-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMutation and Selection of Prions
article has not abstract
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002582
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002582Summary
article has not abstract
Prion Diseases
Prion diseases, or transmissible spongiform encephalopathies (TSEs), occur naturally in several species, including humans, cattle, sheep, and deer, and can be transmitted experimentally to many others. Typically, incubation times are relatively long, extending to 40 years or more in humans; however, after appearance of clinical symptoms, death mostly ensues within less than a year, as a consequence of neurodegeneration accompanied by accumulation of abnormal conformers of the host protein PrP. Natural transmission usually occurs perorally, as exemplified by the kuru epidemic among the Fore people of Papua New Guinea, attributed to cannibalistic practices; the bovine spongiform encephalopathy (BSE) epizootic in the United Kingdom at the end of last century, caused by feeding of contaminated meat-and-bone meal to cattle; or the current epizootic of chronic wasting disease afflicting cervids in 19 states of the United States. Transmission of BSE prions to young humans gave rise to a limited outbreak of a novel illness, variant Creutzfeldt-Jakob disease (vCJD), almost exclusively in the UK. Sporadic cases of prion disease occur at very low frequency in human populations (sCJD) and in cattle herds (atypical BSE), and are attributed to spontaneous generation of prions in the affected individuals. Finally, familial forms of human prion disease are linked to a variety of different, dominant mutations in the PRNP gene, and while afflicted families are rare, penetrance is very high.
Replication of Prions
Prions consist mainly, if not solely, of PrPSc (scrapie prion protein), aggregated conformers of the GPI-linked host glycoprotein PrPC (cellular prion protein). PrPSc propagates by converting PrPC to a replica of itself (Figure 1A). PrPC may exist as an equilibrium mixture of conformers, some of which can accrete to PrPSc “seeds” at a critical rate [1], [2]. This seeding model is supported by the protein misfolding cyclic amplification (PMCA) reaction, in which brain homogenate, as a source of PrPC, is spiked with a seed of infected brain homogenate and subjected to multiple cycles of sonication and incubation, ultimately yielding a vast excess of infectious prions [3]. Infectious prions arose spontaneously in PMCA-mediated, cell-free reactions from defined components [4], in particular from recombinant PrP, a phospholipid, and poly(A) or poly(dT) [5], definitively laying to rest the perennial proposal that the infectious agent is a virus-like entity [6]. Prion-like, seeded conversion into an aggregated state has been proposed for several mammalian proteins such as Abeta, α-synuclein, or serum amyloid, which underlie protein misfolding diseases, and for several fungal, in particular yeast, proteins.
Fig. 1. Propagation, mutation, and selection of prions in cultured cells. 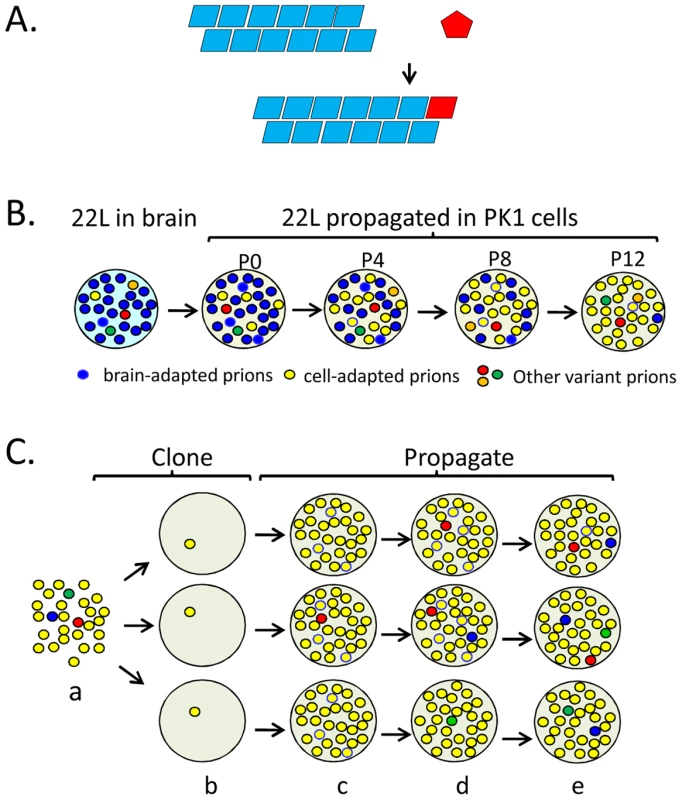
(A) The seeding model of prion propagation predicates that PrPC monomers add to the termini of PrPSc fibrils and in doing so, adopt the conformation of the constituent PrPSc subunits. (B) Prion populations are thought to constitute quasi-species, consisting of a major species and numerous variants at low levels. Brain-adapted 22L prions are resistant to swainsonine treatment when assayed on PK1 cells and are able to infect R33 cells (R33 competent). When propagated in PK1 cells, swainsonine-sensitive, R33-incompetent prions gradually (passages P0 to P12) become the major species in the population because they multiply faster. (C) PK1 cell-adapted 22L prions (a) were cloned (b) in PK1 cells. The populations become heterogeneous as mutations arise during propagation (c–e). The red circles represent swainsonine-resistant prions; when challenged with the drug, some populations (top and middle row) acquire the capacity to become resistant while others (bottom row) do not. Schematic representation of data from reference [16]. Prion Strains
