-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenetic Variants at Chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 Influence the Risk of Breast Cancer in Men
Male breast cancer accounts for approximately 1% of all breast cancer. To date, risk factors for male breast cancer are poorly defined, but certain risk factors and genetic features appear common to both male and female breast cancer. Genome-wide association studies (GWAS) have recently identified common single nucleotide polymorphisms (SNPs) that influence female breast cancer risk; 12 of these have been independently replicated. To examine if these variants contribute to male breast cancer risk, we genotyped 433 male breast cancer cases and 1,569 controls. Five SNPs showed a statistically significant association with male breast cancer: rs13387042 (2q35) (odds ratio (OR) = 1.30, p = 7.98×10−4), rs10941679 (5p12) (OR = 1.26, p = 0.007), rs9383938 (6q25.1) (OR = 1.39, p = 0.004), rs2981579 (FGFR2) (OR = 1.18, p = 0.03), and rs3803662 (TOX3) (OR = 1.48, p = 4.04×10−6). Comparing the ORs for male breast cancer with the published ORs for female breast cancer, three SNPs—rs13387042 (2q35), rs3803662 (TOX3), and rs6504950 (COX11)—showed significant differences in ORs (p<0.05) between sexes. Breast cancer is a heterogeneous disease; the relative risks associated with loci identified to date show subtype and, based on these data, gender specificity. Additional studies of well-defined patient subgroups could provide further insight into the biological basis of breast cancer development.
Published in the journal: . PLoS Genet 7(9): e32767. doi:10.1371/journal.pgen.1002290
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002290Summary
Male breast cancer accounts for approximately 1% of all breast cancer. To date, risk factors for male breast cancer are poorly defined, but certain risk factors and genetic features appear common to both male and female breast cancer. Genome-wide association studies (GWAS) have recently identified common single nucleotide polymorphisms (SNPs) that influence female breast cancer risk; 12 of these have been independently replicated. To examine if these variants contribute to male breast cancer risk, we genotyped 433 male breast cancer cases and 1,569 controls. Five SNPs showed a statistically significant association with male breast cancer: rs13387042 (2q35) (odds ratio (OR) = 1.30, p = 7.98×10−4), rs10941679 (5p12) (OR = 1.26, p = 0.007), rs9383938 (6q25.1) (OR = 1.39, p = 0.004), rs2981579 (FGFR2) (OR = 1.18, p = 0.03), and rs3803662 (TOX3) (OR = 1.48, p = 4.04×10−6). Comparing the ORs for male breast cancer with the published ORs for female breast cancer, three SNPs—rs13387042 (2q35), rs3803662 (TOX3), and rs6504950 (COX11)—showed significant differences in ORs (p<0.05) between sexes. Breast cancer is a heterogeneous disease; the relative risks associated with loci identified to date show subtype and, based on these data, gender specificity. Additional studies of well-defined patient subgroups could provide further insight into the biological basis of breast cancer development.
Introduction
Breast cancer does not exclusively affect females. Around 300 men in the UK and 1,900 men in the US are diagnosed with the disease each year [1]. The average age at incidence of male breast cancer is somewhat different to that seen for female breast cancer, with the disease typically affecting men 5–10 years later than women. Perhaps because male breast cancer is not common, few risk factors have been demonstrated to influence disease risk, but tentative associations with obesity, lack of exercise, excess alcohol consumption, gynaecomastia, past benign breast disease, past liver disease, infertility, diabetes and exposure to ionising radiation have been suggested [2], [3].
Investigation of susceptibility genes for male breast cancer has been limited. It has however been shown that approximately 10% of men with breast cancer carry BRCA2 mutations, while mutations in BRCA1 are exceedingly rare [4]. The relative risk of breast cancer in men associated with BRCA2 mutations is high [5]. Recently the CHEK2 1100delC variant has been found to give a 10-fold risk of male breast cancer independent of BRCA1 or BRCA2 [6]. Mutations in these genes are rare in the general population and it is likely that much of the genetic contribution to female breast cancer risk can be attributed to the co-inheritance of multiple low risk common variants [7]. Recent genome-wide association studies (GWAS) have shown associations between single nucleotide polymorphisms (SNPs) mapping to a dozen or more loci and female breast cancer risk in European populations, each conferring odds ratios (ORs) of 1.04–1.43 [8]–[14]. To explore the possibility that the same risk variants influence male breast cancer risk, we conducted a case-control study of male breast cancer, genotyping 12 SNPs annotating the loci that have the strongest and most consistent associations with female breast cancer.
