-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
Many tissues are sustained by adult stem cells, which replace lost cells by differentiation and maintain their own population through self-renewal. The mechanisms through which adult stem cells maintain their identity are thus important for tissue homeostasis and repair throughout life. Here, we show that a histone variant, His2Av, is required cell autonomously for maintenance of germline and cyst stem cells in the Drosophila testis. The ATP-dependent chromatin-remodeling factor Domino is also required in this tissue for adult stem cell maintenance possibly by regulating the incorporation of His2Av into chromatin. Interestingly, although expression of His2Av was ubiquitous, its function was dispensable for germline and cyst cell differentiation, suggesting a specific role for this non-canonical histone in maintaining the stem cell state in these lineages.
Published in the journal: . PLoS Genet 9(11): e32767. doi:10.1371/journal.pgen.1003903
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003903Summary
Many tissues are sustained by adult stem cells, which replace lost cells by differentiation and maintain their own population through self-renewal. The mechanisms through which adult stem cells maintain their identity are thus important for tissue homeostasis and repair throughout life. Here, we show that a histone variant, His2Av, is required cell autonomously for maintenance of germline and cyst stem cells in the Drosophila testis. The ATP-dependent chromatin-remodeling factor Domino is also required in this tissue for adult stem cell maintenance possibly by regulating the incorporation of His2Av into chromatin. Interestingly, although expression of His2Av was ubiquitous, its function was dispensable for germline and cyst cell differentiation, suggesting a specific role for this non-canonical histone in maintaining the stem cell state in these lineages.
Introduction
Many adult tissues with short-lived, highly differentiated cells such as blood and skin replace cells lost to turnover through the proliferation and differentiation of adult stem cells. Adult stem cells must also self-renew to maintain a source of differentiating cells in the long term. The mechanisms that control the balance between self-renewal and differentiation need to be tightly regulated to maintain homeostasis of adult tissues. Although recent work has focused on signals from the local microenvironment of the stem cell niche, responses to these signals take place in the context of cell autonomous properties of the stem cell state that influence the ability of adult stem cells to maintain their identity. Likely candidates for such cell autonomous properties include the state of chromatin at key regulatory genes that influence stem cell maintenance.
The basic unit of eukaryotic chromatin, the nucleosome, is formed by DNA wrapped around an octamer containing two copies each of histones H2A, H2B, H3, and H4. Access to DNA by transcription factors and RNA polymerase is achieved by factors that control the post-translational modifications of core histones [1] and/or remodel nucleosomes [2]. The replacement of canonical histones with histone variants has recently emerged as an additional mechanism regulating chromatin accessibility [3]. Variants of the canonical histone H2A are highly conserved across species and play roles in transcriptional control, formation of heterochromatin boundaries, lineage commitment, and DNA repair. In yeast and mammals, H2AX is involved in recruiting factors to the sites of DNA damage [4] and H2A.Z is implicated in transcriptional regulation [5], [6]. In Drosophila, His2Av, the only known variant of H2A, assumes functions of both H2AX and H2A.Z [7]. Drosophila His2Av and His2A share 55% of their amino acid sequences, with the C-terminal region of His2Av considerably longer than that of His2A [8].
Here, we show that the histone variant His2Av is required cell autonomously for the maintenance of two adult stem cell populations in the Drosophila testis. The stem cell-niche microenvironment at the apical tip of the Drosophila testis consists of the germline stem cells (GSCs), which give rise to sperm [9]; the cyst stem cells (CySCs), which give rise to the cyst cells that enclose germ cells as they differentiate [10], [11]; and the post-mitotic somatic hub cells, to which GSCs and CySCs attach [12], [13]. His2Av function is required for both GSC and CySC maintenance; however, its function was dispensable for the differentiation program in the germ and cyst cell lineages. Our results suggest that in the absence of DNA damaging agents, the transcriptional role of His2Av may be required to regulate the delicate balance between self-renewal and differentiation states in adult stem cells.
Results
His2Av is required cell autonomously for GSC maintenance
Immunostaining of wild-type adult testes revealed His2Av protein expression in many cell types in the adult testis of Drosophila. At the apical tip of the testis, His2Av localized to the nuclei of somatic cells of the hub, GSCs (Fig. 1A) and CySCs (Fig. 1C). In differentiating spermatocytes, His2Av was concentrated on the autosomal and sex bivalent chromosomes within the nucleus (Fig. 1B, inset). His2Av also localized to the nuclei of differentiating somatic cyst cells associated with spermatocyte cysts (Fig. 1D).
Fig. 1. His2Av is required cell autonomously for GSC maintenance. 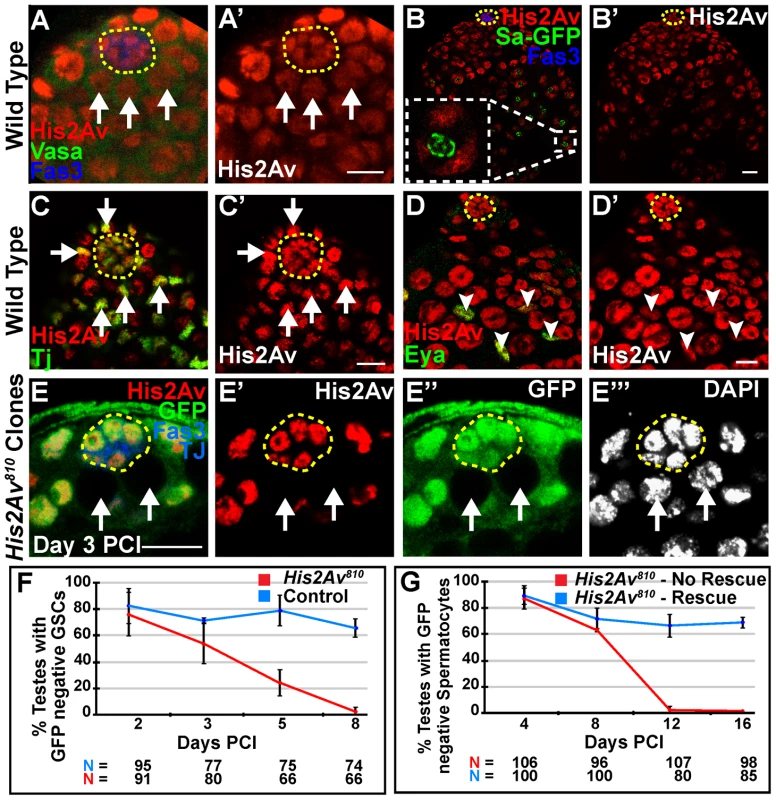
(A–A′): Apical tip of wild-type testis immunostained with anti-His2Av (red), anti-Vasa (green), and anti-Fas3 (blue). GSCs (arrows) identified as Vasa positive cells adjacent to the hub (yellow dashed line). (B–B′): Testes expressing Sa-GFP (green) to mark spermatocytes, immunostained with anti-His2Av (red), anti-GFP (green), and anti-Fas3 (blue). Inset shows spermatocyte nucleus with Sa-GFP marking the nucleolus and His2Av localized to the chromosomes; hub (yellow dashed line). (C–D′): Apical tip of wild-type testes immunostained with anti-His2Av (red, C–D′), anti-Tj (green, C) to mark CySCs (arrows, C, C′) adjacent to the hub (yellow dashed line) or anti-Eya (green, D) to mark cyst cells (arrowheads, D, D′). (E–E′″): Testes day 3 PCI immunostained with anti-GFP (green) to identify homozygous His2Av810 mutant GSCs (arrows), anti-His2Av (red), anti- Fas3 and Tj (blue) and DAPI (E′″); hub (yellow dashed line). Scale bars: 10 µm (F): Percentage of testes with His2Av810 mutant (red) or FRT 82B control (blue) GSCs scored at indicated times PCI.(G): Percentage of testes with His2Av810 mutant spermatocyte cysts in a genetic background either with (blue line, rescue) or lacking (red line, no rescue) a His2Av-mRFP genomic rescue transgene. Data shows average ± S.D. Clonal analysis revealed that His2Av function is required cell autonomously for stem cell maintenance in the Drosophila male germline. Negatively marked GSCs lacking His2Av function were generated in adult fly testes by mitotic recombination using the FLP/FRT system in a His2Av810/+ background [14]. GSCs at day 3 post clonal induction (PCI) (Fig. 1E) and later germline clones at day 8 PCI (Fig. S1A) homozygous mutant for His2Av810 did not exhibit His2Av staining, indicating specificity of the antibody towards His2Av protein and a sharp decline in protein levels in His2Av mutant GSCs by at least day 3 PCI. At day 2 PCI, GSCs homozygous mutant for His2Av810 were detected in 75% of the testes scored, similar to the 81.4% observed in controls (Fig. 1F). By day 8 PCI, the percentage of testes with at least one marked GSC clone dropped to 2% for the His2Av810 mutant (Fig. 1F), suggesting a defect in GSC maintenance upon loss of His2Av function, while 64.8% of control testes had at least one marked GSC.
