-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHuman Genetic Susceptibility to Invasive Aspergillosis
article has not abstract
Published in the journal: . PLoS Pathog 9(8): e32767. doi:10.1371/journal.ppat.1003434
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003434Summary
article has not abstract
Introduction
Aspergillosis includes a wide spectrum of diseases caused by fungi of the genus Aspergillus with clinical manifestations that range from colonization (e.g., aspergilloma), to allergic bronchopulmonary aspergillosis, to disseminated forms of infection. Invasive aspergillosis (IA) has been estimated to occur in 10% of acute myeloid leukemia patients during post-induction aplasia or consolidation therapy and after 5–15% of allogeneic hematopoietic stem cell transplants (HSCT) [1], [2]. Additional persons at risk for IA include recipients of solid organ transplants and patients with chronic granulomatous disease (CGD). Despite the significant progress attained in the management of this severe infection, its prevention, diagnosis, and therapy remain extremely difficult, rendering it a leading cause of death among immunocompromised patients. Additionally, concerns over antimold prescription and the remarkably high healthcare costs owing to its chronic course and mortality rates have been diverting clinicians from universal prophylaxis to risk stratification and preemptive approaches. This has inspired the search for novel individual prognostic factors, particularly genetic, to apply in the categorization of those most vulnerable to infection.
Immune Recognition of Aspergillus : PAMPs, DAMPs, and Beyond
The physical barrier of the respiratory tract affords the first line of resistance against inhaled conidia of Aspergillus. In the event these escape the ciliated epithelium, conidia will then be challenged by cells of the innate immune system, including resident alveolar macrophages and dendritic cells (DCs), as well as recruited inflammatory cells, mainly polymorphonuclear neutrophils. These cells express a vast repertoire of pattern recognition receptors (PRRs) that sense pathogen-associated molecular patterns (PAMPs) and drive the secretion of proinflammatory cytokines and chemokines that arbitrate innate and adaptive immune responses. In the case of fungi, the cell wall is the main source of PAMPs owing to its dynamic composition and structural properties according to morphotype, growth stage, and environmental conditions [3]. Toll-like receptors (TLR)-2 (in cooperation with TLR1 and TLR6), TLR3, TLR4, and TLR9, and the C-type lectin receptors dendritic cell-specific intercellular adhesion molecule 3 grabbing nonintegrin (DC-SIGN), mannose receptor, and dectin-1 are among the PRRs recognizing fungal PAMPs including mannan, β-glucan, and nucleic acids [4]. Fungal sensing is further assisted by the action of collectins, ficolins, pentraxins, and complement components that act as opsonins and facilitate the interaction of phagocytes with fungi. Mammalian PRRs are also able to respond to products released from damaged host cells, including nucleic acids and alarmin proteins, collectively known as danger-associated molecular patterns (DAMPs). The unexpected convergence of PAMP - and DAMP-mediated molecular pathways raised the question of whether and how the host discriminates between them and the relative involvement of either one in inflammation, immune homeostasis, and mechanisms of repair during infection. Recent evidence has demonstrated that the immune system distinguishes fungus - and danger-induced immune responses, a mechanism relying on the spatiotemporal regulation of TLRs and the receptor for advanced glycation endproducts (RAGE) by the S100B alarmin [5].
Despite their undisputed relevance to pathogen resistance, innate immune mechanisms of pathogen detection may behave as double-edged swords, becoming detrimental as the result of hyperproduction of proinflammatory cytokines facilitating tissue damage or impairing protective immunity. This may explain why neutrophils, although indispensable in the implementation of the acute inflammatory response, may instead hamper resolution of infection through an excessive release of oxidants and proteases that may injure organs and promote fungal sepsis. Thus, although inflammation may serve to restrain infection, an overzealous reaction may contribute to pathogenicity and, paradoxically, favor disease progression. Along this line, genomic and transcriptomic approaches have revealed that fungal pathogenicity depends on mechanisms regulating fungal metabolism, morphogenesis, and response to stress in adaptation to the host environment. In particular, the ability of Aspergillus to adapt to hypoxic microenvironments has been found to involve modifications in fungal metabolism leading to the production of secondary metabolites that promote lung inflammation, exacerbate infection, and influence subsequent host immune responses [6].
