-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHost Immune Response to Intestinal Amebiasis
article has not abstract
Published in the journal: . PLoS Pathog 9(8): e32767. doi:10.1371/journal.ppat.1003489
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003489Summary
article has not abstract
Introduction
Entamoeba histolytica is an invasive enteric protozoan parasite that causes amebiasis. Globally, diarrheal disease is second only to pneumonia as a leading cause of death in children under five, and intestinal amebiasis is one of the top ten causes of severe diarrhea in the developing world. Amebiasis is more common in malnourished children, a state that afflicts approximately one-third of children in the developing world. In the critical first year of life, 11% of Bangladeshi infants living in poverty suffer from E. histolytica diarrhea [1], [2]. There is currently no vaccine for this devastating disease, thus an understanding of the human immune response toward the parasite would greatly enhance the ability to develop effective immunotherapies. The host deploys a series of immune defenses against the parasite as it invades the colon. The ameba, however, has developed complex strategies to evade host defenses and promote its own survival. Here, we summarize the dynamics of the interaction of parasite with host and its importance in the pathogenesis of amebiasis (Figure 1).
Fig. 1. A. Host Immune Response to Intestinal Amebiasis. 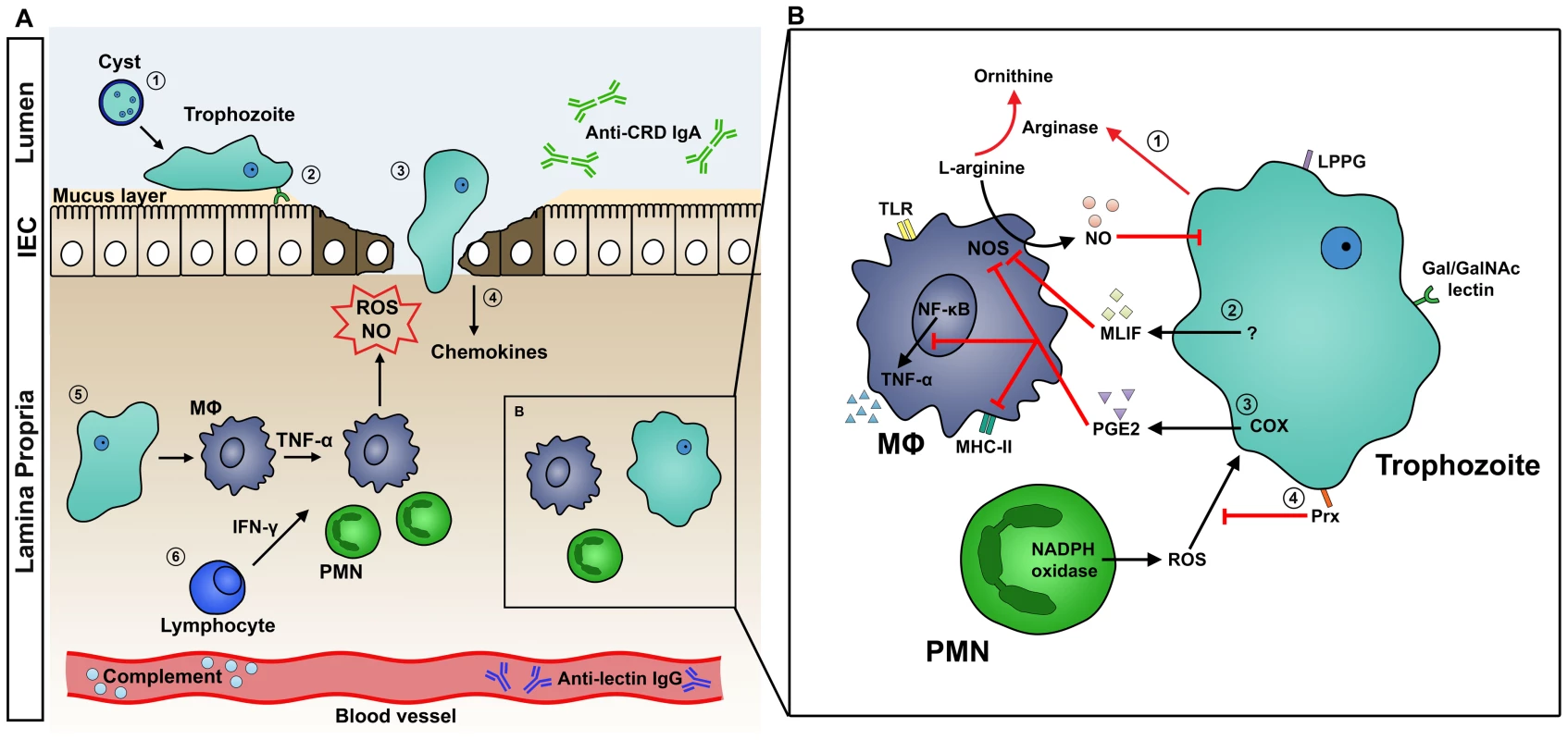
(1) Stomach acid serves as a first line of defense against enteropathogens, but amebic cysts are highly resistant and excyst in the lumen of the intestine. (2) Mucin, a glycoprotein secreted by goblet cells and submucosal glands, is the main constituent of the protective mucus layer. Trophozoites attach to the host tissue surface via Gal/GalNAc lectin. (3) Amebae secrete cysteine proteases, which disrupt the mucus layer and facilitate tissue invasion. (4) Injured IECs release potent chemokines to recruit immune cells to the site of invasion. (5) Activated macrophages release TNF-α, stimulating PMNs and macrophages to release ROS and NO, which kill the parasite. ROS and NO may also contribute to tissue destruction. (6) IFN-γ released by lymphocytes activates macrophages and PMNs. B. Mechanisms of Host Immune Evasion. (1) E. histolytica trophozoites inhibit the respiratory burst of MΦ using arginase, which converts L-arginine, a substrate of NOS, to L-ornithine. This depletes the L-arginine supply that macrophages use to produce NO. (2) MLIF produced by ameba suppresses NO production. (3) COX in ameba or ameba-exposed macrophages produces