Consistent with the loss of mutant GSCs, His2Av810 mutant spermatocytes were not maintained over time after clone induction. At day 4 PCI, His2Av810 spermatocytes were observed in 86% of testes. However, by day 12 PCI, His2Av810 mutant spermatocytes were no longer observed (Fig. 1G). A genomic transgene carrying the His2Av coding sequence under control of its endogenous promoter and fused to the mRFP coding sequence (His2Av-mRFP) [15] rescued the loss of spermatocytes, indicating that the failure to maintain GSCs and their differentiating progeny was due to loss of His2Av function (Fig. 1G, Fig. S1B, C).
Knockdown of His2Av function specifically in GSCs and early germ cells by expression of a RNAi hairpin for His2Av using the nanos-GAL4-VP16 (NGVP16) driver also indicated a cell autonomous role for His2Av in GSC maintenance. By day 3 after RNAi expression, induced by shifting flies from 18°C to 30°C, His2Av protein levels in GSCs dropped considerably compared to controls (Fig. S2A, B). At day 4 after RNAi induction, visualization of testes by phase contrast microscopy revealed the presence of spermatocytes and elongated spermatids in testes expressing His2Av RNAi and in controls (Fig. 2A, B). By day 12, however, testes expressing His2Av RNAi exhibited germ cell loss and did not contain spermatocytes or elongated spermatids (Fig. 2D), while control testes at day 15 still had both cell types (Fig. 2C). Quantitation of GSC number revealed that the loss of germ cells observed 12 days after RNAi induction was due to a failure to maintain GSCs. At day 0, His2Av RNAi expressing and control testes had an average of 7 and 8.2 GSCs, respectively (Fig. 2E, F, I). By day 12, the number of GSCs adjacent to the hub in testes expressing His2Av RNAi had dropped to 0, while control testes contained an average of 7.8 GSCs per testis hub (Fig. 2G, H, I).
Fig. 2. RNAi knockdown of His2Av function in early germ cells results in GSC loss. 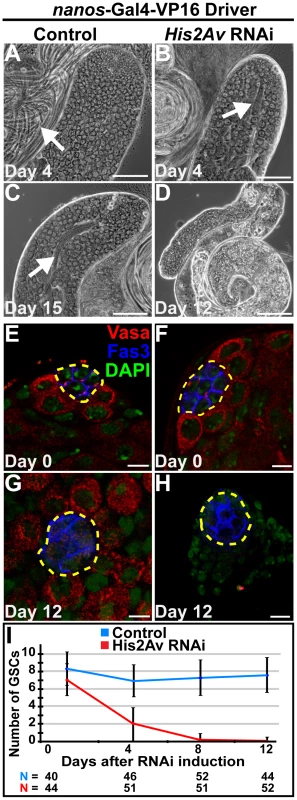
(A–D): Phase images of testes with RNAi knockdown of His2Av in GSCs and early germ cells using the NG4VP16 driver (B, D) and sibling control (A, C) at day 4 (A, B), day 12 (D) and day 15 (C) after RNAi induction. Arrows point to elongated spermatids. Scale Bars: 50 µm (E–H): Apical tips of testes with RNAi knockdown of His2Av in the germline (F, H) and sibling control (E, G) at day 0 (E, F) and day 12 (G, H) after RNAi induction and immunostained with anti-Vasa (red), anti-Fas3 (blue), and DAPI (green); hub (yellow dashed line). Scale bars (A–E): 10 µm(I): Quantification of GSC number over days after His2Av RNAi induction (red line) and sibling control (blue line). Data shows average ± S.D. His2Av is not required for germ cell differentiation
In contrast to its role in GSCs, His2Av was not required cell autonomously for germ cell differentiation. Germline clones homozygous mutant for His2Av810 differentiated into spermatocytes (Fig. 3A and Fig. S1A) and round and elongating spermatids (Fig. 3B, C), as observed 8 days after clone induction. Mutant onion stage round spermatids had the normal size and 1∶1 ratio of nuclei to mitochondrial derivatives, indicating successful progression through meiotic divisions (Fig. 3B). Knockdown of His2Av in late spermatogonial cysts by RNAi expressed under the control of the bam-Gal4 driver confirmed that His2Av function is dispensable for the differentiation program of germ cells at the later stages. His2Av protein levels were greatly reduced in spermatocytes upon expression of RNAi (Fig. S2C, D), yet spermatocytes lacking His2Av protein for 8 days after RNAi induction were still able to differentiate, undergo meiosis, and give rise to elongated spermatids (Fig. 3D, E).
Fig. 3. His2Av is not required for germ cell differentiation. 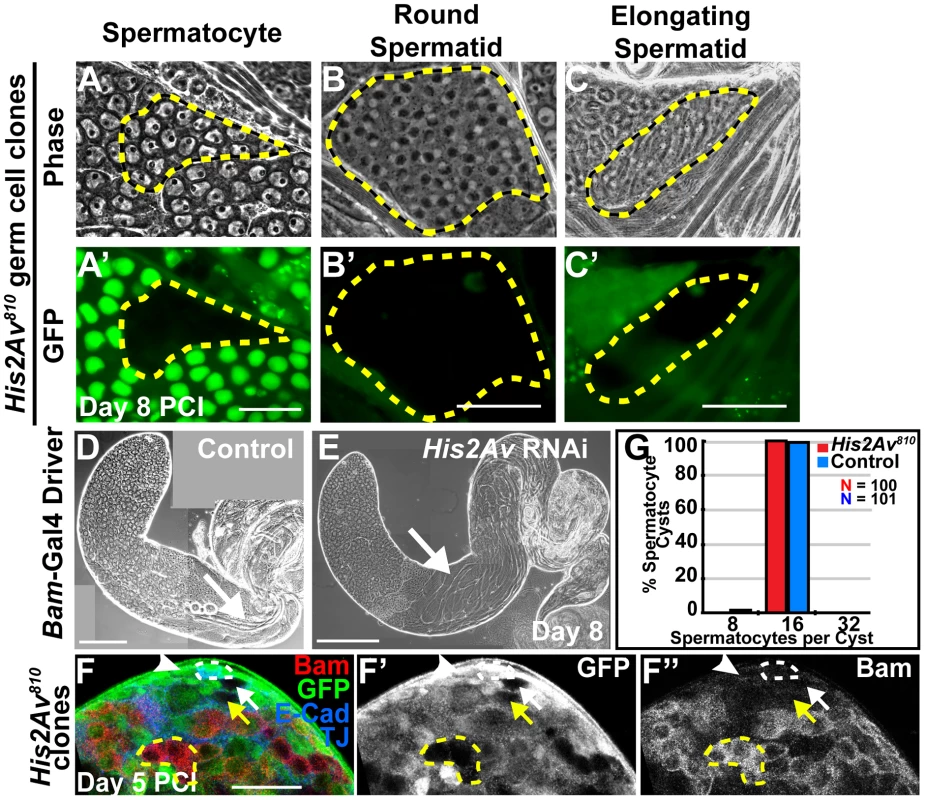
(A–C′): Phase (A, B, C) and GFP (A′, B′, C′) images of GFP-negative His2Av810 spermatocyte (A, A′), round spermatid (B, B′), and elongating spermatid (C, C′) cysts at day 8 PCI. Mutant clones (yellow dashed line). (D, E): Phase images of testes with RNAi knockdown of His2Av in spermatocytes using the Bam-Gal4 driver (E) and sibling control (D) at day 8 after RNAi induction. Arrows point to elongated spermatids. Scale Bars (A–E): 50 µm (F–F″): Immunostaining with anti-Bam (F, red and F″), anti-GFP (F, green and F′), anti-E-Cad and TJ (F, blue) at day 5 PCI. His2Av810 mutant GSC (white arrow), His2Av810 mutant gonialblast (yellow arrow), His2Av810 heterozygous GSC (white arrowhead), His2Av810 mutant 4-cell spermatogonial cyst (yellow dashed line) and hub (white dashed line). Scale bar: 25 µm (G): Number of spermatocytes per cyst of His2Av810 heterozygous (blue) and mutant (red) clones counted at day 8 PCI. Although His2Av mutant GSCs were lost to differentiation, they did not appear to do so by accumulating Bam protein earlier than their heterozygous counterparts. The accumulation of Bam protein in transit-amplifying spermatogonial cells stops proliferation and initiates differentiation to spermatocytes [16]. Immunostaining for Bam protein 5 days PCI revealed that neither heterozygous His2Av810/+ nor homozygous His2Av810 mutant GSCs or gonialblasts expressed Bam protein (Fig. 3F). Bam protein did accumulate at the correct time during the differentiation program in His2Av mutant cells, at the 4-cell spermatogonial stage (Fig. 3F), similar to in wild-type spermatogonial cysts. Consistent with the correct temporal accumulation of Bam protein, germ cells lacking His2Av function underwent 4 rounds of spermatogonial divisions, producing cysts with 16 spermatocytes (Fig. 3G).
