-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
Why gene order is conserved over long evolutionary timespans remains elusive. A common interpretation is that gene order conservation might reflect the existence of functional constraints that are important for organismal performance. Alteration of the integrity of genomic regions, and therefore of those constraints, would result in detrimental effects. This notion seems especially plausible in those genomes that can easily accommodate gene reshuffling via chromosomal inversions since genomic regions free of constraints are likely to have been disrupted in one or more lineages. Nevertheless, no empirical test has been performed to this notion. Here, we disrupt one of the largest conserved genomic regions of the Drosophila genome by chromosome engineering and examine the phenotypic consequences derived from such disruption. The targeted region exhibits multiple patterns of functional enrichment suggestive of the presence of constraints. The carriers of the disrupted collinear block show no defects in their viability, fertility, and parameters of general homeostasis, although their odorant perception is altered. This change in odorant perception does not correlate with modifications of the level of expression and sex bias of the genes within the genomic region disrupted. Our results indicate that even in highly rearranged genomes, like those of Diptera, unusually high levels of gene order conservation cannot be systematically attributed to functional constraints, which raises the possibility that other mechanisms can be in place and therefore the underpinnings of the maintenance of gene organization might be more diverse than previously thought.
Published in the journal: . PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002475
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002475Summary
Why gene order is conserved over long evolutionary timespans remains elusive. A common interpretation is that gene order conservation might reflect the existence of functional constraints that are important for organismal performance. Alteration of the integrity of genomic regions, and therefore of those constraints, would result in detrimental effects. This notion seems especially plausible in those genomes that can easily accommodate gene reshuffling via chromosomal inversions since genomic regions free of constraints are likely to have been disrupted in one or more lineages. Nevertheless, no empirical test has been performed to this notion. Here, we disrupt one of the largest conserved genomic regions of the Drosophila genome by chromosome engineering and examine the phenotypic consequences derived from such disruption. The targeted region exhibits multiple patterns of functional enrichment suggestive of the presence of constraints. The carriers of the disrupted collinear block show no defects in their viability, fertility, and parameters of general homeostasis, although their odorant perception is altered. This change in odorant perception does not correlate with modifications of the level of expression and sex bias of the genes within the genomic region disrupted. Our results indicate that even in highly rearranged genomes, like those of Diptera, unusually high levels of gene order conservation cannot be systematically attributed to functional constraints, which raises the possibility that other mechanisms can be in place and therefore the underpinnings of the maintenance of gene organization might be more diverse than previously thought.
Introduction
Collinearity conservation, i.e. conservation of local gene order, across distantly related phyla is often viewed as the result of functional constraints that prevent the occurrence of breaks of chromosomal rearrangements during evolution. The nature of these constraints is still poorly understood. They may merely reflect the presence of yet-to-be annotated protein and nonprotein-coding genes in intergenic regions [1]–[3]. A second type of constraints may be linked to the existence of regulatory domains, i.e. genomic regions associated with complex regulatory inputs. These regulatory domains can adopt at least two forms. One common form of complex regulatory inputs corresponds to genes that are coordinately expressed or repressed [4]–[7]. Alternatively, regulatory constraints can adopt the form of long-range regulatory interactions, which often involve the interdigitation of cis-regulatory sequences with genes that are not their targets [8]–[10]. These regulatory domains are enriched for noncoding DNA (highly conserved noncoding elements or HCNEs) with putative regulatory potential [11]–[16]. These HCNEs tend to be found in the vicinity of protein-coding genes that participate in key processes during development, such as regulation of gene expression and signal transduction [10], [12], [17]. Disruption of genomic regions under constraints can be accompanied by alteration of gene activity, as illustrated by chromosomal rearrangements that modify gene expression as a result of the separation of a gene from its regulatory sequences [18]–[20]. These alterations in gene activity may have a detrimental effect, which would lead to conservation of gene organization [19], [21].
Highly rearranged genomes, such as those of the Diptera, are especially suitable for analyzing the presence of regulatory-based constraints that preserve collinearity since regions free of them are likely to have been disrupted in one or more lineages. Gene order comparisons have helped to delineate collinear blocks [22] across nine Drosophila species that represent ∼380 million years (myr) of total divergence time [23], [24]. A minimum of ∼6,100 chromosomal breakpoints have been estimated to have occurred in 2,688 intergenic regions [22] scattered across the main chromosomal elements (the so-called Muller's elements A–E) that constitute the Drosophila genome [25], [26]. The analysis of the expected patterns of evolution of gene organization under different evolutionary scenarios indicate that fragile regions, i.e. those that accumulate most chromosomal breaks during evolution [27], are the main factor that explains the patterns of gene arrangement across Drosophila species [22]. Constraints nevertheless may be responsible for ∼15% of gene order conservation and their presence is positively correlated with the size of the collinear block [22]. The top 1% largest collinear blocks, or ultraconserved regions [22], are enriched for genes associated with particular expression profiles, but the functional signature most prominently found in ultraconserved regions are stretches of DNA with multiple HCNEs (14.5% of the 145 HCNE peaks mapped as compared to 6.7% expected).
To our knowledge, only two empirical tests for the presence of functional constraints have been performed in eukaryotic genomes [20], [28]. In both cases, engineered chromosomal inversions were induced to disrupt clusters of genes with shared expression attributes and, subsequently, the phenotypic consequences of such disruptions monitored. For example, the disruption of the mouse Hoxd cluster, which is conserved across vertebrates, helped determine the presence of two functional subdomains and two long-range enhancers on either side of the cluster. This functional organization of the Hoxd cluster was proposed as the underlying cause that kept the cluster intact during the evolution of vertebrate lineages. In Diptera, three gene neighborhoods expressed in testes and one gene neighborhood expressed during embryogenesis of D. melanogaster have been disrupted [28], but no modification of the expression of the genes included in the neighborhoods examined was detected. These neighborhoods are conserved within the D. melanogaster species subgroup but disrupted in some other Drosophila lineages (Table S1), which does not clarify the phylogenetic scope of the putative constraints tested. The phenotypic consequences of disrupting a collinear region conserved throughout the genus Drosophila, which is presumably maintained by constraints, are presently unknown.
Here, we used chromosome engineering to disrupt an ultraconserved region located on Muller's element C of the Drosophila genome (Figure 1A). This ultraconserved region is delimited by the genes CG15121 and CG16894 and is ∼701 kb long in D. melanogaster [22]. Importantly, this ultraconserved region is, based on current information, the one with the largest number of functional signatures, suggestive of the presence of regulatory-based constraints [22]. After disrupting the ultraconserved region CG15121–CG16894, we examined the resulting phenotypic consequences both by performing a variety of genetic and competition experiments that tested for differences in viability, fertility, and relevant parameters of general homeostasis and by characterizing levels of mRNA abundance in both sexes. Our results indicate that, contrary to the prevalent view, the extraordinary conservation of some of the largest collinear blocks in eukaryotic genomes might not necessarily derive only from functional constraints that result in severe detrimental effects.
