-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrA
Many taxonomically diverse prokaryotes enzymatically modify their DNA by replacing a non-bridging oxygen with a sulfur atom at specific sequences. The biological implications of this DNA S-modification (phosphorothioation) were unknown. We observed that simultaneous expression of the dndA-E gene cluster from Streptomyces lividans 66, which is responsible for the DNA S-modification, and the putative Streptomyces coelicolor A(3)2 Type IV methyl-dependent restriction endonuclease ScoA3McrA (Sco4631) leads to cell death in the same host. A His-tagged derivative of ScoA3McrA cleaved S-modified DNA and also Dcm-methylated DNA in vitro near the respective modification sites. Double-strand cleavage occurred 16–28 nucleotides away from the phosphorothioate links. DNase I footprinting demonstrated binding of ScoA3McrA to the Dcm methylation site, but no clear binding could be detected at the S-modified site under cleavage conditions. This is the first report of in vitro endonuclease activity of a McrA homologue and also the first demonstration of an enzyme that specifically cleaves S-modified DNA.
Published in the journal: . PLoS Genet 6(12): e32767. doi:10.1371/journal.pgen.1001253
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001253Summary
Many taxonomically diverse prokaryotes enzymatically modify their DNA by replacing a non-bridging oxygen with a sulfur atom at specific sequences. The biological implications of this DNA S-modification (phosphorothioation) were unknown. We observed that simultaneous expression of the dndA-E gene cluster from Streptomyces lividans 66, which is responsible for the DNA S-modification, and the putative Streptomyces coelicolor A(3)2 Type IV methyl-dependent restriction endonuclease ScoA3McrA (Sco4631) leads to cell death in the same host. A His-tagged derivative of ScoA3McrA cleaved S-modified DNA and also Dcm-methylated DNA in vitro near the respective modification sites. Double-strand cleavage occurred 16–28 nucleotides away from the phosphorothioate links. DNase I footprinting demonstrated binding of ScoA3McrA to the Dcm methylation site, but no clear binding could be detected at the S-modified site under cleavage conditions. This is the first report of in vitro endonuclease activity of a McrA homologue and also the first demonstration of an enzyme that specifically cleaves S-modified DNA.
Introduction
The sequence of the DNA bases contains the genetic information that is copied with great accuracy and inherited by successive generations. DNA may also carry so called epigenetic modifications that are added by host enzymes after replication. These modifications are lost or changed after transfer of the DNA to a new host by conjugation, transformation or transfection. Epigenetic modifications can also change in response to changing environmental conditions, and they are generally important for eukaryotic gene regulation including carcinogenesis [1].
In bacteria, epigenetic modifications have more specialized roles including the protection of self DNA against restriction endonucleases (REases) which cleave foreign, differently modified DNA. Potential invaders, such as bacteriophages, have developed DNA modifications and other measures to overcome these restriction barriers, and bacteria have evolved to restrict even the modified foreign DNA. One such strategy is for bacteria to restrict methylated DNA [2].
There are many methyl-specific REases. Some cleave DNA at specific methylated recognition sequences. Others make contact with their target DNA at a specific methylated recognition sequence, and then move along the DNA before cleaving at an undefined site. These Type IV methyl-specific REases are very diverse in their amino acid (aa) sequences, and they may consist of one or several peptides [3].
There are about 1303 putative Type IV REases in REBASE (http://rebase.neb.com), but only 3 have been biochemically characterized, whereas others are predicted based on bioinformatic analysis of DNA sequences. Quite unusually, some Type IV REases recognize methylated and also hydroxymethylated or glucosyl-hydroxymethylated DNA [4], [5].
We have discovered a Type IV REase from a bacterium of the genus Streptomyces that cleaves methylated and phosphorothioated (S-modified) DNA that has a non-bridging oxygen of the phosphate group in the DNA backbone replaced by sulfur. The DNA S-modification is sequence specific, and a dnd (DNA degradation) gene cluster encoding four or five proteins is responsible for the S-modification [6], [7].
Streptomycetes are filamentous soil bacteria that produce many chemically diverse antibiotics. The 8.7 Mb linear genome of Streptomyces coelicolor A3(2) has been fully sequenced and annotated [8]. S. coelicolor contains at least four methyl-specific restriction endonucleases that restrict (reduce or prevent) the introduction of methylated DNA e.g. from Dam+ Dcm+ Hsd+ E. coli K-12 strains [9]. Therefore, DNA is generally passaged through a non-methylating dam dcm hsd E. coli host before introduction into S. coelicolor [10], [11].
Alternatively, the less restricting Streptomyces lividans 66 has been used as a recipient for methylated DNA from E. coli K. The genes of S. lividans and S. coelicolor are very similar and highly syntenous except for several genomic islands (GIs), some of which are mobile elements [12], [13].
One of these methyl-specific endonucleases, ScoA3McrA (Sco4631) [4] is the focus of this report. As shown schematically in Figure 1, ScoA3McrA is located on the 17.1 kb mobile element Gi11, which is also known as the conjugative and integrative plasmid SLP1 [14].
Fig. 1. Streptomyces and E. coli strains used as sources of recombinant DNA. 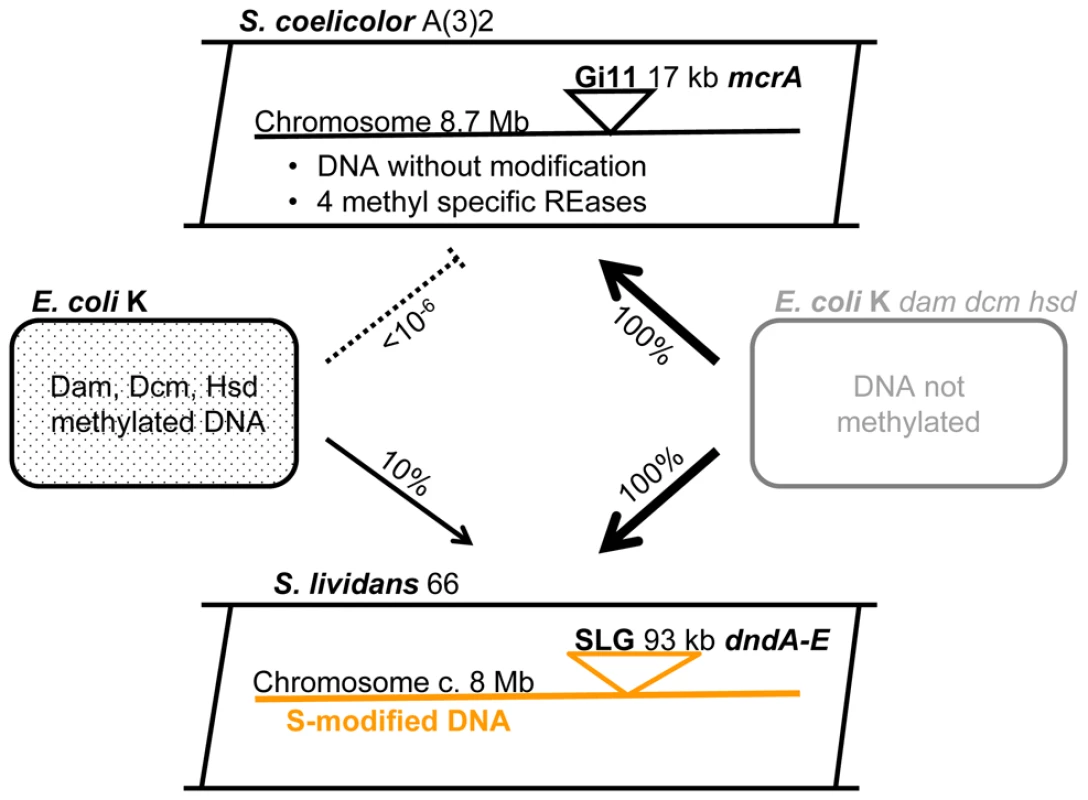
Gi11 ( = SLP1) and SLG are genomic islands (DNA inserts) which are unique to S. coelicolor A(3)2 and S. lividans 66, respectively. The numbers on the arrows between the E. coli and Streptomyces cartoons indicate the approximate relative efficiency of plasmid transfer by protoplast transformation as reported by Gonzalez-Ceron et al. (2009). McrA (ScoA3McrA) is remotely similar to the Type IV restriction endonucleases EcoKMcrA and has been shown to restrict methylated DNA in vivo in Stretpomyces [9]. Dam, Dcm and Hsd are sequence-specific DNA methylases of E. coli. dndA-E is a gene cluster of S. lividans responsible for the sequence-specific S-modification in this strain (only c. 1 in 6000 bp contain sulfur; dnd stands for DNA degradation during electrophoresis). The Type IV REase EcoKMcrA restricts DNA methylated at the sequences C5mCGG and has been shown to bind to this sequence, but in vitro DNA cleavage has not been observed [15]–[17]. Sequence comparison and phylogenetic analysis showed that the ScoA3 and EcoK McrA proteins have very little aa sequence similarities apart from the region surrounding the conserved HNH motif (Figure S7B).
About one in 6000 phosphate groups of the backbone of S. lividans genomic DNA contain sulfur instead of a non-bridging oxygen at specific sequences [7], [18]. The stereospecific S-modification (phosphorothioation) renders purified DNA susceptible to oxidative double-strand cleavage by Tris peracid which is generated at the anode during electrophoresis [19]. Five genes, dndA-E located on the apparently non-transmissible 93 kb SLG genomic island [13], are involved in DNA S-modification. Evidence for DNA S-modification has been discovered in several Streptomyces species, and in phylogenetically diverse prokaryotes including several economically important species [13], [20].
The DNA S-modification of S. lividans does not seem to be accompanied by a cognate restriction endonuclease that could defend the strain against the invasion of bacteriophages that lack this modification, and S. lividans has traditionally been used as a permissive host for the isolation of many Streptomyces phages [11].
In this report we show that S-modified DNA (as well as methylated DNA) is restricted in vivo by the S. coelicolor methyl-specific endonucleases ScoA3McrA. We also report site-specific in vitro cleavage of S-modified DNA by the purified enzyme. This is the first indication of a biological role of DNA S-modification, and also the first report of in vitro DNA cleavage by a McrA homologue.