Prion populations may present as distinct strains: these differ in their phenotypic properties but are associated with PrPSc having the same amino acid sequence. Murine prion strains, originally characterized by the incubation time and the neuropathology they elicit, can be propagated indefinitely in mice homozygous for the PrP gene. Many “classical” strains currently propagated in mice and hamsters, such as 79A, 22L, and ME7, originated from scrapie-infected sheep or goats [7] and were cloned by endpoint dilution in mice.
Strain-specific properties of the prion are believed to be enciphered in the conformation of the cognate PrPSc [8], and indeed, distinct strains are often associated with PrPSc species differing in physicochemical properties. Experiments with yeast prion strains have shown that specific conformations can be propagated in vitro by pure, unglycosylated proteins [9]. Nonetheless, in view of the vast multiplicity of mammalian prion strains and their tropism for particular cell lines, it is conceivable that post translational modifications of PrP, such as glycosylation or association with some cellular components, might favor certain PrP conformations and hence account for cell-specific preferential propagation of particular strains.
The Species Barrier
In general, there is a considerable barrier to transmission of prions between animal species, in that even massive intracerebral trans-species inoculation causes disease at only low frequency (low “attack rate”) and/or only after very long incubation times, if at all. This barrier was abolished in some instances by replacing the PrP gene of the recipient by its counterpart from the donor, but clearly factors other than mismatch of PrP sequences contribute to the incompatibility. Importantly, when prions are serially transmitted from the initial trans-species recipients to further animals of the same species, attack rates increase and incubation times decrease, reflecting “adaptation” to the new host [10]. “Adaptation” implies as a first step accretion of PrPC from the recipient host to the incoming PrPSc seed, which may be a very inefficient process if the amino acid sequence of the host PrP entrains a spectrum of conformations that are poorly compatible with that of the seed. Efficient propagation may only be enabled when the conformation of the seed changes, perhaps initially at the “growing end” [11], resulting in a “mutation” at the conformational level. Subsequently, prions may evolve to replicate more rapidly in the new host, accounting for the striking reduction of their incubation period as they are sequentially transferred within the new species.
In some instances, transfer of a prion strain from one species to another, followed by several passages in the original host species, led to emergence of mutant strains. For example, when cloned murine 139A prions were passaged through hamster and subsequently passaged repeatedly in mouse a new strain, 139A-H2M, was recovered; however, ME7 subjected to the same procedure remained apparently unchanged [12].
Evolution of Prions
The finding that many murine prion strains replicated efficiently in selected murine cell lines created important new experimental opportunities. In particular, the slow, expensive, and imprecise mouse-based bioassay for murine prions could be replaced by a humane, rapid, and precise cell-based procedure, the standard scrapie cell assay (SSCA) [13]. The differential susceptibility of cell lines to various prion strains provided the basis of the cell panel assay (CPA), which rapidly differentiates between various prion strains on the basis of their cell tropism and their susceptibility to various drugs, such as swainsonine or kifunensine [14], [15].
