-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Complex Small RNA Repertoire Is Generated by a Plant/Fungal-Like Machinery and Effected by a Metazoan-Like Argonaute in the Single-Cell Human Parasite
In RNA silencing, small RNAs produced by the RNase-III Dicer guide Argonaute-like proteins as part of RNA-induced silencing complexes (RISC) to regulate gene expression transcriptionally or post-transcriptionally. Here, we have characterized the RNA silencing machinery and exhaustive small RNAome of Toxoplasma gondii, member of the Apicomplexa, a phylum of animal - and human-infecting parasites that cause extensive health and economic damages to human populations worldwide. Remarkably, the small RNA-generating machinery of Toxoplasma is phylogenetically and functionally related to that of plants and fungi, and accounts for an exceptionally diverse array of small RNAs. This array includes conspicuous populations of repeat-associated small interfering RNA (siRNA), which, as in plants, likely generate and maintain heterochromatin at DNA repeats and satellites. Toxoplasma small RNAs also include many microRNAs with clear metazoan-like features whose accumulation is sometimes extremely high and dynamic, an unexpected finding given that Toxoplasma is a unicellular protist. Both plant-like heterochromatic small RNAs and metazoan-like microRNAs bind to a single Argonaute protein, Tg-AGO. Toxoplasma miRNAs co-sediment with polyribosomes, and thus, are likely to act as translational regulators, consistent with the lack of catalytic residues in Tg-AGO. Mass spectrometric analyses of the Tg-AGO protein complex revealed a common set of virtually all known RISC components so far characterized in human and Drosophila, as well as novel proteins involved in RNA metabolism. In agreement with its loading with heterochromatic small RNAs, Tg-AGO also associates substoichiometrically with components of known chromatin-repressing complexes. Thus, a puzzling patchwork of silencing processor and effector proteins from plant, fungal and metazoan origin accounts for the production and action of an unsuspected variety of small RNAs in the single-cell parasite Toxoplasma and possibly in other apicomplexans. This study establishes Toxoplasma as a unique model system for studying the evolution and molecular mechanisms of RNA silencing among eukaryotes.
Published in the journal: . PLoS Pathog 6(5): e32767. doi:10.1371/journal.ppat.1000920
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000920Summary
In RNA silencing, small RNAs produced by the RNase-III Dicer guide Argonaute-like proteins as part of RNA-induced silencing complexes (RISC) to regulate gene expression transcriptionally or post-transcriptionally. Here, we have characterized the RNA silencing machinery and exhaustive small RNAome of Toxoplasma gondii, member of the Apicomplexa, a phylum of animal - and human-infecting parasites that cause extensive health and economic damages to human populations worldwide. Remarkably, the small RNA-generating machinery of Toxoplasma is phylogenetically and functionally related to that of plants and fungi, and accounts for an exceptionally diverse array of small RNAs. This array includes conspicuous populations of repeat-associated small interfering RNA (siRNA), which, as in plants, likely generate and maintain heterochromatin at DNA repeats and satellites. Toxoplasma small RNAs also include many microRNAs with clear metazoan-like features whose accumulation is sometimes extremely high and dynamic, an unexpected finding given that Toxoplasma is a unicellular protist. Both plant-like heterochromatic small RNAs and metazoan-like microRNAs bind to a single Argonaute protein, Tg-AGO. Toxoplasma miRNAs co-sediment with polyribosomes, and thus, are likely to act as translational regulators, consistent with the lack of catalytic residues in Tg-AGO. Mass spectrometric analyses of the Tg-AGO protein complex revealed a common set of virtually all known RISC components so far characterized in human and Drosophila, as well as novel proteins involved in RNA metabolism. In agreement with its loading with heterochromatic small RNAs, Tg-AGO also associates substoichiometrically with components of known chromatin-repressing complexes. Thus, a puzzling patchwork of silencing processor and effector proteins from plant, fungal and metazoan origin accounts for the production and action of an unsuspected variety of small RNAs in the single-cell parasite Toxoplasma and possibly in other apicomplexans. This study establishes Toxoplasma as a unique model system for studying the evolution and molecular mechanisms of RNA silencing among eukaryotes.
