-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTranscription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
The F region downstream of the mecI gene in the SCCmec element in hospital-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) contains two bidirectionally overlapping open reading frames (ORFs), the fudoh ORF and the psm-mec ORF. The psm-mec ORF encodes a cytolysin, phenol-soluble modulin (PSM)-mec. Transformation of the F region into the Newman strain, which is a methicillin-sensitive S. aureus (MSSA) strain, or into the MW2 (USA400) and FRP3757 (USA300) strains, which are community-acquired MRSA (CA-MRSA) strains that lack the F region, attenuated their virulence in a mouse systemic infection model. Introducing the F region to these strains suppressed colony-spreading activity and PSMα production, and promoted biofilm formation. By producing mutations into the psm-mec ORF, we revealed that (i) both the transcription and translation products of the psm-mec ORF suppressed colony-spreading activity and promoted biofilm formation; and (ii) the transcription product of the psm-mec ORF, but not its translation product, decreased PSMα production. These findings suggest that both the psm-mec transcript, acting as a regulatory RNA, and the PSM-mec protein encoded by the gene on the mobile genetic element SCCmec regulate the virulence of Staphylococcus aureus.
Published in the journal: . PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1001267
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001267Summary
The F region downstream of the mecI gene in the SCCmec element in hospital-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) contains two bidirectionally overlapping open reading frames (ORFs), the fudoh ORF and the psm-mec ORF. The psm-mec ORF encodes a cytolysin, phenol-soluble modulin (PSM)-mec. Transformation of the F region into the Newman strain, which is a methicillin-sensitive S. aureus (MSSA) strain, or into the MW2 (USA400) and FRP3757 (USA300) strains, which are community-acquired MRSA (CA-MRSA) strains that lack the F region, attenuated their virulence in a mouse systemic infection model. Introducing the F region to these strains suppressed colony-spreading activity and PSMα production, and promoted biofilm formation. By producing mutations into the psm-mec ORF, we revealed that (i) both the transcription and translation products of the psm-mec ORF suppressed colony-spreading activity and promoted biofilm formation; and (ii) the transcription product of the psm-mec ORF, but not its translation product, decreased PSMα production. These findings suggest that both the psm-mec transcript, acting as a regulatory RNA, and the PSM-mec protein encoded by the gene on the mobile genetic element SCCmec regulate the virulence of Staphylococcus aureus.
Introduction
Staphylococcus aureus is a pathogenic bacterium that causes various diseases in humans. The emergence of methicillin resistant S. aureus (MRSA), vancomycin resistant S. aureus, and community acquired MRSA (CA-MRSA) is a serious clinical problem [1], [2], [3], [4]. These MRSA species are thought to have evolved by acquiring mobile genetic elements that carry antibiotic resistance genes or virulence genes [5]. CA-MRSA is more virulent than hospital-associated MRSA (HA-MRSA) [4], [6], which is isolated from hospitalized patients or patients having risk factors for HA-MRSA, such as catheter use, recent surgery, drug use, etc. [7], [8]. The different virulence phenotypes of these two MRSAs is suggested to be due to Panton-Valentine leukocidin (PVL) [9], [10], which is encoded on lysogenized bacteriophages, a mobile genetic element [11], or phenol-soluble modulin α (PSMα), which is encoded in the core genome [12]. The psmα operon exists in all S. aureus genomes sequenced to date and encodes PSMα1, α2, α3, and α4, with lytic activity against neutrophils [12]. The expression of psmα is elevated in most prevalent CA-MRSA strains, including LAC (USA300) and MW2 (USA400) [12]. The psmα-deleted mutant of CA-MRSA strains exhibits attenuated virulence in a mouse systemic infection model and a mouse skin infection model [12]. The molecular mechanisms that cause the elevated expression of PSMα protein in CA-MRSA strains, however, are not known.
We previously reported that S. aureus possesses the ability to spread on soft agar plates, a process we called “colony spreading” [13], which requires cell wall teichoic acids [13] and a cell envelope-associated protein, MsrR [14]. HA-MRSA strains have low colony-spreading ability, whereas CA-MRSA strains have high colony-spreading ability [15]. The structure of SCCmec, a mobile genetic element that confers methicillin resistance to MRSA strains, is different between HA-MRSA and CA-MRSA strains [16]. In a type-II SCCmec element, we identified a genomic region that explains the difference in the colony-spreading ability between CA-MRSA and HA-MRSA, locating downstream of the mecI ORF (+285 to +859 from the translational initiation site of mecI) [15]. We call this region the “F region” in the present study. The F region exists in type-II and type-III SCCmec, which are found on most HA-MRSA, but not in the type-IV SCCmec, which is found in most CA-MRSA [15]. Introduction of the F region into Newman, a methicillin-sensitive S. aureus (MSSA) strain, suppresses the colony spreading and attenuates virulence in a mouse systemic infection model [15]. The 575-bp F region contains an open reading frame (ORF) that putatively encodes 70 amino acids, which we named fudoh [15]. Recently, Queck et al. reported that the psm-mec ORF, which encodes a cytolytic peptide, exists on the opposite strand of the fudoh ORF [17].
In the present study, we aimed to clarify the molecular mechanism of the F region by which S. aureus virulence is decreased and found that the F region suppresses PSMα production and promotes biofilm formation. Thus, we hypothesized that the absence of the F region in CA-MRSA strains is a reason for the elevated PSMα protein production. Furthermore, to examine which ORFs in the F region are responsible for the S. aureus phenotype, we introduced stop codon mutations into the fudoh and psm-mec ORFs and found that both the transcription and translation products of the psm-mec ORF contribute to negatively regulate staphylococcal virulence, whereas the fudoh ORF does not contribute to suppress colony spreading and virulence.
Results
The fudoh ORF is not necessary to suppress colony spreading
We previously proposed that the fudoh ORF, located downstream of the mecI gene in the type-II SCCmec region, suppresses S. aureus colony spreading [15]. The existence of the psm-mec ORF (69 bp) in the opposite strand of the fudoh ORF (210 bp) was recently reported (Fig. 1A) [17]. The 575-bp F region contains these two ORFs on the opposite strands. We constructed base substitution mutations of the F region to clarify whether the fudoh ORF or psm-mec ORF inhibits colony-spreading activity.
Fig. 1. Analysis of nucleotide substitutions in the F region. 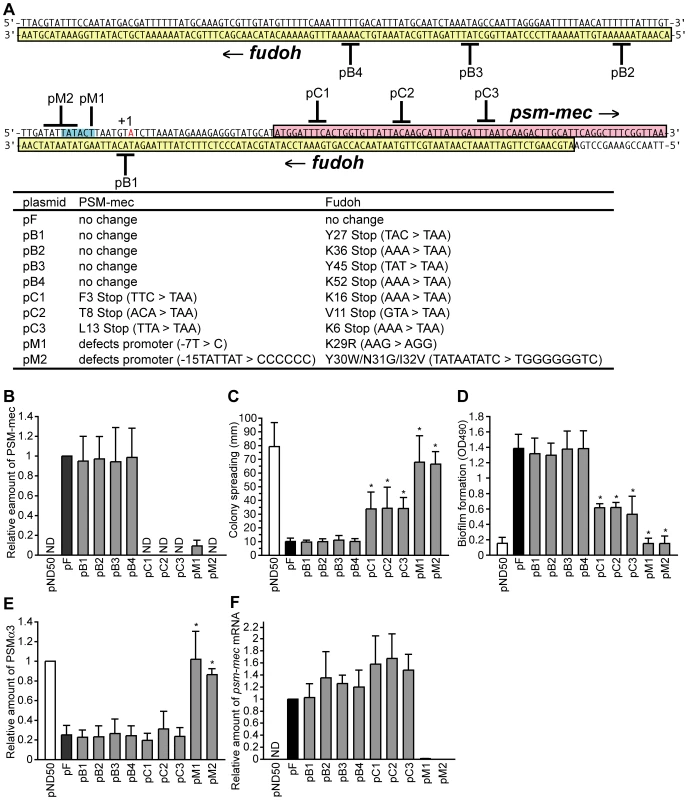
(A) Nucleotide sequences of the psm-mec ORF and fudoh ORF are shown. The psm-mec ORF (magenta-colored) is encoded from left to right, whereas the fudoh ORF (yellow-colored) is encoded from right to left. Black bold lines indicate the substituted nucleotides. Red-colored nucleotide indicates the transcription start site (+1) of the psm-mec ORF, which was determined in this study (Fig. S3). Blue-colored nucleotides are a putative −10 region for transcription of the psm-mec ORF. Table shows the amino acid substitutions caused by the nucleotide substitutions. For pM1 and pM2, the nucleotide substitutions are presented, numbered from transcription start site. (B) The PSM-mec productions of Newman strains transformed with various plasmids harboring nucleotide substitutions in the psm-mec ORF and fudoh ORF were examined by HPLC. The data are presented as the means ± standard deviations from at least three independent experiments. ND, not detected. (C) The colony-spreading abilities of Newman strains transformed with various plasmids harboring nucleotide substitutions in the psm-mec ORF and fudoh ORF were examined. Plates were incubated for 8 h at 37°C and the means ± standard deviations of the halo diameters from at least three independent experiments are shown. The asterisk indicates a p-value of less than 0.05, calculated by Student's t-test, between the sample and the pF-transformed Newman strain. (D) Biofilm formations on polystyrene microplates of Newman strains transformed with various plasmids harboring nucleotide substitutions in the psm-mec ORF and fudoh ORF were examined. The asterisk indicates a p-value of less than 0.05, calculated with the Student's t-test, between the sample and the pF-transformed Newman strain. (E) The PSMα3 production of Newman strains transformed with various plasmids harboring nucleotide substitutions in the psm-mec ORF and fudoh ORF was examined by HPLC. The data shown represent the means ± standard deviations from at least three independent experiments. The asterisk indicates a p-value of less than 0.05, calculated with Student's t-test, between the sample and the pF-transformed Newman strain. (F) The amounts of the psm-mec mRNA in Newman strains transformed with various plasmids harboring nucleotide substitutions in the psm-mec ORF and fudoh ORF were measured by quantitative reverse transcription-PCR. The data are presented as the means ± standard deviations from at least three independent experiments. ND, not detected. pB1, pB2, pB3, and pB4 contain stop codons in the fudoh ORF that do not alter the amino acid sequence of the translation product of the psm-mec ORF (Fig. 1A). pC1, pC2, and pC3, however, contain stop codons in both the psm-mec ORF and fudoh ORF (Fig. 1A). We measured the amount of the psm-mec ORF translation product in the bacterial strains transformed with these plasmids by an established method using HPLC [17]. Newman strains transformed with pB1, pB2, pB3, and pB4, which harbor the same psm-mec coding sequence as pF, produced amounts of PSM-mec comparable to those in the pF-transformed Newman strain (Fig. 1B). On the other hand, Newman strains transformed with pC1, pC2, and pC3 did not produce PSM-mec (Fig. 1B).
