-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
The human double-homeodomain retrogene DUX4 is expressed in the testis and epigenetically repressed in somatic tissues. Facioscapulohumeral muscular dystrophy (FSHD) is caused by mutations that decrease the epigenetic repression of DUX4 in somatic tissues and result in mis-expression of this transcription factor in skeletal muscle. DUX4 binds sites in the human genome that contain a double-homeobox sequence motif, including sites in unique regions of the genome as well as many sites in repetitive elements. Using ChIP-seq and RNA-seq on myoblasts transduced with DUX4 we show that DUX4 binds and activates transcription of mammalian apparent LTR-retrotransposons (MaLRs), endogenous retrovirus (ERVL and ERVK) elements, and pericentromeric satellite HSATII sequences. Some DUX4-activated MaLR and ERV elements create novel promoters for genes, long non-coding RNAs, and antisense transcripts. Many of these novel transcripts are expressed in FSHD muscle cells but not control cells, and thus might contribute to FSHD pathology. For example, HEY1, a repressor of myogenesis, is activated by DUX4 through a MaLR promoter. DUX4-bound motifs, including those in repetitive elements, show evolutionary conservation and some repeat-initiated transcripts are expressed in healthy testis, the normal expression site of DUX4, but more rarely in other somatic tissues. Testis expression patterns are known to have evolved rapidly in mammals, but the mechanisms behind this rapid change have not yet been identified: our results suggest that mobilization of MaLR and ERV elements during mammalian evolution altered germline gene expression patterns through transcriptional activation by DUX4. Our findings demonstrate a role for DUX4 and repetitive elements in mammalian germline evolution and in FSHD muscular dystrophy.
Published in the journal: . PLoS Genet 9(11): e32767. doi:10.1371/journal.pgen.1003947
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003947Summary
The human double-homeodomain retrogene DUX4 is expressed in the testis and epigenetically repressed in somatic tissues. Facioscapulohumeral muscular dystrophy (FSHD) is caused by mutations that decrease the epigenetic repression of DUX4 in somatic tissues and result in mis-expression of this transcription factor in skeletal muscle. DUX4 binds sites in the human genome that contain a double-homeobox sequence motif, including sites in unique regions of the genome as well as many sites in repetitive elements. Using ChIP-seq and RNA-seq on myoblasts transduced with DUX4 we show that DUX4 binds and activates transcription of mammalian apparent LTR-retrotransposons (MaLRs), endogenous retrovirus (ERVL and ERVK) elements, and pericentromeric satellite HSATII sequences. Some DUX4-activated MaLR and ERV elements create novel promoters for genes, long non-coding RNAs, and antisense transcripts. Many of these novel transcripts are expressed in FSHD muscle cells but not control cells, and thus might contribute to FSHD pathology. For example, HEY1, a repressor of myogenesis, is activated by DUX4 through a MaLR promoter. DUX4-bound motifs, including those in repetitive elements, show evolutionary conservation and some repeat-initiated transcripts are expressed in healthy testis, the normal expression site of DUX4, but more rarely in other somatic tissues. Testis expression patterns are known to have evolved rapidly in mammals, but the mechanisms behind this rapid change have not yet been identified: our results suggest that mobilization of MaLR and ERV elements during mammalian evolution altered germline gene expression patterns through transcriptional activation by DUX4. Our findings demonstrate a role for DUX4 and repetitive elements in mammalian germline evolution and in FSHD muscular dystrophy.
Introduction
The transcription factor DUX4 is a member of a small family of double-homeodomain genes that also includes paralogs DUXA, DUXB, DUXBL, DUXC and murine Dux [1]–[3]. The primate-specific DUX4 gene likely arose from retrotransposition of the DUXC mRNA, with subsequent deletion of the DUXC gene from the primate genome [3]. DUX4 exists in the primate genome as part of a ∼3.3 kb repeat unit called D4Z4, found in macrosatellite arrays in the subtelomeric regions of human chromosomes 4 and 10. Poorly studied arrays are also found in other genomic regions and appear to contain interrupted versions of the DUX4 ORF [4], [5]. The number of D4Z4 units in each array varies between human individuals [6], and array size and number of genomic loci vary between different primate species [7].
DUX4 is expressed in germ-line cells of the testis and is epigenetically repressed in somatic tissues [8]–[11], likely in part through repeat-mediated repression [12]. Deletions of the D4Z4 array to fewer than 10 repeat units or mutations in SMCHD1, a gene necessary for repeat-mediated epigenetic repression, result in decreased epigenetic repression of DUX4 in skeletal muscle, causing a human muscle disease, facioscapulohumeral muscular dystrophy (FSHD; OMIM #158900, #158901) [6], [9], [13]–[15]. The decreased epigenetic repression results in occasional bursts of DUX4 expression in a subset of nuclei in FSHD muscle cells [9], [16], which appears to cause death of expressing muscle cells [17]–[19]. Our prior work showed, using microarrays and ChIP-seq, that mis-expression of DUX4 in human skeletal muscle activates the expression of many genes normally expressed in the germline and that DUX4 binds a double-homeobox motif at ∼60,000 sites in the mappable genome [10]. Although many DUX4 binding sites overlap non-repetitive regulatory elements of its activated genes, more than half of the ChIP-seq peaks overlap repeat elements of the MaLR class [10].
MaLRs, or mammalian apparent LTR-retrotransposons, are distantly related to endogenous retroviruses (ERVs), and comprise ∼3.7% of the human genome with ∼350,000 copies of various subclasses of MaLR elements [20]–[23]. Like ERVs, MaLRs are selfish elements that spread in the genome by a copy-paste mechanism called retrotransposition (transcription, reverse transcription and reintegration). ERVs and MaLRs have a pair of long-terminal repeats (LTRs) that act as strong promoters and that flank one or more open reading frames (ORFs). Post-insertion deletions mediated by homology between the two LTRs of an ERV or MaLR element often leave single (or “solo”) LTR elements in the genome. The internal ORFs of conventional ERVs encode the gag, pro, pol, and sometimes env proteins needed for replication and re-integration, whereas the single MaLR ORF encodes a protein with an ∼90-aa stretch of homology to the ERVL gag protein, suggesting that MaLRs derived from ERVL-like retrotransposons [24]. The homology to the ERVL gag protein and the absence of the other proteins necessary for ERV replication suggest that MaLRs might rely on concomitantly expressed ERVs to provide these other proteins. Most human ERV and MaLR elements inserted into the primate genome before the divergence of Old and New World monkeys and are generally thought to be no longer capable of retrotransposition (unlike in rodents, where ERVs and MaLRs are still active). However, reports of very rare polymorphic ERV and MaLR insertions in humans suggest that occasional transposition events still occur in the human population [25], [26].
Retrotransposition provides a way to spread regulatory sequences to large numbers of new genomic locations in short evolutionary time. Some families of repetitive elements appear to have been involved in large-scale rewiring of transcriptional networks [27], [28] and a number of cellular transcription factors have been shown to bind repetitive elements [29]–[31]. Repeats, especially LTRs, can be co-opted (or “exapted”) to act as promoters for genes of the host genome [32], [33]. For example, when placentation evolved during early mammalian divergence, a large number of genes acquired expression in endometrial cells via upstream insertion of the eutherian-specific MER20 transposon [34]. In another example, binding of the OCT4 and NANOG transcription factors to various primate-specific repeats explains many of the differences observed between the transcriptional networks of human and mouse ES cells [31]. To date, no such repeat-mediated rewiring of the male germ cell transcriptional network has been reported, yet testis expression patterns are known have evolved rapidly in mammals [35].
To determine whether DUX4 binding to repetitive elements can affect transcriptional networks, we analyzed ChIP-seq and RNA-seq datasets in skeletal muscle cells that ectopically express DUX4, as well as RNA-seq data from FSHD patient and control muscle cells. We find that repetitive elements are bound and activated by DUX4, including primate-specific MaLR and ERV LTRs, and that some full-length repeat elements are transcribed in response to DUX4. In addition, some DUX4-bound LTRs form novel first exons of annotated genes, long-noncoding (lnc) RNAs, and antisense RNAs. Together, our findings demonstrate that DUX4's transcriptional network is mediated in part through binding to repetitive elements, including some primate-specific retroelements that could contribute to lineage-specific patterns of gene expression [35].
Results
DUX4 binds LTR elements, especially of the MaLR family
In previously published work, we observed that many of the ∼60,000 DUX4 binding sites identified by ChIP-seq following expression of DUX4 in human myoblasts overlap MaLR LTR elements [10]. Using a more recent version of the human genome assembly (hg19) and including the X and Y chromosomes, we identified 63,795 DUX4 binding-sites (Table S1). Overall, ∼2/3 of sites are in a repetitive element, more than expected given that ∼45% of the human genome is recognizable as repeats (Figure 1A) [21]. Furthermore, ∼1/3 of DUX4 binding sites are in a MaLR element, ∼10-fold more than would be expected if binding sites were dispersed randomly throughout the genome (Figure 1B). Because DUX4 binds an AT-rich sequence motif, its binding sites might show repeat enrichment based simply on GC content; however, when we randomly sampled genomic locations with similar AT-content to the DUX4 binding site (see Methods), we find that only ∼49% overlapped repeats of any class, and only ∼3% overlapped MaLR elements. In order to determine which subclasses of MaLRs are responsible for the enrichment, and to explore which other repetitive elements might also bind DUX4, we systematically analyzed ChIP-seq data using two complementary approaches.
