-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStructural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
The intracellular pathogen Legionella pneumophila hijacks the endoplasmic reticulum (ER)-derived vesicles to create an organelle designated Legionella-containing vacuole (LCV) required for bacterial replication. Maturation of the LCV involved acquisition of Rab1, which is mediated by the bacterial effector protein SidM/DrrA. SidM/DrrA is a bifunctional enzyme having the activity of both Rab1-specific GDP dissociation inhibitor (GDI) displacement factor (GDF) and guanine nucleotide exchange factor (GEF). LidA, another Rab1-interacting bacterial effector protein, was reported to promote SidM/DrrA-mediated recruitment of Rab1 to the LCV as well. Here we report the crystal structures of LidA complexes with GDP - and GTP-bound Rab1 respectively. Structural comparison revealed that GDP-Rab1 bound by LidA exhibits an active and nearly identical conformation with that of GTP-Rab1, suggesting that LidA can disrupt the switch function of Rab1 and render it persistently active. As with GTP, LidA maintains GDP-Rab1 in the active conformation through interaction with its two conserved switch regions. Consistent with the structural observations, biochemical assays showed that LidA binds to GDP - and GTP-Rab1 equally well with an affinity approximately 7.5 nM. We propose that the tight interaction with Rab1 allows LidA to facilitate SidM/DrrA-catalyzed release of Rab1 from GDIs. Taken together, our results support a unique mechanism by which a bacterial effector protein regulates Rab1 recycling.
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002528
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002528Summary
The intracellular pathogen Legionella pneumophila hijacks the endoplasmic reticulum (ER)-derived vesicles to create an organelle designated Legionella-containing vacuole (LCV) required for bacterial replication. Maturation of the LCV involved acquisition of Rab1, which is mediated by the bacterial effector protein SidM/DrrA. SidM/DrrA is a bifunctional enzyme having the activity of both Rab1-specific GDP dissociation inhibitor (GDI) displacement factor (GDF) and guanine nucleotide exchange factor (GEF). LidA, another Rab1-interacting bacterial effector protein, was reported to promote SidM/DrrA-mediated recruitment of Rab1 to the LCV as well. Here we report the crystal structures of LidA complexes with GDP - and GTP-bound Rab1 respectively. Structural comparison revealed that GDP-Rab1 bound by LidA exhibits an active and nearly identical conformation with that of GTP-Rab1, suggesting that LidA can disrupt the switch function of Rab1 and render it persistently active. As with GTP, LidA maintains GDP-Rab1 in the active conformation through interaction with its two conserved switch regions. Consistent with the structural observations, biochemical assays showed that LidA binds to GDP - and GTP-Rab1 equally well with an affinity approximately 7.5 nM. We propose that the tight interaction with Rab1 allows LidA to facilitate SidM/DrrA-catalyzed release of Rab1 from GDIs. Taken together, our results support a unique mechanism by which a bacterial effector protein regulates Rab1 recycling.
Introduction
Rab GTPases play a crucial role in vesicular trafficking through shuttling between cytosol and membranes, a process that is controlled by several regulatory proteins [1]–[4]. GDP dissociation inhibitors (GDIs) preferentially interact with and deliver GDP-bound Rabs to their target membranes, where dissociation of the GDI-Rab complexes is catalyzed by GDI displacement factors (GDFs) [5]–[8]. Prenylation at the C-termini of Rab proteins is essential for their membrane association [9]–[11]. The membrane-localized Rabs are subsequently activated by specific guanine nucleotide exchange factors (GEFs) via promoting their exchange of GDP for GTP [12]–[15]. The activated Rabs then bind their cognate effectors, triggering signaling for vesicle formation [16], [17], vesicle transport [18]–[23], vesicle tethering and fusion of vesicles with target membranes [17], [24]–[27]. GTPase activating proteins (GAPs) catalyze hydrolysis of GTP in the activated Rabs and return them to the GDP-bound inactive form that is sensitive to membrane retrieval by GDIs, thus replenishing the cytoplasmic pool of Rab proteins [4], [14], [15].
The intracellular bacterial pathogen Legionella pneumophila (L. pneumophila) is the causative agent of pneumonia Legionnaires disease [8], [28]. Following invasion of host cells, L. pneumophila resides in the LCV [29] that escapes endolysosomal destruction [30]. The bacterial effector proteins, delivered by the type IV secretion system (T4SS) of L. pneumophila into the cytosol of host cells [31], actively remodel the LCV to establish an intracellular niche indispensable to bacterial pathogenesis [32]. For example, the early secretory vesicles from ER can be hijacked to the LCV, converting it into an ER-derived organelle that supports bacterial replication [33]–[36]. Rab1, required for vesicle trafficking between ER and the Golgi complex [18], [37], [38], is one of the host proteins recruited to the LCV shortly after uptake of L. pneumophila [39]–[41]. Rab1 recruitment subsequently promotes transport and fusion of ER-derived vesicles with the LCV, thus playing an essential role in the biogenesis of this organelle [42], [43]. The effector protein SidM/DrrA, by acting as a Rab1-sepcific GEF and GDF, is required for LCV recruitment of Rab1 [44], [45]. Further, the AMPylation activity of SidM/DrrA modified Rab1 by covalently adding adenosine monophosphate (AMP) to avoid the GAP recognition [46]. SidD, functions as the Rab1 deAMPylase, generating de-AMPylated Rab1 accessible for inactivation by LepB [43], [47]. Another translocated effector protein LidA is also involved in the recruitment of early secretory vesicles to the LCV [48], [49]. LidA was found to interact with Rab1 as well, regardless of nucleotide binding states, and promote SidM/DrrA activity of transporting Rab1 to the LCV [39], [45]. Unlike SidM/DrrA mutants, however, L. pneumophila mutants lacking LidA displayed a temporal delay but not a loss of Rab1 recruitment to the LCV [39]. Intriguingly, while indispensable to the recruitment of wild type Rab1, SidM/DrrA is not required to accumulate Rab1 mutant (D44N) that loses interaction with GDIs but not with LidA about the LCV [45]. These results suggest that GDIs play a negative role in Rab1 recruitment by the LCV during L. pneumophila infection. Recruitment of this Rab1 mutant, however, is dependent on LidA, suggesting that interaction with Rab1 is important for the role of LidA in delivering Rab1 to the LCV. Currently the mechanisms of how LidA cooperates with SidM/DrrA for Rab1 recruitment are not well understood.
Here, we present the crystal structures of LidA (residues 224-559) in complex with a GDP-bound Rab1 mutant (S25N; residues 1-176) [50], [51] and LidA (residues 188-449) in complex with GTP-bound Rab1 (residues 1-176). Unexpectedly, the structures showed that GDP-Rab1(S25N), a “constitutively” inactive mutant, adopted an active conformation when bound by LidA. In agreement with the structural observation, biochemical assays demonstrated that GDP-Rab1(S25N) and GTP-Rab1(Q70L) [52] exhibited a similar and exceptionally high binding affinity for LidA. Coupled with previous observations, data presented in current study support a unique mechanism by which LidA interferes with the host secretory vesicular trafficking.
Results
LidA binds both GDP and GTP bound Rab1 with a similar affinity
The Rab1-interacting domain of LidA used in our study is similar to the one (residues 191-600) necessary and sufficient to disrupt the secretory pathway when overexpressed in COS1 cells [49]. Consistently, In vitro data shows truncation of 188 residues from the N-terminal side or 155 residues from the C-terminal side of LidA generated no effect on the formation of such a complex (Figure S1). To further validate the interaction between LidA and Rab1, we measured their binding affinity using isothermal titration calorimetry (ITC) technique. The ITC results showed that the LidA fragment 188-580 containing the predicted coiled-coil domain interacted with the constitutively active mutant Rab1(Q70L) with a dissociation constant of 7.5 nM (Figure 1A). Surprisingly, the same protein also bound nearly equally well to the constitutively GDP-bound mutant of Rab1(S25N), with a dissociation constant of 7.6 nM (Figure 1B). A preference of LidA for the GTP-Rab1 could result from a lower abundance of GDP-Rab1 in cells.
