-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCancer and Neurodegeneration: Between the Devil and the Deep Blue Sea
Cancer and neurodegeneration are often thought of as disease mechanisms at opposite ends of a spectrum; one due to enhanced resistance to cell death and the other due to premature cell death. There is now accumulating evidence to link these two disparate processes. An increasing number of genetic studies add weight to epidemiological evidence suggesting that sufferers of a neurodegenerative disorder have a reduced incidence for most cancers, but an increased risk for other cancers. Many of the genes associated with either cancer and/or neurodegeneration play a central role in cell cycle control, DNA repair, and kinase signalling. However, the links between these two families of diseases remain to be proven. In this review, we discuss recent and sometimes as yet incomplete genetic discoveries that highlight the overlap of molecular pathways implicated in cancer and neurodegeneration.
Published in the journal: . PLoS Genet 6(12): e32767. doi:10.1371/journal.pgen.1001257
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1001257Summary
Cancer and neurodegeneration are often thought of as disease mechanisms at opposite ends of a spectrum; one due to enhanced resistance to cell death and the other due to premature cell death. There is now accumulating evidence to link these two disparate processes. An increasing number of genetic studies add weight to epidemiological evidence suggesting that sufferers of a neurodegenerative disorder have a reduced incidence for most cancers, but an increased risk for other cancers. Many of the genes associated with either cancer and/or neurodegeneration play a central role in cell cycle control, DNA repair, and kinase signalling. However, the links between these two families of diseases remain to be proven. In this review, we discuss recent and sometimes as yet incomplete genetic discoveries that highlight the overlap of molecular pathways implicated in cancer and neurodegeneration.
Introduction: Epidemiological Data
At first glance, cancer and neurodegeneration seem to have little in common. Although neurodegeneration results in the death of post-mitotic neurons, cancer cells are characterised by an enhanced resistance to cell death. However, the more we learn about the molecular genetics and cell biology of cancer and neurodegeneration, the greater the overlap between these disorders appears. Many of the recent findings in both fields offer new avenues of study for these two age-related conditions, addressing an urgent need for therapeutic options, especially for patients with advanced disease.
Many epidemiological studies have linked cancer and neurodegenerative disorders. A growing body of evidence suggests an inverse correlation between the risk of developing cancer and a neurodegenerative disorder, in particular Parkinson's disease (PD). Several case-control and cohort studies have reported a reduced risk of almost all cancers, both smoking-related and non-smoking-related, among individuals with PD [1]. The exception to this is a suggestion of an increased risk of malignant melanoma associated with a PD diagnosis [2]–[7]. Additional work has also identified a possible association between melanoma and amyotrophic lateral sclerosis (ALS), a form of motor neuron disease (MND) [8], [9]. Nevertheless, a recent study showed no significant association between cancer and either MND or multiple sclerosis [10], in contrast to previous reports [11]–[17]. Fewer data are available linking cancer and either Alzheimer's disease (AD) or Huntington's disease (HD). It has been shown that, after adjustment for age, a diagnosis of AD was associated with a 60% reduced risk of cancer, and a history of cancer was associated with a 30% reduced risk of AD [18], [19]. Concerning HD, a lower incidence of cancer was observed among patients with the disease [20].
There is, of course, a difference between association and causality, and it has been proposed that the association between PD and skin cancer could be linked to treatment for PD (e.g., Levodopa therapy) rather than with the disease itself. However, recent reviews of the evidence do not support such a causal association [21], [22]. Additionally, it has been suggested that the decreased incidence of cancer in patients with PD is linked to the negative association between PD and smoking [23]. Although this may account for much of the risk reduction regarding smoking-related cancers, it fails to explain the decrease of non-smoking-related cancers.
The origins of the association and interplay between cancer and neurodegeneration are still a matter of debate, but increasing evidence suggests that new discoveries in genetics of these two conditions may help scientists solve the cancer–neurodegeneration enigma in the coming decade. A number of studies show that the genes causing neurodegeneration are often mutated or abnormally expressed in cancer. In the following sections we use a series of examples to illustrate the emerging genetic evidence linking cancer and neurodegeneration. We discuss whether genes that predispose to cancer also cause neurodegeneration and vice versa. Moreover, we review the genomic means of unravelling the emerging molecular pathways linking cancer and neurodegeneration.
Proven Genetic Factors Implicated in Both Cancer and Neurodegeneration: The ATM Gene
The vast majority of cancers and neurodegenerative disorders in the general population are sporadic in nature but a small proportion of these (5%–10%) are inherited in a Mendelian fashion. The search for the genes responsible for these familial forms of disease has been dominated over the last 20 years by the identification of genes that cause monogenic forms of disease. Such mutations have been discovered predominantly through linkage studies, which typically find high penetrance, but rare, genetic variants. Several genes have been unambiguously shown to cause rare familial forms of neurodegeneration [24], [25] and cancer syndromes [26]. AT-mutated (ATM) provides the closest genetic link between neurodegeneration and cancer thus far.
Ataxia-telangiectasia (AT) is a rare neurodegenerative autosomal recessive disease characterised by chromosomal instability, immunodeficiency, and a predisposition to cancer. This disease is caused by mutations in the ATM gene that leads to a total loss of the ATM protein kinase, which is part of the phosphatidylinositol-3 kinase (PI3K) superfamily, and plays a central role in cell division and DNA repair. Mutations in other DNA repair genes have been shown to cause both cancer and neurodegeneration [27]. Whether DNA repair is a causal link between cancer and neurodegeneration remains, however, to be proven. Nearly 40% of ATM homozygotes will develop cancer, usually childhood leukaemia or lymphoma [28]–[30]. Strikingly, ATM-heterozygote germline mutations were also shown to contribute to breast cancer susceptibility [31], [32]. It is noteworthy that the kinase encoded by the ATM gene is a prominent activator of p53 [27], a key tumour suppressor protein mutated and inactivated in approximately 50% of human cancers [33]–[37]. ATM is a good example of a gene that functions as a tumour suppressor but whose inactivation also leads to neuronal loss when the mutations are in the germline [38], [39].
