-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIn Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
Single-stranded polyanions ≥40 bases in length facilitate the formation of hamster scrapie prions in vitro, and polyanions co-localize with PrPSc aggregates in vivo [1], [2]. To test the hypothesis that intact polyanionic molecules might serve as a structural backbone essential for maintaining the infectious conformation(s) of PrPSc, we produced synthetic prions using a photocleavable, 100-base oligonucleotide (PC-oligo). In serial Protein Misfolding Cyclic Amplification (sPMCA) reactions using purified PrPC substrate, PC-oligo was incorporated into physical complexes with PrPSc molecules that were resistant to benzonase digestion. Exposure of these nuclease-resistant prion complexes to long wave ultraviolet light (315 nm) induced degradation of PC-oligo into 5 base fragments. Light-induced photolysis of incorporated PC-oligo did not alter the infectivity of in vitro-generated prions, as determined by bioassay in hamsters and brain homogenate sPMCA assays. Neuropathological analysis also revealed no significant differences in the neurotropism of prions containing intact versus degraded PC-oligo. These results show that polyanions >5 bases in length are not required for maintaining the infectious properties of in vitro-generated scrapie prions, and indicate that such properties are maintained either by short polyanion remnants, other co-purified cofactors, or by PrPSc molecules alone.
Published in the journal: . PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1002001
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002001Summary
Single-stranded polyanions ≥40 bases in length facilitate the formation of hamster scrapie prions in vitro, and polyanions co-localize with PrPSc aggregates in vivo [1], [2]. To test the hypothesis that intact polyanionic molecules might serve as a structural backbone essential for maintaining the infectious conformation(s) of PrPSc, we produced synthetic prions using a photocleavable, 100-base oligonucleotide (PC-oligo). In serial Protein Misfolding Cyclic Amplification (sPMCA) reactions using purified PrPC substrate, PC-oligo was incorporated into physical complexes with PrPSc molecules that were resistant to benzonase digestion. Exposure of these nuclease-resistant prion complexes to long wave ultraviolet light (315 nm) induced degradation of PC-oligo into 5 base fragments. Light-induced photolysis of incorporated PC-oligo did not alter the infectivity of in vitro-generated prions, as determined by bioassay in hamsters and brain homogenate sPMCA assays. Neuropathological analysis also revealed no significant differences in the neurotropism of prions containing intact versus degraded PC-oligo. These results show that polyanions >5 bases in length are not required for maintaining the infectious properties of in vitro-generated scrapie prions, and indicate that such properties are maintained either by short polyanion remnants, other co-purified cofactors, or by PrPSc molecules alone.
Introduction
Infectious prion diseases such as Creutzfeldt Jakob disease (CJD) and other related human disorders, chronic wasting disease (CWD), bovine spongiform encephalopathy (BSE), and scrapie are associated with the conversion of a host-encoded glycoprotein (PrPC) into a misfolded conformer, PrPSc [3]. Several biochemical studies utilizing the protein misfolding cyclic amplification (PMCA) technique have shown that PrPSc is an essential component of the infectious agent [4], [5], [6].
Interestingly, a variety of prion strains with distinctive infectious phenotypes, such as selective neurotropism and characteristic disease incubation times, have been isolated and propagated in vivo and in vitro [7], [8]. In some cases, infection with specific prion strains produces PrPSc molecules with distinctive biochemical characteristics, suggesting that multiple self-propagating PrPSc conformations may provide the structural basis for the existence of multiple prion strains [9], [10].
Several biochemical and cell culture studies have implicated polyanions such as single stranded nucleic acid and glycosaminoglycan (GAG) molecules as potent cofactors in the process of infectious prion propagation [1], [5], [6], [11], [12], [13], [14], [15], [16], [17]. Moreover, it has been shown that polyanions are selectively incorporated into physical complexes with purified PrP molecules in infectious hamster prions, and that a minimum length corresponding to a 40 base single stranded oligonucleotide is required for a polyanion to facilitate hamster prion formation in vitro [1]. Neuropathological analysis of scrapie-infected animals has shown that nucleic acids and GAG-containing proteoglycans co-localize with PrPSc aggregates in situ [1], [2]. Collectively, these observations raise the possibility that moderately long polyanions might be needed to maintain hamster prion infectivity or strain properties by acting as a structural support for PrPSc molecules.
Nuclease-resistant RNA molecules have been detected in purified prion preparations [18], and analytical methods suggest that the average size of polynucleotides in such preparations is ≤25 nucleotides, assuming a ratio of 1 polynucleotide molecule per infectious unit [19]. More recently, Jeong et al. reported reduction of prion infectivity and PrPSc by treatment of hamster 263K scrapie brain homogenates with LiAlH4 (lithium aluminum hydride), a highly reactive reducing agent capable of degrading RNA molecules [20]. However, because LiAlH4 is a non-specific reducing agent, which can react with a variety of biological molecules other than RNA, including proteins, it is not possible to ascribe definitively the detrimental effect of LiAlH4 on prion infectivity to the degradation of RNA.
