-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNew Insights into spp.: A Potential Link with Irritable Bowel Syndrome
article has not abstract
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002545
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002545Summary
article has not abstract
What Are Blastocystis spp.?
Blastocystis spp. belong to the phylum Stramenopila, a complex and heterogeneous evolutionary assemblage of heterotrophic and photosynthetic protozoa [1]. Interestingly, this is the only stramenopile living in the lower digestive tract of humans, and it also lives in other mammals, birds, reptiles, amphibians, and insects [1]. Even though isolates were reported to be morphologically indistinguishable, an extensive genetic variation among isolates from both humans and animals has been observed. Thirteen subtypes (ST1–ST13), with the first nine being found in humans, have been identified based on genes coding for the small-subunit ribosomal RNA [2]. Preferential repartition of STs exists among animals that appear to constitute the main reservoir for environmental dissemination and human contamination [1].
Four forms of Blastocystis spp. (vacuolar, granular, amoeboid, and cyst) were described in stools and/or in vitro cultures [1]. Studies in animals demonstrated that the water - and environmentally resistant infective cyst undoubtedly represents the transmissible stage of this parasite [1]. Blastocystis spp. prevalence in humans often exceeds 5% in industrialized countries and can reach as high as 76% in developing countries [1], [3]. However, prevalence data are largely dependent on the methods used for detection, quantitative PCR being the most sensitive method, meaning that infections by Blastocystis spp. are likely underestimated [4].
Lately, Blastocystis spp. have been included in the water sanitation and health programs of the World Health Organization [5]. Increasing interest of scientific and medical communities for Blastocystis spp. was coupled with new data about epidemiology, pathogenicity, and, more recently, the first whole genome of a human isolate. Accumulating in vivo, in vitro, and in silico data has enabled researchers to assess the potential impact of Blastocystis spp. in human health.
Are Blastocystis spp. Pathogens?
In vivo endoscopy and biopsy analyses in symptomatic patients indicated that Blastocystis spp. do not invade the colonic mucosa, but lead to disturbances on the barrier function and permeability [1], [6]. Experiments on immunocompetent BALB/c mice revealed intense inflammatory-cell infiltration in the mucosa of some specimens, but not in all mice, suggesting that some host factors could be involved [1]. Subsequently, the infectivity of human isolates obtained from both asymptomatic and symptomatic patients on rats was assessed by Hussein et al. [7]. Interestingly, the moderate and severe degrees of pathological changes were only found in rats infected by isolates from symptomatic patients, and differences in severity were observed among the different STs of Blastocystis, suggesting the existence of some more virulent strains.
To understand cellular mechanisms, in vitro experiments were performed to investigate the cytopathic effects of Blastocystis spp. on mammalian cell cultures (Figure 1). A first study showed in the rat epithelial cell line IEC-6 that Blastocystis ST4 can induce apoptosis in a contact-independent manner, increasing epithelial permeability [8]. The pro-inflammatory effect of Blastocystis ST1 culture filtrates was demonstrated on HT-29 and T-84 human colonic epithelial cells with production of interleukin 8 (IL-8) and granulocyte-macrophage colony stimulating factor (GM-CSF) [1]. Cysteine proteases of Blastocystis ST4 were shown to induce IL-8 production via an NF-κB pathway [9]. Proteases released in culture supernatants of both Blastocystis ST4 and ST7 were also shown to be able to cleave human-secreted immunoglobulin A (IgA) and then modulate the immune response of the host [1]. A surface-located cysteine protease was recently shown to be involved in a pro-survival role in Blastocystis ST7 and may activate other proteases [10]. Nevertheless, in vitro studies are limited by the lack of tools to study Blastocystis spp. Indeed, few strains of Blastocystis spp. are available in axenic cultures, and growth rates in culture are fluctuating and quite low. In addition, growth of this parasite is realized in anaerobic chambers that limit the possibility of long-term exposure in cellular models.
