-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHomeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
The mammalian Msx homeobox genes, Msx1 and Msx2, encode transcription factors that control organogenesis and tissue interactions during embryonic development. We observed overlapping expression of these factors in uterine epithelial and stromal compartments of pregnant mice prior to embryo implantation. Conditional ablation of both Msx1 and Msx2 in the uterus resulted in female infertility due to a failure in implantation. In these mutant mice (Msx1/2d/d), the uterine epithelium exhibited persistent proliferative activity and failed to attach to the embryos. Gene expression profiling of uterine epithelium and stroma of Msx1/2d/d mice revealed an elevated expression of several members of the Wnt gene family in the preimplantation uterus. Increased canonical Wnt signaling in the stromal cells activated β-catenin, stimulating the production of a subset of fibroblast growth factors (FGFs) in these cells. The secreted FGFs acted in a paracrine manner via the FGF receptors in the epithelium to promote epithelial proliferation, thereby preventing differentiation of this tissue and creating a non-receptive uterus refractory to implantation. Collectively, these findings delineate a unique signaling network, involving Msx1/2, Wnts, and FGFs, which operate in the uterus at the time of implantation to control the mesenchymal-epithelial dialogue critical for successful establishment of pregnancy.
Published in the journal: . PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002500
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002500Summary
The mammalian Msx homeobox genes, Msx1 and Msx2, encode transcription factors that control organogenesis and tissue interactions during embryonic development. We observed overlapping expression of these factors in uterine epithelial and stromal compartments of pregnant mice prior to embryo implantation. Conditional ablation of both Msx1 and Msx2 in the uterus resulted in female infertility due to a failure in implantation. In these mutant mice (Msx1/2d/d), the uterine epithelium exhibited persistent proliferative activity and failed to attach to the embryos. Gene expression profiling of uterine epithelium and stroma of Msx1/2d/d mice revealed an elevated expression of several members of the Wnt gene family in the preimplantation uterus. Increased canonical Wnt signaling in the stromal cells activated β-catenin, stimulating the production of a subset of fibroblast growth factors (FGFs) in these cells. The secreted FGFs acted in a paracrine manner via the FGF receptors in the epithelium to promote epithelial proliferation, thereby preventing differentiation of this tissue and creating a non-receptive uterus refractory to implantation. Collectively, these findings delineate a unique signaling network, involving Msx1/2, Wnts, and FGFs, which operate in the uterus at the time of implantation to control the mesenchymal-epithelial dialogue critical for successful establishment of pregnancy.
Introduction
Successful implantation is dependent on a timely progression of a series of biological events during which the embryo undergoes functional interactions with the uterus prepared by the maternal factors [1]–[4]. During implantation, various tissue compartments within the uterus, including luminal epithelium, glandular epithelium, and stroma, undergo sequential proliferation and differentiation as the embryo attaches to the luminal epithelium and invades into the stroma. In mice, the luminal and glandular epithelial cells are initially in a proliferative state on days 1 and 2 of pregnancy. As pregnancy proceeds, these cells exit from the cell cycle and enter a differentiation program that allows their transition to a receptive state. The stromal cells adjacent to the epithelium begin to proliferate on day 3 and this proliferation becomes widespread following embryo attachment to the receptive luminal epithelium on day 4 of pregnancy [1]–[4]. As the embryos invade through the luminal epithelium into the stromal compartment, the stromal cells differentiate into secretory decidual cells, which support further growth and development of the implanted embryos until placentation ensues [1]–[4].
Extensive research over the past decade, using genetically altered mutant mouse models, has identified several factors that critically regulate uterine function in the preimplantation or postimplantation phases of pregnancy [5]–[11]. However, there is only limited insight into the molecular mechanisms and signaling pathways that interconnect the various cellular compartments of the uterus to achieve receptivity to embryo implantation. Recent studies in our laboratory indicated that a subset of fibroblast growth factors (FGFs) produced by the stromal cells act in a paracrine manner to promote luminal epithelial proliferation. The transcription factor Hand2 suppresses the production of these FGFs and inhibits luminal epithelial proliferation at the time of implantation [11]. Studies by Lee et al identified Indian hedgehog (IHH) as an epithelial paracrine factor that acts on the stromal cells to regulate their differentiation [7]. These studies support the concept that maternal competency for implantation is determined by a critical exchange of diffusible signals between the epithelial and stromal compartments, allowing transition of these tissues to proper functional states that permit embryo attachment and invasion. Identification of epithelial or stromal transcription factors and their downstream molecular pathways that control these signals is essential for a clear understanding of the molecular basis of implantation.
It was previously reported that the messenger RNA encoding the homeobox transcription factor MSX1 is expressed in the peri-implantation uterus [12]. We observed that MSX2, another member of MSX family, is expressed in a similar pattern in the epithelial and stromal compartments of the preimplantation uterus during days 1–4 of pregnancy. Expressions of MSX1 and MSX2 were markedly reduced in both compartments following embryo attachment. These findings raised the possibility that the pathways regulated by MSX1 or MSX2 or both regulate the receptive state of the preimplantation uterus. Global deletion of Msx1 and Msx2 gene is embryonic lethal, necessitating the development of conditional deletion of these genes to study their functions during implantation. Conditional ablation of either Msx1 or Msx2 showed only modest impairment in embryo implantation, resulting in sub-fertility of the mutant mice. On the other hand, conditional ablation of both Msx1 and Msx2 in mouse uterus led to complete infertility due to a failure of embryo attachment to the uterine epithelium. We further established that Msx1 and Msx2 function by suppressing the expression of several members of the Wnt family. In Msx1/Msx2-null uterus, continued expression of a subset of WNTs enhances β-catenin signaling in the stroma, which in turn induces the expression of specific members of the fibroblast growth factor (FGF) family in this compartment. One or more of these FGFs act via the FGF receptors in the glandular and luminal epithelial tissues to promote proliferation and prevent differentiation. Lack of differentiation of the glandular epithelial cells results in the failure to express critical factors, such as the leukemia inhibitory factor (LIF), which are critical for implantation. Additionally, undifferentiated luminal epithelial cells exhibit persistent expression of MUC-1, a glycoprotein that serves as a maternal barrier to the attachment of the embryo. This study, therefore, delineated a novel signaling network downstream of Msx1 and Msx2, mediating the stromal-epithelial crosstalk critical for successful establishment of pregnancy.
Results
Msx1 and Msx2 are expressed in the preimplantation uterus
The spatio-temporal profiles of mRNAs and proteins corresponding to Msx1 and Msx2 were examined in the mouse uterus during the preimplantation phase by real-time PCR and immunohistochemistry (IHC), respectively. The expression of both Msx1 and Msx2 mRNAs followed a similar pattern: an increase on days 2–3 of pregnancy followed by a decline on day 4 at the time of embryo implantation (Figure 1A, left panel; Figure 1B, left panel). Both MSX1 and MSX2 proteins were expressed in uterine epithelium on day 1 of pregnancy (Figure 1A, panel a; Figure 1B, panel a). The expression of these proteins increased on days 2 and 3 of pregnancy and was localized to both glandular epithelium and stroma (Figure 1A, panels b and c; Figure 1B, panels b and c). The expression of MSX1 and MSX2 proteins then declined on day 4 at the time of embryo implantation and were undetectable on day 5 (Figure 1A, panels d & e; Figure 1B, panels d & e). Therefore, similar expression of Msx1 and Msx2 was observed in the uterine epithelial and stromal compartments in the preimplantation phase.
Fig. 1. Expression of Msx1 and Msx2 in the uterus during early pregnancy. 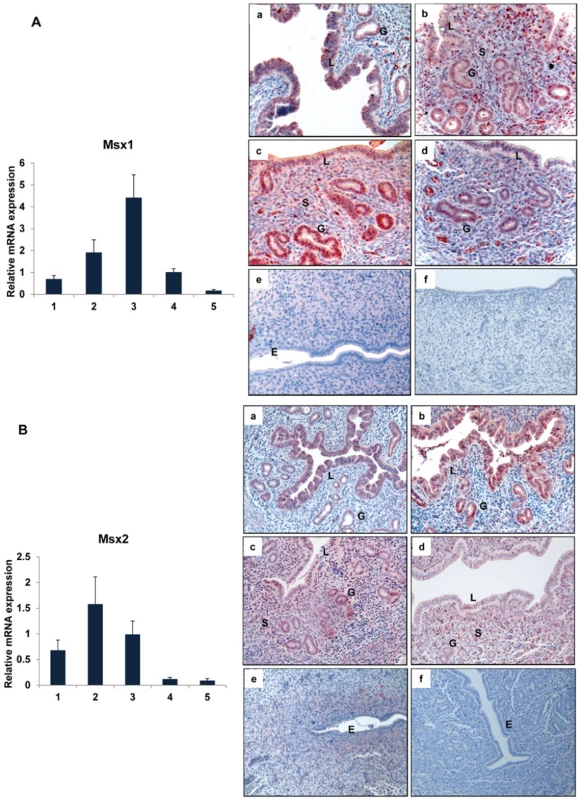
Real-time PCR was performed to monitor the expression of mRNAs corresponding to Msx1 and Msx2 in uterus on days 1 to 5 of gestation. The relative levels of gene expression on different days of pregnancy were determined by setting the expression level of Msx1 mRNA (A, Left panel) and Msx2 mRNA (B, Left panel) on day 1 of pregnancy at 1.0. Rplp0, encoding a ribosomal protein, was used to normalize the level of RNA. Uterine sections from day 1 to day 5 (a–e) of pregnancy were subjected to immunohistochemical analysis using anti-MSX1 (A, Right panel) and anti-MSX2 (B, Right panel) antibodies. Panel f shows uterine sections from day 3 pregnant mice treated with non-immune IgG. L, G and S indicate luminal epithelium, glandular epithelium and stroma, respectively. Ablation of Msx1 and Msx2 in the uterus leads to infertility
To investigate the function of Msx1 and Msx2 in the uterus, we employed the Cre-LoxP strategy to create conditional single knockout of Msx1 or Msx2 or double knockout of Msx1 and Msx2 in the uteri of adult mice. Transgenic mice expressing Cre under the control of progesterone receptor (PR) promoter was previously used to ablate “floxed” genes selectively in cells expressing PR, including uterine cells [7]–[11]. We, therefore, crossed the PR-Cre mice with mice harboring the “floxed” Msx1 or Msx2 or both to create Msx1d/d, Msx2d/d or Msx1d/dMsx2d/d mice. We confirmed the deletion of Msx1 or Msx2 in the uteri of these mutant mice by real-time PCR and IHC. As shown in Figure S1, neither Msx1/Msx2 mRNA nor MSX1/MSX2 protein was detected in uteri of Msx1d/dMsx2d/d mice on day 3 of pregnancy, confirming successful abrogation of both Msx genes in uteri of Msx1d/dMsx2d/d mice.
