-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Single Enhancer Regulating the Differential Expression of Duplicated Red-Sensitive Opsin Genes in Zebrafish
A fundamental step in the evolution of the visual system is the gene duplication of visual opsins and differentiation between the duplicates in absorption spectra and expression pattern in the retina. However, our understanding of the mechanism of expression differentiation is far behind that of spectral tuning of opsins. Zebrafish (Danio rerio) have two red-sensitive cone opsin genes, LWS-1 and LWS-2. These genes are arrayed in a tail-to-head manner, in this order, and are both expressed in the long member of double cones (LDCs) in the retina. Expression of the longer-wave sensitive LWS-1 occurs later in development and is thus confined to the peripheral, especially ventral-nasal region of the adult retina, whereas expression of LWS-2 occurs earlier and is confined to the central region of the adult retina, shifted slightly to the dorsal-temporal region. In this study, we employed a transgenic reporter assay using fluorescent proteins and P1-artificial chromosome (PAC) clones encompassing the two genes and identified a 0.6-kb “LWS-activating region” (LAR) upstream of LWS-1, which regulates expression of both genes. Under the 2.6-kb flanking upstream region containing the LAR, the expression pattern of LWS-1 was recapitulated by the fluorescent reporter. On the other hand, when LAR was directly conjugated to the LWS-2 upstream region, the reporter was expressed in the LDCs but also across the entire outer nuclear layer. Deletion of LAR from the PAC clones drastically lowered the reporter expression of the two genes. These results suggest that LAR regulates both LWS-1 and LWS-2 by enhancing their expression and that interaction of LAR with the promoters is competitive between the two genes in a developmentally restricted manner. Sharing a regulatory region between duplicated genes could be a general way to facilitate the expression differentiation in duplicated visual opsins.
Published in the journal: . PLoS Genet 6(12): e32767. doi:10.1371/journal.pgen.1001245
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001245Summary
A fundamental step in the evolution of the visual system is the gene duplication of visual opsins and differentiation between the duplicates in absorption spectra and expression pattern in the retina. However, our understanding of the mechanism of expression differentiation is far behind that of spectral tuning of opsins. Zebrafish (Danio rerio) have two red-sensitive cone opsin genes, LWS-1 and LWS-2. These genes are arrayed in a tail-to-head manner, in this order, and are both expressed in the long member of double cones (LDCs) in the retina. Expression of the longer-wave sensitive LWS-1 occurs later in development and is thus confined to the peripheral, especially ventral-nasal region of the adult retina, whereas expression of LWS-2 occurs earlier and is confined to the central region of the adult retina, shifted slightly to the dorsal-temporal region. In this study, we employed a transgenic reporter assay using fluorescent proteins and P1-artificial chromosome (PAC) clones encompassing the two genes and identified a 0.6-kb “LWS-activating region” (LAR) upstream of LWS-1, which regulates expression of both genes. Under the 2.6-kb flanking upstream region containing the LAR, the expression pattern of LWS-1 was recapitulated by the fluorescent reporter. On the other hand, when LAR was directly conjugated to the LWS-2 upstream region, the reporter was expressed in the LDCs but also across the entire outer nuclear layer. Deletion of LAR from the PAC clones drastically lowered the reporter expression of the two genes. These results suggest that LAR regulates both LWS-1 and LWS-2 by enhancing their expression and that interaction of LAR with the promoters is competitive between the two genes in a developmentally restricted manner. Sharing a regulatory region between duplicated genes could be a general way to facilitate the expression differentiation in duplicated visual opsins.
Introduction
Gene duplication is a fundamental step in evolution [1]. Most often, one of the resulting daughter genes simply becomes a pseudogene and may be eventually lost from the genome due to functional redundancy between the duplicates and reduction of selective constraint to maintain its function. However, observation of another fate for duplicated genes, such as acquisition of a new function (neofunctionalization) or subdivision of parental gene function between daughter genes (subfunctionalization), implies an evolutionary advantage by the process [2]. Subfunctionalization often involves differentiation of expression pattern between daughter genes and has been a subject of intense scrutiny to understand the regulatory mechanism to achieve the differentiation [3]–[5].
In vertebrates, color vision is enabled by the presence of multiple classes of cone visual cells in the retina, each of which has a different absorption spectrum. The absorption spectrum of a visual cell is mainly determined by the visual pigment it contains. A visual pigment consists of a protein moiety, visual opsin, and a photo-sensing chromophore, either 11-cis retinal or 11-cis 3,4-dehydroretinal [6]. The five types of visual opsins found among extant vertebrates are RH1 (rod opsin or rhodopsin) and four types of cone opsins: RH2 (RH1-like, or green), SWS1 (short wavelength-sensitive type 1, or ultraviolet-blue), SWS2 (short wavelength-sensitive type 2, or blue) and M/LWS (middle to long wavelength-sensitive, or red-green) [7]. The SWS2 and M/LWS type genes are closely located on the same chromosome [8]–[10] and could represent the most ancient gene duplication in vertebrate visual opsin genes, from which other types could have arisen through whole-genome duplications and subsequent gene losses in early vertebrate evolution [7], [11]–[13]. Thus, visual opsin genes represent an excellent case of gene duplication to study the mechanism of neofunctionalization (in absorption spectrum) and subfunctionalization (in expression pattern). While the spectral tuning mechanism of visual opsins has been intensively studied [14]–[18], the regulatory mechanism of their expression differentiation, especially that of cone opsins, has been less explored.
Among vertebrates, fish are known to possess a rich and varied repertoire of visual opsins, including two or more opsin subtypes within the five types by further gene duplications [19]–[21], presumably reflecting their evolutionary adaptation to diverse aquatic light environments [22]. In fish, the eyes continue to grow throughout their lifetime by adding new cells to the peripheral zones, such that the peripheral cells are developmentally younger than central cells [23], [24]. Thus, in the fish retina the timing of gene expression is partly reflected in the region of expression in the retina. All visual opsin genes have been isolated and characterized for zebrafish (Danio rerio) [25], medaka (Oryzias latipes) [26] and cichlids (Family Cichlidae) [27]–[30]. Among them, the expression pattern of visual opsin genes has been best documented for zebrafish.
Zebrafish have nine visual opsin genes consisting of two M/LWS (red), four RH2 (green), and single-copy SWS1 (UV), SWS2 (blue) and RH1 (rod) opsin genes [25]. The red, green, UV and blue opsin genes are expressed in the long-member of double cones (LDCs), the short-member of double cones (SDCs), the short single cones (SSCs) and the long single cones (LSCs), respectively, which are arranged in a regular mosaic pattern in the retina [31], [32]. The two red opsin genes, LWS-1 and LWS-2, are arrayed in a tail to head manner, in this order, and encode photopigments with wavelengths of maximal absorption (λmax) at 558 and 548 nm, respectively [25]. The four green opsin genes, RH2-1, RH2-2, RH2-3 and RH2-4, are also arrayed in a tail to head manner, in this order, and encode photopigments with λmax at 467, 476, 488, and 505 nm, respectively [25]. In both red and green opsins, expression of longer-wave subtypes occurs later in development and is confined to the peripheral, especially ventral-nasal region of the adult retina, whereas expression of shorter-wave subtypes occurs earlier and is confined to the central region of the adult retina, shifted slightly to the dorsal-temporal region [33]. It remains largely unknown how subtypes of an opsin class are directed to express in different regions of the retina while keeping the cell type identical between them. Thus, the zebrafish visual opsins are an excellent model to study the regulatory mechanism of not only cell-type specific expression of opsin types, but also developmental-stage (and thus retinal-region) specific expression of opsin subtypes.
With the feasibility to employ transgenic technology, cis-regulatory regions relevant to the cell-type specific expression of opsin types have been elucidated using a living color reporter such as the green fluorescent protein (GFP) for zebrafish single-copy opsin genes (i.e., rod opsin [34]–[37], UV opsin [38], [39] and blue opsin genes [40]). A regulatory region relevant to not only cell-type specific but also retinal-region specific expression of opsin subtypes has also been reported for the zebrafish green opsin genes [41]. In the present study, we focus on the zebrafish red opsin genes, LWS-1 and LWS-2, and report a cis-regulatory region, “LWS-activating region” (LAR), which is relevant to their expression differentiation.
