-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRelevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
article has not abstract
Published in the journal: . PLoS Pathog 9(8): e32767. doi:10.1371/journal.ppat.1003447
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003447Summary
article has not abstract
Trehalose: A Supporting Act More than a Starring Role
Trehalose, a natural disaccharide, consists of two glucose molecules linked by an α, α-1,1-glucoside bond. Apart from its function as a reserve carbohydrate, trehalose is known for its role as a stress protectant in many organisms across kingdoms. It is present in plants, invertebrates, fungi, and prokaryotes, but not in mammals. Despite its well-known protective roles against desiccation, freezing, starvation, and osmotic stress [1], trehalose has escaped categorization into a specific biological pathway. Interestingly, a role for its metabolism is emerging in the establishment of virulence traits in distantly related microbial species.
In general, although notable exceptions exist, the absence of a complete trehalose metabolism apparatus is associated with a lower pathogenic potential. The mechanisms involved, however, are less clear. Pathogens have engineered several distinct trehalose-associated mechanisms that contribute to cell morphogenesis, cell wall integrity, regulation of metabolism, and evasion from the host immune response.
Trehalose Synthesis Pathways and Virulence: E Pluribus Unum?
Trehalose can be synthesized via different metabolic pathways [2], sometimes coexisting, with both shared and specific functions relevant to pathogenicity (Table 1). Of the three potential trehalose biosynthesis pathways differently contributing to Mycobacterium tuberculosis virulence, the OtsAB pathway (Figure 1) is the dominant one [3]: deletion of OtsA results in severe growth defects, whereas the OtsB gene is essential for viability. Of the two additional pathways, the TreYZ pathway and the TreS pathway, only the latter seems necessary for late development of chronic infections in mice, while absence of the first doesn't cause any apparent defect in microbial growth or virulence.
Fig. 1. Trehalose metabolic pathways. 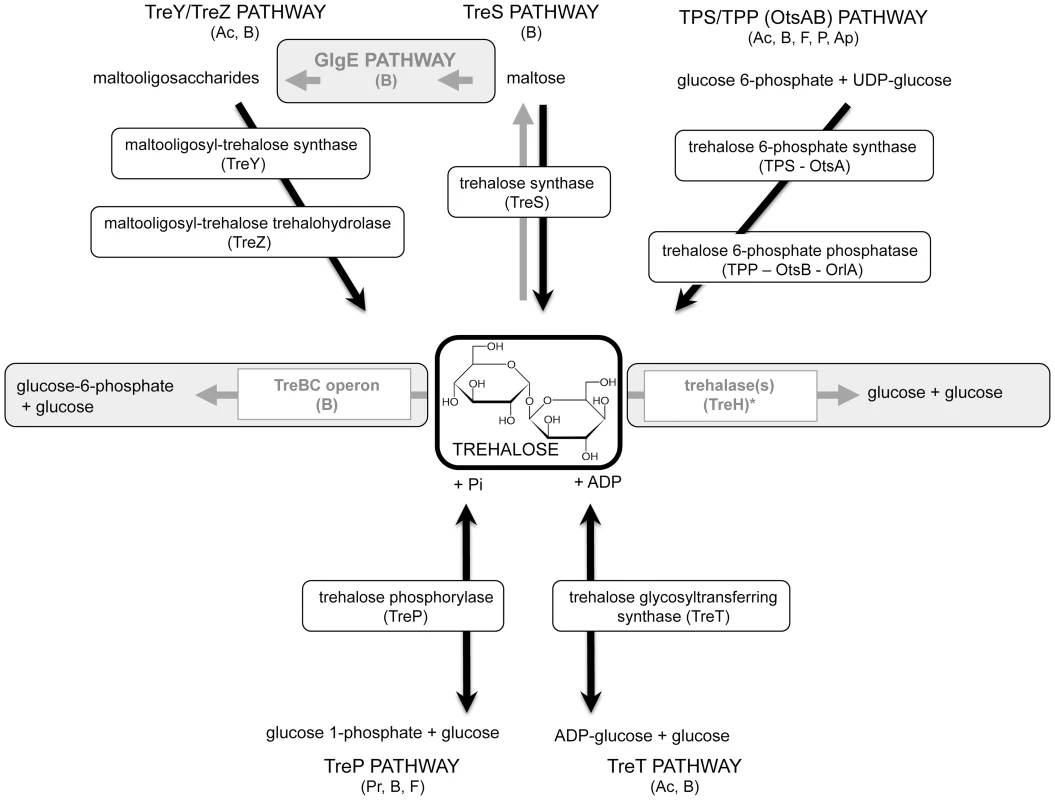