A six-month breeding study demonstrated that the single mutant females, Msx1d/d and Msx2d/d, are subfertile but the double mutant females, Msx1d/dMsx2d/d, are completely infertile (Table 1). The subfertility of the single mutant Msx1d/d or Msx2d/d was likely due to compensation of the function of one Msx gene by the other. Indeed, in Msx1-null uteri, the level of Msx2 expression in the uterus was markedly elevated (Figure S2).
Tab. 1. Ablation of uterine Msx1 and Msx2 leads to female infertility. 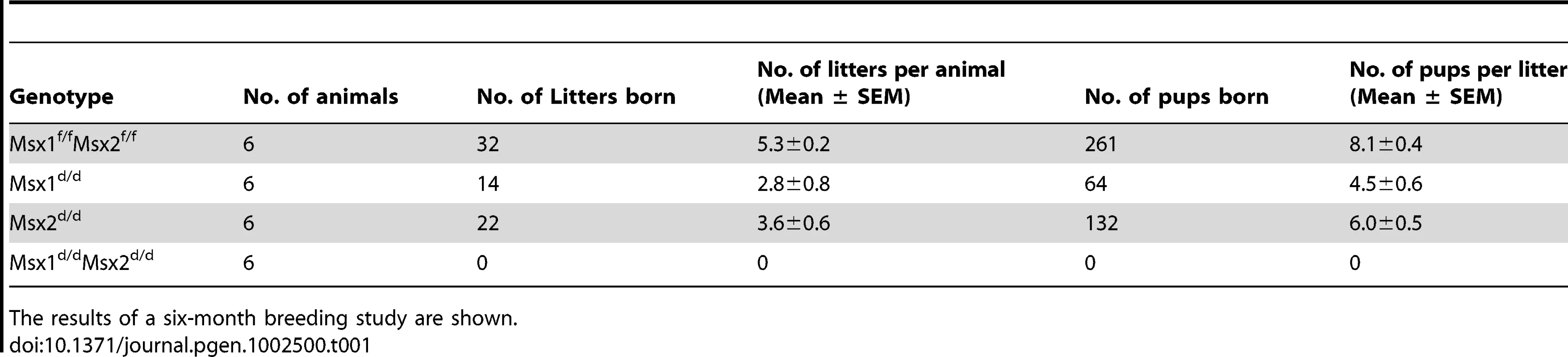
The results of a six-month breeding study are shown. While Msx1f/fMsx2f/f mice exhibited normal litter size and pregnancy rates, the Msx1d/dMsx2d/d females failed to become pregnant when mated with wild-type males. However, copulatory plugs were observed upon mating, indicating normal mating behavior. To investigate the cause of infertility in Msx1d/dMsx2d/d females, we examined their ovarian functions by inducing superovulation. Prepubertal Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice were treated with a regimen of gonadotropin hormones as described in Materials and Methods. We observed that, upon gonadotropin stimulation, the number of eggs produced by Msx1d/dMsx2d/d was comparable to that produced by the Msx1f/fMsx2f/f females (Figure S3A), indicating that ovulation is not affected in the absence of Msx1 and Msx2. To further examine the ovulation and fertilization in these mice under normal physiological conditions, blastocysts were recovered from uteri of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy prior to implantation. Once again, no significant difference was found in either the number or the morphology of the embryos recovered from Msx1f/fMsx2f/f and Msx1d/dMsx2d/d uteri (Figure S3B and Figure 3C). In further support of normal ovarian activity, the serum levels of progesterone and estrogen were comparable in Msx1f/fMsx2f/f and Msx1d/dMsx2d/d females on day 4 of pregnancy (Figure S3D and Figure S3E). Collectively, these results suggested that the infertility of Msx1d/dMsx2d/d females is not due to impairment in the hypothalamic-pituitary-ovarian axis or lack of fertilization, but is likely due to defective implantation or pregnancy failure following implantation.
Ablation of Msx1 and Msx2 in the uterus affects embryo attachment to the luminal epithelium
In mice, the attachment of the embryos to the uterine wall initiates the process of implantation. This is accompanied by increased vascular permeability at the implantation sites, which can be scored visually as distinct blue bands following an intravenous injection of Chicago blue dye [13]. As shown in Figure 2A, Msx1f/fMsx2f/f mice displayed distinct implantation sites on day 5 of pregnancy. In contrast, the Msx1d/dMsx2d/d females did not show any sign of implantation. Implanted embryos were also assessed on days 6 and 7 of pregnancy by visual inspection. Our results indicated that implantation sites are absent in Msx1d/dMsx2d/d uteri (Figure 2A).
Fig. 2. Lack of uterine Msx1 and Msx2 causes implantation failure. 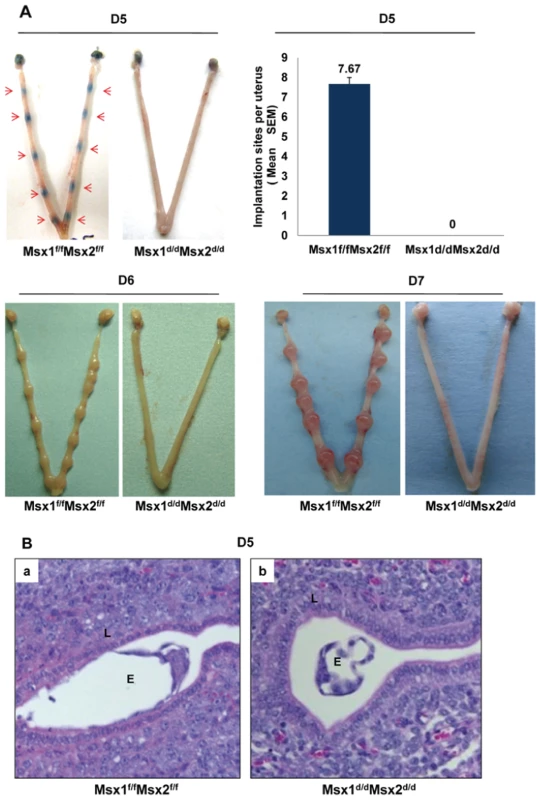
A. Embryo implantation sites were examined in Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice by the vascular permeability assay, which can be scored as distinct blue bands (red arrows) following an injection of Chicago blue dye on day 5 of pregnancy (D5, n = 6) or direct eye-visualization of implanted embryo on day 6 (D6, n = 4) and on day 7 (D7, n = 4) of pregnancy. The graph represents the quantification of implantation sites in Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 5 of pregnancy. B. Failure of embryo attachment in Msx1d/dMsx2d/d uteri. Histological analysis of uterine sections obtained from Msx1f/fMsx2f/f (a) and Msx1d/dMsx2d/d (b) mice on day 5 (n = 3) of pregnancy by Hematoxylin and Eosin staining. Note the intimate contact between embryo and luminal epithelium in Msx1f/fMsx2f/f mice and the free floating embryo in the uterine lumen of Msx1d/dMsx2d/d mice. L and E indicate luminal epithelium and embryo respectively. Histological analysis of Msx1f/fMsx2f/f females on day 5 of pregnancy showed, as expected, a close contact of embryonic trophectoderm with uterine luminal epithelium (Figure 2B, panel a). In contrast, in Msx1d/dMsx2d/d uteri, embryos did not attach to luminal epithelium. Instead, blastocysts remained free-floating in the lumen and were readily recovered by uterine flushing of the Msx1d/dMsx2d/d females (Figure 2B, panel b). Taken together, these results indicated that the loss of Msx1 and Msx2 expression in the uterus resulted in the inability of the luminal epithelium to acquire competency for embryo implantation.
