-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
Differentiation of naïve CD4+ cells into functionally distinct effector helper T cell subsets, characterised by distinct “cytokine signatures,” is a cardinal strategy employed by the mammalian immune system to efficiently deal with the rapidly evolving array of pathogenic microorganisms encountered by the host. Since the TH1/TH2 paradigm was first described by Mosmann and Coffman, research in the field of helper T cell biology has grown exponentially with seven functionally unique subsets having now been described. In this review, recent insights into the molecular mechanisms that govern differentiation and function of effector helper T cell subsets will be discussed in the context of microbial infections, with a focus on how these different helper T cell subsets orchestrate immune responses tailored to combat the nature of the pathogenic threat encountered.
Published in the journal: . PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003905
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1003905Summary
Differentiation of naïve CD4+ cells into functionally distinct effector helper T cell subsets, characterised by distinct “cytokine signatures,” is a cardinal strategy employed by the mammalian immune system to efficiently deal with the rapidly evolving array of pathogenic microorganisms encountered by the host. Since the TH1/TH2 paradigm was first described by Mosmann and Coffman, research in the field of helper T cell biology has grown exponentially with seven functionally unique subsets having now been described. In this review, recent insights into the molecular mechanisms that govern differentiation and function of effector helper T cell subsets will be discussed in the context of microbial infections, with a focus on how these different helper T cell subsets orchestrate immune responses tailored to combat the nature of the pathogenic threat encountered.
Introduction
Bidirectional intercellular communication between innate and adaptive immune systems is crucial for success of immunity to microbial infection. The activation and fate of clonally selected cells of the adaptive immune system is strongly influenced by innate effector cells, and orchestration of adaptive responses to pathogenic microorganisms requires synergistic collaboration with the innate immune system to efficiently resolve infection. Via production of diverse pleiotropic cytokines, effector CD4+ T helper (TH) cells function to direct efficient immune reactions by dictating the actions of both innate and adaptive arms of the immune system. Through their ability to coordinate innate/adaptive effector cell activity, TH cells directly and/or indirectly influence almost every aspect of an immune response: they provide signals to help B cells undergo class switch recombination (CSR), affinity maturation and differentiation, perpetuate CD8+ T cell responses, regulate the recruitment and function of innate effector cells, and contract responses to resolve and/or adjust the magnitude of inflammation.
Pathogen-specific CD4+ T cells coordinate immune responses by differentiating into discrete subsets of effector TH cells defined by production of distinct cytokine “signatures”. The specific differentiated state of effector TH subsets is attributed to their expression of subset-specific transcription factors that programme subset-specific transcriptomes, whilst concomitantly suppressing alternative fates the precursor could have assumed [1]. Induction of these transcriptional programmes is predominantly determined by innate-immune-derived cytokines present during MHC-II-restricted T cell receptor (TCR)-mediated activation released into the “immunological synapse” by antigen-presenting cells, particularly by DCs (examples shown in Figure 1). DCs are themselves instructed to produce cytokines following detection of specific pathogen-associated molecular patterns (PAMPs) on foreign microbes through pattern recognition receptors (PRRs) during pathogen encounter in the periphery [2]. Thus, important information regarding the nature of the specific pathogens can be conveyed to developing effector helper T cells that subsequently differentiate into an effector programme equipped with a particular cytokine-secreting repertoire, thereby eliciting a pathogen-tailored immune response.
Fig. 1. Currently known TH cell subsets. 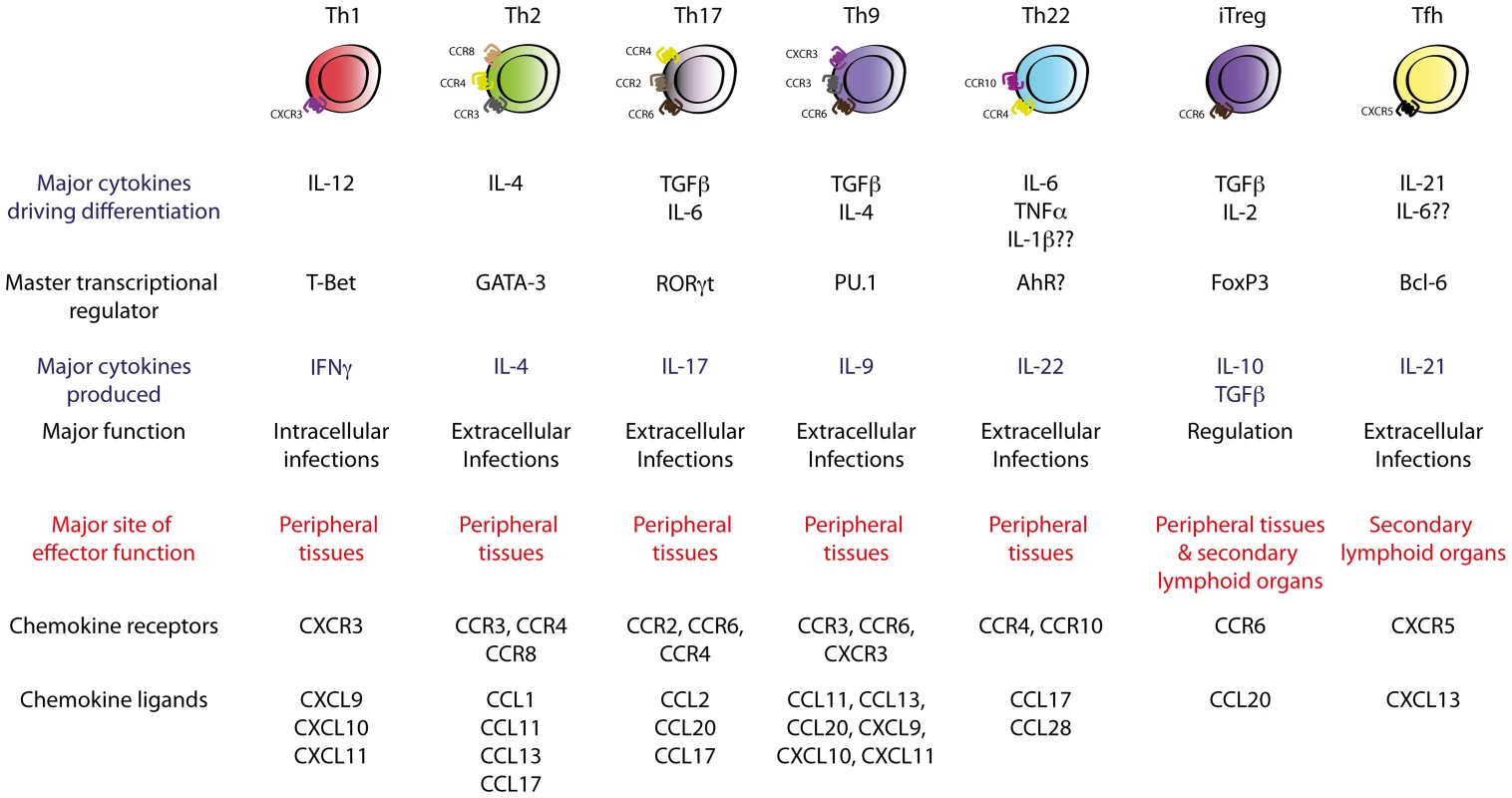
Polarising cytokines encountered during TH cell differentiation drive the expression of subset-specific transcription factors, which imprint subset-specific transcriptomes in the TH cell. These transcription factors define the effector function and migratory capability of the TH cell via regulation of subset-specific cytokines and chemokine receptors. These views of helper T cell differentiation and function were first introduced by Mosmann and Coffman in 1986, who demonstrated that T cell clones were divisible into two subsets, termed TH1 and TH2, based on their mutually exclusive production of interferon (IFN)-γ or interleukin (IL)-4, -5, and -13, respectively [3]. This subdivision was of major significance as IFN-γ-producing TH1 cells were subsequently shown to be critical in host defences against intracellular pathogens by activating cell-mediated immunity, whilst TH2-driven responses were essential for efficient humoral responses against extracellular microbes. The TH1/TH2 paradigm served as a useful conceptual construct for understanding how TH cells controlled different arms of the immune system, and dysregulation of TH1/TH2 responses has since been implicated in the pathogenesis of many immune-related disorders such as autoimmune and allergic disease. Development of techniques such as multi-parameter flow cytometry and engineering of fate-mapping cytokine reporter mice has recently facilitated major progress in TH cell biology, with seven functionally unique TH subsets now described. These comprise TH1, TH2, TH17, follicular helper T cells (TFH), inducible T regulatory cells (iTreg), and the most recently described and least well-characterised subsets, TH9 and TH22 cells, each of which is produced upon antigen presentation in the presence of specific cytokines or sets of cytokines (Figure 1). In this review, recent insights into the mechanisms that govern differentiation, migration, and function of effector TH cells will be discussed in the context of microbial infection, focussing on the contribution of emerging subsets of effector helper T cells, with less emphasis on TH1 and TH2 subsets, whose function has been well-established and is described elsewhere [4]. The function of Tregs in protective immunity will also not be discussed in this review as this has been the subject of recent comprehensive review elsewhere [5].
T Helper 1 (TH1) and T Helper 2 (TH2)
TH1 differentiation from naïve precursors is initiated by signal transducer and activator of transcription (STAT)-1 activation downstream of type 1 interferon, IFN-γ and IL-27 signalling, which induces expression of the TH1-specific master transcription factor T-bet [6]–[9]. This process enables activated CD4+ T cell responsiveness to DC-derived IL-12 via T-bet-mediated induction of the high-affinity IL-12 receptor beta 2 chain on the cell surface [10]–[12]. IL-12 signalling through STAT4, together with T-bet, directly transactivates the Ifng gene, which further promotes TH1 differentiation via STAT1 activation in an autoregulatory feedback loop [13]–[15]. T-bet also drives expression of the chemokine receptor CXCR3, which facilitates TH1 migration to inflamed sites of pathogen encounter where CXCL9, CXCL10, and/or CXCL11 are produced (Figure 1) [12], [15]–[17]. TH1 cells orchestrate the cell-mediated cytotoxic response against intracellular pathogens principally via provision of IFNγ to enhance macrophage activation and promote activation of antigen-specific cytotoxic T lymphocytes (CTLs). Classical infections controlled or cleared by effective TH1 responses include intracellular bacteria such as Listeria monocytogenes, Salmonella species, and Mycobacterium tuberculosis, intracellular parasites such as Leishmania donovani, and a number of viral pathogens [18]–[25]. In addition, TH1-derived production of the pro-inflammatory cytokine IL-21 has also been shown to be a key regulator of the long-term maintenance and functionality of antigen-specific CTLs important for protection against both acute and chronic infection with lymphocytic choriomeningitis virus (LCMV) [26]–[28].
Despite the appreciation of the existence of the TH2 subset for more than 25 years, the molecular mechanisms that govern TH2 differentiation remain controversial. Early reports demonstrated that the TH2 differentiation programme is set up via STAT6 activation downstream of IL-4 signalling, directly transactivating the TH2-lineage-specific transcription factor GATA-3 that in turn induces expression of the TH2-specific cytokine genes Il4, Il5, and Il13 [29]–[36]. However, recent studies suggest that TH2 cell induction may be far more complex than originally described, with reports that TH2 differentiation can occur independently of the STAT6/IL-4 axis [37] and may require additional cytokines including IL-2, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) (reviewed in [4], [38]). Nevertheless, production of IL-4, -5, and -13 by TH2 cells plays important roles in the activation and recruitment of basophils, induction of eosinophilia, regulation of antibody-dependent cell-mediated cytotoxicity (ADCC) mechanisms, and, by acting on resident cells at sites of inflammation, creates a hostile environment that favours extracellular microbial expulsion [4]. TH2 cells express the chemokine receptors CCR3, CCR4, and CCR8, and thus migrate to sites expressing their ligands in response to infection (Figure 1) [39], [40]. Effective TH2 responses are required for host defence against extracellular parasites such as Schistosoma mansoni or Trichuris muris [41]–[44].