His2Av is required cell autonomously for CySC maintenance but not for somatic cyst cell differentiation
Consistent with its expression in the cyst cell lineage, His2Av function was also required cell autonomously for CySC maintenance. Although both His2Av810 mutant and control CySCs were present at comparable frequencies at day 2 PCI, by day 8 PCI almost all testes lacked His2Av810 mutant CySCs, while control CySCs were maintained (Fig. 4A). Consistent with the loss of mutant CySCs, His2Av810 mutant cyst cells expressing the differentiation marker Eya were also lost over time. His2Av810 mutant cyst cells were observed in 100% of testes at day 4 PCI, but by day 12 PCI His2Av810 mutant Eya-positive cyst cells were almost entirely absent (Fig. 4B). The His2Av-mRFP transgene rescued the loss of His2Av810 homozygous mutant CySCs, suggesting that the failure to maintain CySCs was due to loss of His2Av function. At day 2 PCI, an average of 43.2% (n = 32) of testes contained His2Av810 mutant CySCs, while under the same conditions, 67.5% (n = 23) testes from sibling males carrying the His2Av-mRFP transgene contained His2Av mutant CySCs (data not shown). The percentage of testes containing His2Av810 mutant CySCs at day 8 PCI dropped to 3% (n = 26), while 42.1% (n = 31) of testes from males carrying the His2Av-mRFP transgene contained marked CySCs.
Fig. 4. His2Av is required cell autonomously for CySC maintenance but not for somatic cyst cell differentiation. 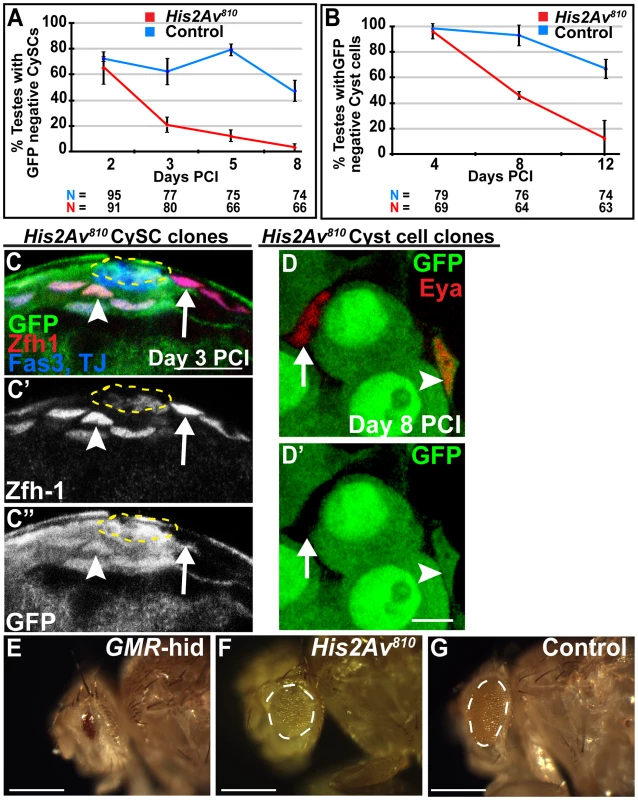
(A, B): Percentage of testes with His2Av810 mutant (red line) or FRT 82B control (blue line) CySCs (A) or cyst cells (B) scored at indicated time points PCI. Data shows average ± S.D. (C–C″): Apical tip of testes 3 days PCI immunostained with anti-GFP (C, green and C″), anti-Zfh-1 (C, red and C′), and anti-Fas3 and TJ (blue). His2Av810 mutant CySCs (arrow), His2Av810 heterozygous CySCs (arrowhead) and hub (yellow dashed line). Scale bar: 12.5 µm (D, D′): Testes from 8 day PCI immunostained with anti-GFP (green) and anti-Eya (red). His2Av810 mutant (arrow) and heterozygous (arrowhead) cyst cells. Scale bar: 10 µm (E–G): Analysis of Drosophila eyes derived from heterozygous GMR-hid/+ (E), homozygous His2Av810 (F), and homozygous FRT control (G) precursor cells. The failure to maintain CySCs was not due to downregulation of the transcriptional repressor Zinc-finger homology-1 (Zfh-1), which is expressed in CySCs and is required for CySC maintenance [17]. At day 3 PCI, when only 20% of testes scored had homozygous mutant CySCs, Zfh-1 expression in His2Av810 homozygous mutant CySCs was comparable to that in neighboring wild-type CySCs (Fig. 4C). As in the germ line, His2Av function was not required for cyst cell differentiation. Cyst cells lacking His2Av function differentiated successfully at least to the stage at which they express the differentiation marker Eya and are associated with differentiating germ cells (Fig. 4D).
In addition to the survival and differentiation of germ cells and cyst cells lacking His2Av, the classic eye test revealed that His2Av function might be dispensable for cell survival in the eye tissue. Eyes composed exclusively of cells lacking His2Av function were generated using the EGUF/hid system [18]. When mitotic recombination was not induced, eye precursor cells expressed the GMR-hid transgene and failed to develop, resulting in adult flies with tiny eyes (Fig. 4E). In contrast, when clones were induced, cells lacking His2Av function produced eyes (Fig. 4F), although they appeared slightly smaller and rougher compared to controls (Fig. 4G), suggesting that His2Av might contribute to proper cell proliferation and/or differentiation in this tissue. Together, the results from clonal and RNAi analysis in the germline, somatic cyst, and eye cell lineages suggest that in the absence of DNA damaging agents, Drosophila His2Av function is required for adult stem cell maintenance but not for cell survival or differentiation.
Loss of His2Av function did not cause defects in STAT-dependent GSC characteristics
Analysis of His2Av mutant GSCs revealed that His2Av function was not required to maintain three previously defined STAT-dependent characteristics of GSCs: 1) attachment to the hub through E-cadherin mediated adherens junctions, 2) oriented cell division [19], and 3) upregulation of STAT92E protein in response to Unpaired (Upd) signaling from the hub. His2Av mutant GSCs localized E-Cadherin-GFP (E-Cad-GFP), expressed in GSCs by the nanos-Gal4 driver and detected 5 days PCI, to the hub-GSC interface similar to neighboring heterozygous GSCs (Fig. 5A) and as previously shown [12]. The expression of E-Cad-GFP in GSCs did not result in an increase in His2Av mutant GSC maintenance; His2Av810 mutant GSCs in testes from sibling males either expressing or lacking the expression of E-Cad-GFP were lost at the same rate (Fig. 5B). His2Av also did not appear to be required for the stereotypical orientation of centrosomes in GSCs that sets up the mitotic spindle orientation and the subsequent asymmetric outcome of GSC division [12]. Analysis of testes 3 days after induction of His2Av810 clones revealed that in GSCs with two centrosomes, one centrosome was found adjacent to the hub-GSC interface in 86.3% of His2Av810 mutant GSCs, similar to neighboring heterozygous His2Av810/+ GSCs (86.42%) and FRT control GSCs (84.62%) (Fig. 5C–E). Loss of His2Av function did not substantially alter the accumulation of STAT92E, an indicator of JAK-STAT activity [20], in GSCs. His2Av810 mutant GSCs remaining adjacent to the hub 5 days PCI had STAT92E protein levels comparable to neighboring heterozygous GSCs (Fig. 5F), suggesting that loss of GSCs in His2Av mutants is not due to failure to express STAT92E. Conversely, GSCs homozygous mutant for either Stat92E06346 (Fig. 5G) or Stat92Ejc46 (data not shown) expressed His2Av protein at levels comparable to neighboring heterozygous GSCs, suggesting that His2Av expression in GSCs was not dependent on STAT92E function.