Genetic Variability of the Host and Susceptibility to IA
The inborn deficiency of the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase leading to CGD is the best known example of primary immunodeficiency with predisposition to IA [7]. As a result of the impaired production of reactive oxygen species, patients with CGD often develop IA, typically within the first decade of life. Of interest, these patients are uniquely susceptible to diseases with the A. nidulans complex, which are weakly virulent molds that rarely cause infection in immunocompromised patients. For most individuals however, genetic propensity to aspergillosis has a polygenic source. A polygenic variant by itself has a negligible effect on phenotype; only in combination with other remarkable predisposing variants (e.g., profound immunosuppression) do sizeable phenotypic effects arise.
In conformity with the crucial requirement of innate immunity for effective antifungal host defense, several studies have uncovered associations between genetic variants in components of the innate immune system and risk for IA [8]–[10] (Table 1). One classical example regards a donor haplotype in TLR4 reported to increase susceptibility to infection after HSCT, especially if combined with cytomegalovirus seropositivity [11]. Despite TLR4 polymorphisms having also been linked with chronic aspergillosis in immunocompetent individuals [12] and fungal colonization in HSCT recipients [13], their prognostic significance remains disputed. The fact that TLR4 ligand(s) in fungi are still unknown and the limited knowledge of the biological consequences of human TLR4 deficiency to antifungal immunity have been hampering the employment of TLR4 genotyping in risk stratification approaches. Most importantly, genetic variants affecting the function of innate receptors other than TLR4 have also been deemed relevant. Indeed, alongside the discovery of TLR3-mediated activation of protective memory CD8(+) T cell responses in experimental aspergillosis, a donor polymorphism impairing the expression of the human receptor was found to predispose to IA due to the inability of human DCs to efficiently prime memory CD8(+) responses to the fungus [14]. Additionally, and given the pivotal role of dectin-1 in fungal sensing, it is also not surprising that human dectin-1 deficiency has been reported to contribute to susceptibility to IA [15]–[17]. Interestingly, a stop codon polymorphism compromising the surface expression and dectin-1-mediated cytokine production displayed a cumulative effect toward risk for infection after HSCT when present concurrently in donors and recipients of stem cell grafts [16], a finding emphasizing the contribution of non-hematopoietic dectin-1 to antifungal immunity. As host damage perception is also fundamental for resolution of infection [5], genetic variants triggering hyperactive DAMP signaling, and presumably leading to uncontrolled inflammatory response to the fungus, were recently found to increase risk for IA [18]. Finally, and although positive associations between genetic variants in cytokine genes and vulnerability to IA have been reported [8], the lack of functional validation and the underpowered design of most studies precludes definite conclusions about the contribution of polymorphisms affecting cytokine production. One exception is the link proposed between a haplotype in CXCL10 and risk for IA in HSCT recipients [19]. Mechanistically, this haplotype was correlated with the inability of DCs to express CXCL10 and, interestingly, patients who survived IA displayed significantly higher CXCL10 levels compared to patients without disease.