Proven Genetic Factors Implicated in Neurodegeneration and Putatively Implicated in Cancer: The PARK2 Gene
The PARK2 gene encodes parkin, an E3 ubiquitin ligase. This gene is the most commonly mutated gene in autosomal recessive PD [40]. PARK2 was a putative candidate for a tumour suppressor gene [41]–[44], with identified whole exon deletions and duplications of this gene in ovarian and other cancers supporting this hypothesis [42], [45]. More recently, chromosomal microarray analysis was used to identify PARK2 somatic mutations and intragenic deletions in glioblastoma, colon cancer, and lung cancer [46]. This suggests that while germline mutations in PARK2 cause PD, somatic mutations in PARK2 contribute to cancer. However, PARK2 is a very large gene prone to deletions and mutations, and whether somatic mutations in parkin are primarily involved in the tumour development remains to be confirmed. Homozygous or compound heterozygous PARK2 mutations unambiguously cause PD [40]. Several lines of evidence suggest that heterozygous PARK2 mutations also have a role in the development of parkinsonism, although this is a matter of debate [47], [48]. Notably, only a few alterations identified in cancer were homozygous, most being heterozygotes. Strikingly, these mutations sufficiently altered parkin's ability to promote tumour growth. Therefore, these data suggest that, in cancer, PARK2 may act in a haploinsufficient manner.
Interestingly, PARK2 and ATM mutations in cancer sometimes occur at the exact same residue, causing neuronal degeneration [30], [46], [49]. This observation supports the idea that not only similar molecules but also similar genetic mutations within the same molecule can have very different effects, depending on the type of cell in which they occur: a dividing cell in cancer or a post-mitotic neuron in neurodegeneration. Notably, neurons are not the only post-mitotic cells, and yet they are the main cell type affected in neurodegenerative disorders. Rather than mitosis on its own, a combination of neuronal functions is therefore likely to explain the link between cancer and neurodegeneration disorders.
It is not yet clear whether the germline pathogenic mutations in the PARK genes can also increase the risk for cancer. One way to answer this question would be to compare the frequency of tumours in PD patients carrying heterozygote, compound heterozygote, or homozygote mutations in the PARK genes to that of idiopathic patients and controls without PARK mutations. However, this kind of study design is difficult to achieve in an epidemiologically robust fashion. It would require a very large number of cases and other epidemiological data as well as detailed family history and risk factor assessment.
A number of somatic mutations in two other genes unequivocally linked to PD, namely PINK1 and LRRK2 [50]–[52], both of which encode protein kinases, were identified in tissue samples from patients with various tumours [53]. The dysregulation of kinases in cancer and neurodegeneration is discussed in more detail later in the text (A Catalogue Of Somatic Mutations In Cancer can be accessed via the Wellcome Trust Sanger Institute COSMIC Web site at http://www.sanger.ac.uk/genetics/CGP/cosmic/). The PINK1 and LRRK2 somatic mutations identified in cancer were all heterozygous and their pathological effect remains to be determined. The prevalence of LRRK2 G2019S (the most common genetic determinant of PD) is not increased in patients with melanoma [7], [54], but a recent study showed an almost 3-fold increased risk of non-skin cancers in LRRK2 G2019S mutation carriers [55]. Moreover, of the 18 known mutation carriers of a large family with LRRK2 R1441C parkinsonism, four had colon cancer [56]. Nevertheless, further studies will be required to ascertain whether the association between LRRK2 parkinsonism and cancer is real or coincidental. Given the frequency of the G2019S mutation in Ashkenazi Jews and Arab Berbers with PD, it should be possible to conduct large epidemiological studies looking at cancer incidence in these families [57].
It is noteworthy that the monogenic forms of neurodegeneration and cancer are, on the whole, very rare. While most of what we know about the molecular background of idiopathic diseases is based on information gleaned from the study of rare familial forms of these disorders, one cannot readily assume that any information learnt from the Mendelian forms of a disease can enlighten us about the idiopathic forms of this disease. In light of this, extending a link that might exist between monogenic disorders to the sporadic forms of cancer and neurodegeneration should be attempted with caution.
Proven Genetic Factors Implicated in Cancer and Putatively Implicated in Neurodegeneration
It is not always the case that cancers are less common in patients with neurodegenerative disease. This is exemplified by melanoma, which has a recognised increased incidence in PD patients. A positive family history is a strongly associated risk factor for melanoma [58]–[62], and approximately 50% of affected families have mutations in one of the three following genes: cyclin-dependent kinase inhibitor 2A (CDKN2A), alternate reading frame (ARF), and cyclin-dependent kinase 4 (CDK4). These mutations, identified through linkage studies, are inherited in an autosomal dominant manner and have a high penetrance. High-frequency alleles with small effects on melanoma risk have also been identified in a number of genes, including MC1R (Melanocortin 1 Receptor) and TYR (tyrosinase). Moreover, an approximately 2-fold increase in the risk of PD was reported among individuals who reported a family history of melanoma compared with individuals without such a family history. The significant association was independent of several known risk factors for PD, including smoking [63]. No significant associations were observed between a family history of several other common cancers and PD risk [64], suggesting the existence of common genetic determinants between PD and melanoma. There remains, however, the possibility that another unknown environmental factor could contribute to the observed association between a family history of melanoma and PD risk. Other genes, such as the CDKs, for which an increased expression or dysregulation has been observed in melanomas [65] and PD [66], [67], could also play a role in the observed association.
Two genome-wide association studies (GWAS) have recently been performed in melanoma and melanocytic nevi [68]–[70]. One study replicated two previously suggested associations with the disease, MC1R and TYR. In addition to hits near these two genes, a locus flanking the familial melanoma susceptibility locus CDKN2A was identified. The second study demonstrated that methylthioadenosine phosphorylase (MTAP), a gene adjacent to CDKN2A, and another locus encompassing PLA2G6 (a member of the phospholipase A2 gene family) both showed an association with melanoma risk. Interestingly, mutations in the gene encoding the phospholipase PLA2G6 can cause parkinsonism [71]. PLA2G6 is also associated with lung cancer susceptibility [72]. The combination of these accumulating epidemiologic and genetic linkages between melanoma and PD suggest a need for more mechanistic/biological work in this area.
Notably, no major known cancer gene was among the combination of genetic variants identified as risk factors for neurodegenerative disorders. In fact, recent studies from GWAS of AD and PD have mainly identified genes principally implicated in protein accumulation and the complement cascade of the immune system [73].