Fig. 1. Schematic representation of the inversion engineered to disrupt the ultraconserved region CG15121–CG16894 and its salient features. 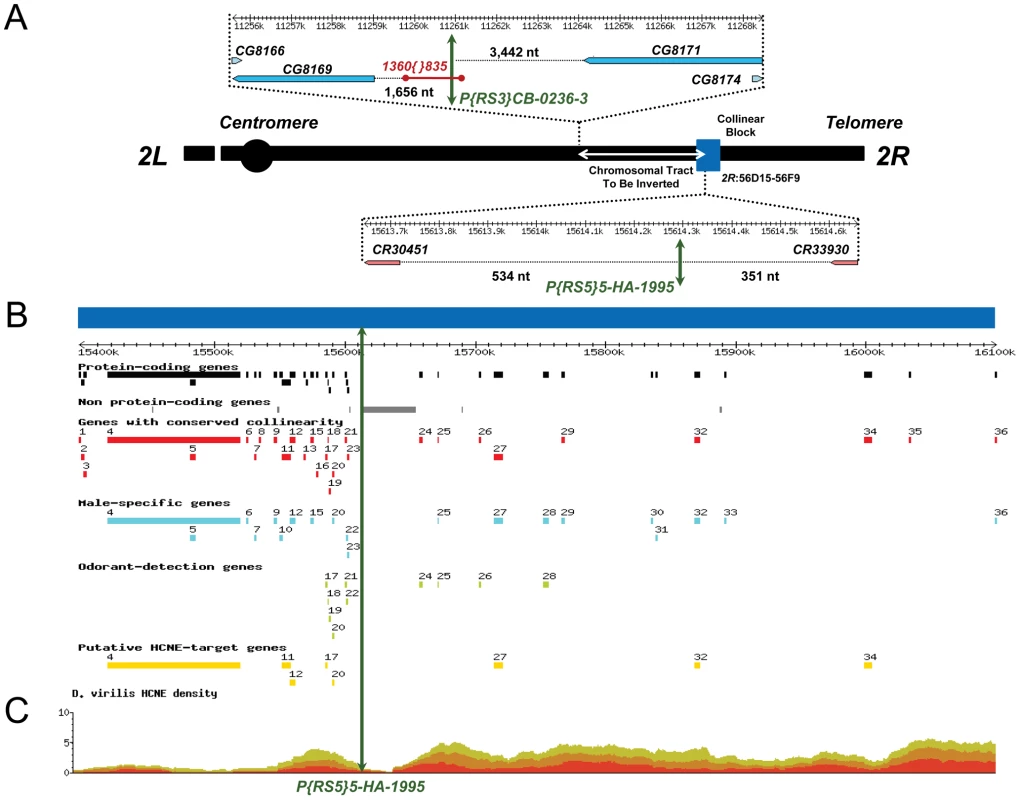
(A) Schematic representation showing the surrounding regions and location of the FRT-bearing TEs P{RS5}5-HA-1995 and P{RS3}CB-0236-3 (green double arrowhead lines) used to generate the inversion In(2R)51F11-56E2 (white double arrowhead line), which disrupts the ultraconserved region CG15121–CG16894 (blue box). The element P{RS3}CB-0236-3 is inserted in a naturally occurring copy of the TE 1360{}835 [43]. Distances between the FRT-bearing TEs and the flanking genes are indicated in nucleotides. Notice that flanking transcription units at the immediate vicinity of the inner breakpoint are two non-protein-coding genes: CR30451, which codes for tRNA:E4:56Fc; and CR33930, which codes for snoRNA:185. (B) Annotation of the ultraconserved region CG15121–CG16894 using D. melanogaster as a reference. From top to bottom: 36 protein-coding genes; 122 non-protein-coding genes (8 miRNAs, 13 tRNAs, 1 snoRNA, and 100 5S rRNAs); 29 protein-coding genes whose collinearity is maintained across nine species of the genus Drosophila; 20 protein-coding genes with male-biased gene expression using mRNA levels as a proxy; 10 protein-coding genes related to odor-guided behavior; and 8 putative targets of Highly Conserved Non-coding Elements (HCNEs) based on their expression profile, core promoter predictions, and mutant phenotypes. Details on the protein-coding genes in this region and their annotation are provided in Table S2. (C) Ancora [82] snapshot (http://ancora.genereg.net) of the distribution of HCNEs when genome sequences of D. melanogaster and D. virilis are compared. Green, orange, and red denote 96%, 98%, and 100% nucleotide identity, respectively. Results
Functional and comparative organization of the ultraconserved region CG15121–CG16894
The ultraconserved region CG15121–CG16894 ranked first in length and eleventh in the number of genes encompassed among 2,683 regions of conserved collinearity across nine Drosophila species [22]. Data from another comparative analysis on gene organization in the genus Drosophila [29] are consistent with the overall maintenance of the collinearity in this genomic region. In addition, this region shows statistically significant enrichment for genes encoding proteins involved in chemosensory perception and for genes preferentially expressed in males, many of them showing this same trend across multiple Drosophila species (Figure 1B and Table S2). This higher-than-expected local density of genes with coherent patterns of expression supports the presence of a male-biased gene expression neighborhood, which is intertwined with a smaller chemosensory perception gene neighborhood.
This ultraconserved region is spanned by four HCNE peaks [12] (Figure 1C), more than any other collinear block. Genes responsive to HCNEs have been postulated to be preferentially associated with a particular kind of core promoters. Specifically, using promoter predictions for 42% of the protein-coding genes of D. melanogaster, a significant overrepresentation of genes with some kind of Inr-motif (Inr only, Inr/DPE, or Inr/TATA) was found in HCNE-dense regions [12]. We screened 500 nt upstream of each protein-coding gene in the region under study using McPromoter and obtained reliable predictions for eight genes. Six of these genes were predicted to have a core promoter responsive to HCNEs. These genes are found scattered along the region (Figure 1B and Figure S1) and encode proteins involved in the regulation of gene expression, from mRNA binding proteins (sm), to signal transducers (Toll-7, 18-w), to ubiquitins (Isopeptidase-T-3), and also to others that we did not predict a priori (Obp56a and CG8654). Given the large number of genes for which a prediction was not provided by McPromoter, we checked for the presence of genes that are expressed during key developmental stages using the expression profile characterization generated by modENCODE [2]. Fifteen out of 36 protein-coding genes show a preferential pattern of expression during embryogenesis; among them, there is a cluster of eight genes mostly displaying high levels of expression during the first 16 h of development and moderate expression during the larva-pupa transition (Figure S1). Six of these eight genes are predicted to have core promoters of the Inr-motif type and four are associated with lethal phenotypes, the latter underscoring their functional relevance prior to imago emergence (Figure S1) [30], [31]. Together, these functional features associated with some of the genes in the region are suggestive of regulation by HCNEs.
Examination of the organization of the ultraconserved region CG15121–CG16894 in Anopheles gambiae revealed the presence of orthologues in six different locations (Figure S2A and Table S3). This degree of dispersion is not surprising given the fast differentiation of the Drosophila and Anopheles genomes via the accumulation of chromosomal rearrangements [32]–[34] and the lack of common constraints reflected in the pronounced differences in development, morphology, and ecology of these two Diptera [33], [35], [36]. Nevertheless, we detected a conserved gene arrangement including the gene Toll-7 and the Obp genes, an association that is also present in A. aegypti (Figure S2B). Phylogenetic analyses [37] unambiguously support the close phylogenetic relationship among several Obp genes in the ultraconserved region under study (Obp56a, Obp56d, and Obp56e) and some that are adjacent to Toll-7 in A. gambiae (OBP23, OBP25, OBP26, OBP28), which in turn are closely related to those that cluster nearby Toll-7 in A. aegypti ([38] and this work; Figure S2C). Interestingly, the genes Obp56a, Obp56d and Toll-7 are found within the same expression cluster in D. melanogaster and two of them have core promoter types presumably responsive to HCNE-mediated regulation (Figure S1).
To sum up, the existence of two intertwined gene neighborhoods associated with very marked expression profiles, the enrichment for HCNE peaks and presence of their putative targets, and the detection of a region conserved across Diptera reinforces the possibility that one or more regulatory-based constraints might exist in the ultraconserved region CG15121–CG16894.
Disruption of the ultraconserved region CG15121–CG16894
To assess the importance of the integrity of the ultraconserved region CG15121–CG16894, we aimed to disrupt it and characterize the resulting phenotypic effects. We examined the existence of stocks carrying isolated naturally occurring inversions disrupting the ultraconserved region and none was found. Thus, we generated a disruption of the ultraconserved region CG15121–CG16894 by inducing the inversion In(2R)51F11-56E2 in D. melanogaster, a species in which nonallelic homologous recombination (NAHR) events can be mediated between FLP recombination target-bearing transposable elements (FRT-bearing TEs hereafter) via activation of a heat-inducible flippase-recombinase [28], [39]–[42] (Figure S3, S4, S5). For that, we used two TEs bearing FRT sites in opposite orientation: P{RS5}5-HA-1995, which is inside the ultraconserved region CG15121–CG16894; and P{RS3}CB-0236-3, which is located 4.35 Mb upstream (Figure 1A). The outer element is virtually terminal within a collinear block of 15 genes and is inserted into a naturally occurring copy of the TE 1360{}835 [43].
We adopted several measures to avoid confounding effects that could overlay those of the intended disruption of the ultraconserved region CG15121–CG16894. First, TEs were selected to avoid disrupting any known regulatory sequences of flanking genes and those presumably embedded in HCNE peaks, thus preventing the generation of artifactual position effects. The comparison of the size and sex ratio of the progeny of flies homozygous for each of the TEs confirmed the absence of any obvious detrimental effect associated with particular TE insertions (Figure S6). Second, in addition to strains carrying the inversion In(2R)51F11-56E2 (INV1 and INV2), we generated several control strains to account for further mutations that could have been incidentally generated by our approach [44]. Specifically, three kinds of control strains were constructed: strains carrying two FRT-bearing TEs in cis (REC), i.e. just before inducing the NAHR event that mediates the inversion; strains carrying the standard arrangement as a result of failed induced NAHR events but that were exposed to the same experimental conditions as the INV strains -SIMultaneous controls - (SIM1, SIM2, and SIM3); and strains in which the inverted segment is reverted back to its original orientation -REVertant controls - (REV1 and REV2) (Figure 2A, Figure S3 and S4). The main molecular changes at the inversion breakpoints of all relevant strains, plus those carrying the original FRT-bearing TEs, are depicted in Figure S5. In the absence of any secondary effect of our procedure, SIM, REV, and REC should perform likewise as measured by the size and sex ratio in the progeny of low-density crosses with homozygous flies (Figure S7). INV strains for which no SIM and/or REV control lines could be generated were discarded. For the remaining strains, their putative karyotype was verified at the cytological level and the expected molecular organization at their breakpoint regions confirmed by PCR and Sanger sequencing (Figure 2B–2C and 8). Further expression profiling confirmed the absence of local position effects at the inversion breakpoints (see below). Lastly, engineered chromosomes were maintained in homozygosis thus preventing the otherwise unavoidable accumulation of detrimental mutations if kept in heterozygosis over a balancer chromosome. Most of the strains that were not discarded were included in one or more downstream analyses.