Tab. 1. Genetic variants associated with increased risk for IA and probable pathogenetic mechanism(s) of susceptibility. 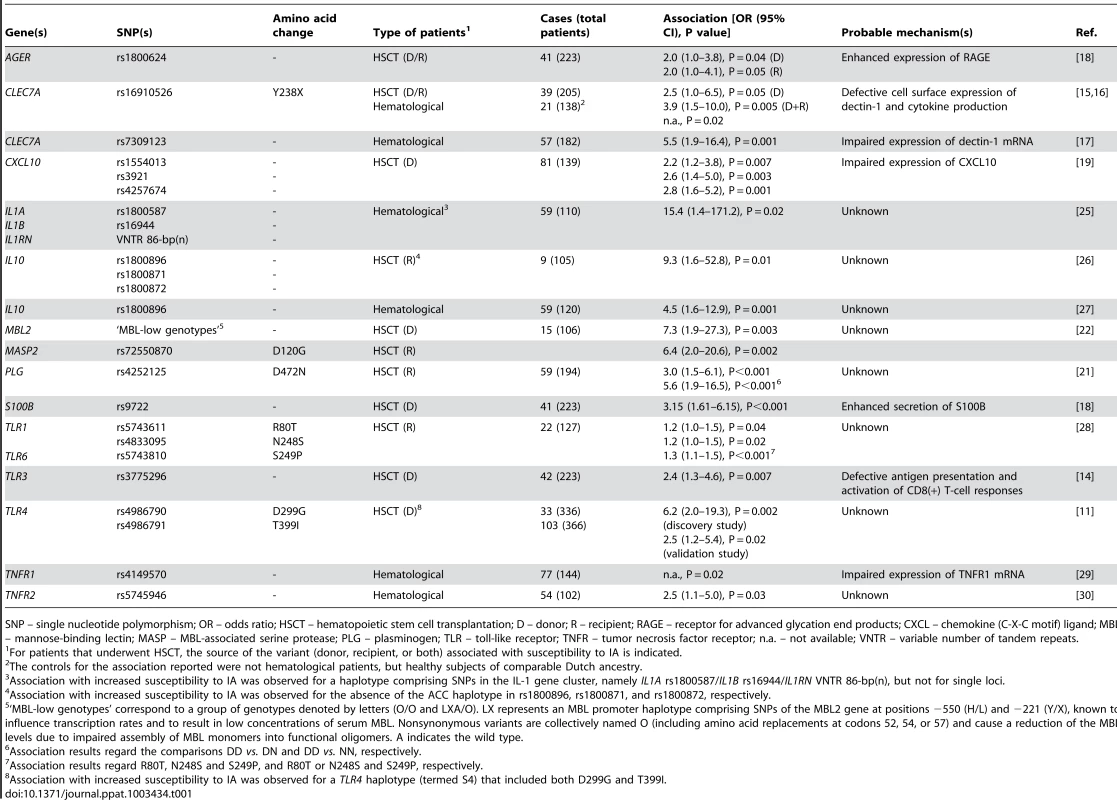
SNP – single nucleotide polymorphism; OR – odds ratio; HSCT – hematopoietic stem cell transplantation; D – donor; R – recipient; RAGE – receptor for advanced glycation end products; CXCL – chemokine (C-X-C motif) ligand; MBL – mannose-binding lectin; MASP – MBL-associated serine protease; PLG – plasminogen; TLR – toll-like receptor; TNFR – tumor necrosis factor receptor; n.a. – not available; VNTR – variable number of tandem repeats. Alternative strategies have been employed to uncover gene candidates for susceptibility to IA other than the “obvious” innate immune receptors and associated cytokines [20], [21]. For example, genetic mapping analysis of survival data of infected mice allowed the identification of plasminogen, a regulatory molecule with opsonic properties, as a fitting contestant for susceptibility [21]. Consequently, a nonsynonymous polymorphism in human plasminogen was found to increase risk for IA in HSCT recipients, particularly late after transplantation. Of interest, genetic and functional deficiency of other molecules with opsonic activity—e.g., mannose-binding lectin [22], [23] and PTX3 (our unpublished data)—have also been disclosed as major determinants of susceptibility to IA, pointing to a foremost contribution of humoral immunity in response to Aspergillus.
Unveiling Human Susceptibility to IA: What Might the Future Hold?
Our existing knowledge of the genetic bases of susceptibility to IA derives from studies screening single variants in candidate genes using small patient cohorts. In addition, statistical issues with multiple comparisons and the lack of validation in larger, independent cohorts or via biological studies of disease mechanisms are further limitations of candidate gene association studies. As cutting-edge “omics” techniques are becoming affordable, multidisciplinary integrative approaches targeting variability in genome-wide association studies or expression in whole-transcriptomics studies may help to identify novel susceptibility signatures in otherwise unsuspected genes or pathways besides confirming those currently acknowledged. As “omics” have contributed to the identification of genetic susceptibility traits in cancer research, these techniques could be ultimately extrapolated with success to the field of invasive fungal diseases. Furthermore, only now are we beginning to fully grasp the significance of the microbiota and its interactions with the mammalian immune system in defining susceptibility to infection. Indeed, the structure and composition of lung microbial communities in patients at-risk was found to diverge significantly from that of healthy individuals [24], thus suggesting a likely susceptibility signature to IA that may involve a host–fungus–microbiota triad. All these state-of-the-art approaches however do not weaken the weight of functional validation. Given that “omics” studies by nature disregard all preceding knowledge about disease pathobiology, studies unveiling useful mechanistic insights into the relevant signatures found, be them genetic or biological, are still essential.