The subdivision of T cells into TH1 and TH2 subsets has utility in understanding how the adaptive immune system tailors responsiveness to different types of pathogens by directing the activation of distinct immune components. Clearly however, the TH1 and TH2 subdivision is an oversimplification as the substantial pathogen diversity warrants more than two broad types of immune response. Therefore, it is perhaps surprising that it was only relatively recently that the TH1 and TH2 paradigm has been expanded to definitively include additional subsets of T cells and the role of these subsets in responses to distinct microbial challenges has been interrogated. In the remainder of this review, these emerging subsets of T cells and their role in protective immunity will be described.
T Helper 17 (TH17)
It was not until 2005 that a third major population of effector TH cells was described on the basis of the observation that peripheral CD4+ T cells could differentiate into a distinct lineage in a GATA-3 – and T-bet-independent fashion [45], [46]. Early reports suggested that these cells did not produce molecules commonly associated with TH1 or TH2 subsets but characteristically expressed the highly pro-inflammatory cytokines IL-17A and IL-17F, and were subsequently designated TH17. However, later studies have demonstrated that subsets of TH17 cells can produce IFN-γ, IL-4, or IL-13 under certain circumstances [47]–[50], although the function of TH17-derived IFN-γ, IL-4, or IL-13 has yet to be explored in infectious models. TH17 differentiation requires IL-6 and is also promoted by the otherwise immunosuppressive cytokine TGF-β1 (Figure 1) [51]–[53]. Signal transduction downstream of these cytokines, including STAT3 activation downstream of the IL-6 receptor, induces expression of the TH17-lineage-specific transcription factor RORγt, which directly transcribes the TH17-lineage-specific cytokines Il17a and Il17f (Figure 1) [54]–[57]. IL-6-mediated induction of IL-21 during TH17 cell differentiation is reported to reinforce TH17-lineage commitment via STAT3 activation downstream of the IL-21 receptor in an autocrine manner [53], [58], [59]. It has also been reported that autocrine TGF-β1 promotes TH17 cell differentiation in vivo [60]. Whilst addition of IL-6 and TGF-β1 into naïve CD4+ T cell cultures does indeed drive TH17 cell differentiation, the in vivo requirements of TH17 cell differentiation are far more complex than these in vitro conditions. Recent reports suggest that TH17 cell differentiation can be induced independent of TGF-β1 signalling when driven by the inflammatory cytokines IL-6, IL-1β, and IL-23 [61]. TH17 cells induced independently of TGF-β1 (termed TH17(23), owing to their requirement for IL-23) appear to possess more inflammatory characteristics than conventional TGF-β1-driven TH17 cells (TH17(β)). Furthermore, it has also recently been shown that IL-6 and TGF-β3 drive differentiation of TH17 cells that are functionally and molecularly distinct to the conventional TH17(β) cell [62]. Thus, it is likely that TH17 cells in any given response may comprise a heterogeneous population of distinct types of TH17 cells that arise in discrete cytokine microenvironments, possess distinct but similar transcriptomes, and subsequently possess different cytokine-secreting repertoires and functions. However, these hypotheses remain to be extensively tested. Characteristically, TH17 cells express the chemokine receptor CCR6 and their homing is thereby regulated by the CCR6 ligand, CCL20, at sites of infection [63]. The near-ubiquitous expression of the IL-17 receptor on non-haematopoietic cells facilitates the broad physiological functions of TH17 cells during inflammation. Through the induction of the inflammatory chemotactic factors CXCL1, CXCL2, CXCL5, and CXCL8 at sites of inflammation via production of IL-17A/F, IL-22, and GM-CSF, TH17-mediated responses are dominated by the inflammatory and phagocytic functions of neutrophils [64]. Other TH17-mediated functions include induction of antimicrobial peptides (including S100 proteins and β-defensins), promotion of granulopoiesis via induction of G-CSF, and enhancement of monocyte and neutrophil activation to promote their phagocytic activity [64].
Whilst TH17 cells represent, in most cases, the major source of adaptive IL-17 during microbial infection, IL-17 elicited from non-TH cell sources can also be a determining factor in host defence. Invariant natural killer T (iNKT) cells, natural killer (NK) cells, γδ-T cells, and type 3 innate lymphoid cells (ILC) (including lymphoid tissue-inducer (LTi) cells) have all been shown to produce protective innate-derived IL-17 in response to infection [65]–[68]. The importance of IL-17 derived from non-TH17 cell origins has recently been reviewed elsewhere [69], [70].
Recent gene-knockout studies have demonstrated the vital importance of IL-17-mediated inflammatory responses for host defences at epithelial barriers, particularly against notoriously persistent extracellular bacteria and fungi (Figure 2, panel A). Seminal work by Ye et al. demonstrated the critical importance of IL-17 for protective immunity in a murine model of extracellular bacterial infection using Klebsiella pneumoniae in IL-17R-deficient mice [71]. These mice were more susceptible to intranasal K. pneumoniae infection relative to WT counterparts, which correlated with significant delay in neutrophil recruitment into the alveolar space and heightened dissemination of bacteria into the circulation [71]. Numerous studies that followed confirmed the essential role of IL-17 in host protection against K. pneumoniae [71]–[73]. Mice infected with other extracellular bacteria including Citrobacter rodentium [74], [75], Bordetella pertussis [76], [77], Porphyromonas gingivalis [78], [79], or Streptococcus pneumoniae [80], [81], for example, also mount protective IL-17 responses, and disruption of IL-17A or its receptor leads to exacerbated bacterial burden or dissemination, increased disease susceptibility resulting from defective induction of CXC chemokines, and impaired neutrophil recruitment to sites of bacterial inoculation. Mice with deficiencies in IL-23, a cytokine axis critical for the stabilisation of the TH17 phenotype [82], also display exacerbated pathology associated with numerous extracellular bacterial infections. IL-23p19-deficient mice, akin to IL-17 – or IL-17R-deficient mice, also fail to effectively mount protective IL-17 responses to C. rodentium [52] and K. pneumoniae [72] infections. In the absence of these components of the TH17 – and IL-17-producing innate cell response, bacterial clearance is impeded, leading to augmented bacterial dissemination and disease susceptibility associated with reduced early IL-17-mediated neutrophil infiltration. Importantly, administration of recombinant IL-17 into IL-23-deficient infected mice restored neutrophilia at sites of inoculation [72], demonstrating the critical importance of the IL-23/IL-17 axis in host defence against various extracellular bacterial infections. Whilst these studies strongly implicate a protective role for the IL-23/IL-17 axis in protection against extracellular infections at epithelial surfaces, the precise cellular origin of IL-17 remains controversial. In the context of C. rodentium infection, both an early innate and late adaptive source of IL-17 is thought to be crucial to host protection. Interestingly, IL-17 responses early during C. rodentium infection were shown to be elicited from a specialised subset of CD4+ T cells present within the lamina propria (LP) [83]. Differentiation of these cells was dependent on the innate immune sensor receptors NOD-1 and NOD-2, which were shown to regulate intestinal DC-derived IL-6 and subsequent differentiation of these LP-resident “early” TH17 cells. Importantly, NOD-1/NOD-2 deficiency did not alter IL-17A production during the late “adaptive” phase of infection suggesting that these sensors specifically regulate early CD4+-T-cell-derived IL-17. Thus, based on their rapid induction and distinct dependency on NOD-1/NOD-2, these early TH17 cells were termed innate TH17 (iTH17) cells. iTH17-derived early IL-17 is not restricted to C. rodentium infection as the same study demonstrated that these cells also contribute to defence against S. typhimurium, another attaching and effacing bacterium [83]. Other cells, including γδ-T and ILC3 cells, have also been shown to produce IL-17 following extracellular bacterial challenge [83]–[86]. Thus, it will be important to delineate the source of IL-17 in the context of extracellular bacterial infections to fully understand the function of TH17 cells in these settings. Experiments where IL-17 is specifically deleted in the T cell compartment will be required to obtain this information.
Fig. 2. Novel TH subsets in inflammation. 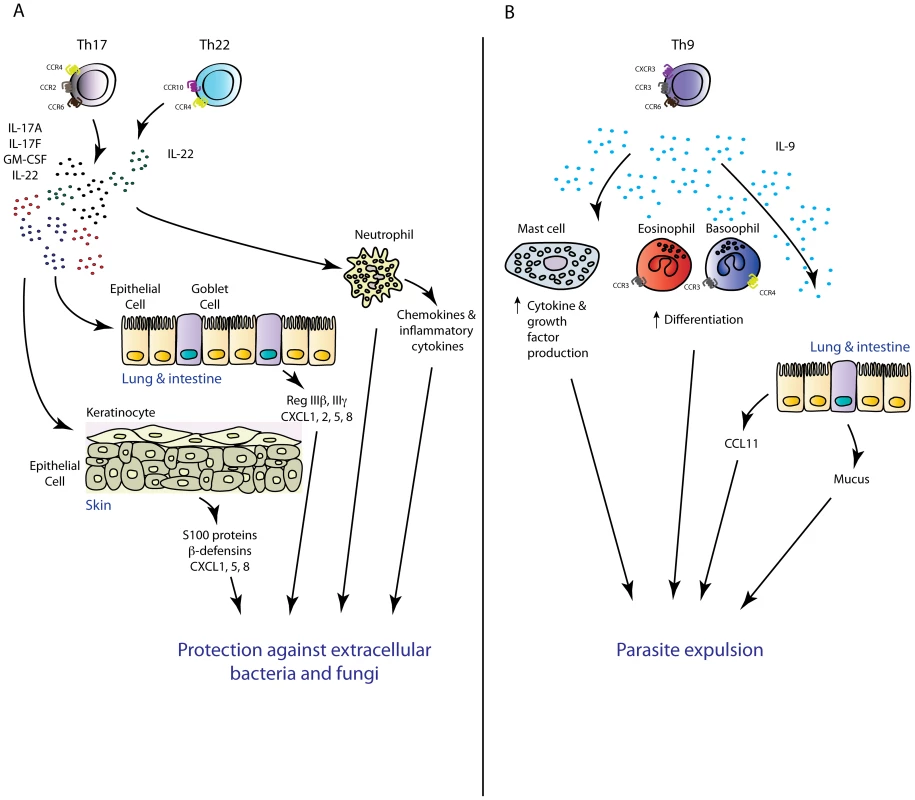
(A) TH17 and TH22 cells have overlapping functions in the mouse. Via production of the inflammatory mediators IL-17A, IL-17F, GMCSF (TH17), and IL-22 (TH22), these TH subsets mediate protective immunity against extracellular pathogens intimately associated with mucosal barriers. (B) TH9-cell-derived IL-9 may play an important role in antiparasitic immunity via mediating mast cell activation and mastocytosis, increasing the chemotactic potential of an inflammatory site via regulation of inflammatory chemokine production, and promote basophil and eosinophil function. The importance of IL-17-driven inflammation in the context of antifungal host defence has also been established. In mice and men, pathogen-specific TH17 responses have been shown to confer protection against the dimorphic filamentous fungus Candida albicans [87]. Within the memory CD4+ T cell pool of healthy volunteers, Candida-specific TH cells are enriched within the TH17 subset and significantly heightened numbers of IL-17-producing cells in peripheral leukocytes of acute Candida-infected patients have been documented compared to healthy controls upon restimulation with Candida antigens [88]. Moreover, chronic mucocutaneous candidiasis patients have diminished numbers of IL-17A-producing cells within the peripheral leukocyte pool compared with acutely infected patients and healthy controls [89]. These data, and observations that patients with autosomal dominant hyper-IgE syndrome, characterised by defects in TH17 differentiation due to mutations in the TH17-polarising transcription factor STAT3 [90]–[92], are more susceptible to Candida and other fungal infections, support an important role for the TH17 response in effective antifungal immunity [93]. More detailed analyses of the functional role of IL-17 in fungal immunity have come from murine models of experimental fungal infection. In line with human studies, mice with a deficiency in IL-17A or its receptor are more susceptible to experimental fungal infection. A role for the IL-17 axis in antifungal immunity in mice was first described in 2004, in a study in which intravenous infection of IL-17R-deficient mice with Candida led to decreased survival rates and augmented fungal burden in the kidney [94]. In a model of oropharyngeal candidiasis (known as “thrush”), mice with deletions in IL-23p19, IL-17RA, or IL-17RC developed exacerbated thrush lesions associated with augmented fungal burdens, whilst mice deficient in TH1 effector cytokines IFN-γ or TNF-α were resistant to oral infection [95]–[97]. Critical requirements for the IL-23/IL-17 axis in protective immunity have also been described in murine models of dermal candidiasis [98]. Collectively, these studies demonstrate that the IL-17 response is essential for protective immunity against disseminated, skin, or mucosal Candida infection. Whilst it was believed that TH17 cells represent the major cellular source of protective IL-17 against Candida, a recent study demonstrated that ILC3-derived IL-17 was the critical source of this cytokine in an oropharyngeal candidiasis infection model [67]. Immunity to oropharyngeal infection with Candida was not altered in Rag-deficient or T-cell-deficient animals, suggesting that TH17-cell-derived IL-17 was not an important component of host defence. Antibody-mediated depletion of all ILCs, as well as deletion of ILC3 cells using Rorc-deficient mice, led to enhanced susceptibility to Candida infection, implicating ILC3 cells as the crucial cellular source of protective IL-17 in this model. Further investigation is required to determine the contribution of innate and adaptive sources of IL-17 in other models of primary Candida infection or other fungal pathogen models where IL-17 has been shown to confer protection including Cryptococcus neoformans [99], Aspergillus fumigatus [100], and Pneumocystis carinii [101]. Taken together, recent data have suggested that both TH17 – and innate-cell-derived IL-17 play important roles in the context of extracellular bacterial and fungal infections. It is likely that innate IL-17 is crucial as the first line of defence whilst the pathogen-specific TH17 cell response plays more prominent roles during the late phase of infection and in recall challenges. In support, TH17 cell recall responses are required for effective clearance of Candida and K. pneumoniae infection [102], [103]. TH17 responses have also been shown to be required for vaccine-induced protection to the endemic fungal pathogens Coccidioides posadasii, Histoplasma capsulatum, and Blastomyces dermatitidis [104] as well as a number of other mucosa-associated pathogens [105].
