-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHost Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
Bacteria lose or gain genetic material and through selection, new variants become fixed in the population. Here we provide the first, genome-wide example of a single bacterial strain's evolution in different deliberately colonized patients and the surprising insight that hosts appear to personalize their microflora. By first obtaining the complete genome sequence of the prototype asymptomatic bacteriuria strain E. coli 83972 and then resequencing its descendants after therapeutic bladder colonization of different patients, we identified 34 mutations, which affected metabolic and virulence-related genes. Further transcriptome and proteome analysis proved that these genome changes altered bacterial gene expression resulting in unique adaptation patterns in each patient. Our results provide evidence that, in addition to stochastic events, adaptive bacterial evolution is driven by individual host environments. Ongoing loss of gene function supports the hypothesis that evolution towards commensalism rather than virulence is favored during asymptomatic bladder colonization.
Published in the journal: . PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001078
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001078Summary
Bacteria lose or gain genetic material and through selection, new variants become fixed in the population. Here we provide the first, genome-wide example of a single bacterial strain's evolution in different deliberately colonized patients and the surprising insight that hosts appear to personalize their microflora. By first obtaining the complete genome sequence of the prototype asymptomatic bacteriuria strain E. coli 83972 and then resequencing its descendants after therapeutic bladder colonization of different patients, we identified 34 mutations, which affected metabolic and virulence-related genes. Further transcriptome and proteome analysis proved that these genome changes altered bacterial gene expression resulting in unique adaptation patterns in each patient. Our results provide evidence that, in addition to stochastic events, adaptive bacterial evolution is driven by individual host environments. Ongoing loss of gene function supports the hypothesis that evolution towards commensalism rather than virulence is favored during asymptomatic bladder colonization.
Introduction
Microbes have adapted many fascinating strategies to co-evolve with their hosts. The specific immune response to surface antigens drives the structural changes in influenza virus hemagglutinin and serotype [1], the antigenic drift in trypanosomes [2] and the immune evasion mechanisms in malaria [3]. Similar mechanisms operate in bacteria, forcing them to vary their surface antigens and to maintain critical functions encoded by those genes, even in the presence of a fully functional immune response [4]. While such host-modulated microbial elements have been extensively studied, less is known about microbial adaptation to environmental signals inside individual patients. Most importantly, a host-specific approach to the analysis of genome-wide alterations has not been taken.
Urinary tract infections (UTIs) present an interesting and highly relevant model for studying microbial adaptation. After establishing significant numbers, the bacteria either cause severe and potentially life threatening disease, or an asymptomatic carrier state resembling the normal flora at other mucosal sites. Patients with asymptomatic bacteriuria (ABU) may carry the same strain for months or years and this outcome is advantageous for the microbe as it can persist in a favored niche with little microbial competition. ABU is also favorable for the host who may be protected from re-infection if the carrier strain outcompetes new invaders [5], [6]. In our previous work, we reported that at least 50% of ABU strains have evolved from virulent uropathogenic E. coli (UPEC) strains by genome reduction, i.e. inactivation of genes encoding virulence-associated factors, either by the accumulation of point mutations or by deletions [7], [8]. These observations suggest that bacteria adapt to the urinary tract environment and that this human host niche is suitable for understanding the mechanisms involved. The determinants of long-term bacterial persistence and adaptation to the host environment are, however, still poorly understood. For these reasons, we looked at real-time evolution by sequencing the progenitor strain E. coli 83972 and then analyzing its re-isolates from several patients.
The prototypic ABU E. coli strain 83972 has been extensively used for therapeutic urinary bladder colonization in patients with chronic UTI. After intravesical inoculation, the strain establishes ABU and this approach has proven to be safe and to protect the patient from super-infection with more virulent strains [6], [9]. Here, we compare the genomes, transcriptomes and proteomes of E. coli 83972 to re-isolates from patients deliberately colonized with this strain. We provide evidence that the pattern of genetic and phenotypic changes was distinct for each host and that it involves a limited number of genes, including regulators, metabolic genes and virulence factors.
Results
Complete genome sequence of the protype ABU E. coli 83972
To characterize the prototype ABU E. coli 83972, we solved the chromosomal DNA sequence and compared it to genomes from other UPEC strains (CFT073, UTI89, 536), enterohemorrhagic E. coli (EHEC) strain O157:H7 Sakai and E. coli K-12 strain MG1655. The E. coli 83972 genome, which was originally isolated from the urinary tract of a schoolgirl [5], comprises a 5,131,397-bp chromosome and a small 1,565-bp cryptic plasmid (Figure 1). According to the genome sequence, E. coli 83972 was most closely related to the UPEC strains in particular to CFT073, sharing four chromosomal regions with only this strain (Figure 1, Table 1). Notably, large parts of region 2 and 4 are identical to genomic islands I and II of non-pathogenic E. coli strain Nissle 1917, a close relative of UPEC strain CFT073 that evolved by reductive evolution [10]. Six other islands were also shared with other UPEC, but not with EHEC or E. coli K-12 (Figure 1, Table 1). These genomic regions encode virulence and fitness-associated factors, including iron-uptake systems, adhesins, toxins, the K5 capsule, different secretion systems, as well as metabolic traits and transporters (Table 1). Other island-encoded traits shared with UPEC and EHEC included type 1 fimbriae, mannonate hydrolase (required for hexuronate degradation) and a C4-dicarboxylate transporter.
Fig. 1. Genetic map of the E. coli 83972 chromosome and the small plasmid pABU. 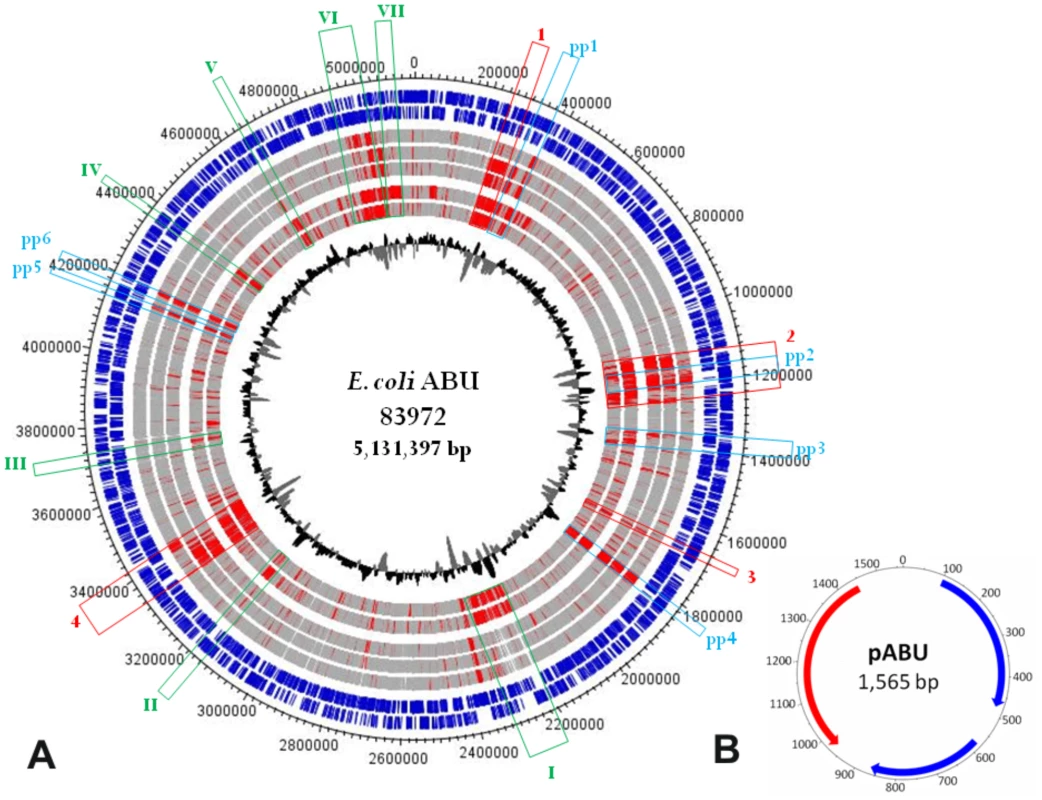
Nucleotide sequence analysis of the E. coli 83972 chromosome a): The two most outer circles represent all putative open reading frames (ORFs), depending on ORF orientation. The following five circles report the results of a two-way genome comparison between E. coli 83972 and one of the following E. coli strains: CFT073 (UPEC), 536 (UPEC), UTI89 (UPEC), MG1655 (K-12) and Sakai (EHEC O157:H7). Genes shared between the strain pair compared are indicated in grey and variable genome regions are indicated in red. The innermost circle represents the G+C distribution. Genomic regions only present in strains ABU83972 and CFT073 are framed in red. Chromosomal segments framed in green or blue are only present in the ABU isolate and pathogenic E. coli or represent bacteriophage-related DNA, respectively. Details on the gene content of these regions are compiled in Table 1. UPEC, uropathogenic E. coli; EHEC, enterohemorrhagic E. coli. Nucleotide sequence analysis of plasmid pABU b): putative predicted ORFs have been indicated. Tab. 1. Genomic islands and prophages in the E. coli 83972 genome. 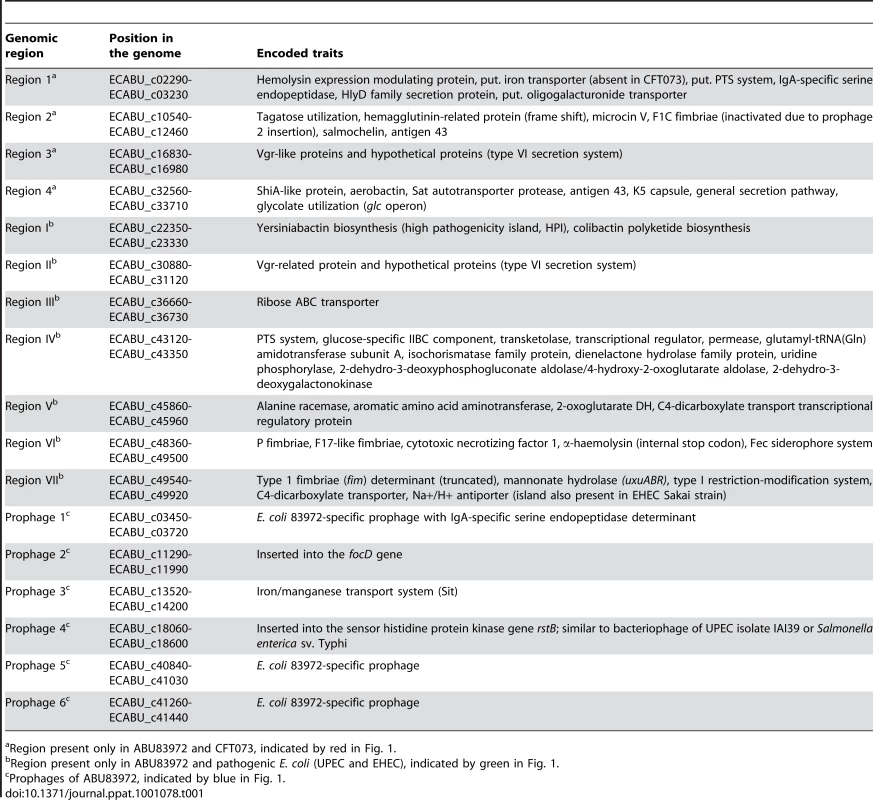
Region present only in ABU83972 and CFT073, indicated by red in Fig. 1. Six prophages were identified which were unique in type or chromosomal localization for E. coli 83972. Two of these are of particular interest. We found that prophage 4 was similar to prophages so far only described in the genomes of UPEC strain IAI39 (accession no. CU928164) or Salmonella enterica serovar Typhi (accession no. AE014613 or AL627270). In strain 83972, it was inserted into the rstB gene which encodes for the sensor histidine kinase RstB of the RstAB two-component system. The RstAB system controls the expression of genes involved in diverse processes relevant for bladder colonization, such as acid tolerance, curli formation and anaerobic respiration [11], [12]. Prophage 2 was similar to EHEC prophages, disrupting focD and thus the F1C fimbrial determinant in E. coli 83972.