Results
P1 artificial chromosome (PAC) clones contain a sufficient regulator for expression of the two red opsin genes of zebrafish
In the two PAC clones we obtained [LWS-PAC(E) and LWS-PAC(H)], the first exons of LWS-1 and LWS-2 were replaced after their initiation codons with DNA segments encoding green and red fluorescent proteins (GFP and RFP), respectively (Figure 1A). The modified clones were designated LWS1/GFP-LWS2/RFP-PAC(E) and LWS1/GFP-LWS2/RFP-PAC(H), respectively. One transgenic zebrafish line was established using each construct: Tg(LWS1/GFP-LWS2/RFP-PAC(E))#1229 and Tg(LWS1/GFP-LWS2/RFP-PAC(H))#430.
Fig. 1. Recapitulation of the LWS-1 and LWS-2 expression in the zebrafish retina by the fluorescent markers in the PAC clones. 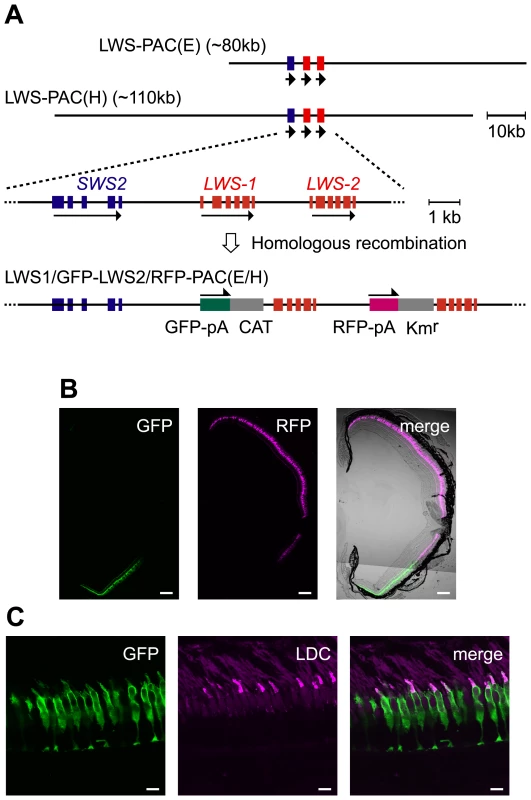
(A) Construction of the LWS1/GFP-LWS2/RFP-PAC clones. The two PAC clones, LWS-PAC(E) and LWS-PAC(H), both encompass the two red opsin genes, LWS-1 and LWS-2, in addition to the blue opsin gene, SWS2. In the expanded view, the blue and red boxes indicate the exons of the blue and red opsin genes, respectively. The orientation of transcription is given by the arrows for each gene. In both the LWS1/GFP-LWS2/RFP-PAC(E) and LWS1/GFP-LWS2/RFP-PAC(H) clones, the first exons of LWS-1 and LWS-2 were replaced with the GFP-polyA-CAT and RFP-polyA-Kmr cassettes by site-specific homologous recombination, respectively. (B) A transverse section of the retina of an adult Tg(LWS1/GFP-LWS2/RFP-PAC(E))#1229 fish. The dorsal side is oriented at the top of each panel and the ventral side is at the bottom. GFP (green) is expressed in the ventral region (left), whereas RFP (magenta) is expressed in the central to dorsal region (middle). The right panel shows the merge of the left two panels with the transmitted light image taken by the differential interference contrast microscopy (DIC image). (C) A vertical section of the photoreceptor layer in the ventral retina of an adult Tg(LWS1/GFP-LWS2/RFP-PAC(H))#430 fish. The outer segments of LDCs are immunostained with an antibody against the zebrafish red opsin (magenta). Note that the GFP (green) is found mostly in the cell body of LDCs. Scale bars = 100 µm for (B), 10 µm for (C). Adult fish of both lines expressed RFP in the central-dorsal-temporal region of the retina and GFP in the peripheral-ventral-nasal region of the retina circumscribing the RFP region (Figure 1B). It was also confirmed that the expression was specific to the LDCs, which were immunostained by an antibody against the zebrafish red opsin (Figure 1C). Thus, in both transgenic lines, the expression of GFP and RFP reporter genes recapitulated the expression of LWS-1 and LWS-2, respectively, demonstrating that both the LWS-PAC(E) and LWS-PAC(H) clones contain sufficient regulatory region(s) for the proper expression of the two red opsin genes.
The upstream region of LWS-1 regulates expression of not only LWS-1, but also LWS-2
Next, we used only the intergenic region between the stop codon of SWS2 and the initiation codon of LWS-1, designated LWS1up2.6kb, and the region between the stop codon of LWS-1 and the initiation codon of LWS-2, designated LWS2up1.8kb. We created a double-reporter construct consisting of the LWS1up2.6kb, GFP reporter, LWS2up1.8kb and RFP reporter, in this order (LWS1up2.6kb:GFP-LWS2up1.8kb:RFP, Figure 2A), and obtained three transgenic lines: Tg(LWS1up2.6kb:GFP-LWS2up1.8kb:RFP)#1464, 1631, and 1640. In two of the three lines, #1631 (Figure 2B, 2C) and #1640, the GFP and the RFP recapitulated the expression patterns of LWS-1 and LWS-2, respectively. In the third line, #1464, the expression of RFP was weaker and sparser but still confined to the central region of the retina and the expression of GFP appeared to be identical to the other two lines (Figure S1). The expression pattern of LWS-1 was also recapitulated when only the LWS1up2.6kb was used with GFP (LWS1up2.6kb:GFP) in all three transgenic lines obtained: Tg(LWS1up2.6kb:GFP)#1508, 1509 (Figure 2A, 2D–2F), and 1515. On the other hand, when only the LWS2up1.8kb was used with GFP (LWS2up1.8kb:GFP), no GFP signal was observed in the transgenic line obtained: Tg(LWS2up1.8kb:GFP)#1433. These results suggest that the LWS1up2.6kb contains a regulatory region not only for LWS-1, but also for LWS-2.
Fig. 2. Regulatory ability of the 5′ flanking regions of LWS-1 and LWS-2. 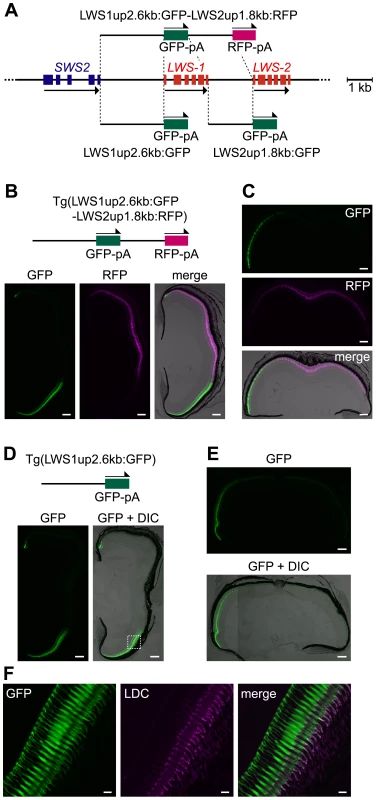
(A) Schematic representation of the GFP or RFP expression constructs. (B) A transverse section of an adult Tg(LWS1up2.6kb:GFP-LWS2up1.8kb:RFP)#1631 retina. The dorsal side is oriented at the top of each panel and the ventral side is at the bottom. The left and middle panels show the GFP (green) and RFP (magenta) images, respectively. The right is the merge of the two panels with the DIC image. (C) A horizontal section of the retina from the same adult individual shown in (B). The nasal side is shown on the left and the temporal side is on the right. The upper and middle panels show the GFP (green) and RFP (magenta) images, respectively, and the lower one is the merge of the two panels with the DIC image. (D, E) Transverse (D) and horizontal (E) sections of an adult Tg(LWS1up2.6kb:GFP)#1509 retina. The left (D) and top (E) panels show images of GFP signals (green) and the right (D) and bottom (E) panels show their overlays with the DIC images. The dorsal side is oriented at the top of each panel and the ventral side is at the bottom in (D). The nasal side is oriented on the left and the temporal side is on the right in (E). (F) A vertical view of the photoreceptor layer magnified from the dashed rectangle in (D). The left panel shows the GFP signals (green) and the middle panel shows the signals of immunostaining against the zebrafish red opsin in the outer segments of LDCs (magenta). The right panel shows a merge of the left two panels. The overlap of the two signals appears as white. Scale bars = 100 µm for (B–E), 10 µm for (F). An “LWS-activating region” (LAR) was found in the upstream region of LWS-1
In order to search for the regulatory region from the LWS1up2.6kb, we employed the transient transgenic assay in which the regulatory activity of a GFP-reporter construct was evaluated in the fish injected with the construct. This was done by examining the incidence of fish bearing GFP-expressing eyes at a larval stage. As in previous studies [39]–[41], the expression level of GFP was graded into four categories, +++, ++, +, −, at 7 days post-fertilization (dpf) (Figure 3). First, we used a whole PAC clone, LWS-PAC(E), and modified it to LWS1/GFP-PAC(E) and LWS2/GFP-PAC(E), in which the first exon of LWS-1 and LWS-2, respectively, was replaced after its initiation codon with GFP-encoding DNA (Figure 3A left). We confirmed that the GFP expression pattern from the two PAC constructs was consistent with the expression patterns of LWS-1 and LWS-2, respectively, at the larval stage (Figure 3A right) (i.e., LWS-2 is expressed predominantly and LWS-1 is expressed only faintly in the retina [33]).