Newman strains transformed with pC1, pC2, and pC3 showed more colony spreading than the pF-transformed Newman strain, although their colony spreading was much less than that of the vector (pND50)-transformed Newman strain (Fig. 1C). On the other hand, the colony spreading of Newman strains transformed with pB1, pB2, pB3, and pB4, which contain stop codons in the fudoh ORF, were suppressed to levels similar to those of the pF-transformed Newman strain (Fig. 1C). Thus, interruption of translation of the fudoh ORF by stop codons did not affect the inhibition of colony spreading (pB1, pB2, pB3, pB4), whereas interruption of the translation of the psm-mec ORF by stop codons attenuated the inhibition of colony spreading (pC1, pC2, pC3). These results suggest that colony spreading is not inhibited by the translation product of the fudoh ORF, but rather by the PSM-mec protein, the translation product of the psm-mec ORF. There were also no differences between pF-transformed Newman and pB1, pB2, pB3, and pB4-transformed Newman strains in the F-dependent phenotypes that were newly discovered and are explained later in this manuscript (Fig. 1D, E). Moreover, we did not detect transcripts and translation products of the fudoh ORF in pF-transformed Newman strain (data not shown), indicating that the fudoh ORF is not functional. Interruption of the translation of the psm-mec ORF by stop codons (pC1, pC2, pC3) did not completely abolish the inhibition of colony spreading, indicating that factors other than the translated product of the psm-mec ORF contributed to inhibit colony spreading.
Introduction of the F region into MSSA and CA-MRSA strains decreased their mouse-killing ability, colony-spreading ability, and the production of extracellular PSMs, but promoted biofilm formation
To understand the effect of the F region against S. aureus virulence, we examined the phenotypes of S. aureus strains that were transformed with the F region. In a mouse systemic infection model, mice injected with the F region-introduced MW2 (USA400) strain or the F region-introduced FRP3757 (USA300) strain, which are CA-MRSA strains, survived longer than mice injected with empty vector-introduced parent strains (Fig. 2A, B). Therefore, the F region decreased the virulence of the MW2 and FRP3757 strains in mice. We then examined whether introducing the F region into CA-MRSA strains decreases colony-spreading ability. Both the F region-introduced MW2 and F region-introduced FRP3757 strains showed decreased colony-spreading ability compared with the empty vector-introduced parent strains (Fig. 2C, D). These results suggest that absence of the F region underlies the virulence of CA-MRSA strains in causing systemic diseases as well as colony spreading ability.
Fig. 2. Introduction of the F region into CA-MRSA strains attenuates virulence in a mouse systemic infection model and decreases colony-spreading ability. 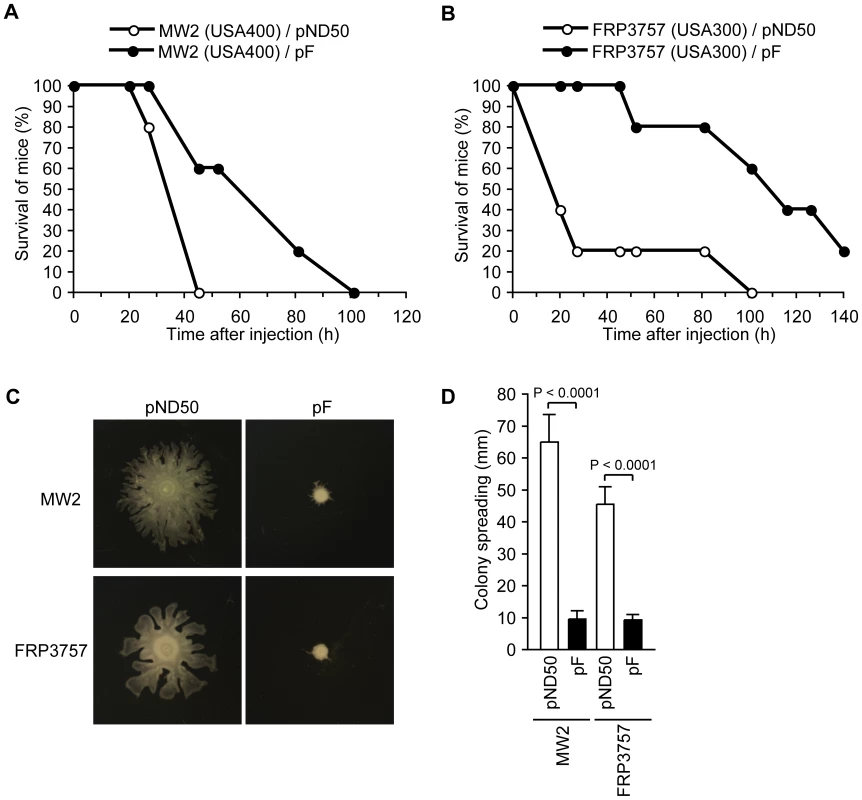
(A) CD-1 mice (n = 5) were intravenously injected with MW2 transformed with pND50 or pF (2×108 CFU) and survival was monitored. Statistical analysis was performed with the Kaplan-Meier test. The P-value between pND50 and pF is 0.0411. (B) CD-1 mice (n = 5) were intravenously injected with FRP3757 transformed with pND50 or pF (2×108 CFU) and survival was monitored. Statistical analysis was performed with the Kaplan-Meier test. The P-value between pND50 and pF is 0.0142. (C) Overnight cultures of MW2 harboring pND50 or pF and FRP3757 harboring pND50 or pF were spotted onto soft agar plates and incubated for 8 h at 37°C. (D) The means ± standard deviations of the halo diameters of at least three independent experiments are presented. S. aureus produces various extracellular proteins that affect its virulence [18], [19]. To understand the molecular mechanism underlying the effect of the F region to suppress mouse systemic infection and colony spreading, we tested our hypothesis that introducing the F region affects the expression of extracellular proteins. Analysis of exoproteins of F region-introduced Newman, an MSSA strain, by sodium-dodecyl sulfate polyacrylamide gel electrophoresis revealed an increase in the amount of a 90-kDa protein, whereas the amount of a protein that migrated faster than the tracking dye was decreased compared with the empty vector-introduced Newman strain (Fig. 3A). Liquid chromatography-tandem mass spectrometry experiments identified the 90-kDa protein as fibronectin binding protein A (FnbA) and the lower molecular-weight protein as a cytolytic peptide, PSMβ1 [12] (Table S1). MSSA produces PSM subtypes α1, α2, α3, α4, β1, β2, and Hld, which are all small hydrophobic polypeptides [12]. We examined whether introducing the F region into the Newman strain affected the amount of these PSMs by high performance liquid chromatography (HPLC) analysis of the culture supernatants [12], [20]. Not only the amount of PSMβ1, but also the amounts of α1+Hld, α2, α3, and α4 were decreased in the culture supernatants of the F region-introduced Newman strain (Fig. 3B, C). To determine whether the decrease in the amount of PSMαs and the increase in FnbA was due to the altered amount of mRNA, we measured the amount of the transcript of the psmα operon and the fnbA gene in the F region-introduced Newman strain by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis. In the F region-introduced Newman strain, the amounts of psmα1-2 and psmα3-4 mRNA were decreased to much less than that in the empty vector-introduced Newman strain (Fig. 3D, E), indicating that the decrease in PSMαs induced by introducing the F region was caused by a decrease in the amount of psmα mRNA. The amount of fnbA mRNA was increased in the F region-introduced Newman strain (Fig. 3E), indicating that the increase in extracellular FnbA is caused by an increase in the amount of fnbA mRNA. To examine whether decreased expression of the psmα mRNA in the F region-introduced Newman strain was caused by decreased promoter activity, we measured the psmα promoter activity. The psmα promoter activity was decreased in the F region-introduced Newman strain (Fig. 3F), indicating that the F region decreased transcriptional initiation of the psmα operon. To examine whether the F-region affects the expression of other virulence genes, we also measured the amount of transcripts of the hla gene encoding α-hemolysin; RNAIII, which is a regulatory RNA transcribed from the agr locus and globally regulates virulence gene expression [21]; agrA, a response regulator that positively regulates psmα expression [22]; and sarS, a transcription factor for virulence genes [23], [24], [25]. Although hla, agrA, and sarS expression was not altered, the amount of RNAIII was decreased in the F region-introduced Newman strain compared with the empty vector-introduced Newman strain (Fig. 3E), indicating that the F-region has an inhibitory effect on the RNAIII regulation of virulence genes.