Fig. 1. Many repeat types are enriched among DUX4 binding sites. 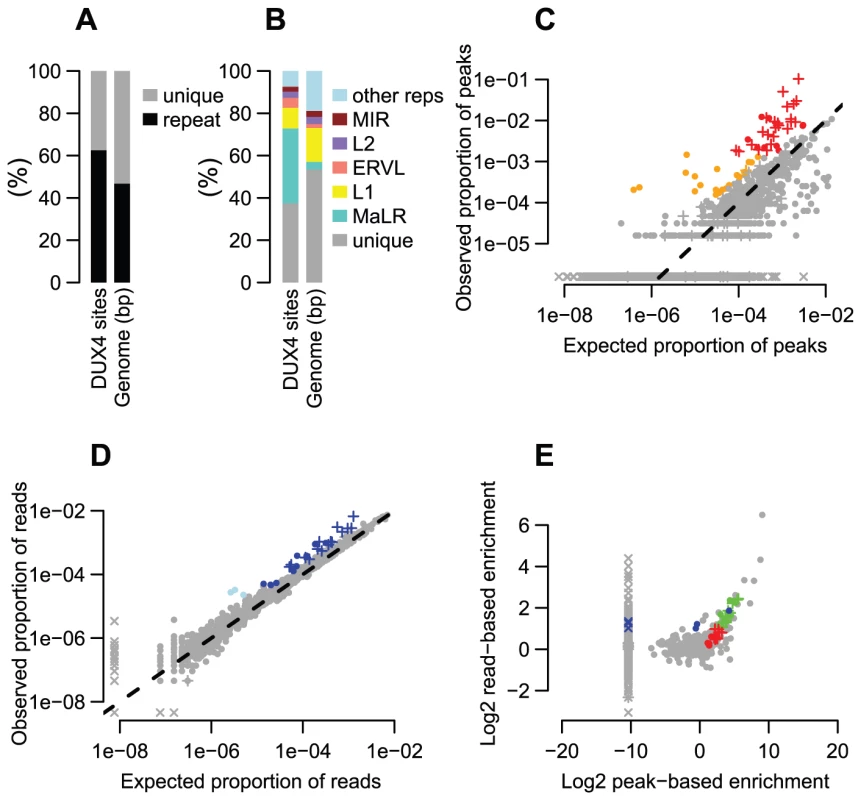
(A) ∼2/3 of DUX4 binding-sites are in repetitive elements, compared to ∼45% of the human genome. (B) Comparing repeat family proportions among DUX4 binding-sites with genome-wide fractions shows ∼10-fold MaLR enrichment. (C) A peak-based method of estimating repeat enrichment uses uniquely-mapped reads, so is blind to recently active repeats; however, it ignores background reads so provides a more sensitive enrichment measure than the read-based estimate (Figures 1D, 1E). 32 repeat types (red) are enriched ≥2-fold with ≥100 peaks (arbitrary thresholds) (Tables 1, S2 and S3); 21 (orange) are rarer in the genome (10–99 peaks) but enriched ≥4-fold. The log10-scaled x-axis shows the proportion of peaks expected to overlap each repeat type if DUX4 binding sites had uniform genomic distribution; the log10-scaled y-axis shows observed proportions. The dashed line represents no enrichment. In all panels, “+” symbols represent MaLR elements and “x” datapoints represent repeat types for which no peaks/reads were observed – these are given an arbitrary low (non-zero) value to ensure visibility on log-scaled plots. (D) The read-based enrichment estimation method examines highly similar repeats as well as uniquely-mappable sequences, but gives a “dampened” enrichment measure due to background reads in ChIP-seq samples (see Methods). 25 repeat types (dark blue) are enriched ≥2-fold, with ≥1000 reads (arbitrary thresholds); 3 (light blue) are rarer among ChIP-seq reads (100–999 reads) but enriched ≥4-fold. (E) The peak-based (x-axis) and read-based (y-axis) methods yield similar results. 19 repeat types (green datapoints, thresholds as in Figures 1A and B for red and dark blue points) were enriched in both analyses; 13 enriched only by the peak-based method (red), and 6 enriched only by the read-based method (blue). Additional repeats (gray datapoints, upper-right quadrant) appear enriched by both methods, but are rare in the genome so do not exceed our arbitrary peak/read thresholds. An analysis of repetitive element content among DUX4 ChIP-seq peaks shows that enrichment is heavily biased towards LTR elements (Figure 1, Table 1, S2 and S3), particularly those of the MaLR class: of 32 enriched repeat types (using arbitrary thresholds of ≥2-fold enrichment and ≥100 DUX4-bound instances), 30 are in the LTR class, and of those, 23/30 are MaLRs. Many subtypes of MaLR-LTRs contribute to the MaLR family-wide enrichment, including the LTRs of THE1 elements (A, B, C and D subfamilies) that were active early in the primate lineage [20] and MLT1 subtypes that were active before mammalian radiation. Outside of the MaLR family, other LTR subtypes also show enrichment, including primate-specific ERVL-LTRs (MLT2A1 and MLT2A2) and hominoid-specific ERVK-LTRs (MER11B and MER11C) (Table 1). Some non-LTR repeats are also enriched among DUX4 binding sites, including the HSAT5 and HSATII satellite repeats and some simple repeats like the (CAAT)n tetranucleotide repeat, although with fewer mappable DUX4 binding sites (<100 DUX4-bound instances). In almost all cases, the consensus sequences for enriched repeats contain at least one DUX binding motif (Figure S1). Analysis of ENCODE ChIP-seq data for 161 other regulatory factors (see Methods) shows that these DUX4-bound regions are not generally bound by other transcription factors (the factor with most overlapping peaks, Runx3, only bound 0.9% of DUX4-bound repeat regions).
Tab. 1. Highly enriched repeat types among DUX4 binding sites. 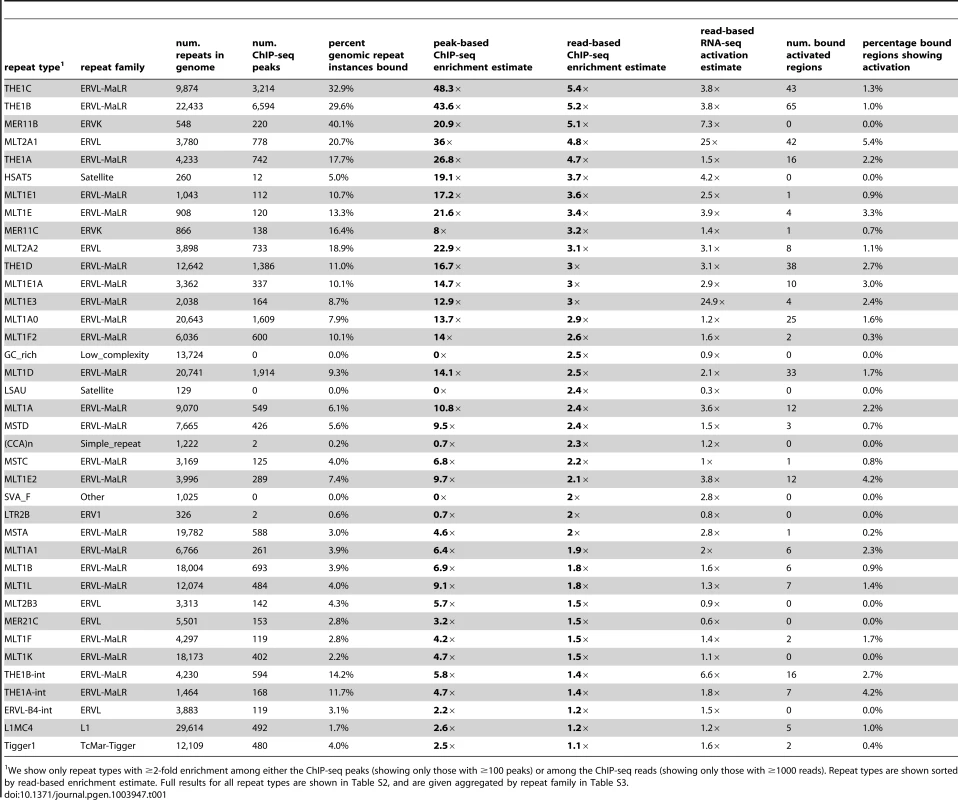
We show only repeat types with ≥2-fold enrichment among either the ChIP-seq peaks (showing only those with ≥100 peaks) or among the ChIP-seq reads (showing only those with ≥1000 reads). Repeat types are shown sorted by read-based enrichment estimate. Full results for all repeat types are shown in Table S2, and are given aggregated by repeat family in Table S3. It remains challenging to accurately map sequence reads to repetitive elements with high sequence identity [36], and the peak-based ChIP-seq analysis we described above is relatively blind to recently mobilized repeats because it only uses sequence reads that map uniquely in the human genome. Therefore we also used a complementary approach, adapting a previously published method [37] that estimates enrichment regardless of whether individual ChIP-seq reads map uniquely in the genome (Figure 1D, Methods). The method aggregates read counts over all genomic copies of each repeat class rather than trying to map reads uniquely to individual repeat instances. Read counts for a test sample are then compared with counts for a control sample to determine enrichment. Although this read-based method can look at recently mobilized repeats, it gives a “dampened” measure of repeat enrichment due to background reads in ChIP-seq samples and is therefore less sensitive to modest levels of enrichment (see Methods). This read-based method identified a similar set of repetitive elements as the peak-based method (Figure 1E), and, in addition, revealed enrichment of a small number of repeat types, such as the SVA_F subfamily, that were not detected using the peak-based method (Figure 1E, Tables 1, S2 and S3). SVAs are composite retroelements that include segments derived from SINE, VNTR and Alu repeats, and have been very recently active in the human, chimpanzee and gorilla genomes [38].
To examine whether DUX4 binding sites are functionally important, especially those in repetitive elements, we determined whether they are evolutionarily conserved. We find that DUX4-bound motifs have tolerated fewer sequence changes than flanking sequences during the evolution of placental mammals (Figure 2A), primates (Figure 2B), and humans (Figure 2C). These findings hold true even if we consider only motifs in repetitive sequences (Figure 2, gray datapoints), demonstrating that at least a subset of DUX4 binding sites in repetitive elements are conserved.
Fig. 2. DUX4-bound motifs are evolutionarily conserved. 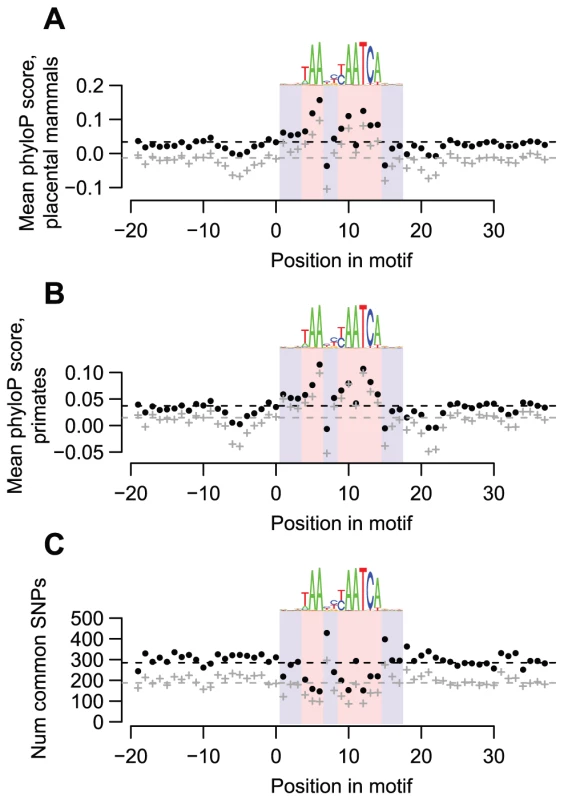
(A) DUX4 motifs are conserved among placental mammals. We obtained phylogenetic conservation scores for placental mammals (phyloP scores) via the UCSC genome browser for the top-scoring DUX4 motif in each of the 63,795 DUX4 ChIP-seq peaks, along with scores for 20 bp flanking regions on each side. For each position in the motif, we show the mean phyloP score across all 63,795 motifs (black datapoints) or across just motifs overlapping repetitive elements (gray datapoints). The dashed lines show overall mean scores for the entire motif plus their flanking regions. We use blue and red background shading to indicate the 17 nucleotide positions that comprise the DUX4 motif, with light red shading showing the least variable nucleotide positions of the motif. A sequence logo of the motif is shown above each graph. It is clear that most of the nucleotide positions that are least variable in the DUX4 motif have higher conservation scores than the surrounding positions. (B) DUX4 motifs are conserved among primates. Primate phyloP scores, plotted as in panel A. (C) Common SNPs are under-represented in DUX4-bound motifs. We used the UCSC genome browser to determine the locations of common SNPs (≥1% minor allele frequency) within any of the 63,795 DUX4-bound motifs and their flanking regions. We plot the number of SNPs found at each position in any of the 63,795 DUX4-bound motifs (black datapoints), or in just motifs overlapping repetitive elements (gray datapoints). It is clear that there are fewer SNPs in most of the nucleotide positions that are least variable in the DUX4 motif than in the surrounding positions. DUX4 activates transcription from bound repetitive elements
In order to determine whether DUX4 binding to repetitive elements results in their transcriptional activation, we generated RNA-seq data (100 nt single-end reads) from DUX4-transduced and control myoblasts. In a conservative analysis, we identified 738 DUX4-activated transcripts within 1 kb on either side of a DUX4 ChIP-seq peak and six DUX4-repressed transcripts (Figure 3A, ≥2-fold change, FDR-adjusted p-value<0.1). These 738 bound and activated regions comprise 1.2% of the 63,795 DUX4-bound sites. Peaks with multiple DUX4-binding sites are more likely to initiate transcripts than those with a single motif (Figure 3B; 2.5% of peaks with more than one site were associated with lenti-DUX4-transcriptional induction, compared to only ∼0.8% peaks with 1 motif; p<10−15, chi-squared test). Peaks with more than one motif also show greater ChIP-seq peak height, a proxy measurement for DUX4 occupancy (Figure 3C).