As outlined above, host defences against intracellular pathogens are classically considered to be coordinated by the TH1 domain. However, recent data have also revealed a potential role for TH17 cells in the context of intracellular microbial infection [106]. Pulmonary infection of mice with the intracellular bacterium Francisella tularensis induced a protective TH17 response [107], [108], and deletion of the IL-23/IL-17A axis, but not IL-17F or IL-22, increased susceptibility to pulmonary tularemia [108]. Interestingly, the reported biological function of IL-17A in this model was induction of IL-12 and IFN-γ production from APCs, subsequently promoting antigen-specific TH1 responses [108]. The ability of IL-17A to regulate TH1 responses in the context of microbial infections is not limited to the tularemia model, with reports that IL-17 can influence adaptive immunity to pulmonary Chlamydia muridarum [109] and Mycobacterium bovis BCG [110] infection via similar mechanisms. However, impaired TH17-mediated neutrophil recruitment is also likely to contribute to these observed phenotypes [108]. Indeed, IL-23/TH17-dependent neutrophil responses are important components of protective immunity to other intracellular bacteria infections such as Mycoplasma pneumonia [111] and Salmonella enterica serotype Typhimurium [112], [113]. Taken together, these studies suggest that TH17 cells function in concert with TH1 cells to efficiently resolve some intracellular bacterial infections. The molecular and cellular basis of how these pathogens elicit TH17 cell responses despite induction of a priming environment dominated by TH1-polarising cytokines that antagonize TH17 differentiation remains to be determined. Owing to inherent differences in PRR stimulation by various bacteria, it is possible that certain bacteria effectively induce potent IL-12/IFN-γ responses, whilst other bacterial pathogens require an additional IL-17-dependent mechanism for host IL-12/IFN-γ production to resolve intracellular infection. Moreover, the requirement of neutrophil responses to intracellular infection poses something of a paradox, as these cells are not thought to elicit robust effector responses against intracellular pathogens. However, it may be that TH17-driven neutrophil responses in these settings are active against the extracellular phase of a pathogen's life cycle, i.e., trans-epithelial bacterial entry.
Virus-specific IL-17-producing CD4+ T cells have also been detected in mice following herpes simplex virus (HSV) [114], Theiler's murine encephalomyelitis virus (TEMV) [115], and vaccinia virus (VV) [116] infection, among others [20], albeit at a lower magnitude than the prototypical antiviral TH1 response. In these settings, TH17 responses appear to be detrimental to the host as IL-17R-deficiency, or neutralisation of IL-17A, reduced HSV-induced stromal keratitis [114]. Furthermore, neutralisation of IL-17 during chronic TEMV infection increased viral clearance and enhanced cytotoxic T cell responses [115], and neutralisation of IL-17 during VV infection decreased the size of primary and satellite lesions, and promoted viral clearance [116]. Nevertheless, protective roles have also been ascribed to TH17-derived cytokines in some viral infections [117]. This precarious balance of protective versus harmful effector functions of TH17 cells has also been observed in the context of parasitic infections where IL-17 has been shown to promote host defence against intestinal Toxoplasma gondii infection at the expense of heightened immunopathology [118] and contribute to control of S. mansoni infection in the lung [119], yet enhance immunopathology associated with schistosomiasis when mice are infected with S. mansoni in the liver [120], [121].
Thus, it is apparent that the TH17 subset is predominantly associated with host defence against extracellular pathogens via IL-17-mediated mobilisation of neutrophil responses. TH17 cells may also serve as an important adjunct to TH1 responses in certain intracellular infections by inducing neutrophil responses that may function during the extracellular life cycle of intracellular pathogens, or by influencing APCs to promote TH1 polarisation in some intracellular bacterial infections that fail to induce efficient pathogen-specific TH1 cell differentiation. Conversely, the TH17 response can be detrimental to the host in that it can contribute to viral persistence and promote pathological inflammation associated with parasitic infection, which thereby presents these cells as a potential therapeutic target to limit pathology in these settings. Given that optimal immunity to extracellular infections is highly dependent on the function of antibodies, the critical importance of generating pathogen-specific memory TH17 cells in vaccine development under conditions where a primary live infection elicits protective TH17 cell responses is often overlooked. Further study of this system will facilitate design of vaccines that result in improved memory TH17 cell development in synergy with robust antibody responses such that both humoral and cell-mediated arms of the immune system enter into immunological memory. Such strategies are indeed currently under development as is evident by promising vaccine candidates that elicit pathogen-specific TH17 cells in the context of anti–S. pneumoniae immunity [122].
T Helper 22 (TH22)
Recently, a subset of human TH cells dedicated to production of the cytokine IL-22 has been described and proposed to be a separate lineage of TH cell, designated TH22 [123], [124]. IL-22 is a pro-inflammatory member of the IL-10 family of cytokines that appears to be particularly important for driving inflammatory responses at cutaneous and mucosal surfaces [125]. TH22 differentiation from naïve precursors has been reported to be IL-6 – and TNF-α-dependent [123] (Figure 1) and studies suggest that IL-22 production by these cells is transcriptionally regulated by the ligand-activated transcription factor aryl-hydrocarbon receptor (AhR) [123], [124], [126], [127] (Figure 1). Expression of the IL-22 receptor is restricted to stromal cells of the skin, intestine, liver, kidney, pancreas, and lung [128], [129], implicating TH22 cells as an important mediator of the interaction of the immune system with the non-hematopoietic environment. TH22 cells have been shown to express the chemokine receptors CCR4, CCR6, and CCR10 [123], [124], the ligands of which are known to regulate homing to these organs.
In mice, IL-22 elicited from TH cells appears to be restricted to the TH17 cell subset [130], with very few studies having detected bona fide IL-17A− TH22 cells in vivo. For this reason, evaluating the function of this potential novel human T cell subset using murine models of microbial infections currently presents significant challenges. The TH22 response clearly shares many similar features with the TH17 response, as evidenced by the lack of obvious divergence between these responses in mice, and the common reliance on AhR signalling for aspects of their function [126]. Akin to the function of other TH17-derived cytokines, IL-22 ligation with its receptor markedly induces expression of multiple antimicrobial peptides including the S100 proteins S100A7–A9, β-defensins, the intestinal antimicrobial peptides RegIII-β and -γ, and stimulates production of protective mucus elicited from goblet cells (Figure 2, panel A) [129]–[132]. IL-22 also upregulates expression of the inflammatory chemokines CXCL1, CXCL2, and CXCL5, which act in synergy with IL-17 to induce a chemotactic environment that promotes neutrophilia at sites of infection [133]. In addition to its antimicrobial and pro-inflammatory effects, IL-22 also plays an important role in tissue regeneration and wound healing by promoting epithelial cell proliferation and inducing expression of anti-apoptotic proteins [134].
IL-22 appears to play a dichotomous role in host defence depending on the nature of the pathogen and site of infection. Protective functions of IL-22 have been described in the context of extracellular pathogen infection of the lung and intestine including K. pneumoniae and C. rodentium. In most cases, IL-22 was essential for control of bacterial replication and dissemination, most likely in part due to the ability of this cytokine to potently induce antimicrobial peptide production by epithelial cells at these barrier surfaces [132], [133]. In line with the protective role for TH17 responses in antifungal immunity, IL-22-producing CD4+ T cells have also been detected within the Candida-specific memory T cell pool of healthy patients [135], and are defective in patients with chronic mucocutaneous candidiasis [89], [136], [137]. However, the function of IL-22 in experimental C. albicans infection remains controversial [98], [138]. In a murine model of oropharyngeal candidiasis, host defence was predominantly mediated by IL-17, not IL-22 [96], whilst protective immunity to C. albicans infected intragastrically was dependent on IL-22-mediated production of antimicrobial peptides including S100A8 and S100A9, which prevented yeast dissemination to the kidneys and stomach [139].
Numerous studies have suggested that immunity to intracellular pathogens or parasites does not appear to rely on IL-22. Host defence against the intracellular pathogens Mycobacterium avium and L. monocytogenes, or the parasitic pathogen S. mansoni, is IL-22-independent [140]–[142]. Unlike K. pneumoniae and C. rodentium, these pathogens are not intimately associated with mucosal or cutaneous barriers, which may underlie the redundant role of IL-22 in these settings. Furthermore, IL-22 has been shown to be detrimental in a murine model of oral T. gondii infection [140], [143]. In this model, IL-23 promoted development of ileitis in an IL-22-dependent manner. Whilst no difference in protozoan burden was documented between WT and IL-22-deficient mice, WT mice succumbed to infection due to intestinal necrosis, whereas IL-22-deficient mice displayed increased survival rates with only minor inflammation evident. Flow cytometric analyses implicated CD4+ T cells in the lamina propria as the major source of IL-22, which contributed to T. gondii–induced panileitis principally via an immune response directed against gut microbiota rather than the protozoan pathogen. These data suggest that particular microbial agents can induce detrimental IL-22-mediated pathogenic inflammation [143]. On the contrary, studies have implicated protective roles for IL-22 in certain intracellular pathogen infections including experimental influenza and dengue infectious models [144]–[146]. Despite reports that IL-22 deficiency or neutralisation does not alter the outcome of M. tuberculosis infection in mice [140], [147], Scriba et al. demonstrated that a substantial proportion of mycobacteria-specific TH cells from healthy M. tuberculosis–exposed individuals produce IL-22 and are distinct from TH17 and TH1 cells, implicating IL-22 as an important cytokine axis in human anti-mycobacterial immunity [148]. These reported differences between mice and men in M. tuberculosis infection support the notion that CD4+-T-cell-derived IL-22 plays a more prominent role in the human than murine immune system, at least under certain circumstances.