Fig. 1. Hypothetical model of pathogeny for Blastocystis spp. from genomic (*) and experimental (**) data. 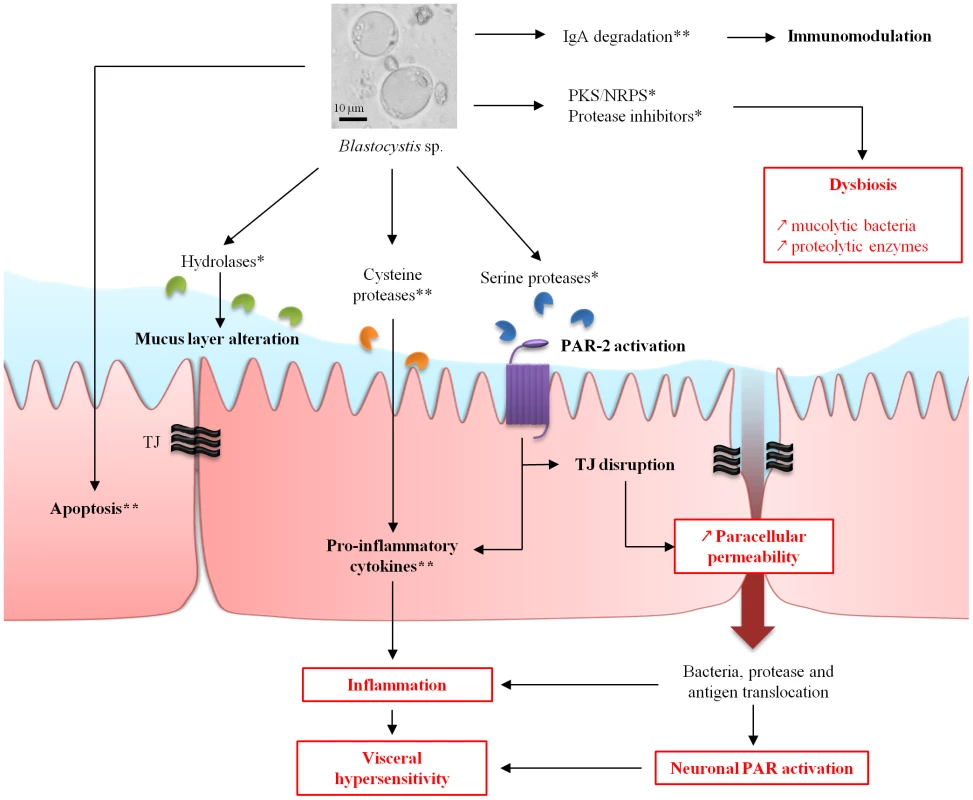
Potential link to IBS pathophysiology mechanisms (in red). IgA, immunoglobulin A; NRPS, non-ribosomal polyketide synthase; PAR, protease-activated receptor; PKS, polyketide synthase. Is There a Link between Blastocystis spp. and IBS?
Some studies have suggested an association of Blastocystis spp. with acute or chronic digestive disorders such as irritable bowel syndrome (IBS) [1]. IBS is a functional gastrointestinal disorder characterized by abdominal discomfort and/or pain associated with changes in bowel habits, affecting 5%–24% of people in industrialized countries with impairment on quality of life [11]. In 1997, Hussain et al. highlighted that sera from IBS patients were characterized by higher IgG antibody levels to Blastocystis spp. when compared to healthy populations [12]. However, the first relevant epidemiological report about a possible link between Blastocystis spp. and IBS was provided two years later by Giacometti et al. [13]. When comparing the prevalence of Blastocystis spp. in individuals with gastrointestinal symptoms and classified as affected or not by IBS, the authors found that it was significantly present in IBS patients. Other more recent studies also argued for a higher prevalence of Blastocystis spp. among IBS patients (Table 1) compared to healthy populations or to patients suffering from other gastrointestinal disorders [3], [13]–[17]. However, three studies failed to demonstrate an association between Blastocystis spp. and IBS [18]–[20]. Possible explanations could be the small IBS cohort studied or the parasitological diagnostic methods used. Two of these three studies were from Thailand and one from Mexico, whereas studies arguing for a link between Blastocystis spp. and IBS were from the Middle East (Table 1) and Europe. IBS is a functional disorder of multifactorial origin, and some genetic, environmental, and microbiological factors could also explain this discrepancy. Nevertheless, overall studies missed an opportunity to provide a therapeutic trial with a follow up of IBS symptoms.
