-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
Proper assignment of cellular fates relies on correct interpretation of Wnt and Hedgehog (Hh) signals. Members of the Wnt Inhibitory Factor-1 (WIF1) family are secreted modulators of these extracellular signaling pathways. Vertebrate WIF1 binds Wnts and inhibits their signaling, but its Drosophila melanogaster ortholog Shifted (Shf) binds Hh and extends the range of Hh activity in the developing D. melanogaster wing. Shf activity is thought to depend on reinforcing interactions between Hh and glypican HSPGs. Using zebrafish embryos and the heterologous system provided by D. melanogaster wing, we report on the contribution of glypican HSPGs to the Wnt-inhibiting activity of zebrafish Wif1 and on the protein domains responsible for the differences in Wif1 and Shf specificity. We show that Wif1 strengthens interactions between Wnt and glypicans, modulating the biphasic action of glypicans towards Wnt inhibition; conversely, glypicans and the glypican-binding “EGF-like” domains of Wif1 are required for Wif1's full Wnt-inhibiting activity. Chimeric constructs between Wif1 and Shf were used to investigate their specificities for Wnt and Hh signaling. Full Wnt inhibition required the “WIF” domain of Wif1, and the HSPG-binding EGF-like domains of either Wif1 or Shf. Full promotion of Hh signaling requires both the EGF-like domains of Shf and the WIF domains of either Wif1 or Shf. That the Wif1 WIF domain can increase the Hh promoting activity of Shf's EGF domains suggests it is capable of interacting with Hh. In fact, full-length Wif1 affected distribution and signaling of Hh in D. melanogaster, albeit weakly, suggesting a possible role for Wif1 as a modulator of vertebrate Hh signaling.
Published in the journal: . PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002503
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002503Summary
Proper assignment of cellular fates relies on correct interpretation of Wnt and Hedgehog (Hh) signals. Members of the Wnt Inhibitory Factor-1 (WIF1) family are secreted modulators of these extracellular signaling pathways. Vertebrate WIF1 binds Wnts and inhibits their signaling, but its Drosophila melanogaster ortholog Shifted (Shf) binds Hh and extends the range of Hh activity in the developing D. melanogaster wing. Shf activity is thought to depend on reinforcing interactions between Hh and glypican HSPGs. Using zebrafish embryos and the heterologous system provided by D. melanogaster wing, we report on the contribution of glypican HSPGs to the Wnt-inhibiting activity of zebrafish Wif1 and on the protein domains responsible for the differences in Wif1 and Shf specificity. We show that Wif1 strengthens interactions between Wnt and glypicans, modulating the biphasic action of glypicans towards Wnt inhibition; conversely, glypicans and the glypican-binding “EGF-like” domains of Wif1 are required for Wif1's full Wnt-inhibiting activity. Chimeric constructs between Wif1 and Shf were used to investigate their specificities for Wnt and Hh signaling. Full Wnt inhibition required the “WIF” domain of Wif1, and the HSPG-binding EGF-like domains of either Wif1 or Shf. Full promotion of Hh signaling requires both the EGF-like domains of Shf and the WIF domains of either Wif1 or Shf. That the Wif1 WIF domain can increase the Hh promoting activity of Shf's EGF domains suggests it is capable of interacting with Hh. In fact, full-length Wif1 affected distribution and signaling of Hh in D. melanogaster, albeit weakly, suggesting a possible role for Wif1 as a modulator of vertebrate Hh signaling.
Introduction
The extracellular space provides an important milieu for the regulation of signaling by Wnt and Hedgehog (Hh) morphogens. Several factors are known that bind secreted Wnts or Hhs and regulate either their extracellular levels, their movement through tissues, or their access to receptors. Members of the Wnt Inhibitory Factor-1 (WIF1) family of secreted proteins are unusual, however, because they can impact either the Wnt or Hh pathways. Vertebrate WIF1 binds Wnts and inhibits Wnt signaling [1], while the Drosophila melanogaster WIF1 homolog Shifted (Shf, NCBI Gene ID: 31617) binds Hh and promotes Hh signaling [2], [3]. This study investigates the mechanism of vertebrate WIF1 action, and the basis of the different activities of the vertebrate and D. melanogaster WIF1 family proteins.
Human WIF1 (NCBI Gene ID: 11197) binds vertebrate Wnts and the D. melanogaster Wnt Wingless (Wg, NCBI Gene ID: 34009) and, in gain-of-function assays, WIF1 inhibits vertebrate Wnt and D. melanogaster Wg signaling [1], [3]–[7]. Morpholino-induced knockdown of wif1 in zebrafish (Danio rerio) embryos results in shortening along the anterior-posterior axis, defective somites and increased canonical Wnt signaling in the developing swimbladder [8]. Blocking WIF1 function also increases rod production in cultures of dissociated rat retinas, similar to the effects of increasing Wnt4 signaling [6]. And while knocking out Wif1 in mice does not lead to obvious developmental defects, it does increase the growth of radiation-induced osteosarcomas [9]. Human WIF1 is also epigenetically silenced in many tumors that have heightened Wnt signaling, and addition of exogenous WIF1 to such tumors reduces Wnt signaling, slows tumor growth and increases apoptosis [9]–[20].
Shf is the only D. melanogaster member of the WIF1 family but, unlike vertebrate WIF1, Shf cannot inhibit Wg signaling. Instead, Shf binds Hh (NCBI Gene ID: 42737), and loss of Shf reduces both the accumulation of extracellular Hh and the range of Hh signaling in the D. melanogaster wing disc [2], [3]. Shf appears to mediate these effects by stabilizing interactions between Hh and the glypican family of membrane-bound Heparan Sulfate Proteoglycans (HSPGs). Glypicans are anchored to the cell surface by glycosylphosphatidylinositol (GPI) linkages, and regulate signaling by binding a variety of signaling and signal-binding molecules, including Hh and Shf [2], [21], [22]. Removing the two D. melanogaster glypicans, Dally (NCBI Gene ID: 39013) and Dally-like protein (Dlp, NCBI Gene ID: 39596), or blocking synthesis of their HS glycosaminoglycan sidechains, reduces the extracellular accumulation of extracellular Shf [2]; binding between HS and WIF1 family members is direct [7]. Loss of dally and dlp or HS synthesis in D. melanogaster wing discs mimics the loss of Shf, similarly reducing the accumulation of extracellular Hh and the range of Hh signaling in wing discs [23]–[28]. Thus, the interaction between Hh and the glypicans appears to be weakened or eliminated by the loss of Shf; conversely, Shf function depends in large part on the presence of the glypicans [3].
While binding has been demonstrated between HS and vertebrate WIF1 [7], the function of this binding is unknown. An important question is therefore whether (and how) HSPGs contribute to the Wnt-inhibiting functions of vertebrate WIF1. Glypicans have complex effects on Wnt signaling [21], [22], [29]. In some contexts, the loss of glypicans reduces Wnt signaling, consistent with a co-receptor-like role, or a less direct effect on Wnt accumulation or movement; however, in other contexts the loss of glypicans increases Wnt signaling, suggesting that glypicans can sequester Wnts away from their receptors [29]–[37]. Indeed, the ability of both D. melanogaster and vertebrate glypicans to promote or inhibit Wnt signaling is “biphasic”, depending in part on their concentration; low levels promote and high levels inhibit [30], [35], [38], [39] (see Discussion).
We will provide evidence that in at least two contexts, the exogenous assay provided by the wing disc of D. melanogaster, and the early embryo of zebrafish, the inhibitory activity of the zebrafish WIF1 homolog (Wif1, Entrez Gene ID: 30476) is greatly facilitated by its ability to act as a bridge between Wnts and glypicans. In this sense, Wif1 can bias the biphasic activity of glypicans, increasing their ability to inhibit Wnt signaling.
We have also examined the structural basis for this interaction. All WIF1 family members (including Shf) are composed of two distinct regions. At the N-terminal end is the Wnt-binding ‘WIF’ domain [1]. This is followed by five ‘EGF-like’ domains; we will provide evidence that these are required for interactions between Wif1 and glypicans, consistent with recent biochemical data [7].
Finally, given the similarities between Wif1 and Shf, what controls their pathway specificity, and does vertebrate Wif1 have any overlapping activity in the promotion of Hh activity? To answer these questions we swapped domains between Wif1 and Shf. Our results show that the ‘EGF-like’ domains, but not the ‘WIF’ domains, of Wif1 and Shf are largely interchangeable for the inhibition of Wnt signaling. The ‘EGF-like’ domains are not, however, interchangeable for the promotion of Hh signaling, while the ‘WIF’ domains are. We will also show that Wif1 can affect the accumulation and, weakly, the activity of D. melanogaster Hh, suggesting that vertebrate WIF1 proteins have the potential to regulate vertebrate Hh signaling.
Results
Zebrafish Wif1 inhibits Wg signaling in D. melanogaster
In order to analyze the function of Wif1, we first made use of the in vivo assays and genetic manipulations provided by the developing wing of D. melanogaster. Three D. melanogaster Wnt family members, Wg, Wnt4 (NCBI Gene ID: 34007) and Wnt6 (NCBI Gene ID: 34010), are co-expressed in a narrow stripe of cells along the prospective wing margin in mid-to-late third instar wing discs, but Wnt4 and Wnt6 are not known to affect wing margin development [40]–[43]. Wg is, however, necessary and sufficient for the development of dorsal and ventral rows of sensory and non-sensory bristles that arise adjacent to the Wnt-expressing cells; strong loss of Wg signaling also eliminates more proximal tissues, leading to reduced wings with a scalloped margin [44]–[46] (Figure S1). Driving expression of UAS-wif1 with the wing blade driver nubbin-Gal4 (nub-Gal4) produced adult wing phenotypes indicative of reduced Wg activity (compare Figure 1A, 1B to Figure S1). In wing discs, distal Wg induces adjacent dorsal and ventral rows of anti-Senseless (Sens) staining (Figure 1C) [47], [48], and we found that driving expression of UAS-wif1 with dpp-Gal4, whose expression is limited to anterior cells near the compartment boundary [49], eliminated anti-Sens staining not only in the anterior, and also non-autonomously in nearby posterior cells, indicating that the secreted Wif1 can act over several cell diameters (Figure 1D). Thus, zebrafish Wif1 can inhibit signaling by the D. melanogaster Wnt Wg, much like human WIF1 [1], [3].
