-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRaf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
Non-coding transcription can trigger histone post-translational modifications forming specialized chromatin. In fission yeast, heterochromatin formation requires RNAi and the histone H3K9 methyltransferase complex CLRC, composed of Clr4, Raf1, Raf2, Cul4, and Rik1. CLRC mediates H3K9 methylation and siRNA production; it also displays E3-ubiquitin ligase activity in vitro. DCAFs act as substrate receptors for E3 ligases and may couple ubiquitination with histone methylation. Here, structural alignment and mutation of signature WDxR motifs in Raf1 indicate that it is a DCAF for CLRC. We demonstrate that Raf1 promotes H3K9 methylation and siRNA amplification via two distinct, separable functions. The association of the DCAF Raf1 with Cul4-Rik1 is critical for H3K9 methylation, but dispensable for processing of centromeric transcripts into siRNAs. Thus the association of a DCAF, Raf1, with its adaptor, Rik1, is required for histone methylation and to allow RNAi to signal to chromatin.
Published in the journal: . PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002499
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002499Summary
Non-coding transcription can trigger histone post-translational modifications forming specialized chromatin. In fission yeast, heterochromatin formation requires RNAi and the histone H3K9 methyltransferase complex CLRC, composed of Clr4, Raf1, Raf2, Cul4, and Rik1. CLRC mediates H3K9 methylation and siRNA production; it also displays E3-ubiquitin ligase activity in vitro. DCAFs act as substrate receptors for E3 ligases and may couple ubiquitination with histone methylation. Here, structural alignment and mutation of signature WDxR motifs in Raf1 indicate that it is a DCAF for CLRC. We demonstrate that Raf1 promotes H3K9 methylation and siRNA amplification via two distinct, separable functions. The association of the DCAF Raf1 with Cul4-Rik1 is critical for H3K9 methylation, but dispensable for processing of centromeric transcripts into siRNAs. Thus the association of a DCAF, Raf1, with its adaptor, Rik1, is required for histone methylation and to allow RNAi to signal to chromatin.
Introduction
Silencing mechanisms mediated by small RNAs occur in most eukaryotes. RNA interference (RNAi) can reduce gene expression post-transcriptionally by cleaving homologous transcripts or by inhibiting their translation [1]. Small RNAs can also act in the nucleus, inducing transcriptional gene silencing (TGS) [2], [3]. Although siRNA-directed modification of homologous chromatin is widespread in eukaryotes, the details of how siRNAs mediate such events remain limited in most systems. [4], [5]. In plants and fungi, the link between siRNA-directed DNA/chromatin modification and heterochromatin formation is well established [2], [3]. The fission yeast, Schizosaccharomyces pombe, is a powerful system in which to study RNAi-directed heterochromatin formation in part because it contains single non-essential genes encoding each of the key RNAi components.
In fission yeast, siRNAs are important for heterochromatin formation on the centromeric outer repeats (composed of dg and dh elements) and other chromosomal locations. Although marker genes inserted at these loci are silenced [6], centromeric repeats are bi-directionally transcribed by RNAPII, producing double-stranded RNA (dsRNA) [2], [3], [7], [8], [9]. Dicer (Dcr1) cleaves these non-coding transcripts into siRNAs that guide the Argonaute (Ago1)-containing RITS complex to homologous nascent transcripts by sequence complementarity. Chromatin-bound-RITS recruits the histone methyltranferase Clr4SuVar3-9 (a CLRC component; see below) to centromeric repeats via the linker protein Stc1 [10]. Clr4 methylates lysine 9 of histone H3 (H3K9me), providing binding sites for the chromodomain proteins Swi6, Chp1 (a RITS component) and Chp2 [11], [12], [13], [14]. Two different non-mutually exclusive models have been proposed to explain how heterochromatin, once assembled, silences centromere repeat transcription. In the ‘transcriptional gene silencing’ (TGS) model, heterochromatin factors directly repress transcription [15]. In the ‘co-transcriptional gene silencing’ (CTGS) model the repeats are continuously transcribed and silencing is due to the efficient processing of transcripts to siRNA [16]. In wild-type cells, siRNA production and H3K9 methylation are coupled processes: deletion of genes encoding CLRC components results not only in loss of H3K9 methylation but also in loss of siRNA [17], [18], [19]. Similarly, deletion of genes encoding RNAi components abrogates siRNA production and reduces H3K9me [9]. However, cells expressing only mutant histone H3 (H3K9R) produce some detectable siRNAs, even though H3K9 can not be methylated [20], [21]. Moreover, tethering the Rik1 CLRC component to ura4 RNA triggers silencing of the ura4+ gene independently of other CLRC subunits, but requires RNAi [21]. Thus CLRC itself, rather than its substrate H3K9, promotes siRNA production independently of H3K9. However, it is not known how CLRC integrates histone methylation with siRNA generation.
CLRC is composed of the histone methyltransferase Clr4, the β-propeller protein Rik1, the cullin protein Cul4, the WD-40 protein Raf1 (Clr8/Cmc1/Dos1) and Raf2 (Clr7/Cmc2/Dos2), which contains a RFTS domain [17], [22], [23], [24], [25]. Although it is not known whether CLRC acts as an E3-ubiquitin ligase in vivo, CLRC purified from cells exhibits E3 ligase activity in vitro [24]. E3 ligases regulate a variety of biological processes by bridging the E2 ubiquitin conjugating enzyme to specific substrates, allowing their ubiquitination (for review see [26]). Histone methylation is frequently regulated by ubiquitination but the mechanistic details remain unknown [27].
The Cullin-RING ligases (CRLs) are the largest family of multi-subunit E3 ligases in eukaryotes and are formed by a neddylated member of the cullin family scaffold (CUL1-CUL5), a RING finger protein (RBX1 or RBX2) and an adaptor protein that bridges the CUL/RING scaffold to substrate receptors [28]. Amongst CRLs, CRL4 is known to ubiquitinate histones [29], [30]. In the CRL4 complex, DDB1 acts as the adaptor. Affinity purification of DDB1 from human cells identified various WD-40 proteins as possible substrate receptors termed DCAFs (DDB1 and CUL4 Associated Factors) [31], [32], [33], [34]. Several DCAFs (WDR5, RBP5 and EED) not only interact with DDB1 but are also members of histone methyltransferase complexes [31], [32], [33], [34]. Although it is not known whether the interaction between these DCAFs and CUL4/DDB1 leads to ubiquitination of substrates, it might have important functional consequences since the knock-down of CUL4 or DDB1 reduces histone methylation [31]. Many DCAFs contain a specific WDxR motif that is important for their association with DDB1 [31], [32], [33], [34].
