-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRemoval of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
Stringent steric exclusion mechanisms limit the misincorporation of ribonucleotides by high-fidelity DNA polymerases into genomic DNA. In contrast, low-fidelity Escherichia coli DNA polymerase V (pol V) has relatively poor sugar discrimination and frequently misincorporates ribonucleotides. Substitution of a steric gate tyrosine residue with alanine (umuC_Y11A) reduces sugar selectivity further and allows pol V to readily misincorporate ribonucleotides as easily as deoxynucleotides, whilst leaving its poor base-substitution fidelity essentially unchanged. However, the mutability of cells expressing the steric gate pol V mutant is very low due to efficient repair mechanisms that are triggered by the misincorporated rNMPs. Comparison of the mutation frequency between strains expressing wild-type and mutant pol V therefore allows us to identify pathways specifically directed at ribonucleotide excision repair (RER). We previously demonstrated that rNMPs incorporated by umuC_Y11A are efficiently removed from DNA in a repair pathway initiated by RNase HII. Using the same approach, we show here that mismatch repair and base excision repair play minimal back-up roles in RER in vivo. In contrast, in the absence of functional RNase HII, umuC_Y11A-dependent mutagenesis increases significantly in ΔuvrA, uvrB5 and ΔuvrC strains, suggesting that rNMPs misincorporated into DNA are actively repaired by nucleotide excision repair (NER) in vivo. Participation of NER in RER was confirmed by reconstituting ribonucleotide-dependent NER in vitro. We show that UvrABC nuclease-catalyzed incisions are readily made on DNA templates containing one, two, or five rNMPs and that the reactions are stimulated by the presence of mispaired bases. Similar to NER of DNA lesions, excision of rNMPs proceeds through dual incisions made at the 8th phosphodiester bond 5′ and 4th–5th phosphodiester bonds 3′ of the ribonucleotide. Ribonucleotides misinserted into DNA can therefore be added to the broad list of helix-distorting modifications that are substrates for NER.
Published in the journal: . PLoS Genet 9(11): e32767. doi:10.1371/journal.pgen.1003878
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003878Summary
Stringent steric exclusion mechanisms limit the misincorporation of ribonucleotides by high-fidelity DNA polymerases into genomic DNA. In contrast, low-fidelity Escherichia coli DNA polymerase V (pol V) has relatively poor sugar discrimination and frequently misincorporates ribonucleotides. Substitution of a steric gate tyrosine residue with alanine (umuC_Y11A) reduces sugar selectivity further and allows pol V to readily misincorporate ribonucleotides as easily as deoxynucleotides, whilst leaving its poor base-substitution fidelity essentially unchanged. However, the mutability of cells expressing the steric gate pol V mutant is very low due to efficient repair mechanisms that are triggered by the misincorporated rNMPs. Comparison of the mutation frequency between strains expressing wild-type and mutant pol V therefore allows us to identify pathways specifically directed at ribonucleotide excision repair (RER). We previously demonstrated that rNMPs incorporated by umuC_Y11A are efficiently removed from DNA in a repair pathway initiated by RNase HII. Using the same approach, we show here that mismatch repair and base excision repair play minimal back-up roles in RER in vivo. In contrast, in the absence of functional RNase HII, umuC_Y11A-dependent mutagenesis increases significantly in ΔuvrA, uvrB5 and ΔuvrC strains, suggesting that rNMPs misincorporated into DNA are actively repaired by nucleotide excision repair (NER) in vivo. Participation of NER in RER was confirmed by reconstituting ribonucleotide-dependent NER in vitro. We show that UvrABC nuclease-catalyzed incisions are readily made on DNA templates containing one, two, or five rNMPs and that the reactions are stimulated by the presence of mispaired bases. Similar to NER of DNA lesions, excision of rNMPs proceeds through dual incisions made at the 8th phosphodiester bond 5′ and 4th–5th phosphodiester bonds 3′ of the ribonucleotide. Ribonucleotides misinserted into DNA can therefore be added to the broad list of helix-distorting modifications that are substrates for NER.
Introduction
In order to preserve the properties and functions of a living organism, the genetic information encoded in its DNA should be kept essentially unchanged. In reality, DNA is constantly subjected to numerous attacks from endogenous and exogenous sources changing its chemical composition and structure. If left unrepaired, these changes could have potentially serious cytotoxic and/or mutagenic consequences for the cell. Among all abnormalities in DNA, the presence of ribonucleotides in the DNA backbone appears to be one of the most common threats to genomic stability. Because of the reactive 2′-hydroxyl group on the sugar moiety, rNMPs embedded in the chromosome make the DNA strand susceptible to spontaneous and enzymatically-catalyzed hydrolytic cleavage [1]. They can also cause B - to A-form helical transition in DNA that would interfere with normal binding of various DNA-interacting proteins and disrupt a range of DNA transactions [2], [3]. Moreover, unrepaired ribonucleotides can lead to replication stress and genome instability [4]–[7].
RNA primers synthesized during the initiation of lagging-strand replication are a major source of rNMPs in DNA. These primers must be excised from DNA prior to joining of Okazaki fragments into an intact lagging strand. Several nucleases have been implicated in this process [8]. Among these are enzymes specifically hydrolyzing the phosphodiester bond between ribo - and deoxyribonucleotides, i.e. ribonucleotide-specific endonucleases, ribonucleases HI and HII (RNase H), which appeared to be ideally suited to play a primary role in RNA primer removal. However, subsequent studies revealed that the RNase H-initiated pathway is not the major mechanism leading to the removal of RNA primers (reviewed in [8]), although the enzymes are nevertheless essential for many key cellular processes requiring degradation of RNA from RNA/DNA hybrids. In particular, it has been shown that the RNase H pathway is indispensable for the removal of errant ribonucleotides randomly misinserted by DNA polymerases during replication and repair synthesis [9]–[11].
The role of Saccharomyces cerevisiae (S. cerevisiae) and Escherichia coli (E. coli) ribonucleases in ribonucleotide excision repair (RER) has been described in several recent publications [10]–[13]. In both organisms rNMP removal is primarily initiated by an RNase H type 2 enzyme; RNase H2 encoded by rnh2 in eukaryotes and RNase HII encoded by rnhB in prokaryotes. Ribonucleases of this type possess a broad cleavage specificity effectively hydrolyzing phosphodiester bonds at the RNA-DNA junction on the templates containing RNA fragments, as well as isolated rNMPs embedded into double-stranded (ds) DNA. In contrast, type 1 ribonucleases, such as RNase H1 encoded by rnh1 in eukaryotes and RNase HI encoded by rnhA in prokaryotes, require a tract of at least four consecutive ribonucleotides within the DNA strand for the efficient cleavage.
Biochemical analysis using yeast purified recombinant proteins revealed that RNase H1 cannot substitute for RNase H2 in the RER pathway [10]. On the other hand, using an in vivo approach, we have recently shown that RNase HI substitutes for RNase HII in ΔrnhB cells thus limiting the mutagenic consequences of excessive ribonucleotide accumulation in E.coli genome [11]. The apparent discrepancy between these two studies is most likely explained by differences in sugar selectivity of the polymerases responsible for rNMPs insertion, rather than by differences in substrate specificities, or other biochemical properties of yeast and bacterial type 1 ribonucleases that govern the participation of the enzymes in the RER pathway. Indeed, both yeast replicative polymerases, pol δ and pol ε effectively discriminate between rNTPs and dNTPs and incorporate ribonucleotides into DNA at low frequencies (1 per ∼600–900 nt; [10]). It is therefore highly unlikely that either pol δ or pol ε would catalyze synthesis of DNA containing several consecutive ribonucleotides, which would be a potential substrate for RNase HI. In contrast, E. coli pol V (UmuD′2C heterotrimer) appears to be one of the most indiscriminate polymerases for sugar selection [14]. In the presence of rNTPs, it is able to synthesize remarkably long RNA products [14]. A Y11A substitution in the steric gate of UmuC not only further reduces the selectivity against single rNTP incorporation, but also essentially converts the resulting mutant into a bona fide primer-dependent RNA polymerase that synthesizes RNA products at a 3-fold faster rate relative to the wild-type enzyme [14]. It is not surprising, therefore, that the mutant pol V catalyzes synthesis of DNA strands containing not only scattered single rNMPs, but also continuous RNA fragments that could be cleaved by both RNase HI and RNase HII [14]. Thus, while RNase HII plays a major role in keeping the E. coli chromosome free from errant ribonucleotides, in its absence RNase HI functions as an effective substitute to reduce genomic instability promoted through frequent ribonucleotide misincorporation.
In contrast, in the absence of a proper substitute for yeast RNase H2, replicative stress occurs and leads to genome instability [12]. This instability depends on the activity of topoisomerase 1 (Top1), whose primary function in the cell is to regulate DNA supercoiling by creating transient single-strand (ss) breaks. When Top1 cleaves the phosphodiester bond at the sites of incorporated rNMP, the ss break that is created is irreversible because of the presence of a 2′-OH group of the ribose ring. This leads to the formation of a 2′–3′-cyclic phosphate that is refractory to re-ligation [12]. Indeed, substitution of RNase H2 by Top1 in the processing of rNMPs has distinct mutagenic consequences, i.e. accumulation of 2 - to 5-bp deletions within tandem repeat sequences [12].
There is no doubt that RER initiated by type 2 RNase H is the major pathway removing errant rNMPs from ds DNA. However, as with other cellular processes, it is anticipated that alternative mechanisms limiting the impact of ribonucleotides in the genome have evolved. Indeed, as noted above, that is the case when rnhB is inactivated and rnhA helps sanitize the E.coli genome of errantly incorporated ribonucleotides [11]. However, whilst umuC_Y11A-dependent mutagenesis relative to wild-type pol V increased from ∼7% in rnh+ strains to ∼39% in ΔrnhB strains and further increased to ∼74% in the ΔrnhB ΔrnhA strain, it was still significantly lower than that promoted by wild-type pol V, despite the fact the enzyme exhibits the same low-base selectivity in vitro [14]. These data suggest that additional mechanisms exist which target the ribonucleotides incorporated by umuC_Y11A for repair and in the process misincorporated deoxyribonucleotides are also removed from the E.coli genome.
We have taken advantage of the pol V phenotype to investigate the contribution of mismatch repair (MMR), base excision repair (BER) and nucleotide excision repair (NER) to ribonucleotide excision repair (RER) in E.coli. We find no evidence for a significant role of either MMR or BER in back-up RER pathways. Somewhat surprisingly, we discovered that there was a major contribution to RER by NER in vivo. By using in vitro assays, we confirm that NER is able to recognize and excise an isolated ribonucleotide, as well as multiple rNMPs within a short RNA fragment in dsDNA in vitro. Efficient ribonucleotide repair in E.coli and most likely other prokaryotes is, therefore, achieved though the concerted actions of rnhB, rnhA and NER.