Fig. 3. Some DUX4-bound repetitive elements are transcriptionally activated by DUX4. 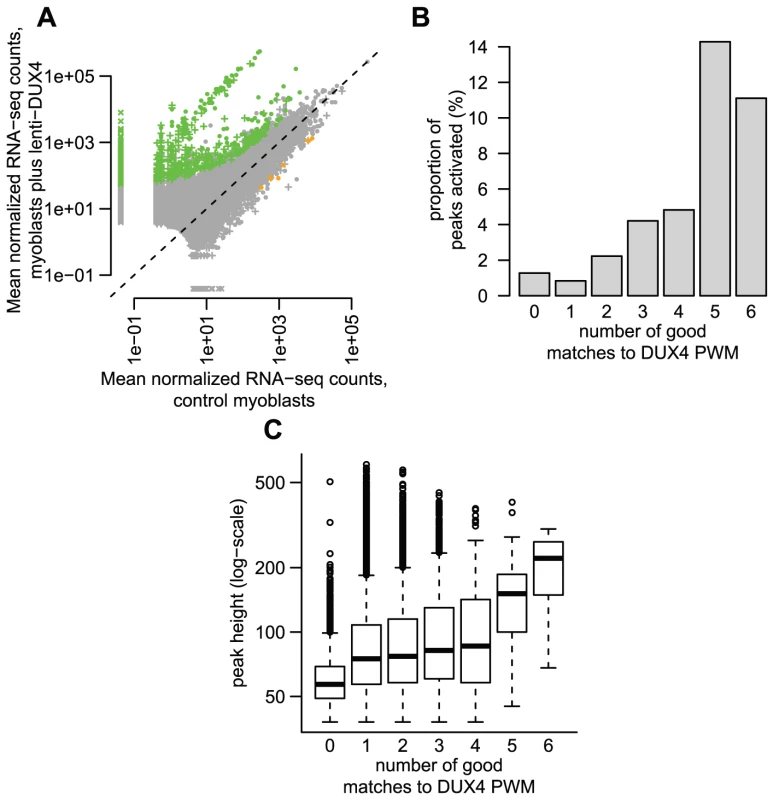
(A) ∼1% of DUX4-bound regions show statistically-significant activation in response to DUX4 in our conservative analysis. We show normalized RNA-seq read counts within an arbitrary distance of 1 kb from DUX4-bound regions (peaks), comparing counts averaged over two myoblast lines that do not express DUX4 (x-axis) to counts averaged over two myoblast lines transduced with lentiviral DUX4 (y-axis). Only regions with at least 10 reads summed over all four samples are shown, and counts are plotted on a log scale. The 738 regions shown as green points show statistically significant activation in response to DUX4 (≥2-fold activation, FDR-adjusted p-value≤0.1) and the 6 orange points show significant repression (same thresholds). Regions surrounding DUX4-bound repeats are shown as “+” symbols, with dots representing DUX4-bound unique regions. Some regions (“x” symbols) had normalized counts of 0 in one condition and are plotted at an arbitrary low value so they appear on a log-scale. (B) DUX4-bound regions with more predicted DUX4 binding motifs are more likely to be transcriptionally activated. The y-axis value gives the percentage of regions that are transcriptionally activated by DUX4 (≥2-fold, FDR-adjusted p-value≤0.1). (C) DUX4-bound regions with more predicted DUX4 binding motifs have greater DUX4 occupancy, using ChIP-seq peak height as a proxy for DUX4 occupancy (shown on a log-scale). DUX4-bound repetitive elements are just as likely to initiate a transcript as DUX4 binding sites in unique sequence (∼1.2% of both classes show activation), with 454/738 (∼62%) of DUX4-initiated transcripts arising in or near a repetitive element. The DUX4-bound repeat types most likely to be transcribed are HSATII elements (23% of bound HSATII repeats show activation) and MLT2A1 ERVL-LTRs (5.4% of bound MLT2A1 LTRs are activated) (Tables 1, S2 and S3). Some of this effect might be explained by the number of DUX4-binding sites within each peak: 65% of MLT2A1 elements contain more than one good DUX4-motif, compared to only 9% of a class of elements that are less frequently activated, the THE1B-MaLRs (1.0% bound THE1Bs are activated).
At least 180 MaLR and ERV internal regions are activated by DUX4
To explore the biological significance of DUX4's ability to bind and activate various repetitive elements, we used RNA-seq data to examine the types of transcripts that result from repeat activation. First, we asked whether full-length repetitive elements are activated. 100,864 regions of the human genome assembly are annotated as internal regions of ERV or MaLR elements, or fragments thereof, and many of these repetitive elements are old enough to have acquired sequence changes that allow unique mapping of short sequence reads. RNA-seq data from DUX4-transduced myoblasts shows that 184 of these regions are activated in the presence of DUX4 (≥2-fold, FDR-adjusted p-value≤0.1), of which 120 are MaLRs and the rest a mix of other ERV classes. These activated internal MaLR/ERV regions tend to be flanked on one or both sides by a DUX4-bound LTR, where transcription seems to initiate. Repeats whose internal regions are activated tend to be younger than repeats that do not show obvious activation, considering only repeats flanked by at least one DUX4-bound LTR (Figure S2). It is tempting to suggest that this finding indicates that repeats have been evolving towards better DUX4 response, but might more likely reflect the fact that, because less evolutionary time has elapsed, younger repeats have retained sequence elements needed to produce stable transcripts, like TATA boxes and polyadenylation signals.
Using RT-PCR, we were able to verify DUX4-mediated activation of a THE1C-MaLR element and one ERVL element, but not of a second ERVL element (Table 2, Figures S3, S4 and S5). Our inability to confirm activation of one of the ERVLs could be explained if mismapping of RNA-seq reads among highly related ERVL elements misled our choice of an individual element from which to design primers for this assay.
Tab. 2. RT-PCR confirms novel transcripts and shows their presence in testis and FHSD patient cells. 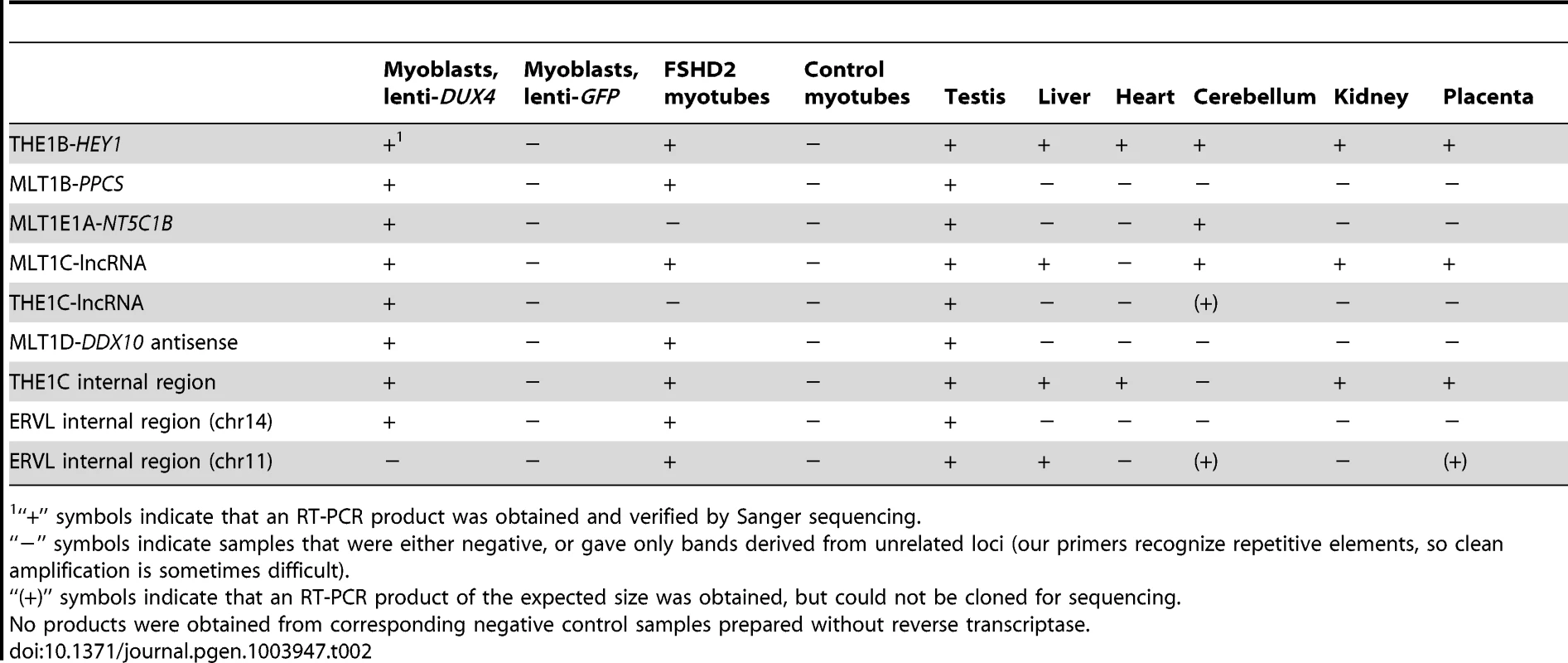
“+” symbols indicate that an RT-PCR product was obtained and verified by Sanger sequencing. Of these 184 transcriptionally activated ERV or MaLR internal elements, only one contains an ORF exceeding 300 amino acids in length. This full-length THE1D-MaLR internal region aligns to the THE1 consensus sequence without stops or frameshifts, and encodes a 464 amino acid ORF. Because the function of MaLR-encoded proteins is unknown, it is difficult to interpret this finding, but we note that this chromosome 7 THE1 is the only THE1 element in the genome that encodes an uninterrupted ORF and thus is a candidate “active” MaLR element. To examine the possibility that this ORF might be preserved as a domesticated protein, we collected its sequence from other primate genomes. We found that although the ORF is also maintained in chimpanzee and gorilla, it is interrupted by stop codons and/or frameshifts in orangutan, macaque, baboon, and marmoset. The lack of conservation among primates suggests that the ORF does not confer selective advantage. 17 other internal regions contain one or more ORFs exceeding 200 amino acids (arbitrary threshold) and encode fragments of various THE1 internal ORFs as well as fragments of some ERV gag and pol ORFs, although none encode full-length proteins.
DUX4-bound repetitive elements form alternative promoters for human genes
Repetitive elements, especially LTRs, can be co-opted as alternative promoters for mammalian genes [32], [33] and can rewire transcriptional networks during evolution [27], [28], [31]. Therefore, acquisition of the DUX4 retrogene and the spread of repetitive elements around the genome had the potential to alter germ cell promoter usage and the germ cell transcriptional network during primate evolution. Analysis of spliced RNA-seq reads that join a DUX4-bound region with a gene sequence identified 238 previously unannotated DUX4-activated promoters for human genes (Table S4) with 144 of those promoters in repetitive elements. Neither GO nor GREAT analyses [39] of these genes and regions revealed striking enrichment of particular functional classes, although we note that germ cell genes (especially primate-specific genes) are likely very poorly annotated. We selected three genes (HEY1, PPCS and NT5C1B) for validation (Figures 4, S6, S7 and S8, Table 2).