Fig. 3. Localization of LAR. 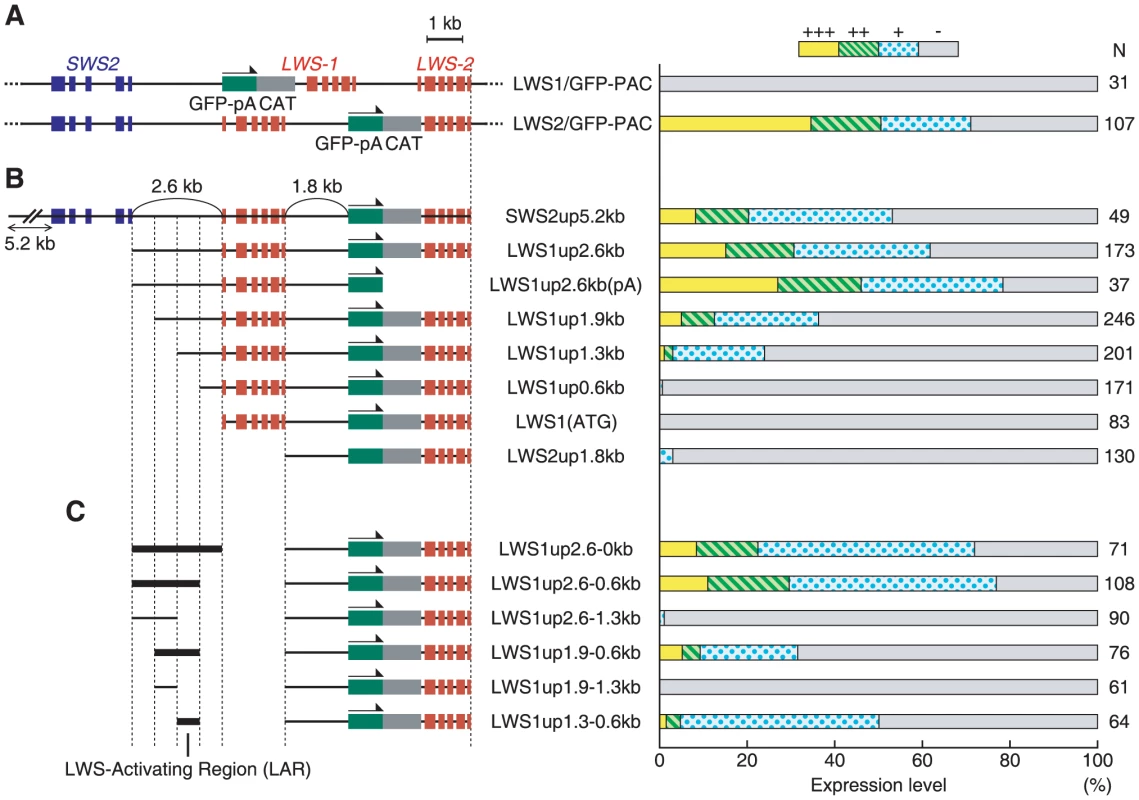
The GFP expression constructs used in the transient transgenic assay are depicted with their names on the left side and the corresponding expression levels of GFP in the retina at 7 dpf are indicated in the histogram on the right side. The histogram shows the percentage of eyes graded into four levels (+++, ++, + and −) according to the number of GFP-expressing cells in the retina. The numbers to the right of the histogram show the total number of eyes examined for each of the constructs. (A) The entire PAC clones were tested using LWS1/GFP-PAC and LWS2/GFP-PAC. (B) A series of DNA regions from the LWS2/GFP-PAC were tested. (C) A variety of DNA segments from the LWS1up2.6kb region (segment names given in the panel) were co-injected with the LWS2up1.8kb-GFP-LWS-2 region. Next, as shown in Figure 3B left, we isolated from LWS2/GFP-PAC(E) a series of DNA regions consisting basically of the LWS2up1.8kb-GFP-LWS-2 region and varying ranges of its upstream region. The GFP signal was apparent when the upstream region contained 1.3-kb or more upstream of LWS-1, but was almost undetectable when it contained 0.6-kb or less upstream of LWS-1 or when only the LWS2up1.8kb-GFP-LWS-2 region was used (Figure 3B right). This implies that the LWS2up1.8kb region does not contain a sufficient regulatory region for the expression of LWS-2, consistent with the absence of a GFP signal in the transgenic line Tg(LWS2up1.8kb:GFP) described above. This also suggests that the regulatory region is located in the 0.6-kb region between 1.3-kb and 0.6-kb upstream of LWS-1.
To test if the 0.6-kb region plays a regulatory role by itself for the expression of LWS-2, a coinjection protocol was employed using mixed concatamers of separate DNA fragments formed upon integration into the genome [42]. The LWS2up1.8kb-GFP-LWS-2 region was injected together with a variety of DNA segments from the LWS1up2.6kb region (Figure 3C left). GFP expression was apparent in the retina only when the segment contained the 0.6-kb region (Figure 3C right). We thus designated the 0.6-kb region as an “LWS-activating region” (LAR).
The LAR enhances LDC–specific gene expression in a position-dependent manner relative to genes
Using a DNA construct consisting of LAR and LWS2up1.8kb:GFP, designated LAR:LWS2up1.8kb:GFP (Figure 4A), we obtained five transgenic lines: Tg(LAR:LWS2up1.8kb:GFP)#1481, 1491, 1496, 1499, and 1501. In one line, #1499, a GFP signal was observed specifically in the LDCs but across the entire outer nuclear layer, not confined to the central-temporal-dorsal region (Figure 4B–4D). The absence of retinal region specificity is in sharp contrast to the case in which the double reporter construct, LWS1up2.6kb:GFP-LWS2up1.8kb:RFP, was used (Figure 2B, 2C). This suggests that the relative position of the LAR to the gene is relevant to the regional specificity of the retina.
