-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCrystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
The HIV-1 envelope glycoprotein (Env) composed of the receptor binding domain gp120 and the fusion protein subunit gp41 catalyzes virus entry and is a major target for therapeutic intervention and for neutralizing antibodies. Env interactions with cellular receptors trigger refolding of gp41, which induces close apposition of viral and cellular membranes leading to membrane fusion. The energy released during refolding is used to overcome the kinetic barrier and drives the fusion reaction. Here, we report the crystal structure at 2 Å resolution of the complete extracellular domain of gp41 lacking the fusion peptide and the cystein-linked loop. Both the fusion peptide proximal region (FPPR) and the membrane proximal external region (MPER) form helical extensions from the gp41 six-helical bundle core structure. The lack of regular coiled-coil interactions within FPPR and MPER splay this end of the structure apart while positioning the fusion peptide towards the outside of the six-helical bundle and exposing conserved hydrophobic MPER residues. Unexpectedly, the section of the MPER, which is juxtaposed to the transmembrane region (TMR), bends in a 90°-angle sideward positioning three aromatic side chains per monomer for membrane insertion. We calculate that this structural motif might facilitate the generation of membrane curvature on the viral membrane. The presence of FPPR and MPER increases the melting temperature of gp41 significantly in comparison to the core structure of gp41. Thus, our data indicate that the ordered assembly of FPPR and MPER beyond the core contributes energy to the membrane fusion reaction. Furthermore, we provide the first structural evidence that part of MPER will be membrane inserted within trimeric gp41. We propose that this framework has important implications for membrane bending on the viral membrane, which is required for fusion and could provide a platform for epitope and lipid bilayer recognition for broadly neutralizing gp41 antibodies.
Published in the journal: . PLoS Pathog 6(5): e32767. doi:10.1371/journal.ppat.1000880
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000880Summary
The HIV-1 envelope glycoprotein (Env) composed of the receptor binding domain gp120 and the fusion protein subunit gp41 catalyzes virus entry and is a major target for therapeutic intervention and for neutralizing antibodies. Env interactions with cellular receptors trigger refolding of gp41, which induces close apposition of viral and cellular membranes leading to membrane fusion. The energy released during refolding is used to overcome the kinetic barrier and drives the fusion reaction. Here, we report the crystal structure at 2 Å resolution of the complete extracellular domain of gp41 lacking the fusion peptide and the cystein-linked loop. Both the fusion peptide proximal region (FPPR) and the membrane proximal external region (MPER) form helical extensions from the gp41 six-helical bundle core structure. The lack of regular coiled-coil interactions within FPPR and MPER splay this end of the structure apart while positioning the fusion peptide towards the outside of the six-helical bundle and exposing conserved hydrophobic MPER residues. Unexpectedly, the section of the MPER, which is juxtaposed to the transmembrane region (TMR), bends in a 90°-angle sideward positioning three aromatic side chains per monomer for membrane insertion. We calculate that this structural motif might facilitate the generation of membrane curvature on the viral membrane. The presence of FPPR and MPER increases the melting temperature of gp41 significantly in comparison to the core structure of gp41. Thus, our data indicate that the ordered assembly of FPPR and MPER beyond the core contributes energy to the membrane fusion reaction. Furthermore, we provide the first structural evidence that part of MPER will be membrane inserted within trimeric gp41. We propose that this framework has important implications for membrane bending on the viral membrane, which is required for fusion and could provide a platform for epitope and lipid bilayer recognition for broadly neutralizing gp41 antibodies.
Introduction
HIV-1 employs its trimeric env glycoprotein, composed of the receptor binding domain gp120 and the membrane anchored fusion protein subunit gp41 to enter host cells. Gp120 interacts sequentially with its cellular receptors CD4 and coreceptor CCR5 or CXCR4 [1], which induce a cascade of conformational changes in gp120 and gp41 [2], [3]. As a consequence the core of gp41 folds into a six helical bundle structure that leads to the apposition of viral and cellular membranes [4], [5].
Gp41 catalyses membrane fusion and current models suggest that receptor binding leads to the exposure of the gp41 fusion peptide (FP), which interacts with the target cell membrane producing an intermediate, pre-hairpin state bridging two membranes. This pre-hairpin has a relatively long half-life [6] and constitutes the target for inhibitory peptides [7], [8], [9] and neutralizing antibodies directed against HR1 [10][11] and MPER [12], [13]. Potentially at this stage, MPER was hypothesized to be membrane embedded based on the reactivity of broadly neutralizing MPER-specific antibodies [14], [15], [16], [17], [18]. The pre-hairpin then refolds into the six-helix bundle core structure [4], [5] and it is this transition that catalyzes membrane fusion [19]. Six-helix bundle core formation is achieved before fusion pore opening [20]. Experimental evidence [6], [19], [21] suggest that fusion proceeds via lipidic intermediate states, a membrane stalk, opening of the fusion pore and its expansion [22]. Mutagenesis analyses indicate that both linkers to the membrane anchors, FPPR and MPER, are implicated in fusion [23], [24] and the TMRs play an important role in fusion pore enlargement [22], [25], [26].
The energy released during gp41 refolding is used to overcome the kinetic barrier [3], [27], which is underlined by the high thermostability of gp41 core structures [28], [29] constituting a common feature of viral fusion proteins [30][31][32][26]. Although the free energy liberated during refolding of one trimer might be sufficient for fusion [26] consistent with experimental evidence [33], other studies imply that cooperativity of several trimers is required [34].
In order to understand the structural basis of MPER and FPPR in the context of gp41 trimers and their potential contribution to stabilize the gp41 post fusion conformation, we have assembled gp41 containing FPPR and MPER (gp41528–683). Thermostability measurements show that inclusion of FPPR and MPER increases the melting temperature (Tm) substantially compared to the gp41 core, suggesting that the gain of free energy can be directly coupled to membrane fusion. The crystal structure of gp41528–683 shows helical refolding of FPPR and part of MPER as well as the potential membrane insertion of MPER adjacent to the TMR. The structure thus indicates for the first time that part of MPER can insert into the viral membrane within trimeric gp41 and supports the hypothesis that a number of neutralizing gp41 antibodies recognize MPER in a membrane environment.