Despite the fact that TH22 cells are not clearly distinguishable from the TH17 subset in mice, experiments to specifically evaluate the significance of T-cell-derived IL-22 in models of microbial infection have been performed. TH22 cells have been detected in experimental coxsackievirus-B3-induced myocarditis where they appear to exacerbate acute viral-induced myocarditis associated with increased cardiac viral replication, heightened cardiomyopathy, and reduced survival rates [149]. More recently, a study by Basu et al. demonstrated, for the first time, the function and protective efficacy of TH22-derived IL-22 in the context of microbial infection [150]. Significant expansion of IL-22-producing CD4+ TH cells that lacked expression of IL-17A occurred in the colonic lamina propria during the late phase of C. rodentium infection, implicating TH22 cells as the predominate TH subset mediating host protection to this enteropathogenic bacterium. Infection of IL-6-deficient mice led to profound defects in lamina-propria-resident TH22 cell numbers, but not IL-22 production from other cells, relative to IL-23-deficient mice or WT counterparts, illustrating the importance of IL-6 in regulating TH22 differentiation. The importance of T-cell-derived IL-22 in protective immunity to this pathogen was reflected in the marked decline in survival of mice treated with neutralising antibodies to IL-22 administered after the peak of the innate immune response. Moreover, adoptive transfer of in vitro–generated TH22 cells, but not in vitro–generated TH17 cells, into Il22−/− mice rescued recipient mice from pathogenic inflammation [150]. These experiments are the first to definitively demonstrate the existence and function of TH22 cells during enteropathogenic bacterial infection. Further detailed studies are required to explore the function of TH-cell-derived IL-22 in other infectious models.
It is important to appreciate that, similar to other TH-cell-derived cytokines, IL-22 production is not restricted to the CD4+ T cell compartment. Various other cells, including γδ-T, NKT, and CD8+, have the ability to produce IL-22 that participates in host defence against microbes [70], [132], [139], [142]. More specifically, ILC3s have been shown to be a dominant innate source of IL-22 during infection [125]. Thus, in order to delineate the function of TH22-derived IL-22 in the context of microbial infection, mice with T-cell-specific deletions of IL-22 will be required. However, to our knowledge, these reagents have yet to be developed.
Given the recent discovery of the TH22 subset, limited studies have been carried out to date regarding the function of TH22 cells in host defence to microbes. As discussed above, current data suggest that the TH22 subset, in most cases, has overlapping functions with the TH17 lineage in mice, in contrast to the human system where IL-22-secreting T cells potentially form a distinct lineage. It is now important to dissect how and why certain infections elicit IL-22 responses that are favoured over IL-17-mediated immunity in humans. The results of such studies may provide crucial insights into how the balance of TH22/TH17 cells defends against certain pathogens and may lead to the development of vaccines tailored to particular microbial threats.
T Helper 9 (TH9)
IL-9 represents one of the most understudied cytokines in the field of TH cell biology despite its diverse biological effects on numerous cell types of myeloid, lymphoid, and stromal origin [151]. IL-9 was first associated with TH2-mediated responses following reports that IL-9 expression in T cells was high in TH2-prone BALB/c mice relative to the TH1-prone C57Bl/6 mouse strain during the course of L. major infection [152]. Subsequent studies implicated a protective role for IL-9 in TH2-driven responses during murine parasitic infections [153], [154], with IL-9 levels in mesenteric lymph nodes correlating with expansion of TH2 cell populations and a requirement of IL-9 for CSR to the “type-2” antibody isotypes IgG1 and IgE [155], [156]. Furthermore, an in vivo requirement for IL-4, a crucial mediator of TH2 differentiation, for induction of IL-9 expression by T cells was later demonstrated in L. major–infected BALB/c mice [152]. The results of these studies led to the classification of IL-9 as a TH2-derived pro-inflammatory cytokine. However, despite the clear association between IL-9 and TH2 responses, recent reports of high-level IL-9 production in macrophage – and neutrophil-dominated inflammatory settings were counter to previous conceptions that IL-9 was elicited from TH2 cells [157]. These findings have recently been reconciled with the discovery that naïve T cell priming in the presence of IL-4 and TGF-β drives differentiation of a functionally disparate subset of IL-9-secreting TH cells, designated TH9 (Figure 1) [158], [159]. Subsequent studies have called into question the requirement of IL-4 in TH9 differentiation with reports that IL-4R signalling induces expression of suppressor of cytokine signalling (SOCS) family member cytokine-induced SH-2 protein (CIS), which inhibits STAT5/STAT6 signalling and subsequent TH9 cell differentiation [160]. Indeed, IL-9 production in T cells has been shown to be independent of IL-4 when activated in the presence of TGF-β1 and IL-1α [161]. In vivo, the molecular requirements for TH9 cell induction may involve many additional stimuli including IL-25, TSLP, 1,25-dihydroxyvitamin D3, programmed cell death ligand (PD-L) 2, cyclooxygenase (COX)-2, and tumor necrosis factor receptor superfamily member 4 (OX40) [162]. Furthermore, data suggesting that the TH9 programme is unstable and highly prone to plasticity have raised questions as to whether this IL-9-secreting CD4+ T cell indeed represents a distinct differentiation lineage. These aspects of TH9 cell biology will not be discussed in this review but have been recently reviewed elsewhere [162], [163]. Differentiation of this subset is thought to require the transcription factors PU.1, IRF4, and BATF [164]–[166]. We have recently examined chemokine receptor expression by TH9 cells and have shown that these cells express a broad range of trafficking receptors, including CCR3, CCR6, and CXCR3 [167]. Notably, these receptors are also characteristically expressed by other TH cell subsets (TH2, TH17, and TH1, respectively) suggesting that TH9 cells have the capability of being recruited to, and contributing to multiple, functionally distinct forms of inflammatory lesions. Whilst CCR4 expression by TH9 cells generated in our models was not detected, recent work has suggested that these cells also express CCR4 and CCR8, which would presumably allow these cells to traffic to cutaneous sites of inflammation [166].
Given that the description of this new TH cell subset came years after initial studies into the role of IL-9 in the context of microbial infections were carried out, the function of IL-9 in protective immunity will be discussed with conjecture on the role of bona fide TH9 cells in these settings. Studies using IL-9 transgenic (IL-9Tg) mice have emphasized the importance of this cytokine in the control of certain intestinal parasitic infections (summarized in Figure 2, panel B). Following infection with Trichinella spiralis or T. muris, IL-9Tg mice developed enhanced intestinal mastocytosis and augmented pathogen-specific IgG1 responses, which led to rapid parasitic expulsion from the gut [153], [154]. Furthermore, treatment of mice with neutralising antibodies to IL-9 during the course of T. muris infection diminished immunity to this pathogen [168]. In line with the protective phenotypes observed in IL-9Tg mice, a specific role for TH9-derived IL-9 in protective immunity to intestinal nematode infection was more recently assessed using mice in which TGF-β signalling, a crucial mediator of TH9 differentiation, was specifically deleted in the CD4+ T cell compartment. Infection of these mice with T. muris augmented worm burden and reduced IL-9 but not IL-13 production in mesenteric lymph nodes [159]. In these models, IL-9 appears to predominantly function via activation of mast cells, the inflammatory mediators from which promote seminal processes required for effective parasite expulsion such as induction of eosinophilia, increased intestinal permeability and contractility, and mucus production. Recent work by Licona-Limon et al. using novel IL-9 reporter mice (termed Interleukin Nine Fluorescent Reporter: INFER) and newly generated IL-9-deficient mice on a BALB/c background has revealed a critical and nonredundant role for TH9 and IL-9 in host defence to N. brasiliensis [169]. GFP reporter activity was detected in CD4+ T cells and type-2 ILCs (ILC2: a known prominent source of IL-9 in numerous type-2 models) in both lungs and mediastinal lymph nodes during the course of N. brasiliensis infection. IL-9-GFP detection in CD4+ T cells in the lung peaked early and declined during the course of infection, whilst IL-9-GFP+ ILC2s were detectable early and remained present throughout, suggesting a transient window of CD4+-T-cell-derived IL-9 in this model. Adoptive transfer of TH9 cells into IL-9-deficient mice led to enhanced worm expulsion, demonstrating that TH9-derived IL-9 was an important contributor to IL-9-dependent immunity in this model. The results of this study also elucidated numerous other unknown aspects of TH9 cell biology including the demonstration of functional differences between TH2 and TH9 cells in host protection, despite prior reports concluding that the function of these two subsets in other models substantially overlapped. Surprisingly, transferred TH9 cells, but not TH2 cells, into infected Rag2-deficient hosts decreased worm burden. TH9-mediated protection correlated with increased numbers of mast cells and basophils in lungs and spleens of infected mice, implicating these innate effector cells as the key responding cell types to IL-9-mediated immunity. Moreover, recent findings by Turner et al., using IL-9 fate mapper reporter mice (termed IL-9CreR26ReYFP mice, which permanently label cells with enhanced YFP (eYFP) that have expressed IL-9 irrespective of their current IL-9 expression status) and IL-9R-deficient animals, support the notion that IL-9 plays a critical role in host defence against N. brasiliensis infection [170]. Both eYFP+ CD4+ T cells and ILC2 cells were detected in the lung during infection. IL-9 in this setting was demonstrated to positively regulate IL-5 and IL-13 responses, likely ILC2-derived IL-5 and IL-13 as TH2 cell numbers were unchanged in IL-9R-deficient animals, promote ILC2 cell survival, drive lung tissue repair mechanisms, and promote eosinophil recruitment and alternative activation of macrophages. These studies highlight the critical importance of both TH9 and ILC2-derived IL-9 in host defence to N. brasiliensis. However, the results of these studies are not in keeping with a prior study, which demonstrated that IL-9-deficient mice on a mixed genetic background (129×C57Bl/6 (F2)) effectively control infection with N. brasiliensis [171]. The conflicting results of these studies warrant further investigation of IL-9 function in anti–N. brasiliensis immunity but suggest that the overall importance of IL-9 depends on complex multi-genetic factors. IL-9 deficiency, using 129×C57Bl/6 (F2) Il9−/− mice backcrossed six times onto a BALB/c background, led to modest reductions in mast cell numbers but did not alter the outcome of infection with the flagellated intestinal protozoan parasite Giardia lamblia [172]. Moreover, IL-4 has been shown to control intestinal parasitic infections in an IL-5/IL-9/IL-13 triple-knockout mouse [173]. Collectively, these data suggest that the TH9 subset may serve as an important adjunct to the TH2 response in certain parasitic infections; however, it appears to be superfluous in certain circumstances where TH2 responses suffice. In support, IL-9 has been shown to precede and regulate TH2-associated cytokine responses in certain parasitic infections [169], [170]. Given that TH9 cells have been reported to produce the CCR4 ligands CCL17 and CCL22 [164], and studies that demonstrate that IL-9 can induce expression of the inflammatory chemokine CCL11 by smooth muscle cells [174], [175], early IL-9 responses may be important for amplifying CCR3+ eosinophil [176] or CCR3+/CCR4+ TH2 cell responses at sites of microbial infection.
The function of IL-9 in other microbial infections is less well-defined with conflicting conclusions having been reached to date. Following reports that bronchial secretions from infants with respiratory syncitial virus (RSV) bronchiolitis contained high levels of IL-9 [177], the function of IL-9 was specifically investigated in a murine model of RSV vaccination and infection. Antibody-mediated neutralisation of IL-9 in these models resulted in enhanced viral clearance from the lungs and had varied effects on pathology depending on the timing of IL-9 depletion [178]. In contrast to the detrimental roles of IL-9 in the RSV model, prophylactic administration of recombinant IL-9 into mice infected with a lethal dose of Pseudomonas aeruginosa enhanced survival via suppression of inflammatory cytokines including IFN-γ and TNF-α, and induction of the immunomodulatory cytokine IL-10 [179]. Endogenous IL-9 induction was detected in spleens of mice infected with sublethal, but not lethal, doses of P. aeruginosa; however, the precise cellular source of this IL-9 was not explored [179]. IL-9-secreting CD4+ T cells have also been detected in humans with M. tuberculosis infection [180]; however, the functional significance of these cells in immunity to this pathogen has yet to be investigated. It is clear that a great deal of work is still required to delineate the function of TH9 cells in the context of microbial infections. Owing to the recent discovery of this subset, the majority of studies investigating the function of IL-9 in protective immunity have utilised systemic means of IL-9 blockade/neutralisation such as the use of antibody-mediated neutralisation or IL-9-deficient mice. However, IL-9 can be elicited from multiple cell types including ILCs, mast cells, Tregs, and natural killer T cells [181]. Therefore, more refined studies making use of the recently described INFER mice [169], which will be useful to detect IL-9-expressing cells in real time, IL-9 fate-mapping reporter mice [182], or mice with specific deletions of the TH9-specific transcription factor PU.1 in the T cell compartment [164] are required to determine which microbial infections elicit a TH9 response and whether this response is protective.