In S. pombe, Cul4 associates with the canonical adaptor Ddb1 [35] and with Rik1 (which shares similarity with Ddb1; [36]) in CLRC [17], [22], [23], [24]. Both Cul4-Ddb1 complex and CLRC affect heterochromatin integrity and neddylation of Cul4 is required for H3K9 methylation of heterochromatic regions [22], [37]. This suggests that E3 ligase activity is involved in heterochromatin formation. However, heterochromatin defects observed in neddylation-defective Cul4 (cul4-K680R) could arise either from impaired Cul4-Ddb1 activity or from defective CLRC function. Furthermore, although Rik1 exhibits some similarity to Ddb1 and thus might act as an adaptor, recent analyses suggest that Rik1 is primarily an RNA binding protein which associates with centromeric transcripts via its CPSF-A domain [21]. Thus, it remains to be determined how CLRC contributes to heterochromatin integrity and whether CLRC acts as a DDB1-related E3 ligase which promotes heterochromatin formation in vivo.
Here we demonstrate that the CLRC components Rik1 and Raf1 can be structurally aligned with the human adaptor DDB1 and its DCAF DDB2, respectively. Our analyses are consistent with Raf1 acting as a DCAF for CLRC that contributes to siRNA amplification and H3K9 methylation by two distinct and separable routes. Our findings provide mechanistic insights into how siRNA production is integrated with chromatin modification via CLRC.
Results
Raf1 is a chromatin associated component of the CLRC complex
Components involved in heterochromatin formation are typically localised in distinct chromatin-associated foci [14], [38]. In contrast, previous studies overexpressing GFP-Raf1 from a strong promoter showed that it is a nuclear protein with no obvious chromatin localisation [23], however, this might not reflect its true subcellular localisation. Indeed, the genome-wide distribution of Raf1 suggests that it interacts predominantly with heterochromatic loci [13]. To reassess Raf1 localisation, we examined cells expressing GFP-Raf1 from its native promoter and found that it decorates several distinct chromatin-associated foci, the largest of which lies adjacent to the clustered kinetochores (Figure 1A). ChIP confirmed that FLAG-Raf1 associates with centromeric repeats (Figure 1B). We conclude that Raf1 is a chromatin-associated protein concentrated at heterochromatin.
Fig. 1. Raf1 is a chromatin-associated CLRC component. 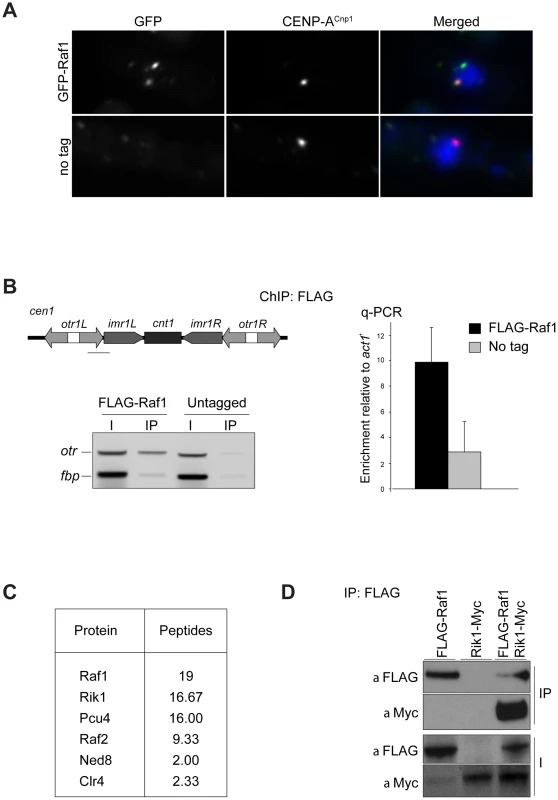
(A) Immunolocalisation of GFP-Raf1 (top panel) or untagged control (bottom panel) localisation. Representative images of fixed cells: GFP-Raf1 (green), centromere specific protein CENP-ACnp1 (red), DAPI stained DNA (blue). (B) FLAG-Raf1 ChIP. Diagram shows position of cen1 primers used (black bar). FLAG-Raf1 or untagged controls cells were analysed by multiplex PCR (otr enrichment relative to fbp1+ control - left) or by qPCR (otr enrichment relative to act1+ - right). I: input; IP: immunoprecipitation. (C) Proteins reproducibly detected in FLAG-Raf1 IPs by LC-MS/MS. Average peptide numbers identified in replicas is shown. (D) Raf1-FLAG IP analysed with anti-FLAG or with anti-Myc to detect Rik1-Myc. Biochemical purification of CLRC components Rik1, Raf2 and Clr4 identified Raf1 as an interacting partner [17], [22], [24]. In contrast, two-step purification of TAP-Raf1 identified the histone demethylase Lid2 plus Cul4 and Rik1, but apparently not Raf2 and Clr4, known CLRC subunits [39]. This raises the possibility that Raf1 is a component of two distinct complexes: CLRC (Cul4/Rik1/Raf1/Raf2/Clr4) and an alternative Lid2/Cul4/Rik1/Raf1 complex. To further investigate this, we affinity selected FLAG-Raf1 from cell lysates and identified associated proteins by mass spectrometry. Silencing assays indicate that FLAG-tagged Raf1 is functional (Figure S1A). Single step FLAG affinity purification is less stringent than the two-step TAP affinity purification and was performed in mild conditions to identify as many Raf1 interacting proteins as possible. Although we detect all CLRC components, Lid2 peptides were absent (Figure 1C). Moreover, while Rik1 coimmunoprecipitated with Raf1 (co-IP; Figure 1D), Rik1 could not be detected in Lid2-TAP IPs (Figure S1B). We conclude that Raf1 mainly associates with the known CLRC components.
Raf1 is a DDB2-like WDxR protein
In CRL4 complexes DDB1 is the adaptor that recruits WD-40-containing DCAF proteins [28]. In CLRC, Rik1 is a DDB1-related protein that interacts with Raf1 [23]. Thus, Rik1 and Raf1 might represent the adaptor and the DCAF, respectively, for CLRC. To test these possibilities, we performed structural alignments of Rik1 and Raf1 and found that they can be modelled on DDB1 and on the DCAF DDB2 (Rik1: DOPE score −11385; Raf1: GA431 score 0.6 respectively) (Figure 2A and 2B). This model reveals that, like human DDB1, Rik1 is composed of three WD-40 β-propeller domains (BPA, BPB and BPC) composed of 21× WD-40 repeats and a C-terminal helical domain (Figure 2B and S2A). Raf1, like DDB2, is predicted to contain an N-terminal helical domain and a 7-bladed WD-40 β-propeller domain forming a ring (Figure 2A and 2B). Unlike DDB2, the helical and WD-40 domains of Raf1 are separated by an additional domain of unknown function (Figure S2B). As in the DDB1/DDB2 complex, our model predicts that the Raf1 helical domain can be inserted into the Rik1 BPA-BPC cleft and the ‘top’ of the β-propeller ring interacts with the ‘bottom’ surface of Rik1 (Figure 2B).