Fig. 3. Introduction of the F region decreases the amount of extracellular PSMs and increases the amount of extracellular FnbA. 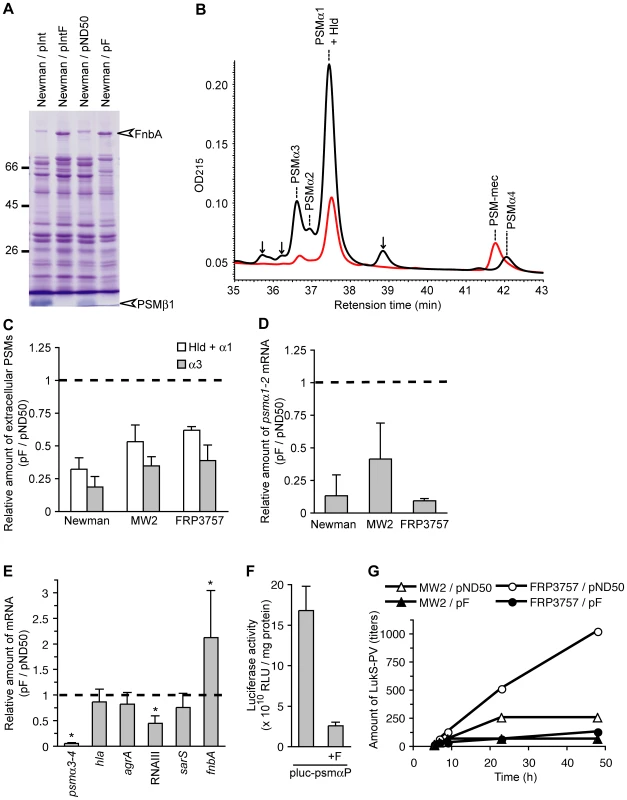
(A) The Newman strain was transformed with an integration plasmid pInt, pInt harboring the F region (pIntF), a multicopy plasmid pND50, or pND50 harboring the F region (pF). Extracellular proteins at the stationary phase were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue. The white arrowhead indicates the excised band for LC-tandem MS analysis and was identified as FnbA and PSMβ1 (Table S1). (B) Measurement of the amount of extracellular PSMs by HPLC. Overnight cultures of the Newman strain harboring pND50 (black line) or pF (red line) were subjected to HPLC and absorbance at 215 nm was obtained. Respective PSMs were identified by LC/MS (Fig. S2). Hld and PSMα1 were contained in the same peak in this assay condition. Arrows indicate unidentified molecules. (C) Amount of PSMs in the pF-transformed strain relative to that in the pND50-transformed strain in Newman, FRP3757, and MW2 genetic backgrounds is presented. (D) Expression of the psmα1-2 mRNA was measured by quantitative reverse transcription-PCR (qRT-PCR) in Newman, FRP3757, and MW2 strains. Amount of the psmα1-2 mRNA in the pF-transformed strains relative to that in the pND50-transformed strains is presented. (E) Expression of the psmα3-4, hla, agrA, RNAIII, sarS, and fnbA were measured by qRT-PCR in pF-transformed and pND50-transformed Newman strains. The asterisks indicate a p-value of less than 0.05, calculated with Student's t test, between pND50- and the pF-transformed Newman strains. (F) Promoter activity of the psmα operon was measured by a luciferase-based reporter assay in the Newman strain. The Newman strain was transformed with pluc-psmαP or pluc-psmαP-F. The means ± standard deviations of three independent experiments are presented. (G) Amounts of LukS-PV during growth in brain heart infusion (BHI) medium were measured. Cells were cultured in 10 ml BHI-medium using an Advantec TN2612 photorecorder. Aliquots of the culture were centrifuged at 3000 rpm for 20 min, and amounts of PVL in the culture supernatant were estimated using anti-LukS-PV monoclonal antibody-coated latex particles, developed by Denka Seiken, Co. Ltd, Niigata, Japan [51]. Representative data from three experiments are shown. Furthermore, we examined whether introducing the F region into CA-MRSA strains that lack the F region decreased PSMα production, as in case of the Newman strain. The F region-introduced MW2 (USA400) and F region-introduced FRP3757 (USA300) strains produced less PSMαs in the culture supernatant and the amount of psmα1-2 mRNA was less than that in empty vector-introduced parent strains (Fig. 3C, D). In addition, we examined whether the production of PVL, a cytolytic toxin composed of LukS-PV and LukF-PV that is one of the virulent determinants of the CA-MRSA strains, is influenced by the presence of the F-region. FRP3757 and MW2 strains carrying pF produced smaller amount of LukS-PV than those carrying an empty vector (Fig. 3G). Thus, the absence of the F region underlies the increased expression of PSMαs and PVL in CA-MRSA strains.
To determine whether the decreased amount of extracellular PSMs in the F region-introduced Newman and CA-MRSA strains contributed to the decreased colony-spreading ability, we examined colony-spreading of null mutants for the psmα operon encoding PSMα1, PSMα2, PSMα3, and PSMα4, and for the psmβ operon encoding PSMβ1 and PSMβ2. The psmα-deleted mutant did not show colony-spreading activity (Fig. 4A, B). Moreover, introduction of the plasmid harboring the psmα operon restored colony-spreading ability of the psmα-deleted mutant (Fig. 4C). These results indicate that the psmα operon is required for S. aureus colony spreading. Thus, the decreased expression of the psmα operon is at least one reason for the decreased colony-spreading ability of the F region-introduced Newman or CA-MRSA strains. In contrast, the psmβ-deleted mutant showed colony-spreading activity similar to that of the parent strain (Fig. 4A, B). Thus, a decrease in the amount of PSMβ1 did not contribute to decrease the colony-spreading ability.
Fig. 4. The psmα operon is required for colony spreading. 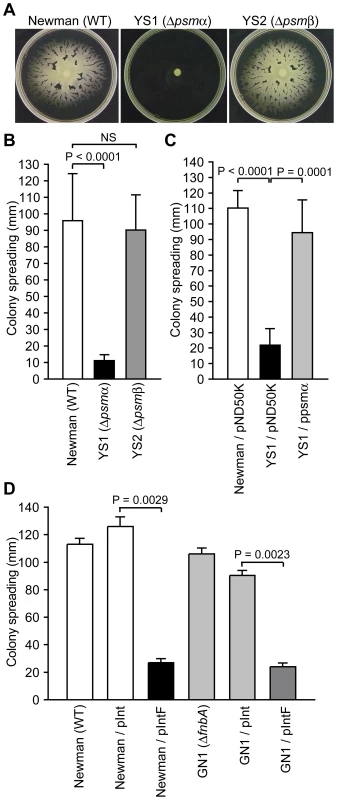
(A) Overnight cultures of Newman, YS1 (Δpsmα), and YS2 (Δpsmβ) were spotted onto soft agar plates and incubated for 10 h at 37°C. (B) The means ± standard deviations of the halo diameters of at least three independent experiments are presented. (C) YS1 was transformed with pND50K or ppsmα. Overnight cultures were spotted onto soft agar plates and incubated for 10 h at 37°C. The halo diameters are presented. (D) The colony-spreading ability of Newman, the fnbA-disrupted mutant (GN1), and the F region introduced GN1 was examined. The halo diameters are presented. Next, to determine whether the increase in the amount of extracellular FnbA in the F region-introduced Newman strain decreased colony-spreading ability, we constructed an fnbA-disrupted mutant. The fnbA-disrupted mutant exhibited colony-spreading activity similar to that of the parent strain (Fig. 4D). In addition, introduction of the F region into the fnbA-disrupted mutant decreased the colony-spreading ability to a level similar to that in the parent strain (Fig. 4D). Therefore, the increased amount of extracellular FnbA caused by introduction of the F region did not contribute to decrease colony-spreading ability.
Our previous observation that water in soft agar plates stimulates colony spreading of S. aureus and that a gene responsible for synthesizing cell wall teichoic acids is required for colony-spreading suggests that the interaction between the cell surface and the soft agar surface is important for colony spreading [13]. Our findings that introduction of the F region into Newman and CA-MRSA strains suppressed colony-spreading and altered the expression of extracellular proteins suggest that the presence of the F region affects the extracellular environment and cell surface structure. The extracellular environment and cell surface structure affects biofilm formation, which is an important phenotype for bacterial pathogenicity [26], [27], [28]. S. aureus forms a biofilm on polypropylene medical devices [29]. Introduction of the F region into the Newman, MW2, and FRP3757 strains promoted bacterial adherence on the internal surfaces of polypropylene tubes (Fig. 5A). We also examined the effect of the F region on S. aureus biofilm formation using polystyrene microplates. Introduction of the F region into the Newman and FRP3757 strains promoted biofilm formation on polystyrene, whereas the F region-introduced MW2 strain did not show increased biofilm formation (Fig. 5B, C). Thus, the F region-promoted S. aureus biofilm formation on polystyrene is dependent on the genetic background.