Human colonization and the in vitro continuous culture
Here, we have established asymptomatic carriage of a single bacterial strain in different human hosts and then, using re-isolates obtained from these individuals, studied the host-specific genome-wide changes. Therapeutic bacteriuria was established in six patients by intravesical inoculation of E. coli 83972 (Figure 2A). Afterwards, re-isolates obtained from each host at different times (in vivo re-isolates) were subjected to genetic and phenotypic analyses (Figure 2B). This was possible as E. coli 83972 establishes a monoculture in the human urinary tract and because bacteriuria often lasts for months or years. To distinguish genetic changes driven by the host environment from random events, we cultured E. coli 83972 in vitro in pooled human urine for more than 2000 generations and included corresponding isolates in the analysis.
Fig. 2. Therapeutic urinary tract inoculation with E. coli 83972. 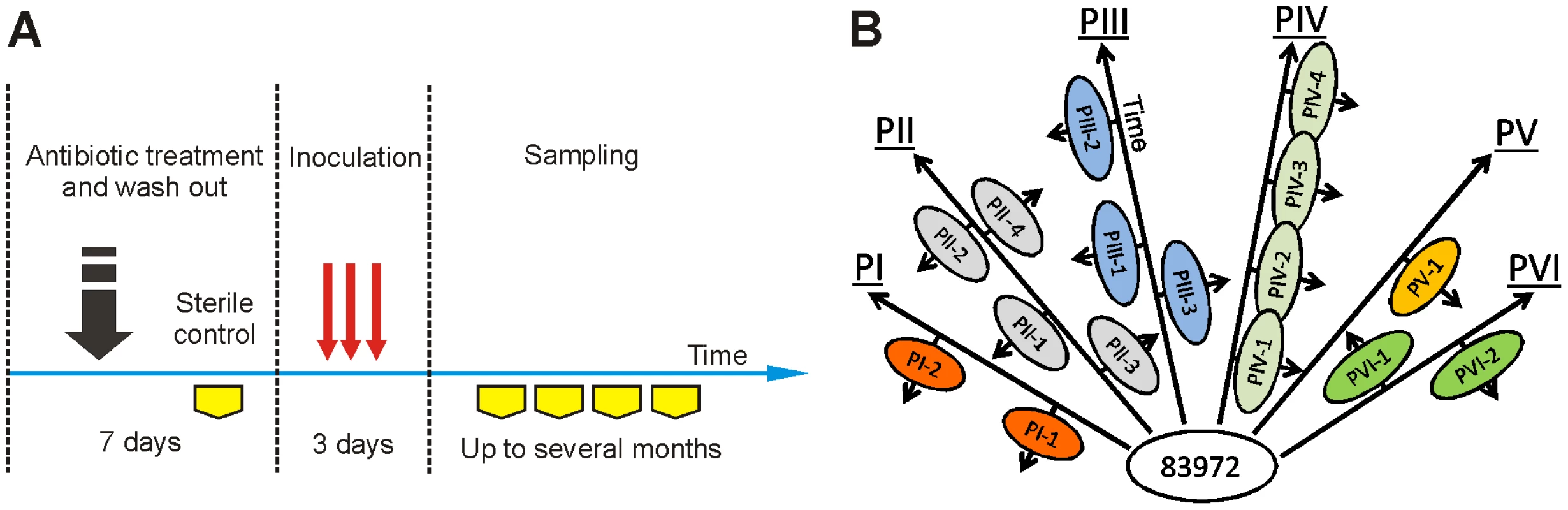
(A) Colonization scheme. Six patients received E. coli 83972 on three consecutive days and bacteriuria was established. Re-isolates from urine were obtained at different time points after inoculation. (B) Schematic representation of the sampling during human colonization. Arrows illustrate the time of colonization. Re-isolates obtained from different inoculations of the same patient are represented on opposite sides of an arrow. Genome structure of re-isolates
By pulsed-field gel electrophoresis (PFGE), we observed alterations in overall genome structure in 31% (5/16) of individual in vivo re-isolates. The exhibited restriction pattern alterations differed in comparison to the progenitor strain and also among themselves (Figure S1A). In contrast, 17 independent isolates from long-term in vitro cultivation showed no change in genome structure, indicating that genomic alterations depended on individual hosts rather than on preexisting hot spots of genomic variability (Figure S1B). Larger changes in the genome size of in vivo re-isolates were not observed, as analyzed by PFGE following I-CeuI digestion, with the exception of strain PII-4 displaying a reduction in genome size (Figure S2A). Analysis of multiple colonies from the corresponding urine samples confirmed that the genome variations were representative for each host and time of sampling (Figure S2B).
Sequencing of re-isolates from inoculated patients and in vitro control cultures
From the above candidates, we chose for genome sequencing three re-isolates with altered PFGE pattern from three patients and one randomly chosen in vitro propagated 83972 variant (E. coli 83972-4.9). Complete genome coverage was obtained and raw sequences were mapped on the chromosome of the progenitor strain E. coli 83972. After verification by single locus Sanger sequencing, 37 loci in the four sequenced re-isolates were confirmed to be polymorphic as compared to the parent strain. We found that genomic alterations occurred within conserved and flexible parts of the bacterial chromosome (Figure 3), and with only three exceptions, these affected coding regions. The majority of the alterations were single nucleotide polymorphisms (SNPs) (2 synonymous vs. 27 non-synonymous substitutions), but one inversion of 1,731-bp, one large 27-kb deletion and four small deletions of 1, 5, 12 or 165 bp were also detected. Many altered genes encoding proteins with regulatory functions (Figure 3, Table S1) were independently acquired in multiple individual re-isolates but not after in vitro culture and thus seemed to represent adaptational hotspots in vivo. They included the BarA/UvrY two-component system that controls a global regulatory network affecting a multitude of cellular functions and that has been proposed as a virulence trait in UTI [13], and mdoH encoding a glycosyl transferase involved in osmoregulated periplasmic glucan synthesis [14] as well as genes involved in oxidative stress responses (frmR) [15].