Results
In vivo system to identify repair pathways contributing to the removal of ribonucleotides misincorporated into the E. coli genome
E. coli pol V is a highly error-prone Y-family DNA polymerase best characterized for its capacity to replicate damaged DNA [15], [16]. However, in certain genetic backgrounds, such as in recA730 strains in which the RecA protein is in a so-called constitutively “activated state” (RecA*) that both favors the formation of pol V (UmuD′2C) [17], [18], and also increases its stability [19], [20], pol V can compete with the cell's replicase (pol III) for access to undamaged genomic DNA. Since pol V has much lower fidelity than pol III, this is manifested as a dramatic increase in spontaneous mutagenesis [21], [22]. We have previously taken advantage of this phenotype to elucidate pathways of ribonucleotide repair in E.coli. To do so, we generated a steric-gate Y11A mutant in the catalytic UmuC subunit of pol V, which has significantly reduced sugar discrimination. As a result, the mutant pol V enzyme incorporates ribonucleotides into DNA nearly as efficiently as deoxyribonucleotides [14]. In contrast, the base-selection fidelity of the Y11A mutant was largely unchanged and like wild-type pol V, umuC_Y11A frequently misincorporated the wrong base into nascent DNA in vitro [14]. However, to our surprise, the spontaneous mutation frequency in a recA730 lexA(Def) ΔdinB ΔumuDC strain expressing umuC_Y11A was an order of magnitude lower than that of the isogenic strain expressing wild-type pol V [23]. The apparent discrepancy was explained by the umuC_Y11A-dependent incorporation of rNMPs into the E.coli genome that triggered efficient ribonucleotide excision repair (RER) pathways and concomitantly removed misincorporated deoxyribonucleotides. umuC_Y11A-dependent spontaneous mutagenesis increased significantly in a ΔrnhB background and to a much larger extent when both rnhB and rnhA were inactivated [11] (Fig. 1). However, mutagenesis was still lower than that of wild-type pol V suggesting that other back-up RER pathways exist in E.coli that operate to remove errantly incorporated ribonucleotides. We hypothesized that similar to our earlier observation, where the extent of umuC_Y11A-dependent mutagenesis relative to wild-type pol V increased when rnhA was inactivated in an ΔrnhB strain, we would also observe an increase in umuC_Y11A-dependent mutagenesis in other genetic backgrounds that are compromised for RER. We therefore constructed a series of isogenic recA730 lexA(Def) ΔdinB ΔumuDC rnhB+/ΔrnhB strains in which mismatch repair (MMR; ΔmutL, ΔmutH, ΔmutS, ΔuvrD), base excision repair (BER; Δung, Δxth, Δnfo), or nucleotide excision repair (NER; ΔuvrA, uvrB5, ΔuvrC, Δcho, ΔuvrD) were inactivated and assayed for pol V-dependent spontaneous mutagenesis (Fig. 1). As noted previously, despite being isogenic, the strains expressing wild-type pol V exhibit quite different levels of spontaneous mutagenesis. We believe that this phenotype is due to effects on the constitutive activation of the RecA protein, which is an absolute requirement for high levels of pol V-dependent spontaneous mutagenesis [21]. As a consequence, we report the extent of umuC_Y11A-dependent mutagenesis relative to wild-type pol V, since any indirect effect on RecA activation would be the same for both mutant and wild-type pol V, with the only difference being their respective ability to efficiently incorporate ribonucleotides into genomic DNA. As a control, we monitored spontaneous mutagenesis in the isogenic strains lacking pol V (containing the plasmid vector, pGB2). Since the number of His+ revertants in these cells is pol V-independent, it should remain constantly low in all genetic backgrounds with the exception of the MMR deficient strains, where a ∼10-fold increase in mutation frequency is anticipated.
Fig. 1. Effect of inactivating RER, MMR, BER and NER on spontaneous mutagenesis in recA730 lexA(Def) ΔdinB strains ΔumuDC. 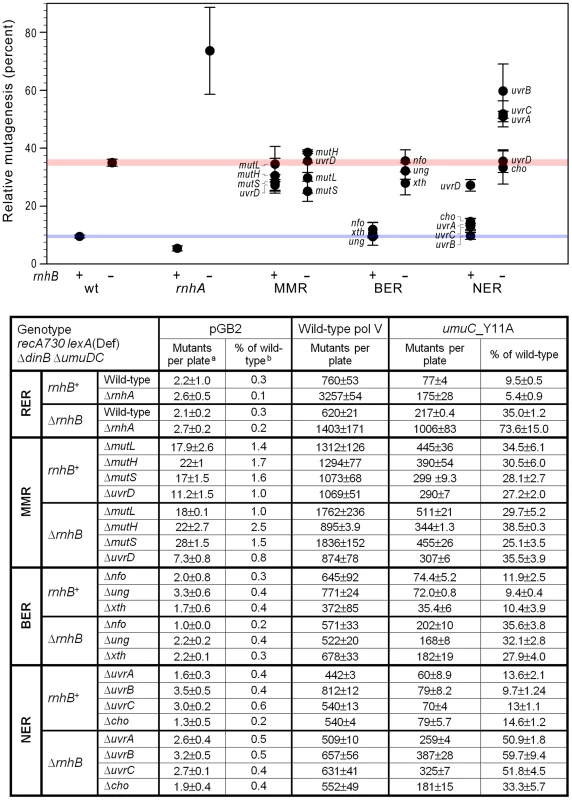
We constructed a series of isogenic recA730 lexA(Def) ΔdinB ΔumuDC rnhB+/ΔrnhB strains in which ribonucleotide excision repair (RER; ΔrnhA), mismatch repair (MMR; ΔmutL, ΔmutH, ΔmutS, ΔuvrD), base excision repair (BER; Δung, Δxth, Δnfo), or nucleotide excision repair (NER; ΔuvrA, uvrB5, ΔuvrC, Δcho, ΔuvrD) were inactivated. The extent of spontaneous mutagenesis in strains harboring the control vector, pGB2, or plasmids expressing wild-type pol V, or umuC_Y11A was determined by assaying reversion of the hisG4 (ochre) allele (leading to histidine prototrophy). The average number of spontaneously arising His+ mutants per plate ± standard error of the mean (a) and the relative spontaneous mutagenesis in cells containing pGB2 vector or expressing the umuC_Y11A variant (b) are shown in the table below the graph. The data plotted on the graph represent the relative extent of spontaneous mutagenesis in cells expressing umuC_Y11A. The level of mutagenesis was calculated by subtracting the number of pre-existing mutants that grew on plates lacking histidine from the number of His+ mutants spontaneously arising on plates containing 1 µg/ml histidine. The relative amount of pol V-independent or umuC_Y11A-dependent mutagenesis was calculated as a percentage of the mutagenesis in cells expressing wild-type pol V. All experiments were performed in triplicate. Standard errors were calculated taking into account variability in spontaneous mutagenesis in each strain. The horizontal blue and red strips on the graph provide a reference baseline corresponding to the level of umuC_Y11A -dependent mutagenesis in repair-proficient rnhB+ and ΔrnhB cells, respectively. Data for wild-type and ΔrnhA strains was taken from [11] and is shown for comparison. As clearly observed, inactivation or rnhA and NER (ΔuvrA, uvrB5, ΔuvrC) in a ΔrnhB background leads to a dramatic increase in umuC_Y11A-dependent mutagenesis, indicating potential roles in RER. Contribution of MMR to the removal of ribonucleotides from the E.coli genome
Inactivation of MMR (via ΔmutL, ΔmutH, or ΔmutS alleles) results in an ∼3.5-fold increase in the relative amount of spontaneous His+ mutagenesis promoted by plasmid encoded umuC_Y11A compared to wild-type pol V (Fig. 1). These data appear to implicate MMR in the repair of at least a subset of ribonucleotides (mis)incorporated by umuC_Y11A and such observations are consistent with an earlier study reporting an effect of MMR on ribonucleotide repair [13]. However, there was no additional increase in the relative amount of umuC_Y11A mutagenesis in ΔrnhB ΔmutL, ΔrnhB ΔmutH, or ΔrnhB ΔmutS strains (Fig. 1), which would be expected if MMR participates in repair pathways that substitute for the RNase HII-dependent RER of ribonucleotides misincorporated by umuC_Y11A. We hypothesize that the increase in relative mutagenesis in the rnhB+ MMR-deficient strains expressing umuC_Y11A is not caused by a reduction of rNMP repair, but rather reflects misincorporations made by a different DNA polymerase participating in the re-synthesis step of ribonucleotide repair. To examine this possibility, we determined the spectra of mutations generated in rnhB+/ΔrnhB strains expressing umuC_Y11A (Fig. 2). As expected, the ΔmutL rnhB+ spectrum (Fig. 2A) was dominated by transition events. In contrast, the ΔmutL ΔrnhB strain exhibited a different mutagenic spectrum that included many more transversions (Table S1), which are “signatures” of error-prone pol V [24], [25].
Fig. 2. Spectra of spontaneously arising rpoB mutations in recA730 lexA(Def) ΔumuDC ΔdinB strains expressing umuC_Y11A and proficient- or deficient- in MMR and RNase HII- mediated RER. 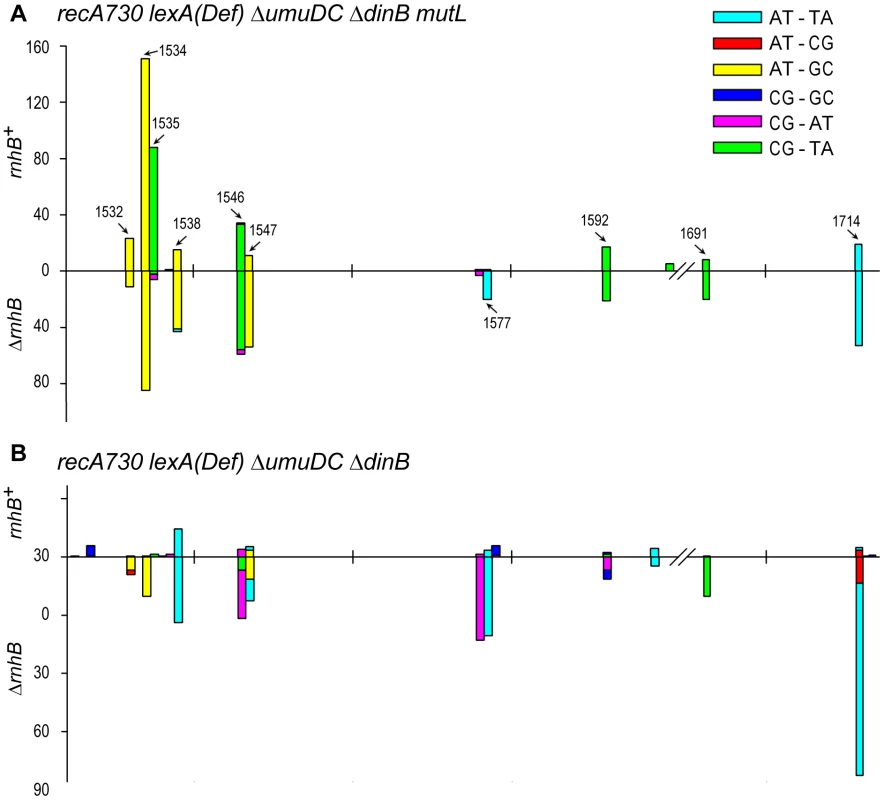
(A), Types of base-pair substitutions generated in the rpoB gene of mismatch repair defective recA730 lexA(Def) ΔdinB ΔumuDC mutL rnhB+/− strains. The arrows indicate mutagenic hot spots within rpoB. The spectra for the rnhB+ strain (RW710) are taken from [14] and are shown for direct comparison with the spectra for the isogenic ΔrnhB strain (RW942). The forward mutation rates in the MMR− strains was assayed by measuring resistance to rifampicin and were calculated as 1.22±0.2×10−6 for RW710 and 1.82±0.2×10−6 for RW942. As expected, because of defects in MMR, the spectra are dominated by transition mutations. However, we have previously reported that low-fidelity pol V makes a significant number of transversion mutations compared to other E.coli DNA polymerases [25] and we note a 4-fold increase in the number of transversion mutations in the ΔrnhB mutL strain expressing umuC_Y11A compared to the mutL rnhB+ strain (see Table S1) consistent with pol V-dependent errors. The rnhB+ mutL strain lacks these transversion mutations, as it undergoes active pol I-dependent RER and as a consequence, the spectrum generated in this strain actually reflects uncorrected pol I-dependent errors, rather than pol V-dependent errors. (B), Types of base-pair substitutions generated in the rpoB gene of mismatch repair proficient recA730 lexA(Def) ΔdinB ΔumuDC rnhB+/− strains. The forward mutation rates in the MMR+ strains assayed by measuring resistance to rifampicin and were calculated as 4.8±0.8×10−8 for RW698 and 2.5±0.4×10−7 for RW838. As expected, because the strains are proficient in MMR and transitions are repaired efficiently, the spectra are dominated by the poorly repaired transversion mutations. Approximately 300 RifR mutants were analyzed for each strain (Table S1). However, the spectrum of the rnhB+ strain has been normalized to reflect the overall ∼6-fold lower mutation rate compared to the ΔrnhB strain. One can clearly observe that the spectra are very different in the two strains, with changes in both the types of mutations and locations of mutagenic hot-spots. Again, we believe the data support the model that in the MMR+ strains, pol I is responsible for low-levels of mutagenic events generated during RNase HII-pol I dependent RER, whilst in the absence of RER, umuC_Y11A mutations characterized by frequent transversion events persist and lead to a 6-fold increase in mutation rate. To further extend our hypothesis that the mutations in the rnhB+ and ΔrnhB strains are generated by two different polymerases operating in two different pathways, we assayed the spectrum of rpoB mutations in mismatch repair proficient rnhB+ and ΔrnhB strains expressing umuC_Y11A (Fig. 2B). In contrast to the MMR− strains, where the spectrum was dominated by transitions, the majority of base substitutions in the MMR+ strains were transversion events, which is consistent with the efficient repair of transition mutations by the mismatch repair machinery (Table S1). In agreement with our hypothesis, not only was the mutation rate of the rnhB+ strain 6-fold lower compared to the ΔrnhB strain, the mutagenic hot-spots varied considerably, suggesting that the mutations were generated by different DNA polymerases. In particular, in the ΔrnhB strain where umuC_Y11A misincorporations are likely to persist, the spectrum was dominated by AT→TA transversions (at positions 1547, 1577 and 1714) that are characteristic of pol V [24].