Fig. 4. LDC–specific expression of GFP in the entire retina by LAR:LWS2up1.8kb:GFP. 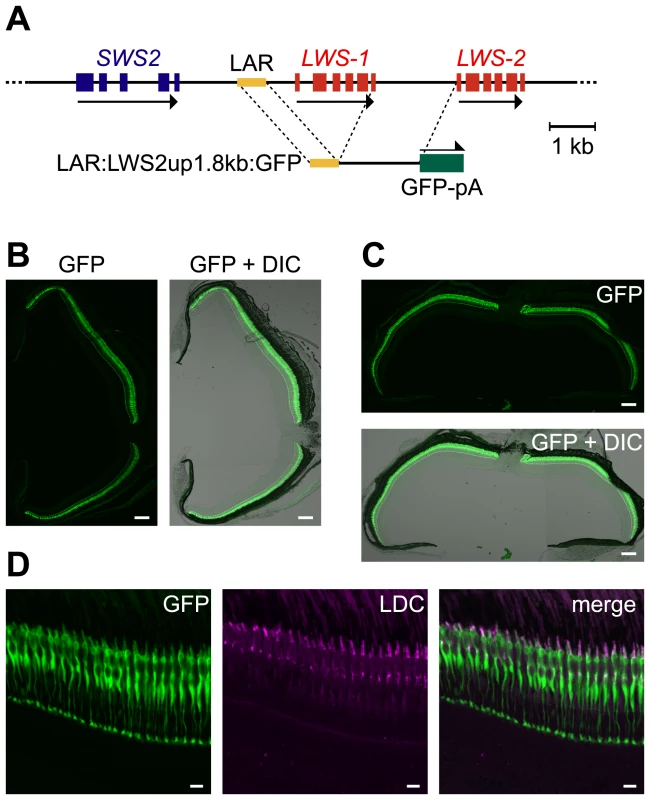
(A) Schematic representation of the construction of LAR:LWS2up1.8kb:GFP. (B, C) Transverse (B) and horizontal (C) sections of an adult Tg(LAR:LWS2up1.8kb:GFP)#1499 retina. The left (B) and top (C) panels show images of GFP signals (green) and the right (B) and bottom (C) panels show their overlays with the DIC images. The dorsal side is oriented at the top of each panel and the ventral side is at the bottom in (B). The nasal side is on the left and the temporal side is on the right in (C). (D) A vertical and expanded view of the photoreceptor layer from the same eye sample as shown in (B). The left panel shows the GFP signals (green) and the middle panel shows the signals of immunostaining against the zebrafish red opsin in the outer segments of LDCs (magenta). The right panel shows a merge of the left two panels. The overlap of the two signals appears as white. Scale bars = 100 µm for (B, C), 10 µm for (D). In another line of Tg(LAR:LWS2up1.8kb:GFP)#1501, the GFP signal also appeared throughout the retina, but was sparser (Figure S2A). At a finer level, the signal appeared not only in LDCs but also weakly in some bipolar cells (Figure S2B). In the other three Tg(LAR:LWS2up1.8kb:GFP) lines, the GFP signal was not detectable. This instability of the reporter signal among the transgenic lines could be attributed not only to the general effect of their insertion sites in the genome, but also to the dependency of LAR to work cooperatively with its adjacent regions in the LWS1up2.6kb and LWS2up1.8kb. Consistently, as in the transient transgenic assay shown in Figure 3B, GFP expression level was much higher when the entire LWS1up2.6kb region was used than when only the proximal 1.3 kb region was used.
To examine if the LDC-specificity of the GFP expression was attributed to LAR itself, we tested the 564-bp adjacent upstream region of a non-retinal keratin 8 gene [43], designated krt8up564bp [41]. The krt8up564bp induces gene expression specifically in the epithelial tissues, but not in the retina, and has been used for enhancer trapping as a basal promoter [44]. When krt8up564bp was conjugated to the LAR and GFP reporter (LAR:krt8up564bp:GFP), no GFP expression was observed in the retina of the two transgenic lines obtained: Tg(LAR:krt8up564bp:GFP)#1469 and 1477. This is in sharp contrast to the case in which krt8up564bp was conjugated to the RH2-LCR and GFP expression was observed in the SDCs throughout the zebrafish retina in our previous study [41]. This suggests that LAR itself is not capable of determining the cell-type specificity of gene expression, unlike RH2-LCR, but works as an enhancer which interacts with cell-type determining regions that should reside in both LWS1up2.6kb and LWS2up1.8kb.
The LAR affects the expression level of both LWS-1 and LWS-2
Next, we removed the LAR from LWS1/GFP-LWS2/RFP-PAC(E) and LWS1/GFP-LWS2/RFP-PAC(H) (designated ΔLAR-LWS1/GFP-LWS2/RFP-PAC(E) and ΔLAR-LWS1/GFP-LWS2/RFP-PAC(H), respectively) (Figure 5A). Two transgenic lines were found for each of the two constructs: Tg(ΔLAR-LWS1/GFP-LWS2/RFP-PAC(E))#1143 (Figure 5C) and 1166 and Tg(ΔLAR-LWS1/GFP-LWS2/RFP-PAC(H))#1107 (Figure 5D, 5E) and 1100. All four of these transgenic lines showed a similar expression pattern of the reporters in the retina with the LAR-bearing Tg(LWS1/GFP-LWS2/RFP-PAC(E)) or Tg(LWS1/GFP-LWS2/RFP-PAC(H)) (Figure 5B) line. The reporter-expressing cells were confined to LDSs (Figure 5E). The GFP and RFP signals were observed in the ventral and dorsal regions of the retina, respectively (Figure 5B–5D). However, the fluorescent signal in each cell was lowered. The number of the reporter-expressing cells decreased and their spatial distribution was restricted to a narrow range in both of these regions (Figure 5B–5E). These results support the deduced role of LAR as an enhancer but not as the cell-type determining factor from the experiments thus far. This experiment also provided the first direct evidence that LWS-1 expression is affected by LAR.
Fig. 5. A negative effect of LAR deletion in the reporter gene expression from the LWS1/GFP-LWS2/RFP-PAC clones. 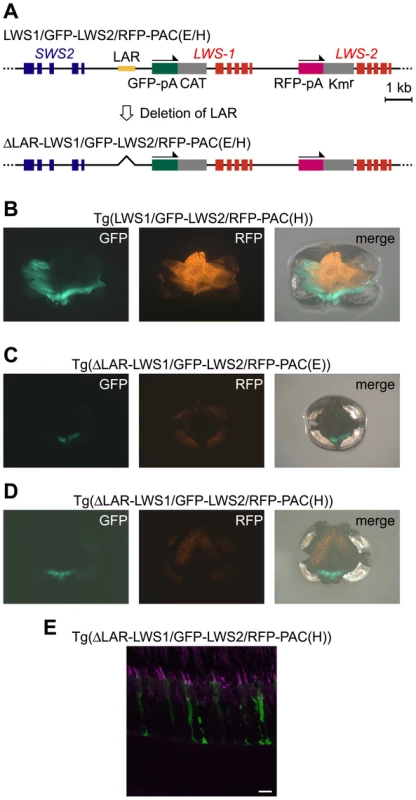
(A) Schematic representation of the construction of ΔLAR-LWS1/GFP-LWS2/RFP-PAC(E) and (H). (B, C, D) Images of a whole mount retina of an adult Tg(LWS1/GFP-LWS2/RFP-PAC(H))#430 (B), Tg(ΔLAR-LWS1/GFP-LWS2/RFP-PAC(E))#1143 (C), and Tg(ΔLAR-LWS1/GFP-LWS2/RFP-PAC(H))#1107 (D) fish. The dorsal side is oriented at the top of each panel, and the nasal side is on the left. The GFP signals appear as green and the RFP signals appear as orange. The right panel is the overlay of the left and center panels. (E) A vertical and expanded view of the photoreceptor layer from the same transgenic line as shown in (D). The LDCs were immunostained with the antibody against the zebrafish red opsin (magenta). The GFP signals appear as green. Overlap of the two signals appears as white. Scale bars = 10 µm. Discussion
The present study identified a 0.6-kb regulatory region, named LAR, for the expression of the duplicated red opsin genes of zebrafish, LWS-1 and LWS-2, in the upstream of the gene array. The LAR functions to enhance the LDC-specific expression of both genes but does not determine the cell-type specificity of the gene expression. The regulatory region for the cell-type specificity of the gene expression appears to reside in the 2.6-kb and 1.8-kb upstream regions of the two genes. The relative position of LAR to a gene is relevant to the retinal region specificity of the expression of the gene.