Fig. 2. Raf1 is the DCAF for CLRC with WDxR motifs essential for heterochromatin formation. 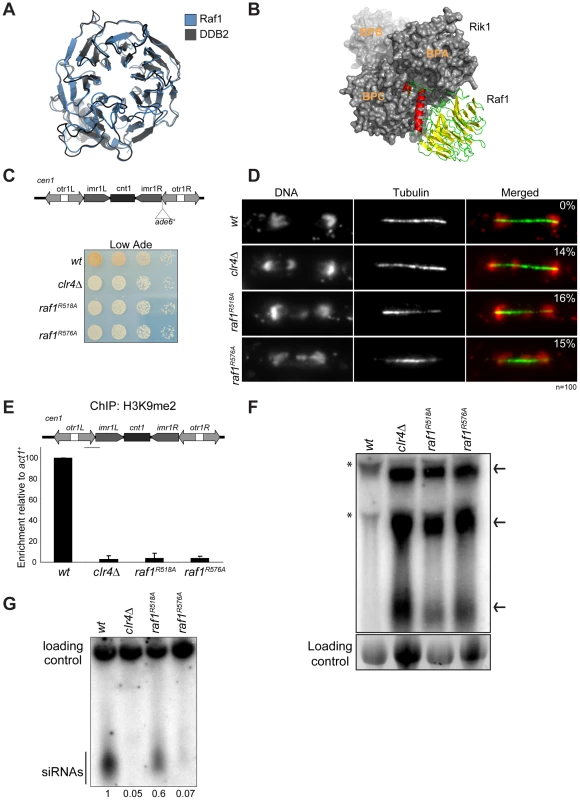
(A) Structural alignment of Raf1 (blue) with human DCAF DDB2 (grey). (B) Structural model of Rik1 (based on DDB1: grey) associated with Raf1 (red, green and yellow). In Raf1, the N-terminal helix (red) and the β-propeller (green and yellow) make specific interaction with Rik1. (C) Centromere silencing assay. Position of ade6+ marker gene in cen1. Wild-type cells with silenced cen1:ade6+ form red colonies; loss of silencing causes white colonies. (D) Lagging chromosomes in anaphase. Representative images of fixed cells stained with DAPI (red) and anti-tubulin (green) and % anaphase cells with lagging chromosomes. (E) H3K9me2 ChIP; levels associated with cen-dg relative to act1+, normalised to wild-type. Error bars: standard deviation (SD). (F) Northern: unprocessed otr transcripts (arrows) in wt, clr4Δ, raf1-R518A and raf1-R576A cells. Loading control: rRNA. (*): rRNA background. (G) Northern: centromeric siRNAs in wt, clr4Δ, raf1-R518A and raf1-R576A cells. Loading control: snoRNA58. Alignment of the Raf1 WD-40 repeats with those of other known DCAFs revealed that Raf1 contains two WDxR motifs (1: aa515–518 and 2: aa573–576) (Figure S3A). WDxR motifs represent a ‘DCAF signature’ and are important for docking DCAFs to DDB1 [31], [32], [33], [34]. Our Raf1 model indicates that these two WDxR motifs are located on the surface of the β-propeller ring and might provide specific sites for interactions with other factors (Figure S3E). To test the importance of Raf1 WDxR motifs, we mutated the endogenous raf1 gene in S. pombe to express FLAG-Raf1-R518A or FLAG-Raf1-R576A. Western analysis indicated that both mutant proteins are expressed at levels similar to wild-type FLAG-Raf1 (Figure S3B). All components of CLRC are required for heterochromatin integrity and consequently for the transcriptional silencing of marker genes placed within centromeric heterochromatin, and centromere function [6]. Like clr4Δ cells, heterochromatin-mediated silencing of cen1:ade6+ is disrupted in raf1-R518A and raf1-R576A mutants, as indicated by white/expressing rather than red/silent colonies (Figure 2C). Moreover, both mutations impair heterochromatin dependent silencing at the mating type locus (Figure S3D).
Centromeric heterochromatin mediates robust sister-centromere cohesion and is therefore required for accurate chromosome segregation during mitosis [40], [41]. Defective heterochromatin causes a quantifiable increase in the frequency of lagging chromosomes on late anaphase spindles [42]. Cells bearing the raf1-R518A or raf1-R576A mutation exhibit a frequency of lagging chromosomes equivalent to clr4Δ cells, indicating that this centromeric function of heterochromatin is disrupted (Figure 2D).
Heterochromatin formation requires processing of centromeric transcripts into siRNA and methylation of H3K9 (H3K9me). Deletion of any gene encoding a CLRC component results in loss of H3K9me, accumulation of centromeric transcripts and a dramatic reduction of siRNA levels [17], [18], [19]. ChIP analyses indicate that H3K9me2 is reduced to background levels in raf1-R518A and raf1-R576A cells (Figure 2E). Importantly, Clr4 levels are equivalent to wild-type in both mutants (Figure S3C). Consistent with this loss of H3K9me2 and as observed in clr4Δ cells, high levels of centromeric transcripts also accumulate (Figure 2F). This is due to increased transcription given that higher levels of RNAPII are detected on centromeric repeats in both mutants compared to wild-type cells (Figure S5E). However, raf1-R576A cells contain low levels of centromeric siRNAs whereas raf1-R518A cells retain high levels of these siRNAs (Figure 2G and S3F). Our structural model predicts that R518 resides on the surface of Raf1 that interacts with Rik1 and predicts that the R518A mutation may specifically impair Raf1-Rik1 interactions (Figure S3G). In contrast, R576 is more distantly located from Rik1 and may have more profound structural effects.
Together our findings demonstrate that the CLRC complex is likely to adopt a canonical Cul4-E3 ligase architecture and that Raf1 is a DCAF for CLRC. Moreover, we show that WDxR motifs are required for heterochromatin integrity and that the raf1-R518A mutation separates the function of CLRC in methylating H3K9 from its role in generating siRNAs.
The raf1-1 mutation conditionally disrupts heterochromatin without affecting siRNA generation
To dissect mechanisms governing heterochromatin assembly and maintenance, we screened for temperature-sensitive mutants that disrupt heterochromatin at the restrictive temperature (36°C) but not the permissive temperature (25°C) and isolated the raf1-1 mutation. raf1-1 produces a protein with a missense mutation (T495I) in the third WD-40 repeat; this residue is conserved in fungi suggesting that it is important for Raf1 function (Figure S3A). Similar levels of mutant FLAG-Raf1-1 and wild-type FLAG-Raf1 proteins are detected at 36°C thus phenotypes are not due to Raf1-1 degradation (Figure S4A). Marker gene silencing within centromeric repeats (cen1:ade6+ or cen1:ura4+), the mating type locus (mat3-M:ura4+) and at telomeres (tel1L:his3+) is alleviated in raf1-1 cells at 36°C, but not 25°C (Figure 3A and Figure S4B, S4C). Consistent with defective centromeric heterochromatin integrity, a high frequency of lagging chromosomes is observed in late anaphase raf1-1 cells at 36°C (Figure 3B). Moreover, H3K9me2 levels on centromeric repeats in raf1-1 cells are similar to wild-type cells at 25°C but negligible at 36°C (Figure 3C). Consistent with reduced H3K9me2, Swi6 localisation and unprocessed centromere repeat transcript accumulation is temperature dependent in raf1-1 cells (Figure 4A and 4B). Importantly, Clr4 levels are unaffected by the raf1-1 mutation (Figure S4B).
