-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRetrotransposon Silencing During Embryogenesis: Cuts in LINE
article has not abstract
Published in the journal: . PLoS Genet 9(11): e32767. doi:10.1371/journal.pgen.1003944
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003944Summary
article has not abstract
Fossilised mobile genetic elements, including Long Interspersed Element-1 (LINE-1 or L1) retrotransposons, comprise at least two-thirds of the human genome [1]. Their molecular history is reminiscent of speciation and natural selection, where, as noted by Carl Sagan, “Extinction is the rule. Survival is the exception” [2]. Broadly, the life cycle of a retrotransposon begins with innovation to evade host genome surveillance, followed by “copy-and-paste” retrotransposition and, finally, quiescence as a result of host defence adaptation. Before being tamed, a new or newly reactivated retrotransposon can undergo massive copy number amplification. For instance, more than one million copies of the primate-specific Short Interspersed Element (SINE) Alu comprise 11% of the human genome [3]. Even more impressively, approximately 500,000 copies of a single retrotransposon superfamily, Gypsy, occupy nearly half of the maize genome [4]. Thus, retrotransposons can overrun a genome within a brief evolutionary period, making their suppression a high host priority.
Retrotransposition requires transcription of an RNA template for DNA-primed reverse transcription. Several cellular defence mechanisms have evolved to hinder this process, including: 1) promoter methylation and heterochromatinisation, 2) degradation of retrotransposon transcripts via RNA interference (RNAi), and 3) host factor prevention or destabilisation of reverse transcription. To describe in detail just one of a myriad of specific inhibitory pathways, repeat associated small interfering RNAs (rasiRNAs) are present in plant, worm, fly, fish, and mouse gametes and, therefore, represent a highly conserved defence against germ line retrotransposition [5]–[8]. A plausible model of rasiRNA biogenesis involves bidirectional transcription of opposed retrotransposon promoters [9], [10], resulting in the formation of double-stranded RNAs (Figure 1). These are cleaved by Dicer (DCR) and then assembled with Argonaute (AGO) and other proteins into the RNA-induced