Complexes
- A New Role for the GARP Complex in MicroRNA-Mediated Gene Regulation
- RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Loss of DNMT1o Disrupts Imprinted X Chromosome Inactivation and Accentuates Placental Defects in Females
- Inhibition of the Smc5/6 Complex during Meiosis Perturbs Joint Molecule Formation and Resolution without Significantly Changing Crossover or Non-crossover Levels
- Disruption of Lipid Metabolism Genes Causes Tissue Overgrowth Associated with Altered Developmental Signaling
- Translation Initiation Factors eIF3 and HCR1 Control Translation Termination and Stop Codon Read-Through in Yeast Cells
- Recruitment of TREX to the Transcription Machinery by Its Direct Binding to the Phospho-CTD of RNA Polymerase II
- MYB97, MYB101 and MYB120 Function as Male Factors That Control Pollen Tube-Synergid Interaction in Fertilization
- Oct4 Is Required ∼E7.5 for Proliferation in the Primitive Streak
- Contrasted Patterns of Crossover and Non-crossover at Meiotic Recombination Hotspots
- Transposable Prophage Mu Is Organized as a Stable Chromosomal Domain of
- Ash1l Methylates Lys36 of Histone H3 Independently of Transcriptional Elongation to Counteract Polycomb Silencing
- Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects
- Genomic Mechanisms Accounting for the Adaptation to Parasitism in Nematode-Trapping Fungi
- Decoding a Signature-Based Model of Transcription Cofactor Recruitment Dictated by Cardinal Cis-Regulatory Elements in Proximal Promoter Regions
- Removal of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
- Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
- Activin Signaling Targeted by Insulin/dFOXO Regulates Aging and Muscle Proteostasis in
- Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans
- Demographic Divergence History of Pied Flycatcher and Collared Flycatcher Inferred from Whole-Genome Re-sequencing Data
- Recurrent Tissue-Specific mtDNA Mutations Are Common in Humans
- The Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
- The Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
- Reconstructing the Population Genetic History of the Caribbean
- and Are Required for Growth under Iron-Limiting Conditions
- Whole Genome, Whole Population Sequencing Reveals That Loss of Signaling Networks Is the Major Adaptive Strategy in a Constant Environment
- Neuron-Specific Feeding RNAi in and Its Use in a Screen for Essential Genes Required for GABA Neuron Function
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
- Mouse BAZ1A (ACF1) Is Dispensable for Double-Strand Break Repair but Is Essential for Averting Improper Gene Expression during Spermatogenesis
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- DUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
- Pathways-Driven Sparse Regression Identifies Pathways and Genes Associated with High-Density Lipoprotein Cholesterol in Two Asian Cohorts
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- and Are Required for Growth under Iron-Limiting Conditions
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