the immunoregulatory molecule PGE2. PGE2 suppresses macrophage effector functions by elevating cAMP levels, which in turn inhibits NO production, MHC-II expression, and TNF-α production. (4) Amebic Prx, a 29-kDa surface protein, confers resistance to neutrophil reactive oxygen species. Abbreviations: COX, cyclooxygenase; IEC, intestinal epithelial cells; IFN-γ, interferon-gamma; MΦ, macrophage; MHC-II, major histocompatibility complex class 2; MLIF, monocyte locomotion factor; NO, nitric oxide; NOS, nitric oxide synthase; PGE2, prostaglandin E2; PMN, polymorphonuclear leukocytes; Prx, peroxiredoxin; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha. Innate Immunity
Stomach acid serves as an important first line of defense against enteropathogens through its ability to kill acid-sensitive microorganisms. However, infectious amebic cysts are highly resistant and survive passage through the acidic environment of the stomach. In the intestine, the next layer of innate defense may be the mucus layer, which is thought to act as a protective barrier, preventing E. histolytica from invading intestinal epithelial cells (IECs). Mucin, a major constituent of the intestinal mucus layer, is a glycoprotein secreted by goblet cells and submucosal glands. Mucin glycoproteins bind to and inhibit the Gal/GalNAc adherence lectin of the parasite, preventing in vitro adherence and killing of CHO cells [3]. Trophozoites, however, can disrupt the mucus layer and intestinal barrier by secreting cysteine proteases (CPs) and glycosidases to allow for penetration of the colonic mucosa. Specifically, E. histolytica cysteine protease-A5 (EhCP-A5) degrades mucin-2 (MUC2) and extracellular matrix (ECM) proteins [4]. The importance of cysteine proteases was demonstrated by an ex vivo human intestinal model, where EhCP-A5–silenced parasites failed to penetrate into the colonic lamina propria [5].
IECs exposed to E. histolytica trophozoites secrete potent chemokines, such as IL-8, resulting in immune cell recruitment and infiltration of the lamina propria and intestinal epithelium [6]. Neutrophils are one of the first immune cells to respond to amebic invasion. Neutrophils activated by interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), or lipopolysaccharides (LPS) carry out amebicidal activity in vitro by releasing reactive oxygen species (ROS) [7], [8]. Depletion of neutrophils with anti-Gr-1 antibodies resulted in exacerbated intestinal disease in murine models, supporting the protective role of neutrophils in amebiasis [9]. It should be noted, however, that anti-Gr-1 antibodies can deplete other granulocytes such as eosinophils.
