-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
Entomopathogenic fungi represent a promising class of bio-insecticides for mosquito control. Detailed knowledge of molecular mechanisms governing anti-fungal immune response in mosquitoes is essential. CLSP2 composed of serine protease and lectin domains functions as a modulator of the mosquito immune system during the anti-fungal response. Transcriptome analysis indicated that the Toll pathway and melanization genes are highly up-regulated in CLSP2 RNA interference depleted mosquitoes infected with the fungus Beauveria bassiana. A thioester-containing protein TEP22, a member of α2-macroglobulin family, is involved in the CLSP2-modulated mosquito antifungal defense. Our study has contributed to the understanding of immune-modulating mechanisms in mosquitoes.
Published in the journal: . PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004931
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004931Summary
Entomopathogenic fungi represent a promising class of bio-insecticides for mosquito control. Detailed knowledge of molecular mechanisms governing anti-fungal immune response in mosquitoes is essential. CLSP2 composed of serine protease and lectin domains functions as a modulator of the mosquito immune system during the anti-fungal response. Transcriptome analysis indicated that the Toll pathway and melanization genes are highly up-regulated in CLSP2 RNA interference depleted mosquitoes infected with the fungus Beauveria bassiana. A thioester-containing protein TEP22, a member of α2-macroglobulin family, is involved in the CLSP2-modulated mosquito antifungal defense. Our study has contributed to the understanding of immune-modulating mechanisms in mosquitoes.
Introduction
Female mosquitoes require repeated blood feedings during their life cycle to satisfy their reproductive nutritional needs and, as a consequence, they serve as vectors of numerous human diseases [1]. Malaria, transmitted by the Anopheles genus, is the most devastating vector-borne human disease and causes about one million deaths per year. The annual number of cases of Dengue fever, a viral disease transmitted by Aedes aegypti, has reached over a hundred million. Major reasons for this serious situation include the lack of effective vaccines against major mosquito-borne diseases, rapidly developing drug and insecticide resistance, and socio-economic problems in endemic countries.
It is imperative to design novel specific biological pesticides, since mosquitoes have developed resistance to most currently used chemical insecticides [2]. While entomopathogenic fungi Beauveria bassiana and Metarhizium anisophliae infect insects by direct penetration of the cuticle, bacteria and viruses often need to be ingested, which makes fungi more promising as pesticides. However, fungal pathogens still require improvements due to the relatively low virulence when compared with chemical pesticides [3]. Detailed studies of antifungal immunity in mosquitoes are essential for future improvements of fungal biocontrol agents.
Multicellular organisms have evolved complex and powerful systems of immune responses to counteract continuous attacks of various pathogens. An essential feature of the immune system in any organism is its capacity to sustain equilibrium between reactivity and quiescence [4]. A loss of such a balance leads to severe consequences, such as autoimmune and inflammatory diseases in humans. Inhibitory receptor systems modulating immune responses have been identified in vertebrates [4,5]. However, the detail mechanism of the analogous system in insect is still not very clear. Our studies have revealed that CLSP2 functions as a key modulator of the mosquito immune system and contributed to a better understanding of immune modulating mechanisms in insects.
In insects, Toll is the principal innate immune pathway responsible for the anti-fungal response [6,7,8]. The Toll pathway is induced by fungal β1,3-glucan and also by Gram-positive bacteria harboring Lys-type peptidoglycan [7]. This pathway is crucial in activating immune responses especially in production of antimicrobial and anti-fungal peptides (AMPs) [6,9,10]. Gram-negative binding protein 3 (GNBP3), a member of the β-1, 3-glucan recognition protein (βGRP) family, binds to fungal cell components and initiates the Toll pathway [11]. Two Clip domain serine proteases (CLIPs)—Persephone and Späetzle-processing enzyme (SPE)—are components of an extracellular serine protease (SP) cascade and cause the cleavage of a cysteine knot cytokine, Späetzle (Spz) [12,13]. The cleaved Spz then functions as a ligand of the Toll receptor, which in turn passes the signals into the intracellular signal cascade consisting of MyD88, Tube, Pelle and TRAF6. Mosquitoes have a single orthologue of Dorsal, Rel1 [6]. Activation of the intracellular signal cascade by Toll results in the phosphorylation and degradation of Cactus, which is an inhibitor of the NF-кB transcription factors Dorsal and Dif [14]. Removal of Cactus releases and causes nuclear translocation of Dorsal and Dif, which eventually leads to the expression of AMPs, including Drosomycin, an antifungal peptide. Previously, Ae. aegypti orthologues of Drosophila genes of the Toll pathway—Spz1C, Toll5A, CLIPB5, and CLIPB29—have been identified and shown to mediate the Toll pathway in response to fungal infection [6,15]. In mosquitoes, Cecropins and Defensins are the major AMPs involved in the systemic antifungal immune responses [16].
Melanization represents a second immune pathway that is essential in the systemic antifungal immune responses [17]. It is the arthropod-specific defense mechanism that plays an essential role in wound healing and innate immunity [17]. The key enzymes for this reaction are prophenoloxidases (PPOs), which, once activated, catalyze the formation of toxic melanin. Melanin is then deposited around the wound or invading pathogens, including fungi. CLIPs constitute a cascade for amplification of a signal triggered by pathogen infection that results in PPO cleavage into an active PO by a melanization protease (MP). The melanization cascade is tightly regulated by serine protease inhibitors (SRPNs), which prevent spontaneous initiation of the reaction. The analysis of the mosquito genomes has shown that genes encoding immune signaling and effector molecules, and the number of melanization pathway genes have undergone major expansion [16]. For example, there are 10 PPO genes in the Ae. aegypti genome [13]. However, the precise roles of each PPO in melanization process are poorly understood. Our previous study revealed a novel level of complexity in the melanization cascade of the mosquito Ae. aegypti. Namely, we identified that there are several independent pathways leading to melanization, each requiring a different protease/SRPN regulatory module [15]. Of particular interest is a clear separation of tissue melanization, represented by melanin tumors often associated with the damage of host tissues, and immune melanization involved in the recognition and killing of pathogens, including fungi [13]. The melanization response has also been shown to significantly retard the growth and dissemination of B. bassiana in the An. gambiae mosquito [18].
Previously, we have identified an immune factor in Ae. aegypti, CLSP2 (AAEL011616), that is composed of an elastase-like serine protease (ESP) and CTL-type domains [19]. In this study, we show that CLSP2 is the key negative modulator of immune responses during anti-fungal infection. The expression of the CLSP2 gene is elevated upon B. bassiana infection. CLSP2 is cleaved upon challenge with B. bassiana and the liberated CLSP2 CTL-type domain binds to fungal cell components. Moreover, RNAi depletion of CLPS2 (iCLSP2) significantly increases the resistance to the fungal challenge. RNA-sequencing (RNA-seq)-based transcriptome analysis indicated that the Toll pathway and melanization genes are highly up-regulated in CLSP2 RNAi-silenced mosquitoes infected with B. bassiana (iCLSP2Bb). TEP22, a member of α2-macroglobulin family, was identified to be regulated by CLSP2 and to participate in the antifungal immune response in the Ae. aegypti mosquitoes.
Results
CLSP2 responses to fungal infection
We investigated the CLSP2 responses to fungal infection at the gene and protein levels. Real-time RT-PCR (qPCR) analysis showed that the CLSP2 mRNA level in mosquitoes was significantly up-regulated after septic injections of conidia of the fungus B. bassiana (S1A Fig). This result was consistent with our previously reported Northern results [19], indicating a CLSP2 response to infections at the gene level.
Aedes CLSP2 consists of two domains: the N-terminal elastase-like serine protein (ESP) and the C-terminal galactose-type C-type lectin (CTL), which includes a signature QPD sequence. CLSP2 includes a signal peptide and no transmembrane domain, suggesting that it is a secreted peptide. To investigate the infection effect on the CLSP2 protein composition, hemolymph samples were resolved on SDS-PAGE and subjected to immunoblot analysis utilizing anti-CLSP2 polyclonal antibodies. In the hemolymph of control mosquitoes injected with sterile phosphate buffered saline (PBS), CLSP2 remained as a single band of about 47 kDa, which disappeared in RNA-interference (RNAi) CLSP2 silenced mosquitoes (iCLSP2) (Fig 1A). However, two bands, corresponding to the 33-kDa ESP and 14-kDa lectin domains, were detected in the mosquito hemolymph after infection with B. bassiana conidia (Fig 1A). This suggested that CLSP2 was cleaved upon immune challenge. When we used the hemolymph from mosquitoes with silenced CLSP2, no bands were evident in the immunoblot, indicating that the 33-kDa and 14-kDa bands observed in the mosquito hemolymph after infection with B. bassiana conidia belong to CLSP2. Additional controls for the specificity of anti-CLSP2 antibodies are presented in S1B and S1C Fig.
