-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAsparagine Repeats in Proteins: Good for Nothing?
article has not abstract
Published in the journal: . PLoS Pathog 9(8): e32767. doi:10.1371/journal.ppat.1003488
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003488Summary
article has not abstract
Malaria is a deadly parasitic human disease that poses a significant health risk for about 3.3 billion people in the tropical and subtropical regions of the world [1]. The past decade has seen significant progress in our understanding of the biology of the most deadly parasite species, Plasmodium falciparum. The groundwork for this progress was laid by genome sequencing efforts that revealed a number of surprising features [2], [3]. One striking aspect of this extreme AT-rich genome is the abundance of trinucleotide repeats (predominantly AAT) coding for asparagine [3]. The wealth of low-complexity regions in P. falciparum proteins had been known prior to sequencing of the genome but not the overabundance of simple amino acid repeats [4].
Amino Acid Repeat Prevalence
The random expansion of large amino acid repeats or low-complexity regions in proteomes are usually disfavored. While the effect of these repeats on the structure of the host protein depends on their amino acid compositions, low-complexity regions have a propensity to form loops or disordered structures [5]. Most sequenced genomes have a low abundance of amino acid repeats. However, there are exceptions, notably the social amoeba Dictyostelium discoideum and the deadly human malaria parasite Plasmodium falciparum [2], [6].
The proteome of D. discoideum is rich in polyglutamine and polyasparagine runs of 20 or more residues, found in more than 2,000 proteins [6]. These repeats are overrepresented in certain protein families such as kinases, transcription factors, RNA helicases, and spliceosome components, leading to the suggestion that the expansion of low-complexity regions may be under positive selection [6]. In the case of P. falciparum, the repeats are present in about 30% of the proteome and are primarily composed of asparagine residues [7]. The average size of such low-complexity regions is about 37 residues [8]. The asparagine-rich, low-complexity regions in P. falciparum are found in all protein families in all developmental stages; they are underrepresented in heat shock proteins and surface antigens [7]. Interestingly, asparagine repeats are rare in other Plasmodium species, even though some are as AT-rich as P. falciparum. The exception (see below) is the P. falciparum–related chimpanzee malaria species P. reichenowi, whose genome has not yet been completed, but has nearly identical asparagine repeats where comparisons have been done.
Consequences of Asparagine Repeats in Proteins
Proteins with large stretches of asparagine repeats have a propensity to form insoluble aggregates, even more so than those with glutamine repeats [9]. Indeed, a large-scale survey of prionogenic proteins in yeast found several proteins that contain asparagine-rich, low-complexity regions and form intracellular aggregates [10]. Formation of insoluble aggregates is not deleterious in all cases [11], [12]. Such protein aggregates have been shown to be important for mediating the inheritance of several phenotypes in yeast [13], persistence of synaptic facilitation in mammals [14], and antiviral innate immunity [15].
In contrast, unregulated aggregation of proteins is harmful and has been associated with various neurodegenerative diseases, systemic amyloidosis, and type II diabetes [12]. Aggregation can lead to cell death by propagating aberrant interactions and by puncturing the cell membrane [16], [17]. In light of this data, the rampant presence of asparagine repeat sequences in 20–30% of all P. falciparum proteins is puzzling.
Heat Shock and Asparagine Repeat–Containing Proteins in P. falciparum
The life cycle of P. falciparum forces the organism through drastic changes in its environment. Every time the parasite passes from its mosquito vector to its human host and vice versa it faces changing temperatures, with the insect at room temperature and the human at 37°C. A hallmark feature of malaria is cyclical fevers that can last several hours and can exceed 40°C. Heat shock increases the propensity of proteins to unfold and misfold, leading to the formation of aggregates. Given the abundance of asparagine repeat–containing proteins in P. falciparum, if all or most of them aggregated during heat shock, it would certainly lead the parasite's demise. However, that is not the case as this is a very successful parasite that has learned to expertly navigate the changing temperature landscape despite the handicap of its asparagine repeat–rich proteome. Learning how the parasite does this holds great promise since it could inform research on how to prevent aggregation of proteins associated with human diseases.