Fig. 5. Loss of His2Av function did not cause defects in STAT dependent GSC characteristics. 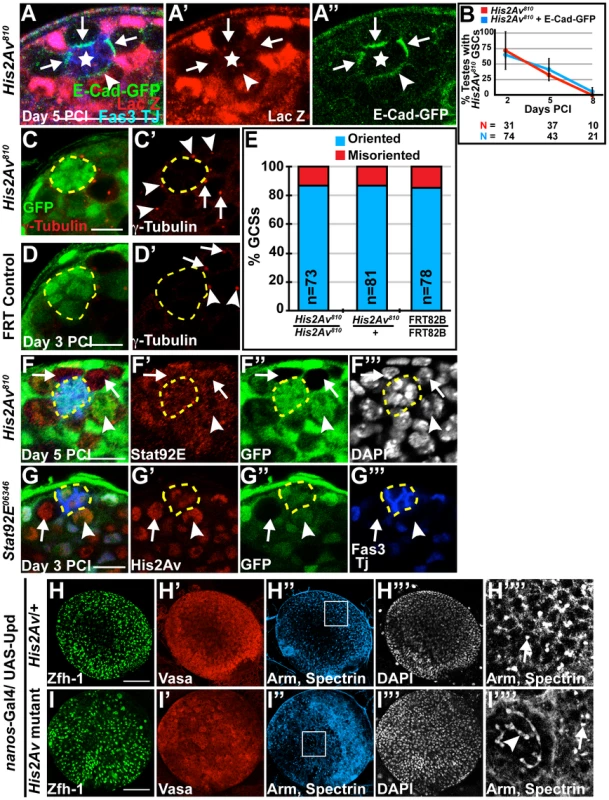
(A–A″): His2Av810/+ testes at 5 days PCI and expressing E-Cadherin-GFP under the control of the nanos-Gal4 driver immunostained with anti-GFP (green), anti-β-galactosidase (LacZ, red) and anti-Fas3 and TJ (blue). His2Av810 mutant GSCs (arrows), His2Av810 heterozygous GSC (arrowhead) and hub (star). Scale bar: 25 µm (B): Percentage of testes with His2Av810 (red) or His2Av810, UAS-E-Cad-GFP (blue) GSCs scored at indicated times after clonal induction. Data shows average ± S.D.(C–D′): Immunostaining of testes 3 days after clonal induction with anti-©-tubulin (red) and anti-GFP (green) in His2Av810 (C–C′) and FRT control (D–D′) GSCs. Centrosomes of GFP negative GSCs (arrows), heterozygous GFP-positive GSCs (arrowheads) and hub (yellow dashed line). Scale bars: 10 µm (E): Percentage of oriented (blue) and misoriented (red) centrosomes in His2Av810 mutant, His2Av810 heterozygous, and FRT 82B control GSCs. (F–G′″): Immunostaining of His2Av810/+ testes (F–F′″) 5 days PCI with anti-GFP (green), anti-STAT92E (red) and anti-Fas3 and TJ (blue) and STAT92E06346/+ testes (G–G′″) 3 days PCI with anti-GFP (green), anti-His2Av (red) and anti-Fas3 and TJ (blue). DAPI (F′″) in white. His2Av810 (F–F′″) and STAT92E06346 (G–G′″) mutant GSCs (arrows), His2Av810 (F–F′″) and STAT92E06346 (G–G′″) heterozygous GSCs (arrowhead) and hub (yellow dashed line). Scale bar: 12.5 µm (F) and 10 µm (G). (H–I″″): Larval testes expressing Upd under the nanos-Gal4 driver and either heterozygous for His2Av (H–H″″) or mutant for His2Av (I–I″″) immunostained with anti-Zfh-1 (H, I, green), anti-Vasa (H′, I′, red), anti-Arm and anti-spectrin (H″, I″, blue and H″″, I″″) and counterstained with DAPI (H′″, I′″). H″″ and I″″ are magnifications of the regions enclosed in H″ and I″, respectively. Dot fusomes (arrows) and branched spectrosomes (arrowhead). Scale bar: 100 µm. Loss of His2Av function did not suppress the overproliferation of CySC-like and GSC-like cells in testes with forced activation of the JAK-STAT pathway. When the Upd ligand was expressed ectopically in early germ cells under the control of the nanos-Gal4 driver, larval testes heterozygous for His2Av had an abundance of small Vasa-positive GSC-like cells with dot spectrosomes and Zfh-1 positive CySC-like cells (Fig. 5H). Under the same conditions, testes from sibling nos-Gal4/UAS-Upd; His2Av810/Df(3R) BSC524 larvae also exhibited an abundance of GSC-like and CySC-like cells (Fig. 5I), although there were subtle signs of differentiating germ cells. In the absence of His2Av function, 16 out of 37 (43.24%) nos-Gal4; UAS-Upd larval testes had a few germ cell cysts containing branched fusomes (Fig. 5I″″). In the same experiment, only 1 out of 37 (2.7%) testes from nos-Gal4/UAS-Upd; His2Av/+ larvae exhibited branched fusomes. Thus, in the absence of His2Av function, a small population of His2Av mutant germ cells appears to initiate the differentiation program even under conditions of high JAK-STAT activation.
The chromatin remodeling factor Domino appears to function in the His2Av pathway to maintain GSCs and CySCs
Clonal analysis suggested that the chromatin remodeling factor Domino, the homolog of yeast Swr1 [21], which exchanges His2A variant for His2A in yeast [22], [23], is required for stem cell maintenance. In the Drosophila testis, when negatively marked clones of domk08108 were generated using the FLP/FRT system, the percentage of testes carrying marked domk08108 homozygous GSCs or CySCs was indistinguishable from the control at day 2 PCI (Fig. 6A, B). However, the percentage of testes carrying marked domk08108 homozygous GSCs steadily decreased over time after clonal induction and dropped to zero by day 8 (Fig. 6A). Similarly, the percentage of testes with domk08108 mutant CySCs dropped from 74% at day 2, to 14.5% at day 4, to 0% at day 15 (Fig. 6B). Immunostaining analysis revealed that Domino function might be required for the localization of His2Av to chromatin in GSCs, similar to the function of the corresponding Swr1 complex in yeast. At day 6 PCI, nuclei in GSCs lacking Domino function had reduced levels of His2Av protein compared to control GSCs (Fig. 6C–C′″). Quantification of His2Av immunofluorescence intensity revealed that the loss of domino function reduced His2Av protein levels in GSCs by an average of 2-fold. The average ratio of His2Av immunostaining per unit area in GSCs that were homozygous for domk08108 compared to His2Av immunostaining per unit area in GSCs heterozygous for domk08108 within a testis (n = 28 testes) was 0.56. In contrast, in FRT 42D control (n = 18 testes), the ratio of His2Av immunostaining per unit area in GFP negative to GFP positive GSCs was 1.12 (Fig. 6D). Consistent with a role for Domino in His2Av incorporation and function in GSC maintenance, the loss of His2Av810 mutant GSC clones increased in a domk08108/+ genetic background (Fig. 6E). At day 2 PCI, 65.5% of testes contained His2Av810 homozygous mutant GSC clones, while under the same conditions, only 51.1% of testes from sibling males carrying the dom allele had marked GSC clones, possibly due to reduced incorporation of His2Av into chromatin before clonal induction. Under the same conditions at day 2 PCI, an average of 92% of testes from both domk08108/+; His2Av810 and sibling His2Av810 males had spermatocyte clones, suggesting that clonal induction occurred at the same rate in both genetic backgrounds (data not shown). The percentage of testes with marked His2Av mutant GSCs was also lower at days 3 and 5 PCI in males with the domk08108/+ allele compared to sibling males without the dom allele.