In the primate L/M opsin genes, the locus control region (LCR) is located at ∼3.5-kb upstream of the gene array and is necessary for the expression of both L and M opsin genes [45], [46]. Although there is no clear overall similarity between the zebrafish LAR and primate L/M opsin LCR, LAR contains two OTX (A/GGATTA) and one OTX-like (TGATTA) sequences (Figure S3) which are also present in the primate L/M opsin LCR [47], [48]. These sequences, or their reverse complement sequences, are the binding sites of the cone-rod homeobox (Crx) protein, a member of the Otx family of the paired-like homeodomain proteins and a key trans-acting regulatory factor responsible for the gene expression in the retina and pineal organ [47], [48]. The mammalian Crx is produced predominantly in both the retinal photoreceptors and pineal cells and regulates expression of retinal photoreceptor-specific genes and of pineal-specific genes [47]–[50]. In zebrafish, Otx5, a paralog of Crx, is produced in the retina and pineal organ and regulates genes that show circadian expression in the pineal organ [51]. The OTX or OTX-like sequences have also been found in the upstream region of the zebrafish SWS2 [40] and in the RH2-LCR [41]. Thus, the LAR could be orthologous to the primate L/M opsin LCR and also be paralogous to the SWS2 regulatory region and the RH2-LCR (see ref. [12] for a similar discussion).
The primate L/M opsin LCR interacts with only the most proximal or the second proximal gene of the array, often L and M opsin genes respectively, through their proximal promoters [46], [52], [53]. The choice of the promoters by the LCR is largely a stochastic process [54], [55]. These characteristics enable the mutually exclusive expression of the L and M opsin genes and nearly a random distribution of the L and M cone photoreceptor cells in the primate retina. In zebrafish, the expression of LWS-1 and LWS-2 is also nearly mutually exclusive in the retina [33]. Unlike the primate L/M opsin system, however, the expression of the two zebrafish red opsin genes is temporally and spatially organized and not random in the retina [33]. Whereas expression of LWS-2 is first observed at 40 hours post-fertilization (hpf) and spread throughout the retina by 72 hpf, initial expression of LWS-1 is observed at 3.5–5.5 days post-fertilization (dpf) in the marginal side of the ventral retina [33]. In sexually mature adults, LWS-2 is expressed in the central-dorsal-temporal region of the retina. Expression of LWS-1 is complementary to the LWS-2 observed in the peripheral-ventral-nasal region of the rest of the retina, although cells at the boundary of the two fields appear to express both gene subtypes and LWS-1 is sparsely expressed in the LWS-2 zone [33].
In this study, the spatially restricted patterns of gene expression were recapitulated by fluorescent reporters for both LWS-1 and LWS-2 in the adult retina of Tg(LWS1up2.6kb:GFP-LWS2up1.8kb:RFP) (Figure 2B, 2C). The expression pattern of LWS-1 was also recapitulated in Tg(LWS1up2.6kb:GFP) (Figure 2D, 2E), whereas that of LWS-2 was not, and GFP was expressed throughout the adult retina in Tg(LAR:LWS2up1.8kb:GFP) (Figure 4B, 4C). This suggests that the LWS1up2.6kb contains a region susceptive to a developmental control that represses gene expression in the early stage or activates it in the later stage in LDCs, while the LWS2up1.8kb allows LDC-specific expression throughout development with the aid of LAR. This also suggests that LAR, which is shared by LWS-1 and LWS-2, interacts with the LWS-2 promoter during the time LWS-1 expression is repressed (or not activated) in the early stage and then interacts with the LWS-1 promoter once the LWS-1 expression is enabled. This preference in interaction of LAR for LWS-1 over LWS-2 could be attributed to the closer distance of LAR to LWS-1, as in the case of the primate L/M opsin LCR [46], [52], [53] and the zebrafish RH2-LCR [41].
Sharing a regulatory region among duplicated genes is a common feature among the zebrafish M/LWS (red) and RH2 (green) and the primate M/LWS (L and M) opsin genes. This system should be advantageous in facilitating differential (i.e., mutually exclusive) expression of duplicated opsin genes by using the regulatory region in a competitive manner between the duplicated genes. If the competition is largely stochastic, an intermingled pattern of photoreceptor cells expressing different daughter genes can be expected in the retina, as in the case of primate L/M opsin genes. The trichromatic color vision is enabled by this stochastic-type system in primates. If the competition is developmentally controlled, for example, so that the regulatory region interacts with a proximal gene in an early stage and shifts the interaction target to a distal gene, the proximal gene would be expressed in the central region and the distal gene in the peripheral region of the retina as in the case of the zebrafish green opsin genes. In the case of the zebrafish red opsin genes, the interaction would start with the distal gene and switch to the proximal gene. In fish, such a control is feasible because the retina continues to grow throughout their lifetime by adding new cells to the peripheral zone [24]. Expression of different opsin genes among different retinal regions results in sights with varying wavelength sensitivity as a function of visual angles, which could be advantageous in the aquatic light environment where wavelength composition differs depending on directions [56]. This could explain why many examples of gene duplication have been found in fish visual opsin genes. Further studies of the regulatory mechanism of differential expression of fish visual opsin genes should contribute to our understanding of the adaptive significance of gene duplications in general.
Materials and Methods
Ethics statement
All animal protocols were approved by the University of Tokyo animal care and use committee.
LWS-PAC clones
Through the screening service of the Resource Center Primary Database (RZPD, Germany; https://www.rzpd.de) of a zebrafish PAC library (no. 706, originally created by C. Amemiya), two clone DNAs (BUSMP706E19271Q9 and BUSMP706H1397Q9), designated LWS-PAC(E) and LWS-PAC(H), were obtained using the LWS-2 cDNA as a probe. Both clones encompass SWS2, LWS-1 and LWS-2 in their ∼80-kb and ∼110-kb inserts, respectively (Figure 1A). Sequencing both ends of the inserts revealed that the nucleotide sequences of LWS-PAC(E) and LWS-PAC(H) correspond to the nucleotide positions 25222648–25311454 and 25174505–25295119 of chromosome 11 in the Ensembl zebrafish assembly version 8, respectively (http://www.ensembl.org/Danio_rerio/Info/Index).
Modification of the LWS-PAC clones by homologous recombination
The I-SceI meganuclease system [57] was used for efficient transgenesis of the PAC-derived constructs. Two I-SceI recognition sites (5′-TAGGGATAACAGGGTAAT-3′) were introduced into the vector backbone of the LWS-PAC clones as follows. The ampicillin-resistance (Ampr) gene was PCR-amplified from the pUC18 plasmid using primers harboring the I-SceI recognition site at their 5′ ends to create the I-SceI-Ampr-I-SceI segment (see “I-SceI-Ampr-I-SceI” in Table S1 for primers). The I-SceI-Ampr-I-SceI segment was inserted into the EcoRV site of pBluescript II (SK-) plasmid vector (Stratagene, Tokyo). The I-SceI-Ampr-I-SceI segment was isolated from the pBluescript clone using primers harboring the flanking sequences of the kanamycin-resistance (Kmr) gene site of the LWS-PAC clones to create the I-SceI-Ampr-I-SceI cassette (see “Kmr<>I-SceI-Ampr-I-SceI” in Table S1 for primers). The Kmr of the LWS-PAC clones was replaced with the I-SceI-Ampr-I-SceI cassette by the site-specific homologous recombination system coupled with drug selection using the E. coli strain EL250 [58] as in our previous study [41].
The first exon after the initiation codon of LWS-1 and LWS-2 in the LWS-PAC clones was replaced with the GFP or the RFP gene as follows. The chloramphenicol acetyl transferase (CAT) and the Kmr gene fragments were PCR-amplified from pBR328 and pCYPAC6 plasmids, respectively (see “CAT” and “Kmr” in Table S1 for primers). The CAT gene was inserted into the pEGFP-1 plasmid vector (BD Biosciences Clontech, Tokyo) at the AflII site, which is located immediately downstream of the SV40 polyadenylation signal (polyA), linked downstream of the GFP coding sequence to create the GFP-polyA-CAT segment. Similarly, the Kmr gene was inserted into the pDsRed-1 or pDsRed-Express-1 plasmid vector (BD Biosciences Clontech, Tokyo) at the AflII site to create the RFP-polyA-Kmr segment. For the LWS1/GFP-LWS2/RFP-PAC(H), the pDsRed-1 was used. For the other RFP-containing constructs (LWS1/GFP-LWS2/RFP-PAC(E), ΔLAR-LWS1/GFP-LWS2/RFP-PAC(E) and ΔLAR-LWS1/GFP-LWS2/RFP-PAC(H)), the pDsRed-Express-1 was used. The GFP-polyA-CAT segment was isolated from the pEGFP-1 construct by PCR using primers harboring the flanking sequences of the exon 1 of LWS-1 or LWS-2 to create the GFP-polyA-CAT cassette (see “LWS-1<>GFP-polyA-CAT” and “LWS-2<>GFP-polyA-CAT” in Table S1 for primers). The RFP-polyA-Kmr segment was isolated from the pDsRed-1 or the pDsRed-Express-1 construct by PCR using primers harboring the flanking sequences of the exon 1 of LWS-2 to create the RFP-polyA-Kmr cassette (see “LWS-2<>RFP-polyA-Kmr” in Table S1 for primers). These cassettes were replaced with the exon 1 of LWS-1 or LWS-2 in the LWS-PAC clones by the site-specific homologous recombination system in EL250.
