-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated Loci
Diabetes impacts approximately 200 million people worldwide, of whom approximately 10% are affected by type 1 diabetes (T1D). The application of genome-wide association studies (GWAS) has robustly revealed dozens of genetic contributors to the pathogenesis of T1D, with the most recent meta-analysis identifying in excess of 40 loci. To identify additional genetic loci for T1D susceptibility, we examined associations in the largest meta-analysis to date between the disease and ∼2.54 million SNPs in a combined cohort of 9,934 cases and 16,956 controls. Targeted follow-up of 53 SNPs in 1,120 affected trios uncovered three new loci associated with T1D that reached genome-wide significance. The most significantly associated SNP (rs539514, P = 5.66×10−11) resides in an intronic region of the LMO7 (LIM domain only 7) gene on 13q22. The second most significantly associated SNP (rs478222, P = 3.50×10−9) resides in an intronic region of the EFR3B (protein EFR3 homolog B) gene on 2p23; however, the region of linkage disequilibrium is approximately 800 kb and harbors additional multiple genes, including NCOA1, C2orf79, CENPO, ADCY3, DNAJC27, POMC, and DNMT3A. The third most significantly associated SNP (rs924043, P = 8.06×10−9) lies in an intergenic region on 6q27, where the region of association is approximately 900 kb and harbors multiple genes including WDR27, C6orf120, PHF10, TCTE3, C6orf208, LOC154449, DLL1, FAM120B, PSMB1, TBP, and PCD2. These latest associated regions add to the growing repertoire of gene networks predisposing to T1D.
Published in the journal: . PLoS Genet 7(9): e32767. doi:10.1371/journal.pgen.1002293
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002293Summary
Diabetes impacts approximately 200 million people worldwide, of whom approximately 10% are affected by type 1 diabetes (T1D). The application of genome-wide association studies (GWAS) has robustly revealed dozens of genetic contributors to the pathogenesis of T1D, with the most recent meta-analysis identifying in excess of 40 loci. To identify additional genetic loci for T1D susceptibility, we examined associations in the largest meta-analysis to date between the disease and ∼2.54 million SNPs in a combined cohort of 9,934 cases and 16,956 controls. Targeted follow-up of 53 SNPs in 1,120 affected trios uncovered three new loci associated with T1D that reached genome-wide significance. The most significantly associated SNP (rs539514, P = 5.66×10−11) resides in an intronic region of the LMO7 (LIM domain only 7) gene on 13q22. The second most significantly associated SNP (rs478222, P = 3.50×10−9) resides in an intronic region of the EFR3B (protein EFR3 homolog B) gene on 2p23; however, the region of linkage disequilibrium is approximately 800 kb and harbors additional multiple genes, including NCOA1, C2orf79, CENPO, ADCY3, DNAJC27, POMC, and DNMT3A. The third most significantly associated SNP (rs924043, P = 8.06×10−9) lies in an intergenic region on 6q27, where the region of association is approximately 900 kb and harbors multiple genes including WDR27, C6orf120, PHF10, TCTE3, C6orf208, LOC154449, DLL1, FAM120B, PSMB1, TBP, and PCD2. These latest associated regions add to the growing repertoire of gene networks predisposing to T1D.
Introduction
Diabetes impacts approximately 200 million people worldwide [1], with microvascular and cardiovascular disease being the primary complications. Approximately 10% of cases are type 1 diabetes (T1D) sufferers, with ∼3% increase in the incidence of T1D globally per year [2]. It is expected that the incidence is 40% higher in 2010 than in 1998 [3].
T1D is a clear example of a complex trait that results from the interplay between environmental and genetic factors. There are many lines of evidence that there is a strong genetic component to T1D, primarily due to the fact that T1D has high concordance among monozygotic twins [4] and runs strongly in families, together with a high sibling risk [5].
Prior to the era of GWAS, only five loci had been fully established to be associated with T1D. However, the majority of the other reported associations in the pre-GWAS era [6]–[8] remain highly doubtful, where an initial report of association does not hold up in subsequent replication attempts by other investigative groups. This previous hazy picture of the genetics of T1D can be put down to the use of the only methodologies that were available at the time and which were much more limited than GWAS i.e. the candidate gene approach (where genomic regions were studied based on biological reasoning) and family-based linkage methodologies. Inconsistent findings can also be attributed to small sample sizes i.e. when power is low the false discovery rate tends to be high; GWAS per se has not improved consistency, rather it has leveraged large, well powered sample sizes combined with sound statistical analyses.
It has been long established that approximately half of the genetic risk for T1D is conferred by the genomic region harboring the HLA class II genes (primarily HLA-DRB1, -DQA1 and -DQB1 genes), which encode the highly polymorphic antigen-presenting proteins. Other established loci prior to the application of GWAS are the genes encoding insulin (INS) [9]-[12], cytotoxic T-lymphocyte-associated protein 4 (CTLA4) [13]–[16], protein tyrosine phosphatase, non-receptor type 22 (PTPN22) gene [17], [18], interleukin 2 receptor alpha (IL2RA) [19]–[21] and ubiquitin-associated and SH3 domain-containing protein A (UBASH3A) [22].
