-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAn Effector Peptide Family Required for Toll-Mediated Immunity
Dedicated defense systems in the bodies of humans and other animals protect against dangerous microbes, such as bacteria and fungi. We study these processes in the fruit fly Drosophila, which can be readily grown and manipulated in the laboratory. In this animal, as in humans, protective activities are triggered when fragments of bacteria or fungi activate a system for defense gene regulation known as the Toll signaling pathway. The result is the large-scale production of defense molecules and, in many cases, clearance of the infection and survival of the animal. Although the systems for recognizing and initiating responses are well described, the role of many defense molecules is not understood. We have identified a group of closely related defense molecules in flies and used state-of-the-art genomic engineering to simultaneously eliminate most of the genes in the group. By comparing the effect of fungal or bacterial infection on the genetically altered flies and normal siblings, we find that this group of defense molecules is essential for disease resistance.
Published in the journal: . PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004876
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004876Summary
Dedicated defense systems in the bodies of humans and other animals protect against dangerous microbes, such as bacteria and fungi. We study these processes in the fruit fly Drosophila, which can be readily grown and manipulated in the laboratory. In this animal, as in humans, protective activities are triggered when fragments of bacteria or fungi activate a system for defense gene regulation known as the Toll signaling pathway. The result is the large-scale production of defense molecules and, in many cases, clearance of the infection and survival of the animal. Although the systems for recognizing and initiating responses are well described, the role of many defense molecules is not understood. We have identified a group of closely related defense molecules in flies and used state-of-the-art genomic engineering to simultaneously eliminate most of the genes in the group. By comparing the effect of fungal or bacterial infection on the genetically altered flies and normal siblings, we find that this group of defense molecules is essential for disease resistance.
Introduction
Constant interaction with microbes is a fact of life, and sometimes death, for animals. Many microbes are neutral or beneficial to the host’s health. Some, however, are pathogenic and threaten the host’s viability. In vertebrates and invertebrates alike, immune responses are initiated by recognition of pathogen associated molecular patterns (PAMPs) following invasion of host tissues [1, 2]. This recognition of conserved microbial products triggers innate immune signaling pathways that are closely related in species as divergent as flies and humans [3–5]. In each case, pathway activation initiates a transcriptional program encoding an array of effector peptides and proteins.
In the fruit fly Drosophila melanogaster, Toll and Imd proteins define the two major immune signaling pathways [6–11]. Fragments of fungal cell walls and bacterial peptidoglycan serve as PAMPs for these pathways. Toll signaling is triggered by the β-1,3-glucans of fungal cell walls or by Lys-type peptidoglycan [12–16]. In contrast, the Imd pathway is activated by DAP-type peptidoglycan [17–21]. Upon activation, Toll and Imd direct expression of distinct but overlapping effector gene repertoires. These effector genes bring about the humoral immune response via factors, including antimicrobial peptides (AMPs), that circulate throughout the fly hemolymph. Effector genes also support other immune processes by, for example, upregulating genes promoting melanization and wound healing [22].
The Drosophila immune effector repertoire has been characterized by microarray, RNA-seq, and mass spectrometry experiments [23–27]. The most highly upregulated genes include most known AMPs, but also many as yet uncharacterized effector peptides. For both the characterized and novel effectors, delineation of in vivo requirements based on loss-of-function phenotypes is largely lacking.
Here, we describe the application of recent advances in genome engineering technology to the genetic dissection of innate immune effector function. Generating a designer deletion of multiple members of an effector gene family, we demonstrate an essential role for these genes in Toll-mediated defense against microbial pathogens.
Results
The Bom genes encode a family of short, secreted, Toll-regulated peptides
Carrying out sequence comparisons among Drosophila melanogaster loci induced by the Toll pathway [24], we identified a family of twelve genes encoding secreted peptides lacking similarity to known AMPs. Each of the twelve peptides contains one or two copies of a 16 amino acid-long motif that includes a CXXC bend surrounded by a region of high sequence conservation (Fig 1A). All orthologs identified to date are from members of the Drosophila genus. We propose naming this family of genes the Bomanins (Boms), after Hans Boman, who carried out pioneering work in peptide-mediated innate immunity [28–31].
Fig. 1. Bom genes share a conserved 16-aa motif. 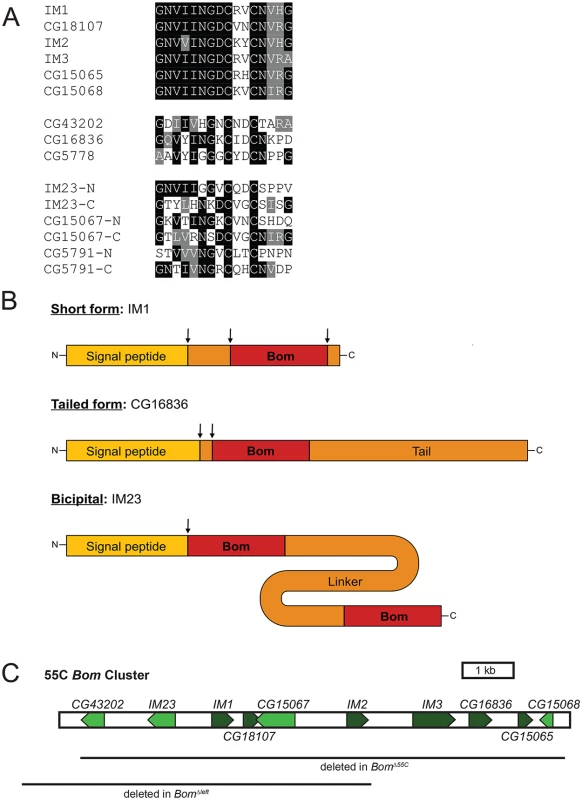
(A) Alignment of Bom motifs. Top. Mature Bom peptide sequences of the short-form Boms. Middle. Bom peptide motifs of the tailed Boms. Bottom. Bom peptide motifs from the N- and C-terminal ends of the three bicipital Boms. Shading indicates sequence identity (black) or similarity (gray). (B) Schematic of the three Bom peptide forms. ‘Bom’ represents the conserved 16-aa motif depicted in Fig 1A. Drawings are to scale and arrows indicate sites of cleavage. (C) Schematic of 55C Bom gene cluster on chromosome 2R. Lines beneath schematic demarcate areas deleted in BomΔ55C and BomΔleft chromosomes. The proximal end of the gene cluster is shown to the left. Several Bom peptides belong to the set of Immune-induced Molecules (IMs) first identified in mass spectrometry studies carried out by Bulet, Hoffmann, and colleagues [23, 25]. Combining those findings with detailed sequence comparisons reveals post-translational processing events: signal peptide cleavage and, often, removal of additional residues at the amino-terminal end as well as carboxyl-terminal amidation (Fig 1B).