The LAR was removed from the LWS-PAC clones by the site-specific homologous recombination system and by the flpe-FRT recombination system for excision of a DNA region sandwiched by FRT sequences in EL250 [41], [58] as follows. The CAT gene was PCR-amplified from the pBR328 using primers harboring the FRT sequences to create the FRT-CAT-FRT segment (see “FRT-CAT-FRT” in Table S1 for primers). The FRT-CAT-FRT segment was inserted into the EcoRV site of pBluescript II (SK-) plasmid. Then, the FRT-CAT-FRT segment was isolated by PCR using primers harboring the flanking sequences of the LAR to create the FRT-CAT-FRT cassette (see “LAR<>FRT-CAT-FRT” in Table S1 for primers). The LAR was replaced with the FRT-CAT-FRT cassette in the LWS-PAC clones by the site-specific homologous recombination system in EL250. The FRT-CAT-FRT cassette was then excised from the modified LWS-PAC clones in EL250 by the flpe-FRT recombination system for excision of a DNA region sandwiched by FRT sequences, leaving one FRT sequence in this region of the clones [41], [58].
Reporter constructs for Tol2-mediated transgenesis
A plasmid construct, pT2AL200R150G [59], was modified as follows. The pT2AL200R150G contains a GFP-expression cassette between XhoI and BglII sites surrounded respectively by the L200 and R150 minimum recognition sequences of the Tol2 transposase. The Tol2 transposase excises the DNA region between the recognition sequences from the plasmid and integrates it into the host genome as a single copy with the recognition sequences attached as in the plasmid [60]. The GFP-expression cassette contains a promoter sequence of a ubiquitously expressed gene (the Xenopus elongation factor (EF) 1α), the rabbit β-globin intron, GFP gene and the SV40 polyA signal. The construct contains two Not I sites, one in the junction between the GFP gene and the SV40 polyA and another upstream of the L200 in the vector backbone. We first removed the Not I site in the vector backbone by eliminating a DNA segment between a Sac I site and L200 encompassing the NotI site. Next, we removed the promoter from the construct by replacing the region from the EF1α promoter to the GFP gene (from XhoI to NotI sites) with a DNA segment in the pEGFP-1 vector consisting of a part of the multiple cloning site (MCS) and the GFP gene (from XhoI to NotI sites of pEGFP-1). Finally, we replaced the SV40 polyA signal in the construct (from NotI to BglII sites) with the polyA signal sequence derived from the herpes simplex virus thymidine kinase (HSV-TK), which was PCR-isolated from the pEGFP-1 vector using a forward primer harboring NotI site and a reverse primer harboring BglII site (“HSV-TK-polyA” in Table S2). This modified construct was designated pT2GFP-TKPA. The replacement of the polyA signal from SV40 to HSV-TK was done to facilitate, in a later stage, the insertion of a DNA fragment containing the SV40 polyA in an appropriate orientation into the pT2GFP-TKPA by avoiding a possible interaction between the two SV40 polyA sequences.
Using the pT2GFP-TKPA as a transfer vector, we constructed the LWS1up2.6kb:GFP and the LWS2up1.8kb:GFP in Figure 2A as the followings. The region from LWS1up2.6kb to GFP in the LWS1/GFP-PAC(E) clone and the region from LWS2up1.8kb to GFP in the LWS2/GFP-PAC(E) clone were isolated by PCR using forward primers harboring a SalI site and reverse primers harboring a NotI site (“LWS1up2.6kb:GFP” and “LWS2up1.8kb:GFP” in Table S2). A DNA segment in the pT2GFP-TKPA from SalI in MCS to NotI in the junction between GFP and HSV-TK polyA was replaced with those segments isolated from the PAC constructs through restriction digestion and ligation at the SalI and NotI sites. In the resulting constructs, the region from LWS1up2.6kb to GFP and that from LWS2up1.8kb to GFP were connected to the HSV-TK polyA (LWS1up2.6kb:GFP and the LWS2up1.8kb:GFP, respectively) at the NotI site.
The LWS1up2.6kb:GFP-LWS2up1.8kb:RFP (Figure 2A) was constructed as follows. The SV40 polyA in the pEGFP-1 vector was isolated together with a NotI site located at its 5′ side by PCR using a forward primer harboring a KpnI site and a reverse primer harboring a SalI site (“SV40-polyA” in Table S2). The isolated fragment was cloned into the pBluescript II (SK-) vector at KpnI and SalI sites. The region from the LWS2up1.8kb to the RFP gene including a NotI site located just downstream of the RFP gene in the LWS1/GFP-LWS2/RFP-PAC(E) clone was isolated with a SalI site attached to the 5′ end of the LWS2up1.8kb. The region was connected to the 3′ side of the SV40 polyA cloned in the pBluescript II (SK-) at SalI site. Then, from the pBluescript construct, the region consisting of the SV40 polyA, LWS2up1.8kb, and RFP gene (from the Not I site at 5′ side of the SV40 polyA to the NotI site at 3′ side of the RFP gene) was inserted into the LWS1up2.6kb:GFP construct in the pT2GFP-TKPA at the NotI site located between the GFP gene and the HSV-TK polyA. This results in the LWS1up2.6kb-GFP segment connected to 5′ side of the SV40 polyA and the LWS2up1.8kb-RFP segment connected to 5′ side of the HSV-TK polyA in the pT2GFP-TKPA (LWS1up2.6kb:GFP-LWS2up1.8kb:RFP).
The LAR:LWS2up1.8kb:GFP and the LAR:krt8up564bp:GFP (see Figure 4A and Results section) were constructed as follows. The LAR was isolated from the LWS-PAC(E) clone by PCR using a forward primer harboring a HindIII site and a reverse primer harboring an EcoRI site (“LAR” in Table S2) and was inserted into the HindIII/EcoRI sites in the MCS of pT2GFP-TKPA. For making the LAR:LWS2up1.8kb:GFP, the GFP gene region in the LAR-inserted pT2GFP-TKPA construct (from the SalI site in MCS to the NotI site at the 3′ side of the GFP gene) was replaced with the region from the LWS2up1.8kb to the GFP gene in LWS2up1.8kb:GFP construct in the pT2GFP-TKPA (from the SalI site at the 5′ side of LWS2up1.8kb to the NotI site at the 3′ side of the GFP) by restriction digestion and ligation at the SalI and NotI sites. Similarly, for making the LAR:krt8up564bp:GFP, the GFP gene region in the LAR-inserted pT2GFP-TKPA was replaced with the region from the krt8up564bp to the GFP gene in LCR:krt8 construct reported in ref. [41] by restriction digestion and ligation at the SalI and NotI sites.
Reporter constructs for transient transgenic assay
A series of the GFP-reporter constructs and DNA fragments for the transient transgenic assay (Figure 3B, 3C) were obtained by PCR from LWS-2/GFP-PAC(E) using primers listed in Table S3. These DNA fragments were purified through gel extraction before the microinjection.
Transgenic fish
Zebrafish were maintained at 28.5°C in a 14-h light/10-h dark cycle as described by ref. [61]. The LWS-PAC derived constructs bearing the I-SceI recognition sequence were injected into the cytoplasm of embryos at the one-cell stage at 20 ng/µl with I-SceI meganuclease (0.5 units/µl) (New England Biolabs, Beverly, MA) in a solution of 0.5× commercial meganuclease buffer with tetramethyl-rhodamin dextran tracer [57].