Together, our data indicate that the prokaryotic MMR pathway, while possessing the capacity to recognize mispaired bases, does not selectively recognize ribonucleotide mispairs over deoxyribonucleotides mispairs and therefore does not contribute significantly to RER.
Contribution of BER to the removal of ribonucleotides from the E.coli genome
To continue our search of a RER backup pathway, we turned to base excision repair (BER), which targets a variety of base lesions. Of particular relevance, are dUMPs frequently misincorporated by DNA polymerases [26], or formed through the spontaneous hydrolytic deamination of cytosines and which are released through BER initiated by uracil DNA glycosylase (encoded by the ung gene). Ung recognizes the lesion and hydrolyzes the N-glycosylic bond between the uracil base and sugar ring converting uracil into an abasic site. The next major step of BER is cleavage of the abasic site by one of the class II apurinic/apyrimidinic (AP) endonucleases, such as exonuclease III (encoded by the xthA gene), or endonuclease IV (encoded by nfo), which together account for the vast majority of AP endonuclease activity in E.coli [27], [28]. Similar to the proposed RNase HII-dependent RER pathway, processing of the BER intermediates involves strand-displacement DNA synthesis with replication products ranging from just one base-pair to several hundred nucleotides [29]–[31]. We therefore considered the possibility that BER might operate to remove misincorporated rUMPs, which would provide a mechanism to reduce the mutagenic potential of umuC_Y11A-dependent spontaneous mutagenesis. However, inactivation of ung, xth, or nfo had no discernible effect on the relative extent of umuC_Y11A mutagenesis in either rnhB+ or ΔrnhB strains (Fig. 1). Based upon these observations, we conclude that BER is unlikely to participate in any RER back-up pathway in E.coli.
Contribution of NER to the removal of ribonucleotides from the E.coli genome
We recently reported that ΔuvrA and ΔuvrC strains expressing umuC_Y11A are as UV-resistant as those expressing wild-type pol V. This is in dramatic contrast to uvr+ strains in which umuC_Y11A confers minimal UV-resistance [23] despite being as proficient as wild-type pol V in its ability to traverse a UV-induced cyclobutane pyrimidine dimer (CPD) [23]. Such phenotypes were attributed to the dual actions of RNase HII nicking the nascent TLS strand and the concomitant actions of the NER proteins on the opposite CPD-containing strand to generate lethal double-strand breaks [11]. In the present study, we analyzed the effect of NER on RER of undamaged DNA.
To do so, we determined spontaneous mutagenesis in strains carrying mutations in genes encoding key proteins that mediate damage recognition and excision steps of the E. coli NER pathway (uvrA, uvrB, uvrC, cho and uvrD). It should be noted that the uvrA, uvrB, cho and uvrD genes are normally regulated at the transcriptional level by the LexA repressor [32]. However, since the strains used for the mutagenesis assays carry the recA730 lexA(Def) alleles which lead to derepression of all genes in the LexA-regulon, the UvrA, UvrB, Cho and UvrD proteins are all expected to be expressed at fully derepressed levels and as a consequence, NER is active in the absence of exogenous DNA damage. Despite this fact, inactivation of uvrA, uvrB, cho or uvrC (which is not under lexA control), in the rnhB+ background had little effect on the overall low level of mutagenesis promoted by umuC_Y11A relative to wild-type pol V (Fig. 1). The ΔuvrD strain exhibited somewhat higher levels of spontaneous mutagenesis. However, this phenotype is probably unrelated to NER, but is instead more in line with its dual functions in MMR [33], [34]. Interestingly, there was a significant increase in the extent of umuC_Y11A-dependent spontaneous mutagenesis in the ΔuvrA ΔrnhB, uvrB5 ΔrnhB and ΔuvrC ΔrnhB strains (Fig. 1). In contrast, the relative extent of spontaneous mutagenesis remained essentially unchanged in the Δcho ΔrnhB, or ΔuvrD ΔrnhB strains. Together, these observations imply that NER is able to remove ribonucleotides from DNA, and this process occurs via the “classical” NER pathway mediated by the UvrABC proteins and not through an alternate UvrAB/Cho-dependent pathway [35]. In addition, the lack of an apparent uvrD phenotype in RER is consistent with a previous study showing that UvrD is not necessary for the lesion removal under SOS conditions [36].
Furthermore, inactivation of NER (by ΔuvrA) in the ΔrnhB ΔrnhA strain resulted in a dramatic increase in umuC_Y11A-dependent mutagenesis, such that it actually became greater than that produced by wild-type pol V (Fig. 3), indicating that RER is completely inactivated in this genetic background. These findings confirm that UvrABC-dependent NER serves as a bona fide back-up to RNase HII-mediated RER.
Fig. 3. Effect of deleting rnhA, rnhB and/or uvrA alone, or in various combinations, on the extent of umuC_Y11A-dependent spontaneous mutagenesis in recA730 lexA(Def) ΔumuDC ΔdinB strains. 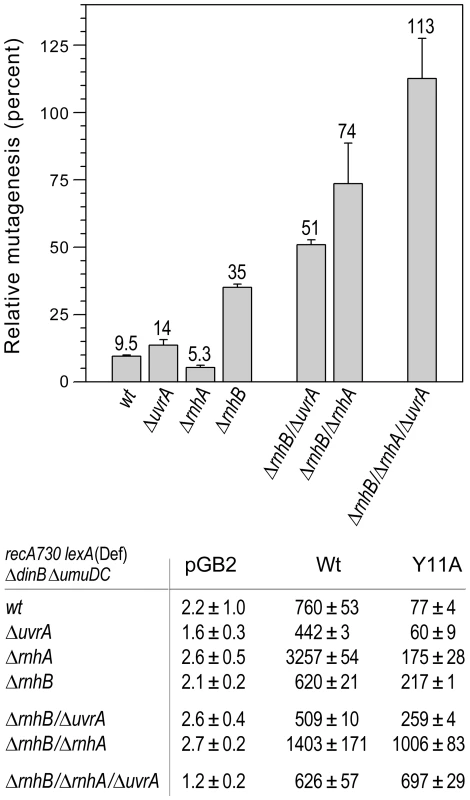
We constructed a series of isogenic recA730 lexA(Def) ΔdinB ΔumuDC strains with ΔrnhA, ΔrnhB or ΔuvrA alleles alone, or in various combinations and assayed pol V-dependent spontaneous mutagenesis in strains harboring plasmids expressing wild-type pol V or umuC_Y11A by assaying reversion of the hisG4 (ochre) allele. The data plotted on the graph represent the relative extent of spontaneous mutagenesis in cells expressing umuC_Y11A calculated as a percentage of the spontaneous mutagenesis in cells expressing wild-type pol V. All experiments were performed in triplicate. Standard errors were calculated taking into account variability in spontaneous mutagenesis in each strain. Data for the wild-type, ΔrnhA, ΔrnhB and the ΔrnhA ΔrnhB strains were taken from [11] and are shown for comparison. As clearly observed, in contrast to the ΔrnhA or ΔuvrA strains, which exhibit roughly the same extent of umuC_Y11A-dependent mutagenesis as the wild-type strain, the ΔrnhB strain exhibited significantly higher levels of spontaneous mutagenesis, suggesting that the rnhB-encoded RNase HII repair pathway is the primary defense against errant ribonucleotide incorporation in E.coli. However, in contrast to the rnhB+ strains, deletion of either rnhA or uvrA in the ΔrnhB strain background leads to a further increase in umuC_Y11A-dependent spontaneous mutagenesis, suggesting that both enzymes participate in back-up pathways of ribonucleotide repair. Furthermore, umuC_Y11A-dependent spontaneous mutagenesis in the ΔrnhA ΔrnhB ΔuvrA triple mutant strain is actually higher than in the isogenic strain expressing wild-type pol V. These data imply that all major pathways specific for ribonucleotide repair are blocked in this strain background. In summary, our in vivo studies do not indicate a significant role for MMR or BER in ribonucleotide repair in E.coli. In contrast, inactivation of RER via mutations in rnhB resulted in an increase in umuC_Y11A-dependent mutagenesis in ΔrnhA and NER-deficient (ΔuvrA, uvrB5 and ΔuvrC) strains, suggesting that RNase HI and the UvrABC proteins function in alternative ribonucleotide repair pathways in E.coli (Fig. 1).