Pathways of trehalose synthesis are highlighted in black, and pathways of degradation in grey. Major conserved enzymes are included. The TPS/TPP (OtsAB) is a two-step pathway. Glucose 6-phosphate and UDP-glucose are converted into trehalose 6-phosphate (T6P) by the trehalose 6-phosphate synthase enzymes. T6P is then converted into trehalose by the T6P phosphatase enzymes. The TreYZ pathway yields trehalose from glucans. The first step, operated by TreY (maltooligosyltrehalose synthase), mediates the inversion of the reducing-end glucosyl residue of α-1,4 glucan into α, α-1,1-linked non-reducing trehalosyl disaccharide end. This is followed by the cleavage of free trehalose from the glucan chain by TreZ enzyme (maltooligosyltrehalose trehalohydrolase). The TreS pathway consists of the trehalose synthase enzyme (TreS), and converts trehalose into maltose and vice versa. The TreS and the GlgE pathways are linked by the Pep2 enzyme, a maltokinase, that converts maltose into maltose 1-phosphate. The novel GlgE pathway converts trehalose via the TreS pathway into glucans and glycogen in Mycobacterium tuberculosis [33]. From maltose 1-phosphate, the essential maltosyl-transferase GlgE extends glucan chains, and the essential GlgB enzyme introduces α-1,6-linked branches to linear glucans. The TreBC operon consists of TreB, the trehalose PTS permease, which transports extracellular trehalose inside the cells and converts it into T6P. T6P is then converted into glucose 6-phosphate and glucose by TreC enzymes encoding T6P hydrolases. Trehalase enzymes degrade trehalose into two molecules of glucose, and have been categorized as cytoplasmic (or neutral) and extracellular (or acid) enzymes. The role of the TreP and TreT pathways in pathogenicity has not been demonstrated thus far. The TreP pathway is the reversible hydrolysis of trehalose in presence of inorganic phosphate by the trehalose phosphorylase enzyme (TreP). The reversible TreT pathway consists of the formation of trehalose and ADP from ADP-glucose and glucose by the trehalose glycosyltransferring synthase enzyme (TreT). Ac, Archea; B, Bacteria; F, Fungi; P, Plants; Ap, Arthropods; Pr, Protists. * Trehalase is present in all kingdoms, including mammals. Tab. 1. Divergence in the relevance of the three main trehalose biosynthetic pathways in the viability and pathogenicity of prokaryotes. 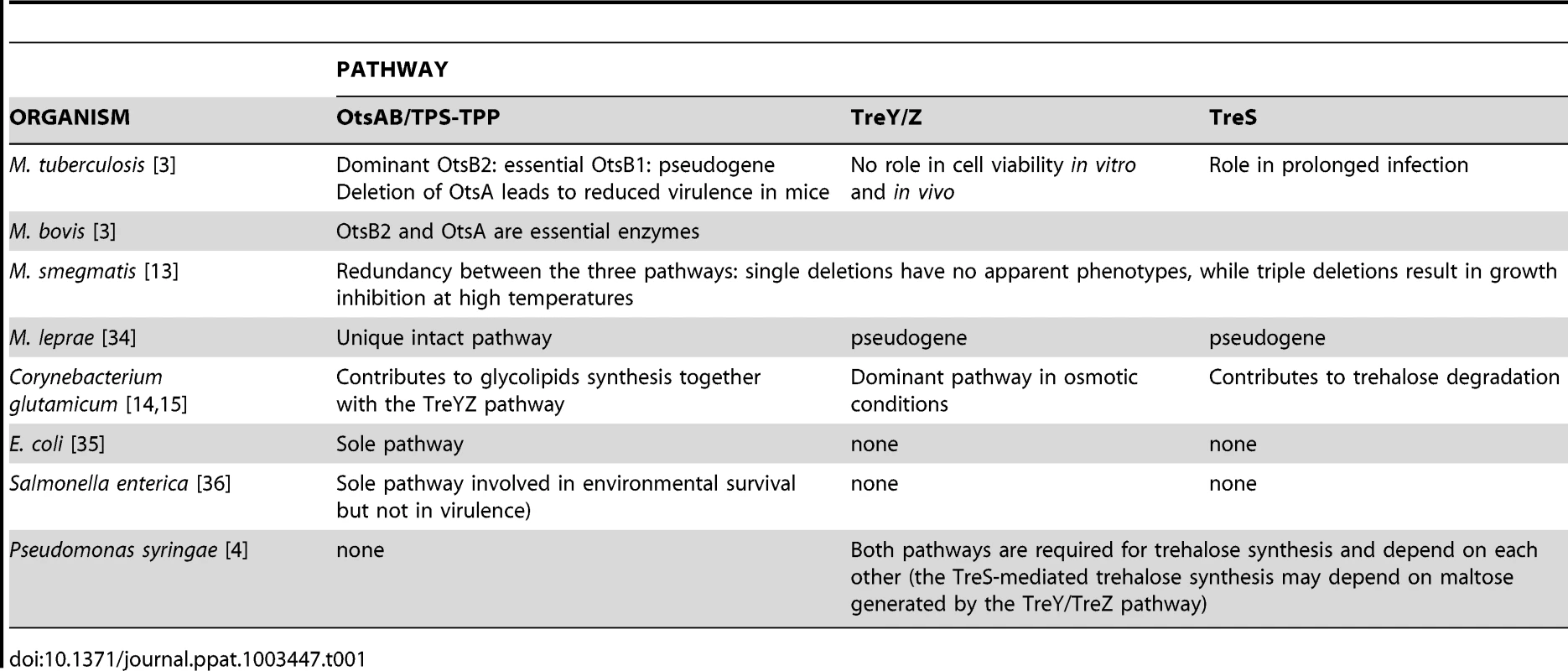