Taken together, it appears that the TH9 response may play significant roles in immunity to certain intestinal parasites and contribute to host protection via amplification of the infectious site's chemotactic potential and mediating mast cell and basophil activation. Although less well-understood, the TH9 response may also participate in a diverse array of other infections; however, it appears that these cells play a role supplementary to TH2 cells and in some cases a potentially detrimental role in host defences.
T Follicular Helper (TFH)
The TH1, TH2, TH17, TH22, and TH9 subsets represent populations of effector helper T cells that contribute to immune responses at peripheral sites of infection. However, within secondary lymphoid organs, populations of effector CD4+ T cells interact with clonally selected B cells to produce humoral immunity by providing crucial signals that regulate B-cell survival, proliferation, affinity maturation, CSR, and differentiation into memory B – or long-lived plasma cells. This molecular cross-talk between B and T cells occurs in two waves: first at the T-B border where CD4+-T-cell-derived cytokines instruct developing B cells to switch to an appropriate isotype and actively form specialised structures known as germinal centres (GC), followed by further interactions within the GC that ultimately determine the quality of antibody response generated [183]. Prior to 2004, CD4+ T cells localised to GCs were thought to be a branch of canonically derived TH1 or TH2 cells that migrated into B cell follicles to coordinate CSR to IgG2A and IgG1 via IFN-γ and IL-4, respectively [184]. However, recent data have demonstrated that these GC-localised T cells, now referred to as follicular TH cells (TFH), are in fact a distinct differentiation lineage (Figure 1 and Figure 3). These cells are characterised by expression of the lineage-specific master regulator Bcl6 (the transcription factor c-Maf is also crucial to this subset) [185]–[187], as well as the ability to produce a range of cytokines including IL-4, IFN-γ, IL-21, and IL-17A [188]–[195] (Figure 1 and Figure 3). TFH cells are also characterised by high expression of the chemokine receptor CXCR5, which mediates migration into B cell follicles and GCs that are rich in CXCL13 [196], [197].
Fig. 3. Mechanism of action of TFH cells. 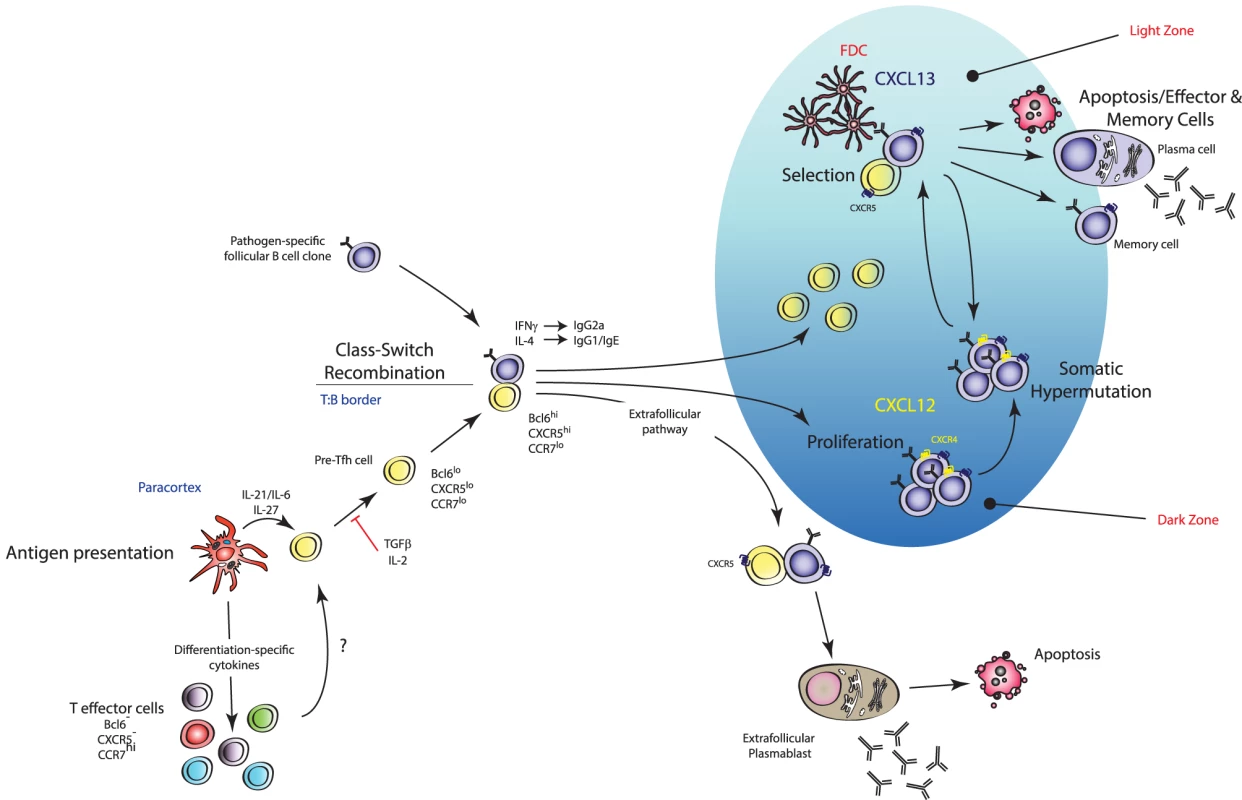
TFH cells are effector TH cells that govern the quality and magnitude of an antibody response via regulation of B cell selection, differentiation, proliferation, and class switch recombination. TFH cells execute these effector functions via expression of various cell surface proteins and cytokines (including IL-21). They are generated during antigen presentation in the T cell areas of secondary lymphoid organs in the presence of IL-21 and IL-6, which is thought to upregulate their master transcription factor Bcl6 (pre-TFH), after which they migrate to the T∶B border where interaction with cognate B cells regulates a number of processes including promoting survival of recently activated B cells, regulating the fate decision of a B cell down extrafollicular plasmablast or germinal center (GC) B cell differentiation pathways, and induction of class switch recombination in GC B cells. Stable interactions with cognate B cells at this border also consolidate the TFH cell programme (pre-TFH to TFH cell differentiation) with further upregulation of Bcl6 and entry into developing GCs. Within GCs, TFH cells are crucial for the regulation of affinity maturation, development of memory B cell populations, and high-affinity antibody responses via regulation of long-lived plasma cell differentiation. Current models of TFH cell development describe a “two-wave” theory of differentiation: DC-instructed commitment to the TFH cell lineage (i.e., pre-TFH cell differentiation) followed by B-cell-instructed consolidation of the TFH cell programme (i.e., GC-resident TFH cells) [198]. It is thought that TFH cells selectively differentiate from naïve precursors with the highest affinity to any given antigen [199], consistent with reports that the magnitude of TFH cell generation is dependent on the dose of Ag made available to the T cell during its interaction with a DC [200]. STAT3 activation downstream of IL-6 and/or IL-21 has also been shown to promote early commitment to the TFH cell lineage via induction of their master transcriptional regulator Bcl6 [193], [201], [202]. Interestingly, STAT1 and STAT4 activation downstream of IL-6 and IL-12 signalling respectively, two transcription factors known to promote TH1 cell differentiation, have been shown to induce expression of Bcl6 in CD4+ T cells [203]–[206]. Nakayamada et al. provided evidence that TH1 cell differentiation may occur through a TFH-cell-like transitional state where T-bet and Bcl6 are co-expressed in the same cell [207]. T-bet was shown to eventually outcompete Bcl6 function when IL-12 signalling persists leading to downregulation of Bcl6, and TH1 lineage fate commitment then predominating [207]. Thus, the earliest events of DC-instructed commitment to the TFH cell lineage are complex and likely involve the integration of numerous microenvironmental signals [208]. Importantly, differentiation of TFH cells appears to be independent of the other known effector subsets as mice deficient in genes critical to TH1, TH2, and TH17 lineage development do not display marked differences in TFH cell induction [193]. Early TFH cell commitment is coupled with the downregulation of CCR7 (the ligands of which are in highest concentration in T cell zones of SLOs) and upregulation of CXCR5 (the ligands of which are in highest concentration in the B cell zones), facilitating the movement of the pre-TFH cell to the T-B border [209]. Stable interactions with cognate B cells at this border are required for the terminal differentiation of the TFH cell programme and are coupled with further upregulation of Bcl6, thus promoting expression of genes required for B cell help, and CXCR5, which facilitates the migration of these cells deeper into the B cell zone and into GCs where they execute their predominant effector function [198], [210].
Given that the ability of the host to generate high-affinity neutralising/opsonising antibody responses via the GC reaction is seminal for defence against a number of ever-evolving pathogens, the function of TFH cells is predominantly associated with regulating the process of antibody affinity maturation through governance of selection, survival, proliferation, and differentiation of high-affinity, pathogen-specific B cell clones. TFH cells execute these effector functions via the wealth of cell-surface and soluble proteins that they express (reviewed in [184]) including CD40L, which interacts with GC-B-cell-expressed CD40 and imprints an anti-apoptotic transcriptome in the responding B cell [211], [212], and IL-21, which promotes the proliferation of GC B cells [190], [213], [214] and drives their differentiation toward the plasma cell compartment in both mice [215] and humans [216]–[218]. Therefore, it is perhaps of no surprise that individuals with mutations in these factors exhibit defects in generating isotype-switched high-affinity antibody responses and, thus, are more prone to opportunistic infection. For instance, patients with mutations in Cd40lg develop the primary immunodeficiency hyper-immunoglobulin M syndrome, characterised by a severe deficit in GC development and lack of circulating isotype-switched immunoglobulin, and, subsequently, an increase in susceptibility to recurrent bacterial infections and are unresponsive to vaccination [214], [219], [220]. Similarly, patients with a mutation in the SLAM-associated protein (SAP) encoding gene Sh2d1a, a signalling protein expressed by TFH cells critically required for the formation of stable T∶B conjugates [221], [222] and GC-TFH cell expression of IL-4 [223], develop X-linked lymphoproliferative (XLP) disease characterised by an increased susceptibility to a number of pathogens (particularly Epstein-Barr virus infection, which can be fatal in children) due to abortive B cell responses [224].
Central to the ability of the host to generate an effective antibody response to a given pathogen is the decision of B cells to class switch to an appropriate antibody isotype for maximum effector function tailored to the nature of the microbe. CSR to IgG2A is driven by IFN-γ and plays a vital role in the neutralisation of viruses, complement fixation, and opsonisation of microbes via the Fc portion. Conversely, IL-4-mediated CSR to IgG1 and IgE is essential for antibody-mediated cell cytotoxicity mechanisms in the context of antiparasitic immunity. Despite the clear demonstration by multiple groups that differentiation of Bcl6+ TFH cells in vivo is independent of TH1, TH2, or TH17 differentiation pathways [185]–[187], how TFH cells could coordinate CSR during particular infections via IL-4 and/or IFN-γ posed something of a paradox. Using reporter systems and other elegant approaches, recent work has revealed that TFH cells can differentiate in a variety of priming environments and that the cytokine milieu present during T cell activation likely favours the production of IFN-γ or IL-4 by TFH cells in the context of TH1 – or TH2-polarising infections, respectively (Figure 3). Using 4get/KN2 dual reporter mice, which faithfully report cells that have actively transcribed from the Il4 locus and cells actively producing IL-4 [225], Reinhardt and colleagues demonstrated that during infection with the type-2 pathogens L. major or N. brasilienis, IL-4 production in draining lymph nodes was restricted to bona fide TFH cells that were phenotypically and functionally distinct from canonical TH2 cells [226]. TFH-cell-derived IL-4 was important for driving parasite-specific IgG1 responses as GC B cells sorted from IL-4-producing TFH∶GC B cell conjugates were actively undergoing CSR to IgG1 [226]. Using IFN-γ reporter mice, the same study demonstrated that GC B cells sorted from IFN-γ-producing TFH∶GC B cell conjugates were actively undergoing CSR to IgG2A [226]. Consistent with these observations, Lutjhe and colleagues recently demonstrated that TFH cells favoured the production of IFN-γ following influenza infection [227]. However, development of IgG2C+ (C57Bl/6 mouse equivalent of IgG2A) GC B cells was unperturbed in chimeric mice reconstituted with IFN-γ-deficient CD4+ T cells [227]. Collectively, the results of these studies suggest that TFH-derived IL-4 is seminal for generation of pathogen-specific IgG1 antibody responses whilst TFH-derived IFN-γ is not essential for an IgG2A antibody response, although it may play a supplementary role in this process.
