-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAdenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
article has not abstract
Published in the journal: . PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004821
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004821Summary
article has not abstract
Introduction
Despite lingering safety concerns [1] and potential restrictions imposed by the host immune response, including the innate immune pattern recognition receptors (PRR), replication-defective and conditionally replicating human and nonhuman adenovirus (AdV) vectors continue to be a favorite vehicle for short-term (e.g., vaccine) and long-term gene delivery. This is due in part to several desirable features of AdV including their broad tissue tropism, their ample capacity for foreign gene insertion, and their LEGO-like structural adaptability (Fig 1A) to add, delete, and swap proteins and motifs from other viruses or host molecules.
Fig. 1. Adenovirus structure and trafficking. 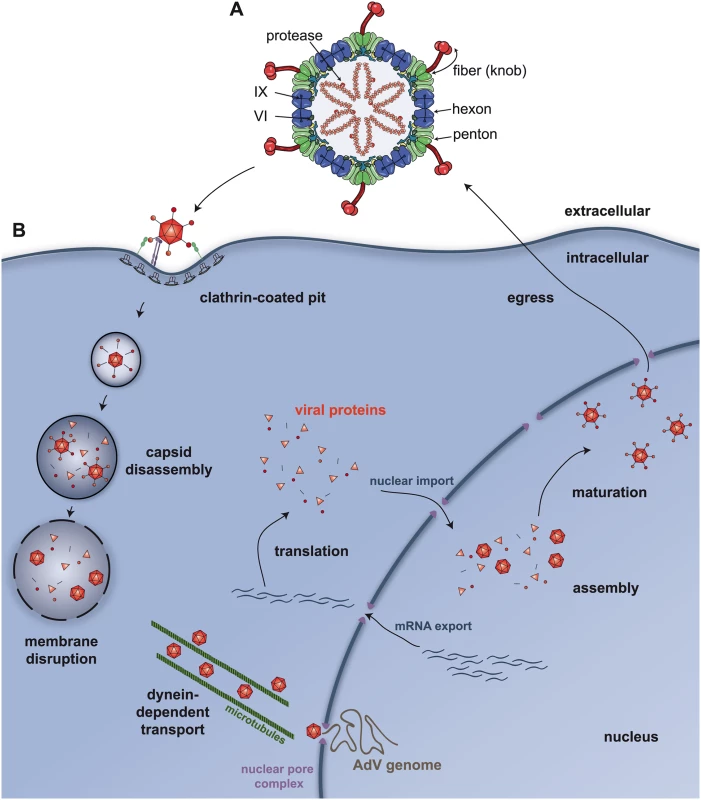
A) An illustration of the cross-section of a prototype 90 nm AdV capsid showing the location of the principal capsid proteins (hexon, penton, protein VI, protein IX, protease, and the fibre—the knob is the globular head of the fibre) involved in trafficking. B) An illustration showing the quintessential steps of AdV trafficking in epithelial cells. Via the knob region of the fiber, the capsid engages the cellular receptor. In some cell types, fibres are lost from the metastable* capsid during internalization in clathrin-coated pits. Postinternalization, the capsid continues to dissociate and releases protein VI, which allows the capsid access to the cytosol and interaction with dynein, then dynein-dependent transport along microtubule to the nuclear pore complex. *Metastable is a common term used to describe the biophysical state of fully mature nonenveloped virions. Overall, the particle is stable to the environment; however, it is able to respond to cellular cues to undergo conformational changes during cell entry. While much is known about the molecular genetics and replication of AdVs, many investigators are continuing to decipher the captivating intracellular events of the first thirty minutes in the virus life cycle. This Pearl accentuates the strikingly diverse mechanisms for AdV entry, comparing human AdV type 5 (HAdV-C5) in epithelial cells and canine type 2 (CAdV2, or commonly referred to as CAV-2) in neurons. Similar viral and cellular proteins are used, and although the function of the cellular protein varies among cell types, these cell protein—virus associations promote similar outcomes. We also highlight some outstanding questions and hurdles needed to improve vector-mediated gene and vaccine delivery and treatments for AdV disease. The take home message is that one may be able to take advantage of a better understanding of these cell entry variations to control AdV pathogenesis and vector tropism for gene therapy.