Results
Thermal denaturation of the extracellular domain of gp41
We assembled the extracellular domain of gp41 from two fragments containing residues 528 (lacking 16 N-terminal gp41 residues including FP) to 581 (FPPR-heptad repeat 1, HR1) and residues 629 to 683 (HR2-MPER) (gp41528–683) (Fig. 1A and Fig. S1). Both chains contain N-terminal Flag-tags to produce a soluble and monodisperse complex (Fig. S2). Circular dichroism analysis reveals a high helical content of ∼90% (Fig. S3A) and a melting temperature (Tm) of 87.6°C (Fig. 1B). In comparison, the core fragment of gp41 composed of HR1 and HR2 [5] (gp41541–665) containing N-terminal Flag-tags shows a Tm of 75.1°C (Fig. 1B). Thus FPPR and MPER interact and impart most likely increased trimer stability.
Fig. 1. FPPR and MPER increase the melting temperature of gp41. 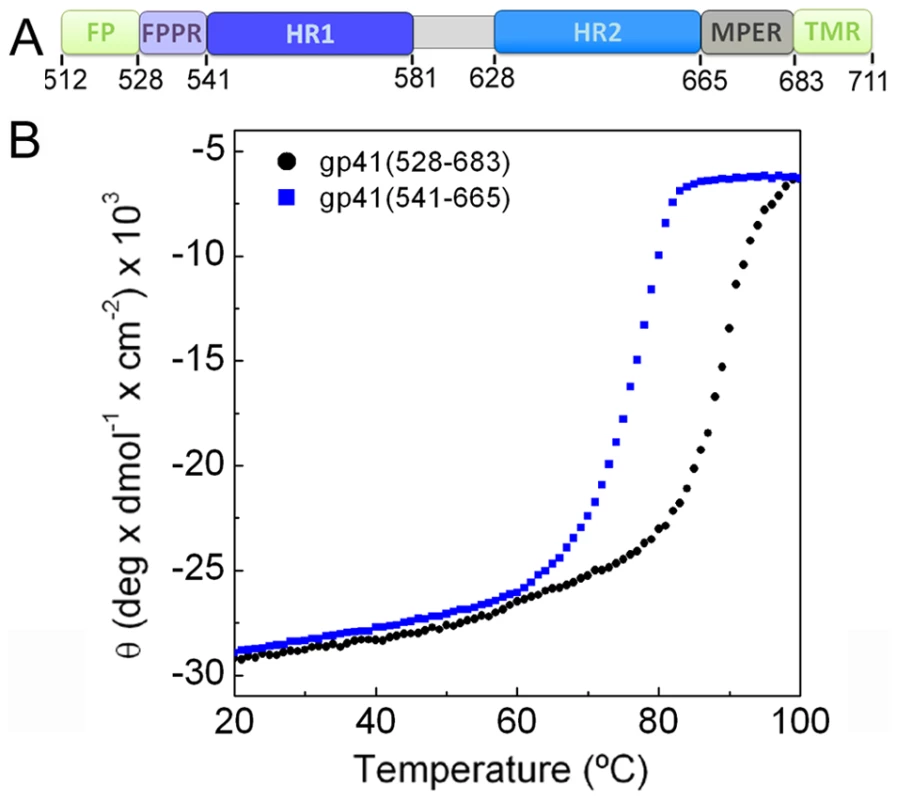
A) Schematic overview of gp41; FP, fusion peptide; FPPR, fusion peptide proximal region; HR1, heptad repeat 1; HR2, heptad repeat 2; MPER, membrane proximal external region; TMR, transmembrane region. B) Unfolding of gp41528–683 and gp41541–665 monitored by circular dichroism spectroscopy at 222 nm. Crystal structure of gp41 and MPER membrane insertion
Gp41528–683 was crystallized in space group P63. The structure was solved by molecular replacement and refined to a resolution of 2 Å (Table 1). The crystal structure composed of residues 531–581 and 629–681 plus 5 N-terminal Flag-tag residues reveals the six helical bundle core [4], [5] with FPPR and MPER extending in a helical conformation resulting in an 88 Å-long rod-like structure (Fig. 2A). A striking feature of the structure is a ∼90° turn of the MPER chain at Asn 677 which positions the remaining residues including Trp 678, Trp 680 and Tyr 681 perpendicular to the rod (Fig. 2B). Two disordered C-terminal residues must connect gp41 into the TMR in the membrane (Fig. S4). As a consequence, Trp 678, Trp 680 and Tyr 681 are exposed towards the membrane and well positioned to insert their side chains into the bilayer (Fig. S4). In order to calculate the membrane curvature generated by a shallow embedding of these MPER residues into the outer leaflet of a bilayer, we used a model for membrane bending by hydrophobic insertions [35]. This suggests that one gp41 chain produces local curvature of ∼0.65 nm−1; thus a gp41 trimer might stabilize a membrane cylinder of about 15 nm diameter, which would facilitate fusion considerably [36].