Estrogen receptor activity is elevated in uterine epithelium of Msx1d/dMsx2d/d mice at the time of implantation
In mice, the window of uterine receptivity is critically regulated by the steroid hormones 17β-estradiol (E) and progesterone (P), acting through their cognate nuclear receptors. We, therefore, examined the expression levels of progesterone receptor (PGR), estrogen receptor alpha (ESR1), and their downstream genes in the uteri of Msx1d/dMsx2d/d mice by immunohistochemistry and real-time PCR analyses. As shown in Figure 3A, the expression levels of PGR (top panel) and ESR1 (middle panel) proteins in the luminal epithelium or stromal compartment of Msx1d/dMsx2d/d uteri were comparable to those of Msx1f/fMsx2f/f controls. However, we noted that the expression of the transcriptionally active form of ESR1, phosphorylated at serine 118 [14], was markedly up-regulated in the luminal epithelial cells of Msx1d/dMsx2d/d uteri, indicating that ER activity is elevated in the uterine epithelia of these mice (lower panel). This observation indicated that the pathways directed by Msx1/Msx2 play an important role in controlling the ESR1 activity, which is normally suppressed in uterine epithelium during the receptive phase [15]–[17]. Consistent with this up-regulation of transcriptional activity of ESR1, expression of mRNAs corresponding to well-known E-regulated genes, such as lactotransferrin (Ltf) [18], chloride channel, calcium activated, family member 3 (Clca3) [19], lipocalin 2 [20] and mucin 1 (Muc-1) [21], was significantly elevated in uterine epithelium of Msx1d/dMsx2d/d uteri on day 4 of pregnancy (Figure 3B).
Fig. 3. Enhanced ESR1 activity in the luminal epithelium of Msx1d/dMsx2d/d uteri. 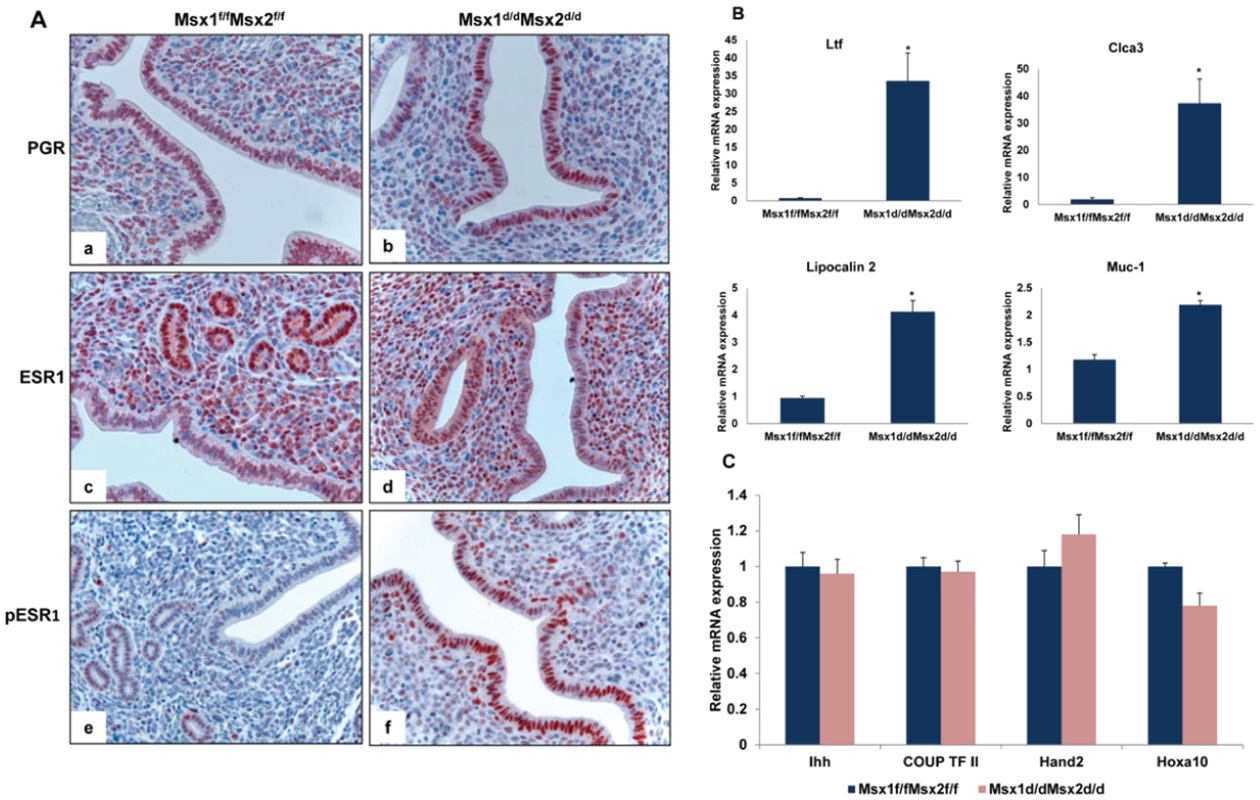
A. Uterine sections obtained from Msx1f/f Msx2f/f (left panel) and Msx1d/dMsx2d/d (right panel) mice on day 4 of pregnancy were subjected to IHC using antibodies against PGR (top panel, a and b), ESR1 (middle panel, c and d) and phospho-ESR1 (lower panel, e and f). B. Real-time PCR was performed to analyze the expression of E-regulated genes, lactotransferrin (Ltf), Clca3, lipocalin2 and Muc-1 in uteri of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy. The level of Ck18 was used as internal control to normalize gene expression. The data are represented as the mean fold induction ± SEM, *p<0.05. C. Real-time PCR was performed to analyze the expression of P-regulated genes, Ihh, COUP-TF II, Hand2 and Hoxa10, in uteri of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy. The level of Rplp0 or Ck18 was used as internal control to normalize gene expression. In contrast, the expression of Ihh, a P-responsive gene in uterine epithelium [7] remained unaltered in Msx1d/dMsx2d/d uteri. Additionally, the mRNA levels of Hand2 [11] and Hoxa10 [3], well-known P-regulated genes in uterine stroma, and that of chicken ovalbumin upstream promoter-transcription factor II (COUP-TF II), a downstream target of IHH in the uterine stroma [9], were unaffected in the uteri of Msx1d/dMsx2d/d mice (Figure 3C). These results indicated that the loss of Msx1 and Msx2 did not impact on the transcriptional activity of PGR, but resulted in an enhancement of the epithelial ESR1 function.
A hallmark of the receptive state of normal pregnant uterus is the cessation of epithelial cell proliferation prior to implantation [1]–[4], [22]. Therefore, in Msx1f/fMsx2f/f mice, immunostaining of Ki67, a cell proliferation marker, was undetectable in the uterine luminal and glandular epithelium on day 4 of pregnancy (Figure 4A, panels a and c). However, uterine sections of Msx1d/dMsx2d/d mice exhibited robust immunostaining for Ki67 in the luminal and glandular epithelia (Figure 4A, panels b and d), indicating persistent epithelial cell proliferation on day 4 in the absence of Msx1 and Msx2. Previous studies indicated that the ability of the glandular epithelium to undergo differentiation and produce factors, including leukemia inhibitory factor (LIF) [23], Foxa2 [24], and Spink3 [25], is critical for implantation. As shown in Figure 4B, the expression of these factors was drastically reduced in uteri deficient of Msx1 and Msx2. Collectively, these findings indicated that persistent proliferation of luminal and glandular epithelia results in impaired epithelial transition from a proliferative to a non-proliferative state that allows proper differentiation. This impairment is a major contributor to implantation failure in Msx1d/dMsx2d/d mice.