Mi Casa Es Su Casa: The Cellular Determinants That Dictate AdV Tropism
Of the more than 60 human AdV (HAdV) types that make up the current seven species (denoted as A–G), the most extensively studied are species C type 2 (HAdV-C2) and HAdV-C5. Many human and some nonhuman AdVs, including CAV-2, use the coxsackievirus and adenovirus receptor (CAR) [2–4] for high affinity attachment to host cells via the capsid fibre protein. On polarized epithelial cells, the predominant CAR isoform is targeted to the basolateral surface and in tight junctions. A minor exon 8-containing CAR isoform may be targeted to the apical surface [5] of some epithelial cells and allow easier access of CAR-tropic virus attachment. Other HAdV types from species B use desmoglein 2 or CD46, a member of the complement regulatory protein family, while species D HAdVs appear to use CAR, sialic acid, the GD1a glycan, and/or CD46 (for a recent review on AdV receptors see [6]). For HAdVs that use CAR as an attachment molecule on epithelial cells, engagement of the αv integrin is needed for efficient internalization (Fig 1B). This engagement occurs through association of the integrin with a consensus integrin interacting motif (RGD in most AdVs) located on an extended loop on the penton base [7]. Integrin ligation triggers signaling events that promote virus entry into early endosomes via clathrin-mediated endocytosis (Fig 1B). In epithelial cells, it seems that CAR facilitates attachment but not cell entry [8]. However, it is still unclear how significantly the integrin repertoire involved in membrane penetration influences different AdV types. Moreover, when injected intravenously in mice, some AdVs can interact with specific coagulation factors [9] that alter tissue tropism by preventing binding of naturally occurring antibodies and then by acting as a bridge to attach to proteoglycans on liver cells [10]. That coagulation factors influence HAdV tropism in rodents is clear, but its relevance for HAdV disease and HAdV vector administration in humans is unknown. As discussed below, AdV trafficking into neurons follows a pathway different from that of epithelial cells. Thus, the routes and modes of AdV cell entry are variable and cell-type dependent.
Houston, We Have a Problem: The Escape Route HAdV Uses to Reach the Nucleus
Internalization of AdV particles is a primordial event for infection—but it is only the beginning of the journey to the nuclear pore complex (NPC). The ligation of CAR and integrins on the cell surface induces distinct membrane trafficking processes that produce a mechanical force to initiate partial capsid disassembly [11]. Analyses using atomic force microscopy are consistent with this model and indicate that integrin ligation by the virus is sufficient to loosen the vertex region(s) of the capsid [12]. Once inside most cells, the “metastable” virions ultimately need to escape a vesicular compartment to be translocated via a dynein-dependent mechanism to the NPC (Fig 1B). Removal of the vertex region—composed of the penton base, fibre [13], and likely the peripentonal hexons—allows release of the membrane lytic protein VI from the inner surface of the virus capsid [14]. Interestingly, α defensins HD5 and HNP1 bind to the vertex region of the virus capsid and prevent its disassembly. This restricts release of protein VI and membrane destruction. Exposure of the inner core of the virus apparently starts in the endosome as monitored by antibody detection of the viral genome at early time points [15]. Protein VI release is associated with increased endosomal membrane destruction [13], and a single point mutation (L40Q) in the amphipathic helical domain of protein VI significantly attenuates membrane insertion, membrane destruction, and cell infection [16]. Protein VI insertion into a lipid bilayer causes positive membrane curvature [17], and this may impart stress and global membrane destruction allowing passage of the partially disassembled capsid into the cytosol. If there are structural rearrangements that occur in protein VI after its release from the HAdV capsid, these could be druggable targets.
Partially uncoated virions also escape endocytic vesicle concomitant with a drop in pH. Yet, the precise role of pH in virus disassembly and/or engagement of molecular motors is enigmatic [18]. Partially uncoated virions can associate with dynein motor proteins that recognize the hexon [19] or possibly protein VI [20]. This association is instrumental in transporting the virions along microtubules, and numerous laboratories have seen that, in superinfected cells, AdV capsids can accumulate at the microtubule-organizing center. However, it is unclear whether the microtubule-organizing center is a launching pad for NPC engagement, an artifact of superinfected cells, or a cul-de-sac. The association of the virion with proteins at the NPC likely facilitates further uncoating of the virion, thereby allowing the genome to be translocated into the nucleus, although the precise mechanisms involved are still being investigated. Afterwards, the AdV genome is delivered to the nucleus to initiate a new round of propagation.
Whether capsid disassembly is initiated at a unique or specific vertex—for example, a “portal vertex” where the DNA is inserted during particle maturation—is unknown. Could a unique portal vertex be used for initial disassembly or protein VI release and nuclear import during infection and DNA packaging? If a single vertex pops off from the disassembled HAdV-C5 capsids, how would the genome become available to detection by cytosolic PRRs in some cell types? Clearly, cell type-specific trafficking influences the interaction with PRRs and therefore may provide an opportunity for vector optimization.