Fig. 1. Measurement of binding affinity between LidA and GTP-bound or GDP-bound Rab1 by ITC. 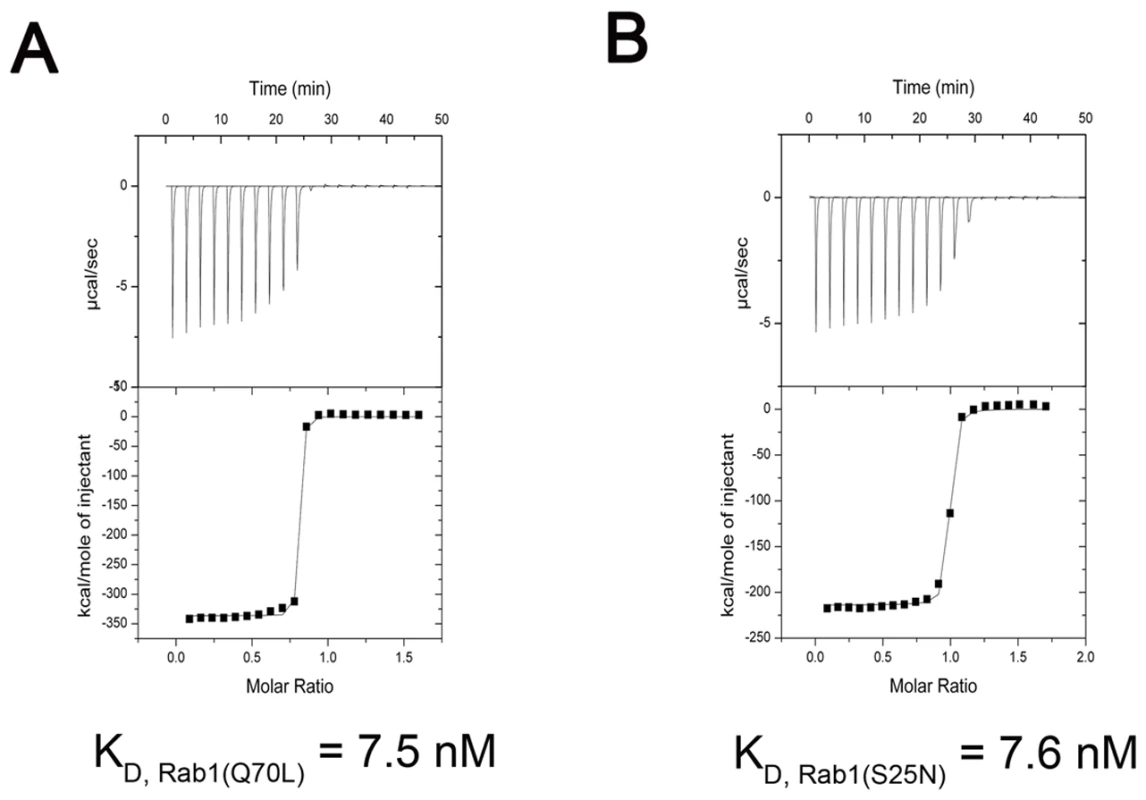
(A) Raw ITC data. Top panel: twenty injections of GTP-bound Rab1(Q70L) solutions were titrated into LidA(188-580) solution in ITC cell. The area of each injection peak corresponds to the total heat released for that injection. Bottom panel: the binding isotherm for GTP-bound Rab1(Q70L) and LidA(188-580) interaction, the integrated heat is plotted against the stoichiometry of 1∶1, data fitting revealed a binding affinity of 7.5 nM. (B) Top panel: twenty injections of GDP-bound Rab1(S25N) solutions were titrated into LidA(188-580) solution in ITC cell. The area of each injection peak corresponds to the total heat released for that injection. Bottom panel: the binding isotherm for GDP-bound Rab1(S25N) and LidA(188-580) interaction, the integrated heat is plotted against the stoichiometry of 1∶1, data fitting revealed a binding affinity of 7.6 nM. Both titrations were performed in the absence of added Mg2+ and GDP/GTP. Overall structures of LidA and its complex with Rab1
To reveal the molecular basis for the LidA-Rab1 interaction, we first solved the crystal structure of LidA(224-559) in complex with a constitutively GDP-bound mutant Rab1(S25N; 1-176) at 1.73 Å using molecular replacement (Table S1 in supporting information).
The overall LidA-Rab1 complex adopts a compact and globular structure (Figures 2A,B). The interaction between LidA and Rab1 resulted in a 1∶1 stoichiometric complex and buried 41% (4031 Å2/9845 Å2) of the Rab1. The exceptionally large buried surface generated by LidA-Rab1 interaction is consistent with their strong binding affinity. In the structure, Rab1 is held in a large and pronounced groove made by the four “fingers” with an extensive, though not complete, charge and surface complementarities. Contacts between LidA and Rab1 are established through a combination of hydrogen bonds and hydrophobic interactions involving the switch I, switch II and the interswitch region of Rab1 (Figure 2B).
Fig. 2. Overall structures of LidA and its complex with GDP-bound Rab1. 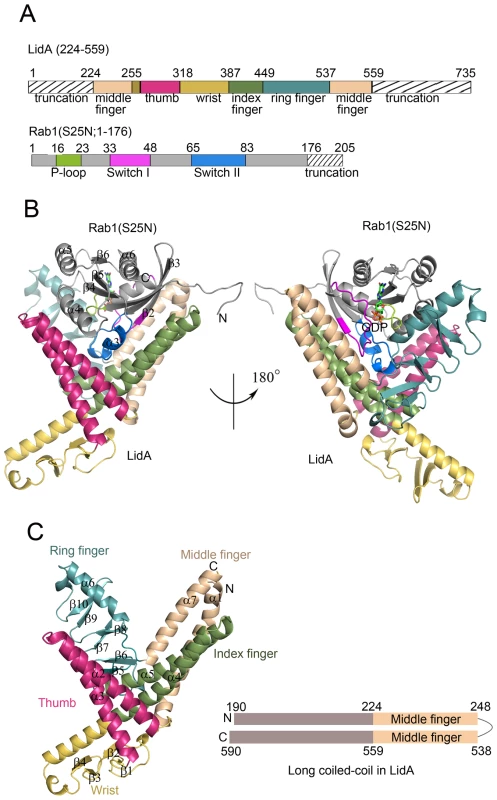
(A) Schematic representation of LidA fingers and Rab1 function regions, sequences not included in the crystallized proteins are marked with hatched lines and labeled as truncation. (B) Cartoon representation of the overall structure of LidA(224-559)-Rab1(S25N; 1-176) complex. Rab1(S25N) is shown in gray, LidA is colored by fingers as shown in schematic representation, GDP is depicted in sticks. (C) Cartoon representation of the LidA fingers (left) and schematic representation of LidA terminal long coiled-coil domain (right), wheat color represents coiled-coil region in crystal structure, gray color represents extend α-helix predicted by secondary structure analysis. N, N terminus; C, C terminus. LidA (residues 224-559) is composed of seven α-helices and ten β-strands, which arrange into a structure resembling a hand with the baby finger buried in the palm and the remaining four straightened (Figure 2C). Six of the seven α-helices form three extended two-stranded α-helical coiled-coils. This structural observation supports a previous prediction that LidA is a coiled-coil rich structure [48], [49]. Two of them, made by α4/α5 and α1/α7, are the “index finger” and the “middle finger”, respectively. Interaction between them is primarily mediated by packing of α1 against α5. The “thumb” stems from a two-helix coiled-coil formed by α2 and α3 helices which interact with the base of “index finger” through packing against α4. The isolated helix α6 and its following loop make extensive hydrophobic contacts with three anti-parallel β-sheets (β5/β6, β7/β8 and β9/β10), resulting in formation of the “ring finger”. The N-terminal side of α4, which is not involved in the formation of coiled-coil structures, tightly contacts the two anti-parallel β-sheets (β1/β2 and β3/β4) located between α3 and α4, thus making the “wrist”. Interestingly, the α1 and α7 stay closely together and form the middle finger. The terminal parallel helix of LidA extends to the outside of interacting region, forms an antiparallel long coiled-coil (Figure 2C). So we suppose the N - and C-terminal domains of LidA may stay spatially close cooperate with each other to perform specific function.
