-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEndopeptidase-Mediated Beta Lactam Tolerance
Inhibition of bacterial cell wall synthesis by antibiotics such as penicillin can lead to unbalanced activity of a poorly defined set of lytic enzymes, termed ‘autolysins,’ which degrade the cell wall and typically cause cell lysis. Here, we report that in Vibrio cholerae (the cause of cholera), inhibition of cell wall synthesis results in the formation of viable spheres rather than cell lysis. Paradoxically, sphere formation requires the activity of cell wall degradative enzymes. Inhibition of cell wall synthesis in additional pathogens also leads to sphere formation. These findings expand our understanding of the cellular responses to cell wall acting antibiotics, demonstrating that cell wall degradative enzymes not only function as autolysins, but can also mediate cell survival in the face of cell wall insufficiency.
Published in the journal: . PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004850
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004850Summary
Inhibition of bacterial cell wall synthesis by antibiotics such as penicillin can lead to unbalanced activity of a poorly defined set of lytic enzymes, termed ‘autolysins,’ which degrade the cell wall and typically cause cell lysis. Here, we report that in Vibrio cholerae (the cause of cholera), inhibition of cell wall synthesis results in the formation of viable spheres rather than cell lysis. Paradoxically, sphere formation requires the activity of cell wall degradative enzymes. Inhibition of cell wall synthesis in additional pathogens also leads to sphere formation. These findings expand our understanding of the cellular responses to cell wall acting antibiotics, demonstrating that cell wall degradative enzymes not only function as autolysins, but can also mediate cell survival in the face of cell wall insufficiency.
Introduction
Nearly all bacteria are surrounded by a rigid cell wall, a structure that maintains cell shape and ensures cellular integrity in the face of potentially extreme osmotic stresses in the environment. The principal component of the cell wall is peptidoglycan (PG), a complex polymer that consists of a polysaccharide web with cross linked peptide sidechains found outside of the cytoplasmic membrane. PG biosynthesis is a multi-step process that begins in the cell cytoplasm, where precursor molecules are built [1]. Once precursors are exported outside the cell membrane, they are assembled into PG by Penicillin Binding Proteins (PBPs), enzymes that catalyze the polymerization of polysaccharide chains and crosslinking of peptide sidechains. Beta lactam antibiotics (penicillins, cephalosporins and carbapenems), which are among the most important antibiotics in current use, covalently bind to and inactivate PBPs [2]. PG’s importance for bacterial survival becomes evident when its synthesis is inhibited by beta lactams or antibiotics that block earlier steps in cell wall synthesis—cells routinely lyse.
It was initially hypothesized that beta lactam-induced lysis was caused by the mechanical force generated by increased turgor pressure that arose upon cessation of PG expansion while the cell maintained other cell growth programs. However, studies in both Gram - positive and Gram-negative organisms indicate that lysis is mediated by enzymatic activity [3,4]. PG cleavage mediated by cell wall hydrolases, also known as autolysins, is presumed to be excessive and/or dysregulated in the absence of ongoing PG synthesis, and the resulting breaches in the cell wall are thought to lead to lysis. Most bacteria contain multiple copies of at least 3 classes of potential autolysins—amidases, lytic transglycosylases and endopeptidases—and all 3 ordinarily play important roles in PG homeostasis [5–8]. An accumulation of degradation products from these enzymes were detected in Escherichia coli cells treated with beta lactam antibiotics [9], consistent with the possibility that lysis after inhibition of cell wall synthesis may be associated with the activity of multiple autolysins. However, multiple autolysins are not always important for beta lactam-induced lysis; e.g., in Streptococcus pneumoniae, deletion of a single amidase (Atl) renders this gram-positive pathogen completely tolerant to beta lactam-induced lysis [3].
In E. coli, beta lactam-induced lysis usually starts from the cell septum [10,11], suggesting that amidases, which are recruited to and activated at the site of cell division, might initiate PG cleavage associated with lysis. Supporting this idea, deletion of multiple amidases leads to a lower rate of lysis after exposure to beta-lactam antibiotics [10,12]. In contrast, there is contradictory evidence regarding the role of lytic transglycosylases in the lysis process. Mutants lacking multiple lytic transglycosylases are typically more susceptible to beta lactam antibiotics [13,14], suggesting that these enzymes promote, rather than impair, survival after inhibition of cell wall synthesis. However, overexpression of bifunctional PBPs containing an inactive transpeptidase active site, which mimics exposure to beta lactam antibiotics, results in E. coli lysis via a process that is largely dependent on LTGs [15]. None of the other predicted cell wall lytic enzymes in E. coli have been definitively linked to beta lactam-induced lysis. Efforts to define the full set of gene products that mediate bacterial lysis after inhibition of cell wall synthesis or the relative importance of their activities have been thwarted by the fact that the observed phenotype (lysis) is typically rapid, potentially masking differences between mutants, and that most lytic enzymes are highly redundant.
Likely because of the prevalence of cell-wall acting antibiotics in their natural habitats [16], bacteria employ multiple strategies to cope with the dangers associated with inhibition of cell wall synthesis. The most well-studied of these strategies is resistance e.g. by beta lactamases, which inactivate beta lactams. A more passive strategy is dormancy (e.g., formation of persister cells), which allows cells to survive exposure to any normally lethal antibiotic. Persistence is mediated by activation of multiple toxin-antitoxin modules [17,18], which stop growth of a small fraction of bacterial populations and thus confer tolerance to antibiotics that are only active on growing cells [19]. Bacteria that are not replicating due to reaching high cell densities also tend to be tolerant to cell wall-acting antibiotics [20] as do bacteria exposed to factors thought to stabilize the outer membrane [11]. It is unclear what other strategies might exist to survive exposure to cell wall synthesis inhibitors.