Much controversy still exists in the field of TFH cell biology regarding the origin of these cells during pathogen encounter. Although it is apparent that the differentiation programmes of T helper cells with B-cell helper function are separate to other effector TH cell subsets as described above, studies have demonstrated that non-TFH effector T cells can re-differentiate to the TFH lineage [228], [229] (Figure 3). TFH cells have been described to arise from TH1 in the context of LCMV infection [230]; from TH2 cells in the context of Heligmosomoides polygyrus, S. mansoni, and N. brasiliensis infection [188], [226], [229]; and from TH17 cells in the Peyer's patches, which was shown to be critical for antigen-specific IgA responses [231]. From a clinical perspective, the ability of the host to generate high-quality antibody responses governs the success of most currently available vaccination strategies; therefore, understanding the differentiation pathways and cytokine-secreting repertoires of TFH cells under different immunising conditions is imperative to the design of vaccines that generate high-affinity antibody responses with the appropriate dominant antibody isotype tailored to the nature of the microbe of interest. The recent development of cytokine fate-mapping reporter mice and generation of Bcl6 and IL-21 reporter mice that faithfully map TFH cells during an immune response [227], [228] should facilitate the collection of important information regarding these processes.
Concluding Remarks
The adaptive immune response has a broad array of strategies to combat infectious, potentially pathogenic agents. One of the most important strategies utilised is to tailor the immune response to combat particular classes of microbial agents, and TH cell subsets play a crucial role in this process. Significant progress has dramatically improved our understanding of TH cell biology with a number of new subsets recently being identified and discussed in this review, as well as emerging effector TH cell phenotypes, such as granzyme B-expressing cytolytic CD4+ T cells found in certain viral infections [232] and subsets of TH cells dedicated to production of IL-21 (TH21) [233], that have not yet been shown to be a distinct effector lineage and are not discussed here but warrant further study. Together, these cells give the adaptive immune response the potential to deliver antigen-specific inflammatory responses that instruct and complement pathogen-tailored innate inflammatory responses. These TH responses differentially combat extracellular pathogens, enhance cell-mediated immunity required to combat intracellular pathogens, and promote humoral immunity to produce antibodies that target pathogens. Future challenges include further dissection of this system to identify other potentially important subsets and identifying ways in which to utilise this knowledge to develop better strategies to combat infectious pathogens. Ascertaining such knowledge will be crucial for determining whether future vaccination strategies: i) elicit robust TFH cell responses with the appropriate cytokine-secreting repertoire to induce an antibody response tailored to the nature of the pathogen; and ii) activate the appropriate components of the innate immune system that induce a priming microenvironment driving differentiation of the desired pathogen specific-effector TH cell subset and/or -CTL activation such that upon contact with the live pathogen, all aspects of the adaptive immune system are armed to promote effective, pathogen-tailored clearance of the infectious agent. Thus, increased understanding of the complex dynamics of TH differentiation in the context of microbial infections should lead to improved vaccine efficacy for a wide range of human pathogens.
Zdroje
1. ZhuJ, PaulWE (2010) Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev 238 : 247–262.
2. JoffreO, NolteMA, SporriR, Reis e SousaC (2009) Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev 227 : 234–247.
3. MosmannTR, CherwinskiH, BondMW, GiedlinMA, CoffmanRL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136 : 2348–2357.
4. OkoyeIS, WilsonMS (2011) CD4+ T helper 2 cells–microbial triggers, differentiation requirements and effector functions. Immunology 134 : 368–377.
5. RoweJH, ErteltJM, WaySS (2012) Foxp3(+) regulatory T cells, immune stimulation and host defence against infection. Immunology 136 : 1–10.
6. TakedaA, HamanoS, YamanakaA, HanadaT, IshibashiT, et al. (2003) Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol 170 : 4886–4890.
7. LighvaniAA, FruchtDM, JankovicD, YamaneH, AlibertiJ, et al. (2001) T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A 98 : 15137–15142.
8. HibbertL, PflanzS, De Waal MalefytR, KasteleinRA (2003) IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res 23 : 513–522.
9. KamiyaS, OwakiT, MorishimaN, FukaiF, MizuguchiJ, et al. (2004) An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol 173 : 3871–3877.
10. LucasS, GhilardiN, LiJ, de SauvageFJ (2003) IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A 100 : 15047–15052.
11. AfkarianM, SedyJR, YangJ, JacobsonNG, CerebN, et al. (2002) T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol 3 : 549–557.
12. SzaboSJ, SullivanBM, StemmannC, SatoskarAR, SleckmanBP, et al. (2002) Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295 : 338–342.
13. ThierfelderWE, van DeursenJM, YamamotoK, TrippRA, SarawarSR, et al. (1996) Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382 : 171–174.
14. KaplanMH, SunYL, HoeyT, GrusbyMJ (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382 : 174–177.
15. SzaboSJ, KimST, CostaGL, ZhangX, FathmanCG, et al. (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100 : 655–669.
16. LordGM, RaoRM, ChoeH, SullivanBM, LichtmanAH, et al. (2005) T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 106 : 3432–3439.
17. GroomJR, LusterAD (2011) CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89 : 207–215.
18. HsiehCS, MacatoniaSE, TrippCS, WolfSF, O'GarraA, et al. (1993) Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260 : 547–549.
19. KempM, KurtzhalsJA, BendtzenK, PoulsenLK, HansenMB, et al. (1993) Leishmania donovani-reactive Th1 - and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun 61 : 1069–1073.
20. SwainSL, McKinstryKK, StruttTM (2012) Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol 12 : 136–148.
21. MutisT, CornelisseYE, OttenhoffTH (1993) Mycobacteria induce CD4+ T cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol 23 : 2189–2195.
22. HaanenJB, de Waal MalefijtR, ResPC, KraakmanEM, OttenhoffTH, et al. (1991) Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med 174 : 583–592.
23. ZhangM, GatelyMK, WangE, GongJ, WolfSF, et al. (1994) Interleukin 12 at the site of disease in tuberculosis. J Clin Invest 93 : 1733–1739.
24. de JongR, AltareF, HaagenIA, ElferinkDG, BoerT, et al. (1998) Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280 : 1435–1438.
25. AltareF, DurandyA, LammasD, EmileJF, LamhamediS, et al. (1998) Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280 : 1432–1435.
26. FrohlichA, KisielowJ, SchmitzI, FreigangS, ShamshievAT, et al. (2009) IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324 : 1576–1580.
27. YiJS, DuM, ZajacAJ (2009) A vital role for interleukin-21 in the control of a chronic viral infection. Science 324 : 1572–1576.
28. YiJS, IngramJT, ZajacAJ (2010) IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J Immunol 185 : 4835–4845.
29. SwainSL, WeinbergAD, EnglishM, HustonG (1990) IL-4 directs the development of Th2-like helper effectors. J Immunol 145 : 3796–3806.
30. Le GrosG, Ben-SassonSZ, SederR, FinkelmanFD, PaulWE (1990) Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med 172 : 921–929.
31. KaplanMH, SchindlerU, SmileyST, GrusbyMJ (1996) Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4 : 313–319.
32. ShimodaK, van DeursenJ, SangsterMY, SarawarSR, CarsonRT, et al. (1996) Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380 : 630–633.
33. TakedaK, TanakaT, ShiW, MatsumotoM, MinamiM, et al. (1996) Essential role of Stat6 in IL-4 signalling. Nature 380 : 627–630.
34. ZhuJ, GuoL, WatsonCJ, Hu-LiJ, PaulWE (2001) Stat6 is necessary and sufficient for IL-4's role in Th2 differentiation and cell expansion. J Immunol 166 : 7276–7281.
35. ZhengW, FlavellRA (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89 : 587–596.
36. ZhangDH, CohnL, RayP, BottomlyK, RayA (1997) Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem 272 : 21597–21603.
37. OuyangW, LohningM, GaoZ, AssenmacherM, RanganathS, et al. (2000) Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity 12 : 27–37.
38. PaulWE (2010) What determines Th2 differentiation, in vitro and in vivo? Immunol Cell Biol 88 : 236–239.
39. MikhakZ, FukuiM, FarsidjaniA, MedoffBD, TagerAM, et al. (2009) Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol 123 : 67–e63, 67-73, e63.
40. SallustoF, MackayCR, LanzavecchiaA (1997) Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 277 : 2005–2007.
41. PearceEJ, CasparP, GrzychJM, LewisFA, SherA (1991) Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med 173 : 159–166.
42. ElseKJ, HultnerL, GrencisRK (1992) Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology 75 : 232–237.
43. SchrammG, HaasH (2010) Th2 immune response against Schistosoma mansoni infection. Microbes Infect 12 : 881–888.
44. MaizelsRM, HewitsonJP, SmithKA (2012) Susceptibility and immunity to helminth parasites. Curr Opin Immunol 24 : 459–466.
45. HarringtonLE, HattonRD, ManganPR, TurnerH, MurphyTL, et al. (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6 : 1123–1132.
46. ParkH, LiZ, YangXO, ChangSH, NurievaR, et al. (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6 : 1133–1141.
47. GalloE, KatzmanS, VillarinoAV (2012) IL-13-producing Th1 and Th17 cells characterize adaptive responses to both self and foreign antigens. Eur J Immunol 42 : 2322–2328.
48. RaymondM, VanVQ, WakaharaK, RubioM, SarfatiM (2011) Lung dendritic cells induce T(H)17 cells that produce T(H)2 cytokines, express GATA-3, and promote airway inflammation. J Allergy Clin Immunol 128 : 192–e196, 192-201, e196.
49. BonifaceK, BlumenscheinWM, Brovont-PorthK, McGeachyMJ, BashamB, et al. (2010) Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol 185 : 679–687.
50. ZielinskiCE, MeleF, AschenbrennerD, JarrossayD, RonchiF, et al. (2012) Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 484 : 514–518.
51. VeldhoenM, HockingRJ, AtkinsCJ, LocksleyRM, StockingerB (2006) TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24 : 179–189.
52. ManganPR, HarringtonLE, O'QuinnDB, HelmsWS, BullardDC, et al. (2006) Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441 : 231–234.
53. ZhouL, IvanovII, SpolskiR, MinR, ShenderovK, et al. (2007) IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8 : 967–974.
54. YangXO, PanopoulosAD, NurievaR, ChangSH, WangD, et al. (2007) STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282 : 9358–9363.
55. HarrisTJ, GrossoJF, YenHR, XinH, KortylewskiM, et al. (2007) Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol 179 : 4313–4317.
56. IvanovII, McKenzieBS, ZhouL, TadokoroCE, LepelleyA, et al. (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126 : 1121–1133.
57. YangXO, PappuBP, NurievaR, AkimzhanovA, KangHS, et al. (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28 : 29–39.
58. WeiL, LaurenceA, EliasKM, O'SheaJJ (2007) IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 282 : 34605–34610.
59. NurievaR, YangXO, MartinezG, ZhangY, PanopoulosAD, et al. (2007) Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448 : 480–483.
60. GutcherI, DonkorMK, MaQ, RudenskyAY, FlavellRA, et al. (2011) Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity 34 : 396–408.
61. GhoreschiK, LaurenceA, YangXP, TatoCM, McGeachyMJ, et al. (2010) Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467 : 967–971.
62. LeeY, AwasthiA, YosefN, QuintanaFJ, XiaoS, et al. (2012) Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13 : 991–999.
63. YamazakiT, YangXO, ChungY, FukunagaA, NurievaR, et al. (2008) CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol 181 : 8391–8401.
64. YuJJ, GaffenSL (2008) Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci 13 : 170–177.
65. LockhartE, GreenAM, FlynnJL (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177 : 4662–4669.
66. TakatoriH, KannoY, WatfordWT, TatoCM, WeissG, et al. (2009) Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206 : 35–41.