It is possible that the parasite asparagine repeat proteins somehow intrinsically do not aggregate. We tested this hypothesis by expressing a parasite protein that contains an 83-residue asparagine repeat (PF3D7_0923500, Figure 1) in mammalian cells and comparing its aggregation properties to the well-studied asparagine/glutamine-rich prion-forming domain from the yeast translation termination factor, Sup35. Both proteins formed cellular aggregates at 37°C and even more so when incubated at 40°C for a few hours [18]. When both proteins were episomally expressed in P. falciparum, no aggregate formation was observed at either temperature, suggesting that parasite chaperones are exceptionally good at preventing aggregation. Indeed, the cytoplasmic P. falciparum heat shock protein 110 (PfHsp110c) was 15 to 30 times better than its yeast or human orthologs at preventing aggregation of asparagine and glutamine repeat proteins in mammalian cells [18]. When the function of PfHsp110c was knocked down in parasites, they were unable to survive even brief heat shock or prevent aggregation of asparagine repeat–containing proteins [18]. It is likely that PfHsp110c isn't the only parasite chaperone that is better than its homologs at preventing aggregation. Chaperones always act in concert, so it is possible that other P. falciparum heat shock proteins have also evolved to be exceptionally good at preventing protein aggregation. Further research will shed light on this. The data also shows that malarial chaperones may be excellent targets for drug development, as shutting them down will turn the parasite proteome against itself. This work has shown us how the parasite is able to thrive in its fluctuating environment with such a dangerous proteome, but it does not answer why it has such a weird proteome.
Fig. 1. The protein with the largest single continuous stretch of asparagines in the proteome of P. falciparum. 
A protein with distant homology to the regulatory subunit of cell cycle kinase, CDK2, has a stretch of 83 asparagines. Asparagine residues constitute 42% of the primary sequence and it has two asparagine-rich regions (highlighted in blue). Is There a Functional Role for Asparagine Repeats in P. falciparum Proteins?
Why does P. falciparum have a proteome abundant in proteins containing asparagine repeats? One obvious answer is that there is a selective advantage or a function that these repeats provide to the parasite. Several hypotheses have been posed. It has been suggested that asparagine repeats act as tRNA sponges [19] or that they have a role in immune evasion and antigenic variation [20], [21] or protein-protein interactions [22]. However, a functional answer seems unlikely since these repeats are present in almost all protein families involved in every metabolic pathway. If there were a specific function for asparagine repeats, they should be enriched in proteins involved in that pathway.
There has been one experimental study that looked at the cellular function of an asparagine repeat in a specific protein. Parasite lines were generated in which a single 28-asparagine repeat in the essential proteasome component, Rpn6, was deleted from the genomic locus [23]. We did not observe any difference between wild-type Rpn6 and the deletion mutant when comparing their expression profiles, protein lifetime, cellular localization, function, and protein-protein interactions. Stressing these parasite lines via heat shock did not reveal any differences either [23]. This suggests that, at least in the case of Rpn6, the asparagine repeat does not have a cellular role.
Evolution of Asparagine Repeats in the P. falciparum Genome
Bioinformatics analyses suggest that asparagine repeats are primarily being spread in the genome via a DNA-based mechanism, most likely unequal crossover and replication slippage that happens due to the AT-rich nature of the repeats [8], [24]. It is possible then that the abundance of asparagine repeats in the parasite proteome is just an accident of evolution that happened because there is no selective pressure against its propagation. This seems unlikely given the preponderance of evidence showing that asparagine repeat–containing proteins have a greater propensity to aggregate [9] and given the ability of P. falciparum to delete unwanted genes and many introns [25]. Others have suggested based on statistical analyses that there is positive selective pressure that specifically promotes the expansion of asparagine repeats within the proteome of P. falciparum [26]. Our data suggests that the parasite chaperones are exceptionally good at preventing aggregation and therefore neutralize the negative selective pressure against the expansion of asparagine-rich regions in the proteome of P. falciparum. We hypothesize that the P. falciparum Hsp110 acts as a capacitor for positive evolutionary change, allowing the propagation of asparagine repeats in the proteome that can then evolve into new protein domains with novel functions (Figure 2) [18], [27]. In the absence of evolutionary and functional evidence, one is left to imagine that repeats like the first one in Figure 1 have started this journey.