Here, we report that Vibrio cholerae, the causative agent of the diarrheal disease cholera, routinely tolerates antibiotic-induced inhibition of cell wall synthesis. Similar to most bacteria, V. cholerae loses the structural integrity of its cell wall following exposure to a wide variety of cell wall synthesis inhibitors. However, in contrast to many other bacteria, this treatment results in formation of viable (though non-dividing) spherical cells, rather than cell lysis. Surprisingly, genetic analyses revealed that V. cholerae sphere formation depends on the activity of M23 family endopeptidases that are required for cell elongation under conditions of normal growth; in contrast, its amidase and lytic transglycosylases are not required for formation of viable spheres. Furthermore, we found that other important pathogens, including Pseudomonas aeruginosa and Acinetobacter baumannii, also fail to respond to beta lactam exposure with lysis under certain growth conditions, suggesting that intrinsic, population-wide beta lactam tolerance may be more widespread than currently appreciated.
Results and Discussion
Vibrio cholerae is highly tolerant to cell-wall acting antibiotics
We observed that mid - to late exponential phase cultures of V. cholerae treated with high doses of penicillin G or ampicillin (100 μg/ml, 20 x MIC) failed to divide, but did not show a decline in viable cells (i.e., cfu) (Fig 1A, S1A Fig). Similarly, inhibition of early steps in PG synthesis by D-cycloserine, an inhibitor of D-Ala-D-Ala ligase (100 μg/ml, 2 x MIC), or phosphomycin, an inhibitor of MurA (100 μg/ml, 2x MIC), did not appreciably affect the survival of V. cholerae. Thus, although antibiotics targeting cell wall synthesis are effective in preventing V. cholerae proliferation, they do not induce the cell death typically observed in dividing cells of other species. Due to V. cholerae’s “tolerance” of these chemotherapeutic agents, their effects are not irreversible.
Fig. 1. Inhibition of cell-wall synthesis in V. cholerae leads to sphere formation. 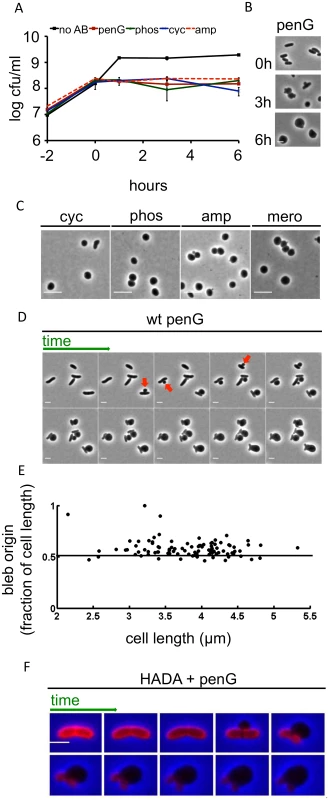
(A) Kinetics of viable V. cholerae cell counts after cells were exposed to various antibiotics that inhibit cell wall synthesis. Cells were grown to ~ 2–3 x 108 cfu/ml and then exposed to 100 μg/ml of penicillin G (penG), phosphomycin (phos), D-cycloserine (cyc), ampicillin (amp) or no antibiotic (no AB) (T0). Data shown are averages of two independent experiments; error bars represent standard deviation. (B) Images of V. cholerae cells at different time points after exposure to penicillin G from the experiment shown in (A). (C) Morphology of wt exponential phase cells exposed to 100 μg/ml cyc, phos, amp or 1 μg/ml meropenem (mero) for 3 h. (D) Time lapse images of wt cells plated on an agarose pad containing 100 μg/ml pen G. Frames are 5 min apart, scale bar = 2 μm. Arrowheads point to selected blebs. (E) Localization of blebs after exposure to pen G. Three different time lapse series with ~ 40 cells each were obtained as described in (D) and bleb location as the ratio of distance from an arbitrarily chosen pole divided by cell length was measured using ImageJ software. (F) Time lapse images of wt cells stained with the fluorescent D amino acid analogue HADA (50 μM) for 30 min and washed 2 x to remove excess dye prior to imaging on an agarose pad containing pen G. Images were brightness/contrast-adjusted to compensate for photobleaching. Frames are 5 min apart, scale bar = 2 μm. HADA stain is false-colored in red. In liquid medium, V. cholerae lost its rod-shape and eventually assumed a spherical morphology after exposure to the previously mentioned antibiotics or to meropenem (10 μg/ml, 100x MIC) (Fig 1B and Fig 1C). Thus, inhibition of V. cholerae cell wall synthesis results in the loss of PG’s ‘exoskeletal’ function to maintain cell shape. This is reminiscent of so-called L-forms (artificially-induced cell wall deficient bacteria, [21,22]); however, while L-forms proliferate in the absence of a functional cell wall, V. cholerae spheres did not divide in the presence of antibiotics (Fig 1A). Moreover, E. coli L-form generation requires the use of osmotically stabilizing media (e.g., containing high concentrations of sucrose) while V. cholerae survived exposure to cell wall acting antibiotics in diverse media lacking stabilizing agents, such as LB broth and rabbit cecal fluid (see below).
V. cholerae sphere formation appears to be independent of which step in cell wall synthesis is inhibited, since a variety of cell wall synthesis inhibitors yielded spheres. Importantly, penicillin also induced formation of viable, spherical V. cholerae in cecal fluid that was collected from infant rabbits with cholera-like diarrhea (S1B Fig), demonstrating that V. cholerae’s absence of lysis in response to inhibitors of cell wall synthesis is not due to stabilizing agents present in artificial growth medium, but instead has in vivo relevance. Sphere formation typically initiated with blebbing from the midcell (Fig 1D), although we did observe rare instances (~ 3% of cells) where blebbing started closer to the cell poles (Fig 1D and 1E). As blebs became large, the remainder of the cell became smaller, until only the poles of the original cell structure remained. Ultimately, poles were assimilated into spheres as well, although this process occurred more slowly.