Fig. 2. Disruption of the ultraconserved region CG15121–CG16894. 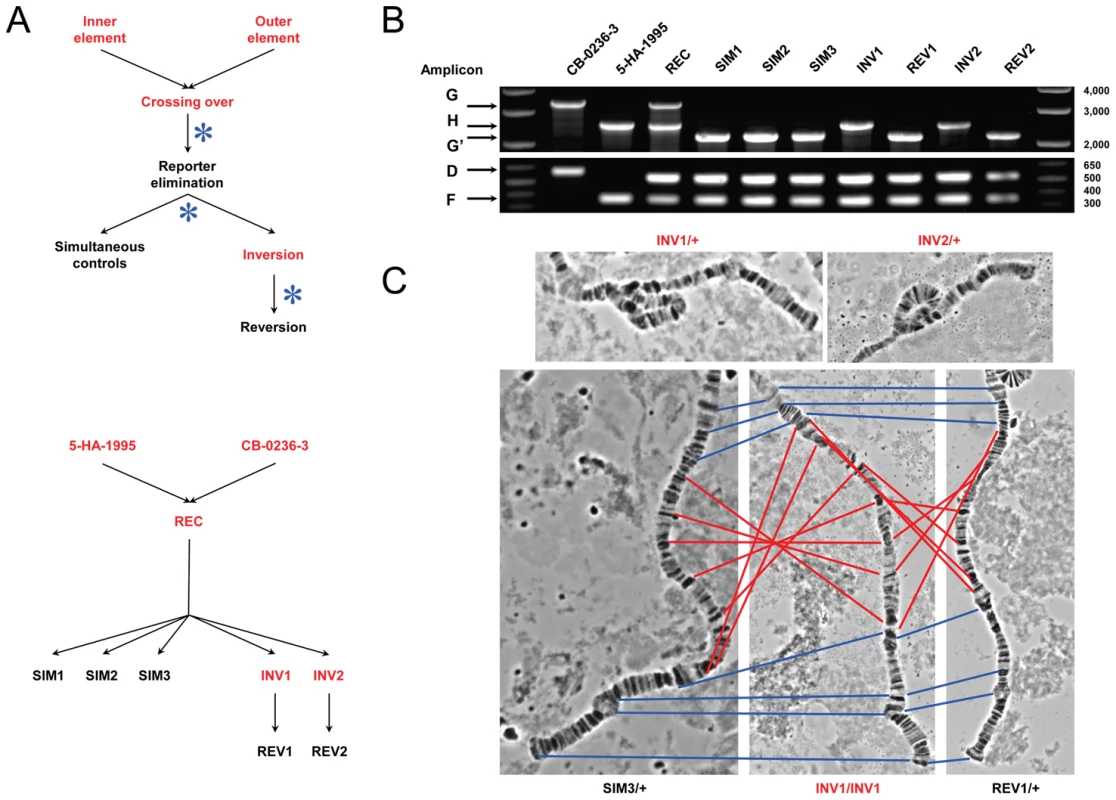
(A) Outline of the main steps used to obtain the strains carrying the ultraconserved region CG15121–CG16894 in its disrupted and intact form (Figures S3 and S4 for details). The strains 5-HA-1995 and CB-0236-3 carrying the FRT-bearing TEs [42] were used to induce the inversion In(2R)51F11-56E2, which disrupts the ultraconserved region (strains INV1 and INV2). Control strains carrying the ultraconserved region in its intact form are also shown (REC, SIM1-SIM3, REV1-REV2; Table S4). Black and red denote w− and w+ phenotype, respectively; asterisk, heat-shock induced FLP expression. (B) Distinctive banding pattern for the PCR products of five different amplicons (D, F, G/G′, H) run simultaneously for each of the strains generated (Material and Methods, Figure S5, and Tables S5 and S6). Two µl from each individual PCR product were combined. Strains 5-HA-1995 and CB-0236-3 show the presence of only one of the FRT-bearing TEs in their genomes while the REC strain shows the presence of both. SIM and REV strains show the successful amplification of the amplicon G′, which denotes the presence of a derivative of P{RS3}CB-0236-3 at the outer breakpoint, but not of the amplicon H, indicating that they carry the 2R standard chromosomal arrangement. INV strains show the opposite pattern; the detection of amplicon H denotes the presence of the inverted chromosomal arrangement. (C) Representative polytene chromosome squashes. Top, an inversion loop is observed for the heterozygote progeny of INV1 and INV2 parentals when crossed with individuals from the strain w1118 thus confirming the presence of the inverted arrangement. Bottom, no inversion loop is observed in the progeny of similar crosses involving SIM3 and REV1. Red and blue lines connect the same polytene band inside and outside of the inverted fragment, respectively, between carriers for the standard arrangement (SIM3 and REV1) and homozygotes for the induced inversion (INV1; center). Apart from the inversion In(2R)51F11-56E2 in the strains INV1 and INV2, no other gross chromosome alteration was detected for the strains shown in (B). Phenotypic consequences of disrupting the ultraconserved region CG15121–CG16894
The disruption of the ultraconserved region CG15121–CG16894 did not lead to viability impairment in the progeny of the carriers (INV1, INV2) as compared to that of non-carriers based on two proxies examined (Figure S7). Cursory examination of embryos, larva, and pupa did not detect any obvious morphological defect either. We then explored the possibility of a detrimental effect in heterozygous condition before reaching adult eclosion, either because of the disruption of the collinear block, meiotic distortion due to the presence of a chromosomal inverted rearrangement, or both. The comparison of different chromosome combinations revealed no departures from the expected Mendelian ratios (1∶2∶1) and absence of sex-specific effects (Figure S9). Further, we assessed differential viability during early stages (i.e. prior to imago emergence), when competition among individuals is specially intense [45], [46], and when most of the genes putatively targeted by HCNEs exhibit high levels of expression. Differences among carriers of different 2R chromosomes were detected in frequency-dependent competition experiments involving different pairwise combinations of embryos, but those differences were not consistently shown by the carriers of the disrupted ultraconserved region (INV1, INV2) compared to those that carry it in its intact form (REC) across different genetic backgrounds (Figure S10). Together, these results do not point out to any obvious detrimental effect on viability, and therefore on stages of the life cycle that encompass key developmental transitions, as a result of disrupting the ultraconserved region CG15121–CG16894.
Next, we examined the effect of disrupting the ultraconserved region CG15121–CG16894 after imago emergence since many of the protein-coding genes included in the region under study are expressed during adulthood, often in a sex-dependent fashion (Figure S1). Specifically, the region under study is populated with male-biased genes in expression, 11 of them preferentially expressed in testes [22] and four, all of them Obp genes, present in the seminal fluid (Figure 1; Table S2). We tested for differences in several parameters of male fertility: progeny size, sperm performance, and mating ability. The comparison of males from the strains INV1, INV2, and REC, which are all red-eyed so that differences due to differential pigmentation can be factored out, revealed that although there are differences in progeny size (Figure S11A), strains INV1 and INV2 are more different from each other than either of them is to strain REC. Double-mating experiments did not reveal any substantial difference in sperm performance (Figure S11B) and mating ability (Figure S12). Further, given the presence of nine odorant-binding and one odorant-receptor protein-coding genes in the ultraconserved region CG15121–CG16894, we analyzed the odorant abilities of the different strains. We examined the response to three volatile compounds (ethanol, acetone, and benzaldehyde) and found statistically significant differences in five out of 12 sex-by-strain combinations. Importantly, for females exposed to ethanol at a concentration of 10−3 and males exposed to acetone at a concentration of 10−4.5, strains INV1 and INV2 exhibited a coherent pattern of differentiation from strain REC (Figure 3; Table S14), involving in all cases an attenuated attraction to the chemical in question. This attenuated response does not result from an overall impairment of the odorant abilities of the flies as shown by the response to the repellent compound benzaldehyde. Analyses of different proxies for the general adult homeostasis (negative gravitaxis, heat-shock resistance, desiccation resistance, and starvation resistance) did not uncover any other difference between the strains with and without the disrupted ultraconserved region CG15121–CG16894 (Figure S13).
