-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCharacterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
Influenza viruses resistant to antiviral drugs emerge frequently. Not surprisingly, the widespread treatment in many countries of patients infected with 2009 pandemic influenza A (H1N1) viruses with the neuraminidase (NA) inhibitors oseltamivir and zanamivir has led to the emergence of pandemic strains resistant to these drugs. Sporadic cases of pandemic influenza have been associated with mutant viruses possessing a histidine-to-tyrosine substitution at position 274 (H274Y) in the NA, a mutation known to be responsible for oseltamivir resistance. Here, we characterized in vitro and in vivo properties of two pairs of oseltaimivir-sensitive and -resistant (possessing the NA H274Y substitution) 2009 H1N1 pandemic viruses isolated in different parts of the world. An in vitro NA inhibition assay confirmed that the NA H274Y substitution confers oseltamivir resistance to 2009 H1N1 pandemic viruses. In mouse lungs, we found no significant difference in replication between oseltamivir-sensitive and -resistant viruses. In the lungs of mice treated with oseltamivir or even zanamivir, 2009 H1N1 pandemic viruses with the NA H274Y substitution replicated efficiently. Pathological analysis revealed that the pathogenicities of the oseltamivir-resistant viruses were comparable to those of their oseltamivir-sensitive counterparts in ferrets. Further, the oseltamivir-resistant viruses transmitted between ferrets as efficiently as their oseltamivir-sensitive counterparts. Collectively, these data indicate that oseltamivir-resistant 2009 H1N1 pandemic viruses with the NA H274Y substitution were comparable to their oseltamivir-sensitive counterparts in their pathogenicity and transmissibility in animal models. Our findings highlight the possibility that NA H274Y-possessing oseltamivir-resistant 2009 H1N1 pandemic viruses could supersede oseltamivir-sensitive viruses, as occurred with seasonal H1N1 viruses.
Published in the journal: . PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001079
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001079Summary
Influenza viruses resistant to antiviral drugs emerge frequently. Not surprisingly, the widespread treatment in many countries of patients infected with 2009 pandemic influenza A (H1N1) viruses with the neuraminidase (NA) inhibitors oseltamivir and zanamivir has led to the emergence of pandemic strains resistant to these drugs. Sporadic cases of pandemic influenza have been associated with mutant viruses possessing a histidine-to-tyrosine substitution at position 274 (H274Y) in the NA, a mutation known to be responsible for oseltamivir resistance. Here, we characterized in vitro and in vivo properties of two pairs of oseltaimivir-sensitive and -resistant (possessing the NA H274Y substitution) 2009 H1N1 pandemic viruses isolated in different parts of the world. An in vitro NA inhibition assay confirmed that the NA H274Y substitution confers oseltamivir resistance to 2009 H1N1 pandemic viruses. In mouse lungs, we found no significant difference in replication between oseltamivir-sensitive and -resistant viruses. In the lungs of mice treated with oseltamivir or even zanamivir, 2009 H1N1 pandemic viruses with the NA H274Y substitution replicated efficiently. Pathological analysis revealed that the pathogenicities of the oseltamivir-resistant viruses were comparable to those of their oseltamivir-sensitive counterparts in ferrets. Further, the oseltamivir-resistant viruses transmitted between ferrets as efficiently as their oseltamivir-sensitive counterparts. Collectively, these data indicate that oseltamivir-resistant 2009 H1N1 pandemic viruses with the NA H274Y substitution were comparable to their oseltamivir-sensitive counterparts in their pathogenicity and transmissibility in animal models. Our findings highlight the possibility that NA H274Y-possessing oseltamivir-resistant 2009 H1N1 pandemic viruses could supersede oseltamivir-sensitive viruses, as occurred with seasonal H1N1 viruses.
Introduction
Since its emergence in early spring of 2009, 2009 pandemic influenza A (H1N1) viruses have been circulating worldwide [1]. Although most infected individuals have exhibited an uncomplicated, mild respiratory disorder, the pathogenicity of this virus is clearly higher than that of seasonal influenza viruses in animal models [2]–[4] and humans, including those who do not have underlying illness [5].