Results
Failure to introduce the DNA sulfur modification gene cluster into S. coelicolor
The dndA-E gene cluster of S. lividans is responsible for the DNA S-modification (phosphorothioation) of this strain [6]. We wanted to express this gene cluster in S. coelicolor which is closely related to S. lividans but lacks these particular genes. In order to generate a stable recombinant S. coelicolor strain, we cloned the dndA-E gene cluster into the φC31-derived integrative vector pSET152 which has an apramycin resistance marker for selection, and an origin of transfer for highly efficient conjugative transfer from E. coli ET12567/pUZ8002 (dam dcm hsd tra+) to S. coelicolor M145. The plasmid vector without cloned DNA (pSET152) produced as expected c. 105 apramycin resistant S. coelicolor exconjugants per experiment (one Petri dish). Unexpectedly, pSET152::dndA-E (pHZ1904) produced only an average of nine apramycin resistant exconjugants per Petri dish (Figure 2, Panel 1).
Fig. 2. Restriction of plasmids expressing the dndA-E gene cluster by Streptomyces strains containing sco4631. 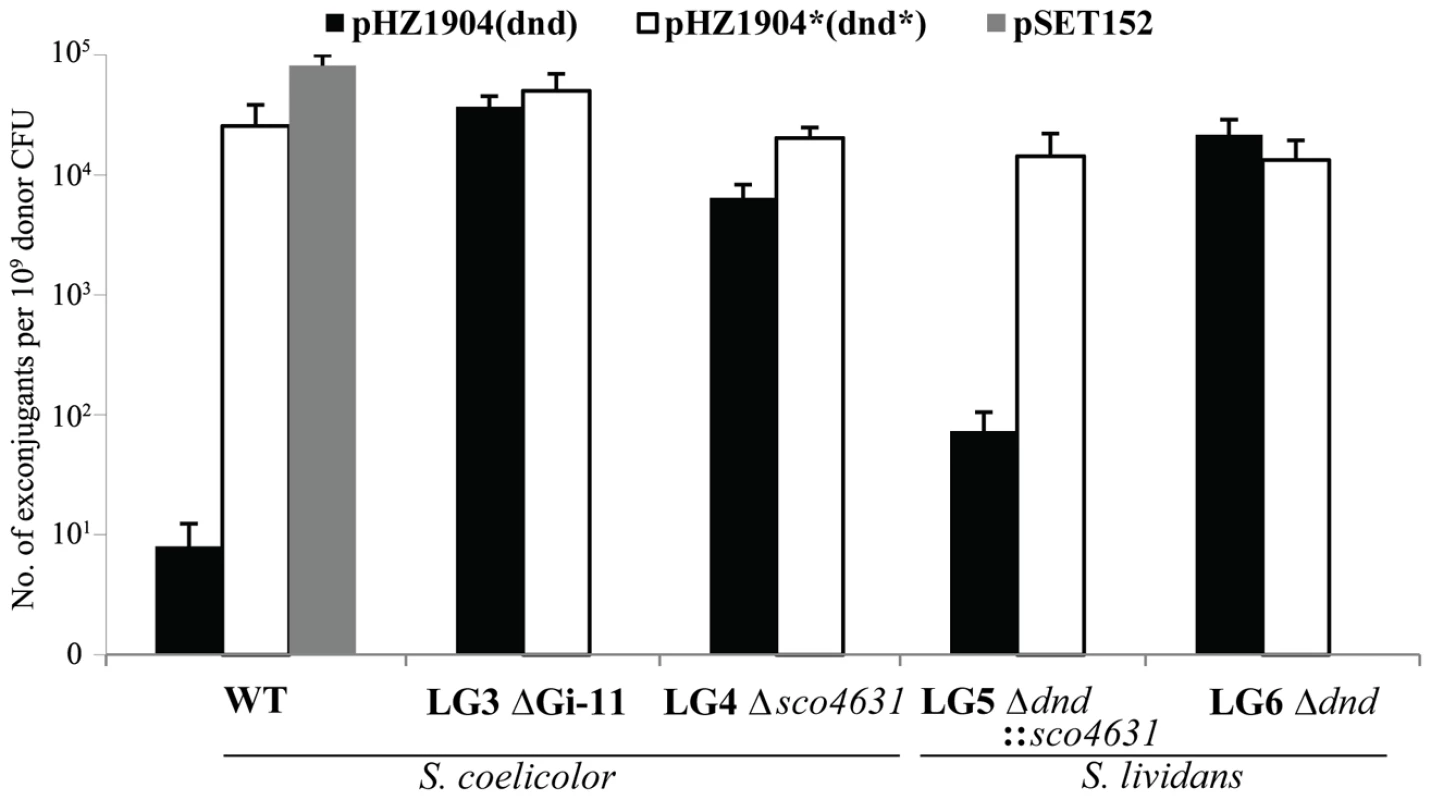
Graph showing the number of apramycin resistant S. coelicolor (1–3) and S. lividans (4 and 5) exconjugants that were obtained in matings with E. coli ET12567/pUZ8002 containing mobilizable (oriT) plasmids. WT, plasmid-free, wild-type S. coelicolor M145 strain; LG3, S. coelicolor mutant lacking the entire genomic island Gi11 ( = SLP1) which includes sco4631; LG4, S. coelicolor mutant from which only sco4631 was deleted; LG5, S. lividans derivative which lacks the dnd gene cluster and contains a cloned copy of the S. coelicolor gene sco4631; LG6 S. lividans derivative which lacks the dnd gene cluster. The graph shows that pHZ1904 which contains the dndA-E gene cluster (causes DNA S-modification; black bars), is specifically restricted by the wild type S. coelicolor strain. pHZ1904* (white bars), which differs from pHZ1904 by an inactivating frame shift point mutation in dndE, was not restricted. The cloning vector pSET152 without insert (grey bar) served as an additional, not restricted control. These apramycin resistant pSET152::dndA-E exconjugants were expected to express the dnd genes and generate S-modified S. coelicolor DNA that is visibly degraded during agarose gel electrophoresis (methods used in [21]). Only 49 out of 100 exconjugants showed the expected DNA degradation (Dnd+ phenotype; Dnd stands for DNA degradation). The remaining 51 contained stable, S-free DNA (Dnd− phenotype, Figure S1A).
The dndA-E gene cluster of three Dnd− exconjugants was analysed and each contained a different mutation that was likely to abolish the S-modification activity (Figure S1B).
One of these plasmids, pHZ1904* contained the entire dndA-E gene cluster with a single base insertion (frameshift mutation) in dndE which is required for DNA S-modification (Figure S1B). pHZ1904* was excised in circular form from S. coelicolor and introduced into non-methylating E. coli ET12567/pUZ8002. In interspecific matings pHZ1904* produced about 3×104 apramycin resistant S. coelicolor M145 exconjugants, almost as many as pSET152 without cloned DNA (Figure 2, Panel 1).
We speculated that S-modification of S. coelicolor DNA by the intact dndA-E gene cluster was not tolerated because of a hypothetical S. coelicolor endonuclease that restricts S-modified DNA. The above 51 Dnd− exconjugants would therefore all contain a mutant, inactive dndA-E gene cluster, and the 49 S-modified (Dnd+) exconjugants may have kept the dndA-E gene cluster intact but may have lost the hypothetical S. coelicolor sulfur-specific REase.
We therefore set out to cure the integrated pSET152::dndA-E from one of the 49 apramycin resistant Dnd+ S. coelicolor exconjugants and expected that the resulting strain would be a mutant that allows the reintroduction of fresh pSET152::dndA-E at high frequency.
Unfortunately, we could not detect spontaneously apramycin sensitive derivatives of S. coelicolor pSET152::dndA-E. Instead, we cloned the dndA-E cluster into the highly unstable autonomously replicating Streptomyces plasmid pJTU412 [22] to generate pJTU1651. Introducing pJTU1651 into S. coelicolor M145 by conjugation from E. coli produced thiostrepton resistant Dnd+ exconjugants. One of these was randomly selected and plated on non-selective medium to allow loss of pJTU1651. LG3, one of the cured (thiostrepton sensitive, Dnd−) S. coelicolor gave c. 5×104 apramycin resistant exconjugants both with pHZ1904 and with pHZ1904* (Figure 2, Panel 2). As expected, the pHZ1904 exconjugants were Dnd+.
These results proved that the process of introducing the dndA-E gene cluster into wild-type S. coelicolor selected for rare mutant derivatives that no longer restricted the establishment of this gene cluster.
Identification of the endonuclease gene that prevented the establishment of the dndA-E–expressing plasmid
Several putative endonucleases genes had been identified in the S. coelicolor genome sequence [12]. The identification of the correct one was aided by the availability of the complete genome sequence of Streptomyces avermitilis which contains dndA-E homologues and produces S-modified DNA like S. lividans [23]. We were thus searching for a putative endonucleases gene of S. coelicolor that does not have a counterpart in S. avermitilis and found Sco4631 which is encoded by the genomic island Gi11 (also known as SLP1) and known to be absent from S. lividans [23]. Sco4631 was shown to restrict methylated DNA in vivo in Streptomyces [9] and is listed in REBASE as ScoA3McrA because it has limited similarity (37% identity including a HNH endonucleases motif in a 91 aa overlap) to EcoKMcrA from E. coli which restricts methylated and hydroxymethylated DNA in vivo, but has not been demonstrated to cleave DNA in vitro [4].
The following two experiments tested whether Sco4631 (ScoA3McrA) was indeed the gene that prevented the establishment of pHZ1904 in S. coelicolor: single crossover gene disruption using an internal fragment of sco4631 on a suicide vector plasmid (thiostrepton resistance) was used to produce S. coelicolor LG4 (Figure S2). Both pHZ1904 (dndA-E+) and pHZ1904* (dndE-) were introduced by conjugation from E. coli ET12567/pUZ8002 with equal high frequency into strain LG4 (c. 104 apramycin-resistant exconjugants per plate, Figure 2, Panel 3).