Fig. 4. Examples of DUX4-bound repeats that function as alternative promoters for annotated genes. 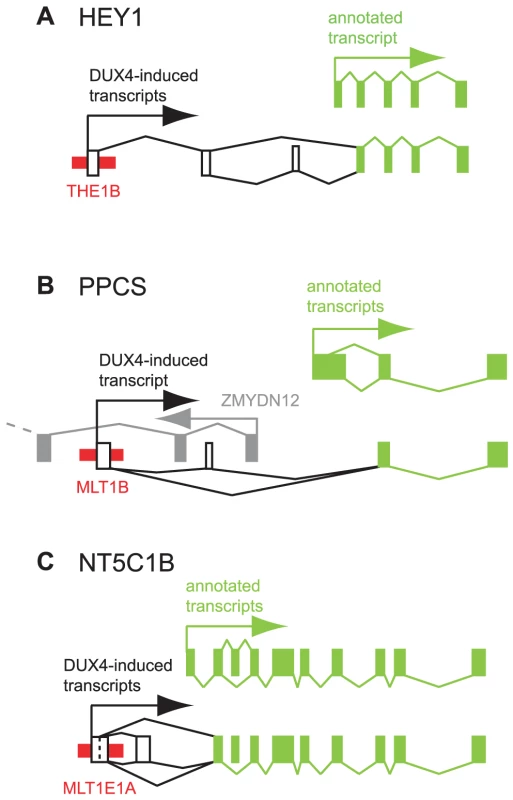
In each panel, thin red boxes depict DUX4-bound repetitive elements. Exons of previously annotated transcripts are depicted as green boxes, with alternative DUX4-induced exons as empty boxes (not to scale). Arrows show the direction of transcription. Diagonal lines show splicing, with alternative splice forms shown above and below the exons. (A) HEY1. (B) PPCS. An upstream, divergently transcribed gene, ZMYDN12, is shown in gray. (C) NT5C1B. HEY1 (hairy/enhancer-of-split related with YRPW motif 1) inhibits myogenesis by repressing myogenin and Mef2C [40], and its ∼13-fold activation by DUX4 in muscle cells could inhibit muscle differentiation in FSHD. RNA-seq data from DUX4-expressing myoblasts suggests the existence of transcripts that initiate ∼40 kb upstream of HEY1's first annotated exon in a THE1B-MaLR retrotransposon and splice via two additional exons to the second exon of HEY1 (Figure 4A). These chimeric transcripts encode an ORF with an in-frame start codon in exon 2 that lacks the first 38 amino acids of the full-length HEY1 protein. We verified this THE1B-HEY1 fusion transcript by RT-PCR, 5′-RACE and Sanger sequencing and showed that its presence in myoblasts is DUX4-dependent (Table 2, Dataset S1). This THE1B element is present at the syntenic location in Old and New World monkeys but not in more distant genomes, demonstrating that it inserted in our genome ∼40–75 million years ago [41], long after the origin of the HEY1 gene.
A DUX4-bound MLT1B-MaLR element initiates DUX4-dependent transcripts ∼6 kb upstream of the phosphopantothenoylcysteine synthetase gene (PPCS) that splice to exon 2 of PPCS, as suggested by RNA-seq data and verified by RT-PCR, 5′-RACE and Sanger sequencing (Figure 4B, Table 2, Dataset S1). The predicted translation start codon of the chimeric transcript in exon 2 is also used in the shorter of the two annotated PPCS isoforms (RefSeq NP_001070915). This MLT1B element is found in the syntenic location in diverse mammalian genomes including those of primates, rodents, carnivores and bats, indicating that it inserted in our genome at least 90 million years ago.
A DUX4-bound MLT1E1A-MaLR element initiates transcripts ∼6 kb upstream of the NT5C1B (5′-nucleotidase, cytosolic IB) gene and uses either of two donor sites to splice to exon 2 of NT5C1B (Figure 4C). This fusion transcript encodes either an ORF lacking the first 14 amino acids of NT5C1B, or a chimeric ORF with the first 10 amino acids of NT5C1B replaced by 17 amino acids encoded in the MLTE1A sequence. Our RNA-seq data shows that the gene is transcribed at low levels in control myoblasts, but is induced ∼300-fold in the presence of DUX4; RT-PCR, 5′-RACE and Sanger sequencing confirm the novel MLT1E1A-NT5C1B fusion transcript (Table 2, Dataset S1). This MLT1E1A element is found at the syntenic location in diverse placental mammalian genomes, so must have inserted into the ancestral genome at least 98 million years ago.
Repetitive elements comprise promoters for lncRNAs and antisense transcripts
In addition to creating novel first exons for protein-coding genes, our RNA-seq data revealed that DUX4-bound repeats can also create promoters for long non-coding RNAs (lncRNAs). Comparison of DUX4 binding and activated transcripts to a recently published dataset of lncRNAs [42] shows that 18 DUX4-bound sites initiate transcripts for lncRNAs, of which 13 are in repetitive elements (Table S5). We used RT-PCR and Sanger sequencing to verify two of these activated lncRNAs; one initiates in an MLT1C-MaLR element shared among many mammals, and one in a primate-specific THE1C-MaLR element (Figures 5A, 5B, S9, S10). lncRNA catalogs are incomplete and more instances of DUX4-initiated lncRNAs are likely to exist. Two very recent reports [43], [44] show that repetitive elements are enriched at the transcription start sites of lncRNAs. Only 56 of the 2045 repeat-initiated lncRNAs (2.7%) described in one of those reports [43] start in DUX4-bound repeats. We note that the catalogs of lncRNAs used in these two recent studies include transcripts expressed in a diverse set of tissues; if suitable data are available in future, it will be interesting to determine whether DUX4-bound repeats comprise a greater proportion of lncRNA transcription start sites in germ cells than in other tissues.
Fig. 5. Examples of DUX4-bound repeats that function as alternative promoters for lncRNAs or antisense transcripts. 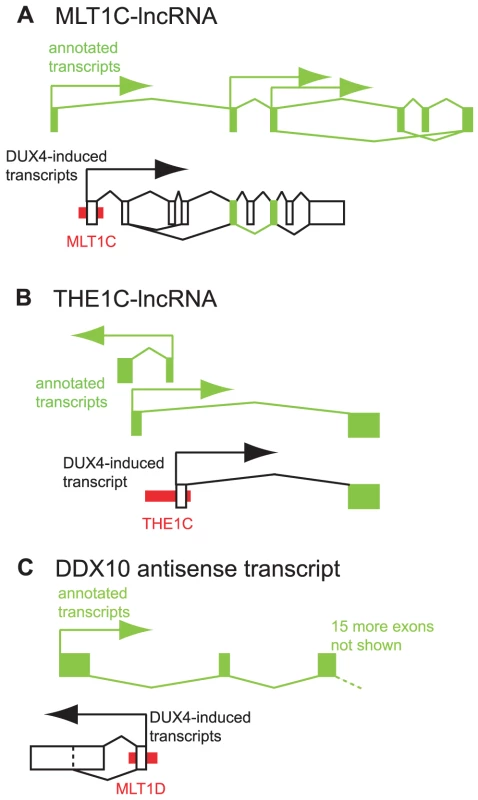
In each panel, thin red boxes depict DUX4-bound repetitive elements. As in Figure 4, exons of previously annotated transcripts are depicted as green boxes, with alternative DUX4-induced exons as empty boxes (not to scale). Arrows show the direction of transcription. Diagonal lines show splicing, with alternative splice forms shown above and below the exons. (A) A lncRNA initiated at an MLT1C element. Two exons of the DUX4-activated lncRNA overlap with exons of previously described lncRNAs TCONS_00003193, TCONS_00002742, TCONS_00002660 and TCONS_00003194 [42]. (B) A lncRNA initiated at an THE1C element that shares the second exon of lncRNA TCONS_00022347 [42]. Additional lncRNAs on the opposite strand also initiate in this region - for clarity, only one is depicted here. (C) An antisense RNA initiated at a DUX4-bound MLT1D element that overlaps the first exon of DDX10. DUX4-bound sites also initiate transcripts antisense to annotated genes. For example, a transcript that initiates in an MLT1D-MaLR element overlaps the first exon of the DDX10 gene in the antisense orientation (Figures 5C, S11), and is confirmed by RT-PCR and Sanger sequencing. We are not aware of any genome-wide catalog of antisense transcripts and thus did not perform a systematic analysis of these RNAs.
Copies of the pericentromeric satellite HSATII are massively activated by DUX4
The analysis above relies on RNA-seq reads that map to fewer than 20 genomic locations and is blind to highly repeated sequences. Therefore, we also used an alternative read-based method to calculate repeat enrichment among DUX4-activated transcripts (see above, and Methods). We identified many of the same repeat classes already highlighted by our analyses of uniquely mappable reads, indicating that many DUX4-bound and activated repeats have diverged enough that standard methods are effective (Figure 6A, Tables S2 and S3). However, as with ChIP-seq data, the read-based analysis uncovered additional classes of activated repeats that were not obviously enriched when we used uniquely mapped reads, including a number of LTR elements, mostly of the ERV1 and ERVK families (e.g. MER52D, LTR12D, MER50B) (Table 1, S2 and S3). Most notably, however, copies of the pericentromeric satellite repeat HSATII are massively activated in the presence of DUX4. Combining the two DUX4-transduced myoblast RNA-seq datasets, HSATIIs are activated ∼860-fold with ∼6700 reads per million in DUX4-expressing cells compared to only ∼8 reads per million in control samples (Figure 6B), a baseline consistent with low HSATII expression (0.2–17 HSATII sequences per million reads; median 0.9 reads per million) in a panel of sixteen normal tissues sequenced by Illumina (the “Body Map 2.0” dataset, GEO accession GSE30611). We aligned HSATII RNA-seq reads to the HSATII consensus sequence, finding that multiple variant sequences (and therefore multiple repeat units) are transcribed (Figure S12).
Fig. 6. Read-based enrichment analysis of RNA-seq data shows activation of several repeat types. 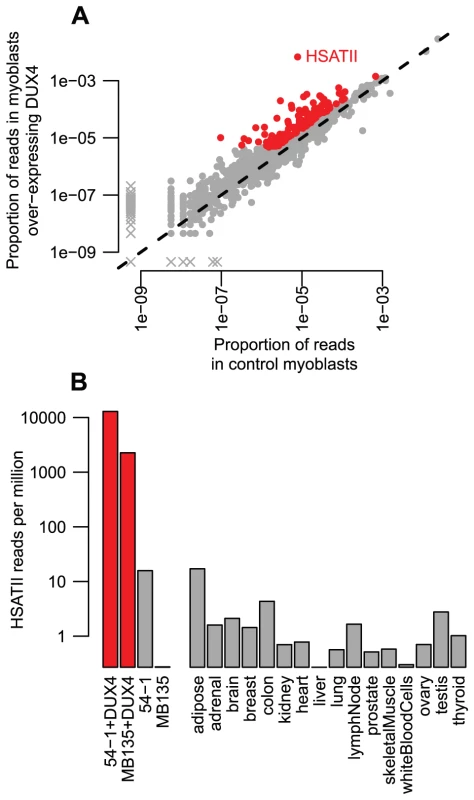
(A) For each repeat type, we show the proportion of RNA-seq reads in two myoblast cell lines transduced with lentiviral DUX4 (y-axis) compared to the proportion from the same two myoblast cell lines without DUX4 transduction (x-axis). Repeats plotted in red show ≥2-fold activation and ≥1000 reads summed across the two DUX4-expressing myoblast lines. The HSATII repeat is labeled. As in Figure 1A, to ensure all repeat types are shown on this log-scaled plot, zero values are replaced with an arbitrarily low value and points shown with an “x”. (B) HSATII is massively activated by DUX4. For our four RNA-seq samples, and sixteen “Body Map” tissues sequenced by Illumina, we show the proportion of reads that match HSATII pericentromeric satellite repeats on a log10-scale. DUX4-expressing cell lines are shown in red, and have a much higher level of HSATII expression than any other sample surveyed. Our ChIP-seq data also demonstrated that HSATIIs are bound by DUX4, with ∼1.9-fold enrichment of HSATII sequences among individual reads and 30 DUX4 peaks in mappable HSATII regions (∼5-fold enrichment). Furthermore, each HSATII ChIP-seq peak appears to derive from multiple tandemly-arranged DUX4 binding sites. The 30 HSATII peaks span bigger genomic regions (median peak width 1.2 kb) than other ChIP-seq peaks (median width 0.4 kb) and contain multiple matches to DUX4's consensus motif - the 299 annotated HSATII regions in the human genome assembly contain a total of 820 DUX4 motifs.
DUX4-bound repeats are promoters in testis and FSHD patient myotubes
We show above that a large number of repeat-initiated transcripts are induced in myoblasts over-expressing DUX4. To determine whether these transcripts are expressed in normal germ cell biology and in FSHD muscle, we used RT-PCR to assay for their presence in FSHD patient cells and various tissues from healthy individuals, using Sanger sequencing to confirm that each amplified product derives from the expected locus (Table 2). We found that most of the repeat-initiated transcripts we tested are expressed in myotube cells derived from an FSHD2 patient where disease-causing mutations result in de-repression of endogenous DUX4, whereas we did not observe these transcripts in control myotubes that do not express DUX4. This indicates that the low level of endogenous DUX4 present in FSHD muscle cells is sufficient to transcriptionally activate the same endogenous repetitive elements identified by our over-expression studies described above.