Materials and Methods
457 cases of male breast cancer were recruited in a population-based case-control study of the genetic, environmental and behavioral causes of male breast cancer being conducted in England and Wales. Potential cases were all men resident in these countries aged 18–79 with newly diagnosed breast cancer since January 1st, 2005, identified through notifications by treatment centres and systematic regular listings of cases from regional cancer registries. 98% of cases for whom registry data has been received have been histologically confirmed. The median age at diagnosis of cases was 65.5 years (interquartile range: 59–72).
A total of 1608 unmatched controls were available for genotyping; 535 men were ascertained through our ongoing breast cancer studies and a further 1073 were healthy male and female individuals from the UK Genetic Lung Cancer Predisposition Study (GELCAPS) [15]. The decision to include a second control set was made a priori, with the aim of increasing statistical power. We saw no evidence for an effect of control group on the overall effect estimate for each SNP. Collection of blood samples from all subjects was undertaken with informed consent and relevant ethical review committee approval.
DNA was extracted from venous blood samples using conventional methodologies and quantified by Picogreen (Invitrogen, Carlsbad CA). SNPs were chosen for analysis on the basis of validated associations with female breast cancer from recent GWAS [8]–[14]. Genotyping of rs11249433, rs13387042, rs4973768, rs10941679, rs16886165, rs9383938, rs13281615, rs865686, rs2981579, rs3817198, rs3803662 and rs6504950 was performed by allele-specific PCR using KASPar chemistry (Kbioscience, Hertfordshire, UK). Each DNA plate contained 5% sample duplication to assess genotyping concordance between duplicate pairs. We attempted to genotype 2119 samples (including duplicates, n = 54) and excluded samples (n = 49; 11 cases, 34 controls and four members of a duplicate pair) in which no-calls were observed for two or more SNPs. Genotyping QC statistics were therefore computed on 2070 samples (Figure S1). Final locus and sample completion rates were >99.9%. The mean genotype concordance between duplicate pairs was 99.8%. We excluded a further 18 subjects due to self-reported non-European ancestry (13 cases and 5 controls). No SNP genotypes showed significant deviation from the proportions expected under Hardy-Weinberg equilibrium in controls (Table S1).
ORs and 95% confidence intervals (CI) were calculated using unconditional logistic regression. The odds ratio for each SNP was determined by fitting multiplicative and unconstrained genetic models. P-values were computed from likelihood ratio test statistics. Case-only unconditional logistic regression was used to test the significance of association with age at diagnosis. Deviation of genotype proportions from Hardy-Weinberg equilibrium was assessed in controls using an exact test [16]. To compare formally the ORs in males with the equivalent published ORs for female disease, we assumed both sets of ORs were log-normally distributed. Then under the null hypothesis that the OR in males is equal to the OR in females, the difference between the estimated log ORs is normally distributed with mean zero and variance equal to the sum of the squared standard errors of the two estimates. From this we obtained a χ2 statistic for each comparison (1 degree of freedom [d.f.]) and from the sum of the χ2 statistics a global test for all comparisons (12 d.f.). Statistical analyses were performed using the Genotype Libraries and Utilities (GLU) package (http://code.google.com/p/glu-genetics) and R [17].
Results/Discussion
433 male breast cancer cases and 1569 controls were successfully genotyped according to our predefined QC criteria. The majority of cases were diagnosed with invasive breast cancer (n = 399 (92%)) while a further 31 (7%) were ductal carcinoma in situ. Three cases (<1%) were of unknown histology. Table 1 shows the OR for male breast cancer associated with each of the 12 SNPs previously reported to be associated with female breast cancer risk. For five SNPs, rs13387042 (2q35), rs10941679 (5p12), rs9383938 (6q25.1), rs2981579 (FGFR2) and rs3803662 (TOX3), the risk allele for female breast cancer was associated with increased risk of male breast cancer (p<0.05). Two SNPs, rs13387042 (2q35) and rs3803662 (TOX3), remained significant below the Bonferroni adjusted threshold for independent tests of p<4.12×10−3.