Fig. 3. Olfactory response of strains with (INV1, INV2) and without (REC) the disrupted ultraconserved region CG15121–CG16894 to three different volatile compounds. 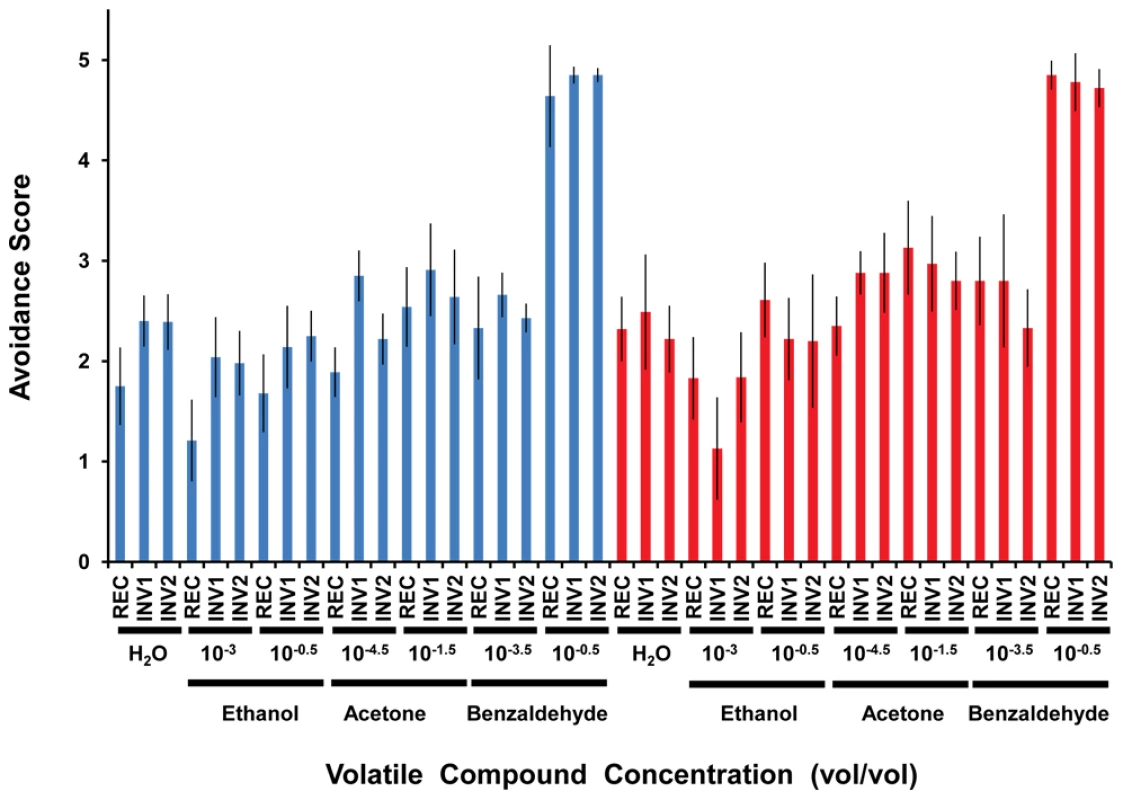
Blue, females; red, males. The “dipstick” method was used in all cases. An avoidance score equal to 2.5 indicates indifference while values <2.5 and >2.5 are interpreted as attractant and repellent responses, respectively. Ethanol, acetone, and benzaldehyde were assayed at two different concentrations (vol/vol) deferring by several orders of magnitude in order to test the response of the strains in very different conditions. Distilled water was used as a reference for the default response when no compound is added. For females, we found statistically significant differences (Kruskal-Wallis, d.f. = 2 in all cases) across strains in response to distilled water (P<0.0107), ethanol (concentration: 10−3, P<0.0056), ethanol (concentration: 10−0.5, P<0.0230), and acetone (concentration: 10−4.5, P<0.0002), while in males, the differences were confined to ethanol (concentration: 10−3, P<0.0183) and acetone (concentration: 10−4.5, P<0.0182). INV1 and INV2 showed significant differences in the same direction in relation to REC after accounting for multiple tests in the case of distilled water (females), ethanol (concentration: 10−3, females), and acetone (concentration: 10−4.5, males) (Table S14). Error bars indicate 95% CI. Lastly, we tested whether the disruption of the ultraconserved region CG15121–CG16894 resulted in a perturbation of gene expression. We performed a microarray-based characterization of the transcriptome of six lines (REC, INV1, INV2, SIM1, REV1, and REV2) during adulthood, the stage in which we found evidence of phenotypic differences associated with the disruption generated. At FDR 0.01, we found a very limited number of differentially expressed transcripts both in males and females (0.07% -11/16,637 - and 6.2% -1,033/16,637-, respectively; Dataset S1 and Table S16). Further analyses confirmed the similarity of the expression profiles between equivalent strains generated by our procedure (first three planned contrasts in Table S17; Datasets S1 and S2). Likewise, these analyses indicated that the most statistically significant differences in mRNA abundance found are associated with differences in pigmentation (last three planned contrasts in Table S17; Dataset S2), in good agreement with the clustering of expression profiles among strains (Figure 4). The inspection of the chromosomal distribution of the differences in gene expression showed that a few of them were related to genes in the ultraconserved region. However, these alterations in mRNA levels are in fact the result of pigmentation differences, given that these alterations were found invariably between red-eyed and white-eyed strains, regardless of whether the former carry the disrupted (INV1 and INV2) or the intact form (REC) of the ultraconserved region (Figures S14 and S15). Searches for biologically coherent patterns among differentially expressed genes indicated that, for example for females, statistically significant enrichment was found for functional classes related to perception of visual stimuli (Table S18). Further, patterns of sex bias in gene expression were not affected either (Figure S16; Dataset S3). Hence, no discernible effect on the levels of mRNA of genes both inside and outside of the ultraconserved region CG15121–CG16894 was detected as a result of its disruption.
Fig. 4. Two-way hierarchical clustering of the average levels of expression estimated for the 1% transcripts exhibiting the lowest P values across the six strains under study according to one-way ANOVA. 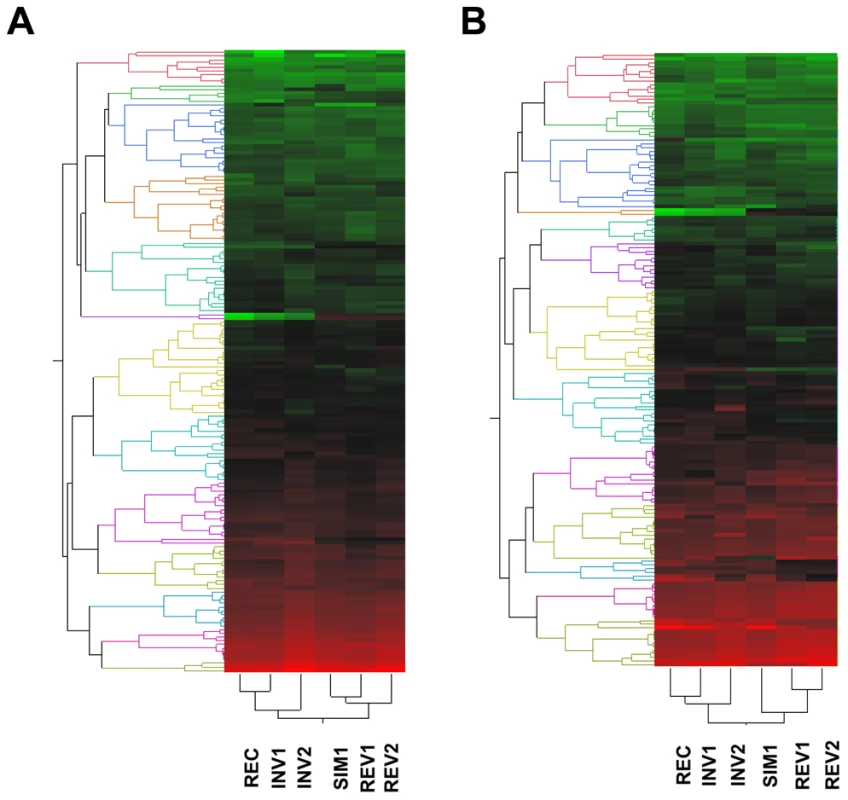
(A) Males; (B) females. For both sexes, the patterns of expression among red-eyed strains, i.e. those with (INV1, INV2) or without (REC) the disrupted ultraconserved region CG15121–CG16894, are more similar to each other than either of them is to any of the white-eyed strains (SIM1, REV1, REV2), all of them carrying the ultraconserved region in intact condition. Thirty-six one-color hybridizations were performed involving three biological replicates for each sex-by-strain combination. Red, high expression; green, low expression. Discussion
The interplay among organization, function, and evolution of eukaryotic chromosomes is still poorly understood. Collinearity conservation is an important genomic feature that can conflate all three aspects, especially in genomes characterized by their ease in accommodating structural variation. Up to date, no empirical evaluation has been performed on the effects of disrupting the integrity of one of the largest genomic regions whose overall gene organization has been preserved over a large time scale. Importantly, this conservation entails species with very different behaviors and ecologies and therefore subject to very different selective pressures [46], [47]. Our results show an absence of detrimental effects on the carriers of the disrupted ultraconserved region for a variety of traits associated with viability and fertility. Although the absence of detrimental phenotypic effects could result from a limited ability to detect differences as statistically significant, the comparison of actual and estimated ideal samples sizes shows that this potential limitation could be the explanation in only a few cases (Table S19). Therefore, at least in Diptera genomes, our results show that an unusually high degree of collinearity conservation coupled with enrichment for functional coherent patterns is not necessarily associated with severe detrimental effects upon perturbation [20], [48].