We used the fluorescent D-amino acid analogue HADA [23] to visualize changes within the V. cholerae cell wall during the process of sphere formation. In V. cholerae, D-amino acids and analogs like HADA can be incorporated into the cell wall via peptide sidestem modification by penicillin-insensitive L,D transpeptidases in the periplasm [23,24]. Thus, at least in V. cholerae, HADA can be employed as a general cell wall label, even in the presence of cell-wall acting antibiotics.
In antibiotic-free cells, HADA staining was initially distributed evenly over the cell; however, the blebs induced by antibiotics lacked a HADA signal. In contrast, HADA staining was evident in the remainder of the cell for at least 20 min after blebbing commenced, consistent with maintenance of a PG-based cell structure (Fig 1F). Thus, inhibition of cell wall synthesis in V. cholerae does not result in a sudden and uniform disintegration of the cell wall; instead, it seems likely that locally confined cuts in PG allow for formation of blebs (which are presumed not to contain cell wall material), followed by gradual degradation of the remaining cell wall material. The ability of PG-deficient cells to survive suggests that the inner and outer bacterial membranes may collectively be able to withstand cellular turgor pressure in the absence of support from PG.
To further characterize sphere anatomy, we used fluorescently labeled proteins with previously demonstrated, distinct subcellular localization patterns [25–27]. In spheres, the periplasm was condensed into one compartment (S2A Fig), consistent with loss of PG’s exoskeletal function. The cytoplasm/inner membrane filled most of each sphere but appeared to often be pushed aside by the condensed periplasmic compartment, while the outer membrane was mostly circular. However, the integrity of the outer membrane in spheres appears to be reduced; spheres were highly susceptible to the membrane-acting agents triton X-100 and polymyxin B (S2B Fig), to which El Tor strains of V. cholerae are normally resistant.
Neither amidases nor the majority of lytic transglycosylases are necessary for sphere formation
In E. coli, lysis following exposure to cell-wall acting antibiotics appears to be partially dependent on the redundant PG amidases AmiA, AmiB, and AmiC, whose cleavage of septal PG enables daughter-cell separation [12]. The V. cholerae genome encodes a single PG amidase (AmiB), and an amiB deletion mutant forms long chains of unseparated daughter cells comparable to those of amidase-deficient E. coli [28]. Unexpectedly, V. cholerae amidase-deficient cells were more, rather than less, susceptible than wild type cells to killing by penicillin, phosphomycin and D-cycloserine (Fig 2A). However, this susceptibility was not associated with the bacterial lysis seen in drug-treated E. coli. We observed no significant decrease in culture density (OD600) of the V. cholerae amiB mutant in response to cell-wall acting antibiotics (S3 Fig), and bacterial lysis was rarely observed using light microscopy. Instead, antibiotic treatment ultimately resulted in formation of spherical cells that were similar to those formed by wild type bacteria (Fig 2B and 2C). We speculate that the ultimate decline in viability of the ΔamiB mutant under these conditions may reflect its previously noted compromised cell envelope [28]. It is important to note that interpretation of cfu and OD600 data is complicated by the fact that the mutant’s multi-cell chains disintegrate into single spheres upon beta lactam treatment (Fig 2B and 2C; discussed below). This is likely the cause of the observed increase in cfu directly after addition of antibiotic in Fig 2A, which may somewhat obscure a loss of viability. The mutant’s increased lag phase compared to the wild type further complicated direct comparisons based on culture density and cfu. Therefore, to directly assess AmiB’s role in the sphere formation process, we turned to single cell analysis.
Fig. 2. V. cholerae amiB is not required for sphere formation in response to inhibition of cell wall synthesis. 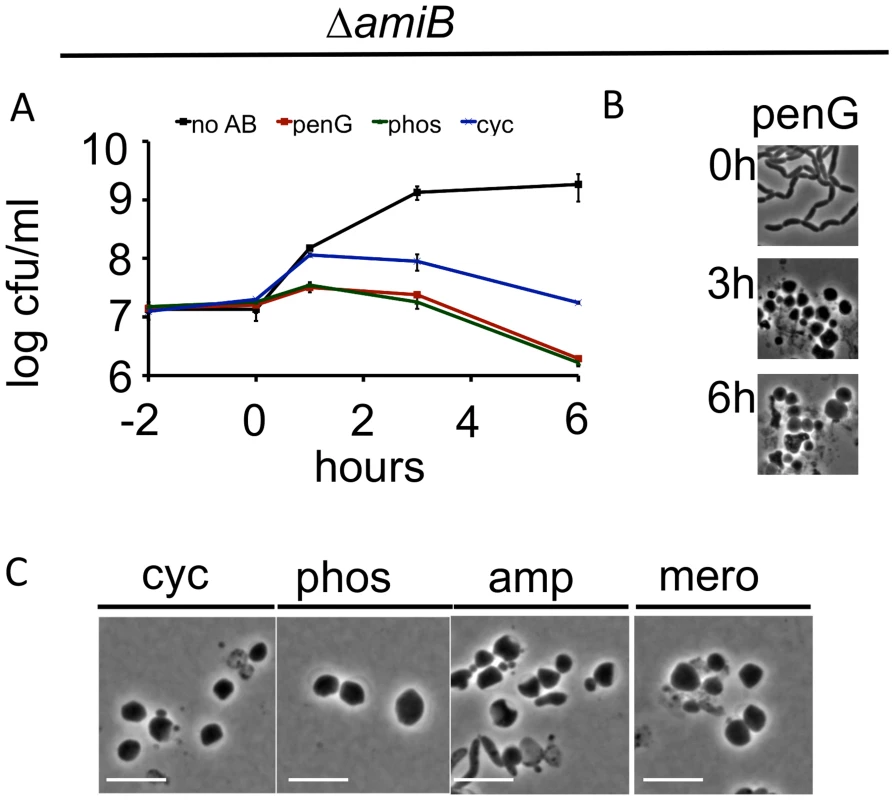
(A-C) A V. cholerae ΔamiB mutant was treated as described in Fig 1 A-C. Scale bar = 5 μm. No AB = no antibiotic added. Time lapse analysis of PenG-induced sphere formation in the ΔamiB mutant showed that blebs formed more slowly than in wt cells (compare S4A Fig to Fig 1D), however, we cannot exclude that this is merely due to the decreased growth rate of the ΔamiB strain. Moreover, blebbing of ΔamiB cells often appeared to originate from outside the midcell (S4A Fig). While the exact location of blebs relative to the septum is difficult to define in this chain-forming mutant, we also noticed blebbing from almost exclusively extraseptal locations when we treated a PBP1A-deficient mutant with the antibiotic cefsulodin, which in V. cholerae inhibits only PBP1B [26] (S4B Fig and S4C Fig). Since AmiB is presumably active at the septum only [28–30], the occurrence of blebbing outside of the septum in these cells provides additional evidence that enzymes other than AmiB can initiate sphere formation in V. cholerae. In aggregate, our data suggest that AmiB may play an initiating and facilitating role in sphere formation after inhibition of cell wall synthesis in V. cholerae, but that other enzymes can partially compensate for its absence.