Fig. 6. Domino function is required for the association of His2Av with chromatin. 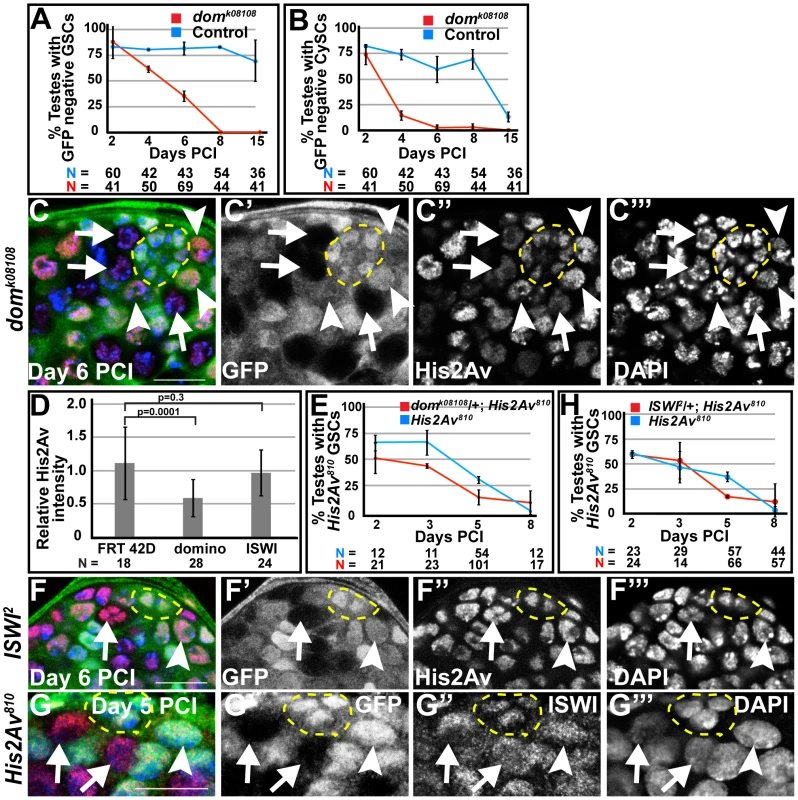
(A, B): Percentage of testes with domk08108 mutant (red) or FRT 42D control (blue) GSCs (A) and CySCs (B) scored at indicated times after clonal induction. Data shows average ± S.D. (C–C′″): Apical tip of domk08108/+ testes at day 6 PCI immunostained with anti-GFP (C, green and C′) and anti-His2Av (C, red and C″) and counterstained with DAPI (blue, C and C′″). domk08108 mutant GSCs (arrows), domk08108 heterozygous GSCs (arrowheads) and hub (yellow dashed line). Scale bar: 12.5 µm (D): Average ratio of His2Av intensity per unit area in GSC homozygous for FRT 42D, domk08108 or ISWI2 to neighboring GSCs heterozygous for FRT 42D, domk08108 or ISWI2, respectively. Data shows average ± S.D. P-values of Student's t-test (2-tailed) are shown. (E): Percentage of testes with His2Av810 (blue) or His2Av810 ;domk08108/+ (red) GSCs scored at indicated times after clonal induction. Data shows average ± S.D. (F–G′″): Apical tip of ISWI2/+ (F–F′″) testes at day 6 PCI or His2Av810/+ (G–G′″) testes at day 5 PCI immunostained with anti-GFP (F, G, green and F′, G′) and anti-His2Av (F, red and F″) or anti-ISWI (G, red and G″) and counterstained with DAPI (blue, F, G and F′″, G′″). ISWI2 (F–F′″) or His2Av810 (G–G′″) mutant GSCs (arrows), ISWI2 (F–F′″) or His2Av810 (G–G′″) heterozygous GSCs (arrowheads) and hub (yellow dashed line). Scale bars: 12.5 µm (H): Percentage of testes with His2Av810 (blue) or His2Av810 ;ISWI2/+ (red) GSCs scored at indicated times after clonal induction. Data shows average ± S.D. In contrast to the function of Domino, the chromatin remodeling factor ISWI, which also functions in GSC and CySC maintenance [24], did not appear to be required for the localization of His2Av to chromatin in GSCs. Immunostaining for His2Av protein in testes containing ISWI2 homozygous mutant GSCs 6 days PCI revealed that the levels of His2Av protein in ISWI mutant GSCs were comparable to neighboring ISWI2/+ GSCs (Fig. 6F). The average ratio of His2Av immunostaining intensity per unit area for GSCs homozygous mutant for ISWI2 to neighboring ISWI2/+ GSCs within the same testis (n = 24 testes) was 0.96 (Fig. 6D). Similarly, ISWI protein levels in the nuclei of His2Av810 mutant GSCs were comparable to that in heterozygous GSCs (Fig. 6G), suggesting that His2Av might not be required to recruit or maintain ISWI on chromatin. ISWI did not exhibit a strong genetic interaction with His2Av to maintain GSCs in the adult testes. The percentage of testes with marked His2Av810 mutant GSC clones in ISIW2/+; His2Av810 testes was comparable to that in testes lacking the ISWI allele at days 2, 3, and 8 PCI, only falling slightly at day 5 PCI (Fig. 6H).
Loss of His2Av function did not globally alter levels of the epigenetic marks associated with transcriptional state in GSCs. Immunostaining with antibodies that recognize H3K4 tri-methyl (H3K4me3) (Fig. 7A), mostly associated with transcriptionally active/poised chromatin regions, and H3K27 tri-methyl (H3K27me3) (Fig. 7B), mostly associated with transcriptionally inactive regions of chromatin [1] on testes with His2Av810 mutant GSCs 5 days PCI revealed that the levels of these epigenetic marks were comparable in mutant and heterozygous GSCs. Likewise, the protein levels of Scrawny (Scny), a histone H2B deubiquitinase required to prevent premature expression of differentiation genes in adult stem cells [25], were also not altered in His2Av mutant GSCs (data not shown). Furthermore, His2Av protein levels scored 6 days PCI were unaltered in GSCs homozygous mutant for scny02331 (Fig. 7C) or scnye00340 (data not shown) compared to neighboring scny/+ heterozygous GSCs. Although scny mutant follicle cells in the Drosophila ovary exhibit elevated levels of H3K4me3 [25], male GSCs homozygous mutant for either scny02331 (Fig. 7D) or scnye00340 (data not shown) did not exhibit changes in H3K4me3 levels compared to neighboring heterozygous GSCs. These data suggest that loss of His2Av or Scny function was not associated with dramatic changes in transcription in the testis, at least when assayed at a global level by immunostaining for histone marks.
Fig. 7. His2Av mutant GSCs do not exhibit dramatic changes in epigenetic markers of transcriptional state. 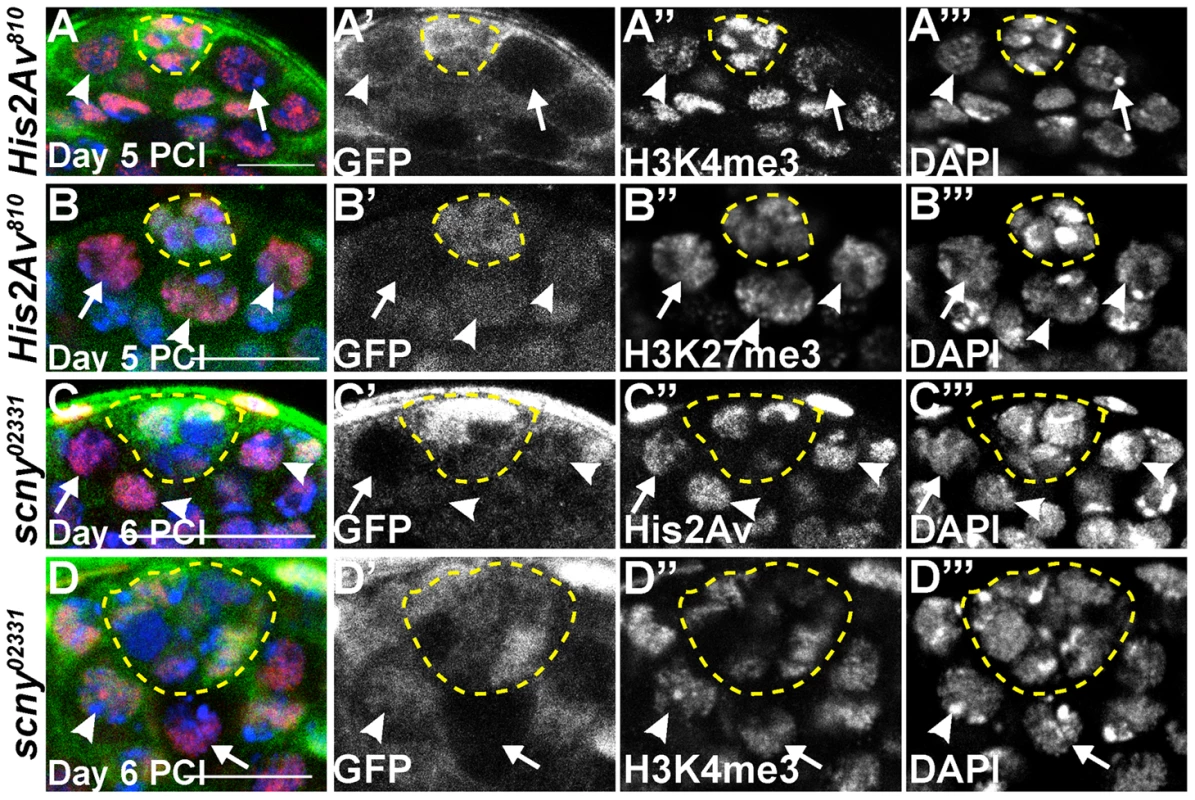
(A–B′″): His2Av810/+ testes 5 days PCI immunostained with anti- GFP (A, B, green and A′, B′), anti-H3K4me3 (A, red and A″) or anti-H3K27me3 (B, red and B″) and counterstained with DAPI (A, B, blue and A′″, B′″). His2Av810 mutant GSCs (arrows), His2Av810 heterozygous GSCs (arrowhead) and hub (yellow dashed line). Scale bar: 10 µm (A) and 12.5 µm (B). (C–D′″): scny02331/+ testes 6 days PCI immunostained with anti- GFP (green, C, D and C′, D′), anti-His2Av (red, C and C″) or anti-H3K4me3 (red, D and D″) and counterstained with DAPI (blue, C, D and C′″, D′″). scny02331 mutant GSCs (arrows), scny02331 heterozygous GSCs (arrowheads) and hub (yellow dashed line). Scale bar: 25 µm (C) and 12.5 µm (D). Discussion
Our results reveal that the histone variant His2Av is required cell autonomously for maintenance of two different adult stem cell types, GSCs and CySCs, in the Drosophila male gonad, but not for the differentiation of the progeny in these two stem cell lineages. The specific requirement for His2Av for adult stem cell maintenance suggests that His2Av may play critical role(s) in the mechanisms that maintain the ability of adult stem cells to self-renew rather than differentiate. His2Av function has been implicated in both transcriptional repression and transcriptional activation. His2Av could maintain adult stem cells by either favoring repression of pro-differentiation genes and/or activation of genes necessary for stem cell identity and function. In yeast, H2A.Z occupies transcriptionally inactive genes and intergenic regions [26], while in Drosophila, His2Av is required for the establishment of heterochromatin and transcriptional repression [27]. Conversely, studies indicate that in Drosophila, yeast, and chicken, His2Av is enriched at nucleosomes downstream of the transcription start site of active or poised genes [28], [29], [30]. Nucleosomes and histone dimers containing H2A.Z appear to be less stable than nucleosomes containing the canonical histone H2A [31], [32], [33]. This lower stability may favor a more open chromatin, giving transcriptional activators or repressors better access to the DNA. Consistent with this model, a recent study showed that H2A.Z promotes self-renewal and pluripotency of murine embryonic stem cells (ESCs) by facilitating the binding of Oct4 to its target genes and the Polycomb repressive complex 2 to differentiation genes [34]. However, in ESCs, unlike in Drosophila male GSCs and CySCs, His2A.Z function was also required for the expression of differentiation genes when ESCs were grown under conditions that induce differentiation [34], [35].