Fig. 4. Enhanced proliferation in the uterine epithelium and lack of receptivity in Msx1d/dMsx2d/d mice. 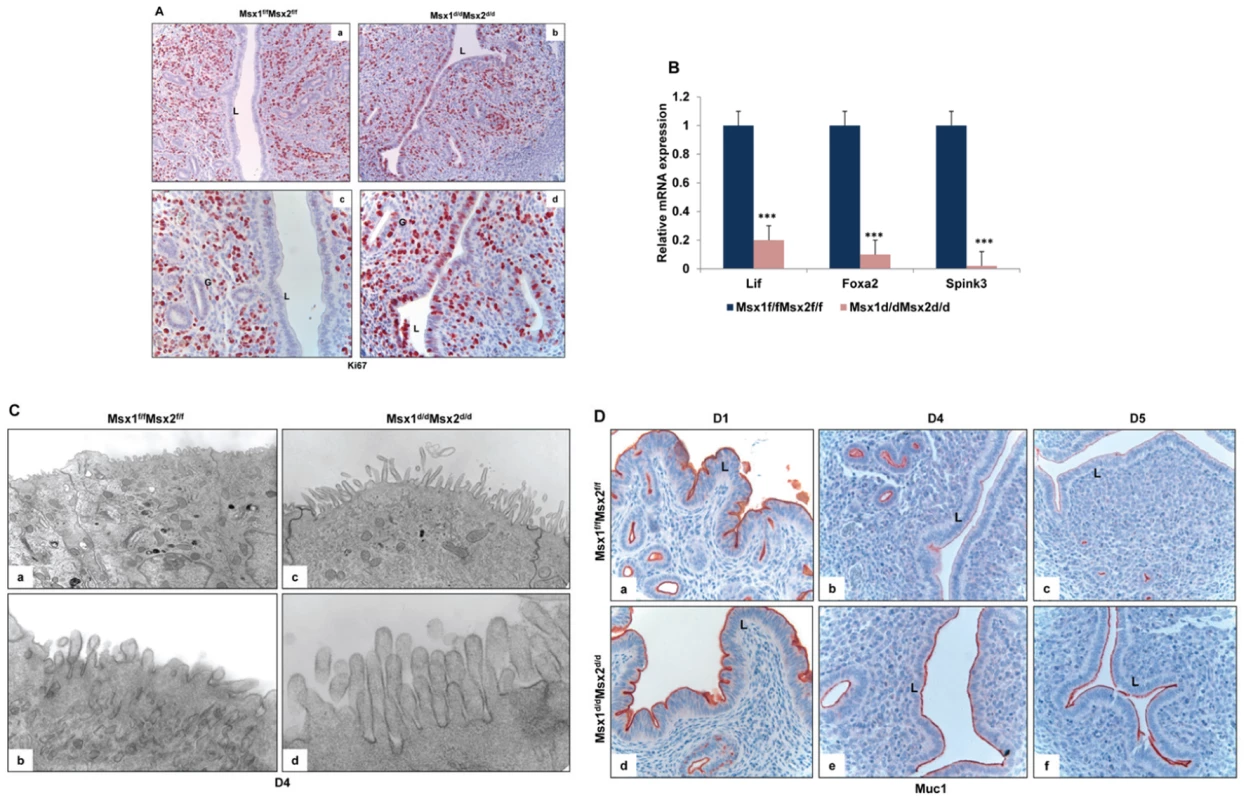
A. Immunohsitochemical localization of Ki67 in the uterine sections of Msx1f/fMsx2f/f (left panel, a and c) and Msx1d/dMsx2d/d (right panel, b and d) mice on day 4 of pregnancy. Panels a and b indicate lower magnification (20×) and c and d indicate higher magnification (40×). L and G indicate luminal epithelium and glandular epithelium respectively. B. Real-time PCR was performed to analyze the expression of glandular factors, Lif, Foxa2 and Spink3 in uteri of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy. The level of Ck18 was used as internal control to normalize gene expression. The data are represented as the mean fold induction ± SEM, ***p<0.0001. C. Transmission electron microscopy of uterine sections obtained from Msx1f/f Msx2f/f (left panel, a and b) and Msx1d/dMsx2d/d (right panel, c and d) mice on day 4 of pregnancy. Panels a and c indicate lower magnification (5Kx) and b and d indicate higher magnification (30Kx). D. Immunohistochemical analysis of Muc-1 expression in the uterine sections of Msx1f/fMsx2f/f (upper panel) and Msx1d/dMsx2d/d (lower panel) mice on day 1 (a and d), day 4 (b and e) and day 5 (c and f) of pregnancy. L indicates luminal epithelium. Another important parameter of receptive uterus is the membrane transformation of uterine epithelium at the time of implantation. The presence of long microvilli, containing a thick layer of glycoprotein known as the glycocalyx, on the uterine epithelium is indicative of the nonreceptive stage. A marked flattening of these microvilli occurs in the receptive phase prior to implantation [26]. Transmission electron microscopy (TEM) revealed that, in contrast to the control epithelium, the epithelia of Msx1d/dMsx2d/d uteri fail to undergo appropriate remodeling to promote microvilli flattening, indicating impaired uterine receptivity in these mice (Figure 4C).
The impaired functional state of uterine epithelium in Msx1d/dMsx2d/d mice was further confirmed when we analyzed the expression of MUC-1 protein, a major component of the endometrial glycocalyx, during early pregnancy. The expression status of MUC-1 is considered an important indicator of uterine receptivity [21]. As the luminal epithelium differentiates and the uterus achieves receptivity, MUC-1 expression is down regulated in this tissue. Persistent MUC-1 expression is indicative of a non-receptive uterus, which is not conducive to embryo implantation. As shown in Figure 4D, prominent expression of MUC-1 was detected in the uterine epithelia of control Msx1f/fMsx2f/f mice on day 1 of pregnancy (panel a). As the pregnancy advanced to days 4 (panel b) and 5 (panel c), Muc-1 was progressively down regulated in uterine epithelia of these mice, consistent with the attainment of receptive status. In contrast, an intense expression of MUC-1 was observed in uteri of Msx1d/dMsx2d/d mice on days 4 and 5 (panels d–f). Therefore, elevated epithelial ESR1 signaling, which likely triggered persistent expression of MUC-1 in luminal epithelium, disrupted uterine receptivity, resulting in implantation failure in Msx1d/dMsx2d/d mice.
Msx1/Msx2 regulates WNT and FGF signaling in the uterus
To gain insights into the mechanisms underlying the implantation defect of uteri lacking Msx1 and Msx2, we isolated luminal epithelial and stromal cells from Msx1f/fMsx2f/f and Msx1d/dMsx2d/d uteri on day 4 of pregnancy and performed compartment-specific gene expression profiling, using Affymetrix Mouse GeneChip arrays. Interestingly, our study revealed up-regulation of two distinct classes of signaling factors, WNTs and FGFs, in Msx1d/dMsx2d/d uteri compared to Msx1f/fMsx2f/f uteri. The microarray data (GEO accession #GSE30969) were validated by real-time PCR analysis. In the epithelial compartment of Msx1d/dMsx2d/d uteri, we observed stimulated expression of mRNAs corresponding to several Wnts, including Wnt4, Wnt7a and Wnt7b (Figure 5A). In the stromal cells of Msx1d/dMsx2d/d uteri, we observed marked up-regulation of Wnt4 and Wnt5a mRNAs (Figure 5B). In addition, the levels of mRNAs encoding several members of the FGF family, such as Fgf1, Fgf10, Fgf18 and Fgf21, were elevated in uterine stromal cells as a consequence of deletions of Msx1 and Msx2 (Figure 5C). The expression of mRNAs corresponding to several other FGF family members as well as other growth factors, such as HBEGF, EGF, IGF-1, and, HGF which are expressed in the uterus during pregnancy, was not significantly altered in the uterine stroma of Msx1d/dMsx2d/d mice (Figure 5C and Figure S4).
Fig. 5. Wnt/β-catenin signaling controls FGF synthesis in uterine stromal cells. 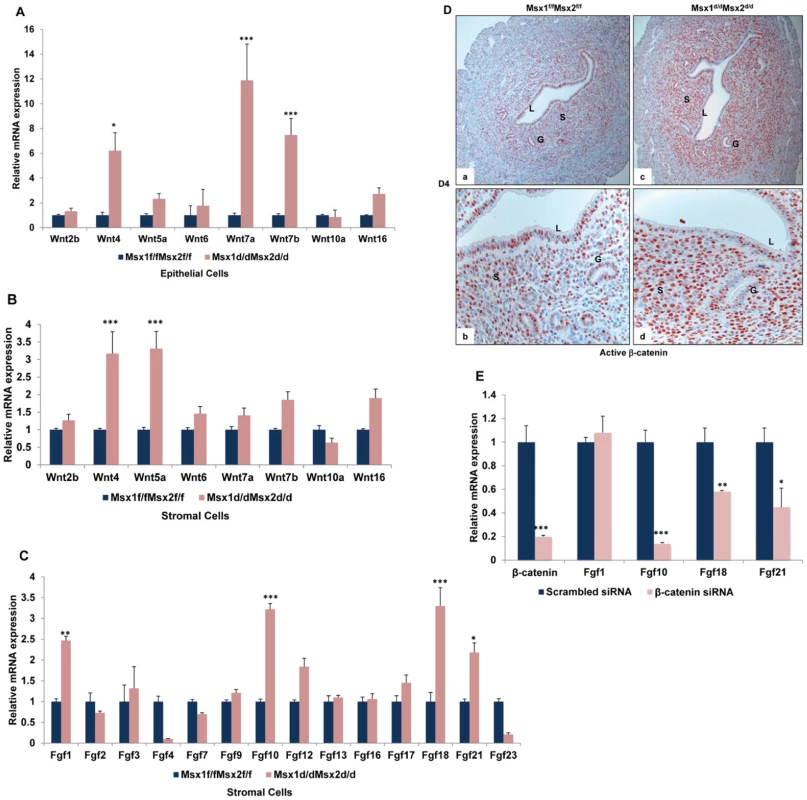
A. Real-time PCR was performed to analyze the expression of Wnt ligands in uterine epithelial cells of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy. The level of Ck18 was used as internal control to normalize gene expression. The data are represented as the mean fold induction ± SEM, *p<0.01, ***p<0.0001. B. Real-time PCR was performed to analyze the expression of Wnt ligands in uterine stromal cells of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy. C. Real-time PCR was performed to analyze the expression of Fgf family members in uterine stromal cells of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy. The level of Rplp0 was used as internal control to normalize gene expression. The data are represented as the mean fold induction ± SEM, *p<0.01, **p<0.001, ***p<0.0001. D. The level of active β-catenin in uterine sections of Msx1f/fMsx2f/f (left panel) and Msx1d/dMsx2d/d (right panel) mice on day 4 of pregnancy was analyzed by IHC. (Magnification: a and c: 10×, b and d: 40×) E. Primary stromal cells were isolated from uteri of Msx1d/dMsx2d/d mice on day 3 of pregnancy and transfected with siRNA targeted to the β-catenin mRNA. Total RNA was isolated 24 h after transfection to analyze the expression of Fgf family members by Real-time PCR. The level of Rplp0 was used as an internal control to normalize gene expression. The data are represented as the mean fold induction ± SEM, *p<0.01, **p<0.001, ***p<0.0001. We next investigated whether the increased expression of the Wnt ligands in the uteri of Msx1d/dMsx2d/d mice is translated into increased activation of the Wnt signaling pathway. Wnt signals are transduced via the canonical Wnt/β-catenin-dependent pathway or the non-canonical β-catenin-independent pathways [27]–[29]. When we examined the expression of active β-catenin in uterine sections of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy, we noted comparable levels of nuclear expression of active β-catenin in luminal and glandular epithelium in both genotypes (Figure 5D). However, a marked increase in the level of nuclear β-catenin was observed in the stromal cells of Msx1Msx2-null uteri, indicating that canonical β-catenin signaling is markedly enhanced in the Msx1Msx2-ablated stroma.