Although two classes of anti-influenza drugs – M2 ion channel blockers (amino-adamantines; amantadine and rimantadine) and neuraminidase (NA) inhibitors (oseltamivir and zanamivir) – are licensed, the 2009 H1N1 pandemic viruses, including the earliest isolate, are already amino-adamantine-resistant [1]. By contrast, most of the currently circulating pandemic viruses are susceptible to NA inhibitors [2], [6], and therefore, pandemic influenza patients are treated with NA inhibitors in many countries. This widespread administration, however, raises concerns over the emergence and global spread of NA inhibitor-resistant 2009 H1N1 pandemic viruses.
Studies with seasonal H1N1, H3N2, and highly pathogenic avian H5N1 viruses revealed that single amino acid substitutions at several positions in or around the NA active site confer resistance to viruses against NA inhibitors [7]–[11]. Among these NA substitutions, a histidine-to-tyrosine substitution at position 274 (N2 numbering, H274Y) is one of the best characterized oseltamivir-resistant markers. Recently, the NA H274Y substitution has been detected in sporadic cases of oseltamivir-treated and -untreated patients infected with 2009 H1N1 pandemic viruses [12]–[14]: however, the pathogenicity and transmissibility of viruses possessing this NA substitution remain unknown.
To better assess the risk of the 2009 H1N1 pandemic viruses that possess the NA H274Y substitution, we studied the in vitro and in vivo properties of two NA H274Y-possessing isolates that emerged independently: A/Osaka/180/2009 (H1N1; O180r) and A/Vietnam/HN32060/2009 (H1N1; VN32060r).
Results/Discussion
In vitro sensitivity of viruses to NA inhibitors
To test the in vitro sensitivity to various NA inhibitors of 2009 H1N1 pandemic viruses possessing the NA H274Y substitution, we measured the NA activity of O180r and VN32060r exposed to oseltamivir carboxylate (the active metabolite of oseltamivir), zanamivir, or R-125489 (the active metabolite of the experimental NA inhibitor CS-8958) and determined IC50 values. These IC50 values were compared with those of A/Osaka/164/2009 (O164s) and A/Vietnam/HCM9727/2009 (VN9727s), both of which do not possess the NA substitution (Supplementary Table S1) and thus served as control strains (Table 1). O164s and O180r were isolated on similar dates, circulating in the same community and were genetically similar. Likewise, VN9727s and VN32060r were similar with respect to their isolation history (temporally and geographically) and their genetic properties. The IC50 values of O180r and VN32060r were about 500-fold higher than those of O164s and VN9727s for oseltamivir, while the sensitivity to zanamivir and R-125489 was comparable for all viruses tested.