Fig. 5. Introduction of the F region promotes biofilm formation. 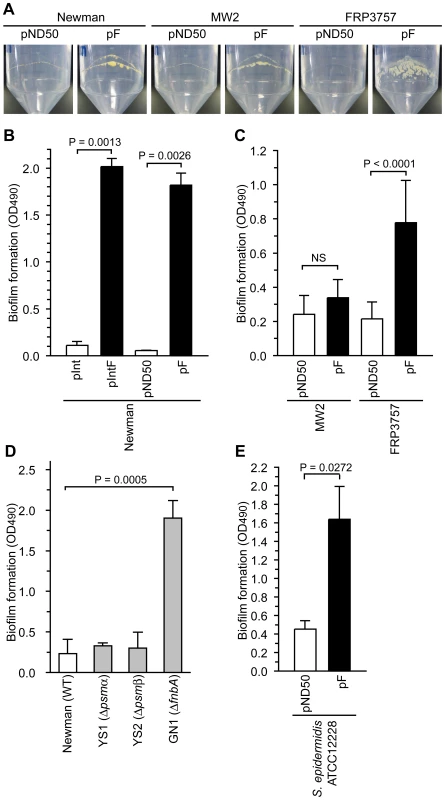
(A) Newman harboring pND50 or pF, MW2 harboring pND50 or pF, and FRP3757 harboring pND50 or pF were cultured in a 50-ml polypropylene tube for 3 days. After removing bacterial cultures, the bacterial adherence to the inner surface of the tubes was observed. (B) The Newman strain was transformed with an integration plasmid pInt, pInt harboring the F region (pIntF), a multicopy plasmid pND50, or pND50 harboring the F region (pF). The bacterial strains were cultured in polystyrene microplates and the bacterial cells that adhered to the plates were stained with safranin. The OD490 was measured. (C) Biofilm formation of MW2 harboring pND50 or pF and FRP3757 harboring pND50 and pF onto polystyrene microplates was examined. (D) Biofilm formation of Newman strain, the psmα-deleted mutant (YS1), the psmβ-deleted mutant (YS2), and the fnbA-disrupted mutant (GN1) onto polystyrene microplates was measured. (E) Biofilm formation of S. epidermidis ATCC12228 harboring pND50 or pF onto polystyrene microplates was measured. To determine whether the decrease in the amount of PSMαs and PSMβ1, or the increase in the amount of FnbA in the F region-introduced Newman strain promotes biofilm formation, we examined biofilm formation of psmα, psmβ, and fnbA mutants. Both the psmα-deletion mutant and the psmβ-deletion mutant formed low levels of biofilm that were indistinguishable from that of the parent strain (Fig. 5D). Therefore, the increased biofilm formation by the F region-introduced Newman strain was not due to the decrease in PSMαs and PSMβ1. On the other hand, the fnbA-disrupted mutant had higher biofilm formation than the parent strain (Fig. 5D). Thus, the fnbA gene repressed biofilm formation in the Newman strain. This means that the increased biofilm formation in the F region-introduced Newman strain was not due to the increased amount of extracellular FnbA. These results suggest that biofilm formation promoted by the F region was caused by mechanisms other than the expression of psmα, psmβ, and fnbA. The Newman strain has a truncated fnbA gene and secretes FnbA, which is a rare phenotype among S. aureus strains [30]. The negative effect of fnbA on biofilm formation might be due to truncation of the fnbA gene in the Newman strain.
PSM-mec, a translation product of the psm-mec ORF encoded in the F region, contributes to promote biofilm formation, but not to inhibit PSMα production
We constructed various types of domain deletions of the F region (Fig. 6A) and base substitution mutations of the F region (Fig. 1A) to clarify whether the translation product of psm-mec ORF inhibits PSMα production, and stimulates the biofilm formation caused by introduction of the F region.
Fig. 6. Analysis of domain deletions of the F region. 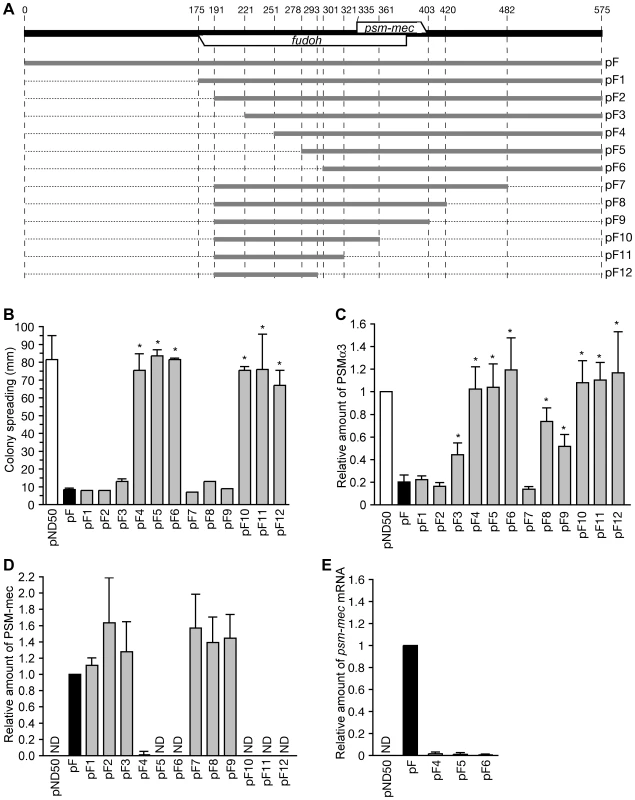
(A) The 575-bp F region is indicated by a bold black line. The fudoh ORF exists at the opposite strand of the psm-mec ORF. Domain deletions of the F region indicated by bold grey lines were cloned into plasmids. The names of the plasmids are shown on the right side. (B) The colony-spreading abilities of Newman strains transformed with various plasmids harboring domain deletions of the F region were examined. Plates were incubated for 8 h at 37°C and the means ± standard deviations of the halo diameters from at least three independent experiments are shown. The asterisk indicates a p-value of less than 0.05, calculated with Student's t-test, between the sample and the pF-transformed Newman strain. (C) The PSMα3 productions of Newman strains transformed with various plasmids harboring domain deletions of the F region were examined by HPLC. The data were the means ± standard deviations from at least three independent experiments. The asterisks indicate a p-value of less than 0.05, calculated with Student's t test, between the sample and the pF-transformed Newman strain. (D) PSM-mec production of the Newman strains transformed with various plasmids harboring domain deletions of the F region were examined by HPLC. The data were the means ± standard deviations from at least three independent experiments. ND, not detected. (E) The amounts of the psm-mec mRNA in Newman strains transformed with pF4, pF5, and pF6 were measured by qRT-PCR. The data are presented as the means ± standard deviations from at least three independent experiments. pF3 with deletion of 0–221 bp and pF9 with deletion of 403–575 bp inhibited colony spreading as well as pF harboring the intact F region (Fig. 6B). In contrast, pF4 with deletion of 0–251 bp and pF10 with deletion of 361–575 bp did not inhibit colony-spreading activity (Fig. 6B). Therefore, the 221–251 bp and 361–403 bp regions are required to inhibit colony spreading. Although pF3 with deletion of 0–221 bp and pF8 with deletion of 420–575 bp inhibited colony-spreading activity to the same extent as pF (Fig. 6B), these plasmids decreased the inhibition of PSMα production (α3, Fig. 6C; Hld + α1, Fig. S1A). In contrast, pF2 with deletion of 0–191 bp and pF7 with deletion of 482–575 bp inhibited PSMα production (α3, Fig. 6C; Hld + α1, Fig. S1A). Therefore, neither the 191–221 bp region nor the 420–482 bp region, which locate outside of the psm-mec ORF, were required to inhibit colony spreading, but contributed to inhibit PSMα production.
Although Newman strains transformed with pF1, pF2, pF3, pF7, pF8, and pF9 produced the same amount of PSM-mec as the Newman strain transformed with pF, Newman strains transformed with pF4, pF5, pF6, pF10, pF11, and pF12 produced little PSM-mec (Fig. 6D). The Newman strains transformed with pF4, pF5, and pF6 (Fig. 6E) contained little psm-mec mRNA, indicating that the 221–251 bp-region (84–114 nt upstream of the translation start of the psm-mec) is important for the transcription of psm-mec. Thus, the colony-spreading inhibition correlated with the amount of PSM-mec. This finding confirms that the PSM-mec protein was involved in the inhibition of colony spreading, as indicated by base substitution experiments (Fig. 1C). In contrast, the inhibition of PSMα production did not correlate with the amount of PSM-mec in strains transformed with pF3, pF8, and pF9, indicating that factors other than PSM-mec inhibited PSMα production.
We also examined stop codon mutations into the psm-mec ORF of the F region (Fig. 1A, pC1; pC2; pC3) and attempted to determine the factor in the F region responsible for inhibiting PSMα production, and for stimulating biofilm formation. Newman strains transformed with pC1, pC2, and pC3 showed decreased PSMα production, similar to pF-transformed Newman (α3, Fig. 1E; Hld+α1, Fig. S1B). This result suggests that the factor that inhibits PSMα production is not the translation product of the psm-mec ORF.
Newman strains transformed with pC1, pC2, and pC3 formed less biofilm than the pF-transformed Newman, although much more biofilm was formed than in the empty vector-transformed Newman strain (Fig. 1D). This result suggests that the translation product of the psm-mec ORF, PSM-mec, promoted biofilm formation and that factors other than the translation product of the psm-mec ORF also contributed to stimulate biofilm formation.
