-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStructural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
Three closely related bacterial species within the genus Neisseria are of importance to human disease and health. Neisseria meningitidis is a major cause of meningitis, while Neisseria gonorrhoeae is the agent of the sexually transmitted disease gonorrhea and Neisseria lactamica is a common, harmless commensal of children. Comparative genomics have yet to yield clear insights into which factors dictate the unique host-parasite relationships exhibited by each since, as a group, they display remarkable conservation at the levels of nucleotide sequence, gene content and synteny. Here, we discovered two rare alterations in the gene encoding the CcoP protein component of cytochrome cbb3 oxidase that are phylogenetically informative. One is a single nucleotide polymorphism resulting in CcoP truncation that acts as a molecular signature for the species N. meningitidis. We go on to show that the ancestral ccoP gene arose by a unique gene duplication and fusion event and is specifically and completely distributed within species of the genus Neisseria. Surprisingly, we found that strains engineered to express either of the two CcoP forms conditionally differed in their capacity to support nitrite-dependent, microaerobic growth mediated by NirK, a nitrite reductase. Thus, we propose that changes in CcoP domain architecture and ensuing alterations in function are key traits in successive, adaptive radiations within these metapopulations. These findings provide a dramatic example of how rare changes in core metabolic proteins can be connected to significant macroevolutionary shifts. They also show how evolutionary change at the molecular level can be linked to metabolic innovation and its reversal as well as demonstrating how genotype can be used to infer alterations of the fitness landscape within a single host.
Published in the journal: . PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001055
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001055Summary
Three closely related bacterial species within the genus Neisseria are of importance to human disease and health. Neisseria meningitidis is a major cause of meningitis, while Neisseria gonorrhoeae is the agent of the sexually transmitted disease gonorrhea and Neisseria lactamica is a common, harmless commensal of children. Comparative genomics have yet to yield clear insights into which factors dictate the unique host-parasite relationships exhibited by each since, as a group, they display remarkable conservation at the levels of nucleotide sequence, gene content and synteny. Here, we discovered two rare alterations in the gene encoding the CcoP protein component of cytochrome cbb3 oxidase that are phylogenetically informative. One is a single nucleotide polymorphism resulting in CcoP truncation that acts as a molecular signature for the species N. meningitidis. We go on to show that the ancestral ccoP gene arose by a unique gene duplication and fusion event and is specifically and completely distributed within species of the genus Neisseria. Surprisingly, we found that strains engineered to express either of the two CcoP forms conditionally differed in their capacity to support nitrite-dependent, microaerobic growth mediated by NirK, a nitrite reductase. Thus, we propose that changes in CcoP domain architecture and ensuing alterations in function are key traits in successive, adaptive radiations within these metapopulations. These findings provide a dramatic example of how rare changes in core metabolic proteins can be connected to significant macroevolutionary shifts. They also show how evolutionary change at the molecular level can be linked to metabolic innovation and its reversal as well as demonstrating how genotype can be used to infer alterations of the fitness landscape within a single host.
Introduction
The genus Neisseria comprises Gram-negative, oxidase-positive diplococci that are frequently isolated from the mucosal surfaces of humans and two closely related species are important pathogens of man [1]. Neisseria gonorrhoeae is the etiologic agent of gonorrhea that remains one of the most common sexually transmitted diseases contributing to worldwide morbidity, mortality and infertility. Although treatable with antibiotics, no vaccine is currently available against the gonococcus. Neisseria meningitidis is primarily a commensal of the human oropharynx that, under incompletely understood circumstances, causes invasive disease and meningitis. Most cases of meningococcal disease are caused by clonal complexes of related sequence types (STs), the so-called hyper-invasive lineages [2]. These lineages are underrepresented in healthy carriers and significant numbers of individuals are colonized with carriage isolates belonging to an array of STs that rarely cause disease [3].
Despite their differing host interactions, mechanisms of transmission and ecology, N. gonorrhoeae and N. meningitidis display remarkable conservation and uniformity at the levels of coding sequences, gene content and synteny. Nonetheless, comparative genome analyses have identified genes and gene clusters unique to either N. gonorrhoeae or N. meningitidis but few if any of the corresponding products can be specifically connected to the differential host interactions observed. A prime example of this situation would be the genes required for biosynthesis of polysaccharide capsule, which is essential to systemic meningococcal disease, and that are absent in N. gonorrhoeae. However, only a limited subset of capsular serogroups is associated with disease and 16–20% of meningococcal carriage isolates do not possess these genes [4]. A recent study documented that the presence of the insertion sequence IS1655 is restricted to N. meningitidis but how this element might relate to speciation or specifically to meningococcal biology remains unclear [5]. Attempts to reconcile the relationships between genotype and lifestyle of N. meningitidis and N. gonorrhoeae are further complicated by the existence of the closely related species Neisseria lactamica, a harmless commensal found predominantly in the upper respiratory tracts of infants and children [6]. In contrast to N. meningitidis in which carriage is low during infancy and rises to high levels in adolescents and young adults, carriage of N. lactamica is high in young children but declines with age [7]. Microarray-based genome hybridization studies showed that the majority of coding sequences are highly conserved in all three species although some genes unique to both N. gonorrhoeae and N. meningitidis were identified [8], [9], [10]. Included in the latter are the iga1 and pptA genes encoding an extracellular endopeptidase and a protein targeting phosphoethanolamine transferase [11], [12]. Validation of the impact of putative virulence components has been hampered by a lack of relevant animal models for neisserial disease.
The highly conserved genetic structure and human host restriction observed for N. gonorrhoeae and N. meningitidis are most consistent with allopatric divergence from a single common ancestor. Such a model was first proposed by Vazquez and colleagues based on the relatively reduced diversity of N. gonorrhoeae strains measured by multilocus enzyme electrophoresis (vs N. meningitidis) of house keeping genes and the fact that the primary niche for all human Neisseria species other than N. gonorrhoeae is the oropharynx [13]. Specifically, it was suggested that N. gonorrhoeae arose as a clone of N. meningitidis that could colonize the urogenital tract. Prolonged physical isolation, niche specialization and genetic isolation would thus have driven speciation. This model was further supported by the analyses of the porA gene (encoding the class 1 outer membrane porin protein PorA) that is found in all strains of N. meningitidis and N. gonorrhoeae but absent in N. lactamica and other commensal Neisseria species [14]. While PorA is a major constituent of the outer membrane of most meningococcal isolates, all strains of N. gonorrhoeae examined to date carry an identical frameshift mutation that disrupts the integrity of the porA ORF [15]. Two other genes unique to the pathogenic Neisseria, ggt and adhC (encoding gamma-glutamyl transpeptidase and S-nitrosoglutathione oxidoreductase respectively), are also intact in N. meningitidis but inactivated due to frameshift mutations in N. gonorrhoeae [16], [17]. While the presence of these three pseudogenes in N. gonorrhoeae denotes descent from an organism carrying active forms of the genes, it remains unknown how extant isolates of N. meningitidis and N. gonorrhoeae might relate to such an ancestral population. Despite the potentially confounding contributions of shared ancestry and genetic exchange, a recent study utilizing multilocus sequence typing (MLST) demonstrated the ability to readily categorize isolates of N. meningitidis, N. gonorrhoeae and N. lactamica into three distinct species [18].
Oxygen reductase members of the heme-copper superfamily act as terminal oxidases in all domains of life and play a central role in aerobic energy generation and conservation [19]. Given their critical function, alterations in their structure are likely to have important consequences and therefore be targets of natural selection. Adaptive changes in cytochrome c oxidases have been implicated in major evolutionary transitions in anthropoid primates and carnivorous plants [20], [21]. Most bacteria utilize branched electron-transfer networks that enable them to use diverse electron donors and/or electron acceptors in respiration. The sole oxygen reductase catalysing the reduction of dioxygen to water encoded in Neisseria genomes is of the c-family or cytochrome cbb3 oxidase type [22]. Canonical cytochrome cbb3 oxidases consist of four subunits encoded by tandemly arranged genes (Figure 1A) [23]. CcoN is the highly conserved, catalytic subunit that contains a dinuclear centre formed by the iron of a high-spin heme and an associated copper ion (CuB) where dioxygen is reduced [24]. CcoO and CcoP are both membrane-bound c-type cytochromes believed to channel electrons to the dinuclear center [25]. CcoQ is a small, single-spanning membrane protein believed to function in stabilizing the interaction of CcoP with the CcoNO complex [26]. The roles of CcoQ and CcoP may vary between species as active forms of the complex can be detected in their absence in some but not all cases [27], [28]. Cytochrome cbb3 oxidase in some organisms has a high affinity for oxygen and is often associated with growth under conditions of oxygen-restriction [22] and both N. gonorrhoeae and N. meningitidis inhabit niches associated with low oxygen tension [29], [30]. Importantly, cytochrome cbb3 oxidases couple oxygen reduction to translocating protons across the inner membrane such that metabolic energy is conserved for subsequent ATP synthesis [31].
Fig. 1. Organization of the neisserial ccoNOQP locus and domain architectures of c-type multiheme CcoPs. 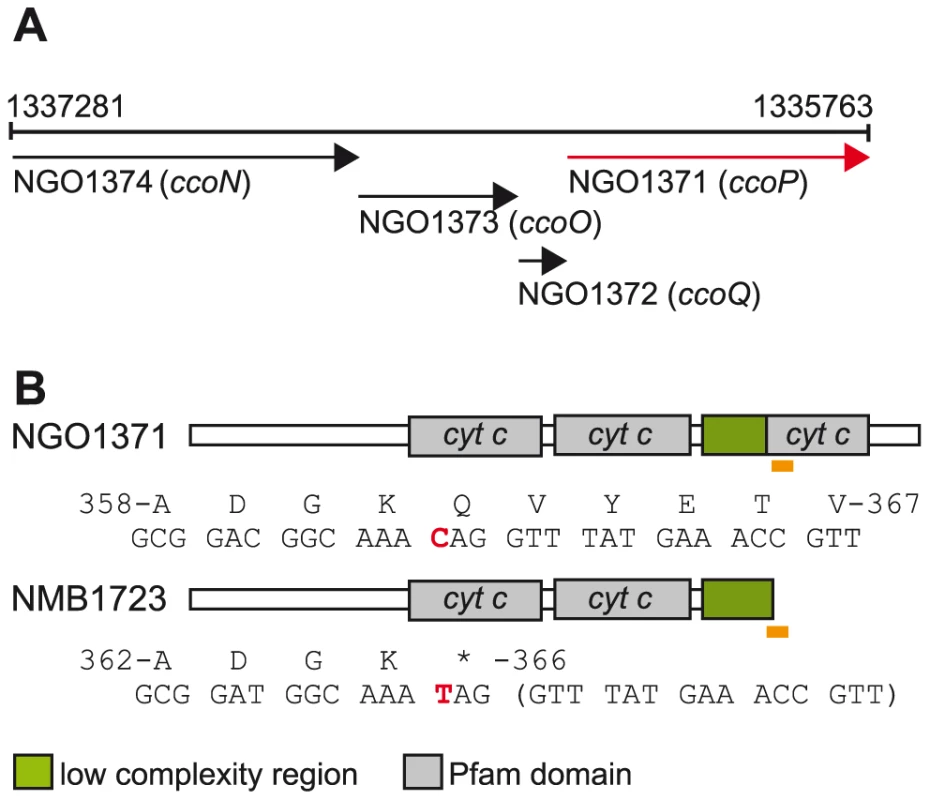
(A) The organization of the neisserial ccoNOQP gene cluster. Numbers denote the nucleotide sequence coordinates from the genome sequence of N. gonorrhoeae strain FA1090 (Genbank AE004969). (B) Modular structures of the cognate neisserial tri-heme CcoP and the di-heme form associated with isolates of N. meningitidis. The region encompassing the premature stop codon resulting in CcoP truncation and the corresponding region in tri-heme CcoP are indicated by yellow rectangles and the specific residues and the corresponding codons are numbered according to those of intact polypeptides. The single base change creating a premature stop codon in ccoPNme (CAG to TAG) is indicated in red. Note that the codon involved corresponds to residue 366 in N. meningitidis but to residue 362 in gonococcal CcoP owing to a 12 base pair deletion in the N. gonorrhoeae alleles (Figure S1). N. gonorrhoeae and N. meningitidis also possess a truncated denitrification pathway in which nitrite (NO2−) is first reduced to nitric oxide (NO) by NirK (AniA) that is then reduced to nitrous oxide (N2O) by NorB [32], [33], [34], [35]. The linked nirK and norB genes are differentially controlled by a number of transcriptional regulators that are responsive to changes in the levels of oxygen, NO2− and NO [36], [37], [38], [39], [40], [41]. Although previously considered to be an anaerobic process, denitrification under oxic conditions has been documented in many species including N. gonorrhoeae and N. meningitidis [35], [42]. Taken together, the latter species appear to be adapted to similar growth environments at distinct ecological sites (i.e. urogenital versus oropharyngeal mucosal sites) and have highly related respiratory chains that allow them to co-metabolize oxygen and nitrite as electron acceptors under microaerobic conditions.