The application of genome wide association studies (GWAS) has robustly revealed dozens of genetic contributors to T1D [23]–[29], the results of which have largely been independently replicated [30]–[36]. The most recently reported meta-analysis of this trait identified in excess of forty loci [29], including 18 novel regions plus confirmation of a number of loci uncovered through cross-disease comparisons [34]–[36]. As such, the risks conferred by these additional loci are relatively modest compared to the ‘low-hanging fruit’ described in the first studies and could only be ultimately uncovered when larger sample sizes were utilized.
We sought to expand further on this mode of analysis by combining our cohort with all publically released genome wide SNP datasets to identify additional loci contributing to the etiology of T1D. Unfortunately, there is a relative paucity of control genotype data in these publically available sources. To circumvent this problem, we combined individual level data from each available cohort and we then compared the cases with controls from two sources. We next separated all the individual level data into two groups, characterized by the type of genotyping platform that was used to genotype the samples, which would later be recombined using inverse-variance meta-analysis. The 6,523 cases genotyped on an Illumina BeadChip included subjects from McGill University, The Children's Hospital of Philadelphia (CHOP), The Diabetes Control and Complications Trial – Epidemiology of Diabetes Interventions and Complications (DCCT-EDIC) cohort, and the Type 1 Diabetes Genetics Consortium (T1DGC), which in turn were compared with 6,648 similarly genotyped controls recruited at CHOP. The 3,411 cases genotyped on Affymetrix arrays included subjects from the Genetics of Kidneys in Diabetes Study (GoKinD) and the Wellcome Trust Cases Control Consortium (WTCCC) that were then compared with 10,308 similarly genotyped controls, including being derived from non-autoimmune disease related cases from the WTCCC, as well as from the British 1958 Birth Cohort and the UK National Blood Service [24].
Results
We compared the power of our meta-analysis to that of the previous largest meta-analysis to date. We have more than double the power of the Barrett et al. meta-analysis to find variants with a relative risk of 1.2 and approximately three times the power to detect variants with a relative risk of 1.1 [29] (Figure S1).