Interestingly, previous studies indicated that the production of FGFs, particularly FGF10 and FGF18, is stimulated downstream of canonical Wnt signaling during certain cellular processes, such as chick embryo development, bone development and human hepatocellular carcinoma [30]–[32], raising the possibility that the enhanced β-catenin signaling seen in uterine stromal cells of Msx1d/dMsx2d/d mice may drive the increased FGF synthesis in these cells. To test this possibility, primary stromal cells were isolated from uteri of Msx1d/dMsx2d/d mice on day 3 of pregnancy and transfected with siRNA targeted specifically to the β-catenin mRNA. We observed that treatment with this siRNA resulted in more than 80% reduction in β-catenin mRNA expression compared to cells transfected with control (scrambled) siRNA (Figure 5E). Most importantly, as shown in Figure 5E, siRNA-mediated down regulation of β-catenin in the stromal cells led to a significant reduction in expression of FGF10, FGF18, and FGF21. However, the expression of FGF1 remained unaltered in cells treated with β-catenin siRNA. These results indicated that canonical Wnt signaling via β-catenin regulates the expression of a specific subset of FGF family members in the uterine stromal cells.
We next investigated whether the increased production of FGFs downstream of Wnt signaling leads to enhanced FGF receptor (FGFR) signaling in the uteri of Msx1d/dMsx2d/d mice. Stimulation of the cell surface FGFRs by FGF ligands leads to phosphorylation of specific tyrosine residues in a critical docking protein, FGFR substrate 2 (FRS2), which guides the assembly of distinct multi-protein complexes, leading to the activation of either MAP kinase or AKT signaling cascades [33]–[35]. We, therefore, investigated the state of activation of the FGFR signaling pathway in the uteri of Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice by monitoring the level of phospho-FRS2. We observed only low level of phospho-FRS2 in the uterine luminal or glandular epithelium or stroma of Msx1f/fMsx2f/f mice on day 4 of pregnancy (Figure 6A, panels a–c). In contrast, a marked elevation in the level of phospho-FRS2 was observed specifically in the luminal and glandular epithelium, but not in the stroma (Figure 6A, panels d–f) of Msx1Msx2-null uteri, indicating that FGFR signaling is increased in uterine epithelium in the absence of Msx1/Msx2. Since the FGFs are produced in the stroma of these mutant uteri, this finding suggests that they act in a paracrine fashion via the FGFRs on the epithelial cells.
Fig. 6. Enhanced FGFR signaling in the epithelium of Msx1d/dMsx2d/d uteri. 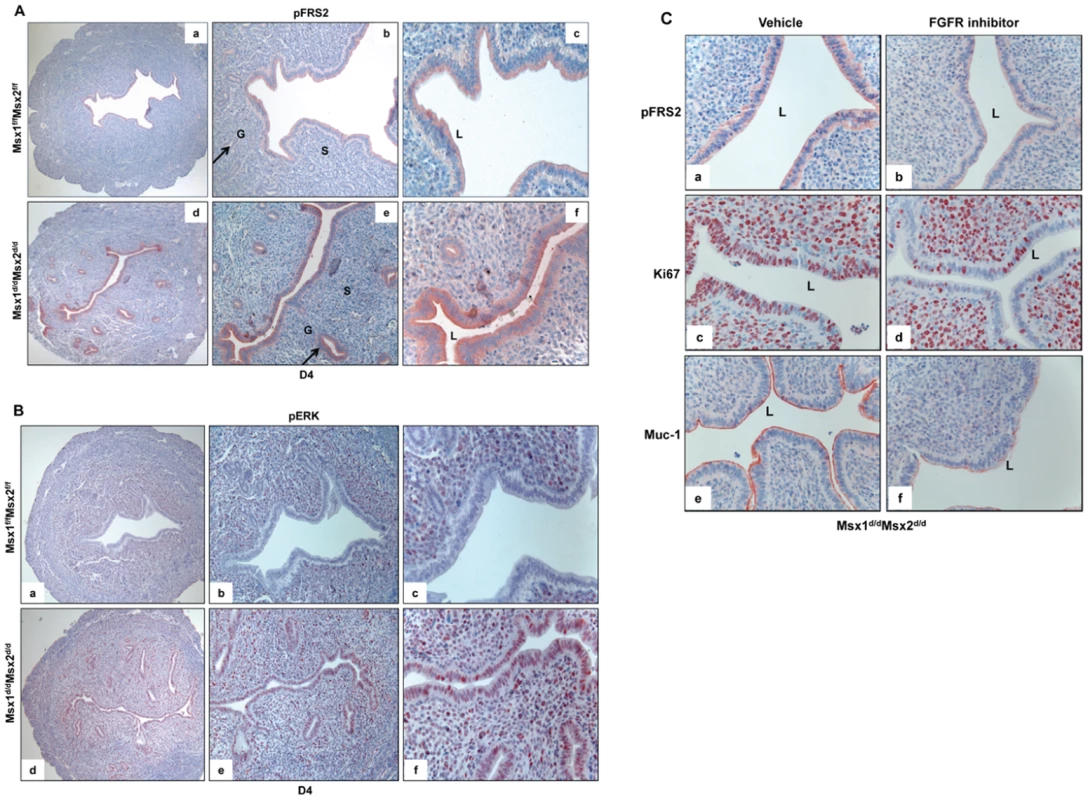
A. The level of p-FRS2 was examined in the uterine sections of Msx1f/fMsx2f/f (upper panel) and Msx1d/dMsx2d/d (lower panel) mice on day 4 of pregnancy by immunohistochemistry. Magnification: a and d: 10×, b and e: 20×, c and f: 40×. B. The level of p-ERK was examined in the uterine sections of Msx1f/fMsx2f/f (upper panel) and Msx1d/dMsx2d/d (lower panel) mice on day 4 of pregnancy by immunohistochemistry. Magnification: a and d: 10×, b and e: 20×, c and f: 40×. L, G and S indicate luminal epithelium, glandular epithelium, and stroma respectively. C. FGFR-specific inhibitor PD173074 was applied to one uterine horn of Msx1d/dMsx2d/d (n = 3) mice on day 3 of pregnancy. The other horn served as vehicle-treated control. Uterine horns were collected on day 4 morning and sections were subjected to immunohistochemistry to detect p-FRS2, Ki67, and Muc-1. The kinases ERK1/2 and/or PI3K/AKT are known to be activated downstream of FGF receptor signaling [33]. We, therefore, investigated whether these pathways were activated in the epithelia of Msx1Msx2-ablated uteri. As shown in Figure 6B, phospho-ERK1/2 (pERK) was undetectable in the uterine epithelium of Msx1f/fMsx2f/f mice on day 4 of pregnancy (panels a–c). However, a dramatic increase in the immunostaining of pERK1/2 was seen in epithelium of Msx1Msx2-null uteri on day 4 of pregnancy (panels d–f). In contrast, the expression of phospho-AKT was undetectable in both of these genotypes (data not shown), suggesting that the ERK1/2 pathway, rather than the PI3K/AKT pathway, is the key downstream mediator of enhanced FGFR signaling in Msx1Msx2-null uteri.
To examine whether the elevated mitogenic activity in the luminal epithelium of Msx1d/dMsx2d/d uteri on day 4 of pregnancy is indeed a result of the enhanced FGF signaling, we administered PD173074, a FGFR-specific inhibitor [36], or vehicle into uterine horns of Msx1d/dMsx2d/d mice in the pre-implantation phase. As shown in Figure 6C, the epithelia of vehicle-treated uterine horns of these mice showed strong expression of phospho-FRS2 on day 4 of pregnancy (panel a). Treatment with the FGFR inhibitor led to a marked reduction in the level of phospho-FRS2 in the uterine epithelium (Figure 6C, panel b). Concomitant with this down regulation of FGFR signaling, we observed a decline in the proliferative activity of Msx1Msx2-null uterine epithelia as well as down-regulation of MUC-1 expression (Figure 6C, panels c–f). Collectively, these results are consistent with the hypothesis that increased FGF production, downstream of Wnt-β-catenin pathway in Msx1Msx2-null uterine stroma, stimulates epithelial proliferation by activating FGFR-ERK1/2 signaling pathway. The proliferative epithelium fails to undergo differentiation, resulting in persistent expression of MUC-1, which acts as a major barrier to embryo attachment and implantation.
Discussion
Members of Msx family of homeobox genes, comprising of Msx1, Msx2, and Msx3, are critical regulators of tissue morphogenesis [37]–[39]. While Msx1 and Msx2 are expressed in several tissues during embryonic development, Msx3 expression is mostly restricted to neural tube [40], [41]. The present study describes the expression of Msx1 and Msx2 in adult uterus and addresses their roles in female reproduction. Using mutant mouse models harboring conditional deletion of Msx1 and/or Msx2 in the uterus, we established that these factors play critical roles in regulating uterine function during implantation. Due to the overlapping spatio-temporal expression of these two transcription factors in uterine epithelium and stroma, it is not surprising that female mice carrying deletion of either Msx1 or Msx2 are subfertile, while those lacking both Msx1 and Msx2 are infertile. We observed that the expression of Msx2 is markedly elevated in Msx1-null uterus during early pregnancy (Figure S2), supporting the concept that the loss of function of one Msx gene during early pregnancy is partially compensated by the other.