Fig. 1. Zebrafish Wif1 inhibits D. melanogaster Wg. 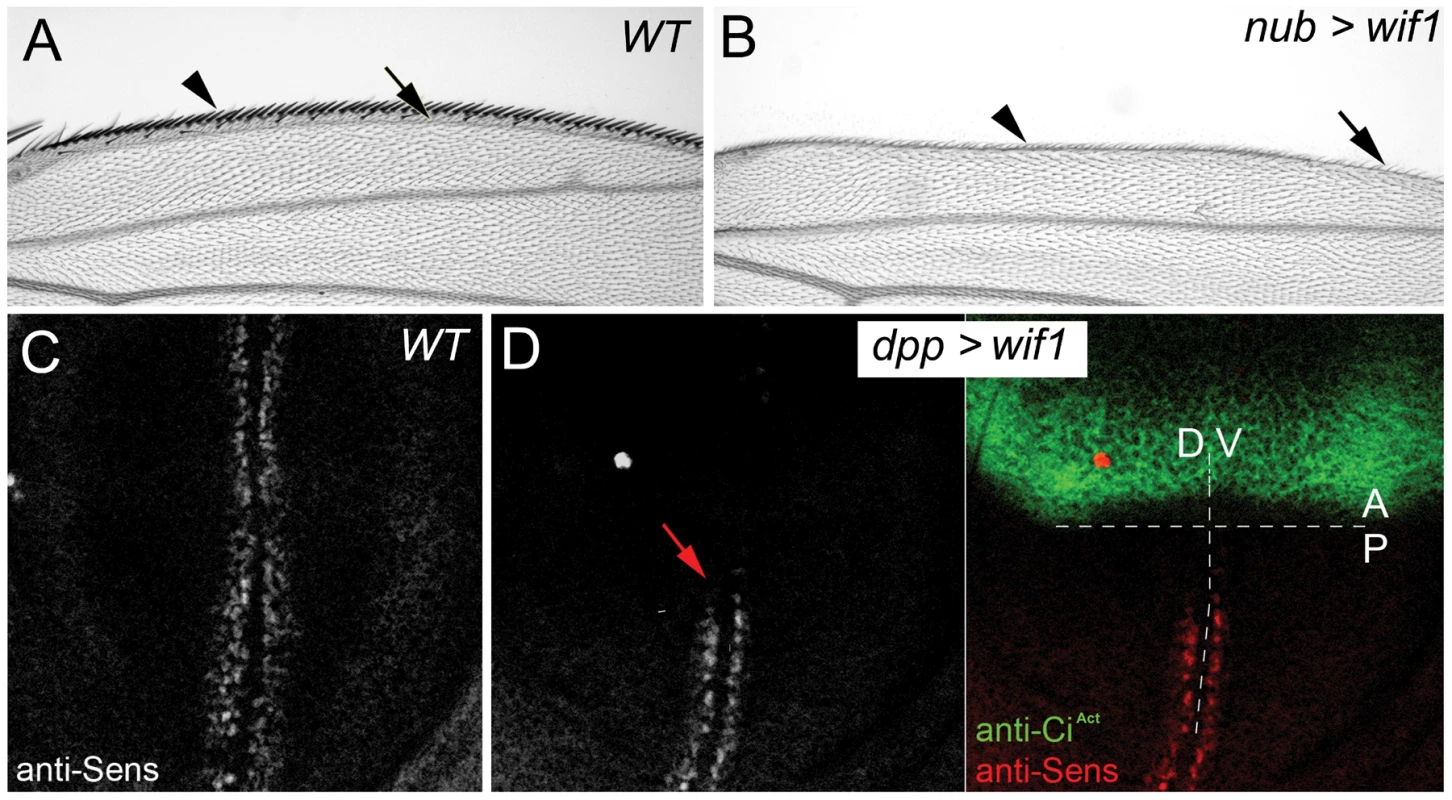
(A) Anterior margin of wild-type (WT) wing shows a dense array of sensory bristles (arrowhead). First longitudinal vein (L1, arrow) marks the anterior edge of the wing blade. (B) nub-Gal4-driven expression of UAS-wif1 eliminates anterior bristles (arrowhead) and disrupts L1 (arrow). (C) Anti-Sens staining along the presumptive wing margin in wild type late third instar wing disc. (D) dpp-Gal4-driven expression of UAS-wif1 in anterior cells of late third instar wing disc (marked green by anti-CiAct) eliminates anti-Sens staining locally and in adjacent posterior cells (arrow). In these and the remaining figures anterior is up. In adult wings distal is to the right, in wing discs ventral is to the right. Wif1 increases the accumulation of extracellular Wg on Dlp-expressing cells
Conventional anti-Wg staining suggests that expressing human WIF1 in wing discs reduces Wg internalization, perhaps by reducing receptor-mediated endocytosis [3]. Little is known, however, about how WIF1 affects extracellular Wg, which is poorly visualized by conventional staining. We therefore used an alternate method that stains extracellular Wg (ex-Wg), and that reveals a gradual gradient of ex-Wg in wild type discs from the distal, wg-expressing cells to proximal cells that lack wg expression (Figure 2A, 2B) [50].
Fig. 2. Wif1 stabilizes Wg on Dlp-expressing cells in late third instar wing discs. 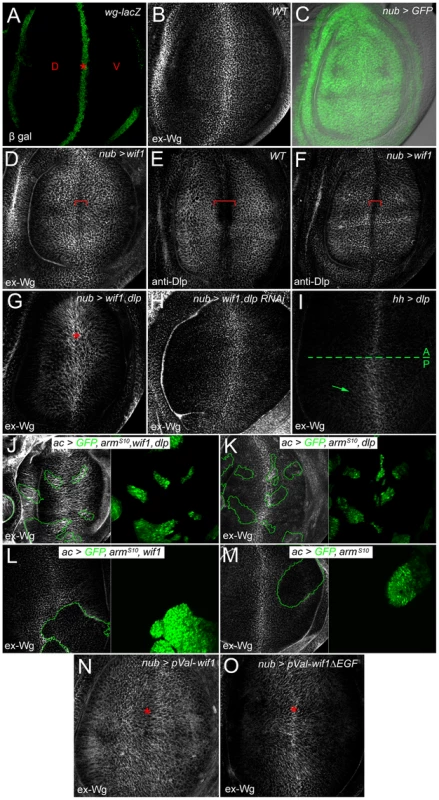
Wing pouch regions of wing imaginal discs. (A) wg-lacZ expression along the prospective wing margin (asterisk) where prospective dorsal (D) and ventral (V) wing blade surfaces abut. (B) Extracellular Wg (ex-Wg) from the wg-expressing cells, which is high distally and lower proximally. (C) Pattern of nub-Gal4 expression, marked by UAS-GFP. In all subsequent panels, except for I-M, nub-Gal4 is used to drive UAS-transgene expression. (D) ex-Wg after expression of UAS-wif1. ex-Wg is higher on proximal cells than on distal ones (red bracket). (E) Anti-Dlp staining in wild-type wing disc. Dlp expression is downregulated in distal cells of the prospective wing margin (red bracket). (F) Anti-Dlp staining after UAS-wif1 expression. The width of the prospective wing margin region with reduced staining (red bracket) is narrowed compared to anti-Dlp staining in the wild-type disc in E. (G) ex-Wg staining after co-expression of UAS-wif1 and UAS-dlp. ex-Wg is increased at the wing margin (asterisk). (H) ex-Wg staining after co-expression of UAS-wif1 and UAS-dlp RNAi is similar to that in the wild-type disc in E. (I) Posterior expression of UAS-dlp (using hh-Gal4). ex-Wg accumulates in the posterior compartment. (J-M) Flpout actin-Gal4 (ac) clones marked with UAS-GFP (green). (J) High ex-Wg levels inside and, to a lesser extent, outside clones expressing: UAS-armS10, UAS-wif1, and UAS-dlp. (K) Low, largely unchanged ex-Wg levels inside clones expressing UAS-armS10 and UAS-dlp. (L) Reduced ex-Wg levels in clones expressing UAS-wif1 and UAS-armS10. zWIF1 increases ex-Wg outside the clone. (M) Reduced ex-Wg levels in clones expressing ArmS10. (N) After expression of pVal-UAS-wif1, ex-Wg staining is high proximally and low along the wing margin (asterisk). (O) Expression of pVal-UAS-wif1ΔEGF does not lead to a strong increase in proximal ex-Wg, and does not decrease ex-Wg along the wing margin (asterisk). When we used nub-Gal4 to express zebrafish wif1 throughout the presumptive wing blade (Figure 2C), the pattern of wild-type ex-Wg distribution was inverted: ex-Wg levels were greatly elevated proximally, and distal levels near the prospective wing margin were reduced (compare Figure 2D, 2B). The Wif1-induced ex-Wg pattern was strikingly similar to the distribution of the glypican Dlp, which is high within the wing pouch but reduced along the prospective wing margin (Figure 2E) [33], [35]. Although the region of reduced ex-Wg at the margin of wif1-expressing discs was somewhat narrower than the normal Dlp-deficient zone of wild-type discs, anti-Dlp staining also revealed that the zone with diminished Dlp was narrower in nub-Gal4, UAS-wif1 discs (Figure 2F). This change in Dlp expression is most likely a consequence of the reduced Wg activity caused by Wif1; a previous study showed that Wg signaling downregulates Dlp levels [33]. Thus, the distribution of ex-Wg after wif1 expression strongly resembles that of Dlp.
To test the role of Dlp in the distribution of ex-Wg, we first co-expressed dlp and wif1 using nub-Gal4, and found that ex-Wg now accumulated on the cells of the presumptive wing margin, likely because of the ex-Wg is bound by high levels of distal Dlp (Figure 2G). Next, we simultaneously expressed wif1 and knocked-down endogenous Dlp levels. In nub-Gal4, UAS-wif1, UAS-dlp RNAi discs, the ex-Wg gradient reverted (Figure 2H) to resemble the wild-type ex-Wg gradient (e.g. Figure 2B). Thus, Dlp is both sufficient and necessary for much of the Wif1-induced redistribution of ex-Wg.
The above data indicates that Wif1 increases the levels of Dlp-bound Wg, thereby increasing the accumulation of ex-Wg on cell surfaces that have high levels of Dlp. We hypothesize that this also reduces the levels of free, diffusible Wg around proximal cells, creating a diffusion “sink” that in turn reduces the levels of Wg around the distal, Wg-secreting cells where Dlp levels are low.