Fig. 3. Localization of genomic alterations within the re-isolates' genomes relative to parent E. coli 83972 as revealed by whole genome sequencing. 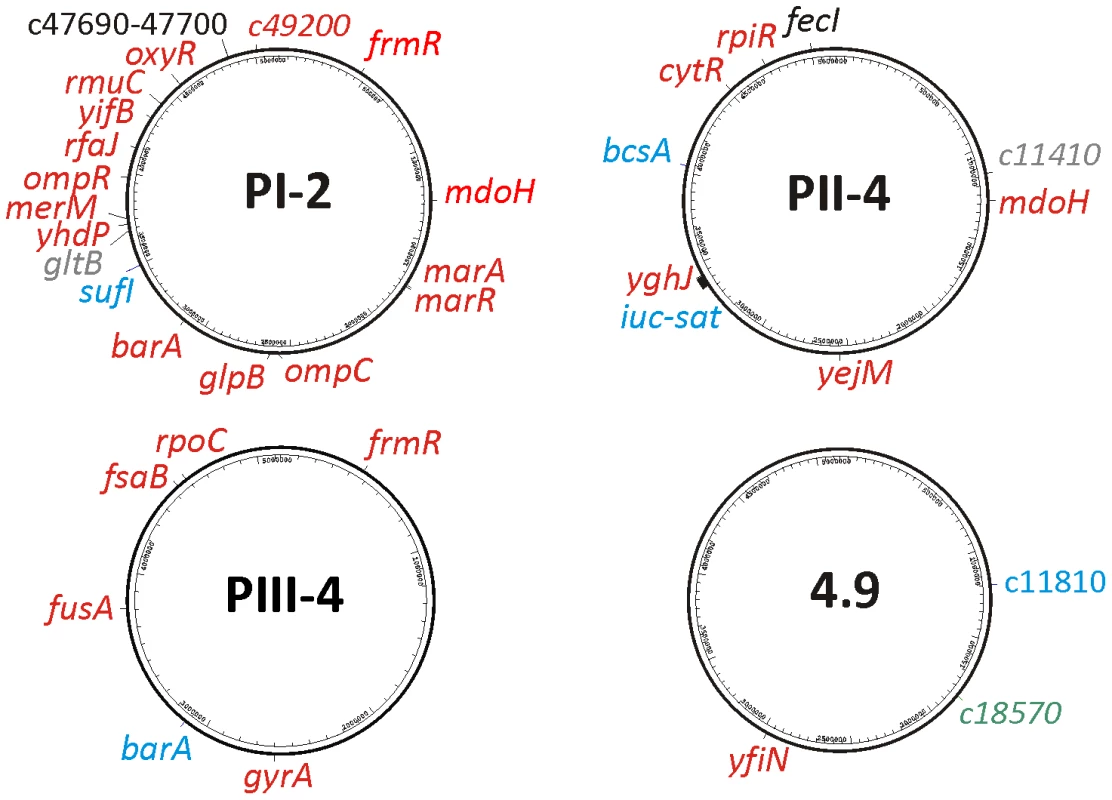
The nature of mutation is indicated by color: red- non-synonymous, grey- synonymous, black- intergenic, blue- deletion, green- inversion. In re-isolate PI-2, we found that nineteen different genomic loci were mutated relative to the progenitor strain, and 89% of these resulted in an altered amino acid sequence of the encoded proteins. Interestingly, 35% of the above mutations were stop codons and frame shifts. Furthermore, many of the mutations impacted pleiotropic regulatory genes involved in adaptation to different stress conditions including oxidative stress and/or resistance to antibiotics (frmR, marR, oxyR) [16]. Osmolarity, and virulence - or fitness-associated traits were also affected (barA, ompR, ompC, mdoH). The genes barA and ompR are part of the two-component systems OmpR/EnvZ and BarA/UvrY which regulate flagella and adhesin expression, biofilm formation, and glycolytic or gluconeogenic utilization of different carbon sources [17], [18].
In re-isolate PII-4, we found nine genomic alterations including five non-synonymous SNPs, a frame shift in the gene encoding for cellulose synthase bcsA, as well as huge deletion and one mutation in a non-coding region. Most intriguingly, the last two mutations affected iron uptake systems: aerobactin (iuc) and the ferric citrate uptake system (fec). The aerobactin gene cluster was lost due to a 27-kb partial deletion of a pathogenicity island (Figure S2C) and the fecI upstream region required for ferric citrate uptake was polymorphic (T to C substitution). In addition, we detected sequence alterations in genes encoding the transcriptional repressor of ribonucleoside metabolism (cytR) and the transcriptional repressor of ribose catabolism (rpiR).
In re-isolate PIII-4, we also observed mutations in barA and frmR. In this strain, all six mutations affected coding sequences of housekeeping genes, four of which were non-synonymous, one nonsense mutation, and one was an internal deletion. Surprisingly, we found SNPs in rpoC and gyrA, which was consistent with previous studies of long-term in vitro experimental evolution [19], [20].
In contrast to the in vivo re-isolates, the in vitro-propagated strain 4.9 showed only three genomic alterations: one predicted diguanylate cyclase (yfiN) and in two phage-related genes (Figure 3; Table S1).
Genomic alterations in re-isolates obtained after a second inoculation of each patient
To address the hypothesis that the host selects specific mutants or ‘imprints’ the pathogen during bladder colonization, we sequenced selected genomic regions of the E. coli 83972 genome in re-isolates from a second, independent inoculation of each patient. Therapeutic inoculations were repeated for medical reasons, urine cultures were obtained at monthly intervals and five independent bacterial colonies from the last sampling time point were subjected to Sanger sequencing. Specifically, we examined chromosomal loci, which were altered in E. coli 83972 re-isolates from the first inoculation event in PI-2, PII-4 and PIII-4.
Several loci were repeatedly altered in re-isolates of strain 83972 from the same host (Table S2). This included the fecIR promoter region where the re-isolate of the second bladder colonization of patient PII carried a point mutation 23 nucleotides upstream of the SNP previously detected in strain PII-4. Re-isolates from the first and second inoculation in patients PI and PIII had different point mutations in the frmR gene. The mdoH gene was mutated in isolates PI-2 and PII-4 from the first inoculation and mutations were detected in re-isolates from the second inoculation in all three patients. In contrast, these genomic alterations did not occur in five isolates from two independent in vitro urine cultures of E. coli 83972, further suggesting that the host environment may drive seletion of these genomic changes.
Stability of genomic alterations, examined in repeat re-isolates
To examine if the genetic alterations might represent adaptive changes that are cyclic in nature and that, in different patients, the re-isolates were picked at different cycles, we obtained E. coli 83972 re-isolates at a time point distant from that of PI-2 and PII-4 and subjected them to single locus Sanger sequencing. Most of the SNPs (17/19), in isolate PI-2 were still present in its progeny after an additional 126 days of bladder colonization. In descendants of PII-4, 4 out of 9 genomic changes (mdoH, rpiR, fecI, yejM) remained after an additional 125 days propagation time. Interestingly, all detected alterations in the later re-isolates were identical to those found in re-isolates PI-2 or PII-4. Isolate PIII-4 was the last sequential isolate derived from the inoculation of patient PIII and comparisons could not be performed.
Characterization of individual bacterial adaptation by transcriptome and proteome analysis of E. coli 83972 re-isolates
By comparing the three individual in vivo re-isolates and the in vitro-propagated variant 4.9 to the progenitor E. coli 83872, we observed differences in the respective phenotypes. Although growth characteristics in pooled human urine did not reveal major variations between re-isolates (Figure S3A), these strains differed in both motility and biofilm formation. The re-isolate PIII-4 was more motile than the parent strain (Figure S3B). Regarding biofilm formation, PI-2 formed significantly less biofilm than the parent strain while PII-4 showed significantly more (Figure S3C).
To further determine whether stable genomic changes of sequenced re-isolates (see previous section) affected the gene and protein expression level, we subjected them to transcriptome and outer membrane proteome (OMP) analysis. For this reason, prior to either RNA or protein isolation bacteria were grown in vitro in pooled human urine. Overall, the number of de-regulated genes, as implicated by the transcriptome, was higher in the patient re-isolates than in the in vitro-propagated variant 4.9 (Table 2). In each strain, we identified distinct gene expression patterns matching the proteome and genome data (Figure 4).