Msx1 and Msx2 are critical regulators of the receptive state of the uterus during implantation. Particularly interesting is the finding that uterine expression of Msx1/Msx2 influences the activity of β-catenin in the stroma, which in turn regulates epithelial activity during early pregnancy. The identity of the factors that function downstream of Msx1/Msx2 to regulate stromal-epithelial cross-talk during implantation was revealed by compartment-specific gene expression profiling of epithelial and stromal cells collected from control and Msx1Msx2-ablated uteri. We found that, in the absence of Msx1 and Msx2 in the uterus, the expression of several WNT ligands was up-regulated in uterine epithelial and stromal cells. While the expression of WNT4, WNT7a, and WNT7b was elevated in the epithelium, that of WNT4 and WNT5a increased in the stroma. With the exception of WNT5a, these WNTs are known to signal via the canonical pathway to release β-catenin from a complex with GSK3β, leading to its stabilization and nuclear accumulation [27]–[29]. Nuclear β-catenin then associates with TCF/LEF family transcription factors to regulate cellular gene expression. Consistent with this scenario, a marked increase in the level of active β-catenin was observed in uterine stromal cells of Msx1d/dMsx2d/d uteri, while the active β-catenin levels remained unaltered in the surface epithelium. Our results indicated that canonical Wnt signaling is specifically enhanced in the stromal cells as a consequence of Msx1Msx2 ablation. How does Msx1/Msx2 regulate the WNTs and whether the β-catenin activation in the stromal cells is driven by WNTs originating in the epithelium or stroma, is unclear.
An important finding of this paper is that, in addition to WNTs, the expression of several members of the FGF family is stimulated in the stromal cells of Msx1d/dMsx2d/d uteri. The FGFs belong to a large family of growth factors, comprising 23 distinct members [33]–[35]. We observed that a subset of FGFs, including FGF1, FGF10, FGF18 and FGF21, exhibited marked up-regulation in uterine stroma of Msx1d/dMsx2d/d mice, indicating that the expression of these growth factors are normally suppressed by Msx1/Msx2. Interestingly, previous studies have shown that TCF/LEF, activated downstream of WNT-β-catenin signaling in colorectal cancer cells, binds to the promoter regions of FGF18 and FGF20 [30], [31], [42]. Studies have also shown that, in the chick embryo, WNT-β-catenin signaling triggered the synthesis of FGF8 and FGF10, which control the initiation of limb development. These previous findings suggested that WNT-activated β-catenin regulates the expression of a subset of FGFs [30]. In the present study, we provide direct evidence that active β-catenin regulates the synthesis of the FGFs, particularly FGF10, FGF18, and FGF21, in the stromal cells, uncovering a link between the WNT and FGF signaling pathways in the endometrium. The precise mechanism by which active β-catenin regulates the expression of these FGFs in uterine stromal cells remains to be determined.
The FGFs exert their paracrine responses by binding to FGFRs on the surface of the target cells and activating the receptor tyrosine kinase pathway. It is well documented that signaling via FGFRs leads to tyrosine phosphorylation of the docking protein FRS2, followed by the recruitment of multiple distinct complexes, which results in activation of Ras/ERK/MAP kinase and/or PI3 kinase/AKT signaling pathways in a variety of cell types [33], [35]. In uteri lacking Msx1 and Msx2, the accumulation in the uterine epithelium of phospho-FRS2, a key indicator of FGF signaling, indicated activation of FGFR signaling. Bazer and his coworkers have previously reported that the FGFRs are activated in ovine uterine epithelia of sheep in response to the secretion of FGF7 and FGF10 from the progesterone-primed mesenchyme and proposed that these factors are potential regulators of the maternal-fetal interactions [43], [44]. However, in the mouse uterus, the expression of FGF10, FGF18, and FGF21 is suppressed during the receptive phase of implantation. The expression of these factors is induced in the absence of Msx1 and Msx2, and the consequent increase in FGFR signaling is associated with the lack of uterine receptivity and implantation failure in Msx1d/dMsx2d/d mice.
The central hypothesis of this paper is that Msx1/Msx2 controls uterine receptivity at the time of embryo implantation by regulating epithelial function. During normal pregnancy in mice, the uterus attains receptive status on day 4 of gestation when the luminal and glandular epithelia cease to proliferate and begin to differentiate. Our study suggests that, in the absence of Msx1 and Msx2, the uterine stroma produces a subset of FGFs, which act via the FGFRs to stimulate the ERK1/2 kinase pathway in both luminal and glandular epithelia. As a consequence, the uterine epithelia of Msx1d/dMsx2d/d mice remain proliferative and fail to undergo transformation to the receptive state that allows embryo attachment to initiate implantation. The activation of the ERK1/2 pathway in the epithelium also triggers phosphorylation of epithelial ESR1 at serine-118. It is well established that this phosphorylation event is critical for the transcriptional activation of ESR1 [14]. An elevated ESR1 signaling in the epithelium is, however, detrimental to the implantation process. For example, ESR1 promotes the expression of Muc-1, a well-known cell surface glycoprotein, which creates a barrier that prevents embryo attachment. In mice, high levels of MUC-1 are present in the non-receptive uterus on days 1 and 2 of pregnancy. As the pregnancy progresses, MUC-1 expression declines in the epithelium and it is drastically reduced on day 4 at the time of implantation [21]. Therefore, the reduction of MUC-1 expression is considered a sign of uterine receptivity in mice. The persistence of high levels of MUC-1 in the Msx1d/dMsx2d/d uteri on day 4 of pregnancy is indicative of hyperestrogenic activity in the luminal epithelium and, consequently, reflects a lack of uterine receptivity.
Pathways downstream of Msx1 and Msx2 also control the synthesis of glandular factors critical for uterine receptivity at the time of implantation. While the uterine luminal epithelium is the initial site of embryo attachment, the glandular epithelium is an important source of paracrine factors required for the establishment and maintenance of pregnancy [45]. As uterus acquires competency for implantation, the glandular epithelial cells cease to proliferate and undergo differentiation to express factors, such as LIF and FOXA2, which are critical for embryo implantation [23], [24]. Presumably due to enhanced WNT and FGF signaling in Msx1d/dMsx2d/d uteri, the glandular epithelial cells remain proliferative and fail to express LIF and FOXA2. Consistent with this hypothesis, a recent study has shown that expression of constitutively active β-catenin in mouse endometrium leads to enhanced proliferation and glandular hyperplasia [46].
We recently reported that the transcription factor HAND2 suppresses the production of a subset of FGFs, which act in a paracrine manner to stimulate the proliferation of the luminal epithelium [11]. Conditional deletion of Hand2 in the uterus also results in the failure of implantation due to impaired uterine receptivity caused by increased production of FGFs in the stroma. The uterine phenotype of Hand2 deletion is remarkably similar to those of Msx1/Msx2 ablation. We, therefore, examined whether Hand2 is regulated by Msx1/Msx2 or vice versa. Surprisingly, our studies showed that Msx1/Msx2 expression is unaltered in Hand2–null uteri (Figure S5). Similarly, the loss of Msx1 or Msx2 or both did not affect Hand2 expression in the uterus during implantation (Figure S6). Furthermore, while Hand2 coordinately suppresses the expression of FGF1, FGF2, FGF9 and FGF18, Msx1/Msx2 inhibits the expression of FGF1, FGF10, FGF18, and FGF21. Although these results suggest that one or more of these FGFs act in a paracrine manner through the epithelial FGFRs to promote epithelial cell proliferation, the contribution of each these FGFs is unclear and, therefore, it remains to be determined whether Hand2 and Msx1/Msx2 function via similar or distinct mechanisms.
In summary, we have uncovered a novel mechanism by which Msx1/Msx2 regulates epithelial function at the time of implantation. In normal pregnancy, these factors act to repress WNT and β-catenin signaling and inhibit FGF synthesis in the uterine stroma, thereby attenuating the paracrine mechanisms that promote epithelial proliferation. It is also evident that the activation of ERK1/2 kinase pathway downstream of FGFR signaling in the epithelium of Msx1Msx2-ablated uteri activates transcriptional function of ESR1, contributing to the non-receptive status of the uterus (Figure 7). Continued analysis of the mechanisms by which Msx1 and Msx2 control the WNT-β-catenin-FGF pathway to direct uterine stromal-epithelial communication will clarify our understanding of the molecular events that underlie uterine receptivity.