Previous photo-irradiation studies of naturally occurring prions [21], [22] suggested that specific nucleic acid sequences are not necessary for prion infectivity, and this conclusion was confirmed by the de novo formation of purified prions using synthetic, homopolymeric poly(A) RNA molecules [5]. However, these prior studies did not exclude a structural, non-coding role for polyanions such as RNA in maintaining the infectious conformation of PrPSc since ultraviolet (UV) light mutates pyrimidine bases but does not significantly degrade the polynucleotide backbone of nucleic acids or other types of polyanions such as GAGs.
Here we report the results of studies using purified synthetic prions containing a photocleavable (dT)100 oligonucleotide (PC-oligo), which can be selectively degraded in situ into 5-mers by exposure to long wave UV light. This approach directly addresses the question of whether polyanions might serve as a structural backbone for infectious prions in a chemically defined model system.
Materials and Methods
Reagents
Hamster prion strain Sc237 was kindly provided by Dr. Stanley Prusiner (UCSF, San Francisco, CA). Three-week-old female Golden Syrian hamsters used in inoculation experiments and seven-week-old Golden Syrian hamsters used to make brain homogenates were purchased from Charles River (Wilmington, MA). Monoclonal antibody (mAb) 6D11 was purchased from Covance (Princeton, NJ). PC-oligo was chemically synthesized by placing a photocleavable group in between every five base of (dT)100, using (3-(4,4′-Dimethoxytrityl)-1-(2-nitrophenyl)-propan-1-yl-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite). PC-oligowas synthesized by and purchased from Gene Link (Hawthorne, NY). Stock solutions of PC-oligo were made by dissolving lyophilized powder into 50% DMSO/ 50% TE pH 8.0 (10 mM Tris 8.0, 1 mM EDTA) to a final concentration of 5 mg/ml. The dT-oligo was synthesized by and purchased from Operon (Huntsville, AL). Wild type mouse recombinant PrP was expressed in E. Coli and purified as previously described [6]. Benzonase nuclease (50-230-8706) was purchased from EMD Chemicals (Gibbstown, NH).
Serial Protein Misfolding Cyclic Amplification (sPMCA)
sPMCA reactions were performed as previously described [4], [5]. Reaction tubes were subjected to PMCA for 24 h using a Misonix s3000 programmable sonicator equipped with a microplate horn (Misonix, Farmingdale, NY) containing 350 ml of water. The temperature was maintained inside the horn by a circulating water bath, pumping warmed water through aluminum coils surrounding the horn. Sample tubes were held in a plastic rack which prevents lid opening and hold tubes ∼3 mm from the surface of the horn. After 24 h, the tubes were removed from the sonicator, and 10 µl of the reaction mixture was added to a new tube containing fresh substrate.
Western Blotting
A portion of each sample was treated with 25 µg/ml proteinase K for 30 min at 37°C shaking at 750 rpm in an Eppendorf Thermomixer (Fisher Scientific, Pittsburg, PA). An equal volume of 2x sodium dodecyl sulfate (SDS) loading buffer was added to each tube and then boiled at 95°C for 10 min. SDS-polyacrylamide gel electrophoresis (PAGE) was then performed using 1.5 mm 12% polyacrylamide gels (29∶1 acrylamide∶bisacrylamide) The gel was transferred to a methanol-charged polyvinylidene difluoride membrane (Millipore, Billerica, MA) using a Transblot SD semidry transfer cell (Bio-Rad, Hercules, CA). The transfer was set to achieve 3 mA/cm2 for 30 min. After transfer, the membrane was incubated in 20% (v/v) instant nonfat dry milk (Nestle, Vevey, Switzerland) dissolved in TBST (10 mM Tris pH 7.1, 150 mM NaCl, 0.1% Tween 20). The membrane was then incubated overnight at 4°C with mAb 6D11 in TBST (final concentration 80 ng/ml). The membrane was washed 3 times with TBST for 10 min and then incubated for 1 h with horseradish peroxidase-labeled anti-mouse immunoglobulin G secondary antibody conjugate (GE Healthcare) diluted 1∶5,000 in TBST. Again the membrane was washed 4 times for 10 min in TBST. Blots were developed with West Pico (Pierce, Rockford, IL) chemiluminescence substrate. Images were captured using a Fuji LAS-3000 chemiluminescence documentation system (Fujifilm, Tokyo, Japan). Relative molecular masses were determined by comparison to prestained standards from Fermentas (Hanover, MD).
Preparation of Purified PrPSc Molecules
Immunopurified PrPC was prepared as described previously [5]. To produce substrate mixtures, 37.5 µl of immunopurified PrPC was mixed with 10 µl of 10x reaction buffer (200 mM MOPS pH 7.5, 10% Triton X-100, 500 mM imidazole, 50 mM EDTA, 1.5 M NaCl), 7.5 µl of dT-oligo or PC-oligo (0.3 mg/ml for a total of 2.25 µg) and 35 µl of molecular grade water (Mediatech, Herndon, VA). On the first day, 10 µl of Sc237 brain homogenate was added to a 0.5 ml thin-walled PCR tube (Axygen, Union City, CA) and subjected to sPMCA as described above. The sonicator delivered bursts of 30 s every 30 min at output 6.5. sPMCA was continued for 15 days, effectively diluting the original inocula 1015 fold. The negative control line was treated as stated above with the omission of nucleic acid from the substrate cocktail.