Post-Translational Modifications—Strongly Implicated in Cancer, with an Emerging Role in Neurodegeneration?
Post-translational modifications also play a role in the association between cancer and neurodegeneration. For example, protein alterations that predispose the cell toward cell death might lead to a decreased risk of cancer and an increased risk of neurodegeneration, whereas conditions that favour cell growth might lead to an increased risk of cancer and a decreased risk in neurodegeneration [74]–[77]. Indeed, the same molecules are often used for different purposes in the control of cell division, cell differentiation, and cell death. Depending on whether the cell is an actively dividing or a post-mitotic neuron, responses to alterations in these molecules and pathways may differ, ultimately leading to either cancer or neurodegeneration (for a comprehensive overview of the genes implicated in neurodegeneration and cancer, see Table 1).
Tab. 1. Genetic determinants at the interface of cancer and neurodegeneration. 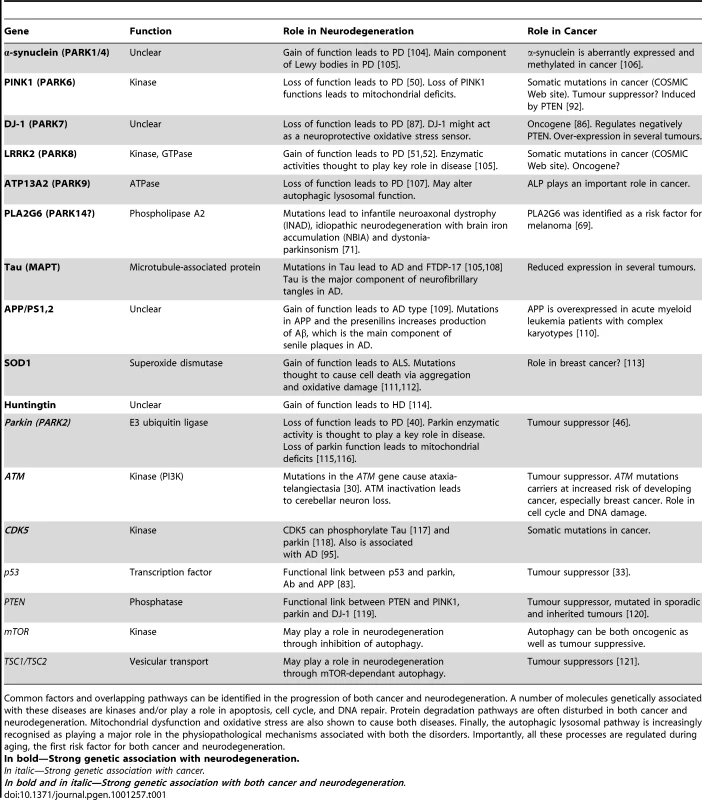
Common factors and overlapping pathways can be identified in the progression of both cancer and neurodegeneration. A number of molecules genetically associated with these diseases are kinases and/or play a role in apoptosis, cell cycle, and DNA repair. Protein degradation pathways are often disturbed in both cancer and neurodegeneration. Mitochondrial dysfunction and oxidative stress are also shown to cause both diseases. Finally, the autophagic lysosomal pathway is increasingly recognised as playing a major role in the physiopathological mechanisms associated with both the disorders. Importantly, all these processes are regulated during aging, the first risk factor for both cancer and neurodegeneration. Many proteins when abnormally expressed or aberrantly regulated have been linked to cancer or neurodegeneration; in particular, proteins implicated in cell cycle regulation [75]. For example, many human cancers have lost the function of p53, a key tumour suppressor transcription factor playing an important role in cell cycle arrest in response to DNA damage and apoptosis [33]–[37]. Increasing evidence supports the contribution of transcriptional inhibition to neurotoxicity of DNA damage [78]. Interestingly, p53 is associated with several neurodegenerative disorders, including HD, AD, and PD [35], [37]. P53 protein can regulate huntingtin (htt) expression at transcriptional level [79]. Moreover, p53 provides strong protection from neurotoxicity associated with the mutant htt with expanded polyglutamine in HD fly and mouse models [80]. The PD-associated protein parkin can repress p53 transcriptional activity that is impaired by the PARK2 mutations associated with PD [81], [82]. Finally, p53 regulates and is regulated by AD-associated proteins such as the members of the γ-secretase complex [83]. A recent review discusses the role of p53 as a potential candidate that may explain the inverse association between AD and cancer [84]. It would be interesting to determine whether patients with Li-Fraumeni syndrome, characterised by germline mutations in the p53 gene [85], have an altered risk for neurodegeneration. Cancer-related proteins can cause neurodegeneration when abnormally expressed or regulated and the opposite is also true. A number of genes associated with neurodegeneration were investigated in cancer research before their role in neurodegeneration was identified, but whether these genes are true oncogenes or tumour suppressors remains to be proven. For example, DJ-1 was identified as an oncogene before it was linked to autosomal recessive PD [86], [87]. This gene was initially cloned as a cMyc interactor. It is expressed at high levels in lung and prostate cancer biopsies and in the sera of breast cancer patients [88]–[90]. DJ-1 was shown to suppress the function of the tumour suppressor PTEN [91], a gene shown to induce PINK1 when overexpressed [92]. However, DJ-1 showed a weak transforming activity by itself, throwing into doubt its oncogenic function [86].
Protein kinases, when abnormally expressed or dysregulated, can lead to cancer. Because of the key apical role of kinases in the control of key signal transduction networks that impact normal cellular physiology and pathological conditions, the development of small molecule kinase inhibitors as potential cancer therapeutics is an area of intense research. A subset of these agents target CDK activity. Interest in the therapeutic potential of CDK inhibitors has expanded to include neurodegenerative diseases [93]. Specifically, there is growing evidence suggesting that CDK5, an important modulator of neuronal activity and a critical player in a number of cancers, is involved in various physiological roles within the central nervous system and a number of neurodegenerative disorders such as AD, ALS, HD, and PD [94]. Interestingly, variations in the CDK5 gene are associated with AD [95]. Finally, as a result of their putative kinase function, PINK1 and LRRK2 are attractive potential targets in the treatment of PD and cancer even though their potential influence in tumour growth remains mostly indirect and suggestive thus far (see Table 1; [1], [96], [97]).