Fig. 2. Crystal structure of gp41528–683 reveals a 90 Å long rod-like structure. 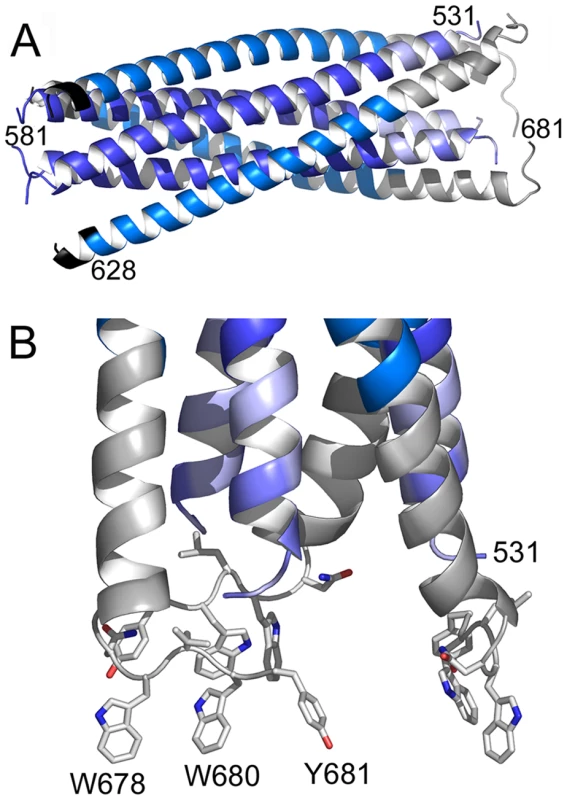
A) Ribbon representation of gp41. The previously determined core is colored dark blue (HR1) and marine blue (HR2). The flag sequence present at the N-terminus of HR2 is shown in black. FPPR is colored in light blue and MPER in grey. Note that the N-terminus of FPPR (residue 531) points towards the outside of the rod. B) Close up of the MPER and FPPR region shows the exposure of aromatic side chains Trp 678, Trp 680 and Tyr 681 towards the membrane. Tab. 1. Crystallographic statistics. 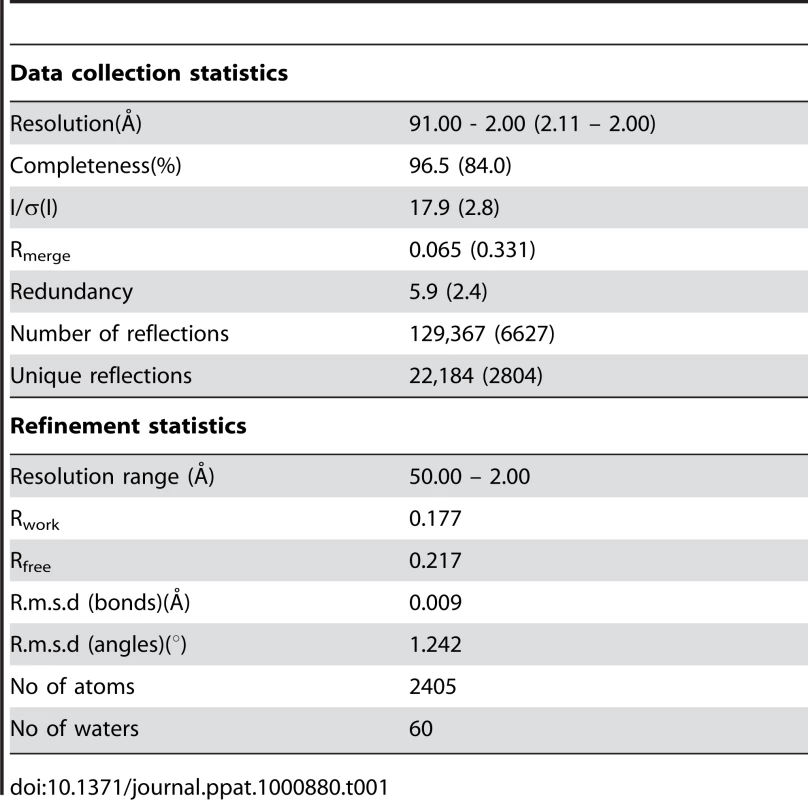
Structure of FPPR and MPER
Both FPPR and MPER extend HR1 and HR2 as continuous helices, but neither extension shows the regular knobs into holes packing reminiscent of classical coiled coils. Instead the FPPR region splays the inner core apart starting from Leu 545 (a position) (Fig. 3A). The distance between Arg 579 residues at the HR1 C-terminus is 12.5 Å while the one at the extreme N-terminus opens up to 22.7 Å (between Gly residues 531). As a consequence HR1 heptad positions are too far apart for interaction (Fig. 3A). The FPPR-MPER region is only stabilized by few hydrophobic contacts between adjacent chains, including interactions of Gly 531 - Leu679, Ala 533 - Trp 670, Met 535-Ile 675/Asn 671, Thr 536/Leu 537 - Trp 666 and one hydrogen bond between the carbonyl of Ala 533 and NE1 of Trp 670 (Fig. 3B). At position of MPER residue Asn 676, the N-terminus of FPPR-HR1 points towards the outside of the rod (Fig. 2A) facilitating fusion peptide (residues 512–530) membrane interaction or further refolding of FP with MPER and possibly TMR. Another striking feature of the structure is the solvent exposure of a stretch of hydrophobic MPER residues (Trp 666, Leu 669, Trp670, Trp 672, Phe 673) that generate a hydrophobic surface patch (Fig. S5).
Fig. 3. The FPPR-MPER regions are splayed apart. 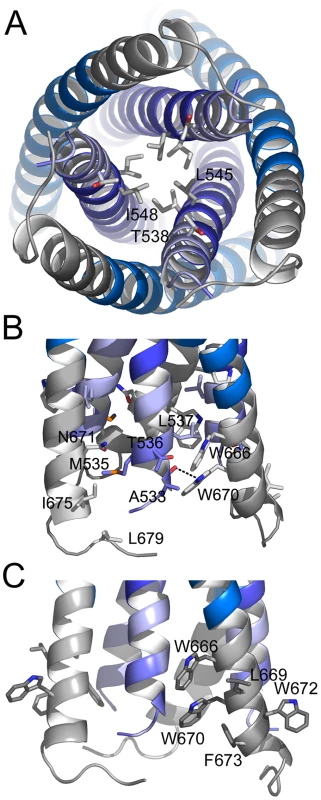
A) Close-up view from the bottom showing residue Leu 545 as the last coiled coil interacting residue of the HR1 core of gp41. The preceding potential heptad positions are Ala 541 and Thr 538. B) Close up view revealing mostly hydrophobic interactions between FPPR and MPER and only one hydrogen bond between the carbonyl of Ala and NE1 of Trp 670. C) Close-up of solvent exposed hydrophobic MPER residues. Since the crystals were grown at a high MPD concentration, we tested the effect of MPD on the structure in solution. MPD does not change the overall helical content of gp41528–683, which is ∼90% in the absence and presence of high MPD concentrations (Fig. S3A). However, MPD reduced the Tm of gp41528–683 to 82.2°C (5% MPD) and 74.7°C (10% MPD) as well as that of the gp41541–665 core (Fig. S3B). Therefore, we cannot exclude the possibility that MPD might have destabilized the rod resulting in the ‘open’ structure (Fig. 3A) and FPPR and MPER might pack tighter in the absence of MPD.