The Bom peptides fall into three distinct groups (Fig 1B and S1 Table). For six of the twelve, the mature peptide is just 16 or 17 amino acids long. The sequences of these six short-form peptides are highly similar and correspond to the conserved, CXXC-containing region that we have defined as the Bom motif (see Fig 1A and 1B). Three other Bom peptides have a tailed form—a Bom motif followed by a C-terminal extension or tail, 15 to 82 amino acids in length. The remaining three peptides have a Bom motif at each end, connected by a linker region of 43 to 103 amino acids. We refer to these peptides as two-headed or bicipital. Sequence identity and similarity within the Bom motif is reduced, but still significant, in the tailed and bicipital forms (see Fig 1A). In contrast, the tail and linker regions in these two classes are rich in homopolymeric stretches and contain no appreciable sequence conservation either with each other or with other proteins in available databases.
Published microarray, RNA-seq, and mass spectrometry experiments document robust expression of the Bom transcripts and peptides after bacterial or fungal infection [22–27]. Indeed, induced expression of many Boms is at levels equal to or greater than those of AMP loci. Furthermore, Bom peptides, like AMPs, are abundant in the hemolymph of infected flies [23, 32].
Ten of the twelve D. melanogaster Bom genes are clustered on chromosome 2 at cytogenetic position 55C (henceforth 55C Bom cluster, Fig 1C). The two remaining Bom genes, CG5791 and CG5778, reside in a mini-cluster on chromosome 3 and encode a bicipital and a tailed Bom peptide, respectively. Because the predicted mature Bom peptides are highly similar and hence potentially overlapping in function, we began our investigation of the Boms by precisely deleting the ten genes of the 55C Bom cluster using a TALEN-based approach. The deletion, henceforth BomΔ55C, is 9 kb long and removes no annotated loci other than the Bom genes.
The 55C Bom cluster is specifically required for the Toll-mediated immune defense
Because Toll signaling induces Bom expression, we challenged BomΔ55C adults with Enterococcus faecalis, a bacterium that has Lys-type peptidoglycan and therefore specifically induces the Toll pathway. Using septic wounding, we systemically infected adult flies and then monitored survival. In control experiments, we found that flies lacking a functional Toll pathway (MyD88-) were much more susceptible to E. faecalis infection than were flies with wild-type immune competence (w1118), as reported previously [7, 33]. Following infection, more than 50% of MyD88- flies died within one day and nearly all (>90%) were dead within two days (Fig 2A). In contrast, more than 95% of wild-type adults were alive one day post-infection and more than 50% survived two days or longer.
Fig. 2. The 55C Boms are essential for Toll-mediated defense. 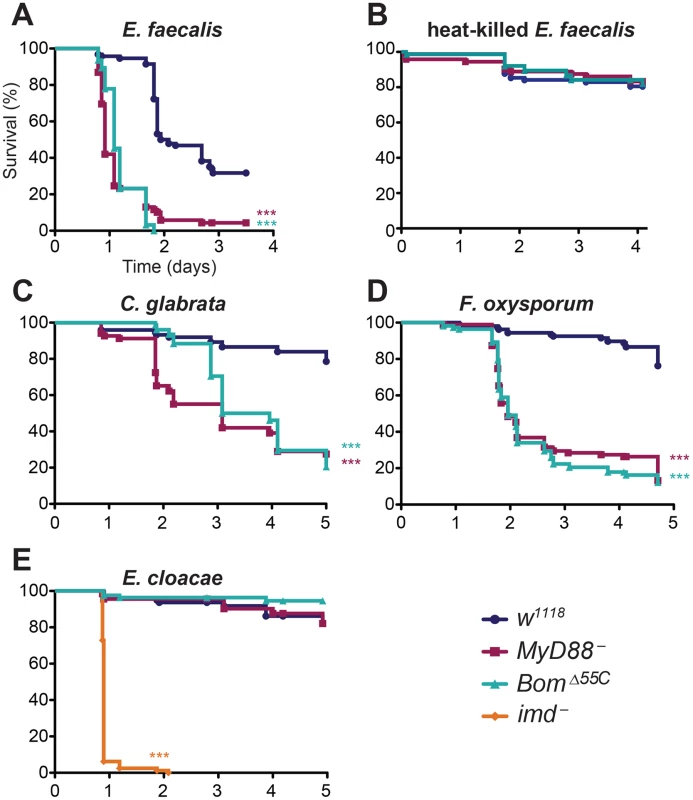
Graphs indicate survival at indicated intervals post-infection with (A) E. faecalis, (B) heat-killed E. faecalis, (C) C. glabrata, (D) F. oxysporum, and (E) E. cloacae. Each curve represents the pooled results of at least three independent experiments involving 20 or more flies per genotype. Survival curves were compared using the Gehan-Breslow-Wilcoxon test. Significance is shown relative to the wild type (w1118) and adjusted for multiple comparisons (*** p<0.0003, n.s. = not significant, p>0.0167). Strikingly, BomΔ55C flies were as susceptible to E. faecalis infection as MyD88- flies. Indeed, the survival curves of BomΔ55C and MyD88- flies were almost indistinguishable, suggesting that loss of the 55C Bom cluster is as detrimental to defense against this bacterial pathogen as is loss of Toll signaling entirely.
Having observed that BomΔ55C flies rapidly succumb to septic wounding with E. faecalis (see Fig 2A), we wondered if this phenotype reflected a defective response to wounding or stress rather than infection per se. To test this idea, we wounded wild-type and mutant flies with a clean needle or with one dipped in a suspension of heat-killed E. faecalis. The survival of BomΔ55C flies was markedly better for either challenge compared to septic wounding over the same time period. Specifically, upon either clean wounding or wounding with heat-killed bacteria, more than 75% of BomΔ55C flies survived for four or more days, comparable to the wild type (Fig 2B and S1 Fig). We conclude that active infection, rather than wounding itself or response to PAMP recognition, causes the rapid death of BomΔ55C flies challenged with live E. faecalis.
Toll mediates resistance not only to a number of bacteria, but also to fungi, including yeast [6, 34]. To determine if this Toll activity is also Bom-dependent, we assayed the effect of deleting the 55C Bom genes on survival after infection with the yeast Candida glabrata. Wild-type flies exhibit significant resistance to C. glabrata, with over 80% of wild-type flies surviving five days after infection (Fig 2C). In contrast, 50% of MyD88- flies succumbed just two days after being infected. BomΔ55C flies were similarly affected. Although it took BomΔ55C flies slightly longer than MyD88- flies to drop to 50% survival (three days), survival rates were nearly coincident at later time points.