Transcription product of the psm-mec ORF inhibits PSMα production as a regulatory RNA molecule
We previously demonstrated that pM1 harboring the base displacement of −33T with C from the translation start of the psm-mec ORF lost inhibition of colony spreading [15] (Fig. 1A and 1C). The mutated nucleotide locates outside of the psm-mec ORF. We examined the possibility that the nucleotide substitution affects the expression of the psm-mec ORF. The pM1-transformed Newman strain produced little PSM-mec compared with the pF-transformed Newman strain (Fig. 1B). Moreover, the amount of psm-mec mRNA was considerably lower in the pM1-transformed Newman compared with the pF-transformed Newman strain (Fig. 1F). Therefore, the loss of the colony spreading inhibitory activity in pM1 was due to the inhibition of transcription of the psm-mec ORF by the mutation. The pM1-transformed Newman strain showed completely restored colony-spreading ability, in contrast to the Newman strain transformed with pC1, pC2, or pC3 (Fig. 1C). This finding led us to hypothesize that not only the translation product but also the transcription product of the psm-mec ORF contributed to inhibit colony spreading.
We then examined whether the psm-mec mRNA acts as a regulatory RNA to inhibit colony spreading, PSMα production, and stimulate biofilm formation. By performing a primer extension analysis (Fig. S3), we determined the transcription start site of messenger RNA encoding the psm-mec ORF, indicated by red letters in Fig. 1A. In addition to pM1, we constructed pM2 harboring 6 nucleotide substitutions from −15 to −10 of the transcription start site (Fig. 1A). The psm-mec mRNA and PSM-mec protein were not detected in the pM2-transformed Newman strain (Fig. 1B, F), indicating that the psm-mec ORF promoter was disrupted in pM2. The Newman strain transformed with pM1 or pM2 did not exhibit decreased colony spreading ability, whereas the pF-transformed Newman did (Fig. 1C). Moreover, the pM1 - or pM2-transformed Newman strain did not exhibit decreased PSMα production (α3, Fig. 1E; Hld+α1, Fig. S1B) and did not show enhanced biofilm formation (Fig. 1D). Newman strain transformed with pC1, pC2, or pC3 showed decreased PSMα production, although the Newman strain transformed with pM1 or pM2 did not show decreased PSMα production (Fig. 1E and S1B), indicating that it is not the translation product but the transcription product of the psm-mec ORF that acted as a regulatory RNA molecule contribute to inhibit PSMα production.
Newman strains transformed with pC1, pC2, or pC3 did not completely lose their colony spreading inhibitory activity (Fig. 1C) or biofilm formation ability (Fig. 1D), whereas Newman strains transformed with pM1 and pM2 completely lost these activities. These results suggest that not only the translation product but also the transcription product of the psm-mec ORF, as a regulatory RNA molecule, contribute to inhibit colony spreading and stimulate biofilm formation.
To further examine whether the psm-mec ORF transcript functions as a regulatory RNA molecule, we constructed pFP, which harbors synonymous codon substitutions in the psm-mec ORF (Fig. 7A). The mutated psm-mec ORF harbors 20 nucleotide substitutions within 69 bases of the psm-mec ORF, changing the secondary structure of its mRNA (data not shown), as estimated by the M. Zuker Mfold program (http://mfold.bioinfo.rpi.edu/cgi-bin/rna-form1.cgi) [31]. The pFP-transformed Newman strain produced approximately twice the amount of psm-mec mRNA and nearly the same amount of PSM-mec as the pF-transformed Newman strain (Fig. 7B, C). The pFP-transformed Newman strain showed higher colony-spreading ability (Fig. 7D) and produced more PSMα than the pF-transformed Newman strain (α3, Fig. 7E; Hld+α1, Fig. S1C). Moreover, the pFP-transformed Newman strain formed little biofilm compared with the pF-transformed Newman strain (Fig. 7F). Thus, pFP, which harbors synonymous codon substitutions in the psm-mec ORF, showed decreased inhibition of colony-spreading, PSMα production, and stimulation of biofilm formation. To further address whether the psm-mec ORF transcript contributes to inhibit PSMα production, we placed the psm-mec ORF under an anhydrotetracycline-inducible promoter. Induction of psm-mec transcription with anhydrotetracycline decreased PSMα production, whereas induction of the synonymous codon-substituted psm-mec did not inhibit PSMα production (Fig. 7G). These results also suggest that the psm-mec ORF transcript functioned as a regulatory RNA for these phenomena.
Fig. 7. Analysis of synonymous codon substitutions in the psm-mec ORF. 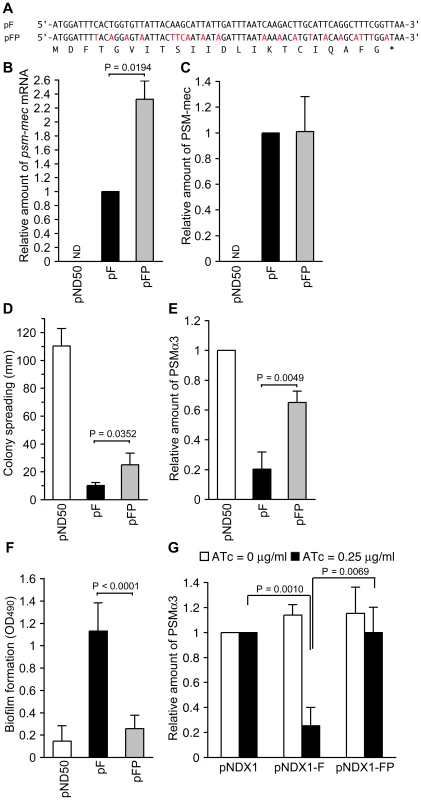
(A) The nucleotide sequence of the psm-mec ORF in pF and the synonymous codon substituted sequence of the psm-mec ORF in pFP are shown. The substituted nucleotides are colored in red. The amino acid sequence of PSM-mec protein is shown below the nucleotide sequence. (B) The amounts of psm-mec mRNA in Newman strains harboring pND50, pF, and pFP were measured. The data are presented as the means ± standard deviations from at least three independent experiments. ND, not detected. (C) The PSM-mec production of Newman strains harboring pND50, pF, or pFP was examined by HPLC. The data are presented as the means ± standard deviations from at least three independent experiments. ND, not detected. (D) The colony-spreading abilities of Newman strains harboring pND50, pF, or pFP were examined. Plates were incubated for 8 h at 37°C and the means ± standard deviations of the halo diameters from at least three independent experiments are shown. (E) The PSMα production of Newman strains harboring pND50, pF, or pFP was examined by HPLC. The data are presented as the means ± standard deviations from at least three independent experiments. (F) Biofilm formation onto polystyrene microplates of Newman strains harboring pND50, pF, or pFP was examined. (G) The PSMα production of Newman strains harboring the psm-mec gene-inducible plasmid (pNDX1-F) was examined. pNDX1-FP harbors the synonymous codon-substituted sequence of the psm-mec ORF in (A). ATc, anhydrotetracycline. Hfq is an RNA chaperone that mediates the interaction between small RNA and mRNA. S. aureus hfq has a global regulatory role for virulence genes in the NCTC8325-4 strain [32] but not in the Newman strain [33]. To verify whether the hfq gene is required for the effect of the psm-mec ORF on colony spreading, PSMα production, and biofilm formation, we constructed an hfq-deleted mutant of Newman and NCTC8325-4 that were transformed with pF. pF inhibited colony spreading and PSMα production, whereas it increased biofilm formation in the hfq-deleted mutant of the Newman strain as well as in the Newman strain (Fig. S4A, B, C). pF also inhibited PSMα1 + Hld production, whereas it increased biofilm formation in the hfq-deleted mutant of NCTC8325-4 as well as in NCTC8325-4 (Fig. S4B, C). These results suggest that the psm-mec ORF inhibits colony spreading and PSMα production, whereas it increases biofilm formation in an hfq-independent manner.
Discussion
In the present study, we found that the translation product as well as the transcription product of the psm-mec ORF in the F region suppresses colony spreading and promotes biofilm formation in S. aureus (Fig. 8A). We also revealed that the transcription product of the psm-mec ORF decrease the production of PSMα, which is core genome-encoded [12], [17] (Fig. 8A). We previously reported that introduction of the F region into the Newman strain decreases its virulence in a mouse systemic infection model [15]. In the present study, introducing the F region into MW2 and FRP3757, which are CA-MRSA strains, also decreased their virulence in a mouse systemic infection model. Thus, the absence of the psm-mec ORF in CA-MRSA strains, a distinguishable feature from HA-MRSA strains harboring type-II SCCmec, restores PSMα production and contributes to the high virulence phenotype (Fig. 8A, B).