Concluding Remarks
The identification of patient-specific prognostic signatures of susceptibility to IA in high-risk patients is currently one major priority in the fields of hematology and microbiology. Ultimately, the discovery of reliable markers of susceptibility consistently associated with risk for IA and functionally correlated with impaired antifungal mechanisms of the host may be a turning point toward innovative stratification strategies based on genetic screening or immune profiling to predict risk and severity of disease, efficacy of antifungal prophylaxis and therapy, and eventually contribute to the successful design of antifungal vaccines.
Zdroje
1. KontoyiannisDP, MarrKA, ParkBJ, AlexanderBD, AnaissieEJ, et al. (2010) Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50 : 1091–1100.
2. PaganoL, CairaM, CandoniA, OffidaniM, MartinoB, et al. (2010) Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica 95 : 644–650.
3. LatgeJP (2010) Tasting the fungal cell wall. Cell Microbiol 12 : 863–872.
4. RomaniL (2011) Immunity to fungal infections. Nat Rev Immunol 11 : 275–288.
5. SorciG, GiovanniniG, RiuzziF, BonifaziP, ZelanteT, et al. (2011) The danger signal S100B integrates pathogen - and danger-sensing pathways to restrain inflammation. PLoS Pathog 7: e1001315 doi:10.1371/journal.ppat.1001315
6. GrahlN, PuttikamonkulS, MacdonaldJM, GamcsikMP, NgoLY, et al. (2011) In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog 7: e1002145 doi:10.1371/journal.ppat.1002145
7. VinhDC (2011) Insights into human antifungal immunity from primary immunodeficiencies. Lancet Infect Dis 11 : 780–792.
8. CunhaC, AversaF, BistoniG, CasagrandeA, RodriguesF, et al. (2011) Immunogenetic profiling to predict risk of invasive fungal diseases: where are we now? Immunol Invest 40 : 723–734.
9. CunhaC, RomaniL, CarvalhoA (2010) Cracking the Toll-like receptor code in fungal infections. Expert Rev Anti Infect Ther 8 : 1121–1137.
10. van der VeldenWJFM, BlijlevensNMA, DonnellyJP (2011) Genetic variants and the risk for invasive mould disease in immunocompromised hematology patients. Curr Opin Infect Dis 24 : 554–563.
11. BochudPY, ChienJW, MarrKA, LeisenringWM, UptonA, et al. (2008) Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med 359 : 1766–1777.
12. CarvalhoA, PasqualottoAC, PitzurraL, RomaniL, DenningDW, et al. (2008) Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis 197 : 618–621.
13. CarvalhoA, CunhaC, CarottiA, AloisiT, GuarreraO, et al. (2009) Polymorphisms in Toll-like receptor genes and susceptibility to infections in allogeneic stem cell transplantation. Exp Hematol 37 : 1022–1029.
14. CarvalhoA, De LucaA, BozzaS, CunhaC, D'AngeloC, et al. (2012) TLR3 essentially promotes protective class I-restricted memory CD8(+) T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 119 : 967–977.
15. ChaiLY, de BoerMG, van der VeldenWJ, PlantingaTS, van SprielAB, et al. (2011) The Y238X stop codon polymorphism in the human beta-glucan receptor dectin-1 and susceptibility to invasive aspergillosis. J Infect Dis 203 : 736–743.
16. CunhaC, Di IanniM, BozzaS, GiovanniniG, ZagarellaS, et al. (2010) Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient - and donor-dependent mechanisms of antifungal immunity. Blood 116 : 5394–5402.
17. SainzJ, LupiáñezCB, Segura-CatenaJ, VazquezL, RíosR, et al. (2012) Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS ONE 7: e32273 doi:10.1371/journal.pone.0032273
18. CunhaC, GiovanniniG, PieriniA, BellAS, SorciG, et al. (2011) Genetically-determined hyperfunction of the S100B/RAGE axis is a risk factor for aspergillosis in stem cell transplant recipients. PLoS ONE 6: e27962 doi:10.1371/journal.pone.0027962
19. MezgerM, SteffensM, BeyerM, MangerC, EberleJ, et al. (2008) Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells. Blood 111 : 534–536.
20. DurrantC, TayemH, YalcinB, CleakJ, GoodstadtL, et al. (2011) Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res 21 : 1239–1248.