Comparison of MPER conformations
The NMR structures of MPER peptides show kinked or straight helical conformations [17], [37], which superimpose partly with MPER present in the crystal structure (Fig. S6A and B). Three broadly neutralizing antibodies (nAb) target MPER and utilize diverse structural motifs for recognition. NAb 2F5 recognizes a beta-hairpin [15] and Z13e1 binds to a short kinked helix [38]. Both epitopes refold into a straight helix in the gp41 structure (Fig. 4A and B). The epitope of nAb 4E10 is helical [16]; although it is present and exposed in the gp41 crystal structure (Fig. 4C) nAb 4E10 does not interact with gp41528–683 (data not shown), due to clashes with the helical conformation of HR2. However, if we consider only MPER and its membrane orientation and dock the 4E10 structure onto its epitope, nAb 4E10 could present its heavy chain CDR3 loop implicated in bilayer interaction [14], [18] towards the membrane, lined up with the gp41 membrane embedded residues W678, W680 and Y681 (Fig. S7). The comparison of the peptide epitope structures and gp41 corroborate that nAbs 2F5 and Z13e1 block the refolding process of gp41 at early steps. In contrast the 4E10 epitope might be present throughout gp41 refolding from a native conformation as evident by its presence in the late fusion intermediate conformation.
Fig. 4. Comparison of MPER conformations. 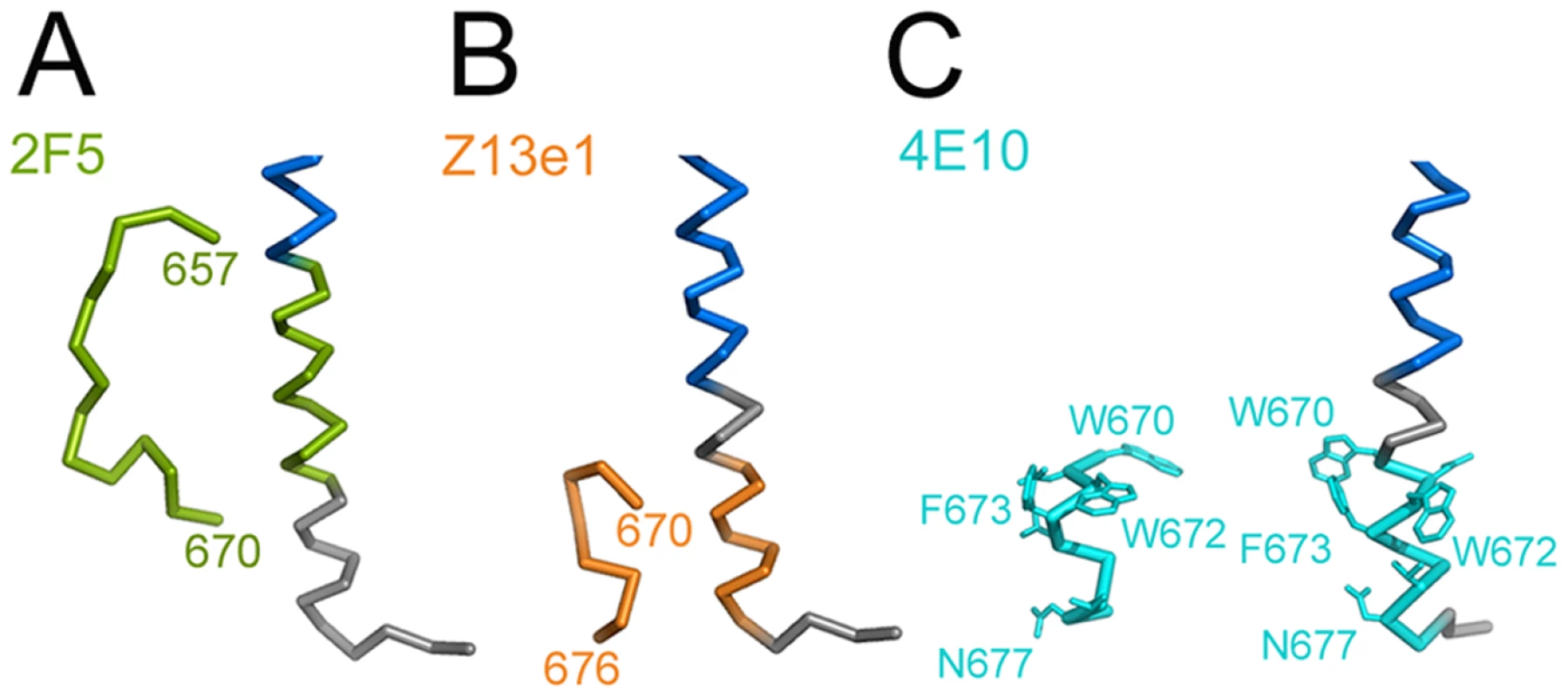
MPER conformations as determined in complex with broadly neutralizing antibodies (A) 2F5 [15], (B) Z13e1 [38] and (C) 4E10 [16] are shown in comparison to MPER within trimeric gp41. The corresponding MPER segments are colored equally and residues contacting the 4E10 Fab are shown as sticks. (blue, HR2). Discussion
Although the core structure of the HIV-1 fusion protein has been solved [4], [5], detailed structural information on the regions linking up to the membrane anchors (FPPR and MPER) has been lacking. We crystallized gp41(528–683), which has a similar N - terminal end as a proteolytic fragment of HIV-2 gp41 [39] and N - and C-terminal ends as determined by peptide studies [40] and solved its structure. FPPR and most of MPER extend in a helical fashion from the gp41 core and interact with each other as indicated by peptide studies performed at pH 3.2 [40]. Although the interactions are mostly hydrophobic, they are not classical coiled-coil interactions. The TMR-juxtaposed region of MPER positions three aromatic side chains per monomer towards the membrane. We calculate that membrane insertion of these residues could induce membrane curvature in the outer leaflet of the viral lipid bilayer [35], which would facilitate fusion based on previous studies [36]. Membrane fusion models postulate that fusion proteins induce local bending of both bilayers into “nipples” projecting toward each other to reduce the energy requirement for initial stalk formation [22], [41], [42]. Bending on the target-cell side can be stabilized by insertion of the fusion peptide [43] or hydrophobic residues of fusion loops [26]. The present structure suggests that bending on the viral side may be stabilized by membrane-embedded MPER residues. We suggest that MPER membrane insertion may occur early during the conformational transition of gp41 and persist through the process of refolding. Alternatively this segment of MPER may adopt a straight helical conformation [37] in continuity with TMR in the final postfusion conformation. Such a continuous helical structure was observed for the linker sequences that connect the core SNARE complex to its TMRs [44].