We also assessed the role of lytic transglycosylases (LTGs) in V. cholerae’s response to antibiotics that inhibit PG synthesis. The V. cholerae genome encodes 6 predicted LTGs (mltA, mltB, mltC, mltD, mltF, and slt70). We found that a strain lacking 5 of these (ΔmltABDFΔslt70; Δ5LTG) was viable; however, we were unable to obtain a mutant lacking all six. Exposure of Δ5LTG to penicillin resulted in a ~1 log reduction in viability that was not accompanied by lysis, whereas phosphomycin or D-cycloserine did not reduce this strain’s viability (Fig 3A and Fig 3B). Similarly, deletion of certain lytic transglycosylases sensitizes other bacteria to beta lactam antibiotics [13,14], and this has recently been proposed to reflect the LTGs’ role in a quality control mechanism during cell wall synthesis [31].
Fig. 3. Deletion of multiple lytic transglycosylases alters the kinetics of V. cholerae sphere formation after inhibition of cell wall synthesis. 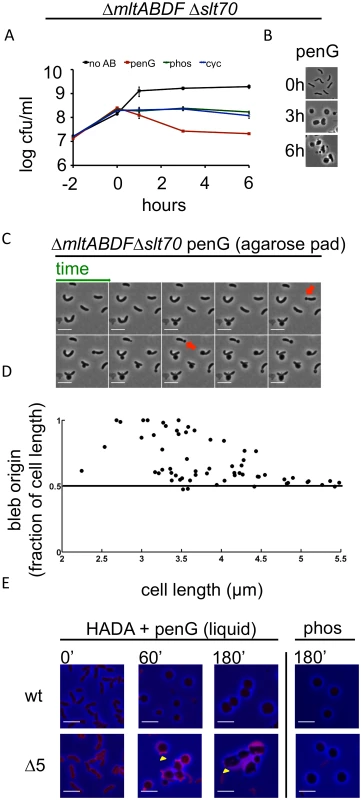
(A and B) The Δ5LTG mutant (ΔmltABDFΔslt70) was treated as described in Fig 1A and Fig 1B. No AB = no antibiotic added. (C) Time lapse images of Δ5LTG cells plated on agarose pads containing 100 μg/ml pen G. Frames are 5 min apart, scale bar = 5 μm; red arrowheads point to polar blebs. (D) Quantification of bleb location for time lapses described in C. For details, see Methods. (E) HADA incorporation during exposure to cell wall synthesis inhibitors in broth culture. Cells were grown to exponential phase in the presence of HADA (50 μM), then exposed to 100 μg/ml pen G or phos with HADA remaining in the growth medium. At the indicated time points, samples were washed twice prior to imaging. Images were minimally processed (background subtraction) and are comparable to each other, but not to the one depicted in Fig 1F. Scale bar = 5 μm. Yellow arrowheads point to polar appendages containing PG. Penicillin treatment of Δ5LTG or a single disruption of mltC, the sixth LTG, yielded spherical cells similar in appearance to those observed after treatment of the wild type strain (Fig 3B and S5 Fig), but the dynamics of sphere formation in Δ5LTG cells were altered. Unlike penicillin-treated wt cells, on agarose pads, a significant proportion (~ 40%) of penicillin-treated Δ5LTG cells started blebbing from sites close to or at the cell poles (Fig 3C and 3D), suggesting that in the absence of LTGs, there is less cleavage of septal PG, consistent with the proposed auxiliary role for these enzymes in cell separation in other bacteria [32,33]. Those cells that initiated blebbing from midcell retained long polar appendages for the entire duration of the experiment (Fig 3C), indicating that the comprehensive disruption of polar and lateral PG observed after beta lactam exposure of wild type cells depends upon one or more LTGs. This was also observed in liquid medium, albeit to a lesser degree, where HADA staining revealed the presence of polar appendages 1–3 hours after exposure to penicillin in Δ5LTG but not in the wt strain (Fig 3E). Additionally, HADA stained Δ5LTG cells much more intensely than wt cells before exposure to penicillin (Fig 3E and S6 Fig) and the mutant cells also retained more fluorescent material in the periplasm during exposure to the antibiotic. Thus, the Δ5LTG mutant appears to have reduced PG turnover, a deficiency that is accentuated by exposure to beta lactam antibiotics. Since beta lactams inhibit the transpeptidase activity of PBPs, but presumably leave their transglycosylase activity intact, it is possible that the periplasmic accumulation of HADA stain in the Δ5LTG mutant reflects the build-up of minimally cross linked (and HADA-labeled) PG strands that would ordinarily be degraded by lytic transglycosylases. Consistent with this possibility, we found that neither the Δ5LTG mutant nor wt cells treated with phosphomycin (an antibiotic that affects precursor synthesis and thus should not allow any PG synthesis) accumulated HADA-labeled material (Fig 3E). In summary, lytic transglycosylases, at least MltABDF and Slt70 in combination, or MltC alone, are not required for sphere formation but appear to be involved in downstream processes of cell wall degradation.