Fig. 1. CLSP2 responses to infection by the fungus B. bassiana and isolation of rLectin. 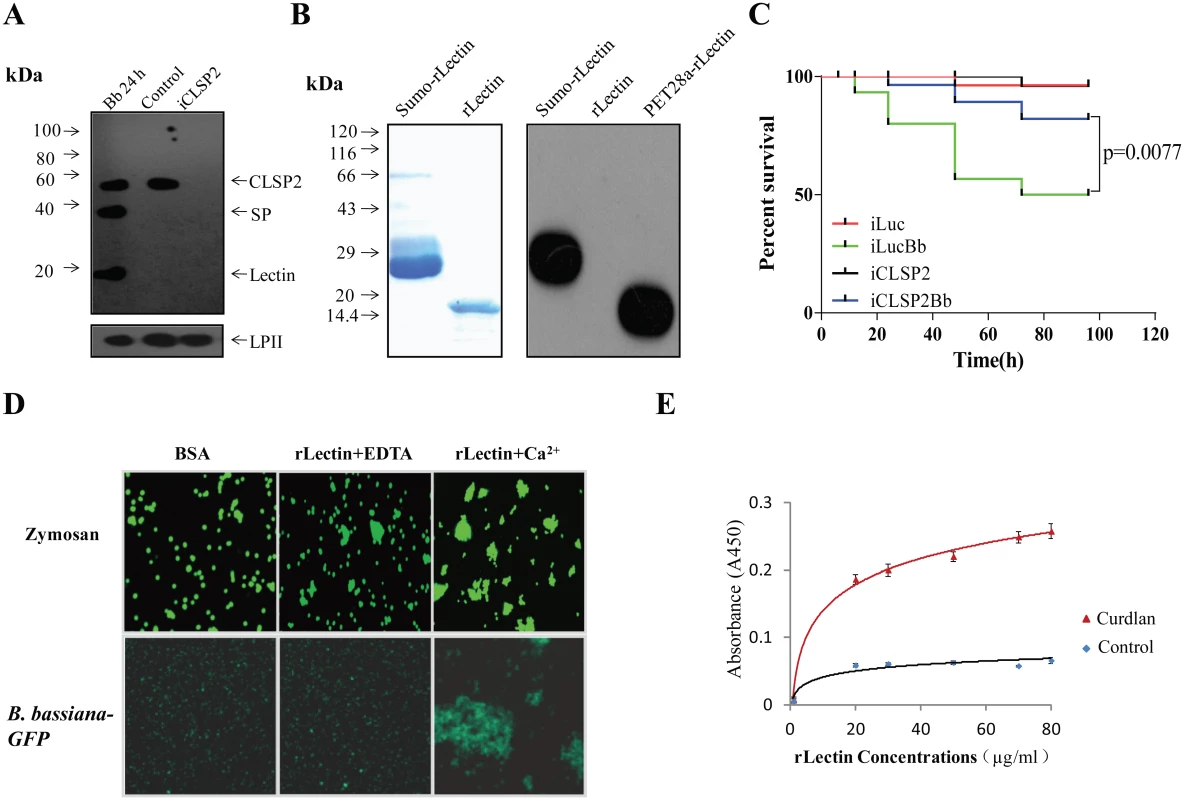
A) Immunoblot analysis showing the composition and cleavage of CLSP2 in iCLSP2, and mosquitoes after fungal challenge. Hemolymph was isolated from mosquitoes 24 h after treatment with B. bassiana (Bb 24h). Control, mosquitoes were treated with (PBS). iCLSP2, CLSP2 RNAi depleted mosquitoes injected with PBS. The 14-kDa lectin domain and the 33-kDa SP domain bands were present in hemolymph samples from Bb 24h mosquitoes, but not those from Naïve ones. CLSP2 polyclonal antibodies were used in the immunoblot analysis; Ae. aegypti Lipophorin II was used as the loading control. B) Purified sumo-rLectin and rLectin analyzed on 10% SDS-PAGE. To generate rLectin, the sumo-rLectin was cleaved by SUMO protease, and sumo tag was removed using a nickel affinity column. SDS-PAGE with Coomassie blue staining of Sumo-rLectin and rLectin (left panel). Western blot analysis of purified sumo-rLectin, rLectin, and PET28a-rLectin, which is cloned in PET28a and contains His-tag (right panel). Sumo-rLectin and PET28a-rLectin were detected using the anti-Histidine monoclonal antibody. C) Survival rate of mosquitoes infected with B. bassiana conidia was partially rescued by CLSP2 RNAi depletion (iCLSP2Bb). The survival rate of iLucBb mosquitoes was significantly different from that of the iLuc and iCLSP2Bb mosquitoes (p < 0.01). Each experiment was performed in three replicates. iLucBb, iLuc-depleted mosquitoes infected with B. bassiana; iCLSP2Bb, CLSP2 RNAi depleted mosquitoes infected with B. bassiana; iCLSP2, CLSP2 RNAi depleted mosquitoes injected with PBS; iLuc, luciferase RNAi-treated control mosquitoes injected with sterile phosphate buffered saline (PBS). See Materials and Methods for details. D) The agglutination assay testing interaction of rLectin with zymosan and GFP-conjugated B. bassiana. Purified rLectin samples (Fig 1B) were incubated with FITC-labeled zymosan or B. bassiana-GFP in addition of either 0.5 mM EDTA (rLectin + EDTA) or 5 mM CaCl2 (rLectin + Ca2+) for 45 min. The left panel is a control in which zymosan and B. bassiana-GFP were incubated with 1 mg/ml BSA. E) ELISA test of rLectin binding to the fungal cell wall component, curdlan. rLectin was prepared at different concentrations (0–80 μg/ml) in binding buffer containing 5 mM CaCl2 and BSA for 20 min. rLectin was added to curdlan-coated microtiter plates, and the binding assay was performed as described in Materials and Methods. The control was tested by pre-immune antiserum. Each point represents the mean of four individual measurements ± SEM. In an attempt to understand the biochemical properties of CLSP2, we cloned and produced its lectin domain, designated as rLectin, using an Escherichia coli expression system. Myc tag rLectin fused with hexahistidine-tagged SUMO was purified using an affinity Ni-NTA agarose column. The isolated product was cleaved by SUMO protease, and then reloaded onto the Ni-NTA column, so that rLectin was in the flow-through fraction and the His-tagged protease was retained on the column. The purified rLectin migrated as a single band with the expected molecular weight (MW) of 14 kDa on SDS-PAGE (Fig 1B) that was not recognized by the anti-Histidine monoclonal antibody (Fig 1B).
To address the CLSP2 role in immune responses, we investigated the susceptibility of iCLSP2 mosquitoes to fungal infections. Three days after CLSP2 dsRNA injection, mosquitoes were infected with B. bassiana and their survival rate was evaluated. The survival rate of iCLSP2 mosquitoes challenged with B. bassiana (iCLSP2Bb) was significantly higher than that of mosquitoes infected with this fungus alone in the iLuc background (iLucBb). Mosquitoes with Luciferase (Luc) gene silencing served as a control (iLuc) (Fig 1C). qPCR and immunoblotting tests confirmed efficiency of CLSP2 RNAi (S2A–S2C) Fig. Thus, silencing of CLSP2 in mosquitoes led to an increased resistance to fungal infection, suggesting a role of CLSP2 in modulating immune activation.