Given the normal expression of DUX4 in testis, we assayed these repeat-initiated transcripts in human testis RNA from an individual unaffected by FSHD. We found that all tested repeat-initiated transcripts that respond to DUX4 in skeletal muscle are normally expressed in testis (Table 2), demonstrating that DUX4-repeat binding likely regulates transcription in the male germline.
It is possible that factors other than DUX4 might also regulate transcription from these repetitive elements, perhaps explaining why we also detected some of these transcripts in other normal somatic tissue samples where DUX4 is not expressed (Table 2). For example, the internal regions of some ERVL and THE1C full-length repeats appear expressed in many tissues – we note that our primers recognize many copies of these repeats, and expression of only a single copy would enable us to detect transcription. Further research is needed to determine whether other transcription factors bind repetitive element promoters in those tissues. Other DUX family members might fill this role; their expression patterns and binding specificities are currently unknown.
To further assess transcripts of repetitive elements in FSHD muscle cells, we performed a focused analysis of a small dataset of RNA-seq data from myotubes cultured from three control muscle and two FSHD1 muscle biopsies (Yao et al., manuscript in preparation). RNA-seq profiles show that most of the genes, lncRNAs and internal repeat regions we tested using RT-PCR are expressed in FSHD myotubes but not controls (Figures S3, S4, S5, S6, S7, S8, S9, S10, S11). In regions within 1 kb of DUX4-bound sites (both bound repetitive elements and unique sites), ratios of expression in FSHD myotubes versus controls are well-correlated with the activation levels we found in lenti-DUX4 transduced myoblasts (Figure 7A, Spearman's rho = 0.38, p<10−15). Expression ratios of internal MaLR/ERV regions in FSHD myotubes versus controls are also well-correlated with activation levels in lenti-DUX4-transduced myoblasts (Spearman's rho = 0.49, p<10−15, Figure 7B). In addition, 13% of the 144 DUX4-bound repetitive elements that form alternative promoters for annotated genes show FSHD-specific transcripts and HSATII is expressed at ∼26-fold higher levels in FSHD myotubes (median ∼2.2 reads per million) than control myotubes (median 0.08 reads per million). Therefore, the endogenous DUX4 that is expressed in just a subset of FSHD muscle cells is sufficient to drive expression from bound repetitive elements. We also performed a similar focused analysis using testis RNA-seq from the Illumina BodyMap data but the expression level of DUX4 was very low. Only the most abundant DUX4 targets (according to our DUX4-transduced myoblast data) were detected in the testis RNA-seq despite our ability to detect all tested transcripts by RT-PCR (see Table 2) most likely because only a small proportion of cells in the testis express DUX4 [9].
Fig. 7. DUX4-bound regions are similarly activated in FSHD patient myotubes and in DUX4-transduced myoblasts. 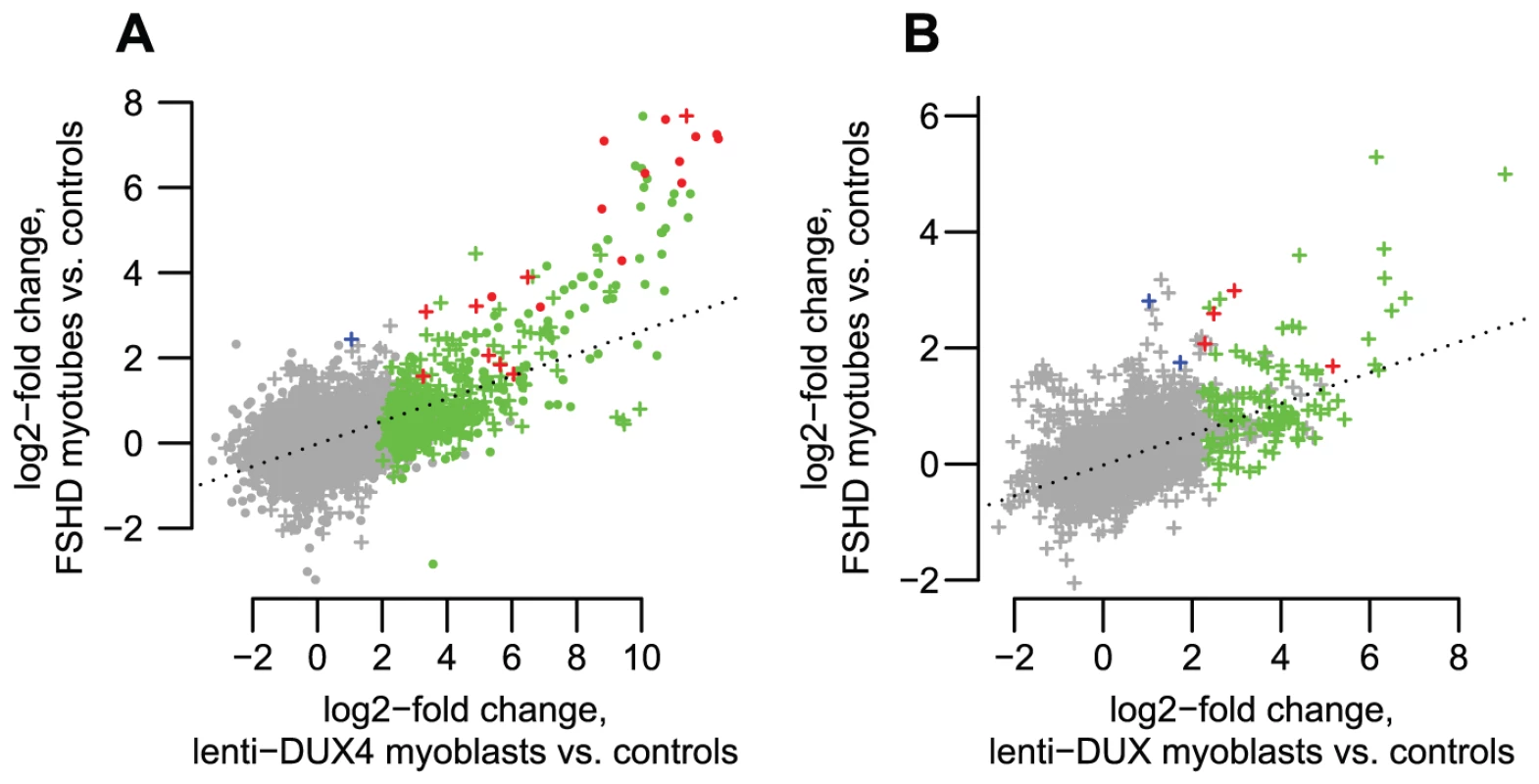
(A) DUX4-bound regions show correlated activation levels in FSHD patient myotubes and in our DUX4-transduced myoblast experimental system. We show log2-activation levels in each system, counting RNA-seq reads within an arbitrary 1 kb of DUX4-bound regions as for Figure 3A. “+” symbols show bound repetitive elements and dot symbols are unique regions. Green symbols show regions that reach statistical significance in only the DUX4-transduced myoblasts, blue symbols are significant in only the FSHD myotubes, and red symbols are significant in both comparisons. The dotted line is a regression line (slope 0.265). (B) Internal regions of ERV and MaLR repeats show correlated activation levels in FSHD patient myotubes and in our DUX4-transduced myoblast experimental system. Colors as in panel A. The dotted line is a regression line (slope 0.270). Discussion
In this study we show that DUX4 binds many LTR repetitive elements of the MaLR and ERV families and initiates transcription at a number of those elements. Some DUX4-bound LTRs produce retrotransposon transcripts and others form previously unrecognized alternative promoters for human protein-coding genes, lncRNAs, and antisense transcripts. DUX4 also binds and activates transcription of the pericentromeric satellite HSATII. We initially identified these DUX4-activated transcripts in myoblasts transduced with lentivirally-expressed DUX4, but show that many of the same loci are transcribed in FSHD but not control muscle cells, indicating that endogenously expressed DUX4 can activate LTR-driven transcription in FSHD muscle. We also show that all loci we tested using RT-PCR are expressed in the testis of an unaffected individual, suggesting that DUX4 drives transcription from at least some repetitive elements during normal development.
Transposable elements can generate evolutionary novelty by exaptation [28], [45]: their protein-coding regions can evolve to form a host gene, for example the mammalian placentation gene syncytin [46], or their regulatory elements can affect the expression patterns or post-transcriptional control of pre-existing host genes [27], [28]. Barbara McClintock initially proposed that transposable elements could alter expression of neighboring genes [47], and her hypothesis is now supported by a growing body of literature describing repetitive elements that regulate transcription of host genes [32], [33]. In some cases, repetitive elements of a particular family are enriched upstream of genes in similar functional classes [27], [31], [34] and may have provided a means to rewire transcriptional networks, distributing new transcription factor binding sites around the genome in short evolutionary time. We find that DUX4-bound MaLR and ERV repeats are used as alternative promoters for host genes and, at least in some cases, modulate gene expression in human testis. Although other TFs have been shown to bind LTR elements [29]–[31], we provide the first demonstration of a transcription factor that binds and activates the MaLR subfamily of LTR elements, and a possible explanation for the rapid evolution of testis expression patterns that has been observed in mammals [35]. In a possible parallel with our results, Peaston et al. found that MaLR elements initiate dozens of genic transcripts in mouse oocytes [48], although they did not identify the transcription factor(s) involved.
DUX4-induced repeat-initiated transcripts also include a number of lncRNAs. Although the human genome contains several thousand lncRNAs, functions have been determined for only a few. Even those few functions appear diverse, including recruitment of chromatin-modifying factors, involvement in enhancer function, organization of nuclear substructures, and control of translation [49], [50]. Many lncRNAs are testis-specific [42], raising the question of whether DUX4 might be responsible for transcription of a subset of lncRNAs in the testis. In the future when lncRNA catalogs are more complete and their functions have begun to be elucidated, it will be interesting to revisit the question of whether DUX4-bound repeats played a role in the evolution of the germline lncRNA transcriptional network.
DUX4 also activates the transcription of relatively intact copies of ERV and MaLR retrotransposons that do not splice to genes or lncRNAs, bringing up the possibility that it had a role in their genomic spread. In order for a retroelement to successfully invade the mammalian genome, it must be active in germ cells. However, because retrotransposition can also be harmful to the host organism [22], [23], retroelements whose activity is strictly restricted to germ cells could have an evolutionary advantage. The germline transcription factors that activated MaLR and ERV retrotransposition are currently unknown: the expression of DUX4 in testis and repression in other tissues together with its ability to bind and activate MaLR and ERV elements could suggest it had a role in repeat mobilization during evolution.
DUX4's activation of relatively intact ERV and MaLR copies might additionally suggest that it currently has a role in their developmental epigenetic silencing. Recent years have yielded an increasing understanding of the mechanisms eukaryotes employ to defend against repetitive elements, including piRNA pathways and mechanisms that establish repressive chromatin marks [51], [52]. These crucial defenses against repetitive elements are particularly active in germ cells and the early embryo and require an initial transcriptional activation of the retrotransposon to feed into the “ping-pong” cycle that produces and amplifies piRNAs that then silence homologous sequences [51]. It would be interesting in future to investigate whether DUX4 is involved in the initial activation of retrotransposon transcription in germ cells. Other pathways also exist to silence repetitive elements. For example, KAP1 controls endogenous retroviral elements by recruiting chromatin-modifying factors [53], [54]; it is targeted to murine leukemia virus LTRs in a sequence-specific fashion by the KRAB-zinc finger protein ZFP809 [55]. To explain KAP1's more general role in ERV silencing, it is assumed that other sequence-specific DNA-binding proteins exist to target it to other ERV classes. The large number of diverse zinc finger proteins present in the mouse and human genomes may fill this role [56], but the sequence-specificity of DUX4 for LTRs might also allow it to recruit repressive factors to repetitive elements in a cell-type specific context.