We used principal components analysis (PCA) [37] in order to minimize the potential impact of population stratification in our case/control sample sets. Eigenstrat 3.0 was employed to remove outliers and to subsequently calculate the principal components in the Illumina and Affymetrix assigned groups separately. The principal components were then used as covariates in a logistic regression, using the software PLINK [38], to compute the P-values, betas and standard errors which were combined in our fixed effects inverse variance meta-analysis. After controlling for population stratification, the λ in the Affymetrix and Illumina cohorts was 1.11 and 1.17, respectively (see Figure 1 for Q-Q plot). A full description of the correlation of each eigenvector with known continental ancestry appears in Text S1. Mach was used to impute ∼2.54 million SNPs, including HapMap Phase 2 SNPs in the Illumina and Affymetrix datasets in order for the statistics to be uniform and amenable to being combined [39]. Results from the meta-analysis of this resulting ‘discovery’ cohort are shown Table 1 and graphically in Figure 2.
Fig. 1. QQ-plot of all previously unassociated regions in the combined meta-analysis discovery cohort. 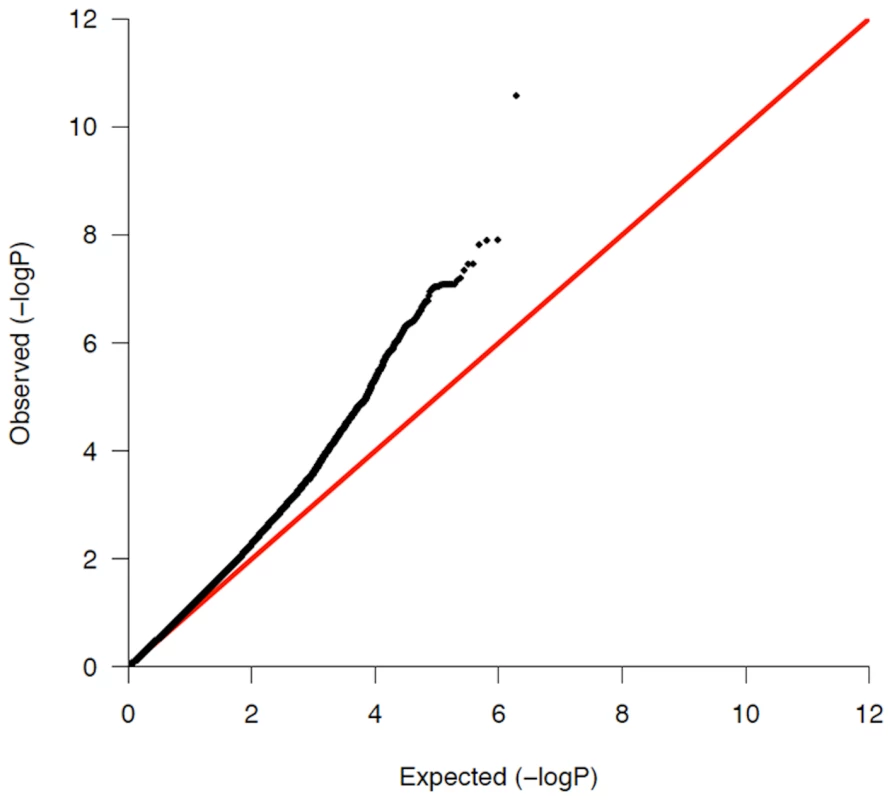
Fig. 2. Fixed effects meta-analysis P-values shown for each SNP in the combined meta-analyzed discovery cohort. 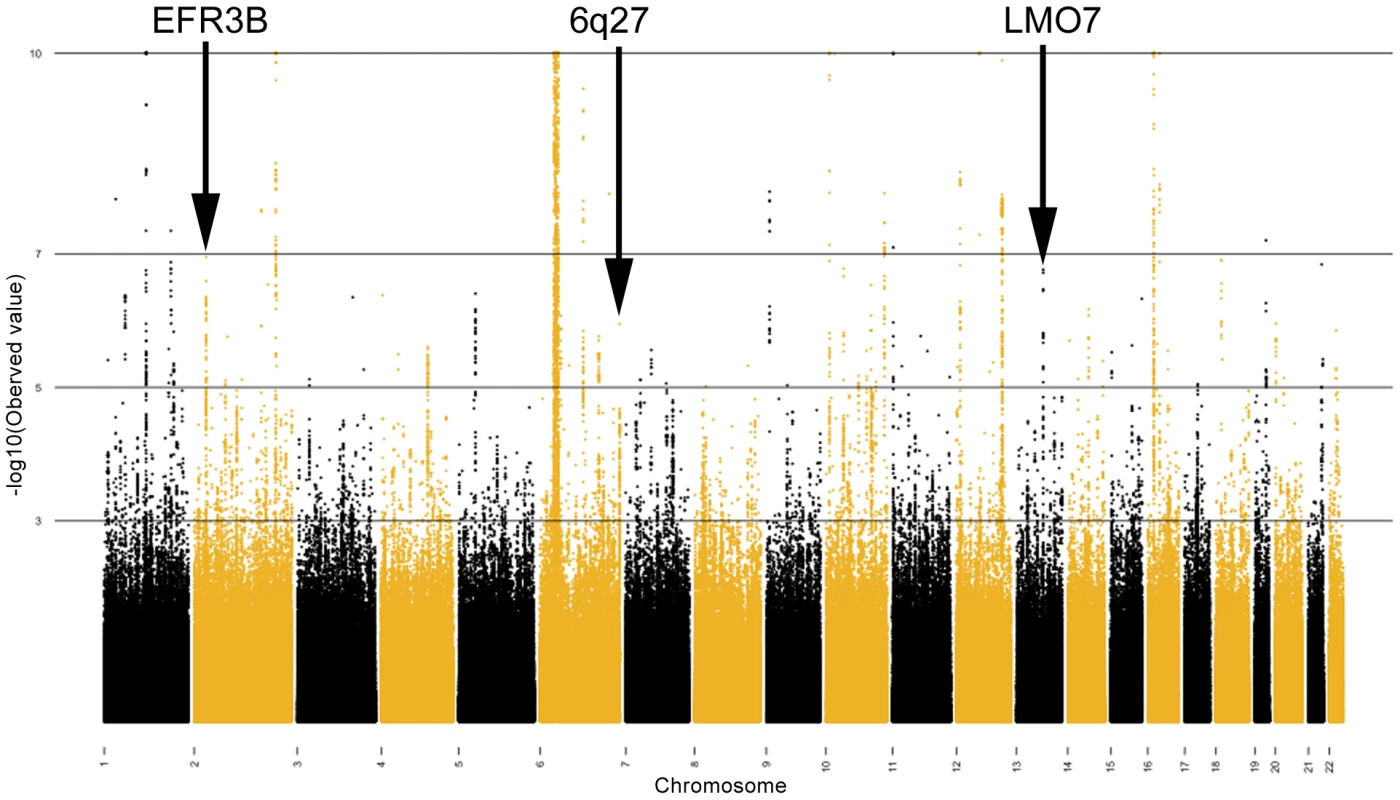
SNPs are sorted by chromosomal location. –log10(P-value) are shown, where the minimum P-value has been capped at 1×10−10. Only the novel loci are indicated. Tab. 1. SNPs are shown with P<0.05 in the replication set. 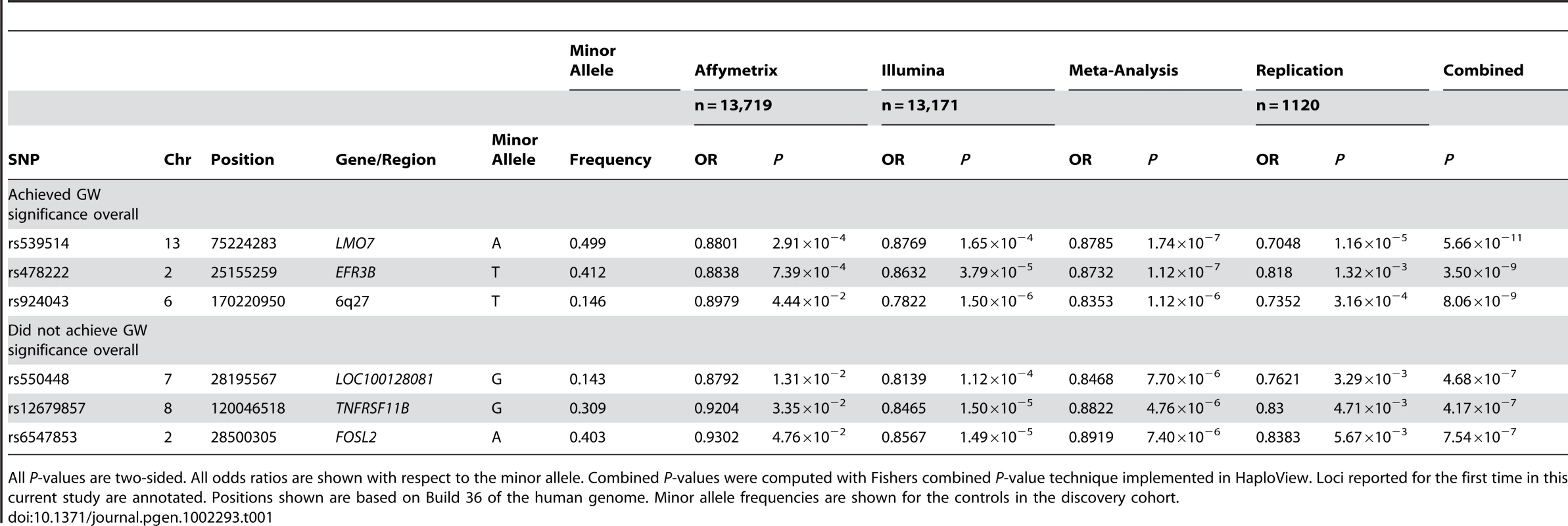
All P-values are two-sided. All odds ratios are shown with respect to the minor allele. Combined P-values were computed with Fishers combined P-value technique implemented in HaploView. Loci reported for the first time in this current study are annotated. Positions shown are based on Build 36 of the human genome. Minor allele frequencies are shown for the controls in the discovery cohort. 