Introduction
Apicomplexa are unicellular eukaryotes that multiply intracellularly in their mammalian hosts. They include parasites of major medical importance like Plasmodium species, the causative agent of malaria, and Toxoplasma gondii, the most widespread apicomplexan parasite, present virtually everywhere on earth. Although usually causing only mild symptoms in the adult, Toxoplasma can cause severe and life-threatening diseases in developing fetuses and in immunocompromised individuals, especially AIDS and transplant patients [1], [2]. Toxoplasma has a complex life cycle that includes infections of more than one host organism, differentiation through several morphologically distinct forms, and both sexual and asexual replication [3]. Changes in gene expression is expected as (i) parasites progress through the cell cycle, (ii) parasites differentiate in specific stages, and (iii) parasites are exposed to the host immune system during infection [4]. How these changes are regulated at the molecular level remains to a large extent unknown. A puzzling feature is the apparent lack, in apicomplexan parasites, of large families of recognizable specific transcription factors (TFs) operating in other eukaryotes [5]. Despite the paucity of recognizable TFs, apicomplexans are endowed with a rich repertoire of enzymes associated with epigenetics and chromatin remodeling, and this observation has fueled the idea that epigenetics could play an important role in the control of gene expression [6], [7].
Small regulatory RNAs are linked to epigenetic regulation of gene expression in several organisms but these are presently understudied in the Apicomplexa. The defining features of small silencing RNAs are their short length (∼20–30 nucleotides) and their association with members of the Piwi/Argonaute (AGO) family of proteins, which they guide to their regulatory targets [8], [9]. Many, albeit not all, small RNAs (sRNA) are produced by the RNase III-related enzyme Dicer. Small interfering RNAs (siRNA) are generated as populations from multiple Dicer cleavages along long dsRNA precursors, whereas microRNAs (miRNA) are discrete species generated from a single Dicer cleavage event of noncoding primary precursor transcripts containing small, imperfect stem–loop structures [10]. These distinct small RNA pathways compete and collaborate as they regulate genes and protect genome integrity from invading nucleic acids including viruses and transposons. They function as guides for effector complexes (RNA-induced silencing complexes, RISCs) that regulate gene expression by degrading mRNA, repressing its translation, or modifying chromatin. RNA silencing is an evolutionary ancient regulatory mechanism, and small RNA pathways in unicellular organisms appear, so far, to be relatively simple. In fission yeast, a single class of endogenous siRNAs has demonstrated roles in epigenetic silencing at centromeres and the initiation of heterochromatin assembly at the mat locus [11]. In the ciliated protozoan Tetrahymena thermophila, small RNAs are involved in developmentally regulated DNA elimination [12], [13] and post-transcriptional gene regulation [14]. Particularly surprising is the recent finding that the unicellular green alga Chlamydomonas produces microRNAs that had been previously associated with developmental regulation and multi-cellularity [15], [16].
Here, we show that the T. gondii genome, unlike in Plasmodium species [17], encodes all core components of an elaborate RNA silencing machinery that has been evolutionary shaped as a patchwork of factors of plant and fungal origin. We establish a comprehensive sRNA landscape of T. gondii through deep sequencing, and unravel that the most abundant sRNA classes are formed by metazoan-like miRNAs as well as plant-like repeat-and-satellite-associated sRNAs coined rdsRNA and satRNA, respectively. Beyond the surprising complexity of the small RNAome, we provide a thorough biochemical characterization of the proteins that associate with the single T. gondii Argonaute protein, Tg-AGO. Unexpectedly these proteins constitute the near-entire cohort of previously identified human and fly miRNA-RISC components. Our data indicate that miRNA-loaded T. gondii argonaute associates with polysome probably to regulate translation of Tg-miRNA predicted targets, many of which include mRNA with perfect or near perfect complementarity. Tg-AGO also co-purifies with chromatin-repressing complexes, suggesting a role in transcriptional silencing, most likely through it demonstrated association with rdsRNAs or satRNAs.
Results/Discussion
The Toxoplasma RNA silencing machinery
Previous analyses have suggested a monophyletic origin for plant and animal Dicer proteins [18]. Sequence analyses show that the Toxoplasma genome (TOXODB release v6.0) [19] encodes only one Dicer-like protein, Tg-Dicer, which displays significant variability in primary sequence and domain organization compared to the Dicer consensus (Figure 1A): Tg-Dicer possesses an RNA helicase domain and two RNaseIII catalytic domains (RNaseIIIa and RNaseIIIb), but it lacks recognizable domains for dsRNA binding (DSRM) and PIWI-ARGONAUTE-ZWILLE (PAZ) functions. This organization is strikingly reminiscent of the DCL1 protein of the single cell algae C. reinhardtii (Figure 1A). Toxoplasma and Chlamydomonas Dicer-like sequences seem, indeed, orthologous, as they form a specific clade supported by a strong bootstrap score (Figure 1B), a consequence of a Drosha-like signature polypeptide that is more related (albeit weakly) to eubacterial RNaseIII enzymes and known to form an out-group with respect to higher plant and animal Dicers [18].