Similar to retroelements, the pericentromeric satellite HSATII is bound and activated by DUX4. HSATII is a pericentromeric satellite sequence, repeated in large tandem arrays close to a subset of human centromeres. Its consensus sequence is 170 bp long [24] and comprises ∼6 imperfect tandem copies of a smaller ∼25–28 bp repeat unit (data not shown). Pericentromeric regions show evidence of transcriptional activity during specific stages of male meiosis [57], and transcription of satellite sequences at early developmental stages appears be an important prerequisite for later establishment of repressive heterochromatin [58]. The activation of both interspersed repetitive elements and HSATII by DUX4 and its expression in germ cells of the testis could suggest a role in establishing repressive heterochromatin at both dispersed transposons and in tandemly repeated sequences near centromeres. Transcription of repetitive elements and satellite sequences in other, less appropriate biological contexts would likely be harmful, perhaps giving a strong evolutionary advantage to the location of the DUX4 retrogene within a high copy-number macrosatellite that can be tightly repressed by similar epigenetic means.
DUX4 is normally expressed in the testis and epigenetically repressed in somatic tissues, but its variegated de-repression in muscle cells causes facioscapulohumeral muscular dystrophy (FSHD) [9], [13]–[15]. Previous work has provided some insight into why DUX4 over-expression is pathogenic [10], [59]–[61]. The repeat-initiated transcripts we describe here could also contribute to FSHD pathogenesis. For example, the HEY1 gene (induced by DUX4-mediated activation of an upstream THE1B element) can inhibit myogenesis by repressing myogenin and Mef2C [40], and its activation might contribute to the muscle deficiencies seen in FSHD. In addition, expression of satellite transcripts can cause genomic instability [62], [63]; DUX4-mediated activation of HSATII might similarly affect FSHD muscle cells. DUX4-induced expression of ERV and MaLR-encoded proteins or protein fragments could also have functional consequences in testis or FSHD muscle cells. Notably, some ERV-encoded env proteins contain a peptide with immunosuppressive properties [64], perhaps contributing to the suppression of innate immunity we observe upon DUX4 over-expression in myoblasts [10]. Conversely, ERV-encoded protein fragments could be antigenic, and might elicit an immune response and some of the inflammation seen in FSHD muscle [65], [66].
Our findings may also have clinical implications for cancer biology. The DUX4 target HSATII is expressed in a number of cancers [67], and it has been shown that mouse cells lacking the genome caretaker gene Brca1 aberrantly transcribe satellite sequences leading to genome instability [62]. A large number of other DUX4 targets are known “cancer testis antigens” (CTAs): genes normally expressed only in testis but de-repressed in some cancers, eliciting an immune response [10]. Furthermore, in Hodgkin's lymphoma cells, a THE1B-MaLR element provides an alternative promoter for the CSF1R proto-oncogene and de-repression of THE1B elements is widespread [68]. Together with the previous finding that the DUX4-containing D4Z4 repeat is hypomethylated in certain tumors [69], these observations raise the question of whether DUX4 de-repression in cancers might mediate the activation of HSATII, CTAs and/or THE1B promoters.
DUX4 is a primate-specific retrogene and a member of a small gene family that has experienced substantial change during mammalian evolution [1]–[3]. Although the binding preferences and functions of primate DUX4 orthologs and of DUX paralogs are still unknown, we note that an alignment of DUX family homeodomain sequences [1] shows that at least some of the residues predicted to determine DNA-recognition preferences [70] are different between DUX4 and the parental DUXC gene. Determining whether other members of the DUX gene family also bind and regulate retrotransposons will illuminate the importance of these largely unstudied genes and retroelements in the biology and evolution of mammalian germ cells and in muscle disease.
Materials and Methods
General methods and genome-wide datasets
All experiments were performed with approval of the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
We wrote a number of custom scripts using R [71], PERL, and several Bioconductor [72], [73] and Bioperl functions [74]. We use the hg19 (GRCh37/February 2009) reference human genome assembly and annotations provided by UCSC Genome Bioinformatics [75], including the RefSeq [76], lncRNA [42] and common SNP tracks, and phyloP scores for primates and placental mammals [77]. We also used RepeatMasker [78] analysis of the human genome assembly obtained via the UCSC site (chromOut.tar.gz, which uses “RELEASE 20090120” of RepBase [24]). This version of RepBase recognizes ∼1400 human repetitive element types, classified into 56 families, with families classified into 21 classes. We obtained repeat consensus sequences from RepBase [24]. We used the Bioconductor GOstats package [79] to test for GO term enrichment, and GREAT analysis [39] was performed online (http://great.stanford.edu).
Estimation of repeat enrichment among ChIP-seq peaks
Our ChIP-seq data were previously published [10] (GEO accession GSE33838). Briefly, these 40 bp ChIP-seq reads derive from chromatin immunoprecipitated with a mix of two DUX4 antibodies. Chromatin was extracted from myoblasts transduced with lentivirus carrying DUX4, or from negative control myoblasts that do not express DUX4. The human genome contains multiple near-identical copies of DUX4: in our experiments, we used the full-length splice form of the most distal DUX4 copy on chromosome 4q35, because this is the copy that appears to be expressed and pathogenic in FSHD patient muscle [9], [10], [13]. This DUX4 isoform is also expressed in testis, along with other copies containing minor sequence variants whose functional consequences are currently unknown [9]. We mapped each ChIP-seq read to the human reference assembly (hg19) using BWA [80]. We eliminated multiply-mapping reads (retaining reads with mapq >15) and determined peak locations [10]. We identified a position weight matrix (PWM) describing a motif that is strongly enriched among DUX4 peaks, using only the ∼24,000 peaks that do not overlap a repetitive element [10]. We determined a score threshold for this PWM of 9.75, above which >97% of ChIP-seq peaks contain at least one motif.
For further analysis of DUX4 binding sites we refined peak locations by identifying the single 17-mer subsequence with highest score to the DUX4 PWM, rather than using the entire peak region (ChIP-seq resolution is limited by fragment size of ∼200 bp), making the simplifying assumption that each peak contains a single DUX4-binding site. We then determined whether each peak's best-scoring subsequence overlaps a repetitive element. To estimate peak-level enrichment for each repeat type, we divided the proportion of all peaks that overlap each repeat type (observed) by the proportion of base-pairs in the sequenced genome within that repeat type (expected). We previously found that DUX4 binding sites are distributed relatively uniformly across different types of genomic regions (promoters, intergenic regions, introns, etc.) [10], so it is not necessary to adjust our “expected” proportions for the different prevalence of various repeats in these region types.
The DUX4 binding motif includes an average of 5.03 G or C residues among its 17 bases. In order to create an AT-matched set of genomic locations, we randomly selected 17-bp regions from the human genome, eliminating any that overlap assembly gaps, retaining those whose sequence contains 4–6 G or C residues, and downsampling the final set to contain 63,795 sites to match the dataset of DUX4 binding sites. We then determined whether these randomly selected 17-mers overlapped repetitive elements, and counted the types of repeats found among overlapping elements. We performed this sampling 10 times, and use the mean fraction of sites overlapping repeats to calculate enrichment measures shown in the columns of Tables S2 and S3 labeled “peak-based ChIP-seq enrichment estimate, compared to randomly sampled AT-matched regions”.
In order to determine whether DUX4-bound repeats also tend to be bound by other regulatory factors, we obtained ENCODE ChIP-seq peak locations for 161 regulatory factors via the UCSC Genome Bioinformatics “wgEncodeAwgTfbsUniform” tables. For each factor, we chose a single representative ENCODE dataset, and determined the number of peaks that overlap DUX4 ChIP-seq peaks assigned as bound repeats (see above). Using the entire peak regions (several hundred base-pairs wide) for both DUX4 and the other TFs (rather than binding sites defined at higher resolution) allows us to ask the biologically relevant question of whether TFs bind in the vicinity of DUX4, rather than asking whether binding sites are exactly overlapping.
Estimation of repeat enrichment among ChIP-seq reads
In our standard ChIP-seq analysis (above), we ignored sequencing reads that map to multiple genomic locations to ensure that called peaks likely represent true binding sites. However, this method is blind to binding in very recently duplicated regions, so we used an alternative bioinformatic method very similar to that of Day et al. [37]. This method examines repeat enrichment among individual sequencing reads, comparing counts of reads matching each repeat type in a ChIP-seq sample to counts in a negative control sample.
In more detail, we first filtered ChIP-seq read datasets to remove low quality sequences, and reads that match our lentivirus-DUX4 constructs, the packaging constructs used during lentivirus preparation, or Illumina adapter sequences. We constructed an alternative repeat-based “reference genome”, where each repeat type is represented by a “chromosome” comprising every genomic instance of that repeat, with an amount of flanking sequence on each side equal to half the length of a sequence read, concatenated together with a intervening stretches of Ns that are each longer than a sequencing read. We then used BWA to map filtered reads to the repeat-based reference genomes, without filtering results for uniquely mapping sequences. We used the samtools idxstats program [81] to determine the proportion of filtered reads mapping to each repeat type. We estimated enrichment by comparing the proportion of reads mapping to each repeat in the ChIP sample with the proportion in the control sample, adding 0.5 to each count to avoid problems that would arise from division by zero.
These enrichment estimates are “dampened” because ChIP samples contain background DNA derived from unbound genomic regions (50–90% of reads); although immunoprecipitation depletes unbound sequences it cannot completely eliminate them. Background proportions differ between experimental and control samples, and background fraction undoubtedly contains many repetitive sequences. These read-based estimates are therefore likely a very conservative measure of true enrichment in the bound DNA fraction.
Transcriptome data
Our RNA-seq data are available from GEO with accessions GSE45883 and GSE51041.
Two human myoblast cell lines (54-1 and MB135) were each transduced with lentivirus carrying DUX4. After 48 hours (54-1 cells) or 24 hours (MB135 cells), RNA was extracted, poly(A) selected, and subjected to Illumina sequencing using standard protocols to generate 100 bp single-end reads. As negative controls, we also sequenced RNA from untransduced 54-1 cells, and from MB135 cells transduced with lentivirus carrying GFP.
In addition, we sequenced RNA from two FSHD1 and three control myotube samples. Primary myoblast cell lines were received from the University of Rochester biorepository (http://www.urmc.rochester.edu/fields-center) and were cultured in DMEM/F-10 media (Gibco) in the presence of 20% heat-inactivated fetal bovine serum (Gibco), 1% penicillin/streptomycin (Gibco). Media was supplemented with 10 ng/ml rhFGF (Promega) and 1 µM dexamethasone (SIGMA). Myoblasts were fused at 80% confluence in DMEM/F-12 Glutamax media containing 2% KnockOut serum replacement formulation (Gibco) for 36 hours. RNA was extracted, poly(A) selected, and subjected to Illumina sequencing using standard protocols to generate 100 bp single-end reads.
Our analyses are conservative, identifying only the elements with greatest activation extents, because we lack statistical power due to small numbers of samples and a minor technical issue with the 54-1 control sample (see below). In addition, we note that we are only examining polyadenylated transcripts and may be ignoring others; however, full-length transcripts of many repetitive elements are polyadenylated, including those of ERVs, L1s and Alu elements [23], [82].
Our analysis of RNA-seq reads falls into two general categories, both described in detail below.
First, we performed a read-based analysis as we had done for ChIP-seq reads, combining read counts across all instances of a particular repeat type; this method does not allow us to determine which instance of a repeat type is activated, merely that one or more elements of that class shows activation, but unlike standard methods, it does allow examination of recently duplicated sequences. We used the same read-based method as we did for the ChIP-seq reads (see above) on our RNA-seq reads. Although this method uses the BWA read-mapping tool and will therefore fail to map spliced reads, it does not suffer from a limitation of tophat that it suppresses mappings for reads mapping to many genomic locations.
Second, we considered individual genomic locations (repeat instances, genes, lncRNAs, etc.) using tophat and DESeq, a method that limits our ability to examine highly similar multicopy sequences. We mapped reads to the genome using tophat [83], allowing up to 20 map locations for multiply-mapping reads (no map location is reported for reads that map to >20 locations). We counted reads overlapping each region of interest (gene, lncRNA, repeat, etc.) using the bedtools coverageBed function [84] with the “split” and “counts” options. We filtered regions to retain only those that had at least 10 mapped reads (summed across the four myoblast datasets). We then used the DESeq Bioconductor package [85] to detect differentially expressed regions. We also repeated these analyses after filtering tophat output to retain only reads that map uniquely to the genome - results were very similar to those we obtained using all map locations (data not shown).