Only the TreS and TreYZ pathways are present in the plant pathogen Pseudomonas syringae, and their absence leads to reduced survival on host tomato leaves [4], an effect that may be attributed, at least in part, to a fitness defect of the corresponding mutants in low-water environments. Inactivation of the same two pathways reduces the pathogenicity of Pseudomonas aeruginosa against plants, restored by co-inoculation of exogenous trehalose, but not against worms, insects, and mice [5].
In fungal pathogens, trehalose is mainly synthesized via the TPS/TPP pathway (Figure 1). Originally demonstrated in Candida albicans [6], the abolishment of trehalose production has a significant negative impact on the in vivo survival of several plant and human pathogens, including Magnaporthe oryzae [7], Stagonospora nodorum [8], Cryptococcus neoformans [9], and Cryptococcus gattii [10]. Aspergillus fumigatus, the causative agent of invasive pulmonary disease in immunocompromised patients, represents an exception to this pattern. In this organism, disruption of the two trehalose 6-phosphate synthase genes, tpsA and tpsB, surprisingly leads to higher resistance to phagocytosis and hypervirulence in mice, despite the complete absence of trehalose accumulation and severely reduced fitness of these mutants [11]. Conversely, deletion of the trehalose 6-phosphate phosphatase OrlA (Figure 1) results in avirulence, despite the absence of growth defects in vitro [12].
Trehalose Is Associated with Several Biological Processes: Sporulation, Germination, Metabolism, and Morphogenesis
Trehalose and trehalose biosynthetic enzymes have emerged as essential players in virulence-associated phenotypes. Interestingly, the hierarchy of trehalose biosynthetic pathways appears to be species-specific. Contrary to the case of M. tuberculosis, described above, in the closely related M. smegmatis the OtsAB, TreYZ, and TreS pathways show a great level of redundancy [13], whereas osmotically regulated trehalose synthesis in Corynebacterium glutamicum is predominantly mediated by the TreYZ pathway (Table 1) [14], [15]. Independently from these differences in metabolic routes, however, impairment in trehalose production results in severe growth defects in all mentioned species. In Pseudomonas syringae, trehalose accumulation is abolished in mutants of either producing pathways, with consequences on growth in hyperosmotic but not in stress-free conditions.