However, since Wg signaling can reduce Dlp levels [33], and Dlp stabilizes Wg [33], [51], [52], it is possible that wif1 expression increases Dlp levels (and thus Dlp-bound Wg) by inhibiting Wg signaling. Our initial results argue against this, since wif1 expression did not obviously affect anti-Dlp staining, except by narrowing the zone with low Dlp levels near the wing margin (Figure 2F). As a more rigorous test we examined the effects of wif1 expression on ex-Wg in cells with fixed levels of Wg signaling and dlp transcription. We drove Wg signaling at high levels by expressing Armadillo (Arm)S10, a constitutively active, Wnt-independent form of the D. melanogaster β-Catenin Arm (NCBI Gene ID: 31151) [53], and drove dlp transcription at high levels using UAS-dlp. To bypass the deformation of wing tissues expected from widespread ArmS10 expression, we used the Gal4 Flpout technique to generate clonal clusters of cells misexpressing UAS-armS10, either alone or in combination with UAS-dlp and/or UAS-wif1. First, we found that ex-Wg levels were much higher within clones expressing UAS-armS10, UAS-wif1 and UAS-dlp than in clones expressing only UAS-armS10 and UAS-dlp (compare clones in Figure 2J, 2K). As expected from the diffusion of Wif1, ex-Wg also accumulated outside the UAS-wif1, UAS-armS10 UAS-dlp clones, albeit not at levels quite as high as inside the clone. These results indicate that Wif1 increased the accumulation of ex-Wg independently of any effects that may have been caused by the repression of Wg signaling. Ex-Wg levels were reduced in clones co-misexpressing UAS-armS10 and UAS-wif1 (Figure 2L) or UAS-armS10 alone (Figure 2M). This is most likely due to the reduction of endogenous Dlp levels by ArmS10 [33], and is consistent with the requirement for Dlp in Wif1-dependent stabilization of ex-Wg (see Figure 2J).
Since WIF1 binds directly to HS and Wnts [7], we propose that Wif1 stabilizes or reinforces the binding between glypican HSPGs and Wnt on the cell surface. This parallels the role proposed for the D. melanogaster Wif1 homolog Shf, which binds Hh and glypicans and is thought to thereby stabilize Hh on cell surfaces [2], [3]. However, in the case of Shf the increased Hh accumulation is accompanied by increased Hh signaling. It was striking that the accumulation of ex-Wg around proximal cells caused by wif1 expression was not accompanied by obvious gains in proximal Wg signaling, since ectopic anti-Sens staining or bristle development in proximal cells was never observed (Figure 1), nor could we detect obvious effects on the low-level Wg target Distal-less (data not shown). The reduction of distal ex-Wg levels caused by wif1 expression might explain the reduced Wg signaling at the presumptive margin. However, distal co-expression of dlp and wif1 increases ex-Wg accumulation at the presumptive wing margin (Figure 2G), yet we will show in the following section that this leads to an even stronger reduction of distal Wg signaling. Thus, ex-Wg depletion from the presumptive wing margin is unlikely to be responsible for the defects in wing margin signaling. Rather, the ex-Wg that accumulates at high levels on the surfaces of Dlp-expressing cells is incapable of activating Wg receptors.
This data shows a strong role for Wif1 and Dlp in Wg accumulation, but does not rule out a partially redundant role for the other D. melanogaster glypican, Dally. dally is transcribed at slightly higher levels along the distal margin [33], [54], which would not be consistent with the pattern of extracellular Wg accumulation induced by wif1 expression. However, since there are no antisera to Dally its pattern of extracellular accumulation is unknown. Evidence suggests that Dally's levels may actually be reduced at the margin: the GPI-linkage between Dally and cell membranes might be cleaved along the distal wing margin by distally-expressed Notum, as suggested both by genetic interactions [33] and the modification of Dally by Notum in vitro [51], although overexpressed Dally-HA accumulates uniformly in wing discs [35]. Below we will show genetic interactions consistent with redundant activities of both glypicans.
Glypicans promote Wif1-dependent inhibition of Wnt signaling
We next asked how glypicans modulate the effects of Wif1 on Wnt signaling, testing first the effects of dlp overexpression. In control, nub-Gal4, UAS-dlp wings we found a very slight reduction of margin bristles compared to wild type (Figure S3), but wing blades were largely of normal size and showed no signs of scalloping at the margin (Figure 3A and 3B). Nonetheless, expression of UAS-dlp strongly enhanced the effects of a moderately strong UAS-wif1 genomic insertion, increasing the extent of wing scalloping and bristle loss (Figure 3C, 3D). We next re-tested this interaction using a weaker UAS-wif1 construct inserted into a viral integrase site in the genome (pVal-UAS-wif1). nub-Gal4-driven expression of pVal-UAS-wif1 did not cause any margin scalloping or wing blade reduction on its own, but did so when co-misexpressed with UAS-dlp (Figure 3I, 3J and Figure S3). This indicates that the genetic interaction between dlp and wif1 was not simply additive, but synergistic.
Fig. 3. Dlp and Dally enhance the effects of Wif1 expression in Drosophila wings. 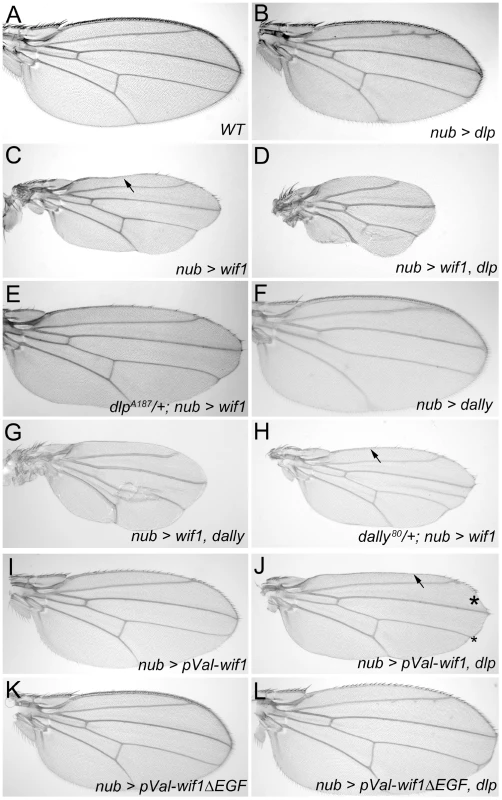
(A–L) nub-Gal4 is used to drive transgene expression. (A) Wild-type wing. (B) Overexpression of UAS-dlp results in only slightly fewer bristles along the wing margin, no loss of L1 and no reduction in wing size. For a detailed comparison of bristle numbers, see Figure S3. (C) Expression of UAS-wif1 eliminates many bristles, interrupts L1 (arrow) and somewhat reduces wing size. (D) Co-expression of UAS-dlp and UAS-wif1 almost completely eliminates wing margin bristles and L1, and strongly reduces wing size. (E) Expression of UAS-wif1 causes much weaker wing margin defects in dlpA187/+ heterozygotes. (F) Overexpression of UAS-dally results in only very slightly fewer wing margin bristles and no obvious reductions in wing size. For detailed comparison of bristle numbers see Figure S3. (G) Combined expression of UAS-dally and UAS-wif1 almost completely eliminates wing margin bristles and L1, and further reduces wing size. (H) Expression of UAS-wif1 causes weaker wing margin defects in dally80/+ heterozygotes (e.g. more complete anterior L1; compare arrows in C and H). (I–L) EGF-depleted Wif1 is less effective at inhibiting Wg signaling and does not interact with Dlp. Control wings expressing pVal-UAS-wif1 show modest defects in margin development (I), which is synergistically enhanced by UAS-dlp; the arrow marks the interruption of L1 and the asterisks mark scalloping of the margin (J). pVal-UAS-wif1ΔEGF is less effective at reducing number of margin bristles than pVal-UAS-wif1 and its effects on wing margin development are not enhanced by co-expression of UAS-dlp (J). See Figure S3 for comparison of bristle numbers. To test whether the genetic interaction between dlp and wif1 was specific, we examined the effects of co-misexpressing UAS-dlp with a different extracellular Wg inhibitor, a GPI-linked extracellular fragment of the D. melanogaster Frizzled 2 (DFz2, NCBI Gene ID: 40090) Wnt receptor (UAS-Dfz2-GPI) that binds ex-Wg but cannot transduce Wg signal [55]. The effects of nub-Gal4-driven UAS-Dfz2-GPI expression were not strengthened by co-expression of UAS-dlp (Figure S2C, S2E). Dfz2-GPI-expressing wings were still sensitive to further reductions in Wg function, however, since co-expression with UAS-wif1 reduced their size (Figure S2D).
We next examined whether reducing Dlp levels altered the effects of wif1 expression. Since dlp null mutants are lethal and defective in several signaling pathways, we instead reduced Dlp levels using dlp heterozygotes or expression of UAS-dlp RNAi. In both cases, the effects of nub-Gal4-driven expression of the moderately strong UAS-wif1 insertion were greatly decreased; scalloping of the adult wing margin was almost completely eliminated and more margin bristles were retained (Figure 3E and data not shown). These results demonstrate that the glypican Dlp increases the effectiveness of Wif1.
We also found similar genetic interactions between Wif1 and the D. melanogaster glypican Dally. Overexpression of Dally alone did not induce wing margin scalloping, and had weaker effects on bristle number than overexpression of Dlp (Figure 3F; quantified in Figure S3), in agreement with weaker effects of Dally overexpression on extracellular Wg [33], [50]. Nonetheless, Dally overexpression synergized the effects of Wif1 expression, causing additional scalloping and bristle loss (Figure 3G). Moreover, while removing dally weakens Wg signaling along the wing margin [36], the reduction of Wg signaling observed after Wif1 expression was partially reversed in a dally heterozygote background (Figure 3H). This suggests that Wif1 binds to more than one kind of HSPG, consistent with the observed interactions between WIF1 family members and the HS sidechains attached to all glypicans [2], [7]
To test if similar relationships exist between Wif1 and glypicans in the context of zebrafish Wnt signaling, we analyzed genetic interactions between Wif1 and zebrafish Glypican 4 (Gpc4, also known as Knypek, NCBI Gene ID: 118437), which is similar to Dally and Dlp and interacts with zebrafish Wnts and Wnt-binding proteins [38], [56]. WIF1 binds Wnts that stimulate both canonical β-catenin-mediated and non-canonical planar cell polarity (PCP) signaling [1], [7], [57], and WIF1 overexpression inhibits canonical Wnt signaling in several contexts [1], and PCP in the rat inner ear [58]. We found that injection of wif1 mRNA intro zebrafish embryos inhibited canonical Wnt signaling, as indicated by the expression of the Tg(TOP:dGFP) reporter line [59] in the dorsal hindbrain (Figure S4). wif1 injection also inhibited posterior development (Figure S4), phenocopying the posterior defects caused by reduced canonical signaling [56], [60]–[62], or by reduced PCP signaling and the resultant defects in convergent-extension movements [38], [63]–[67]. The most extreme phentoypes included slightly enlarged forebrains, indicative of decreased canonical signaling, but not the enlarged heads caused by very strong reductions in canonical signaling. While defects in posterior development and convergent extension movements can also be caused by changes in BMP signaling [68], [69], WIF1 does not interact with BMP signaling in frog embryos [1]. wif1 expression in zebrafish did not induce the ventral fin defects typical of BMP-regulated changes in the dorsal-ventral axis [70], and in D. melanogaster Wif1 did not induce the changes in wing vein development typical of altered BMP signaling [71]. While we show below the Wif1 can affect D. melanogaster Hh signaling, our wif1-injected embryos also do not resemble those with altered Hh signaling [72], [73]. We therefore used shortening of the posterior as a measure of Wnt inhibition.