53 SNPs were brought forward to the replication stage based on satisfying the following criteria; however one of these SNPs consistently failed genotyping in the replication effort. The most significantly associated SNP at a given locus if the meta-analysis P-value was less than 1×10−5 (an independent locus was defined as a region for a given focal SNP, where we extended the region in both directions until either 250 kb had been traversed or until reaching another SNP with P<10−5), the Cochran's Q statistic P-value was greater than 0.05 and the locus had not been already reported from a previous GWAS of T1D. A table outlining the results for all previously described T1D associated SNPs plus our strongest associations for known regions associated with the disease are shown in Table 2 and Table S1, respectively. The replication cohort consisted of additional T1D affected trios from the T1DGC and McGill which had not been part of the original discovery cohort. The replication cohort was genotyped using the Sequenom iPLEX system and the results were analyzed using the transmission disequilibrium test in PLINK. Results for both the discovery and replication cohorts for the six SNPs that replicated with P≤0.05 are shown in Table 1 (the full outcomes for all SNPs tested are in Table S2).
Tab. 2. Discovery set P-values and odd ratios are shown for known T1D associated autosomal SNPs. 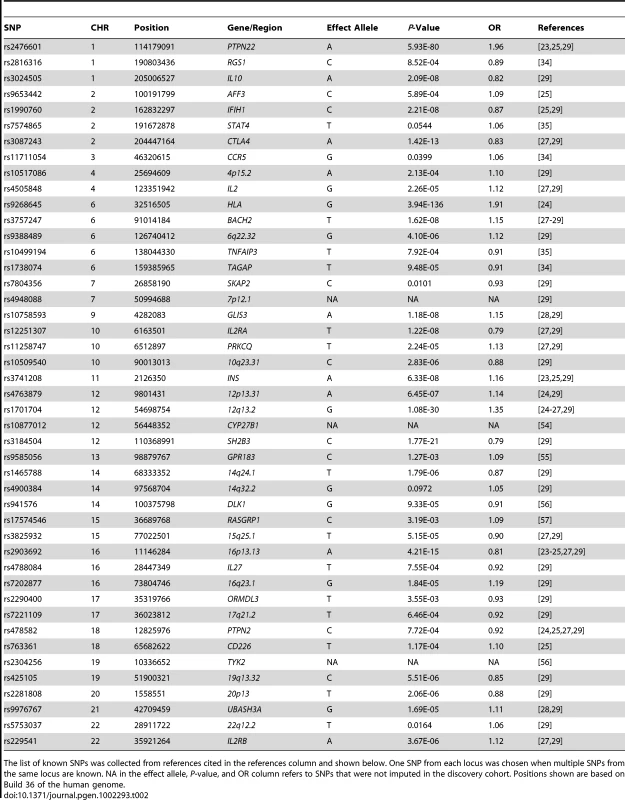
The list of known SNPs was collected from references cited in the references column and shown below. One SNP from each locus was chosen when multiple SNPs from the same locus are known. NA in the effect allele, P-value, and OR column refers to SNPs that were not imputed in the discovery cohort. Positions shown are based on Build 36 of the human genome. We combined the ‘discovery’ and ‘replication’ meta-analysis P-values using Fisher's combined P-value method implemented in Haploview, comparable to what has been previously employed by others [40]. Three of the SNPs, namely rs539514, rs478222 and rs924043, had combined P-values <5×10−8, the statistical threshold for genome wide significance, while the remaining three, namely rs550448, rs12679857 and rs6547853, failed to reach this bar but were suggestive of association as the alleles yielded both a consistent direction of effect and P-values <0.05 in the replication cohort. These two categories of outcome are summarized in Table 1; in addition, these six SNPs were further investigated with respect to adjustments of the discovery and met-analysis P-values based on the lambdas of each respective cohort (Table S3).