Fig. 7. Mechanism of Msx1 and Msx2 action in mouse uterus. 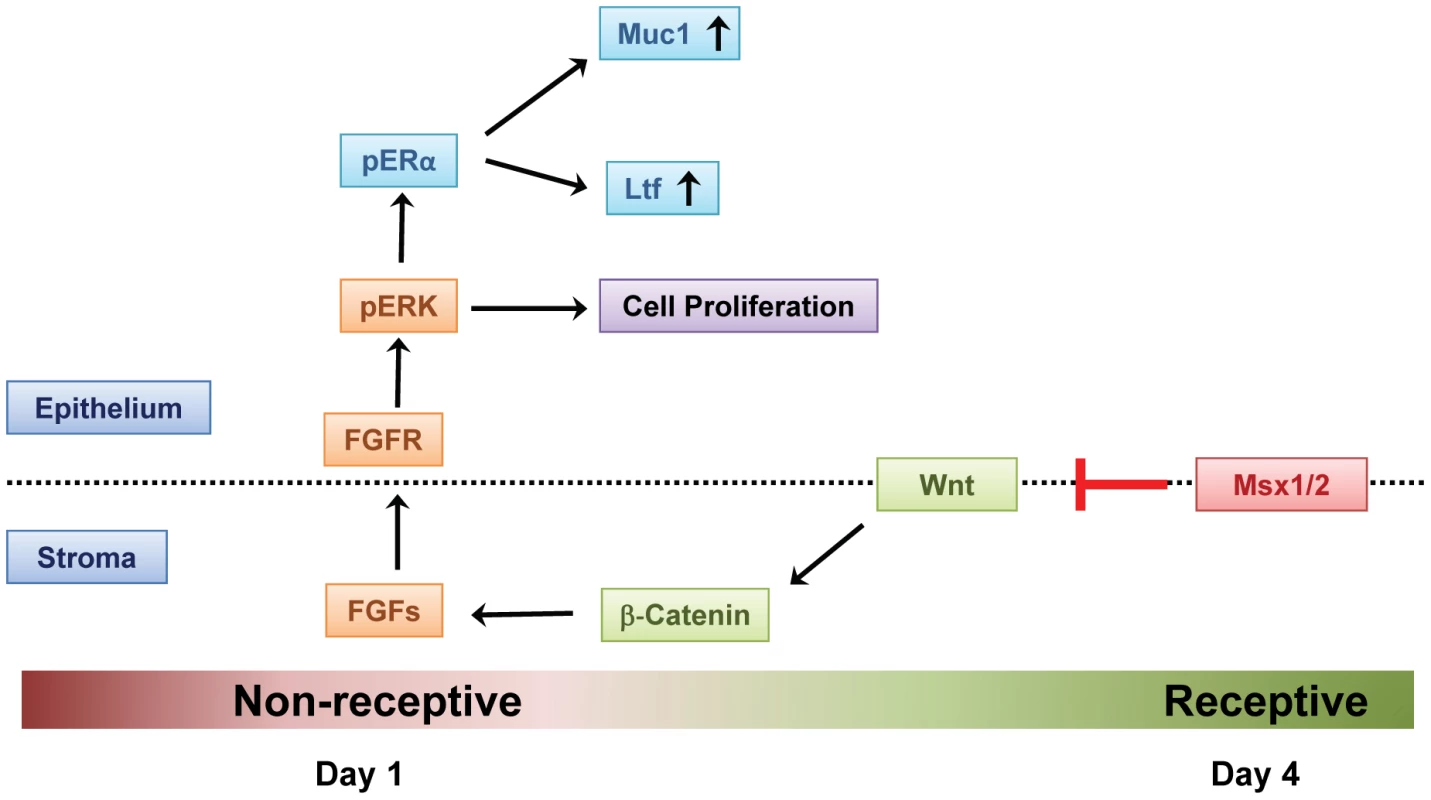
In normal pregnancy, MSX1 and MSX2 act to repress WNT and β-catenin signaling and inhibit FGF synthesis in the uterine stroma, thereby suppressing stromal-epithelial cross-talk. In the absence of MSX1 and MSX2, FGFs are induced, activating the epithelial FGFR-ERK1/2 pathway, and promoting epithelial proliferation. Activated ERK1/2 then phosphorylates epithelial ESR1. This triggers transcriptional activation of ESR1 and expression of its target genes, such as Muc-1, which prevent the functional transformation of the luminal epithelium to receptive state, blocking embryo implantation. Materials and Methods
Animals
Mice were maintained in the designated animal care facility at the College of Veterinary Medicine of the University of Illinois, Urbana-Champaign, according to the institutional guidelines for the care and use of laboratory animals. To generate the conditional Msx1Msx2-null mice (Msx1d/dMsx2d/d), Msx1Msx2-floxed (Msx1f/fMsx2f/f) [47] mice were mated with PR-Cre knock-in mice [48].
For breeding studies, cycling Msx1d/dMsx2d/d and Msx1f/fMsx2f/f female mice (C57BL/6 genetic background) were housed with wild-type C57BL/6 male mice (Charles Rivers) for 6 months. The presence of a vaginal plug after mating was designated as day 1 of pregnancy. The number of litters and pups born were recorded at birth to assess the fertility status.
To induce superovulation, 3-week old female mice were administered intraperitoneally with 5 IU of pregnant mare serum gonadotrophin (PMSG, Sigma St. Louis, MO) followed by 5 IU of human chorionic gonadotropin (hCG, Sigma St. Louis, MO) 48 hours later. The mice were killed 16–18 hours post-hCG administration and the oocytes were recovered from the ampulla and counted.
To collect blastocysts, 8-week old female mice were mated with wild-type males. To assess the pre - implantation development of embryos, blastocysts were flushed from day 4 pregnant uteri and examined for their quality under a stereo-zoom microscope.
For certain experiments, the FGFR-specific inhibitor, PD173074 (Selleck Chemicals Co., Ltd., London ON, Canada), was dissolved in DMSO and was diluted with HBSS. Ten microlitre of inhibitor (50 µM) was injected intraluminally in one horn and vehicle was injected in the other horn of Msx1d/dMsx2d/d mice on day 3 of pregnancy. Uterine tissues were collected on day 4 of pregnancy.
Transmission electron microscopy
Uterine tissues isolated from Msx1f/fMsx2f/f and Msx1d/dMsx2d/d female mice on day 4 of pregnancy were fixed in 2.0% paraformaldehyde and 2.5% glutaraldehyde in buffer containing 0.1 M sodium cacodylate. Tissues were then washed and fixed with 1.0% aqueous osmium tetroxide in 0.1 M sodium cacodylate buffer. Following dehydration with ethanol and propylene oxide, the tissues were embedded in 100% Polybed 812 mixture. Sections (80 nm) were cut with an Ultramicrotome, stained and examined under a Philips CM 200 Transmission Electron Microscope.
Isolation of uterine epithelial and stromal cells
Uterine epithelial cells were isolated as previously described [49]. Briefly, uterine horns were dissected into 3–4 mm pieces and incubated in a solution of 1% trypsin (Difco, Dertroit, MI) in Hank's balanced salt solution (HBSS) for 90 min at 4°C and then for 30 min at room temperature. The tissues were then rinsed with 10% FBS. Under a dissecting microscope, each enzyme treated piece of uterus was squeezed by forceps to separate the epithelium from the rest of the uterine tissue. Uterine stromal cells were isolated as previously described [50]. Briefly, uterine horns of pregnant mice were dissected and placed in HBSS containing 6 g/liter dispase and 25 g/liter pancreatin for 1 h at room temperature and then 15 min at 37°C to remove the endometrial epithelial clumps. The tissues were then placed in HBSS containing 0.5 g/liter collagenase for 45 min at 37°C to disperse the stromal cells. After vortexing, the contents were passed through a 70-µm gauze filter (Millipore). The filtrate contained the stromal cells.
Culture of uterine stromal cells
The uterine stromal cells, isolated as described above, were diluted in Dulbecco's modified Eagle's Medium-F12 medium (DMEM-F12; with 100 unit/liter penicillin, 0.1 g/liter streptomycin, 1.25 mg/liter Fungizone) with 2% heat-inactivated fetal calf serum. The live cells were counted by trypan blue staining using a hemocytometer. Cells were then seeded in 6-well cell culture plates. The unattached cells were removed by washing several times with HBSS after 2 h, and cell culture was continued after addition of fresh medium supplemented with P (1 µm) and E (10 nm).
Quantitative real-time PCR analysis (qPCR)
Uterine tissue was homogenized and total RNA was extracted by using TRIZOL reagent, according to the manufacturer's protocol. cDNA was prepared by standard protocols. The cDNA was amplified to quantify gene expression by quantitative PCR, using gene-specific primers and SYBR Green (Applied Biosystems, Warringtom, UK). The expression level of RPLP0 (36B4) or Cytokeratin 18 (Ck18) was used as the internal control. For each treatment, the mean Ct and standard deviation were calculated from individual Ct values obtained from three replicates of a sample. The normalized ΔCt in each sample was calculated as mean Ct of target gene subtracted by the mean Ct of internal control gene. ΔΔCt was then calculated as the difference between the ΔCt values of the control and treatment sample. The fold change of gene expression in each sample relative to a control was computed as 2−ΔΔCt. The mean fold induction and standard errors were calculated from three or more independent experiments.
Immunohistochemistry
Uterine tissues were processed and subjected to immunohistochemistry as described previously [13]. Briefly, paraffin-embedded tissues were sectioned at 5 µm and mounted on microscopic slides. Sections were deparaffinized in xylene, rehydrated through a series of ethanol washes, and rinsed in water. Antigen retrieval was performed by immersing the slides in 0.1 M citrate buffer solution, pH 6.0, followed by microwave heating for 25 min. The slides were allowed to cool and endogenous peroxidase activity was blocked by incubating sections in 0.3% hydrogen peroxide in methanol for 15 min at room temperature. After washing with PBS for 15 min and the slides were incubated in a blocking solution for 1 h before incubating them in primary antibody overnight at 4°C with antibodies specific for MSX1 (Abcam, ab73883), MSX2 (Santa Cruz, sc-15396), MUC1 (Novus biological, NB120-15481), Ki67 (BD Pharmingen, 550609), ESR1 (Santa Cruz, sc-7207), p-ESR1 (Santa Cruz, sc-12915), PGR (Neomarkers MS-194-PO), HAND2 (Santa Cruz sc-9409), phospho-FRS2 (R&D systems AF5126) and active β-catenin (PY489, Developmental Studies Hybridoma Bank, Iowa City, IA 52242). The slides were incubated with the biotinylated secondary antibodies at room temperature for 1 h, followed by incubation with horseradish peroxidase-conjugated streptavidin (Invitrogen Corp., MD 21704). The sections were stained in 3-amino-9-ethylcarbazole chromogen (AEC) solution until optimal signal was developed. Sections were counterstained with Mayer's Hematoxylin and examined by bright field microscopy.
siRNA transfection
Control (scrambled) siRNA and siRNA targeted to β-catenin (s438) were purchased from Ambion Inc. The transfection was performed using SilentFect™ Reagent (Bio-Rad), according to the manufacturer's protocol. The stromal cells were isolated from uteri of Msx1d/dMsx2d/d mice on day 3 of pregnancy and transfected with siRNA after 5–6 h of culture. The cells were harvested 24 h following transfection and RNA was isolated.