We observed synergy between the effects of overexpressing wif1 and gpc4. Control embryos injected with low (5 pg/nL) doses of gpc4 message were morphologically indistinguishable from wild-type individuals and showed normal levels of canonical TOP:dGFP expression in the hindbrain (Figure 4 and Figure S4). As shown by others, even much higher doses of gpc4 mRNA produced morphologically wild-type embryos [38]. Nonetheless, injecting embryos with 5 pg/nL of gpc4 message enhanced the effects of injecting low (10 pg/nL) or high (60 pg/nL) levels of wif1 message, as indicated by an increase in both the fraction of short-tailed embryos and the severity of the defects (Figure 4). Since the dose gpc4 message we used has no effect on its own, these data indicate that Gpc4 enhances the activity of Wif1, consistent with the binding observed between human WIF1 and glypican HS sidechains [7]. We were not able to test the effects of removing gpc4 on the wif1-overexpression phenotype because gpc4 mutants already have strong axis defects, likely due to loss of a co-receptor-like activity in Wnt signaling [38].
Fig. 4. Gpc4 enhances the effects of full-length Wif1 in zebrafish embryos. 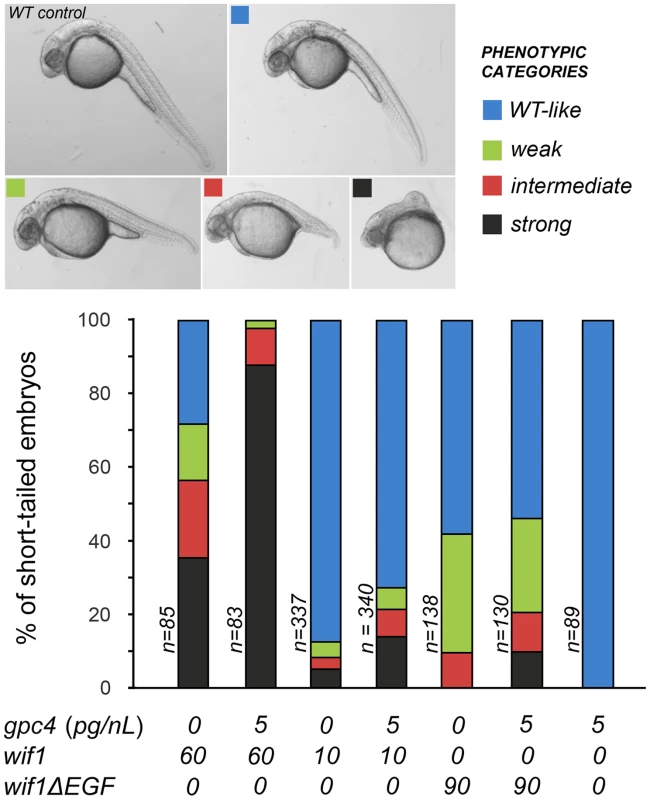
Approximately 2 nL of mRNA of a given concentration was injected into one cell stage embryos, and embryos were scored at 28–30 hours post-fertilization. Images show representative examples of the penetrance of the short-tailed phenotype compared to an uninjected control. In top panels dorsal is up and anterior is to the left. Bar graphs show percentage of short-tailed embryos. The data is pooled from two independent experiments; frequencies were scored and their percentages were averaged. See Materials and Methods for information on RNA preparation. Interactions between Wif1 and glypicans require EGF-like domains
D. melanogaster Shf is stabilized in the extracellular space by glypicans [2]. For instance, the levels of endogenous Shf are reduced in clones lacking the glypican Dally, and are increased in cells overexpressing it (Figure S5A, S5B). These interactions require the presence of a normal ‘EGF-like’ domain, since shf2 mutants that harbor a mutation in the third ‘EGF-like’ domain do not respond to changes in Dally levels (Figure S5C, S5D). Completely removing Shf's ‘EGF-like’ domains also greatly reduces its activity: UAS-shfΔEGF was unable to fully rescue Hh activity in shf nulls [2]. Similarly, the ‘EGF-like’ domains of WIF1 have been shown to bind HS in vitro [7].
We therefore tested the activities of a wif1 construct lacking the ‘EGF-like’ domains (wif1ΔEGF). To bypass the variability in transgene transcription frequently caused by different genomic insertion sites, we used pVal-UAS-wif1 and pVal-UAS-wif1ΔEGF constructs that integrate into a single, pre-selected genomic location [74]. The absence of the ‘EGF-like’ domains is unlikely to alter the stability of the recombinant protein, since both the Wif1 and Wif1ΔEGF were secreted in vitro by D. melanogaster S2 cells at equal levels (Figure S6).
pVal-UAS-wif1ΔEGF was substantially less effective at disrupting wing margin development than pVal-UAS-wif1: driving pVal-UAS-wif1ΔEGF expression with nub-Gal4 caused a much smaller reduction in the number of wing margin bristles (Figure 3I, 3K; quantified in Figure S3). Moreover, the effects of combining pVal-UAS-wif1ΔEGF with UAS-dlp on bristle numbers were additive, rather than synergistic (Figure 3L; quantified in Figure S3). Co-misexpression of pVal-UAS-wif1ΔEGF with UAS-dlp did not induce the synergistic wing margin scalloping that was observed after co-misexpression of pVal-UAS-wif1 and UAS-dlp (Figure 3J, 3L). In addition, Wif1ΔEGF did a much poorer job than full length Wif1 at increasing the accumulation of ex-Wg on the surfaces of wing disc cells expressing high levels of endogenous Dlp: in nub-Gal4, pVal-UAS-wif1ΔEGF discs, ex-Wg remained high along the prospective wing margin and low proximally (Figure 2N, 2O).
In the context of zebrafish Wnt signaling, Wif1ΔEGF was also much less effective, and did not show comparable synergistic increases in its activity when with co-injected with gpc4 (Figure 4). Therefore, the ‘EGF-like’ domains of Wif1 are important for interactions with glypicans in both D. melanogaster and zebrafish. However, it was recently reported that the ‘EGF-like’ domains also contribute to Wnt binding, providing an additional mechanism for the reduced activity of the Wif1ΔEGF constructs [7].
The EGF-like domains are interchangeable between Wif1 and Shf
Unlike vertebrate WIF1, D. melanogaster Shf cannot inhibit Wg signaling [2], [3]. To investigate the domains responsible for this difference, we generated chimeric constructs in which we swapped the ‘WIF’ and ‘EGF-like’ domains between Wif1 and Shf (Figure 5A). WIFWif1-EGFShf denotes a construct bearing zebrafish ‘WIF’ domain fused to the ‘EGF-like’ repeats of Shf (Figure 5). This construct affected Wg in a manner similar to that of full-length Wif1, causing wing margin defects in the adult wings, and redistributing ex-Wg in wing discs. All three UAS-WIFwif1-EGFshf transgenic lines tested caused adult wing defects comparable to that of our strongest UAS-wif1 lines (Figure 5B), and also synergized with UAS-dlp (Figure 5C). Only two pieces of evidence indicate that the WIFWif1-EGFShf chimera is not as potent as the full-length Wif1 : 1) in wing discs nub-Gal4-driven expression of UAS-WIFwif11-EGFshf did not extend ex-Wg as far proximally as UAS-wif1 (Figure 5D, 5E), and 2) en-Gal4-driven expression of UAS-WIFwif1-EGFshf yielded adult escapers, but en-Gal4-driven expression of UAS-wif1 did not. Nonetheless, these data show that the ‘EGF-like’ domains of Wif1 and Shf are largely interchangeable during Wnt inhibition, suggesting that either can interact with HSPGs.
Fig. 5. The EGF-like domains are interchangeable between Wif1 and Shf during Wif1-dependent Wg inhibition. 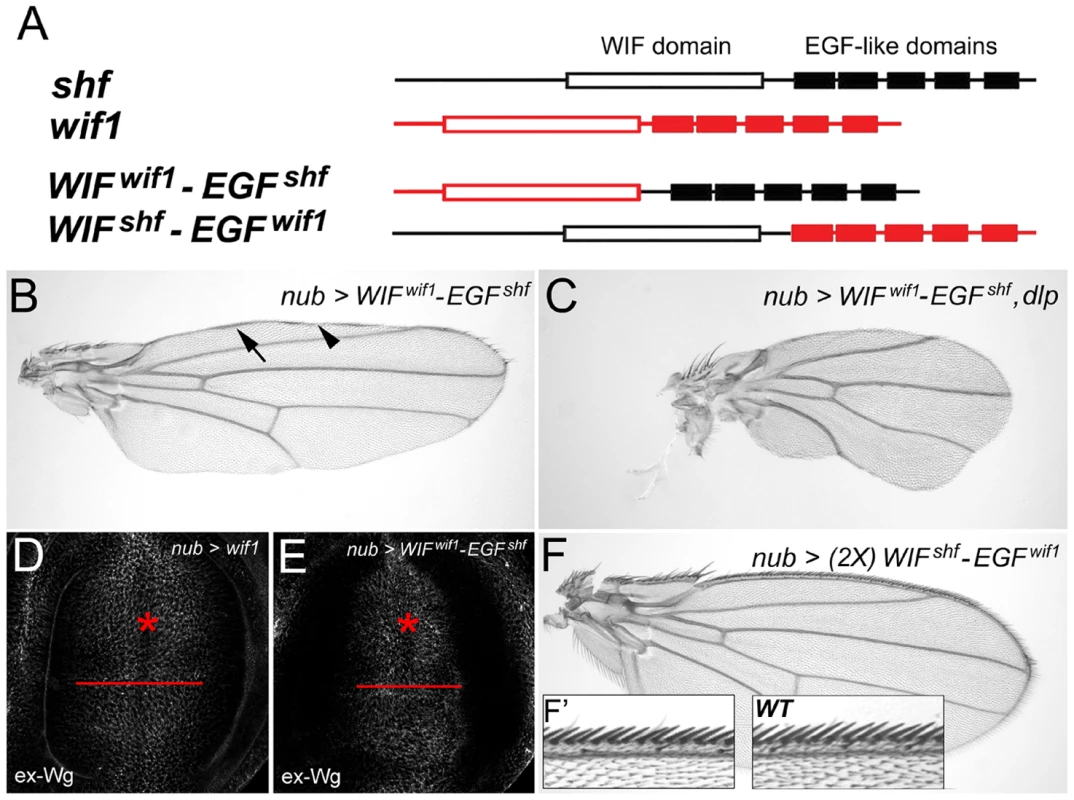
(A) Domain compositions of Wif1, Shf and the two chimeric constructs. Open boxes show the ‘WIF’ domain, filled boxes the EGF-like domains. (B–F) nub-Gal4-driven misexpression of respective transgenes. (B) UAS-WIFwif1-EGFshf strongly reduces the density of anterior wing margin bristles and interrupts L1. Arrow and arrowheads denote L1 or lack of thereof, respectively. (C) Co-expression of UAS-WIFwif1-EGFshf and UAS-dlp almost completely eliminates wing margin bristles and L1, and reduces the size of the wing. (D, E) Expression of either UAS-wif1 or UAS-WIFwif1-EGFshf similarly reduces ex-Wg levels on the surface of prospective margin cells (asterisks) and increases levels proximally. However, compared to UAS-wif1, UAS-WIFwif1-EGFshf expression does not increase ex-Wg as far proximally (compare red bars). (F, F′) Expression of two copies of UAS-WIFshf-EGFwif1 does not alter wing shape or size, and has no measurable effect on margin bristles (anterior margin details in F′). In contrast, expression of one or even two copies of the reciprocal chimera, UAS-WIFshf-EGFwif1, had no effect on Wg signaling (Figure 5F, 5F′). This indicates that the ‘WIF’ domain of Shf cannot interact with Wg strongly enough to inhibit signaling, even in the presence of the Wif1's ‘EGF-like’ domains. This also indicates that the ‘EGF-like’ domains of Wif1 cannot interact with Wg strongly enough to inhibit signaling, despite the presence of an orthologous ‘WIF’ domain. Thus, the specificity for Wnt inhibition resides in the WIF domain of Wif1.