Fig. 4. Host-specific changes in gene expression patterns of E. coli 83972 revealed by transcriptome analysis. 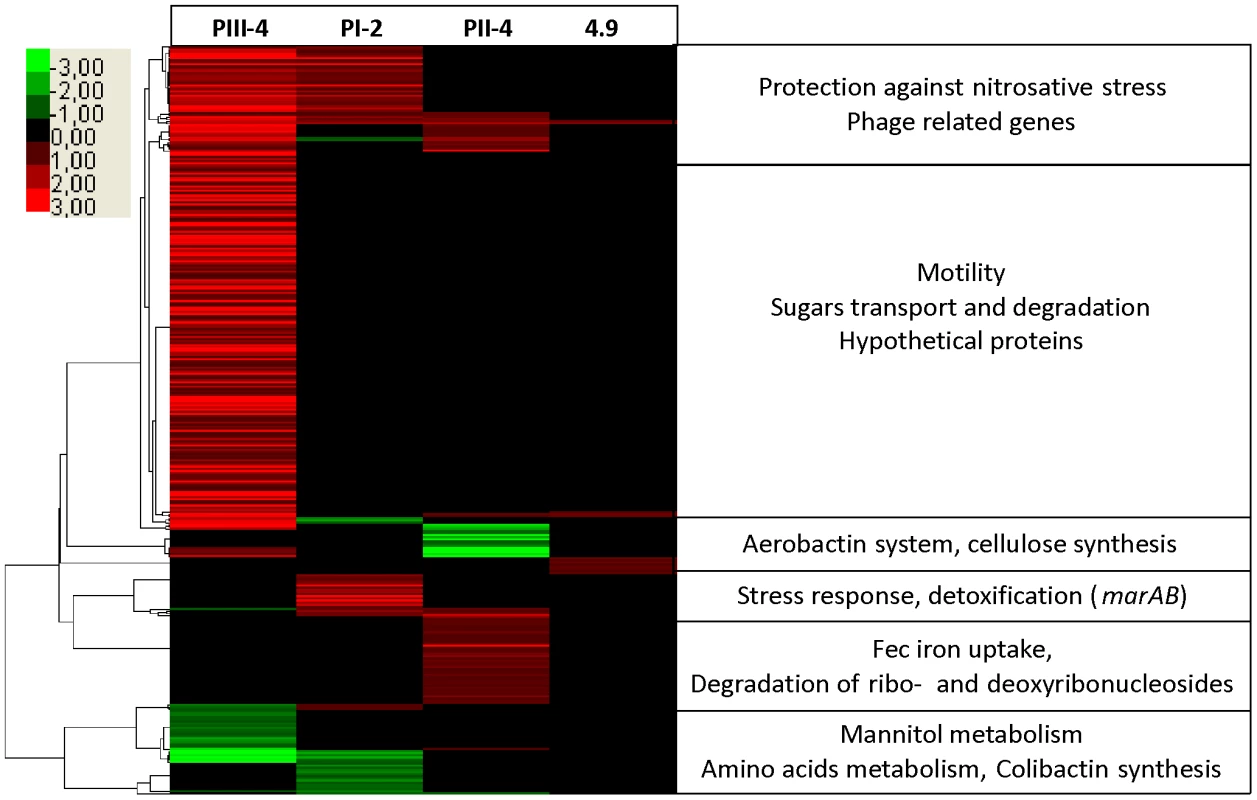
Hierarchical clustering of de-regulated genes in in vivo re-isolates PI-2, PII-4 and PIII-4 and in vitro grown strain 4.9 relative to parent E. coli 83972 upon in vitro growth in pooled human urine. Each horizontal line represents one gene; expression is given relative to the intensity bar (log 2-fold, mean values of > three experiments). Unaffected genes are shown in black (p-value >0.09). Tab. 2. Summary of global genomic alterations upon prolonged in vivo or in vitro growth of E. coli 83972. 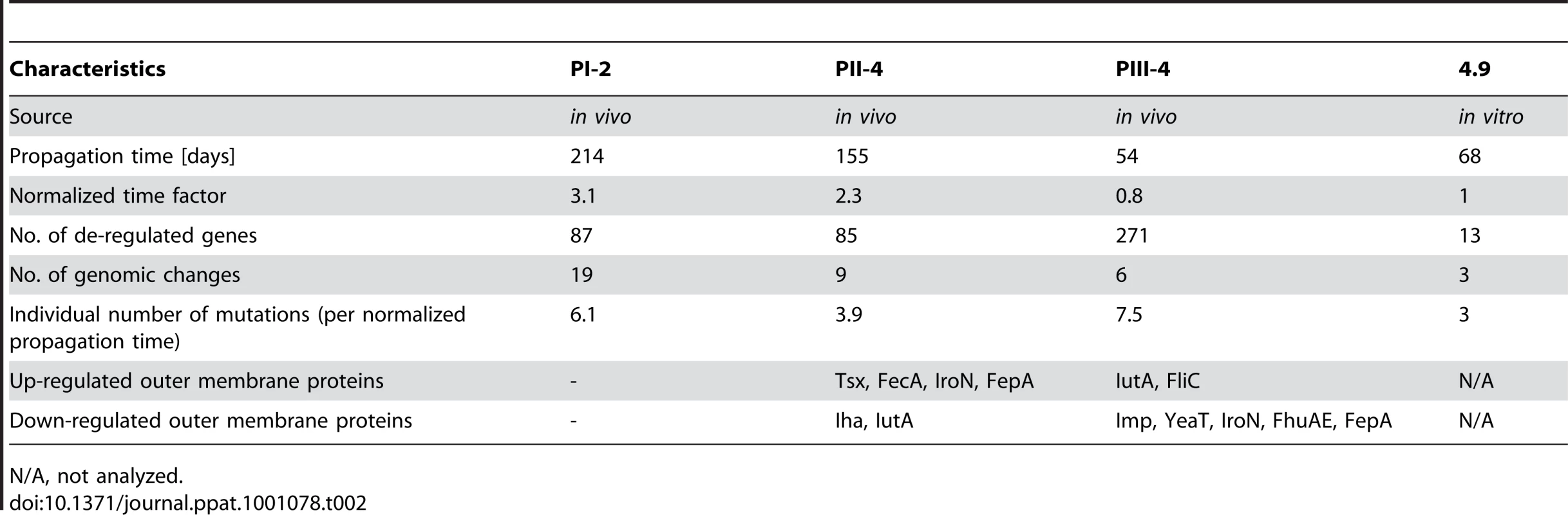
N/A, not analyzed. Studying re-isolate PIII-4, we found that its metabolism, motility and stress responses were affected when compared to the progenitor strain 83972. As already indicated by phenotypic tests, this isolate showed increased motility. Indeed, flagellum and chemotaxis determinants made up 32 of the upregulated genes (Figure 4). OMP analysis further corroborated these results and FliC was the most upregulated protein on the bacterial surface (Figure 4). Against the background of generally impaired virulence gene expression in E. coli 83972 and its re-isolates, this is the first observation that expression of an immunogenic and functional virulence factor, i.e. flagella, is increased in E. coli 83972 upon prolonged in vivo growth. With regard to metabolic adaptations, we detected 68 upregulated genes involved in diverse processes, suggesting nutrition adaptation, e.g. sugar and sugar acid uptake fuelling glycolysis (galacturonate, glucuronate, sialic acid, arabinose and mannose), (see Figure S5A). Upregulated D-serine uptake and its deamination pathway in PIII-4, together with reduced glutamine uptake and degradation (downregulated glnALG and glnHPQ operons, see Figure S5B), mirror adaptation to urine as it is a nitrogen and D-serine-rich environment [21], [22]. Utilization of the RNA degradation product pseudouridine, a nucleoside present in human urine [23], was also upregulated in strain PIII-4 as indicated by increased yeiC and yeiN gene expression. In addition to multiple metabolic alterations, we observed that genes frmAB were up-regulated when compared to the progenitor strain. Accordingly, genome analysis of this re-isolate demonstrated a corresponding point mutation in the frmR gene.
In the second re-isolate (PI-2), we found that the majority of the deregulated genes were also connected to growth and stress responses (Figure 4). Growth-related genes required for peptide/amino acid transport and utilization (degP, metNIQ, pepD, oppD, and artJ), that have been reported to be essential for bacterial multiplication in urine [24], were upregulated. As in the previous re-isolate (PIII-4), the genes frmAB, which were proposed to provide protection against oxidative or nitrosative stress [15], were upregulated. In addition, marAB expression was upregulated, corresponding to the genome sequence in which both marR and marA displayed point mutations (Table S1). This is important because the MarAB proteins are known to respond to oxidative/nitrosative stress as well as to antimicrobial peptides [25]. We also found that the expression of ribonucleotide-diphosphate reductase required for DNA synthesis, replication and repair was increased. It should be noted that expression of the ribonucleotide-diphosphate reductase 2-encoding genes nrdHIEF, which are increased by oxidative stress, is indirectly regulated by OxyR, and that oxyR was mutated in re-isolate PI-2 (Table S1).
In isolate PII-4 we mainly identified alterations in central intermediary metabolism and iron uptake, in contrast to the possible stress adaptations in previous re-isolates. Upregulation of the tsx, cdd, udp and deoABCD genes in re-isolate PII-4 (Figure 4, S7 and S8) indicated that ribo - and deoxyribonucleoside utilization was enhanced. Resulting ribose-5-phosphate or deoxyribose-5-phosphate could be channeled into the non-oxidative branch of the pentose phosphate or the TCA cycle, respectively. Derepression of this catabolic pathway was probably due to a SNP in cytR coding for a transcriptional repressor of the above-mentioned determinants (Table S1). It may be hypothesized that such adaptations could improve bacterial fitness as substantial amounts of nucleic acids are accessible in urine due to bacterial disintegration, exfoliation and lysis of bladder epithelial cells [26]. We also found that iron homeostasis was affected. Expression of ferric aerobactin receptor IutA was drastically reduced in re-isolate PII-4 (Figure 4) what could be explained by the loss of the aerobactin determinant through a 27-kb genomic deletion (Figure S2C). As IutA is highly immunogenic [27], this deletion may provide an adaptive advantage given the asymptomatic lifestyle of E. coli 83972. Moreover, transcriptome analysis indicated that fec transcript levels were significantly increased in this strain (Figure 4). This was further corroborated by OMP analysis showing that ferric dicitrate transporter FecA expression was upregulated relative to the parent strain (Figure S7). Comparing genomes of the 83972 progenitor and its descendant PII-4, we found a SNP in the putative binding site of the ferric uptake regulator (Fur) upstream of the fecIR regulatory genes (Table S1, Figure 3). Reporter gene assays with the wild type or the PII-4 fecIR upstream region that was fused with the promoterless luciferase gene cluster uncovered 8-fold increase of the re-isolate promoter activity (Figure 5A, S9). This result suggested differences in the binding efficiency of the Fur protein to the polymorphic fecIR promoter site of E. coli PII-4. To assess the molecular mechanisms underlying increased fec expression on the DNA/protein binding level, electrophoretic mobility shift assays (EMSA) were performed. We found that this point mutation decreases binding efficiency of Fur dimers to the altered Fur box on one DNA strand. Consequently, in the re-isolate PII-4 strong Fur tetramer-mediated repression of fecIR transcription was weakened resulting in upregulation of the ferric dicitrate uptake (Figure 5B and C). Interestingly, in the second inoculation re-isolate PII-B, we found another SNP again present within the Fur binding site (Figure 5D).