Tab. 1. Summary of studies investigating the association between Blastocystis spp. and IBS. 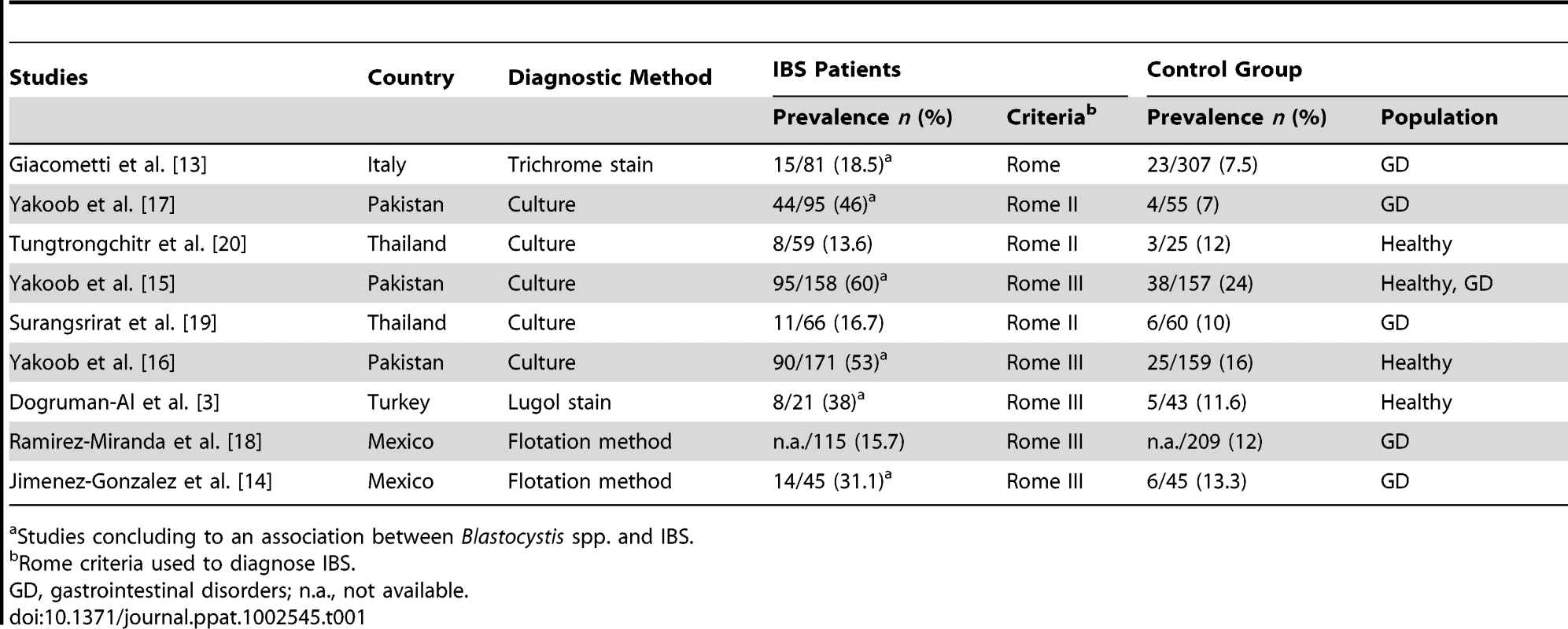
Studies concluding to an association between Blastocystis spp. and IBS. Recent studies suggest that visceral pain associated with IBS could be explained by alterations of the epithelial barrier, resulting in bowel motility and sensitivity disorders (Figure 1). Indeed, in vitro studies on colonic biopsies from patients with IBS showed an increase of paracellular permeability associated with perturbations of tight junctions (TJs) [21]. On the other hand, it is now well recognized that there is a low-grade inflammation of the mucosa in IBS patients [22]. Then, protease-activated receptor type 2 (PAR-2) was proposed to be involved in both an increase of permeability and low-grade inflammation [23]. PAR-2 are activated by serine-proteases that cleave the N-terminal domain of the receptor. Then, the released peptide may act as a ligand and turn on the receptor to enhance TJ opening and trigger inflammation. Increase of paracellular permeability allows diffusion of both antigens and bacteria to sub-mucosa, participating in inflammation. PARs are also present at the surface of intestinal neurons. The activation of some members of the PAR family could contribute to abdominal pain [24]. Studies also showed that stools from IBS patients present higher proteolytic activity than healthy controls [25]. Experimental data have shown a protease activity of supernatants from axenic cultures of both Blastocystis ST4 and ST7 [1]. This is supported by the prediction of 22 secreted proteases from genomic data of Blastocystis ST7 (detailed below) [26]. These experimental and genomic data suggest a possible involvement of parasite proteases in gastrointestinal disturbances. Thus, proteases from bacteria or Blastocystis spp., such as metalloproteases, cysteine, or serine proteases, could play a key role in IBS genesis [24]. This perturbation could be linked to a modification of lumen microbiota in IBS patients compared to healthy patients [27]. Then, dysbiosis may take part in low-grade inflammation of the mucosa and IBS symptoms.
How Can Genomic Data Support the Blastocystis spp./IBS Association?