We did, however, find sensitized contexts in which Shf weakly affected Wg signaling, albeit in an unexpected direction: co-expression of UAS-shf slightly improved the adult wing margin defects caused by expression of UAS-wif1 or UAS-Dfz2-GPI, although not the wing margin defects caused by expression of UAS-wg RNAi (Figure S7 and data not shown). Thus, Shf weakly promotes Wg signaling in these contexts, the opposite of Wif1. Conversely, while expressing UAS-wif1 with nub-Gal4 yielded viable adults, shf2 larvae expressing UAS-wif1 did not survive to adulthood; this suggests that endogenous Shf can counteract the otherwise lethal Wnt-inhibitory effects of Wif1. We will present possible mechanisms for these effects in the Discussion.
The WIF domain of Wif1 can regulate Hh signaling
Previous results suggested that neither human WIF1 nor its fish homolog could promote strong Hh signaling in D. melanogaster [2], [3]. Through a more careful examination, however, we found that Wif1 can alter Hh signaling, albeit weakly. In the wing disc, Hh is produced by the cells of posterior (P) compartment and signals to adjacent cells of the anterior (A) compartment [75]. Antibodies to the activated form of the Gli-family transcription factor Cubitus interruptus (CiAct, NCBI Gene ID: 43767), and to the Hh receptor Patched (Ptc, NCBI Gene ID: 35851), measure low - and high-threshold Hh responses, respectively [76], [77]. Signaling can also be measured in the adult wing, since the anterior-posterior distance between the longitudinal wing veins L3 and L4 is regulated via transcription of knot (also known as collier), a high-threshold Hh target [76], [78]–[82]. In shf adult wings the spacing between L3 and L4 is greatly reduced, and in wing discs the normally broader domains of anti-CiAct and anti-Ptc staining regress to thin stripes [2], [3] (Figure 6A, 6B, 6D, 6E and Figure 7A, 7B). shf is also required for the extracellular accumulation and movement of Hh: when GFP-tagged Hh (Hh-GFP) is expressed in dorsal cells using ap-Gal4, it accumulates in adjacent ventral cells (Figure 6C), but in shf mutants the ventral accumulation of dorsally-expressed Hh-GFP is largely lost (Figure 6F). Because Shf is quite diffusible, all of these defects can be rescued by expression of UAS-shf from any domain in the wing disc [2], [3].
Fig. 6. Wif1 affects Hh activity in shf discs. 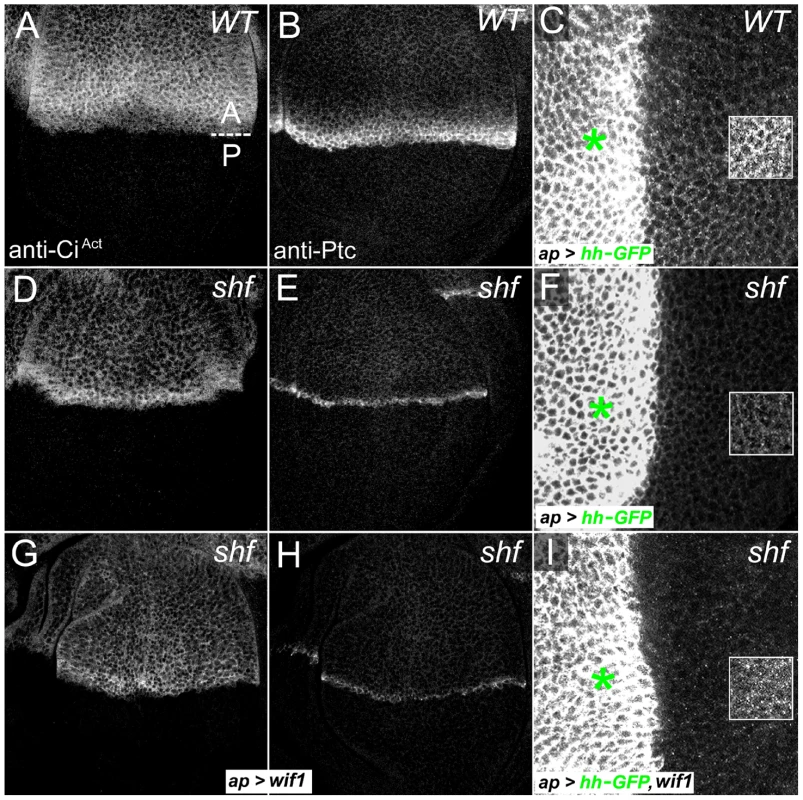
(A–C) Wild-type wing discs, showing the regions with high levels (red bars) of CiAct (A) or Ptc (B), or showing the ventral accumulation of Hh-GFP in the posterior compartment after dorsal, ap-Gal4-driven expression of UAS-hh-GFP (C). (D–F) shf/Y wing discs show reductions in the width of domains expressing high levels of CiAct (D) or Ptc (E), and also show reduced ventral accumulation of Hh-GFP in the posterior compartment after dorsal ap-Gal4-driven expression of UAS-hh-GFP (F). (G–I) shf/Y wing discs with ap-Gal4-driven expression of UAS-wif1 have a broader domain of less intense anti-CiAct staining (G) and lower levels of anti-Ptc staining (H), but improve the ventral accumulation of Hh-GFP in the posterior compartment after ap-Gal4-driven expression of UAS-hh-GFP (I). Ventral Hh-GFP was more punctuate than in wild type discs (compare to C). To make it easier to see the differences in ventral Hh-GFP accumulation, levels were increased equally in the boxed regions in C, F, and I. Hh-GFP levels are quantified in Figure S9. Fig. 7. Vertebrate WIF domain regulates long-range Hh signaling. 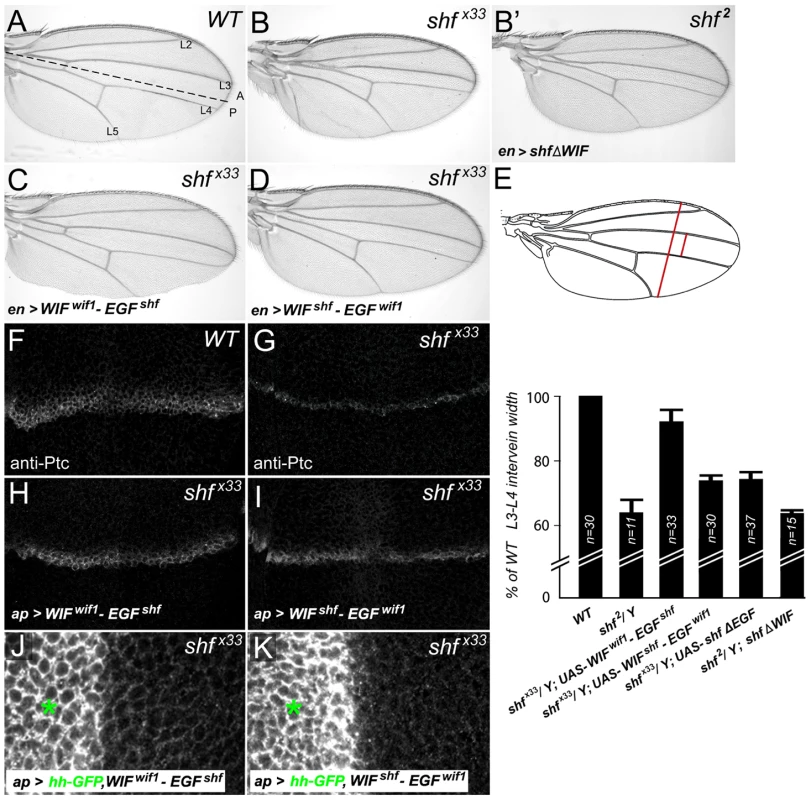
(A) Wild type wing showing the positions of the first through fifth longitudinal veins (L1–L5) and the position of the A/P compartment boundary (dashed line). (B) Reduced L3–L4 spacing in shf mutant wing (B) is not improved upon expression of UAS-shfΔWIF (B′). (C, D) Posterior, en-Gal4-driven expression of UAS-WIFwif1-EGFshf (C) or UAS-WIFshf-EGFwif1 (D) in shfx33/Y wings. UAS-WIFwif1-EGFshf strongly improved and UAS-WIFshf-EGFwif1 weakly improved L3–L4 spacing. (E) Comparison of L3–L4 spacing in wild type, shf2, and shfx33/Y with en-Gal4-driven UAS-construct expression. To compensate for differences in overall wing size, we normalized the L3–L4 distance to the distance between the anterior and posterior margins (red bars). In all but one case we presented the experimental normalized L3–L4 distances as percentages of the wild type normalized distances. However, since expression of UAS-WIFwif1-EGFshf reduced the size of the posterior compartment, and thus the distance between the anterior and posterior margins, we compared the normalized L3–L4 distance in shfx33 UAS-WIFwif1-EGFshf wings to the normalized L3–L4 distance in non-shf UAS-WIFwif1-EGFshf siblings (n = 31). Bars denote standard deviation. Two-tailed Student's t test showed no significant differences between UAS-WIFshf-EGFwif1 and UAS-shfΔEGF, or between shf2 and shf2, UAS-shfΔWIF. Differences between the other conditions were significant (p<0.0001). (F–I) Anti-Ptc staining in wild type (F), shfx33/Y (G), and shfx33/Y wing discs with ap-Gal4-driven expression of UAS-WIFwif1-EGFshf (H) or UAS-WIFshf-EGFwif1 (I). The improvement in the width of Ptc expression in shfx33 discs was stronger after expression of UAS-WIFwif1-EGFshf than UAS-WIFshf-EGFwif1. (J, K) shfx33/Y wing discs with ap-Gal4-driven expression of UAS-hh-GFP and UAS-WIFwif1-EGFshf (J) or UAS-hh-GFP and UAS-WIFshf-EGFwif1 (K). UAS-WIFwif1-EGFshf strongly improved the ventral accumulation of Hh-GFP, while the improvement with UAS-WIFshf-EGFwif1 was more modest (quantifications in Figure S9). We were unable to test the effects of Wif1 on L3–L4 spacing in the adult shf mutant wings, since expressing UAS-wif1 in shf mutants using any of several drivers, including nub-Gal4, caused pupal lethality (see below). Nonetheless, expression of UAS-wif1 using ap-Gal4 significantly increased the ventral accumulation of dorsally expressed Hh-GFP in wing discs mutant for the null allele of shf (shfx33) (Figure 6I; quantified in Figure S7). This accumulation was not normal, however: it appeared more punctate than the ventral Hh-GFP accumulation observed in control discs. This abnormal Hh accumulation may account for its effects on signaling. Instead of the narrow domain of intense anti-CiAct staining normally observed anterior the compartment boundary of shf discs, the staining now appeared less intense, broader and more uniform after wif1 expression (Figure 6G). In some shfx33 discs, UAS-wif1 expression also appeared to reduce the intensity of the high-threshold Hh target Ptc, although the width of the anti-Ptc staining was not affected (data not shown).