A cardinal feature of N. gonorrhoeae and N. meningitidis is their highly recombinogenic nature that results from inter - and intraspecies genetic exchange [43]. Efficient lateral gene transfer in these instances is mediated through natural competence for transformation requiring specific uptake sequences in the donor DNA [44]. As these DNA uptake sequences are dramatically overrepresented only in the genomes of Neisseria species, this largely restricts imports to donors from within the genus. In the particular case of N. meningitidis, interspecies recombination at numerous loci encoding surface antigens has been widely documented [45], [46], [47]. Still as N. gonorrhoeae, N. meningitidis and N. lactamica species represent cohesive, differentiated entities [18], gene flow must be constrained at some level in order to limit convergence and despeciation [48]. However, the molecular and ecological factors constraining genetic structuring in these instances remain to be determined. Given these circumstances, the phylogenetic basis for the evolution of human Neisseria species remains poorly understood. Moreover, it remains unclear what genetic elements dictate the unique human interactions exhibited by each species.
Rare genomic changes, signature mutations occurring in the genomes of particular clades, have frequently been employed to resolve phylogenetic uncertainties [49]. Here, we report the identification of a single nucleotide polymorphism (SNP) that results in a molecular marker for the species N. meningitidis as it is found in all strains examined and absent from all isolates of N. gonorrhoeae and N. lactamica and of other commensals tested. Although this mutation results in the truncation of an essential component of the cytochrome cbb3 oxidase, the sole neisserial respiratory oxidase, it conditionally affects nitrite consumption by the nitrite reductase that functions in the denitrification pathway. These findings provide evidence that an alteration in the circuitry of respiratory electron-transfer networks is associated with N. meningitidis speciation.
Results
A SNP leading to truncation of the CcoP subunit of cytochrome cbb3 oxidase is associated with the species N. meningitidis
In the course of characterizing a general O-linked protein glycosylation system in N. gonorrhoeae strain MS11 [50], we discovered that its CcoP is a glycoprotein possessing a tri-heme c-type cytochrome domain architecture as opposed to the di-heme form found in all other CcoP proteins (as annotated by possessing the IPR004678 domain of the InterPro database) (Figure 1B). When the status of N. meningitidis CcoP was examined using the genome sequences for 5 strains available, the nucleotide and deduced amino acid sequences were highly related (greater than 97%) to that in N. gonorrhoeae save for the presence of transitional substitution in codon 366 (CAG to TAG resulting in a premature stop codon). The N. meningitidis forms would then be predicted to be truncated at the end of the AlaSerPro-rich, low complexity region (LCR) that separates the second and third c-type heme domains and encompasses the glycan attachment site [50]. DNA sequencing of ccoP from 78 additional N. meningitidis isolates used in this study (Table S1) further supported the presence of the SNP seen in the genomic sequences resulting in CcoP truncation. In contrast, ccoP DNA sequencing and accessing genome sequencing projects for which ccoP data were available revealed the absence of the SNP from 26 N. gonorrhoeae strains, 13 N. lactamica strains and 11 commensal Neisseria strains encompassing 8 species (Table S2). Thus, the association of the SNP with the species N. meningitidis was complete. Furthermore, we conclude that the tri-heme encoding ccoP allele is ancestral to the N. meningitidis alleles.
Phylogenetic examination of ccoP genes using the majority rule consensus tree constructed with Clonalframe revealed five distinct branches corresponding to each of the Neisseria species: N. meningitidis, N. gonorrhoeae, N. lactamica and other Neisseria including N. cinerea, N. polysaccharea, N. sicca, N. subflava and N. flavescens further differentiating these isolates from one another. Within each clade, ccoP genes were well conserved (p-distances: N. lactamica = 0.010, N. meningitidis = 0.004, N. gonorrhoeae = 0.002) although ccoP genes belonging to commensal isolates other than N. lactamica were much more diverse (p-distance = 0.165) (Figure 2). This was apparent in the alignment of polymorphic sites (Figure S1) where synonymous and non-synonymous substitutions along the ccoP gene were more abundant when these neisserial commensals were included in the analysis further confirming the homology of ccoP genes belonging to N. meningitidis, N. gonorrhoeae and N. lactamica isolates. Recombination events among ccoP genes were uncommon with a total of five events predominantly among ccoP genes belonging to commensal Neisseria. One horizontal gene transfer event from a meningococcal isolate and gonococcal isolate to N. cinerea was also detected with high probability by ClonalFrame (Table S3).
Fig. 2. Genealogical representation of the ccoP gene among Neisseria strains using ClonalFrame. 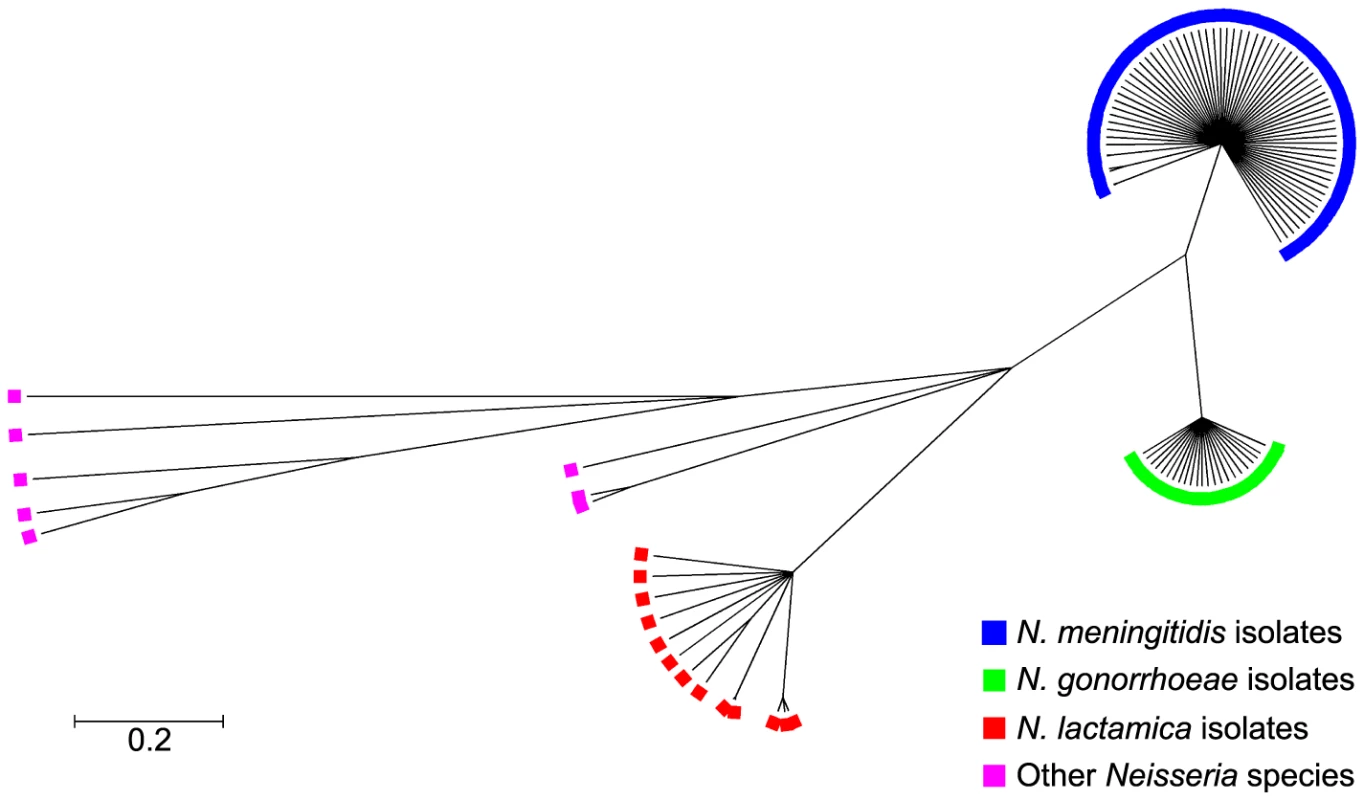
Phylogenetic trees were constructed using ClonalFrame version 1.1 available at http://www.xavierdidelot.xtreemhost.com/clonalframe.htm [74]. In the present study, over 300,000 iterations and 100,000 burn-ins were performed with every hundredth tree sampled after which, a 95% consensus tree was derived. Annotation was then undertaken by importing the tree into the Molecular Evolutionary Genetics Analysis software package (MEGA v4.0) [73]. The extended, third c-type heme domain of CcoP impacts on denitrification respiratory flux
Given the unique association between the SNP resulting in expression of the truncated, di-heme CcoP form and N. meningitidis, we sought to determine what phenotypic consequences might ensue from such an alteration. We therefore took a comparative approach to addressing this point by constructing strains of N. meningitidis MC58 expressing the tri-heme CcoP form found in N. gonorrhoeae and strains of N. gonorrhoeae VD300 expressing the truncated di-heme form from N. meningitidis (Figures 3A and B, respectively). This was done using transposon insertions mapping 3′ of ccoP as selectable markers in trans-species transformation experiments. Direct sequencing of ccoP was used to ensure that the entire, specific ORFs were transferred and exchanged in the recombinants used. In addition, a strain of N. gonorrhoeae was constructed in which solely the SNP responsible for CcoP truncation in N. meningitidis was incorporated into the otherwise unaltered N. gonorrhoeae allele. When subjected to a variety of aerobic growth conditions, we observed no differences between isogenic strains expressing the di - and tri-heme forms in either N. meningitidis or N. gonorrhoeae (Figures S2A, B and S3A, B respectively, data not shown). Thus, the skewed distribution of CcoP forms was not a consequence of gross metabolic incompatability as measured in vitro.