Challenges for the Future
Although many epidemiologic studies have found a relationship between cancer and neurodegeneration, in particular in PD, the results have been inconsistent. Variations in the design, methods, and quality of the studies on cancer risk among patients with PD have made it difficult to ascertain the link between the two disorders. In the next section, we discuss the means of exploring this link in order to accelerate progress in the next few years. Our understanding of the control of signalling pathways is further advanced in cancer studies compared to neurodegeneration. As a result, many small molecule inhibitors, such as histone deacetylase inhibitors and kinase inhibitors, have been approved as anticancer agents or are currently being tested in clinical trials [98]. Thus, discoveries in cancer research are likely to provide a solid base upon which scientists will study the pathophysiology of neurodegenerative diseases.
The results of the many epidemiologic studies that have found patients with a neurodegenerative disease to be associated with a modified incidence of cancer have varied in their consistency. Diversity in the design and quality of the studies exploring cancer risk in patients with neurodegenerative disease has made it difficult to confirm the relationship between the two diseases with certainty. The GWAS approach has effected a step change in human genetic research by linking a number of variants to complex diseases. Each variant robustly linked to a disease offers a possible route to unravelling the molecular pathways associated with the disease. GWAS have been performed for most cancers and neurodegenerative disorders; a catalog of published GWAS is available online at http://www.genome.gov/. However, the results of GWAS have also been variable [73], and it is likely that much larger epidemiologic and genetic studies and meta-analysis will be required to determine if there is a real association between cancer and neurodegeneration. A quantitative analysis of several independent studies has confirmed the overall lower cancer risk ratio among patients with PD [99].
Although the variants that have been identified thus far confer only a small risk of the disease, identifying additional variants that contribute to the pathogenesis of the disease is likely to help the scientific community to move forward in understanding the link between these two disorders. With this in mind, a second generation of GWAS will be performed using new chips targeting variants throughout the genome at even lower frequencies. Additionally, as sequencing technology becomes cheaper, an explosion of targeted gene-sequencing studies looking for rarer risk variants is to be expected. The use of approaches such as array-based comparative genomic hybridisation, high-throughput sequencing, and transcriptome analysis has already enabled the identification of common variants for cancer and neurodegeneration, for example PARK2 in cancer [46].
The next generation of sequencing is also likely to help with the understanding of the link between cancer and neurodegeneration. Exome sequencing may represent only an intermediary step before whole-genome sequencing becomes widely available. However, this technology may still be able to shed light on important coding mutations in these disorders. It is important to note that this approach can miss potentially important non-coding changes (e.g., regulatory regions or miRNAS), which will require the systematic approach offered by whole-genome sequencing. Some major cancer genome screening projects aim to eventually sequence the full genomes of thousands of tumour samples and those of people from whom the tumours were taken. Currently, most laboratories investigating these diseases are carrying out exome sequencing, although whole-genome sequences of a patient with acute myeloid leukaemia have already been obtained [100].
Finally, it is becoming increasingly clear that a multitude of complex and interconnected epigenetic modifications such as miRNAs, DNA acetylation, and DNA methylation can conspire with genetic alterations in disease pathogenesis [101]. As a result, methodologies like genome-wide promoter DNA methylation profiling could reveal specific patterns that are associated with the disease [102].
Conclusion
Both cancer and neurodegeneration are thought to be the result of the interaction of genetic and environmental factors [103]. Age is the single most important risk factor for both cancer and neurodegeneration and, although the exact mechanisms of aging are not yet completely defined, age is likely to play an important role in the link between the two disorders. Both cancer and neurodegeneration are also characterised by the contribution of the inheritance of mutated genes. Research showing that cancer and neurodegenerative disorders share some of the same genes and molecular mechanisms strengthens the idea that individuals affected by a neurodegenerative disease may have a decreased risk of some cancers. Despite a number of intriguing pointers, little is known about the genetic association between cancer and neurodegeneration. Although a large number of genes have been implicated in the genesis of cancer and neurodegeneration, only two, parkin and ATM, have been shown to strongly overlap (Figure 1). Given the large number of signalling molecules that crosstalk in multiple pathways, one cannot exclude that these overlaps could be coincidental. Further, large genetic and epidemiological studies looking at cancer incidence in the population afflicted with neurodegenerative disease (and vice versa) will be required to find putative new genes at the interface of the two diseases and to ascertain that the genetic link between these two disorders is not coincidental. Unravelling the precise molecular processes that may be involved in both disorders is likely to be enlightening. Most degenerative diseases of the brain are incurable and the study of tissue from the brains of people with significant neurodegeneration should be approached with caution because the neuronal cells that are dysregulated and likely to be most informative are already dead. However, cancer research has been extremely prolific over the past two decades, and one could imagine that research in neurodegeneration will benefit from breakthrough studies in cancer. Therefore, the extensive therapeutic developments in cancer research may allow the identification of prognostic markers for cancer and neurodegeneration that could result in improved treatments for both disorders.