Fig. 3. raf1-1 conditionally disrupts heterochromatin. 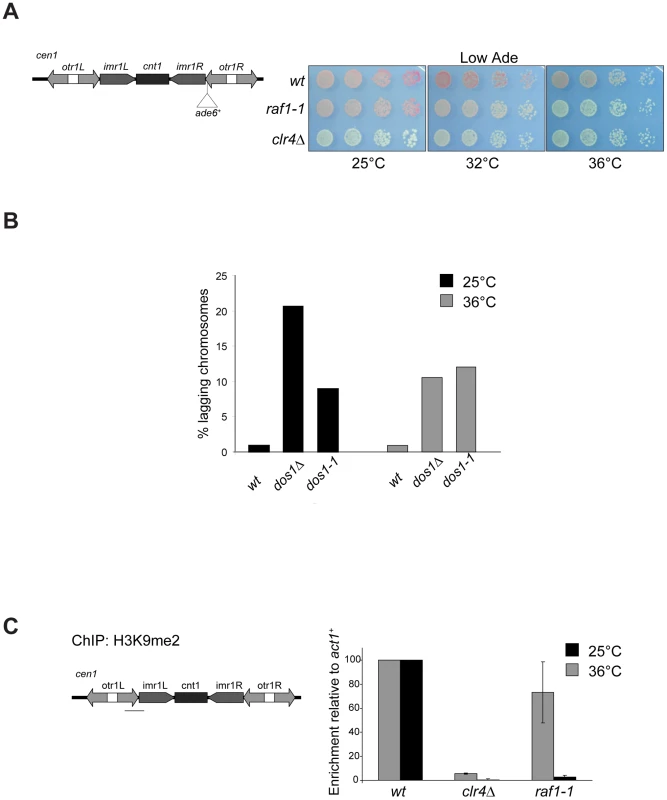
(A) cen1:ade6+ silencing assay in wt, clr4Δ or raf1-1 cells at 25°, 32° or 36°C. (B) Percentage of lagging chromosomes in anaphase of wt, raf1Δ and raf1-1 cells at 25°C or 36°C. Cell lacking heterochromatin display higher rates of chromosome missegregation at lower temperature [42]. (C) H3K9me2 ChIP: levels associated with cen-dg relative to act1+ in clr4Δ and raf1-1 cells normalised to wild-type at 25° or 36°C. Error bars: SD. Fig. 4. Heterochromatin, but not siRNA production, is erased in raf1-1 cells. 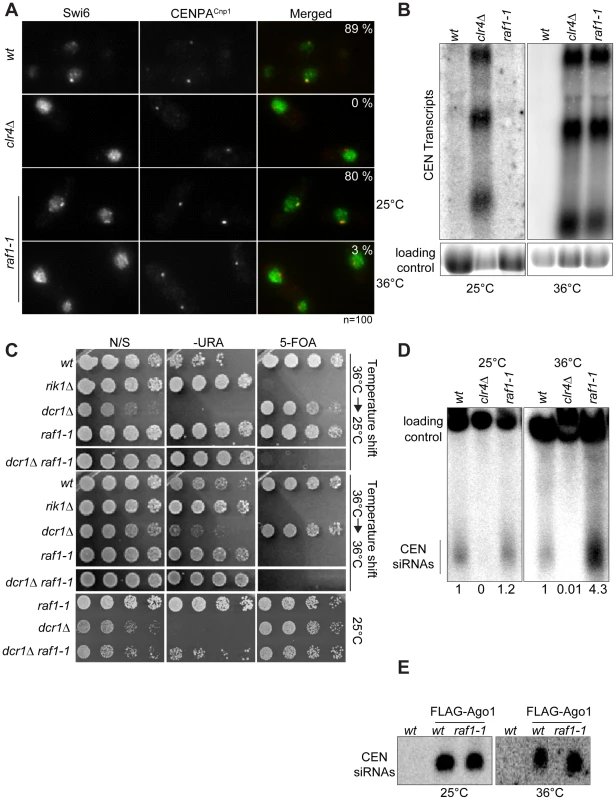
(A) Swi6 localisation in indicated cells at 25° or 36°C. Representative images of fixed cells with Swi6 (green) and CENP-ACnp1(red). % cells with Swi6 localised at centromeres. (B) Northern: unprocessed otr transcripts in indicated cells at 25° or 36°C. Loading control: rRNA. (C) mat3-M:ura4+ silencing assay of cells grown at 36°C and shifted to 25°C (top panel) or kept at 36°C (bottom panel). non-selective (N/S), –URA or 5-FOA plates indicated. Silencing (wt, dcr1Δ and raf1-1 at 25°C) allows little growth on -URA but good growth on 5-FOA. Loss of silencing (rik1Δ, raf1-1 at 36°C and raf1-1 dcr1Δ at 25° or 36°C) results in good growth on –URA and 5-FOA sensitivity. (D) Northern: centromeric siRNA in indicated cells at 25° or 36°C. Loading control: snoRNA58. (E) Northern: FLAG-Ago1-associated siRNA from indicated cells at 25° or 36°C. Although we detect no H3K9me2 on centromere repeats in raf1-1 at 36°C, it is possible that residual levels of H3K9me2 remain. To determine if heterochromatin is erased at 36°C, a heterochromatin establishment assay was performed. At the mating type locus, active RNAi is required to establish heterochromatin, but once assembled, RNAi is not required to maintain heterochromatin [43]. Hence, loss of dcr1/RNAi has no impact on mating type locus silencing (i.e. mat3-M:ura4+). However, when presented with a naive template, dcr1Δ cells fail to assemble heterochromatin de novo and are unable to silence mat3-M:ura4+. To test whether the raf1-1 mutant erases heterochromatin structures, a raf1-1 dcr1Δ double mutant was generated at 36°C and mat3-M:ura4+ silencing assessed after shifting cells to 25°C. Silencing of mat3-M:ura4+ could not be established in this double mutant following a shift down from 36° to 25°C, but remained intact in the raf1-1 dcr1Δ double mutant generated at 25°C (Figure 4C). This demonstrates that raf1-1 cells are unable to form heterochromatin without RNAi and indicates that heterochromatin is completely erased by the raf1-1 mutation at 36°C.
Surprisingly, unlike clr4Δ and raf1Δ mutants, centromeric siRNAs are produced at wild-type levels in raf1-1 cells at both temperatures (Figure 4D, Figure S4E and S5D). It is possible that siRNA levels remain high because raf1-1 inhibits the degradation of pre-existing siRNAs. However, high siRNA levels remain in raf1-1 cells which have undergone 144 divisions at 36°C; pre-existing siRNA would be diluted out (Figure S4E). Thus, the continual synthesis of siRNAs from centromere repeat transcripts must be unaffected by the defect in raf1-1. Moreover, these siRNAs are loaded into Ago1/RITS as indicated by their association with FLAG-Ago1 (Figure 4E). We conclude that as with raf1-R518A, the raf1-1 mutation uncouples siRNA production from H3K9 methylation.