The presence of FPPR and MPER splay the “membrane-anchor” end of the rod apart, which may be required to accommodate FP whose chain direction points to the exterior of the structure. The missing part of FP (residues 512–530) could thus contact the membrane and/or interact with the kinked membrane embedded MPER or with a straight helical MPER conformation. Since the thermostability measurements indicate that the MPD crystallization conditions could influence the stability of gp41 in solution, it is possible that FPPR and MPER pack tighter in the absence of MPD. We thus propose that the structure represents a late fusion intermediate state rather than the final postfusion conformation, although the latter possibility cannot be excluded.
MPER contains a number of hydrophobic residues, which are conserved in the majority of HIV and SIV isolates, namely Trp666, Trp672, Phe673 and Ile675. Single Ala mutations of these residues do not affect cell-cell fusion but reduce viral infectivity significantly [24]. Interestingly all residues are mostly exposed in the crystal structure and/or contribute to hydrophobic interactions with FPPR. Mutation of FPPR Leu537, which makes a hydrophobic contact with Trp666, in combination with mutations of conserved MPER residues Trp666 or Trp672 or Phe673 or Ile675, reduces virus infectivity further thus confirming the important interplay between FPPR and MPER during fusion [24]. Analysis showed that the defect of mutant Leu537-Trp666 is at the level of lipid mixing [24]. Another study demonstrated that mutations of the five conserved tryptophan residues (Trp666, Trp670, Trp 672, Trp678, Trp680) alone or in combination or deletion mutants within MPER affect syncytium formation thus supporting the importance of MPER for fusion [45]. Reduction in viral infectivity was also reported for pseudoviruses containing alanine mutations of hydrophobic MPER residues (Leu 669, Ile675, Leu679) exposed within MPER in addition to the conserved tryptophan residues [46]. The hydrophobic surface generated by the conserved MPER residues as shown here might induce clustering of several gp41 trimers at the site of fusion although the number of env trimers required for fusion is still debated [33], [34]. Such a function may be consistent with mutagenesis data showing that single tryptophan exchanges within MPER affect cell-cell fusion, while combinations of tryptophan mutations abrogate cell-cell fusion completely [45]. Thus mutagenesis of multiple tryptophans may reduce the hydrophobicity of the exposed patch sufficiently to affect the clustering function.
Six-helix bundle formation leads to fusion pore opening [20] and an intact six-helix bundle is required for its enlargement [47]. Since FPPR and MPER folding most likely follows six-helix bundle formation its hydrophobic patch may further support pore enlargement together with the essential role of TMRs [22], [25], [26]. This suggestion is in agreement with data on mutagenesis of all 5 tryptophan residues within MPER; these mutations do not affect fusion pore opening, but inhibit fusion pore expansion [23]. Finally the linker region that connects the SNARE complex with its TMR exposes a similar patch of hydrophobic residues [44] underlining functional similarities between viral fusion protein and SNARE-mediated membrane fusion processes.
Fusion proteins utilize the free energy released during their refolding to draw two membranes into close apposition and catalyze membrane fusion [26]. The thermostability measurement of the gp41 core compared to the crystal structure reveals a 12°C increase of the melting temperature, which can translate into an increase in ΔG that can be directly coupled to membrane fusion. Notably, folding of the complete SNARE complex versus the core produces a similar increase in Tm that can convert into energy for fusion [44].
MPER harbors the epitopes of three broadly neutralizing antibodies, 2F5, Z13e1 and 4E10. The epitopes of 2F5 and Z13e1 [15], [38] adopt a straight helical conformation, indicating that both antibodies neutralize by blocking the transition into the trimeric gp41 structure. In contrast the epitope of 4E10 [16] is still present and exposed, although nAb 4E10 does not interact with gp41528–683 due to clashes with the helical conformation of HR2. NAb 4E10 has a long CDR3 region that does not contact the epitope, but was proposed to interact with the membrane [16] based on its reactivity with lipids [14]. If we consider only the 4E10 epitope and the membrane embedded part of MPER, 4E10 could orient its CDR3 towards the membrane and insert its aromatic residues into the bilayer as required for neutralization [18]. Thus stabilization of a peptide in the conformation of the MPER as present in the crystal structure should prove useful to generate an immunogen capable of inducing 4E10-like antibody responses.
Based on the crystal structure we suggest the following extension to our picture of the fusion process. Receptor binding induced conformational changes exposes FP, which interacts and bends the target cell membrane. Concomitantly, TMR and MPER dissociate, potentially from a native MPER coiled-coil structure [48] and a few aromatic MPER residues insert into and bend the outer leaflet of the viral membrane. This then generates the functional epitope for nAb 4E10. Part of MPER stays membrane associated throughout the folding of the gp41 core that leads to fusion pore opening. Subsequently FPPR and the soluble part of MPER interact, releasing more energy for fusion. Alternatively, we cannot exclude the possibilities that (i) membrane insertion of MPER is already present in the native env trimer or (ii) that membrane insertion of MPER is not important for the generation of membrane curvature and exerts another role during the fusion process. Finally, although the conformational state of gp41 observed in the crystal structure is no longer targeted by neutralizing antibodies, the development of small molecules targeting the FPPR-MPER conformation could block further gp41 refolding required for membrane fusion.
Materials and Methods
Protein constructs
The gp41 proteins were assembled from different fragments of gp41 (Fig. S1): FPPR-HR1-HR2-MPER (Ser528 to Leu581 and Met628 to Lys683; gp41528–683), HR1-HR2 (Ala541 to Leu581 and Met628 to Lys665; gp41541–665). DNA sequencing and MALDI TOF Mass Spectrometry confirmed all constructs.
Protein expression and purification
Fragments of HIV-1 gp41 HXB2 group M subtype B were amplified by standard PCR techniques and cloned either into pETM-MBP-1a (EMBL, Heidelberg), pETM-20 (thioredoxin fusion, EMBL, Heidelberg) or pET11 (His-tag). HR1 and HR2 containing constructs were N-terminally fused to the Flag-tag sequence (ASP-ASP-ASP-ASP-Lys) to improve solubility (Fig. S1).