Fig. 1. Common pathways to cancer and neurodegeneration? 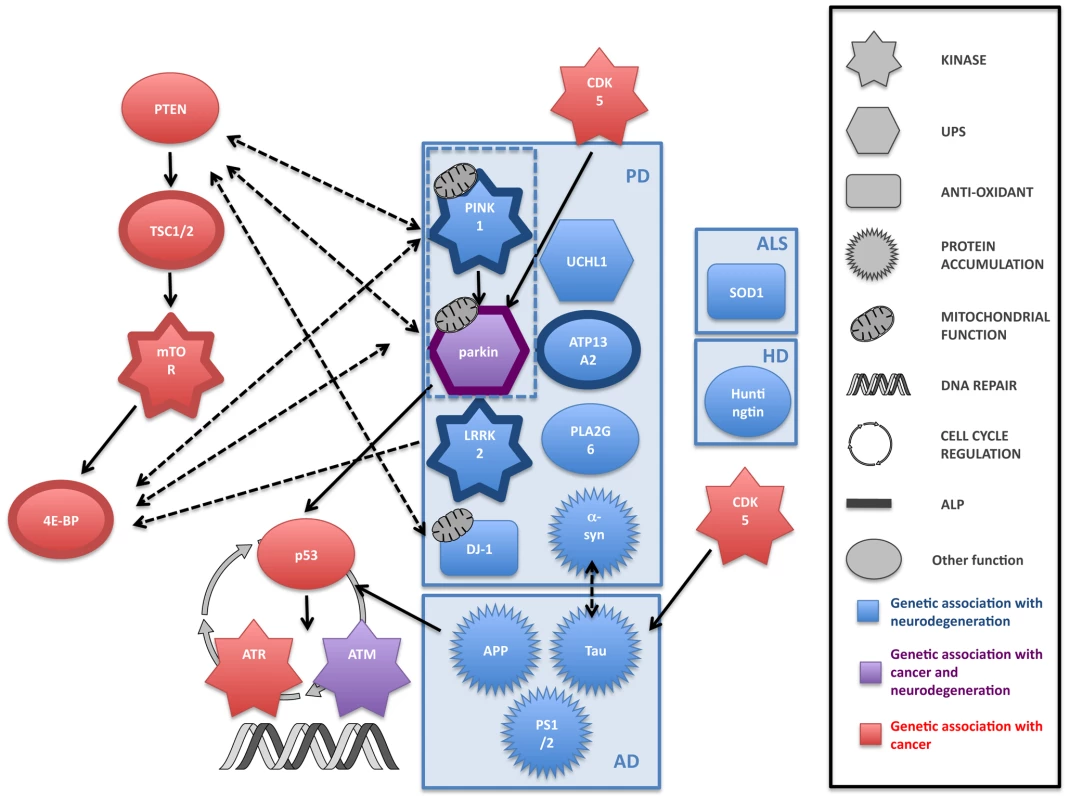
An illustration of some of the genes that are linked to cancer and neurodegeneration, and the crosstalk plus overlap between them. Although the links between genes involved in the individual disorders themselves are not yet completely clear (for example, there is evidence that there may be several parallel pathways leading to cell loss in the substantia nigra and the clinical symptom of parkinsonism), there is an intriguing picture emerging of fundamental links between cell proliferation and cell death. ALP, autophagy-lysosome pathway; UPS: ubiquitin-proteasome system.
Zdroje
1. InzelbergR
JankovicJ
2007 Are Parkinson disease patients protected from some but not all cancers? Neurology 69 1542 1550
2. MollerH
MellemkjaerL
McLaughlinJK
OlsenJH
1995 Occurrence of different cancers in patients with Parkinson's disease. BMJ 310 1500 1501
3. DriverJA
LogroscinoG
BuringJE
GazianoJM
KurthT
2007 A prospective cohort study of cancer incidence following the diagnosis of Parkinson's disease. Cancer Epidemiol Biomarkers Prev 16 1260 1265
4. OlsenJH
FriisS
FrederiksenK
2006 Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology 17 582 587
5. OlsenJH
FriisS
FrederiksenK
McLaughlinJK
MellemkjaerL
2005 Atypical cancer pattern in patients with Parkinson's disease. Br J Cancer 92 201 205
6. OlsenJH
TangerudK
WermuthL
FrederiksenK
FriisS
2007 Treatment with levodopa and risk for malignant melanoma. Mov Disord 22 1252 1257
7. InzelbergR
Israeli-KornSD
2009 The particular relationship between Parkinson's disease and malignancy: a focus on skin cancers. J Neural Transm 116 1503 1507
8. FreedmanDM
TravisLB
GridleyG
KunclRW
2005 Amyotrophic lateral sclerosis mortality in 1.9 million US cancer survivors. Neuroepidemiology 25 176 180
9. BaadePD
FritschiL
FreedmanDM
2007 Mortality due to amyotrophic lateral sclerosis and Parkinson's disease among melanoma patients. Neuroepidemiology 28 16 20
10. FoisAF
WottonCJ
YeatesD
TurnerMR
GoldacreMJ
2010 Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson's disease: record linkage studies. J Neurol Neurosurg Psychiatry 81 215 221
11. BrainL
CroftPB
WilkinsonM
1965 Motor neurone disease as a manifestation of neoplasm (with a note on the course of classical motor neurone disease). Brain 88 479 500
12. SadotE
CarluerL
CorciaP
DelozierY
LevyC
2007 Breast cancer and motor neuron disease: clinical study of seven cases. Amyotroph Lateral Scler 8 288 291
13. NielsenNM
RostgaardK
RasmussenS
Koch-HenriksenN
StormHH
2006 Cancer risk among patients with multiple sclerosis: a population-based register study. Int J Cancer 118 979 984
14. HjalgrimH
RasmussenS
RostgaardK
NielsenNM
Koch-HenriksenN
2004 Familial clustering of Hodgkin lymphoma and multiple sclerosis. J Natl Cancer Inst 96 780 784
15. VineisP
CrosignaniP
ViganoC
FontanaA
MasalaG
2001 Lymphomas and multiple sclerosis in a multicenter case-control study. Epidemiology 12 134 135
16. MidgardR
GlattreE
GronningM
RiiseT
EdlandA
1996 Multiple sclerosis and cancer in Norway. A retrospective cohort study. Acta Neurol Scand 93 411 415
17. AndersonM
HughesB
JeffersonM
SmithWT
WaterhouseJA
1980 Gliomatous transformation and demyelinating diseases. Brain 103 603 622
18. BennettDA
LeurgansS
2010 Is there a link between cancer and Alzheimer disease? Neurology 74 100 101
19. RoeCM
FitzpatrickAL
XiongC
SiehW
KullerL
2010 Cancer linked to Alzheimer disease but not vascular dementia. Neurology 74 106 112
20. SorensenSA
FengerK
OlsenJH
1999 Significantly lower incidence of cancer among patients with Huntington disease: An apoptotic effect of an expanded polyglutamine tract? Cancer 86 1342 1346
21. FialaKH
WhetteckeyJ
ManyamBV
2003 Malignant melanoma and levodopa in Parkinson's disease: causality or coincidence? Parkinsonism Relat Disord 9 321 327
22. ZanettiR
LoriaD
RossoS
2006 Melanoma, Parkinson's disease and levodopa: causal or spurious link? A review of the literature. Melanoma Res 16 201 206
23. HernanMA
TakkoucheB
Caamano-IsornaF
Gestal-OteroJJ
2002 A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 52 276 284
24. BurchellVS
GandhiS
DeasE
WoodNW
AbramovAY
2010 Targeting mitochondrial dysfunction in neurodegenerative disease: Part II. Expert Opin Ther Targets 14 497 511
25. BurchellVS
GandhiS
DeasE
WoodNW
AbramovAY
2010 Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert Opin Ther Targets 14 369 385
26. GarberJE
OffitK
2005 Hereditary cancer predisposition syndromes. J Clin Oncol 23 276 292
27. MorrisLG
VeeriahS
ChanTA
2010 Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene 29 3453 3464
28. BallLG
XiaoW
2005 Molecular basis of ataxia telangiectasia and related diseases. Acta Pharmacol Sin 26 897 907
29. Gumy-PauseF
WackerP
MailletP
BettsDR
SappinoAP
2006 ATM variants and predisposition to childhood T-lineage acute lymphoblastic leukaemia. Leukemia 20 526 527; author reply 527
30. MavrouA
TsangarisGT
RomaE
KolialexiA
2008 The ATM gene and ataxia telangiectasia. Anticancer Res 28 401 405
31. BroeksA
UrbanusJH
FlooreAN
DahlerEC
KlijnJG
2000 ATM-heterozygous germline mutations contribute to breast cancer-susceptibility. Am J Hum Genet 66 494 500
32. AhmedM
RahmanN
2006 ATM and breast cancer susceptibility. Oncogene 25 5906 5911
33. VogelsteinB
LaneD
LevineAJ
2000 Surfing the p53 network. Nature 408 307 310