The reporter constructs in the pT2GFP-TKPA vector were resuspended at a final concentration of 25 ng/µl in 0.1 M KCl and tetramethyl-rhodamin dextran tracer. They were co-injected with mRNA of Tol2 transpsase of 27 ng/µl that was prepared through in vitro transcription from pCS-TP using the mMESSAGE mMACHINE kit (Ambion, Austin, TX) [59], [60].
For generation of transgenic lines, the injected embryos were grown to sexual maturity and crossed with non-injected fish in a pair-wise fashion. Founders and fish of subsequent generations transmitting a reporter transgene were screened by PCR-based genotyping as described in ref. [41]. All the transgenic lines analyzed in this study are listed in Table S4.
The GFP-reporter constructs for the transient transgenic assay (Figure 3B) were microinjected with 0.1 M KCl and tetramethyl-rhodamin dextran at a final concentration of 25–50 ng/µl. The LWS2up1.8kb-GFP-LWS-2 region was injected together with a variety of DNA segments from the LWS1up2.6kb region (Figure 3C) at a final concentration of approximately 25–50 ng/µl each in 0.1 M KCl and tetramethyl-rhodamin dextran tracer.
For transient transgenic assay of GFP expression, embryos injected with the GFP-expression constructs were grown in 0.003% 1-phenyl–2-thiourea after 12–24 hpf to disrupt pigment formation. One eye per injected embryo was examined at 7 dpf for GFP fluorescence under a dissecting fluorescent microscope. The eyes were scored as “+++”, “++”, “+”, and “−” when GFP was expressed in more than 50 cells, in 11–50 cells, in 1–10 cells, and in no cell per eye, respectively [39]–[41].
Immunohistochemistry
Immunostaining was carried out against adult retinal sections following the procedure of ref. [39]. An antibody against the zebrafish red opsin raised in rabbits [32] was used to stain LDCs. The Cy3-conjugated anti-rabbit IgG was used as a secondary antibody. Images of GFP, RFP and Cy3 fluorescence of the sections were captured using a Zeiss 510 laser-scanning confocal microscope (Zeiss, Thornwood, NY).
Supporting Information
Zdroje
1. OhnoS
1970
Evolution by Gene Duplication.
Berlin
Springer-Verlag
2. ZhangJ
2003
Evolution by gene duplication: an update.
Trends Ecol Evol
18
292
298
3. ForceA
LynchM
PickettFB
AmoresA
YanYL
1999
Preservation of duplicate genes by complementary, degenerative mutations.
Genetics
151
1531
1545
4. WagnerA
2000
Decoupled evolution of coding region and mRNA expression patterns after gene duplication: implications for the neutralist-selectionist debate.
Proc Natl Acad Sci USA
97
6579
6584
5. GuZ
NicolaeD
LuHH
LiWH
2002
Rapid divergence in expression between duplicate genes inferred from microarray data.
Trends Genet
18
609
613
6. NathansJ
1987
Molecular biology of visual pigments.
Annu Rev Neurosci
10
163
194
7. YokoyamaS
2000
Molecular evolution of vertebrate visual pigments.
Prog Retin Eye Res
19
385
419
8. YokoyamaR
YokoyamaS
1993
Molecular characterization of a blue visual pigment gene in the fish Astyanax fasciatus.
FEBS Lett
334
27
31
9. KawamuraS
BlowNS
YokoyamaS
1999
Genetic analyses of visual pigments of the pigeon (Columba livia).
Genetics
153
1839
1850
10. DaviesWL
CarvalhoLS
CowingJA
BeazleyLD
HuntDM
2007
Visual pigments of the platypus: a novel route to mammalian colour vision.
Curr Biol
17
R161
163
11. NordstromK
LarssonTA
LarhammarD
2004
Extensive duplications of phototransduction genes in early vertebrate evolution correlate with block (chromosome) duplications.
Genomics
83
852
872
12. WakefieldMJ
AndersonM
ChangE
WeiKJ
KaulR
2008
Cone visual pigments of monotremes: filling the phylogenetic gap.
Vis Neurosci
25
257
264
13. DaviesWL
CollinSP
HuntDM
2009
Adaptive gene loss reflects differences in the visual ecology of basal vertebrates.
Mol Biol Evol
26
1803
1809
14. YokoyamaS
StarmerWT
TakahashiY
TadaT
2006
Tertiary structure and spectral tuning of UV and violet pigments in vertebrates.
Gene
365
95
103
15. HuntDM
CarvalhoLS
CowingJA
ParryJW
WilkieSE
2007
Spectral tuning of shortwave-sensitive visual pigments in vertebrates.
Photochem Photobiol
83
303
310
16. AltunA
YokoyamaS
MorokumaK
2008
Quantum mechanical/molecular mechanical studies on spectral tuning mechanisms of visual pigments and other photoactive proteins.
Photochem Photobiol
84
845
854
17. YokoyamaS
TadaT
ZhangH
BrittL
2008
Elucidation of phenotypic adaptations: Molecular analyses of dim-light vision proteins in vertebrates.
Proc Natl Acad Sci USA
105
13480
13485
18. YokoyamaS
YangH
StarmerWT
2008
Molecular basis of spectral tuning in the red - and green-sensitive (M/LWS) pigments in vertebrates.
Genetics
179
2037
2043
19. TreziseAE
CollinSP
2005
Opsins: evolution in waiting.
Curr Biol
15
R794
796
20. BowmakerJK
2008
Evolution of vertebrate visual pigments.
Vision Res
48
2022
2041
21. HofmannCM
CarletonKL
2009
Gene duplication and differential gene expression play an important role in the diversification of visual pigments in fish.
Integr Comp Biol
49
630
643
22. LevineJS
MacNicholEFJr
1982
Color vision in fishes.
Sci Am
246
140
149
23. La VailMM
RapaportDH
RakicP
1991
Cytogenesis in the monkey retina.
J Comp Neurol
309
86
114
24. MoshiriA
CloseJ
RehTA
2004
Retinal stem cells and regeneration.
Int J Dev Biol
48
1003
1014
25. ChinenA
HamaokaT
YamadaY
KawamuraS
2003
Gene duplication and spectral diversification of cone visual pigments of zebrafish.
Genetics
163
663
675
26. MatsumotoY
FukamachiS
MitaniH
KawamuraS
2006
Functional characterization of visual opsin repertoire in Medaka (Oryzias latipes).
Gene
371
268
278
27. TeraiY
MayerWE
KleinJ
TichyH
OkadaN
2002
The effect of selection on a long wavelength-sensitive (LWS) opsin gene of Lake Victoria cichlid fishes.
Proc Natl Acad Sci USA
99
15501
15506
28. ParryJW
CarletonKL
SpadyT
CarbooA
HuntDM
2005
Mix and match color vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids.
Curr Biol
15
1734
1739
29. TeraiY
SeehausenO
SasakiT
TakahashiK
MizoiriS
2006
Divergent selection on opsins drives incipient speciation in Lake Victoria cichlids.
PLoS Biol
4
2244
2251
30. SeehausenO
TeraiY
MagalhaesIS
CarletonKL
MrossoHD
2008
Speciation through sensory drive in cichlid fish.
Nature
455
620
626
31. RaymondPA
BarthelLK
RounsiferME
SullivanSA
KnightJK
1993
Expression of rod and cone visual pigments in goldfish and zebrafish: a rhodopsin-like gene is expressed in cones.
Neuron
10
1161
1174
32. VihtelicTS
DoroCJ
HydeDR
1999
Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins.
Vis Neurosci
16
571
585
33. TakechiM
KawamuraS
2005
Temporal and spatial changes in the expression pattern of multiple red and green subtype opsin genes during zebrafish development.
J Exp Biol
208
1337
1345
34. KennedyBN
VihtelicTS
CheckleyL
VaughanKT
HydeDR
2001
Isolation of a zebrafish rod opsin promoter to generate a transgenic zebrafish line expressing enhanced green fluorescent protein in rod photoreceptors.