We next tested the survival of BomΔ55C flies after infection with a filamentous fungus, Fusarium oxysporum, that also triggers a Toll-dependent immune response. Among wild-type flies, roughly 80% survived for four or more days (Fig 2D). In contrast, both MyD88- and BomΔ55C flies succumbed much more quickly. Specifically, BomΔ55C flies had a median survival of just over two days post-infection, nearly identical to MyD88- flies. We conclude that the 55C Boms are also essential for Toll-mediated defense against both a unicellular and a filamentous fungus.
Because the Imd pathway does not appear to regulate Bom expression [24], we predicted that the Bom genes would be dispensable for Imd-mediated defenses. We could test this hypothesis with Enterobacter cloacae, a bacterium that has a DAP-type peptidoglycan and therefore triggers Imd signaling. We used E. cloacae to infect BomΔ55C flies, as well as control flies lacking imd function. Whereas more than 90% of imd- flies died within 24 hours of septic wounding with E. cloacae, greater than 80% of BomΔ55C, MyD88-, and wild-type flies survived for four or more days (Fig 2E). Taken together, these studies indicate that the 55C Bom peptides are specifically required in the Toll-mediated, acute phase defense against systemic infection.
Bom peptides are not required to maintain, protect, or amplify Toll signaling
Given the similarity in phenotypes between BomΔ55C and MyD88- flies, we wondered if loss of the 55C Boms disrupts Toll signaling. These Boms might, for example, be required to counteract pathogen virulence factors that target Toll signaling. They might also provide positive feedback, spreading and amplifying Toll signaling after initial pathogen detection. According to such models, induction of Toll-responsive genes should be reduced in BomΔ55C flies relative to the wild type. To test this idea, we infected flies with E. faecalis and used qRT-PCR to measure induction of marker loci. For this purpose, we chose two genes that are strongly expressed upon Toll activation but that lie outside of the 55C cluster: IM4 and Drosomycin (Drs). Six hours after E. faecalis infection, we detected robust expression of IM4 and Drs in the wild type but, as expected, negligible induction in MyD88- (Fig 3A and 3B). In BomΔ55C flies, induction of both Toll-responsive genes was comparable to that in the wild type. In fact, expression of IM4 was greater in BomΔ55C flies than wild-type flies, perhaps reflecting the enhanced induction of Toll by an unchecked infection.
Fig. 3. Toll-mediated activation of immune genes is normal in BomΔ55C flies. 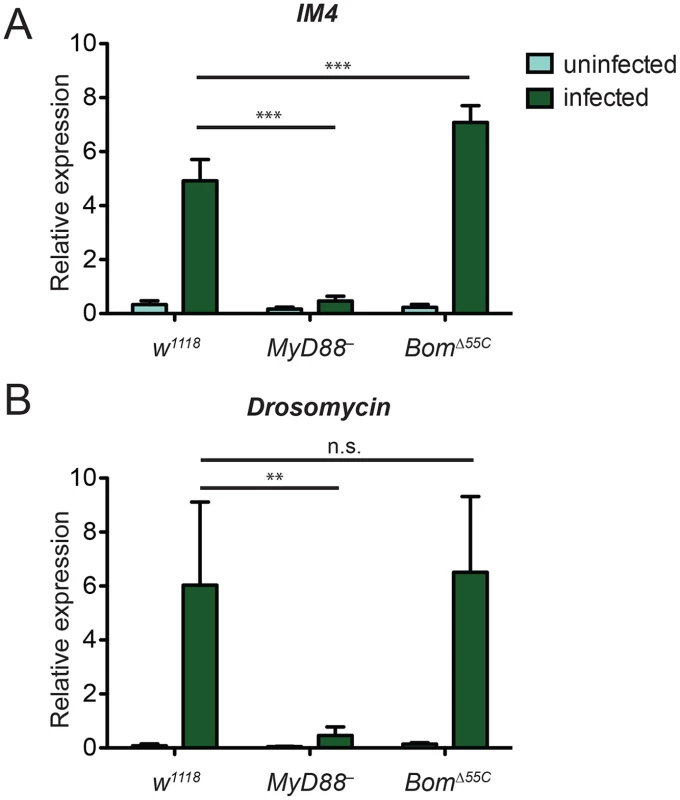
Transcript levels of the Toll-responsive genes (A) IM4 and (B) Drs in flies were measured in the absence of infection and six hours after infection with E. faecalis. Expression was measured by qRT-PCR and normalized to that of the ribosomal protein gene rp49 (rp49 = 1). Error bars represent SEM. Significance was measured by two-way ANOVA (** p<0.01, *** p<0.001, n.s. = not significant, p>0.05). These experiments reveal that the susceptibility of BomΔ55C flies to microbial infection does not reflect a general block in Toll signaling. Further, they strongly suggest that flies lacking Bom gene function have increased susceptibility to E. faecalis, C. glabrata, and F. oxysporum despite normal, Toll-mediated induction of AMP genes.
Bom peptides mediate infection resistance, not tolerance
Infection resistance is defined as the ability to clear microbes, while infection tolerance is the ability to endure the presence of microbes [35]. Expression of Toll-responsive genes appears unaffected in BomΔ55C flies. Is it the case that AMPs and other Toll-induced effectors kill pathogens in BomΔ55C flies, but the flies nevertheless die due to an inability to tolerate the infection? Alternatively, do BomΔ55C flies succumb because Bom peptides are in fact required to control and clear infections? We set out to distinguish between these hypotheses.
To assess resistance and tolerance, we assayed bacterial load over the course of an E. faecalis infection, using wild-type, MyD88-, and BomΔ55C flies in parallel. Because BomΔ55C and MyD88- flies have a median survival after E. faecalis infection of about 23 hours, time points were taken at intervals up to 18 hours. At two hours post-infection, all flies had similar bacterial loads (Fig 4A). At later time points, however, differences emerged. At six hours, the bacterial load was on average 3-fold greater in BomΔ55C than in the wild type. The bacterial load of MyD88- flies was similarly elevated relative to wild-type flies. At 18 hours, both BomΔ55C and MyD88- flies had a bacterial load at least 20-fold greater than did wild-type flies. This elevation in bacterial load in MyD88- and BomΔ55C flies over the course of infection suggests that Toll signaling in general, and Bom peptides specifically, contribute to resistance.