An endopeptidase enables sphere formation
Since amidases and the 5 LTGs were not critical for sphere formation, we turned our focus towards endopeptidases, two of which (ShyA and ShyC) are synthetically lethal and essential for cell elongation in V. cholerae [25]. The V. cholerae genome encodes five periplasmic M23 endopeptidases and one P60 family endopeptidase (NlpC). We constructed a mutant that lacks all predicted non-PBP endopeptidases and expresses inducible shyA from a neutral chromosomal locus (ΔshyA ΔshyB ΔshyC ΔnlpC ΔtagE1 ΔtagE2 Ptac:shyA; Δendo). In this background, depletion of shyA slowed growth, similar to our previous observations with a ΔshyA ΔshyC Ptac:shyA strain, which is defective in cell elongation but proficient in cell division [25](Fig 4A). Importantly, untreated Δendo cells did not lyse even after extended ShyA depletion (Fig 4C and “control”). Unexpectedly, however, exposure of ShyA-depleted Δendo cells to penicillin G resulted in rapid loss of viability and concomitant lysis of the majority of the population (Fig 4A and 4B). Lysis could be prevented and sphere formation restored by expression of shyA (Fig 4C). A similar pattern was observed with D-cycloserine and phosphomycin (S7 Fig). Notably, analysis of the dynamics of cell lysis using time lapse microscopy revealed that cell disintegration did not proceed through a spherical intermediate (Fig 4C). These results suggest that, paradoxically, the presence of a D,D endopeptidase (ShyA), a putative ‘autolysin’, prevents lysis and enables formation of viable spheres after exposure of V. cholerae to a beta lactam antibiotic.
Fig. 4. V. cholerae endopeptidase are required to prevent lysis in response to inhibition of cell wall synthesis. 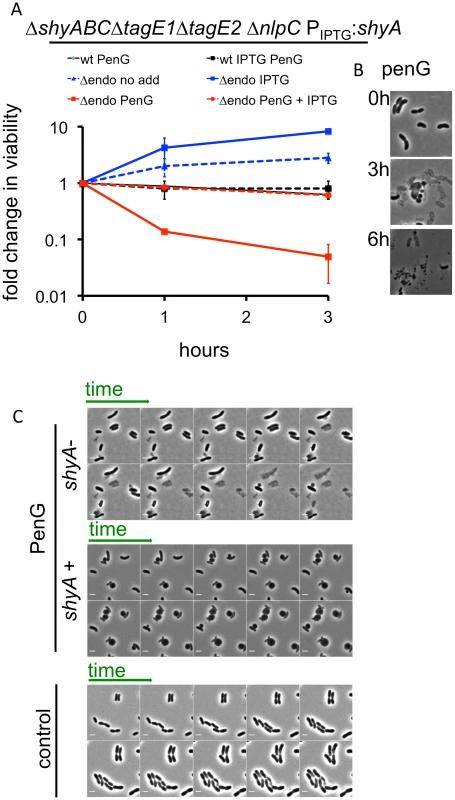
(A) Wild type V. cholerae and a Δendo (ΔshyABC ΔnlpC ΔtagE1 ΔtagE2 Ptac:shyA) mutant were grown in the presence or absence of 200 μM IPTG for 1.5 h (= T0) and then exposed to pen G. Cfu/ml at the indicated time points were normalized to cfu/ml at T0 and shown as fold change in viability. Data are mean of two biological replicates; error bars represent standard deviation. (B) Representative images of V. cholerae Δendo cells at different time points after exposure to penicillin G from the experiment shown in (A). (C) Time lapse images of Δendo cells initially grown for 1.5 h in either the presence or absence of IPTG (25 μM) and subsequently imaged on agarose pads containing 100 μg/ml pen G. The pad for ShyA+ cells also contained IPTG. The control panel depicts cells applied to an agarose pad containing neither antibiotic nor IPTG. Frames in the lower (control) panel are 10 min apart; in the other panels, frames are 5 min apart. Scale bar = 2 μm. The lysis phenotype was also observed in a ShyA-depleted ΔshyA ΔshyB ΔshyC Ptac:shyA (S8 Fig) strain, and expression of ShyC but not ShyB could at least partially prevent lysis of ShyA-depleted Δendo (S8 Fig), demonstrating that either one of the paralogues ShyA and ShyC must be present for beta lactam tolerance and formation of viable spheres, while the other M23 endopeptidases and NlpC are dispensable. We also observed penicillin G-induced lysis of Δendo ΔamiB cells when ShyA was depleted (S8 Fig), as well as in Δendo cells with additional mutations in LTG genes (Δendo ΔmltBΔmltD and Δendo ΔmltB Δslt70, which were the only strains with multiple LTG disruptions that we were able to make in the Δendo background). These results indicate that neither amidase activity nor MltB, MltD or Slt70 activity are necessary to cause lysis of Δendo cells. Lastly, ShyA-depleted Δendo cells were not more susceptible to membrane-acting agents than ShyA-replete cells (S9 Fig), suggesting that the observed lysis phenotype is not simply the consequence of a general weakness of the cell envelope.
Since ShyA could prevent beta lactam-mediated lysis in V. cholerae, we tested whether it could protect a heterologous organism from lysis after inhibition of cell wall synthesis. We overproduced ShyA in the EHEC isolate EDL933 and measured its survival after exposure to meropenem. Overexpression of shyA alone did not influence EHEC growth. However, expression of this endopeptidase increased EHEC’s capacity to survive meropenem exposure by ~10-fold compared to an empty vector control, overexpression of yebA, E. coli’s ShyA homologue (Fig 5A) or overexpression of ShyA carrying an active site mutation (H375A). Meropenem-treated EDL933 expressing ShyA formed spheres, while the control cells carrying the empty vector rapidly lysed (Fig 5B). These data suggest that ShyA activity is linked to survival in the presence of beta lactam antibiotics, and that ShyA-mediated cleavage of the cell wall may differ from YebA-mediated cleavage events, although it is theoretically possible that these results only reflect differences in the expression levels for the two proteins.