Light Treatment of Oligonucleotides
dT-oligo and PC-oligo were placed in their own ProxiPlate 96-well plate (PerkinElmer, Waltham, MA). The plates were placed into a UV Stratalinker 2400 (Stratagene, La Jolla, CA) equipped with bulbs that emit light at 315 nm. The 96-well plates were placed on top of tip boxes and racks so samples were ∼2.5 cm from the light source. Each light treated sample received 4 pulses of light at an energy level of 500,000 µJ. Non-light treated samples were also placed in their own ProxiPlate 96-well plate but were then placed inside a drawer to protect from light. Treated and non-treated oligos were either added to sPMCA reactions as described above, or directly analyzed as described below. For benzonase protection assays 6 µg of either dT-oligo or PC-oligo were incubated for 10 min at 37°C and shaking at 750 rpm with or without 300 µg recPrP. After incubation samples were sonicated for one 30 s pulse followed by another 10 min incubation at 37°C and 750 rpm. After second incubation 7 µl of benzonase was added (175 units) and further Incubated for 1 h at 37°C and 750 rpm. The oligonucleotides were then extracted as described below.
Light Treatment of PrPSc Samples
Concentrations of PrPSc containing either dT-oligo or PC-oligo were compared by Western blot and diluted to achieve equal concentrations. Of the normalized material, 500 µl of each sample was placed in its own ProxiPlate 96-well plate (PerkinElmer, Waltham, MA). Samples were then treated as described above with one modification. In between each light pulse, samples were removed from the 96-well plate, placed in a 1.5 ml tube and subjected to sonication in a cold sonicator. Briefly, 350 ml of chilled water was placed in the microplate horn, and tubes were positioned in a rack as described above and subjected to a 20 s pulse at output 6.5. Samples were returned to new wells in the 96-well plate and subjected to another round of light or dark treatment.
DNA Recovery and Analysis
To analyze the effect of light on the nucleic acids 150 µl of each sample was brought to 250 µl by adding 1x reaction buffer. An equal volume of Phenol∶Chloroform∶Isoamyl alcohol 25∶24∶1 (Sigma, St. Louis, MO) was added to each tube and vortexed. Samples were spun at 14,000× g for 2 min. The top aqueous phase was carefully removed and the process repeated twice more. After three extractions with the Phenol∶Chloroform∶Isoamyl alcohol, an equal volume of chloroform was added to each tube, vortexed and spun as above. The top aqueous phase was removed and to it 25 µl of 3 M sodium acetate pH 7.0 was added followed by 750 µl of 95% EtOH. Tubes were vortexed and incubated at −20°C for 15 min. After incubation tubes were spun at 14,000× g for 10 min to pellet the DNA. The pellet was washed with 80% EtOH, spun and dried. The pellet was resuspended in equal volumes of TE (10 mM Tris pH 7.0, 1 mM EDTA) and 2x TBE-Urea sample buffers (Invitrogen, Carlsbad, CA). Samples were boiled at 95°C for 5 min and then loaded into pre-cast 15% TBE-Urea acrylamide gels (Invitrogen, Carlsbad, CA). Gels were run in a X-Cell SureLock apparatus (Invitrogen, Carlsbad, CA) at 200 V for 50 min. Molecular weight ladder was made by mixing oligo dT 100mer, oligo dT 30mer and oligo dT 10mer (10 mg/ml) in a ratio of 1∶3∶9 with 2x TBE-Urea sample buffer (oligos purchased from Operon, Huntsville, AL). Gels were then stained for 1 h with Sybr Gold (Invitrogen, Carlsbad, CA) diluted 1∶10,000 in TBE (89 mM Tris-base, 89 mM Boric Acid, 2 mM EDTA pH 8.3). After staining, gels were visualized on a transilluminator and photographed with a Canon Power Shot A650 IS (Canon, Tokyo, Japan) equipped with proper filters.
sPMCA End Point Titration
A 10% hamster brain homogenate (w/v) was made in conversion buffer (PBS with 150 mM NaCl, 1% Triton X-100, 4 mM EDTA) plus a Complete PI tablet with EDTA (Poche, Indianapolis, IN) using a polytron tissue grinder. The homogenate was then spun at 200× g for 30 s. The supernatant was then aliquotted into 0.5 ml thin-walled PCR tubes (90 µl reactions) and frozen at −70°C until used. PrPSc molecules treated with or without light were serially diluted into conversion buffer (PBS with 150 mM NaCl, 1% Triton X-100, 5 mM EDTA) to create 10 titers ranging from 10−1–10−10. Each titer was used to start a reaction by adding 10 µl to 90 µl of brain homogenate prepared as described above. Samples were subjected to 3 rounds of sPMCA as described above. The sonicator for this experiment was set to deliver 30 s pulses at output 8.5. Each sample was Western blotted as described above.