In vitro reconstitution of NER targeted to rNMPs embedded in a dsDNA substrate
To our knowledge, our data present the first biological evidence for the participation of prokaryotic NER proteins in ribonucleotide repair. We therefore wanted to test the ability of the UvrABC complex to remove rNMPs from DNA directly. To do so, we performed an in vitro incision assay to determine whether the reconstituted NER complex has the capacity to remove ribonucleotides from double-stranded (ds) DNA. For these experiments, we chose to utilize highly purified Bacillus caldotenax UvrA and UvrB and Thermatoga maritima UvrC proteins. The UvrABC proteins from the thermophilic bacteria while having extremely high level of sequence similarity with the E. coli proteins are remarkably more stable [37]–[41]. Furthermore, previous studies have demonstrated that individual subunits of the NER complex from Gram-positive bacteria are able to efficiently substitute for the components of the E. coli nuclease in various in vitro excision reactions [37]–[39], [42]–[46]. We have shown earlier that the UmuC_Y11A polymerase readily extends primers by very efficient (mis)incorporation of ATP opposite a variety of different template bases [14]. Therefore, the double-stranded DNA oligonucleotide used as a substrate in the in vitro assays contained either a single, or two consecutive rAMPs. In addition, since UmuC_Y11A is also known to replicate DNA with very low base-substitution fidelity producing both transitions and transversions, in some of the substrates either one (3′), or both rAMPs, were mispaired with either cytosine or adenine on the complementary DNA strand. The UmuC_Y11A enzyme is also characterized by an extraordinary ability to synthesize long RNA strands within minutes of engaging the primer-terminus [14]. As a consequence, we also wanted to examine whether the UvrABC proteins are able to initiate repair of DNA containing multiple ribonucleotides and for this purpose, we utilized oligonucleotides with five sequential rNMPs.
In the current study, we used double-stranded 50-mer oligonucleotides with rNMPs embedded into a 5′ or 3′ end-labeled DNA strand, which allows us to monitor incisions at both sides of the modified base(s) (Fig. 4). Furthermore, the duplex oligonucleotides were modeled upon a nearly identical fluorescein adducted substrate, which has previously been shown to be a good substrate for the UvrABC endonuclease in vitro [39] (Fig. 4A, lanes 2,3) thereby allowing us to directly compare the efficiency of lesion-mediated incision to ribonucleotide-mediated incision.
Fig. 4. NER cleavage reaction products generated using various DNA-RNA-DNA hybrid substrates. 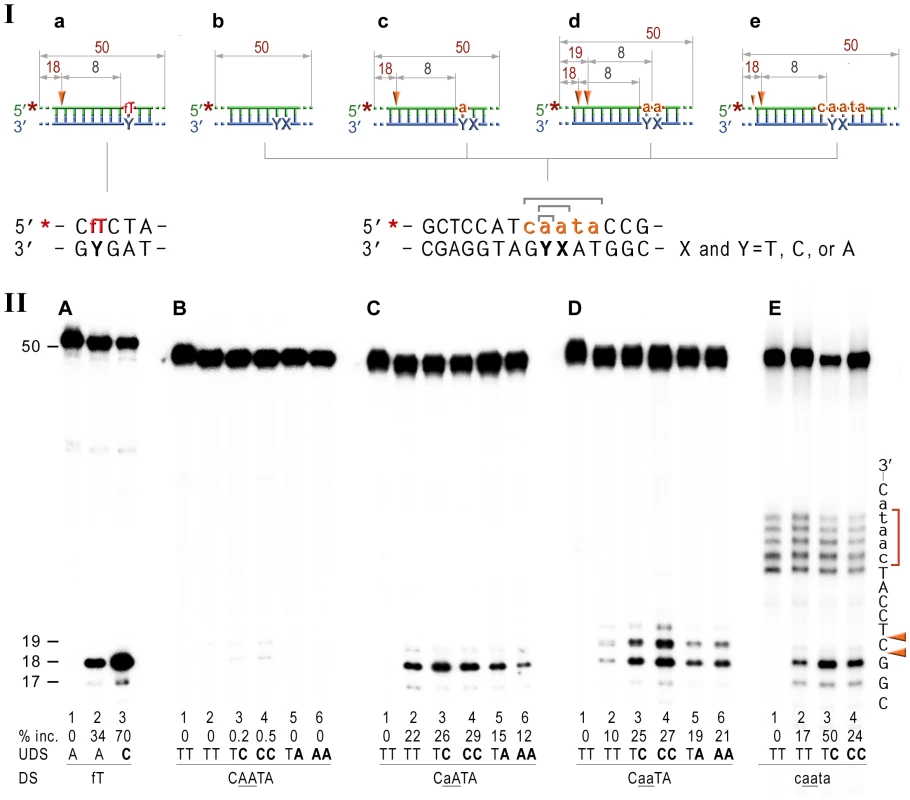
I; Cartoon of the synthetic substrates used in the in vitro assays, with the sites of incision and expected product size indicated, along with the DNA sequence containing rNMP(s) and mismatched nucleotides. II; The 50-mer duplexes (10 nM) in which the modified strand was 5′ end-labeled (indicated by *), were incubated with the NER proteins at concentrations of 40 nM (UvrA), 200 nM (UvrB), and 100 nM (UvrC) for 60 min at 55°C in the presence of 1 mM ATP. UvrABC-dependent incision on the fluorescein adducted 50-bp duplex (fT) was used as a control NER activity of the purified proteins (panel A). The DNA duplexes (panel B) as well as DNA-RNA-DNA hybrids either containing a single rAMP (panels C), two consecutive rAMPs (panel D), or five rNMPs (panels E) were assayed. The reaction products were separated under denaturing conditions by 15% polyacrylamide gel electrophoresis (PAGE). The efficiency of UvrABC-dependent incision was determined as a percentage of the radioactivity in the incised products relative to the total signal of the substrate (% inc.). The data below the gels are mean values calculated from at least two independent experiments. The DNA sequence containing rNMP(s) is shown alongside the gels where DNA and RNA are represented by uppercase and lowercase letters, respectively. Orange arrows indicate the cleavage sites. The red bracket indicates spontaneous ribonucleotide cleavage. UDS, refers to the undamaged strand, and DS, is damage/ribonucleotide-containing strand. The in vitro assays reveal that the NER proteins incise the DNA backbone 8 base pairs 5′ of ribonucleotides and that the reactions are stimulated by base mispairs. As expected, the undistorted templates remained intact (Fig. 4B, lane 2), while reactions with duplexes containing A:C and AA:CC mispairs yielded barely detectible bands corresponding to the incisions made at the 8th bond 5′ to the mispair (Fig. 4B, lanes 3,4). In contrast, A:A and AA:AA mismatches did not attract UvrABC mediated incisions (Fig. 4B, lanes 5,6). Our finding is in good agreement with previously published reports of low levels (0.03–0.5%) of mismatch repair by the bacterial and human NER proteins [47], [48]. Consistent with our in vivo observations for a role of NER in RER, incubation of the 5′-labeled rAMP-containing DNA with UvrA, UvrB, and UvrC proteins resulted in the robust oligonucleotide cleavage (Fig. 4C, lane 2), which was only minimally less efficient than cleavage of the DNA with a single fluorescein-adducted thymine (c.f. Fig. 4A vs. 4C, lane 2). As with the lesions-containing substrate, incision of the ribonucleotide-containing substrate was made at the 8th phosphodiester bond 5′ to the RNA–DNA junction producing an 18 bp product. Reactions with oligonucleotides having two sequential AMPs used as substrates for the UvrABC endonuclease yielded two bands, 18 - and 19-mers (Fig. 4D, lane 2) that correspond to incisions made at the 8th bond on the 5′ side of each ribonucleotide. Incision of the substrates with five rNMPs in a row mainly generated an 18 nucleotide fragment (corresponding to an incision 7 bases 5′ to the first rAMP), although a small amount of 17-mer, which is produced by the incisions of the 8th bond 5′ to the RNA/DNA junction, was also observed (Fig. 4E, lane 2). In contrast and as expected, no cleavage was observed when the complementary DNA strand lacking rNMPs was labeled (Fig. S1).
It has been reported that NER prefers compound lesions consisting of a base damage placed opposite one or more mispaired bases, over the correctly paired lesions (Fig. 4A, lane 3) [49]–[51]. Similarly, the efficiency with which the rNMP-containing template was incised by the UvrABC endonuclease, was also determined by the type of the mispaired base (Fig. 4, B–E). Mispairing of rA with dC potentiated incision of the substrates containing one, two, or five ribonucleotides (lane 3 on the Fig. 4C, D, and E). In contrast, formation of a dA:rA mispair inhibited removal of the nucleotide with an incorrect sugar (lane 5 on the Fig. 3B, C, and D). The number of mismatches did not affect the incision efficiency for the substrates with one or two rAMPs (lanes 4 and 6 in Fig. 4C and D), but in case of the longer ribonucleotide fragment, the presence of an additional mispair (Fig. 4E, lane 4) counteracted the stimulatory effect of a single mismatch (lane 3). Among all DNA/RNA hybrids tested, the greatest incision by UvrABC was observed on the substrate with a tract of five rNMPs and one C:rA mispair (Fig. 4E, lane 3). In contrast to the UvrABC reactions, RNase HII-catalyzed cleavage 5′ to the rAMP was not stimulated by a mispaired base, but was identical for all the substrates with one or two ribonucleotides embedded into ds DNA (Fig. 5).
Fig. 5. RNase HII cleavage reaction products generated using various DNA-RNA-DNA hybrid substrates. 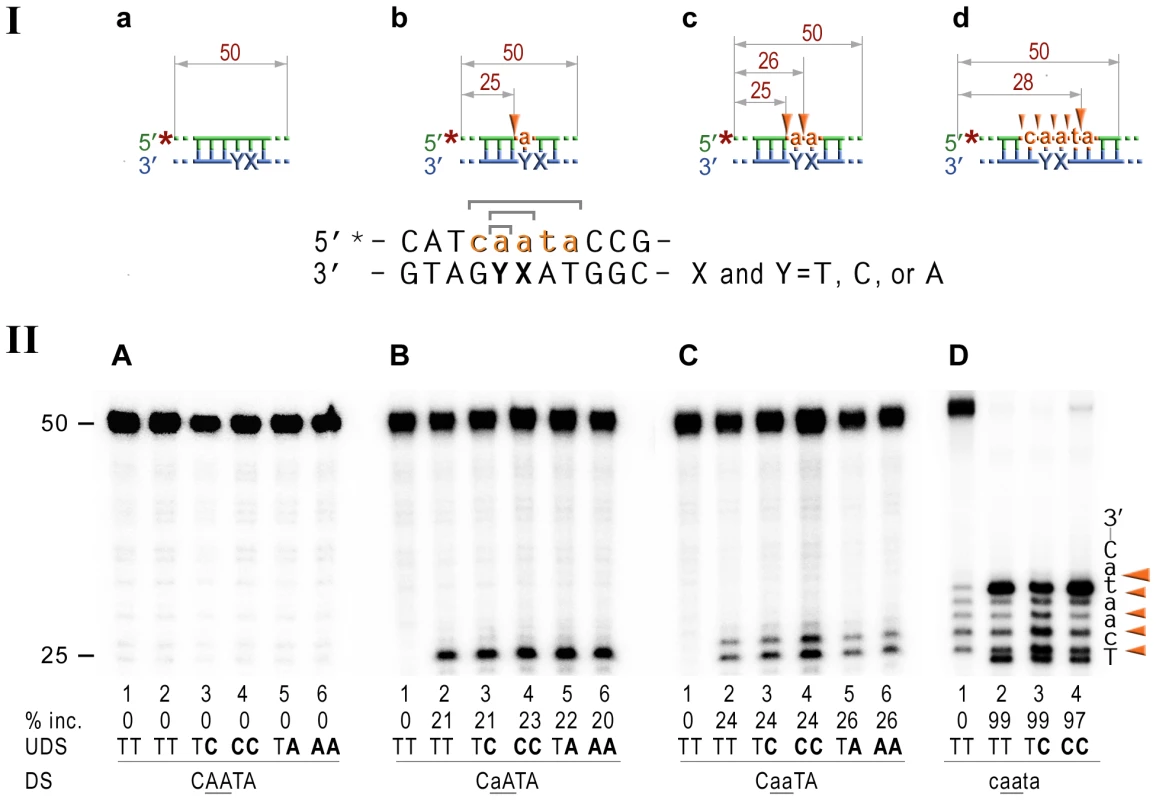
I; Cartoon of the synthetic substrates used in the in vitro assays, with the sites of incision and expected product size indicated, along with the DNA sequence containing rNMP(s) and mismatched nucleotides. II; The 50-mer duplexes (10 nM) in which the modified strand was 5′ end-labeled (indicated by *), were incubated with Rnase HII for 60 min at 37°C. The DNA duplexes (panel A) as well as DNA-RNA-DNA hybrids containing either single rAMP (panel B), two consecutive rAMPs (panel C), or five rNMPs (panel D) were assayed. The reaction products were separated under denaturing conditions by 15% polyacrylamide gel electrophoresis (PAGE). The efficiency of RNase HII-dependent incision was determined as a percentage of the radioactivity in the incised products relative to the total signal of the substrate (% inc.). The data below the gels are mean values calculated from at least two independent experiments. The DNA sequence containing rNMP(s) is shown alongside the gels where DNA and RNA are represented by uppercase and lowercase letters, respectively. Orange arrows indicate the cleavage sites. The in vitro assays confirm that E.coli RNase HII nicks the DNA backbone 5′ of ribonucleotides embedded in DNA and shows that the efficiency of the reaction is largely unaffected by base-mispairs. Analysis of the NER reactions using templates with 3′-labeled rNMP-containing strands indicated that the incision was made at the 5th phosphodiester bond 3′ to the RNA–DNA junction producing a 20 bp (Fig. 6A), or a 19 bp (Fig. 6B) product. In contrast to the 5′ incision activity, the efficiency of the cleavage 3′ to the rNMP(s) was mainly independent of the presence of base mismatches and type and number of mispaired bases, although templates with the rAs were cleaved somewhat more efficiently than templates containing a single rAMP (Fig. 6).