The CLSP2 lectin domain is responsible for fungal recognition
In order to decipher the interaction between CLSP2 and fungi, we examined binding properties of rLectin by means of the agglutination assay. As fungal representatives, we tested zymosan, which is a component of the Saccharomyces cerevisiae cell wall composed of β-glucans and mannan, and GFP-conjugated B. bassiana in the rLectin agglutination assay (Fig 1D). Neither zymosan nor GFP-conjugated B. bassiana aggregates were observed in the presence of bovine serum albumin (BSA) used as a control. Only minor aggregates were found in the presence of EDTA. However, large aggregates were observed in the presence of Ca2+, indicating that it was required for the agglutination reaction by rLectin of either zymosan or GFP-conjugated B. bassiana (Fig 1D).
Next, we performed enzyme-linked immunosorbent assay (ELISA) to test whether rLectin directly bound to the fungal cell component curdlan. Different amounts of rLectin (20, 30, 50, 70 and 80 μg/ml; 50 μl each) were added to microtiter plate wells coated with curdlan, and the bound rLectin was detected using Myc antibodies. ELISA has demonstrated that rLectin effectively binds to curdlan (Fig 1E). Taken together, these results suggest that the CLSP2 lectin domain is capable of recognizing and binding to fungal carbohydrate surface molecules in a saturable and Ca2+ dependent manner.
CLSP2 modulates expression of a large array of genes involved in innate immunity
Next, CLSP2 effect on expression of immune genes was elucidated by means of the RNA-sequence-based transcriptome analysis (RNA-seq) linked with RNAi screens. For this analysis, we used mosquitoes after three different treatments: control iLuc infected with B. bassiana (iLucBb), silenced with CLSP2 RNAi (iCLSP2), silenced with CLSP2 RNAi and infected B. bassiana (iCLSP2Bb). iLuc mosquitoes served as a control. Regulated immune gene repertoire (fold change ≥ 1.5) in different groups is shown in S1–S3 Tables. The hierarchical clustering analysis has revealed a stunning up-regulation of major immune gene transcripts in iCLSP2Bb mosquitoes (Fig 2A and S2D Fig).
Fig. 2. Transcriptome analysis of CLSP2 modulation of Ae. aegypti immune genes. 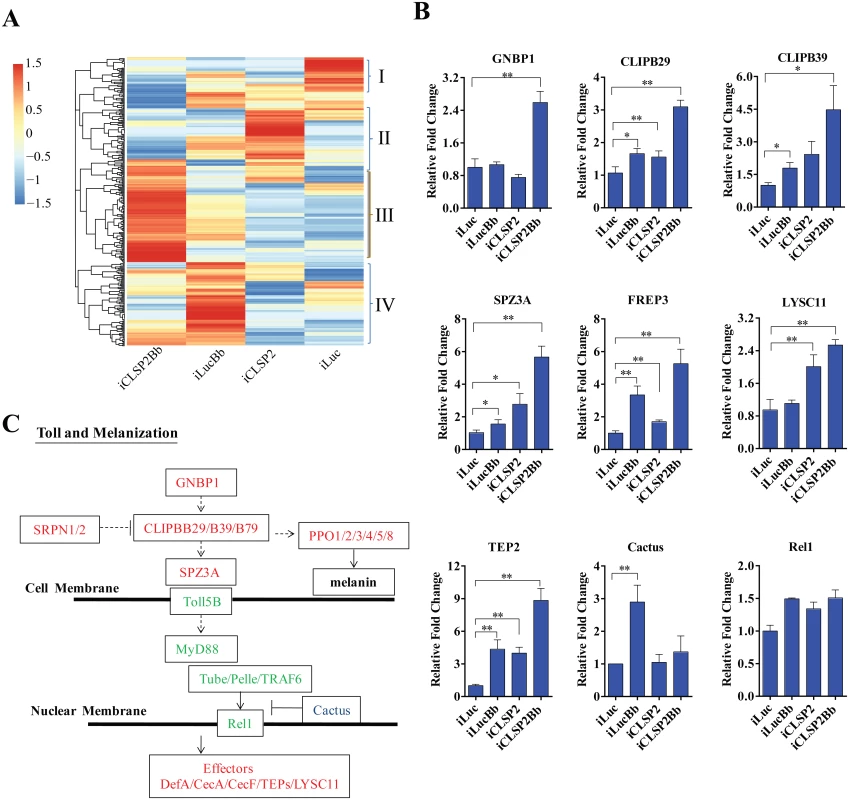
A) Hierarchical cluster analysis of gene transcripts in mosquito tissue (abdominal carcasses) after four different treatments: iLuc, iCLSP2, iCLSP2Bb, and iLucBb (left panel). Clusters I-IV represent gene cohorts that are specifically up-regulated in iLuc (Cluster I), iCLSP2 (Cluster II), iCLSP2Bb (Cluster III) and iLucBb (Cluster IV). Hierarchical cluster analysis of Cluster III immunity-related genes up-regulated in iCLSP2Bb mosquitoes is shown in S2 Fig. Transcript abundance of each gene was calculated using the RSEM software package (Trinity) and heat maps generated by R software. The color scale indicates the abundance deviation from the median for each gene. The list of up-regulated genes in each group was shown in S1–S3 Tables. B) Real-time RT-PCR validation of transcript levels of selected immune genes. Data were normalized to the expression level of iLuc. Data were shown as mean ± SEM. * p < 0.05; ** p < 0.01. iLucBb, iLuc mosquitoes infected with B. bassiana (24 h post infection); iCLSP2, CLSP2 dsRNA-treated mosquitoes infected with B. bassiana (24 h post infection); iCLSP2, mosquitoes injected with CLSP2 dsRNA. Control group (iLuc) was injected with luciferase dsRNA. C) A schematic diagram of the Toll and melanization pathways. Red represents genes up-regulated in iCLSP2Bb (ratios ≥ 1.5 fold), while those that were not significantly affected are shown in green. Cactus, which inhibited in iCLSP2Bb, is in blue. Serine proteases (SPs) play important roles in a wide range of biological processes, including innate immunity. They constitute an integral part of immune reactions, such as the Toll and melanization cascades in arthropods [7,14]. There were 40 CLIPs (almost half of Ae. aegypti genome CLIPs) in the iCLSP2Bb upregulated transcriptome (S3 Table). A high elevation of expression levels of several immune genes in iCLSP2Bb mosquitoes was confirmed by means of the qPCR analysis (Fig 2B and S2E Fig). Our previous study has shown that CLIPB5 and CLIPB29 are involved in the activation of Toll pathway by fungal infection or by infection-independent manner, respectively [15]. Indeed, we found that both CLIPB5 and CLIPB29 were moderately up-regulated in iCLSP2Bb (S3 Table). Two Toll pathway regulators—Spz2 and Spz3A—were also dramatically up-regulated in the iCLSP2Bb mosquitoes (Fig 2A and 2B and S2E Fig). Moreover, the gene encoding the pattern recognition receptor GNBP1 was also significantly activated in iCLSP2Bb mosquitoes. Thus, our results have shown that CLSP2 modulates the transcriptional expression of the Toll pathway upstream genes (Fig 2C). However, the expression of genes encoding intracellular components of the intracellular Toll pathway signaling, including Rel1, was not significantly affected in these mosquitoes (Fig 2B and 2C). Interestingly, Cactus was elevated as a result of B. bassiana infection, however its transcript was reduced in iCLSP2Bb (Fig 2B).
Genes encoding pattern recognition receptors from the fibrinogen-related protein family (FREP) represented another highly elevated group of genes in the iCLSP2Bb mosquitoes (Fig 2B and S2E Fig, S3 and S4 Tables). FREP3, FREP5 and FREP10 were particularly up-regulated. The FREPs are an evolutionarily conserved immune gene family found in mammals and invertebrates [20]. It is the largest pattern recognition receptor gene family in mosquitoes, with 59 putative members in An. gambiae [20] and 35 in Ae. aegypti [16]. Genes encoding thioester-containing proteins, TEP2, TEP3 and TEP22 were also up-regulated. These data suggest that CLSP2 is an immune factor working upstream of the pattern-recognition receptor system, modulating their responses.