Preparation of Inocula
For each of the lines injected, 20 µl of day 15 material was spiked into each of 5 reaction tubes containing 200 µl of fresh substrate cocktail and subjected to 1 cycle of PMCA as described above. After 24 h tubes were removed and combined. This material was then treated with or without light as described above. After treatments, 100 µl of each sample was diluted into 900 µl of sterile PBS with 5 mg/ml bovine serum albumin (BSA) to prepare the inoculum. The DNA alone inoculum was prepared by mixing 2.25 µg of each nucleic acid into 100 µl of 1x reaction buffer. This material was then diluted 1∶10 into 5 mg/ml BSA, as described above.
Scrapie Inoculation and Diagnosis
Injections were performed in laminar-flow biosafety cabinets using disposable syringes, gloves and surfaces. Intracerebral inoculations were performed using 28-gauge hypodermic needles inserted into the parietal lobe. Each animal received 50 µl of inoculum. Hamsters were examined daily by veterinary staff blinded to experimental groups. Standard diagnostic criteria were used to identify animals showing signs of scrapie.
Neuropathology
Animals were euthanized by CO2 inhalation until death was imminent. Their brains were quickly removed using sterile dissection instruments on disposable surfaces. Brains were immersed in 10% buffered formalin for 1 week. Sagittal and parasagittal sections were made and brains placed into tissue-processing cassettes. The cassettes were immersed into 90% formic acid for 1 hr and then placed into 10% formalin. Tissues were next embedded in paraffin and cut into 4 µm sections. Sections were stained with hematoxylin and eosin. A single neuropathologist, who was blinded to the experimental groups examined all slides and scored them according to the degree of vacuolation. Scores were made according to previously published standards [5].
Ethics Statement
All animals were handled in strict accordance with good animal practice, as defined by the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Dartmouth College Institutional Animal Care and Use Committee approved the animal work (assurance number A3259-01). Inoculations were performed under isoflurane anesthesia, and all efforts were made to minimize suffering.
Results
We chemically synthesized a 100-base poly-dT molecule containing with a UV-photocleavable group (PC-oligo) every five bases (Figure 1). To confirm that PC-oligo can support PrPSc propagation, sPMCA reactions containing purified Syrian hamster PrPC supplemented with either PC-oligo or dT-oligo were seeded with Sc237 brain homogenate. The PrPC substrate used in these studies has been previously characterized extensively by a variety of analytical methods, and was found to contain stoichiometric quantities of co-purified endogenous lipids; but no other proteins, nucleic acids, or specific metal ions [5]. The results of our preliminary experiment show that both PC-oligo and dT-oligo support PrPSc propagation through three rounds of sPMCA reactions whereas control reactions without added polyanions failed to propagate (Figure S1 in Supporting Information S1). After proteinase K (PK) digestion, PrPSc molecules formed with either oligonucleotide showed a similar shift in electrophoretic mobility to 27–30 kD (Figure S1 in Supporting Information S1).
Fig. 1. Schematic showing the composition of the PC-oligo. 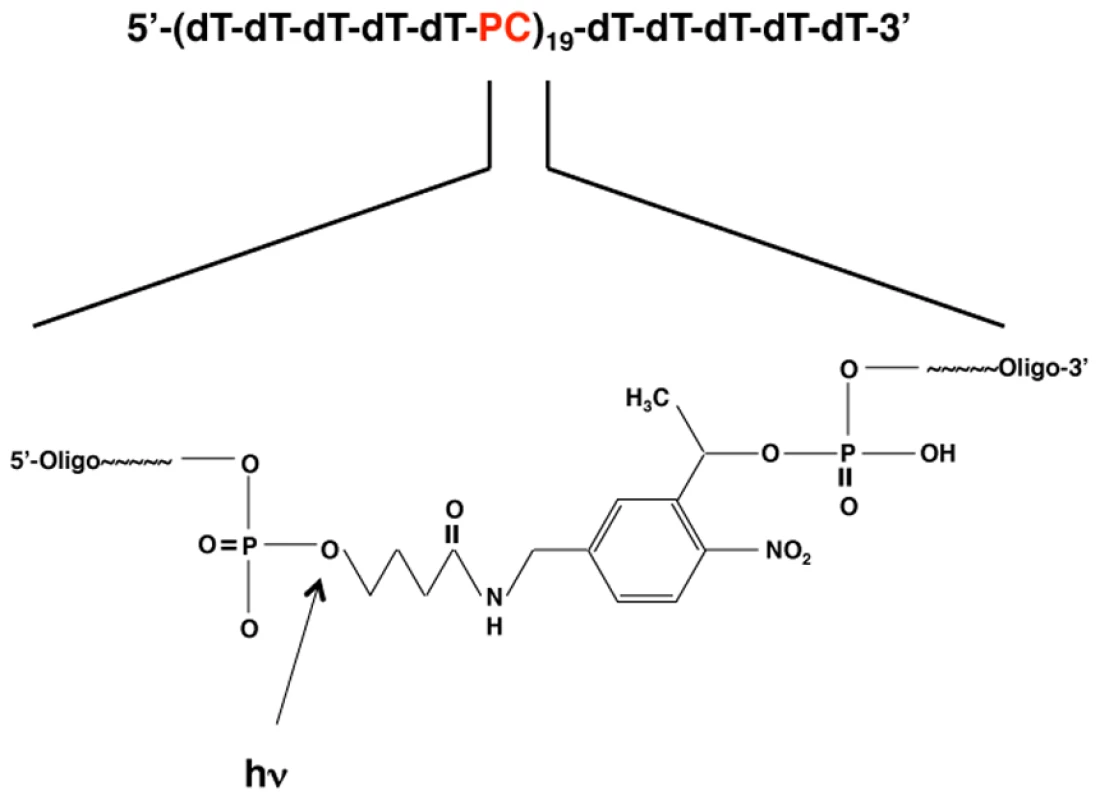
Repeating units of five deoxythymine residues were followed by a photocleavable (PC) linker as shown. Enlarged inset shows the chemical structure of the PC linker (3-(4,4′-Dimethoxytrityl)-1-(2-nitrophenyl)-propan-1-yl-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite). The arrow indicates the location of the cleavage site (the phosphodiester bond between the linker and the 5′ phosphate of the oligonucleotide). To determine the optimal irradiation conditions for the photolysis of PC-oligo, pure solutions of each oligonucleotide in buffer were treated with varying amounts of UV light (315 nm) and analyzed by acrylamide gel electrophoresis. Prior to light treatment, PC-oligo can be seen a 5-base ladder, representing the collection of full length and incomplete products formed during the sequential conjugation chemical synthesis protocol (Figure S2 in Supporting Information S1, lane 3). Upon exposure to two Joules of light, PC-oligo was completely degraded to 5-base oligonucleotide units (Figure S2 in Supporting Information S1, compare lanes 3 and 7) while dT-oligo remained intact (Figure S2 in Supporting Information S1, compare lanes 1 and 2).