Fig. 5. Inhibition of cell wall synthesis in other bacteria does not always lead to cell lysis. 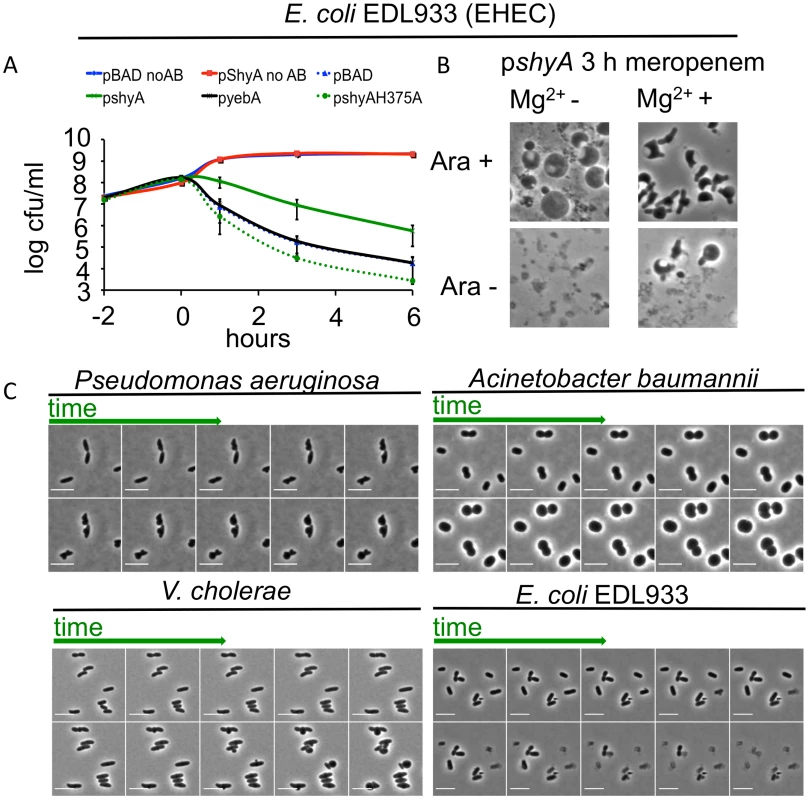
(A) Influence of shyA or yebA overexpression on viability of E. coli EDL933 treated with meropenem. EDL933 carrying either pBAD33 (plasmid vector) or its derivatives encoding yebA or shyA was grown to exponential phase in the presence of arabinose, then meropenem (1 μg/ml) was added at (T0) and viable cell counts determined at the indicated times. Data are average of three independent experiments; error bars represent standard deviation. (B) Representative images from the 3 h time point of an experiment similar to the one depicted in (A), with or without the addition of 10 mM Mg2+ in the growth medium. (C) Time lapse images of P. aeruginosa, A. baumannii, V. cholerae and EDL933 cells exposed to meropenem. Cells were grown in LB until OD600 ~ 0.5 and then applied to an agarose pad (10% LB/PBS) containing meropenem (4 μg/ml) as well as MgSO4 (10 mM)/CaCl2 (1 mM) for P. aeruginosa and A. baumannii only. Frames are 9 min apart, scale bar = 5 μm. No AB = no antibiotic added. When EDL933 was grown in the presence of Mg2+, which is thought to have a stabilizing effect on the outer membrane [11], cells carrying an empty (control) plasmid formed spheres at a low but detectable frequency after meropenem exposure. However, in this medium, visible lysis was still markedly reduced and sphere formation concomitantly enhanced after overexpression of plasmid-encoded shyA (Fig 5B). ShyA’s protective effect can thus apparently be enhanced by additional stabilization of the outer membrane, suggesting that multiple processes can contribute to beta lactam tolerance.
Finally, a recent report showed that Pseudomonas aeruginosa could assume a spherical morphology in response to carbapenem antibiotics, especially in media fortified with Mg2+ and Ca2+ [34], suggesting that the absence of cell lysis after inhibition of cell wall synthesis may not be limited to V. cholerae.
P. aeruginosa turned into spheroid forms on agarose pads containing meropenem via steps that resemble V. cholerae’s transformation into spheres after exposure to this antibiotic (Fig 5C), suggesting that the mechanisms underlying sphere formation may be similar in these bacteria. Notably, we observed that under the same experimental conditions, the multidrug resistant Acinetobacter baumannii clinical isolate Lac-4 also failed to lyse in response to meropenem, albeit in a process unlike that observed in V. cholerae and P. aeruginosa (Fig 5C). Thus, population-wide lysis in response to inhibition of cell wall synthesis may not be the norm for many bacteria.
Conclusions
In summary, we found that inhibition of cell wall synthesis does not inexorably lead to cell lysis and death, as occurs in the model organism E. coli. Some pathogenic bacteria survive blockade of PG synthesis, and instead form viable spheres. Surprisingly, in V. cholerae, sphere formation/survival depends on the activity of PG hydrolases (autolysins), particularly the D,D endopeptidase ShyA. Thus, our findings suggest that although the pathways and enzymes that mediate PG degradation after inhibition of PG synthesis are critical for determining bacterial fate under these conditions, such fates are variable: in different organisms, enzymes that ordinarily degrade PG can either lead to lysis or promote survival when PG synthesis is blocked by antibiotics.
In V. cholerae, our data suggests that there is an ordered series of steps that lead to sphere formation following interference with cell wall synthesis. In the majority of cells, the first cell wall lesion from which blebbing commences is likely generated by AmiB, perhaps with some assistance from LTGs. The latter enzymes play a more critical role in downstream processes that lead to PG resorption. The procession from blebs to spheres rather than lysis requires the presence of an endopeptidase, either ShyA or ShyC. Our findings suggest that the specificity, rate or location of PG hydrolysis mediated by such D,D endopeptidases is important for preventing cell lysis; however, the exact mechanism by which these proteins prevent lysis and death requires further investigation. It is tempting to speculate that while the end result of cell wall synthesis inhibition may differ between bacteria, the hierarchy of cell wall lytic events might be conserved.