We then studied the effect of CLSP2 on mRNA levels of anti-microbial effector peptides (AMPs). Defensin A (DefA) and Cecropin A (CecA) represent the major mosquito AMPs [16], which also convey anti-Plasmodium activity [21]. As shown using qPCR, the mRNA levels of AMP genes, DefA, CecA, CecE, and CecF were induced in B. bassiana infected mosquitoes at 20 - to 40-fold levels. Impressively, much higher induction levels of DefA, CecA, CecE, and CecF were observed in the iCLSP2Bb mosquitoes (Fig 3A). Thus, the CLSP2 played essential role in systemic immunity in mosquitoes by preventing the spontaneous transcription activation of downstream AMP immune genes. Next, we investigated interrelationship of CLSP2 and Rel1, which is a factor directly controlling the expression of AMP genes. As expected, the high expression of DefA and CecA brought by iCLSP2 was significantly decreased in mosquitoes with double CLSP2 and Rel1 RNAi silencing (Fig 3B). Moreover, the extremely high level of AMP expression in iCLSP2Bb mosquitoes was almost completely eliminated in mosquitoes with double knockdown of CLPS2Bb and Rel1 (Fig 3C). These experiments indicate that the action of CLSP2 due to modulation of upstream regulatory factors of the Toll immune cascade. According to the result from our RNA-seq analysis and qPCR, the induced immune genes belong mainly to the Toll pathway (Fig 2C), whereas the genes involved in the IMD and JAK/STAT pathways were not affected by the depletion of CLSP2 (S3A Fig). The only exception is PGRP-LC, which was influenced by CLSP2 and surprisingly by fungal challenge (S3B Fig). However, unlike GNBP1, it was not up-regulated the in iCLSP2Bb mosquitoes, thus pointing to the lack of modulation of this gene encoding the IMD pattern-recognition receptor by CLSP2. Moreover, CLSP2 had no effect on expression of the Rel2 gene, the principal regulator of the IMD pathway (S3A and S3B Fig). Toll and IMD share regulation of AMPs [22]. However, our experiments strongly suggest that CLSP2 effect on expression of the AMP genes is likely solely due to its influence on the Toll pathway.
Fig. 3. The effect of CLSP2 on the expression of AMPs. 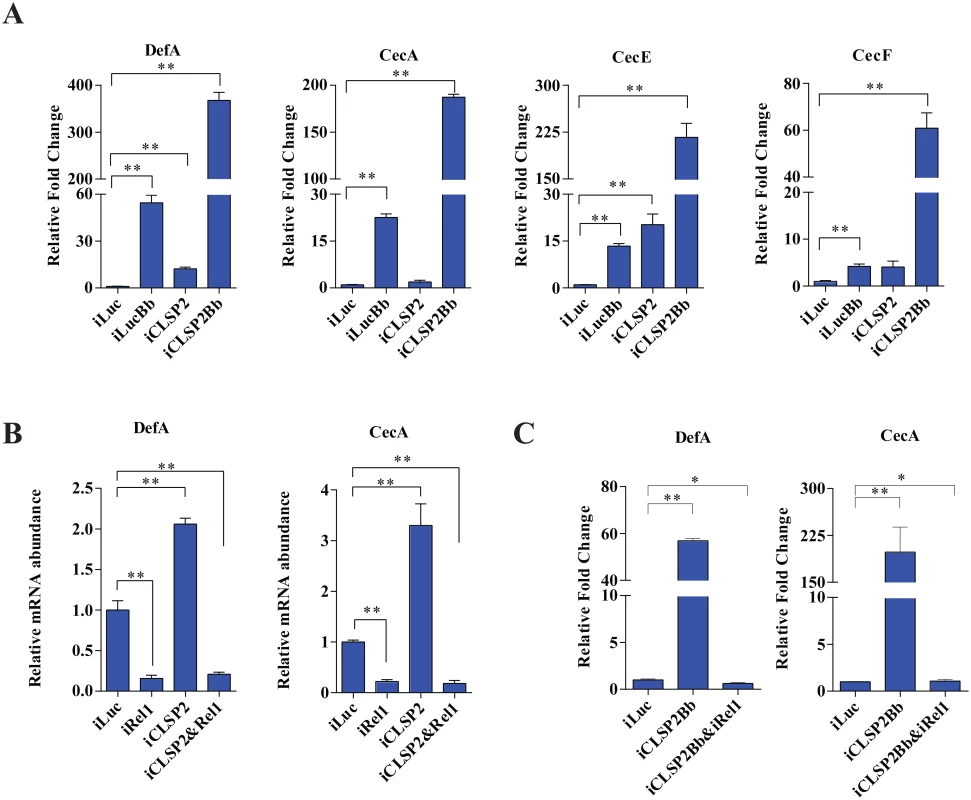
A) The mRNA levels of DefA, CecA, CecE, and CecF were determined using real-time RT-PCR. Data are presented as mean ± SEM. *, p < 0.05; **, p < 0.01. The experiments were repeated four times. Data were normalized to the expression level of iLuc. iLucBb, iLuc-depleted mosquitoes infected with B. bassiana; iCLSP2Bb, CLSP2 RNAi depleted mosquitoes infected with B. bassiana; iCLSP2, CLSP2 RNAi depleted mosquitoes; iLuc, luciferase RNAi-treated control mosquitoes. B-C) The effect of double-knockdown of CLSP2 and Rel1 on the expression of AMPs (B) and the response to fungal challenge (C). The mRNA levels of DefA, CecA were determined using qRT-PCR. Data are presented as mean ± SEM. **, p < 0.01. Data were normalized to the expression level of iLuc. iCLSP2, mosquitoes treated with CLSP2 dsRNA; iRel1, mosquitoes treated with Rel1 dsRNA; iCLSP2&Rel1, mosquitoes treated with CLSP2 and Rel1 dsRNA; iLuc, luciferase RNAi-treated control mosquitoes. iCLSP2Bb&Rel1 and iCLSP2Bb, CLSP2 and Rel1 RNAi depleted mosquitoes infected with B. bassiana. We next selected up-regulated gene cohorts from mosquitoes after three different treatments—iCLSP2 (72 genes), iLucBb (93 genes) and iCLSP2Bb (108 genes) for further analysis. Forty immune genes were induced under all three experimental conditions (Fig 4A and S4 Table). The ontology analysis demonstrated that, except for several effector genes, the majority of co-upregulated genes belonged to regulatory categories located upstream of immune cascades (Fig 4B and S4 Table). The hierarchical clustering indicated that transcript levels of most of these genes are considerably higher in iCLSP2Bb than in the other two groups (Fig 4B). These results further suggest that CLSP2 is the modulator of the immune response involved in the anti-fungal infection.