We next tested the effect of light-induced degradation on the ability of PC-oligo to facilitate prion propagation in sPMCA reactions. As anticipated, dT-oligo supported propagation of Sc237 prions in sPMCA reactions using immunopurified hamster PrPC substrate even when the control oligonucleotide was pretreated with UV light (Figure 2, left gels). PC-oligo pretreated with UV light did not support propagation (Figure 2, bottom right gel), whereas PC-oligo mock-treated in the dark supported Sc237 propagation in sPMCA for three rounds (Figure 2, top right gel). These results confirm our previously published results that an oligonucleotide must be at least 40 bases long to support propagation, and that our light treatment protocol completely degrades PC-oligo to a length smaller than that required for efficient propagation in sPMCA.
Fig. 2. Western blot showing the effect of degrading PC-oligo on its ability to stimulate prion conversion in sPMCA. 
Lanes 1 and 6 show non-digested PrPC used as substrate (-PK). The remaining samples were subjected to limited proteolysis with proteinase K. We previously found that single stranded nucleic acids form a nuclease-resistant complex with both PrPC and PrPSc molecules during sPMCA reactions[1]. To study the penetration of UV light into nucleoprotein complexes, we combined a stoichiometric excess of full-length recombinant mouse PrP (recPrP) with our test oligonucleotides. Following PMCA sonication, both PC-oligo and dT-oligo became completely protected from nuclease digestion, whereas both oligonucleotides were nuclease-sensitive in the absence of PrP (Figures 3A and 3B). UV irradiation induced the degradation of PC-oligo, but not dT-oligo, within these nuclease-resistant complexes containing excess PrP (compare Figure 3A, lanes 7–8 and Figure 3B, lanes 7–8). Thus, UV light is able to penetrate PrP nucleoprotein complexes, providing us with a unique opportunity to control the degradation of PC-oligo in situ within purified prions.
Fig. 3. Acrylamide gel electrophoresis showing the effects of benzonase and light treatment on oligonucleotide integrity. 
A. Experiments performed with dT-oligo. B. Experiments performed with PC-oligo. In both panels, treatments were performed on oligonucleotides in the presence or absence of 1.5 mg/ml recPrP, as indicated. Having established conditions for UV light penetration of PrP nucleoprotein complexes, we sought to determine the effect of light-induced degradation of PC-oligo in situ (i.e. already incorporated into complexes PrPSc molecules) on the conformational stability, sPMCA seeding activity, and biological infectivity of synthetic PrPSc molecules. PrPSc molecules containing either PC-oligo or control dT-oligo were generated by performing sPMCA for 15 rounds in order to dilute the initial prion seeds several orders of magnitude beyond the calculated endpoint [4], [5], and then either treated with UV light or mock-treated in the dark. A PK digestion assay revealed no significant differences in PrPSc protease-resistance between light-treated versus mock-treated samples (Figure S3 in Supporting Information S1). To measure sPMCA seeding activity, samples were serially titrated, and the resulting dilutions were used to seed three rounds of sPMCA using fresh hamster brain homogenate as substrate, and samples from the final round of sPMCA were analyzed for the presence of PrPSc by Western blot. The results of this quantitative end-point titration assay show that PrPSc molecules containing either dT-oligo or PC-oligo were equally susceptible to UV irradiation; both sets of samples displayed ∼1 log decrease in seed titer upon light treatment, presumably due to non-specific effects (Figure 4). A small amount of each sample was concurrently analyzed by acrylamide gel electrophoresis to confirm that UV treatment was successful in degrading the PC-oligo below detection limits (Figure S4 in Supporting Information S1). Taken together, the results indicate that light-induced degradation of incorporated PC-oligo into 5-mers had no specific effect on the ability of PrPSc to seed sPMCA reactions of normal brain homogenate.