Macrophages also play a crucial role in the host response against intestinal amebiasis. Macrophages are amebicidal after stimulation with IFN-γ or TNF-α [10], [11]. Several amebic antigens are known to activate these cells via pattern recognition receptors. Toll-like receptor (TLR)-2 expression in macrophages is upregulated when exposed to the Gal/GalNAc lectin of E. histolytica, triggering pro-inflammatory cytokine production via NF-κB activation [12]. Macrophages that lack TLR-2 and TLR-4 displayed impaired response to E. histolytica lipopeptidophosphoglycan (LPPG), suggesting that pattern recognition is essential to the immune response [13]. Additionally, E. histolytica DNA can activate macrophages through interacting with TLR-9 [14]. Amebicidal activity of macrophages is contributed to by the production of nitric oxide (NO) from L-arginine, which is mediated by macrophage nitric oxide synthase. Inducible nitric oxide synthase (iNOS)–deficient mice were more susceptible to amebic liver abscess and to E. histolytica–induced hepatocytic apoptosis, implicating a critical role for NO in the host defense against amebiasis [15].
Adaptive Immunity: Mucosal Immunoglobulin A and Cell-Mediated Response
The Gal/GalNAc lectin is the major amebic surface adhesion molecule and mediates binding to the colonic mucus layer as well as carbohydrate determinants on a variety of host cells including epithelial cells. The heavy chain of Gal/GalNAc lectin contains the carbohydrate recognition domain (CRD) that is responsible for binding. In both mice and baboons vaccinated against E. histolytica, IgA antibodies against Gal/GalNAc lectin correlated with protection [16], [17]. In a cohort of preschool children in Dhaka, Bangladesh, mucosal IgA directed at the CRD domain was associated with protection of children from E. histolytica infection and disease. Conversely, serum anti-lectin IgG was not associated with protection, but instead was associated with an increased frequency of new E. histolytica infections [18].
Cell-mediated interferon gamma (IFN-γ) appears to provide protection from amebiasis through its ability to activate neutrophils and macrophages to kill the parasite. In a prospective study, children's peripheral blood mononuclear cells (PMBCs) were stimulated with soluble amebic extract and IFN-γ levels were measured. Children with higher IFN-γ production had a significantly lower incidence of future E. histolytica diarrhea [19]. This finding was supported by murine vaccination studies, which showed that vaccine-induced protection against E. histolytica infection could be passively transferred to naïve animals by IFN-γ–producing T cells. In addition to IFN-γ, IL-17 was also shown to contribute to vaccine-induced protection in murine studies [20], [21]. These findings suggest an important role for cell-mediated cytokine production in protection from amebiasis.
Mechanisms of Parasite Evasion
Although the host deploys a robust immune response against E. histolytica, the parasite has developed a remarkable number of mechanisms to evade these attacks (Figure 1B). While neutrophils are capable of killing ameba, virulent ameba are far more effective at lysing and phagocytosing neutrophils: in vitro, one trophozoite can kill upwards of 3,000 neutrophils [8]. In addition, amebic peroxiredoxin, a 29-kDa surface protein with antioxidant properties, protects the parasite from neutrophil reactive oxygen defenses [22]. E. histolytica has also developed several strategies to modulate macrophage responses. Amebic arginase converts L-arginine, a substrate of macrophage NOS, to L-ornithine, thereby limiting NO production by macrophages [23]. Cyclooxygenase (COX) in amebae can produce prostaglandin E2 (PGE2), which elevates the cyclic adenosine monophosphate (cAMP) levels in macrophages [24]. This, in turn, inhibits protein kinase C (PKC)–mediated expression of class II MHC [25]. Furthermore, monocyte locomotion inhibitory factor (MLIF) has been implicated to modulate macrophage function by inhibiting NO [26].
Complement may act to prevent dissemination of trophozoites and extraintestinal disease. Once amebae activate the complement system, membrane attack complexes (MACs) form and lyse the parasites. The parasite has at least two mechanisms to resist complement. Gal/GalNAc lectin, which shares sequence similarity and antigenic cross-reactivity with the MAC-inhibitory protein CD59, inhibits the formation of the C5b-9 complex, and thus prevents lysis by MACs [27]. Additionally, CPs can cleave complement factors [28].