The whole genome of a Blastocystis ST7 isolate has been sequenced [26]. Interestingly, candidate proteins potentially involved in the pathogenicity of Blastocystis spp. were identified by in silico analyses of the predicted proteome and secretome (Figure 1).
Blastocystis ST7 likely uses hydrolases to attack host tissues for its nutrient supply. Fucosidase, hexosaminidase, and β-galactosidase were identified in the Blastocystis ST7 predicted secretome. Blastocystis ST7 may participate in this process by degrading host glycoproteins, especially those that constitute the mucus [26]. In addition, cysteine proteases could also degrade mucins [24]. Thus, these enzymes may allow Blastocystis spp. to use mucus as a carbohydrate and protein source and enable them to survive within the intestinal environment by creating their own micro-environment. Interestingly, 22 proteases, including 20 cysteine proteases, one serine protease, and one aspartic protease, were predicted to be secreted [26]. The impairment of mucus may be the initial step to inflammatory and allergic disturbance caused by chronic exposure to luminal antigens. Then, proteases from Blastocystis spp. and/or gut bacteria can also target receptors at the intestinal cell surface. These proteolytic enzymes are known to be involved in paracellular permeability, inflammation, and hypersensitivity [24]. The Blastocystis ST7 serine protease could therefore have the ability to target PAR-2 (Figure 1), inducing inflammation and TJ disruption as frequently seen in IBS. Once the TJs are opened, luminal proteases can have access to submucosal ganglia, activate PARs on enteric neurons (Figure 1), and be responsible for hypersensitivity in IBS patients [24]. Penetration of luminal bacteria or antigens could participate in the establishment of a chronic low-grade inflammation in submucosa by stimulation of innate immunity.
Blastocystis ST7–secreted glycosyltransferases could also participate in TJ disruption in IBS. This can be illustrated by the lymphostatin (toxin) of Citrobacter rodentium, which possesses a glycosyltransferase activity that primarily influences localization of ZO-1 and occludin at intestinal cell TJs, compromising epithelial barrier function [24]. This is also the case for Clostridium spp., which exhibit toxins with glycosyltransferase activities [24]. These proteins act by inactivating Rho proteins that are known to be important in maintaining TJs.
Concerning the intestinal protease balance that regulates gut functioning, protease inhibitors released by enteric pathogens or parasites can modulate the activity of host proteases and disturb intestinal homeostasis [28]. Genes coding for protease inhibitors are also present in the Blastocystis ST7 genome, and some are predicted to be secreted, including cystatin, type 1-protease inhibitor, and endopeptidase inhibitor-like protein [26]. Moreover, dysbiosis was shown to occur during IBS [27]. Genomic data revealed that a polyketide synthase (PKS) and a non-ribosomal polyketide synthase (NRPS) are present in Blastocystis ST7. These enzymes are known to produce non-ribosomal peptides and polyketides with various biological properties, such as antibiotics or immunomodulatory molecules that could participate in dysbiosis and inflammation [29].
Finally, we can assume that Blastocystis spp.–secreted proteins have the potential to modulate host defenses and to facilitate nutrient acquisition and parasite colonization. Moreover, Blastocystis spp. would be able to alter integrity of gut epithelia and probably participate in dysbiosis.
What Is Needed to Improve the Knowledge on Blastocystis spp. Biology?
A consortium including the most relevant researchers on this topic is being set up. The first aim is to develop efficient standardized tools to study Blastocystis spp., including the improvement of cultural methods (in particular, axenization protocols of the other STs) and development of new molecular markers for epidemiological studies. The role of STs in pathogenicity needs to be elucidated, notably by sequencing the whole genome of other STs. Comparative genomic analyses will then be useful for both identification and validation of in silico–predicted virulence factors. Animal models are also needed to go further in the understanding of the role of Blastocystis spp. in gut dysfunctions. Another aim is to develop some reverse genetic tools for functional characterization of genes of interest. Considering the clinical implication of Blastocystis spp. in IBS, none of the published studies provided complete clinical data about IBS classification and Blastocystis STs. Thus, well-designed multi-centric studies are required, and studies on Blastocystis spp. susceptibility to treatment have to be used to suggest guidelines for the eradication of this infection [30]. The role of Blastocystis spp. in dysbiosis and the exact interactions with bacteria also have to be highlighted. This approach seems more relevant because of the increasing interest in microbiota disturbances in the genesis of various gastrointestinal dysfunctions.