Discussion
We have carried out the largest meta-analysis of genome wide genotyped datasets for T1D to date. The replication of three loci using the stratification-free TDT with minimal Mendelian error clearly indicates that they are not false positives due to artifacts such as uncorrected systematic error from stratification or genotyping bias.
The most significantly associated SNP (rs539514, P = 5.66×10−11) resides in an intronic region of the LMO7 (LIM domain only 7) gene on 13q22. We investigated the associated region using LocusZoom [41] and determined that it is the only gene residing within the block of linkage disequilibrium harboring the signal (Figure S3). Regional plots showing P-values, linkage disequilibrium, and recombination rate for all SNPs in Table 1 are outlined in the Figures S2, S3, S4, S5, S6, S7. LMO7 encodes a protein that contains multiple domains, including a calponin homology domain, a PDZ domain and a LIM domain. There are multiple LMO7 isoforms already known but their full nature and the actual extent of different isoforms remains unclear [42]. Mice with homozygous deletions of LMO7 display retinal, muscular, and growth retardation [43]. Although the function of LMO7 doesn't clearly relate to the etiology of T1D, LMO7 is expressed in pancreatic islets and thus is a possible biological candidate at this locus [44]; however it should be noted that the retinal, muscular development and islet patterns are a key element in Emery-Dreifuss Muscular Dystrophy, caused by mutations in LMO7 [45], but bears very little similarity to T1D.
The second most significantly associated SNP (rs478222, P = 3.50×10−9) resides in an intronic region of the EFR3B (protein EFR3 homolog B) gene on 2p23; however the region of linkage disequilibrium is approximately 800 kb and harbors additional multiple genes, including 3NCOA1, C2orf79, CENPO, ADCY3, DNAJC27, POMC, and DNMT3A. (Figure S2). A previous meta-analysis of a subset of the data used in this current study found suggestive association with T1D in the same LD block with the independent SNP, rs2165738(r2 = 0.115), but did not achieve genome wide significance at that time (P = 3.65×10−6) [27]; however, we only found modest evidence of association with rs2165738 (P = 4.78×10−3) in our discovery cohort. There has also been association to inflammatory bowel disease [46] height [47], [48] and BMI [49] reported at this locus, where in both cases the risk allele for increased height or BMI was protective for T1D risk.
The third most significantly associated SNP (rs924043, P = 8.06×10−9) lies in an intergenic region on 6q27, where the region of association is approximately 900 kb and harbors multiple genes including WDR27, C6orf120, PHF10, TCTE3, C6orf208, LOC154449, DLL1, FAM120B, PSMB1, TBP and PCD2 (Figure S5). In addition, despite not reaching the bar for genome wide significance, we did observe evidence for association at three additional loci (Table 1) containing the candidate genes LOC100128081, TNFRSF11B and FOSL2. Of these, it is notable that TNFRSF11B is a strongly associated locus with bone mineral density, also as a consequence of GWAS [50], [51]. In addition, the locus harboring LOC100128081 has also been reported in the context of a GWAS of SLE [52]. Further work will be required to fully validate the role of these particular loci in the pathogenesis of T1D.
The Barrett et al. meta-analysis was able to use British controls with British cases and American controls with American cases [29]. We did not have the same control data to be able to make the same comparisons. In the case of the Affymetrix analysis, some American cases were analyzed with purely British controls and, in the case of the Illumina analysis, some British cases with purely American controls. As such, we were forced to make our corrections using eigenvectors as covariates in our analysis; this will have the effect of modestly weakening the level of significance for associations that vary in allele frequency between the cases and controls, as now the case and controls will both vary with the eigenvectors to some degree. This in effect will make our analysis overly conservative with estimating the true effect of a SNP, and in fact every SNP that had a P-value less than 0.05 in the replication set did indeed have a greater effect than that which was estimated from the discovery set.
In summary, we provide convincing evidence for the existence of three additional loci associated with the T1D, adding to the repertoire of over 50 loci already demonstrated to be associated with the disease.
Materials and Methods
Ethical statement
The study was approved by the institutional review board and the ethics committee of each institution. Written informed consent was obtained from each participant in accordance with institutional requirements and the Declaration of Helsinki Principles.
Samples
Cases in the discovery set were obtained from four publically available resources and combined with those from a previous publication for the meta-analysis. Samples descriptions are available on dbgap (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap) for the T1DGC (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000180.v1.p1), GoKinD (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000088.v1.p1), and DCCT-EDIC (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000086.v2.p1) patients. The WTCCC sample information is available from [24]. Samples from the T1D segment of the WTCCC were used as cases, while controls were derived from the 1958 Birth Cohort, UK Blood Service, Bipolar disorder, Coronary heart disease, Hypertension, and Type 2 Diabetes segments. The remaining cases used in the meta-analysis were previously described [23].
The total number of individuals used in the meta-analysis discovery set was 26,890 (9,934 cases/16,956 controls). The replication set consisted of 1120 case-parent trios from the T1DGC and those identified through pediatric diabetes clinics in Canada. The replication set was identical to that used in Hakonarson et al. with an extension of patients identified through pediatric diabetes clinics in Montreal, Toronto, Ottawa, and Winnipeg. All individuals were of Caucasian ancestry. A breakdown of the number of samples in each cohort in the discovery phase and a comparison with the numbers used in the Barrett et al. meta-analysis are shown in Table 3 [29]. The minor variation in the number of cases reflects that, despite using slight differences in both quality control and methods for dealing with population stratification, we have comparable numbers of cases from this cohort remaining in our analysis. Primarily, this small difference is due to the fact that we strictly accounted for relatedness and duplicates within and across cohorts in this current setting.