silencing complex (RISC) that, in turn, produces RNAi against retrotransposon transcripts [11]. The suppressive influence of rasiRNAs, in concert with other pathways, may explain why retrotransposition is more common during embryogenesis than in gametes [12], [13]. Importantly, although rasiRNAs have been found in stem cells and soma, their capacity to suppress retrotransposition during development is relatively unexplored [14]–[16].
Fig. 1. rasiRNAs inhibit LINE-1 expression in mESCs. 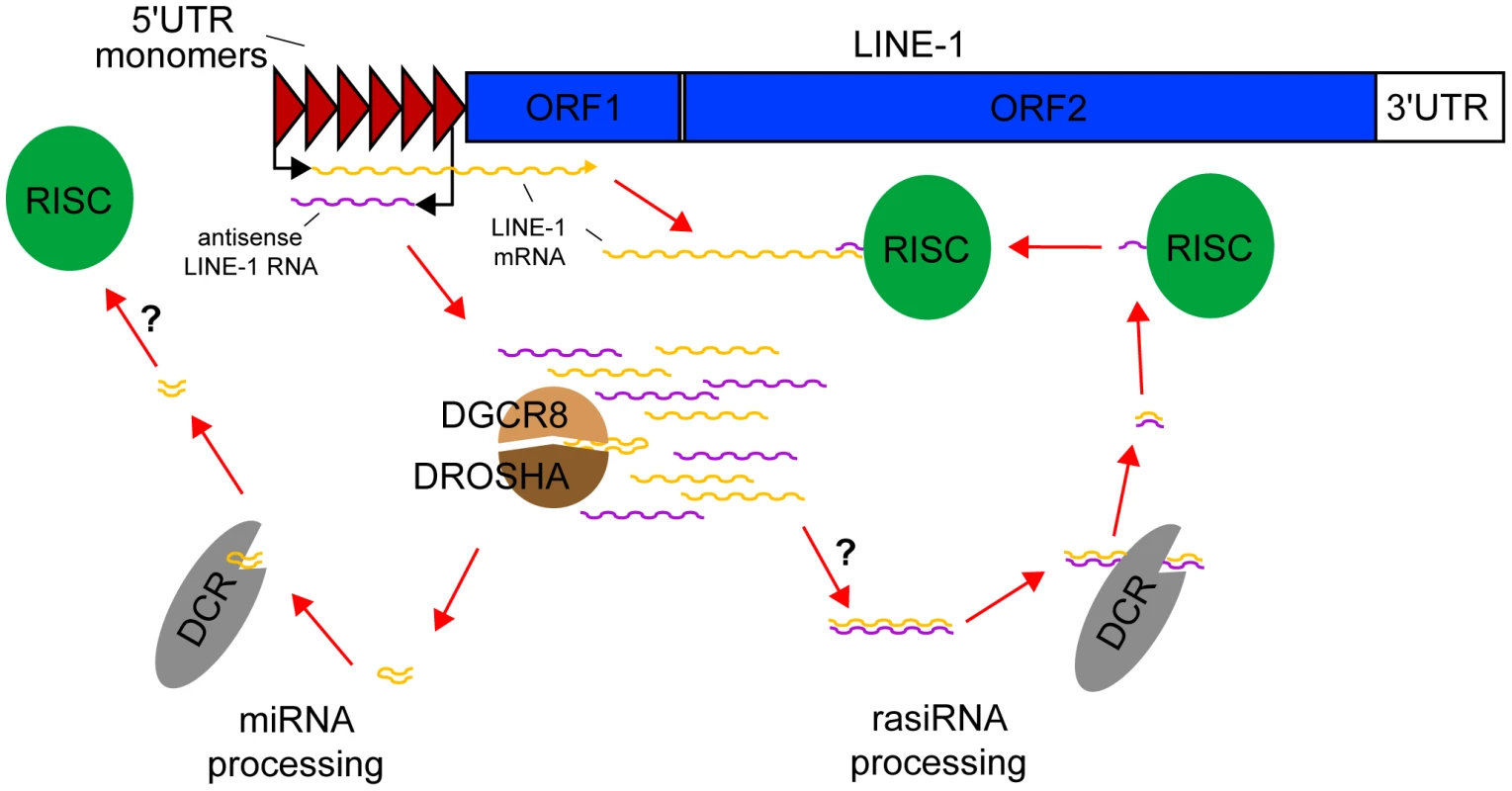
Mouse LINE-1s are comprised of two ORFs flanked by 5′ and 3′UTRs. Several monomers in the 5′UTR provide promoter activity. Following the LINE-1 expression and copy number variation data of Ciaudo et al., bidirectional transcription of the 5′UTR generates sense and antisense LINE-1 RNAs. The Drosha-DGCR8 Microprocessor cleaves these precursors into pre-miRNAs, which are processed into miRNAs by Dicer, but may not be loaded into the RISC complex. By contrast, double-stranded RNAs potentially formed by sense/antisense pairing of LINE-1 RNAs are also cleaved by Dicer but here generate rasiRNAs, loaded into the RISC complex, which degrade canonical LINE-1 mRNAs. Dicer also appears to mediate LINE-1 promoter methylation (not shown). In this issue of PLOS Genetics, Ciaudo et al. [17] describe rasiRNA-mediated suppression of LINE-1 activity in mouse embryonic stem cells (mESCs). Focusing on the L1-Tf subfamily, where they previously described an unusual rasiRNA signature mapping to the 5′UTR [15], Ciaudo et al. observed that knock-out of Dicer markedly decreases L1-Tf promoter methylation and increases L1-Tf transcription, translation, and copy number in cultured mESCs. In particular, DCR−/− mESCs accumulate a remarkable 860 L1-Tf copies (greater than five megabases of genomic DNA) per cell over 20 passages, versus 255 copies per cell in DCRFlx/Flx controls, based on SYBR-Green qPCR targeting the L1-Tf 5′UTR. High-throughput small RNA sequencing then confirmed that DCR−/− mESCs were depleted of approximately 22 nt molecules found in wild-type mESCs, immunoprecipitated with AGO2 and aligned to L1-Tf, and therefore resembling rasiRNAs. Hence, LINE-1 activation in DCR−/− mESCs coincides with rasiRNA depletion and is also possibly influenced by ablation of Dicer-mediated LINE-1 promoter methylation.