The Penthouse Please: How Does CAV-2 Enter Neurons?
We all know that cells differ in their morphology and functions, and it would be surprising if they interacted with pathogens in identical manners. A distinct variation on AdV trafficking has been observed in neurons. CAV-2 has found an unlikely niche as a vector for gene transfer to central (brain and spinal cord) and peripheral (e.g., motor and sensory) neurons [21–23]. The basis of CAV-2’s preferential targeting to neurons is likely due to the exclusive use of CAR as both the attachment and entry molecule, as well as the selective expression of CAR on neurons in the brain parenchyma (versus microglia, astrocytes, and oligodendrocytes) and at neuromuscular junctions [24]. In addition to the preferential neuronal tropism, CAV-2 vectors are efficiently taken up at axon termini and transported back to the soma via retrograde transport [24,25]. While numerous studies of AdV trafficking in epithelial-like cells (including CAV-2 [26]) have led to a well-recognized pathway, CAV-2 trafficking in neurons accentuates AdV trafficking adaptability.
CAV-2, like the two members of HAdV species F, does not contain an identifiable integrin-interacting motif in the penton base [27]. CAV-2 uses its trimeric fibre knob at the end of a double-hinged shaft [28] to attach to CAR with very high (1 nM) affinity. At axon termini, CAR is located in lipid rafts [29], and CAR-mediated internalization of CAV-2 appears to be induced by the disruption of intracellular homodimeric CAR interactions [29]. The number of CAR molecules needed to induce internalization is unknown, nor whether a torsional force is applied to the CAV-2 capsid during entry to induce the release of protein VI. It is also unclear whether neurons induce partial CAV-2 disassembly during internalization at, or near, the plasma membrane. The CAR-CAV-2 complex likely stays in lipid rafts, and internalization depends on actin reorganization, dynamin function, and—in contrast to HAdV-C5 internalization in epithelial cells—is clathrin-independent. Immediately postinternalization, the CAR-CAV-2 complex can be found in static Rab5+ vesicles that mature into Rab7+ vesicles, where active retrograde transport starts (Fig 2) [20].
Fig. 2. Axonal transport of CAV-2 in neurons. 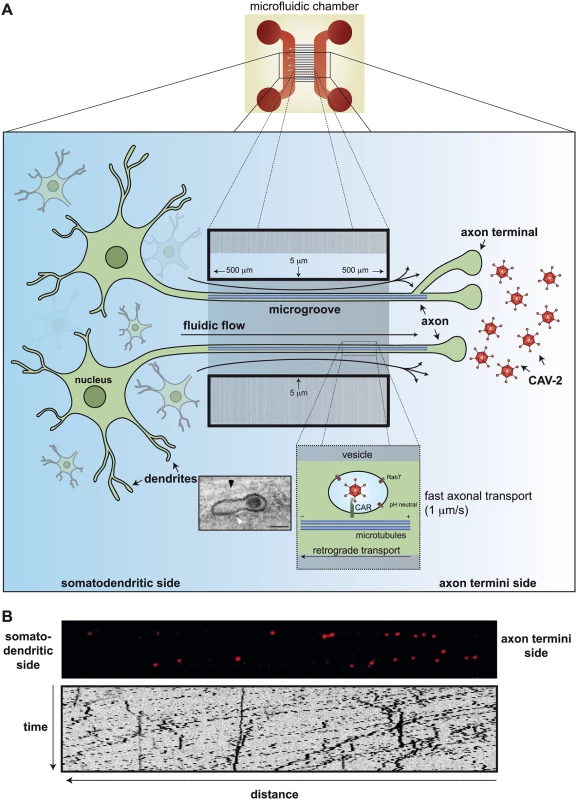
A) A schema showing the assays used to record CAV-2 directionality and speed in murine dorsal root ganglion (DRG) neurons. Neurons are cultivated in microfluidic chambers (top center) in which the microfluidic flow is from left to right. This flow of the medium in the 5-micron-wide and 500-micron-long microgroove prevents diffusion of particles and allows a physical separation between cell bodies (left) and axon termini (right). CAV-2, covalently labeled with a fluorophore (Cy3), were added for 90 min in the axonal compartment before the video was started, and axons in the middle of the microgroove were imaged at one frame/second. The rate of retrograde transport of CAV-2 in these conditions is approximately 1–2 microns/s (insert: ultrastructural electron micrograph of CAV-2 vesicular transport in motor neurons from Salinas et al. [31]). CAV-2 was mainly present in vesicular structures (white arrow) near microtubule tracks (black arrow). B) Still images of a microgroove of the chambers containing Cy3-labeled CAV-2 (red puncta) 90 min postincubation on the axon termini side. Below is a kymograph, which gives a graphical representation of the spatial position over time, of the corresponding movie (S1 Video). Scale bars in the micrograph = 100 nm. The sorting platform for endocytosed vesicles is complex at the synapse: does CAV-2 influence the content of the vesicles it ends up in? Does this influence vesicular trafficking and targeting in neurons? And here is another difference between epithelial-like cells and neurons: CAV-2 does not escape from these CAR+/pH-neutral/Rab7+ multitasking compartments [30]. Of note, in epithelial cells, Rab7+ is a hallmark of late endosomes that have an acidic lumen. What appears to be an intact CAV-2 (Fig 2A insert) is transported to the soma of the neurons in a vesicular structure. This long, protected retrograde journey in motor neuron axons could be more than 1 meter in humans. When the CAR-CAV-2 complex reaches the soma, CAV-2 vesicular escape also coincides with a drop in the pH [31]. Whether the pH drop and endolysosome escape of CAV-2 in neurons are mechanistically linked has not been formally tested.