The CPA revealed that serial propagation of brain-derived 22L prions in PK1 cells led to progressive change in their properties; while initially able to propagate in R33 cells (“R33 competent”) or in PK1 cells in the presence of swainsonine (“swainsonine resistant”), the prions gradually became completely R33 incompetent and swainsonine-sensitive (Figure 1B). When these “cell-adapted” prions were returned to mouse brain, they gradually re-acquired their former properties and became indistinguishable from the original 22L strain [16]. Along similar lines, when swainsonine-sensitive prions were propagated in PK1 cells in the presence of the drug, a swainsonine-resistant prion population emerged after a few passages, documenting adaptation to the new environment. After withdrawal of the drug, further propagation for several splits again yielded drug-sensitive prions [16]. These findings suggested that prion populations constitute so-called quasispecies [17], that is, they are composed of a variety of conformational variants, each present at a low level; when the environment changes, the most efficiently replicating variant becomes the predominant component of the population, which then constitutes a distinct sub-strain [1], [16], [18]. Indeed, PK1 cell-adapted 22L populations were found to contain about 0.5% swainsonine-resistant variants before ever being exposed to the drug [16]. Because the 22L prions used in these experiments had been cloned by endpoint dilution years earlier, heterogeneity must have arisen by a mutation-like process in the interim. Mutations in the case of prions represent conformational changes and not modifications at the level of the protein sequence, because PrP is encoded by the host genome and the mutation is inherent to the proteinaceous particle. To verify whether heterogeneity of prion populations came about by mutation, swainsonine-sensitive prions were cloned by endpoint dilution into PK1 cells, and the infected cells were propagated serially for up to 100 doublings and challenged with swainsonine to determine at which stage the prion populations acquired the capacity for becoming resistant to the drug. Early after cloning the populations were incapable of doing so, but most clones developed this capability after 31–86 doublings (Figure 1C). However, at least one of nine populations failed to do so even after 116 doublings, suggesting that the prions were heterogeneous in regard to their ability to develop swainsonine resistance [11], [16]. Acquisition of drug resistance by murine prions has also been reported by Ghaemmaghami et al. [19] and by yeast prions by Shorter [20]. Most if not all of the prion variants, or sub-strains, described above were reversible, suggesting that the underlying conformations were readily interconvertible. In contrast, strains are very stable, at least as long as they are propagated in the same species. As shown in Figure 2, this suggests a low activation energy barrier between sub-strains, readily surmountable under physiological conditions, while high activation energy barriers prevent conversion between strains.
Fig. 2. Conjectural free energy landscape for prion strains and sub-strains. 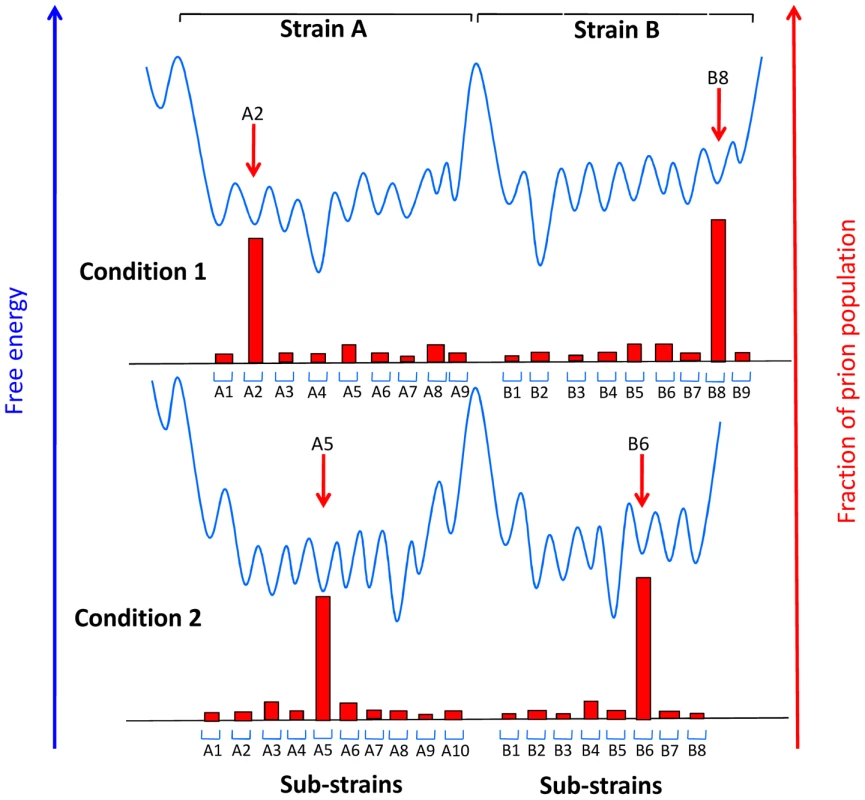