Impairment of trehalose homeostasis results in increased susceptibility to oxidative stress in Aspergillus nidulans [16], defects in melanin synthesis, capsule production, mating, and cell wall integrity in Cryptococcus gattii but not in C. neoformans [9], [10], and in poor sporulation in S. nodorum and M. oryzae [7], [8]. In the latter species, Tps1 governs the process of carbon catabolite repression, via glucose 6-phosphate sensing, favouring the utilization of glucose over other carbon sources [17]. Multiple functions for the trehalose 6-phosphate synthase Tps1 were uncovered in M. oryzae. Besides trehalose biosynthesis, Tps1 regulates nitrogen source utilization, hence contributing to adaptation of the pathogen to the plant host [18]. Recently, a similar process has been identified in P. aeruginosa, in which trehalose itself was shown to promote the acquisition of nitrogen-containing nutrients upon infection of plants [5]. M. oryzae Tps1 also controls transcriptional effectors linked to virulence factors via NADPH-binding during appressorium-mediated rice infection [19].
In A. fumigatus, disruption of the two trehalose 6-phosphate synthase genes, tpsA and tpsB, leads to the complete absence of trehalose accumulation, and to defects in spore germination, growth at high temperature, and susceptibility to oxidative stress [11]. Deletion of the trehalose 6-phosphate phosphatase gene OrlA in this organism results in unaltered levels of trehalose and increased levels of trehalose 6-phosphate. Two putative trehalose phosphorylases (TreP) (Figure 1) were found to be encoded by the genome of A. fumigatus [12]. Functionality of the TreP pathway in this organism might explain the unexpected accumulation of trehalose and trehalose 6-phosphate in orlA mutants. However, this hypothesis is awaiting experimental validation.
In C. albicans, absence of a functional trehalose synthesis machinery causes defects in the yeast to hyphal transition [6], whereas high levels of trehalose correlate with deficient Hsp90-dependent cell elongation in response to elevated temperature [20]. Trehalose accumulation is induced by addition of amphotericin B in C. albicans [21]. This ergosterol-targeting antifungal promotes trehalose synthesis activity and deactivation of the neutral trehalase; however, it is not known whether high trehalose can reduce the fungal susceptibility to amphotericin B.
Fluctuations and Turnover of Trehalose Content during Infection Processes: It's All about Timing
Trehalose is essential at certain stages of development or in certain environmental conditions, and its abundance varies from undetectable levels to up to 10% of dry weight in response to stress, in late phases of the life cycle, or during the infection process. Increased trehalose contents were suggested to indicate adaptation of microorganisms to adverse or stressful conditions. Recycling of released trehalose is essential for virulence of M. tuberculosis [22]. The trehalose moiety of the glycolipid trehalose monomycolate is released extracellularly, and its re-uptake, mediated via an ABC transporter, is essential to virulence. In Salmonella enterica, mutants with a defect in trehalose biosynthesis are still virulent. Interestingly, however, failure to import the compatible solute glycine betaine in ΔopuA-D mutants leads to a strong increase in trehalose biosynthesis under stress conditions, ultimately resulting in increased tolerance to stress conditions mimicking the host innate immunity. The trehalose-dependent higher proficiency of ΔopuA-D mutants to colonize host tissues, as compared to the wild type, indicate that the OpuA-D transporter represses trehalose biosynthesis, therefore limiting virulence in favour of chronic infections [23]. Thus, while the absence of trehalose has no known effect in vivo, high levels of the disaccharide may promote more aggressive infections. In P. aeruginosa, trehalose, like other metabolites such as amino acids and acetate, is differentially abundant during the evolution of lung infections in patients affected by cystic fibrosis [24]. In Klebsiella pneumoniae, expression of the treB and treC genes, involved in trehalose uptake and hydrolysis, respectively (Figure 1), is induced during early stages of biofilm formation, and the absence of either gene impairs biofilm development [25]. Proper trehalose utilization and catabolism into glucose and glucose 6-phosphate is required for capsule formation, known to play a role in biofilm formation in vitro as well as in a murine model of gastrointestinal tract colonization.