Et Alors: The Implications for AdV-Mediated Gene Transfer
The modularity of the AdV capsid allows enormous adaptability for practical and theoretical vector design. With the more than 200 different AdVs partially or totally characterized to date, we have a rich reserve to create vectors for short - or long-term gene transfer. CAV-2 vector use in the brain is a perfect example [32]. Few would have predicted that CAV-2—which normally causes respiratory tract infection in some Canidae (e.g., dogs, wolves, foxes, bears, etc.)—would be a valuable tool with which to probe higher order brain function or as a vector to treat neurodegenerative diseases that affect the entire brain [33]. A CAV-2 vector eliminated the neuropathology in the mucopolysaccharidosis type VII (MPS VII) mouse and the MPS VII dog brain—a brain that is one-third the size of a two-year-old child. This therapeutic potential is due to the low immunogenicity, long-term transgene expression, ample cloning capacity [25], preferential transduction of neurons, and the use of the interconnectivity of neurons to reach structures throughout the brain. CAV-2 vector potential was unpredictable 20 years ago.
So, what are we waiting for? Carpe momentum.
Supporting Information
Zdroje
1. Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J Exp Med. 2008 24;205(12):2717–25. PMID: ISI:000261295300006. doi: 10.1084/jem.20081786 18981239
2. Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–3 9036860
3. Roelvink PW, Mi Lee G, Einfeld DA, Kovesdi I, Wickham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286(5444):1568–71 10567265
4. Seiradake E, Lortat-Jacob H, Billet O, Kremer EJ, Cusack S. Structural and mutational analysis of human Ad37 and canine adenovirus 2 fiber heads in complex with the D1 domain of coxsackie and adenovirus receptor. J Biol Chem. 2006 3;281(44):33704–16. 16923808
5. Excoffon KJ, Gansemer ND, Mobily ME, Karp PH, Parekh KR, Zabner J. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS ONE. 2010;5(3):e9909. 20361046. doi: 10.1371/journal.pone.0009909
6. Arnberg N. Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol Sci. 2012 33(8):442–8. 22621975. doi: 10.1016/j.tips.2012.04.005
7. Stewart PL, Chiu CY, Huang S, Muir T, Zhao Y, Chait B, et al. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 1997;16(6):1189–98. 9135136
8. Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73(2):309–19. 8477447
9. Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008 8;132(3):397–409. 18267072 doi: 10.1016/j.cell.2008.01.016
10. Xu Z, Qiu Q, Tian J, Smith JS, Conenello GM, Morita T, et al. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat Med. 2013 19(4):452–7. 23524342 doi: 10.1038/nm.3107
11. Burckhardt CJ, Suomalainen M, Schoenenberger P, Boucke K, Hemmi S, Greber UF. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe. 2011 18;10(2):105–17. 21843868 doi: 10.1016/j.chom.2011.07.006
12. Snijder J, Reddy VS, May ER, Roos WH, Nemerow GR, Wuite GJ. Integrin and defensin modulate the mechanical properties of adenovirus. J Virol. 2013 Mar;87(5):2756–66. 23269786 doi: 10.1128/JVI.02516-12
13. Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005 79(4):1992–2000. 15681401.