Tab. 1. In vitro virus susceptibility to NA inhibitors. 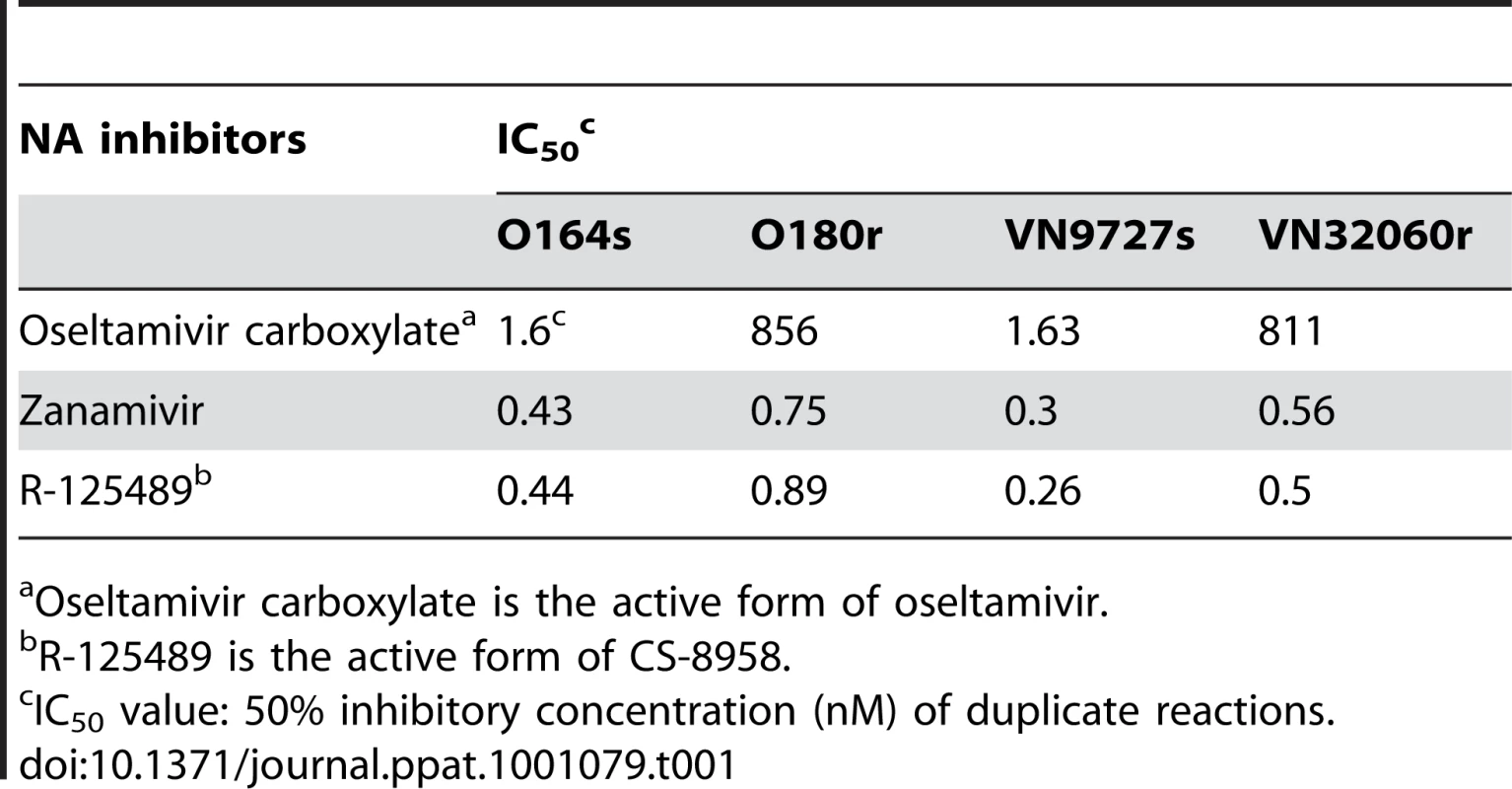
Oseltamivir carboxylate is the active form of oseltamivir. To further assess the in vitro sensitivity of oseltamivir-resistant 2009 H1N1 pandemic viruses to the NA inhibitors, we investigated the growth kinetics of the two pairs of oseltaimivir-sensitive and -resistant viruses in cultured cells in the presence of oseltamivir carboxylate, zanamivir, or R-125489 (Supplementary Fig. S1). While the growth of O164s and VN9727s was severely impaired by oseltamivir carboxylate, O180r and VN32060r replicated efficiently even in the presence of oseltamivir carboxylate. By contrast, both zanamivir and R-125489 inhibited the replication of all of the viruses tested. These results indicate that the NA H274Y substitution reduces the sensitivity of 2009 H1N1 pandemic viruses to oseltamivir, as has been shown with seasonal H1N1, H3N2, and highly pathogenic avian H5N1 viruses [7]–[11], [15], since the other amino acid variations among the viruses tested (Supplementary Table S1) did not account for their reduced oseltamivir sensitivity: the lower growth of O180r compared to that of O164s in the absence of antivirals (Supplementary Fig. S1) might be due to these additional amino acid differences.