J Biol Chem
276
14037
14043
35. HamaokaT
TakechiM
ChinenA
NishiwakiY
KawamuraS
2002
Visualization of rod photoreceptor development using GFP-transgenic zebrafish.
Genesis
34
215
220
36. AsaokaY
ManoH
KojimaD
FukadaY
2002
Pineal expression-promoting element (PIPE), a cis-acting element, directs pineal-specific gene expression in zebrafish.
Proc Natl Acad Sci USA
99
15456
15461
37. KawamuraS
TakeshitaK
TsujimuraT
KasagiS
MatsumotoY
2005
Evolutionarily conserved and divergent regulatory sequences in the fish rod opsin promoter.
Comp Biochem Physiol B
141
391
399
38. TakechiM
HamaokaT
KawamuraS
2003
Fluorescence visualization of ultraviolet-sensitive cone photoreceptor development in living zebrafish.
FEBS Lett
553
90
94
39. LuoW
WilliamsJ
SmallwoodPM
TouchmanJW
RomanLM
2004
Proximal and distal sequences control UV cone pigment gene expression in transgenic zebrafish.
J Biol Chem
279
19286
19293
40. TakechiM
SenoS
KawamuraS
2008
Identification of cis-acting elements repressing blue opsin expression in zebrafish UV cones and pineal cells.
J Biol Chem
283
31625
31632
41. TsujimuraT
ChinenA
KawamuraS
2007
Identification of a locus control region for quadruplicated green-sensitive opsin genes in zebrafish.
Proc Natl Acad Sci USA
104
12813
12818
42. MullerF
WilliamsDW
KobolakJ
GauvryL
GoldspinkG
1997
Activator effect of coinjected enhancers on the muscle-specific expression of promoters in zebrafish embryos.
Mol Reprod Dev
47
404
412
43. GongZ
JuB
WangX
HeJ
WanH
2002
Green fluorescent protein expression in germ-line transmitted transgenic zebrafish under a stratified epithelial promoter from keratin8.
Dev Dyn
223
204
215
44. ParinovS
KondrichinI
KorzhV
EmelyanovA
2004
Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo.
Dev Dyn
231
449
459
45. NathansJ
DavenportCM
MaumeneeIH
LewisRA
HejtmancikJF
1989
Molecular genetics of human blue cone monochromacy.
Science
245
831
838
46. WangY
MackeJP
MerbsSL
ZackDJ
KlaunbergB
1992
A locus control region adjacent to the human red and green visual pigment genes.
Neuron
9
429
440
47. ChenS
WangQL
NieZ
SunH
LennonG
1997
Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes.
Neuron
19
1017
1030
48. FurukawaT
MorrowEM
CepkoCL
1997
Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation.
Cell
91
531
541
49. FreundCL
Gregory-EvansCY
FurukawaT
PapaioannouM
LooserJ
1997
Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor.
Cell
91
543
553
50. LiX
ChenS
WangQ
ZackDJ
SnyderSH
1998
A pineal regulatory element (PIRE) mediates transactivation by the pineal/retina-specific transcription factor CRX.
Proc Natl Acad Sci USA
95
1876
1881
51. GamseJT
ShenYC
ThisseC
ThisseB
RaymondPA
2002
Otx5 regulates genes that show circadian expression in the zebrafish pineal complex.
Nat Genet
30
117
121
52. WinderickxJ
BattistiL
MotulskyAG
DeebSS
1992
Selective expression of human X chromosome-linked green opsin genes.
Proc Natl Acad Sci USA
89
9710
9714
53. HayashiT
MotulskyAG
DeebSS
1999
Position of a ‘green-red’ hybrid gene in the visual pigment array determines colour-vision phenotype.
Nat Genet
22
90
93
54. WangY
SmallwoodPM
CowanM
BleshD
LawlerA
1999
Mutually exclusive expression of human red and green visual pigment-reporter transgenes occurs at high frequency in murine cone photoreceptors.
Proc Natl Acad Sci USA
96
5251
5256
55. SmallwoodPM
WangY
NathansJ
2002
Role of a locus control region in the mutually exclusive expression of human red and green cone pigment genes.
Proc Natl Acad Sci USA
99
1008
1011
56. LevineJS
MacNicholEFJr
1979
Visual pigments in teleost fishes: effects of habitat, microhabitat, and behavior on visual system evolution.
Sens Processes
3
95
131
57. ThermesV
GrabherC
RistoratoreF
BourratF
ChoulikaA
2002
I-SceI meganuclease mediates highly efficient transgenesis in fish.
Mech Dev
118
91
98
58. LeeEC
YuD
Martinez de VelascoJ
TessarolloL
SwingDA
2001
A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA.
Genomics
73
56
65
59. UrasakiA
MorvanG
KawakamiK
2006
Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition.
Genetics
174
639
649
60. KawakamiK
TakedaH
KawakamiN
KobayashiM
MatsudaN
2004
A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish.
Dev Cell
7
133
144
61. WesterfieldM
1995
The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio).
Eugene
University of Oregon Press
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome DeletionsČlánek Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable EpiallelesČlánek A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular StressČlánek Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis inČlánek The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 inČlánek Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrAČlánek Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 12
-
Všechny články tohoto čísla
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Whole-Genome and Chromosome Evolution Associated with Host Adaptation and Speciation of the Wheat Pathogen
- Association of Variants at 1q32 and with Ankylosing Spondylitis Suggests Genetic Overlap with Crohn's Disease
- Initiator Elements Function to Determine the Activity State of BX-C Enhancers
- Identification of Genes Required for Neural-Specific Glycosylation Using Functional Genomics
- A Young Duplicate Gene Plays Essential Roles in Spermatogenesis by Regulating Several Y-Linked Male Fertility Genes
- The EpsE Flagellar Clutch Is Bifunctional and Synergizes with EPS Biosynthesis to Promote Biofilm Formation
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
- Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable Epialleles
- A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular Stress
- GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells
- A Single Enhancer Regulating the Differential Expression of Duplicated Red-Sensitive Opsin Genes in Zebrafish
- Investigation and Functional Characterization of Rare Genetic Variants in the Adipose Triglyceride Lipase in a Large Healthy Working Population
- Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis in
- Noisy Splicing Drives mRNA Isoform Diversity in Human Cells
- The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 in
- Thymus-Associated Parathyroid Hormone Has Two Cellular Origins with Distinct Endocrine and Immunological Functions
- An ABC Transporter Mutation Is Correlated with Insect Resistance to Cry1Ac Toxin
- Role of Individual Subunits of the CSN Complex in Regulation of Deneddylation and Stability of Cullin Proteins
- The C-Terminal Domain of the Bacterial SSB Protein Acts as a DNA Maintenance Hub at Active Chromosome Replication Forks
- The DNA Damage Response Pathway Contributes to the Stability of Chromosome III Derivatives Lacking Efficient Replicators
- Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrA
- LaeA Control of Velvet Family Regulatory Proteins for Light-Dependent Development and Fungal Cell-Type Specificity
- Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
- Distinct Functions for the piRNA Pathway in Genome Maintenance and Telomere Protection
- MOS11: A New Component in the mRNA Export Pathway
- Self-Mating in the Definitive Host Potentiates Clonal Outbreaks of the Apicomplexan Parasites and
- A Role for ATF2 in Regulating MITF and Melanoma Development
- Ancestral Regulatory Circuits Governing Ectoderm Patterning Downstream of Nodal and BMP2/4 Revealed by Gene Regulatory Network Analysis in an Echinoderm
- Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea
- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Linking Crohn's Disease and Ankylosing Spondylitis: It's All about Genes!
- Genomics Meets Glycomics—The First GWAS Study of Human N-Glycome Identifies HNF1α as a Master Regulator of Plasma Protein Fucosylation
- Continuous and Periodic Expansion of CAG Repeats in Huntington's Disease R6/1 Mice
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Endocytic Sorting and Recycling Require Membrane Phosphatidylserine Asymmetry Maintained by TAT-1/CHAT-1
- Histone Deacetylases Suppress CGG Repeat–Induced Neurodegeneration Via Transcriptional Silencing in Models of Fragile X Tremor Ataxia Syndrome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