We encountered a minor technical issue: the RNA-seq read dataset for one sample, the untreated 54-1 negative control, has very low levels of contaminating reads from a lentivirus-DUX4 treated sample. A small number of reads match the lentivirus backbone, the DUX4 insert, and the lentivirus vector-DUX4 junction. In addition, we find small numbers of reads for genes (and repeats) that are activated to very high levels in DUX4-expressing cells but are “off” in cells that do not express DUX4, very consistently at about 1/1000 of the number of reads found in 54-1 cells over-expressing DUX4. The most likely explanation is that a small amount of RNA from another sample contaminated the untreated 54-1 cell RNA sample before sequencing. Although this issue only affects genes expressed to very high levels, it causes a technical problem for the DESeq statistical analysis method we used, because contaminating reads for genes expressed to high levels make dispersal estimation difficult (data not shown). This issue contributes to the conservative nature of our conclusions.
In order to examine the age of activated internal repeat regions relative to internal repeat regions that do not show obvious activation (Figure S2), we first applied the following filters to the full dataset of 100,864 regions of the human genome assembly annotated as internal regions of ERV or MaLR elements (these regions were obtained via UCSC's track of RepeatMasker data). We first selected repeats flanked by DUX4-bound LTRs, requiring a ChIP-seq peak within 5 kb of the internal repeat region whose best DUX4 motif is within an LTR-type repeat element. We then filtered the dataset to only retain repeat regions spanning ≥500 bp, because repeat ages estimated from shorter regions are likely unreliable. This filtered dataset contains 10,190 internal repeat regions, including 92 of the 184 activated regions. We use the “milliDiv” statistic reported in UCSC's RepeatMasker track (divergence from consensus sequence, per 1000 sites examined) as a proxy for repeat age (lower divergence = younger), dividing the number by 1000 to present a more intuitive per-site divergence measure.
To analyze diversity of transcribed HSATII repeat units among RNA-seq reads (Figure S12), we first extracted all reads that mapped to any HSATII copy in our alternative repeat-based reference genome (see above, in “Estimation of repeat enrichment among ChIP-seq reads” section). We then re-aligned those reads to a consensus-based HSATII reference sequence, comprising a full copy of the 170 bp consensus sequence from RepBase, concatenated to a second partial copy (bases 1–98), because HSATII is found in the genome in large tandemly repeated blocks, and we wanted to ensure we captured any RNA-seq reads that begin in the end of one repeat unit and continue into the beginning of the next unit. We used blastn [86] to align reads to this consensus HSATII sequence, tolerating mismatches, and used a custom PERL script to convert blastn output to sam format so that we could use the IGV browser [87] to view the resulting large alignment.
Identification of transcripts that initiate at DUX4-bound repeats and splice to annotated genes or lncRNAs
In order to identify DUX4-bound regions that are used as previously unannotated promoters for genes or lncRNAs, we first used bedtools' intersectBed function [84] on tophat's genomic mappings to filter RNA-seq datasets to retain only reads that overlap DUX4 ChIP-seq peaks (DUX4-bound regions). We additionally filtered reads to retain only those that contain an intron of at least 20 bp and that overlap annotated genes (or lncRNAs). After these filtering steps, we created a table of peak-gene (or peak-lncRNA) pairs, counting the number of reads for each peak-gene pair in each RNA-seq dataset. We eliminated any peak-gene pairs where the peak and the gene themselves overlapped, and further focused on pairs linked by at least one read in both of the DUX4-overexpressing myoblast cell lines (or in both of the FSHD patient myotube samples). For each peak-gene (or peak-lncRNA) pair, we estimated a DUX4 activation ratio, by comparing the proportion of reads linking that peak and gene in the two DUX4-expressing myoblasts (or two FSHD patient myotubes) with the proportion of reads in the two control myoblast samples (or three control myotubes). Again, we added 0.5 to each read count to avoid problems with division by zero. We then filtered the peak-gene (or peak-lncRNA) list to only include pairs with a DUX4 activation ratio of ≥2.
RNA samples used for RT-PCR and 5′-RACE of selected transcripts
Human tissue RNAs were purchased from BioChain (Hayward, CA) and had been DNase-treated by the supplier.
Primary human myoblasts (54-1 and MB135, neither of which has an FSHD mutation, and MB200, from an individual with FSHD2) were collected and cultured as previously described (Snider et al., 2010). 54-1 primary myoblasts were transduced with a lentiviral vector expressing either DUX4 or GFP (as in our RNA-seq experiments). 24 hours after transduction, RNA was harvested for RT-PCR or 5′ RACE. Non-transduced 54-1 and MB200 cells were differentiated into myotubes by growing to 100% confluency and adding differentiation media for 48 hours (Dulbecco's Modified Eagle Medium, 1% penicillin-streptomycin, 1% horse serum, 0.1% insulin, 0.1% transferrin).
Total RNA was isolated from cultured cells using the RNeasy Mini Kit (Qiagen) followed by Invitrogen's protocol for DNase I (Amplification Grade) treatment with the addition of RNaseOUT (Invitrogen) to the reaction. DNase I reaction components were removed using the RNeasy Mini Kit (Qiagen). RNA was eluted using 50 µl of RNase-free water, and the volume was reduced using a SpeedVac.
RT-PCR
cDNA synthesis was performed using 1 µg of RNA, SuperScript III reverse transcriptase (Invitrogen) and random hexamers (Roche) according to the manufacturer's instructions (50°C 30 min and then 55°C 30 min). Reactions were cleaned using the QIAquick (Qiagen) PCR purification system and eluted with 50 µl of water. Negative control samples corresponding to each cDNA sample were prepared by omitting reverse transcriptase.
PCR reactions were performed with 10% PCRx Enhancer solution (Invitrogen) and Platinum Taq polymerase (Invitrogen) using 10% of the cDNA reaction as template in a total reaction volume of 20 µl in thin-walled MicroAmp reaction tubes (Applied Biosystems). Primers are listed in Table S6. PCR cycling conditions for cell culture samples were 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 55°C for 30 s and 68°C for 2 min, followed by a final extension of 7 minutes at 68°C. Cycling conditions for human tissue samples were the same, except that 45 cycles were used. PCR products were examined on 1% UltraPure (Invitrogen) agarose gels in TBE, cloned and sequenced using BigDye Terminators (Applied Biosystems).
5′ RACE
5′ RACE for the THE1B-HEY1, MLT1B-PPCS, and MLT1E1A-NT5C1B transcripts was performed on total RNA using the GeneRacer kit (Invitrogen). Prior to PCR with gene-specific primers and GeneRacer 5′ primers, the RT reaction was cleaned using QIAquick spin columns (Qiagen) as described above. Gene-specific reverse primers are listed in Table S6. PCR products were gel purified, cloned into TOPO 4.0 (Invitrogen) and sequenced using BigDye Terminators (Applied Biosystems).
Supporting Information
Zdroje
1. ClappJ, MitchellLM, BollandDJ, FantesJ, CorcoranAE, et al. (2007) Evolutionary conservation of a coding function for D4Z4, the tandem DNA repeat mutated in facioscapulohumeral muscular dystrophy. Am J Hum Genet 81 : 264–279.
2. LeidenrothA, HewittJE (2010) A family history of DUX4: phylogenetic analysis of DUXA, B, C and Duxbl reveals the ancestral DUX gene. BMC Evol Biol 10 : 364.
3. LeidenrothA, ClappJ, MitchellLM, ConeyworthD, DeardenFL, et al. (2012) Evolution of DUX gene macrosatellites in placental mammals. Chromosoma 121 : 489–497.
4. LyleR, WrightTJ, ClarkLN, HewittJE (1995) The FSHD-associated repeat, D4Z4, is a member of a dispersed family of homeobox-containing repeats, subsets of which are clustered on the short arms of the acrocentric chromosomes. Genomics 28 : 389–397.
5. SchmidtJ, KirschS, RappoldGA, SchemppW (2009) Complex evolution of a Y-chromosomal double homeobox 4 (DUX4)-related gene family in hominoids. PLoS ONE 4: e5288.
6. WijmengaC, HewittJE, SandkuijlLA, ClarkLN, WrightTJ, et al. (1992) Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet 2 : 26–30.
7. RuddMK, EndicottRM, FriedmanC, WalkerM, YoungJM, et al. (2009) Comparative sequence analysis of primate subtelomeres originating from a chromosome fission event. Genome Res 19 : 33–41.
8. SniderL, AsawachaicharnA, TylerAE, GengLN, PetekLM, et al. (2009) RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy. Hum Mol Genet 18 : 2414–2430.
9. SniderL, GengLN, LemmersRJ, KybaM, WareCB, et al. (2010) Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene. PLoS Genet 6: e1001181.
10. GengLN, YaoZ, SniderL, FongAP, CechJN, et al. (2012) DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy. Dev Cell 22 : 38–51.
11. KromYD, ThijssenPE, YoungJM, den HamerB, BalogJ, et al. (2013) Intrinsic epigenetic regulation of the D4Z4 macrosatellite repeat in a transgenic mouse model for FSHD. PLoS Genet 9: e1003415.
12. van der MaarelSM, TawilR, TapscottSJ (2011) Facioscapulohumeral muscular dystrophy and DUX4: breaking the silence. Trends Mol Med 17 : 252–258.
13. LemmersRJ, van der VlietPJ, KloosterR, SacconiS, CamanoP, et al. (2010) A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 329 : 1650–1653.
14. LemmersRJ, TawilR, PetekLM, BalogJ, BlockGJ, et al. (2012) Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet 44 : 1370–1374.
15. RichardsM, CoppeeF, ThomasN, BelayewA, UpadhyayaM (2012) Facioscapulohumeral muscular dystrophy (FSHD): an enigma unravelled? Hum Genet 131 : 325–340.
16. JonesTI, ChenJC, RahimovF, HommaS, ArashiroP, et al. (2012) Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: evidence for disease modifiers and a quantitative model of pathogenesis. Hum Mol Genet 21 : 4419–4430.
17. KowaljowV, MarcowyczA, AnsseauE, CondeCB, SauvageS, et al. (2007) The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul Disord 17 : 611–623.
18. BosnakovskiD, XuZ, GangEJ, GalindoCL, LiuM, et al. (2008) An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies. EMBO J 27 : 2766–2779.
19. BlockGJ, NarayananD, AmellAM, PetekLM, DavidsonKC, et al. (2013) Wnt/beta-catenin signaling suppresses DUX4 expression and prevents apoptosis of FSHD muscle cells. Hum Mol Genet E-pub ahead of print.
20. SmitAF (1993) Identification of a new, abundant superfamily of mammalian LTR-transposons. Nucleic Acids Res 21 : 1863–1872.
21. LanderES, LintonLM, BirrenB, NusbaumC, ZodyMC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409 : 860–921.
22. FeschotteC, GilbertC (2012) Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet 13 : 283–296.
23. BannertN, KurthR (2006) The evolutionary dynamics of human endogenous retroviral families. Annu Rev Genom 7 : 149–173.
24. JurkaJ, KapitonovVV, PavlicekA, KlonowskiP, KohanyO, et al. (2005) Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res 110 : 462–467.
25. TurnerG, BarbulescuM, SuM, Jensen-SeamanMI, KiddKK, et al. (2001) Insertional polymorphisms of full-length endogenous retroviruses in humans. Curr Biol 11 : 1531–1535.
26. LeeE, IskowR, YangL, GokcumenO, HaseleyP, et al. (2012) Landscape of somatic retrotransposition in human cancers. Science 337 : 967–971.
27. FeschotteC (2008) Transposable elements and the evolution of regulatory networks. Nat Rev Genet 9 : 397–405.
28. CowleyM, OakeyRJ (2013) Transposable elements re-wire and fine-tune the transcriptome. PLoS Genet 9: e1003234.
29. WangT, ZengJ, LoweCB, SellersRG, SalamaSR, et al. (2007) Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc Natl Acad Sci USA 104 : 18613–18618.