Replication and antiviral sensitivity of viruses in mice
To evaluate the effect of the NA H274Y substitution on the replication of the 2009 pandemic viruses in vivo, we infected BALB/c mice with either 102 or 103 plaque-forming units (PFU) of O164s, VN9727s, O180r, or VN32060r and determined virus titers in the lungs of mice on days 3 and 6 post-infection (pi) (Fig. 1, see “Control” samples). We found that oseltamivir-resistant viruses replicated significantly better than oseltamivir-sensitive viruses at both day 3 and day 6 pi with one exception: on day 6 pi, the virus titer in the lungs of mice infected with 103 PFU of VN9727s was significantly higher than that of VN32060r-infected mice. These results indicate that the NA H274Y substitution does not negatively affect virus replication in the mouse lung in most settings.
Fig. 1. Antiviral sensitivity of viruses in mice. 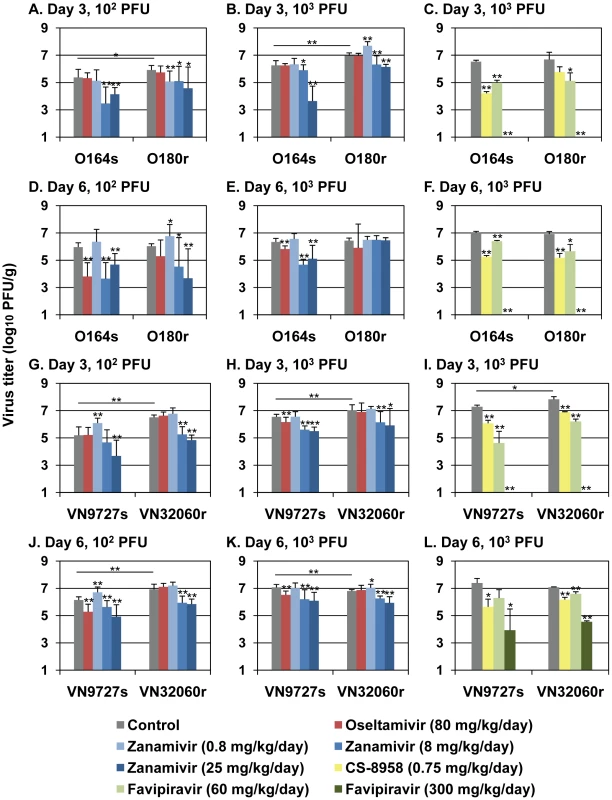
Mice were intranasally inoculated with 102 (A, D, G, and J) or 103 PFU of O164s, O180r (A–F), VN9727s, or VN32060r (G–L). At one hour post-infection (pi), mice were administered oseltamivir, zanamivir, CS-8958, favipiravir, or distilled water and PBS (control). Ten (A, B, D, E, G, H, J, and K) or three (C, F, I, and L) mice per group were euthanized on days 3 and 6 pi and virus titers in the lungs were determined by plaque assays in MDCK cells (detection limit: 1.0 log10 PFU/g). Error bars indicate the standard deviations of viral titers detected in the lungs of ten or three mice. Statistical significance was assessed by use of the Student's t-test: *, p<0.05, **, p<0.01. Next, we tested virus sensitivity to anti-influenza drugs in mice (Fig. 1). One hour pi, mice received oseltamivir, zanamivir, CS-8958, or an experimental viral RNA polymerase inhibitor favipiravir (also known as T-705). When mice were treated with oseltamivir (80 mg/kg/day dose), the replication of the NA H274Y-possessing viruses O180r and VN32060r was not inhibited, whereas the virus titers of their oseltamivir-sensitive counterparts O164s and VN9727s were significantly reduced. These results support the results of the in vitro NA inhibition assays and growth kinetics in MDCK cells indicates that the NA H274Y substitution confers oseltamivir resistance to viruses in mice. Although the efficacy of zanamivir against an oseltamivir-resistant virus (O180r) was not prominent when mice were infected with 103 PFU of viruses (Fig. 1), at a lower infectious dose (102 PFU) of viruses, its efficacy was apparent. CS-8958 and favipiravir significantly reduced the replication of all viruses tested, indicating that these 2009 H1N1 pandemic isolates, including oseltamivir-resistant strains, are as susceptible to these experimental antiviral drugs as one of the initial isolates A/California/04/2009 (H1N1) [2].