Methylation of H3K9, but not siRNA production, depends on an intact CLRC complex
The interaction between the adaptor DDB1 and its DCAFs is known to require intact WDxR motifs in the DCAF [32], [33], [34]. Our structural alignment predicts that the raf1-1 T495I mutation is located on the ‘top’ surface of the WD-40 ring that interacts with Rik1 (Figure S5A). It is therefore possible that our specific raf1 mutations impair the ability of Raf1 to associate with Rik1 and that this interaction is essential for establishing and maintaining H3K9 methylation on heterochromatic loci. Indeed two-hybrid assays reveal that the binding of Raf1 to Rik1 [23] is disrupted in both WDxR motif mutants and the raf1-1 mutation (Figure 5A). All three mutations also impair co-immunoprecipitation of Rik1 with Raf1 from S. pombe extracts (Figure 5B). In addition, the association of the Clr4 H3K9 methyltransferase with Raf1 and Rik1 is lost in raf1-R518A, raf1-R576A and raf1-1 mutants (Figure 5C and Figure S5B). Recently we identified Stc1 as protein that links the RNAi machinery to CLRC [10]. Consistent with this, these same three specific raf1 mutations abolish the association of the Rik1 CLRC component with Stc1 (Figure S5C). Furthermore, ChIP analyses indicate that FLAG-Raf1-R518A, FLAG-Raf1-R576A and FLAG-Raf1-1 do not associate with centromeric repeats (Figure 5D). We conclude that binding of Raf1 to Rik1 is required to allow Raf1 to associate with centromeric repeats. This interaction is required to assemble an active CLRC complex in order to establish and maintain H3K9 methylation and hence functional heterochromatin.
Fig. 5. Raf1 mutations disrupt interaction with Rik1. 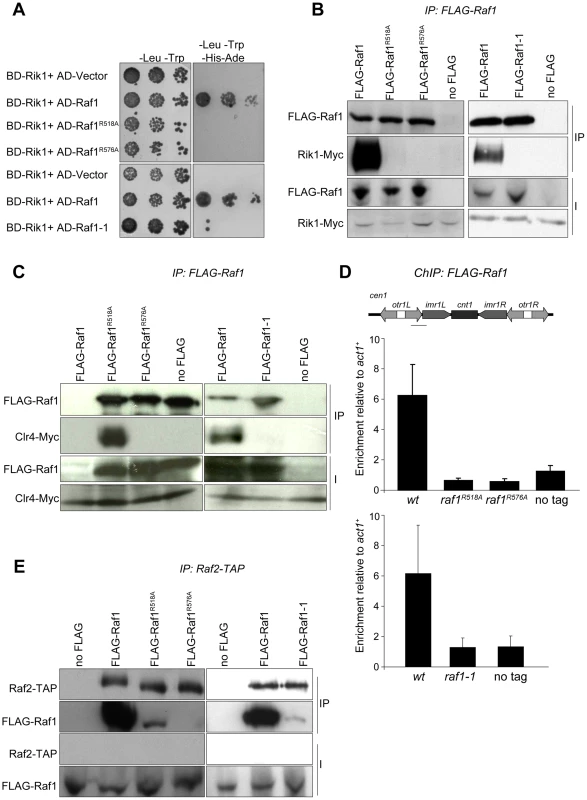
(A) Yeast two-hybrid assay. Interaction of Raf1 with Rik1 is indicated by growth on -Leu, -Trp, -His, -Ade plates. BD and AD: GAL4 Binding or Activation Domain fusions, respectively. (B) Western of FLAG-Raf1, FLAG-Raf1-R518A FLAG-Raf1-R576A and FLAG-Raf1-1 IPs analysed for FLAG-Raf1 and Rik1-Myc (right: cells grown at 36°C). (C) Western of FLAG-Raf1, FLAG-Raf1-R518A FLAG-Raf1-R576A and FLAG-Raf1-1 IPs analysed for FLAG-Raf1 and Clr4-Myc (right: cells grown at 36°C). (D) FLAG-Raf1, FLAG-Raf1-R518A, FLAG-Raf1-R576A and FLAG-Raf1-1 ChIP. Diagram shows cen1 primers used (black bar). Enrichment of FLAG-tagged proteins or untagged control were analysed by qPCR of otr relative to act1+(bottom: cells grown at 36°C). Error bar SD. (E) Westerns of Raf2-TAP IP analysed for FLAG-Raf1, FLAG-Raf1-1, FLAG-Raf1-R518A and FLAG-Raf1-R576A (right: cells grown at 36°C). The coupling of H3K9 methylation and siRNA production could be achieved by bringing the distinct activities required for both processes together in the same complex. Since the raf1Δ and raf1-R576A mutations results in loss of siRNAs, Raf1 is clearly required for siRNA production (Figure S5D and Figure 2G). However, the raf1-R518A and raf1-1 mutants lose H3K9 methylation without affecting siRNA synthesis (Figure 2E, 2G; Figure 3C; Figure 4D; Figures S4E and S5D). This suggests that specific interactions between Raf1 and individual proteins in CLRC and/or other unknown factors are sufficient to allow siRNA generation in the absence of H3K9 methylation. An intact CLRC complex may not be required. Indeed, co-immunoprecipitation experiments show that a weak but detectable interaction between Raf1 and Raf2 remains in raf1-1 and raf1-R518A, but not raf1-R576A cells (Figure 5E). We conclude that although an intact CLRC complex is dispensable for siRNA synthesis, a minimal Raf1-Raf2 interaction might be sufficient to allow the processing of centromere repeat transcripts to siRNAs.
Discussion
The interaction between the E3 ligase adaptor Rik1 and the DCAF Raf1 correlates with methylation of lysine 9 on histone H3
The CLRC complex, harbouring the histone methyltransferase Clr4Suvar3-9, performs an essential role in the establishment and maintenance of heterochromatic structures. In addition to Clr4, the complex contains the components Cul4, Rik1, Raf1 and Raf2.
Rik1 is a WD-40 repeat protein that shares homology with the E3 ligase adaptor DDB1 [36]. DDB1 contains 21 WD-40 repeats that form the seven blades of three β-propellers that mediate association with Cul4 and DCAFs. This supports the possibility that Cul4-Rik1 form the core of an E3 ligase [24]. However, the C-terminus of Rik1 also shows homology with the β-propeller domain of cleavage and polyadenylation factor CSPF-A a well-known RNA binding protein [18]. Based on this CPSF-A homology, it has been suggested that Rik1 might bind RNA through this domain [21]. Although we cannot exclude that Rik1 is a bifunctional protein, our manual alignment detects 21 WD-40 repeats within Rik1 allowing it to be structurally aligned along its entire length with DDB1 (Figure 2B and Figure S2A). In our model, the CSPF-A homology domain corresponds to the β-propeller BPC involved in the interaction with the DCAF Raf1 (Figure 2B and Figure S2A). Moreover, we also show that Raf1 can be structurally aligned to the DCAF DDB2, the partner of DDB1 (Figure 2A, 2B and Figure S2B). As expected for a bona fide DCAF, Raf1 associates directly with the putative substrate adaptor Rik1 and contains two signature WDxR motifs which we show are required for the association of Raf1 with Rik1 and for H3K9 methylation and heterochromatin formation (Figure 2B and Figure S3). This suggests that the CLRC complex can adopt the architecture of a Cul4 E3 ligase in which Rik1 is the adaptor protein bridging the interaction between Cul4 and the DCAF Raf1 (Figure 6A). In addition, we show that the association of the DCAF Raf1 with Rik1 within an intact CLRC complex is critical for H3K9 methylation but it is dispensable for the processing of centromeric transcripts into siRNAs (Figure 2, Figure 4, and Figure 5).