Fig. 4. Western blot showing the final samples from 3 round sPMCA reactions. 
Samples containing dT-oligo or PC-oligo were seeded with a dilution of PrPSc which was either subjected to light treatment or not, as indicated. All samples were subjected to limited proteolysis with proteinase K. Light - and dark-treated PrPSc containing either dT-oligo or PC-oligo were injected intracerebrally into Golden Syrian hamsters for bioassay. In addition, negative control samples consisting of either the original Sc237 seed propagated for 15 rounds in PrPC substrate without nucleic acid (designated PrPC) or a cocktail containing the dT-oligo and PC-oligo (designated dT/PC-oligo alone) were inoculated in parallel. The scrapie incubation time for all 4 experimental groups was ∼150 days, with all of the inoculated animals developing similar clinical signs of ataxia, trembling, and circling (Table 1). Animals injected with the PrPC and dT/PC-oligo control samples did not develop signs of clinical scrapie during the time frame of the experiment (Table 1 and figures 5A and 5B). Neuropathological analysis showed significant vacuolization throughout the brains of animals in all 4 groups, including the brainstem and hippocampus (Figure 5A and 5B), and a blinded comparison of vacuolization scores from different brain regions showed no difference between the experimental groups (Figure 6). Biochemical analysis showed no differences in the protease sensitivity, glycoform ratio, or electrophoretic mobility of the PrPSc molecules produced in the brains of animals from all 4 experimental groups (Figure S5 in Supporting Information S1). There was also no apparent difference in the conformational stability of PrPSc molecules in the brains of animals injected with light-treated versus control inocula, as determined by a urea denaturation assay (Figure S6 in Supporting Information S1). Therefore, these results indicate that degradation of an incorporated polyanion does not significantly alter the biological infectivity or the strain-dependent properties of purified Sc237 prions.
Fig. 5. Representative histological fields of brainstem and hippocampus. 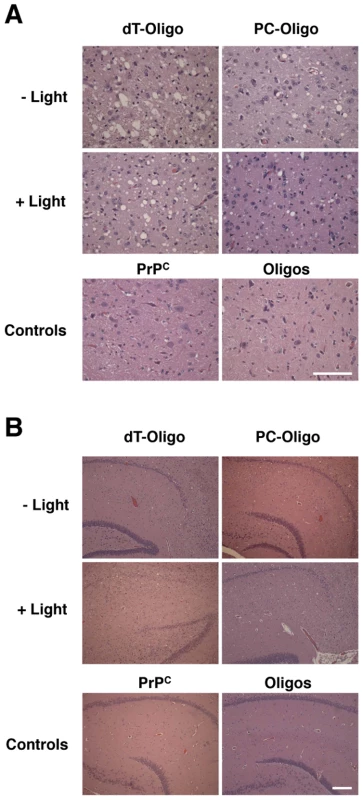
Hemotoxylin and Eosin staining from animals injected with in vitro generated PrPSc containing dT-oligo or PC-oligo treated with or without light. Top row: animals injected with prions treated in dark. Middle row: animals injected with prions treated with light. Bottom row: control animals. A. Brain stem. (Scale bar, 100 µm). B. Hippocampus. (Scale bar, 200 µm). Fig. 6. Regional neuropathology of hamsters infected with light- or dark-treated inocula. 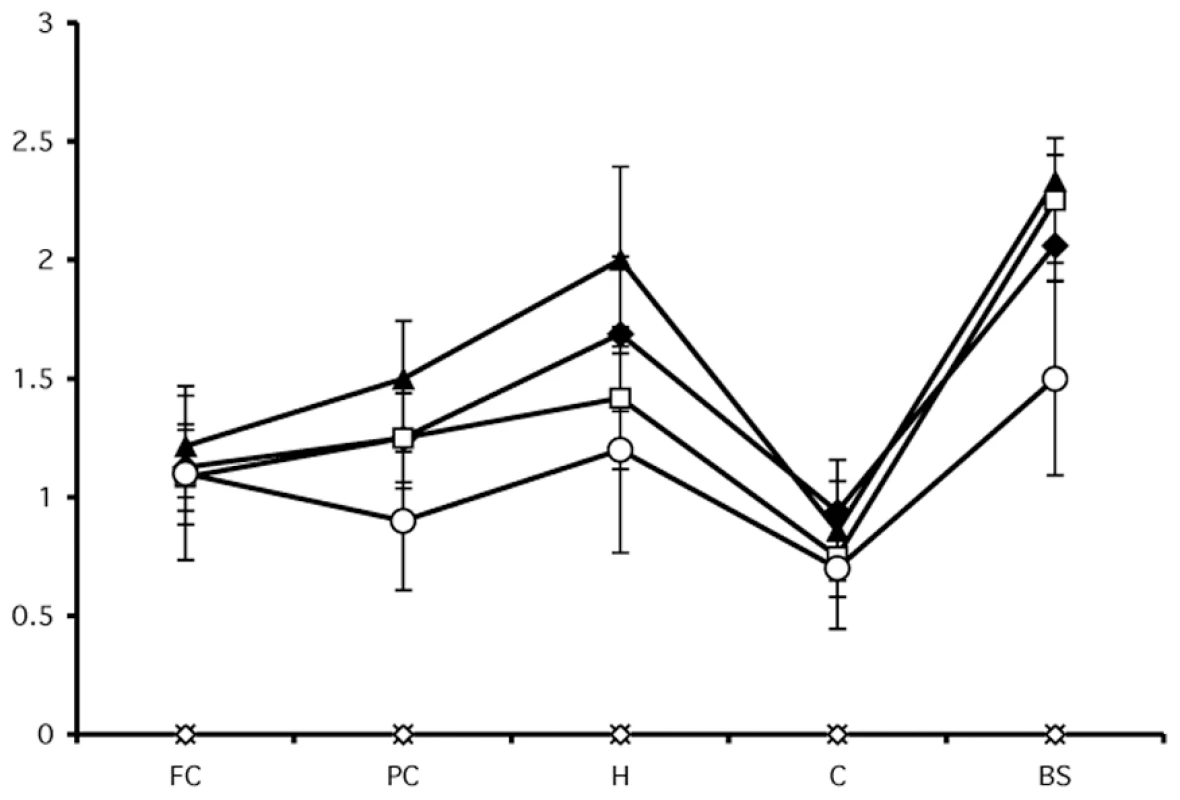
Tab. 1. Prion incubation times in hamsters inoculated intracerebrally with sPMCA generated prions. 
The PrPC sample was generated by serially propagating the original Sc237 seed for 15 rounds in PrPC substrate alone (without polyanion) at a 1∶10 ratio in each round. The dT/PC-Oligo alone inoculum contains a mixture of both oligonucleotides as described in Methods. Discussion
The major finding of this work is that selective degradation of an incorporated photocleavable polyanion cofactor does not alter the catalytic activity, infectivity, or strain properties of in vitro generated prions.