Fig. 3. Heme-stained protein blots showing altered expression of c5 and CcoP in defined backgrounds. 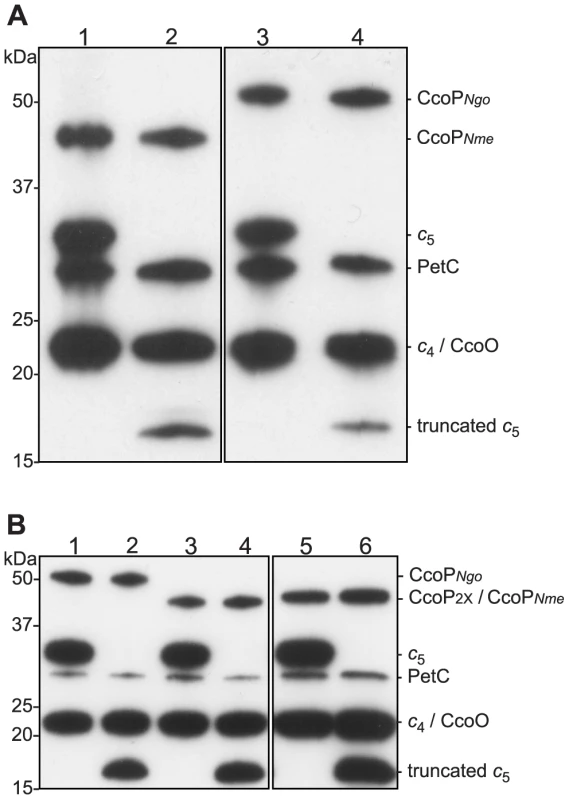
Samples of total cell extracts were separated by 12% SDS-PAGE, blotted and stained for heme-dependent peroxidase activity. (A) N. meningitidis strains: 1, wild type (MC58); 2, cycB (c5-); 3, ccoPNgo (KS348); 4, cycB, ccoPNgo (KS349). (B) N. gonorrhoeae strains: 1, wild-type (VD300); 2, cycB (KS336); 3, ccoP2x (KS335); 4, ccoP2x, cycB (KS337); 5, ccoPNme (KS340); 6, ccoPNme cycB (KS341). In another approach, BLAST searches were performed using the third heme encompassing domain immediately C-terminal of the major AlaSerPro-rich LCR in N. gonorrhoeae CcoP as a query. This segment was highly related to the C-terminally localized domain in cytochrome c5, a di-heme c-type cytochrome encoded by the cycB gene (NMB1677 in N. meningitidis and NGO1328 in N. gonorrhoeae). Like CcoP, c5 is predicted to be membrane-associated and also possesses an AlaSerPro-rich LCR that encompasses the attachment sites of its O-linked glycan between its two c-type heme domains [50]. In N. meningitidis, a tetracycline resistance gene cassette insertion mutation in cycB that disrupts the integrity of the ORF distal to the first heme domain was shown to abolish nitrite reduction and nitrite-dependent growth under microaerobic conditions [51]. In N. gonorrhoeae, a cycB null mutation was reported to result in increased sensitivity to growth inhibition by excess oxygen and small decreases in respiratory capacity [52]. Thus, c5 is a prime candidate to act as an electron carrier in pathways ultimately targeting both cytochrome cbb3 oxidase and NirK.
Given the structural similarities between c5 and the tri-heme CcoP form, we examined what genetic interactions might exist between cycB and the different ccoP alleles with regard to the truncated dentrification pathway. To this end, a cycB null mutation was generated in which the entire open reading frame was deleted. Derivatives of N. gonorrhoeae carrying this allele were distinctive in that they exhibited a severe growth defect manifested as poor plating efficiency and slow growth that was not seen in equivalent strains carrying the previously characterized cycB insertion mutation (data not shown). While profiling of c-type heme proteins confirmed the absence of intact c5 in both backgrounds, strains carrying the cycB insertion mutation expressed a c-type heme protein whose migration corresponded to that predicted for a truncated, mono-heme c5 form resulting from disruption of the open reading frame at residue 171 (Figures 3A, B and Figure S4). As the cycB null mutation was pleiotropic, the cycB insertion mutation was used in this study. As previously reported [51], introduction of cycB insertion mutation into N. meningitidis MC58 led to a clear defect in nitrite-dependent, microaerobic growth and measurements from culture medium showed that this growth defect was associated with an inability to reduce nitrite (Figures 4A and B). In contrast, the strain carrying both the ccoPNgo and cycB alleles was remarkably similar to the wildtype strain and that carrying only the ccoPNgo allele in these phenotypes (Figures 4A and B). No differences in growth for any of these strains were seen under aerobic and microaerobic conditions (Figures S2A and B, data not shown). Thus, the ccoPNgo allele was epistatic to cycB demonstrating that the CcoP tri-heme isoform and c5 overlap functionally in supporting NirK-mediated, nitrite reduction.
Fig. 4. Effects of CcoP domain alterations on microaerobic growth and nitrite reduction in N. meningitidis. 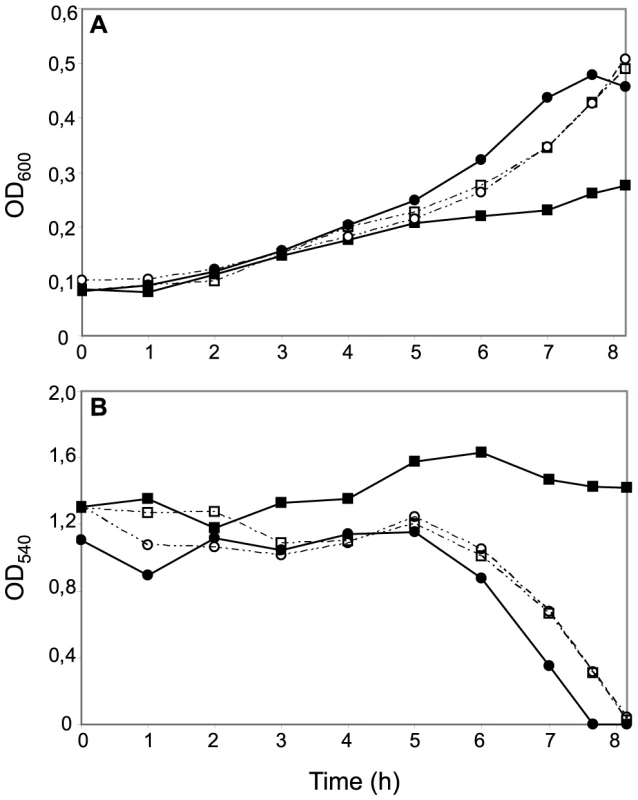
Cultures of wild-type (MC58) (open squares); ccoPNgo (KS348) (open circles); cycB (c5-), (filled squares) and ccoPNgo, cycB (KS349) (filled circles) growing under microaerobic conditions plus 5 mM nitrite (A). Growth was monitored by measuring OD600. (B) Nitrite reduction was monitored for microaerobic cultures from (A). The results shown are representative of three independent experiments. Reciprocal experiments were carried out in N. gonorrhoeae VD300 using backgrounds carrying either endogenous ccoP or alleles encoding truncated di-heme CcoP forms. Here again, no significant differences in growth for any of these strains were seen under aerobic and microaerobic conditions (Figures S3A and B). Both N. gonorrhoeae strains expressing di-heme CcoP forms were reduced in rates of growth under nitrite supplemented, microaerobic conditions relative to the strain expressing the tri-heme form and this property was paralleled by delayed nitrite consumption (Figures 5A and B). These results were similar to those reported elsewhere in which missense mutations predicted to disrupt the integrity of the third c-type heme domain of N. gonorrhoeae CcoP were associated with diminished nitrite-dependent, microaerobic growth and reduced capacities to reduce nitrite [42]. These same phenotypes were exhibited by the N. gonorrhoeae strain with the cycB insertion mutation alone. However, when combined with the alleles encoding di-heme CcoP, the cycB mutation led to a complete defect in nitrite-dependent, microaerobic growth (Figure 5A). Measurements from culture medium showed that this growth defect was associated with the inability to reduce nitrite (Figure 5B). These phenotypes were not attributable to a defect in NirK expression, as the latter was detected at similar levels in all backgrounds (Figure S5). To ensure that the defect in nitrite reduction in these backgrounds was not due to a spurious mutation or genetic alteration arising during strain construction or propagation, a wildtype copy of cycB was reintroduced at an ectopic site. This led to restoration of nitrite-dependent, microaerobic growth and nitrite reduction in the cycB, ccoP2X (di-heme form of N. gonorrhoeae CcoP made by changing codon number 362 from CAG to the stop codon TAG) backgrounds (data not shown). Taken together, these results document clear differences in the requirements for nitrite reduction associated with di - and tri-heme encoding ccoP alleles. Accordingly, the findings strongly suggest that strains of N. meningitidis are fundamentally distinct from those of other neisserial species with regard to respiratory denitrification.
Fig. 5. Effects of CcoP domain alterations on microaerobic growth and nitrite reduction in N. gonorrhoeae. 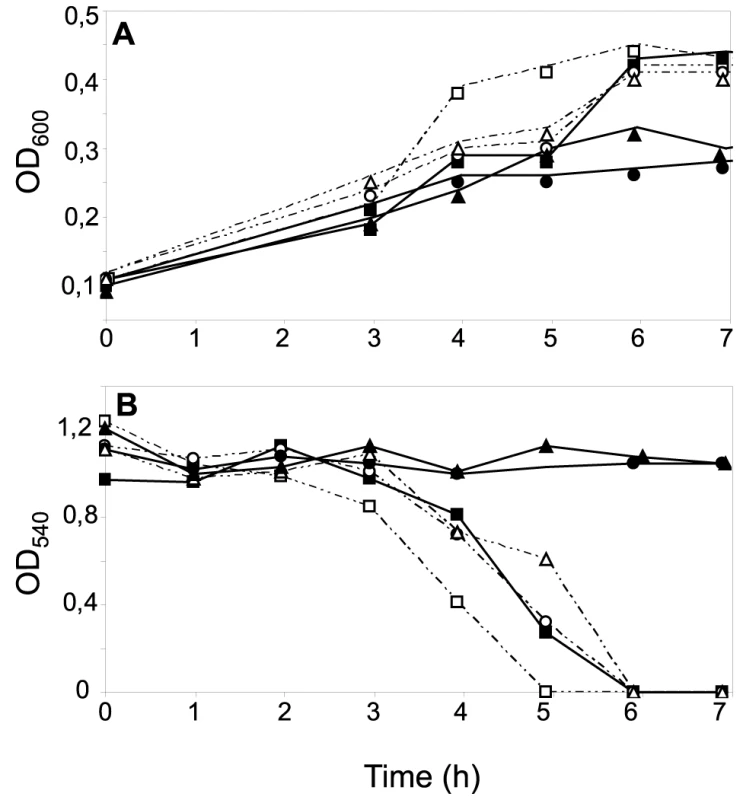
Cultures of wild-type (VD300) (open squares); and mutants ccoP2x (KS335) (open circles); cycB (KS336) (filled squares); ccoP2x, cycB (KS337) (filled circles); ccoPNme (KS340) (open triangles); ccoPNme, cycB (KS341) (filled triangles) growing under microaerobic conditions plus 5 mM nitrite (A). Growth was monitored by measuring OD600. (B) Nitrite reduction was monitored for microaerobic cultures from (A). The results shown are representative of three independent experiments. The di-heme CcoP form is essential to microaerobic growth in N. meningitidis and N. gonorrhoeae
To examine the role of CcoP in N. meningitidis, transposon insertion mutations were generated in the cloned ccoP gene that were then introduced into a wildtype background. However, transformants were only recovered for those insertions that mapped 3′ to the ORF encompassing the second c-type heme domain (i.e. within the LCR and third c-type heme domain). To ensure that this selectivity was not due to some peculiarity of the constructs themselves, a strain carrying a second active copy of ccoP at an ectopic site was created. Transformants for all insertion mutations were recovered using this merodiploid strain (Figure 6A). A similar approach was taken to examining CcoP function in N. gonorrhoeae with transposon insertion mutations being generated in the cloned gene that were then introduced into a wildtype background. As seen in the N. meningitidis wildtype background, transformants were only recovered for those mutations that mapped 3′ to the ORF encoding the second c-type heme domain (i.e. within the LCR and third c-type heme domain). Here as a control, a strain carrying a tandem duplication of ccoP (by Campbell-type plasmid integration) was used in which the distal gene copy was non-functional due to a deletion of the promoter and translational initiation sites. Transformants for all insertion mutations were recovered using this strain and importantly, all those that could not be recovered in the wildtype background mapped exclusively in the non-expressed ccoP copy (Figure 6B). As mutations that disrupt the integrity of the di-heme form are conditionally defective in microaerobic growth, CcoP appeared to be an essential gene product in both N. meningitidis and N. gonorrhoeae. Moreover, the structural features and constraints for minimal CcoP function (imparted by the di-heme form) were likely identical in both species.