Tab. 3. A comparison of the number of samples used in each discovery cohort from the current meta-analysis and those used in the previously reported meta-analysis <em class="ref">[29]</em> . ![A comparison of the number of samples used in each discovery cohort from the current meta-analysis and those used in the previously reported meta-analysis <em class="ref">[29]</em> .](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/d6aa9d2fe355c12329a1c0a682bb87d0.png)
Power analysis
Power analysis was performed with the genetic analysis calculator which can be found at (http://pngu.mgh.harvard.edu/~purcell/gpc/) [53]. Various assumption were made included perfect LD between the causative variant and the markers that were genotyped, an additive genetic model, a disease prevalence of 0.0033 and an alpha of 1×10−5.
Genotyping, quality control, and imputation
Discovery samples from Philadelphia, Canada, T1DGC, and DCCT-EDIC were genotyped on a mixture of the Illumina HumanHap 550v1, 2, and 3, whereas samples from GoKinD and WTCCC were genotyped on the Affymetrix 500 K Chip. Sequenom iPlex was used to replicate the findings of the meta-analysis in 1,120 affected offspring trios from the T1DGC and from Canada.
All individuals needed an individual genotyping call rate greater than 0.98 to be included in the analysis pre-imputation and individuals were removed that showed evidence of cryptic relatedness and duplication within and across cohorts using identity-by-state. SNP quality control was performed on all samples pre-imputation. SNPs were excluded from the analysis if the minor allele was below 1%, the genotyping call rate was less than 95%, or the Hardy Weinberg equilibrium P-value was less than 0.00001.
To control for population stratification, Eigenstrat 3.0 was used to compute the top 10 principal components of the individuals genotyped on the Illumina SNP chips and the Affymetrix SNP chips separately [37]. Individuals were removed from the analysis if they were 6 standard deviations away from the mean of one of the top 10 principal components. After controlling for population stratification, the estimated lambda in the Affymetrix data was 1.11 and 1.17 in the Illumina data.
Mach 1.0 was used to impute ∼2.54 millions SNPs from the HapMap CEU panel for all individuals [39]. SNPs were excluded after imputation if they had a minor allele frequency less than 0.01 and an r2 value less than 0.3.
Genome-wide association and meta-analysis
PLINK [38] was used to perform a logistic regression using the 10 principal components as covariates, T1D status as the outcome, and in the case of the Affymetrix cohort, an extra dummy covariate specifying WTCCC or GoKinD cohort membership. Results from the logistic regression of 2,436,110 SNPs from the Affymetrix samples and 2,062,307 SNPs from the Illumina samples separately were combined using inverse-variance meta-analysis in PLINK. A fixed effects meta-analysis was performed and 53 SNPs were chosen for replication who had a fixed effects P-value <0.00001, a Cochran's Q statistic P-value greater than 0.05 and were not previously known to be associated with type 1 diabetes. However one of the SNPs consistently failed during the replication effort.
Supporting Information
Zdroje
1. SteynNPLambertEVTabanaH 2009 Conference on "Multidisciplinary approaches to nutritional problems". Symposium on "Diabetes and health". Nutrition interventions for the prevention of type 2 diabetes. Proc Nutr Soc 68 55 70
2. EURODIAB ACE Study Group 2000 Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet 355 873 876
3. OnkamoPVaananenSKarvonenMTuomilehtoJ 1999 Worldwide increase in incidence of Type I diabetes-the analysis of the data on published incidence trends. Diabetologia 42 1395 1403
4. RedondoMJYuLHawaMMackenzieTPykeDA 2001 Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia 44 354 362
5. ClaytonDG 2009 Prediction and interaction in complex disease genetics: experience in type 1 diabetes. PLoS Genet 5 e1000540 doi:10.1371/journal.pgen.1000540
6. GuoDLiMZhangYYangPEckenrodeS 2004 A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nat Genet 36 837 841
7. MirelDBValdesAMLazzeroniLCReynoldsRLErlichHA 2002 Association of IL4R haplotypes with type 1 diabetes. Diabetes 51 3336 3341
8. Biason-LauberABoehmBLang-MuritanoMGauthierBRBrunT 2005 Association of childhood type 1 diabetes mellitus with a variant of PAX4: possible link to beta cell regenerative capacity. Diabetologia 48 900 905
9. BellGIHoritaSKaramJH 1984 A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes 33 176 183
10. BennettSTLucassenAMGoughSCPowellEEUndlienDE 1995 Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet 9 284 292
11. VafiadisPBennettSTToddJANadeauJGrabsR 1997 Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 15 289 292
12. BarrattBJPayneFLoweCEHermannRHealyBC 2004 Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes 53 1884 1889
13. KristiansenOPLarsenZMPociotF 2000 CTLA-4 in autoimmune diseases-a general susceptibility gene to autoimmunity? Genes Immun 1 170 184
14. UedaHHowsonJMEspositoLHewardJSnookH 2003 Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423 506 511
15. AnjosSMTessierMCPolychronakosC 2004 Association of the cytotoxic T lymphocyte-associated antigen 4 gene with type 1 diabetes: evidence for independent effects of two polymorphisms on the same haplotype block. J Clin Endocrinol Metab 89 6257 6265