Tab. 1. Risk estimates for male breast cancer conferred by 12 loci identified through GWAS of female breast cancer. 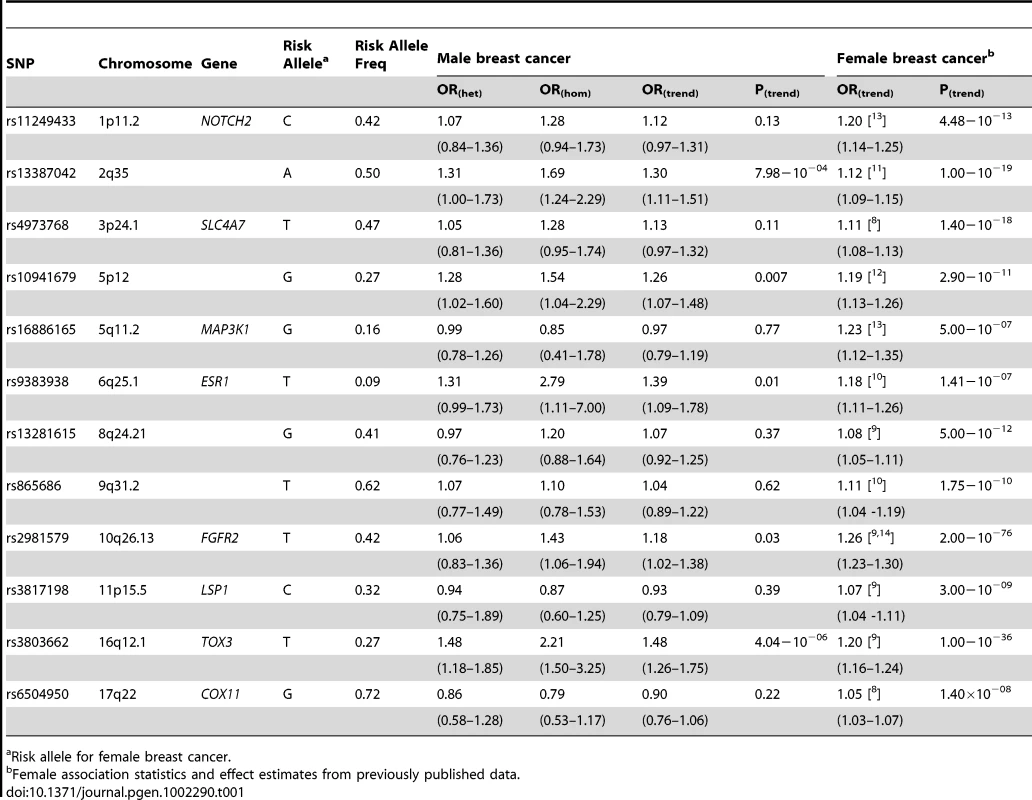
Risk allele for female breast cancer. Comparing ORmale estimates with those for female breast cancer (ORfemale) there were two SNPs, rs13387042 (2q35) and rs3803882 (TOX3) for which the ORmale was significantly higher than the ORfemale, albeit not after adjusting for multiple testing (rs13387042, ORmale:ORfemale p = 0.03; rs3803882, ORmale:ORfemale p = 0.04; Table 2). rs3803662 (TOX3) showed the strongest association with male breast cancer (ORmale = 1.48; 95% CI 1.26–1.75, p = 4.04×10−6) with an excess relative risk that was more than twice the female estimate (ORfemale = 1.20; 95% CI 1.16–1.24) [9]. Similarly, the excess risk conferred by rs13387042 (2q35) in males (ORmale = 1.30; 95% CI 1.11–1.51, p = 7.98×10−4) was more than double that observed in females (ORfemale = 1.12; 95% CI 1.09–1.15) [11].