To make sure that the change in anti-CiAct staining induced by Wif1 was not an indirect effect caused by reduced Wg signaling, we reduced Wg signaling in shf discs using UAS-Dfz2-GPI. This did not alter anti-CiAct staining (Figure S8), despite the strong effects of UAS-Dfz2-GPI on Wg signaling and wing margin development (Figure S2C). This is consistent with previous findings that changes in Wg signaling do not obviously affect anti-CiAct staining or Hh signaling in the wing [2], [3], [45].
We next used the chimeric constructs described above to investigate the protein domains responsible for the different Hh signaling activities of Wif1 and Shf, again measuring their ability to rescue the shf mutant phenotypes. Expression of UAS-WIFwif1-EGFshf in shfx33 mutants restored the wing vein phenotype to nearly wild-type (Figure 7A–7C, 7E) and greatly increased both the width of the region expressing the high-threshold Hh target Ptc and the ventral accumulation of dorsally-expressed Hh-GFP (Figure 7F–7H, 7J; quantified in Figure S9). Since expression of the Shf's ‘EGF-like’ domains on their own (ShfΔWIF) cannot improve shf2 mutant defects [83] (Figure 7B′, 7E), these results reveal that the zebrafish ‘WIF’ domain can be highly active in Hh signaling, as long as it is coupled to the EGF-like domains of D. melanogaster Shf.
In contrast, the effects of the UAS-WIFshf-EGFwif1 chimera on L3–L4 spacing, anti-Ptc staining and Hh-GFP movement in shf wings and discs were much more modest (Figure 7A, 7B, 7D, 7E, 7G, 7I, 7K; quantified in Figure S9), which was very similar to the weak effects of Shf's WIF domain alone (UAS-shfΔEGF, Figure 7E) (A.A. and S.S.B submitted). This indicates that Wif1's ‘EGF-like’ domains contribute little to the regulation of Hh signaling.
Discussion
Wif1's role in regulating Wnt-glypican interactions
We have shown that, in the model system provided by the developing D. melanogaster wing, the inhibition of Wg signaling caused by Wif1 expression is accompanied by the accumulation of extracellular Wg on the surfaces of cells that have high concentrations of the glypican Dlp. Dlp was necessary and sufficient for much of this effect: the accumulation of extracellular Wg is largely eliminated by reducing Dlp levels and increased by increasing Dlp levels; genetic interactions also suggest a partly redundant role for the other D. melanogaster glypican, Dally (see below). WIF1 binds both Wnts, largely through its ‘WIF’ domain [1], and the HS sidechains of glypicans, largely through its ‘EGF-like’ domains [7]. Thus, WIF1 likely reinforces Wnt-glypican binding by forming a complex with both, similar to the role proposed for the D. melanogaster Wif1 homolog Shf, which binds Hh and glypicans and stabilizes Hh on cell surfaces [2], [3].
But while the stabilization of Hh-glypican interactions by Shf is accompanied by an increase in Hh signaling, our evidence shows that the increase in Wnt-glypican binding caused by WIF1 does not increase signaling. In fact, the glypican interactions strongly potentiate Wif1's Wnt-inhibiting activity: the D melanogaster glypicans Dlp and Dally increase the effectiveness of Wif1 in developing wings, while the zebrafish glypican Glp4 (also known as Knypek) increases the effectiveness of Wif1 in zebrafish embryos. Previous studies suggest that human WIF1 expression reduces the receptor-mediated endocytosis of Wg in the wing disc [3], so the complex of Wif1 and glypicans apparently either sterically blocks Wg's ability to bind receptors, or sequesters Wif1-bound Wg into an extracellular, glypican-rich domain with less access to the receptors. HSGP interactions are similarly thought to potentiate the modulatory effects of BMP-binding proteins, such as Noggin [84], Chordin [85] and Crossveinless-2/BMPER (Cv-2) [86]–[88].
In vitro evidence suggests that WIF1 binds the HS sidechains of HSPGs through its ‘EGF-like’ domain [7]. This is consistent with our data on the D. melanogaster Wif1 ortholog Shf; Shf accumulation in wing discs is sensitive to glypican and HS levels, but that sensitivity is lost after a missense mutation in Shf's third ‘EGF-like’ domain [2]. We show that the loss of Wif1's ‘EGF-like’ domains, and thus HS binding, greatly reduces Wif1's ability to increase Wg accumulation on glypican-expressing cells, consistent with direct role for a Wg-Wif1-glypican complex.
We also found that loss of the ‘EGF-like’ domains greatly reduces Wif1's effectiveness in both D. melanogaster and zebrafish embryos, and that removing the ‘EGF-like’ domains reduced the synergism between Wif1 and Dlp in wing discs, and between Wif1 and the zebrafish glypican Gpc4 in zebrafish embryos. A recent study also found that removing the ‘EGF-like’ domains reduced human WIF1's effectiveness in an in vitro assay; however, the ‘EGF-like’ domains were also reported to reinforce the binding of Wnts to the WIF domain [7], so it is uncertain whether the reduction of Wif1 activity can be attributed wholly to the loss of HS binding. Interestingly, in other published assays loss of the ‘EGF-like’ domains did not obviously reduce WIF1 activity [1]. This suggests that the HS interactions are not important for the inhibition of Wnt signaling in all contexts. This parallels the activity of the BMP-binding and glypican-binding protein Cv-2, since removal of its HSGP-binding domain greatly reduces its ability to inhibit BMP signaling in some assays, but not others [86]–[89].
The biphasic effects of the glypicans on Wnt signaling
One consequence of the interaction between Wif1 and glypicans is that it alters the effects of glypicans on signaling. Increasing the levels of Dlp or Dally in the presence of WIF1 synergistically decreases Wg signaling, and reducing the levels of endogenous Dlp or Dally increases Wg signaling. The latter result is particularly telling, because in the absence of WIF1 removing endogenous Dally reduces Wg signaling in the wing [32], [33], [36]. Thus, WIF1 can change a glypican's role from the stimulation to the inhibition of Wnt signaling. We observed similar genetic interactions in zebrafish embryos between WIF1 and zebrafish Gpc4.
This underscores the complexity of the role glypicans play in regulating signaling. In some settings the effects of the glypicans on Wnt are known to be biphasic. While endogenous Dally weakly stimulates Wg signaling in the wing disc [32], [33], [36], we and others have shown that overexpression of Dally can inhibit Wg-dependent signaling in the embryo and during wing margin development [90], [91]. Endogenous Dlp inhibits signaling close to the distal, wg-expressing cells of the wing margin, but stimulates signaling in proximal cells distant from the wing margin [30]–[35], [39]. One explanation proposed for these different effects is spatial: Dlp may sequester excess Wg from its receptors distally, near the site of Wg secretion, but increase the movement of Wg from distal to proximal cells, increasing the amount of Wg that is available for proximal signaling. But Dlp can also both stimulate and inhibit Wg signaling in vitro, where all cells are likely to have access to the Wg in the culture medium [30], [39]. Low levels of Dlp stimulate Wg signaling, while high levels inhibit; the biphasic effects of Dlp are also influenced by the levels of Wg and the DFz2 receptor, favoring stimulation when the levels of Wg are low and the levels of DFz2 are high, but favoring inhibition when the levels of Wg are high and the levels of DFz2 are low.
Vertebrate glypicans such as Gpc4 can be similarly biphasic. Removing Gpc4 inhibits non-canonical Wnt signaling in zebrafish embryos, indicating a positive role in signaling, and while overexpression of Gpc4 does not inhibit signaling on its own, at high levels it makes wnt11 mRNA less effective at rescuing zygotic wnt11 mutants [38]. Other vertebrate glypicans have also been reported to inhibit Wnt activity in various contexts [92].
A mathematical model using a different cell surface ligand-binding protein, the BMP-binding protein Cv-2, provides one way of explaining such biphasic effects [87]. If a ligand-binding protein can exchange ligand directly with the receptor, it may either provide more ligand for the receptor or sequester ligand from the receptor. The model suggests that, within certain ranges of binding constants, the signaling outcome will be positive with lower concentrations of the ligand-binding protein, and negative with higher concentrations.
The ability of glypicans to interact with other Wnt-binding molecules provides another way of altering the biphasic activity of glypicans. Since Wif1 increases the amount of Wnt binding to the glypican, this should increase the glypican's effective concentration, biasing its biphasic activity towards inhibition. Thus, the presence or absence of proteins that bind both glypicans and Wnts may provide an explanation for some of context-specific activities of vertebrate glypicans.
Pathway specificity of Wif1 and Shifted
The ‘WIF’ domain of WIF1 does not bind HS sidechains, but is sufficient for Wnt binding; the ‘EGF-like’ domains show only weak binding to Wnts on their own, but appear to strengthen Wnt binding to the ‘WIF’ domain [1], [7]. But while the D. melanogaster WIF1 homolog Shf contains both ‘WIF’ and ‘EGF-like’ domains, it does not inhibit Wg signaling; instead, it increases the levels or range of Hh signaling [2], [3]. We found that a construct containing Shf's ‘WIF’ domain and the zebrafish Wif1's ‘EGF-like’ domains also cannot inhibit Wnt signaling, while the reciprocal construct with Wif1's ‘WIF’ domain and Shf's ‘EGF-like’ domain can. Similar results have been obtained with constructs made from Shifted and human WIF1 (I. Guerrero, personal communication). Thus, the ability to inhibit Wg activity, and likely to bind significant levels of Wg, resides in the different ‘WIF’ domains of Wif1 and Shf.