21. ZaasAK, LiaoG, ChienJW, WeinbergC, ShoreD, et al. (2008) Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet 4: e1000101 doi:10.1371/journal.pgen.1000101
22. GranellM, Urbano-IspizuaA, SuarezB, RoviraM, Fernandez-AvilesF, et al. (2006) Mannan-binding lectin pathway deficiencies and invasive fungal infections following allogeneic stem cell transplantation. Exp Hematol 34 : 1435–1441.
23. LambourneJ, AgranoffD, HerbrechtR, TrokePF, BuchbinderA, et al. (2009) Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin Infect Dis 49 : 1486–1491.
24. CharlsonES, DiamondJM, BittingerK, FitzgeraldAS, YadavA, et al. (2012) Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med 186 : 536–545.
25. SainzJ, PerezE, Gomez-LoperaS, JuradoM (2008) IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol 28 : 473–485.
26. SeoKW, KimDH, SohnSK, LeeNY, ChangHH, et al. (2005) Protective role of interleukin-10 promoter gene polymorphism in the pathogenesis of invasive pulmonary aspergillosis after allogeneic stem cell transplantation. Bone Marrow Transplant 36 : 1089–1095.
27. SainzJ, HassanL, PerezE, RomeroA, MoratallaA, et al. (2007) Interleukin-10 promoter polymorphism as risk factor to develop invasive pulmonary aspergillosis. Immunol Lett 109 : 76–82.
28. KeshS, MensahNY, PeterlongoP, JaffeD, HsuK, et al. (2005) TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann N Y Acad Sci 1062 : 95–103.
29. SainzJ, Salas-AlvaradoI, Lopez-FernandezE, OlmedoC, CominoA, et al. (2010) TNFR1 mRNA expression level and TNFR1 gene polymorphisms are predictive markers for susceptibility to develop invasive pulmonary aspergillosis. Int J Immunopathol Pharmacol 23 : 423–436.
30. SainzJ, PerezE, HassanL, MoratallaA, RomeroA, et al. (2007) Variable number of tandem repeats of TNF receptor type 2 promoter as genetic biomarker of susceptibility to develop invasive pulmonary aspergillosis. Hum Immunol 68 : 41–50.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits fromČlánek X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Biosecurity Implications of New Technology and Discovery in Plant Virus Research
- Pathogenic Yeasts Deploy Cell Surface Receptors to Acquire Iron in Vertebrate Hosts
- Asparagine Repeats in Proteins: Good for Nothing?
- Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from
- Intact Type I Interferon Production and IRF7 Function in Sooty Mangabeys
- Prion Replication Occurs in Endogenous Adult Neural Stem Cells and Alters Their Neuronal Fate: Involvement of Endogenous Neural Stem Cells in Prion Diseases
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
- HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women
- Crystal Structure of the Full-Length Japanese Encephalitis Virus NS5 Reveals a Conserved Methyltransferase-Polymerase Interface
- Quantitative Models of the Dose-Response and Time Course of Inhalational Anthrax in Humans
- Stabilization of Myc through Heterotypic Poly-Ubiquitination by mLANA Is Critical for γ-Herpesvirus Lymphoproliferation
- The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Biofilms
- A Novel Role for Pro-Coagulant Microvesicles in the Early Host Defense against
- Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits from
- CD36 Recruits αβ Integrin to Promote Cytoadherence of -Infected Erythrocytes
- X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Chikungunya Virus 3′ Untranslated Region: Adaptation to Mosquitoes and a Population Bottleneck as Major Evolutionary Forces
- Mucin Gene (PoMuc) Expression: Epigenetic Control to Shape Adaptation to a New Host
- Determinants of GPI-PLC Localisation to the Flagellum and Access to GPI-Anchored Substrates in Trypanosomes
- The Smallest Capsid Protein Mediates Binding of the Essential Tegument Protein pp150 to Stabilize DNA-Containing Capsids in Human Cytomegalovirus
- Understanding Human Variation in Infectious Disease Susceptibility through Clinical and Cellular GWAS
- Human Genetic Susceptibility to Invasive Aspergillosis
- Host Immune Response to Intestinal Amebiasis
- Fungi Infecting Plants and Animals: Killers, Non-Killers, and Cell Death
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Host Immune Response to Intestinal Amebiasis
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