Fig. 6. The CLRC complex couples H3K9me and siRNA production. 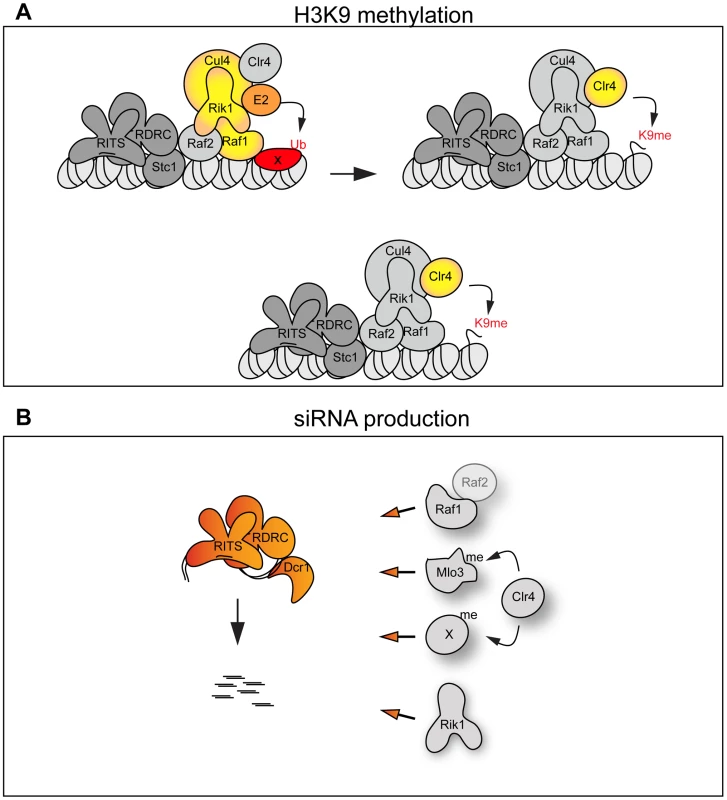
(A) Top panel: The CLRC complex is an active E3 ligase. The DCAF Raf1 binds a specific substrate (X) allowing its ubiquitination. This is essential for methylation of H3K9. Bottom panel: The CLRC complex is not an active E3 ligase. Cul4, Rik1, Raf1 and Raf2 form a protein scaffold allowing the correct targeting of the histone methyltransferase Clr4. (B) Individual CLRC components stimulate processing of centromeric transcripts into siRNAs independently of CLRC complex integrity. Raf1-Raf2, methylation of Mlo3 or other substrates and Rik1 (left) are required for processing centromeric transcripts into siRNAs. We envisage two alternative scenarios that can explain how the Cul4-Rik1-Raf1 complex might mediate H3K9 methylation (Figure 6A). First, it is possible that the CLRC is an active E3 ligase and that mono - or poly-ubiquitination of specific factors must occur to allow methylation of H3K9. Thus the DCAF Raf1 would be essential for the recognition and ubiquitination of key specific substrate(s) (Figure 6A top panel). In other systems, Cul4-E3 ligases have been shown to ubiquitinate histones [29], [30], it is possible that a specific histone residue needs to be ubiquitinated to allow methylation of H3K9. E3 ligase activity has been shown to associate with affinity selected CLRC in vitro [24], however, we have been unable to detect this activity in similar experiments. This suggests that CLRC E3 ligase activity may be particularly inefficient and/or the ubiquitination events that it mediates are transient.
The alternative scenario is that the CLRC complex is not an active E3 ligase in vivo and that Cul4, Rik1, Raf1 and Raf2 just form a protein scaffold that acts to target Clr4 methyltransferase to heterochromatic repeats, independently of ubiquitination (Figure 6A bottom panel). In this case the specific mutations which impair the Rik1-Raf1 interaction may just disrupt the scaffold so that Raf1 is not targeted to the heterochromatic repeats and Clr4 no longer associates with Rik1 and Raf1. Interestingly, the DCAF DDB2 has been shown to bind DNA with the ‘bottom’ surface of its WD-40 ring [44]. Similarly, the WD-40 ring of the DCAF WDR5 specifically binds the histone H3 N-terminal tail methylated on lysine 4 [45], [46], [47]. Thus, it is possible that the WD-40 ring of the DCAF Raf1 also binds DNA or histones to allow the correct targeting of the histone methyltransferase Clr4 to heterochromatic repeats.
Raf1 function in H3K9 methylation and RNAi are separable
CLRC plays a dual role in heterochromatin formation: it harbours the histone methyltransferase Clr4 and hence it is responsible for H3K9 methylation, it also mediates siRNA production since cells lacking any single CLRC subunit have low centromeric siRNA levels (Figure S5D and [17], [18]). Therefore, in wild-type fission yeast H3K9 methylation and siRNAs synthesis are coupled. This ensures formation of heterochromatin at centromeres, telomeres, and the mating type locus, and prevents promiscuous silencing at other chromosomal regions. However, cells expressing only mutant histone H3 (H3K9R) have been shown to produce some detectable siRNAs, even though H3K9 can not be methylated [20], [21]. Such analyses implicate CLRC itself, rather than its known substrate H3K9, in promoting siRNA production independently of H3K9.
Here we have isolated two mutations in the raf1 gene (raf1-1 and raf1R518A) that destroy CLRC integrity without affecting siRNA levels. Although we cannot exclude that a minimal CLRC complex is still present in these mutants, our results indicate that individual CLRC components can stimulate processing of centromeric transcripts into siRNAs independently of CLRC complex integrity (Figure 6B). It remains to be determined how CLRC components promote this H3K9 methylation-independent siRNA production. Our analyses of specific raf1 mutants suggests that the putative E3 ligase activity of the CLRC complex is not required for siRNA synthesis given that in raf1-1 and raf1-R518A mutants (predicted to impair the E3 ligase activity of the complex) siRNA levels remain high (Figure 2G and Figure 4D). One possibility is that Clr4 can methylate specific substrates independently of CLRC integrity and that this is a key event in siRNA production [21]. In accordance with this, Clr4 was recently shown to methylate the RNA processing factor Mlo3 and this methylation correlates with high siRNA levels [48]. However, Clr4-mediated methylation of specific substrates cannot be the only event required to trigger siRNA production since the loss of any CLRC component results in dramatic reduction of siRNA levels. We find that high siRNA levels correlate with a low but detectable Raf1-Raf2 interaction. Complete disruption of this Raf1-Raf2 interaction (as observed in raf1-R576A) cuts siRNA production to undetectable levels. Interestingly, centromeric repeats are transcribed preferentially in S-phase and Raf2 has been recently shown to interact with Cdc20 (the catalytic subunit of DNA-polymerase ε) [49], [50], [51]. Coordination of DNA replication, siRNA generation and methylation of specific substrates may be essential for heterochromatin establishment and maintenance of heterochromatic structures.