Surprisingly, Shf did show a weak ability to improve Wg signaling in sensitized backgrounds expressing either Wif1 or the dominant negative DFz2-GPI construct. While we have never detected any obvious effect of Shf on ex-Wg levels, it may weakly interact with Wg in a manner that reduces the levels bound to Wif1 or DFz2-GPI and increases the levels available for the Wg receptors. Consistent with this interpretation, UAS-shf did not alleviate margin defects caused by expression of UAS-wg RNAi, even though UAS-Dfz2-GPI and UAS-wg RNAi show a very comparable impact on Wg activity. Alternatively, Shf's effect on Wnt signaling might be due to interactions with the Wnt4 or Wnt6 expressed along the wing margin, which may have redundant roles in wing margin development [42] that are only obvious in a sensitized background. Indirect effects via Hh signaling are unlikely, as Shf overexpression does not further increase Hh signaling [2], [3].
The situation with Hh signaling is more complex. First, vertebrate WIF1's are not known to regulate vertebrate Hh signaling, but we found that zebrafish Wif1 can weakly affect the reduced movement or accumulation of Hh normally observed in shf mutant wing discs. The Hh-GFP accumulation is abnormal, however, appearing more punctuate than in normal wing discs, perhaps accounting for its ability to reduce the expression of Hh targets.
Placing WIF domain of zebrafish Wif1 in the context of Shf's ‘EGF-like’ domains in a chimeric WIFWif1−EGFShf construct almost fully rescues loss of shf function, something not observed after expression of the Shf ‘EGF-like’ domains alone. Together, these data suggest that the ‘WIF’ domains of both Shf and zebrafish Wif1 are capable of interacting with Hh. Like Wnts, Hh is palmitoylated [93], and it has been suggested that these palmitates might bind a hydrophobic pocket found in the WIF domain [94], [95], although this has been recently questioned [7]. The activity of ‘WIF’ domains in Hh signaling may also vary between different vertebrates, since unlike the WIFWif1–EGFShf construct made using zebrafish ‘WIF’ domains, a similar construct made using the ‘WIF’ domain from human WIF1 does not rescue loss of shf function (I. Guerrero, personal communication).
The Shf ‘EGF-like’ domains are necessary to confer a Shf-like level of Hh-promoting activity to the ‘WIF’ domains of zebrafish Wif1. The Hh-promoting activity of Wif1's ‘WIF’ domain is increased by placing it in the context of Shf's ‘EGF-like’ domains, and the low Hh-promoting activity of Shf's ‘WIF’ domain is not changed by placing it in the context of Wif1's ‘EGF-like’ domains. It is unlikely that the ‘EGF-like’ domains of Shf and Wif1 differ significantly in their HSPG-binding activities, since Wif1 and WIFWif1-EGFShf differ only slightly in their ability to inhibit Wnt signaling and interact genetically with Dlp. We therefore favor the alternative hypothesis that Shf's ‘EGF-like’ domains contribute to Hh signaling through a mechanism independent of glypican binding. While the Shf ‘EGF-like’ domains alone (ShfΔWIF) cannot increase Hh signaling, we have found that they can increase the levels of extracellular Hh (A.A. and S.S.B., submitted), suggesting that they contribute to Hh binding, much as the ‘EGF-like’ domains of WIF1 do to Wnt binding [7].
Since Wif1 can alter Hh distribution and, more weakly, signaling in D. melanogaster, an important question is whether it can also do so in vertebrates. Because of its strong effects on Wnt signaling, vertebrate WIF1 family proteins have rarely been assayed for their effects on other pathways, so a weak modulation of one of the vertebrate Hhs remains a possibility.
Materials and Methods
Ethics statement
Animals were handled in accordance with guidelines set forth by NIH and IACUC. Our animal use protocols were approved by the University of Wisconsin and Tufts University Institutional Animal Care and Use Committees
Molecular constructs and transgenic flies
pUAS-wif1 and pVal-UAS-wif1 were generated by PCR from full-length wif1 template [2]. pVal-wif1ΔEGF terminates at R177; both the pVal inserted constructs also contain a C-terminal V5 epitope. Constructs were expressed in S2 cells as described [96], and checked for expression on Western blots using standard procedures. The chimeric Shf/Wif1 coding sequences were generated using PCR and were spliced between the end of the ‘WIF’ domain and the beginning of the first ‘EGF-like’ domains with junctions at E282:Q180 (WIFShf-EGFWif1) and T174:C261 (WIFzWIF1-EGFShf). WIFShf-EGFWif1 also contains a V5 epitope N-terminal to the WIF domain. The identically tagged full-length Shf localized and functioned like endogenous Shf protein (A.A., S.H., S.S.B; unpublished). Most transgenes were subcloned into pUAST, but for the comparison between Wif1 and Wif1ΔEGF they were subcloned into pValium1 and intergrated into identical attP2 genomic sites [74]. Construct DNA was injected into D. melanogaster embryos by Injection Services, Inc. (Sudbury, MA).
Zebrafish experiments
gpc4 open reading frame was generously provided by L. Solnica-Krezel. mRNA was prepared in vitro from linearized plasmids using mMessage mMachine Kit (Ambion), purified and kept at −80 C° in frozen aliquots. Before injection, freshly thawed mRNA was diluted to stock concentrations depicted in Figure 4 in 0.1 M KCl containing small amounts of phenol-red for tracing purposes. Approximately 2 nL of injection mixture was injected into one cell stage embryos as described [97]. Injected embryos were allowed to recover at 28.5°C in embryo medium and 28–30 hour old embryos were evaluated for defects in posterior development. To evaluate canonical Wnt signaling, homozygous Tg(TOP:dGFP) fish were crossed with wild-type and their F1 progeny were injected with the chosen mRNAs as described above. To measure GFP fluorescence 30 hour old embryos were mounted in low melting point agarose on coverslips and photographed using an EM-CCD camera (Photometrix) under constant exposure settings. Calculations of GFP intensity were performed in ImageJ and presented in arbitrary units and compared to the levels of the wild-type controls that were set at 100% after normalization.
D. melanogaster strains and genetics
Flies were maintained at 25°C. Mutant analyses used shfx33 [2], shf2 [98]; dally80 and dlpA187 [26]. Mutant clones were generated using FRT-mediated mitotic recombination [99]. UAS-transgenes were expressed using ap-Gal4, dpp-Gal4, en-Gal4, or nub-Gal4 (Bloomington, IN) [100], or in Flpout-Gal4 clones using y,w, hs-Flp; Act>y+>Gal4 UAS-GFP and a 60 minute 37°C heat shock at three days after egg laying. In addition to the UAS lines generated above, we used: UAS-dally [101], UAS-dlp [34], UAS-wif1, UAS-shf, UAS-shfΔEGF, UAS-shfΔWIF [2], UAS-armS10 [53], UAS-Dfz2-GPI [102], UAS-hh-GFP [103], UAS-dlp RNAi (Vienna Drosophila RNAi Center 10299) and UAS-wg RNAi (Transgenic RNAi Project JF01480).
Immunohistochemistry
Late third instar discs were dissected in ice cold PBS and fixed in EM-grade 4% formaldehyde/PBS for 30 minutes at 4°C, rinsed in PBS containing 0.2% Triton X-100, and incubated with primary antibodies overnight at 4°C. Primary antibodies and their working dilutions were: mouse anti-Wg (1∶50; Development Studies Hybridoma Bank [DSHB], Iowa City) [104], rabbit anti-GFP (1∶200, MBL International), mouse anti-GFP (1∶200, Chemicon), mouse anti-V5 (1∶200, Invitrogen), rabbit anti-V5 (1∶200, Bethyl), mouse ant-Dlp (1∶200; DSHB) [105], guinea pig anti-Sens (1∶200) [47], rat anti-CiAct (1∶20) [77], and/or mouse anti-Ptc (1∶200; DSHB) [106], followed by incubation with fluorescent secondary antibodies (Jackson Immunoresearch). Images were acquired using laser scanning confocal microscopy.
Extracellular Wg was detected by incubating discs with anti-Wg (1∶3) for 30–45 minutes at 4°C in Shields and Sang M3 culture medium, briefly rinsing in PBS, fixing for 30 min in 4% formaldehyde/PBS and staining with secondary antisera in PBS [50]. In some cases this was followed by staining for additional antigens as described above.