The ultraconserved region CG15121–CG16894 harbors at least three sets of protein-coding genes that may be associated with regulatory-based constraints. The genes fall into the following broad categories: detection of chemical stimuli; sperm manufacturing and performance; and developmental processes. The only phenotype detected in association with our disruption was the more attenuated odorant response to attractant volatile compounds. Although variation in olfactory response occurs both within and between species [49], [50], it has been shown that the altered function of Obp genes, including some in the region studied, can affect fitness components in D. melanogaster [51]. Transcriptome characterization of adult flies did not uncover any obvious consequences in the activity of the Obp genes due to our disruption as measured by mRNA abundance, and magnitude and direction of sex bias. This lack of evidence for misregulation cannot rule out though that some other expression attributes, such as the spatial distribution of transcripts, had not been altered since they would have gone unnoticed by our approach. The evolutionary relevance of the maintenance of the clustering of Obp genes throughout the genus Drosophila is reinforced by the presence of an orthologous arrangement in different mosquito species to an extent not explained by chance [37]. In fact, the maintenance of the arrangement that includes a core cluster of Obp genes and the gene Toll-7 for ∼970 myr was unexpected due to the extent of rearrangement undergone by the genomes of the Diptera involved [22], [33], [34] (Figure S2B). Regardless, the conservation of the cluster of Obp genes and Toll-7 would not explain the collinearity conservation observed elsewhere in the ultraconserved region under study.
In relation to the two other biological signatures suggestive of constraints, we detected no evidence that our perturbation resulted in a detectable phenotype. The genes included in the two intertwined gene expression neighborhoods of the ultraconserved region CG15121–CG16894 do not show evidence of altered mRNA levels or malfunction that could result in impaired male fertility. Whether the constituent genes are under a tight coordinated regulation [52] that could affect the long-term stability of the region does not seem to be upheld by our results. This conclusion would be reinforced by the genus-wide lability shown by clusters of male-biased genes in expression [22]. The absence of misregulation, as measured by mRNA abundance, is consistent with the results obtained when D. melanogaster male-specific gene neighborhoods spanning over hundred of kb were disrupted [28].
Further, protein-coding genes presumably responsive to long-range regulation mediated by HCNEs during development are found scattered across the ultraconserved region CG15121–CG16894 at both sides of the disruption. The lack of a detrimental phenotype could reflect that the region is a composite of several autonomous genomic regulatory domains, each of them under the control of a particular HCNE peak. Under this scenario, our disruption would have separated different genomic regulatory domains without affecting any long-range interaction between HCNEs and their targets. Nevertheless, marked autonomy among HCNE peaks should result in certain regionalization of the expression profiles, i.e. physically closer HCNE targets should exhibit more similar expression profiles. In fact, we find putative targets of HCNEs located at both sides of the disruption, such as CG9854 and CG8896, showing very similar expression profiles (Figure 1 and Figure S1). If this similarity denotes the existence of long-range interactions, the latter are not associated with detrimental effects that explain the collinearity conservation. Alternatively, some degree of regulatory redundancy could exist at both sides of the disruption, which would explain the lack of phenotypic effect but not the collinearity conservation.
A different view of collinearity conservation entails factors other than functional constraints. DNA stretches with sufficient sequence identity can mediate NAHR events that give rise to chromosomal rearrangements [53]. Large collinear regions could be depleted of this type of sequences thus explaining why this ultraconserved region has maintained its integrity. A search for well-annotated sequences with the potential to mediate NAHR events [54] in the studied region across Drosophila species revealed that these sequences are present (Table S20). These sequences include ncRNA genes such as tRNAs [55], [56], rRNAs genes [57], [58], and snoRNAs [59]. Equivalent comparative analysis focusing on TEs, once properly annotated, will enable to test whether the region under study is particularly depleted for these sequences, which would decrease its propensity of being rearranged. Further, recent findings indicate that orthologous landmarks harboring genes that bind to the nuclear periphery are significantly larger than those that do not harbor any suggesting that particular intranuclear localizations might provide molecular environments associated with higher levels of genome stability [60]. The ultraconserved region CG15121–CG16894 is known to establish some contact with the nuclear periphery [61]. Specifically, it contains at least five protein-coding genes (CG16716, CG13872, CG10822, CG8654, CG16898) that exhibit statistically significant association with the B-type Lamin protein, a key component of the inner nuclear membrane [60], [62]. This pattern leaves open the possibility that some regulatory-based constraints had evolved under the enhanced evolutionary stability enjoyed by genomic regions associated with the nuclear periphery, and therefore functional constraints would not be the only mechanism contributing to their collinearity conservation.
Regardless how extensive the characterization of individuals carrying engineered genomic regions could be, our ability to detect phenotypic effects will always be contingent to the experimental setting used and the timescale in which the assays are performed. Nevertheless, our results raise the possibility that, at least in Diptera genomes, the mechanistic basis of collinearity conservation might be much more subtle and diverse than previously thought and that regulatory-based interactions might not suffice to account for the patterns of extensive conservation seen in some genomic regions [22]. Only further empirical tests for this and other ultraconserved regions can shed light on the scope of our observations.
Materials and Methods
Fly husbandry
Table S4 describes the strains used. Fly cultures were grown and maintained on dextrose-cornmeal-yeast medium at room temperature. Unless otherwise stated, all phenotypic assays were performed at 25°C in a temperature-controlled chamber and fly manipulation, sorting, and scoring were carried out under CO2 anesthesia. Strains generated are available upon request.
Inversion generation
Strains carrying the original FRT-bearing TE insertions (5-HA-1996 and CB-0236-3) were selected from the DrosDel collection and examined at different levels prior to their use in the generation of the chromosomal inversion In(2R)51F11-56E2. Sanger sequencing confirmed the insertion point of the FRT-bearing TEs used. Low-density crosses using homozygous flies were also performed to test for potential detrimental effects associated with the TE insertions. Briefly, six sexually mature individuals (three four-day-old females and three two-day-old males) were used per cross and strain; each cross was replicated five times. Males were discarded after 24 hours while females were transferred twice every three days until discarded on the ninth day.
To generate the inversion In(2R)51F11-56E2, we followed essentially [42] with slight modifications (John Roote, pers. comm.; Figure S3A–S3B). Three types of strains are generated under this procedure (REC, INV, and SIM); a fourth type of strain (REV; Figure S3C) was generated by reverting the inversion In(2R)51F11-56E2 [40]. The strains generated were scrutinized for evidence of side effect associated with our procedure (see below). Individuals from strains deemed as valid were crossed with those from the strain w1118, which possesses the standard arrangement for all chromosomes, and the third-instar salivary gland polytene chromosomes of the progeny examined. Chromosome squashes were stained with orcein and inspected with a Zeiss AX10 Imager M1 microscope. Cytological analysis was performed using the photographic polytene maps of D. melanogaster as a reference [63]. Diagnostic DNA stretches at the breakpoint regions were PCR amplified and their identity verified by Sanger sequencing; sequences were deposited at GenBank (http://www.ncbi.nlm.nih.gov/genbank/; JN805541–JN805602). Amplicons C, D, E, and F confirmed the presence of FRT-bearing TEs and their derivatives at the breakpoint regions. Amplicon G, or G′ after excision of one of the exons of the reporter gene, and H correlate with the absence and presence of the inversion, respectively (Tables S5 and S6 for further details on primers used and amplicons). Genomic DNA used in PCR genotyping was extracted from 50 individuals of each strain as described [39] and quantified using a NanoDrop 8000 Spectrophotometer. Takara Taq and Takara Ex Taq, depending on the size of the DNA fragment to be amplified, were used according to manufacturer conditions. PCR products were resolved on 1% agarose gels and visualized in an AlphaImager HP system. Amplicon sequences were examined for point mutations and indels that could have been generated incidentally during the course of our procedure.