Fig. 2. Proposed role of P. falciparum Hsp110 and other chaperones as capacitors for evolutionary change. 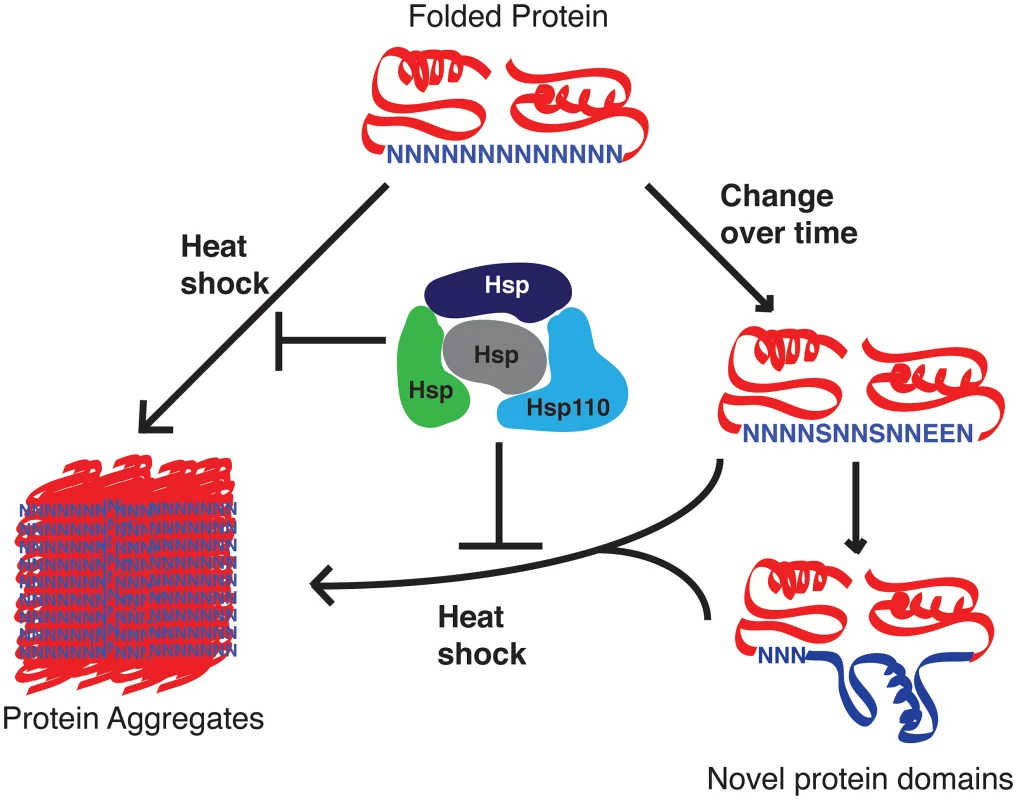
Malaria parasite proteins with asparagine repeat–containing sequences have a greater risk of aggregation. P. falciparum Hsp110c, possibly with the help of other chaperones, negates much of this risk, thereby allowing these loop-like regions to mutate. Over time these mutations can give rise to novel protein domains, allowing the parasite to develop new functionalities such as drug resistance and new pathogenic factors. It is likely that the last common ancestor of P. falciparum and P. reichenowi was the origin of these expanded asparagine repeats. Completion of P. reichenowi genome sequencing and annotation will shed light on their evolution. Detailed informatics analysis of the few polymorphisms in P. falciparum repeats between different strains of this species may also tell us if these features are hotspots for evolutionary change. Understanding the expansion of asparagine repeats will provide vital insights into parasite evolution that could be utilized to tackle this deadly disease.
Zdroje
1. CibulskisRE, AregawiM, WilliamsR, OttenM, DyeC (2011) Worldwide incidence of malaria in 2009: estimates, time trends, and a critique of methods. PLoS Med 8: e1001142 doi:10.1371/journal.pmed.1001142
2. GardnerMJ, HallN, FungE, WhiteO, BerrimanM, et al. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419 : 498–511.
3. AravindL, IyerLM, WellemsTE, MillerLH (2003) Plasmodium biology: genomic gleanings. Cell 115 : 771–785.
4. KempDJ, CoppelRL, AndersRF (1987) Repetitive proteins and genes of malaria. Annu Rev Microbiol 41 : 181–208.
5. WoottonJC (1994) Non-globular domains in protein sequences: automated segmentation using complexity measures. Comput Chem 18 : 269–285.
6. EichingerL, PachebatJA, GlöcknerG, RajandreamMA, SucgangR, et al. (2005) The genome of the social amoeba Dictyostelium discoideum. Nature 435 : 43–57.
7. SinghGP, ChandraBR, BhattacharyaA, AkhouriRR, SinghSK, et al. (2004) Hyper-expansion of asparagines correlates with an abundance of proteins with prion-like domains in Plasmodium falciparum. Mol Biochem Parasitol 137 : 307–319.
8. ZilversmitMM, VolkmanSK, DePristoMA, WirthDF, AwadallaP, et al. (2010) Low-complexity regions in Plasmodium falciparum: missing links in the evolution of an extreme genome. Mol Biol Evol 27 : 2198–2209.
9. HalfmannR, AlbertiS, KrishnanR, LyleN, O'DonnellCW, et al. (2011) Opposing effects of glutamine and asparagine govern prion formation by intrinsically disordered proteins. Mol Cell 43 : 72–84.
10. AlbertiS, HalfmannR, KingO, KapilaA, LindquistS (2009) A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137 : 146–158.
11. FowlerDM, KoulovAV, BalchWE, KellyJW (2007) Functional amyloid – from bacteria to humans. Trends Biochem Sci 32 : 217–224.