Our analyses suggest that integration of DNA replication, siRNA production and methylation of H3K9 is achieved by bringing the distinct activities required for these processes together in the same protein complex (CLRC). This ensures the assembly of robust heterochromatin structures.
Cotranscriptional gene silencing plays a subsidiary role in repression of heterochromatic repeats
Two different models have been proposed to explain how heterochromatin could silence centromeric repeats. In the ‘TGS’ model, heterochromatin factors have been proposed to repress transcription at centromeres [15] whereas the ‘CTGS’ model suggests that centromere repeats are continuously transcribed and silencing is caused by the efficient cleavage of centromeric transcripts by RNAi into siRNA [16]. Our analyses demonstrate that the RNAi machinery is active in raf1-1 cells, so that centromeric transcripts are processed into siRNAs independently of heterochromatin integrity. raf1-1 cells produce siRNAs at levels similar to wild-type cells and they are loaded into Ago1. However, despite this, high levels of unprocessed centromeric transcripts persist, indicating that RNAi fails to destroy them. The fact that heterochromatin is absent in raf1-1 and raf1-R518A cells and transcripts remain high, even though high levels of homologous Ago1-associated centromeric siRNAs are present, is more compatible with a TGS model where heterochromatin directly represses RNAPII transcription of centromere repeats.
In agreement with this model, RNAPII ChIP clearly shows an increase of RNAPII occupancy in the raf1 mutants compared to wild-type cells (Figure S5E). This observation is also consistent with the fact that tethered Clr4 can generate heterochromatin and silence marker genes independently of RNAi [52].
The coupling of non-coding transcription with chromatin modification to establish and maintain distinct epigenetic states is a general mechanism of gene regulation in eukaryotes. Links between Cul4-dependent ubiquitination and histone methylation are continuing to emerge in different systems. Further analyses will determine how these activities are integrated to regulate specific chromatin states.
Materials and Methods
Strain and plasmid construction
Standard procedures were used for bacterial and fission yeast growth and genetic manipulations [53]. S. pombe strains used in this study are described in Table S1. Primer sequences are listed in Table S2.
Structural modelling
The homology model of Raf1 was created with the program Modeller (9v8) using the structure of DDB2 (PDB code 3EI4) [44]. Iterative rounds of alignment adjustment of the two protein sequences and model building were attempted until the Modeller scores (diagnostic of model quality) were optimised. The homology model of Rik1 was created with the program Modeller (9v8) using 5 templates (PDB code: 2B5L; 317N; 3189; 318C; 3E0C). The generated model (85.2) had the following model scores: RMSD: 1.536; MolPDF: 453.98; DOPE: −11385.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described [54] with the following modifications. Cells were fixed in 1% PFA/15 min for H3K9me2 ChIP or in 1% PFA/20 min for RNA Polymerase II ChIP. For ChIP analyses of raf1-R518A and raf1-R576A strains, cells were grown at 32°C. For ChIP analyses of raf1-1 strain, cells were kept at the indicated temperature (25°C, 32°C or 36°C) for at least 96 hours. One microliter of monoclonal H3K9me2 antibody (m5.1.1), two microliter of anti-FLAG M2 monoclonal antibody (Sigma, F1804) or five microliters of RNA Polymerase II 8WG16 antibody (COVANCE, MMS-126R) was used per ChIP. Duplex PCR was performed to analyse ChIP samples using oligonucleotides specific to the regions of interest and to the control gene fbp1 (Table S2). Real-time PCR (qPCR) was performed using the LightCycler 480 SYBR Green I Master (Roche) on a LightCycler 480 Instrument (Roche). qPCR analysis primers are in Table S2. Relative enrichments were calculated as the ratio of product of interest to control product (act1+ or tRNA) in IP over input. Histograms represent data from three biological replicates analysed in parallel.
Immunoaffinity purification
Immunoaffinity purifications (IP) for LC-MS/MS analysis were performed as described [55], with the following modifications: 5 g of cells were resuspended in ice-cold-lysis buffer (50 mM Hepes pH7.5, 150 mM KCl, 0.1% NP40). Immunoprecipitation was performed using proteinG Dynabeads resin (Life Technologies) coupled to anti-FLAG M2 antibody (Sigma, F1804) for 15 min. The IP'd material was treated with 500 U Benzonase, washed, subjected to on-bead Tryptic digestion, and prepared for LC-MS/MS analysis as described previously [56].
Co-IPs for Western analysis were performed on 2 g of cells as above but for 1 hr. IP'd material was washed four times with ice-cold lysis buffer (50 mM Hepes pH7.5, 150 mM KCl, 0.1% NP40), resuspended in SDS sample buffer and analysed by SDS-PAGE. For tandem affinity purification (TAP)-tagged strains, Dynabeads coupled to IgG were used (gift from K. Hardwick). For Western analysis the following antibodies were used: anti-FLAG M2 (Sigma, F1804), anti-HA 12CA5 (gift from K. Samejima) and anti-myc (A14) (Santa Cruz sc-789), all at 1∶1000. Further details of IP protocol are provided in Text S1.
RNA analysis
Northern analysis of long non-coding centromeric transcripts and centromeric siRNAs were performed as described previously [56]. RNA probes are listed in Table S2.
Note: Further details of all experimental procedures are provided in Text S1.