Supporting Information
Zdroje
1. HsiehJCKodjabachianLRebbertMLRattnerASmallwoodPM 1999 A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398 431 436
2. GliseBMillerCACrozatierMHalbisenMAWiseS 2005 Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Dev Cell 8 255 266
3. GorfinkielNSierraJCallejoAIbanezCGuerreroI 2005 The Drosophila ortholog of the human Wnt inhibitor factor Shifted controls the diffusion of lipid-modified Hedgehog. Dev Cell 8 241 253
4. NakayaNLeeHSTakadaYTzchoriITomarevSI 2008 Zebrafish olfactomedin 1 regulates retinal axon elongation in vivo and is a modulator of Wnt signaling pathway. J Neurosci 28 7900 7910
5. BuermansHPvan WijkBHulskerMASmitNCden DunnenJT 2010 Comprehensive gene-expression survey identifies wif1 as a modulator of cardiomyocyte differentiation. PLoS ONE 5 e15504 doi:10.1371/journal.pone.0015504
6. HunterDDZhangMFergusonJWKochMBrunkenWJ 2004 The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci 27 477 488
7. MalinauskasTAricescuARLuWSieboldCJonesEY 2011 Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nat Struct Mol Biol 18 886 893
8. YinAKorzhVGongZ 2011 Perturbation of zebrafish swimbladder development by enhancing Wnt signaling in Wif1 morphants. Biochim Biophys Acta doi:10.1016/j.bbamcr.2011.09.018
9. KansaraMTsangMKodjabachianLSimsNATrivettMK 2009 Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 119 837 851
10. CebratMStrzadalaLKisielowP 2004 Wnt inhibitory factor-1: a candidate for a new player in tumorigenesis of intestinal epithelial cells. Cancer Lett 206 107 113
11. ClementGGuilleretIHeBYagui-BeltranALinYC 2008 Epigenetic alteration of the Wnt inhibitory factor-1 promoter occurs early in the carcinogenesis of Barrett's esophagus. Cancer Sci 99 46 53
12. DengYYuBChengQJinJYouH 2010 Epigenetic silencing of WIF-1 in hepatocellular carcinomas. J Cancer Res Clin Oncol 136 1161 1167
13. GaoZXuZHungMSLinYCWangT 2009 Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. Anticancer Res 29 2025 2030
14. GaoZXuZHungMSLinYCWangT 2009 Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol Rep 22 1479 1484
15. HeBReguartNYouLMazieresJXuZ 2005 Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 24 3054 3058
16. LinYCYouLXuZHeBYangCT 2007 Wnt inhibitory factor-1 gene transfer inhibits melanoma cell growth. Hum Gene Ther 18 379 386
17. MazieresJHeBYouLXuZLeeAY 2004 Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res 64 4717 4720
18. RubinEMGuoYTuKXieJZiX Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol Cancer Ther 9 731 741
19. TaniguchiHYamamotoHHirataTMiyamotoNOkiM 2005 Frequent epigenetic inactivation of Wnt inhibitory factor-1 in human gastrointestinal cancers. Oncogene 24 7946 7952
20. WissmannCWildPJKaiserSRoepckeSStoehrR 2003 WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol 201 204 212
21. FilmusJCapurroMRastJ 2008 Glypicans. Genome Biol 9 224
22. KirkpatrickCASelleckSB 2007 Heparan sulfate proteoglycans at a glance. J Cell Sci 120 1829 1832
23. BellaicheYTheIPerrimonN 1998 Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature 394 85 88
24. DesbordesSCSansonB 2003 The glypican Dally-like is required for Hedgehog signalling in the embryonic epidermis of Drosophila. Development 130 6245 6255
25. HanCBelenkayaTYKhodounMTauchiMLinX 2004 Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development 131 1563 1575
26. HanCBelenkayaTYWangBLinX 2004 Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development 131 601 611
27. TakeiYOzawaYSatoMWatanabeATabataT 2004 Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 131 73 82
28. BornemannDJDuncanJEStaatzWSelleckSWarriorR 2004 Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development 131 1927 1938
29. YanDLinX 2009 Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol 1 a002493
30. BaegGHSelvaEMGoodmanRMDasguptaRPerrimonN 2004 The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol 276 89 100
31. CapurroMIXiangYYLobeCFilmusJ 2005 Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 65 6245 6254
32. Franch-MarroXMarchandOPiddiniERicardoSAlexandreC 2005 Glypicans shunt the Wingless signal between local signalling and further transport. Development 132 659 666
33. HanCYanDBelenkayaTYLinX 2005 Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development 132 667 679
34. KirkpatrickCADimitroffBDRawsonJMSelleckSB 2004 Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell 7 513 523
35. KreugerJPerezLGiraldezAJCohenSM 2004 Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell 7 503 512
36. LinXPerrimonN 1999 Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 400 281 284
37. TsudaMKamimuraKNakatoHArcherMStaatzW 1999 The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature 400 276 280
38. TopczewskiJSepichDSMyersDCWalkerCAmoresA 2001 The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell 1 251 264
39. YanDWuYFengYLinSCLinX 2009 The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev Cell 17 470 481
40. BakerNE 1988 Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development 102 489 497
41. CohenEDMariolMCWallaceRMWeyersJKamberovYG 2002 DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev Cell 2 437 448
42. GieselerKWilderEMariolMCBuratovitchMBerengerH 2001 DWnt4 and wingless elicit similar cellular responses during imaginal development. Dev Biol 232 339 350
43. JansonKCohenEDWilderEL 2001 Expression of DWnt6, DWnt10, and DFz4 during Drosophila development. Mech Dev 103 117 120
44. BlairSS 1994 A role for the segment polarity gene shaggy-zeste white 3 in the specification of regional identity in the developing wing of Drosophila. Dev Biol 162 229 244
45. CousoJPBishopSAMartinez AriasA 1994 The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120 621 636
46. PhillipsRGWhittleJR 1993 wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development 118 427 438
47. NoloRAbbottLABellenHJ 2000 Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102 349 362
48. ParkerDSJemisonJCadiganKM 2002 Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129 2565 2576
49. WuJMlodzikM 2008 The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell 15 462 469
50. StriginiMCohenSM 2000 Wingless gradient formation in the Drosophila wing. Curr Biol 10 293 300
51. GiraldezAJCopleyRRCohenSM 2002 HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev Cell 2 667 676
52. MaroisEMahmoudAEatonS 2006 The endocytic pathway and formation of the Wingless morphogen gradient. Development 133 307 317
53. PaiLMOrsulicSBejsovecAPeiferM 1997 Negative regulation of Armadillo, a Wingless effector in Drosophila. Development 124 2255 2266
54. FujiseMIzumiSSelleckSBNakatoH 2001 Regulation of dally, an integral membrane proteoglycan, and its function during adult sensory organ formation of Drosophila. Dev Biol 235 433 448
55. CadiganKMFishMPRulifsonEJNusseR 1998 Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell 93 767 777
56. CaneparoLHuangYLStaudtNTadaMAhrendtR 2007 Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev 21 465 480
57. Surmann-SchmittCWidmannNDietzUSaegerBEitzingerN 2009 Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J Cell Sci 122 3627 3637
58. DabdoubADonohueMJBrennanAWolfVMontcouquiolM 2003 Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 130 2375 2384
59. DorskyRISheldahlLCMoonRT 2002 A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol 241 229 237
60. ErterCEWilmTPBaslerNWrightCVSolnica-KrezelL 2001 Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development 128 3571 3583
61. KieckerCNiehrsC 2001 The role of prechordal mesendoderm in neural patterning. Curr Opin Neurobiol 11 27 33
62. LekvenACThorpeCJWaxmanJSMoonRT 2001 Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1 103 114
63. HeisenbergCPTadaMRauchGJSaudeLConchaML 2000 Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405 76 81
64. MatsuiTRayaAKawakamiYCallol-MassotCCapdevilaJ 2005 Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev 19 164 175
65. RauchGJHammerschmidtMBladerPSchauerteHEStrahleU 1997 Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol 62 227 234
66. RoszkoISawadaASolnica-KrezelL 2009 Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol 20 986 997
67. KilianBMansukoskiHBarbosaFCUlrichFTadaM 2003 The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev 120 467 476
68. von der HardtSBakkersJInbalACarvalhoLSolnica-KrezelL 2007 The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol 17 475 487
69. HammerschmidtMMullinsMC 2002 Dorsoventral patterning in the zebrafish: bone morphogenetic proteins and beyond. Results Probl Cell Differ 40 72 95
70. LittleSCMullinsMC 2004 Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development 131 5825 5835
71. BlairSS 2007 Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol 23 293 319
72. HammerschmidtMBitgoodMJMcMahonAP 1996 Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev 10 647 658
73. BarresiMJStickneyHLDevotoSH 2000 The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development 127 2189 2199
74. MarksteinMPitsouliCVillaltaCCelnikerSEPerrimonN 2008 Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet 40 476 483
75. TabataTKornbergTB 1994 Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76 89 102
76. StriginiMCohenSM 1997 A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124 4697 4705
77. MotznyCKHolmgrenR 1995 The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech Dev 52 137 150
78. CrozatierMGliseBVincentA 2002 Connecting Hh, Dpp and EGF signalling in patterning of the Drosophila wing; the pivotal role of collier/knot in the AP organiser. Development 129 4261 4269
79. MohlerJSeecoomarMAgarwalSBierEHsaiJ 2000 Activation of knot (kn) specifies the 3–4 intervein region in the Drosophila wing. Development 127 55 63
80. MullorJLCallejaMCapdevilaJGuerreroI 1997 Hedgehog activity, independent of decapentaplegic, participates in wing disc patterning. Development 124 1227 1237
81. NestorasKLeeHMohlerJ 1997 Role of knot (kn) in wing patterning in Drosophila. Genetics 147 1203 1212
82. VervoortMCrozatierMValleDVincentA 1999 The COE transcription factor Collier is a mediator of short-range Hedgehog-induced patterning of the Drosophila wing. Curr Biol 9 632 639
83. GliseBJonesDLInghamPW 2002 Notch and Wingless modulate the response of cells to Hedgehog signalling in the Drosophila wing. Dev Biol 248 93 106
84. Paine-SaundersSVivianoBLEconomidesANSaundersS 2002 Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem 277 2089 2096
85. JasujaRAllenBLPappanoWNRapraegerACGreenspanDS 2004 Cell-surface heparan sulfate proteoglycans potentiate chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of chordin. J Biol Chem 279 51289 51297
86. RentzschFZhangJKramerCSebaldWHammerschmidtM 2006 Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133 801 811
87. SerpeMUmulisDRalstonAChenJOlsonDJ 2008 The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell 14 940 953
88. KelleyRRenRPiXWuYMorenoI 2009 A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol 184 597 609
89. ZhangJLQiuLYKotzschAWeidauerSPattersonL 2008 Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell 14 739 750
90. TakeoSAkiyamaTFirkusCAigakiTNakatoH 2005 Expression of a secreted form of Dally, a Drosophila glypican, induces overgrowth phenotype by affecting action range of Hedgehog. Dev Biol 284 204 218
91. MolineMMDierickHASouthernCBejsovecA 2000 Non-equivalent roles of Drosophila Frizzled and Dfrizzled2 in embryonic wingless signal transduction. Curr Biol 10 1127 1130
92. SongHHShiWXiangYYFilmusJ 2005 The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem 280 2116 2125
93. PepinskyRBZengCWenDRayhornPBakerDP 1998 Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem 273 14037 14045
94. LiepinshEBanyaiLPatthyLOttingG 2006 NMR structure of the WIF domain of the human Wnt-inhibitory factor-1. J Mol Biol 357 942 950
95. MalinauskasT 2008 Docking of fatty acids into the WIF domain of the human Wnt inhibitory factor-1. Lipids 43 227 230
96. HanK 1996 An efficient DDAB-mediated transfection of Drosophila S2 cells. Nucleic Acids Res 24 4362 4363
97. MalickiJJoHWeiXHsiungMPujicZ 2002 Analysis of gene function in the zebrafish retina. Methods 28 427 438
98. ConleyCASilburnRSingerMARalstonARohwer-NutterD 2000 Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127 3947 3959
99. BlairSS 2003 Genetic mosaic techniques for studying Drosophila development. Development 130 5065 5072
100. BrandAHPerrimonN 1993 Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401 415
101. JacksonSMNakatoHSugiuraMJannuziAOakesR 1997 dally, a Drosophila glypican, controls cellular responses to the TGF-beta-related morphogen, Dpp. Development 124 4113 4120
102. RulifsonEJWuCHNusseR 2000 Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell 6 117 126
103. TorrojaCGorfinkielNGuerreroI 2004 Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development 131 2395 2408
104. BrookWJCohenSM 1996 Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila Leg. Science 273 1373 1377
105. LumLYaoSMozerBRovescalliAVon KesslerD 2003 Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science 299 2039 2045
106. CapdevilaJParienteFSampedroJAlonsoJLGuerreroI 1994 Subcellular localization of the segment polarity protein patched suggests an interaction with the wingless reception complex in Drosophila embryos. Development 120 987 998
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 2
-
Všechny články tohoto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