Interaction interfaces between LidA and Rab1
In the complex, switch I (residues 33-48) of Rab1 is mainly sandwiched between the “middle finger” and the “ring finger” by making contacts with α1, α7 and α6. It also contacts the “index finger” α5 (Figures 3A,B). Close packing of Ile44Rab1 from switch I and Phe73Rab1 from switch II (residues 65-83) against Leu541LidA, Val542LidA, Val538LidA, Leu436LidA, Ala439LidA, Y243LidA and the aliphatic portion of Asn432LidA (from α5) appears to dominate the interactions around this interface. Additional van der Waals interactions result from contacts of Tyr532LidA with the alpha carbon atom of Gly21Rab1 and Tyr40Rab1, and Ile41Rab1 with Leu548LidA and Glu549LidA in LidA. Hydrogen bonds further strengthen this interface. In particular, in addition to forming a salt bridge with Asp443LidA, Arg72Rab1 from the switch II region makes a hydrogen bonds with the backbone carbonyl of Met536LidA. Through an intramolecular hydrogen bond, Arg72Rab1 is stabilized by the catalytically important residue Gln70Rab1, which in turn mediates a bifurcated polar interaction with the carbonyl oxygen of Thr534LidA and Glu533LidA from LidA.
Fig. 3. Interaction interfaces between LidA and Rab1. 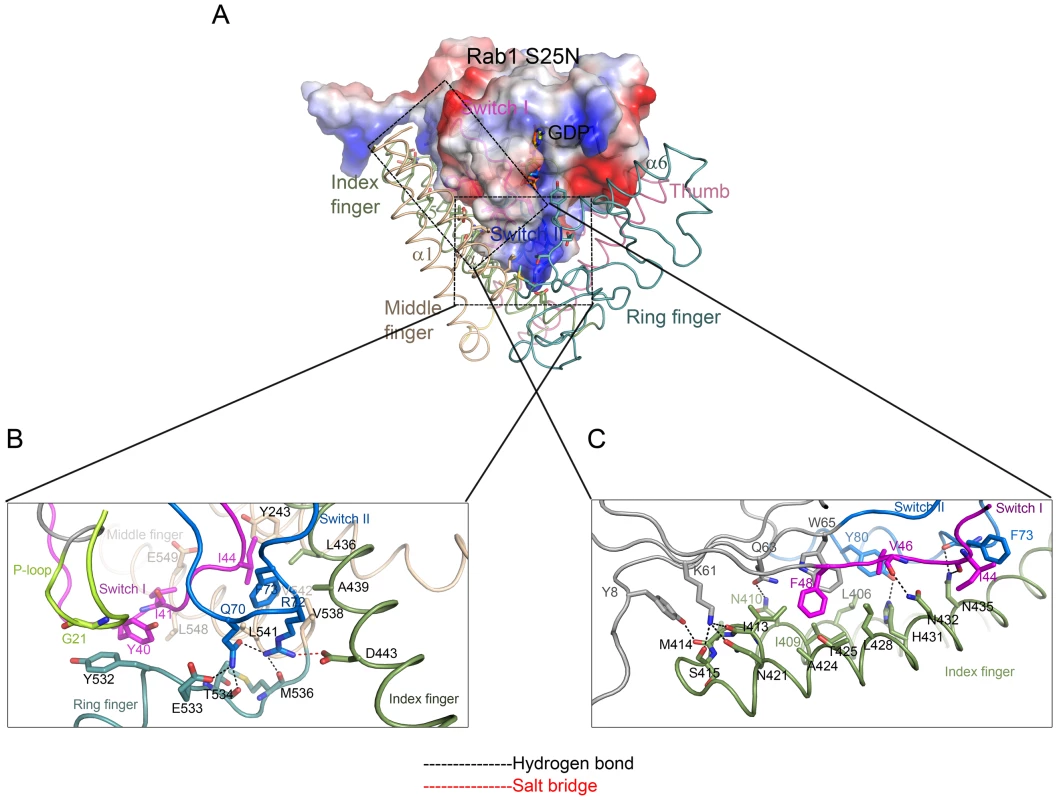
(A) Rab1(S25N) is shown with electrostatic surface potentials. Blue and red represent the positive and negative charge potential, respectively. The extensive interactions between Rab1(S25N) switch regions and LidA index finger (smude), middle finger (wheat) and ring finger (light teal) are shown. This Figure is in the same orientation as the right panel of Figure 2B. (B) The detailed interactions between Rab1 switch I (magentas), switch II (marine), P-loop (limon) and LidA middle finger and ring finger. (C) The detailed interactions between Rab1 switch II and LidA index finger. The interacting residues of Rab1 and LidA are shown in sticks, residues labels are color-coded as corresponding cartoon chains, respectively. Hydrogen bonds are indicated by black dashed lines. Salt bridge is indicated by red dashed lines. The C-terminal portion of the switch II, together with the region defined by β2, β3 and β4, makes extensive contacts with the base of “index finger” (Figures 3A,C). Center to this interface are the van der Waals interactions made by Phe48Rab1 and Trp65Rab1 from Rab1 with a cluster of surrounding hydrophobic residues of LidA. At periphery, a number of hydrogen bonds flank the hydrophobic interaction center. Among these, Lys61Rab1 from Rab1 forms three hydrogen bonds with Asn421LidA and the carbonyl oxygen atoms of Ile413LidA and Ser415LidA. Other hydrogen bonds include those formed by Tyr8Rab1 with the carbonyl oxygen atom of Met414LidA, Gln63Rab1 with Asn410LidA, the carbonyl oxygen atoms of Val46Rab1, ILE44Rab1 and Phe73Rab1 with Asn432LidA and Asn435LidA, respectively. In addition to hydrogen bonding with His431LidA, Tyr80Rab1 also engages hydrophobic contact with Leu406LidA.
The GDP-bound Rab1(S25N) is held in the active conformation upon LidA binding
Our biochemical assay (Figures 1A,B) indicates that LidA binding is independent of the Rab1 nucleotide-binding states. To understand the underlying molecular mechanisms, we solved the crystal structure of LidA (residues 188-449) in complex with the Rab1(WT; residues 1-191) at 2.2 Å. The structure of the complex is highly similar to that of LidA(224-559)-Rab1(S25N) with an r.m.s.d of 0.678 Å for Rab1; 1.496 Å for LidA, respectively (Figure 4A). As anticipated, GTP is well defined in the crystal structure (Figure 4A) and its interactions with Rab1 are conserved in other active Rabs (Figure S2) [53]–[55]. Surprisingly, structural comparison revealed that the conformation of LidA-bound Rab1(S25N) is essentially identical with that of LidA-bound GTP-Rab1 (Figure 4B), indicating that Rab1(S25N) is in the active conformation following LidA binding. While the switch II region in the free GDP-Rab1 is completely disordered (Figure 4C), it is well defined when bound by LidA. As with GTP, LidA binding induces striking structural remodeling around the switch I region of Rab1(S25N), which swings from the edge of β-sheet to the nucleotide-binding site, with the largest displacement of 9.2 Å at the Cα atom of Ile. Relocation of switch I results in formation of an extended β-strand β2 at its N-terminal side and hydrophobic packing against switch II (Figure 4C).