Tab. 2. Ratio of ORmale:ORfemale for 12 risk loci identified by genome-wide association studies of female breast cancer. 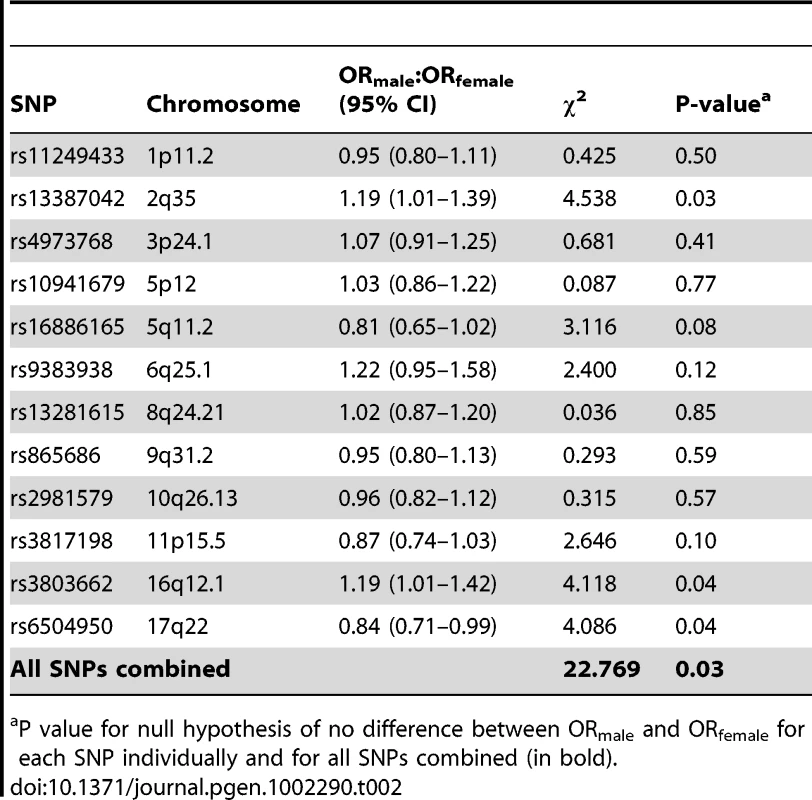
P value for null hypothesis of no difference between ORmale and ORfemale for each SNP individually and for all SNPs combined (in bold). For one SNP (rs6504950, COX11) the ORmale was in the opposite direction to that reported for female breast cancer (ORmale = 0.90; 95% CI 0.76–1.06, ORfemale = 1.05; 95% CI 1.03–1.07) [8], [9] and was inconsistent with the female estimate (ORmale:ORfemale p = 0.04; Table 2). For the other nine SNPs that we tested the ORmale estimates were consistent with the ORfemale estimates. Comparing the combined estimates of all 12 SNPs, however, there was nominal evidence that the male ORs differed from the female ORs (p = 0.03; Table 2).
The frequency of female breast tumors that are estrogen receptor (ER) positive varies, particularly according to menopausal status at diagnosis [18]. Based on a sample of almost 3,000 patients the proportion is typically between 64% and 79% [18]. In contrast, male breast tumors, tend to be overwhelmingly ER-positive (>90%) [19]. In the current study estrogen receptor status was known for 251 male breast cancer cases, 246 (98%) of which had ER-positive tumors. For nine of the 12 SNPs that we genotyped, ORfemale estimates stratified according to ER status have been reported for Caucasian populations (Tables S2a and S2b). In females, the OR for ER-positive disease is stronger than the OR for ER-negative disease for all nine of these loci and this difference is significant for all but two of them (rs16886165 (MAP3KI) and rs3817198 (LSP1) [8], [11], [20]. Given the predominance of ER-positive tumors in male disease we also compared the ORmale with the ORfemale for ER-positive disease (Table S2a). There was nominally significant evidence overall that the male ORs differed from those for ER-positive female disease (p = 0.05). We also tested for a difference between the ORmale estimates for these nine SNPs and the ORfemale estimates for ER-negative disease (Table S2b); there was stronger evidence of a difference (p = 0.01).
Finally, we assessed the relationship between genotype and age at onset of male breast cancer (Table S3) for each of the 12 loci. There was no evidence for a trend with age at diagnosis.
We have shown, for the first time that common genetic variants influence susceptibility to male breast cancer. Furthermore we have demonstrated that for at least a subset of known susceptibility loci the risk allele for female breast cancer is also associated with increased risk of disease in males. To our knowledge these 433 male breast cancer cases represent the largest single series to date; despite this, we lacked power to detect modest relative risks for all but the most common variants. For example we had only 40% power to detect an OR of 1.15 for a variant with a minor allele frequency of 30% at a significance level of 5%. The lack of a statistically significant association with male breast cancer risk for seven of the 12 SNPs that we tested may, therefore, simply reflect a lack of power.