In addition, sco4631 including the upstream promoter was cloned into the pSAM2-derived integrating vector pPM927, resulting in pJTU1654, and introduced into HXY16, a S. lividans derivative that does not S-modify its DNA because it lacks the entire SLG genomic island including dndA-E. The resulting strain, LG5, gave only c. 100 apramycin resistant exconjugants with pHZ1904 but c. 104 exconjugants with pHZ1904* (Figure 2, Panel 4), while LG6, a control strain containing pPM927 without the cloned sco4631 gene, accepted both pHZ1904 and 1904* with equal high frequency (Figure 2, Panel 5).
These results proved that Sco4631/ScoA3McrA without the need for any other gene on Gi11 (SLP1) restricted the establishment of pHZ1904 which confers S-modification on its host.
Introduction of dndA-E into S. coelicolor resulted in the loss of the entire SLP1 sequence
Since Sco4631 is encoded by Gi11, which is also known as the 17.1 kb conjugative plasmid SLP1, we wondered whether the above 49 Dnd+ pHZ1904 exconjugants might have lost the entire SLP1 sequence. PCR amplification of LG3 and ten similar exconjugants using outside flanking primers showed the same band. Sequencing of the PCR product of LG3 showed the entire Gi11 sequence was excised precisely, restoring the tRNATyr sequence into which SLP1 had originally inserted (Figure S3A).
This was again consistent with the hypothesis that Sco4631 was responsible for restricting the establishment of pHZ1904 in S. coelicolor, and it demonstrated that SLP1 can be lost spontaneously from S. coelicolor (Figure S3A). This has not been observed before because SLP1 is a highly efficient conjugative plasmid that would immediately reinfect cured strains in the absence of the dndA-E genes.
Expression of sco4631 in S. lividans resulted in the loss of the genomic island that contains the dndA-E gene cluster
pJTU1654 (pPM927 derivative expressing sco4631, see above) was introduced by conjugation into wild type S. lividans 1326 and into S. lividans HXY16 (lacks SLG including dndA-E). A high frequency of 3×104 thiostrepton exconjugants were obtained per plate with S. lividans HXY16, and 300-fold fewer exconjugants were obtained with wild type (Dnd+) S. lividans 1326. The control pPM927 without cloned DNA transformed both strains with equal frequency (Figure S4).
Ten randomly selected, independent exconjugants were examined using PCR amplification and sequencing of the amplified product. All of them had suffered a precise deletion of the entire 93 kb S. lividans SLG genomic island that contains the dndA-E gene cluster (Figure S3B). These results again support the hypothesis that sco4631 and the dnd gene cluster are unable to coexist in the same host.
Cloning and expression of sco4631 and its mutant derivative in E. coli
The coding sequence of sco4631 including the stop codon was cloned into the expression vector pET28a, generating pJTU1655 for producing amino-terminally His6-tagged Sco4631. A similar plasmid, pSco4631H508A, was constructed that contains an inactive mutant protein because the first histidine residue of the conserved motif (H508-N521-H529) was changed to alanine. The two plasmids were introduced into E. coli BL21 (DE3)/pLysE and His6-Sco4631 and its mutant derivative were overexpressed for 5 h at 30°C. About 70% percent of the overexpressed protein was soluble in the supernatants and 30% was in the precipitates (Figure S5). The soluble proteins were purified using a Ni affinity column and stored at −20°C in Tris-Cl buffer pH 8.0 containing 50% glycerol.
His-tagged Sco4631 cleaves Dcm methylated DNA near the site of methylation
In the course of these experiments we noticed that the pET28a derivative containing the cloned sco4631 gene could not be transformed into Dam+ Dcm+ E. coli DH10B but the plasmid was stably maintained in the Dcm- E. coli BL21(DE3)/pLysE expression host and also in the non-methylating E. coli ET12567 (see Table S1).
This gave us the idea to test the in vitro DNA cleaving activity using as a substrate dcm methylated (Cm5CWGG) EcoRV pre-linearized pOJ260 DNA isolated from E. coli DH10B.
No DNA cleavage was observed using standard restriction NEB buffers 1–4 without and with BSA or ATP. Endonuclease activity was, however, observed when Mn2+ or Co2+ was included in the reaction buffer (Figure 3). Optimal cleavage with minimal unspecific star activity was achieved at 30°C for 5 min using 20 mM Tris-Cl pH 9.0, 50 mM NaCl, 1 mM Mn2+.
Fig. 3. Divalent cation ion requirements analysis for the cleavage of S. lividans 1326 total DNA by Sco4631. 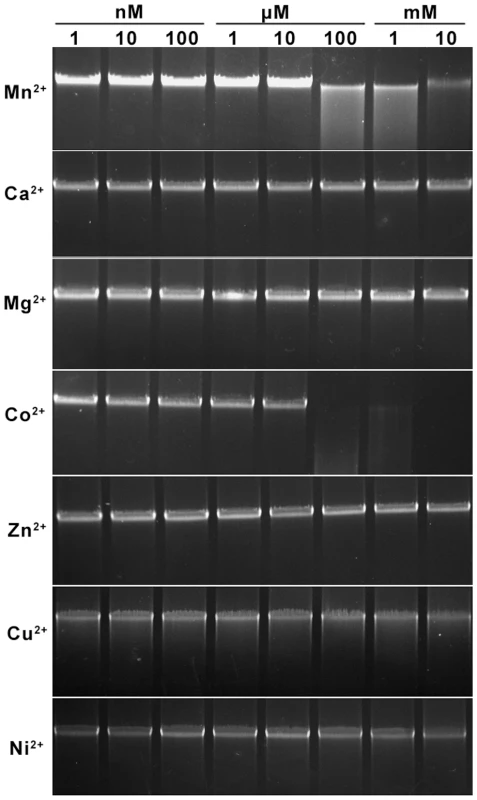
Total S. lividans 1326 DNA (100 ng) prepared by the Kirby mix procedure [11] was used as a substrate to react with 500 ng purified His6-Sco4631 protein at 30°C for 5 min in a total reaction volume of 20 µl. The metal ions added to the reaction buffer are indicated to the left, and the concentrations are above the lanes. The reactions were stopped by adding loading buffer containing 1% SDS, 50% glycerol and 0.05% bromophenol blue (Takara). After the reaction, the DNA samples were examined by 0.75% agarose gel electrophoresis. The gel was run at 5 V/cm for 30 min and visualized by staining with ethidium bromide. The strong bands are high molecular weight DNA; the smear observed with Mn2+ and Co2+ concentrations ≥100 µM indicates DNA cleavage by Sco4631. Under optimal conditions, about 25% of 100 ng pOJ260 was cleaved and produced at least five bands of differing intensity, indicative of a partial digest (Figure 4A). The precise position of eight cleavage sites was determined by blunt-end ligating the digested pOJ260 DNA to a DNA fragment containing the bla gene conferring ampicillin resistance (see Materials and Methods). The cleavage/ligation sites were sequenced using PCR primers SeqF and SeqR reading away from the ends of the bla fragment. Each of eight cleavage sites was between 12 and 16 bp away from a C5mCWGG Dcm methylation site. pOJ260 has ten Dcm methylation sites and six of them had nearby cut sites (Figure 4C).
Fig. 4. Sco4631-mediated in vitro cleavage of Dcm-methylated plasmid DNA. 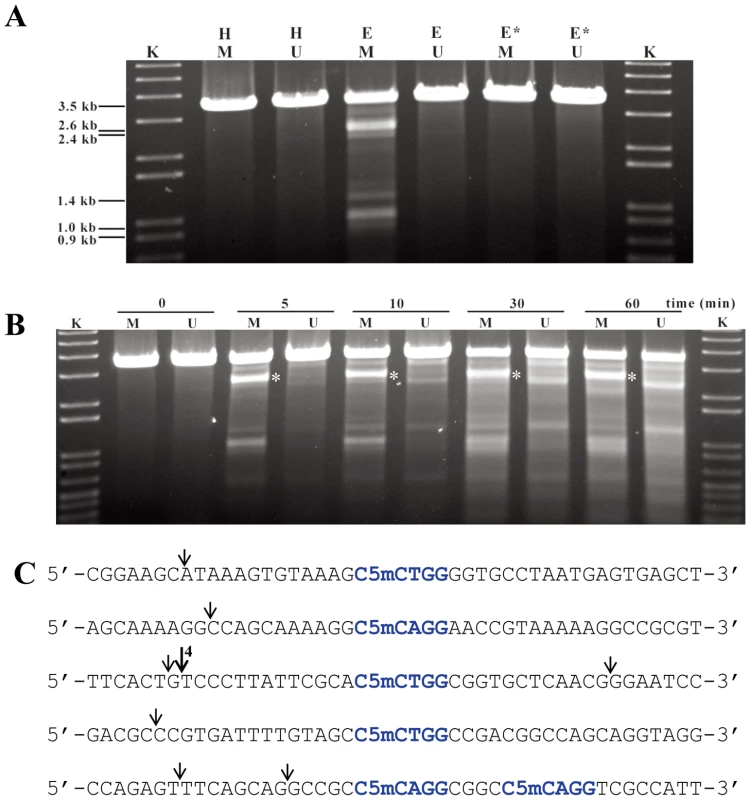
A. Agarose gel showing that DNA cleavage by His6-Sco4631 requires DNA methylation. The ethidium bromide-stained agarose gel shows EcoRV linearized pOJ260 DNA treated as indicated above the lanes. Incubation was for 5 min at 30°C in buffer containing 1 mM Mn2+. H, heat inactivated His6-Sco4631 used as a control; E (Enzyme) and E*, His6-Sco4631 and inactive His6-Sco4631(H508A), respectively, purified from E. coli BL21(DE3); K, kb ladder DNA standard; M (methylated), indicates Dcm methylated pOJ260 that was prepared from Dam+ Dcm+ host E. coli DH10B. U (unmethylated), indicates pOJ260 that was isolated from non-methylating E. coli ET12567. The 0.9–2.6 kb bands generated by Sco4631 digestion of methylated DNA are relatively faint, indicating partial digestion. B. Prolonged incubation with His6-Sco4631 causes non-specific DNA degradation, also of unmethylated DNA. EcoRV linearized pOJ260 DNA (like in panel A) was incubated for 5–60 min as indicated above the gel. M, methylated DNA; U, unmethylated DNA. C. Sequences of the cleavage sites including the nearby Dcm methylation sites (C5mCWGG, shown in blue). Vertical arrows point to the cleaved bonds, most cuts were observed once except for the cut marked by a larger arrow that was observed in four independent samples. Note, there are four additional C5mCWGG sequences in pOJ260 (not shown) where no cleavage was observed. These results were consistent with our expectation that Sco4631 is a Type IV restriction endonuclease that cleaves near rather than precisely at its methylated DNA recognition site. (Note that EcoKMcrA does not restrict Dcm-methylated DNA.)