Fig. 4. The complexed GDP-bound Rab1 (S25N) is held in the active conformation. 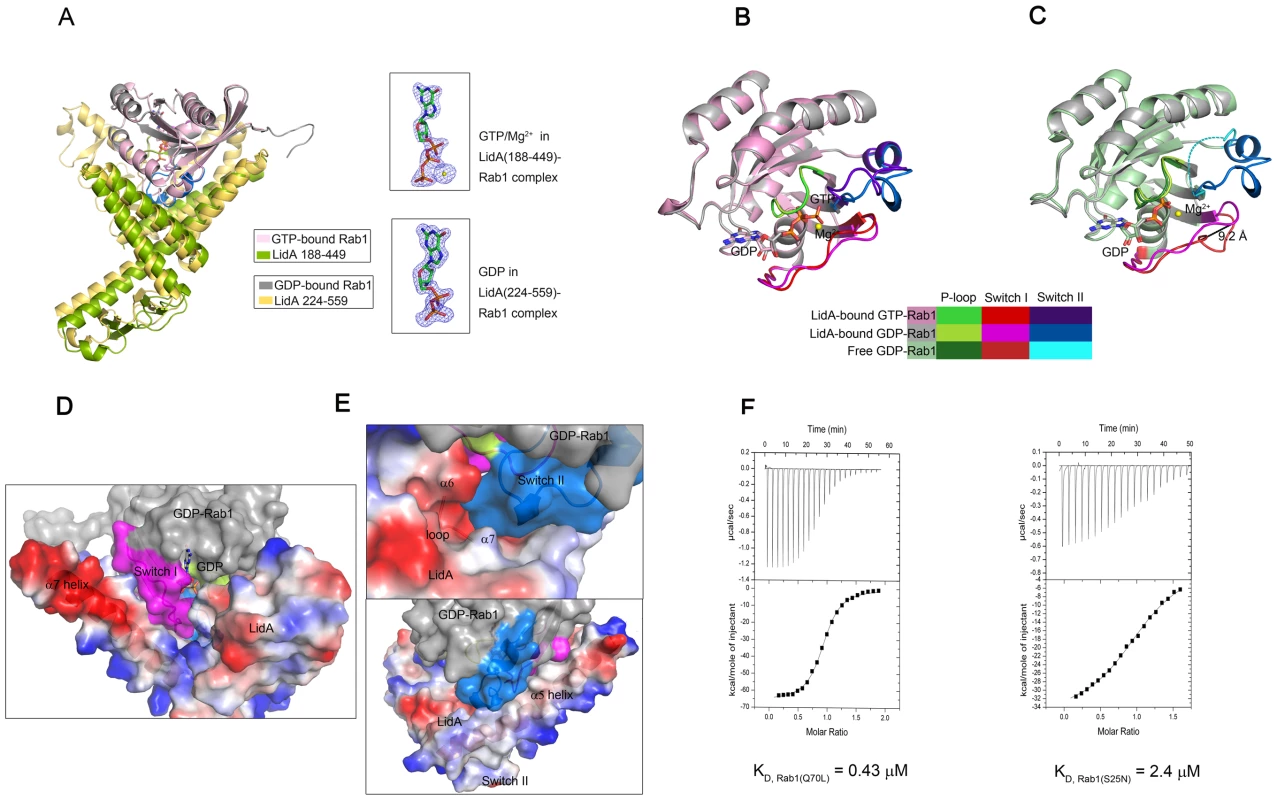
(A) Superimposition of GDP-bound Rab1(S25N)-LidA complex and GTP-bound Rab1-LidA complex (left). The Fo-Fc electron density of GDP and GTP/Mg2+ in the two complexes is shown (right), respectively. The Figure demonstrates the quality of the electron density (blue mesh). Both of the electron density are depicted at 3.0 σ. (B) Superimposition of LidA-bound GDP-Rab1(S25N) and LidA-bound GTP-Rab1(WT). (C) Superimposition of LidA-bound GDP-Rab1(S25N) and free GDP-Rab1 (PDB ID code 2FOL). The largest displacement of 9.2 Å at the Cα atom of Ile in Switch I is shown. The disordered broken switch II in 2FOL is shown by blue dashed lines. The switch regions and P-loops are highlighted in different colors indicated in the color-box to contrast their conformational changes. (D and E) The C-terminal region of LidA contributes to maintain the GDP-bound Rab1(S25N) in active conformation. LidA(418-559) is shown with electrostatic surface potentials. (D) GDP-Rab1 switch I region is stabilized through the interaction with LidA α7 helix. (E) Switch II region is stabilized both by polar interaction with the loop linking LidA α6 and α7 and hydrophobic contacts with LidA α5 helix; Blue and red represent the positive and negative charge potential, white represent the hydrophobic contacts. Switch regions are color-coded as before. (F) Quantitative analysis of two ITC runs: titration of GTP-bound Rab1(Q70L) (left) and GDP-bound Rab1(S25N) (right) into LidA(188-449), respectively. Both titrations were performed in the absence of added Mg2+ and GDP/GTP. The KD value is 0.43 µM for GTP-bound Rab1(Q70L) and 2.4 µM for GDP-bound Rab1(S25N), respectively. Our structural analyses suggest that LidA utilizes a similar mechanism to GTP for maintaining Rab1 in the active conformation. The hydrogen bonding interactions between the γ phosphate group of GTP with Thr43 and Gly69, from switch I and switch II of Rab1 respectively, are highly conserved among Rab proteins and important for stabilizing their active state (Figure S2). Despite loss of the γ phosphate group-mediated interactions, the switch I region in Rab1(S25N) is stabilized through its interaction with α7 of LidA (Figure 4D). Stabilization of the switch II region in Rab1(S25N) is via its polar interaction with the loop linking α6 and α7 as well as hydrophobic contacts with α5 of LidA (Figure 4E). Deletion of the switch-stabilizing structural elements would be expected to disfavor LidA interaction with the GDP-bound Rab1 as compared to the GTP-bound Rab1. We therefore quantified LidA (residues 188-449) interaction with Rab1(S25N) and Rab1(Q70L) using an ITC assay. In support of the structure-based prediction, the assay showed that the LidA fragment exhibited a higher binding affinity (0.43 µM) to the GTP-bound Rab1 than to the GDP-bound Rab1 (2.4 µM) (Figure 4F).
Interaction of Rab1-LidA shares similar features with that of Rab4-Rabenosyn-5
Interaction of the anti-parallel coiled-coil formed by the helices α4 and α5 with the switch and interswitch regions of Rab1 is reminiscent of the structure of Rab4-Rabenosyn-5 complex [54]. A superposition of the overall structures of these two complexes revealed significant structural similarity (Figure 5A). Some subtle structural differences surrounding the switch I and II are consistent with the notion that the switch regions adopt specific conformations in the GTP-binding form of Rab proteins [55]. Rabenosyn-5 forms a similar anti-parallel coiled-coil to LidA and binds the switch and interswitch regions of Rab4. Interestingly, although these two peptides have reverse orientations, they contact a significant subset of the highly conserved resides between Rab1 and Rab4, though the detailed molecular interactions differ. For example, in both complexes the highly conserved residues Phe48Rab1 (Phe45Rab4) and Trp65Rab1 (Trp62Rab4) are buried in a cluster of hydrophobic residues despite their different side chain rotamer conformations, whereas Lys61Rab1 (Lys58Rab4) and Tyr80Rab1 (Tyr77Rab4) are involved in polar interactions (Figures 5B,C).