Notably, for two of the three SNPs for which the ORmale was inconsistent with the ORfemale (rs13387042 (2q35) and rs3803882 (TOX3)) the association in males was stronger than that in females. While the ORmale estimates were slightly closer to the ORs for ER-positive disease in females, it is noticeable that these are the two SNPs that show the largest effects on ER-negative disease risk in females. Although the significance of this observation, if any, is not yet clear, our data on male breast cancer alongside the published associations with female breast cancer, clearly implicate the 2q35 and 16q12.1 loci in the aetiology of breast cancer, irrespective of gender and tumor pathology.
Given that the majority of female breast cancer risk loci identified to date demonstrate a degree of specificity for ER-positive or ER-negative disease [8], [11], [20], [21] it seems likely that subtype specific GWAS will lead to the identification of additional risk loci. Our analyses suggest that GWAS of male breast cancer may also lead to the identification of novel breast cancer risk loci in males and that these should provide further insight into the biological basis of male and female breast cancer development.
Supporting Information
Zdroje
1. JemalASiegelRWardEHaoYXuJ 2009 Cancer statistics, 2009. CA Cancer J Clin 59 225 249
2. SascoAJLowenfelsABPasker-de JongP 1993 Review article: epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer 53 538 549
3. WeissJRMoysichKBSwedeH 2005 Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev 14 20 26
4. Gomez-RaposoCZambrana TevarFSereno MoyanoMLopez GomezMCasadoE 2010 Male breast cancer. Cancer Treat Rev 36 451 457
5. ThompsonDEastonD 2001 Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet 68 410 419
6. Meijers-HeijboerHvan den OuwelandAKlijnJWasielewskiMde SnooA 2002 Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31 55 59
7. FletcherOHoulstonRS 2010 Architecture of inherited susceptibility to common cancer. Nat Rev Cancer 10 353 361
8. AhmedSThomasGGhoussainiMHealeyCSHumphreysMK 2009 Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41 585 590
9. EastonDFPooleyKADunningAMPharoahPDThompsonD 2007 Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447 1087 1093
10. FletcherOJohnsonNOrrNHoskingFJGibsonLJ 2011 Novel Breast Cancer Susceptibility Locus at 9q31.2: Results of a Genome-Wide Association Study. J Natl Cancer Inst
11. MilneRLBenitezJNevanlinnaHHeikkinenTAittomakiK 2009 Risk of estrogen receptor-positive and -negative breast cancer and single-nucleotide polymorphism 2q35-rs13387042. J Natl Cancer Inst 101 1012 1018
12. StaceySNManolescuASulemPThorlaciusSGudjonssonSA 2008 Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 40 703 706
13. ThomasGJacobsKBKraftPYeagerMWacholderS 2009 A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat Genet 41 579 584
14. TurnbullCAhmedSMorrisonJPernetDRenwickA 2010 Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 42 504 507
15. EisenTMatakidouAHoulstonR 2008 Identification of low penetrance alleles for lung cancer: the GEnetic Lung CAncer Predisposition Study (GELCAPS). BMC Cancer 8 244
16. WiggintonJECutlerDJAbecasisGR 2005 A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet 76 887 893
17. Team RDC 2010 R: A language and envirnoment for statistical computing. Vienna R Foundation for Statistical Computing 2.11.1 ed.