Viability tests
Low-density crosses using homozygous flies were set up for each strain, as described above for the evaluation of the TE insertions, to confirm absence of differences among control strains and to test for differences among the latter and the strains carrying the disrupted ultraconserved region. Additional crosses, five per strain, evaluated the effects of the disruption in heterozygous condition with different chromosomes carrying the standard arrangement (SIM1, REV1, REV2); 10 sexually mature individuals of each sex were used per cross. Further, adapted frequency-dependent experiments [64] evaluated differences in viability of mixtures of embryos for two different genotypes. Two types of mixtures were prepared. In both, w+ individuals (INV1, INV2, or REC; the tested chromosome) compete with w− individuals carrying the ultraconserved region in its intact form. The only difference between the mixtures was the condition of the tested chromosome (heterozygosis, e.g. INV1/SIM1 versus SIM1/SIM1; homozygosis, e.g. INV1/INV1 versus SIM1/SIM1). For both types of mixtures, three sets of experiments were done varying the standard arrangement of the competing embryo (SIM1, REV1, REV2; the tester embryo), which was always in homozygosis. Embryos were collected from grape juice-sucrose-agar plates supplemented with yeast paste using FlyStuff Small Embryo Collection Cages. Using dissecting needles, 100 embryos were deposited on small grape juice-sucrose-agar cube according to one of three starting proportions for the two competing embryos (1∶3, 1∶1, 3∶1). The cube was subsequently introduced into a vial of fresh medium; each assay was replicated 10 times. Separate previous pilot experiments for the strains REC, SIM1, and INV1, using 100 embryos, indicated that the rate of survival was approximately 40% and therefore enough to detect differences between the strains to be compared; each assay was replicated 10 times. In total, 42 different competition settings were set up (7 tester by tested combinations×2 genotype conditions for the tested chromosome×3 starting proportions). Given the number of embryos used across competition experiments (42,000), only those involving the same tester w− embryo (e.g. SIM1/SIM1) were performed simultaneously, which determined how the contrasts were done (three for each genotype condition of the tested chromosome and particular starting proportion, i.e. 18 in total). No bias in sex ratio was assumed in all cases. Progenies from each cross were scored after 15 days to ensure the emergence of all surviving imagoes and the relative viability between the two competing genotypes estimated as (n1′×n2)/(n1×n2′), where n1 and n2, and n1′ and n2′, are the number of embryos and imagoes, respectively, of the two competing genotypes. Values of relative viability were log2-transformed.
Male fertility tests
The effects of the induced disruption on male fertility were assayed by examining progeny size, sperm performance, and mating ability. For the first test, we exposed single four-day-old virgin females carrying the ultraconserved region CG15121–CG16894 in its intact form (SIM1, REV1, REV2) to single two-day-old males with (INV1, INV2) or without (REC) the disrupted ultraconserved region. Males were discarded after 24 hours while females were transferred daily to vials with fresh food over a 10-day period. The number of replicates ranged from seven to nine. To evaluate sperm performance in the carriers of the disrupted ultraconserved region, we followed a similar experimental design to that for monitoring progeny size with the exception that the females mated in the first day were exposed to single males of their own strain in subsequent days. The progeny sired by the first and second males was scored based on eye color. For those days during which progeny from both parents were detected, i.e. those oviposited roughly the same day, we estimated the fraction of red-eyed individuals (necessarily sired by INV1, INV2, or REC males) in relation to the total. To account for the effect of the order of the males used, we performed identical experiments but this time the first male possessed the same genotype as the female while the second male was from the strains INV1, INV2, or REC. The number of replicates for each combination of genotypes ranged from four to nine. As for the comparison of mating abilities, we exposed single two-day-old males to 10 four-day-old virgin females for different time periods (1 hr, 3 hr, 6 hr). Afterwards, the females were transferred individually into vials with fresh food. After 15 days, 10 vials were examined for the presence of progeny, which indicates that at least one successful fertilization event occurred, and the number of females successfully fertilized recorded. Ten replicates were done per strain and time period combination.
Response to volatile compounds
The “dipstick” method was used [65]. Briefly, virgin individuals from INV and REC strains were separated by sex and transferred by aspiration in groups of five to marked empty plastic vials (O.D.×H: 25×95 mm) 24 hr after emergence. The vials were marked at 3 and 6 cm from the bottom. Fisherbrand Q-tips dipped into the odorant dilutions to be tested were introduced into the vials up to the 6 cm mark, and secured with a cotton plug to avoid contact with the walls of the vial. After a 15-second recovery period, the number of flies in the bottom compartment was recorded 10 times every five seconds and the avoidance scored estimated as the average over those 10 measurements. A score of 2.5 indicates indifference to the odorant tested while values >2.5 and <2.5 indicate repulsion and attraction, respectively. All tests were performed between 2 and 6 pm after starving the flies for no less than four hours, and the vials were always placed sideways to prevent interfering with the geotactic response. For each strain, 10 groups of five individuals for each of the sexes were analyzed. We tested three odorants, two of them usually considered to elicit an attracting response (ethanol, Gold Shield; acetone, Fisher Chemical) while the other is considered to be a repellant (benzaldehyde; Sigma Aldrich B1334 Benzaldehyde-ReagentPlus). Since odorant response can be concentration dependent, we tested two concentrations deferring by several orders of magnitude. These concentrations (vol/vol) were: ethanol, 10−3 and 10−0.5; acetone, 10−4.5 and 10−1.5; and benzaldehyde, 10−3 and 10−0.5. Only fresh and thoroughly mixed dilutions were used to prevent oxidation, which is particularly relevant in the case of benzaldehyde. As a control, we used distilled water, which is known to attract starved flies [65].
General homeostasis tests
Four proxies, including gravity response and survival to three stressors (heat-shock, desiccation, and starvation), were assayed. In all cases, males and females were separated after emergence and left for 24 hours in vials with fresh food. Subsequently, all individuals were transferred to new vials by aspiration for performing the pertinent tests. Negative gravitaxis was measured essentially as reported [66]. Twenty flies per sex and strain were transferred individually into a 250 ml glass volumetric cylinder. Flies were knocked down by tapping the cylinder ten times on a pad, and the height reached by each fly in 20 s recorded using the volumetric scale, which was divided by the maximum height. In our experience, flies performed more consistently after some training, reason why only measurements from a third trial were recorded. For the heat-shock resistance test [67], five groups of 20 flies per sex and strain were transferred in pairs into 3 ml Pyrex vials and then incubated in water baths at 35°C for 30 m (as a pre-conditioning step) and then at 39°C for another 30 m. The mobility of the flies was restricted to the submerged portion of the vial with cotton plugs. After the heat-shock treatment, flies were collected in vials with food that had been incubated at 25°C overnight. Next morning, the fraction of flies alive within each group was recorded. For the desiccation resistance test [68], groups of five flies, from a total of 20 per sex and strain, were transferred into empty vials. The mobility of the flies was restricted to the lower third of the vial using foam plugs, over which 3 g of Drierite desiccant were added. Next, the vials were sealed with Parafilm to maintain low humidity. Flies were checked every hour and the elapsed time-to-death recorded. As for the starvation resistance test, 20 flies per sex and strain were transferred into glass vials containing 2 ml of 1% agar dissolved in water to ensure normal humidity conditions. Flies were examined every 12 hr and the elapsed time-to death recorded.
Statistical analysis of non-molecular phenotypes
For each phenotype, normality of the data was visually inspected using normal quantile plots and precisely determined with the Shapiro-Wilk test. Homogeneity of variances was estimated using the Levene's test. Parametric tests were used if the departure from the assumptions of normality and homoscedasticity was absent or negligible. The Welch ANOVA was used if only heterodasticity was detected. Different transformations (log2 or arcsine square root, depending on the test) of the measurements were calculated for some phenotypes to improve fit to normality although this had a very little effect. Alternatively, non-parametric tests (e.g. Kruskal-Wallis) were used. When multiple post-hoc contrasts were necessary, appropriate tests that account for multiple comparisons were used (e.g. Steel-Dwass). In the case of the departure from expected Mendelian ratios in frequency-dependent competition experiments, the G-test for goodness of fit, upon applying the William's correction, was used. Statistical contrasts were performed with JMP 4.1 (SAS Institute); the evaluation of the sensitivity to detect statistically significant differences was done with GPower 3.1.3 when needed [69].