Fig. 4. Deletion of the 55C Bom genes impairs resistance to E. faecalis infection rather than tolerance. 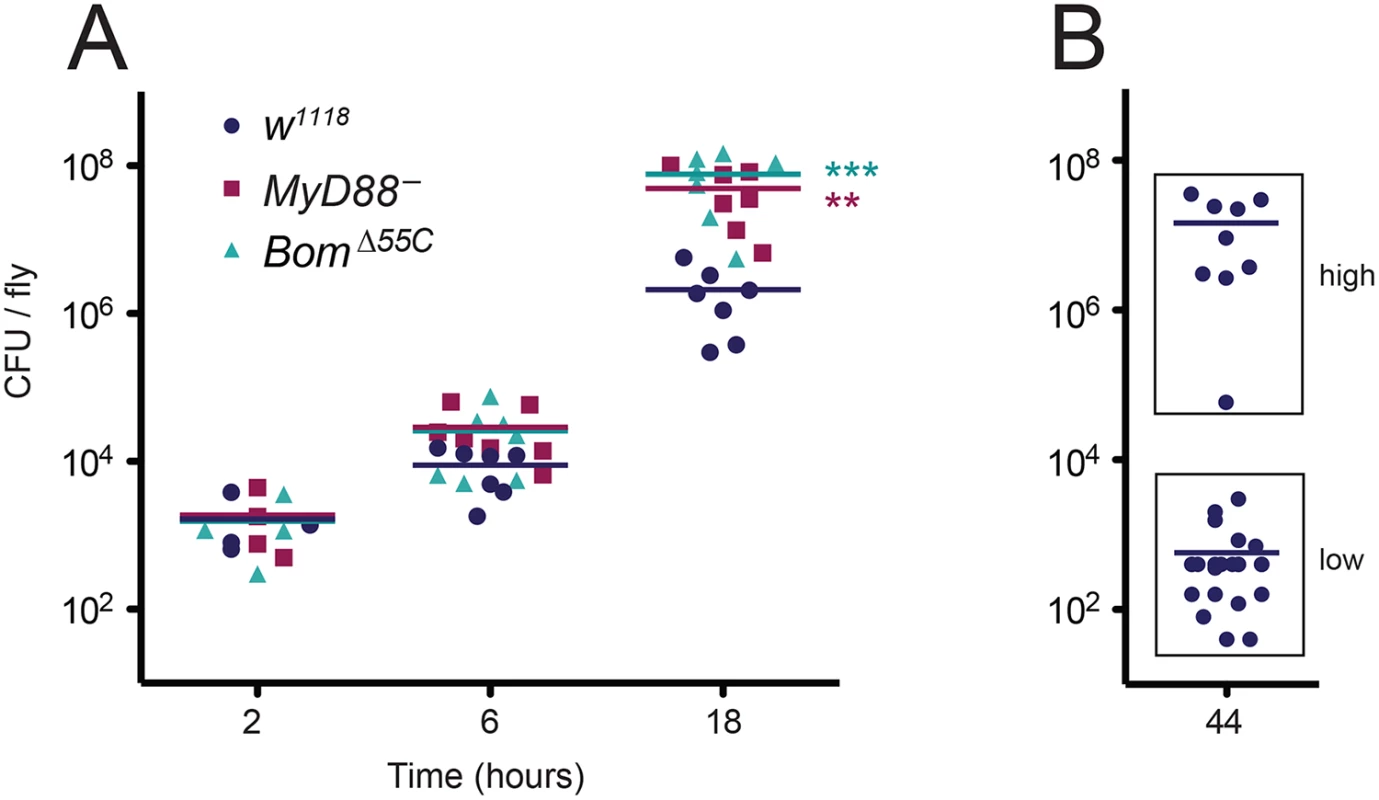
(A) Points indicate the mean CFU/fly from individual experiments using pools of 5–10 flies per genotype after E. faecalis infection at the indicated time point. Horizontal bars represent the means of the (four or more) independent experiments shown. Significance was measured by two-way ANOVA and is relative to the wild type (w1118) at the same time point (** p<0.01, *** p<0.001). (B) CFU of individual wild-type (w1118) flies at 44 hours post-infection. Horizontal bars represent means. “Low” (<3800 CFU/fly) and “high” (>3800 CFU/fly) populations were measured simultaneously during four independent collections of individual flies. Data were binned (indicated by boxes), and means were calculated separately. To further explore the questions of resistance and tolerance, we measured bacterial load in wild-type flies at 44 hours post-infection, an interval slightly shorter than their median survival time (48 hours). If Boms contribute to resistance rather than tolerance, the bacterial load in wild-type flies at 44 hours post-infection should be similar to the bacterial load of BomΔ55C and MyD88- flies at 18 hours post-infection.
The response of the wild type to E. faecalis infection necessitated a minor modification in our protocol for assaying bacterial load. In particular, some wild-type flies appear to clear E. faecalis infection, as evident in survival curves that do not reach 0% survival, but instead level out at an intermediate value (see, for example, Fig 2A). Foreseeing a bimodal distribution of bacterial loads among wild-type flies at 44 hours—some clearing infection and others not—we measured bacterial load for this time point in individual flies, rather than in groups. Measured in this way, there were indeed two groups with quite distinct bacterial loads. Of 32 wild-type flies still alive at this time point, 23 had a bacterial load less than 4,000 colony forming units (CFU) (Fig 4B, “low”). These low CFU flies presumably represent the fraction of the population that survives infection. The other nine wild-type flies had bacterial loads at 44 hours ranging from 60,000 to 36,000,000 CFU, comparable to those of BomΔ55C and MyD88- flies at 18 hours (compare Fig 4B, “high” to Fig 4A, 18h). Thus wild-type flies that succumb to infection do so at a bacterial load comparable to that in the mutants. We conclude that the BomΔ55C flies succumb to infection more quickly than the wild type due to a defect in resistance.
Deleting a subset of 55C Bom genes reveals overlap in Bom gene activity
Our experiments with BomΔ55C flies demonstrate that the 55C Bom cluster is required to provide Toll-mediated resistance to our test set of pathogens. Is the entire gene cluster required? If not, to what extent do the 55C genes overlap in function? To address these questions, we set out to assay how flies expressing a subset of the 55C Bom genes fare when infected with the same test set.
In the course of investigating the 55C cluster, we came across a publically available stock carrying an insertion in the 3’ UTR of IM2 of a MiMIC (Minos-mediated integration cassette) transposon [36]. By inducing the excision of this MiMIC element, we obtained two chromosomes that had lost the insertion. In one case the excision was imprecise. The resulting chromosome, hereafter BomΔleft, lacks IM2 and the five Bom genes to the left (proximal) of IM2, but retains the four Bom genes to the right (distal) of IM2 (see Fig 1C). The other chromosome, hereafter IM2ΔMi, had undergone a precise excision and thus provided a valuable control for subsequent studies.
To assay the activity of the four 55C Bom genes present in the imprecise excisant, we challenged BomΔleft, IM2ΔMi, and BomΔ55C flies by infection and monitored survival. For all pathogens tested, we defined the phenotype of BomΔ55C flies as lacking resistance and that of IM2ΔMi flies as having full resistance over a four to five day post-infection interval. Based on this scale, BomΔleft flies lacked resistance to E. faecalis, exhibited partial resistance to F. oxysporum, and had full resistance to C. glabrata (Fig 5A–5C). The BomΔleft chromosome thus provided a subset of the 55C Bom cluster immune activity.