12. ChitiF, DobsonCM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75 : 333–366.
13. PatinoMM, LiuJJ, GloverJR, LindquistS (1996) Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273 : 622–626.
14. SiK, ChoiY-B, White-GrindleyE, MajumdarA, KandelER (2010) Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell 140 : 421–435.
15. HouF, SunL, ZhengH, SkaugB, JiangQ-X, et al. (2011) MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146 : 448–461.
16. OlzschaH, SchermannSM, WoernerAC, PinkertS, HechtMH, et al. (2011) Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144 : 67–78.
17. SeuringC, GreenwaldJ, WasmerC, WepfR, SaupeSJ, et al. (2012) The mechanism of toxicity in HET-S/HET-s prion incompatibility. PLoS Biol 10: e1001451 doi:10.1371/journal.pbio.1001451
18. MuralidharanV, OksmanA, PalP, LindquistS, GoldbergDE (2012) Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat Commun 3 : 1310.
19. FrugierM, BourT, AyachM, SantosMAS, Rudinger-ThirionJ, et al. (2010) Low complexity regions behave as tRNA sponges to help co-translational folding of plasmodial proteins. FEBS Lett 584 : 448–454.
20. VerraF, HughesAL (1999) Biased amino acid composition in repeat regions of Plasmodium antigens. Mol Biol Evol 16 : 627–633.
21. HughesAL (2004) The evolution of amino acid repeat arrays in Plasmodium and other organisms. J Mol Evol 59 : 528–535.
22. KarlinS, BrocchieriL, BergmanA, MrazekJ, GentlesAJ (2002) Amino acid runs in eukaryotic proteomes and disease associations. Proc Natl Acad Sci U S A 99 : 333–338.
23. MuralidharanV, OksmanA, IwamotoM, WandlessTJ, GoldbergDE (2011) Asparagine repeat function in a Plasmodium falciparum protein assessed via a regulatable fluorescent affinity tag. Proc Natl Acad Sci U S A 108 : 4411–4416 doi:10.1073/pnas.1018449108
24. XueHY, ForsdykeDR (2003) Low-complexity segments in Plasmodium falciparum proteins are primarily nucleic acid level adaptations. Mol Biochem Parasitol 128 : 21–32.
25. AndersonTJC, PatelJ, FerdigMT (2009) Gene copy number and malaria biology. Trends Parasitol 25 : 336–343.
26. DalbyAR (2009) A comparative proteomic analysis of the simple amino acid repeat distributions in Plasmodia reveals lineage specific amino acid selection. PLoS ONE 4: e6231 doi:10.1371/journal.pone.0006231
27. RutherfordSL, LindquistS (1998) Hsp90 as a capacitor for morphological evolution. Nature 396 : 336–342.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits fromČlánek X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Biosecurity Implications of New Technology and Discovery in Plant Virus Research
- Pathogenic Yeasts Deploy Cell Surface Receptors to Acquire Iron in Vertebrate Hosts
- Asparagine Repeats in Proteins: Good for Nothing?
- Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from
- Intact Type I Interferon Production and IRF7 Function in Sooty Mangabeys
- Prion Replication Occurs in Endogenous Adult Neural Stem Cells and Alters Their Neuronal Fate: Involvement of Endogenous Neural Stem Cells in Prion Diseases
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
- HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women
- Crystal Structure of the Full-Length Japanese Encephalitis Virus NS5 Reveals a Conserved Methyltransferase-Polymerase Interface
- Quantitative Models of the Dose-Response and Time Course of Inhalational Anthrax in Humans
- Stabilization of Myc through Heterotypic Poly-Ubiquitination by mLANA Is Critical for γ-Herpesvirus Lymphoproliferation
- The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Biofilms
- A Novel Role for Pro-Coagulant Microvesicles in the Early Host Defense against
- Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits from
- CD36 Recruits αβ Integrin to Promote Cytoadherence of -Infected Erythrocytes
- X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Chikungunya Virus 3′ Untranslated Region: Adaptation to Mosquitoes and a Population Bottleneck as Major Evolutionary Forces
- Mucin Gene (PoMuc) Expression: Epigenetic Control to Shape Adaptation to a New Host
- Determinants of GPI-PLC Localisation to the Flagellum and Access to GPI-Anchored Substrates in Trypanosomes
- The Smallest Capsid Protein Mediates Binding of the Essential Tegument Protein pp150 to Stabilize DNA-Containing Capsids in Human Cytomegalovirus
- Understanding Human Variation in Infectious Disease Susceptibility through Clinical and Cellular GWAS
- Human Genetic Susceptibility to Invasive Aspergillosis
- Host Immune Response to Intestinal Amebiasis
- Fungi Infecting Plants and Animals: Killers, Non-Killers, and Cell Death
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Host Immune Response to Intestinal Amebiasis
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