Fig. 6. Distribution of ccoP transposon insertion mutations recovered in wildtype and merodiploid backgrounds. 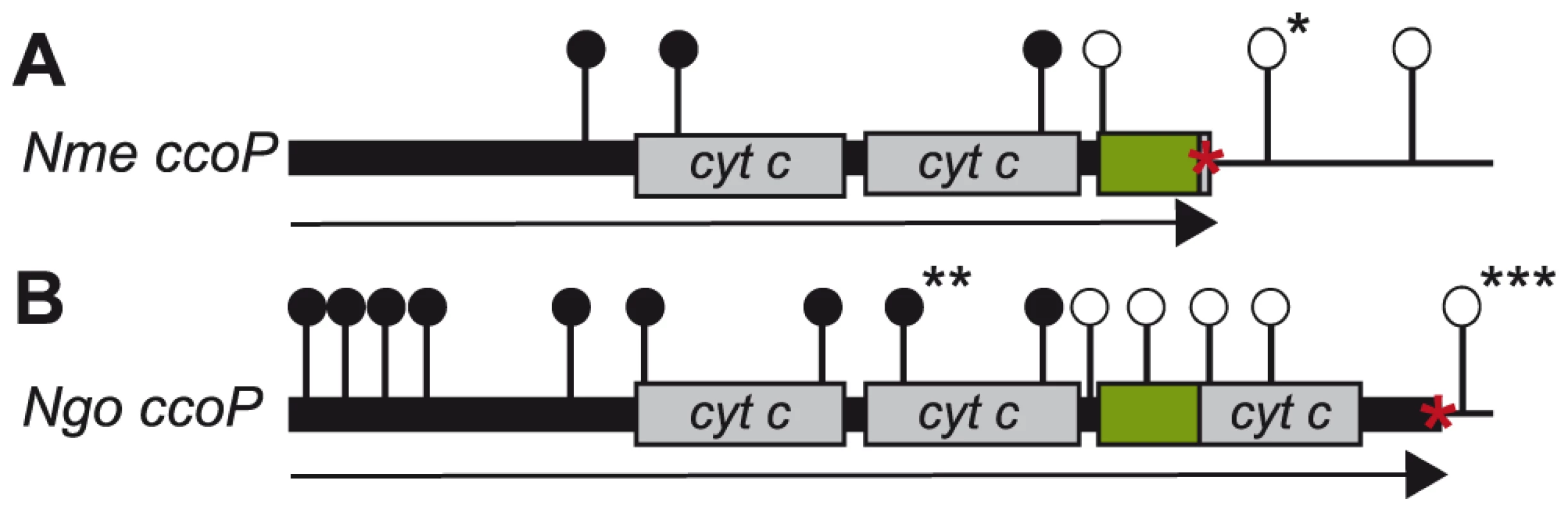
Shown are the sites of mTn insertions with open circles denoting those recoverable in N. meningitidis (A) and N. gonorrhoeae (B) wildtype backgrounds. Filled circles denote those that were only recoverable in strains carrying a second copy of ccoP. In the case of N. meningitidis merodiploid background in which both gene copies were active, insertions denoted by filled circles were recovered at both loci. In the case of N. gonorrhoeae merodiploid background, the second ccoP copy was inactive and all insertions denoted by filled circles mapped in the non-expressed copy. Symbols: arrows indicate the ccoP open reading frame, grey boxes represent regions encoding c-type heme domains, green boxes show AlaSerPro-rich, low complexity regions and red asterisks indicate stop codons. Insertions marked with single and triple black asterisks were used to swap ccoP alleles between N. meningitidis and N. gonorrhoeae. The insertion marked with double black asterisks was used to inactivate the endogenous N. gonorrhoeae allele in the background carrying the de-repressible ccoP allele at an ectopic site (see text). To confirm the essentiality of ccoP, a transcriptional fusion construct was made in which expression was linked to tac-UV5 control sequences and introduced at an ectopic site in the neisserial genome. As this construct does not include an associated lacIQ gene, repression requires a background expressing LacIQ. For practical purposes, we therefore carried out these experiments in N. gonorrhoeae which enabled us to first introduce the ectopic de-repressible ccoP allele in a background expressing di-heme CcoP from the endogenous locus (Figure 7A). Transposon mutations previously established as abrogating CcoP function were then introduced into these backgrounds and transformants were selected in the presence of IPTG. Those that acquired IPTG dependent growth were readily identified in N. gonorrhoeae and in each instance, the transposon mapped to the endogenous ccoP gene (Figure 7B). It was quite easy to pick out revertants in which growth became IPTG-independent and in these instances, this phenotype was associated with restoration of de-regulated CcoP expression (data not shown). These findings demonstrated that CcoP was essential to growth under the microareobic conditions examined and reinforced the hypothesis that this effect was due to its role in cytochrome cbb3 oxidase activity.
Fig. 7. Conditional expression of CcoP confirms its essential role in growth. 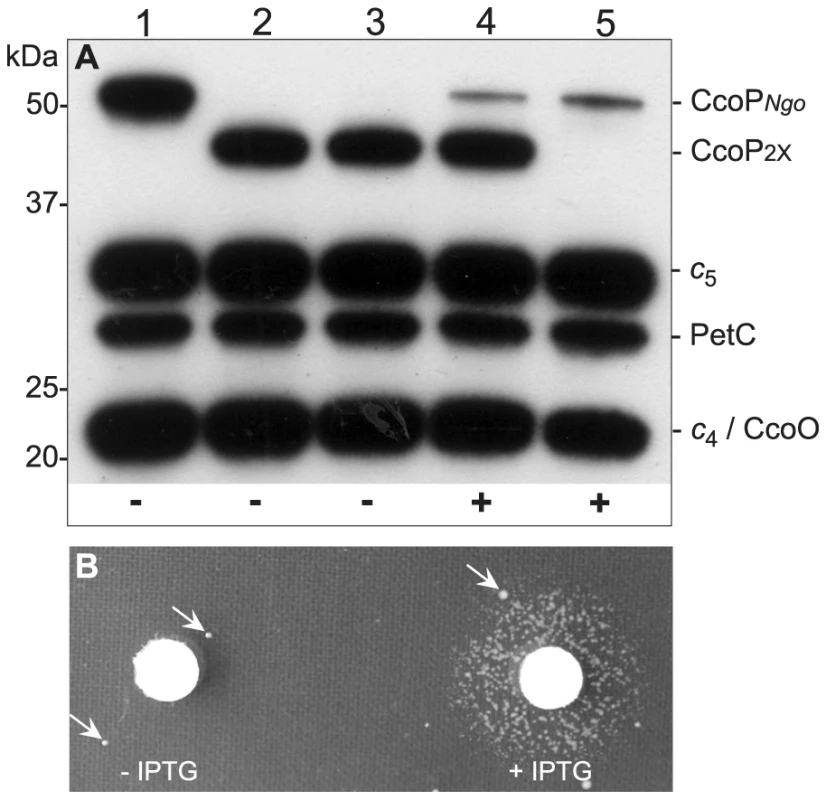
(A) Heme-stained blot of N. gonorrhoeae extracts showing profiles of c-type heme proteins. Total cell extracts from wildtype and mutants were separated by 10% SDS-PAGE, transferred to membrane and stained for heme-dependent peroxidase activity. Strains: 1, wildtype (KS101); 2, ccoP2x (KS351); 3 and 4, ccoP2x, ccoPind (KS345); 5, ccoP2x::mTnerm26, ccoPind (KS346). CcoPNgo and CcoP2x mark the relative mobility of the tri-heme and di-heme forms. Note that strain ccoP2x::mTnerm26, ccoPind (lane 5) expresses only the inducible CcoP form. ‘+’ and ‘−’ at bottom denote the presence and absence of IPTG respectively in the culture media. As shown in (B), the strain expressing solely the inducible CcoP form requires IPTG for growth on solid medium. Arrows indicate the presence of suppressor mutants that are associated with IPTG-independent CcoP expression. A c5-CcoP hybrid supports nitrite reduction and related phenotypes
The genetic interactions and degree of shared structural identity suggested that the C-terminal c-type heme domains of c5 and CcoP have related functions in chanelling electrons to support nitrite reduction activity. Therefore, a translational fusion consisting of the amino-terminus of c5 (encompassing the first c-type heme domain) and the third heme domain of N. gonorrhoeae CcoP was constructed. This was done in a manner such that the relative spacing between the two heme domains in the hybrid was maintained as seen for wildtype c5 and was facilitated by using a conserved stretch of residues in the AlaSerPro-rich linker domains (Figure S6). This gene fusion including the endogenous cycB promoter sequences was then introduced at an ectopic site in the N. gonorrhoeae background expressing the truncated cycB allele (from its endogenous site) and a di-heme expressing ccoP allele. The c5-CcoP chimaera was detected as a novel cytochrome c species that migrated with a mobility equivalent to that seen for endogenous c5 (Figure 8A). This hybrid gene restored both nitrite reduction and nitrite-dependent microareobic growth in this background (Figures 8B and C). These findings formally demonstrate the functional equivalence of the terminal heme domains of N. gonorrhoeae CcoP and c5 when displayed in the context of otherwise structurally identical polypeptides.
Fig. 8. Ectopic expression of a c5-CcoP hybrid protein complements a defect in nitrite-dependent, microaerobic growth in N. gonorrhoeae. 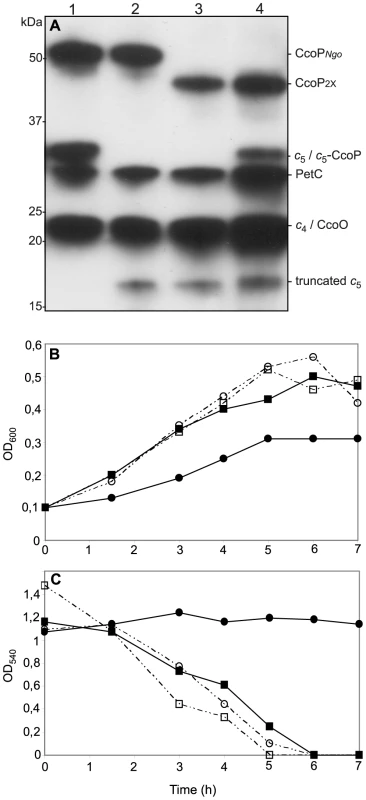
(A) Samples of total cell extracts from wildtype and mutants separated by SDS-PAGE, blotted and stained for c-type heme-dependent peroxidase activity. Strains: 1, wildtype (VD300); 2, cycB (KS336); 3, cycB, ccoP2x (KS337); 4, cycB, ccoP2x, cycB-ccoP (KS347). (B) Cultures of wild-type (VD300) (open squares); and mutants cycB (KS336) (filled squares); cycB, ccoP2x (KS337) (filled circles) and cycB, ccoP2x, cycB-ccoP (KS347) (open circles) growing under microaerobic conditions supplemented with 5 mM nitrite. (C) Nitrite depletion was monitored for microaerobic cultures from (B). The results shown are representative of three independent experiments. A modular domain fusion with c5 accounts for the genesis of the tri-heme CcoP form
Given its unusual activity and unique distribution within Neisseria species, the potential evolutionary origin of the tri-heme CcoP was examined. As the vast majority of CcoP forms identified to date are di-heme forms, a simple model might involve the amplification of the tandemly arrayed, heme encoding gene segments. However, this mechanism is inconsistent with the observed lack of nucleotide sequence identity encoding the spacer domains between the first and second heme units and the second and third heme units. Instead, the most parsimonious scenario entails a non-reciprocal recombination event involving the 3′ ends of the c5 gene and a primordial di-heme CcoP. In this model, unequal crossing over within the homologous sequences of the c5 di-heme - encoding and CcoP second heme - encoding segments would provide gene and protein structures most reconciliable with that of extant tri-heme CcoP forms (Figure 9). This hypothesis is particularly well supported by the high degree of structural conservation between the carboxy-terminal segment (encompassing the third heme domain) of CcoP and the two heme domains of c5. The last 81 residues of CcoP (359–439) share 74% sequence identity with the corresponding C-terminal segment of c5 and 47% with the first heme domain of c5. Such a scenario would account for the presence of the AlaSerPro-rich region between the second and third CcoP heme domains. Although it is difficult to establish amino acid identity within these AlaSerPro rich regions (due to their underlying low complexity), both the lengths of the AlaSerPro stretches (34 residues in c5 versus 42 in CcoP) and overall alanine richness (47.1% Ala residues in c5 versus 47.6% in CcoP) are similar between the two. Moreover, these stretches each bear serine occupancy sites for the glycans associated with the general O-linked glycosylation system in N. gonorrhoeae [50]. Taken together, the conserved structural features and shared functional attributes of c5 and the tri-heme CcoP provide strong evidence for modular-based evolution underlying CcoP neofunctionalization.