Fig. 5. Interaction of Rab1-LidA shares similar features with that of Rab4-Rabenosyn-5. 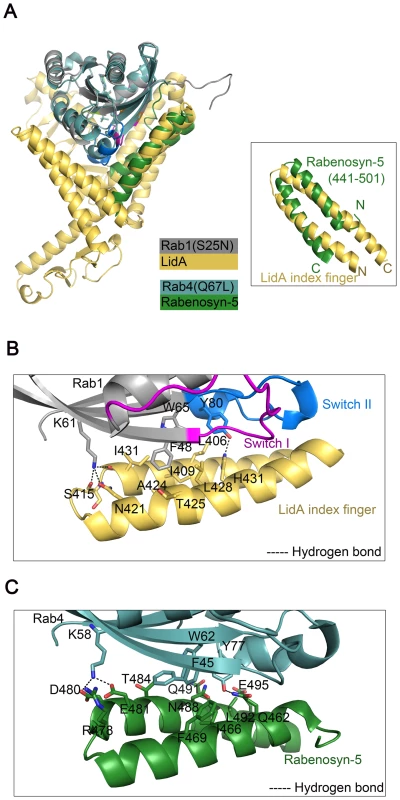
(A) Superimposition of Rab1(S25N)-LidA(224-559) complex with Rab4-Rabenosyn-5 complex (PDB ID code 1Z0K). The superimposition of the similar anti-parallel coiled-coil regions is shown. Rab1(S25N) is in gray and LidA is in yellow orange; Rab4 is in light blue and Rabenosyn-5 is in green. (B and C) Similar interfaces of the two complexes. (B) represents the detailed interactions between Rab1(S25N) and LidA. (C) represents the detailed interactions between Rab4 and Rabenosyn-5. The residues involved in interaction are shown as stick representations and denoted by black labels. LidA interacts with multiple Rabs
In addition to Rab1, LidA has been shown to be able to interact with other Rab member proteins such as Rab6a and Rab8b [39]. Indeed, many LidA-interacting residues in Rab1 are highly conserved in these two Rabs as well as other Rab members (Figure 6A) suggesting that LidA may have promiscuity of binding Rab proteins. To further experimentally test this, we purified 15 GST-fused Rab family members (His-fused Rab20 is exceptional) and tested their interaction with LidA using pull down assay. As shown in Figure 6B, thirteen out of the fifteen Rabs tested interacted with LidA. The binding affinities of LidA for other Rabs, including Rab2, Rab4, Rab6, Rab7, Rab9, Rab11, Rab20 and Rab22 have been quantified by ITC. All give KD values in the micromolar range. Compared with them Rab1 gives the strongest binding affinity (The KD is in nanomolar range). Thus Rab1 should be the preferred substrate of LidA compared with other Rabs (Figure 7). Thus, although previous LCV analysis identified interacting Rab GTPases such as Rab1, Rab7, Rab8, and Rab14 as novel LCV components [56], the biological relevance of the interactions await further investigation. To make clear how LidA choose substrate from so many Rabs, we attempted to disrupt the interactions of Rab1 and LidA by making single or double mutations of Rab1 and LidA along the surface of the interactions. However, none of the mutants we have tested by the pull-down assay lose their interaction with LidA. We also tested such mutants in other Rabs and did not observe an effect. So it remains undetermined how LidA selects different Rabs. Nonetheless, it is expected that the mechanism of Rab1 recognition by LidA is applicable to other Rab members given the high conservation in the LidA-interacting surface.
Fig. 6. 13 kinds of Rab GTPase family members could be recognized by LidA in vitro. 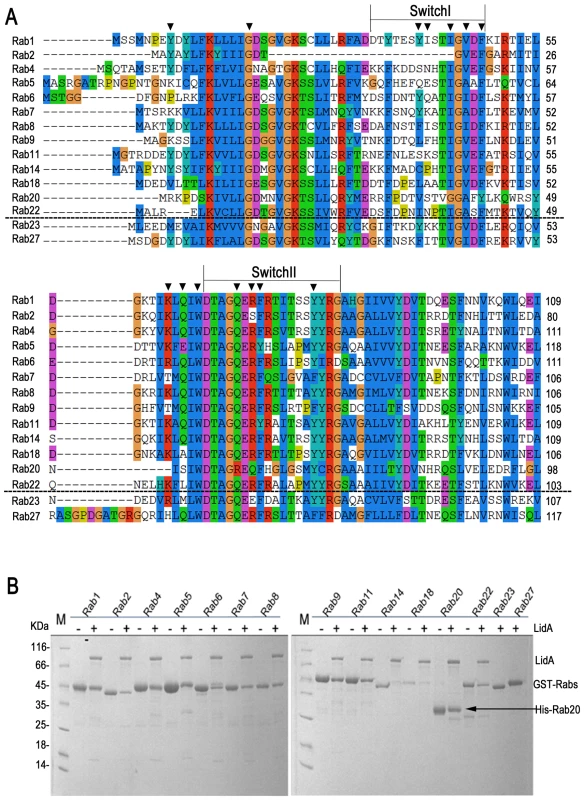
(A) Sequence alignment of fifteen different Rabs. The LidA-interacting residues in Rab1 are depicted by triangles. The two sequences underneath the black dashed lines correspond to the members unbound to LidA. (B) The GST-pull down of LidA(FL) by beads-immobilized 15 kinds of GST-Rab family members, Rab20 was His-tagged for exceptional. Fig. 7. Measurement of binding affinity between LidA and 9 kinds of Rabs by ITC. 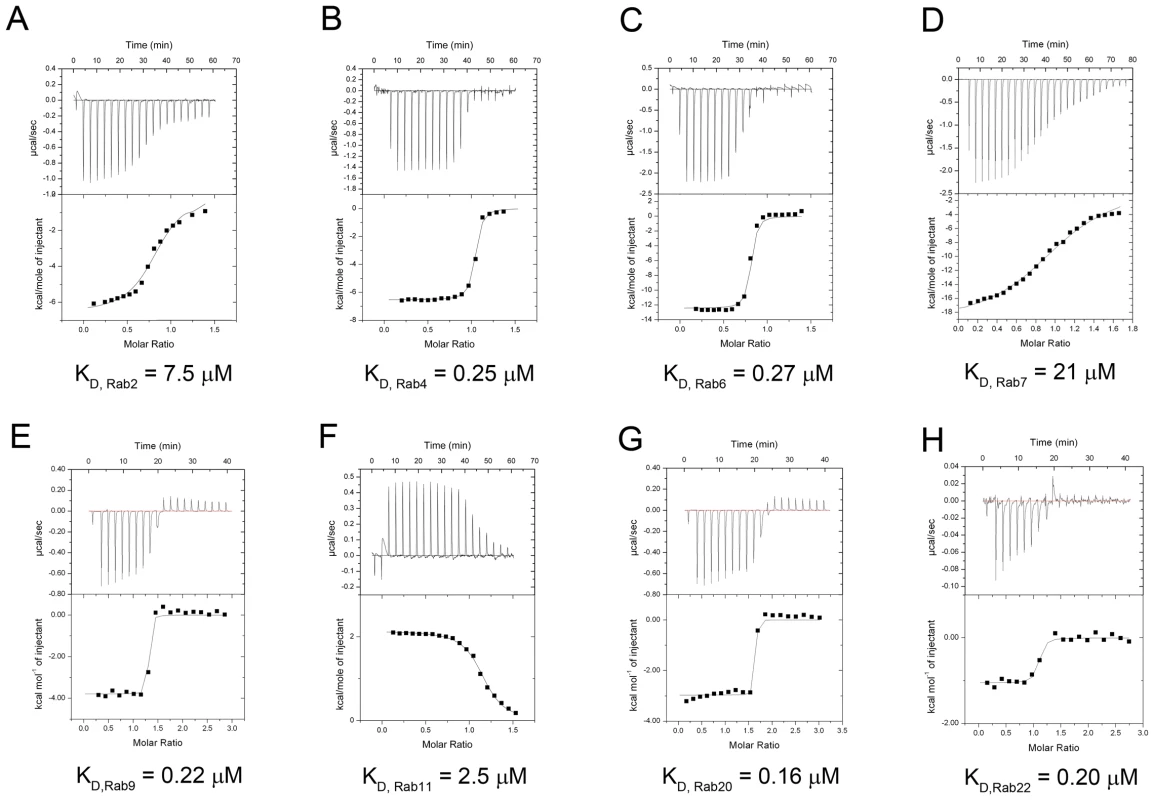
(A–G) Raw ITC data. Top panel: twenty injections of Rab2 (A), Rab4 (B), Rab6 (C), Rab7 (D), Rab9 (E), Rab11 (F), Rab20 (G) solutions were titrated into LidA(188-580) solution in ITC cell. The area of each injection peak corresponds to the total heat released for that injection. Bottom panel: the binding isotherm for these Rabs and LidA(188-580) interaction, the integrated heat is plotted against the stoichiometry of 1∶1, data fitting revealed a binding affinity as shown. (H) Raw ITC data. Top panel: twenty injections of Rab22 (H) solutions were titrated into LidA(FL) solution ITC cell, the experiment conditions was exactly the same as the top one unless the LidA is full-length. The KD values of these Rabs and LidA are shown. Discussion
The crystal structures presented in current study revealed that the GDP-bound Rab1 is held in the active conformation through associating with LidA. Consistent with this structural observation, quantification assay using ITC demonstrated that LidA binds strongly to both GDP - and GTP-Rab1 with a nearly equal affinity (Figures 1A, B). These results indicate that Rab1 recognition by LidA is independent of its cycling between GDP - and GTP-bound states. To LidA, the switch mechanisms of Rab1 are not operational and thus Rab1 is rendered persistently active in interaction with LidA. Such a unique function of LidA provides explanations for a body of previous observations.