30. BourqueG, LeongB, VegaVB, ChenX, LeeYL, et al. (2008) Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res 18 : 1752–1762.
31. KunarsoG, ChiaNY, JeyakaniJ, HwangC, LuX, et al. (2010) Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat Genet 42 : 631–634.
32. CohenCJ, LockWM, MagerDL (2009) Endogenous retroviral LTRs as promoters for human genes: a critical assessment. Gene 448 : 105–114.
33. FaulknerGJ, KimuraY, DaubCO, WaniS, PlessyC, et al. (2009) The regulated retrotransposon transcriptome of mammalian cells. Nat Genet 41 : 563–571.
34. LynchVJ, LeclercRD, MayG, WagnerGP (2011) Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat Genet 43 : 1154–1159.
35. BrawandD, SoumillonM, NecsuleaA, JulienP, CsardiG, et al. (2011) The evolution of gene expression levels in mammalian organs. Nature 478 : 343–348.
36. TreangenTJ, SalzbergSL (2012) Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet 13 : 36–46.
37. DayDS, LuquetteLJ, ParkPJ, KharchenkoPV (2010) Estimating enrichment of repetitive elements from high-throughput sequence data. Genome Biol 11: R69.
38. WangH, XingJ, GroverD, HedgesDJ, HanK, et al. (2005) SVA elements: a hominid-specific retroposon family. J Mol Biol 354 : 994–1007.
39. McLeanCY, BristorD, HillerM, ClarkeSL, SchaarBT, et al. (2010) GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28 : 495–501.
40. BuasMF, KabakS, KadeschT (2010) The Notch effector Hey1 associates with myogenic target genes to repress myogenesis. J Biol Chem 285 : 1249–1258.
41. HedgesSB, DudleyJ, KumarS (2006) TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 22 : 2971–2972.
42. CabiliMN, TrapnellC, GoffL, KoziolM, Tazon-VegaB, et al. (2011) Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 25 : 1915–1927.
43. KelleyD, RinnJ (2012) Transposable elements reveal a stem cell-specific class of long noncoding RNAs. Genome Biol 13: R107.
44. KapustaA, KronenbergZ, LynchVJ, ZhuoX, RamsayL, et al. (2013) Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet 9: e1003470.
45. SmitAFA (1999) Interspersed repeats and other mementos of transposable elements in mammalian genomes. Curr Opin Genet Dev 9 : 657–663.
46. MiS, LeeX, LiX, VeldmanGM, FinnertyH, et al. (2000) Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403 : 785–789.
47. McClintockB (1950) The origin and behavior of mutable loci in maize. Proc Natl Acad Sci U S A 36 : 344–355.
48. PeastonAE, EvsikovAV, GraberJH, de VriesWN, HolbrookAE, et al. (2004) Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev Cell 7 : 597–606.
49. NaganoT, FraserP (2011) No-nonsense functions for long noncoding RNAs. Cell 145 : 178–181.
50. CarrieriC, CimattiL, BiagioliM, BeugnetA, ZucchelliS, et al. (2012) Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 491 : 454–457.
51. SiomiMC, SatoK, PezicD, AravinAA (2011) PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol 12 : 246–258.
52. RoweHM, TronoD (2011) Dynamic control of endogenous retroviruses during development. Virology 411 : 273–287.
53. MatsuiT, LeungD, MiyashitaH, MaksakovaIA, MiyachiH, et al. (2010) Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature 464 : 927–931.
54. RoweHM, JakobssonJ, MesnardD, RougemontJ, ReynardS, et al. (2010) KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 463 : 237–240.
55. WolfD, GoffSP (2009) Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 458 : 1201–1204.
56. ThomasJH, SchneiderS (2011) Coevolution of retroelements and tandem zinc finger genes. Genome Res 21 : 1800–1812.
57. KhalilAM, DriscollDJ (2010) Epigenetic regulation of pericentromeric heterochromatin during mammalian meiosis. Cytogenet Genome Res 129 : 280–289.
58. ChanFL, WongLH (2012) Transcription in the maintenance of centromere chromatin identity. Nucleic Acids Res 40 : 11178–11188.
59. WallaceLM, GarwickSE, MeiW, BelayewA, CoppeeF, et al. (2011) DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann Neurol 69 : 540–552.
60. WuebblesRD, LongSW, HanelML, JonesPL (2010) Testing the effects of FSHD candidate gene expression in vertebrate muscle development. Int J Clin Exp Pathol 3 : 386–400.
61. VanderplanckC, AnsseauE, CharronS, StricwantN, TassinA, et al. (2011) The FSHD atrophic myotube phenotype is caused by DUX4 expression. PLoS ONE 6: e26820.
62. ZhuQ, PaoGM, HuynhAM, SuhH, TonnuN, et al. (2011) BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature 477 : 179–184.
63. Bouzinba-SegardH, GuaisA, FrancastelC (2006) Accumulation of small murine minor satellite transcripts leads to impaired centromeric architecture and function. Proc Natl Acad Sci U S A 103 : 8709–8714.
64. CiancioloGJ, CopelandTD, OroszlanS, SnydermanR (1985) Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science 230 : 453–455.
65. MolnarM, DioszeghyP, MechlerF (1991) Inflammatory changes in facioscapulohumeral muscular dystrophy. Eur Arch Psychiatry Clin Neurosci 241 : 105–108.
66. FrisulloG, FruscianteR, NocitiV, TascaG, RennaR, et al. (2011) CD8(+) T cells in facioscapulohumeral muscular dystrophy patients with inflammatory features at muscle MRI. J Clin Immunol 31 : 155–166.
67. TingDT, LipsonD, PaulS, BranniganBW, AkhavanfardS, et al. (2011) Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science 331 : 593–596.
68. LamprechtB, WalterK, KreherS, KumarR, HummelM, et al. (2010) Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med 16 : 571–579.
69. FragaMF, BallestarE, Villar-GareaA, Boix-ChornetM, EspadaJ, et al. (2005) Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37 : 391–400.
70. NoyesMB, ChristensenRG, WakabayashiA, StormoGD, BrodskyMH, et al. (2008) Analysis of homeodomain specificities allows the family-wide prediction of preferred recognition sites. Cell 133 : 1277–1289.
71. IhakaR, GentlemanR (1996) A language for data analysis and graphics. J Comput Graph Stat 5 : 299–314.
72. GentlemanRC, CareyVJ, BatesDM, BolstadB, DettlingM, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80.
73. LawrenceM, HuberW, PagesH, AboyounP, CarlsonM, et al. (2013) Software for computing and annotating genomic ranges. PLoS Comput Biol 9: e1003118.
74. StajichJE, BlockD, BoulezK, BrennerSE, ChervitzSA, et al. (2002) The Bioperl toolkit: Perl modules for the life sciences. Genome Res 12 : 1611–1618.
75. DreszerTR, KarolchikD, ZweigAS, HinrichsAS, RaneyBJ, et al. (2012) The UCSC Genome Browser database: extensions and updates 2011. Nucleic Acids Res 40: D918–923.
76. PruittKD, TatusovaT, MaglottDR (2005) NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 33: D501–504.
77. PollardKS, HubiszMJ, RosenbloomKR, SiepelA (2010) Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 20 : 110–121.
78. Smit AF, Hubley R, Green P (1996–2004) RepeatMasker Open-3.0. http://www.repeatmasker.org.
79. FalconS, GentlemanR (2007) Using GOstats to test gene lists for GO term association. Bioinformatics 23 : 257–258.
80. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
81. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
82. CordauxR, BatzerMA (2009) The impact of retrotransposons on human genome evolution. Nat Rev Genet 10 : 691–703.
83. TrapnellC, PachterL, SalzbergSL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25 : 1105–1111.
84. QuinlanAR, HallIM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26 : 841–842.
85. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106.
86. AltschulSF, MaddenTL, SchafferAA, ZhangJ, ZhangZ, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 : 3389–3402.
87. RobinsonJT, ThorvaldsdottirH, WincklerW, GuttmanM, LanderES, et al. (2011) Integrative genomics viewer. Nat Biotechnol 29 : 24–26.
88. KentWJ, SugnetCW, FureyTS, RoskinKM, PringleTH, et al. (2002) The human genome browser at UCSC. Genome Res 12 : 996–1006.
Štítky
Genetika Reprodukční medicína
Článek Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis inČlánek Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 11
-
Všechny články tohoto čísla
- Molecular Recognition by a Polymorphic Cell Surface Receptor Governs Cooperative Behaviors in Bacteria
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis in
- Retrotransposon Silencing During Embryogenesis: Cuts in LINE
- Roles of XRCC2, RAD51B and RAD51D in RAD51-Independent SSA Recombination
- Parallel Evolution of Chordate Regulatory Code for Development
- A Genetic Approach to the Recruitment of PRC2 at the Locus
- Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
- Elevated Mutagenesis Does Not Explain the Increased Frequency of Antibiotic Resistant Mutants in Starved Aging Colonies
- Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
- Interplay between Active Chromatin Marks and RNA-Directed DNA Methylation in
- Recombinogenic Conditions Influence Partner Choice in Spontaneous Mitotic Recombination
- Crosstalk between NSL Histone Acetyltransferase and MLL/SET Complexes: NSL Complex Functions in Promoting Histone H3K4 Di-Methylation Activity by MLL/SET Complexes
- A New Role for the GARP Complex in MicroRNA-Mediated Gene Regulation
- RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Loss of DNMT1o Disrupts Imprinted X Chromosome Inactivation and Accentuates Placental Defects in Females
- Inhibition of the Smc5/6 Complex during Meiosis Perturbs Joint Molecule Formation and Resolution without Significantly Changing Crossover or Non-crossover Levels
- Disruption of Lipid Metabolism Genes Causes Tissue Overgrowth Associated with Altered Developmental Signaling
- Translation Initiation Factors eIF3 and HCR1 Control Translation Termination and Stop Codon Read-Through in Yeast Cells
- Recruitment of TREX to the Transcription Machinery by Its Direct Binding to the Phospho-CTD of RNA Polymerase II
- MYB97, MYB101 and MYB120 Function as Male Factors That Control Pollen Tube-Synergid Interaction in Fertilization
- Oct4 Is Required ∼E7.5 for Proliferation in the Primitive Streak
- Contrasted Patterns of Crossover and Non-crossover at Meiotic Recombination Hotspots
- Transposable Prophage Mu Is Organized as a Stable Chromosomal Domain of
- Ash1l Methylates Lys36 of Histone H3 Independently of Transcriptional Elongation to Counteract Polycomb Silencing
- Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects
- Genomic Mechanisms Accounting for the Adaptation to Parasitism in Nematode-Trapping Fungi
- Decoding a Signature-Based Model of Transcription Cofactor Recruitment Dictated by Cardinal Cis-Regulatory Elements in Proximal Promoter Regions
- Removal of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
- Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
- Activin Signaling Targeted by Insulin/dFOXO Regulates Aging and Muscle Proteostasis in
- Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans
- Demographic Divergence History of Pied Flycatcher and Collared Flycatcher Inferred from Whole-Genome Re-sequencing Data
- Recurrent Tissue-Specific mtDNA Mutations Are Common in Humans
- The Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
- The Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
- Reconstructing the Population Genetic History of the Caribbean
- and Are Required for Growth under Iron-Limiting Conditions
- Whole Genome, Whole Population Sequencing Reveals That Loss of Signaling Networks Is the Major Adaptive Strategy in a Constant Environment
- Neuron-Specific Feeding RNAi in and Its Use in a Screen for Essential Genes Required for GABA Neuron Function
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
- Mouse BAZ1A (ACF1) Is Dispensable for Double-Strand Break Repair but Is Essential for Averting Improper Gene Expression during Spermatogenesis
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- DUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
- Pathways-Driven Sparse Regression Identifies Pathways and Genes Associated with High-Density Lipoprotein Cholesterol in Two Asian Cohorts
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- and Are Required for Growth under Iron-Limiting Conditions
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