Fig. 4. Comparative transcriptome analysis of CLSP2 modulation of immune genes and the role of TEP22 in anti-fungal defense. 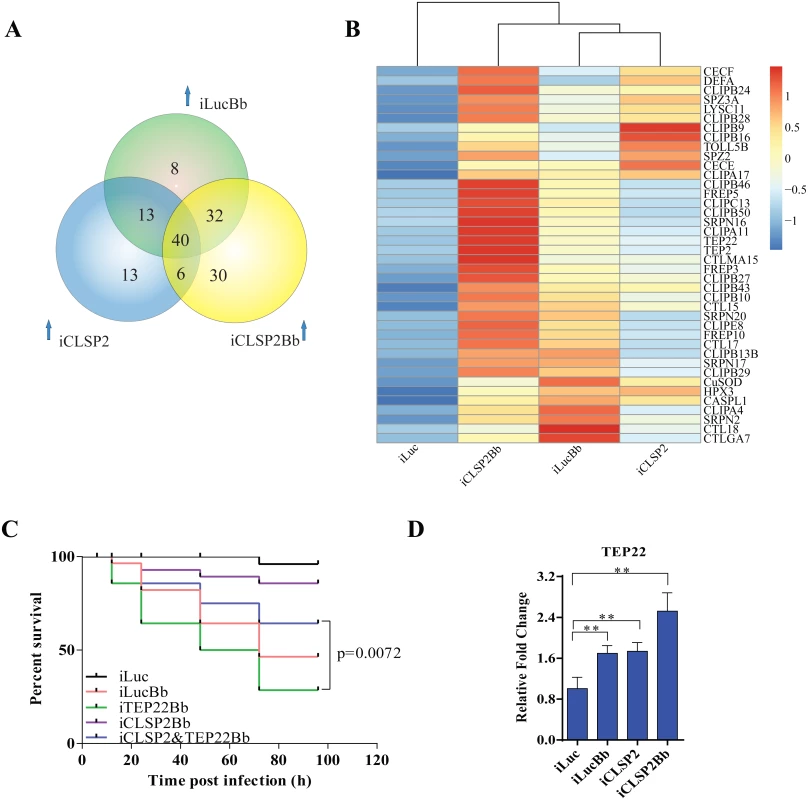
A) Venn diagram representing unique and shared up-regulated immune genes (1.5 fold changes compared with control) in iLucBb, iCLSP2, and iCLSP2Bb mosquitoes. B) Expression profiles of immune genes that are concurrently up-regulated in iLucBb, iCLSP2, and iCLSP2Bb mosquitoes. Transcript abundance of each gene was calculated using the RSEM software package (Trinity) and heat maps generated by R software. The color scale indicates the abundance deviation from the median for each gene. Expression levels of common up-regulated genes are also shown in S4 Table. C) The survival rate of mosquitoes showed that concomitant depletion of TEP22 and CLSP2 (iCLSP2&iTEP22Bb) enhanced the capacity of mosquitoes to defend B. bassiana (p < 0.01) compared with single depletions of iTEP22Bb, or control iLucBb. Each experiment was performed in three replicates. Green line—iTEP22Bb, TEP22 dsRNA-treated mosquitoes infected with B. bassiana; purple line—double knockdown iCLSP2&TEP22 mosquitoes infected with B. bassiana; blue line—iCLSP2 mosquitoes injected with B. bassiana conidia; red line—iLucBb, iLuc mosquitoes injected with the fungus; black line—iLuc control mosquitoes. D) mRNA abundance of TEP22 was up-regulated in iCLSP2Bb mosquitoes. Normalized expression level of iLuc was shown. Data were shown as mean ± SEM. * p < 0.05; ** p < 0.01. iLucBb, iLuc mosquitoes infected with B. bassiana (24 h post infection); iCLSP2, CLSP2 dsRNA-treated mosquitoes infected with B. bassiana (24 h post infection); iCLSP2, mosquitoes injected with CLSP2 dsRNA. Control group (iLuc) was injected with luciferase dsRNA. Thioester-containing protein (TEP22) is involved in the CLSP2-modulated mosquito antifungal defense
Our analysis suggests that the modulating factor CLSP2 acts upstream of immune cascades, possibly interacting with other factors. To explore this possibility, we analyzed eight genes selected from those co-up-regulated in iLucBb, iCLSP2 and iCLSP2Bb mosquitoes (S5 Table), and their functions were studied by means of RNAi depletions in a combination with B. bassiana infection. After treatment with TEP22 dsRNA, a member of α2-macroglobulin family (S4 Fig), mosquitoes became extremely sensitive to the B. bassiana infection, and the survival rate dramatically decreased. However, the survival of affected mosquitoes could be partially rescued after the knockdown of CLSP2 was performed simultaneously with that of TEP22 (Fig 4C). Additionally, TEP22 was significantly regulated in iCLSP2Bb mosquitoes (Fig 4D). The results indicate that TEP22 is required for the anti-fungal response in a mosquito. Moreover, these results suggested that CLSP2 is likely mediated the response to fungal infection via interaction with TEP22 as a recognition molecule. Seven other tested genes from the iCLSP2Bb did not yield a similar phenotype indicating that they were not involved in the CLSP2 immune modulation directly (S5 Fig).
CLSP2 differentially modulates the transcription of PPO genes
CLSP2 has been shown to be a negative modulator of hemolymph melanization [19]. To examine whether CLSP2 was involved in regulation of PPO gene expression, we utilized CLSP2 RNAi silencing in combination with B. bassiana infection (Fig 5A). The RNA abundance of 10 Aedes PPOs was investigated by means of qPCR analysis. Whereas transcript abundance of PPO genes did not change significantly after infection with B. bassiana alone, a highly pronounced activation of several PPO genes was observed in the iCLSP2Bb mosquitoes. PPO1 transcript increased dramatically by 9-fold, while levels of PPO2, PPO3, PPO4, PPO5 and PPO8 were elevated to about 4 - to 6 - fold. These results suggest that CLSP2 is an essential modulator of PPO gene expression. Moreover, this modulation is highly specific to just a few PPO genes that are most likely involved in immune responses during fungal infection. In addition to PPO genes, according to the results of RNA-seq, we also found that transcripts of several genes involved in melanization were up-regulated in iCLSP2Bb mosquitoes, including CLIPB9 and SRPN2 (S3 Table). The elevation of these gene transcripts in iCLSP2Bb was confirmed by qPCR (S2E Fig).
Fig. 5. The effect of CLSP2 on transcriptional activation of PPO genes. 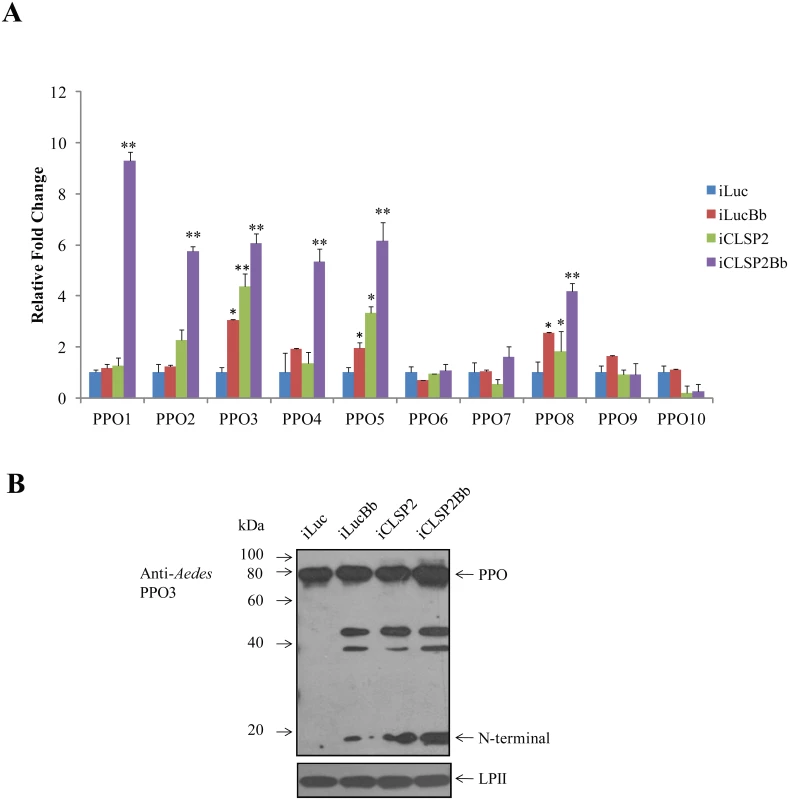
A) The effect of CLSP2 on the expression of 10 PPO genes in the mosquito carcass. The mRNA levels of PPOs were determined using real-time RT-PCR. Data were represented as mean ± SEM. *, p < 0.05; **, p < 0.01. The experiments were repeated four times. iLucBb, iLuc mosquitoes injected with B. bassiana; iCLSP2Bb, CLSP2 dsRNA-treated mosquitoes injected with B. bassiana; iCLSP2, mosquitoes injected with CLSP2 dsRNA; iLuc, luciferase RNAi-treated control mosquitoes. B) Immunoblot analysis of hemolymph PPO cleavage. Hemolymph was isolated at 24 h post-infection from four groups (iLuc, iLucBb, iCLSP2, and iCLSP2Bb, see Material and Methods) of mosquitoes, separated by SDS-PAGE, and then probed with Ae. aegypti PPO3 polyclonal antibodies. A ~20-kDa band appeared in samples of iLucBb, iCLSP2 and iCLSP2Bb. Ae. aegypti Lipophorin II was used as the loading control. iLucBb, iLuc mosquitoes infected with B. bassiana; iCLSP2Bb, CLSP2 dsRNA-treated mosquitoes infected with B. bassiana; iCLSP2, mosquitoes treated with CLSP2 dsRNA; iLuc, luciferase dsRNA treated control mosquitoes. We selected PPO3 for further protein analysis, because its gene transcript was elevated in response to the fungal infection in iLucBb and was also highly upregulated in the iCLSP2Bb mosquitoes. Proteolytic cleavage of hemolymph PPO3 was detected by immunoblotting using polyclonal antibodies against Aedes PPO3. There was only a precursor PPO band in the iLuc control mosquitoes (Fig 5B). However, it was cleaved in the hemolymph of B. bassiana-infected and CLSP2-silenced mosquitoes as marked by the appearance of around 20-kDa-protein band (Fig 5B). The PPO3-derived cleavage proteins were also observed in iCLSP2Bb mosquitoes. At present we cannot assume that CLSP2 is responsible for cleavage of only PPO3 as we lack specific antibodies to other PPOs to investigate this question. The cleavage of PPO is likely not a direct effect of CPLS2 and occurs as a consequence of activation of melanization pathway factors by iCPLS2 and/or infection with B. bassiana as shown above. However, this experiment demonstrates importance of CLSP2 as a modulating factor working upstream in the PPO cascade.