Fig. 9. Model for the origin of the tri-heme CcoP in the genus Neisseria. 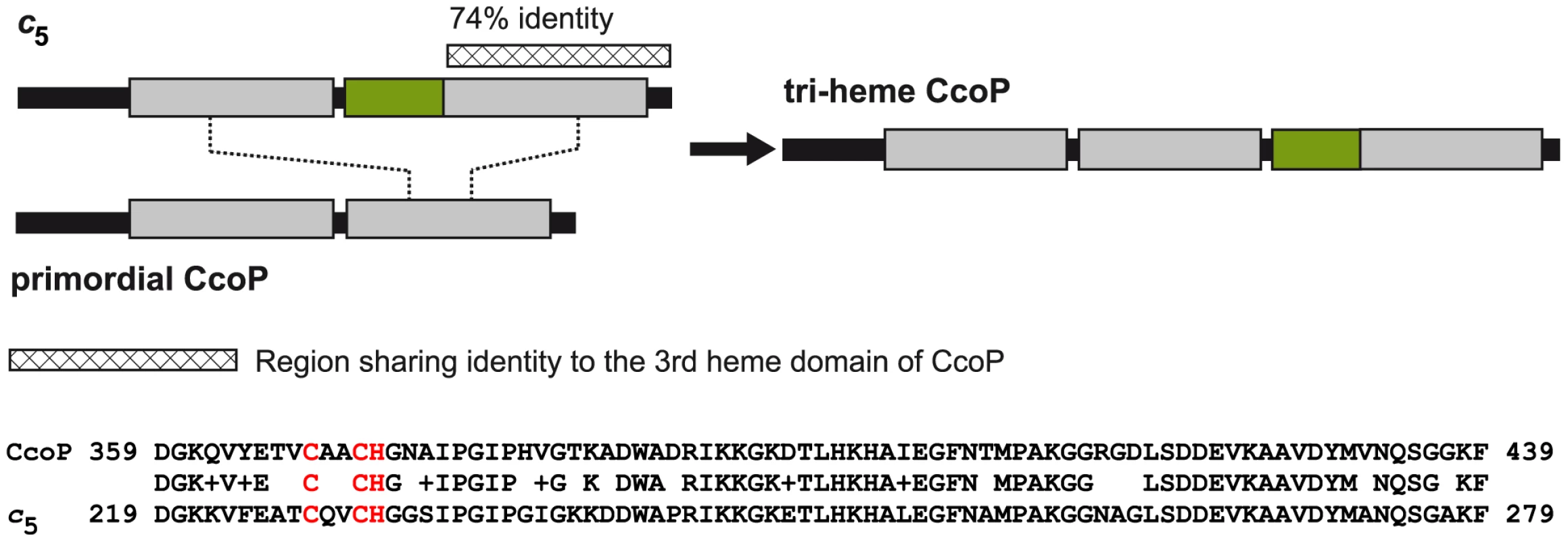
Based on the shared domain architecture, high degrees of primary sequence identity and function, the most parsimonious model invokes a gene fusion event between cycB (encoding c5) and a primordial c-type di-heme encoding ccoP gene (top panel). This hypothesis is particularly well supported by the high degree of structural conservation between the carboxy-terminal segment (encompassing the third heme domain) of CcoP and the heme domains of c5 (heme domains in grey). Specifically, residues 359–439 of CcoP share 74% identity with the corresponding C-terminal segment of c5 and 47% with the first heme domain of c5 (bottom panel, the two cysteine and single histidine residues found in a CXXCH motif required for disulfide bonding to the vinyl groups of heme are shown in red). Secondly, such a scenario would account for the presence of the AlaSerPro-rich region (in green) between the second and third CcoP heme domains that is similar in sequence composition and length to the equivalent interdomain segment of c5. Finally, both of these same stretches each bear serine occupancy sites for the O-linked glycosylation [50]. Discussion
Three closely related species within the genus Neisseria represent an intriguing model system in which to investigate the evolution trajectories of related pathogenic and commensal bacteria in a single host. Here, we report the identification of a SNP unique in its association with the species N. meningitidis that leads to truncation of the c-type heme protein CcoP, an essential component of its sole, terminal respiratory cytochrome oxidase. The dichotomy in neisserial phylogeny revealed through this altered molecular character raises the obvious question as to why this change appears to be so adaptive on the one hand and yet at the same time, so restricted in its distribution. From the sole viewpoint of aerobic respiration, CcoP functions by supporting cytochrome cbb3 oxidase activity. However, we failed to discern any phenotypic alterations in either the N. gonorrhoeae or N. meningitidis strains varying solely in expression of the di - versus tri-heme forms under standard lab conditions. Therefore, a key factor here may be the altered circuitry of electron transfer favoring microaerobic (oxygen reduction by cytochrome cbb3 oxidase) versus combined microaerobic denitrifying (oxygen reduction by cytochrome cbb3 oxidase supplemented by nitrite reduction by NirK) respiration. For example, the ability of di-heme CcoP to promote solely cytochrome cbb3 oxidase activity (at the expense of reduced electron carriage to NirK) could be adaptive under more aerobic conditions or in situations where nitrite levels might be diminished. Moreover, nitrite reduction could come with significant metabolic cost as it generates nitric oxide that can be toxic and growth inhibitory [39]. Although N. meningitidis strains express the NorB nitric oxide reductase (reducing nitric oxide to innocuous nitrous oxide), toxic NO can accumulate so rapidly that growth inhibition occurs before sufficient NorB activity has been expressed (as it is specifically de-repressed by NO acting on the nitric oxide responsive repressor NsrR) [37], [39], [40]. This effect can be detected in N. meningitidis as NirK-dependent, nitrite sensitivity occurring under a number of growth conditions [53]. Additionally, there is strong evidence that N. meningitidis strains are under relaxed selective constraints with regard to NirK-mediated nitrite reduction. Specifically, nirK was not detected in 7 of 26 N. meningitidis strains examined by microarray-based genome hybridization technology [10]. Moreover, two reports examining nirK found frameshift and inactivating missense mutations in 5 of 23 and 8 of 31 strains respectively [54], [55]. Whilst the selection pressure for maintaining a functional NirK is relaxed in N. meningitidis, this is not the case in N. gonorrhoeae and other Neisseria species. In the latter strains, alleles encoding intact NirK are found in all available genomic and individual sequences (6 commensal neisserial strains and 36 strains of N. gonorrhoeae including those presented in Figure 2, Table S2 and in [54]. Thus, the fixation of the SNP - associated ccoP allele is consistent with the hypothesis that N. meningitidis is an organism in the process of evolving into a microaerobe that no longer supplements growth via NirK-mediated denitrification.
Another extraordinary aspect of the N. meningitidis ccoP allele is the fact that it represents a reversal of a previous rare genetic change in CcoP domain architecture. We provide a data-based model for how the tri-heme encoding ccoP arose by a homologous recombination event between c5 and a primordial, di-heme encoding ccoP allele and suggest that like the case of the SNP mutation, this event occurred only once and radiated by vertical or lateral transmission due to its adaptive value. Thus, the ccoP tri-heme allele (in its wildtype and derivative SNP variant forms) is a molecular marker of species within the genus Neisseria. The ability of tri-heme CcoP to act as a redox partner in a pathway that ultimately can transfer electrons to NirK would dramatically reshape the organization of electron transport chain, providing broader connectivity to electrons emanating from bc1 through c4 as well as c5 (Figure 10) [51], [52]. Reprogramming of electron flow circuitry might facilitate fine-tuning of the partition of electrons between the oxygen and nitrite reduction pathways. Such a system might be particularly useful when both electron acceptors are available but vary dynamically in relative abundance. Further studies of ccoP status in neisserial species are warranted as the number of strains examined here was restricted and some findings may be subject to sample bias. This concern may be particularly relevant to the N. meninigitidis isolates carrying capsule null loci (cnl) and N. lactamica isolates used here that were derived from carriage studies within the United Kingdom as well to other commensal species for which the data set is small. Nonetheless, the ccoP tri-heme allele is present in all Neisseria species for which data are available and it is conspicuously absent in any other species including members of the most closely related genera in the family Neisseriaceae such as Chromobacterium, Eikenella, Kingella, and Laribacter and more distant relatives within Betaproteobacteria.
Fig. 10. Proposed pathway for electron transfer from NADH to nitric oxide, oxygen and nitrite in N. meningitidis and N. gonorrhoeae. 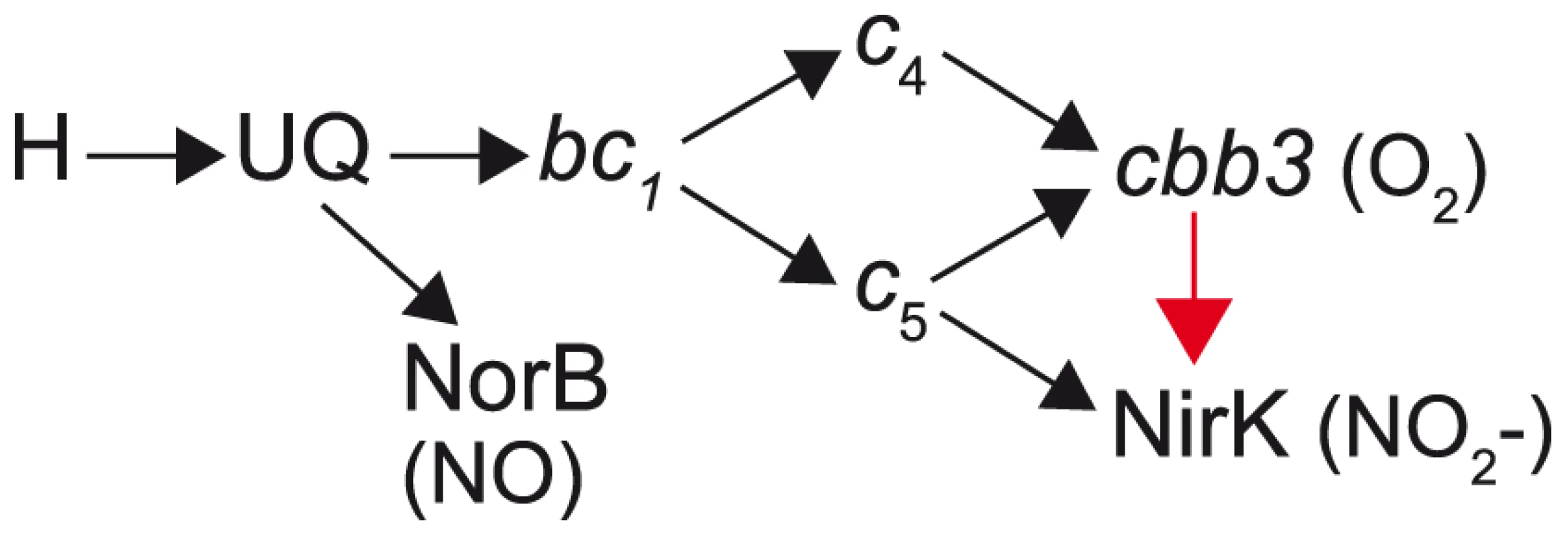
Electron flow from cytoplasmic reductants (NADH, succinate, etc. shown as [H]) to terminal electron acceptors NO, O2, and NO2 are shown by arrows. The unique pathway associated with the tri-heme CcoP form in N. gonorrhoeae (absent in N. meningitidis) is designated by the red arrow. UQ, ubiquinone; NirK, nitrite reductase; NorB, nitric oxide reductase. The model is based on data from this work and that found in [51], [52], [78]. The SNP-associated ccoP allele suggests that N. meningitidis as a clade has either undergone a relatively recent, dramatic reduction in population size (with a ccoP SNP -bearing strain first to pass the bottleneck) or that there has been a selective sweep of the SNP allele through the population via natural genetic transformation. Both scenarios are plausible given the relatively short time period over which sequential bottlenecks associated with epidemic spread can lead to clonal descent [43], [56] and the well - established capacity for frequent intraspecies recombination [57], [58]. N. meningitidis, N. lactamica and other commensal Neisseria species inhabit the same apparent oropharyngeal niche and bi-directional, interspecific genetic exchange of some loci occurs frequently [59]. We assume therefore that strains of N. meningitidis expressing tri-heme CcoP and of N. lactamica and other commensal species expressing di-heme CcoP arise but are purged due to reduced fitness. Consistent with this idea, the low p-distances observed by ClonalFrame for ccoP genes belonging to N. meningitidis, N. lactamica and N. gonorrhoeae are indicative of the conserved nature within species such that diversity may result in reduced fitness of the organism. The lack of recombination observed by RDP3 analysis (Table S3) among N. meningitidis, N. lactamica and N. gonorrhoeae (when compared to those found in other Neisseria species which exhibited higher p-distance values) further support this hypothesis. Therefore, other as yet unidentified differences in gene repertoire or expression are likely epistatic to ccoP.