The reaction buffer used in the above experiment resembles buffers that induce star activity (reduction of sequence specificity) in many Type II REases [24], [25]. It might thus be that not all Dcm methylated sites are cleaved in vivo by Sco4631. The fact that cutting near one of the sites was observed six times independently suggested that the DNA recognition sequence of Sco4631 may extend beyond the sequence C5mCWGG, or that Dcm methylation of the plasmid was incomplete as was observed by H. ZHOU (in preparation). Also, the in vivo results of Gonzalez-Ceron et al. [9] showed that Sco4631 restricts DNA containing other DNA methylations.
His-tagged Sco4631 protein binds and cleaves in vitro a synthetic oligonucleotide containing a single Dcm-methylated site
The above results were consistent with the in vivo observations suggesting that Sco4631 is a methyl-directed REase, but we could not be absolutely sure that E. coli DH10B had not added an unknown, additional modification to pOJ260 that was necessary for target selection by Sco4631. To test whether indeed the Dcm methylation alone was sufficient for cleavage by Sco4631, we synthesized two 164 bp double stranded DNA fragment that contained centrally the above preferred presumptive methylated site, and a 5′32P end label either at the top or the bottom strand so that nicking of both strands could be observed. The labelled DNA fragments were used to test for binding of Sco4631 using DNase I footprinting, and for detecting endonucleolytic activity. Figure 5A and 5B show a staggered cut in the position where four independent clones showed DNA cleavage (Figure 4C, third sequence). Overexposed gels showed two additional, faint bands corresponding to single strand nicks on the bottom strand only on the right side of the Dcm methylation site as it is shown in Figure 5B and 5C. All these bands were absent in the controls using an unmethylated form of the oligonucleotide or heat treated Sco4631.
Fig. 5. In vitro DNase I protection and cleavage of a double-stranded 164 bp Dcm-methylated synthetic oligonucleotide by purified His6-Sco4631. 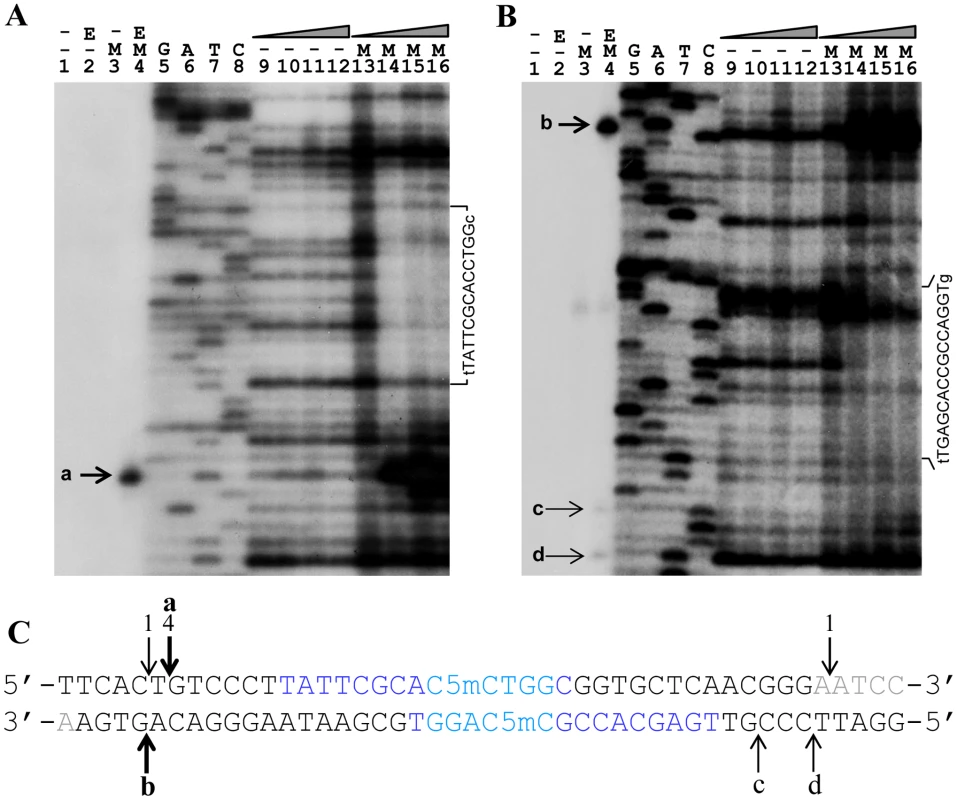
A. Analysis of 5′ labeled top strand DNA, and B, 5′ labeled bottom strand DNA. Bands a-d on these autoradiographs represent cleavage of the DNA strands by His6-tagged Sco4631. Lanes 1–3 are controls lacking either the enzyme (E) or the Dcm methylation (M). The samples in lanes 4 contained both methylated DNA and active His6-Sco4631. Lanes 5–8 are sequencing ladders. Lanes 9–12 are controls containing unmethylated DNA and increasing amounts of active His6-Sco4631. Lanes 13–16 contained methylated DNA and increasing amounts of the active His6-Sco4631. Lanes 9 and 13 contained no enzyme, and lanes 10–12 and lanes 14–16 contained 1.1, 4.5 and 18 µM enzyme, respectively. The vertical sequence to the right of each gel picture indicates the DNA regions that were partially protected from cleavage by DNase I. The horizontal lines point to bases that are not protected and shown in lower case letters. (Please note, panel A shows the correct sequence of the top strand, but in panel B there is a compression of the sequence with the bold bases in the sequence CCAGGTGCGAATAAG not visible.) C. Central sequence of the 164 bp double-stranded oligonucleotide containing the Dcm methylated sequence C5mCWGG (dark blue) and additional sequences protected against DNase I activity (light blue). The fat vertical arrows labeled a and b are the major cut sites, and the thin arrows labeled c and d are minor cut sites indicated in the gels A and B. Arrows with numbers indicate cut sites that were identified by cloning and sequencing (see Figure 3). Bases printed light grey are not visible on the gel sections shown. There may be a trivial explanation for the lack of faint bands, indicating secondary cleavage sites in the upper strand: most the DNA molecules that were cleaved at one of the secondary sites in the upper strand will also be cleaved at the primary site. Because of the 5′ end-labeling, secondary cleavage cannot be detected.
DNase I footprinting revealed protection of 14 nucleotides centred around the Dcm methylation site C5mCTGG on the top strand (light blue nucleotides in Figure 5C), and weaker, asymmetric protection of the bottom strand containing the sequence C5mCAGG.
These results confirm that Sco4631 binds specifically to the Dcm methylated DNA and covers additional bases outside the C5mCWGG sequences.
His-tagged Sco4631 protein cleaves in vivo S-modified DNA
S. lividans DNA is S-modified (phosphorothioated) at specific positions. Only about one in 6000 DNA backbone phosphates contain sulfur in a non-bridging position. S-modification of S. lividans DNA seems to be incomplete, and certain sequences are preferentially S-modified. The Streptomyces multicopy plasmid pHZ209 contains such a preferentially modified site which was chosen for our study.
S-modified pHZ209 was isolated from Dnd+ S. lividans 1326 and either cleaved oxidatively using Tris-peracid or using His-tagged Sco4631 protein and the same buffer that was used for the cleavage of Dcm methylated DNA. After digestion using EcoRV, which cuts pHZ209 once, three prominent bands and additional fainter bands were observed in both the Tris-peracid and the Sco4631-treated S-modified pHZ209 samples (Figure 6A). The largest fragment represents 5.4 kb linearised pHZ209 generated from plasmids that were not cleaved by Tris-peracid or Sco4631. The other two smaller bands were of the sizes expected if pHZ209 was cleaved at or near the preferential S-modification site. Controls with pHZ209 that was isolated from a dnd− host did not produce these two smaller bands.