Fig. 5. Loss of a subset of the 55C Bom genes has an intermediate effect on survival. 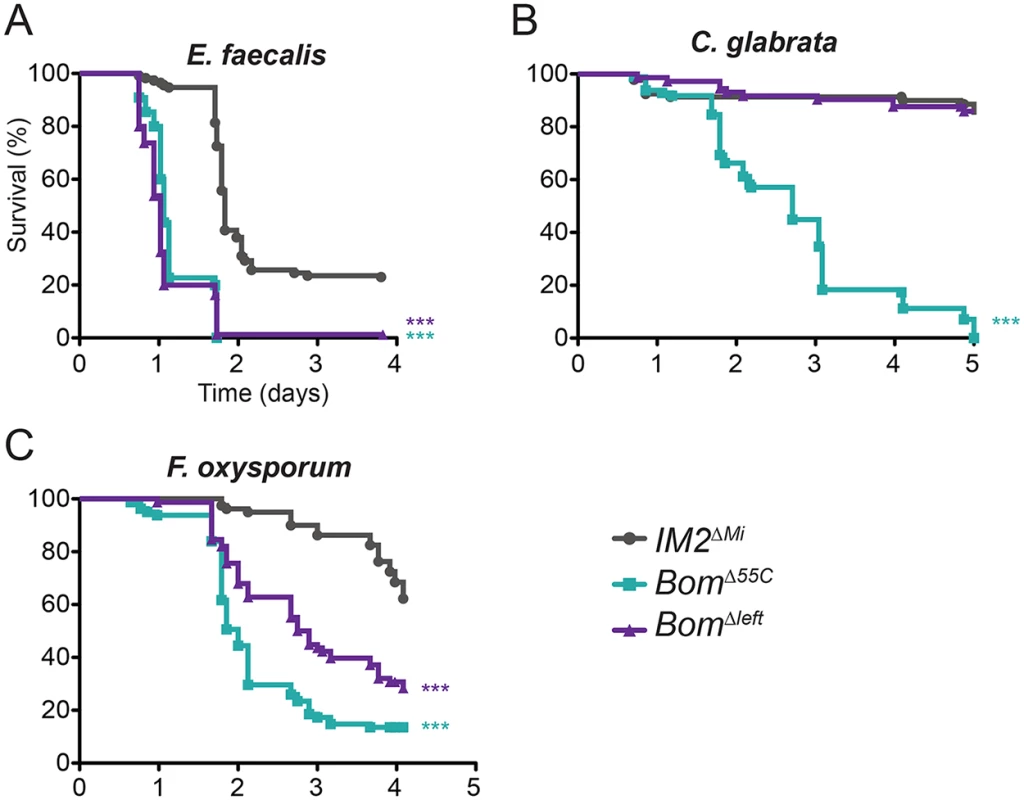
Graphs indicate survival at indicated intervals post-infection with (A) E. faecalis, (B) C. glabrata, and (C) F. oxysporum. Each curve represents the pooled results of at least three independent experiments involving 20 or more flies per genotype. Survival curves were compared using the Gehan-Breslow-Wilcoxon test. Significance is relative to IM2ΔMi and adjusted for multiple comparisons (*** p<0.0003, n.s. = not significant, p>0.0167). We draw three conclusions from the experiments shown in Fig 5. First, the wild-type resistance of BomΔleft flies to challenge with C. glabrata demonstrates that the complete 55C Bom gene set is not a prerequisite for Bom function. Second, the fact that BomΔleft flies have partial resistance to F. oxysporum indicates that at least some Bom genes overlap in specificity. Third, resistance to some pathogens requires more than one Bom peptide. In particular, wild-type resistance to F. oxysporum must require at least one of the genes present in BomΔleft but deleted in BomΔ55C, as well as one or more of the genes deleted in in BomΔleft. In and of themselves, these studies do not reveal whether Bom peptides have a narrow - or broad-spectrum of activity, but do provide clues in this regard, as addressed in the discussion.
Discussion
A gene family essential for infection resistance
We report here that Toll-mediated defenses against a bacterium, yeast, or filamentous fungus require Bom gene function. Having reached this conclusion based on loss-of-function phenotypes, we note that such an approach has only rarely been applied to the role of innate immune effectors [37–40]. The paucity of such studies has several likely causes. First, many effector genes, such as the Boms and the known AMP genes, encode peptides that are sufficiently small as to be relatively refractory to random mutagenesis. Second, large-scale screens that rely on reporter genes are useful for identifying lesions that block pathogen recognition or response pathway signaling, but opaque to disruptions in more downstream processes.
Perhaps the biggest obstacle, real or imagined, to loss-of-function studies of immune effectors has been the existence of families of closely related genes. One might reasonably expect significant overlap in gene function, meaning that multiple family members would need to be inactivated to uncover reliable phenotypes. Instead, researchers interested in knockout phenotypes have typically focused on those examples where paralogs are absent. Thus, for example, the loss-of-function study demonstrating that disruption of a mouse cathelicidin gene promoted invasive skin infection with Group A Streptococcus [40] relied on the fact that mice, unlike some other mammals, encode only one member of this gene family.
Bomanin peptide function
How do Bom peptides promote infection resistance? One possibility is that the Bom peptides support cellular immune function. To explore this question, we infected BomΔ55C flies with Staphylococcus aureus. Defense against S. aureus has been shown to involve cellular immune activities to a greater extent than for some other Toll-activating bacteria, including E. faecalis [41–46]. Although BomΔ55C flies succumbed to S. aureus infection more quickly than did the wild type, BomΔ55C survival was indistinguishable from that of MyD88- and the genetic background control, IM2ΔMi (S2A Fig). We thus found no evidence that the 55C Bom gene cluster contributes to Toll-independent cellular mechanisms of resistance. In additional studies of cellular immune functions, we found neither defects in wound site melanization in BomΔ55C adults nor any deficiency in hemocyte number in BomΔ55C larvae (S2B and S2C Fig). These experiments do not, however, preclude a role for for the Boms in Toll-dependent cellular immunity.
A likely alternative is that the Bom genes encode antimicrobial peptides (AMPs). Like many AMPs, Bom peptides are short, secreted, have intramolecular disulfide bonds, and undergo post-translational processing. Although short-form Bom peptides would be the shortest characterized Drosophila AMP, the mature form of Drosocin is just three amino acids longer [47]. Furthermore, both the Boms and the known AMPs populate the upper echelons of the sets of genes most highly upregulated upon activation of the Toll pathway. Specifically, at 12, 24, and 96 hours after natural infection by the fungus Beauveria bassiana, the 30 most highly upregulated genes include five or more Bom family members and five or more known AMP genes [24]. Additionally, mass spectrometry data indicate that a number of Bom peptides are as abundant as known AMPs, which after infection reach concentrations of 10–100 μM in the hemolymph [23, 32].