Discussion
Innate immune responses are initiated by the interaction between pathogen surface molecules and pathogen-related receptors (PRRs). C-type lectin recognition receptors (CTL or CTR) comprise a large family of PRRs that are engaged in the recognition of a broad spectrum of pathogens. They are also defined as Ca2+—dependent carbohydrate (lectin) binding proteins identified in a wide range of animal groups [23]. CTLs interact with glycans on cell surfaces, in the extracellular matrix, or on soluble secreted glycoproteins, mediating processes such as cell adhesion, cell-cell interactions and pathogen recognition [23].
CTLs have been implicated in pathogen evasion of a host immune system. In mammals, a large family of C-type lectin receptors modulating immune responses has been characterized[24]. Two CTLs (CTL4 and CTLMA2) have been shown to act as protective agonists during the development of Plasmodium ookinetes to oocysts in the mosquito An. gambiae [25]. Similarly, another C-type lectin, mosGCTL-1, an equivalent to CTLMA15, was found to facilitate the infection of West Nile virus in the mosquito Ae. aegypti [26]. Although CTLs have been identified as negative regulators of the immune response against malaria parasites and virus [25,26], details of mosquito CTL-based pathways are still unknown. Mosquitoes with the CLSP2 RNAi depletion displayed an elevated resistance to B. bassiana infection as compared to those with pathogens alone. Previously, we have also demonstrated that CLSP2 also functions during Plasmodium infection [17]. Thus, our study has uncovered an important role of CLSP2 as a factor modulating immune responses in the mosquito Ae. aegypti.
Ae. aegypti CLSP2 is a composite protein consisting of an elastase-like SP (ESP) domain located at the N-terminal and a CTL-type domain at the C-terminal. Composite immune proteases, such as Manduca sexta HP14 and the factor C of the horseshoe crab Tachypleus tridentatus, undergo cleavage after immune challenge [27,28]. Our experiments have shown that CLSP2 is also cleaved upon challenge with B. bassiana. Two bands were detected corresponding to the 14-kDa lectin and 33-kDa ESP domains in the hemolymph samples of mosquitoes with fungal infection by means of immunoblot analysis utilizing anti-CLSP2 antibodies. We have provided two lines of evidence clearly showing that the CLSP2 CTL-type domain binds to fungal sugar cell components. The purified recombinant CTL-type domain (rLectin) agglutinates zymosan and B. bassiana conidia in a calcium-dependent manner. Moreover, ELISA has shown that rLectin directly binds to the polysaccharide fungal cell component, curdlan, and this binding is saturable. However, whether the CTL domain binding to fungal surface molecules occurred before or after the CLSP2 cleavage in the hemolymph could not be determined. It also remains to be clarified whether upon infection-induced cleavage the CLSP2 domains undergo conformational changes still remaining as a single molecule in its native state or yielding completely separate molecules corresponding to the C-Type Lectin and SP domains.
Our study of the CLSP2-mediated immune activation using RNAseq-based transcriptome analysis further supports a hypothesis that CLSP2 is a modulator of the transcription responses involved in innate immunity and suggests that CLSP2 acts upstream of extracellular pathogen-recognition factors. CLSP2 depletion affected genes encoding FREP pattern recognition receptors and TEPs indicating that CLSP2 is an immune factor working upstream of the pattern-recognition receptor system, modulating their responses.
We identified TEP22 as an important player of the anti-fungal response in Aedes mosquitoes. TEPs are immune effectors genes that are conserved from insects to mammals. TEP molecules contain a motif harboring an intra-chain β-cysteinyl-γ-glutamyl thioester bond, which binds to target surfaces and prompts a series of complement cascades against microbes and parasites [29]. Knockdown of AgTEP1 in the resistant strain of An. gambiae led to a massive increase in the number of Plasmodium oocysts [30]. AgTEP1 is essential for blocking oocyst development in the midgut of An. gambiae by forming complex with two proteins from leucine-rich repeat family, LRIM1 and APL1C [31,32,33]. Nine TEP genes have been identified in the Ae. aegypti genome [16]. Our phylogenetic analysis has shown that Aedes TEP20, 22, 23, and 25 form an independent clade supported by high bootstrap value (S4 Fig). Transcript levels of TEP20, 22, and 23 were elevated in the fat body Rel1 and Rel2 gain-of-function transgenic mosquitoes and also in response to the Plasmodium infection [22]. We have shown in the present study that the TEP22 expression is dramatically elevated in iCLSP2Bb mosquitoes. Furthermore, TEP22-depeleted mosquitoes are extremely sensitive to B. bassiana infection, while CLSP2 knockdown in these mosquitoes rescues their survival. Thus, our findings suggest that TEP22 is involved in the antifungal immune pathway and it could interact with CLSP2 in this immune response. This interaction would be reminiscent of the mannose-binding lectin (MBL) triggered complement activation in mammals [34] or TEP1/LRIM1/APL1C complex in An. gambiae [30–33]. However, the detailed mechanism of complement-like factor action in the anti-fungal immunity and TEP22 association with the immune modulating factor CLSP2 requires further mechanistic study.
Our study has demonstrated that the intracellular signal transduction components of the Toll pathway are not regulated by CLSP2 at the transcriptional level. In vertebrates, inhibitory receptor systems modulating immune responses depend on the intracellular phosphorylation pathway and not regulation at the transcription level [4,5]. Similar mechanism is also identified in the negative regulation of Toll-like receptor mediated pathways [35,36]. Interestingly, we observed that the activation of Cactus, the Rel1 inhibitor in Toll-mediated infection [37], brought by fungal infection was abolished by the RNAi depletion of CLSP2. This iCLSP2 effect on Cactus is completely opposite from those on other immune genes. Although Cactus target Rel1 is not affected by CLSP2, the downstream gene cohorts highly activated in the iCLSP2Bb mosquitoes include those encoded effector molecules such as AMPs. The unique interaction of CLSP2 with Cactus suggests that it contributes in the control of AMP gene activation. Moreover, the abolishment of activation of AMPs, brought by iCLSP2 by the double knockdown of CLSP2 and Rel1, indicates that Rel1 mediates the action of CLSP2 on these immune genes.
We also have uncovered the CLSP2 role in modulating the melanization pathway in Ae. aegypti. represents a second immune pathway that is essential in the systemic antifungal immune responses [17]. CLSP2 not only modulates the hemolymph activation of PPO, but also negatively regulates the expression of PPO genes. The melanization cascade is tightly regulated by serine protease inhibitors (SRPNs), which prevent spontaneous initiation of the reaction. The analysis of the mosquito genomes has shown that genes encoding immune signaling and effector molecules, and the number of melanization pathway genes have undergone major expansion [16]. For example, there are 10 PPO genes in the Ae. aegypti genome [13]. However, the precise roles of each PPO in melanization process are poorly understood. Our previous study revealed a novel level of complexity in the melanization cascade of the mosquito Ae. aegypti. Namely, we identified that there are several independent pathways leading to melanization, each requiring a different protease/SRPN regulatory module [15]. Of particular interest is a clear separation of tissue melanization, represented by melanin tumors often associated with the damage of host tissues, and immune melanization involved in the recognition and killing of pathogens, including fungi [13]. The melanization response has also been shown to significantly retard the growth and dissemination of B. bassiana in the An. gambiae mosquito [18].
Multicellular organisms have evolved complex and powerful systems of immune responses to counteract continuous attacks of various pathogens. An essential feature of the immune system in any organism is its capacity to sustain equilibrium between reactivity and quiescence [4]. A loss of such a balance leads to severe consequences, such as autoimmune and inflammatory diseases in humans. Inhibitory receptor systems balancing immune responses have been identified in vertebrates [4,5]. Our study has revealed that CLSP2 functions as a key modulator of the mosquito immune system and contributes to a better understanding of immune mechanisms in insects.