34. BodeAM
DongZ
2004 Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4 793 805
35. DavenportCM
SevastouIG
HooperC
PocockJM
2010 Inhibiting p53 pathways in microglia attenuates microglial-evoked neurotoxicity following exposure to Alzheimer peptides. J Neurochem 112 552 563
36. DunysJ
SevalleJ
GiaimeE
Pardossi-PiquardR
VitekMP
2009 p53-dependent control of transactivation of the Pen2 promoter by presenilins. J Cell Sci 122 4003 4008
37. JacobsWB
KaplanDR
MillerFD
2006 The p53 family in nervous system development and disease. J Neurochem 97 1571 1584
38. EngC
2003 PTEN: one gene, many syndromes. Hum Mutat 22 183 198
39. ShilohY
RotmanG
1996 Ataxia-telangiectasia and the ATM gene: linking neurodegeneration, immunodeficiency, and cancer to cell cycle checkpoints. J Clin Immunol 16 254 260
40. KitadaT
AsakawaS
HattoriN
MatsumineH
YamamuraY
1998 Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392 605 608
41. CesariR
MartinES
CalinGA
PentimalliF
BichiR
2003 Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25–q27. Proc Natl Acad Sci U S A 100 5956 5961
42. DenisonSR
WangF
BeckerNA
SchuleB
KockN
2003 Alterations in the common fragile site gene Parkin in ovarian and other cancers. Oncogene 22 8370 8378
43. PicchioMC
MartinES
CesariR
CalinGA
YendamuriS
2004 Alterations of the tumor suppressor gene Parkin in non-small cell lung cancer. Clin Cancer Res 10 2720 2724
44. WangF
DenisonS
LaiJP
PhilipsLA
MontoyaD
2004 Parkin gene alterations in hepatocellular carcinoma. Genes Chromosomes Cancer 40 85 96
45. DenisonSR
CallahanG
BeckerNA
PhillipsLA
SmithDI
2003 Characterization of FRA6E and its potential role in autosomal recessive juvenile parkinsonism and ovarian cancer. Genes Chromosomes Cancer 38 40 52
46. VeeriahS
TaylorBS
MengS
FangF
YilmazE
2010 Somatic mutations of the Parkinson's disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat Genet 42 77 82
47. KleinC
Lohmann-HedrichK
RogaevaE
SchlossmacherMG
LangAE
2007 Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol 6 652 662
48. Abou-SleimanPM
MuqitMM
McDonaldNQ
YangYX
GandhiS
2006 A heterozygous effect for PINK1 mutations in Parkinson's disease? Ann Neurol 60 414 419
49. VeeriahS
MorrisLG
SolitD
ChanTA
2010 The familial Parkinson disease gene PARK2 is a multisite tumor suppressor on chromosome 6q25.2–27 that regulates cyclin E. Cell Cycle 9 1451 1452
50. ValenteEM
Abou-SleimanPM
CaputoV
MuqitMM
HarveyK
2004 Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304 1158 1160
51. ZimprichA
BiskupS
LeitnerP
LichtnerP
FarrerM
2004 Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44 601 607
52. Paisan-RuizC
JainS
EvansEW
GilksWP
SimonJ
2004 Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44 595 600
53. GreenmanC
StephensP
SmithR
DalglieshGL
HunterC
2007 Patterns of somatic mutation in human cancer genomes. Nature 446 153 158
54. Hassin-BaerS
LaitmanY
AziziE
MolchadskiI
Galore-HaskelG
2009 The leucine rich repeat kinase 2 (LRRK2) G2019S substitution mutation. Association with Parkinson disease, malignant melanoma and prevalence in ethnic groups in Israel. J Neurol 256 483 487
55. Saunders-PullmanR
BarrettMJ
StanleyKM
LucianoMS
ShankerV
2010 LRRK2 G2019S mutations are associated with an increased cancer risk in Parkinson disease. Mov Disord 5 2536 2541
56. StrongoskyAJ
FarrerM
WszolekZK
2008 Are Parkinson disease patients protected from some but not all cancers? Neurology 71 1650; author reply 1650–1651
57. BressmanS
GiladiN
MarderK
Orr-UrtregerA
2009 Parkinson's disease, Ashkenazi Jews and LRRK2–a consortium proposal [abstract]. The Michael J Fox Foundation for Parkinson's Research searchable database of funded grants. Available: http://www.michaeljfox.org/research_MJFFfundingPortfolio_searchableAwardedGrants_3.cfm?ID=559. Accessed 22 November 2010
58. FordD
BlissJM
SwerdlowAJ
ArmstrongBK
FranceschiS
1995 Risk of cutaneous melanoma associated with a family history of the disease. The International Melanoma Analysis Group (IMAGE). Int J Cancer 62 377 381
59. GandiniS
SeraF
CattaruzzaMS
PasquiniP
AbeniD
2005 Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer 41 28 44
60. GandiniS
SeraF
CattaruzzaMS
PasquiniP
PicconiO
2005 Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer 41 45 60
61. GandiniS
SeraF
CattaruzzaMS
PasquiniP
ZanettiR
2005 Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer 41 2040 2059
62. NoeM
SchroyP
DemierreMF
BabayanR
GellerAC
2008 Increased cancer risk for individuals with a family history of prostate cancer, colorectal cancer, and melanoma and their associated screening recommendations and practices. Cancer Causes Control 19 1 12
63. GaoX
SimonKC
HanJ
SchwarzschildMA
AscherioA
2009 Family history of melanoma and Parkinson disease risk. Neurology 73 1286 1291
64. GaoX
SimonKC
HanJ
SchwarzschildMA
AscherioA
2009 Genetic determinants of hair color and Parkinson's disease risk. Ann Neurol 65 76 82
65. TangL
LiG
TronVA
TrotterMJ
HoVC
1999 Expression of cell cycle regulators in human cutaneous malignant melanoma. Melanoma Res 9 148 154
66. VerdaguerE
JordaEG
StrangesA
CanudasAM
JimenezA
2003 Inhibition of CDKs: a strategy for preventing kainic acid-induced apoptosis in neurons. Ann N Y Acad Sci 1010 671 674
67. AlviraD
TajesM
VerdaguerE
de ArribaSG
AllgaierC
2007 Inhibition of cyclin-dependent kinases is neuroprotective in 1-methyl-4-phenylpyridinium-induced apoptosis in neurons. Neuroscience 146 350 365