Pathogenicity and transmissibility of viruses in ferrets
To assess the transmissibility of oseltamivir-resistant 2009 H1N1 pandemic viruses, on day 1 pi we co-housed naïve ferrets in perforated cages next to ferrets inoculated with 106 PFU of O164s, VN9727s, O180r, or VN32060r and collected nasal swabs from inoculated and contact ferrets on days 1, 3, 5, 7, 9, and 11 after infection and co-housing, respectively, for virological analysis (Table 2). For all viruses tested, all contact ferrets were infected with viruses via respiratory droplets from infected ferrets, although the detection of O180r in contact ferrets was delayed by up to two days in all three pairs. Sequencing of the NA gene of the oseltamivir-resistant viruses (i.e., O180r and VN32060r) revealed that all of the viruses isolated from the contact ferrets retained the oseltamivir resistance-conferring tyrosine at position 274 in NA. Collectively, there were no substantial differences in transmissibility between oseltamivir-sensitive and -resistant viruses in ferrets.
Tab. 2. Virus titers in the nasal swabs of inoculated and contact ferretsa. 
For pairs of ferrets, one animal was intranasally inoculated with 106 PFU of virus (500 µl) (inoculated ferret, i) and one day later, a naïve ferret was placed in an adjacent cage (contact ferret, c). Nasal swabs were collected from all animals every other day for virus titration. To evaluate the pathogenicity of NA H274Y-possessing oseltamivir-resistant 2009 H1N1 pandemic viruses, we infected ferrets with 106 PFU of O164s, VN9727s, O180r, or VN32060r and monitored them daily for changes in body temperature and weight, and for clinical signs. On day 6 pi, nasal turbinates, trachea, and lungs were removed for pathological analysis. All viruses tested caused respiratory symptoms such as sneezing, although no marked changes in body temperature or weight were observed (data not shown). We found no remarkable pathological differences in the extent and manifestation of disease between ferrets infected with oseltamivir-sensitive or -resistant viruses (Fig. 2). All ferrets examined had severe inflammatory changes in their respiratory tracts: rhinitis, tracheitis and bronchopneumonia with bronchadenitis (Fig. 2A and B). In addition, we found limited bronchadenitis lesions with viral antigens in four ferrets, two of which were infected with oseltamivir-sensitive viruses (either O164s or VN9727s) while the other two were infected with oseltamivir-resistant VN32060r (Fig. 2C and D). These results indicate the comparable pathogenicity of the oseltamivir-resistant viruses to their oseltamivir-sensitive counterparts in ferrets. These results also suggest that the oseltamivir-resistant 2009 H1N1 pandemic viruses have the potential to be readily transmitted among humans without loss of pathogenicity.
Fig. 2. Pathological findings in the lungs of infected ferrets. 