Materials and Methods
Experimental animals
The UGAL strain of Ae. aegypti mosquitoes was maintained in the laboratory as described previously [38]. Adults were fed continuously on water and 10% sucrose solution. To initiate egg development, mosquitoes were blood fed on chickens. All procedures for using vertebrate animals were approved by the Institute of Zoology Animal Care and Use Committee.
Fungal culture and septic injury
B. bassiana strain ARSEF 2680 and B. bassiana strain expressed GFP were cultured on potato dextrose agar plates at 25°C and 80% humidity [39]. B. bassiana strain ARSEF 2680 was used in immune challenge and the strain 252-GFP was used in the agglutination assay. Conidia (fungal spores), used for mosquito challenge were harvested from 3 - to 4-week-old cultures and diluted to 5×107 conidia/ml in PBS. Septic injures were carried out by pricking the rear part of the mosquito abdomen with an acupuncture needle dipped into fungal conidia suspension [6].
For the immune response of CLSP2 to fungal infection, 3 days old adult mosquitoes were divided into two groups (30 adults / group): the control group (control) was challenged with PBS; the experiment group (Bb 24h) with B. bassiana spores. Tissue samples were collected 24 h later.
For the RNA-seq, immune genes expression and survival rate analysis, new emergence mosquitoes were divided into four groups (30 adults / group): two groups (luciferase groups) were injected with luciferase dsRNA; another two groups (CLSP2 groups) were injected with CLSP2 dsRNA. 3 days later, one of the luciferase groups were challenged with PBS (iLuc), and the other one were challenged with B. bassiana spores (iLucBb). One of the CLSP2 groups was challenged with PBS (iCLSP2), and the other one with B. bassiana spores (iCLSP2Bb). The same treatments were also used in the survival rate analysis of TEP22 and other immune genes.
Synthesis and micro-injection of dsRNA
cDNA templates of target genes were generated by means of RT-PCR using both sense and antisense primers fused with T7-phage promoter sequences. RT-PCR was performed using the cDNA samples as templates to generate 400-bp to 1-kb gene-specific cDNA fragments. Synthesis of dsRNA was accomplished by simultaneous transcription of both strands of template DNA using T7 RNA polymerase from the T7 RiboMAX Express RNAi kit (Promega). The luciferase gene was used to generate control iLuc dsRNA. A Nanoliter 2000 injector (World Precision Instrument) was used to introduce corresponding dsRNA into the thorax of CO2-anesthetized mosquito females within 1 day post-eclosion. Primers used for generating dsRNA are listed in S6 Table. The transcripts of specific genes decreased to 50–70% 1 week after dsRNA injection, confirmed by real-time RT-PCR.
Survival analysis
At 3 days after eclosion, 30 female mosquitoes were challenged with B. bassiana conidia [6]. The mosquitoes were maintained in individual containers and fed continuously on water and 10% sucrose solution. The survival curves were compared using Kaplan-Meier, and the threshold of p value was calculated with a Log-rank or Mantel Cox test, and p < 0.01 were considered to be statistically significant. Graphpad 6.0 software was used in all statistical analyses.
Immunoblot analysis
Hemolymph from 20 decapitated mosquitoes was collected into 20 μl of 1×protease inhibitor cocktail (Roche) by centrifugation at 5,000 rpm for 5 min with Qiashredder column (QIAGEN) [15]. Aliquots of hemolymph samples were resolved on 4–15% gradients SDS-polyacrylamide gels (Bio-Rad) and electrotransferred to PVDF membranes (Invitrogen). After blocking, the membranes were incubated with the primary antibody against CLSP2 or PPO3 overnight at 4°C. We used polyclonal antibodies against Ae. aegypti Lipophorin II [15,40] and β-actin (Sigma) as the loading controls. Immune complexes were visualized by means of SuperSignal West Pico Substrate (Pierce).
Preparation of rLectin and antibody preparation
rLectin (Lectin domain of CLSP2) was amplified by RT-PCR from cDNA with specific primers (S6 Table). The PCR product was subcloned into PSFM (a kind gift from Dr. Haobo Jiang, Oklahoma State University), a vector with a Sumo at the N-terminal, which increases the solubility of the fusion protein and can be removed by SUMO protease afterwards. The N-terminal FLAG and the C-terminal Myc are short sequences for detection of the expression of fusion protein and its cleavage products using commercially available monoclonal antibodies against these two tags, respectively. SUMO-rLectin was first purified on a Ni-NTA (nickel-nitrilotriacetic acid, Qiagen) agarose column. Then, SUMO-rLectin was cleaved using SUMO protease, as per the manufacturer’s protocol (GeneCopoeia), and re-purified on the Ni-NTA agarose column. Monoclonal antibodies were prepared against KLH-peptide from CLSP2 (Beijing Protein Innovation). Polyclonal antibodies were prepared against recombinant CLSP2 and recombinant PPO3 (Beijing Protein Innovation). Specificity tests of these antibodies are presented in S1 Fig.
Agglutination and binding analysis of rLectin
FITC-conjugated zymosan (Molecular Probes) or GFP-conjugated B. bassiana conidia suspended in Tris-buffered saline (TBS) (25 mM Tris-HCl, 137 mM NaCl and 3 mM KCl, pH 7.0) were incubated with purified rLectin (80 μg/ml) for the agglutination assay, as described by Yu et al. [41]. After incubation for 45 min at RT, samples were examined using fluorescence confocal microscopy (Zeiss 710).
For binding assay, wells of a flat-bottom, 96-well plate (Nunc, Fisher Scientific) were coated with 2 mg (50 μl of 40 mg/ml per well) of curdlan (Sigma) as described [41]. The plate was then blocked with BSA (100 μl/well of 1 mg/ml) for 2 h at 37°C and rinsed with binding buffer (50 mM Tris-HCl, 50 mM NaCl, pH 8.0) (200 μl/well). rLectin diluted with binding buffer containing 5 mM CaCl2 and 0.1 mg/ml BSA was adjusted to 50 μl/well binding at RT for 4 h. The plates were rinsed as before, and bound rLectin was measured using mouse anti-C-myc antibody (1 : 1000), and horseradish peroxidase (HRP) conjugated antibodies against mouse. The pre-immune antiserum was used as control. Soluble TMB Substrate Solution (100 μl/well, Tiangen) was added to react for 20 min, and then stopped with 8.5 M acetic acid. Absorbance at 450 nm of the samples in each well was determined using a microplate reader (Molecular Devices).
RNA-sequencing transcriptome experiments
To investigate the immune response to B. bassiana infection in the mosquito Ae. aegypti, we used a high-throughput sequencing (HTS) platform (HiSeq 2000) to analyze gene expression in carcasses of fungal infected mosquitoes. Four fat body libraries were built from iLucBb, iCLSP2, iCLSP2Bb, and iLuc mosquitoes. Three replicates of each sample (25 mosquitoes/sample) were pooled for analysis and 100 ng of total mRNA from each sample was used to construct libraries with an Illumina kit v2.
Bioinformatics analysis
Raw reads generated from the sequencing were preprocessed using in-house perl scripts, including adaptor removing and low quality reads filtering. Those with average quality lower than 20 and read length shorter than 35 bp were discarded automatically. To minimize the sequencing noise from other species, we mapped the filtered reads against both the bacteria and virus databases in NCBI, and made sure the remainder was highly reliable. The genome of Ae. aegypti was downloaded from VectorBase (https://classic.vectorbase.org/genomes), as was the annotation file. The clean reads were mapped to the genome using GSNAP to estimate the expression level of all of the transcripts [42]: three mismatches were tolerated during processing, and the parameter of new transcript finding was shut down to guarantee the precise matching. We used flux-capacitor to calculate the FPKM of the transcripts, and DEGseq package in R Scripts to determine the DEGs [43]. P values less than 0.05 indicated genes were differentially expressed. All immune genes were then assigned according to immunodb [16]. Hierarchical clustering of gene expression intensity was performed using Pearson distance as the distance measure between genes and libraries [44]. Cross comparison performing within each treated sample was normalized by their reads count (FPKM), and iLuc sample was considered as background value while the ratio of fold change was calculated. Phylogenetic trees were constructed using MEGA6 by the neighbor-joining method [45].