These results contrast with those of Jeong et al., who showed that treatment of scrapie-infected brain homogenates with LiAlH4 caused degradation of endogenous RNA and increased prion incubation time from ∼100 to ∼340 days [20]. There are several possible reasons for the discrepancy between the two studies: (1) Although both LiAlH4 and UV light treatment are capable of reacting non-specifically with non-polyanionic molecules, under the conditions used for each study, it is possible that LiAlH4 treatment is more non-specifically damaging than UV light. For instance, it could reduce PrPSc molecules and/or directly damage protein structure. (2) The work of Jeong et al. used scrapie brain homogenate as the starting infectious material, whereas the experiments reported here used purified prions formed from native PrPC substrate in vitro using sPMCA. Such purified prions are composed only of PrP, stoichiometric amounts of an endogenous lipid molecule containing 20 carbon fatty acids, and a synthetic polyanion [5]. (3) Whereas LiAlH4 is capable of hydrolyzing RNA molecules completely, UV light degrades the PC-oligo used in our study to (dT)5 oligonucleotides. Although such small nucleic acids do not support prion propagation in vitro, our results cannot formally exclude the possibility that short oligonucleotides, once incorporated, might be able to act as a reinforcing backbone for PrPSc. (4) Although we did not detect any intact PC-oligo after light treatment using a highly sensitive intercalating agent, it is possible that a small, undetectable amount of PC-oligo was protected from UV-irradiation by virtue of being complexed with PrPSc molecules. This possibility is difficult to address experimentally because it is technically difficult to recover and detect such small amounts of DNA that cannot be amplified. It is worth noting that UV light degrades PC-oligo quantitatively within nuclease-resistant complexes with recombinant PrP.
Our results are compatible with the scenario in which prion infectivity might be exclusively encoded by PrPSc structure in the absence of non-proteinaceous cofactors, as proposed by the “protein only” hypothesis [23], [24]. However, this hypothesis cannot be confirmed in our experimental system because both endogenous lipid and remnant (dT)5 oligonucleotide molecules remain present in purified prions generated with PC-oligo following light treatment. Nonetheless, we are able to conclude that it is unnecessary for prions to contain polyanions >5 bases in length to maintain infectivity, whereas polyanions ≥40 bases are required for the process of prion formation in vitro. This conclusion places a significant geometric constraint on the mechanism by which polyanionic cofactors could be theoretically used to maintain PrPSc architecture. Stoichiometric quantities of endogenous lipids containing 20-carbon fatty acids co-purify with the PrPC substrate used in these studies [5], and therefore these lipid molecules could also become incorporated into the infectious PrPSc product during PMCA reactions. Future studies will be required to test whether endogenous lipid molecules or other non-nucleic acid cofactors, such as those recently described in mouse brain homogenates [25], [26], might be required to maintain prion infectivity and strain phenotypes.