Fig. 8. Model of the alteration of S. aureus virulence phenotype exerted by psm-mec mRNA and PSM-mec protein. 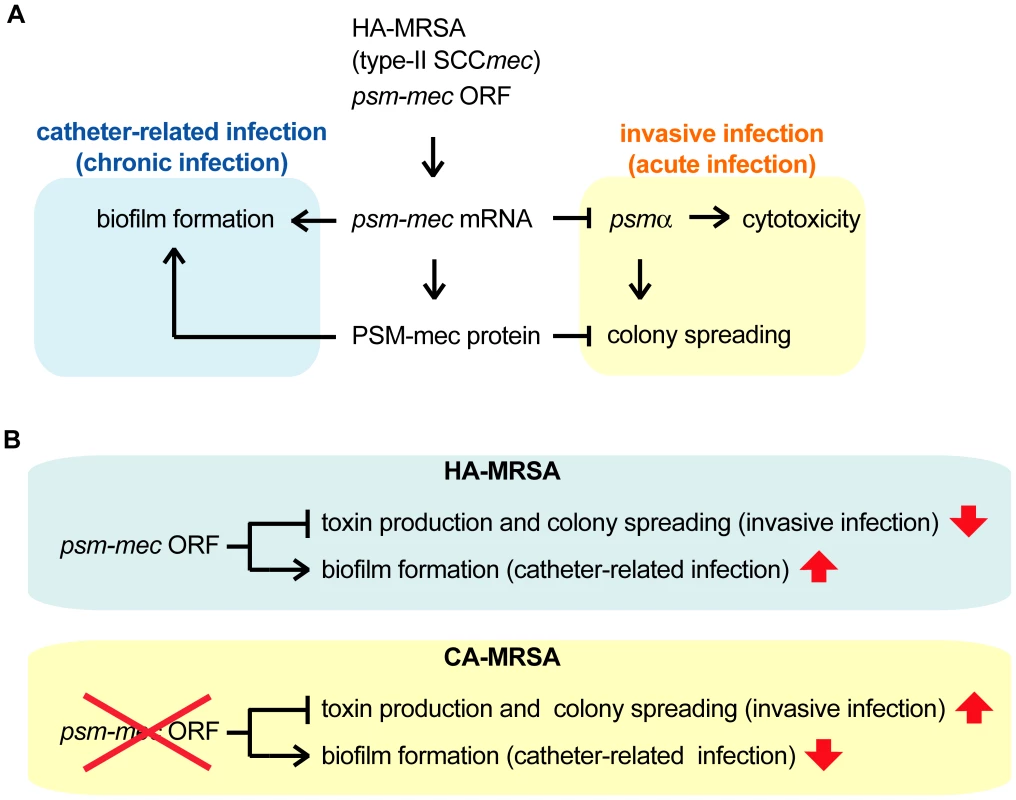
(A) The transcript of the psm-mec ORF inhibits the expression of psmα, contributing to the decreased colony spreading and decreased cytotoxicity. The translation product of the psm-mec ORF inhibits colony spreading, whereas it promotes biofilm formation. These altered phenotypes have decreased S. aureus virulence, which leads to invasive and acute infections, whereas increased S. aureus virulence leads to chronic infection, including catheter-related infections. (B) In CA-MRSA strains, absence of the psm-mec ORF leads to increased virulence, causing invasive infections, whereas decreased virulence causes catheter-related infections. We assume that the attenuated virulence of the F region-transformed S. aureus strains in a mouse systemic infection model is caused by a decrease in both colony spreading and PSMα production. The decreased colony-spreading ability might lead to defective S. aureus dissemination into various organs in the animal body, resulting in attenuated virulence (Fig. 8A). It is not clear, however, whether colony spreading is directly involved in the S. aureus virulence in animals. Further experiments are needed to address this point.
Queck et al. reported that the psm-mec-deleted mutant of the MSA890 strain, in which the production of PSM-mec is higher than other genome-encoded PSMs, showed attenuated virulence in a mouse systemic infection model and decreased cytolytic activity against neutrophils [17]. Whereas the psm-mec-deleted strains of S. aureus, in which PSM-mec production is lower than other genome-encoded PSMs, did not show decreased cytolytic activity against neutrophils [17]. Based on these observations, Queck et al. proposed that PSM-mec has a positive effect on the virulence of S. aureus strains, in which a higher amount of PSM-mec is produced compared to other genome-encoded PSMs [17]. On the other hand, we demonstrated that introduction of the psm-mec ORF into any of the Newman, MW2, and FRP3757 strains has negative effects on virulence in a mouse systemic infection model. According to the proposal by Queck et al., expression of PSM-mec is expected to be much lower than PSMαs in these strains. The difference in the genetic backgrounds of S. aureus, for example mutations in the promoters of psm-mec and psmα, might affect the ratio of the expression levels of PSM-mec and PSMαs. If too much PSM-mec is produced, the positive effect of PSM-mec on virulence may be dominant.
We demonstrated that introducing the psm-mec ORF into Newman, MW2, and FRP3757 strains increases biofilm formation. Biofilm formation by S. aureus is considered to be important for catheter-related infections [26], [34], [35], [36]. Thus, the psm-mec ORF is postulated to have positive effects on catheter-related S. aureus infections, which are associated with biofilm formation (Fig. 8A). Using a psm-mec-deleted mutant of the MSA890 strain, Otto et al. also demonstrated that the psm-mec gene stimulates biofilm formation [17]. Therefore, the psm-mec ORF in HA-MRSA inhibits the virulence properties that lead to invasive infections accompanied by PSMα production and colony spreading, whereas it promotes the virulence properties for chronic infections such as catheter-related infections (Fig. 8A, B). Thus, the psm-mec ORF may regulate the virulence property of S. aureus. The function of the psm-mec ORF may be beneficial for HA-MRSA to establish long-lasting infection in the human body. In contrast, in CA-MRSA strains, the absence of regulation by the psm-mec ORF leads to increased virulence properties that cause invasive and acute infections in humans (Fig. 8B). Our proposed mechanism may explain a number of observations that CA-MRSA causes more severe invasive infections, such as hemorrhagic necrotizing pneumonia, septicemia, and necrotizing fasciitis, than HA-MRSA [37], [38], [39], [40], [41], [42]. In addition, the mutation (−7T>C) in the psm-mec promoter that decreases the amount of psm-mec mRNA is found in 25% of HA-MRSA strains isolated in Japan [15]. These strains might have different virulence properties compared with most Japanese HA-MRSA strains carrying the intact psm-mec promoter. Future studies with psm-mec-deleted mutants should address whether endogenous psm-mec regulates the virulence of HA-MRSA strains or whether HA-MRSA strains have already adapted to the presence of psm-mec. HA-MRSA strains do not necessarily possess the psm-mec ORF. MRSA strains having type-I SCCmec or type-IV SCCmec, which do not carry the psm-mec ORF, have been isolated from hospitals in European countries and Australia [43], [44]. Further studies are needed to determine whether the absence of the psm-mec in these HA-MRSA strains affects the virulence properties.
We propose that both the transcription product and translation product of the psm-mec ORF, which is a cytolytic peptide gene encoded on the mobile genetic element SCCmec, alter the virulence properties of the pathogen. To our knowledge, this is the first report that a transcription product of a toxin gene, acting as a regulatory RNA that is encoded on the mobile genetic element, suppresses the expression of core genome-encoded toxin genes. RNAIII is one of the regulatory RNAs in S. aureus that is encoded in the agr locus and regulates the expression of various virulence genes [21]. RNAIII contains an ORF encoding Hld, a PSM [45]. Thus, both RNAIII and the psm-mec transcript encode PSM. Further studies are needed to determine how the psm-mec transcript exerts its regulatory function as an RNA. The inhibitory effect of psm-mec on PSMα production is probably not due to a direct interaction between psm-mec RNA and psmα mRNA, but rather to an interference of the transcriptional regulatory pathways of the psmα operon, because the promoter activity of the psmα operon was inhibited by introduction of the psm-mec (Fig. 3F). The SCCmec region containing the psm-mec ORF is also found in S. epidermidis [15]. The psm-mec ORF stimulated biofilm formation in S. epidermidis (Fig. 5E). The regulation of virulence properties by both transcript and translation products of the psm-mec ORF may not be specific for S. aureus, but are presumably conserved among pathogens carrying the psm-mec ORF on the mobile genetic element SCCmec.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendation in the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, 2006. All mouse protocols followed the Regulations for Animal Care and Use of the University of Tokyo and were approved by the Animal Use Committee at the Graduate School of Pharmaceutical Science at the University of Tokyo (approval number: 19–28).
Bacterial strains and growth conditions
The JM109 strain of Escherichia coli was used as the host for pND50, pKOR3a, and pSF151, and their derivatives. E. coli strains transformed with the plasmids were cultured in Luria-Bertani broth containing 25 µg/ml chloramphenicol or 50 µg/ml kanamycin. S. aureus strains were aerobically cultured in tryptic soy broth at 37°C in a 50-ml disposable tube (FALCON 352070, Becton, Franklin Lakes, NJ), and 12.5 µg/ml chloramphenicol or 50 µg/ml kanamycin was added to the medium if required. Details of the bacterial strains and plasmids used in the present study are shown in Table 1.
Tab. 1. A list of bacterial strains and plasmids used. 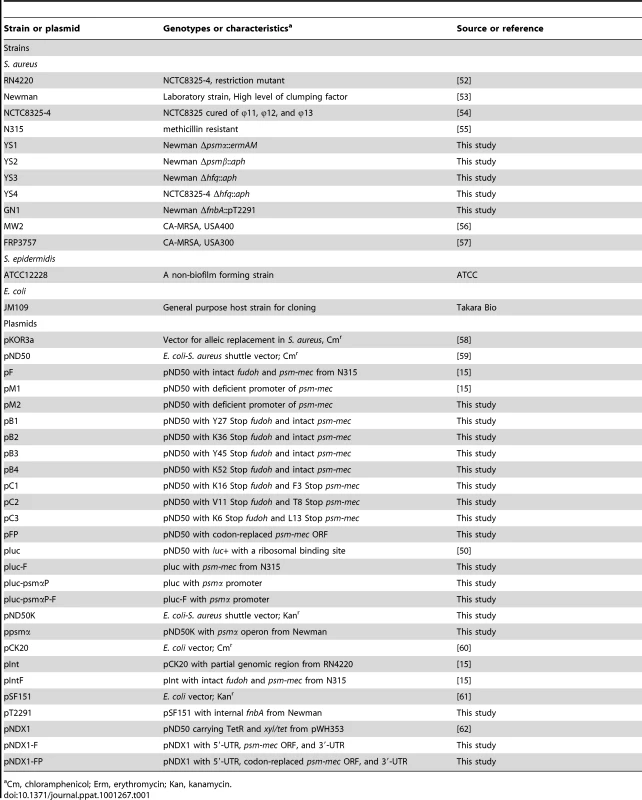
Cm, chloramphenicol; Erm, erythromycin; Kan, kanamycin. Mouse infection experiment
Bacterial overnight cultures were centrifuged and cells were suspended in phosphate buffered saline. The bacterial suspension (100 µl) was injected into the tail vein of 8-week-old female CD-1 mice. Survival after the injection was monitored.