RNA preparation, RT-PCR, and real-time RT-PCR analysis
Total RNA samples were prepared from dissected abdominal carcasses of 10–15 individual mosquitoes. Malpighian tubules, midguts, and ovaries were removed, then abdominal carcasses with adhered fat body tissue and sessile hemocytes were rinsed in PBS, transferred into TRI reagent (Sigma), and homogenized using a motor-driven pellet pestle mixer (Kontes, Vineland, NJ). A 2-μg sample of total RNA was treated with DNase I (Invitrogen) to remove contaminating genomic DNA, and then used for cDNA synthesis (M-MLV reverse transcriptase kit, Promega). Actin was used as an internal standard to normalize the templates in a preliminary PCR experiment. After template adjustment, PCRs were performed to detect relative levels using specific primers. Primers were designed by software Primer5. Real-time RT-PCR (qPCR) reaction was performed on the MX3000P system (Stratagene, CA), and we used a SYBR green PCR Master Mix (Tiangen, Beijing) for these reactions. Thermal cycling conditions were: 94°C, 20 s; 59°C, 20 s; 68°C 20 s. Quantitative measurements were performed in triplicate and normalized to the internal control of S6 ribosomal protein mRNA for each sample. Primers and gene accession numbers are listed in S6 and S7 Tables, online. Real-time RT-PCR data were collected and exported to EXCEL for analysis. Values were represented as the mean ± SEM, and the statistically significant difference between samples was calculated using the Student-t test (Graphpad 6.0).
Supporting Information
Zdroje
1. Attardo GM, Hansen IA, Raikhel AS (2005) Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochemistry and Molecular Biology 35 : 661–675. 15894184
2. Ramirez JL, Garver LS, Dimopoulos G (2009) Challenges and approaches for mosquito targeted malaria control. Curr Mol Med 9 : 116–130. 19275622
3. Fang W, Azimzadeh P, Leger RJ St (2012) Strain improvement of fungal insecticides for controlling insect pests and vector-borne diseases. Curr Opin Microbiol 15 : 232–238. doi: 10.1016/j.mib.2011.12.012 22245564
4. Ravetch JV, Lanier LL (2000) Immune inhibitory receptors. Science 290 : 84–89. 11021804
5. Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, et al. (1997) Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell 90 : 293–301. 9244303
6. Shin SW, Kokoza V, Bian G, Cheon HM, Kim YJ, et al. (2005) REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem 280 : 16499–16507. 15722339
7. Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25 : 697–743. 17201680
8. Tanaka H, Ishibashi J, Fujita K, Nakajima Y, Sagisaka A, et al. (2008) A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem Mol Biol 38 : 1087–1110. doi: 10.1016/j.ibmb.2008.09.001 18835443
9. Roh KB, Kim CH, Lee H, Kwon HM, Park JW, et al. (2009) Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J Biol Chem 284 : 19474–19481. doi: 10.1074/jbc.M109.007419 19473968
10. Ferrandon D, Imler JL, Hetru C, Hoffmann JA (2007) The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7 : 862–874. 17948019
11. Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, et al. (2006) Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 127 : 1425–1437. 17190605
12. Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM (2002) Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297 : 114–116. 12098703
13. Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, et al. (2006) A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell 10 : 45–55. 16399077
14. Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA (1996) The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell 86 : 973–983. 8808632
15. Zou Z, Shin SW, Alvarez KS, Kokoza V, Raikhel AS (2010) Distinct melanization pathways in the mosquito Aedes aegypti. Immunity 32 : 41–53. doi: 10.1016/j.immuni.2009.11.011 20152169
16. Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, et al. (2007) Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316 : 1738–1743. 17588928
17. Kanost MR, Jiang H, Yu XQ (2004) Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev 198 : 97–105. 15199957
18. Yassine H, Kamareddine L, Osta MA (2012) The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog 8: e1003029. doi: 10.1371/journal.ppat.1003029 23166497
19. Shin SW, Zou Z, Raikhel AS (2011) A new factor in the Aedes aegypti immune response: CLSP2 modulates melanization. EMBO Rep 12 : 938–943. doi: 10.1038/embor.2011.130 21760616
20. Dong Y, Dimopoulos G (2009) Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J Biol Chem 284 : 9835–9844. doi: 10.1074/jbc.M807084200 19193639
21. Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, et al. (2010) Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proceedings of the National Academy of Sciences 107 : 8111–8116. doi: 10.1073/pnas.1003056107 20385844
22. Zou Z, Souza-Neto J, Xi Z, Kokoza V, Shin SW, et al. (2011) Transcriptome analysis of Aedes aegypti transgenic mosquitoes with altered immunity. PLoS Pathog 7: e1002394. doi: 10.1371/journal.ppat.1002394 22114564
23. Fujita T, Matsushita M, Endo Y (2004) The lectin-complement pathway—its role in innate immunity and evolution. Immunol Rev 198 : 185–202. 15199963
24. Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, et al. (2013) C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39 : 324–334. doi: 10.1016/j.immuni.2013.05.017 23911656
25. Osta MA, Christophides GK, Kafatos FC (2004) Effects of mosquito genes on Plasmodium development. Science 303 : 2030–2032. 15044804
26. Cheng G, Cox J, Wang P, Krishnan MN, Dai J, et al. (2010) A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell 142 : 714–725. doi: 10.1016/j.cell.2010.07.038 20797779
27. Ji C, Wang Y, Guo X, Hartson S, Jiang H (2004) A pattern recognition serine proteinase triggers the prophenoloxidase activation cascade in the tobacco hornworm, Manduca sexta. J Biol Chem 279 : 34101–34106. 15190055
28. Jiang H, Kanost MR (2000) The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol 30 : 95–105. 10696585
29. Volohonsky G, Steinert S, Levashina EA (2010) Focusing on complement in the antiparasitic defense of mosquitoes. Trends Parasitol 26 : 1–3. doi: 10.1016/j.pt.2009.10.003 19853513
30. Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, et al. (2004) Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116 : 661–670. 15006349
31. Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, et al. (2009) Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5 : 273–284. doi: 10.1016/j.chom.2009.01.005 19286136
32. Riehle MM, Markianos K, Niare O, Xu J, Li J, et al. (2006) Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science 312 : 577–579. 16645095
33. Povelones M, Waterhouse RM, Kafatos FC, Christophides GK (2009) Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324 : 258–261. doi: 10.1126/science.1171400 19264986
34. Vasta GR, Quesenberry M, Ahmed H, O'Leary N (1999) C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev Comp Immunol 23 : 401–420. 10426431
35. Liew FY, Xu D, Brint EK, O'Neill LA (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5 : 446–458. 15928677
36. Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, et al. (2000) Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol 165 : 6682–6686. 11120784
37. De Gregorio E, Spellman PT, Rubin GM, Lemaitre B (2001) Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A 98 : 12590–12595. 11606746
38. Hays AR, Raikhel AS (1990) A Novel Protein Produced by the Vitellogenic Fat-Body and Accumulated in Mosquito Oocytes. Rouxs Archives of Developmental Biology 199 : 114–121.
39. Xiao G, Ying SH, Zheng P, Wang ZL, Zhang S, et al. (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep 2 : 483. doi: 10.1038/srep00483 22761991
40. Cheon HM, Shin SW, Bian G, Park JH, Raikhel AS (2006) Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aegypti. J Biol Chem 281 : 8426–8435. 16449228
41. Yu XQ, Kanost MR (2000) Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J Biol Chem 275 : 37373–37381. 10954704
42. Wu TD, Watanabe CK (2005) GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 21 : 1859–1875. 15728110
43. Wang L, Feng Z, Wang X, Zhang X (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26 : 136–138. doi: 10.1093/bioinformatics/btp612 19855105
44. Chen X, Hu Y, Zheng H, Cao L, Niu D, et al. (2012) Transcriptome comparison between honey bee queen - and worker-destined larvae. Insect Biochem Mol Biol 42 : 665–673. doi: 10.1016/j.ibmb.2012.05.004 22659440
45. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30 : 2725–2729. doi: 10.1093/molbev/mst197 24132122
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