DNA microarray analysis
Uterine epithelial and stromal cells were isolated from Msx1f/fMsx2f/f and Msx1d/dMsx2d/d mice on day 4 of pregnancy. Total RNA was prepared from these cells, and hybridized to Affymetrix GeneChip Mouse Genome 430 2.0 array as previously described [6]. They were processed and analyzed according to the Affymetrix protocol.
Measurement of serum E and P levels
The levels of E and P in the serum were measured by radioimmunoassay (RIA) performed at the Ligand Core facility of the University of Virginia at Charlottesville.
Statistical analysis
Statistical analysis was performed by t-test or ANOVA. The values were expressed as mean ± SEM and considered significant if p<0.05.
Supporting Information
Zdroje
1. CarsonDDBagchiIDeySKEndersACFazleabasAT 2000 Embryo Implantation. Developmental Biology 223 217 237
2. DeySKLimHDasSKReeseJPariaBC 2004 Molecular Cues to Implantation. Endocr Rev 25 341 373
3. RamathalCYBagchiICTaylorRNBagchiMK 2010 Endometrial Decidualization: Of Mice and Men. Semin Reprod Med 28 17 26
4. RubelCAJeongJ-WTsaiSYLydonJPDeMayoFJ 2010 Epithelial-Stromal Interaction and Progesterone Receptors in the Mouse Uterus. Semin Reprod Med 28 027 035
5. LeeKYJeongJ-WTsaiSYLydonJPDeMayoFJ 2007 Mouse models of implantation. Trends in Endocrinology & Metabolism 18 234 239
6. MantenaSRKannanACheonY-PLiQJohnsonPF 2006 C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proceedings of the National Academy of Sciences of the United States of America 103 1870 1875
7. LeeKJeongJKwakIYuC-TLanskeB 2006 Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 38 1204 1209
8. LeeKYJeongJ-WWangJMaLMartinJF 2007 Bmp2 Is Critical for the Murine Uterine Decidual Response. Mol Cell Biol 27 5468 5478
9. KuriharaILeeD-KPetitFGJeongJLeeK 2007 COUP-TFII Mediates Progesterone Regulation of Uterine Implantation by Controlling ER Activity. PLoS Genet 3 e102 doi:10.1371/journal.pgen.0030102
10. LawsMJTaylorRNSidellNDeMayoFJLydonJP 2008 Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development 135 2659 2668
11. LiQKannanADeMayoFJLydonJPCookePS 2011 The Antiproliferative Action of Progesterone in Uterine Epithelium Is Mediated by Hand2. Science 331 912 916
12. DaikokuTSongHGuoYRiesewijkAMosselmanS 2004 Uterine Msx-1 and Wnt4 Signaling Becomes Aberrant in Mice with the Loss of Leukemia Inhibitory Factor or Hoxa-10: Evidence for a Novel Cytokine-Homeobox-Wnt Signaling in Implantation. Molecular Endocrinology 18 1238 1250
13. LiQCheonYPKannanAShankerSBagchiIC 2004 A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor gamma regulates implantation in mice. J Biol Chem 279 11570 11581
14. KatoSEndohHMasuhiroYKitamotoTUchiyamaS 1995 Activation of the Estrogen Receptor Through Phosphorylation by Mitogen-Activated Protein Kinase. Science 270 1491 1494
15. BagchiICCheonYPLiQBagchiMK 2003 Progesterone receptor-regulated gene networks in implantation. Front Biosci 8 s852 861
16. BagchiICLiQCheonYP 2001 Role of steroid hormone-regulated genes in implantation. Ann N Y Acad Sci 943 68 76
17. PanHDengYPollardJW 2006 Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A 103 14021 14026
18. McMasterMTTengCTDeySKAndrewsGK 1992 Lactoferrin in the mouse uterus: analyses of the preimplantation period and regulation by ovarian steroids. Molecular Endocrinology 6 101 111
19. JeongJ-WLeeKYLydonJPDeMayoFJ 2006 Steroid hormone regulation of Clca3 expression in the murine uterus. Journal of Endocrinology 189 473 484
20. HuangHChuSChenY 1999 Ovarian steroids regulate 24p3 expression in mouse uterus during the natural estrous cycle and the preimplantation period. Journal of Endocrinology 162 11 19
21. SurveyorGAGendlerSJPembertonLDasSKChakrabortyI 1995 Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 136 3639 3647
22. FinnCAMartinL 1974 The control of implantation. Journal of Reproduction and Fertility 39 195 206
23. StewartCLKasparPBrunetLJBhattHGadiI 1992 Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359 76 79
24. JeongJ-WKwakILeeKYKimTHLargeMJ 2010 Foxa2 Is Essential for Mouse Endometrial Gland Development and Fertility. Biology of Reproduction 83 396 403
25. ChenWHanB-CWangR-CXiongG-FPengJ-P Role of secretory protease inhibitor SPINK3 in mouse uterus during early pregnancy. Cell and Tissue Research 341 441 451
26. MurphyCR 2004 Uterine receptivity and the plasma membrane transformation. Cell Res 14 259 267
27. LoganCYNusseR 2004 The WNT signaling pathway in development and diseasse. Annual Review of Cell and Developmental Biology 20 781 810
28. GordonMDNusseR 2006 Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. Journal of Biological Chemistry 281 22429 22433
29. AngersSMoonRT 2009 Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol advanced online publication
30. KawakamiYCapdevilaJBüscherDItohTEstebanCRg 2001 WNT Signals Control FGF-Dependent Limb Initiation and AER Induction in the Chick Embryo. Cell 104 891 900
31. ShimokawaTFurukawaYSakaiMLiMMiwaN 2003 Involvement of the FGF18 Gene in Colorectal Carcinogenesis, as a Novel Downstream Target of the β-Catenin/T-Cell Factor Complex. Cancer Research 63 6116 6120
32. ReinholdMINaskiMC 2007 Direct Interactions of Runx2 and Canonical Wnt Signaling Induce FGF18. Journal of Biological Chemistry 282 3653 3663
33. EswarakumarVPLaxISchlessingerJ 2005 Cellular signaling by fibroblast growth factor receptors. Cytokine & Growth Factor Reviews 16 139 149
34. BeenkenAMohammadiM 2009 The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8 235 253
35. TurnerNGroseR 2010 Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10 116 129
36. KoziczakMHolbroTHynesNE 0000 Blocking of FGFR signaling inhibits breast cancer cell proliferation through downregulation of D-type cyclins. Oncogene 23 3501 3508
37. DavidsonD 1995 The function and evolution of Msx genes: pointers and paradoxes. Trends in Genetics 11 405 411
38. MaasRChenYPBeiMWooISatokataI 1996 The Role of Msx Genes in Mammalian Development. Annals of the New York Academy of Sciences 785 171 181
39. AlappatSZhangZYChenYP 2003 Msx homeobox gene family and craniofacial development. Cell Res 13 429 442
40. WangWChenXXuHLufkinT 1996 Msx3: a novel murine homologue of the Drosophila msh homeobox gene restricted to the dorsal embryonic central nervous system. Mechanisms of Development 58 203 215
41. RamosCRobertB 2005 msh/Msx gene family in neural development. Trends in Genetics 21 624 632
42. ChamorroMNSchwartzDRVonicaABrivanlouAHChoKR 2005 FGF-20 and DKK1 are transcriptional targets of [beta]-catenin and FGF-20 is implicated in cancer and development. EMBO J 24 73 84
43. ChenCSpencerTEBazerFW 2000 Fibroblast Growth Factor-10: A Stromal Mediator of Epithelial Functionin the Ovine Uterus. Biology of Reproduction 63 959 966
44. SatterfieldMCHayashiKSongGBlackSGBazerFW 2008 Progesterone Regulates FGF10, MET, IGFBP1, and IGFBP3 in the Endometrium of the Ovine Uterus. Biology of Reproduction 79 1226 1236
45. GrayCABartolFFTarletonBJWileyAAJohnsonGA 2001 Developmental Biology of Uterine Glands. Biology of Reproduction 65 1311 1323
46. JeongJWLeeHSFrancoHLBroaddusRRTaketoMM 2008 [beta]-catenin mediates glandular formation and dysregulation of [beta]-catenin induces hyperplasia formation in the murine uterus. Oncogene 28 31 40
47. FuHIshiiMGuYMaxsonR 2007 Conditional alleles of Msx1 and Msx2. genesis 45 477 481
48. SoyalSMMukherjeeALeeKYSLiJLiH 2005 Cre-mediated recombination in cell lineages that express the progesterone receptor. genesis 41 58 66
49. BigsbyRMCookePSCunhaGR 1986 A simple efficient method for separating murine uterine epithelial and mesenchymal cells. American Journal of Physiology - Endocrinology And Metabolism 251 E630 E636
50. LiQKannanAWangWDeMayoFJTaylorRN 2007 Bone Morphogenetic Protein 2 Functions via a Conserved Signaling Pathway Involving Wnt4 to Regulate Uterine Decidualization in the Mouse and the Human. Journal of Biological Chemistry 282 31725 31732
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 2
-
Všechny články tohoto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