Expression data and analysis
Three low-density crosses were set up for the strains REC, INV1, INV2, SIM1, REV1, and REV2. The resulting virgin progeny was collected, separated by sex, and allowed to age for 5–7 days. Fifty individuals for each sex were snap frozen in liquid nitrogen at the same time of the day within a 2 hours window and subsequently stored at −80°C. Total RNA from biological samples was extracted using TRIzol Reagent (Invitrogen) and purified using the RNeasy Mini Kit (Qiagen). Concentration, quality and integrity of the RNA samples were estimated using a NanoDrop 8000 Spectrophotometer and the RNA 6000 Nano Chip Kit (Agilent Technologies) with an Agilent 2100 Bioanalyzer. Ten µg of total RNA were reverse transcribed into cDNA using the SuperScript Double-Stranded cDNA Labeling Kit (Invitrogen). Probe labeling, hybridization, array scanning, and data extraction were performed by Roche NimbleGen Service Group in Iceland. We used the oligonucleotide NimbleGen 12×135k D. melanogaster arrays, which contain 135,000 probes including different types of controls and 16,637 transcripts of protein-coding genes as annotated in release 5.7. The experiment consisted of 36 one-color hybridizations (6 samples×2 sexes×3 biological replicates). Raw fluorescence intensity values of probe pairs were summarized for each transcript using the median value after log transformation. Subsequent data analysis was performed using the tools implemented in the online pipeline WebArrayDB [70]. Data were normalized between arrays using the scale method [71] implemented in the LIMMA package [72]. Data for males and females were analyzed separately. Statistically significant differences across strains were assessed using a fixed-effect model ANOVA and multiple testing was performed with the Benjamini-Hochberg correction [73]. Similarity in expression profiles across genes and strains was assessed by hierarchical clustering using Ward's minimum variances as a distance metric. The first principal component was used to assist in the sorting. Six biologically meaningful planned contrasts defined a priori, all of them orthogonal, were done likewise by pooling the appropriately expression data of different strains as necessary (Table S17). Scrutiny of the differences in post-hoc comparisons among strains was done using the “multcomp” R package [74]. Functional information for relevant genes was obtained from FlyBase [30] and enrichment for particular Gene Ontology term categories (biological processes, molecular functions, and cellular components), KEGG pathways, and InterPro protein domains was evaluated using DAVID [75]. Benjamini-Hochberg correction [73] was applied to account for multiple tests. We proceeded likewise, but for each strain separately, to evaluate statistically significant differences between the sexes. Raw microarray data were deposited at the Gene Expression Omnibus database (GSE31120). In relation to the characterization of the expression profiles of the genes in the region under study during the life cycle of D. melanogaster, RPKM expression values across 30 timepoints and conditions [2] were extracted from FlyBase [30], log transformed, and compared using hierarchical clustering as above.
Promoter analysis
Core promoter predictions for all the transcripts of protein-coding genes examined were done with McPromoter using the most stringent parameter values [76]. We inspected 500 nt upstream of the 5′ UTR start of each transcripts as annotated in FlyBase [30]. In the absence of an annotated 5′UTR, a stretch of DNA of equal length upstream of the first nucleotide annotated was examined. Categorization of genes as responsive to HCNEs was based on the prediction of having an Inr core promoter type.
Comparative organization of the ultraconserved region CG15121–CG16894
We retrieved mapping coordinates of protein-coding genes included in the ultraconserved region under study in A. gambiae (AgamP3 assembly) using Biomart [77]. Any orthologous mapping information that did not conform to a one-to-one relationship between species was discarded. The global gene organization nearby the ortholog of Toll-7 in A. gambiae and A. aegypti was examined through VectorBase [78]. Phylogenetic relationships among Obp-related amino acid sequences encoded by genes in the same region that harbors the gene Toll-7 from the two mosquito species were conducted in MEGA 5.0 [79]. Amino acid sequences were downloaded from VectorBase [78] and aligned with CLUSTALW [80]. The best evolutionary model of amino acid evolution was found to be WAG and the consensus tree was built using the Maximum Likelihood method [81]. A discrete Gamma distribution was used to model evolutionary rate differences among sites (+G; 4 categories). Bootstrapping was performed to determine the confidence of the branches (1,000 replicates).
Supporting Information
Zdroje
1. KapranovPChengJDikeSNixDADuttaguptaR 2007 RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316 1484 1488
2. GraveleyBRBrooksANCarlsonJWDuffMOLandolinJM 2010 The developmental transcriptome of Drosophila melanogaster. Nature
3. RoySErnstJKharchenkoPVKheradpourPNegreN 2010 Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330 1787 1797
4. SharpeJNonchevSGouldAWhitingJKrumlaufR 1998 Selectivity, sharing and competitive interactions in the regulation of Hoxb genes. Embo J 17 1788 1798
5. SpitzFGonzalezFDubouleD 2003 A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell 113 405 417
6. BulgerMGroudineM 1999 Looping versus linking: toward a model for long-distance gene activation. Genes Dev 13 2465 2477
7. CavodeassiFModolellJGomez-SkarmetaJL 2001 The Iroquois family of genes: from body building to neural patterning. Development 128 2847 2855
8. MackenzieAMillerKACollinsonJM 2004 Is there a functional link between gene interdigitation and multi-species conservation of synteny blocks? Bioessays 26 1217 1224
9. SpitzFDubouleD 2008 Global control regions and regulatory landscapes in vertebrate development and evolution. Adv Genet 61 175 205
10. MonginEDewarKBlanchetteM 2009 Long-range regulation is a major driving force in maintaining genome integrity. BMC Evol Biol 9 203
11. KikutaHLaplanteMNavratilovaPKomisarczukAZEngstromPG 2007 Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Res 17 545 555
12. EngstromPGHo SuiSJDrivenesOBeckerTSLenhardB 2007 Genomic regulatory blocks underlie extensive microsynteny conservation in insects. Genome Res 17 1898 1908
13. HuftonALMathiaSBraunHGeorgiULehrachH 2009 Deeply conserved chordate noncoding sequences preserve genome synteny but do not drive gene duplicate retention. Genome Res 19 2036 2051
14. de la Calle-MustienesEFeijooCGManzanaresMTenaJJRodriguez-SeguelE 2005 A functional survey of the enhancer activity of conserved non-coding sequences from vertebrate Iroquois cluster gene deserts. Genome Res 15 1061 1072
15. PennacchioLAAhituvNMosesAMPrabhakarSNobregaMA 2006 In vivo enhancer analysis of human conserved non-coding sequences. Nature 444 499 502
16. EllingsenSLaplanteMAKonigMKikutaHFurmanekT 2005 Large-scale enhancer detection in the zebrafish genome. Development 132 3799 3811
17. WoolfeAGoodsonMGoodeDKSnellPMcEwenGK 2005 Highly conserved non-coding sequences are associated with vertebrate development. PLoS Biol 3 e7 doi:10.1371/journal.pbio.0030007
18. ZunigaAMichosOSpitzFHaramisAPPanmanL 2004 Mouse limb deformity mutations disrupt a global control region within the large regulatory landscape required for Gremlin expression. Genes Dev 18 1553 1564
19. KleinjanDAvan HeyningenV 2005 Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet 76 8 32
20. SpitzFHerkenneCMorrisMADubouleD 2005 Inversion-induced disruption of the Hoxd cluster leads to the partition of regulatory landscapes. Nat Genet 37 889 893
21. NavratilovaPBeckerTS 2009 Genomic regulatory blocks in vertebrates and implications in human disease. Brief Funct Genomic Proteomic 8 333 342
22. von GrotthussMAshburnerMRanzJM 2010 Fragile regions and not functional constraints predominate in shaping gene organization in the genus Drosophila. Genome Res 20 1084 1096
23. TamuraKSubramanianSKumarS 2004 Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol 21 36 44
24. PowellJRDeSalleR 1995 Drosophila molecular phylogenies and their uses. HechtMKMacIntireRJCleggMT Evolutionary Biology New York Plenum 87 138
25. MullerHJ 1940 Bearings of the Drosophila work on systematics. HuxleyJ The New Systematics Oxford Clarendon Press 185 268