67. GladiatorA, WanglerN, Trautwein-WeidnerK, LeibundGut-LandmannS (2013) Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol 190 : 521–525.
68. DoisneJM, SoulardV, BecourtC, AmniaiL, HenrotP, et al. (2011) Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1 - invariant NKT cells to bacteria. J Immunol 186 : 662–666.
69. CuaDJ, TatoCM (2010) Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 10 : 479–489.
70. RubinoSJ, GeddesK, GirardinSE (2012) Innate IL-17 and IL-22 responses to enteric bacterial pathogens. Trends Immunol 33 : 112–118.
71. YeP, GarveyPB, ZhangP, NelsonS, BagbyG, et al. (2001) Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 25 : 335–340.
72. HappelKI, DubinPJ, ZhengM, GhilardiN, LockhartC, et al. (2005) Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 202 : 761–769.
73. WangF, XuJ, LiaoY, WangY, LiuC, et al. (2011) Tim-3 ligand galectin-9 reduces IL-17 level and accelerates Klebsiella pneumoniae infection. Cell Immunol 269 : 22–28.
74. IshigameH, KakutaS, NagaiT, KadokiM, NambuA, et al. (2009) Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30 : 108–119.
75. IvanovII, AtarashiK, ManelN, BrodieEL, ShimaT, et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139 : 485–498.
76. HigginsSC, JarnickiAG, LavelleEC, MillsKH (2006) TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol 177 : 7980–7989.
77. AndreasenC, PowellDA, CarbonettiNH (2009) Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS ONE 4: e7079.
78. YuJJ, RuddyMJ, WongGC, SfintescuC, BakerPJ, et al. (2007) An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109 : 3794–3802.
79. YuJJ, RuddyMJ, ContiHR, BoonanantanasarnK, GaffenSL (2008) The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infect Immun 76 : 4206–4213.
80. LuY-J, GrossJ, BogaertD, FinnA, BagradeL, et al. (2008) Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4: e1000159.
81. ZhangZ, ClarkeTB, WeiserJN (2009) Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119 : 1899–1909.
82. McGeachyMJ, ChenY, TatoCM, LaurenceA, Joyce-ShaikhB, et al. (2009) The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10 : 314–324.
83. GeddesK, RubinoSJ, MagalhaesJG, StreutkerC, Le BourhisL, et al. (2011) Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med 17 : 837–844.
84. BuonocoreS, AhernPP, UhligHH, IvanovII, LittmanDR, et al. (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464 : 1371–1375.
85. PriceAE, ReinhardtRL, LiangH-E, LocksleyRM (2012) Marking and quantifying IL-17A-producing cells in vivo. PLoS ONE 7: e39750.
86. SuttonCE, MielkeLA, MillsKH (2012) IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol 42 : 2221–2231.
87. Hernandez-SantosN, GaffenSL (2012) Th17 cells in immunity to Candida albicans. Cell Host Microbe 11 : 425–435.
88. Acosta-RodriguezEV, RivinoL, GeginatJ, JarrossayD, GattornoM, et al. (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8 : 639–646.
89. EyerichK, FoersterS, RomboldS, SeidlHP, BehrendtH, et al. (2008) Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol 128 : 2640–2645.
90. MilnerJD, BrenchleyJM, LaurenceA, FreemanAF, HillBJ, et al. (2008) Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452 : 773–776.
91. MaCS, ChewGY, SimpsonN, PriyadarshiA, WongM, et al. (2008) Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 205 : 1551–1557.
92. MinegishiY, SaitoM, TsuchiyaS, TsugeI, TakadaH, et al. (2007) Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448 : 1058–1062.
93. GrimbacherB, HollandSM, GallinJI, GreenbergF, HillSC, et al. (1999) Hyper-IgE syndrome with recurrent infections–an autosomal dominant multisystem disorder. N Engl J Med 340 : 692–702.
94. HuangW, NaL, FidelPL, SchwarzenbergerP (2004) Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190 : 624–631.
95. FarahCS, HuY, RimintonS, AshmanRB (2006) Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol Immunol 21 : 252–255.
96. ContiHR, ShenF, NayyarN, StocumE, SunJN, et al. (2009) Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206 : 299–311.
97. HoAW, ShenF, ContiHR, PatelN, ChildsEE, et al. (2010) IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol 185 : 1063–1070.
98. KagamiS, RizzoHL, KurtzSE, MillerLS, BlauveltA (2010) IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol 185 : 5453–5462.
99. AntachopoulosC, WalshTJ (2012) Immunotherapy of Cryptococcus infections. Clin Microbiol Infect 18 : 126–133.
100. ZelanteT, BozzaS, De LucaA, D'AngeloC, BonifaziP, et al. (2009) Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol 47 Suppl 1: S162–169.
101. RudnerXL, HappelKI, YoungEA, ShellitoJE (2007) Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun 75 : 3055–3061.
102. Hernandez-SantosN, HupplerAR, PetersonAC, KhaderSA, McKennaKC, et al. (2013) Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol 6 : 900–910.
103. ChenK, McAleerJP, LinY, PatersonDL, ZhengM, et al. (2011) Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35 : 997–1009.
104. WuthrichM, GernB, HungCY, ErslandK, RoccoN, et al. (2011) Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 121 : 554–568.
105. KumarP, ChenK, KollsJK (2013) Th17 cell based vaccines in mucosal immunity. Curr Opin Immunol 25 : 373–380.
106. KhaderSA, GopalR (2010) IL-17 in protective immunity to intracellular pathogens. Virulence 1 : 423–427.
107. WoolardMD, HensleyLL, KawulaTH, FrelingerJA (2008) Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun 76 : 2651–2659.
108. LinY, RitcheaS, LogarA, SlightS, MessmerM, et al. (2009) Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31 : 799–810.
109. BaiH, ChengJ, GaoX, JoyeeAG, FanY, et al. (2009) IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol 183 : 5886–5895.
110. UmemuraM, YahagiA, HamadaS, BegumMD, WatanabeH, et al. (2007) IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 178 : 3786–3796.
111. WuQ, MartinRJ, RinoJG, BreedR, TorresRM, et al. (2007) IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect 9 : 78–86.
112. GodinezI, RaffatelluM, ChuH, PaixaoTA, HanedaT, et al. (2009) Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun 77 : 387–398.
113. RaffatelluM, SantosRL, VerhoevenDE, GeorgeMD, WilsonRP, et al. (2008) Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14 : 421–428.
114. SuryawanshiA, Veiga-PargaT, RajasagiNK, ReddyPB, SehrawatS, et al. (2011) Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol 187 : 1919–1930.
115. HouW, KangHS, KimBS (2009) Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med 206 : 313–328.
116. OyoshiMK, ElkhalA, KumarL, ScottJE, KoduruS, et al. (2009) Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A 106 : 14954–14959.
117. McKinstryKK, StruttTM, BuckA, CurtisJD, DibbleJP, et al. (2009) IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol 182 : 7353–7363.
118. KellyMN, KollsJK, HappelK, SchwartzmanJD, SchwarzenbergerP, et al. (2005) Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun 73 : 617–621.
119. TallimaH, SalahM, GuirguisFR, El RidiR (2009) Transforming growth factor-beta and Th17 responses in resistance to primary murine schistosomiasis mansoni. Cytokine 48 : 239–245.
120. RutitzkyLI, Lopes da RosaJR, StadeckerMJ (2005) Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol 175 : 3920–3926.
121. RutitzkyLI, StadeckerMJ (2006) CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem Inst Oswaldo Cruz 101 Suppl 1 : 327–330.
122. MoffittKL, GierahnTM, LuY-J, GouveiaP, AldersonM, et al. (2011) T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 9 : 158–165.
123. DuhenT, GeigerR, JarrossayD, LanzavecchiaA, SallustoF (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10 : 857–863.
124. TrifariS, KaplanCD, TranEH, CrellinNK, SpitsH (2009) Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 10 : 864–871.
125. SonnenbergGF, FouserLA, ArtisD (2011) Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 12 : 383–390.
126. RamirezJ-M, BrembillaNC, SorgO, ChicheporticheR, MatthesT, et al. (2010) Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol 40 : 2450–2459.
127. BrembillaNC, RamirezJ-M, ChicheporticheR, SorgO, SauratJ-H, et al. (2011) In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PLoS ONE 6: e18741.
128. TachiiriA, ImamuraR, WangY, FukuiM, UmemuraM, et al. (2003) Genomic structure and inducible expression of the IL-22 receptor alpha chain in mice. Genes Immun 4 : 153–159.
129. WolkK, KunzS, WitteE, FriedrichM, AsadullahK, et al. (2004) IL-22 increases the innate immunity of tissues. Immunity 21 : 241–254.
130. LiangSC, TanXY, LuxenbergDP, KarimR, Dunussi-JoannopoulosK, et al. (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203 : 2271–2279.
131. WolkK, WitteE, WallaceE, DockeWD, KunzS, et al. (2006) IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36 : 1309–1323.
132. ZhengY, ValdezPA, DanilenkoDM, HuY, SaSM, et al. (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14 : 282–289.
133. AujlaSJ, ChanYR, ZhengM, FeiM, AskewDJ, et al. (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14 : 275–281.
134. WitteE, WitteK, WarszawskaK, SabatR, WolkK (2010) Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev 21 : 365–379.
135. LiuY, YangB, ZhouM, LiL, ZhouH, et al. (2009) Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans. Eur J Immunol 39 : 1472–1479.
136. NgWF, von DelwigA, CarmichaelAJ, ArkwrightPD, AbinunM, et al. (2010) Impaired T(H)17 responses in patients with chronic mucocutaneous candidiasis with and without autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Allergy Clin Immunol 126 : 1006–1004, 1006-1015, 1015, e1001-1004.
137. van der MeerJW, van de VeerdonkFL, JoostenLA, KullbergBJ, NeteaMG (2010) Severe Candida spp. infections: new insights into natural immunity. Int J Antimicrob Agents 36 Suppl 2: S58–62.
138. EyerichS, WagenerJ, WenzelV, ScarponiC, PenninoD, et al. (2011) IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol 41 : 1894–1901.
139. De LucaA, ZelanteT, D'AngeloC, ZagarellaS, FallarinoF, et al. (2010) IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 3 : 361–373.
140. WilsonMS, FengCG, BarberDL, YarovinskyF, CheeverAW, et al. (2010) Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol 184 : 4378–4390.
141. ZenewiczLA, YancopoulosGD, ValenzuelaDM, MurphyAJ, KarowM, et al. (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27 : 647–659.
142. GuoH, TophamDJ (2010) Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol 84 : 7750–7759.
143. MunozM, HeimesaatMM, DankerK, StruckD, LohmannU, et al. (2009) Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med 206 : 3047–3059.
144. IvanovS, RennesonJ, FontaineJ, BarthelemyA, PagetC, et al. (2013) Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol 87 : 6911–6924.
145. PociaskDA, SchellerEV, MandalapuS, McHughKJ, EnelowRI, et al. (2013) IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 182 : 1286–1296.
146. GuabirabaR, BesnardAG, MarquesRE, MailletI, FagundesCT, et al. (2013) IL-22 modulates IL-17A production and controls inflammation and tissue damage in experimental dengue infection. Eur J Immunol 43 : 1529–1544.
147. BehrendsJ, RenauldJ-C, EhlersS, HölscherC (2013) IL-22 is mainly produced by IFNγ-secreting cells but is dispensable for host protection against Mycobacterium tuberculosis infection. PLoS ONE 8: e57379.
148. ScribaTJ, KalsdorfB, AbrahamsDA, IsaacsF, HofmeisterJ, et al. (2008) Distinct, specific IL-17 - and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol 180 : 1962–1970.
149. QingK, FengWW, FanY, LiLY, MinXY, et al. (2012) Increased Expressions of IL-22 and Th22 cells in the coxsackievirus B3-Induced mice acute viral myocarditis. Virol J 9 : 232.
150. BasuR, O'QuinnDB, SilbergerDJ, SchoebTR, FouserL, et al. (2012) Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37 : 1061–1075.