Fig. 1. Domain organization and phylogenetic analysis of Dicer and RDR proteins. 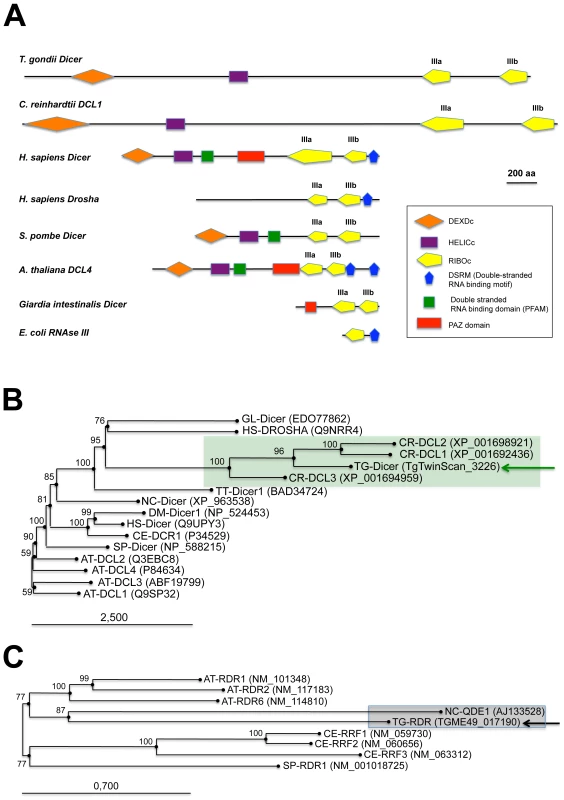
(A) Dicer proteins cleave dsRNA precursors into characteristic lengths through the action of two RNase III domains (in yellow). The domain arrangement of several Dicer enzymes is compared to Toxoplasma Tg-Dicer (TgTwinScan_3226, TOXODB v4.3). Evolutionary relationships between Dicer (B) and RDR (C) proteins from the following species: AT: Arabidopsis thaliana, GL: Giardia lambdia, SP: Schizosaccharomyces pombe, DM: Drosophila melanogaster, HS: Homo sapiens, CE: Caenorhabditis elegans, CR: Chlamydomonas reinhardtii, NC: Neurospora crassa, TT: Tetrahymena thermophila, TG: Toxoplasma gondii. Tg-Dicer and Tg-RDR are shown by a green and black arrows respectively and their branch family are shaded in green and gray. Numbers above the nodes indicate neighbor-joining bootstrap percentages. Ago-related proteins are divided into the Ago-like and Piwi-like subfamilies (Figure 2A); a third clade, termed ‘Group 3 Argonautes’, is worm-specific [20]. Toxoplasma Argonaute (Tg-AGO) is represented at a single genomic locus; there is no evidence for Toxoplasma-encoded Piwi proteins. Tg-AGO belongs to the Ago-like family but only with weak bootstrap support, suggesting that the protein diverges significantly from its metazoan and plant counterparts. Nonetheless, the two key signature domains of the AGO family —the PAZ domain and the C-terminal PIWI domain — are conserved in Tg-AGO (Figure 2B). Overall, the PIWI domain shows the highest degree of conservation, but the Asp-Asp-Glu/Asp catalytic triad required for slicer activity is not found, suggesting that the protein lacks endonucleolytic cleavage capacity (Figure 2B). The second signature motif, the PAZ domain, contains only a few residues that are strictly conserved, while the middle (MID) domain of Tg-AGO harbors the residues (Y596, K600, Q610 and K644; Figure 2C) required to bind the characteristic 5′ phosphate group of guide small RNA strands [21], [22]. We also noted the presence, in the amino terminal part of Tg-AGO, of a stretch of repeated RGG residues (amino acids 1–68), in which the arginines have the potential to undergo methylation (Figure 2B). This feature is found in metazoan and plant AGO-related proteins and was shown to alter their stability and/or sub-cellular distribution, ultimately impacting their function [23], [24], [25].