16. NisticoLBuzzettiRPritchardLEVan der AuweraBGiovanniniC 1996 The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry. Hum Mol Genet 5 1075 1080
17. BottiniNMusumeciLAlonsoARahmouniSNikaK 2004 A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 36 337 338
18. SmythDCooperJDCollinsJEHewardJMFranklynJA 2004 Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes 53 3020 3023
19. VellaACooperJDLoweCEWalkerNNutlandS 2005 Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet 76 773 779
20. QuHQMontpetitAGeBHudsonTJPolychronakosC 2007 Toward further mapping of the association between the IL2RA locus and type 1 diabetes. Diabetes 56 1174 1176
21. LoweCECooperJDBruskoTWalkerNMSmythDJ 2007 Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet 39 1074 1082
22. ConcannonPOnengut-GumuscuSToddJASmythDJPociotF 2008 A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes 57 2858 2861
23. HakonarsonHGrantSFABradfieldJPMarchandLKimCE 2007 A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 448 591 594
24. Wellcome Trust Case Control Consortium 2007 Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 661 678
25. ToddJAWalkerNMCooperJDSmythDJDownesK 2007 Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39 857 864
26. HakonarsonHQuHQBradfieldJPMarchandLKimCE 2008 A novel susceptibility locus for type 1 diabetes on Chr12q13 identified by a genome-wide association study. Diabetes 57 1143 1146
27. CooperJDSmythDJSmilesAMPlagnolVWalkerNM 2008 Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat Genet 40 1399 1401
28. GrantSFQuHQBradfieldJPMarchandLKimCE 2009 Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes 58 290 295
29. BarrettJCClaytonDGConcannonPAkolkarBCooperJD 2009 Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41 703 707
30. QuHQBradfieldJPLiQKimCFrackeltonE 2010 In silico replication of the genome-wide association results of the Type 1 Diabetes Genetics Consortium. Hum Mol Genet 19 2534 2538
31. WangKBaldassanoRZhangHQuHQImielinskiM 2010 Comparative genetic analysis of inflammatory bowel disease and type 1 diabetes implicates multiple loci with opposite effects. Hum Mol Genet 19 2059 2067
32. CooperJDWalkerNMSmythDJDownesKHealyBC 2009 Follow-up of 1715 SNPs from the Wellcome Trust Case Control Consortium genome-wide association study in type I diabetes families. Genes Immun 10 Suppl 1 S85 94
33. BarrettJCClaytonDGConcannonPAkolkarBCooperJD 2009 Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet
34. SmythDJPlagnolVWalkerNMCooperJDDownesK 2008 Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 359 2767 2777
35. FungEYSmythDJHowsonJMCooperJDWalkerNM 2009 Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun 10 188 191
36. CooperJDWalkerNMHealyBCSmythDJDownesK 2009 Analysis of 55 autoimmune disease and type II diabetes loci: further confirmation of chromosomes 4q27, 12q13.2 and 12q24.13 as type I diabetes loci, and support for a new locus, 12q13.3-q14.1. Genes Immun 10 Suppl 1 S95 120
37. PriceALPattersonNJPlengeRMWeinblattMEShadickNA 2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
38. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
39. LiYWillerCSannaSAbecasisG 2009 Genotype imputation. Annu Rev Genomics Hum Genet 10 387 406
40. BarrettJCFryBMallerJDalyMJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 263 265
41. PruimRJWelchRPSannaSTeslovichTMChinesPS 2010 LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26 2336 2337
42. PruittKDTatusovaTMaglottDR 2005 NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 33 D501 504
43. SemenovaEWangXJablonskiMMLevorseJTilghmanSM 2003 An engineered 800 kilobase deletion of Uchl3 and Lmo7 on mouse chromosome 14 causes defects in viability, postnatal growth and degeneration of muscle and retina. Hum Mol Genet 12 1301 1312
44. KutluBBurdickDBaxterDRasschaertJFlamezD 2009 Detailed transcriptome atlas of the pancreatic beta cell. BMC Med Genomics 2 3
45. HolaskaJMRais-BahramiSWilsonKL 2006 Lmo7 is an emerin-binding protein that regulates the transcription of emerin and many other muscle-relevant genes. Hum Mol Genet 15 3459 3472
46. FrankeAMcGovernDPBarrettJCWangKRadford-SmithGL 2010 Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42 1118 1125
47. Lango AllenHEstradaKLettreGBerndtSIWeedonMN 2010 Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467 832 838
48. GudbjartssonDFWaltersGBThorleifssonGStefanssonHHalldorssonBV 2008 Many sequence variants affecting diversity of adult human height. Nat Genet 40 609 615
49. SpeliotesEKWillerCJBerndtSIMondaKLThorleifssonG 2010 Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42 937 948
50. RichardsJBRivadeneiraFInouyeMPastinenTMSoranzoN 2008 Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371 1505 1512
51. RivadeneiraFStyrkarsdottirUEstradaKHalldorssonBVHsuYH 2009 Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41 1199 1206
52. GatevaVSandlingJKHomGTaylorKEChungSA 2009 A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41 1228 1233
53. PurcellSChernySSShamPC 2003 Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19 149 150
54. BaileyRCooperJDZeitelsLSmythDJYangJH 2007 Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 56 2616 2621
55. HeinigMPetrettoEWallaceCBottoloLRotivalM A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 467 460 464
56. WallaceCSmythDJMaisuria-ArmerMWalkerNMToddJA The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat Genet 42 68 71
57. QuHQGrantSFBradfieldJPKimCFrackeltonE 2009 Association of RASGRP1 with type 1 diabetes is revealed by combined follow-up of two genome-wide studies. J Med Genet 46 553 554