The structure and sequence of the 55C Bom cluster suggests that the Bom family arose by multiple gene duplications. Such events are enriched among loci involved in pathogen resistance and provide the opportunity for divergence in gene function driven by positive selection [48, 49]. One example is the Peptidoglycan Recognition Protein (PGRP) gene family, which consists of 13 genes, some clustered, encoding 19 proteins. While all PGRPs share a peptidoglycan-recognition domain, the functions of the proteins vary considerably [50]. Some activate the Toll or Imd signaling pathways and promote phagocytosis, autophagy, and melanization. Others suppress the Imd pathway, protecting commensal gut bacteria. A third class has direct bactericidal activity.
Gene duplications need not, however, result in functional divergence. Rather, there are circumstances in which gene duplications instead lead to changes in the level, location, or timing of expression of what is essentially the same gene product. Minor sequence variation will arise, but in general the coding regions will not bear the hallmarks of positive selection [51–53].
Comparing the effects of eliminating some or all Bom genes in the Bom 55C cluster, we observe differential effects on resistance to particular pathogens. How does this observation fit with the alternative potential outcomes for gene duplication?
If functional divergence has occurred, we would expect that at least some Bom peptides have a narrow-spectrum effector activity, i.e., are specific for a particular pathogen or set of pathogens. One or more of the six Bom genes deleted in the BomΔleft chromosome would be specific for E. faecalis, while one or more of the four 55C Bom genes remaining in BomΔleft would protect specifically against C. glabrata. There would need to be at least two peptides specific for F. oxysporum: one or more of the six genes deleted in the BomΔleft chromosome, as well as one or more of the four remaining 55C Bom genes.
Although our data can accommodate a narrow-spectrum activity model, we favor the idea that Bom peptides have a common, broad-spectrum activity. In this scenario, resistance to different pathogens would require different total Bom peptide levels, as could be produced by variation in the numbers of Bom genes. In particular, defense against E. faecalis would require the greatest level of Bom activity, while C. glabrata would require the least. Defense against F. oxysporum would require a level intermediate to that required for E. faecalis and C. glabrata.
When considered in toto, our survival data support this broad-spectrum effector model: the predicted hierarchy of Bom activity levels required for particular pathogens parallels the overall virulence levels for these pathogens (E. faecalis > F. oxysporum > C. glabrata). This holds true when virulence is measured either by the proportion of wild-type flies that succumb to infection or by the rates at which the mutants succumb after infection (compare Fig 2A, 2C and 2D). The parallel between required Bom activity level and pathogen virulence makes sense if we make the reasonable assumption that the broad-spectrum activity of Bom peptides is more efficient and rapid at higher concentrations.
Future comprehensive consideration of the Bom genes will necessitate taking into account four additional loci. Two of these, CG5778 and CG5791, are Bom genes located outside the 55C cluster. The remaining two, IM4 and IM14, encode peptides that lack a CXXC motif, but nevertheless exhibit sequence similarity with Bom family members. Furthermore, the IM4 and IM14 peptides, like the Bomanins listed in Fig 1A, are small, secreted, specific to the Drosophila genus, and robustly induced by Toll.
The functional relationship of Bom peptides and Drosomycin
Lemaitre and colleagues have reported that overexpression of a UAS-Drs construct using a ubiquitous GAL4 driver restores F. oxysporum resistance to flies lacking both Toll and Imd pathway function [54]. In our studies, however, we found that BomΔ55C flies induce Drs expression upon infection (see Fig 3B), but succumb as rapidly as flies lacking Toll signaling (see Fig 2). Why was a requirement for Bom gene function not apparent in the Lemaitre study? One possibility is that loading the flies with high levels of Drosomycin prior to infection obviates the need for additional Toll-induced loci, including the Bom genes. An alternative explanation lies in the fact only inducible Bom expression was blocked in the Lemaitre study, whereas our study eliminated all Bom gene function. It might be that the synergistic activity of both Drosomycin and Boms is required to defend against F. oxysporum, but that a basal, Toll-independent level of Bom expression is sufficient for this synergy. In support of this idea, RNA-seq data from modENCODE demonstrate that expression of many Bom genes is robust even in the absence of infection [55].
Contribution of Bom activity to Toll-mediated defense
Given that innate immune signaling pathways direct expression of large batteries of effector genes upon infection, including many AMPs, one might have expected that disabling a small subset of that repertoire would have only minor effects on the overall immune response. That is not what we observe. Instead, elimination of Bom activity is indistinguishable in phenotype from loss of the entire Toll-mediated immune defense for the pathogens tested, although Toll signaling is intact. We envision at least four explanations for the essential role of the Bom gene family:
The Boms have a unique and central role in Toll-mediated defense. This might seem unlikely, given that the Bom family is apparently specific to the Drosophila genus and thus represents a relatively young family of effectors. However, such a model is, in fact, in keeping with recent studies on genes that are essential in the sense of being required for viability. In particular, we now know that essentiality is found in equal proportions among old and young Drosophila genes, where age is measured on the scale of divergence time between species [56]. It could therefore be that a gene family and associated function that arose fairly recently has become a dominant and essential feature of the innate immune response.
The Boms are essential for Toll-mediated responses specific to certain microbial pathogens. We know from a variety of expression studies that the entire Toll effector repertoire is upregulated regardless of the source of the activating signal, PAMP or otherwise. By this model, Toll activates a large number of effectors, each attacking a subset of pathogens, rather than collectively fighting a common target pathogen. If so, there should be additional pathogens for which Toll is required and the Bom peptides are not.
The Bom genes and other effectors synergize, such that loss of just a single factor disrupts defense as strongly as loss of all components. By this model, innate immunity involves a network of effector functions that comprise multiple hubs, each making a vital contribution to defense.
The Bom family and other components of the Toll repertoire are each expressed at the minimal level required for resistance. There is good evidence that immune activation requires an energy tradeoff with metabolic processes [57, 58]. As a result, limiting the resources used by an immune response can be beneficial to overall health.
By either of the last two models, knocking out other effector families should result in the same phenotype observed with the BomΔ55C deletion, i.e., inactivation of Toll defenses. Testing this prediction thus holds promise for a broader understanding of innate immune effector function in vivo.
Materials and Methods
Flies and mutant generation
Flies were raised at 25°C on standard cornmeal agar media. The w1118 strain was used as the wild type. MyD88- flies were MyD88kra1, and imd- flies were imdshadok. All flies were homozygous for the listed mutations.