Colony spreading assay
Tryptic soy broth (Becton, Sparks, MD) supplemented with 0.24% agar (Code 01028-14, Nacalai Tesque Inc., Kyoto, Japan) was autoclaved at 121°C for 15 min. Sterile medium (50 ml) was poured into a petri dish (150-mm diameter, FALCON 351058, Becton). The plates were dried for 20 min in a biologic safety cabinet (MHE-130AJ, SANYO, Tokyo, Japan). Bacterial overnight culture (2 µl) was spotted onto the center of the plates and dried for 20 min in a biologic safety cabinet. The plates were covered and incubated at 37°C.
Biofilm formation assay
Four microliters of bacterial overnight culture were inoculated into 1 ml tryptic soy broth containing 0.25% glucose. An aliquot (200 µl) of the sample was poured into each well of a 96-well polystyrene microplate (3860-096, IWAKI, Tokyo, Japan), and incubated for 3 days at 37°C. The cultures in the plate were discarded and the plate was stained with 0.1% safranin solution. The OD490 was measured using a microplate reader (MTP300, CORONA, Ibaraki, Japan). To observe the biofilm formation on polypropylene, bacterial colonies were inoculated into 5 ml of tryptic soy broth and aerobically cultured for 3 days in 50-ml tubes (352070, Becton Dickinson, Franklin Lakes, NJ) at 37°C.
Measurement of PSMs
Overnight bacterial cultures (50 µl) were inoculated into 5 ml fresh tryptic soy broth and aerobically cultured at 37°C for 14 h without antibiotics. The cultures were filtered with a 0.22-µm polyvinylidene difluoride filter (Millipore, Carrigtwohill, Ireland) and the filtrates were used for analysis by reversed phase-HPLC. Chromatography was performed using SOURCE 5RPC ST 4.6/150 column (GE Healthcare, Tokyo, Japan) and a water/acetonitrile gradient in 0.1% trifluoroacetic acid from 0 to 100% acetonitrile in 50 min at a flow rate of 1 ml/min (600E, Waters, Milford, MA). Absorbance at 215 nm was detected using a 2998 Photodiode Array Detector (Waters). The molecular mass in the respective peak was determined using liquid chromatography-electrospray ionization mass spectrometry (LC/ESI-MS; LC 1100 series, Agilent Technologies, Santa Clara, CA; ESI-MS, Bio-TOFQ, Bruker Daltonics, Billerica, MA) and respective PSMs were identified (Fig. S2). Although there was a difference in the retention time between chromatographies in the LC/MS and HPLC systems (around 4 min faster in the LC/ESI-MS system), the pattern of the respective PSMs was similar. Hld and PSMα1 were not separated in both systems.
DNA manipulation
Transformation of E. coli, extraction of plasmid DNA from E. coli, and PCR were performed as previously described [46]. S. aureus genomic DNA was extracted using a QIAamp DNA Blood Kit (Qiagen Sciences, Germantown, MD) and lysostaphin (Takara Bio). Transformation of S. aureus with plasmid DNA was performed by electroporation [47].
Determination of the transcriptional start site of the psm-mec ORF
Oligonucleotide primer 5AA-F was end-labeled with [γ-32P] ATP using T4 polynucleotide kinase. RNA was reverse-transcribed using the labeled primer and Multiscribe Reverse Transcriptase (Roche, Basel, Switzerland). Sequencing ladder samples were obtained by cycle-sequencing reactions using the labeled primer, DNA fragments of the F region, and Thermo sequencing primer cycle sequencing kit (GE Healthcare). The samples were electrophoresed in a denaturing 7.5% polyacrylamide gel containing 6 M urea in 0.5×TBE buffer [45 mM Tris borate (pH8.3), 1 mM Na2EDTA]. The gels were dried and analyzed by phosphoimaging using BAS-1800II (Fujifilm, Tokyo, Japan) and Image Gauge software v. 4.23 (Fujifilm).
Construction of gene-disrupted mutants for the psmα operon, the psmβ operon, the fnbA gene, and the hfq gene
The upstream region of the psmα operon (966 bp) was amplified by PCR using oligonucleotide primers psma-U-F and psma-U-R, and Newman genomic DNA as the template. The downstream region of the psmα operon (979 bp) was amplified by PCR using oligonucleotide primers psma-D-F and psma-D-R, and Newman genomic DNA as the template. The ermAM gene, conferring erythromycin resistance, was amplified by PCR using oligonucleotide primers ErmF and ErmR, and pMutinT3 as the template. These three DNA fragments were spliced together using splicing by overlap extension-PCR, resulting in a psmα-cassette. The psmα-cassette was inserted into the Sma I site of pKOR3a, resulting in pKOR3a-psmα. S. aureus RN4220 was transformed with pKOR3a-psmα. The transformant was cultured in tryptic soy broth and 103 cells were spread onto tryptic soy agar plates containing 12.5 µg/ml chloramphenicol. The plates were incubated at 43°C overnight. The resulting colonies were cultured in tryptic soy broth at 37°C and spread onto tryptic soy agar plates containing 1 µg/ml anhydrotetracycline and 10 µg/ml erythromycin. The resulting colonies were examined for sensitivity to chloramphenicol. The disruption was transferred to the Newman strain by phage 80α, as reported previously [48], resulting in YS1. The deletion of psmα was confirmed by Southern blot analysis (Fig. S5A, B).
To construct the psmβ-deleted mutant, primers of psmb-U-F, psmb-U-R, psmb-D-F, and psmb-D-R were used for to amplify the upstream (1546 bp) and downstream (1727bp) regions of the psmβ operon and primers of KanF and KanR were used to amplify the kanamycin resistance encoding gene, aph, from pSF151. The amplified DNA fragments were spliced together by splicing by overlap extension-PCR, resulting in a psmβ-cassette. Other procedures were the same with as that for the psmα-deleted mutant. Disruption of psmβ in the YS2 strain was confirmed by Southern blot analysis (Fig. S5A, C).
To construct the fnbA-disrupted mutant, the internal region of fnbA was amplified by PCR using oligonucleotide primers fnbA-F and fnbA-R, and Newman genomic DNA as the template. The amplified DNA fragment was inserted into pSF151, resulting in pT2291. S. aureus RN4220 was transformed with pT2291 and kanamycin-resistant transformants were obtained. The disruption was transferred to the Newman strain by phage 80α, resulting in GN1. The disruption of fnbA was confirmed by Southern blot analysis (Fig. S5D, E).
To construct the hfq-deleted mutant, the upstream (886 bp) and downstream (821 bp) regions of the hfq gene and aph gene were amplified by PCR and spliced together by overlap extension-PCR, resulting in an hfq-cassette. Other procedures were the same as that used for the psmα-deleted mutant. Disruption of hfq in the YS3 and YS4 strains was confirmed by Southern blot analysis (Fig. S5F, G).
Construction of plasmids harboring shortened F-regions, point-mutated F-regions, or psm-mec with inducible promoter
To construct plasmids harboring a shortened F-region, we amplified DNA fragments by PCR using the primers listed in Table S2, pF as a template, and KOD-Plus DNA polymerase (TOYOBO, Tokyo, Japan). The amplified DNA fragments were self-ligated, which resulted in the plasmids harboring the shortened F-region. The desired constructs of the plasmids were confirmed by restriction digestion and sequencing. To construct plasmids harboring point-mutated F-regions, we synthesized mutated DNA strands by thermal cycling using primer pairs in Table S2 and pF as a template. The E. coli JM109 strain was transformed with the synthesized DNA strands after treatment with Dpn I [49]. The plasmids were extracted and sequenced to confirm the desired mutation. To construct pFP, we performed three rounds of nested PCR using primer pairs of onlyP-F and onlyP-R, onlyP-F1 and onlyP-R1, or onlyP-F2 and onlyP-R1 (Table S2), and pF as a template. E. coli JM109 strain was transformed with the amplified DNA fragments. The plasmids were extracted and sequenced to confirm the desired mutation. To construct plasmid harboring psm-mec with xyl/tet promoter, 575 bp F-region was cloned into Sma I site of pNDX1 and the upstream region of the transcription start site of psm-mec was removed by PCR using the primers listed in Table S2. The transcription start site of psm-mec from pNDX1-F was confirmed to be same with that from pF by primer extension analyses (data not shown).
Measurement of gene expression by quantitative real-time PCR analysis
RNA was extracted from exponentially growing S. aureus cells (A600 = 1) using an RNeasy Mini Kit (Qiagen, Gaithersburg, MD). RNA was reverse-transcribed to cDNA using Multiscribe Reverse Transcriptase (Roche). Quantitative real-time PCR was performed using cDNA as template and SYBR Premix ExTaq (Takara Bio, Tokyo, Japan) and primers (Table S2). The signals were detected by ABI PRISM 7700 Sequence Detector (Applied Biosystems, Tokyo, Japan). The reaction mixture was incubated at 95°C for 10 s and at 40 cycles (95°C, 5 s; 60°C, 31 s). The data were normalized to 16S rRNA. To determine the amount of the psm-mec mRNA in Fig. 7, we used primer pairs of psm-mecF2 and psm-mecR2, which hybridized with the outside region of psm-mec ORF. The amplification efficiency was not different between pF and pFP.