Supporting Information
Zdroje
1. FaraziTAJuranekSATuschlT 2008 The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development 135 1201 1214
2. GrewalSI 2010 RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev 20 134 141
3. MoazedD 2009 Small RNAs in transcriptional gene silencing and genome defence. Nature 457 413 420
4. GuangSBochnerAFBurkhartKBBurtonNPavelecDM 2010 Small regulatory RNAs inhibit RNA polymerase II during the elongation phase of transcription. Nature 465 1097 1101
5. Kuramochi-MiyagawaSWatanabeTGotohKTotokiYToyodaA 2008 DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 22 908 917
6. AllshireRCJaverzatJPRedheadNJCranstonG 1994 Position effect variegation at fission yeast centromeres. Cell 76 157 169
7. DjupedalIPortosoMSpahrHBonillaCGustafssonCM 2005 RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes Dev 19 2301 2306
8. KatoHGotoDBMartienssenRAUranoTFurukawaK 2005 RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science 309 467 469
9. VolpeTAKidnerCHallIMTengGGrewalSI 2002 Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297 1833 1837
10. BayneEHWhiteSAKaganskyABijosDASanchez-PulidoL 2010 Stc1: a critical link between RNAi and chromatin modification required for heterochromatin integrity. Cell 140 666 677
11. SadaieMIidaTUranoTNakayamaJ 2004 A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. Embo J 23 3825 3835
12. BannisterAJZegermanPPartridgeJFMiskaEAThomasJO 2001 Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410 120 124
13. ZhangKMoschKFischleWGrewalSI 2008 Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nat Struct Mol Biol 15 381 388
14. PetrieVJWuitschickJDGivensCDKosinskiAMPartridgeJF 2005 RNA interference (RNAi)-dependent and RNAi-independent association of the Chp1 chromodomain protein with distinct heterochromatic loci in fission yeast. Mol Cell Biol 25 2331 2346
15. SugiyamaTCamHPSugiyamaRNomaKZofallM 2007 SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128 491 504
16. BuhlerMVerdelAMoazedD 2006 Tethering RITS to a nascent transcript initiates RNAi - and heterochromatin-dependent gene silencing. Cell 125 873 886
17. HongEJVillenJGeraceELGygiSPMoazedD 2005 A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol 2 106 111
18. MotamediMRVerdelAColmenaresSUGerberSAGygiSP 2004 Two RNAi complexes, RITS and RDRC, physically interact and localise to noncoding centromeric RNAs. Cell 119 789 802
19. NomaKSugiyamaTCamHVerdelAZofallM 2004 RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing. Nat Genet 36 1174 1180
20. DjupedalIKos-BraunICMosherRASoderholmNSimmerF 2009 Analysis of small RNA in fission yeast; centromeric siRNAs are potentially generated through a structured RNA. EMBO J 28 3832 3844
21. GeraceELHalicMMoazedD 2010 The methyltransferase activity of Clr4Suv39h triggers RNAi independently of histone H3K9 methylation. Mol Cell 39 360 372
22. JiaSKobayashiRGrewalSI 2005 Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat Cell Biol 7 1007 1013
23. LiFGotoDBZaratieguiMTangXMartienssenR 2005 Two novel proteins, dos1 and dos2, interact with rik1 to regulate heterochromatic RNA interference and histone modification. Curr Biol 15 1448 1457
24. HornPJBastieJNPetersonCL 2005 A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev 19 1705 1714
25. ThonGHansenKRAltesSPSidhuDSinghG 2005 The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics 171 1583 1595
26. JacksonSXiongY 2009 CRL4s: the CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci 34 562 570
27. ShilatifardA 2006 Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75 243 269
28. PetroskiMDDeshaiesRJ 2005 Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6 9 20
29. KapetanakiMGGuerrero-SantoroJBisiDCHsiehCLRapic-OtrinV 2006 The DDB1-CUL4ADDB2 ubiquitin ligase is deficient in xeroderma pigmentosum group E and targets histone H2A at UV-damaged DNA sites. Proc Natl Acad Sci U S A 103 2588 2593
30. WangHZhaiLXuJJooHYJacksonS 2006 Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol Cell 22 383 394
31. HigaLAWuMYeTKobayashiRSunH 2006 CUL4-DDB1 ubiquitin ligase interacts with multiple WD-40-repeat proteins and regulates histone methylation. Nat Cell Biol 8 1277 1283
32. JinJAriasEEChenJHarperJWWalterJC 2006 A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell 23 709 721
33. AngersSLiTYiXMacCossMJMoonRT 2006 Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 443 590 593
34. HeYJMcCallCMHuJZengYXiongY 2006 DDB1 functions as a linker to recruit receptor WD-40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev 20 2949 2954
35. LiuCPowellKAMundtKWuLCarrAM 2003 Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev 17 1130 1140
36. NeuwaldAFPoleksicA 2000 PSI-BLAST searches using hidden markov models of structural repeats: prediction of an unusual sliding DNA clamp and of beta-propellers in UV-damaged DNA-binding protein. Nucleic Acids Res 28 3570 3580
37. BraunSGarciaJFRowleyMRougemailleMShankarS 2011 The cul4-ddb1(cdt2) ubiquitin ligase inhibits invasion of a boundary-associated antisilencing factor into heterochromatin. Cell 144 41 54
38. PidouxALUzawaSPerryPECandeWZAllshireRC 2000 Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast. J Cell Sci 113 Pt 23 4177 4191
39. LiFHuarteMZaratieguiMVaughnMWShiY 2008 Lid2 is required for coordinating H3K4 and H3K9 methylation of heterochromatin and euchromatin. Cell 135 272 283
40. BernardPMaureJFPartridgeJFGenierSJaverzatJP 2001 Requirement of heterochromatin for cohesion at centromeres. Science 294 2539 2542
41. NonakaNKitajimaTYokobayashiSXiaoGYamamotoM 2002 Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat Cell Biol 4 89 93
42. EkwallKJaverzatJPLorentzASchmidtHCranstonG 1995 The chromodomain protein Swi6: a key component at fission yeast centromeres. Science 269 1429 1431
43. HallIMShankaranarayanaGDNomaKAyoubNCohenA 2002 Establishment and maintenance of a heterochromatin domain. Science 297 2232 2237
44. ScrimaAKonickovaRCzyzewskiBKKawasakiYJeffreyPD 2008 Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell 135 1213 1223
45. HanZGuoLWangHShenYDengXW 2006 Structural basis for the specific recognition of methylated histone H3 lysine 4 by the WD-40 protein WDR5. Mol Cell 22 137 144
46. CoutureJFCollazoETrievelRC 2006 Molecular recognition of histone H3 by the WD-40 protein WDR5. Nat Struct Mol Biol 13 698 703
47. SchuetzAAllali-HassaniAMartinFLoppnauPVedadiM 2006 Structural basis for molecular recognition and presentation of histone H3 by WDR5. EMBO J 25 4245 4252
48. ZhangKFischerTPorterRLDhakshnamoorthyJZofallM 2011 Clr4/Suv39 and RNA quality control factors cooperate to trigger RNAi and suppress antisense RNA. Science 331 1624 1627
49. LiFMartienssenRCandeWZ 2011 Coordination of DNA replication and histone modification by the Rik1-Dos2 complex. Nature 475 244 248
50. ChenESZhangKNicolasECamHPZofallM 2008 Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 451 734 737
51. KlocAZaratieguiMNoraEMartienssenR 2008 RNA interference guides histone modification during the S phase of chromosomal replication. Curr Biol 18 490 495
52. KaganskyAFolcoHDAlmeidaRPidouxALBoukabaA 2009 Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science 324 1716 1719
53. MorenoSKlarANurseP 1991 Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194 795 823
54. PidouxAMelloneBAllshireR 2004 Analysis of chromatin in fission yeast. Methods 33 252 259
55. OeffingerMWeiKERogersRDeGrasseJAChaitBT 2007 Comprehensive analysis of diverse ribonucleoprotein complexes. Nat Methods 4 951 956
56. BayneEHPortosoMKaganskyAKos-BraunICUranoT 2008 Splicing factors facilitate RNAi-directed silencing in fission yeast. Science 322 602 606
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 2
-
Všechny články tohoto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