An important aspect of this topic relates to which redox partners carry out direct electron transfer to neisserial NirK. Although in vitro studies have identified both azurins (members of the cupredoxin family) and c-type heme cytochromes as electron donors to other members of the blue, copper - containing nitrite reductase (CuNIR) family [60], [61], only rarely have the physiological contributions been addressed in vivo. Although both N. meningitidis and N. gonorrhoeae express a lipoprotein form of azurin termed Laz, it has been reported that Laz is nonessential for growth under either aerobic or anerobic conditions (in the presence of nitrite) [62]. A null mutation in the gene encoding Cyt c550 protein was recently shown to disrupt NirK dependent growth and nitrite utilization in Bradyrhizobium japonicum [63]. Also, CuNIRs that carry c-type heme domains translationally fused to the carboxy-termini of the nitrite reductase domain have been identified in other proteobacterial species by genome mining [64]. Perhaps most relevant, the recent high-resolution crystal structure of a blue CuNIR together with a c-type cytochrome provided conclusive evidence for the direct transfer of electrons between these partners [65]. Based on the genetic interactions established between cycB and ccoP here along with related observations [42], we favor a model in which both of the corresponding gene products directly donate electrons to NirK. A prerequisite for such a model is that c5 and CcoP (presumably in the context of cbb3) come in close contact with NirK. However, the NirK lipoprotein in its active trimer form is proposed to be linked to the outer membrane [66], [67] while cytochrome cbb3 oxidases must be integrated into the inner membrane in order to fulfil their role in proton pumping essential to ATP synthesis. While the carboxy terminal extensions (encompassing both the flexible LCR and distal heme domain) found in CcoP and c5 might function to bridge a potential periplasmic gap, more detailed studies of the spacial distribution and organization of the structures involved are needed to address this matter. Regardless of the molecular nature of electron transfer between CcoP and NirK, our finding here that a component of the oxygen reducing respiratory complex can be essential to the activity of an alternative reductase is unprecedented.
In summary, these findings provide a novel perspective on the evolution of species within the genus Neisseria. They also demonstrate that microaerobic denitrification is a metabolic pathway of major influence in these bacteria and support the position that N. gonorrhoeae, N. lactamica and N. meningitidis fully deserve their designation as a distinct species, because they are clearly evolving independently. These findings also provide a dramatic example of how evolutionary change at the molecular level can be linked to metabolic innovation and its reversal as well as demonstrating how genotype can be used to infer alterations of the fitness landscape within a single host.
Materials and Methods
Bacterial strains, plasmids and growth conditions
A total of 71 N. meningitidis isolates initially employed in the evaluation of the (MultiLocus Sequence Typing) MLST typing method were used for ccoP sequence analysis [68]. The collection, which was assembled to be representative of organisms causing endemic and epidemic disease in the latter part of the 20th century, included several isolates from each of seven recognised hyper-invasive clonal complexes: (Sequence Type) ST-1, ST-5, ST-4, ST-11, ST-32, ST-8, and ST-41/44 in addition, to isolates belonging to clonal complexes ST-22, ST-23 and ST-13 (cc269) as well as isolates with unique sequence types. A total of 11 unencapsulated N. meningitidis isolates containing the capsule null locus were also investigated and these included isolates from clonal complexes ST-53, ST-198 and ST-334 (Table S1). The non-pathogenic Neisseria and N. gonorrhoeae isolates used for sequence studies are listed in Table S2. All isolates described above were grown overnight on Mueller Hinton agar supplemented with 5% defibrillated sterile horse blood in a 5% CO2 atmosphere. DNA was extracted using an IsoQuick Nucleic Acid Extraction kit (Orca Research Inc.) according to manufacturer's instructions. The neisserial strains used for functional studies are described in Table 1 and unless otherwise stated, all these strains were cultured on conventional GC plates over night at 37°C, in the presence of 5% CO2 as described previously [69]. Aerobic cultures were carried out in 7.5 ml of broth in 50 ml tubes shaken at 190 r.p.m (SARSTEDT, Nümbrecht, D). Microaerobic culture was carried out using 23 ml of GC broth, supplemented with 10mM NaHCO3, in 25 ml Sterilin McCartney bottle shaken at 190 r.p.m. [35]. Where appropriate, cultures were supplemented with 5mM NaNO2. Growth was monitored by measuring the optical density at 600 nm (OD600) in a WPA biowave CO 8000 Cell Density Meter. E. coli DH5α and HB101 were used for plasmid propagation and cloning experiments and were grown on Luria-Bertani media (LB). Antibiotics were used at the following concentrations for N. gonorrhoeae: chloramphenicol, 10µg/ml; erythromycin, 8µg/ml; tetracycline, 4µg/ml; for N. meningitidis: erythromycin, 8µg/ml; tetracycline, 20µg/ml; for E. coli: chloramphenicol, 30 µg/ml; erythoromycin 300µg/ml; kanamycin 50µg/ml; ampicillin 100µg/ml; tetracycline, 15µg/ml; streptomycin, 100µg/ml. pUP6 is a derivative of pHSS6 that carries two gonococcal DNA uptake sequences [70]. pGCC6 carries a chloramphenicol resistance cassette and a lac promoter/operator at an intergenic chromosomal site located between the gonococcal genes lctP and aspC. Cloning in front of the lac promoter thus allows for chromosomal integration of the insert between lctP and aspC in N. gonorrhoeae and subsequent IPTG inducible expression [71]. Isolation and purification of plasmid DNA was performed using QIAprep Spin Miniprep columns according to manufacturers specifications (Qiagen, Chatsworth, CA, U.S.A). The nucleotide sequences of all clones and constructs were determined from plasmid DNA or from PCR products by ABI 3730 high-throughput capillary electrophoresis sequencing.
Tab. 1. Neisseria strains used in this work. 
Di-heme form of N. gonorrhoeae CcoP made by changing codon number 362 from CAG to the stop codon TAG. Nucleotide sequence determination of ccoP alleles
Amplification and sequencing of ccoP genes from strains described in Tables S1 and S2 were completed using the primers listed in Table S4. PCR amplification consisted of a denaturation step at 94°C for five minutes followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for three minutes. PCR products were PEG purified and sequenced directly by cycle sequencing with BigDye Ready Reaction Mix (Applied Biosystems) according to manufacturer's instructions and using an ABI 377 automated DNA sequencer.
Sequence data manipulation and analysis
Nucleotide sequence data for forward and reverse strands were assembled with the STADEN software package [72], reformatted into the Genetics Computer Group (GCG) format and aligned manually using the Molecular Evolutionary Genetics Analysis (MEGA) v4 software package [73]. Phylogenetic relationships between individual sequences were inferred using ClonalFrame v1.1 [74]. This software is based on a model of genetic diversification that accounts for the way recombination occurs in bacterial populations. This enables the inference of phylogenetic relationships based on sequence data even if they are partly incongruent due to recombination. Sequence data for all isolates were input into ClonalFrame and default values were used for all options. Three independent runs were performed, each consisting of 300,000 iterations with 100,000 burn-in iterations. The convergence of the Markov Chain Monte Carlo (MCMC) in the different runs were judged satisfactory based on the Gelman-Rubin test as implemented in ClonalFrame GUI interface. The samples from the three runs were then concatenated for further analysis with a 95% majority rule consensus tree constructed using the ClonalFrame GUI. The consensus tree was then imported as a newick file into MEGA v4 for further annotation. Calculations of recombination tests were performed using RDP3 [74].
ccoP allele exchange between N. gonorrhoeae and N. meningitidis
To exchange the endogenous ccoP locus of N. meningitidis with the tri-heme ccoP allele of N. gonorrhoeae and vice versa, the endogenous ccoP locus of N. gonorrhoeae with the di-heme ccoP allele of N. meningitidis, the strains MC58 and VD300 were transformed with the plasmids pUP6NGOccoP::mTnerm#22 and pUP6NMEccoP::mTnerm#17 respectively, and selected on GC agar plates containing erythromycin (each plasmid carries a transposon insertion downstream of the ccoP stop codon, see Figures 6A and B).
Construction of a gonococcal strain expressing di-heme CcoP
The premature stop codon was introduced into N. gonorrhoeae ccoP by PCR-based splicing-by-overlap extension (SOE) reactions into an otheriwse wildtype allele. Each pair of PCR fragments containing the mutation was created using primers FE1141 (5′-CGGAATTCGAGCTCTCTTTATCTGTTTCCTGTTAGTAC-3′) in combination with FE1156 (5′-AACGGTTTCGTAGACCTATTTGCCATCCGCTTTGGCGGC-3′), and FE1155 (5′-CGGATGGCAAATAGGTCTACGAAACCGTTTGTGCCGCCTGCC-3′) in combination with Av2045 (5′-TTGGACGACGGACGAAGTCTC-3′) was used to make the second overlapping PCR fragment. Altered base pairs including the novel stop codon and an AccI restriction site (underlined) are shown in bold. The overlapping PCR fragments were spliced together using primers ccoP seq5′ (5′ - TCGGTTATCTGGTTATGTATCC -3′) and Av2044 (5′-GAATACGCTCTCCCTCTTTACC-3′). Purified PCR products were used to genetically transform N. gonorrhoeae strains [75] and correct transformants were screened for by AccI digestion of PCR fragments. Direct DNA sequencing of PCR fragments derived from the transformants was done, using appropriate primer sets, to verify the introduction of the stop codon and the absence of any other alterations.