Fig. 5. Increased fecIR expression due to a T → C transition in the upstream region of fecIR genes in re-isolate PII-4 relative to parent strain 83972. 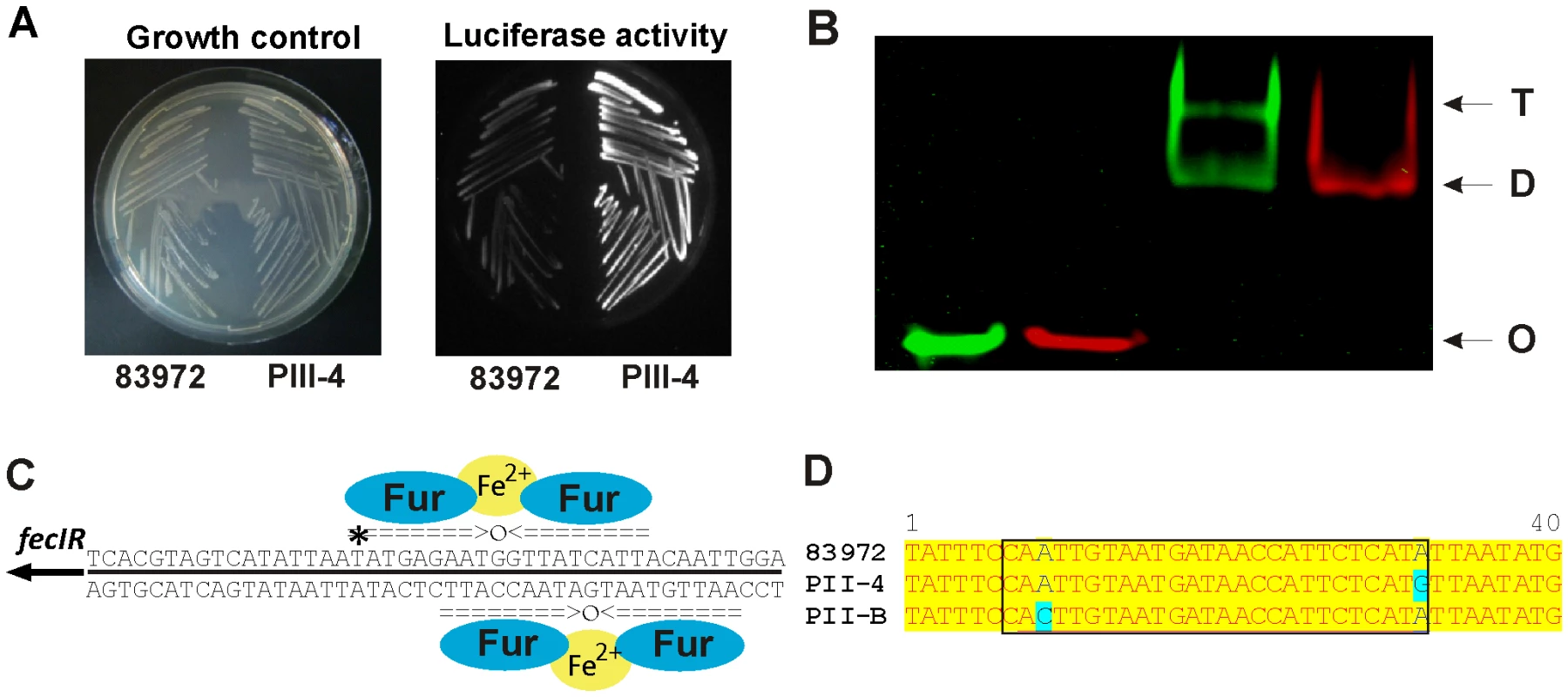
(A) Growth and luciferase activity of E. coli K-12 carrying pACYC184-based transcriptional reporter gene fusions of sequences upstream of fecIR from E. coli 83972 or PII-4, respectively, and the promoterless luciferase gene. (B) Electric mobility shift assay (EMSA) showing that the SNP in the fecIR upstream region of strain PII-4 abolishes tetramer formation of the Fur protein binding to the Fur box. Green, 83972; red, PII-4; D, Fur protein dimer; T, Fur protein tetramer; O, unbound Cy-3- or Cy-5-labeled DNA oligomer. (C) Model describing binding of the Fur protein to the upstream region of fecIR. The nucleotide sequence depicted corresponds to the 45-bp Cy-3- or Cy-5-labeled DNA oligomer comprising the Fur binding site upstream of fecIR used for electrophoretic mobility shift assays. The asterisk indicates the SNP in strain PII-4 relative to parent strain 83972. (D) Alignment of nucleotide sequences of the putative Fur binding site (region in black box) within the fecIR promoter from independent PII re-isolates. Letters in blue indicate two distinct point mutations acquired during independent colonization episodes. Relationship between duration of colonization and genomic alterations
The genomic analysis of re-isolates from the different time points of patient colonization indicated a positive correlation between the number of genetic changes and the colonization time. However, if one normalizes the propagation time of the in vivo re-isolates PI-2, PII-4 and PIII-4 to that of in vitro isolate 4.9, the propagation time of the in vivo re-isolates exceeds that of E. coli 4.9 by factor 3.1, 2.3 and 0.8, respectively. By dividing the number of genetic changes by the normalized propagation time of the isolate, we were able to assess the individual extent of genomic alterations upon in vivo and in vitro growth in urine (Table 2). Our data indicate that the number of mutations was markedly higher in re-isolates PI-2 and PIII-4 (2 - and 2.5-fold, respectively) which, according to their gene expression profiles, were subjected to increased oxidative stress during bladder colonization (Figure 4). In contrast, the mutation rate of E. coli PII-4, which did not show adaptation to oxidative stress, was comparable to that of the in vitro isolate 4.9.
Bacterial adaptation and innate host response
To examine if the host immune status might influence bacterial adaptation, the innate immune response to inoculation was quantified on a monthly basis with regard to Interleukin 6 (IL-6) and Interleukin 8 (IL-8) concentrations and neutrophil infiltration. In addition, urine samples were subjected to extended cytokine/chemokine profiling (Figure 6).
Fig. 6. Innate immune response to inoculation with E. coli 83972. 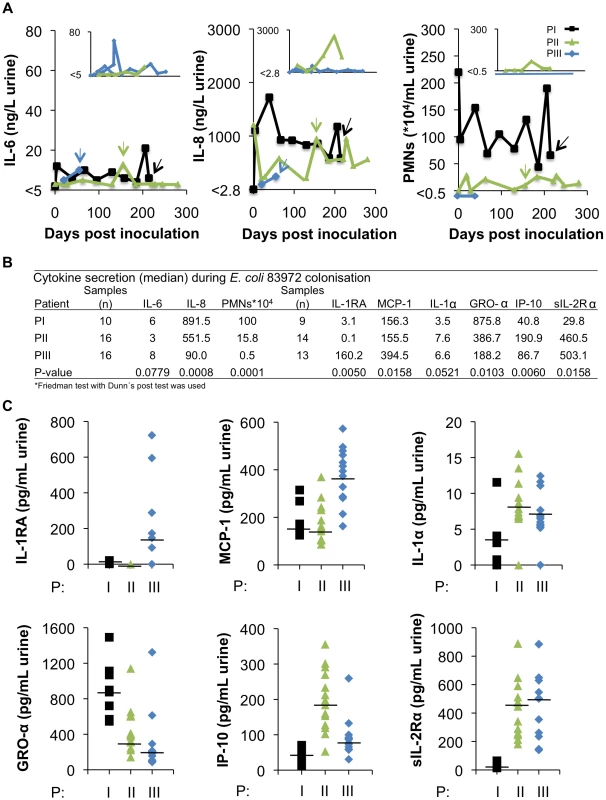
(A) IL-6 and IL-8 concentrations and neutrophil numbers were quantified in urine samples obtained from the three patients throughout the colonization period. Kinetics of the host response and time of collection of re-isolates PI-2, PII-4 and PIII-4. Inset diagrams present the host response parameters to re-inoculations of PII and PIII with E. coli 83972. (B) Median host responses for cytokines/chemokines in urine. (C) Extended cytokine/chemokine analysis, showing significant differences bwetween the three patients, except for IL-1α. Several interesting differences in the innate immune response profile were observed between the patients. PI, with the highest number of genomic alterations showed the highest IL-8 response over time and the strongest neutrophil recruitment (Figure 6A and B). In PIII neutrophil (p<0.0001) and IL-8 (p<0.008) responses were not detected, but this patient showed the highest IL-6 response and had very high concentrations of IL-1RA in urine, compared to PI and PII (p<0.005, Figure 6B and C). Some of the host response differences were reproduced during the second inoculation (Figure 6A). The results suggest that the patients activate different aspects of the innate immune response to infection.