Ferrets intranasally inoculated with 106 PFU of O164s, O180r, VN9727s, or VN32060r were euthanized on day 6 pi. The nasal turbinates, lungs, and trachea were subjected to pathological examination. (A) Bronchopneumonia in a ferret infected with VN9727s (H&E staining). (B) Bronchopneumonia in a ferret infected with VN32060r (H&E staining). (C) Viral antigen (brown pigment) in the lungs of a ferret infected with O164s (immunohistological staining). (D) Viral antigen (brown pigment) in the lungs of a ferret infected with O180r (immunohistological staining). Here, we investigated antiviral sensitivity, pathogenicity, and transmissibility of 2009 H1N1 pandemic influenza viruses possessing the substitution NA H274Y, which is known to reduce the sensitivity of influenza viruses to oseltamivir in cultured cells, mice, and ferrets. Viruses with this NA substitution exhibited reduced sensitivity to oseltamivir with comparable replication, pathogenicity, and transmissibility compared to their oseltamivir-sensitive counterparts: these findings need to be validated in humans. The widespread administration of oseltamivir, and to a lesser extent zanamivir, will clearly contribute to the emergence of NA inhibitor-resistant viruses that retain optimal replication fitness and transmissibility in humans. Given our finding that the oseltamivir-resistant 2009 H1N1 pandemic viruses are as pathogenic and transmissible as their drug-sensitive counterparts, specific criteria for the use of NA inhibitors may need to be reconsidered.
Oseltamivir has been used extensively in patients with 2009 pandemic influenza, especially those who are immunocompromised. Approximately one-third of the oseltamivir-resistant pandemic strains have been isolated from immunocompromised patients (http://www.who.int/csr/disease/swineflu/frequently_asked_questions/antivirals/resistance/en/index.html), a possibility we predicted early in this pandemic [16]. Oseltamivir-resistant viruses readily emerge in immunocompromised patients partly because viruses can replicate to higher titers and for longer periods in immunocompromised hosts compared to those who are immunocompetent hosts [17]. Since the oseltamivir-resistant 2009 pandemic viruses are as pathogenic and transmissible as their drug-sensitive parents, immunocompromised influenza patients require special care - increased drug doses, combination therapy, and possibly isolation. In particular, combination therapy has the potential to reduce the emergence of drug-resistant mutants [18] and provide synergistic effects on the inhibition of virus replication [19]–[21] with no increase in adverse events [22]. Adopting such measures would help prevent the situation experienced with the oseltamivir-resistant seasonal H1N1 virus, which emerged during the 2007-2008 influenza season in Europe [23], spread, and superseded previously circulating oseltamivir-sensitive viruses [24], [25].
Finally, our in vitro and in vivo experiments suggest that the experimental drugs CS-8958 and favipiravir may be promising candidates to combat 2009 H1N1 pandemic viruses.
Materials and Methods
Viruses and cells
We used two pairs of H1N1 pandemic virus clinical isolates; A/Osaka/164/2009 (O164s), A/Osaka/180/2009 (O180r), A/Vietnam/HCM9727/2009 (VN9727s), and A/Vietnam/HN32060/2009 (VN32060r). All of the viruses were isolated in Madin-Darby canine kidney (MDCK) cells, which were maintained in Eagle’s minimal essential medium (MEM) supplemented with 5% newborn calf serum (Sigma, St. Louis, MO) and cultured at 35°C in 5% CO2. Tyrosine at position 275 in the NA of O180r and VN32060r was confirmed by molecular cloning and sequencing of 18 individual clones each, while the rest of the viral genome sequences were determined by direct sequencing of amplified DNA.
Antiviral compounds
Oseltamivir (oseltamivir phosphate) and oseltamivir carboxylate (the active metabolite of oseltamivir; GS4104) were prepared from Tamiflu (Roche Laboratories Inc., Basel, Switzerland). Zanamivir, CS-8958 (an experimental NA inhibitor), and R-125489 (the active metabolite of CS-8958) were synthesized at Daiichi Sankyo Co. Ltd. according to published procedures [26]. Favipiravir (an experimental broad-spectrum viral RNA polymerase inhibitor, also known as T-705), was synthesized at Toyama Chemical Co., Ltd.
NA inhibition assay
NA activity of viruses in the presence of NA inhibitor was measured by an NA inhibition assay as described previously [8], [27], [28]. Briefly, diluted viruses were mixed with various concentrations of NA inhibitor in 2-[N-morpholino]ethanesulfonic acid containing CaCl2 and incubated for 30 min at 37°C. A fluorescent substrate methylumbelliferyl-N-acetylneuraminic acid (Sigma) was added to this mixture, which was then incubated for one hour at 37°C. After adding NaOH in 80% ethanol to the mixture to stop the reaction, the fluorescence of the solution was measured at an excitation wavelength of 360 nm and an emission wavelength of 465 nm and the 50% inhibitory concentration (IC50) was calculated.