Mini-transposon mutagenesis of the ccoP locus
The N. gonorrhoeae ccoP gene along with 133 bp upstream of the gene and 151 bp downstream of the gene was amplified using primers FE1140 (5′-TACTCTATATCGTCTTCAACAGG-3′) and FE1144 (5′-CAAAAATATCAGTCGGTCTGACTGC-3′). The resulting PCR fragments were digested with unique, flanking EcoRI/SacI sites and cloned into the polylinker of pUP6, yielding plasmid pUP6NGOccoP. Similarly, the N. meningitidis ccoP gene along with 133 bp upstream of the gene and 350 bp downstream of the gene was amplified using primers FE1140 and FE1181 (5′-GCGGGATCCGAGCTCTTACAACAAATAGGCAGTCTGCG-3′). The resulting PCR fragments was digested with unique, flanking EcoRI/BamHI and cloned into the polylinker of pUP6, yielding plasmid pUP6NMEccoP. Mini-transposon (mTn) mutagenesis was performed on both pUP6NGOccoP and PUP6NMEccoP as previously described [76]. The series of mTn insertions, conferring resistance to either chloramphenicol (mTncm) or erythromycin (mTnerm), were isolated, mapped by PCR and sequencing using appropriate primer sets, depending on the location of the transposon insertion. Primer sequences and the detailed location of each transposon insertion site are available upon request.
Construction and expression of an ectopic de-repressible ccoP allele
For construction of a gonococcal strain carrying an ectopic de-repressible ccoP gene the coding region of ccoP and 20 bp of upstream DNA, to include the RBS, was amplified by PCR from N. gonorrhoeae strain VD300 by using the forward primer av2226 (5′ - CGAAAACCTTAATTAATGTGATAACGGAGCAAAACAATG - 3′), PacI site underlined, and the reverse primer FE1144 (5′ - CAAAAATATCAGTCGGTCTGACTGC - 3′). The PCR reaction was performed by Advantage HD polymerase (AH diagnostics) to create blunt ends. The resulting PCR product was cut with PacI and cloned into pGCC6, digested with PmeI and PacI, at an intergenic chromosomal site located between the gonococcal genes lctP and aspC and linked to the lac promoter/operator. The resulting plasmid pGCC6NGOccoP was then used to transform strain KS351 (ccoP2x) and transformants were selected for growth on GC agar plates containing chloramphenicol. To inactivate the ccoP allele from the endogenous locus, the N. gonorrhoeae strain carrying the ectopic de-repressible ccoP allele (KS345) was transformed with pUP6NGOccoP::mTnerm#26 (position of this mTn insertion is labelled with two asterisks in Figure 6B) and selected on GC agar plates containing erythromycin and 250µM IPTG.
Construction of ccoP transposon insertion mutants in N. gonorrhoeae
To create an N. gonorrhoeae strain carrying a tandem duplication of ccoP, strain KS101 was transformed with pUP6NGOccoP and transformants were selected on GC agar plates containing kanamycin. PCR using appropriate primer sets was used to confirm the correct (Campbell-type) integration of pUP6NGOccoP on the chromosome. The resulting N. gonorrhoeae strain carrying a tandem duplication of ccoP (KS344) was transformed with a series of characterized pUP6NGOccoP::mTnerm and pUP6NGOccoP::mTncm plasmids and transformants were selected on GC plates containing erythromycin and chloramphenicol respectively. The location of each transposon insertion, to either the expressed or non-expressed copy of ccoP, was determined by PCR using appropriate primer sets (available upon request).
Construction of ccoP transposon insertion mutants in N. meningitidis
For this purpose, the wildtype N. meningitidis strain MC58 was transformed with the plasmid pGCC6NGOccoP to create a strain carrying an ectopic copy of N. gonorrhoeae ccoP. Transformants were selected on GC agar plates containing chloramphenicol and confirmed correct by appropriate PCR primer sets and immunoblotting followed by heme-staining to visualize expression of the tri-heme form of CcoP. The resulting N. meningitidis strain (KS350) carrying the ectopic N. gonorrhoeae ccoP and the wildtype strain MC58 were transformed in parallel with a series of characterized pUP6NMEccoP::mTnerm plasmids and transformants were selected on GC plates containing erythromycin.
Construction of cycB mutants
To generate the cycB insertion mutants, [51] the plasmid cycB::tet was introduced into MC58 and into VD300 by transformation and transformants were selected for growth on GC agar plates containing tetracycline. To obtain a complete deletion of cycB in N. gonorrhoeae the primers FE2223 (5′ - TCCGCAAAGCGGTGGAAATG - 3′) and FE2220 (5′ – GTTTAAACTGTCGCGGAGTTGTTTCATTTG - 3′) were used to amplify an 800 bp fragment of genomic DNA upstream of the cycB gene and the primers FE2221 (5′-TGAAACAACTCCGCGACAGTTTAAACACTATATGGCAAACCAATCCGGTGC -3′) and FE2225 (5′ –TATTTTGACAAACCACCGGAG - 3′) were used to amplify a 900 bp fragment of genomic DNA downstream of the cycB gene. The PCR products contained regions of homology at the 3′ end of the upstream fragment and at the 5′ end of the downstream fragment such that they could be spliced together by PCR-based splicing-by-overlap extension leaving out the entire cycB gene. This was done by using the primers FE2224 (5′–TTCATCCGGACAAACGCGTTG -3′) and FE2222 (5′–AACCTGTCGCTCTACGGCGAAC - 3′). The purified PCR product was used to genetically transform N. gonorrhoeae by a non-selective transformation technique [75], and correct transformants were screened for by PCR using the primers FE2223 and FE2225. The absence of c5 expression, including the truncated c5 form seen in the cycB::tet mutants, was verified by heme-stained blots of whole cell extracts.
Ectopic expression of cycB and a cycB-ccoP hybrid allele in N. gonorrhoeae
Ectopic expression of cycB and the cycB-ccoP hybrid allele was performed by cloning PCR amplified products into a unique SacI restriction site in plasmid p2/16/1 [70], allowing integration into the iga locus of the gonococcal chromosome. The resulting plasmids were then used to transform the mutant KS337 (VD300 ccoP2x, cycB) and transformants were selected for growth on GC agar plates containing erythromycin.
For cloning of the wildtype cycB allele the coding region including about 250 bp of upstream DNA was amplified by PCR from N. gonorrhoeae strain VD300 by using the forward primer c555F_SacI (5′ – TGCAGAGCTCAATTGGCAAAGGTTATCTTGCG - 3′) in combination with the reverse primer FE2200 (5′ – ATTCGAGCTCACACCCATTTGATGTCATTTCC - 3′), SacI sites underlined.
The cycB-ccoP hybrid allele was constructed by PCR-based splicing-by-overlap extension such that the region encoding the amino-terminus of cycB encompassing the first c-type heme domain and about 250 bp of upstream DNA was fused to the region encoding the third heme domain of ccoP. The relative spacing between the two heme domains in the hybrid protein was maintained as seen for wildtype cycB by facilitating a conserved stretch of four residues (Ala-Ala-Pro-Ala) in the C-terminal end of the AlaSerPro-rich linker domains. The cycB part of the hybrid allele, including about 250 bp of upstream DNA, was amplified by PCR using the flanking primer c555F_SacI in combination with the primer FE2216 (5′ – TCCGCTTTGGCCGCAGGGGCTGCCGCACCCTTGTC – 3′). The region encoding the third heme domain of ccoP was amplified by PCR using the flanking primer FE2218 (5′ – CCGGGAGCTCATTCGATATGAATCCGGATTTCTG – 3′), SacI site underlined, in combination with the primer FE2217 (5′ – ACAAGGGTGCGGCCGCACCTGCCGCCAAAGCGGATG – 3′). The two overlapping PCR fragments were spliced together by using the flanking primers c555F_SacI and FE2218.
Nitrite consumption assays
Nitrite concentrations in culture media were measured by a colourimetric assay as previously detailed [51].
Protein gels, immunoblotting and detection of c-type cytochromes
Whole-cell extracts for detection of c-type cytochromes were prepared by harvesting bacteria from plates into 10mM Hepes buffer pH 7.0, subjecting the suspension to 5 cycles of freezing and thawing, and finally suspending the bacteria in 1% w/v n-dodecyl β-D-maltoside (Sigma-Aldrich). After 10 min incubation at 50°C samples were separated on 10% or 12% Criterion XT Precast Gels (Bio-Rad Laboratories, Hercules CA, USA) and blotted onto PVDF membranes. To visualize heme-dependent, peroxidase activity of the c-type cytochromes, the membranes were first incubated with SuperSignal West Pico Chemiluminiscent Substrate, according to manufacturers instructions (PIERCE, Rockford, IL. USA), and then exposed to X-ray film (GE Healthcare, Buckinghamshire, UK) [77]. Immunoblotting was used to detect expression of nitrite reductase NirK. For SDS-PAGE, whole cell extracts were prepared from equivalent numbers of cells by heating cell suspensions to 100°C for 3 min in SDS-sample loading buffer. The primary antibody used to detect NirK was a polyclonal rabbit antibody and was diluted 1∶1000 [51]. Procedures for immunoblotting using alkaline phosphatase-coupled goat anti-rabbit and goat anti-mouse antibodies have been described previously [76].