Intriguingly, a second class of Dicer - and AGO2-independent small RNAs were found to “paint” the L1-Tf 5′UTR. Again, assessing L1-Tf transcription and copy number, Ciaudo et al. found that deletion of XRN2 and DGCR8, respective members of the RNA surveillance and Drosha-DGCR8 Microprocessor pathways, led to increased L1-Tf transcription but not copy number amplification. These observations agree with other recent reports of small RNAs immunoprecipitated with DGCR8 and enriched for LINE-1 sequences [18], as well as evidence of elevated L1-Tf expression in DGCR8−/− mESCs [19]. As a final experiment, Ciaudo et al. complemented DCR−/− mESCs with human Dicer and found that these cells recapitulated wild-type mESC LINE-1 suppression and differentiated normally, unlike DCR−/− mESCs.
Evidence for a reciprocal relationship between rasiRNA depletion and LINE-1 activation significantly advances our understanding of RNAi-mediated control of retrotransposition during mammalian embryogenesis. These data are also important because they address a longstanding question of why rasiRNAs cannot be consistently detected in mammalian somatic cells: small RNAs generated by RNA surveillance and the Microprocessor may cleave the same pool of precursor LINE-1 mRNAs processed by Dicer and obscure rasiRNA detection (Figure 1). As Ciaudo et al. note, it is possible that insertional mutagenesis caused by LINE-1 contributes to the reported differentiation defects for DCR−/− mESCs [20], though it is unclear why lesser but still substantial LINE-1 activity is tolerated by wild-type mESCs. Interestingly, experiments using engineered LINE-1 reporters have shown elsewhere [16], [19] that mutation of Dicer or the Microprocessor increases LINE-1 mobilisation in cancer cells, with the latter result at odds with data generated here from mESCs. Future advances in high-throughput sequencing and single cell genomics should enable characterisation of endogenous LINE-1 mobilisation events in stem cells and further delineate the multifaceted roles of Dicer and other factors in LINE-1 inhibition.
Zdroje
1. de KoningAP, GuW, CastoeTA, BatzerMA, PollockDD (2011) Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet 7: e1002384 doi:10.1371/journal.pgen.1002384
2. Sagan C, Druyan A (2006) The varieties of scientific experience : a personal view of the search for God. New York: Penguin Press. xviii, 284 p. p.
3. LanderES, LintonLM, BirrenB, NusbaumC, ZodyMC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409 : 860–921.
4. SchnablePS, WareD, FultonRS, SteinJC, WeiF, et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326 : 1112–1115.
5. CzechB, MaloneCD, ZhouR, StarkA, SchlingeheydeC, et al. (2008) An endogenous small interfering RNA pathway in Drosophila. Nature 453 : 798–802.
6. SijenT, PlasterkRH (2003) Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature 426 : 310–314.
7. SlotkinRK, VaughnM, BorgesF, TanurdzicM, BeckerJD, et al. (2009) Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136 : 461–472.
8. WatanabeT, TotokiY, ToyodaA, KanedaM, Kuramochi-MiyagawaS, et al. (2008) Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453 : 539–543.
9. SpeekM (2001) Antisense promoter of human L1 retrotransposon drives transcription of adjacent cellular genes. Mol Cell Biol 21 : 1973–1985.
10. ZemojtelT, PenzkoferT, SchultzJ, DandekarT, BadgeR, et al. (2007) Exonization of active mouse L1s: a driver of transcriptome evolution? BMC Genomics 8 : 392.
11. HammondSM, BernsteinE, BeachD, HannonGJ (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404 : 293–296.
12. Garcia-PerezJL, MarchettoMC, MuotriAR, CoufalNG, GageFH, et al. (2007) LINE-1 retrotransposition in human embryonic stem cells. Hum Mol Genet 16 : 1569–1577.
13. KanoH, GodoyI, CourtneyC, VetterMR, GertonGL, et al. (2009) L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev 23 : 1303–1312.
14. BabiarzJE, RubyJG, WangY, BartelDP, BlellochR (2008) Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev 22 : 2773–2785.
15. ChowJC, CiaudoC, FazzariMJ, MiseN, ServantN, et al. (2010) LINE-1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell 141 : 956–969.
16. YangN, KazazianHHJr (2006) L1 retrotransposition is suppressed by endogenously encoded small interfering RNAs in human cultured cells. Nat Struct Mol Biol 13 : 763–771.
17. CiaudoC, JayF, OkamotoI, ChenCJ, SarazinA, et al. (2013) RNAi-dependent and independent control of LINE1 mobility and accumulation in mouse Embryonic Stem Cells. PLoS Genet 9 e1003791 doi: 10.1371/journal.pgen.1003791
18. MaciasS, PlassM, StajudaA, MichlewskiG, EyrasE, et al. (2012) DGCR8 HITS-CLIP reveals novel functions for the Microprocessor. Nat Struct Mol Biol 19 : 760–766.