Discussion
Single bacterial surface antigens or virulence factor profiles are known to vary under host immune pressure. For example, E. coli isolates from recurrent bacteremia or chronic UTI often lose the expression of long chain LPS, capsules or flagella [28], [29] and enterohemorrhagic E. coli may lose major virulence determinants in the course of infection [30]. Data on genome-wide changes and adaptation during long-term growth of E. coli in vitro has only started to accumulate recently [31]. However, genomic alterations involved in bacterial adaptation to individual human host environments have largely not been studied. In this context, only a few studies focused on analyses of sequential isolates obtained from hosts persistently infected with Pseudomonas aeruginosa or Helicobacter pylori [32], [33]. They reported a loss of virulence due to successive alterations in genome content and gene expression, but the extent to which different human hosts modify single bacterial genomes has not been investigated.
In our study, we have examined to which extent host imprinting guides the evolution of adaptive genomic modifications during asymptomatic bacterial carriage by comparing whole genomes, transcriptomes and proteomes of the prototype ABU strain E. coli 83972 before therapeutic inoculation and after re-isolation from several human hosts. The urinary tract inoculation protocol is a safe and efficient way to prevent symptomatic infections in certain patient groups [9] and allowed us to administer the same bacterial strain to multiple hosts rather than relying on natural infections of different hosts with different strains. We also controlled the time of bacterial carriage, thus ensuring that the in vivo adaptation of the bacterial genome was followed from the onset of establishment in each host.
We identified potential molecular adaptation mechanisms based on a limited number of point mutations and small deletions that frequently altered the coding regions (Figure 3, Table S1). Strikingly, some of these adaptation mechanisms appeared to be unique for each host, suggesting that the genomic identity of a bacterial isolate is flexible and relevant in a given host niche. Sequencing of the re-isolates enabled us to analyze the genome-wide extent of bacterial adaptation. As the E. coli strain 83972 was isolated from a young girl, who was colonized for more than three years [5], it was expected to be well-adapted to growth in urine. We observed that the number of genomic alterations increased with prolonged colonization time of the patients, as displayed by the number of mutations as a function of time (Table 2). Suboptimal fitness in the new hosts was apparently tailored by targeting regulators of bacterial metabolism. In consequence, each of the re-sequenced isolates demonstrated unique adaptations potentially resulting in growth advantages in their growth environment (Figure 7). It still remains to be elucidated to what extend growth conditions in the individual hosts contributed to this divergent evolution.
Fig. 7. Different adaptational strategies of E. coli 83972 upon prolonged growth in the urinary bladder of human hosts. 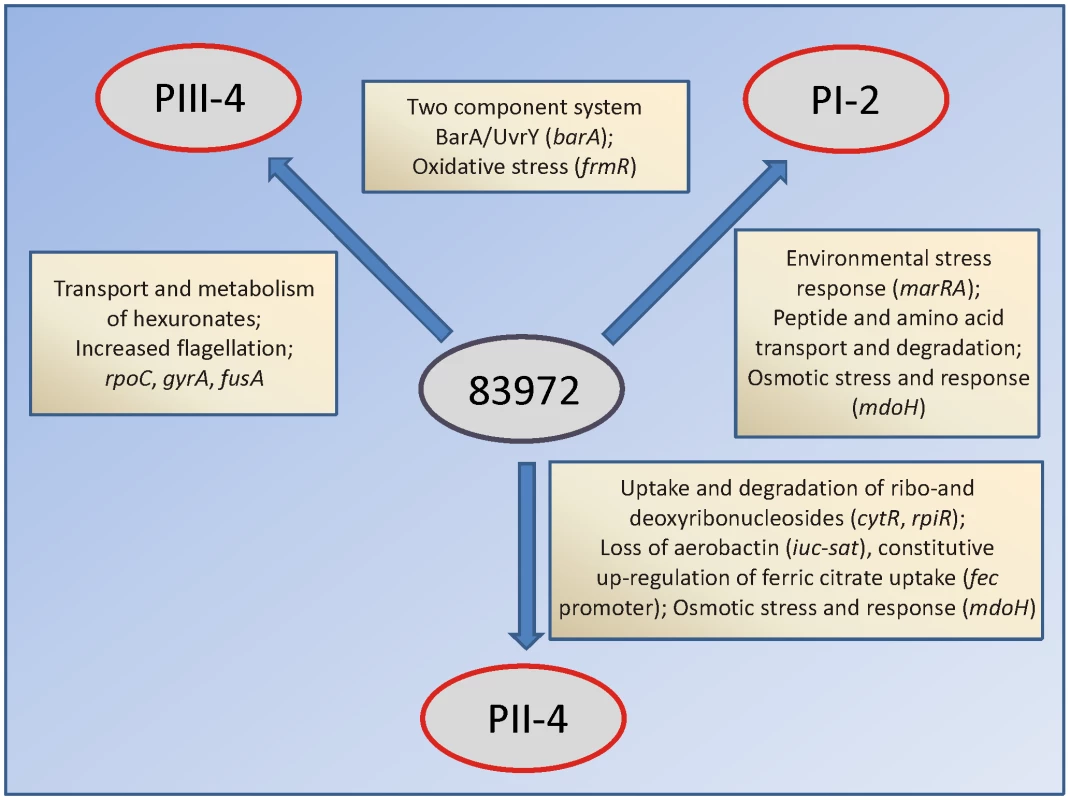
Adaptational strategies were deduced from genomic, transcriptomic and proteomic alterations in re-isolates PI-2, PII-4 and PIII-4. Genes in brackets are mutated in re-isolates relative to their parent E. coli 83972. Adaptation to individual hosts included different metabolic pathways, i.e. utilization of amino acids, hexuronates or (deoxy-) ribonucleosides; iron uptake and stress protection systems. Adaptation patterns of the in vivo re-isolates supported the hypothesis that evolution in individual hosts was driven by positive selection of genetic variants which are better suited to the particular host and to some extend probably also by genetic drift. The results suggest that the genome of prototype ABU isolate E. coli 83972 is relatively stable as only 34 mutations were detected after bladder colonization for 423 patient days. To distinguish host imprinting from stochastic events, we sequenced the polymorphic positions in re-isolates from repeat inoculation events in each patient. The reproducibility of some genetic changes indicates that host-driven genetic change may play an important role in bacterial microevolution. Certain genetic alterations were detected in re-isolates from several hosts or from the same host, after independent inoculations, but not in bacteria propagated in vitro. The number of non-redundant genetic changes observed after repeated inoculations might on the other hand be explained by random mutagenesis. We also examined if the adaptive changes might be cyclic in nature and if, in different patients, the re-isolates were picked at different cycles. In two of the patients, who carried E. coli 83972 for more than a hundred days after the initial re-isolate, we obtained repeat re-isolates and evidence that several genomic changes were stable in the population.
The impact of host-dependent selection of specific mutants (“genomic imprinting”) versus random selection remains to be defined. Non-synonymous mutations were mainly detected suggesting that positive selection for structural changes over silent ones was favored during bladder colonization. Based on the genomic profile and on mechanisms of susceptibility in human hosts, several classes of host molecules may be discussed. Mutations reducing the sensitivity to stress [34], [35] or changing metabolism pointed to specific host processes, as did genes that became redundant and were lost in the new environment [36], [37]. In re-isolates PI-2 and PIII-4, whose gene expression profiles and genomic alterations indicate adaptation to oxidative stress (Figure 4 and 7), the mutation rate was markedly higher than in the in vitro propagated strain 4.9 (Table 2), suggesting that in these cases host response mechanisms, i.e. release of reactive oxygen species may have triggered bacterial adaptation. In line with this, the analysis of re-isolate PII-4 did not point towards pronounced adaptation to oxidative stress and its mutation rate was comparable relative to the in vitro-propagated E. coli 4.9.
Host resistance to UTI is controlled by innate immunity and there are genetic differences in innate immune responses between patients prone to severe, symptomatic infections and those who develop ABU, affecting the IL-8 receptor CXCR1, the IRF3 transcription factor and in TLR4 promoter sequences [38], [39], [40]. Such differences influence the efficiency of bacterial clearance and the extent of tissue damage, thus limiting or promoting the antibacterial host environment [38], [41], [42], [43]. In this study, differences in innate immune responses to inoculation were detected, influencing IL-8 secretion and thus the CXCR1-mediated innate immune response. A second, differentially regulated pathway reflected events downstream of TRIF and IRF3, modifying the IL-1/IL-6 signaling pathways. The results suggest that the patients activate different aspects of the innate immune response to infection and are consistent with such responses driving bacterial adaptation. To understand this complexity is immensely challenging, but our findings illustrate the need to study microbial interactions within individual hosts in symptomatic infections versus asymptomatic carriage. It may be speculated that long-term asymptomatic carriage in a low responder host combined with attenuation of virulence might be an excellent mutual strategy.
In ABU patients, bacteria persist as a privileged monoculture, resembling the normal flora but without the complex microbial competition characteristic of other mucosal sites. Most ABU E. coli strains arise from virulent variants by gene loss, suggesting that attenuation may constitute a survival mechanism for mucosal pathogens [8], [44]. This evolution of commensalism is interesting, as based on early predictions by Haldane [45], microbial populations evolve towards virulence. In this proposal, symptoms caused by the virulent organisms would promote transmission and the resulting increase in host number would be the most successful survival mechanism. The present study suggests that ABU bacteria may evolve towards commensalism rather than virulence, thereby achieving long-term carriage in individual hosts. While it is possible that ABU may favor between-host transmission, such consequences remain to be investigated.