In vitro growth kinetics
MDCK cells were infected with viruses at a multiplicity of infection of 0.0001. One hour later, oseltamivir carboxylate, zanamivir, R-125489 (10 µM each), or nothing (control) was added to the cells. At 8, 16, 24, 32, 40, and 48 h post-infection (pi), the supernatants of the infected cells were harvested and subjected to plaque assays in MDCK cells to determine virus titers.
Experimental infection of mice
Six-week-old female BALB/c mice (Japan SLC Inc., Shizuoka, Japan) were anesthetized with isoflurane and intranasally infected with 102 or 103 plaque-forming units (PFU) (50 µl) of viruses. One hour pi, six mice per group were administered the following antiviral compounds: (1) oseltamivir phosphate: 80 mg per kg per 400 µl (divided into two oral administrations per day) for 5 days; (2) zanamivir: 0.8, 8, or 25 mg per kg per 50 µl in one daily intranasal administration for 5 days; (3) CS-8958 : 0.75 mg per kg per 50 µl in one intranasal administration; (4) Favipiravir: 60 or 300 mg per kg per 400 µl (divided into two oral administrations per day) for 5 days; (5) or distilled water orally (200 µl) and PBS intranasally (50 µl). Three mice per group were euthanized on days 3 and 6 pi and virus titers in the lungs were determined by plaque assays in MDCK cells.
Experimental infection of ferrets
Three-to-four-month-old male ferrets (Marshall Farms, Wolcott, NY) and six-to-eight-month-old female ferrets (Triple F Farms, Sayre, PA) were used; animals from Triple F Farms were used for the transmission study of O164s and O180r, while those from Marshall Farms were used for the rest of studies. All of the animals, which were serologically negative for currently circulating human influenza viruses (including pandemic H1N1 viruses) by haemagglutination inhibition (HI) assay, were anesthetized with ketamine and xylazine (5 mg and 0.5 mg per kg of body weight, respectively) and intranasally infected with 106 PFU (500 µl) of viruses. On day 6 pi, three ferrets per group were euthanized and their nasal turbinates, trachea, and lungs subjected to virological and pathological analyses. For the transmission study, on day 1 pi, three naïve ferrets per group were co-housed in a perforated cage adjacent to an inoculated ferret; ferrets were separated by a perforated divider and did not have direct contact. All ferrets were monitored daily for changes in body temperature and weight, and clinical signs. Nasal swabs of inoculated and contact ferrets were collected on days 1, 3, 5, 7, 9, and 11 after infection and co-housing, respectively, and virus titers were determined by plaque assays in MDCK cells.
Pathological examination
Nasal turbinates, lungs, and trachea of euthanized ferrets were preserved in 10% phosphate-buffered formalin. Tissues were then processed for paraffin embedding and cut into 5 µm-thick sections. One section from each tissue sample was subjected to standard hematoxylin-and-eosin (H&E) staining, while another was processed for immunohistological staining with an anti-influenza virus rabbit antibody (R309; prepared in our laboratory) that reacts comparably with all viruses tested. Specific antigen-antibody reactions were visualized by 3,3'-diaminobenzidine tetrahydrochloride staining using a Dako EnVision system (Dako Co. Ltd., Tokyo, Japan).
Ethics
Our research protocol for the use of mice and ferrets followed the University of Tokyo's Regulations for Animal Care and Use, which was approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval number: 19–28). The committee acknowledged and accepted both the legal and ethical responsibility for the animals, as specified in the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, 2006.