19. HerasSR, MaciasS, PlassM, FernandezN, CanoD, et al. (2013) The Microprocessor controls the activity of mammalian retrotransposons. Nat Struct Mol Biol doi:10.1038/nsmb.2658
20. KanellopoulouC, MuljoSA, KungAL, GanesanS, DrapkinR, et al. (2005) Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 19 : 489–501.
Štítky
Genetika Reprodukční medicína
Článek Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis inČlánek Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 11
-
Všechny články tohoto čísla
- Molecular Recognition by a Polymorphic Cell Surface Receptor Governs Cooperative Behaviors in Bacteria
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis in
- Retrotransposon Silencing During Embryogenesis: Cuts in LINE
- Roles of XRCC2, RAD51B and RAD51D in RAD51-Independent SSA Recombination
- Parallel Evolution of Chordate Regulatory Code for Development
- A Genetic Approach to the Recruitment of PRC2 at the Locus
- Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
- Elevated Mutagenesis Does Not Explain the Increased Frequency of Antibiotic Resistant Mutants in Starved Aging Colonies
- Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
- Interplay between Active Chromatin Marks and RNA-Directed DNA Methylation in
- Recombinogenic Conditions Influence Partner Choice in Spontaneous Mitotic Recombination
- Crosstalk between NSL Histone Acetyltransferase and MLL/SET Complexes: NSL Complex Functions in Promoting Histone H3K4 Di-Methylation Activity by MLL/SET Complexes
- A New Role for the GARP Complex in MicroRNA-Mediated Gene Regulation
- RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Loss of DNMT1o Disrupts Imprinted X Chromosome Inactivation and Accentuates Placental Defects in Females
- Inhibition of the Smc5/6 Complex during Meiosis Perturbs Joint Molecule Formation and Resolution without Significantly Changing Crossover or Non-crossover Levels
- Disruption of Lipid Metabolism Genes Causes Tissue Overgrowth Associated with Altered Developmental Signaling
- Translation Initiation Factors eIF3 and HCR1 Control Translation Termination and Stop Codon Read-Through in Yeast Cells
- Recruitment of TREX to the Transcription Machinery by Its Direct Binding to the Phospho-CTD of RNA Polymerase II
- MYB97, MYB101 and MYB120 Function as Male Factors That Control Pollen Tube-Synergid Interaction in Fertilization
- Oct4 Is Required ∼E7.5 for Proliferation in the Primitive Streak
- Contrasted Patterns of Crossover and Non-crossover at Meiotic Recombination Hotspots
- Transposable Prophage Mu Is Organized as a Stable Chromosomal Domain of
- Ash1l Methylates Lys36 of Histone H3 Independently of Transcriptional Elongation to Counteract Polycomb Silencing
- Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects
- Genomic Mechanisms Accounting for the Adaptation to Parasitism in Nematode-Trapping Fungi
- Decoding a Signature-Based Model of Transcription Cofactor Recruitment Dictated by Cardinal Cis-Regulatory Elements in Proximal Promoter Regions
- Removal of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
- Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
- Activin Signaling Targeted by Insulin/dFOXO Regulates Aging and Muscle Proteostasis in
- Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans
- Demographic Divergence History of Pied Flycatcher and Collared Flycatcher Inferred from Whole-Genome Re-sequencing Data
- Recurrent Tissue-Specific mtDNA Mutations Are Common in Humans
- The Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
- The Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
- Reconstructing the Population Genetic History of the Caribbean
- and Are Required for Growth under Iron-Limiting Conditions
- Whole Genome, Whole Population Sequencing Reveals That Loss of Signaling Networks Is the Major Adaptive Strategy in a Constant Environment
- Neuron-Specific Feeding RNAi in and Its Use in a Screen for Essential Genes Required for GABA Neuron Function
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
- Mouse BAZ1A (ACF1) Is Dispensable for Double-Strand Break Repair but Is Essential for Averting Improper Gene Expression during Spermatogenesis
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- DUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
- Pathways-Driven Sparse Regression Identifies Pathways and Genes Associated with High-Density Lipoprotein Cholesterol in Two Asian Cohorts
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- and Are Required for Growth under Iron-Limiting Conditions
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