It will be interesting to explore whether PG-degrading enzymes important for cell elongation are required for sphere formation in other organisms, as in V. cholerae. Our observations suggest that the absence of lysis after treatment with beta lactam antibiotics may be more common than currently appreciated, but the determinants of such survival have not been identified. Importantly, some reports have suggested that spherical bacteria can be isolated from patients treated with beta lactam antibiotics during chronic infections (e.g. respiratory infections caused by Haemophilus influenzae, [35]). Thus, similar to persister cells [19], population-wide tolerance and sphere formation may represent another fairly widespread way by which bacteria can evade the lethal consequences of beta lactam exposure. New antibiotics that target processes critical for sphere formation (e.g. inhibitors of ShyA) or for sphere survival might exhibit potent synergy with beta lactams and thus provide a novel approach for improved antimicrobial therapeutics.
Materials and Methods
Strain construction
Gene deletions were conducted by standard techniques using suicide plasmid (pCVD442) containing ~600 bp flanking regions of the gene to be deleted [25]. All knockouts are substitutions of the respective open reading frame with the linker sequence 5’-TTATCATTACTCGAGTGCGGCCGCATGAAA-3’.
Overexpression plasmids were constructed by amplifying the gene of interest including its native ribosome binding site and cloning into Sma1-digested pBAD33 using isothermal assembly [36].
Growth and killing experiments
Cells were grown in LB medium at 37°C. Growth curves were conducted in 200 μL volume in 200 well honeycomb plates using a Biotek growth curve machine.
For time-dependent killing experiments, overnight cultures were diluted 1 : 100 into 3 ml LB medium and grown shaking at 37°C until cell density reached ~ 2 x 108 cfu/ml (~OD600 0.3). Antibiotics (Sigma) were added to either 100 μg/ml (penicillin G, ampicillin, phosphomycin, D-cycloserine, cefsulodine) or 10 μg/ml (meropenem). At the indicated time points, cells were serially diluted and spot plated to determine cfu/ml.
For post-penicillin survival assays, cells grown as described above were exposed to penicillin G for three hours; then either nothing, Triton X-100 (1% final concentration) or Polymixin B (40 μg/ml final concentration) were added, followed by 30 min incubation at 37°C and subsequent spot-plating for cfu/ml.
For antibiotic exposure assays in cecal fluid, 200 μL of cecal fluid from infected infant rabbits (which contains a high-density monoculture of V. cholerae, [37]) was collected ~ 16 h post infection, transferred to eppendorf tubes and incubated standing at 37°C for 3 h after addition of antibiotic.
For depletion experiments, overnight cultures of Δendo were diluted 1 : 100 into 3 ml LB medium containing 25 μM IPTG (which is the minimal growth permitting IPTG concentration). These cultures were then grown for 2 h, washed 2 x with fresh medium and then resuspended in LB lacking IPTG and grown for an additional 1.5 h. Cells were then directly applied to agarose pads (see below) containing penicillin G with or without IPTG (100 μM).
MIC determination
For assessment of minimum inhibitory concentrations (MIC), overnight cultures were diluted 1000fold into fresh LB medium, grown for 1 h and again diluted 1 : 1000 in fresh LB medium. 50 μL of this inoculum were applied to 96 well plates containing 50 μL of 2fold serial dilutions of the antibiotic to be tested. The MIC was read as the lowest antibiotic concentration at which no turbidity was visible.
Image acquisition and analysis
Time lapse experiments were conducted on 0.8% agarose pads with 10% LB and PBS. Images were analyzed using ImageJ software, and adjusted by removing background fluorescence (using imageJ’s built-in function, 50 px rolling ball radius) and adjusting brightness/contrast levels where appropriate (i.e. in Fig 1F). Care was taken to use the same adjustment parameters for images that were to be compared directly with each other (i.e. in Fig 3E, wt vs. Δ5).
Supporting Information
Zdroje
1. Typas A, Banzhaf M, Gross CA, Vollmer W (2011) From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 10 : 123–136. doi: 10.1038/nrmicro2677 22203377
2. Tomasz A (1979) From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view. Rev Infect Dis 1 : 434–467. 45147
3. Tomasz A, Waks S (1975) Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A 72 : 4162–4166. 674
4. Kitano K, Tomasz A (1979) Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother 16 : 838–848. 93877
5. Scheurwater E, Reid CW, Clarke AJ (2008) Lytic transglycosylases: bacterial space-making autolysins. Int J Biochem Cell Biol 40 : 586–591. 17468031
6. Lee TK, Huang KC (2013) The role of hydrolases in bacterial cell-wall growth. Curr Opin Microbiol 16 : 760–766. doi: 10.1016/j.mib.2013.08.005 24035761
7. Uehara T, Bernhardt TG (2011) More than just lysins: peptidoglycan hydrolases tailor the cell wall. Curr Opin Microbiol 14 : 698–703. doi: 10.1016/j.mib.2011.10.003 22055466
8. Wyckoff TJ, Taylor JA, Salama NR Beyond growth: novel functions for bacterial cell wall hydrolases. Trends Microbiol 20 : 540–547. doi: 10.1016/j.tim.2012.08.003 22944244
9. Kitano K, Tuomanen E, Tomasz A (1986) Transglycosylase and endopeptidase participate in the degradation of murein during autolysis of Escherichia coli. J Bacteriol 167 : 759–765. 2875060