Supporting Information
Zdroje
1. GeogheganJCValdesPAOremNRDeleaultNRWilliamsonRA 2007 Selective incorporation of polyanionic molecules into hamster prions. J Biol Chem 282 36341 36353
2. SnowADWightTNNochlinDKoikeYKimataK 1990 Immunolocalization of heparan sulfate proteoglycans to the prion protein amyloid plaques of Gerstmann-Straussler syndrome, Creutzfeldt - Jakob disease and scrapie. Lab Invest 63 601 611
3. BaslerKOeschBScottMWestawayDWalchliM 1986 Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell 46 417 428
4. CastillaJSaaPHetzCSotoC 2005 In vitro generation of infectious scrapie prions. Cell 121 195 206
5. DeleaultNRHarrisBTReesJRSupattaponeS 2007 Formation of native prions from minimal componenets in vitro. Proc Natl Acad Sci U S A 104 9741 9746
6. WangFWangXYuanCGMaJ 2010 Generating a Prion with Bacterially Expressed Recombinant Prion Protein. Science 327 1132 1135
7. BruceME 1993 Scrapie strain variation and mutation. Br Med Bull 49 822 838
8. CarlsonGA 1996 Prion strains. Curr Top Microbiol Immunol 207 35 47
9. BessenRAMarshRF 1994 Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol 68 7859 7868
10. TellingGCParchiPDeArmondSJCortelliPMontagnaP 1996 Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274 2079 2082
11. DeleaultNRLucassenRWSupattaponeS 2003 RNA molecules stimulate prion protein conversion. Nature 425 717 720
12. DeleaultNRGeogheganJCNishinaKKascsakRWilliamsonRA 2005 Protease-resistant Prion Protein Amplification Reconstituted with Partially Purified Substrates and Synthetic Polyanions. J Biol Chem 280 26873 26879
13. WongCXiongLWHoriuchiMRaymondLWehrlyK 2001 Sulfated glycans and elevated temperature stimulate PrP(Sc)-dependent cell-free formation of protease-resistant prion protein. Embo J 20 377 386
14. ShakedGMMeinerZAvrahamITaraboulosAGabizonR 2001 Reconstitution of prion infectivity from solubilized protease-resistant PrP and nonprotein components of prion rods. J Biol Chem 276 14324 14328
15. Ben-ZakenOTzabanSTalYHoronchikLEskoJD 2003 Cellular heparan sulfate participates in the metabolism of prions. J Biol Chem 278 40041 40049
16. WarnerRGHundtCWeissSTurnbullJE 2002 Identification of the heparan sulfate binding sites in the cellular prion protein. J Biol Chem 277 18421 18430
17. CaugheyBBrownKRaymondGJKatzensteinGEThresherW 1994 Binding of the protease-sensitive form of PrP (prion protein) to sulfated glycosaminoglycan and congo red [corrected]. J Virol 68 2135 2141
18. AkowitzASklaviadisTManuelidisEEManuelidisL 1990 Nuclease-resistant polyadenylated RNAs of significant size are detected by PCR in highly purified Creutzfeldt-Jakob disease preparations. Microb Pathog 9 33 45
19. SafarJGKellingsKSerbanAGrothDCleaverJE 2005 Search for a prion-specific nucleic acid. J Virol 79 10796 10806
20. JeongBHKimNHJinJKChoiJKLeeYJ 2009 Reduction of prion infectivity and levels of scrapie prion protein by lithium aluminum hydride: implications for RNA in prion diseases. J Neuropathol Exp Neurol 68 870 879
21. AlperT 1985 Scrapie agent unlike viruses in size and susceptibility to inactivation by ionizing or ultraviolet radiation. Nature 317 750
22. Bellinger-KawaharaCCleaverJEDienerTOPrusinerSB 1987 Purified scrapie prions resist inactivation by UV irradiation. J Virol 61 159 166
23. GriffithJS 1967 Self-replication and scrapie. Nature 215 1043 1044
24. CohenFEPanKMHuangZBaldwinMFletterickRJ 1994 Structural clues to prion replication. Science 264 530 531
25. DeleaultNRKascsakRGeogheganJCSupattaponeS 2010 Species-dependent differences in cofactor utilization for formation of the protease-resistant prion protein in vitro. Biochemistry 49 3928 3934
26. GillACAgarwalSPinheiroTJGrahamJF 2010 Structural requirements for efficient prion protein conversion: Cofactors may promote a conversion-competent structure for PrP(C). Prion 4 235 242
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance inČlánek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