Sub-strains are depicted as distinguishable collectives of prions that can interconvert readily because they are separated by activation energy barriers that can be overcome in a particular environment under physiological conditions, while strains are separated by high energy barriers. The extent to which the individual wells are populated (red blocks) is determined by the accumulation rate of the particular sub-strain. When the environment changes, for example when prions are transferred between distinct tissues, different sub-strains may be favored. Adapted from reference [18]. Concluding Thoughts
The finding that prions can acquire resistance to drugs has significant implications for drug design. Drugs targeted to PrPSc may have to be administered in combination, as in the case of viruses, in particular HIV. Alternatively, drugs could be targeted to bind and stabilize PrPC or, in view of the finding that ablation of PrPC, at least in animals, is not detrimental to health [21], [22], to suppress its synthesis. At present no therapeutically useful drugs are available, but deepening insight into the molecular biology of prions may pave the way to novel approaches.
Zdroje
1. CollingeJClarkeAR 2007 A general model of prion strains and their pathogenicity. Science 318 930 936
2. WeissmannC 2009 Thoughts on mammalian prion strains. Folia Neuropathol 47 104 113
3. CastillaJSaaPHetzCSotoC 2005 In vitro generation of infectious scrapie prions. Cell 121 195 206
4. DeleaultNRHarrisBTReesJRSupattaponeS 2007 Formation of native prions from minimal components in vitro. Proc Natl Acad Sci U S A 104 9741 9746
5. WangFZhangZWangXLiJZhaL 2012 Genetic informational RNA is not required for recombinant prion infectivity. J Virol 86 1874 1876
6. ManuelidisL 2010 Transmissible encephalopathy agents: virulence, geography and clockwork. Virulence 1 101 104
7. DickinsonAG 1976 Scrapie in sheep and goats. KimberlinRH Slow virus diseases of animals and man Amsterdam Elsevier/North Holland 209 241
8. PrusinerSB 1991 Molecular biology of prion diseases. Science 252 1515 1522
9. TanakaMCollinsSRToyamaBHWeissmanJS 2006 The physical basis of how prion conformations determine strain phenotypes. Nature 442 585 589
10. KimberlinRHWalkerC 1977 Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol 34 295 304
11. LiJMahalSPDemczykCAWeissmannC 2011 Mutability of prions. EMBO Rep 14 191
12. KimberlinRHWalkerCAFraserH 1989 The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol 70 2017 2025
13. KlohnPCStoltzeLFlechsigEEnariMWeissmannC 2003 A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci U S A 100 11666 11671
14. MahalSPBakerCADemczykCASmithEWJuliusC 2007 Prion strain discrimination in cell culture: the cell panel assay. Proc Natl Acad Sci U S A 104 20908 20913
15. BrowningSBakerCASmithEMahalSPHervaME 2011 Abrogation of complex glycosylation by Swainsonine results in strain - and cell-specific inhibition of prion replication. J Biol Chem 19 19
16. LiJBrowningSMahalSPOelschlegelAMWeissmannC 2010 Darwinian evolution of prions in cell culture. Science 327 869 872
17. EigenM 1971 Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58 465 523
18. WeissmannCLiJMahalSPBrowningS 2011 Prions on the move. EMBO Rep 12 1109 1117
19. GhaemmaghamiSAhnMLessardPGilesKLegnameG 2009 Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog 5 e1000673 doi:10.1371/journal.ppat.1000673
20. ShorterJ 2010 Emergence and natural selection of drug-resistant prions. Mol Biosyst 6 1115 1130
21. BüelerHAguzziASailerAGreinerRAAutenriedP 1993 Mice devoid of PrP are resistant to scrapie. Cell 73 1339 1347
22. RichtJAKasinathanPHamirANCastillaJSathiyaseelanT 2007 Production of cattle lacking prion protein. Nat Biotechnol 25 132 138
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