Fig. 6. NER cleavage reaction products generated using DNA templates with 3′ end-labeled rNMP-containing strands. 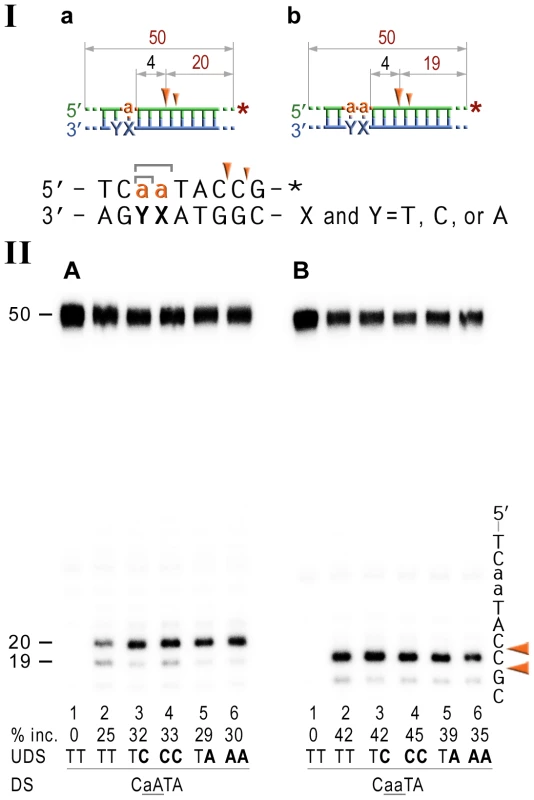
I; Cartoon of the synthetic substrates used in the in vitro assays, with the sites of incision and expected product size indicated, along with the DNA sequence containing rNMP(s) and mismatched nucleotides. II; The 50-mer duplexes (10 nM) in which the modified strand was 3′ end-labeled (indicated by *), were incubated with the NER proteins at concentrations of 40 nM (UvrA), 200 nM (UvrB), and 100 nM (UvrC) for 60 min at 55°C in the presence of 1 mM ATP. The DNA duplexes DNA-RNA-DNA hybrids either containing a single rAMP (panel A), two consecutive rAMPs (panel B) were assayed. The reaction products were separated under denaturing conditions by 15% polyacrylamide gel electrophoresis (PAGE). The efficiency of UvrABC-dependent incision was determined as a percentage of the radioactivity in the incised (% inc.) products relative to the total signal of the substrate. The data below the gels are mean values calculated from at least two independent experiments. The DNA sequence containing rNMP(s) is shown alongside the gels where DNA and RNA are represented by uppercase and lowercase letters, respectively. The full-sized DNA template (50-mer) and incision products of 19–20 bp are indicated. Orange arrows indicate the cleavage sites. The in vitro assays reveal that the NER proteins incise the DNA backbone 4–5 base pairs 3′ of ribonucleotides and that the efficiency of the reaction is largely unaffected by base-mispairs. Discussion
E. coli pol V belongs to the Y-family of DNA polymerases [52], most of which are involved in replication of damaged or distorted DNA [53]. In order to accommodate abnormal nucleotides, the active site of these polymerases is spacious and solvent-exposed [54]. As a consequence, the polymerases exhibit low-fidelity when replicating undamaged DNA and their up-regulation in cells often confers a mutator phenotype [21], [55], [56]. Pol V differs from other Y-family members in that along with low base-substitution fidelity, it is also characterized by exceptionally low sugar selectivity [14].
Even though the majority of DNA polymerases discriminate against nucleotides with a ribose moiety quite efficiently, rNMPs appear to be among the most abundant abnormalities in chromosomal DNA [5], [57]. Nevertheless, investigation of the cellular mechanisms directed at removal of ribonucleotides from DNA has only recently attracted considerable attention. The first line of defense comes from the innate structural features of DNA polymerases themselves. Active sites of most DNA polymerases contain a so-called “steric gate” residue that plays a major role in rNMP exclusion by colliding with the 2′-hydroxyl of an incoming ribonucleotide [58]. The steric gate residue of pol V is Y11 in the UmuC subunit of the polymerase and is among the least efficient barriers against ribonucleotide incorporation since wild-type pol V readily incorporates ribonucleotides into DNA [14]. Substitution of Y11 with a much smaller alanine residue takes sugar indiscretion of the variant polymerase to extremes, since when presented with both types of deoxy - and ribonucleotides, UmuC_Y11A often selects rNTPs during primer extension [14].
Biochemical characterization of UmuC_Y11A and wild-type pol V revealed that besides the differences in sugar selectivity, other properties of the polymerases are similar [14], [23]. Despite a virtually identical base-substitution fidelity in vitro, the mutability of the strains expressing umuC_Y11A is quite low compared to wild-type pol V [23]. The difference in spontaneous mutagenesis is explained by the extent of the accumulation of errant rNMPs into genomic DNA, and the subsequent actions of repair pathways directed at rNMP removal [11]. Although triggered by the presence of nucleotides with the wrong sugar, activation of these pathways also results in the removal of the deoxyribonucleotides base mispairs which happen to lie inside the rNMP repair “patch” [11]. The connection between rNMP repair and levels of spontaneous mutagenesis therefore provides a unique opportunity to elucidate various repair mechanisms aimed at sanitization of errantly incorporated NTPs from the E.coli genome. Thus, changes in the extent of umuC_Y11A-dependent spontaneous mutagenesis compared to wild-type pol V mutagenesis should identify pathways for rNMP removal. Indeed, using such an approach, we have recently demonstrated that the main pathway directed at rNMP excision involves the nicking action of RNase HII [11] and subsequent strand-displacement DNA synthesis by pol I (unpublished observations). In the present study, we have analyzed the contribution of mismatch repair (MMR), base-excision repair (BER) and nucleotide excision repair (NER) to ribonucleotide repair in E.coli.
Although umuC_Y11A is expected to frequently incorporate ribo-UMP into DNA, we found no evidence that deletion of ung, xth or nfo, all of which lead to defects in the uracil glycosylase-mediated BER pathway, has any effect on ribonucleotide repair in E.coli (Fig. 1). Such observations are, therefore, consistent with the limited ability of the uracil glycosylase to remove rU compared to dU in vitro [59]–[61]. Furthermore, given the fact that the level of umuC_Y11A-dependent mutagenesis observed when rnhA, rnhB and NER are all inactivated was even higher than with wild-type pol V (Fig. 3), it seems unlikely that another, as yet unidentified, BER enzyme(s) might contribute to RER, but it cannot be formally excluded.
When compared to the level of mutagenesis exhibited by wild type pol V, mismatch repair-deficient ΔmutL, ΔmutH and ΔmutS cells all exhibited higher levels of umuC_Y11A-dependent mutagenesis (Fig. 1). This was initially assumed to reflect the participation of MMR in the removal of ribonucleotides incorporated by umuC_Y11A, especially if the base was also mispaired [13]. However, there was no further increase in umuC_Y11A mutagenesis in the MMR− strains upon deletion of RNase HII (Fig. 2). Based on our recent finding revealing that the extent of mutagenesis in rnhB+ MMR-deficient strains is dependent upon pol I (unpublished observations), we hypothesize that the increase in mutagenesis observed in the rnhB+ umuC_Y11A MMR− strains actually reflects persisting transition errors which are made by pol I during RNase HII-initiated ribonucleotide repair and which otherwise would be subjected to repair in MMR+ cells (Fig. 2). Overall, our data suggest that even though MMR is able to remove mispaired ribonucleotides from DNA, it has a limited (if any), role in prokaryotic RER in vivo. Conversely, it has recently been shown that RNase HII-dependent RER plays a significant role in MMR in eukaryotes by providing the strand-discrimination signal that identifies the newly synthesized DNA [62], [63].
In contrast to MMR and BER, our present study strongly implicates NER as a backup mechanism for ribonucleotide repair in prokaryotes (Fig. 1). The in vivo data suggest that similar to the RNase HI-dependent pathway, NER is not a primary mechanism of rNMP repair in cells with a functional RNase HII-initiated RER pathway, but plays an important role in the absence of RNase HII. Furthermore, it appears that in ΔrnhB cells both the NER proteins and RNase HI are required for efficient ribonucleotide repair (Fig. 3). While RNase HI specializes in removal of longer RNA fragments, the UvrABC endonuclease is able to eliminate isolated rNMPs, as well as to compete with RNase HI for removal of several sequential ribonucleotides.
In general, it is assumed that NER is the major defense mechanism against bulky DNA adducts, although it is also known to repair relatively minor DNA modifications, such as apurinic sites (reviewed in [40], [41]) and we report here that even misincorporated ribonucleotides that only differ by a single 2′-OH from their deoxyribose counterparts, are also substrates for NER. UvrB/C dependent incisions are made 8 bp 5′ and 4–5 bp 3′ to the ribonucleotide generating a ribonucleotide containing fragment of ∼12–13 bases (Fig. 4 & 6), which is identical to that obtained with DNA damage-mediated NER in E.coli [40].