The definition of commensalism has long been debated, and it is unclear if the relationship identified as commensalism is more likely to be slightly symbiotic or parasitic. The gut flora (“true commensals”) uses nutrients ingested by the host, indicating a slightly parasitic situation but may outcompete possible pathogens, indicating symbiosis. It may also be debated whether asymptomatic carriage of E. coli in the urinary tract should be considered as an infection as it represents the establishment of bacteria at a normally sterile site, or as a condition moving towards symbiosis/commensalism. The term asymptomatic bacteriuria is generally used to distinguish colonization from infection and to emphasize that the presence of bacteria at mucosal surfaces does not always cause symptoms and tissue damage. We have proposed asymptomatic bacteriuria as a model to study mechanisms underlying the development of commensalism. In the gut, a complex bacterial flora makes it technically difficult or impossible to study de novo responses of microbes to the host environment, unless germ free mice are used; in itself an artificial situation. As commensalism is defined as a relationship in which one symbiont, the commensal, benefits while the other (host) is neither harmed nor helped, asymptomatic bacteriuria clearly fulfills the definition in many individuals, while in others the asymptomatic carriage will be beneficial to both partners, thereby perhaps indicating a more symbiotic relationship.
Here we present for the first time the complete genome sequence of an asymptomatic bacteriuria E. coli isolate and the analysis of bacterial microevolution in the human urinary tract. We demonstrate that upon prolonged bladder colonization metabolism, preferentially the exploitation of suitable carbon - and nitrogen sources in urine, iron uptake and stress resistance of E. coli 83972 was affected depending on the colonized host. Future work will analyze the biological relevance of the genomic alterations observed in this study and show if this knowledge can help us to identify potential drug targets to decrease bacterial fitness during symptomatic infections.
Materials and Methods
Ethics statement
The deliberate colonization study has been approved after written informed consent from the patients by the Medical Ethics committee, University of Lund, Sweden (Approval no. LU 742-01/2001).
Patients
Patients with lower urinary tract dysfunctions and recurrent lower UTI (≥3 UTI/year, for two years) were invited to participate in the study [9]. Their UTI history was confirmed by the use of interviews and patient records, and patients with a history of acute pyelonephritis, urological malignancies or corticosteroid treatment were excluded. Enrolled patients underwent renal function tests, upper urinary tract imaging and cystoscopy to exclude renal disease or stone formation. All patients could not completely empty their bladder upon voiding (residual urine ≥100 ml). Bacterial culture records were consulted to acertain that the UTI episodes were accompanied by significant bacteriuria (≥105 cfu/ml) and that the patient experienced improvement after antibiotic therapy. Before inoculation, patients were treated with appropriate antibiotics to sterilize the urine and after an antibiotic free interval, the patients were catheterized. After emptying the bladder, 30 ml of E. coli 83972 (105 cfu/ml) was instilled and the patients were followed according to a defined study protocol [9]. E. coli 83972 was originally isolated from a girl with asymptomatic bacteriuria [5] and its ability to cause long term bacteriuria in patients with dysfunctional voiding is well documented. The inoculated patients developed long-term, asymptomatic bacteriuria, experiencing no discomfort, except for the first 24 hours after catheterization. In a standardized questionnaire addressing symptoms and need for therapeutic intervention, no significant events were recorded [6], [9].
Throughout the colonization period, monthly urine samples were collected and analyzed for IL-6 and IL-8 as well as neutrophil infiltration. For each urine sample urine proteome array analysis was performed to study the specific host response. Bacteria from each urine sample were verified by PCR for presence of a kryptic plasmid unique for strain 83972 and one chromosomal marker (4.7-kb deletion in strain 83972 in the type 1 fimbrial gene cluster). For further analysis, five independent colonies per urine sample were used.
Genome sequencing, assembly and gap closure
Total genomic DNA of E.coli 83972 was mechanically sheared (HydroShear, GeneMachines) for a Sanger sequencing approach. A shotgun library based on pCR4.1-TOPO (Invitrogen) was constructed with the 1.5 - to 3-kb size fraction of DNA fragments. Recombinant plasmids inserts were sequenced using dye terminator chemistry and ABI Prism 3730XL DNA sequencers (Applied Biosystems). Sequences were processed with Phred and assembled with the Phrap assembly tool (www.phrap.org). Additionally, genomic DNA of E.coli 83972 and its re-isolates was pyrosequenced using a 454 Life Sciences GS-FLX sequencer (Roche). The 454 reads were assembled using Newbler (Roche). Sequence editing of shotgun and 454 sequences was done with GAP4 [46]. For correction of misassembled regions and gap closure, PCR or combinatorial multiplex PCR using the Extender System polymerase (5 Prime) or the TempliPhi Sequence Resolver kit (GE Healthcare), and primer walking with recombinant plasmids were applied. For the validation of genetic differences between the re-isolates and the ancestor strain, single locus sequencing (Sanger) was performed.
Open reading frames (ORFs) were predicted with YACOP [47]. For annotation, all proteins were screened against Swiss-Prot data and publicly available protein sequences from other completed genomes. All predictions were then verified and manually modified using the ERGO software package (Integrated Genomics) [48]. Complete genome comparisons were done with ACT [49] based on replicon-specific nucleotide BLAST [50] and with protein based BiBlast comparisons to selected E.coli genomes (Wollherr 2009, personal communication). The 83972 genome sequence reported in this paper has been deposited in the GenBank database (accession number CP001671).
Strain cultivation
E.coli strain 83972 was routinely grown in vitro in pooled sterile human urine at 37°C without agitation. For long-term propagation in vitro, the strain was grown as independent cultures for 68 days (>2000 generations) in pooled sterile human urine at 37°C in a continous culture.
Pulsed Field Gel Electrophoresis (PFGE)
PFGE was done as previously described (Zdziarski et. al, 2007).
Total RNA isolation
Bacteria were harvested from mid-log phase cultures. Samples were treated with RNAprotect (Qiagen) and extracted using the RNeasy mini kit (Qiagen). DNA traces were removed by RNase-free DNase I (New England Biolabs).
Array hybridization and data processing
For expression profiling, custom-tailored oligonucleotide microarrays (Operon Biotechnologies) were used. The custom array contained 10,816 longmer oligonucleotide probes covering the complete genomes of six E.coli strains (non-pathogenic E.coli K-12 strain MG1655, EHEC O157:H7 strains EDL933 and Sakai, UPEC strains CFT073, 536 and UTI89, pOSAK1, pO157_Sakai, pO157_EDL933 and pUTI89).
10 µg of total RNA were reverse transcribed (SuperScript III, Invitrogen) with direct incorporation of fluorescently labelled (Cy3 - or Cy5-) dCTP (GE Healthcare). 160 pmol of each Cy-3 and Cy-5 labelled probe were used for hybridisation. For each experiment, at least three independent hybridizations were performed. Hybridized and washed slides were scanned using a GenePix 4000B Microarray Scanner (GE Healthcare) with a resolution of 10 µm pixel size.
Outer membrane protein isolation
Outer membrane protein (OMP) preparations from bacteria were performed as described previously [27].
Two-dimensional protein gel electrophoresis
Proteome analysis was performed with 300 µg OMP samples as described previously [27]. Coomassie G-250-stained gels were scanned and analyzed with the Delta-2D Software (http://www.decodon.com).
Mass spectrometry
Protein spots were excised from stained 2-D gels. Following tryptic digestion, MALDI-TOF measurement was carried out with the 4800 MALDI TOF/TOF Analyzer (Applied Biosystems). The Mascot search engine version 2.1 (Matrix Science Ltd, London, UK) was used for data base search with a specific E. coli sequence database.
Luciferase measurements
The 485-bp upstream region of fecIR was fused with the promoterless luciferase gene cluster luxABCDE in pACYC184. Plasmids with the transcriptional reporter gene fusions were transformed into E. coli strain DH5α and grown at 37°C in Luria broth. 100 µl samples were withdrawn after 3 hours of growth and light emission was recorded with a luminometer (Berthold). To test the luciferase activity directly on LB agar plates, bacterial luminescence was recorded with the ChemiLux photoimager (Intas).
E. coli Fur expression and purification
E. coli Fur protein was purified with the IMPACT protein purification system (New England Biolabs) according to the manufacturer's instructions. The fur sequence was amplified using primers Fur_up_NdeI (5′-GGTGGTCATATGACTGATAACAATACCGCCC-3′) and Fur_down_SapI (5′-GGTGGTTGCTCTTCCGCATTTGCCTTCGTGCGCGTGCTC-3′).
Electrophoretic Mobility Shift Assay (EMSA)
A 45-bp Cy3 - or Cy5-labeled DNA oligomer (Operon) comprising the Fur binding site upstream of fecIR (tccaattgtaatgataaccattctcatattaatatgactacgtga-Cy3 – 83972; tccaattgtaatgataaccattctcatgttaatatgactacgtga-Cy5 – PII-4) was annealed with an unlabeled complementary 45-bp oligomer in annealing buffer (10 mM Tris-HCl, 50 mM NaCl, 1 mM EDTA; 95°C–5 min, 67°C–20 min, 30°C–1 h). EMSAs were performed as previously described [52]. Gels were subsequently scanned on a Typhoon variable mode imager (Molecular Dynamics).
Innate immune response parameters in urine
Neutrophil numbers were counted in un-centrifuged fresh urine using a Bürker chamber [53]. IL-6 and IL-8 concentrations in fresh urine samples were determined in the Lund University hospital routine lab using an Immulite 1000 (Siemens). The detection limits were 2.8 pg/ml (IL-6) and 5 ng/ml (IL-8). Samples with undetectable cytokine concentrations were assigned the value of lower detection limit. For extended cytokine/chemokine profiling, we used the MILLIPLEX MAP Human Cytokine/Chemokine Panel to detect IL-1RA, MCP-1, IL-1α, GRO-α, IP-10 and sIL-2Rα. The analysis was according to the manufacturer's protocol and measurements were in duplicates on a Luminex 200 instrument (Luminex Corp.).