In fungi, while synthesis of trehalose is required for proficient initial plant infection by M. oryzae, trehalose mobilization plays a role in the colonization step [7]. Deletion of the neutral trehalase Nth1 in C. gattii has no effect on virulence [10], while in C. albicans, deletion of the cell wall–associated acid trehalase, but not of the neutral trehalase, has a negative impact on morphogenesis and virulence in mice [26]. Trehalose mobilization is required for efficient growth resumption in favourable conditions, and this may also be associated with the use of trehalose as a carbon source. Trehalose levels also vary in C. albicans biofilms, where its content increases dramatically in the first six hours of biofilm formation, followed by a decline in mature biofilms [27]. The exact role of these fluctuations remains elusive, however it is tempting to speculate on an antioxidant function of trehalose in the early stages of biofilm formation, and a role as energy supply in mature structures. Alternatively, it is also possible that in the initial phases of biofilm formation, high trehalose levels may prevent filamentation [20] so yeast cells can colonize the whole substrate, and that at later stages, lower trehalose levels allow filament formation. The decline in trehalose at later stages may also indicate a shift in metabolic flux as more UDP-glucose is likely required for β-glucan synthesis during matrix formation, resulting in less substrate for trehalose biosynthesis.
Trehalose and Lipids: A Unique Collaboration
A wealth of information describes the unique relationship between trehalose and mycolic acids in Mycobacteria and the phylogenetically related Corynebacteria [28], [29]. Trehalose and mycolic acid form trehalose 6,6-dimycolate (TDM), or cord factor, which is the most abundant glycolipid in Mycobacteria. Involvement of TDM in several aspects of pathogenicity has been demonstrated, including protection against phagocytes' killing, evasion from the immune response, and reduction of antibiotic effectiveness. Tissue damage and necrosis caused by its association with host lipids were also reported. Recent findings point toward the recognition of TDM by the host innate immune cells via the Mincle C-type lectin receptor, which also recognizes other pathogens such as C. albicans, Malasezzia spp., and Fonsecaea pedrosoi in a TDM-independent manner [30]. Yet, the ligand activity of TDM requires both the sugar and lipid moieties, as both components separately do not activate Mincle-expressing cells [30]. Bacterial cell wall–associated lipids, and in particular TDM, are also important for the infection process in the Gram-positive bacterium Nocardia brasiliensis. Absence of TDM and other hydrophobic compounds abolishes the infection without affecting the pathogen's viability [31].
Besides TDM, trehalose is the precursor of several metabolites and cell-wall glycolipids, the so-called “trehalome” of Mycobacteria. Detection and visualization of the cell surface trehalome were recently described [32]. Probing the trehalome in a pathway-targeted manner, including the recycling pathway, is now possible in several mycobacterial species, including M. tuberculosis. This represents a promising perspective for the study of the dynamics and trafficking of the mycobacterial trehalome during the infection process.
What does the future hold for trehalose and its synthesis, and in particular what does it hold for the field of antimicrobial research? No doubt that the beat goes on! The discovery of a novel pathway from trehalose to glycogen and α-glucans in M. tuberculosis leads the way. The GlgE enzyme is validated as a drug target candidate by the combination of its essentiality in a synthetic lethal pathway, and by the absence of GlgE homologs in humans and commensal gut microflora [33]. Despite or perhaps due to its versatility, trehalose stands out as a major player in the lifestyle of pathogens. The challenge, however, remains in the identification of molecular links between trehalose or its metabolism and pathways regulating the ability of pathogens to infect their host and/or to hide from the host.
Zdroje
1. IturriagaG, SuarezR, Nova-FrancoB (2009) Trehalose metabolism: from osmoprotection to signalling. Int J Mol Sci 10 : 3793–3810.
2. AvonceN, Mendoza-VargasA, MorettE, IturriagaG (2006) Insights on the evolution of trehalose biosynthesis. BMC Evol Biol 6 : 109.
3. MurphyHN, StewartGR, MIschenkoVV, AptAS, HarrisR, et al. (2005) The OtsAB pathway is essential for trehalose biosynthesis in Mycobacterium tuberculosis. J Biol Chem 280 : 14524–14529.
4. FreemanBC, ChenC, BeattieGA (2010) Identification of the trehalose biosynthetic loci of Pseudomonas syringae and their contribution to fitness in the phyllosphere. Environ Microbiol 12 : 1486–1497.
5. DjonovićS, UrbachJM, DrenkardE, BushJ, FeinbaumR, et al. (2013) Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathog 9: e1003217 doi:10.1371/journal.ppat.1003217
6. ZaragozaO, BlazquezMA, GancedoC (1998) Disruption of the Candida albicans TPS1 gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J Bacteriol 180 : 3809–3815.