So how is the ribose moiety recognized as a “lesion”? Based on the analysis of the structure and conformation of the diverse set of DNA lesions that are repaired by the NER machinery, it has been suggested that UvrA2B complex is not targeted by nucleotide damage per se, but rather by damaged-induced conformational changes in DNA; the more the DNA helix deviates from the canonical B-form conformation, the more efficient the NER [40], [41]. This is supported by the fact that NER is much more efficient within the context of distorting base mispairs (e.g., Fig. 3A). Similarly, we show that the ribonucleotide excision activity of UvrABC endonuclease varies depending on the number of ribonucleotides incorporated into DNA, the presence of base mismatches, and the type and number of mispaired bases, suggesting that it the distortion of the DNA around the ribonucleotide that helps target it for NER (Fig. 4). However, we also observed significant incision of a single correctly-paired rNMP embedded in DNA, which is unlikely to cause a major conformational change in the local sequence surrounding the ribonucleotide [3]. Since the ribonucleotide moiety provides a negative electrostatic potential and offers new hydrogen bonding opportunities compared to the deoxynucleotide, it is possible that the local differences between an embedded ribo - vs. deoxynucleotide might lead to NER recognition. Clearly, the mechanisms underlying ribonucleotide recognition by the NER complex is a topic that should be investigated further.
In summary, we show here that a complex network of DNA repair mechanisms is involved in cleansing chromosomal DNA of misincorporated ribonucleotides (Fig. 7). RER initiated by RNase HII plays the leading role in removing isolated rNMPs. When this pathway is overloaded, or inactivated, prominent backup roles are assumed by RNase HI and NER proteins. In general, RNase HI facilitates the removal of stretches of ribonucleotides 4 bp or more in length, while NER can excise single and poly-ribonucleotides embedded in DNA. As a consequence, both RNase HI and NER proteins help to reduce genomic instability generated though errant ribonucleotide misincorporation.
Fig. 7. Various DNA repair pathways compete, cooperate, or substitute for each other in order to sanitize the E.coli chromosome from mispairs, uracils and incorporated rNMPs. 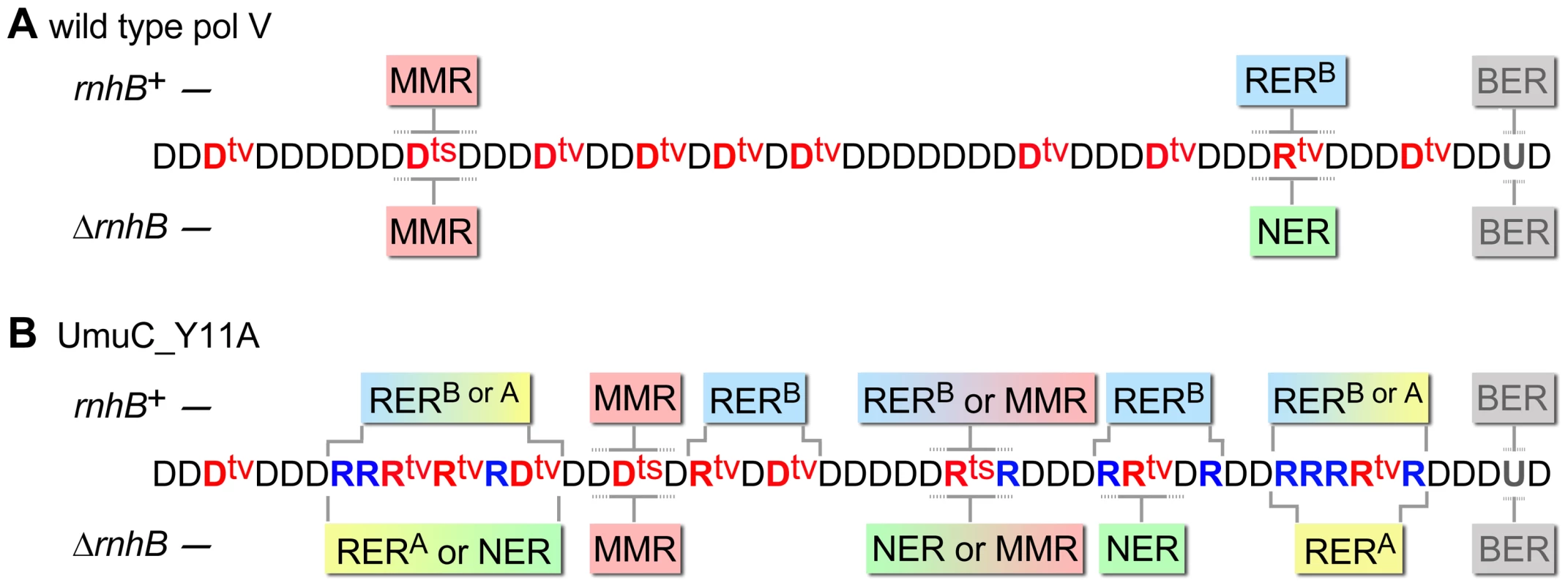
The cartoon helps to explain why spontaneous mutagenesis induced by wild-type pol V (A) differs from Y11A_UmuC-dependent mutagenesis (B) and illustrates the respective roles of MMR (red box), RERA (stands for RNase HI-initiated ribonucleotide excision repair and is indicated in yellow), RERB (RNase HII-initiated ribonucleotide excision repair, indicated in blue), NER (green), and BER (grey) (The competing pathways are indicated by boxes with gradient colors). Misincorporated nucleotides are shown in red (where ts are transitions, tv are transversions), correctly paired ribonucleotides are indicated in blue. For simplicity, all the transversions are shown refractory to MMR and NER while in reality they could be repaired by both pathways although less efficiently than transitions. Both wild-type and mutant pol V make frequent base-substitution errors. The transition mutations are rapidly repaired by MMR, while any errant ribonucleotides (correctly-paired or mispaired) are also efficiently removed by RERB. Ung-dependent BER only operates on dU, not rU, incorporated into the DNA and therefore has no role in RER. In contrast, NER is able to remove rNMPs misincorporated by either wild-type or mutant pol V. Since umuC_Y11A is able to incorporate multiple consecutive rNMPs into DNA, RER involving RNase HI is limited to strains expressing the pol V variant. RERB is normally required for highly efficient removal of errant ribonucleotides, however, in its absence the role of NER and RNase HI becomes apparent. The fact that the level of spontaneous mutagenesis in strains expressing umuC_Y11A with an “unlocked” sugar steric gate is 90% lower than mutagenesis in strains harboring wild-type pol V, implies that numerous errant ribonucleotides are very efficiently excised by the collaborative actions of rNMP-specific repair pathways which concomitantly remove mispaired dNMPs positioned within the repair patch (such as for example two Dtvs in the panel B). In the absence of RNase HI, RNase HII and NER proteins, the majority of the misincorporated rNMPs remains embedded in the chromosomal DNA. As a result, spontaneous mutagenesis in the ΔrnhA ΔrnhB ΔuvrA strain expressing umuC_Y11A is higher than in the isogenic strain expressing wild-type pol V. Materials and Methods
Bacterial strains
Most of the E. coli K-12 strains used in this study are derivatives of RW698 (full genotype: recA730 lexA51(Def) ΔdinB61::ble ΔumuDC596::ermGT thr-1 araD139 Δ(gpt-proA)62 lacY1 tsx-33 glnV44 galK2 hisG4 rpsL31 xyl-5 mtl-1 argE3 thi-1 sulA211) [11]. Repair-deficient “KEIO” strains were obtained from the E.coli Genetic Stock Center and isogenic derivatives of RW698 were generated via generalized transduction using P1vir [64] (Table 1). Where noted, KanS strains were obtained by transforming cells with the temperature sensitive ampicillin and chloramphenicol resistant plasmid, pCP20, which expresses the FLP recombinase [65]. Transformants were selected on LB plates containing the appropriate antibiotics at 25°C and subsequently re-streaked on LB plates lacking ampicillin, chloramphenicol and kanamycin and incubated overnight at 43°C. Colonies from these plates were subsequently confirmed to be ampicillin, chloramphenicol and kanamycin sensitive at 37°C.
Tab. 1. E.coli strains used in this study. 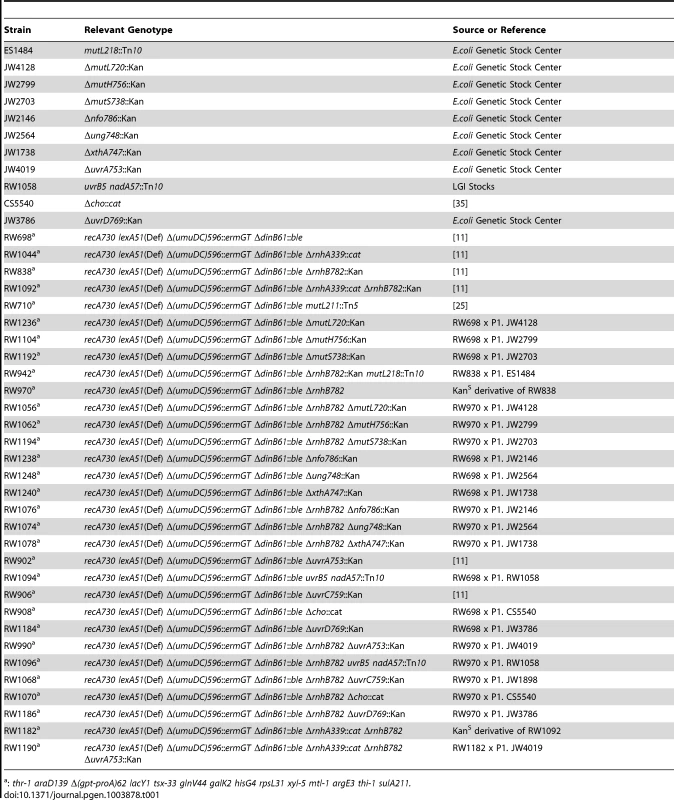
: thr-1 araD139 Δ(gpt-proA)62 lacY1 tsx-33 glnV44 galK2 hisG4 rpsL31 xyl-5 mtl-1 argE3 thi-1 sulA211. The following antibiotics were used for selection; Zeocin (25 µg/ml), Kanamycin (50 µg/ml), Tetracycline (15 µg/ml), Chloramphenicol (20 µg/ml), and Ampicillin (100 µg/ml), Spectinomycin (50 µg/ml).
Plasmids
The low-copy-number spectinomycin resistant plasmids pRW134 and pJM963 which encode E.coli wild-type UmuC and the umuC_Y11A variant, respectively, along with UmuD′ [23] are derived from pGB2 [66] and express the UmuD′C proteins at close to physiological levels from their native promoter [23]. Bacteria harboring plasmids were grown in LB media containing appropriate 50 µg/ml spectinomycin.
Quantitative spontaneous mutagenesis assay
Cells transformed with the vector plasmid, pGB2, or the low-copy number plasmid pRW134 expressing wild-type pol V, or pJM963 expressing the umuC_Y11A variant [23] were grown overnight at 37°C in LB media plus appropriate antibiotics. The next day, cultures were centrifuged and resuspended in an equal volume of SM buffer [64]. To determine the number of spontaneously arising histidine mutants on the plate, the cell cultures were seeded on the Davis and Mingioli minimal agar plates [67] plus glucose (0.4% wt/vol); agar (1.0% wt/vol); proline, threonine, valine, leucine, and isoleucine (all at 100 µg/ml); thiamine (0.25 µg/ml); and either no histidine, or histidine (1 µg/ml). On the plates containing no histidine, only pre-existing His+ mutants grew to form colonies. When ∼4×107 bacteria were seeded on the 1 µg/ml histidine, they grew to form a lawn, concomitantly exhausting the low level of histidine. Spontaneously arising His+ mutants grew up through the lawn and were counted after 4 days incubation at 37°C. Spontaneous mutagenesis is expressed as a frequency (mutants per plate), because the number of mutants arising on the plate is independent of the number of cells plated, but is, instead, dependent upon the limiting amount of nutrient (histidine) in the plate [68]. The relative extent of umuC_Y11A mutagenesis was calculated by first subtracting the number of pre-existing His+ mutants (mutants arising on the plates lacking histidine) and subsequently dividing the number of spontaneously arising mutants on the umuC_Y11A (pJM963) plates by the number of spontaneously arising mutants on the wild-type pol V (pRW134) plates. The data reported in Figs. 1 and 3 represent the average number of His+ mutants from at least 3 separate experiments (± standard error of the mean [SEM]).