Fig. 6. In vitro cleavage of S-modified (phosphorothioated) DNA by Tris-peracid or His6-Sco4631. 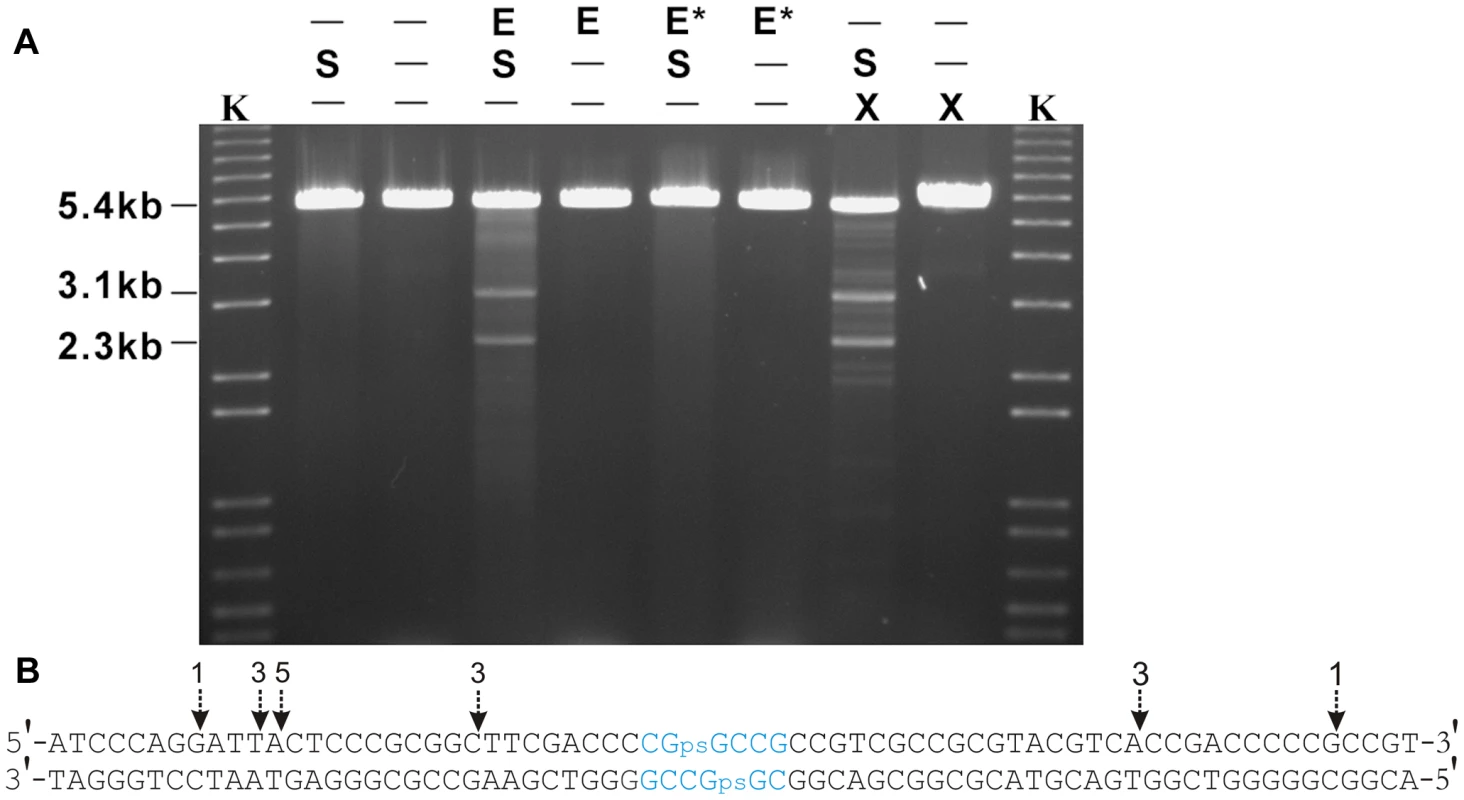
A. Ethidium bromide-stained agarose gel showing EcoRV-linearized pHZ209 DNA (5.4 kb). K, kb ladder DNA size standard; S, S-modified DNA prepared from S. lividans 1326; E, His6-Sco4631; E*, inactive His6-Sco4631(H508A); X, Tris-peracid. Non-phosphorothioated DNA was prepared from S. lividans HXY6. Partial digestion producing strong 3.1 and 2.3 kb bands and additional fainter bands was observed in the samples containing S-modified DNA (S) and either His6-Sco4631 (E), or Tris-peracid. B. Summary of preferential cleavage sites on Dnd-phosphorothioated pHZ209. The two main bands (3.1 kb and 2.3 kb in Figure 5A) from the His6-Sco4631-digested S-modified DNA were cloned into pBluescript SK+, and 16 clones were end-sequenced from both sides using primers T3 and T7. Vertical arrows show where the cuts occurred, and the numbers indicate how many times each particular cut was observed. All 16 cuts occurred near the preferential Dnd-phosphorothioation modification sequence CGpsGCCG shown in blue. The precise Sco4631 cleavage sites were determined by blunt end cloning and sequencing. Tris-peracid cleaves the DNA backbone precisely at the site of the sulfur producing a 1 bp 5′ overhanging staggered cut [19], [21]. The Sco4631-induced cuts were found on both sides between 16 and 28 nt away from the S-modification (dotted line arrows in Figure 6B). This was consistent with the expectation that Sco4631, like other Type IV REases, bound to the S-modification but cleaved some distance away from the cleavage site [26]–[28]. This endonucleolytic cleavage of DNA is, of course, consistent with our initial observations that Sco4631 and dndA-E are unable to coexist in the same host.
Sco4631 cleaves DNA at multiple possible sites on either side of the S-modification
Oxidative cleavage of S-modified DNA breaks specifically the phosphorothioate bonds resulting in a 1 bp 5′ staggered cut. The above sequence data (Figure 6) suggested that Sco4631 cuts S-modified DNA rather imprecisely on either side near the sequence CGpsGCCG. The fact that cleavage occurred on both sides of the sequence suggests that Sco4631 may bind to both strands equally, i.e. the essential binding region may not extend beyond this palindromic sequence.
We could not exclude the possibility that the DNA propagated in S. lividans 1326 contained additional modifications that influenced the results. For this reason, we synthesized a 118 bp double-stranded oligonucleotide containing one phosphorothioate on each strand at the preferred sequence of pHZ209. Again, 5′32P label was used separately on both the top and bottom strand so that cuts in either strand could be observed. Oligonucleotides without S-modified bases were used as controls.
His-tagged Sco4631-specific cleavage was observed in both the top and the bottom strand, and on both sides of the S-modification (Figure 7). (Note that no cleavage occurred in the controls or at the site of S-modification which is marked by ** in Figure 7A and 7B.) Multiple cleavage sites were observed (solid arrows in Figure 7A and 7B), consistent with the data from the cloning and sequencing of individual cleavage sites (dotted arrows).
Fig. 7. In vitro cleavage of a synthetic S-modified (phosphorothioated) double-stranded 118 bp oligonucleotide by purified His6-Sco4631. 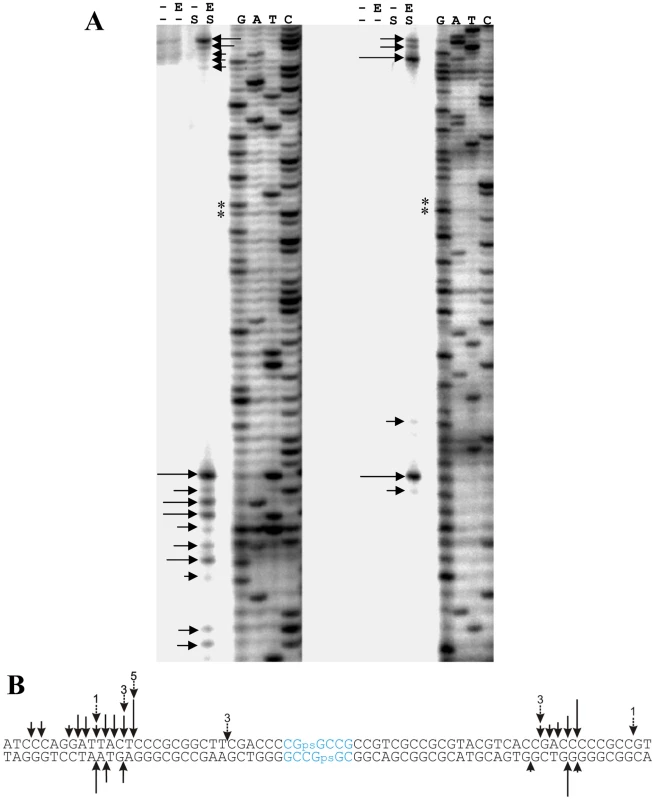
A. Autoradiographs of 5′ labeled top strand DNA (left) and 5′ labeled bottom strand DNA (right). G, A, T, C denote the sequencing ladders. Horizontal arrows indicate cleavage sites, and ** indicates the tandem G residues linked by a phosphorothioate bond. His6-Sco4631-mediated cleavage was observed exclusively in the samples of S-modified DNA (S) that also contained active His6-Sco4631 (E). Samples without DNA S modification or containing heat-inactivated His6-Sco4631 were not cleaved. - indicates the absence of enzyme or S-modification, respectively. B. In vitro cleavage of S-modified DNA by His6-Sco4631. Vertical arrows indicate bonds where cleavage of the 118 bp oligonucleotide was observed. The length of the solid arrows indicates the intensity of the bands in panel A and thus the frequency of cleavage. Dotted arrows indicate in vitro generated cleavage sites of pHZ209 (see Figure 5). The preferred S-modification sequence CGpsGCCG is shown in blue. Surprisingly, no concentration dependent protection by Sco4631 of the S-modified DNA against DNase I was observed (Figure S6), possibly because the S-modified DNA was cleaved too efficiently. This result may not apply precisely to the majority of S-modified sites because this preferred site is a 6 bp palindrome rather than the more usual 4 bp core palindrome. Also, the S-modification in the synthetic oligonucleotides is racemic while the DndA-E proteins add the sulfur stereospecifically in the RP configuration [18].
These results, however, confirmed that His6-tagged Sco4631 could cleave S-modified without interference from hypothetical DNA modifications that might have been present on naturally S-modified pHZ209.
Discussion
Activity of Sco4631
We demonstrated that the S. coelicolor protein Sco4631 is a Type IV REase that cleaves Dcm-methylated and also S-modified (phosphorothioated) DNA in vitro near the respective modification sites. DNA cleavage required a special reaction buffer containing Mn2+ or Co2+ at pH 9.0. These conditions reduce the sequence specificity of some Type II REases [24], [25]. Also the N-terminal His6 tag that was added to facilitate protein purification might have reduced or even altered the sequence specificity of the protein that was used for the in vitro studies. The fact that all the observed DNA cleavage events occurred very specifically near the respective modification sites suggests that neither the chosen buffer nor the His6 tag changed the enzyme specificity. Two other Type IV REases also have special buffer requirements for in vitro activity: GmrSD, encoded by an E. coli prophage, required Ca2+ and UTP for DNA cleavage [5], McrBC required GTP and Mg2+ for DNA binding [29]. Recently, Chan et al. reported that HNH nucleases generally have unusual buffer requirements [30]. Only MspJI from Mycobacterium, a remote homologue of E. coli Mrr, cleaved methylated DNA in a standard REase buffer [31]. (Please note that BseMII and BspLU11III have been reclassified as Type IIG REases [4], [32].The in vivo Dcm-methylated or S-modified DNAs might have contained unknown additional modifications that could have influenced the in vitro target selection. We excluded this possibility by repeating the cleavage experiments using long (164 and 118 bp) double-stranded synthetic oligonucleotides that had the same sequence and DNA modifications as the preferred cleavage sites identified on in vivo methylated or S-modified DNA. The results confirmed the initial data, indicating that there had been no unknown in vivo modifications influencing our results. In addition, using the end-labelled modified oligonucleotides provided better information about the relative frequencies of DNA cleavage in different positions (Figure 5, Figure 7).