7. FosterAJ, JenkinsonJM, TalbotNJ (2003) Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. EMBO J 22 : 225–235.
8. LoweRG, LordM, RybakK, TrengoveRD, OliverRP, et al. (2009) Trehalose biosynthesis is involved in sporulation of Stagonospora nodorum. Fungal Genet Biol 46 : 381–389.
9. PetzoldEW, HimmelreichU, MylonakisE, RudeT, ToffalettiD, et al. (2006) Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect Immun 74 : 5877–5887.
10. NgamskulrungrojP, HimmelreichU, BregerJA, WilsonC, ChayakulkeereeM, et al. (2009) The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect Immun 77 : 4584–4596.
11. Al-BaderN, VanierG, LiuH, GravelatFN, UrbM, et al. (2010) Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect Immun 78 : 3007–3018.
12. PuttikamonkulS, WillgerSD, GrahlN, PerfectJR, MovahedN, et al. (2010) Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pahogen Aspegillus fumigatus. Mol Microbiol 77 : 891–911.
13. WoodruffPJ, CarlsonBL, SiridechadilokB, PrattMR, SenaratneRH, et al. (2004) Trehalose is required for growth of Mycobacterium smegmatis. J Biol Chem 279 : 28835–28843.
14. WolfA, KramerR, MorbachS (2003) Three pathways for trehalose metabolism in Corynebacterium glutamicum ATCC13032 and their significance in response to osmotic stress. Mol Microbiol 49 : 1119–1134.
15. TzvetkovM, KlopproggeC, ZelderO, LieblW (2003) Genetic dissection of trehalose biosynthesis in Corynebacterium glutamicum: inactivation of trehalose production leads to impaired growth and an altered cell wall lipid composition. Microbiology 149 : 1659–1673.
16. FillingerS, ChaverocheMK, van DijckP, de VriesR, RuijterG, et al. (2001) Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 147 : 1851–1862.
17. HarveyPC, WatsonM, HulmeS, JonesMA, LovellM, et al. (2011) Salmonella enterica serovar typhimurium colonizing the lumen of the chicken intestine grows slowly and upregulates a unique set of virulence and metabolism genes. Infect Immun 79 : 4105–4121.
18. WilsonRA, JenkinsonJM, GibsonRP, LittlechildJA, WangZY, et al. (2007) Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. Embo J 26 : 3673–3685.
19. WilsonRA, GibsonRP, QuispeCF, LittlechildJA, TalbotNJ (2010) An NADPH-dependent genetic switch regulates plant infection by the rice blast fungus. Proc Natl Acad Sci U S A 107 : 21902–21907.
20. SerneelsJ, TournuH, Van DijckP (2012) Tight control of trehalose content is required for efficient heat-induced cell elongation in Candida albicans. J Biol Chem 287 : 36873–36882.
21. Gonzalez-ParragaP, Sanchez-FresnedaR, ZaragozaO, ArguellesJC (2011) Amphotericin B induces trehalose synthesis and simultaneously activates an antioxidant enzymatic response in Candida albicans. Biochim Biophys Acta 1810 : 777–783.
22. KalscheuerR, WeinrickB, VeeraraghavanU, BesraGS, JacobsWRJr (2010) Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 107 : 21761–21766.
23. PilonietaMC, NagyTA, JorgensenDR, DetweilerCS (2012) A glycine betaine importer limits Salmonella stress resistance and tissue colonization by reducing trehalose production. Mol Microbiol 84 : 296–309.
24. BehrendsV, RyallB, ZlosnikJE, SpeertDP, BundyJG, et al. (2013) Metabolic adaptations of Pseudomonas aeruginosa during cystic fibrosis chronic lung infections. Environ Microbiol 15 : 398–408.
25. WuM-C, LinT-L, HsiehP-F, YangH-C, WangJ-T (2011) Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE 6: e23500 doi:10.1371/journal.pone.0023500
26. PedrenoY, Gonzalez-ParragaP, Martinez-EsparzaM, SentandreuR, ValentinE, et al. (2007) Disruption of the Candida albicans ATC1 gene encoding a cell-linked acid trehalase decreases hypha formation and infectivity without affecting resistance to oxidative stress. Microbiology 153 : 1372–1381.