Spectra of spontaneous base-pair substitutions in the E.coli rpoB gene
The mutation spectra were generated using the rpoB/RifR mutagenesis assay [69], [70]. A single pair of oligonucleotide primers can be used for PCR amplification and a single primer for DNA sequencing because 88% of all rpoB mutations are localized in the central 202 bp region of the gene [69]. E. coli strains RW710 [relevant genotype: lexA(Def) recA730 ΔdinB ΔumuDC ΔmutL], RW942 [relevant genotype: lexA(Def) recA730 ΔdinB ΔumuDC ΔmutL ΔrnhB ], RW698 [relevant genotype: lexA(Def) recA730 ΔdinB ΔumuDC], and RW838 [relevant genotype: lexA(Def) recA730 ΔdinB ΔumuDC ΔrnhB ], harboring the umuC_Y11A plasmid, pJM963 were diluted from a frozen stock cultures such that the initial inoculum contained <1000 viable cells. Cultures were grown in LB for 24 h at 37°C and appropriate dilutions spread on an LB agar plate containing 100 µg/ml rifampicin. Individual independent RifR colonies were picked from the plate using a pipette tip and subjected to PCR in a 96-well micro-titer plate. An <1 kb central region of the rpoB gene was amplified using the PCR primers RpoB1 : 5′-CAC ACG GCA TCT GGT TGA TAC AG-3′ and RpoF1 : 5′-TGG CGA AAT GGC GGA AAA C-3 by denaturation at 95°C for 3 min, followed by 30 cycles of 94°C for 30 s, 1 min at 59°C, 2 min at 72°C, followed by a final extension step at 72°C for 7 min. The nucleotide sequence of the ∼200 bp target region of rpoB in each PCR amplicon was determined by Beckman Coulter Genomics (Danvers, MA) using WOG923AP01 primer (5′-CAG TTC CGC GTT GGC CTG-3′). Only base-pair substitutions occurring between positions 1516 and 1717 of the rpoB gene were considered during data analysis. Nucleotide sequences obtained were aligned and analyzed using the ClustalW multiple sequence alignment program (Hinxton, UK). Rates for forward mutations to rifampicin resistance (mutations in rpoB) were determined as previously described [25].
Excision of various DNA substrates by the UvrABC complex
The Bacillus caldotenax UvrA and UvrB proteins and the Thermatoga maritima UvrC protein were purified as previously described [39], [71]. E. coli RNase HII was purchased from New England Biolabs (Ipswich, MA, USA).
All oligonucleotides were synthesized by Lofstrand Laboratories (Gaithersburg, MD) and gel purified prior to use. The basic sequence of the 50-mer template is: 5′-GAC TAC GTA CTG TTA CGG CTC CAT caa taC CGC AAT CAG GCC AGA TCT GC-3′. The lowercase letters indicate sites at which substrate dNMPs were replaced by rNMPs. Substrates with a single rAMP (first underlined a on the 5′ side), two consecutive rAMPs (shown in bold), or five rNMPs were tested. A substrate with the site-specifically placed fluorescein adduct (fT) [39] was used as a control for the activity of NER proteins and has the same sequence except that the aa bases (shown in bold) were replaced with the [fT]C sites. The fT-containing oligonucleotide and the DNA-RNA-DNA hybrids were 5′ - or 3′-32P end-labeled and annealed with either completely complementary DNA strands, or with the DNA strands containing one or two mispaired bases. In the case of the fluorescein-adducted template, the damaged T was either correctly paired with A, or mispaired with C. In the case of the DNA-RNA-DNA hybrids, either the 5′A, or both As shown in bold were correctly paired with Ts or mispaired with one, or two, Cs or As. Hybridization was performed at a 1.5 molar excess of the unlabeled strand by heating in an annealing buffer (50 mM Tris-HCl (pH 8), 5 mM MgCl2, 50 µg/ml BSA, 1.42 mM 2-mercaptoethanol) for 10 min at 100°C followed by slow cooling to room temperature. Prior to initiation of the incision assay, the UvrABC proteins were diluted from stock solutions and preheated for 10 min at 55°C. The 10 nM DNA substrates were incubated with UvrA (40 nM), UvrB (200 nM), and UvrC (100 nM) proteins for 1 hour at 55°C in the presence of 1 mM ATP in a 1× reaction buffer (10 mM Tris, pH 7.5, 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.2 mM ATP). Cleavage of the 10 nM DNA substrates by RNase HII was performed according to the manufacturer's instructions. Reactions were terminated by the addition of 2× loading buffer (97% formamide, 10 mM EDTA, 0.1% xylene cyanol, 0.1% bromophenol blue) and the incision products were analyzed on a 15% denaturing polyacrylamide gel. The extent of incision was determined for each substrate and expressed as a percentage of radioactivity in the cleaved products relative to the total signal. Data shown below the gels are the mean values calculated from at least two independent experiments.
Supporting Information
Zdroje
1. LindahlT (1993) Instability and decay of the primary structure of DNA. Nature 362 : 709–715.
2. WahlMC, SundaralingamM (2000) B-form to A-form conversion by a 3′-terminal ribose: crystal structure of the chimera d(CCACTAGTG)r(G). Nucleic Acids Res 28 : 4356–4363.
3. DeRoseEF, PereraL, MurrayMS, KunkelTA, LondonRE (2012) Solution structure of the Dickerson DNA dodecamer containing a single ribonucleotide. Biochemistry 51 : 2407–2416.
4. Nick McElhinnySA, KumarD, ClarkAB, WattDL, WattsBE, et al. (2010) Genome instability due to ribonucleotide incorporation into DNA. Nat Chem Biol 6 : 774–781.
5. ReijnsMA, RabeB, RigbyRE, MillP, AstellKR, et al. (2012) Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell 149 : 1008–1022.
6. LazzaroF, NovarinaD, AmaraF, WattDL, StoneJE, et al. (2012) RNase H and postreplication repair protect cells from ribonucleotides incorporated in DNA. Mol Cell 45 : 99–110.
7. HillerB, AchleitnerM, GlageS, NaumannR, BehrendtR, et al. (2012) Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J Exp Med 209 : 1419–1426.
8. ZhengL, ShenB (2011) Okazaki fragment maturation: nucleases take centre stage. J Mol Cell Biol 3 : 23–30.
9. RydbergB, GameJ (2002) Excision of misincorporated ribonucleotides in DNA by RNase H (type 2) and FEN-1 in cell-free extracts. Proc Natl Acad Sci U S A 99 : 16654–16659.
10. SparksJL, ChonH, CerritelliSM, KunkelTA, JohanssonE, et al. (2012) RNase H2-Initiated Ribonucleotide Excision Repair. Mol Cell 47 : 980–986.
11. McDonaldJP, VaismanA, KubanW, GoodmanMF, WoodgateR (2012) Mechanisms employed by Escherichia coli to prevent ribonucleotide incorporation into genomic DNA by pol V. PLoS Genet 8: e1003030.
12. KimN, HuangSN, WilliamsJS, LiYC, ClarkAB, et al. (2011) Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332 : 1561–1564.
13. ShenY, KohKD, WeissB, StoriciF (2012) Mispaired rNMPs in DNA are mutagenic and are targets of mismatch repair and RNases H. Nat Struc Mol Biol 19 : 98–104.
14. VaismanA, KubanW, McDonaldJP, KarataK, YangW, et al. (2012) Critical amino acids in Escherichia coli responsible for sugar discrimination and base-substitution fidelity. Nucleic Acids Res 40 : 6144–6157.
15. TangM, ShenX, FrankEG, O'DonnellM, WoodgateR, et al. (1999) UmuD′2C is an error-prone DNA polymerase, Escherichia coli, DNA pol V. Proc Natl Acad Sci U S A 96 : 8919–8924.
16. JiangQ, KarataK, WoodgateR, CoxMM, GoodmanMF (2009) The active form of DNA polymerase V is UmuD′2C-RecA-ATP. Nature 460 : 359–363.
17. ShinagawaH, IwasakiH, KatoT, NakataA (1988) RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A 85 : 1806–1810.
18. WoodgateR, EnnisDG (1991) Levels of chromosomally encoded Umu proteins and requirements for in vivo UmuD cleavage. Mol Gen Genet 229 : 10–16.
19. FrankEG, GonzalezM, EnnisDG, LevineAS, WoodgateR (1996) In vivo stability of the Umu mutagenesis proteins: a major role for RecA. J Bacteriol 178 : 3550–3556.
20. FrankEG, EnnisDG, GonzalezM, LevineAS, WoodgateR (1996) Regulation of SOS mutagenesis by proteolysis. Proc Natl Acad Sci U S A 93 : 10291–10296.
21. SweasyJB, WitkinEM, SinhaN, Roegner-ManiscalcoV (1990) RecA protein of Escherichia coli has a third essential role in SOS mutator activity. J Bacteriol 172 : 3030–3036.
22. FijalkowskaIJ, DunnRL, SchaaperRM (1997) Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J Bacteriol 179 : 7435–7445.
23. KubanW, VaismanA, McDonaldJP, KarataK, YangW, et al. (2012) Escherichia coli UmuC active site mutants: effects on translesion DNA synthesis, mutagenesis and cell survival. DNA Repair 11 : 726–732.
24. Watanabe-AkanumaM, WoodgateR, OhtaT (1997) Enhanced generation of A:T->T:A transversions in a recA730 lexA51(Def) mutant of Escherichia coli. Mutat Res 373 : 61–66.
25. CurtiE, McDonaldJP, MeadS, WoodgateR (2009) DNA polymerase switching: effects on spontaneous mutagenesis in Escherichia coli. Mol Microbiol 71 : 315–331.
26. KouzminovaEA, KuzminovA (2008) Patterns of chromosomal fragmentation due to uracil-DNA incorporation reveal a novel mechanism of replication-dependent double-stranded breaks. Mol Microbiol 68 : 202–215.
27. KowYW, WallaceSS (1985) Exonuclease III recognizes urea residues in oxidized DNA. Proc Natl Acad Sci U S A 82 : 8354–8358.
28. CunninghamRP, SaporitoSM, SpitzerSG, WeissB (1986) Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol 168 : 1120–1127.
29. SandigurskyM, FreyerGA, FranklinWA (1998) The post-incision steps of the DNA base excision repair pathway in Escherichia coli: studies with a closed circular DNA substrate containing a single U:G base pair. Nucleic Acids Res 26 : 1282–1287.
30. SandersonRJ, BennettSE, SungJS, MosbaughDW (2001) Uracil-initiated base excision DNA repair synthesis fidelity in human colon adenocarcinoma LoVo and Escherichia coli cell extracts. Prog Nuc Acid Res Mol Biol 68 : 165–188.
31. HouEW, PrasadR, AsagoshiK, MasaokaA, WilsonSH (2007) Comparative assessment of plasmid and oligonucleotide DNA substrates in measurement of in vitro base excision repair activity. Nucleic Acids Res 35: e112.