10. Chung HS, Yao Z, Goehring NW, Kishony R, Beckwith J, et al. (2009) Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery. Proc Natl Acad Sci U S A 106 : 21872–21877. doi: 10.1073/pnas.0911674106 19995973
11. Yao Z, Kahne D, Kishony R (2012) Distinct single-cell morphological dynamics under beta-lactam antibiotics. Mol Cell 48 : 705–712. doi: 10.1016/j.molcel.2012.09.016 23103254
12. Heidrich C, Templin MF, Ursinus A, Merdanovic M, Berger J, et al. (2001) Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol Microbiol 41 : 167–178. 11454209
13. Bonis M, Williams A, Guadagnini S, Werts C, Boneca IG (2012) The effect of bulgecin A on peptidoglycan metabolism and physiology of Helicobacter pylori. Microb Drug Resist 18 : 230–239. doi: 10.1089/mdr.2011.0231 22432710
14. Templin MF, Edwards DH, Holtje JV (1992) A murein hydrolase is the specific target of bulgecin in Escherichia coli. J Biol Chem 267 : 20039–20043. 1400320
15. Meisel U, Holtje JV, Vollmer W (2003) Overproduction of inactive variants of the murein synthase PBP1B causes lysis in Escherichia coli. J Bacteriol 185 : 5342–5348. 12949085
16. Finley RL, Collignon P, Larsson DG, McEwen SA, Li XZ, et al. (2013) The scourge of antibiotic resistance: the important role of the environment. Clin Infect Dis 57 : 704–710. doi: 10.1093/cid/cit355 23723195
17. Gerdes K, Maisonneuve E (2012) Bacterial persistence and toxin-antitoxin loci. Annu Rev Microbiol 66 : 103–123. doi: 10.1146/annurev-micro-092611-150159 22994490
18. Keren I, Shah D, Spoering A, Kaldalu N, Lewis K (2004) Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol 186 : 8172–8180. 15576765
19. Lewis K (2010) Persister cells. Annu Rev Microbiol 64 : 357–372. doi: 10.1146/annurev.micro.112408.134306 20528688
20. Tuomanen E, Gilbert K, Tomasz A (1986) Modulation of bacteriolysis by cooperative effects of penicillin-binding proteins 1a and 3 in Escherichia coli. Antimicrob Agents Chemother 30 : 659–663. 3541782
21. Errington J (2013) L-form bacteria, cell walls and the origins of life. Open Biol 3 : 120143. doi: 10.1098/rsob.120143 23303308
22. Billings G, Ouzounov N, Ursell T, Desmarais SM, Shaevitz J, et al. (2014) De novo morphogenesis in L-forms via geometric control of cell growth. Mol Microbiol 93 : 883–896. doi: 10.1111/mmi.12703 24995493
23. Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, et al. (2012) In Situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D-amino acids. Angew Chem Int Ed Engl 51 : 12519–12523. doi: 10.1002/anie.201206749 23055266
24. Cava F, de Pedro MA, Lam H, Davis BM, Waldor MK (2011) Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J 30 : 3442–3453. doi: 10.1038/emboj.2011.246 21792174
25. Dorr T, Cava F, Lam H, Davis BM, Waldor MK (2013) Substrate specificity of an elongation-specific peptidoglycan endopeptidase and its implications for cell wall architecture and growth of Vibrio cholerae. Mol Microbiol 89 : 949–962. doi: 10.1111/mmi.12323 23834664
26. Dorr T, Moll A, Chao MC, Cava F, Lam H, et al. (2014) Differential requirement for PBP1a and PBP1b in in vivo and in vitro fitness of Vibrio cholerae. Infect Immun 82 : 2115–2124. doi: 10.1128/IAI.00012-14 24614657
27. Dorr T, Lam H, Alvarez L, Cava F, Davis BM, et al. (2014) A novel peptidoglycan binding protein crucial for PBP1A-mediated cell wall biogenesis in Vibrio cholerae. PLoS Genet 10: e1004433. doi: 10.1371/journal.pgen.1004433 24945690
28. Moll A, Dorr T, Alvarez L, Chao MC, Davis BM, et al. (2014) Cell separation in Vibrio cholerae is mediated by a single amidase whose action is modulated by two non-redundant activators. J Bacteriol.
29. Peters NT, Dinh T, Bernhardt TG (2011) A fail-safe mechanism in the septal ring assembly pathway generated by the sequential recruitment of cell separation amidases and their activators. J Bacteriol 193 : 4973–4983. doi: 10.1128/JB.00316-11 21764913
30. Uehara T, Parzych KR, Dinh T, Bernhardt TG (2010) Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J 29 : 1412–1422. doi: 10.1038/emboj.2010.36 20300061
31. Cho H, Uehara T, Bernhardt TG (2014) Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 159 : 1300–1311. doi: 10.1016/j.cell.2014.11.017 25480295
32. Jorgenson MA, Chen Y, Yahashiri A, Popham DL, Weiss DS (2014) The bacterial septal ring protein RlpA is a lytic transglycosylase that contributes to rod shape and daughter cell separation in Pseudomonas aeruginosa. Mol Microbiol 93 : 113–128. doi: 10.1111/mmi.12643 24806796
33. Heidrich C, Ursinus A, Berger J, Schwarz H, Holtje JV (2002) Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J Bacteriol 184 : 6093–6099. 12399477
34. Monahan LG, Turnbull L, Osvath SR, Birch D, Charles IG, et al. (2014) Rapid conversion of Pseudomonas aeruginosa to a spherical cell morphotype facilitates tolerance to carbapenems and penicillins but increases susceptibility to antimicrobial peptides. Antimicrob Agents Chemother 58 : 1956–1962. doi: 10.1128/AAC.01901-13 24419348
35. Roberts D, Higgs E, Rutman A, Cole P (1984) Isolation of spheroplastic forms of Haemophilus influenzae from sputum in conventionally treated chronic bronchial sepsis using selective medium supplemented with N-acetyl-D-glucosamine: possible reservoir for re-emergence of infection. Br Med J (Clin Res Ed) 289 : 1409–1412. 6437576
36. Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, et al. (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6 : 343–345. doi: 10.1038/nmeth.1318 19363495
37. Ritchie JM, Rui H, Bronson RT, Waldor MK (2010) Back to the future: studying cholera pathogenesis using infant rabbits. MBio 1.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