Reporter assay
Pluc was designed to contain a functional ribosomal binding site and translational start codon of luc after a series of stop codons in all reading frames [50]. DNA fragment containing the F region was inserted into EcoR I and Sac I site of pluc vector, resulting in pluc-F harboring the psm-mec ORF that was transcribed in the opposite direction from the luc ORF. The DNA fragment containing the promoter region of the psmα operon [22] was amplified by PCR and inserted into the Kpn I and Xba I sites of pluc and pluc-F. The staphylococcal strains transformed with pluc, pluc-F, and their derivatives were cultured and harvested at A600 = 1. The cells were lysed in a lysis buffer (25 mM KH2PO4 [pH 7.8], 0.04% Triton X-100, 0.1 mM dithiothreitol, 10 µg/ml of lysostaphin, and protease inhibitor cocktail [Roche, Basel, Switzerland]). The supernatant of the cell lysate was incubated with the luciferase substrate (Roche), and luminescence was measured using a luminometer (Berthold Technologies, Bad WildBad, Germany). The promoter activity was calculated as the luminescence unit per milligram of protein subtracted from the value of the cells transformed with the vector, pluc, or pluc-F.
Supporting Information
Zdroje
1. HiramatsuK
2001 Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis 1 147 155
2. HiramatsuK
CuiL
KurodaM
ItoT
2001 The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol 9 486 493
3. DeLeoFR
ChambersHF
2009 Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest 119 2464 2474
4. DeleoFR
OttoM
KreiswirthBN
ChambersHF
2010 Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375 1557 68
5. LindsayJA
2010 Genomic variation and evolution of Staphylococcus aureus. Int J Med Microbiol 300 98 103
6. GravesSF
KobayashiSD
DeLeoFR
2010 Community-associated methicillin-resistant Staphylococcus aureus immune evasion and virulence. J Mol Med 88 109 114
7. SeyboldU
KourbatovaEV
JohnsonJG
HalvosaSJ
WangYF
2006 Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis 42 647 656
8. PopovichKJ
WeinsteinRA
HotaB
2008 Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis 46 787 794
9. Labandeira-ReyM
CouzonF
BoissetS
BrownEL
BesM
2007 Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315 1130 1133
10. VoyichJM
OttoM
MathemaB
BraughtonKR
WhitneyAR
2006 Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis 194 1761 1770
11. OtterJA
KearnsAM
FrenchGL
EllingtonMJ
2010 Panton-Valentine leukocidin-encoding bacteriophage and gene sequence variation in community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 16 68 73
12. WangR
BraughtonKR
KretschmerD
BachTH
QueckSY
2007 Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13 1510 1514
13. KaitoC
SekimizuK
2007 Colony spreading in Staphylococcus aureus. J Bacteriol 189 2553 2557
14. HubscherJ
McCallumN
SifriCD
MajcherczykPA
EntenzaJM
2009 MsrR contributes to cell surface characteristics and virulence in Staphylococcus aureus. FEMS Microbiol Lett 295 251 260
15. KaitoC
OmaeY
MatsumotoY
NagataM
YamaguchiH
2008 A novel gene, fudoh, in the SCCmec region suppresses the colony spreading ability and virulence of Staphylococcus aureus. PLoS ONE 3 e3921
16. ItuMa
ItoT
TiensasitornC
JamklangM
ChongtrakoolP
2002 Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother 46 1147 1152
17. QueckSY
KhanBA
WangR
BachTH
KretschmerD
2009 Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog 5 e1000533
18. SibbaldMJ
ZiebandtAK
EngelmannS
HeckerM
de JongA
2006 Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol Mol Biol Rev 70 755 788
19. KobayashiSD
DeLeoFR
2009 An update on community-associated MRSA virulence. Curr Opin Pharmacol 9 545 551
20. YaoY
SturdevantDE
OttoM
2005 Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis 191 289 298
21. NovickRP
2003 Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48 1429 1449
22. QueckSY
Jameson-LeeM
VillaruzAE
BachTH
KhanBA
2008 RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell 32 150 158
23. CheungAL
SchmidtK
BatemanB
MannaAC
2001 SarS, a SarA homolog repressible by agr, is an activator of protein A synthesis in Staphylococcus aureus. Infect Immun 69 2448 2455
24. CheungAL
BayerAS
ZhangG
GreshamH
XiongYQ
2004 Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol 40 1 9
25. CheungAL
NishinaKA
TrotondaMP
TamberS
2008 The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol 40 355 361
26. OttoM
2008 Staphylococcal biofilms. Curr Top Microbiol Immunol 322 207 228
27. DasT
SharmaPK
BusscherHJ
van der MeiHC
KromBP
2010 Role of Extracellular DNA in Initial Bacterial Adhesion and Surface Aggregation. Appl Environ Microbiol 76 3405 8
28. TielenP
RosenauF
WilhelmS
JaegerKE
FlemmingHC
2010 Extracellular enzymes affect biofilm formation of mucoid Pseudomonas aeruginosa. Microbiology 156 2239 52
29. AydinurazK
AgalarC
AgalarF
CekenS
DuruyurekN
2009 In vitro S. epidermidis and S. aureus adherence to composite and lightweight polypropylene grafts. J Surg Res 157 e79 86
30. O'NeillE
PozziC
HoustonP
HumphreysH
RobinsonDA
2008 A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 190 3835 3850
31. ZukerM
2003 Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31 3406 3415
32. LiuY
WuN
DongJ
GaoY
ZhangX
2010 Hfq is a global regulator that controls the pathogenicity of Staphylococcus aureus. PLoS ONE 5 e13069
33. BohnC
RigoulayC
BoulocP
2007 No detectable effect of RNA-binding protein Hfq absence in Staphylococcus aureus. BMC Microbiol 7 10
34. ArciolaCR
BaldassarriL
MontanaroL
2001 Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol 39 2151 2156
35. BegunJ
GaianiJM
RohdeH
MackD
CalderwoodSB
2007 Staphylococcal biofilm exopolysaccharide protects against Caenorhabditis elegans immune defenses. PLoS Pathog 3 e57
36. Hall-StoodleyL
StoodleyP
2009 Evolving concepts in biofilm infections. Cell Microbiol 11 1034 1043
37. HidronAI
LowCE
HonigEG
BlumbergHM
2009 Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis 9 384 392
38. MongkolrattanothaiK
BoyleS
KahanaMD
DaumRS
2003 Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates. Clin Infect Dis 37 1050 1058
39. HagemanJC
UyekiTM
FrancisJS
JerniganDB
WheelerJG
2006 Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis 12 894 899
40. CastaldoET
YangEY
2007 Severe sepsis attributable to community-associated methicillin-resistant Staphylococcus aureus: an emerging fatal problem. Am Surg 73 684 687; discussion 687–688
41. Boyle-VavraS
DaumRS
2007 Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest 87 3 9
42. Centers for Disease Control and Prevention 1999 Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus–Minnesota and North Dakota, 1997–1999. JAMA 282 1123 1125
43. EnrightMC
RobinsonDA
RandleG
FeilEJ
GrundmannH
2002 The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A 99 7687 7692
44. OkumaK
IwakawaK
TurnidgeJD
GrubbWB
BellJM
2002 Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol 40 4289 4294
45. NovickRP
RossHF
ProjanSJ
KornblumJ
KreiswirthB
1993 Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12 3967 3975
46. SambrookJ
RussellDW
2001 Molecular cloning : a laboratory manual Cold Spring Harbor, N.Y. Cold Spring Harbor Laboratory Press
47. SchenkS
LaddagaRA
1992 Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett 73 133 138
48. NovickRP
1991 Genetic systems in staphylococci. Methods Enzymol 204 587 636
49. KaitoC
MorishitaD
MatsumotoY
KurokawaK
SekimizuK
2006 Novel DNA binding protein SarZ contributes to virulence in Staphylococcus aureus. Mol Microbiol 62 1601 1617
50. MatsumotoY
KaitoC
MorishitaD
KurokawaK
SekimizuK
2007 Regulation of exoprotein gene expression by the Staphylococcus aureus cvfB gene. Infect Immun 75 1964 1972
51. OishiK
BabaT
NakatomiY
ItoT
HiramatsuK
2008 A latex agglutination assay for specific detection of Panton-Valentine leukocidin. J Microbiol Methods 75 411 415
52. PengHL
NovickRP
KreiswirthB
KornblumJ
SchlievertP
1988 Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol 170 4365 4372
53. DuthieES
LorenzLL
1952 Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 6 95 107
54. NovickR
1967 Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33 155 166
55. KurodaM
OhtaT
UchiyamaI
BabaT
YuzawaH
2001 Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357 1225 1240
56. NaimiTS
LeDellKH
BoxrudDJ
GroomAV
StewardCD
2001 Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996–1998. Clin Infect Dis 33 990 996
57. DiepBA
GillSR
ChangRF
PhanTH
ChenJH
2006 Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367 731 739
58. BaeT
SchneewindO
2006 Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55 58 63
59. MatsuoM
KurokawaK
NishidaS
LiY
TakimuraH
2003 Isolation and mutation site determination of the temperature-sensitive murB mutants of Staphylococcus aureus. FEMS Microbiol Lett 222 107 113
60. IchihashiN
KurokawaK
MatsuoM
KaitoC
SekimizuK
2003 Inhibitory effects of basic or neutral phospholipid on acidic phospholipid-mediated dissociation of adenine nucleotide bound to DnaA protein, the initiator of chromosomal DNA replication. J Biol Chem 278 28778 28786
61. TaoL
LeBlancDJ
FerrettiJJ
1992 Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120 105 110
62. OkuY
KurokawaK
MatsuoM
YamadaS
LeeBL
2009 Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J Bacteriol 191 141 151
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance inČlánek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