TALEN mutagenesis was conducted as previously described [59]. In vitro transcription was conducted using the Ambion Megascript kit with a Promega 5’-cap analog. TALEN transcripts were injected into a fly line containing a MiMIC element in the IM2 gene (y1w*; Mi[MIC]IM2MI01019, Bloomington stock center, #32727). The MiMIC element carries the mini-yellow marker. F0 flies were crossed to yw; Sco/CyO and the resulting y- F1 flies were collected and crossed to yw; Sco/CyO. Stocks were established and genotyped using Phusion polymerase and primers flanking the predicted deletion end points. We confirmed the exact endpoints of the deletions by sequencing the PCR product.
Excision of the MiMIC element from Mi[MIC]IM2MI01019 was conducted as described previously [60] with the transposase source coming from stock y1 w*; snaSco/SM6a, P{hsILMiT}2.4 (obtained from Bloomington stock center, stock #36311).
Microbial culture
Microbes were prepared for infection experiments as follows. Enterococcus faecalis strain NCTC 775 (ATCC 19433) and Enterobacter cloacae were cultured in LB media at 37°C and concentrated to OD600 = 10 in 20% glycerol. For heat-killed E. faecalis challenges, cultures were concentrated to OD600 = 10 in 20% glycerol and then boiled for 30 minutes. Staphylococcus aureus subsp. aureus Rosenbach (ATCC 29213) was cultured in LB media at 37°C and concentrated to OD600 = 0.5 in 20% glycerol. Candida glabrata strain CBS 138 (ATCC 2001) was cultured in YPD media at 30°C and concentrated to OD600 = 50 in 20% glycerol for infection. Fusarium oxysporum f. sp. lycopersici (obtained from the Fungal Genetics Stock Center) was cultured on oatmeal agar plates for 7–10 days at 29°C before being strained through steel wool to isolate spores. Purified spores were resuspended in 20% glycerol and stored at -80°C until infection.
Drosophila infection, survival analysis, and bacterial load analysis
For infection, at least 20 2–7 day old, male flies per genotype were anaesthetized and septically wounded in the anterior lateral thorax with a size 000 insect pin dipped in a suspension of pathogen. Survival analysis was conducted essentially as described previously [61]. After infection, flies were incubated at 25°C (live or heat-killed E. faecalis or live S. aureus) or 29°C (clean wounding, F. oxysporum, C. glabrata, and E. cloacae), and the number of dead flies were counted at least once per day for the given time interval. Flies that died within 6 hours of infection were excluded from analysis, except in challenges with a clean needle or heat-killed E. faecalis. Colony forming units (CFUs) were assayed as described previously [62].
Cellular immunity assays
For the wound site melanization assay, 2–7 day old males were wounded as described above with a clean insect pin, incubated for three days at 25°C, and visually inspected for melanization at the wound site. Hemocytes from five wandering third instar larvae per genotype per experiment were obtained and counted as previously described [63].
Gene expression quantitation
RNA was prepared using Trizol (Ambion) from 2–7 day old males, and first-strand cDNA was synthesized with the SuperScript II kit (Invitrogen). Quantitative RT-PCR was performed on an iQ5 cycler (BioRad) using iQ SYBR Green Supermix (BioRad).
Data analysis
GraphPad Prism was used to run statistical analyses. Survival data were plotted on a Kaplan-Meier curve and the Gehan-Breslow-Wilcoxon test was used to determine significance. The Gehan-Breslow-Wilcoxon test is recommended for analysis of infection with sublethal pathogen doses; where a sublethal dose is defined as a dose where some proportion of wild-type flies survive. Hemocyte count data were analyzed by one-way ANOVA using the Bonferroni post method. Quantitative RT-PCR and bacterial load data were analyzed by two-way ANOVA using the Bonferroni post method.
Supporting Information
Zdroje
1. Medzhitov R, Janeway C Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173(44):89–97.
2. Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311 : 1–16. 17048703
3. Leulier F, Lemaitre B. Toll-like receptors—taking an evolutionary approach. Nature reviews Genetics. 2008 Mar;9(3):165–78. doi: 10.1038/nrg2303 18227810
4. Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3(2):121–6. 11812988
5. Wasserman SA. A conserved signal transduction pathway regulating the activity of the rel-like proteins dorsal and NF-κB. Mol Biol Cell. 1993;4(8):767–71. 8241564
6. Lemaitre B, Emmanuelle N, Michaut L, Reichhart J - M, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86 : 973–83. 8808632
7. Rutschmann S, Kilinc A, Ferrandon D. Cutting edge: the toll pathway is required for resistance to gram-positive bacterial infections in Drosophila. J Immunol. 2002 Feb 15;168(4):1542–6. 11823479
8. Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, et al. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92(21):9465–9. 7568155
9. Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001 Oct;1(4):503–14. 11703941
10. Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-kappaB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349 : 25–60. doi: 10.1007/82_2010_107 20852987
11. Valanne S, Wang JH, Ramet M. The Drosophila Toll signaling pathway. J Immunol. 2011 Jan 15;186(2):649–56. doi: 10.4049/jimmunol.1002302 21209287
12. Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001 Dec 13;414(6865):756–9. 11742401
13. Bischoff V, Vignal C, Boneca IG, Michel T, Hoffmann JA, Royet J. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004 Nov;5(11):1175–80. 15448690
14. Gobert V, Gottar M, Matskevich AA, Rutschmann S, Royet J, Belvin M, et al. Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science. 2003 Dec 19;302(5653):2126–30. 14684822
15. Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, et al. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A. 2009 Jul 28;106(30):12442–7. doi: 10.1073/pnas.0901924106 19590012
16. Lindsay SA, Wasserman SA. Conventional and non-conventional Drosophila Toll signaling Dev Comp Immunol. 2013 Jan;42(1):16–24. doi: 10.1016/j.dci.2013.04.011 23632253
17. Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002 Apr 12;296(5566):359–62. 11872802
18. Kaneko T, Silverman N. Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol. 2005 Apr;7(4):461–9. 15760446
19. Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006 Jul;7(7):715–23. 16767093
20. Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002 Apr 11;416(6881):640–4. 11912488
21. Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002 Apr 11;416(6881):644–8. 11912489
22. De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A. 2001 Oct 23;98(22):12590–5. 11606746