18. ClarkGMOsborneCKMcGuireWL 1984 Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol 2 1102 1109
19. WillsherPCLeachIHEllisIOBellJAElstonCW 1997 Male breast cancer: pathological and immunohistochemical features. Anticancer Res 17 2335 2338
20. Garcia-ClosasMHallPNevanlinnaHPooleyKMorrisonJ 2008 Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet 4 e1000054 doi:10.1371/journal.pgen.1000054
21. AntoniouACWangXFredericksenZSMcGuffogLTarrellR 2010 A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet 42 885 892
Štítky
Genetika Reprodukční medicína
Článek Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and BrainČlánek Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-StateČlánek A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated LociČlánek Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional PolymorphismsČlánek Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of ChromosomesČlánek The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and LearningČlánek PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian CellsČlánek Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 9
-
Všechny články tohoto čísla
- Temporal Trends in Results Availability from Genome-Wide Association Studies
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
- Genetic Variants at Chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 Influence the Risk of Breast Cancer in Men
- Large-Scale Gene-Centric Analysis Identifies Novel Variants for Coronary Artery Disease
- Genetic Association for Renal Traits among Participants of African Ancestry Reveals New Loci for Renal Function
- Transcriptome Kinetics Is Governed by a Genome-Wide Coupling of mRNA Production and Degradation: A Role for RNA Pol II
- Conserved Regulation of p53 Network Dosage by MicroRNA–125b Occurs through Evolving miRNA–Target Gene Pairs
- Heterozygous Mutations of Are Associated with an Increased Risk of Isolated Metopic Craniosynostosis in Humans and Mice
- Study of FoxA Pioneer Factor at Silent Genes Reveals Rfx-Repressed Enhancer at and a Potential Indicator of Esophageal Adenocarcinoma Development
- Cholesterol Metabolism Is Required for Intracellular Hedgehog Signal Transduction
- Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and Brain
- Age-Dependent Recombination Rates in Human Pedigrees
- Sequence Conservation and Functional Constraint on Intergenic Spacers in Reduced Genomes of the Obligate Symbiont
- Sex Chromosome Mosaicism and Hybrid Speciation among Tiger Swallowtail Butterflies
- A Negative Feedback Loop That Limits the Ectopic Activation of a Cell Type–Specific Sporulation Sigma Factor of
- Phased Whole-Genome Genetic Risk in a Family Quartet Using a Major Allele Reference Sequence
- Mutations in or near the Transmembrane Domain Alter PMEL Amyloid Formation from Functional to Pathogenic
- Inactivation of Alters Melanosome Shape But Has Only a Subtle Effect on Visible Pigmentation
- Novel Interactions between Actin and the Proteasome Revealed by Complex Haploinsufficiency
- Germline Genetic Variants Disturbing the /LIN28 Double-Negative Feedback Loop Alter Breast Cancer Susceptibility
- Separation of Recombination and SOS Response in RecA Suggests LexA Interaction Sites
- Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-State
- Misregulation of Scm3p/HJURP Causes Chromosome Instability in and Human Cells
- A Noncoding Point Mutation of Causes Multiple Developmental Malformations and Obesity in Twirler Mice
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- A Genome-Wide Metabolic QTL Analysis in Europeans Implicates Two Loci Shaped by Recent Positive Selection
- Bacterial Communities of Diverse Species: Ecological Context of a Host–Microbe Model System
- A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated Loci
- Elongator Complex Influences Telomeric Gene Silencing and DNA Damage Response by Its Role in Wobble Uridine tRNA Modification
- Elevated Proteasome Capacity Extends Replicative Lifespan in
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- MicroRNA Predictors of Longevity in
- An Iterative Genetic and Dynamical Modelling Approach Identifies Novel Features of the Gene Regulatory Network Underlying Melanocyte Development
- Atypical AT Skew in Firmicute Genomes Results from Selection and Not from Mutation
- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- Genomic Analysis of QTLs and Genes Altering Natural Variation in Stochastic Noise
- The Abnormal Phenotypes of Cartilage and Bone in Calcium-Sensing Receptor Deficient Mice Are Dependent on the Actions of Calcium, Phosphorus, and PTH
- Cell Type–Specific Transcriptome Analysis Reveals a Major Role for and miR-200b in Mouse Inner Ear Morphogenesis
- Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of Chromosomes
- IAP1-Mediated Ubiquitylation Controls Activation of the Initiator Caspase DRONC Independent of Protein Degradation
- VANG-1 and PRKL-1 Cooperate to Negatively Regulate Neurite Formation in
- The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and Learning
- Comparative and Functional Genomics of PD630 for Biofuels Development
- Identification of Type 1 Diabetes–Associated DNA Methylation Variable Positions That Precede Disease Diagnosis
- PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian Cells
- Genetic Effects at Pleiotropic Loci Are Context-Dependent with Consequences for the Maintenance of Genetic Variation in Populations
- Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
- Bmp and Nodal Independently Regulate Expression to Maintain Unilateral Nodal Activity during Left-Right Axis Specification in Zebrafish
- Inter-Allelic Prion Propagation Reveals Conformational Relationships among a Multitude of [] Strains
- Emergence and Modular Evolution of a Novel Motility Machinery in Bacteria
- Histone Methyltransferase MET-2 Shields the Male X Chromosome from Checkpoint Machinery and Mediates Meiotic Sex Chromosome Inactivation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