Gp41528–683 and gp41541–665 fusion proteins were expressed in E. coli strain Rosetta 3 (DE3) (Strategene). Cells were grown to an OD600 nm of 0.7 and induced with 1 mM IPTG at 37°C. After 2 hours cells were harvested by centrifugation, resuspended in buffer A (0.02 M Tris pH 8.0, 0.1 M NaCl) and pellets of HR1 and HR2 expressing bacteria were mixed before lysis. Notably, bacteria expressing HR2 were used in excess over HR1 expressing bacteria. The soluble fraction was loaded onto an amylose column (NEB) and eluted in buffer A with 0.01 M maltose. In order to remove fusion proteins, constructs were digested o. n. at 4 C° with TEV (Tobbacco Etch Protease) and the uncleaved material was removed by Ni2+ chromatography. Further purification was achieved by anionic exchange chromatography in buffer A. A final purification step included size exclusion chromatography on a superdex 200 column in buffer A.
Crystallization, data collection and structure determination
Crystals of gp41528–683 were obtained by the vapor diffusion method in hanging drops mixing equal volumes of purified complex and reservoir solution (0.1 M citric acid pH 6, 60% MPD (v/v)). Crystals were improved by macroseeding; briefly crystals grown in the initial conditions (0.1 M citric acid pH 6, 60% MPD (v/v)) were transferred into a new drop equilibrated with 0.1 M citric acid pH 6, 56% MPD (v/v), 1.5% glycerol (v/v). Before data collection, crystals were flash frozen at 100 K using the same reservoir solution supplemented with 10% of glycerol (v/v).
A dataset was collected at the ESRF beam line ID14-EH4 at 100 K. The images were indexed with MOSFLM [49] and scaled with SCALA [50], [51]. The crystals were twinned and analysis with phenix.xtriage [52] revealed space group P63 with twin fractions of 0.45 (Britton) and 0.47 (H test and Maximum likelihood test) and an associated twin law of h, -h-k, -l. The cell parameters are a = b = 57.42 Å, c = 182.76 Å, α = β = 90°, γ = 120°. The structure was solved by molecular replacement using the program Phaser [53] and the model of the gp41 core (PDB ID: 1AIK) by applying the twin law of h, -h-k, -l on the data, revealing 3 molecules in the asymmetric unit. The model was built manually with COOT [54] and refined with the program Phenix [52]. The final structure has an Rfactor of 0.177 and Rfree of 0.217 and good stereochemistry (Table 1). The most complete monomer contains gp41 residues 531–581 and gp41 residues 629–681 plus 5 N-terminal residues (624-DDDDK-628 derived from the Flag/enterokinase cleavage site sequence); this monomer was used to reconstruct the trimer by applying crystallographic symmetry. The second monomer contains residues 538–581 and 629–672 plus 5 N-terminal residues (residues 624-DDDDK-628); the third monomer contains residues 542–580 and 629–665 plus 5 N-terminal residues (residues 623-MDDDDK-628). All molecular graphics figures were generated with Pymol (http://www.pymol.org). Coordinates and structure factors have been deposited in the protein data bank with accession number 2×7r.
CD spectroscopy
CD measurements were performed using a JASCO Spectropolarimeter equipped with a thermoelectric temperature controller. Spectra of each protein were recorded at 20°C in 1 nm steps from 190 to 260 nm in buffer A or buffer A supplemented with MPD as indicated. Spectra were recorded at 222 nm using a bandwidth of 4 nm and averaging time of 4 sec per step. For thermal denaturation experiments, the ellipticity was recorded at 222 nm with 1°C steps from 20° to 100°C with an increment of 80°C h−1 and an averaging time of 30 s/step. Since the unfolding of gp41528–683 was not reversible, two more spectra were recorded with increments of 40°C h−1 and 120°C h−1, which resulted in comparable Tms, indicating that the system was in equilibrium. For data analysis, spectra were corrected for the baseline (recorded with buffer) and the raw ellipticity values were converted to mean residue ellipticity. Thermal melting (Tm) points were calculated with a Boltzmann sigmoid fit using the program OriginLab.
Physical model
The effective shape of a membrane embedding domain consisting of the gp41 hydrophobic residues was approximated by a short cylindrical rod of 16 Å in length and 7 Å in diameter, shallowly inserted up to a 5 Å depth into the outer membrane monolayer (the insertion volume constituting 468.9 Å3). According to the previously developed model of membrane bending by hydrophobic insertions [35] the effective spontaneous curvature of such an insertion equals . The overall membrane curvature generated by the insertions is proportional to their area fraction in the membrane plane whose maximal value is limited by a dense packing of the proteins on the membrane surface. For a gp41 trimer the area of each of the three inserted side chains is 16 Å×7 Å = 112 Å2, while the total area of the trimer projection on the membrane plane is determined by the dimensions of the ectodomains and constitutes, approximately, 800 Å2. Taking into account these numbers, we obtain that a maximal area fraction of the gp41 hydrophobic insertions is which results in a total radius of curvature .