We have thus shown that a few Dcm-methylated sites and one preferentially S-modified DNA sequence were cleaved by Sco4631. Gonzalez-Ceron et al. demonstrated, however, that also other sequence specific 5mC methylations lead to in vivo restriction in Streptomyces strains expressing Sco4631 [9]. We cannot exclude that Sco4631 can cleave DNA with methylated bases or S-modifications in many different possible target sequences. The observation that one of the six available Dcm sites in pOJ260 was cleaved four times while others were cleaved once or not at all could be due to statistical fluctuation, but it could also indicate that the uncut sites may have been undermethylated or that the DNA sequence around the methylation may influence the frequency of cleavage. It is also not possible to speculate about the importance of the surrounding DNA sequence for the enzymatic cleavage near S-modification sites because only a minority of the potential sites receive the sulfur, and some preferred sites are more strongly phosphorothioated than others [33]. Preferential S-modification alone, without differential cutting of sites depending on the surrounding DNA sequence, could have produced the major and minor bands in Figure 7A.
Type IV REases generally have low sequence specificity, and the same enzyme may cleave e.g. methylated and hydroxyl-methylated DNA [17], [27], [34]. A good illustration for this is MspJI that cleaved CmCWGG, CmCGG, AGmCT, GGmCC, GmCGC and mCCGG [31].
Gonzalez-Ceron et al. observed that a S. lividans strain expressing cloned sco4631 restricted pSET152 DNA methylated by Dam or M. TaqI 400-fold and 20-fold, respectively, while Dcm methylated pSET152 was restricted only four fold [9]. It therefore seemed surprising at first that we could introduce pET28a::HisSco4631 into the Dam+Dcm− strain BL21(DE3), but not into the Dam+Dcm+ strain DH10B. Tighter regulation of the lac promoter in the latter strain may have caused the difference rather than the methylation status.
The purified His6-Sco4631 was produced in E. coli that did not contain any other Streptomyces genes. This proved that Sco4631 acted without accessory proteins as are required for endonucleases activity by the McrBC family of Type IV REases [5], [29].
Sco4631 cleaved the Dcm-modified DNA preferentially to the left of the modification site on the sequence shown in Figure 5, indicating that the sequence flanking the 5mC modification may influence where cleavage occurs. The S-modified DNA was cleaved more evenly on both sides of the S-modification (Figure 7), but there was no evidence for simultaneous cleavage on both sites.
It was surprising that the distance between the DNA modification and the cleavage sites was different for Dcm and S-modified DNA. Maybe the enzyme remains locked to the Dcm site producing a near cut 12–16 bp away, but moves away from the S-modified site before cleaving less precisely c. 16–28 bp away.
The above speculation would be consistent with the observation that DNase I protection footprints, which were made under conditions that allowed DNA cleavage, were detectable on both strands of the Dcm-methylated oligonucleotides, but they were absent from the S-modified oligonucleotides (Figure S6). Note that the protection on each strand was asymmetric with respect to the methylated C.
The natural S. lividans DNA S-modification is chiral (P-SR-configuration, [18]) but the S-modification on the synthetic oligonucleotides was racemic and thus only one in four double-stranded oligonucleotides had the natural R-R conformation. It is unknown whether this may have affected protein binding or cleavage activity. The larger distance between the S-modification and the cleavage sites is, however, not an artefact of racemic S-modification because the larger distances were observed both with the naturally S-modified DNA and the synthetic S-modified oligonucleotide (see Figure 7B dotted arrows and solid arrows, respectively, for comparison).
Sco4631 had been identified by Gonzalez-Ceron et al. as a methyl-specific REase that has some limited (37% in a region of 91 aa) amino acid sequence similarity to the 5mC and 5hmC-specific EcoKMcrA [35], [36]. The two enzymes share a consensus HNH protein sequence motif that occurs in homing endonucleases and inteins, and in Group I and Group II introns [37], [38]. In REBASE [4] Sco4631 is listed as ScoA3McrA. It should be noted, however, that EcoKMcrA does not restrict Dcm methylated DNA [16].
Biological functions of Sco4631
Because in vitro cleavage by Type IV REases has been elusive for many years, it was speculated that these enzymes may achieve restriction of modified DNA without DNA cleavage that could easily be reversed by re-ligation. Simple binding to the target DNA might prevent its replication or integration into the host genome [39]. By adding a third example of in vitro DNA cleavage by a Type IV REase we support the original idea that Type IV REases cleave DNA like all the other types of REases.
S. coelicolor restricts Dam, Dcm, Hsd and other methylated DNA very effectively using at least four different methyl-specific REases including Sco4631 [9], [40]. However, S. coelicolor only weakly restricts S-modified DNA from S. lividans [41]. Unexpectedly, the integrating vector pHZ1904 was severely restricted by S. coelicolor even though it did not have any known DNA modification because it had been propagated in the non-methylating E. coli host ET12567 that also did not support expression of the dndA-E DNA S-modification genes. Mutant derivatives of pHZ1904 were, however not restricted, excluding the possibility of an unknown sequence-specific (as opposed to modification-specific) restriction system acting in S. coelicolor.
It seemed therefore likely that expression of the dndA-E gene cluster in S. coelicolor resulted in S-modification of the host DNA. Sco4631 would then cleave near the modified sites resulting in cell death.
S. coelicolor mutants that tolerated S-modification by DndA-E represented about 50% of the rare pHZ1904 exconjugants. We speculated that one of the putative S. coelicolor endonucleases without counterpart in S. lividans might cleave S-modified DNA. Such a nuclease was indeed found to reside on the genomic island Gi11 [12]. Gi11, also known as SLP1 is a 17.1 kb mobile element that can excise from the S. coelicolor genome and transfer by conjugation to S. lividans where it usually forms autonomously replicating plasmids [14]. These plasmids in S. lividans always lack a part of the original Gi11 sequence including sco4631 which would destroy the S-modified S. lividans DNA.
Occasionally, the entire SLP1 sequence integrates into the S. lividans genome [42]. It is not known whether these integrated elements feature a mutant sco4631 or whether dndA-E is inactivated or deleted from these strains.
It seems that Sco4631 does not effectively restrict the entry of S-modified DNA into S. coelicolor [43], but it very effectively prevents the establishment of mobile elements that contain gene clusters like dndA-E that cause S-modification of its DNA. Such suicidal processes are frequently used by plasmids containing stable kil (kill) and unstable kor (kill override) genes resulting in cell death as a result of plasmid loss [44]. Also, restriction-modification (R-M) systems which are often on mobile elements [45] are stabilized in a bacterial population because the R-activity persists longer than the M-activity after the genes have been lost from a cell [46]. Fukuda et al. proposed that an important function of Type IV REases in general is the sacrificial killing of cells that have newly acquired a DNA modification system [39].
It was expected that strains containing a dnd gene cluster would not contain a Sco4631 homologue. This was true for 40 out of 41 fully sequenced strains containing a dnd gene cluster. Pseudomonas fluorescens Pf0-1, however, contains both a complete dnd gene cluster and S-modified DNA, and it also contains two HNH proteins that are, however, very different from Sco4631 (Figure S7A, S7B).
It is not understood why bacteria need such defences against DNA modification systems. Dam methylation reduces the mutation rate by directing the mutHLS pathway to correct errors in the newly synthesized DNA strand, and DNA adenine methylation also has known regulatory roles in bacteria [47]–[49], but the functions of Dcm methylation and phosphorothioation remain unknown.
Importance of Sco4631 and DndA-E for the genomic islands Gi11 of S. coelicolor and SLG of S. lividans
SLP1 in S. coelicolor and SLG in S. lividans are segregationally very stable genomic islands [13], [50]. Spontaneous excision of each element in the absence of selective pressure could, however, be observed using sensitive PCR analysis (Figure S3B) [13].
Our experiments demonstrate that both SLP1 and SLG can be cleanly excised from the respective genomes by natural processes.
The sequence-specific S-modification of DNA prevents DNA cleavage by some Type II REases whose specificity overlaps the S-modification site (Liang J, personal communication), and Salmonella enterica serovar Cerro 87 which has S-modified DNA, also contains a restriction system that cleaves foreign DNA that lacks the cognate S-modification [51]. The fact that S. coelicolor has in Sco4631 an effective defence against invasion by these genes indicates that DNA S-modification may have important biological functions that remain to be discovered.
Materials and Methods
Strains, plasmids and primers used are listed in Table S1 and Table S2, respectively. E. coli and Streptomyces strains were grown in LB [52] and SFM medium [11], respectively, supplemented with 100 µg/ml ampicilin, 30 µg/ml apramycin, or 12 µg/ml thiostrepton as required. Transformation of E. coli DH10B with Streptomyces plasmids was performed using the machine EasyjecT Plus electroporator (EQUIBIO). Conjugative transfer of DNA from E. coli ET12567/pUZ8002 to Streptomyces was performed as described in [11], except that always approximately 1010 recipient spores were used, and tenfold dilutions of donor cells (109 to 105 CFU) were used per 9 cm agar plate. The exconjugants were counted from appropriate dilutions after incubation at 30°C for 3 days on SFM plates. The number of E. coli and Streptomyces CFUs in each experiment was determined by making dilutions and plating LB agar and MS plates, respectively. The conjugation frequencies were expressed as the number of exconjugants per 109 CFU of donors. The calcium chloride transformation frequency of E. coli was determined using 10 ng of CCC plasmid DNA. Each experiment was repeated three times and the final frequency was the mean value. Investigation of Dnd phenotype (degradation of S-modified DNA by Tris-peracid) was carried out as described in [21].