Host Inflammatory Response Contributes to Tissue Damage
An appropriate immune response clears pathogens without causing significant damage to the host tissue. Tissue destruction in amebic colitis arises from both E. histolytica cytolytic factors and the resultant gut inflammatory response. While TNF-α stimulates neutrophils and macrophages to release ROS and NO to fight the parasite, an excess amount of TNF-α can result in direct damage to host tissue.
Higher TNF-α production was recently shown to correlate with E. histolytica diarrhea in children. Each 1,000 pg/mL increase of TNF-α correlated with an 18% increased chance of acquiring E. histolytica diarrhea [29]. Blocking TNF-α with monoclonal antibodies reduced inflammation and intestinal damage in amebic infection in the severe combined immunodeficient mouse-human intestinal xenograft (SCID-HU-INT) model [30].
The anti-inflammatory cytokine, interleukin-10 (IL-10), is an important immunoregulator in the intestinal tract. IL-10 counteracts an exaggerated pro-inflammatory immune response by inhibiting the production of inflammatory mediators such as TNF-α. Disruption of the IL-10 gene in mice results in colitis and is used as a model to study inflammatory bowel disease. Additionally, in a phase I trial, transgenic bacteria expressing IL-10 were shown to decrease disease activity in Crohn's patients [31]. IL-10–deficient C57BL/6 mice are susceptible to amebic colitis, supporting the role of inflammation in E. histolytica pathogenesis [20], [32].
Host Genetics, Immunity, and Susceptibility to Amebiasis
Millions of people worldwide are colonized with E. histolytica, yet only 20% develop symptomatic disease, with highly variable outcomes [33]. Host genetic makeup may explain, in part, why individuals differ in their susceptibility to amebic infection. Leptin, an adipocytokine first characterized for its metabolic effects, is now recognized as an important modulator of the immune system. Normal leptin signaling may mediate resistance to amebiasis via several mechanisms such as stimulating a Th1 response, inducing anti-apoptotic pathways, and promoting tissue repair. In a murine model, leptin-deficient (ob/ob) and leptin receptor–deficient (db/db) mice were highly susceptible to E. histolytica infection, while wild-type C57BL/6 mice were resistant [34]. In a nine year prospective study in a cohort of preschool children in Dhaka, Bangladesh, a single amino acid polymorphism (Q223R) in the leptin receptor was associated with increased susceptibility to E. histolytica infection. Children with two arginine alleles (223R) were nearly four times more likely to suffer E. histolytica infection as compared to those homozygous for glutamine (223Q). Similarly, mice with at least one 223R allele were significantly more susceptible to amebic infection and exhibited greater levels of intestinal epithelial apoptosis and mucosal destruction following infection [35]. The site of leptin-mediated resistance was localized to the intestinal epithelial cells by tissue-specific knockout. An in vitro model showed that leptin signaling protected human epithelial cells from amebic killing via a STAT3-dependent pathway [36].
Human leukocyte antigens (HLAs) play a crucial role in the immune response and are also highly polymorphic. The ability of specific HLA II alleles to present amebic antigens to CD4+ T cells may alter susceptibility to E. histolytica. It was found that children who were heterozygous for the HLA class II DQB1*0601/DRB1*1501 haplotype were more likely to be E. histolytica negative [37].
Future Perspectives
Several decades of research have led to an improved understanding of the host immune response to intestinal amebiasis. Despite these advances, fundamental questions remain unanswered. For example, the immune mechanism that explains why only a subset of exposed individuals develops clinical disease is not fully understood. This is arguably one of the most important knowledge gaps to be filled. Additionally, the emerging field of microbiome science opens new avenues for amebic research. The effect of the microbiota on the immune response to E. histolytica and/or its virulence is not yet known. The answers to these questions may lay the foundation for developing an effective vaccine against this devastating disease.
Zdroje
1. BarteltLA, LimaAAM, KosekM, Peñataro YoriP, LeeG, et al. (2013) “Barriers” to child development and human potential: the case for including the “neglected enteric protozoa” (NEP) and other enteropathy-associated pathogens in the NTDs. PLoS Negl Trop Dis 7: e2125 doi:10.1371/journal.pntd.0002125
2. MondalD, MinakJ, AlamM, LiuY, DaiJ, et al. (2012) Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 54 : 185–192.
3. ChadeeK, PetriWAJr, InnesDJ, RavdinJI (1987) Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest 80 : 1245–1254.
4. LidellME, MoncadaDM, ChadeeK, HanssonGC (2006) Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci U S A 103 : 9298–9303.