68. BishopDT
DemenaisF
IlesMM
HarlandM
TaylorJC
2009 Genome-wide association study identifies three loci associated with melanoma risk. Nat Genet 41 920 925
69. FalchiM
BatailleV
HaywardNK
DuffyDL
BishopJA
2009 Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat Genet 41 915 919
70. YehI
BastianBC
2009 Genome-wide associations studies for melanoma and nevi. Pigment Cell Melanoma Res 22 527 528
71. Paisan-RuizC
BhatiaKP
LiA
HernandezD
DavisM
2009 Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol 65 19 23
72. HosgoodHD3rd
MenasheI
ShenM
YeagerM
YuengerJ
2008 Pathway-based evaluation of 380 candidate genes and lung cancer susceptibility suggests the importance of the cell cycle pathway. Carcinogenesis 29 1938 1943
73. GandhiS
WoodNW
2010 Genome-wide association studies: the key to unlocking neurodegeneration? Nat Neurosci 13 789 794
74. WestAB
DawsonVL
DawsonTM
2005 To die or grow: Parkinson's disease and cancer. Trends Neurosci 28 348 352
75. StaropoliJF
2008 Tumorigenesis and neurodegeneration: two sides of the same coin? Bioessays 30 719 727
76. GarberK
2010 Parkinson's disease and cancer: the unexplored connection. J Natl Cancer Inst 102 371 374
77. KimRH
MakTW
2006 Tumours and tremors: how PTEN regulation underlies both. Br J Cancer 94 620 624
78. HetmanM
VashishtaA
RempalaG
2010 Neurotoxic mechanisms of DNA damage: focus on transcriptional inhibition. J Neurochem 114 1537 1549
79. FengZ
JinS
ZupnickA
HohJ
de StanchinaE
2006 p53 tumor suppressor protein regulates the levels of huntingtin gene expression. Oncogene 25 1 7
80. BaeBI
XuH
IgarashiS
FujimuroM
AgrawalN
2005 p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron 47 29 41
81. da CostaCA
CheclerF
2010 A novel parkin-mediated transcriptional function links p53 to familial Parkinson's disease. Cell Cycle 9 16 17
82. da CostaCA
SunyachC
GiaimeE
WestA
CortiO
2009 Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson's disease. Nat Cell Biol 11 1370 1375
83. CheclerF
DunysJ
Pardossi-PiquardR
Alves da CostaC
2010 p53 is regulated by and regulates members of the gamma-secretase complex. Neurodegener Dis 7 50 55
84. BehrensMI
LendonC
RoeCM
2009 A common biological mechanism in cancer and Alzheimer's disease? Curr Alzheimer Res 6 196 204
85. MalkinD
LiFP
StrongLC
FraumeniJFJr
NelsonCE
1990 Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250 1233 1238
86. NagakuboD
TairaT
KitauraH
IkedaM
TamaiK
1997 DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun 231 509 513
87. BonifatiV
RizzuP
van BarenMJ
SchaapO
BreedveldGJ
2003 Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299 256 259
88. Le NaourF
MisekDE
KrauseMC
DeneuxL
GiordanoTJ
2001 Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res 7 3328 3335
89. MacKeiganJP
ClementsCM
LichJD
PopeRM
HodY
2003 Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIalpha. Cancer Res 63 6928 6934
90. HodY
2004 Differential control of apoptosis by DJ-1 in prostate benign and cancer cells. J Cell Biochem 92 1221 1233
91. KimRH
PetersM
JangY
ShiW
PintilieM
2005 DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 7 263 273
92. UnokiM
NakamuraY
2001 Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene 20 4457 4465
93. MonacoEA3rd
VallanoML
2003 Cyclin-dependent kinase inhibitors: cancer killers to neuronal guardians. Curr Med Chem 10 367 379
94. DhariwalaFA
RajadhyakshaMS
2008 An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol 28 351 369
95. Arias-VasquezA
AulchenkoYS
IsaacsA
van OosterhoutA
SleegersK
2008 Cyclin-dependent kinase 5 is associated with risk for Alzheimer's disease in a Dutch population-based study. J Neurol 255 655 662
96. GreggioE
SingletonA
2007 Kinase signaling pathways as potential targets in the treatment of Parkinson's disease. Expert Rev Proteomics 4 783 792
97. MacKeiganJP
MurphyLO
BlenisJ
2005 Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol 7 591 600
98. CiavarellaS
MilanoA
DammaccoF
SilvestrisF
2010 Targeted therapies in cancer. BioDrugs 24 77 88
99. BajajA
DriverJA
SchernhammerES
2010 Parkinson's disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control 21 697 707
100. LeyTJ
MardisER
DingL
FultonB
McLellanMD
2008 DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456 66 72
101. JonesPA
BaylinSB
2007 The epigenomics of cancer. Cell 128 683 692
102. BullingerL
ArmstrongSA
2010 HELP for AML: methylation profiling opens new avenues. Cancer Cell 17 1 3
103. MiglioreL
CoppedeF
2002 Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat Res 512 135 153
104. PolymeropoulosMH
LavedanC
LeroyE
IdeSE
DehejiaA
1997 Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276 2045 2047
105. DevineMJ
LewisPA
2008 Emerging pathways in genetic Parkinson's disease: tangles, Lewy bodies and LRRK2. FEBS J 275 5748 5757
106. JowaedA
SchmittI
KautO
WullnerU
2010 Methylation regulates alpha-synuclein expression and is decreased in Parkinson's disease patients' brains. J Neurosci 30 6355 6359
107. RamirezA
HeimbachA
GrundemannJ
StillerB
HampshireD
2006 Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 38 1184 1191
108. HuttonM
LendonCL
RizzuP
BakerM
FroelichS
1998 Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17 Nature 393 702 705
109. HardyJ
1997 Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci 20 154 159
110. BaldusCD
LiyanarachchiS
MrozekK
AuerH