Zdroje
1. TanKS 2008 New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21 639 665
2. StensvoldCRSureshGKTanKSThompsonRCTraubRJ 2007 Terminology for Blastocystis subtypes: a consensus. Trends Parasitol 23 93 96
3. Dogruman-AlFSimsekZBooromKEkiciESahinM 2010 Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS ONE 5 e15484 doi:10.1371/journal.pone.0015484
4. PoirierPWawrzyniakIAlbertAEl AlaouiHDelbacF 2011 Development and evaluation of a real-time PCR assay for detection and quantification of blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J Clin Microbiol 49 975 983
5. WHO 2008 Guidelines for drinking-water quality. Third edition. Incorporating first and second addenda Geneva WHO 514
6. DagciHUstunSTanerMSErsozGKaracasuF 2002 Protozoon infections and intestinal permeability. Acta Trop 81 1 5
7. HusseinEMHusseinAMEidaMMAtwaMM 2008 Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res 102 853 860
8. PuthiaMKSioSWLuJTanKS 2006 Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect Immun 74 4114 4123
9. PuthiaMKLuJTanKS 2008 Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-kappaB-dependent manner. Eukaryot Cell 7 435 443
10. WuBYinJTexierCRousselMTanKS 2010 Blastocystis legumain is localized on the cell surface, and specific inhibition of its activity implicates a pro-survival role for the enzyme. J Biol Chem 285 1790 1798
11. LongstrethGFThompsonWGCheyWDHoughtonLAMearinF 2006 Functional bowel disorders. Gastroenterology 130 1480 1491
12. HussainRJaferiWZuberiSBaqaiRAbrarN 1997 Significantly increased IgG2 subclass antibody levels to Blastocystis hominis in patients with irritable bowel syndrome. Am J Trop Med Hyg 56 301 306
13. GiacomettiACirioniOFiorentiniAFortunaMScaliseG 1999 Irritable bowel syndrome in patients with Blastocystis hominis infection. Eur J Clin Microbiol Infect Dis 18 436 439
14. Jimenez-GonzalezDEMartinez-FloresWAReyes-GordilloJRamirez-MirandaMEArroyo-EscalanteS 2011 Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res E-pub ahead of print 26 August 2011. doi:10.1007/s00436-011-2626-7
15. YakoobJJafriWBegMAAbbasZNazS 2010 Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis. Parasitol Res 106 1033 1038
16. YakoobJJafriWBegMAAbbasZNazS 2010 Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107 679 684
17. YakoobJJafriWJafriNKhanRIslamM 2004 Irritable bowel syndrome: in search of an etiology: role of Blastocystis hominis. Am J Trop Med Hyg 70 383 385
18. Ramirez-MirandaMEHernandez-CastellanosRLopez-EscamillaEMoncadaDRodriguez-MagallanA 2010 Parasites in Mexican patients with irritable bowel syndrome: a case-control study. Parasit Vectors 3 96
19. SurangsriratSThamrongwittawatpongLPiyaniranWNaaglorTKhoprasertC 2010 Assessment of the association between Blastocystis infection and irritable bowel syndrome. J Med Assoc Thai 93 Suppl 6 S119 S124
20. TungtrongchitrAManatsathitSKositchaiwatCOngrotchanakunJMunkongN 2004 Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J Trop Med Public Health 35 705 710
21. PicheTBarbaraGAubertPBruley des VarannesSDaineseR 2009 Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58 196 201
22. ChadwickVSChenWShuDPaulusBBethwaiteP 2002 Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 122 1778 1783
23. BuenoLFioramontiJ 2008 Protease-activated receptor 2 and gut permeability: a review. Neurogastroenterol Motil 20 580 587
24. SteckNMuellerKSchemannMHallerD 2011 Bacterial proteases in IBD and IBS. Gut E-pub ahead of print 7 September 2011. doi:10.1136/gutjnl-2011-300775
25. GecseKRokaRFerrierLLevequeMEutameneH 2008 Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57 591 599
26. DenoeudFRousselMNoelBWawrzyniakIDa SilvaC 2011 Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biol 12 R29
27. LeeBJBakYT 2011 Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil 17 252 266
28. RhoadsMLFettererRHHillDEUrbanJFJr 2000 Trichuris suis: a secretory chymotrypsin/elastase inhibitor with potential as an immunomodulator. Exp Parasitol 95 36 44
29. SchwarzerDMarahielMA 2001 Multimodular biocatalysts for natural product assembly. Naturwissenschaften 88 93 101
30. CoyleCMVarugheseJWeissLMTanowitzHB 2012 Blastocystis: to treat or not to treat. Clin Infect Dis 54 105 110
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