Supporting Information
Zdroje
1. MooreJP
TrkolaA
DragicT
1997 Co-receptors for HIV-1 entry. Curr Opin Immunol 9 551 562
2. GalloSA
FinneganCM
ViardM
RavivY
DimitrovA
RawatSS
PuriA
DurellS
BR
2003 The HIV Env-mediated fusion reaction. Biochimica Biophysica Acta 1614 36 50
3. HarrisonSC
2005 Mechanism of membrane fusion by viral envelope proteins. Adv Virus Res 64 231 259
4. WeissenhornW
DessenA
HarrisonSC
SkehelJJ
WileyDC
1997 Atomic structure of the ectodomain from HIV-1 gp41. Nature 387 426 430
5. ChanDC
FassD
BergerJM
KimPS
1997 Core structure of gp41 from the HIV envelope glycoprotein. Cell 89 263 273
6. Munoz-BarrosoI
DurellS
SakaguchiK
AppellaE
BlumenthalR
1998 Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol 140 315 323
7. WildCT
ShugarsDC
GreenwellTK
McDanalCB
MatthewsTJ
1994 Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A 91 9770 9774
8. FurutaRA
WildCT
WengY
WeissCD
1998 Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol 5 276 279
9. ChanDC
KimPS
1998 HIV entry and its inhibition. Cell 93 681 684
10. LuftigMA
MattuM
Di GiovineP
GeleziunasR
HrinR
BarbatoG
BianchiE
MillerMD
PessiA
CarfiA
2006 Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody. Nat Struct Mol Biol 13 740 747
11. CortiD
LangedijkJPM
HinzA
SeamanMS
VanzettaF
Fernandez-RodriguezBM
SilacciC
PinnaD
JarrossayD
Balla-JhagjhoorsinghS
WillemsB
ZekveldMJ
DrejaH
O'SullivanE
PadeC
OrkinC
JeffsSA
MontefioriDC
DavidD
WeissenhornW
McKnightA
HeeneyJL
SallustoF
SattentauQJ
WeissRA
LanzavecchiaA
2010 Analysis of Memory B Cell Responses and Isolation of Novel Monoclonal Antibodies with Neutralizing Breadth from HIV-1-Infected Individuals. PLoS ONE 5 e8805 doi:10.1371/journal.pone.0008805
12. DimitrovAS
JacobsA
FinneganCM
StieglerG
KatingerH
BlumenthalR
2007 Exposure of the membrane-proximal external region of HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion. Biochemistry 46 1398 1401
13. FreyG
PengH
Rits-VollochS
MorelliM
ChengY
ChenB
2008 A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci U S A 105 3739 3744
14. HaynesBF
FlemingJ
St ClairEW
KatingerH
StieglerG
KunertR
RobinsonJ
ScearceRM
PlonkK
StaatsHF
OrtelTL
LiaoHX
AlamSM
2005 Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308 1906 1908
15. OfekG
TangM
SamborA
KatingerH
MascolaJR
WyattR
KwongPD
2004 Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol 78 10724 10737
16. CardosoRM
ZwickMB
StanfieldRL
KunertR
BinleyJM
KatingerH
BurtonDR
WilsonIA
2005 Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22 163 173
17. SunZY
OhKJ
KimM
YuJ
BrusicV
SongL
QiaoZ
WangJH
WagnerG
ReinherzEL
2008 HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity 28 52 63
18. AlamSM
MorelliM
DennisonSM
LiaoH-X
ZhangR
XiaS-M
Rits-VollochS
SunL
HarrisonSC
HaynesBF
ChenB
2009 Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Nat Acad Sci USA 106(48) 20234 9
19. MelikyanGB
MarkosyanRM
HemmatiH
DelmedicoMK
LambertDM
CohenFS
2000 Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol 151 413 423
20. MarkosyanRM
CohenFS
MelikyanGB
2003 HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol Biol Cell 14 926 938
21. KligerY
GalloSA
PeisajovichSG
Munoz-BarrosoI
AvkinS
BlumenthalR
ShaiY
2001 Mode of action of an antiviral peptide from HIV-1. Inhibition at a post-lipid mixing stage. J Biol Chem 276 1391 1397
22. ChernomordikLV
KozlovMM
2008 Mechanics of membrane fusion. Nat Struct Mol Biol 15 675 683
23. Munoz-BarrosoI
SalzwedelK
HunterE
BlumenthalR
1999 Role of the membrane-proximal domain in the initial stages of human immunodeficiency virus type 1 envelope glycoprotein-mediated membrane fusion. J Virol 73 6089 6092
24. Bellamy-McIntyreAK
LayC-S
BaarS
MaerzAL
TalboGH
DrummerHE
PoumbouriosP
2007 Functional Links between the Fusion Peptide-proximal Polar Segment and Membrane-proximal Region of Human Immunodeficiency Virus gp41 in Distinct Phases of Membrane Fusion. J Biol Chem 282 23104 23116
25. FrolovVA
ChoM-S
BronkP
ReeseTS
ZimmerbergJ
2000 Multiple Local Contact Sites are Induced by GPI-Linked Influenza Hemagglutinin During Hemifusion and Flickering Pore Formation. Traffic 1 622 630
26. HarrisonSC
2008 Viral membrane fusion. Nat Struct Mol Biol 15 690 698
27. ChernomordikLV
KozlovMM
2003 Protein-lipid interplay in fusion and fission of biological membranes. Annual Rev Biochem 72 175 207
28. LuM
BlacklowSC
KimPS
1995 A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol 2 1075 1082
29. WeissenhornW
WhartonSA
CalderLJ
EarlPL
MossB
AliprandisE
SkehelJJ
WileyDC
1996 The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J 15 1507 1514
30. KielianM
ReyFA
2006 Virus membrane-fusion proteins: more than one way to make a hairpin. 4 67 76
31. LambRA
JardetzkyTS
2007 Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol 17(4) 427 436
32. RocheS
AlbertiniAA
LepaultJ
BressanelliS
GaudinY
2008 Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell Mol Life Sci 65 1716 1728
33. YangX
KurtevaS
RenX
LeeS
SodroskiJ
2006 Subunit stoichiometry of human immunodeficiency virus type 1 envelope glycoprotein trimers during virus entry into host cells. J Virol 80 4388 4395
34. MagnusC
RusertP
BonhoefferS
TrkolaA
RegoesRR
2009 Estimating the Stoichiometry of Human Immunodeficiency Virus Entry. J Virol 83 1523 1531
35. CampeloF
McMahonHT
KozlovMM
2008 The Hydrophobic Insertion Mechanism of Membrane Curvature Generation by Proteins. Biophys J 95 2325 2339
36. MartensS
KozlovMM
McMahonHT
2007 How Synaptotagmin Promotes Membrane Fusion. Science 316 1205 1208
37. SchibliDJ
MontelaroRC
VogelHJ
2001 The membrane-proximal tryptophan-rich region of the HIV glycoprotein, gp41, forms a well-defined helix in dodecylphosphocholine micelles. Biochemistry 40 9570 9578
38. PejchalR
GachJS
BrunelFM
CardosoRM
StanfieldRL
DawsonPE
BurtonDR
ZwickMB
WilsonIA
2009 A Conformational Switch in Human Immunodeficiency Virus gp41 Revealed by the Structures of Overlapping Epitopes Recognized by Neutralizing Antibodies. J Virol 83 8451 8462
39. LayCS
WilsonKA
KobeB
KempBE
DrummerHE
PoumbouriosP
2004 Expression and biochemical analysis of the entire HIV-2 gp41 ectodomain: determinants of stability map to N - and C-terminal sequences outside the 6-helix bundle core. FEBS Lett 567 183 188
40. NoahE
BironZ
NaiderF
ArshavaB
AnglisterJ