23. Uttenweiler-Joseph S, Moniatte M, Lagueux M, Van Dorsselaer A, Hoffmann JA, Bulet P. Differential display of peptides induced during the immune response of Drosophila: a matrix-assisted laser desorption ionization time-of-flight mass spectrometry study. Proc Natl Acad Sci U S A. 1998 Sep 15;95(19):11342–7. 9736738
24. De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J. 2002 Jun 3;21(11):2568–79. 12032070
25. Levy F, Rabel D, Charlet M, Bulet P, Hoffmann JA, Ehret-Sabatier L. Peptidomic and proteomic analyses of the systemic immune response of Drosophila. Biochimie. 2004 Sep-Oct;86(9–10):607–16. 15589689
26. Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, Reichhart JM, et al. A genome-wide analysis of immune responses in Drosophila. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15119–24. 11742098
27. Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002 Nov;3(5):711–22. 12431377
28. Boman HG, Nilsson I, Rasmuson B. Inducible antibacterial defence system in Drosophila. Nature. 1972 May 26;237(5352):232–5. 4625204
29. Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–8. 7019715
30. Lee JY, Boman A, Sun CX, Andersson M, Jornvall H, Mutt V, et al. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9159–62. 2512577
31. Strominger JL. Animal antimicrobial peptides: ancient players in innate immunity. J Immunol. 2009 Jun 1;182(11):6633–4. doi: 10.4049/jimmunol.0990038 19454654
32. Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, et al. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994 Dec 30;269(52):33159–63. 7806546
33. Tauszig-Delamasure S, Bilak H, Capovilla M, Hoffmann JA, Imler JL. Drosophila MyD88 is required for the response to fungal and Gram - positive bacterial infections. Nat Immunol. 2002;3(1):91–7. 11743586
34. Quintin J, Asmar J, Matskevich AA, Lafarge MC, Ferrandon D. The Drosophila Toll pathway controls but does not clear Candida glabrata infections. J Immunol. 2013 Mar 15;190(6):2818–27. doi: 10.4049/jimmunol.1201861 23401590
35. Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30 : 271–94. doi: 10.1146/annurev-immunol-020711-075030 22224770
36. Venken KJ, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat methods. 2011 Sep;8(9):737–43. 21985007
37. Hamilton C, Bulmer MS. Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Dev Comp Immunol. 2012 Feb;36(2):372–7. doi: 10.1016/j.dci.2011.07.008 21824492
38. Moule MG, Monack DM, Schneider DS. Reciprocal analysis of Francisella novicida infections of a Drosophila melanogaster model reveal host-pathogen conflicts mediated by reactive oxygen and imd-regulated innate immune response. PLoS Pathog. 2010;6(8):e1001065. doi: 10.1371/journal.ppat.1001065 20865166
39. Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 2002 Sep;3(9):852–6. 12189180
40. Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001 Nov 22;414(6862):454–7. 11719807
41. Nehme NT, Quintin J, Cho JH, Lee J, Lafarge MC, Kocks C, et al. Relative roles of the cellular and humoral responses in the Drosophila host defense against three gram-positive bacterial infections. PLoS One. 2011;6(3):e14743. doi: 10.1371/journal.pone.0014743 21390224
42. Binggeli O, Neyen C, Poidevin M, Lemaitre B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 2014 May;10(5):e1004067. doi: 10.1371/journal.ppat.1004067 24788090
43. Defaye A, Evans I, Crozatier M, Wood W, Lemaitre B, Leulier F. Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J Innate Immun. 2009;1(4):322–34. doi: 10.1159/000210264 20375589
44. Haine ER, Moret Y, Siva-Jothy MT, Rolff J. Antimicrobial defense and persistent infection in insects. Science. 2008 Nov 21;322(5905):1257–9. doi: 10.1126/science.1165265 19023083
45. Ulvila J, Vanha-aho LM, Kleino A, Vaha-Makila M, Vuoksio M, Eskelinen S, et al. Cofilin regulator 14-3-3zeta is an evolutionarily conserved protein required for phagocytosis and microbial resistance. J Leukoc Biol. 2011 May;89(5):649–59. doi: 10.1189/jlb.0410195 21208897
46. Charroux B, Royet J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9797–802. doi: 10.1073/pnas.0903971106 19482944
47. Bulet P, Dimarcq JL, Hetru C, Lagueux M, Charlet M, Hegy G, et al. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J Biol Chem. 1993 Jul 15;268(20):14893–7. 8325867
48. Sackton TB, Lazzaro BP, Schlenke TA, Evans JD, Hultmark D, Clark AG. Dynamic evolution of the innate immune system in Drosophila. Nat Genet. 2007 Dec;39(12):1461–8. 17987029
49. Thomas JH. Analysis of homologous gene clusters in Caenorhabditis elegans reveals striking regional cluster domains. Genetics. 2006 Jan;172(1):127–43. 16291650
50. Kurata S. Peptidoglycan recognition proteins in Drosophila immunity. Dev Comp Immunol. 2014 Jan;42(1):36–41. doi: 10.1016/j.dci.2013.06.006 23796791
51. Yang WY, Wen SY, Huang YD, Ye MQ, Deng XJ, Han D, et al. Functional divergence of six isoforms of antifungal peptide Drosomycin in Drosophila melanogaster. Gene. 2006 Sep 1;379 : 26–32. 16824706
52. Tian C, Gao B, Rodriguez Mdel C, Lanz-Mendoza H, Ma B, Zhu S. Gene expression, antiparasitic activity, and functional evolution of the drosomycin family. Mol Immunol. 2008 Sep;45(15):3909–16. doi: 10.1016/j.molimm.2008.06.025 18657321
53. Deng XJ, Yang WY, Huang YD, Cao Y, Wen SY, Xia QY, et al. Gene expression divergence and evolutionary analysis of the drosomycin gene family in Drosophila melanogaster. J Biomed Biotechnol. 2009;2009 : 315423. doi: 10.1155/2009/315423 19888430
54. Tzou P, Reichhart JM, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2152–7. 11854512
55. Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011 Mar 24;471(7339):473–9. doi: 10.1038/nature09715 21179090
56. Chen S, Zhang YE, Long M. New genes in Drosophila quickly become essential. Science. 2010 Dec 17;330(6011):1682–5. doi: 10.1126/science.1196380 21164016
57. Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster—from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014 Nov 25;14(12):796–810. doi: 10.1038/nri3763 25421701
58. Dionne M. Immune-metabolic interaction in Drosophila. Fly. 2014 Apr-May;8(2):75–9. doi: 10.4161/fly.28113 25483252
59. Liu J, Li C, Yu Z, Huang P, Wu H, Wei C, et al. Efficient and specific modifications of the Drosophila genome by means of an easy TALEN strategy. J Genet Genomics. 2012 May 20;39(5):209–15. doi: 10.1016/j.jgg.2012.04.003 22624882
60. Metaxakis A, Oehler S, Klinakis A, Savakis C. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics. 2005 Oct;171(2):571–81. 15972463
61. Neyen C, Bretscher AJ, Binggeli O, Lemaitre B. Methods to study Drosophila immunity. Methods. 2014 Jun 15;68(1):116–28. doi: 10.1016/j.ymeth.2014.02.023 24631888
62. Kuo TH, Handa A, Williams JA. Quantitative measurement of the immune response and sleep in Drosophila. J Vis Exp. 2012 (70):e4355. doi: 10.3791/4355 23242373
63. Kacsoh BZ, Schlenke TA. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS One. 2012;7(4):e34721. doi: 10.1371/journal.pone.0034721 22529929
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