Štítky
Genetika Reprodukční medicína
Článek Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and BrainČlánek Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-StateČlánek Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional PolymorphismsČlánek Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of ChromosomesČlánek The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and LearningČlánek PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian CellsČlánek Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 9
-
Všechny články tohoto čísla
- Temporal Trends in Results Availability from Genome-Wide Association Studies
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
- Genetic Variants at Chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 Influence the Risk of Breast Cancer in Men
- Large-Scale Gene-Centric Analysis Identifies Novel Variants for Coronary Artery Disease
- Genetic Association for Renal Traits among Participants of African Ancestry Reveals New Loci for Renal Function
- Transcriptome Kinetics Is Governed by a Genome-Wide Coupling of mRNA Production and Degradation: A Role for RNA Pol II
- Conserved Regulation of p53 Network Dosage by MicroRNA–125b Occurs through Evolving miRNA–Target Gene Pairs
- Heterozygous Mutations of Are Associated with an Increased Risk of Isolated Metopic Craniosynostosis in Humans and Mice
- Study of FoxA Pioneer Factor at Silent Genes Reveals Rfx-Repressed Enhancer at and a Potential Indicator of Esophageal Adenocarcinoma Development
- Cholesterol Metabolism Is Required for Intracellular Hedgehog Signal Transduction
- Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and Brain
- Age-Dependent Recombination Rates in Human Pedigrees
- Sequence Conservation and Functional Constraint on Intergenic Spacers in Reduced Genomes of the Obligate Symbiont
- Sex Chromosome Mosaicism and Hybrid Speciation among Tiger Swallowtail Butterflies
- A Negative Feedback Loop That Limits the Ectopic Activation of a Cell Type–Specific Sporulation Sigma Factor of
- Phased Whole-Genome Genetic Risk in a Family Quartet Using a Major Allele Reference Sequence
- Mutations in or near the Transmembrane Domain Alter PMEL Amyloid Formation from Functional to Pathogenic
- Inactivation of Alters Melanosome Shape But Has Only a Subtle Effect on Visible Pigmentation
- Novel Interactions between Actin and the Proteasome Revealed by Complex Haploinsufficiency
- Germline Genetic Variants Disturbing the /LIN28 Double-Negative Feedback Loop Alter Breast Cancer Susceptibility
- Separation of Recombination and SOS Response in RecA Suggests LexA Interaction Sites
- Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-State
- Misregulation of Scm3p/HJURP Causes Chromosome Instability in and Human Cells
- A Noncoding Point Mutation of Causes Multiple Developmental Malformations and Obesity in Twirler Mice
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- A Genome-Wide Metabolic QTL Analysis in Europeans Implicates Two Loci Shaped by Recent Positive Selection
- Bacterial Communities of Diverse Species: Ecological Context of a Host–Microbe Model System
- A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated Loci
- Elongator Complex Influences Telomeric Gene Silencing and DNA Damage Response by Its Role in Wobble Uridine tRNA Modification
- Elevated Proteasome Capacity Extends Replicative Lifespan in
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- MicroRNA Predictors of Longevity in
- An Iterative Genetic and Dynamical Modelling Approach Identifies Novel Features of the Gene Regulatory Network Underlying Melanocyte Development
- Atypical AT Skew in Firmicute Genomes Results from Selection and Not from Mutation
- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- Genomic Analysis of QTLs and Genes Altering Natural Variation in Stochastic Noise
- The Abnormal Phenotypes of Cartilage and Bone in Calcium-Sensing Receptor Deficient Mice Are Dependent on the Actions of Calcium, Phosphorus, and PTH
- Cell Type–Specific Transcriptome Analysis Reveals a Major Role for and miR-200b in Mouse Inner Ear Morphogenesis
- Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of Chromosomes
- IAP1-Mediated Ubiquitylation Controls Activation of the Initiator Caspase DRONC Independent of Protein Degradation
- VANG-1 and PRKL-1 Cooperate to Negatively Regulate Neurite Formation in
- The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and Learning
- Comparative and Functional Genomics of PD630 for Biofuels Development
- Identification of Type 1 Diabetes–Associated DNA Methylation Variable Positions That Precede Disease Diagnosis
- PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian Cells
- Genetic Effects at Pleiotropic Loci Are Context-Dependent with Consequences for the Maintenance of Genetic Variation in Populations
- Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
- Bmp and Nodal Independently Regulate Expression to Maintain Unilateral Nodal Activity during Left-Right Axis Specification in Zebrafish
- Inter-Allelic Prion Propagation Reveals Conformational Relationships among a Multitude of [] Strains
- Emergence and Modular Evolution of a Novel Motility Machinery in Bacteria
- Histone Methyltransferase MET-2 Shields the Male X Chromosome from Checkpoint Machinery and Mediates Meiotic Sex Chromosome Inactivation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