26. SturtevantAHNovitskiE 1941 The homologies of the chromosome elements in the genus Drosophila. Genetics 26 517 541
27. PevznerPTeslerG 2003 Human and mouse genomic sequences reveal extensive breakpoint reuse in mammalian evolution. Proc Natl Acad Sci U S A 100 7672 7677
28. MeadowsLAChanYSRooteJRussellS 2010 Neighbourhood continuity is not required for correct testis gene expression in Drosophila. PLoS Biol 8 e1000552 doi:10.1371/journal.pbio.1000552
29. BhutkarASchaefferSWRussoSMXuMSmithTF 2008 Chromosomal rearrangement inferred from comparisons of 12 Drosophila genomes. Genetics 179 1657 1680
30. TweedieSAshburnerMFallsKLeylandPMcQuiltonP 2009 FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res 37 D555 559
31. SwarupSWilliamsTIAnholtRR 2011 Functional Dissection of Odorant Binding Protein Genes in Drosophila melanogaster. Genes Brain Behav
32. RanzJMCasalsFRuizA 2001 How malleable is the eukaryotic genome? Extreme rate of chromosomal rearrangement in the genus Drosophila. Genome Res 11 230 239
33. ZdobnovEMVon MeringCLetunicITorrentsDSuyamaM 2002 Comparative genome and proteome analysis of Anopheles gambiae and Drosophila melanogaster. Science 298 149 159
34. ZdobnovEMBorkP 2007 Quantification of insect genome divergence. Trends Genet 23 16 20
35. HoltRASubramanianGMHalpernASuttonGGCharlabR 2002 The genome sequence of the malaria mosquito Anopheles gambiae. Science 298 129 149
36. PapatsenkoDLevineMGoltsevY 2011 Clusters of temporal discordances reveal distinct embryonic patterning mechanisms in Drosophila and anopheles. PLoS Biol 9 e1000584 doi:10.1371/journal.pbio.1000584
37. VieiraFGRozasJ 2011 Comparative Genomics of the Odorant-Binding and Chemosensory Protein Gene Families across the Arthropoda: Origin and evolutionary history of the chemosensory system. Genome Biol Evol
38. ZhouJJHeXLPickettJAFieldLM 2008 Identification of odorant-binding proteins of the yellow fever mosquito Aedes aegypti: genome annotation and comparative analyses. Insect Mol Biol 17 147 163
39. ParksALCookKRBelvinMDompeNAFawcettR 2004 Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet 36 288 292
40. GolicKGGolicMM 1996 Engineering the Drosophila genome: chromosome rearrangements by design. Genetics 144 1693 1711
41. RyderEBlowsFAshburnerMBautista-LlacerRCoulsonD 2004 The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167 797 813
42. RyderEAshburnerMBautista-LlacerRDrummondJWebsterJ 2007 The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics 177 615 629
43. AdamsMDCelnikerSEHoltRAEvansCAGocayneJD 2000 The genome sequence of Drosophila melanogaster. Science 287 2185 2195
44. O'KeefeLVSmibertPColellaAChatawayTKSaintR 2007 Know thy fly. Trends Genet 23 238 242
45. MarksRW 1982 Genetic Variability for Density Sensitivity of Three Components of Fitness in DROSOPHILA MELANOGASTER. Genetics 101 301 316
46. PowellJR 1997 Progress and prospects in evolutionary biology: the Drosophila model Oxford Oxford University Press 576
47. AshburnerMAGolicKGHawleyRS 2005 Drosophila: A Laboratory Handbook. Cold Spring Harbor Cold Spring Harbor Laboratory Press
48. JeongYEl-JaickKRoesslerEMuenkeMEpsteinDJ 2006 A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development 133 761 772
49. DekkerTIbbaISijuKPStensmyrMCHanssonBS 2006 Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr Biol 16 101 109
50. RuebenbauerASchlyterFHanssonBSLofstedtCLarssonMC 2008 Genetic variability and robustness of host odor preference in Drosophila melanogaster. Curr Biol 18 1438 1443
51. MackayTFHeinsohnSLLymanRFMoehringAJMorganTJ 2005 Genetics and genomics of Drosophila mating behavior. Proc Natl Acad Sci U S A 102 Suppl 1 6622 6629
52. ShevelyovYYLavrovSAMikhaylovaLMNurminskyIDKulathinalRJ 2009 The B-type lamin is required for somatic repression of testis-specific gene clusters. Proc Natl Acad Sci U S A 106 3282 3287
53. LupskiJR 1998 Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet 14 417 422
54. StankiewiczPLupskiJR 2002 Genome architecture, rearrangements and genomic disorders. Trends Genet 18 74 82
55. SzankasiPGyslerCZehntnerULeupoldUKohliJ 1986 Mitotic recombination between dispersed but related rRNA genes of Schizosaccharomyces pombe generates a reciprocal translocation. Molecular General Genetics 202 394 402
56. KellisMPattersonNEndrizziMBirrenBLanderES 2003 Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423 241 254
57. HillCWGrayJA 1988 Effects of chromosomal inversion on cell fitness in Escherichia coli K-12. Genetics 119 771 778
58. LiuSLSandersonKE 1995 Rearrangements in the genome of the bacterium Salmonella typhi. Proc Natl Acad Sci U S A 92 1018 1022
59. GonzalezJCasalsFRuizA 2004 Duplicative and conservative transpositions of larval serum protein 1 genes in the genus Drosophila. Genetics 168 253 264
60. RanzJMDíaz-CastilloCPetersenR 2011 Conserved gene order at the nuclear periphery in Drosophila. Mol Biol Evol
61. MathogDSedatJW 1989 The three-dimensional organization of polytene nuclei in male Drosophila melanogaster with compound XY or ring X chromosomes. Genetics 121 293 311
62. PickersgillHKalverdaBde WitETalhoutWFornerodM 2006 Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet 38 1005 1014
63. LefevreG 1976 A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands. AshburnerMANovitskiE The Genetics and Biology of Drosophila London Academic Press 31 66
64. AndersonWWArnoldJSammonsSAYardleyDG 1986 Frequency-dependent viabilities of Drosophila pseudoobscura karyotypes. Heredity 56 7 17
65. AnholtRRLymanRFMackayTF 1996 Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster. Genetics 143 293 301
66. MiquelJLundgrenPRBenschKGAtlanH 1976 Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev 5 347 370
67. GongWJGolicKG 2006 Loss of Hsp70 in Drosophila is pleiotropic, with effects on thermotolerance, recovery from heat shock and neurodegeneration. Genetics 172 275 286
68. GibbsAGChippindaleAKRoseMR 1997 Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J Exp Biol 200 1821 1832
69. FaulFErdfelderELangAGBuchnerA 2007 G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39 175 191
70. XiaXQMcClellandMPorwollikSSongWCongX 2009 WebArrayDB: cross-platform microarray data analysis and public data repository. Bioinformatics 25 2425 2429
71. YangYHDudoitSLuuPLinDMPengV 2002 Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30 e15
72. SmythGK 2005 Limma: linear models for microarray data. GentlemanRCareyVDudoitSIrizarryRHuberW Bioinformatics and Computational Biology Solutions using R and Bioconductor New York Springer 397 420
73. BenjaminiYHochbergY 1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B 57 289 300
74. HothornTBretzFWestfallP 2008 Simultaneous inference in general parametric models. Biom J 50 346 363
75. Huang daWShermanBTLempickiRA 2009 Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 44 57
76. OhlerU 2006 Identification of core promoter modules in Drosophila and their application in accurate transcription start site prediction. Nucleic Acids Res 34 5943 5950
77. HaiderSBallesterBSmedleyDZhangJRiceP 2009 BioMart Central Portal–unified access to biological data. Nucleic Acids Res 37 W23 27
78. LawsonDArensburgerPAtkinsonPBesanskyNJBruggnerRV 2009 VectorBase: a data resource for invertebrate vector genomics. Nucleic Acids Res 37 D583 587
79. TamuraKPetersonDPetersonNStecherGNeiM 2011 MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol
80. ThompsonJDHigginsDGGibsonTJ 1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
81. WhelanSGoldmanN 2001 A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18 691 699
82. EngstromPGFredmanDLenhardB 2008 Ancora: a web resource for exploring highly conserved noncoding elements and their association with developmental regulatory genes. Genome Biol 9 R34
83. GauntMWMilesMA 2002 An insect molecular clock dates the origin of the insects and accords with palaeontological and biogeographic landmarks. Mol Biol Evol 19 748 761
84. KrzywinskiJGrushkoOGBesanskyNJ 2006 Analysis of the complete mitochondrial DNA from Anopheles funestus: an improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol Phylogenet Evol 39 417 423
85. VieiraFGSanchez-GraciaARozasJ 2007 Comparative genomic analysis of the odorant-binding protein family in 12 Drosophila genomes: purifying selection and birth-and-death evolution. Genome Biol 8 R235
86. KlemenzRWeberUGehringWJ 1987 The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res 15 3947 3959
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 2
-
Všechny články tohoto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