Inactivation and heterologous expression of sco4631
An internal region of sco4631 was amplified by PCR using primers S31DF and S31DR, gel purified and inserted into the EcoRV site of pBluescript prior to transformation into pSET151 to give pJTU1653, which was then transferred from ET12567/pUZ8002 into S. coelicolor M145 by two-parental mating [11]. A 2176 bp fragment containing intact sco4631 was amplified using KOD Plus (TOYOBO) and primers S31HEF (XbaI linker) and S31HER (XbaI linker). The resulting fragment was cut by XbaI and inserted into pBluescript, and then the XbaI insertion was purified from this intermediate and cloned into the XbaI site of pPM927 to give pJTU1654. pJTU1654 and pPM927 were transformed individually into ET12567/pUZ8002 for conjugation into S. lividans strains 1326 and HXY16.
Overexpression and purification of His-tagged Sco4631 and its mutant
sco4631 was amplified by PCR using KOD Plus and primers S31OEF and S31OER, inserted into pBluescript, excised as an NdeI–EcoRI fragment and ligated between cognate sites of pET28a, generating pJTU1655. Site directed mutagenesis of pJTU1655 was carried out by using KOD-Plus-Mutagenesis Kit (TOYOBO) with primers H508A-F and H508A-R, resulting in pSco4631H508A. The expression constructs were then transformed into BL21(DE3)/pLysE respectively. 10 ml of the overnight culture of BL21(DE3)/pLysE pJTU1655 or BL21(DE3)/pLysE pSco4631H508A was inoculated into 1 L LB medium supplied with 50 µg/ml kanamycin and 34 µg/ml chloramphenicol. Then the culture was incubated at 37°C to OD600 = 0.4, cooled to room temperature and 0.4 mM IPTG was added, followed by incubation for another 5 hours at 30°C. Then the cells were harvested and resuspended in 20 ml binding buffer (20 mM Tris-Cl, 20 mM imidazole and 150 mM NaCl pH 8.0) and lysed by sonication in an ice bath. After centrifugation (16000 g for 30 min at 4°C), the supernatant was applied to a HisTrap HP column (GE Healthcare) and purified using an ÄKTA FPLC (GE Healthcare) by eluting with imidazole linear gradient 20–500 mM. The product was desalted by a HiTrap Desalting column (GE healthcare) and stored in Tris-Cl buffer (50 mM, pH 8.0) with 50% glycerol at −20°C. Purified Sco4631 was visualized by Coomassie-stained 12% SDS-PAGE analysis and protein concentration determined using a Bradford Protein Assay Kit (Bio-Rad). The protein purity was determined by Quantity One (Bio-Rad) from the gel, and the purities of Sco4631 and Sco4631(H508A) are about 96.5% and 97.9%, respectively.
Optimization of in vitro conditions for cleavage activity of Sco4631
Total S. lividans 1326 DNA prepared by the Kirby mix procedure was used as a substrate for the Sco4631 cleavage activity assay. Purified Sco4631 protein (500 ng) and 100 ng DNA substrate were incubated at 30°C for 5 min in a total reaction volume of 20 µl. The reaction buffer contained 20 mM Tris-Cl (pH 8.0), 50 mM NaCl and each of seven divalent ions, Ca2+, Co2+, Cu2+, Mg2+, Mn2+, Ni2+ or Zn2+ at a concentration of 1 nM to 10 mM. The reactions were stopped by adding loading buffer (Takara) containing 1% SDS, 50% glycerol and 0.05% bromophenol blue. The DNA after the reaction was examined by 0.75% agarose gel electrophoresis. Mn2+ or Co2+ ions at concentrations between 100 µM and 10 mM gave maximal DNA cleavage. The optimal pH was determined in a similar manner using equivalent buffers of pH 5 to pH 10, supplemented with 1 mM Mn2+; pH 9 was optimal for Sco4631-mediated DNA cleavage.
End sequencing of fragments generated by Sco4631 cleavage of Dcm-methylated or Dnd-phosphorothioated DNA
The plasmids pOJ260 and pHZ209 were isolated from DH10B and S. lividans 1326, respectively, using the QIAGEN Plasmid Midi Kit (Qiagen). One microgram pOJ260 DNA was incubated with Sco4631 under the optimized conditions described above. Linearised pOJ260 was gel purified and blunted using Klenow Fragment (Fermentas) and dNTPs at 37°C for 10 min in a total volume of 20 µl (1×reaction buffer, 0.05 mM dNTP mix, 5 Unit Klenow Fragment), followed by heating at 75°C for 10 min to stop the reaction. The blunted mix was ligated to a 1311 bp amplicon harboring a bla cassette and the ligation mix was transformed into E. coli DH10B. Plasmid DNA was then prepared from randomly selected ampicillin-resistant transformants for insert end-sequencing using the primers seqF and seqR. Similarly, pHZ209 was linearized using EcoRV and then incubated with Sco4631. The resulting DNA fragments were gel purified, blunted using Klenow fragment, and ligated into the EcoRV site of pBluescript for transformation into E. coli DH10B. Plasmid DNA was then prepared from randomly selected transformants for insert end-sequencing using the primers T3 and T7.
Generation of 5′ end-labeled methylated or phosphorothioated DNA substrates for cleavage and DNase I footprinting analyses
A 164 bp PCR amplicon was generated using pOJ260 as a template, and the long primers MF and MR that contained a m5C residue on each strand within the Cm5C(A/T)GG Dcm-modification motif. An unmodified matching amplicon was also generated using primers UMF and UMR. Individual strands of the modified and unmodified amplicons were 5′ end-labeled by pre-tagging the appropriate primer with γ-32P using the following protocol. 10 pmol of primer was incubated with [γ-32P]-ATP (Beijing Furui Co. Ltd) using 10U of T4 polynucleotide kinase (Promega) at 37°C for 30 min, followed by 90°C for 2 min to inactivate the T4 polynucleotide kinase. PCR reactions were performed in a total volume of 50 µl containing 2 ng template DNA, 10 pmol of each primer, 1× PCR buffer, 5% DMSO, and 5U Taq polymerase (Genescript). PCR products were purified using the QIAquick PCR Purification Kit (Qiangen). An identical strategy was used to synthesize and strand-specifically label the phosphorothioate-modified and unmodified pHZ209-derived 118 bp amplicons. The phosphorothioate modification was introduced between the tandem guanine residues in the Dnd-motif (GpsGCC) by prior chemical synthesis of the long PCR primers SF and SR. The matching unmodified amplicon was generated using the primers USF and USR.
Cleavage sites analysis and DNase I footprinting assay
The DNA substrates used in these analyses were as described above. For cleavage sites analysis, the reaction mixture containing 100 cps 32P-labeled DNA substrate (10 nM), 20 mM Tris-Cl (pH 9.0), 50 mM NaCl, 1 mM MnCl2, 5% glycerol and 500 ng Sco4631 in a total volume of 20 µl was incubated at 30°C for 5 min. For the DNase I footprinting assay, 500 cps 32P-labeled DNA substrate (50 nM) was added to 0–24 µg (18 µM) of Sco4631 under the same buffer conditions as above, and the reaction mix was then incubated on ice for 5 min prior to adding 2.5 µl DNase I buffer and 0.3 U of DNase I (Promega), and further incubation at 37°C for 1 min. The reaction was stopped by adding 100 µl stop solution (3 M ammonium acetate, 0.25 M EDTA, 1 mg/ml glycogen), and 50 µl phenol-chloroform. Samples were then denatured at 95°C for 2 min and loaded on an 8% polyacrylamide–urea gel. The DNA sequence ladder was generated using an fmol DNA Cycle Sequencing kit (Promega). After electrophoresis, the gels were dried and exposed to a Kodak X-ray film.
Accession number
The nucleotide sequence of pOJ260 has been deposited in GenBank under Accession number GU270843.