27. ZhuZ, WangH, ShangQ, JiangY, CaoY, et al. (2012) Time course analysis of Candida albicans metabolites during biofilm development. J Proteome Res 12 : 2375–2385.
28. HunterRL, ArmitigeL, JagannathC, ActorJK (2009) TB research at UT-Houston–a review of cord factor: new approaches to drugs, vaccines and the pathogenesis of tuberculosis. Tuberculosis (Edinb) 89 Suppl 1: S18–25.
29. Lopez-MarinLM (2012) Nonprotein structure from Mycobacteria: emerging actors for tuberculosis control. Clin Develop Immunol 2012 : 917860.
30. IshikawaE, IshikawaT, MoritaYS, ToyonagaK, YamadaH, et al. (2009) Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med 206 : 2879–2888.
31. Trevino-VillarrealJH, Vera-CabreraL, Valero-GuillenPL, Salinas-CarmonaMC (2012) Nocardia brasiliensis cell wall lipids modulate macrophage and dendritic responses that favor development of experimental actinomycetoma in BALB/c mice. Infect Immun 80 : 3587–3601.
32. SwartsBM, HolsclawCM, JewettJC, AlberM, FoxDM, et al. (2012) Probing the mycobacterial trehalome with bioorthogonal chemistry. J Am Chem Soc 134 : 16123–16126.
33. KalscheuerR, SysonK, VeeraraghavanU, WeinrickB, BiermannKE, et al. (2010) Self-poisoning of Mycobacterium tuberculosis by targeting GlgE ina alpha-glucan pathway. Nat Chem Biol 6 : 376–384.
34. ColeST, EiglmeierK, ParkhillJ, JamesKD, ThomsonNR, et al. (2001) Massive gene decay in the leprosy bacillus. Nature 409 : 1007–1011.
35. StrømAR, KaasenI (1993) Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol Microbiol 8 : 205–210.
36. HowellsAM, BullifentHL, DhaliwalK, GriffinK, Garcia de CastroA, et al. (2002) Role of trehalose biosynthesis in environmental survival and virulence of Salmonella enterica serovar typhimurium. Res Microbiol 153 : 281–287.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits fromČlánek X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Biosecurity Implications of New Technology and Discovery in Plant Virus Research
- Pathogenic Yeasts Deploy Cell Surface Receptors to Acquire Iron in Vertebrate Hosts
- Asparagine Repeats in Proteins: Good for Nothing?
- Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from
- Intact Type I Interferon Production and IRF7 Function in Sooty Mangabeys
- Prion Replication Occurs in Endogenous Adult Neural Stem Cells and Alters Their Neuronal Fate: Involvement of Endogenous Neural Stem Cells in Prion Diseases
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
- HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women
- Crystal Structure of the Full-Length Japanese Encephalitis Virus NS5 Reveals a Conserved Methyltransferase-Polymerase Interface
- Quantitative Models of the Dose-Response and Time Course of Inhalational Anthrax in Humans
- Stabilization of Myc through Heterotypic Poly-Ubiquitination by mLANA Is Critical for γ-Herpesvirus Lymphoproliferation
- The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Biofilms
- A Novel Role for Pro-Coagulant Microvesicles in the Early Host Defense against
- Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits from
- CD36 Recruits αβ Integrin to Promote Cytoadherence of -Infected Erythrocytes
- X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Chikungunya Virus 3′ Untranslated Region: Adaptation to Mosquitoes and a Population Bottleneck as Major Evolutionary Forces
- Mucin Gene (PoMuc) Expression: Epigenetic Control to Shape Adaptation to a New Host
- Determinants of GPI-PLC Localisation to the Flagellum and Access to GPI-Anchored Substrates in Trypanosomes
- The Smallest Capsid Protein Mediates Binding of the Essential Tegument Protein pp150 to Stabilize DNA-Containing Capsids in Human Cytomegalovirus
- Understanding Human Variation in Infectious Disease Susceptibility through Clinical and Cellular GWAS
- Human Genetic Susceptibility to Invasive Aspergillosis
- Host Immune Response to Intestinal Amebiasis
- Fungi Infecting Plants and Animals: Killers, Non-Killers, and Cell Death
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Host Immune Response to Intestinal Amebiasis
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