5. BansalD, AveP, KerneisS, FrileuxP, BochéO, et al. (2009) An ex-vivo human intestinal model to study Entamoeba histolytica pathogenesis. PLoS Negl Trop Dis 3: e551 doi:10.1371/journal.pntd.0000551
6. YuY, ChadeeK (1997) Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 112 : 1536–1547.
7. DenisM, ChadeeK (1989) Human neutrophils activated by interferon-gamma and tumour necrosis factor-alpha kill Entamoeba histolytica trophozoites in vitro. J Leukoc Biol 46 : 270–274.
8. GuerrantRL, BrushJ, RavdinJI, SullivanJA, MandellGL (1981) Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis 143 : 83–93.
9. AsgharpourA, GilchristC, BabaD, HamanoS, HouptE (2005) Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect Immun 73 : 4522–4529.
10. LinJY, SeguinR, KellerK, ChadeeK (1994) Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect Immun 62 : 1534–1541.
11. GhadirianE, DenisM (1992) Entamoeba histolytica extract and interferon-gamma activation of macrophage-mediated amoebicidal function. Immunobiology 185 : 1–10.
12. KammanadimintiSJ, MannBJ, DutilL, ChadeeK (2004) Regulation of Toll-like receptor-2 expression by the Gal-lectin of Entamoeba histolytica. FASEB J 18 : 155–157.
13. Maldonado-BernalC, KirschningCJ, RosensteinY, RochaLM, Rios-SarabiaN, et al. (2005) The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol 27 : 127–137.
14. IvoryCP, PrystajeckyM, JobinC, ChadeeK (2008) Toll-like receptor 9-dependent macrophage activation by Entamoeba histolytica DNA. Infect Immun 76 : 289–297.
15. SeydelKB, SmithSJ, StanleySLJr (2000) Innate immunity to amebic liver abscess is dependent on gamma interferon and nitric oxide in a murine model of disease. Infect Immun 68 : 400–402.
16. HouptE, BarrosoL, LockhartL, WrightR, CramerC, et al. (2004) Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine 22 : 611–617.
17. Abd AllaMD, WolfR, WhiteGL, KosankeSD, CaryD, et al. (2012) Efficacy of a Gal-lectin subunit vaccine against experimental Entamoeba histolytica infection and colitis in baboons (Papio sp.). Vaccine 30 : 3068–3075.
18. HaqueR, AliIM, SackRB, FarrBM, RamakrishnanG, et al. (2001) Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis 183 : 1787–1793.
19. HaqueR, MondalD, ShuJ, RoyS, KabirM, et al. (2007) Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg 76 : 340–344.
20. GuoX, BarrosoL, BeckerSM, LyerlyDM, VedvickTS, et al. (2009) Protection against intestinal amebiasis by a recombinant vaccine is transferable by T cells and mediated by gamma interferon. Infect Immun 77 : 3909–3918.
21. GuoX, BarrosoL, LyerlyDM, PetriWAJr, HouptER (2011) CD4+ and CD8+ T cell - and IL-17-mediated protection against Entamoeba histolytica induced by a recombinant vaccine. Vaccine 29 : 772–777.
22. DavisPH, ZhangX, GuoJ, TownsendRR, StanleySLJr (2006) Comparative proteomic analysis of two Entamoeba histolytica strains with different virulence phenotypes identifies peroxiredoxin as an important component of amoebic virulence. Mol Microbiol 61 : 1523–1532.
23. ElnekaveK, Siman-TovR, AnkriS (2003) Consumption of L-arginine mediated by Entamoeba histolytica L-arginase (EhArg) inhibits amoebicidal activity and nitric oxide production by activated macrophages. Parasite Immunol 25 : 597–608.
24. DeyI, KellerK, BelleyA, ChadeeK (2003) Identification and characterization of a cyclooxygenase-like enzyme from Entamoeba histolytica. Proc Natl Acad Sci U S A 100 : 13561–13566.
25. WangW, ChadeeK (1995) Entamoeba histolytica suppresses gamma interferon-induced macrophage class II major histocompatibility complex Ia molecule and I-A beta mRNA expression by a prostaglandin E2-dependent mechanism. Infect Immun 63 : 1089–1094.