The prenylated GDI-free Rab1(D44N) was shown to be primarily membrane-localized, indicating that GDI is not absolutely required for membrane targeting of Rab1 [57]. This principle is likely applicable to the delivery of Rab1(D44N) to the LCV [45]. The strong interaction with LidA can be important for the membrane retention of Rab1(D44N), as LidA-deficient mutants displayed a scattered distribution of this Rab1 mutant in COS-1 cells [45]. Because no GDP-Rab1(D44N)-GDI complex is formed and LidA is able to recognize GDP-Rab1, GDF and GEF activity would be made redundant under these conditions, providing an explanation why recruitment of Rab1(D44N) to LCV is independent of SidM/DrrA [45]. To wild type Rab1, the complex of GDP-Rab1-GDI is formed with a high affinity. The direct competition of LidA with GDI for binding GDP-Rab1 will be difficult and inefficient. Thus GDP-Rab1 has to be released from GDI by SidM/DrrA for LidA recognition [39]. These arguments likely hold true with the recruitment of the GDP-restricted Rab1 mutant (S25N) to the LCV. In uninfected cells, the dominant inhibitory effect of Rab1(S25N) derives from the competition of this Rab1 mutant with wild type Rab1 for binding GEFs, resulting in formation of “dead-end” Rab1-GEF complexes [58]. In the case of wild type Rab1 recruitment to the LCV, this situation could be altered, because interaction of GDP-bound Rab1-SidM/DrrA complex is transient and much weaker than that of GDP-bound Rab1-LidA complex [39], [45] (Figure S3). LidA is therefore expected to be able to outcompete with SidM/DrrA for binding GDP-Rab1, which in turn facilitates the release of GDP-Rab1 from GDF (see further discussion below). As GDP-Rab1 is in the active conformation when complexed with LidA, it is conceivable that Rab1(S25N) can still support the functions performed by wild type Rab1. In complete agreement with this hypothesis, Rab1 binding to the LCV precedes the delivery of Sec22b, GDP-locked Rab1(S25N) can delay but not block the recruitment of Sec22b. This Rab1 mutant can support growth of L. pneumophila, at least for the first 10 hours of postinfection [42].
The strong association with LidA can facilitate the recruitment of Rab1 to LCV by SidM/DrrA. Release of GDP-Rab1 from GDIs catalyzed by SidM/DrrA (as a GDF) is a dynamic process and GDP-Rab1-SidM/DrrA is an intermediate of the reaction [59]–[61]. LidA is expected to displace SidM/DrrA from the intermediate because of its much higher binding affinity to GDP-Rab1, thus shifting the equilibrium toward dissociation of GDP-Rab1-GDI complex. In this respect, LidA is similar to GTP in that the latter is able to exchange GDP from the intermediate and form the GTP-bound Rab1, a product that is unfavorable for interaction with GDFs and thereby promotes disruption of GDP-Rab1-GDI complex. It can be imagined that lack of LidA would slow down the release of GDP-Rab1 from GDI. Consistently, deletion of LidA resulted in delayed but not a blocked recruitment of Rab1 to the LCV [39]. The inability of LidA to discriminate between GDP - and GTP-bound Rab1 may also be advantageous for L. pneumophila to retain the recruited Rab1 on the LCV. Because even GTP-bound Rab1 is subjected to hydrolysis by GAPs on LCV [43], [44], the resulting product of GDP-Rab1 is still able to be captured by LidA with a strong binding affinity. Moreover, interaction with LidA would make GDP-Rab1 less susceptible to extraction from the membrane of LCV by GDIs, because they have to outcompete LidA for Rab1 binding.
Unlike other small GTPase effector proteins which mainly binding to Rab1 through interacting with the switch I and II regions that determine nucleotide-dependent interaction, LidA holds Rab1 tightly in its hand like structure with four fingers. It is unlikely that any Rab1 effector can displace LidA from Rab1-LidA complex. LidA has been known to associate with the LCVs for much longer time than SidM/DrrA. However, Rab1 does eventually leave the LCV [44], [48], so that a mechanism is needed to decrease the binding affinity between Rab1 and LidA. Since Rab1 is deeply buried in the complex, the most efficient way to release Rab1 is to force LidA to open its fingers by downstream proteins. The GTP-Rab1-LidA structure shows truncation of the middle and the ring fingers does not disrupt Rab1-LidA complex, so that the thumb or the index fingers, though necessary, may not be sufficient to release Rab1 (Figure 4A). Structure analysis indicates that the thumb and the ring finger do not pack as tightly with other part of LidA as the index and middle finger. This means that LidA's wrist (the N-terminal half of α4 and β1–β4) which is isolated from the central interacting region, the bottom of the ring finger and thumb are accessible for other proteins to trigger a conformational change that releases Rab1 (Figure S4). Opening of the ring finger could be very important since it can expose the switch region immediately to facilitate Rab1 binding by other effectors.
Recent research showed that the post-translationally modified Rab1b by the L. pneumophila effector protein SidM/DrrA retains the ability to interact with LidA and can avoid the GAP recognition [46]. Our data confirmed that the correspondent residue Tyr80 in Rab1a was also AMPylated (Figure S5A). Tyr80 is located on the interface between Rab1a and LidA, involved in both hydrogen bonding with His431LidA and hydrophobic contact with Leu406LidA (Figure 3C), so it is reasonable to speculate that the covalent-bound AMP group on Tyr80 will affect the stability of LidA-Rab1 complex. However, Rab1 interacts with the LidA palm tightly via an extensive surface, so that the interaction is difficult to interrupt. In the structure of GTP-Rab1-LidA we can see even the middle and the ring fingers are truncated, LidA can still holds Rab1 (Figure 4A). Moreover the structural feature of four fingers possesses a certain degree of flexibility. Thus the orientations of the four fingers could adjust a little bit to accommodate the additional AMP group. Consistent with previous reports [46] our ITC results showed that the binding affinity of Rab1a(K62H, 1-176) and LidA(188-580) is not significantly affected by the AMPylation (Figure S5B,C). Thus we presume that LidA can form both the Rab1-LidA and AMP-Rab1-LidA complex in vivo. The ability of LidA to form stable complex with AMPylated Rab1 is consistent with its ability to bind a number of different Rabs, which small differences in their surface residues. L. pneumophila may also benefit from this property. Formation of AMPylated-Rab1-LidA complex may protect Rab1 from the de-AMPylation activity of SidD until Rab1 is released from LidA by still unknown mechanisms.