32. Fernández de HenestrosaAR, OgiT, AoyagiS, ChafinD, HayesJJ, et al. (2000) Identification of additional genes belonging to the LexA-regulon in Escherichia coli. Mol Microbiol 35 : 1560–1572.
33. LuAL, ClarkS, ModrichP (1983) Methyl-directed repair of DNA base-pair mismatches in vitro. Proc Natl Acad Sci U S A 80 : 4639–4643.
34. YamaguchiM, DaoV, ModrichP (1998) MutS and MutL activate DNA helicase II in a mismatch-dependent manner. J Biol Chem 273 : 9197–9201.
35. MoolenaarGF, van Rossum-FikkertS, van KesterenM, GoosenN (2002) Cho, a second endonuclease involved in Escherichia coli nucleotide excision repair. Proc Natl Acad Sci U S A 99 : 1467–1472.
36. CrowleyDJ, HanawaltPC (2001) The SOS-dependent upregulation of uvrD is not required for efficient nucleotide excision repair of ultraviolet light induced DNA photoproducts in Escherichia coli. Mutat Res 485 : 319–329.
37. JiangG, SkorvagaM, Van HoutenB, StatesJC (2003) Reduced sulfhydryls maintain specific incision of BPDE-DNA adducts by recombinant thermoresistant Bacillus caldotenax UvrABC endonuclease. Prot Exp Pur 31 : 88–98.
38. SkorvagaM, TheisK, MandavilliBS, KiskerC, Van HoutenB (2002) The β-hairpin motif of UvrB is essential for DNA binding, damage processing, and UvrC-mediated incisions. J Biol Chem 277 : 1553–1559.
39. CroteauDL, DellaVecchiaMJ, WangH, BienstockRJ, MeltonMA, et al. (2006) The C-terminal zinc finger of UvrA does not bind DNA directly but regulates damage-specific DNA binding. J Biol Chem 281 : 26370–26381.
40. Van HoutenB (1990) Nucleotide excision repair in Escherichia coli. Microbiol Rev 54 : 18–51.
41. TruglioJJ, CroteauDL, Van HoutenB, KiskerC (2006) Prokaryotic nucleotide excision repair: the UvrABC system. Chem Rev 106 : 233–252.
42. LinJJ, SancarA (1990) Reconstitution of nucleotide excision nuclease with UvrA and UvrB proteins from Escherichia coli and UvrC protein from Bacillus subtilis. J Biol Chem 265 : 21337–21341.
43. RuanQ, LiuT, KolbanovskiyA, LiuY, RenJ, et al. (2007) Sequence context - and temperature-dependent nucleotide excision repair of a benzo[a]pyrene diol epoxide-guanine DNA adduct catalyzed by thermophilic UvrABC proteins. Biochemistry 46 : 7006–7015.
44. ChristensenLA, WangH, Van HoutenB, VasquezKM (2008) Efficient processing of TFO-directed psoralen DNA interstrand crosslinks by the UvrABC nuclease. Nucleic Acids Res 36 : 7136–7145.
45. NakanoT, KatafuchiA, ShimizuR, TeratoH, SuzukiT, et al. (2005) Repair activity of base and nucleotide excision repair enzymes for guanine lesions induced by nitrosative stress. Nucleic Acids Res 33 : 2181–2191.
46. CroteauDL, DellaVecchiaMJ, PereraL, Van HoutenB (2008) Cooperative damage recognition by UvrA and UvrB: identification of UvrA residues that mediate DNA binding. DNA Repair 7 : 392–404.
47. HuangJC, HsuDS, KazantsevA, SancarA (1994) Substrate spectrum of human excinuclease: repair of abasic sites, methylated bases, mismatches, and bulky adducts. Proc Natl Acad Sci U S A 91 : 12213–12217.
48. BranumME, ReardonJT, SancarA (2001) DNA repair excision nuclease attacks undamaged DNA. A potential source of spontaneous mutations. J Biol Chem 276 : 25421–25426.
49. MoggsJG, SzymkowskiDE, YamadaM, KarranP, WoodRD (1997) Differential human nucleotide excision repair of paired and mispaired cisplatin-DNA adducts. Nucleic Acids Res 25 : 480–491.
50. SugasawaK, OkamotoT, ShimizuY, MasutaniC, IwaiS, et al. (2001) A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes & Dev 15 : 507–521.
51. MuD, TursunM, DuckettDR, DrummondJT, ModrichP, et al. (1997) Recognition and repair of compound DNA lesions (base damage and mismatch) by human mismatch repair and excision repair systems. Mol Cell Biol 17 : 760–769.
52. OhmoriH, FriedbergEC, FuchsRPP, GoodmanMF, HanaokaF, et al. (2001) The Y-family of DNA polymerases. Mol Cell 8 : 7–8.
53. SaleJE, LehmannAR, WoodgateR (2012) Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol 13 : 142–152.
54. YangW, WoodgateR (2007) What a difference a decade makes: insights into translesion DNA synthesis. Proc Natl Acad Sci U S A 104 : 15591–15598.
55. AlbertellaMR, LauA, O'ConnorMJ (2005) The overexpression of specialized DNA polymerases in cancer. DNA Repair 4 : 583–593.
56. YangJ, ChenZ, LiuY, HickeyRJ, MalkasLH (2004) Altered DNA polymerase ι expression in breast cancer cells leads to a reduction in DNA replication fidelity and a higher rate of mutagenesis. Cancer Res 64 : 5597–5607.
57. Nick McElhinnySA, WattsBE, KumarD, WattDL, LundstromEB, et al. (2010) Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc Natl Acad Sci U S A 107 : 4949–4954.
58. BrownJA, SuoZ (2011) Unlocking the sugar “steric gate” of DNA polymerases. Biochemistry 50 : 1135–1142.
59. ConeR, DuncanJ, HamiltonL, FriedbergEC (1977) Partial purification and characterization of a uracil DNA N-glycosidase from Bacillus subtilis. Biochemistry 16 : 3194–3201.
60. LindahlT, LjungquistS, SiegertW, NybergB, SperensB (1977) DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem 252 : 3286–3294.
61. KrokanH, WittwerCU (1981) Uracil DNA-glycosylase from HeLa cells: general properties, substrate specificity and effect of uracil analogs. Nucleic Acids Res 9 : 2599–2613.
62. LujanSA, WilliamsJS, ClausenAR, ClarkAB, KunkelTA (2013) Ribonucleotides are signals for mismatch repair of leading-strand replication errors. Mol Cell 50 : 437–443.
63. GhodgaonkarMM, LazzaroF, Olivera-PimentelM, Artola-BoranM, CejkaP, et al. (2013) Ribonucleotides misincorporated into DNA act as strand-discrimination signals in eukaryotic mismatch repair. Mol Cell 50 : 323–332.
64. Miller JH (1992) A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press.
65. BabaT, AraT, HasegawaM, TakaiY, OkumuraY, et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 : 2006.0008.
66. ChurchwardG, BelinD, NagamineY (1984) A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene 31 : 165–171.
67. DavisBD, MingioliES (1950) Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol 60 : 17–28.
68. MaronDM, AmesBN (1983) Revised methods for the Salmonella mutagenicity test. Mutat Res 113 : 173–215.
69. GaribyanL, HuangT, KimM, WolffE, NguyenA, et al. (2003) Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair 2 : 593–608.
70. WolffE, KimM, HuK, YangH, MillerJH (2004) Polymerases leave fingerprints: analysis of the mutational spectrum in Escherichia coli rpoB to assess the role of polymerase IV in spontaneous mutation. J Bacteriol 186 : 2900–2905.
71. WangH, DellaVecchiaMJ, SkorvagaM, CroteauDL, ErieDA, et al. (2006) UvrB domain 4, an autoinhibitory gate for regulation of DNA binding and ATPase activity. J Biol Chem 281 : 15227–15237.
Štítky
Genetika Reprodukční medicína
Článek Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis inČlánek Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 11
-
Všechny články tohoto čísla
- Molecular Recognition by a Polymorphic Cell Surface Receptor Governs Cooperative Behaviors in Bacteria
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis in
- Retrotransposon Silencing During Embryogenesis: Cuts in LINE
- Roles of XRCC2, RAD51B and RAD51D in RAD51-Independent SSA Recombination
- Parallel Evolution of Chordate Regulatory Code for Development
- A Genetic Approach to the Recruitment of PRC2 at the Locus
- Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
- Elevated Mutagenesis Does Not Explain the Increased Frequency of Antibiotic Resistant Mutants in Starved Aging Colonies
- Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
- Interplay between Active Chromatin Marks and RNA-Directed DNA Methylation in
- Recombinogenic Conditions Influence Partner Choice in Spontaneous Mitotic Recombination
- Crosstalk between NSL Histone Acetyltransferase and MLL/SET Complexes: NSL Complex Functions in Promoting Histone H3K4 Di-Methylation Activity by MLL/SET Complexes
- A New Role for the GARP Complex in MicroRNA-Mediated Gene Regulation
- RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Loss of DNMT1o Disrupts Imprinted X Chromosome Inactivation and Accentuates Placental Defects in Females
- Inhibition of the Smc5/6 Complex during Meiosis Perturbs Joint Molecule Formation and Resolution without Significantly Changing Crossover or Non-crossover Levels
- Disruption of Lipid Metabolism Genes Causes Tissue Overgrowth Associated with Altered Developmental Signaling
- Translation Initiation Factors eIF3 and HCR1 Control Translation Termination and Stop Codon Read-Through in Yeast Cells
- Recruitment of TREX to the Transcription Machinery by Its Direct Binding to the Phospho-CTD of RNA Polymerase II
- MYB97, MYB101 and MYB120 Function as Male Factors That Control Pollen Tube-Synergid Interaction in Fertilization
- Oct4 Is Required ∼E7.5 for Proliferation in the Primitive Streak
- Contrasted Patterns of Crossover and Non-crossover at Meiotic Recombination Hotspots
- Transposable Prophage Mu Is Organized as a Stable Chromosomal Domain of
- Ash1l Methylates Lys36 of Histone H3 Independently of Transcriptional Elongation to Counteract Polycomb Silencing
- Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects
- Genomic Mechanisms Accounting for the Adaptation to Parasitism in Nematode-Trapping Fungi
- Decoding a Signature-Based Model of Transcription Cofactor Recruitment Dictated by Cardinal Cis-Regulatory Elements in Proximal Promoter Regions
- Removal of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
- Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
- Activin Signaling Targeted by Insulin/dFOXO Regulates Aging and Muscle Proteostasis in
- Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans
- Demographic Divergence History of Pied Flycatcher and Collared Flycatcher Inferred from Whole-Genome Re-sequencing Data
- Recurrent Tissue-Specific mtDNA Mutations Are Common in Humans
- The Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
- The Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
- Reconstructing the Population Genetic History of the Caribbean
- and Are Required for Growth under Iron-Limiting Conditions
- Whole Genome, Whole Population Sequencing Reveals That Loss of Signaling Networks Is the Major Adaptive Strategy in a Constant Environment
- Neuron-Specific Feeding RNAi in and Its Use in a Screen for Essential Genes Required for GABA Neuron Function
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
- Mouse BAZ1A (ACF1) Is Dispensable for Double-Strand Break Repair but Is Essential for Averting Improper Gene Expression during Spermatogenesis
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- DUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
- Pathways-Driven Sparse Regression Identifies Pathways and Genes Associated with High-Density Lipoprotein Cholesterol in Two Asian Cohorts
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- and Are Required for Growth under Iron-Limiting Conditions
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