151. GoswamiR, KaplanMH (2011) A brief history of IL-9. J Immunol 186 : 3283–3288.
152. GessnerA, BlumH, RollinghoffM (1993) Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology 189 : 419–435.
153. FaulknerH, RenauldJ-C, Van SnickJ, GrencisRK (1998) Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect Immun 66 : 3832–3840.
154. FaulknerH, HumphreysN, RenauldJ-C, Van SnickJ, GrencisR (1997) Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol 27 : 2536–2540.
155. DugasB, RenauldJC, PeneJ, BonnefoyJY, Peti-FrereC, et al. (1993) Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol 23 : 1687–1692.
156. Petit-FrereC, DugasB, BraquetP, Mencia-HuertaJM (1993) Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology 79 : 146–151.
157. LiH, RostamiA (2010) IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neuroimmune Pharmacol 5 : 198–209.
158. DardalhonV, AwasthiA, KwonH, GalileosG, GaoW, et al. (2008) IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol 9 : 1347–1355.
159. VeldhoenM, UyttenhoveC, van SnickJ, HelmbyH, WestendorfA, et al. (2008) Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 9 : 1341–1346.
160. YangXO, ZhangH, KimBS, NiuX, PengJ, et al. (2013) The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol 14 : 732–740.
161. UyttenhoveC, BrombacherF, Van SnickJ (2010) TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur J Immunol 40 : 2230–2235.
162. SchmittE, KleinM, BoppT (2013) Th9 cells, new players in adaptive immunity. Trends Immunol E-pub ahead of print.
163. PerumalNB, KaplanMH (2011) Regulating Il9 transcription in T helper cells. Trends Immunol 32 : 146–150.
164. ChangHC, SehraS, GoswamiR, YaoW, YuQ, et al. (2010) The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol 11 : 527–534.
165. StaudtV, BothurE, KleinM, LingnauK, ReuterS, et al. (2010) Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33 : 192–202.
166. JabeenR, GoswamiR, AweO, KulkarniA, NguyenET, et al. (2013) Th9 cell development requires a BATF-regulated transcriptional network. J Clin Invest E-pub ahead of print.
167. KaraEE, ComerfordI, BastowCR, FenixKA, LitchfieldW, et al. (2013) Distinct chemokine receptor axes regulate Th9 cell trafficking to allergic and autoimmune inflammatory sites. J Immunol 191 : 1110–1117.
168. KhanWI, RichardM, AkihoH, BlennerhassetPA, HumphreysNE, et al. (2003) Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun 71 : 2430–2438.
169. Licona-LimonP, Henao-MejiaJ, TemannAU, GaglianiN, Licona-LimonI, et al. (2013) Th9 cells drive host immunity against gastrointestinal worm infection. Immunity 39 : 744–757.
170. TurnerJE, MorrisonPJ, WilhelmC, WilsonM, AhlforsH, et al. (2013) IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med 210 : 2951–2965.
171. TownsendJM, FallonGP, MatthewsJD, SmithP, JolinEH, et al. (2000) IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13 : 573–583.
172. LiE, ZhouP, PetrinZ, SingerSM (2004) Mast cell-dependent control of Giardia lamblia infections in mice. Infect Immun 72 : 6642–6649.
173. FallonPG, JolinHE, SmithP, EmsonCL, TownsendMJ, et al. (2002) IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17 : 7–17.
174. YamasakiA, SalehA, KoussihL, MuroS, HalaykoAJ, et al. (2010) IL-9 induces CCL11 expression via STAT3 signalling in human airway smooth muscle cells. PLoS ONE 5: e9178.
175. GounniAS, HamidQ, RahmanSM, HoeckJ, YangJ, et al. (2004) IL-9-mediated induction of eotaxin1/CCL11 in human airway smooth muscle cells. J Immunol 173 : 2771–2779.
176. LouahedJ, ZhouY, MaloyWL, RaniPU, WeissC, et al. (2001) Interleukin 9 promotes influx and local maturation of eosinophils. Blood 97 : 1035–1042.
177. McNamaraPS, FlanaganBF, BaldwinLM, NewlandP, HartCA, et al. (2004) Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet 363 : 1031–1037.
178. DoddJS, LumE, GouldingJ, MuirR, Van SnickJ, et al. (2009) IL-9 regulates pathology during primary and memory responses to respiratory syncytial virus infection. J Immunol 183 : 7006–7013.
179. GrohmannU, Van SnickJ, CampanileF, SillaS, GiampietriA, et al. (2000) IL-9 protects mice from Gram-negative bacterial shock: suppression of TNF-alpha, IL-12, and IFN-gamma, and induction of IL-10. J Immunol 164 : 4197–4203.
180. YeZ-J, YuanM-L, ZhouQ, DuR-H, YangW-B, et al. (2012) Differentiation and recruitment of Th9 cells stimulated by pleural mesothelial cells in human Mycobacterium tuberculosis infection. PLoS ONE 7: e31710.
181. NoelleRJ, NowakEC (2010) Cellular sources and immune functions of interleukin-9. Nat Rev Immunol 10 : 683–687.
182. WilhelmC, HirotaK, StieglitzB, Van SnickJ, TolainiM, et al. (2011) An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol 12 : 1071–1077.
183. NuttSL, TarlintonDM (2011) Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol 12 : 472–477.
184. CrottyS (2011) Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29 : 621–663.
185. JohnstonRJ, PoholekAC, DiToroD, YusufI, EtoD, et al. (2009) Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325 : 1006–1010.
186. NurievaRI, ChungY, MartinezGJ, YangXO, TanakaS, et al. (2009) Bcl6 mediates the development of T follicular helper cells. Science 325 : 1001–1005.
187. YuD, RaoS, TsaiLM, LeeSK, HeY, et al. (2009) The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31 : 457–468.
188. KingIL, MohrsM (2009) IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med 206 : 1001–1007.
189. HsuHC, YangP, WangJ, WuQ, MyersR, et al. (2008) Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 9 : 166–175.
190. LintermanMA, BeatonL, YuD, RamiscalRR, SrivastavaM, et al. (2010) IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med 207 : 353–363.
191. LeeSK, SilvaDG, MartinJL, PratamaA, HuX, et al. (2012) Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 37 : 880–892.
192. ChtanovaT, TangyeSG, NewtonR, FrankN, HodgeMR, et al. (2004) T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 173 : 68–78.
193. NurievaRI, ChungY, HwangD, YangXO, KangHS, et al. (2008) Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29 : 138–149.
194. KimCH, LimHW, KimJR, RottL, HillsamerP, et al. (2004) Unique gene expression program of human germinal center T helper cells. Blood 104 : 1952–1960.
195. KroenkeMA, EtoD, LocciM, ChoM, DavidsonT, et al. (2012) Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol 188 : 3734–3744.
196. BreitfeldD, OhlL, KremmerE, EllwartJ, SallustoF, et al. (2000) Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 192 : 1545–1552.
197. SchaerliP, WillimannK, LangAB, LippM, LoetscherP, et al. (2000) CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 192 : 1553–1562.
198. MaCS, DeenickEK, BattenM, TangyeSG (2012) The origins, function, and regulation of T follicular helper cells. J Exp Med 209 : 1241–1253.
199. FazilleauN, McHeyzer-WilliamsLJ, RosenH, McHeyzer-WilliamsMG (2009) The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol 10 : 375–384.
200. DeenickEK, ChanA, MaCS, GattoD, SchwartzbergPL, et al. (2010) Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33 : 241–253.
201. EtoD, LaoC, DiToroD, BarnettB, EscobarTC, et al. (2011) IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE 6: e17739.
202. MaCS, AveryDT, ChanA, BattenM, BustamanteJ, et al. (2012) Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 119 : 3997–4008.
203. ChoiYS, EtoD, YangJA, LaoC, CrottyS (2013) Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol 190 : 3049–3053.
204. MaCS, SuryaniS, AveryDT, ChanA, NananR, et al. (2009) Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol 87 : 590–600.
205. SchmittN, BustamanteJ, BourderyL, BentebibelSE, Boisson-DupuisS, et al. (2013) IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood 121 : 3375–3385.
206. SchmittN, MoritaR, BourderyL, BentebibelSE, ZurawskiSM, et al. (2009) Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 31 : 158–169.
207. NakayamadaS, KannoY, TakahashiH, JankovicD, LuKT, et al. (2011) Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35 : 919–931.
208. Ballesteros-TatoA, RandallTD (2014) Priming of T follicular helper cells by dendritic cells. Immunol Cell Biol 92 : 22–27.
209. HaynesNM, AllenCD, LesleyR, AnselKM, KilleenN, et al. (2007) Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol 179 : 5099–5108.
210. BaumjohannD, OkadaT, AnselKM (2011) Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J Immunol 187 : 2089–2092.
211. LiuYJ, JoshuaDE, WilliamsGT, SmithCA, GordonJ, et al. (1989) Mechanism of antigen-driven selection in germinal centres. Nature 342 : 929–931.
212. ChoeJ, KimHS, ZhangX, ArmitageRJ, ChoiYS (1996) Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. Anti-Ig down-regulates Fas expression of CD40 ligand-stimulated germinal center B cells and inhibits Fas-mediated apoptosis. J Immunol 157 : 1006–1016.
213. ZotosD, CoquetJM, ZhangY, LightA, D'CostaK, et al. (2010) IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med 207 : 365–378.
214. AruffoA, FarringtonM, HollenbaughD, LiX, MilatovichA, et al. (1993) The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell 72 : 291–300.
215. OzakiK, SpolskiR, EttingerR, KimHP, WangG, et al. (2004) Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 173 : 5361–5371.
216. EttingerR, SimsGP, FairhurstAM, RobbinsR, da SilvaYS, et al. (2005) IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 175 : 7867–7879.
217. KuchenS, RobbinsR, SimsGP, ShengC, PhillipsTM, et al. (2007) Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol 179 : 5886–5896.
218. BryantVL, MaCS, AveryDT, LiY, GoodKL, et al. (2007) Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol 179 : 8180–8190.
219. DiSantoJP, BonnefoyJY, GauchatJF, FischerA, de Saint BasileG (1993) CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature 361 : 541–543.
220. AllenRC, ArmitageRJ, ConleyME, RosenblattH, JenkinsNA, et al. (1993) CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science 259 : 990–993.
221. QiH, CannonsJL, KlauschenF, SchwartzbergPL, GermainRN (2008) SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455 : 764–769.
222. CannonsJL, QiH, LuKT, DuttaM, Gomez-RodriguezJ, et al. (2010) Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 32 : 253–265.
223. YusufI, KageyamaR, MonticelliL, JohnstonRJ, DitoroD, et al. (2010) Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol 185 : 190–202.
224. RezaeiN, MahmoudiE, AghamohammadiA, DasR, NicholsKE (2011) X-linked lymphoproliferative syndrome: a genetic condition typified by the triad of infection, immunodeficiency and lymphoma. Br J Haematol 152 : 13–30.
225. MohrsK, WakilAE, KilleenN, LocksleyRM, MohrsM (2005) A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23 : 419–429.
226. ReinhardtRL, LiangH-E, LocksleyRM (2009) Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol 10 : 385–393.
227. LuthjeK, KalliesA, ShimohakamadaY, GTTB, LightA, et al. (2012) The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol 13 : 491–498.
228. LiuX, YanX, ZhongB, NurievaRI, WangA, et al. (2012) Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med 209 : 1841–1824, 1841-1852, S1841-1824.
229. ZaretskyAG, TaylorJJ, KingIL, MarshallFA, MohrsM, et al. (2009) T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med 206 : 991–999.
230. FaheyLM, WilsonEB, ElsaesserH, FistonichCD, McGavernDB, et al. (2011) Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med 208 : 987–999.
231. HirotaK, TurnerJE, VillaM, DuarteJH, DemengeotJ, et al. (2013) Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol 14 : 372–379.
232. SoghoianDZ, StreeckH (2010) Cytolytic CD4(+) T cells in viral immunity. Expert Rev Vaccines 9 : 1453–1463.
233. SutoA, KashiwakumaD, KagamiS, HiroseK, WatanabeN, et al. (2008) Development and characterization of IL-21-producing CD4+ T cells. J Exp Med 205 : 1369–1379.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