We propose that the requirement of His2Av for adult stem cell maintenance, but not for differentiation, may reflect a subtle role for His2Av in maintaining expression of genes required for self-renewal versus differentiation. Adult stem cells lie at the cusp of two alternate fate choices, self-renewal and differentiation; the progeny of stem cell division are maintained in a state where they can execute either self-renewal or differentiation programs depending on local cues. The requirement for this balanced, bi-potential state may make adult stem cells more sensitive to the small alterations in the relative levels of key transcripts associated with the loss of His2Av function, tilting the balance from stem cell maintenance to onset of differentiation. Consistent with the model that His2Av may alter transcriptional levels subtly, H2A.Z was shown to be required to fine-tune the transcriptional state of hsp70 and a wide variety of other genes in response to temperature changes in Arabidopsis [36], [37].
The ATP-dependent chromatin-remodeling factor Domino is required for GSC and CySC maintenance in the male germline, as previously shown for somatic follicle stem cells in the female gonad [38]. The yeast Swr1 complex containing the homolog of Drosophila Domino exchanges His2A with Htz1 (the yeast His2A variant) [22], [23], [39] and in Drosophila, Domino - containing Tip60 chromatin remodeling complex has been shown to exchange phospho-His2Av with unmodified His2Av in in vitro assays [40]. Our studies indicate that Domino function is required in vivo in GSCs for the incorporation of His2Av into chromatin. Nuclei of domino mutant GSCs had lowered but still detectable levels of His2Av protein, possibly due to the weak domino allele used in this study. Alternatively, incorporation of His2Av in some regions of the chromatin may occur independently of Domino function, as has been reported in yeast, in which stress-responsive genes exhibit Swr1-independent incorporation of Htz in the coding region [41]. Although ISWI, like His2Av, is required for GSC and CySC maintenance in the male germline [24], these proteins may function in parallel pathways to maintain adult stem cells in the testis. The ISWI containing nucleosome-remodeling factor (NURF) was shown to maintain GSCs and CySCs in the Drosophila testis by positively regulating the JAK-STAT signaling pathway; GSCs mutant for components of the NURF complex exhibited low levels of STAT92E protein [24]. In contrast, as discussed below, His2Av may function independently of the JAK-STAT signaling pathway.
Our results indicate that His2Av may function independently of the JAK-STAT signaling pathway to provide a chromatin environment that allows for stem cell maintenance. Expression of the His2Av and STAT92E proteins in GSCs was not dependent on each other. Our studies indicate that His2Av may not be required for expression of at least one other key STAT-dependent gene in CySCs. Activation of the JAK-STAT signaling pathway in response to the Upd signal from the hub is important for CySC maintenance, possibly in part through STAT-dependent transcription of Zfh-1 [17]. However, CySCs lacking His2Av function still expressed Zfh-1. In GSCs, activation of the JAK-STAT pathway is important for maintaining hub-GSC adhesion and for centrosome orientation [19], both of which appeared unaffected in His2Av mutant GSCs. Loss of His2Av function did not strongly suppress the phenotype associated with ectopic overexpression of Upd in the testis, although a few His2Av mutant germ cells were able to initiate differentiation, possibly due to relatively lower levels of JAK-STAT activation in these cells. Even though loss of His2Av normally resulted in differentiation of GSCs and CySCs, the requirement for His2Av function can be overridden by high levels of activation of the JAK-STAT pathway, possibly maintaining somatic CySCs in a stem cell like state, which may fail to provide a microenvironment for germ cells to initiate differentiation [19], [42].
Materials and Methods
Fly strains and husbandry
Fly stocks were raised on cornmeal/molasses medium at 25°C unless stated otherwise. Stocks are from the Bloomington Stock Center unless specified otherwise. Mutant alleles used in this study include 1) w;FRT82B, His2Av810/TM6B, Tb, carrying a 311 base pair deletion that removes the second exon of the His2Av gene [43], 2) w;FRT82B, His2Av05146/TM3, 3)w;; Df(3R)BSC524/TM6b,Tb, a deletion that encompasses the His2Av gene, 4) the Stat92E alleles, FRT82B, Stat92E06346/TM3 and FRT82B, Stat92EJ6C8/TM3 (gift from E. Matunis), 5) y, w, ey-Flp, GMR-lacZ; FRT42D, domk08108/CyO, y+, a loss of function allele (also known as dom1) with a P-element inserted at the 3′ boundary of the first exon [21] obtained from DGRC, 7) y w;FRT 42D, ISWI2, sp/SM5, Cy, sp , a null allele carrying a nonsense mutation [44], 8) the scrawny alleles: FRT 80B, scnyl(3)02331 and FRT 80B, scnye00340 [25]. w; His2Av-mRFP; FRT82B, His2Av810/TM6B, Tb flies were used to rescue GSC and CySC loss. The His2Av-mRFP construct rescues the lethality of His2Av05146 mutant [15]. The following flies 1) hs-FLP122;;FRT82B, ubi-nGFP, 2) hs-FLP122;nos-GAL4;FRT82B, tub-LacZ (gift from D. Kalderon), 3) hs-FLP122;;FRT80B, ubi-nGFP, 4) hs-FLP122;FRT42D, ubi-nGFP were used to induce marked clones in the testes. y,w;ey-GAL4, UAS-FLP;FRT82B, GMR-hid/TM2 flies were used to induce marked clones in adult eyes. FRT82B, FRT80B and FRT42D were used as wild-type controls for clone induction. Other stocks used include w,sa-GFP [45], UAS-Upd [46], UAS-DEFL #6-1 (UAS-E-Cad-GFP) [47] from DGRC, nanos-GAL4, UAS-Dicer2;; nanos-GAL4VP16 (NG4VP16) and ;; Bam-GAL4. RNAi flies against His2Av (Transformant ID #110598) were obtained from the Vienna Drosophila RNAi Center.