Supporting Information
Zdroje
1. RobertsonKD
2005 DNA Methylation and human disease. Nat Rev Genet 6 597 610
2. BickleTA
KrugerDH
1993 Biology of DNA restriction. Microbiol Rev 57 434 450
3. DrydenDT
MurrayNE
RaoDN
2001 Nucleoside triphosphate-dependent restriction enzymes. Nucleic Acids Res 29 3728 3741
4. RobertsRJ
VinczeT
PosfaiJ
MacelisD
2010 REBASE–a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res 38 D234 236
5. BairCL
BlackLW
2007 A type IV modification dependent restriction nuclease that targets glucosylated hydroxymethyl cytosine modified DNAs. J Mol Biol 366 768 778
6. ZhouX
HeX
LiangJ
LiA
XuT
2005 A novel DNA modification by sulphur. Mol Microbiol 57 1428 1438
7. ZhouX
DengZ
FirminJL
HopwoodDA
KieserT
1988 Site-specific degradation of Streptomyces lividans DNA during electrophoresis in buffers contaminated with ferrous iron. Nucleic Acids Res 16 4341 4352
8. BentleySD
ChaterKF
Cerdeno-TarragaAM
ChallisGL
ThomsonNR
2002 Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417 141 147
9. Gonzalez-CeronG
Miranda-OlivaresOJ
Servin-GonzalezL
2009 Characterization of the methyl-specific restriction system of Streptomyces coelicolor A3(2) and of the role played by laterally acquired nucleases. FEMS Microbiol Lett 301 35 43
10. FlettF
MersiniasV
SmithCP
1997 High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol Lett 155 223 229
11. KieserT
BibbMJ
ChaterKF
ButterMJ
HopwoodDA
2000 Practical Streptomyces genetics. a laboratory manual. Norwich John Innes Foundation, United Kingdom
12. JayapalKP
LianW
GlodF
ShermanDH
HuWS
2007 Comparative genomic hybridizations reveal absence of large Streptomyces coelicolor genomic islands in Streptomyces lividans. BMC Genomics 8 229
13. HeX
OuHY
YuQ
ZhouX
WuJ
2007 Analysis of a genomic island housing genes for DNA S-modification system in Streptomyces lividans 66 and its counterparts in other distantly related bacteria. Mol Microbiol 65 1034 1048
14. BibbMJ
WardJM
KieserT
CohenSN
HopwoodDA
1981 Excision of chromosomal DNA sequences from Streptomyces coelicolor forms a novel family of plasmids detectable in Streptomyces lividans. Mol Gen Genet 184 230 240
15. RaleighEA
WilsonG
1986 Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc Natl Acad Sci U S A 83 9070 9074
16. MulliganEA
DunnJJ
2008 Cloning, purification and initial characterization of E. coli McrA, a putative 5-methylcytosine-specific nuclease. Protein Expr Purif 62 98 103
17. MulliganEA
HatchwellE
McCorkleSR
DunnJJ
2010 Differential binding of Escherichia coli McrA protein to DNA sequences that contain the dinucleotide m5CpG. Nucleic Acids Res 38 1997 2005
18. WangL
ChenS
XuT
TaghizadehK
WishnokJS
2007 Phosphorothioation of DNA in bacteria by dnd genes. Nat Chem Biol 3 709 710
19. RayT
MillsA
DysonP
1995 Tris-dependent oxidative DNA strand scission during electrophoresis. Electrophoresis 16 888 894
20. OuHY
HeX
ShaoY
TaiC
RajakumarK
2009 dndDB: a database focused on phosphorothioation of the DNA backbone. PLoS ONE 4 e5132 doi:10.1371/journal.pone.0005132
21. LiangJ
WangZ
HeX
LiJ
ZhouX
2007 DNA modification by sulfur: analysis of the sequence recognition specificity surrounding the modification sites. Nucleic Acids Res 35 2944 2954
22. SunY
HeX
LiangJ
ZhouX
DengZ
2009 Analysis of functions in plasmid pHZ1358 influencing its genetic and structural stability in Streptomyces lividans 1326. Appl Microbiol Biotechnol 82 303 310
23. EvansM
KaczmarekFS
Stutzman-EngwallK
DysonP
1994 Characterization of a Streptomyces-lividans-type site-specific DNA modification system in the avermectin-producer Streptomyces avermitilis permits investigation of two novel giant linear plasmids, pSA1 and pSA2. Microbiology 140 Pt 6 1367 1371
24. HsuM
BergP
1978 Altering the specificity of restriction endonuclease: effect of replacing Mg2+ with Mn2+. Biochemistry 17 131 138
25. ThielkingV
SelentU
KohlerE
LandgrafA
WolfesH
1992 Mg2+ confers DNA binding specificity to the EcoRV restriction endonuclease. Biochemistry 31 3727 3732
26. JanulaitisA
PetrusyteM
ManelieneZ
KlimasauskasS
ButkusV
1992 Purification and properties of the Eco57I restriction endonuclease and methylase–prototypes of a new class (type IV). Nucleic Acids Res 20 6043 6049
27. StewartFJ
RaleighEA
1998 Dependence of McrBC cleavage on distance between recognition elements. Biol Chem 379 611 616
28. Jurenaite-UrbanavicieneS
KazlauskieneR
UrbelyteV
ManelieneZ
PetrusyteM
2001 Characterization of BseMII, a new type IV restriction-modification system, which recognizes the pentanucleotide sequence 5′-CTCAG(N)(10/8). Nucleic Acids Res 29 895 903
29. StewartFJ
PanneD
BickleTA
RaleighEA
2000 Methyl-specific DNA binding by McrBC, a modification-dependent restriction enzyme. J Mol Biol 298 611 622
30. ChanSH
OpitzL
HigginsL
O'LoaneD
XuSY
2010 Cofactor requirement of HpyAV restriction endonuclease. PLoS ONE 5 e9071 doi:10.1371/journal.pone.0009071
31. ZhengY
Cohen-KarniD
XuD
ChinHG
WilsonG
2010 A unique family of Mrr-like modification-dependent restriction endonucleases. Nucleic Acids Res
32. RobertsRJ
BelfortM
BestorT
BhagwatAS
BickleTA
2003 A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res 31 1805 1812
33. DysonP
EvansM
1998 Novel post-replicative DNA modification in Streptomyces: analysis of the preferred modification site of plasmid pIJ101. Nucleic Acids Res 26 1248 1253
34. SutherlandE
CoeL
RaleighEA
1992 McrBC: a multisubunit GTP-dependent restriction endonuclease. J Mol Biol 225 327 348
35. RaleighEA
TrimarchiR
RevelH
1989 Genetic and physical mapping of the mcrA (rglA) and mcrB (rglB) loci of Escherichia coli K-12. Genetics 122 279 296
36. RaleighEA
BennerJ
BloomF
BraymerHD
DeCruzE
1991 Nomenclature relating to restriction of modified DNA in Escherichia coli. J Bacteriol 173 2707 2709
37. GorbalenyaAE
1994 Self-splicing group I and group II introns encode homologous (putative) DNA endonucleases of a new family. Protein Sci 3 1117 1120
38. DalgaardJZ
KlarAJ
MoserMJ
HolleyWR
ChatterjeeA
1997 Statistical modeling and analysis of the LAGLIDADG family of site-specific endonucleases and identification of an intein that encodes a site-specific endonuclease of the HNH family. Nucleic Acids Res 25 4626 4638
39. FukudaE
KaminskaKH
BujnickiJM
KobayashiI
2008 Cell death upon epigenetic genome methylation: a novel function of methyl-specific deoxyribonucleases. Genome Biol 9 R163
40. MacNeilDJ
1988 Characterization of a unique methyl-specific restriction system in Streptomyces avermitilis. J Bacteriol 170 5607 5612
41. ThompsonCJ
WardJM
HopwoodDA
1982 Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol 151 668 677
42. OmerCA
CohenSN
1984 Plasmid formation in Streptomyces: excision and integration of the SLP1 replicon at a specific chromosomal site. Mol Gen Genet 196 429 438
43. ThompsonCJ
KieserT
WardJM
HopwoodDA
1982 Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene 20 51 62
44. BahlMI
HansenLH
SorensenSJ
2009 Persistence mechanisms of conjugative plasmids. Methods Mol Biol 532 73 102
45. FurutaY
AbeK
KobayashiI
2010 Genome comparison and context analysis reveals putative mobile forms of restriction-modification systems and related rearrangements. Nucleic Acids Res 38 2428 2443
46. AsakuraY
KobayashiI
2009 From damaged genome to cell surface: transcriptome changes during bacterial cell death triggered by loss of a restriction-modification gene complex. Nucleic Acids Res 37 3021 3031
47. CollierJ
2009 Epigenetic regulation of the bacterial cell cycle. Curr Opin Microbiol 12 722 729
48. MarinusMG
CasadesusJ
2009 Roles of DNA adenine methylation in host-pathogen interactions: mismatch repair, transcriptional regulation, and more. FEMS Microbiol Rev 33 488 503
49. ModrichP
1987 DNA mismatch correction. Annu Rev Biochem 56 435 466
50. LeeSC
OmerCA
BraschMA
CohenSN
1988 Analysis of recombination occurring at SLP1 att sites. J Bacteriol 170 5806 5813
51. XuT
YaoF
ZhouX
DengZ
YouD
2010 A novel host-specific restriction system associated with DNA backbone S-modification in Salmonella. Nucleic Acids Res
52. SambrookJ
FritschEF
ManiatisT
1989 Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor, N.Y Cold Spring Harbor Laboratory
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome DeletionsČlánek Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable EpiallelesČlánek A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular StressČlánek Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis inČlánek The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 inČlánek Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 12
-
Všechny články tohoto čísla
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Whole-Genome and Chromosome Evolution Associated with Host Adaptation and Speciation of the Wheat Pathogen
- Association of Variants at 1q32 and with Ankylosing Spondylitis Suggests Genetic Overlap with Crohn's Disease
- Initiator Elements Function to Determine the Activity State of BX-C Enhancers
- Identification of Genes Required for Neural-Specific Glycosylation Using Functional Genomics
- A Young Duplicate Gene Plays Essential Roles in Spermatogenesis by Regulating Several Y-Linked Male Fertility Genes
- The EpsE Flagellar Clutch Is Bifunctional and Synergizes with EPS Biosynthesis to Promote Biofilm Formation
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
- Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable Epialleles
- A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular Stress
- GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells
- A Single Enhancer Regulating the Differential Expression of Duplicated Red-Sensitive Opsin Genes in Zebrafish
- Investigation and Functional Characterization of Rare Genetic Variants in the Adipose Triglyceride Lipase in a Large Healthy Working Population
- Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis in
- Noisy Splicing Drives mRNA Isoform Diversity in Human Cells
- The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 in
- Thymus-Associated Parathyroid Hormone Has Two Cellular Origins with Distinct Endocrine and Immunological Functions
- An ABC Transporter Mutation Is Correlated with Insect Resistance to Cry1Ac Toxin
- Role of Individual Subunits of the CSN Complex in Regulation of Deneddylation and Stability of Cullin Proteins
- The C-Terminal Domain of the Bacterial SSB Protein Acts as a DNA Maintenance Hub at Active Chromosome Replication Forks
- The DNA Damage Response Pathway Contributes to the Stability of Chromosome III Derivatives Lacking Efficient Replicators
- Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrA
- LaeA Control of Velvet Family Regulatory Proteins for Light-Dependent Development and Fungal Cell-Type Specificity
- Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
- Distinct Functions for the piRNA Pathway in Genome Maintenance and Telomere Protection
- MOS11: A New Component in the mRNA Export Pathway
- Self-Mating in the Definitive Host Potentiates Clonal Outbreaks of the Apicomplexan Parasites and
- A Role for ATF2 in Regulating MITF and Melanoma Development
- Ancestral Regulatory Circuits Governing Ectoderm Patterning Downstream of Nodal and BMP2/4 Revealed by Gene Regulatory Network Analysis in an Echinoderm
- Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea
- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Linking Crohn's Disease and Ankylosing Spondylitis: It's All about Genes!
- Genomics Meets Glycomics—The First GWAS Study of Human N-Glycome Identifies HNF1α as a Master Regulator of Plasma Protein Fucosylation
- Continuous and Periodic Expansion of CAG Repeats in Huntington's Disease R6/1 Mice
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Endocytic Sorting and Recycling Require Membrane Phosphatidylserine Asymmetry Maintained by TAT-1/CHAT-1
- Histone Deacetylases Suppress CGG Repeat–Induced Neurodegeneration Via Transcriptional Silencing in Models of Fragile X Tremor Ataxia Syndrome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