In addition to promoting SidM/DrrA for Rab1 recruitment, LidA may have other function(s). LidA interacts with the active conformation of Rab1 and exhibits no enzymatic activity [39]. Structural comparison showed that binding of LidA to Rab1 shares some features of the interaction between the effector protein Rabenosyn-5 with Rab4 [54]. Furthermore, recruitment of Rab1 is essential for delivering ER-derived vesicles to LCV [42], [43], [62]. Collectively, these results suggest that one potential function of LidA is to act as an effector of Rab1 and signal downstream components for remodeling of LCV. LidA was previously predicted and further confirmed in the present study to be a coiled-coil rich protein, one type of the best characterized Rab tethering factors [27], [54], [63]. The terminal parallel helix forms an antiparallel long coiled-coil and extends to the outside of interacting region (Figure 2C). Secondary structure analysis suggests that in full-length LidA, the length of this domain is likely to be longer than 100 Å. It is thus possible that LidA may function as a tethering factor, bridging the ER-derived vesicles and the LCV [49]. One potential advantage of employing its own protein as a tethering factor would allow L. pneumophila to be selective for recruiting ER-derived components for its growth. It is well established that a GTP-bound form of Rab GTPases interacts with their effectors for signaling. The only exception to this rule is Protrudin that interacts with the GDP-bound form of Rab11, regulating Rab11-dependent membrane recycling to promote the directional membrane trafficking [64]. While interacting with GDP-Rab1, LidA appears to be different from protrudin in that it essentially recognizes the active conformation of Rab1 and probably other Rabs [39], [43].
During the peer review of this manuscript, the structure of Rab8-LidA complex was published online. Structure comparison shows that Rab8-LidA complex adopts quite similar conformations with our structure and that the binding interface is highly conserved, consistent with our proposal that lidA recognizes Rabs in a similar way. We note that the authors report a binding affinity of LidA for GTP-bound Rab1 that is roughly one order of magnitude stronger than what we measured using ITC. At this point, it is inconsistent with our ITC data that LidA binds to both GDP - and GTP-Rab1 with a nearly equal affinity.
Materials and Methods
Protein expression purification
The genes of lidA and sidM were amplified from genomic DNA of Legionella pneumophila strain Corby. The DNA fragment encoding human Rab1a (noted Rab1 throughout the text) and other 14 kinds of Rab family members were amplified from a homemade human cDNA library. All the genes encoding the mutant proteins were produced using a two-step PCR procedure. LidA, both full-length (FL) and various fragments, SidM variants include 317-545 and 1-545 were subcloned into the pET15b (Invitrogen), Rab1 variants and Rab2, 4, 6, 7, 8, 11, 14, 18, 23, 27 were subcloned into the pGEX-6P-1 plasmid (GE Healthcare), Rab5, 9, 20, 22 were subcloned into both of the two vectors. Each protein was produced in E. coli BL21 (DE3). Cells grow at 37°C until the OD600 reach to 0.8. LidA proteins were induced with 0.5 mM isopropyl - β-D-Thiogalactopyranoside (IPTG) for 15 h at 15°C, Rab1 and other Rabs were all induced with 0.2 mM IPTG for 15 h at 22°C. All proteins were purified using affinity, anion exchange and gel filtration chromatography. To obtain LidA-Rab1 complexes, LidA and Rab1 proteins were mixed up with a 1∶1 molar ratio at 4°C for 2 hours. The LidA(188-449)-Rab1(1-191) complex and LidA(224-559)-Rab1(S25N; 1-176) complex were further purified using the above mentioned procedure except the GST tag and His tag were removed by homemade PreScission protease digestion before the anion exchange step. Both of the protein complexes have a final concentration of 10 mg/mL. All purification processes were performed at 4°C unless noted otherwise.
Crystallization and data collection
Crystallization conditions for complexes were determined from the sparse matrix screen (Hampton Research). All crystals were obtained using the hanging drop diffusion method at 20°C. LidA(224-559)-Rab1(S25N, 1-176) complex was crystallized by mixing equal volumes of protein with reservoir solution containing 28% Jeffamin ED-2001 and 0.1 M sodium citrate tribasic dihydrate pH 4.8. LidA(224-559)-Rab1(S25N, 1-176) crystals were optimized by microseeding to reach the satisfied diffraction. LidA(188-449)-Rab1(wild-type, WT1-191) complex was crystallized by mixing equal volumes of protein with reservoir solution containing 0.1 M Hepes pH 7.5, 25% PEG3350 and 0.7% butanol. All diffraction data were collected at Shanghai Synchrotron Radiation facility (SSRF) beamline BL17U. To prevent radiation damage, crystals were equilibrated in a cryoprotectant buffer containing 20% ethylene glycol (v/v) plus reservoir buffer and then flash frozen in a 100 K nitrogen stream. The best crystal of LidA(225-559)-Rab1(S25N; 1-176) complex diffracted to 1.73 Å. The best crystal of LidA(188-449)-Rab1(WT; 1-191) complex diffracted to 2.2 Å. Data sets were processed using the HKL2000 software suite [65].
Structure determination
The crystal structures of the two kinds of LidA-Rab1 complexes were determined by molecular replacement using PHASER [66] with the coordinates of Rab1 as the searching models (PDB ID code 2FOL) [67]. The atomic models were built using COOT [68] and refined using PHENIX [69]. Data collection and structure refinement statistics are summarized in Table S1. A SA-composite omit map of LidA index finger shows the good match between the atom skeleton and the electron density map as supplementary Figure S6. All the molecular graphics Figures were generated using PyMol (http://www.pymol.org).
Isothermal titration calorimetry
ITC was employed to measure the binding affinities of various fragments of LidA with Rab1 variants and 9 kinds of Rabs. All protein samples were purified in a buffer containing 20 mM Hepes (pH 8.0) and 100 mM NaCl with tag removed by PreScission protease digestion. The final concentration of LidA(188-580) and LidA(188-449) were 0.2 mM, Rab1(S25N) and Rab1(Q70L) were 2 mM, LidA(FL) was 0.15 mM, Rabs(FL) (include 2, 4, 6, 7, 9, 11, 20, 22) were 1.5 mM. The samples were centrifuged to remove any precipitate before the experiments. All measurements were carried out at 25°C by using a VP-ITC microcalorimeter 200 (MicroCal). Titrations were carried out by titrating Rab1(S25N) and Rab1(Q70L) into LidA(188-580) or LidA(188-449), respectively. Rab2, 4, 6, 7, 9, 11, 20 proteins were titrated into LidA(188-580). Rab22 were titrated into LidA(FL). The titration Data were analyzed using ORIGIN data analysis software (MicroCalSoftware).
In vitro pull down assay
15 kinds of purified Rab family members (WT; FL) with N-terminal GST tag (His-fused Rab20 is exceptional) was immobilized onto 200 µL glutathione-Sepharose resin (GE) or Ni-chelating sepharose (GE healthcare) for His-Rab20. The resin was then washed three times to remove excess unbound protein. Untagged LidA(FL) protein solution was loaded onto the Rabs-immobilized beads and incubated at 4°C for 2 h. The loaded protein dose of LidA and Rabs was at a molar ratio of 2∶1. The resin was washed three times using the wash buffer (25 mM Tris-HCl buffer pH 8.0, 100 mM NaCl) to remove unbound LidA. Proteins binding to the resin were eluted by elution buffer (25 mM Tris-HCl buffer pH 8.0, 100 mM NaCl, 3 mM DTT, 5 mM GSH for glutathione-Sepharose resin and 25 mM Tris-HCl buffer pH 8.0, 100 mM NaCl, 250 mM imidazole for Ni-chelating sepharose). All samples were subjected to SDS-PAGE which was visualized by Coomassie Brilliant Blue staining.
Accession numbers
Crystal structure of LidA(224-559)-Rab1(S25N; 1-176) complex and the structure factor have been deposited in the Protein Data Bank (http://www.rcsb.org/pdb) under ID codes 3SFV. Crystal structure of LidA(188-449)-Rab1(Q70L; 1-191) complex and the structure factor are deposited with access codes 3TKL.