A heteroallelic combination of His2Av810 and Df(3R) BSC524 survives until the third instar larval stage when grown at 25°C for 2 days and then shifted to 29°C. The effects of loss of His2Av function in testes ectopically expressing Upd ligand was analysed in the third-instar larval progeny of nanos-GAL4; Df(3R) BSC524/TM6B,Tb and UAS-Upd; His2Av810/TM6B,Tb. Tb-positive larvae (heterozygous for either His2Av810 or Df(3R) BSC524) expressing UAS-Upd under the nanos-Gal4 driver were used as controls
Immunofluorescence
Testes were dissected in 1× phosphate-buffered saline (PBS) and fixed in 4% formaldehyde in PBS for 20 minutes at room temperature, washed twice for 30 minutes each in PBS with 0.3% Triton X-100 and 0.6% sodium deoxycholate. Testes were incubated overnight at 4°C in primary antibodies against Armadillo (Arm, mouse 1∶10; Developmental Studies Hybridoma Bank (DSHB)) [48], Fas3 (mouse 1∶10; DSHB) [49], α-spectrin (mouse 1∶10; DSHB) [50], Eyes absent (Eya, mouse 1∶10; DSHB) [51], E-cadherin (mouse 1∶10, DSHB) [52], Green Fluorescent protein (GFP, rabbit 1∶400–1∶1000; Invitrogen and Sheep 1∶1000, Abd-Serotec), β-Galactosidase (rabbit 1∶1000; Cappel), Histone H3 lysine 4 trimethyl (H3K4me3, rabbit 1∶200: Cell Signaling), Histone H3 lysine 27 trimethyl (H3K27me3, rabbit 1∶200: Cell Signaling), His2Av (rabbit 1∶1000; gift from R. Glaser) [53], Traffic-jam (Tj, guinea pig 1∶5000; gift from D. Godt) [54], Vasa (goat 1∶50; Santa Cruz Biotechnology), ©-tubulin (mouse 1∶50; Sigma), Zfh-1 (rabbit 1∶5000; gift from R. Lehman), STAT92E (rabbit 1∶1000; gift from E.Bach) [55], Scrawny (guinea pig 1∶200; gift from M. Buszczak) [25] and ISWI (rabbit 1∶100; gift from J.Tamkun) [56]. Secondary antibodies used were from the Alexa Fluor-conjugated series (1∶500; Molecular Probes). Samples were mounted in VECTASHIELD medium containing DAPI to visualize DNA (Vector Labs H-1200). Immunofluorescence images were obtained with a Leica SP2 Confocal Laser Scanning microscope. Phase and clonal analysis images were obtained using a Zeiss Axioskop microscope and SPOT RT3 camera by Diagnostic Instruments or CoolSNAPez camera by Photometrics. Images were processed using Adobe CS4 Photoshop and Illustrator. Comparison of intensity of His2Av staining in GSCs was performed using the ImageJ program [57]. The nuclear area in GSCs was selected based on the DAPI staining and the average intensity of His2Av immunostaining within the nucleus was measured using ImageJ. An average of immunofluorescence intensity per unit area for all GSCs homozygous (identified as GFP negative) or heterozygous (identified as GFP positive) for a given genotype was calculated for each testis. The relative level of His2Av protein was calculated as a ratio of the average immunofluorescence intensity per unit area for homozygous GSC to heterozygous GSC within each testis. Similar results were obtained when anti-His2Av intensity was normalized to the intensity for DAPI staining for each GSC.
Clonal and RNAi analysis
Homozygous His2Av mutant clones in a heterozygous background were generated by crossing either 1) hs-FLP122;;FRT82B, ubi-nGFP, 2) hs-FLP122; FRT42D, domk08108/CyO;FRT82B, ubi-nGFP, 3) hs-FLP122; FRT 42D, ISWI2, sp/CyO;FRT82B, ubi-nGFP, or 4) hs-FLP122;nos-GAL4;FRT82B, tub-LacZ virgin females to w;;FRT82B, w;;FRT82B, His2Av810/TM6B, Tb or w;; UAS-DEFL #6-1, FRT82B, His2Av810/TM6B, Tb [The UAS-DEFL #6-1 (UAS-E-Cad-GFP) containing chromosome was recombined to the FRT82B, His2Av810 chromosome] males. Homozygous domk08108 or ISWI2 mutant clones were obtained by crossing males of the alleles to hs-FLP122;FRT42D, ubi-nGFP virgin females, while males of scny alleles were crossed to hs-FLP122;;FRT80B, ubi-nGFP males. The progeny were raised at 25°C and heat-shocked at 37°C for two hours each on two consecutive days at the pupal stage. GSCs homozygous mutant for His2Av810 or other alleles were identified by their lack of GFP (or β-Galactosidase), presence of the germ cell marker Vasa, and contact with the hub. Homozygous clones of CySCs generated by heat shock induced mitotic recombination were identified by their lack of GFP (or β-Galactosidase) and the germ cell marker Vasa, by the presence of Tj, a marker of the cyst cell lineage, and by their proximity to the hub.
Homozygous mutant germline clones generated in His2Av05146/+ resulted in the loss of mutant GSCs (Fig. S3A) and spermatocytes (Fig. S3B) over time after clone induction. However, this loss of marked cells was not associated with a loss of anti-His2Av staining (Fig. S3C′), and the loss of homozygous mutant germ cells was not rescued by the presence of His2Av-mRFP transgene (Fig. S3B), suggesting that a mutation other than His2Av on the chromosome might be responsible for GSC loss in this line.
FLP-medicated mitotic recombination was induced in eye precursor cells by crossing y,w;ey-GAL4, UAS-FLP;FRT82B, GMR-hid/TM2 virgins to males carrying FRT 82B, His2Av810 (or FRT control). Eye precursor cells carrying one copy of the dominant cell lethal transgene GMR-hid fail to survive, thereby generating eyes composed entirely of cells homozygous for His2Av810 (or the FRT control).
RNAi knockdown experiments were carried out by crossing flies carrying His2Av RNAi hairpin under the UAS regulatory sequence to either UAS-Dicer2;;NG4VP16 females or Bam-GAL4. The progeny were raised at 18°C until eclosion and transferred to and held at 30°C.
Supporting Information
Zdroje
1. BannisterAJ, KouzaridesT (2011) Regulation of chromatin by histone modifications. Cell Res 21 : 381–395.
2. ClapierCR, CairnsBR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78 : 273–304.
3. TalbertPB, HenikoffS (2010) Histone variants–ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol 11 : 264–275.
4. RedonC, PilchD, RogakouE, SedelnikovaO, NewrockK, et al. (2002) Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev 12 : 162–169.
5. RaisnerRM, MadhaniHD (2006) Patterning chromatin: form and function for H2A.Z variant nucleosomes. Curr Opin Genet Dev 16 : 119–124.
6. ZlatanovaJ, ThakarA (2008) H2A.Z: view from the top. Structure 16 : 166–179.
7. van DaalA, WhiteEM, GorovskyMA, ElginSC (1988) Drosophila has a single copy of the gene encoding a highly conserved histone H2A variant of the H2A.F/Z type. Nucleic Acids Res 16 : 7487–7497.
8. BaldiS, BeckerPB (2013) The variant histone H2A.V of Drosophila-three roles, two guises. Chromosoma 122 : 245–258.
9. FullerMT, SpradlingAC (2007) Male and Female Drosophila Germline Stem Cells: Two Versions of Immortality. Science 316 : 402–404.
10. KigerAA, White-CooperH, FullerMT (2000) Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407 : 750–754.
11. TranJ, BrennerTJ, DiNardoS (2000) Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature 407 : 754–757.
12. YamashitaYM, JonesDL, FullerMT (2003) Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301 : 1547–1550.
13. VoogJ, D'AlterioC, JonesDL (2008) Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature 454 : 1132–1136.
14. XuT, RubinGM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 : 1223–1237.
15. SchuhM, LehnerCF, HeidmannS (2007) Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr Biol 17 : 237–243.
16. InscoML, LeonA, TamCH, McKearinDM, FullerMT (2009) Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc Natl Acad Sci U S A 106 : 22311–22316.
17. LeathermanJL, DinardoS (2008) Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3 : 44–54.
18. StowersRS, SchwarzTL (1999) A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152 : 1631–1639.
19. LeathermanJL, DinardoS (2010) Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol 12 : 806–811.
20. ChenHW, ChenX, OhSW, MarinissenMJ, GutkindJS, et al. (2002) mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev 16 : 388–398.
21. RuhfML, BraunA, PapoulasO, TamkunJW, RandsholtN, et al. (2001) The domino gene of Drosophila encodes novel members of the SWI2/SNF2 family of DNA-dependent ATPases, which contribute to the silencing of homeotic genes. Development 128 : 1429–1441.
22. KoborMS, VenkatasubrahmanyamS, MeneghiniMD, GinJW, JenningsJL, et al. (2004) A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol 2: E131.
23. MizuguchiG, ShenX, LandryJ, WuWH, SenS, et al. (2004) ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303 : 343–348.
24. CherryCM, MatunisEL (2010) Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell 6 : 557–567.
25. BuszczakM, PaternoS, SpradlingAC (2009) Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science 323 : 248–251.
26. LiB, PattendenSG, LeeD, GutierrezJ, ChenJ, et al. (2005) Preferential occupancy of histone variant H2AZ at inactive promoters influences local histone modifications and chromatin remodeling. Proc Natl Acad Sci U S A 102 : 18385–18390.