Supporting Information
Zdroje
1. DawoodFS
JainS
FinelliL
ShawMW
LindstromS
2009 Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360 2605 2615
2. ItohY
ShinyaK
KisoM
WatanabeT
SakodaY
2009 In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460 1021 1025
3. MunsterVJ
de WitE
van den BrandJM
HerfstS
SchrauwenEJ
2009 Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325 481 483
4. MainesTR
JayaramanA
BelserJA
WadfordDA
PappasC
2009 Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325 484 487
5. JainS
KamimotoL
BramleyAM
SchmitzAM
BenoitSR
2009 Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 361 1935 1944
6. Centers for Disease Control and Prevention 2009 Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep 58 433 435
7. GubarevaLV
KaiserL
HaydenFG
2000 Influenza virus neuraminidase inhibitors. Lancet 355 827 835
8. KisoM
MitamuraK
Sakai-TagawaY
ShiraishiK
KawakamiC
2004 Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364 759 765
9. ZambonM
HaydenFG
2001 Position statement: global neuraminidase inhibitor susceptibility network. Antiviral Res 49 147 156
10. WardP
SmallI
SmithJ
SuterP
DutkowskiR
2005 Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother 55 Suppl 1 i5 i21
11. LeQM
KisoM
SomeyaK
SakaiYT
NguyenTH
2005 Avian flu: isolation of drug-resistant H5N1 virus. Nature 437 1108
12. Centers for Disease Control and Prevention 2009 Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients - Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep 58 893 896
13. LeungTW
TaiAL
ChengPK
KongMS
LimW
2009 Detection of an oseltamivir-resistant pandemic influenza A/H1N1 virus in Hong Kong. J Clin Virol 46 298 299
14. LeQM
WertheimHF
TranND
van DoornHR
NguyenTH
2010 A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 362 86 87
15. YamashitaM
TomozawaT
KakutaM
TokumitsuA
NasuH
2009 CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob Agents Chemother 53 186 192
16. NeumannG
NodaT
KawaokaY
2009 Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459 931 939
17. IsonMG
MishinVP
BracialeTJ
HaydenFG
GubarevaLV
2006 Comparative activities of oseltamivir and A-322278 in immunocompetent and immunocompromised murine models of influenza virus infection. J Infect Dis 193 765 772
18. IlyushinaNA
BovinNV
WebsterRG
GovorkovaEA
2006 Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res 70 121 131
19. NguyenJT
HoopesJD
LeMH
SmeeDF
PatickAK
Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One 5 e9332
20. IlyushinaNA
HayA
YilmazN
BoonAC
WebsterRG
2008 Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob Agents Chemother 52 3889 3897
21. GovorkovaEA
FangHB
TanM
WebsterRG
2004 Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob Agents Chemother 48 4855 4863
22. MorrisonD
RoyS
RaynerC
AmerA
HowardD
2007 A randomized, crossover study to evaluate the pharmacokinetics of amantadine and oseltamivir administered alone and in combination. PLoS ONE 2 e1305
23. HaugeSH
DudmanS
BorgenK
LackenbyA
HungnesO
2009 Oseltamivir-resistant influenza viruses A (H1N1), Norway, 2007-08. Emerg Infect Dis 15 155 162
24. NicollA
CiancioB
KramarzP
2008 Observed oseltamivir resistance in seasonal influenza viruses in Europe interpretation and potential implications. Euro Surveill 13
25. LackenbyA
ThompsonCI
DemocratisJ
2008 The potential impact of neuraminidase inhibitor resistant influenza. Curr Opin Infect Dis 21 626 638
26. HondaT
KuboS
MasudaT
AraiM
KobayashiY
2009 Synthesis and in vivo influenza virus-inhibitory effect of ester prodrug of 4-guanidino-7-O-methyl-Neu5Ac2en. Bioorg Med Chem Lett 19 2938 2940
27. GubarevaLV
WebsterRG
HaydenFG
2001 Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother 45 3403 3408
28. HatakeyamaS
SugayaN
ItoM
YamazakiM
IchikawaM
2007 Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. Jama 297 1435 1442
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