26. RicoG, LeandroE, RojasS, GimenezJA, KretschmerRR (2003) The monocyte locomotion inhibitory factor produced by Entamoeba histolytica inhibits induced nitric oxide production in human leukocytes. Parasitol Res 90 : 264–267.
27. BragaLL, NinomiyaH, McCoyJJ, EackerS, WiedmerT, et al. (1992) Inhibition of the complement membrane attack complex by the galactose-specific adhesion of Entamoeba histolytica. J Clin Invest 90 : 1131–1137.
28. ReedSL, EmberJA, HerdmanDS, DiScipioRG, HugliTE, et al. (1995) The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a. J Immunol 155 : 266–274.
29. PetersonKM, ShuJ, DuggalP, HaqueR, MondalD, et al. (2010) Association between TNF-alpha and Entamoeba histolytica diarrhea. Am J Trop Med Hyg 82 : 620–625.
30. ZhangZ, MahajanS, ZhangX, StanleySLJr (2003) Tumor necrosis factor alpha is a key mediator of gut inflammation seen in amebic colitis in human intestine in the SCID mouse-human intestinal xenograft model of disease. Infect Immun 71 : 5355–5359.
31. BraatH, RottiersP, HommesDW, HuyghebaertN, RemautE, et al. (2006) A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol 4 : 754–759.
32. HamanoS, AsgharpourA, StroupSE, WynnTA, LeiterEH, et al. (2006) Resistance of C57BL/6 mice to amoebiasis is mediated by nonhemopoietic cells but requires hemopoietic IL-10 production. J Immunol 177 : 1208–1213.
33. HaqueR, DuggalP, AliIM, HossainMB, MondalD, et al. (2002) Innate and acquired resistance to amebiasis in bangladeshi children. J Infect Dis 186 : 547–552.
34. GuoX, RobertsMR, BeckerSM, PoddB, ZhangY, et al. (2011) Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol 4 : 294–303.
35. DuggalP, GuoX, HaqueR, PetersonKM, RicklefsS, et al. (2011) A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest 121 : 1191–1198.
36. MarieCS, VerkerkeHP, PaulSN, MackeyAJ, PetriWAJr (2012) Leptin protects host cells from Entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect Immun 80 : 1934–1943.
37. DuggalP, HaqueR, RoyS, MondalD, SackRB, et al. (2004) Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J Infect Dis 189 : 520–526.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits fromČlánek X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Biosecurity Implications of New Technology and Discovery in Plant Virus Research
- Pathogenic Yeasts Deploy Cell Surface Receptors to Acquire Iron in Vertebrate Hosts
- Asparagine Repeats in Proteins: Good for Nothing?
- Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from
- Intact Type I Interferon Production and IRF7 Function in Sooty Mangabeys
- Prion Replication Occurs in Endogenous Adult Neural Stem Cells and Alters Their Neuronal Fate: Involvement of Endogenous Neural Stem Cells in Prion Diseases
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
- HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women
- Crystal Structure of the Full-Length Japanese Encephalitis Virus NS5 Reveals a Conserved Methyltransferase-Polymerase Interface
- Quantitative Models of the Dose-Response and Time Course of Inhalational Anthrax in Humans
- Stabilization of Myc through Heterotypic Poly-Ubiquitination by mLANA Is Critical for γ-Herpesvirus Lymphoproliferation
- The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Biofilms
- A Novel Role for Pro-Coagulant Microvesicles in the Early Host Defense against
- Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits from
- CD36 Recruits αβ Integrin to Promote Cytoadherence of -Infected Erythrocytes
- X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Chikungunya Virus 3′ Untranslated Region: Adaptation to Mosquitoes and a Population Bottleneck as Major Evolutionary Forces
- Mucin Gene (PoMuc) Expression: Epigenetic Control to Shape Adaptation to a New Host
- Determinants of GPI-PLC Localisation to the Flagellum and Access to GPI-Anchored Substrates in Trypanosomes
- The Smallest Capsid Protein Mediates Binding of the Essential Tegument Protein pp150 to Stabilize DNA-Containing Capsids in Human Cytomegalovirus
- Understanding Human Variation in Infectious Disease Susceptibility through Clinical and Cellular GWAS
- Human Genetic Susceptibility to Invasive Aspergillosis
- Host Immune Response to Intestinal Amebiasis
- Fungi Infecting Plants and Animals: Killers, Non-Killers, and Cell Death
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Host Immune Response to Intestinal Amebiasis
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