TannerSM
2004 Acute myeloid leukemia with complex karyotypes and abnormal chromosome 21: Amplification discloses overexpression of APP, ETS2, and ERG genes. Proc Natl Acad Sci U S A 101 3915 3920
111. ClevelandDW
RothsteinJD
2001 From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2 806 819
112. PasinelliP
BrownRH
2006 Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7 710 723
113. RaoAK
ZieglerYS
McLeodIX
YatesJR
NardulliAM
2008 Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol Endocrinol 22 1113 1124
114. 1993 A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell 72 971 983
115. DeasE
WoodPlun-Favreau NWH
2010 Mitophagy and Parkinson's disease: the PINK1-parkin link. Biochim Biophys Acta E-pub ahead of print 21 August 2010
116. ShimuraH
HattoriN
KuboS
MizunoY
AsakawaS
2000 Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25 302 305
117. BaumannK
MandelkowEM
BiernatJ
Piwnica-WormsH
MandelkowE
1993 Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett 336 417 424
118. AvrahamE
RottR
LianiE
SzargelR
EngelenderS
2007 Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem 282 12842 12850
119. FitzgeraldJC
Plun-FavreauH
2008 Emerging pathways in genetic Parkinson's disease: autosomal-recessive genes in Parkinson's disease–a common pathway? FEBS J 275 5758 5766
120. SalmenaL
CarracedoA
PandolfiPP
2008 Tenets of PTEN tumor suppression. Cell 133 403 414
121. ReilingJH
SabatiniDM
2006 Stress and mTORture signaling. Oncogene 25 6373 6383
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome DeletionsČlánek Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable EpiallelesČlánek A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular StressČlánek Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis inČlánek The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 inČlánek Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrAČlánek Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 12
-
Všechny články tohoto čísla
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Whole-Genome and Chromosome Evolution Associated with Host Adaptation and Speciation of the Wheat Pathogen
- Association of Variants at 1q32 and with Ankylosing Spondylitis Suggests Genetic Overlap with Crohn's Disease
- Initiator Elements Function to Determine the Activity State of BX-C Enhancers
- Identification of Genes Required for Neural-Specific Glycosylation Using Functional Genomics
- A Young Duplicate Gene Plays Essential Roles in Spermatogenesis by Regulating Several Y-Linked Male Fertility Genes
- The EpsE Flagellar Clutch Is Bifunctional and Synergizes with EPS Biosynthesis to Promote Biofilm Formation
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
- Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable Epialleles
- A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular Stress
- GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells
- A Single Enhancer Regulating the Differential Expression of Duplicated Red-Sensitive Opsin Genes in Zebrafish
- Investigation and Functional Characterization of Rare Genetic Variants in the Adipose Triglyceride Lipase in a Large Healthy Working Population
- Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis in
- Noisy Splicing Drives mRNA Isoform Diversity in Human Cells
- The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 in
- Thymus-Associated Parathyroid Hormone Has Two Cellular Origins with Distinct Endocrine and Immunological Functions
- An ABC Transporter Mutation Is Correlated with Insect Resistance to Cry1Ac Toxin
- Role of Individual Subunits of the CSN Complex in Regulation of Deneddylation and Stability of Cullin Proteins
- The C-Terminal Domain of the Bacterial SSB Protein Acts as a DNA Maintenance Hub at Active Chromosome Replication Forks
- The DNA Damage Response Pathway Contributes to the Stability of Chromosome III Derivatives Lacking Efficient Replicators
- Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrA
- LaeA Control of Velvet Family Regulatory Proteins for Light-Dependent Development and Fungal Cell-Type Specificity
- Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
- Distinct Functions for the piRNA Pathway in Genome Maintenance and Telomere Protection
- MOS11: A New Component in the mRNA Export Pathway
- Self-Mating in the Definitive Host Potentiates Clonal Outbreaks of the Apicomplexan Parasites and
- A Role for ATF2 in Regulating MITF and Melanoma Development
- Ancestral Regulatory Circuits Governing Ectoderm Patterning Downstream of Nodal and BMP2/4 Revealed by Gene Regulatory Network Analysis in an Echinoderm
- Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea
- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Linking Crohn's Disease and Ankylosing Spondylitis: It's All about Genes!
- Genomics Meets Glycomics—The First GWAS Study of Human N-Glycome Identifies HNF1α as a Master Regulator of Plasma Protein Fucosylation
- Continuous and Periodic Expansion of CAG Repeats in Huntington's Disease R6/1 Mice
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Endocytic Sorting and Recycling Require Membrane Phosphatidylserine Asymmetry Maintained by TAT-1/CHAT-1
- Histone Deacetylases Suppress CGG Repeat–Induced Neurodegeneration Via Transcriptional Silencing in Models of Fragile X Tremor Ataxia Syndrome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