14. Smith JG, Silvestry M, Lindert S, Lu W, Nemerow GR, Stewart PL. Insight into the mechanisms of adenovirus capsid disassembly from studies of defensin neutralization. PLoS Pathog. 2010;6(6):e1000959. 20585634. doi: 10.1371/journal.ppat.1000959
15. Nguyen EK, Nemerow GR, Smith JG. Direct evidence from single-cell analysis that human {alpha}-defensins block adenovirus uncoating to neutralize infection. J Virol. 2010 84(8):4041–9. 20130047. doi: 10.1128/JVI.02471-09
16. Moyer CL, Wiethoff CM, Maier O, Smith JG, Nemerow GR. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J Virol. 2011 85(6):2631–41. 21209115 doi: 10.1128/JVI.02321-10
17. Maier O, Galan DL, Wodrich H, Wiethoff CM. An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature. Virology. 2010 20;402(1):11–9. 20409568 doi: 10.1016/j.virol.2010.03.043
18. Suomalainen M, Luisoni S, Boucke K, Bianchi S, Engel DA, Greber UF. A direct and versatile assay measuring membrane penetration of adenovirus in single cells. J Virol. 2013 87(22):12367–79. 24027314. doi: 10.1128/JVI.01833-13
19. Bremner KH, Scherer J, Yi J, Vershinin M, Gross SP, Vallee RB. Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit. Cell Host Microbe. 2009 17;6(6):523–35. 20006841 doi: 10.1016/j.chom.2009.11.006
20. Wodrich H, Henaff D, Jammart B, Segura-Morales C, Seelmeir S, Coux O, et al. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010 6(3):e1000808. 20333243 doi: 10.1371/journal.ppat.1000808
21. Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, et al. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci U S A. 2006 6;103(23):8858–63. 16723393.
22. Pivetta C, Esposito MS, Sigrist M, Arber S. Motor-Circuit Communication Matrix from Spinal Cord to Brainstem Neurons Revealed by Developmental Origin. Cell. 2014 30;156(3):537–48. PMID: ISI:000330580800019 doi: 10.1016/j.cell.2013.12.014 24485459
23. Ekstrand MI, Nectow AR, Knight ZA, Latcha KN, Pomeranz LE, Friedman JM. Molecular profiling of neurons based on connectivity. Cell. 2014 22;157(5):1230–42. 24855954 doi: 10.1016/j.cell.2014.03.059
24. Soudais C, Laplace-Builhe C, Kissa K, Kremer EJ. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 2001;15(12):2283–5. 11511531
25. Soudais C, Skander N, Kremer EJ. Long-term in vivo transduction of neurons throughout the rat CNS using novel helper-dependent CAV-2 vectors. FASEB J. 2004 18(2):391–3. 14688208
26. Chillon M, Kremer EJ. Trafficking and propagation of canine adenovirus vectors lacking a known integrin-interacting motif. Hum Gene Ther. 2001 20;12(14):1815–23. 11560774
27. Soudais C, Boutin S, Hong SS, Chillon M, Danos O, Bergelson JM, et al. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J Virol. 2000 74(22):10639–49. 11044108
28. Schoehn G, El Bakkouri M, Fabry CM, Billet O, Estrozi LF, Le L, et al. Three-dimensional structure of canine adenovirus serotype 2 capsid. J Virol. 2008 82(7):3192–203. 18216088 doi: 10.1128/JVI.02393-07
29. Salinas S, Zussy C, Loustalot F, Henaff D, Menendez G, Morton PE, et al. Disruption of the coxsackievirus and adenovirus receptor-homodimeric interaction triggers lipid microdomain - and dynamin-dependent endocytosis and lysosomal targeting. J Biol Chem. 2014 10;289(2):680–95. 24273169 doi: 10.1074/jbc.M113.518365
30. Salinas S, Schiavo G, Kremer EJ. A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol. 2010 8(9):645–55. PMID: ISI:000280855500011 doi: 10.1038/nrmicro2395 20706281
31. Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, et al. CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 2009 5(5):e1000442. 19461877 doi: 10.1371/journal.ppat.1000442
32. Bru T, Salinas S, Kremer EJ. An update on canine adenovirus type 2 and its vectors. Viruses. 2010 Sep;2(9):2134–53. 21994722 doi: 10.3390/v2092134
33. Cubizolle A, Serratrice N, Skander N, Colle MA, Ibanes S, Gennetier A, et al. Corrective GUSB Transfer to the Canine MPS VII Brain. Mol Ther. 2014 22(4):762–73. PMID: ISI:000334061100010 doi: 10.1038/mt.2013.283 24343103
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