Supporting Information
Zdroje
1. TzengYL
StephensDS
2000 Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect 2 687 700
2. YazdankhahSP
KrizP
TzanakakiG
KremastinouJ
KalmusovaJ
2004 Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol 42 5146 5153
3. CaugantDA
2008 Genetics and evolution of Neisseria meningitidis: importance for the epidemiology of meningococcal disease. Infect Genet Evol 8 558 565
4. ClausH
MaidenMC
MaagR
FroschM
VogelU
2002 Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 148 1813 1819
5. SchoenC
BlomJ
ClausH
Schramm-GluckA
BrandtP
2008 Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc Natl Acad Sci U S A 105 3473 3478
6. HollisDG
WigginsGL
WeaverRE
1972 An unclassified gram-negative rod isolated from the pharynx on Thayer-Martin medium (selective agar). Appl Microbiol 24 772 777
7. GoldschneiderI
GotschlichEC
ArtensteinMS
1969 Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med 129 1327 1348
8. HotoppJC
GrifantiniR
KumarN
TzengYL
FoutsD
2006 Comparative genomics of Neisseria meningitidis: core genome, islands of horizontal transfer and pathogen-specific genes. Microbiology 152 3733 3749
9. SnyderLA
SaundersNJ
2006 The majority of genes in the pathogenic Neisseria species are present in non-pathogenic Neisseria lactamica, including those designated as ‘virulence genes’. BMC Genomics 7 128
10. StablerRA
MarsdenGL
WitneyAA
LiY
BentleySD
2005 Identification of pathogen-specific genes through microarray analysis of pathogenic and commensal Neisseria species. Microbiology 151 2907 2922
11. KoomeyJM
GillRE
FalkowS
1982 Genetic and biochemical analysis of gonococcal IgA1 protease: cloning in Escherichia coli and construction of mutants of gonococci that fail to produce the activity. Proc Natl Acad Sci U S A 79 7881 7885
12. NaessanCL
Egge-JacobsenW
HeinigerRW
WolfgangMC
AasFE
2008 Genetic and functional analyses of PptA, a phospho-form transferase targeting type IV pili in Neisseria gonorrhoeae. J Bacteriol 190 387 400
13. VazquezJA
de la FuenteL
BerronS
O'RourkeM
SmithNH
1993 Ecological separation and genetic isolation of Neisseria gonorrhoeae and Neisseria meningitidis. Curr Biol 3 567 572
14. WardMJ
LambdenPR
HeckelsJE
1992 Sequence analysis and relationships between meningococcal class 3 serotype proteins and other porins from pathogenic and non-pathogenic Neisseria species. FEMS Microbiol Lett 73 283 289
15. FeaversIM
MaidenMC
1998 A gonococcal porA pseudogene: implications for understanding the evolution and pathogenicity of Neisseria gonorrhoeae. Mol Microbiol 30 647 656
16. PotterAJ
KiddSP
JenningsMP
McEwanAG
2007 Evidence for distinctive mechanisms of S-nitrosoglutathione metabolism by AdhC in two closely related species, Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun 75 1534 1536
17. TakahashiH
WatanabeH
2005 A gonococcal homologue of meningococcal gamma-glutamyl transpeptidase gene is a new type of bacterial pseudogene that is transcriptionally active but phenotypically silent. BMC Microbiol 5 56
18. BennettJS
JolleyKA
SparlingPF
SaundersNJ
HartCA
2007 Species status of Neisseria gonorrhoeae: evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol 5 35
19. Ferguson-MillerS
BabcockGT
1996 Heme/Copper Terminal Oxidases. Chem Rev 96 2889 2908
20. JobsonRW
NielsenR
LaakkonenL
WikstromM
AlbertVA
2004 Adaptive evolution of cytochrome c oxidase: Infrastructure for a carnivorous plant radiation. Proc Natl Acad Sci U S A 101 18064 18068
21. SchmidtTR
WildmanDE
UddinM
OpazoJC
GoodmanM
2005 Rapid electrostatic evolution at the binding site for cytochrome c on cytochrome c oxidase in anthropoid primates. Proc Natl Acad Sci U S A 102 6379 6384
22. PitcherRS
WatmoughNJ
2004 The bacterial cytochrome cbb3 oxidases. Biochim Biophys Acta 1655 388 399
23. DucluzeauAL
OuchaneS
NitschkeW
2008 The cbb3 oxidases are an ancient innovation of the domain bacteria. Mol Biol Evol 25 1158 1166
24. RauhamakiV
BlochDA
VerkhovskyMI
WikstromM
2009 Active site of cytochrome cbb3. J Biol Chem 284 11301 11308
25. PitcherRS
CheesmanMR
WatmoughNJ
2002 Molecular and spectroscopic analysis of the cytochrome cbb(3) oxidase from Pseudomonas stutzeri. J Biol Chem 277 31474 31483
26. PetersA
KulajtaC
PawlikG
DaldalF
KochHG
2008 Stability of the cbb3-type cytochrome oxidase requires specific CcoQ-CcoP interactions. J Bacteriol 190 5576 5586
27. KochHG
HwangO
DaldalF
1998 Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3 oxidase activity. J Bacteriol 180 969 978
28. ZuffereyR
PreisigO
HenneckeH
Thony-MeyerL
1996 Assembly and function of the cytochrome cbb3 oxidase subunits in Bradyrhizobium japonicum. J Biol Chem 271 9114 9119
29. BrookI
2003 Microbial dynamics of purulent nasopharyngitis in children. Int J Pediatr Otorhinolaryngol 67 1047 1053
30. EskowRN
LoescheWJ
1971 Oxygen tensions in the human oral cavity. Arch Oral Biol 16 1127 1128
31. Toledo-CuevasM
BarqueraB
GennisRB
WikstromM
Garcia-HorsmanJA
1998 The cbb3-type cytochrome c oxidase from Rhodobacter sphaeroides, a proton-pumping heme-copper oxidase. Biochim Biophys Acta 1365 421 434
32. AnjumMF
StevaninTM
ReadRC
MoirJW
2002 Nitric oxide metabolism in Neisseria meningitidis. J Bacteriol 184 2987 2993
33. HouseholderTC
FozoEM
CardinaleJA
ClarkVL
2000 Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect Immun 68 5241 5246
34. MelliesJ
JoseJ
MeyerTF
1997 The Neisseria gonorrhoeae gene aniA encodes an inducible nitrite reductase. Mol Gen Genet 256 525 532
35. RockJD
MahnaneMR
AnjumMF
ShawJG
ReadRC
2005 The pathogen Neisseria meningitidis requires oxygen, but supplements growth by denitrification. Nitrite, nitric oxide and oxygen control respiratory flux at genetic and metabolic levels. Mol Microbiol 58 800 809
36. HeurlierK
ThomsonMJ
AzizN
MoirJW
2008 The nitric oxide (NO)-sensing repressor NsrR of Neisseria meningitidis has a compact regulon of genes involved in NO synthesis and detoxification. J Bacteriol 190 2488 2495
37. IsabellaV
WrightLF
BarthK
SpenceJM
GroganS
2008 cis - and trans-acting elements involved in regulation of norB (norZ), the gene encoding nitric oxide reductase in Neisseria gonorrhoeae. Microbiology 154 226 239
38. IsabellaVM
LapekJDJr
KennedyEM
ClarkVL
2009 Functional analysis of NsrR, a nitric oxide-sensing Rrf2 repressor in Neisseria gonorrhoeae. Mol Microbiol 71 227 239
39. OvertonTW
WhiteheadR
LiY
SnyderLA
SaundersNJ
2006 Coordinated regulation of the Neisseria gonorrhoeae-truncated denitrification pathway by the nitric oxide-sensitive repressor, NsrR, and nitrite-insensitive NarQ-NarP. J Biol Chem 281 33115 33126
40. RockJD
ThomsonMJ
ReadRC
MoirJW
2007 Regulation of denitrification genes in Neisseria meningitidis by nitric oxide and the repressor NsrR. J Bacteriol 189 1138 1144
41. LissendenS
MohanS
OvertonT
ReganT
CrookeH
2000 Identification of transcription activators that regulate gonococcal adaptation from aerobic to anaerobic or oxygen-limited growth. Mol Microbiol 37 839 855
42. HopperA
TovellN
ColeJ
2009 A physiologically significant role in nitrite reduction of the CcoP subunit of the cytochrome oxidase cbb from Neisseria gonorrhoeae. FEMS Microbiol Lett
43. MaidenMC
2008 Population genomics: diversity and virulence in the Neisseria. Curr Opin Microbiol 11 467 471
44. TreangenTJ
AmburOH
TønjumT
RochaEP
2008 The impact of the neisserial DNA uptake sequences on genome evolution and stability. Genome Biol 9 R60
45. LinzB
SchenkerM
ZhuP
AchtmanM
2000 Frequent interspecific genetic exchange between commensal Neisseriae and Neisseria meningitidis. Mol Microbiol 36 1049 1058
46. SprattBG
BowlerLD
ZhangQY
ZhouJ
SmithJM
1992 Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol 34 115 125
47. ZhouJ
BowlerLD
SprattBG
1997 Interspecies recombination, and phylogenetic distortions, within the glutamine synthetase and shikimate dehydrogenase genes of Neisseria meningitidis and commensal Neisseria species. Mol Microbiol 23 799 812
48. SheppardSK
McCarthyND
FalushD
MaidenMC
2008 Convergence of Campylobacter species: implications for bacterial evolution. Science 320 237 239
49. RokasA
HollandPW
2000 Rare genomic changes as a tool for phylogenetics. Trends Ecol Evol 15 454 459
50. VikA
AasFE
AnonsenJH
BilsboroughS
SchneiderA
2009 Broad spectrum O-linked protein glycosylation in the human pathogen Neisseria gonorrhoeae. Proc Natl Acad Sci U S A 106 4447 4452
51. DeeudomM
KoomeyM
MoirJW
2008 Roles of c-type cytochromes in respiration in Neisseria meningitidis. Microbiology 154 2857 2864
52. LiY
HopperA
OvertonT
SquireDJ
ColeJ
2010 Organisation of the electron transfer chain to oxygen in the obligate human pathogen, Neisseria gonorrhoeae: roles for cytochromes c4 and c5, but not cytochrome c2, in oxygen reduction. J Bacteriol 192 2395 2406
53. ThomsonMJ
StevaninTM
MoirJW
2008 Measuring nitric oxide metabolism in the pathogen Neisseria meningitidis. Methods Enzymol 437 539 560
54. KuSC
SchulzBL
PowerPM
JenningsMP
2009 The pilin O-glycosylation pathway of pathogenic Neisseria is a general system that glycosylates AniA, an outer membrane nitrite reductase. Biochem Biophys Res Commun 378 84 89
55. StefanelliP
ColottiG
NeriA
SalucciML
MiccoliR
2008 Molecular characterization of nitrite reductase gene (aniA) and gene product in Neisseria meningitidis isolates: is aniA essential for meningococcal survival? IUBMB Life 60 629 636
56. AchtmanM
1998 Microevolution during epidemic spread of Neisseria meningitidis. Electrophoresis 19 593 596
57. BuckeeCO
GuptaS
KrizP
MaidenMC
JolleyKA
Long-term evolution of antigen repertoires among carried meningococci. Proc Biol Sci
58. JolleyKA
KalmusovaJ
FeilEJ
GuptaS
MusilekM
2000 Carried meningococci in the Czech Republic: a diverse recombining population. J Clin Microbiol 38 4492 4498
59. TangJ
HanageWP
FraserC
CoranderJ
2009 Identifying currents in the gene pool for bacterial populations using an integrative approach. PLoS Comput Biol 5 e1000455
60. KoteishiH
NojiriM
NakagamiT
YamaguchiK
SuzukiS
2009 Cytochrome c551 Is a Mediator of Electron Transfer between Copper-Containing Nitrite Reductase and Azurin in a Denitrifying Bacterium, Achromobacter xylosoxidans. Bull Chem Soc Jpn 82 1003 1005
61. MurphyLM
DoddFE
YousafzaiFK
EadyRR
HasnainSS
2002 Electron donation between copper containing nitrite reductases and cupredoxins: the nature of protein-protein interaction in complex formation. J Mol Biol 315 859 871
62. CannonJG
1989 Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin Microbiol Rev 2 Suppl S1 4
63. BuenoE
RichardsonDJ
BedmarEJ
DelgadoMJ
2009 Expression of Bradyrhizobium japonicum cbb(3) terminal oxidase under denitrifying conditions is subjected to redox control. FEMS Microbiol Lett 298 20 28
64. EllisMJ
GrossmannJG
EadyRR
HasnainSS
2007 Genomic analysis reveals widespread occurrence of new classes of copper nitrite reductases. J Biol Inorg Chem 12 1119 1127
65. NojiriM
KoteishiH
NakagamiT
KobayashiK
InoueT
2009 Structural basis of inter-protein electron transfer for nitrite reduction in denitrification. Nature 462 117 120
66. HoehnGT
ClarkVL
1992 The major anaerobically induced outer membrane protein of Neisseria gonorrhoeae, Pan 1, is a lipoprotein. Infect Immun 60 4704 4708
67. BoulangerMJ
MurphyME
2002 Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: a new class of copper-containing nitrite reductases. J Mol Biol 315 1111 1127
68. MaidenMC
BygravesJA
FeilE
MorelliG
RussellJE
1998 Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 95 3140 3145
69. AasFE
VikA
VeddeJ
KoomeyM
Egge-JacobsenW
2007 Neisseria gonorrhoeae O-linked pilin glycosylation: functional analyses define both the biosynthetic pathway and glycan structure. Mol Microbiol 65 607 624
70. WolfgangM
van PuttenJP
HayesSF
DorwardD
KoomeyM
2000 Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. Embo J 19 6408 6418
71. SkaarEP
LecuyerB
LenichAG
LazioMP
Perkins-BaldingD
2005 Analysis of the Piv recombinase-related gene family of Neisseria gonorrhoeae. J Bacteriol 187 1276 1286
72. StadenR
1996 The Staden sequence analysis package. Mol Biotechnol 5 233 241
73. TamuraK
DudleyJ
NeiM
KumarS
2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
74. DidelotX
FalushD
2007 Inference of bacterial microevolution using multilocus sequence data. Genetics 175 1251 1266
75. GunnJS
SteinDC
1996 Use of a non-selective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol Gen Genet 251 509 517
76. FreitagNE
SeifertHS
KoomeyM
1995 Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol 16 575 586
77. VargasC
McEwanAG
DownieJA
1993 Detection of c-type cytochromes using enhanced chemiluminescence. Anal Biochem 209 323 326
78. DeeudomM
RockJ
MoirJ
2006 Organization of the respiratory chain of Neisseria meningitidis. Biochem Soc Trans 34 139 142
79. KoomeyJM
FalkowS
1987 Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recA mutants. J Bacteriol 169 790 795
80. McGuinnessBT
ClarkeIN
LambdenPR
BarlowAK
PoolmanJT
1991 Point mutation in meningococcal por A gene associated with increased endemic disease. Lancet 337 514 517
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