Supporting Information
Zdroje
1. ZerialMMcBrideH 2001 Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2 107 117
2. PfefferSR 2001 Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol 11 487 491
3. SegevN 2001 Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol 13 500 511
4. SeabraMCWasmeierC 2004 Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol 16 451 457
5. UllrichOStenmarkHAlexandrovKHuberLAKaibuchiK 1993 Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem 268 18143 18150
6. UllrichOHoriuchiHBucciCZerialM 1994 Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature 368 157 160
7. PfefferSRDirac-SvejstrupABSoldatiT 1995 Rab GDP dissociation inhibitor: putting rab GTPases in the right place. J Biol Chem 270 17057 17059
8. Dirac-SvejstrupABSumizawaTPfefferSR 1997 Identification of a GDI displacement factor that releases endosomal Rab GTPases from Rab-GDI. EMBO J 16 465 472
9. Pereira-LealJBHumeANSeabraMC 2001 Prenylation of Rab GTPases: molecular mechanisms and involvement in genetic disease. FEBS Lett 498 197 200
10. CaleroMChenCZZhuWWinandNHavasKA 2003 Dual prenylation is required for Rab protein localization and function. Mol Biol Cell 14 1852 1867
11. GomesAQAliBRRamalhoJSGodfreyRFBarralDC 2003 Membrane targeting of Rab GTPases is influenced by the prenylation motif. Mol Biol Cell 14 1882 1899
12. SiniossoglouSPeak-ChewSYPelhamHR 2000 Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J 19 4885 4894
13. RossmanKLDerCJSondekJ 2005 GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6 167 180
14. BosJLRehmannHWittinghoferA 2007 GEFs and GAPs: critical elements in the control of small G proteins. Cell 129 865 877
15. BarrFLambrightDG 2010 Rab GEFs and GAPs. Curr Opin Cell Biol 22 461 470
16. McLauchlanHNewellJMorriceNOsborneAWestM 1998 A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol 8 34 45
17. KoumandouVLDacksJBCoulsonRMFieldMC 2007 Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol Biol 7 29
18. BatokoHZhengHQHawesCMooreI 2000 A rab1 GTPase is required for transport between the endoplasmic reticulum and golgi apparatus and for normal golgi movement in plants. Plant Cell 12 2201 2218
19. MuslinAJ 2001 Road Rage: Cardiac Rab1 and ER-to-Golgi Traffic. Circ Res 89 1087 1088
20. FilipeanuCMZhouFClaycombWCWuG 2004 Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem 279 41077 41084
21. GrosshansBLOrtizDNovickP 2006 Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 103 11821 11827
22. HaasAKYoshimuraSStephensDJPreisingerCFuchsE 2007 Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J Cell Sci 120 2997 3010
23. YinHLiQQianGWangYLiY 2011 Rab1 GTPase regulates phenotypic modulation of pulmonary artery smooth muscle cells by mediating the transport of angiotensin II type 1 receptor under hypoxia. Int J Biochem Cell Biol 43 401 408
24. AllanBBMoyerBDBalchWE 2000 Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289 444 448
25. MoyerBDAllanBBBalchWE 2001 Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis–Golgi tethering. Traffic 2 268 276
26. BeardMSatohAShorterJWarrenG 2005 A cryptic Rab1-binding site in the p115 tethering protein. J Biol Chem 280 25840 25848
27. SztulELupashinV 2006 Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol 290 C11 26
28. Albert-WeissenbergerCCazaletCBuchrieserC 2007 Legionella pneumophila - a human pathogen that co-evolved with fresh water protozoa. Cell Mol Life Sci 64 432 448
29. HorwitzMA 1983 Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med 158 1319 1331
30. Sturgill-KoszyckiSSwansonMS 2000 Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J Exp Med 192 1261 1272
31. EnsmingerAWIsbergRR 2009 Legionella pneumophila Dot/Icm translocated substrates: a sum of parts. Curr Opin Microbiol 12 67 73
32. DerreIIsbergRR 2004 Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun 72 3048 3053
33. BruggemannHCazaletCBuchrieserC 2006 Adaptation of Legionella pneumophila to the host environment: role of protein secretion, effectors and eukaryotic-like proteins. Curr Opin Microbiol 9 86 94
34. NinioSRoyCR 2007 Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol 15 372 380
35. ShinSRoyCR 2008 Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol 10 1209 1220
36. IsbergRRO'ConnorTJHeidtmanM 2009 The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 7 13 24
37. WuG 2008 Regulation of the trafficking and function of G protein-coupled receptors by Rab1 GTPase in cardiomyocytes. Methods Enzymol 438 227 238
38. ZhuangXAdipietroKADattaSNorthupJKRayK 2010 Rab1 small GTP-binding protein regulates cell surface trafficking of the human calcium-sensing receptor. Endocrinology 151 5114 5123
39. MachnerMPIsbergRR 2006 Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell 11 47 56
40. MurataTDelpratoAIngmundsonAToomreDKLambrightDG 2006 The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol 8 971 977
41. BrumellJHScidmoreMA 2007 Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev 71 636 652
42. KaganJCSteinMPPypaertMRoyCR 2004 Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med 199 1201 1211
43. NeunuebelMRChenYGasparAHBacklundPSJrYergeyA 2011 De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science 333 453 456
44. IngmundsonADelpratoALambrightDGRoyCR 2007 Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 450 365 369
45. MachnerMPIsbergRR 2007 A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 318 974 977
46. MullerMPPetersHBlumerJBlankenfeldtWGoodyRS 2010 The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 329 946 949
47. TanYLuoZQ 2011 Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature 475 506 509
48. ConoverGMDerreIVogelJPIsbergRR 2003 The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol Microbiol 48 305 321
49. DerreIIsbergRR 2005 LidA, a translocated substrate of the Legionella pneumophila type IV secretion system, interferes with the early secretory pathway. Infect Immun 73 4370 4380
50. NuofferCDavidsonHWMattesonJMeinkothJBalchWE 1994 A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol 125 225 237
51. WilsonBSNuofferCMeinkothJLMcCafferyMFeramiscoJR 1994 A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J Cell Biol 125 557 571
52. TisdaleEJBourneJRKhosravi-FarRDerCJBalchWE 1992 GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol 119 749 761
53. StroupeCBrungerAT 2000 Crystal structures of a Rab protein in its inactive and active conformations. J Mol Biol 304 585 598
54. EathirajSPanXRitaccoCLambrightDG 2005 Structural basis of family-wide Rab GTPase recognition by rabenosyn-5. Nature 436 415 419
55. PfefferSR 2005 Structural clues to Rab GTPase functional diversity. J Biol Chem 280 15485 15488
56. UrwylerSNyfelerYRagazCLeeHMuellerLN 2009 Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic 10 76 87
57. WilsonALErdmanRAMalteseWA 1996 Association of Rab1B with GDP-dissociation inhibitor (GDI) is required for recycling but not initial membrane targeting of the Rab protein. J Biol Chem 271 10932 10940
58. StorrieB 2005 Microinjection as a tool to explore small GTPase function. Methods Enzymol 404 26 42
59. SchoebelSOesterlinLKBlankenfeldtWGoodyRSItzenA 2009 RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell 36 1060 1072
60. SuhHYLeeDWLeeKHKuBChoiSJ 2010 Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA. EMBO J 29 496 504
61. ZhuYHuLZhouYYaoQLiuL 2010 Structural mechanism of host Rab1 activation by the bifunctional Legionella type IV effector SidM/DrrA. Proc Natl Acad Sci U S A 107 4699 4704
62. BrombacherEUrwylerSRagazCWeberSSKamiK 2009 Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J Biol Chem 284 4846 4856
63. MarkgrafDFPeplowskaKUngermannC 2007 Rab cascades and tethering factors in the endomembrane system. FEBS Lett 581 2125 2130
64. ShiraneMNakayamaKI 2006 Protrudin induces neurite formation by directional membrane trafficking. Science 314 818 821
65. Otwinowski ZMW 1997 Processing of X-ray Diffration Data Collected in Oscillation Mode. Methods in Enzymology 276 20
66. McCoyAJGrosse-KunstleveRWAdamsPDWinnMDStoroniLC 2007 Phaser crystallographic software. J Appl Crystallogr 40 658 674
67. 1994 The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50 760 763
68. EmsleyPCowtanK 2004 Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126 2132
69. AdamsPDGrosse-KunstleveRWHungLWIoergerTRMcCoyAJ 2002 PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58 1948 1954
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