27. SwaminathanJ, BaxterEM, CorcesVG (2005) The role of histone H2Av variant replacement and histone H4 acetylation in the establishment of Drosophila heterochromatin. Genes Dev 19 : 65–76.
28. MavrichTN, JiangC, IoshikhesIP, LiX, VentersBJ, et al. (2008) Nucleosome organization in the Drosophila genome. Nature 453 : 358–362.
29. RaisnerRM, HartleyPD, MeneghiniMD, BaoMZ, LiuCL, et al. (2005) Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 123 : 233–248.
30. ZhangH, RobertsDN, CairnsBR (2005) Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123 : 219–231.
31. AbbottDW, IvanovaVS, WangX, BonnerWM, AusioJ (2001) Characterization of the stability and folding of H2A.Z chromatin particles: implications for transcriptional activation. J Biol Chem 276 : 41945–41949.
32. PlacekBJ, HarrisonLN, VillersBM, GlossLM (2005) The H2A.Z/H2B dimer is unstable compared to the dimer containing the major H2A isoform. Protein Sci 14 : 514–522.
33. SutoRK, ClarksonMJ, TremethickDJ, LugerK (2000) Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat Struct Biol 7 : 1121–1124.
34. HuG, CuiK, NorthrupD, LiuC, WangC, et al. (2012) H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 12 : 180–192.
35. LiZ, GadueP, ChenK, JiaoY, TutejaG, et al. (2012) Foxa2 and H2A.Z mediate nucleosome depletion during embryonic stem cell differentiation. Cell 151 : 1608–1616.
36. DealRB, HenikoffS (2010) Gene regulation: A chromatin thermostat. Nature 463 : 887–888.
37. KumarSV, WiggePA (2009) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140 : 136–147.
38. XiR, XieT (2005) Stem cell self-renewal controlled by chromatin remodeling factors. Science 310 : 1487–1489.
39. KroganNJ, KeoghMC, DattaN, SawaC, RyanOW, et al. (2003) A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell 12 : 1565–1576.
40. KuschT, FlorensL, MacdonaldWH, SwansonSK, GlaserRL, et al. (2004) Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 306 : 2084–2087.
41. SadeghiL, BonillaC, StralforsA, EkwallK, SvenssonJP (2011) Podbat: a novel genomic tool reveals Swr1-independent H2A.Z incorporation at gene coding sequences through epigenetic meta-analysis. PLoS Comput Biol 7: e1002163.
42. LimJG, FullerMT (2012) Somatic cell lineage is required for differentiation and not maintenance of germline stem cells in Drosophila testes. Proc Natl Acad Sci U S A 109 : 18477–18481.
43. van DaalA, ElginSC (1992) A histone variant, H2AvD, is essential in Drosophila melanogaster. Mol Biol Cell 3 : 593–602.
44. DeuringR, FantiL, ArmstrongJA, SarteM, PapoulasO, et al. (2000) The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol Cell 5 : 355–365.
45. ChenX, HillerM, SancakY, FullerMT (2005) Tissue-specific TAFs counteract Polycomb to turn on terminal differentiation. Science 310 : 869–872.
46. ZeidlerMP, PerrimonN, StruttDI (1999) Polarity determination in the Drosophila eye: a novel role for unpaired and JAK/STAT signaling. Genes Dev 13 : 1342–1353.
47. OdaH, TsukitaS (1999) Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev Biol 216 : 406–422.
48. RigglemanB, SchedlP, WieschausE (1990) Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell 63 : 549–560.
49. PatelNH, SnowPM, GoodmanCS (1987) Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell 48 : 975–988.
50. DubreuilR, ByersTJ, BrantonD, GoldsteinLS, KiehartDP (1987) Drosophilia spectrin. I. Characterization of the purified protein. J Cell Biol 105 : 2095–2102.
51. BoniniNM, LeisersonWM, BenzerS (1993) The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72 : 379–395.
52. OdaH, UemuraT, HaradaY, IwaiY, TakeichiM (1994) A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol 165 : 716–726.
53. LeachTJ, MazzeoM, ChotkowskiHL, MadiganJP, WotringMG, et al. (2000) Histone H2A.Z is widely but nonrandomly distributed in chromosomes of Drosophila melanogaster. J Biol Chem 275 : 23267–23272.
54. LiMA, AllsJD, AvanciniRM, KooK, GodtD (2003) The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol 5 : 994–1000.
55. FlahertyMS, SalisP, EvansCJ, EkasLA, MaroufA, et al. (2010) chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell 18 : 556–568.
56. TsukiyamaT, DanielC, TamkunJ, WuC (1995) ISWI, a member of the SWI2/SNF2 ATPase family, encodes the 140 kDa subunit of the nucleosome remodeling factor. Cell 83 : 1021–1026.
57. SchneiderCA, RasbandWS, EliceiriKW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9 : 671–675.
Štítky
Genetika Reprodukční medicína
Článek Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis inČlánek Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 11
-
Všechny články tohoto čísla
- Molecular Recognition by a Polymorphic Cell Surface Receptor Governs Cooperative Behaviors in Bacteria
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis in
- Retrotransposon Silencing During Embryogenesis: Cuts in LINE
- Roles of XRCC2, RAD51B and RAD51D in RAD51-Independent SSA Recombination
- Parallel Evolution of Chordate Regulatory Code for Development
- A Genetic Approach to the Recruitment of PRC2 at the Locus
- Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
- Elevated Mutagenesis Does Not Explain the Increased Frequency of Antibiotic Resistant Mutants in Starved Aging Colonies
- Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
- Interplay between Active Chromatin Marks and RNA-Directed DNA Methylation in
- Recombinogenic Conditions Influence Partner Choice in Spontaneous Mitotic Recombination
- Crosstalk between NSL Histone Acetyltransferase and MLL/SET Complexes: NSL Complex Functions in Promoting Histone H3K4 Di-Methylation Activity by MLL/SET Complexes
- A New Role for the GARP Complex in MicroRNA-Mediated Gene Regulation
- RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Loss of DNMT1o Disrupts Imprinted X Chromosome Inactivation and Accentuates Placental Defects in Females
- Inhibition of the Smc5/6 Complex during Meiosis Perturbs Joint Molecule Formation and Resolution without Significantly Changing Crossover or Non-crossover Levels
- Disruption of Lipid Metabolism Genes Causes Tissue Overgrowth Associated with Altered Developmental Signaling
- Translation Initiation Factors eIF3 and HCR1 Control Translation Termination and Stop Codon Read-Through in Yeast Cells
- Recruitment of TREX to the Transcription Machinery by Its Direct Binding to the Phospho-CTD of RNA Polymerase II
- MYB97, MYB101 and MYB120 Function as Male Factors That Control Pollen Tube-Synergid Interaction in Fertilization
- Oct4 Is Required ∼E7.5 for Proliferation in the Primitive Streak
- Contrasted Patterns of Crossover and Non-crossover at Meiotic Recombination Hotspots
- Transposable Prophage Mu Is Organized as a Stable Chromosomal Domain of
- Ash1l Methylates Lys36 of Histone H3 Independently of Transcriptional Elongation to Counteract Polycomb Silencing
- Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects
- Genomic Mechanisms Accounting for the Adaptation to Parasitism in Nematode-Trapping Fungi
- Decoding a Signature-Based Model of Transcription Cofactor Recruitment Dictated by Cardinal Cis-Regulatory Elements in Proximal Promoter Regions
- Removal of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
- Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
- Activin Signaling Targeted by Insulin/dFOXO Regulates Aging and Muscle Proteostasis in
- Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans
- Demographic Divergence History of Pied Flycatcher and Collared Flycatcher Inferred from Whole-Genome Re-sequencing Data
- Recurrent Tissue-Specific mtDNA Mutations Are Common in Humans
- The Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
- The Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
- Reconstructing the Population Genetic History of the Caribbean
- and Are Required for Growth under Iron-Limiting Conditions
- Whole Genome, Whole Population Sequencing Reveals That Loss of Signaling Networks Is the Major Adaptive Strategy in a Constant Environment
- Neuron-Specific Feeding RNAi in and Its Use in a Screen for Essential Genes Required for GABA Neuron Function
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
- Mouse BAZ1A (ACF1) Is Dispensable for Double-Strand Break Repair but Is Essential for Averting Improper Gene Expression during Spermatogenesis
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- DUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
- Pathways-Driven Sparse Regression Identifies Pathways and Genes Associated with High-Density Lipoprotein Cholesterol in Two Asian Cohorts
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- and Are Required for Growth under Iron-Limiting Conditions
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