2008 The membrane proximal external region of the HIV-1 envelope glycoprotein gp41 contributes to the stabilization of the six-helix bundle formed with a matching N' peptide. Biochemistry 47 6782 6792
41. KozlovMM
ChernomordikLV
1998 A Mechanism of Protein-Mediated Fusion: Coupling between Refolding of the Influenza Hemagglutinin and Lipid Rearrangements. Biophys J 75 1384 1396
42. KuzminPI
ZimmerbergJ
ChizmadzhevYA
CohenFS
2001 A quantitative model for membrane fusion based on low-energy intermediates. Proc Nat Acad Sci USA 98 7235 7240
43. LiY
TammLK
2007 Structure and Plasticity of the Human Immunodeficiency Virus gp41 Fusion Domain in Lipid Micelles and Bilayers. Biophys J 93 876 885
44. SteinA
WeberG
WahlMC
JahnR
2009 Helical extension of the neuronal SNARE complex into the membrane. Nature 460 525 528
45. SalzwedelK
WestJT
HunterE
1999 A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J Virol 73 2469 2480
46. ZwickMB
JensenR
ChurchS
WangM
StieglerG
KunertR
KatingerH
BurtonDR
2005 Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J Virol 79 1252 1261
47. MarkosyanRM
LeungMY
CohenFS
2009 The six-helix bundle of human immunodeficiency virus Env controls pore formation and enlargement and is initiated at residues proximal to the hairpin turn. J Virol 83 10048 10057
48. LiuJ
DengY
DeyAK
MooreJP
LuM
2009 Structure of the HIV-1 gp41 Membrane-Proximal Ectodomain Region in a Putative Prefusion Conformation. Biochemistry 48 2915 2923
49. LeslieAGW
1992 Recent changes to the MOSFLM package for processing film and image plate data. Jnt CCP4/ESF-EACMB Newslett Protein Crystallogr 26
50. CCP4 1994 The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50 157 163
51. EvansP
2006 Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr 62 72 82
52. AdamsPD
Grosse-KunstleveRW
HungL-W
LoergerTR
McCoyAJ
MoriartyNW
ReadRJ
SacchettiniJC
K.SN
TerwilligerTC
2002 PHENIX: building new software for automated crystallographic structure determination. Acta Cryst D58 1948 1954
53. McCoyAJ
Grosse-KunstleveRW
AdamsPD
WinnMD
StoroniLC
ReadRJ
2007 Phaser crystallographic software. J Appl Crystallogr 40 658 674
54. EmsleyP
CowtanK
2004 Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126 2132
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing ActivityČlánek The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced MucositisČlánek Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The HMW1C Protein Is a Glycosyltransferase That Transfers Hexose Residues to Asparagine Sites in the HMW1 Adhesin
- Analysis of Virion Structural Components Reveals Vestiges of the Ancestral Ichnovirus Genome
- Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing Activity
- Global Migration Dynamics Underlie Evolution and Persistence of Human Influenza A (H3N2)
- The Type III Effectors NleE and NleB from Enteropathogenic and OspZ from Block Nuclear Translocation of NF-κB p65
- VEGF Promotes Malaria-Associated Acute Lung Injury in Mice
- Identification of a Mutant PfCRT-Mediated Chloroquine Tolerance Phenotype in
- The Early Stage of Bacterial Genome-Reductive Evolution in the Host
- Host-Detrimental Role of Esx-1-Mediated Inflammasome Activation in Mycobacterial Infection
- Elevation of Intact and Proteolytic Fragments of Acute Phase Proteins Constitutes the Earliest Systemic Antiviral Response in HIV-1 Infection
- The Pleiotropic CymR Regulator of Plays an Important Role in Virulence and Stress Response
- Alternative Sigma Factor σ Modulates Prophage Integration and Excision in
- Effect of Neuraminidase Inhibitor–Resistant Mutations on Pathogenicity of Clade 2.2 A/Turkey/15/06 (H5N1) Influenza Virus in Ferrets
- Massive APOBEC3 Editing of Hepatitis B Viral DNA in Cirrhosis
- NK Cells and γδ T Cells Mediate Resistance to Polyomavirus–Induced Tumors
- Is Genetically Diverse in Animals and Appears to Have Crossed the Host Barrier to Humans on (At Least) Two Occasions
- Adenylate Cyclase Toxin Mobilizes Its β Integrin Receptor into Lipid Rafts to Accomplish Translocation across Target Cell Membrane in Two Steps
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- HIV-1 Transmitting Couples Have Similar Viral Load Set-Points in Rakai, Uganda
- Few and Far Between: How HIV May Be Evading Antibody Avidity
- Galectin-9/TIM-3 Interaction Regulates Virus-Specific Primary and Memory CD8 T Cell Response
- Perforin Expression Directly by HIV-Specific CD8 T-Cells Is a Correlate of HIV Elite Control
- The Set3/Hos2 Histone Deacetylase Complex Attenuates cAMP/PKA Signaling to Regulate Morphogenesis and Virulence of
- Infidelity of SARS-CoV Nsp14-Exonuclease Mutant Virus Replication Is Revealed by Complete Genome Sequencing
- Combining ChIP-chip and Expression Profiling to Model the MoCRZ1 Mediated Circuit for Ca/Calcineurin Signaling in the Rice Blast Fungus
- Internalin B Activates Junctional Endocytosis to Accelerate Intestinal Invasion
- A Complex Small RNA Repertoire Is Generated by a Plant/Fungal-Like Machinery and Effected by a Metazoan-Like Argonaute in the Single-Cell Human Parasite
- Opc Invasin Binds to the Sulphated Tyrosines of Activated Vitronectin to Attach to and Invade Human Brain Endothelial Cells
- Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa
- PdeH, a High-Affinity cAMP Phosphodiesterase, Is a Key Regulator of Asexual and Pathogenic Differentiation in
- Isolates with Antimony-Resistant but Not -Sensitive Phenotype Inhibit Sodium Antimony Gluconate-Induced Dendritic Cell Activation
- The Microbiota and Allergies/Asthma
- Environmental Factors Determining the Epidemiology and Population Genetic Structure of the Group in the Field
- Prolonged Antigen Presentation Is Required for Optimal CD8+ T Cell Responses against Malaria Liver Stage Parasites
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
- Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
- Effective, Broad Spectrum Control of Virulent Bacterial Infections Using Cationic DNA Liposome Complexes Combined with Bacterial Antigens
- High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men
- The -Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections
- EBV Promotes Human CD8 NKT Cell Development
- Persistent Growth of a Human Plasma-Derived Hepatitis C Virus Genotype 1b Isolate in Cell Culture
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