Statistics
The Freidman test with Dunn's post test was used for comparisons of innate host responses.
Supporting Information
Zdroje
1. ShihAC
HsiaoTC
HoMS
LiWH
2007 Simultaneous amino acid substitutions at antigenic sites drive influenza A hemagglutinin evolution. Proc Natl Acad Sci U S A 104 6283 6288
2. PalmerGH
BraytonKA
2007 Gene conversion is a convergent strategy for pathogen antigenic variation. Trends Parasitol 23 408 413
3. TempletonTJ
2009 The varieties of gene amplification, diversification and hypervariability in the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol 166 109 116
4. DeitschKW
LukehartSA
StringerJR
2009 Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol 7 493 503
5. LindbergU
ClaessonI
HansonLA
JodalU
1978 Asymptomatic bacteriuria in schoolgirls. VIII. Clinical course during a 3-year follow-up. J Pediatr 92 194 199
6. SundénF
HåkanssonL
LjunggrenE
WulltB
2006 Bacterial interference-is deliberate colonization with Escherichia coli 83972 an alternative treatment for patients with recurrent urinary tract infection? Int J Antimicrob Agents 28 Suppl 1 S26 29
7. KlemmP
RoosV
UlettGC
SvanborgC
SchembriMA
2006 Molecular characterization of the Escherichia coli asymptomatic bacteriuria strain 83972: the taming of a pathogen. Infect Immun 74 781 785
8. ZdziarskiJ
SvanborgC
WulltB
HackerJ
DobrindtU
2008 Molecular basis of commensalism in the urinary tract: low virulence or virulence attenuation? Infect Immun 76 695 703
9. SundénF
HåkanssonL
LjunggrenE
WulltB
2010 Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J Urol 184 179 185
10. GrozdanovL
RaaschC
SchulzeJ
SonnenbornU
GottschalkG
2004 Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol 186 5432 5441
11. HirakawaH
NishinoK
HirataT
YamaguchiA
2003 Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J Bacteriol 185 1851 1856
12. MinagawaS
OgasawaraH
KatoA
YamamotoK
EguchiY
2003 Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J Bacteriol 185 3696 3702
13. TomeniusH
PernestigAK
JonasK
GeorgellisD
MollbyR
2006 The Escherichia coli BarA-UvrY two-component system is a virulence determinant in the urinary tract. BMC Microbiol 6 27
14. DebarbieuxL
BohinA
BohinJP
1997 Topological analysis of the membrane-bound glycosyltransferase, MdoH, required for osmoregulated periplasmic glucan synthesis in Escherichia coli. J Bacteriol 179 6692 6698
15. LiuL
HausladenA
ZengM
QueL
HeitmanJ
2001 A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410 490 494
16. GreenbergJT
ChouJH
MonachPA
DempleB
1991 Activation of oxidative stress genes by mutations at the soxQ/cfxB/marA locus of Escherichia coli. J Bacteriol 173 4433 4439
17. JubelinG
VianneyA
BeloinC
GhigoJM
LazzaroniJC
2005 CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J Bacteriol 187 2038 2049
18. PernestigAK
GeorgellisD
RomeoT
SuzukiK
TomeniusH
2003 The Escherichia coli BarA-UvrY two-component system is needed for efficient switching between glycolytic and gluconeogenic carbon sources. J Bacteriol 185 843 853
19. HerringCD
RaghunathanA
HonischC
PatelT
ApplebeeMK
2006 Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat Genet 38 1406 1412
20. PhilippeN
CrozatE
LenskiRE
SchneiderD
2007 Evolution of global regulatory networks during a long-term experiment with Escherichia coli. Bioessays 29 846 860
21. AnforaAT
HalladinDK
HaugenBJ
WelchRA
2008 Uropathogenic Escherichia coli CFT073 is adapted to acetatogenic growth but does not require acetate during murine urinary tract infection. Infect Immun 76 5760 5767
22. MoritzRL
WelchRA
2006 The Escherichia coli argW-dsdCXA genetic island is highly variable, and E. coli K1 strains commonly possess two copies of dsdCXA. J Clin Microbiol 44 4038 4048
23. PreumontA
SnoussiK
StroobantV
ColletJF
Van SchaftingenE
2008 Molecular identification of pseudouridine-metabolizing enzymes. J Biol Chem 283 25238 25246
24. AlteriCJ
SmithSN
MobleyHL
2009 Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog 5 e1000448
25. WarnerDM
LevySB
2009 Different effects of transcriptional regulators marA, soxS, and rob on susceptibility of Escherichia coli to cationic antimicrobial peptides (CAMPs): Rob-dependent CAMP induction of the marRAB operon. Microbiology doi:10.1099/mic.0.033415-0
26. BryzgunovaOE
SkvortsovaTE
KolesnikovaEV
StarikovAV
RykovaEY
2006 Isolation and comparative study of cell-free nucleic acids from human urine. Ann N Y Acad Sci 1075 334 340
27. HaganEC
MobleyHL
2007 Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect Immun 75 3941 3949
28. BettelheimKA
TaylorJ
1969 A study of Escherichia coli isolated from chronic urinary infection. J Med Microbiol 2 225 236
29. OlesenB
KolmosHJ
ØrskovF
ØrskovI
1998 Escherichia coli bacteraemia in patients with and without haematological malignancies: a study of strain characters and recurrent episodes. J Infect 36 93 100
30. BielaszewskaM
KockR
FriedrichAW
von EiffC
ZimmerhacklLB
2007 Shiga toxin-mediated hemolytic uremic syndrome: time to change the diagnostic paradigm? PLoS One 2 e1024
31. BarrickJE
YuDS
YoonSH
JeongH
OhTK
2009 Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature 461 1243 1247
32. JelsbakL
JohansenHK
FrostAL
ThogersenR
ThomsenLE
2007 Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in lungs of cystic fibrosis patients. Infect Immun 75 2214 2224
33. OhJD
Kling-BackhedH
GiannakisM
XuJ
FultonRS
2006 The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc Natl Acad Sci U S A 103 9999 10004
34. BjedovI
TenaillonO
GerardB
SouzaV
DenamurE
2003 Stress-induced mutagenesis in bacteria. Science 300 1404 1409
35. RosenbergSM
2001 Evolving responsively: adaptive mutation. Nat Rev Genet 2 504 515
36. GiraudA
ArousS
De PaepeM
Gaboriau-RouthiauV
BambouJC
2008 Dissecting the genetic components of adaptation of Escherichia coli to the mouse gut. PLoS Genet 4 e2
37. NovakM
PfeifferT
LenskiRE
SauerU
BonhoefferS
2006 Experimental tests for an evolutionary trade-off between growth rate and yield in E. coli. Am Nat 168 242 251
38. LundstedtAC
McCarthyS
GustafssonMC
GodalyG
JodalU
2007 A genetic basis of susceptibility to acute pyelonephritis. PLoS One 2 e825
39. FischerH
LutayN
RagnarsdóttirB
YadavM
JönssonK
2010 IRF3-dependent signalling, human polymorphism and innate resistance to kidney infection. in revision
40. RagnarsdóttirB
JönssonK
UrbanoA
Grönberg-HernandezJ
LutayN
2010 Toll-like receptor 4 promoter polymorphisms: Common TLR4 variants protect against severe urinary tract infection. PLoS One
41. BergstenG
SamuelssonM
WulltB
LeijonhufvudI
FischerH
2004 PapG-dependent adherence breaks mucosal inertia and triggers the innate host response. J Infect Dis 189 1734 1742
42. FrendeusB
GodalyG
HangL
KarpmanD
LundstedtAC
2000 Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J Exp Med 192 881 890
43. RagnarsdottirB
FischerH
GodalyG
Gronberg-HernandezJ
GustafssonM
2008 TLR - and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections. Eur J Clin Invest 38 Suppl 2 12 20
44. BergstenG
WulltB
SvanborgC
2005 Escherichia coli, fimbriae, bacterial persistence and host response induction in the human urinary tract. Int J Med Microbiol 295 487 502
45. HaldaneJB
1949 Suggestions as to quantitative measurement of rates of evolution. Evolution 3 51 56
46. StadenR
BealKF
BonfieldJK
2000 The Staden package, 1998. Methods Mol Biol 132 115 130
47. TechM
MerklR
2003 YACOP: Enhanced gene prediction obtained by a combination of existing methods. In Silico Biol 3 441 451
48. OverbeekR
LarsenN
WalunasT
D'SouzaM
PuschG
2003 The ERGO genome analysis and discovery system. Nucleic Acids Res 31 164 171
49. CarverT
BerrimanM
TiveyA
PatelC
BohmeU
2008 Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics 24 2672 2676
50. AltschulSF
MaddenTL
SchafferAA
ZhangJ
ZhangZ
1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389 3402
51. EisenMB
SpellmanPT
BrownPO
BotsteinD
1998 Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95 14863 14868
52. de LorenzoV
GiovanniniF
HerreroM
NeilandsJB
1988 Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol 203 875 884
53. JodalU
1987 The natural history of bacteriuria in childhood. Infect Dis Clin North Am 1 713 729
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