Fig. 2. Domain organization and phylogenetic analysis of Argonaute and PIWI proteins. 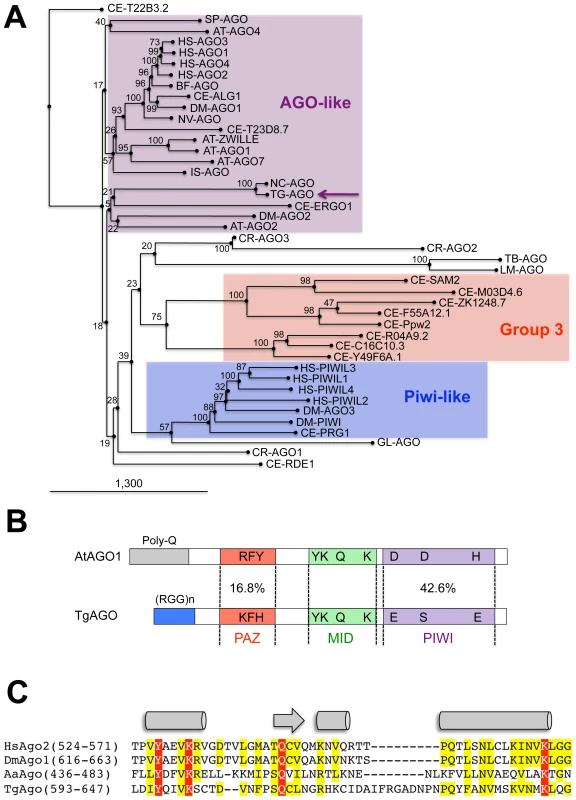
(A) Evolutionary relationships between Argonaute and Piwi proteins from the following species: AT: Arabidopsis thaliana, GL: Giardia lambdia, SP: Schizosaccharomyces pombe, DM: Drosophila melanogaster, HS: Homo sapiens, CE: Caenorhabditis elegans, CR: Chlamydomonas reinhardtii, NC: Neospora caninum, TT: Tetrahymena thermophila, TG: Toxoplasma gondii. Tg-AGO (accession number: GU046561) is pointed out by a pink arrow. Argonaute (in pink), Piwi (in blue) and group 3 (in red) branch family are represented as shaded boxes. Numbers above the nodes indicate neighbor-joining bootstrap percentages. (B) Schematic depiction of Toxoplasma Tg-AGO and Arabidopsis At-AGO1 (GeneID: 841262) domain according to archaeon Aquifex aeolicus X-ray crystal structure. The amino-terminal domain (grey or blue) is linked to the PAZ domain (red). The MID domain (green) connects the PAZ domain with the PIWI domain (pink) at the carboxy-terminal end of the protein. In the PAZ domain, residues important for binding of small RNA 3′ ends are indicated. In the MID domain, the residues required for 5′ end binding to small RNAs and binding to the 7-methylguanine (m7G) cap of target mRNAs are shown. The conserved residues for RNase H cleavage activity (DDH) in the PIWI domain are also shown. RGG indicates the domain of Tg-AGO containing arginine-glycine-glycine repeats. Poly Q, polyglutamine-containing domain in Arabidopsis Ago1. (C) Sequence alignment of conserved 5′–phosphate binding residues (MID domain) across various species of Argonaute proteins. Prefix Hs, Homo sapiens; Dm, Drosophila melanogaster; Aa, Aquifex aeolicus. Conserved residues are shaded in red. A phylogenetic tree constructed by aligning the stereotypical RNA-dependent RNA polymerase (RDR) domain supports the monophyletic origin of the proteins found in C. elegans, fungi and Arabidopsis [26]. Inspection of the Toxoplasma genome showed the presence of a single RDR-like gene, suggesting the existence of an amplified RNA silencing machinery in this organism. Tg-RDR is closely related to Neurospora crassa RDR, QDE1, and forms a specific clade with plant RDRs, which itself constitutes an out-group from the RDRs of metazoans and from the fission yeast S. pombe (Figure 1C). We conclude from this analysis that a patchwork of factors of plant and fungal origin form the core processor components of the Toxoplasma RNA silencing machinery. This finding can be rationalized partly by the fact that the apicomplexa ancestor is a presumed endosymbiont of red algae [27]. We note, however, the moderate or poor phylogenetic relationship observed between Tg-Dicer, Tg-AGO and the corresponding paralogous proteins of its mammalian hosts.
Complexity of the Toxoplasma small RNAome
Having established that the Toxoplasma genome encodes all core components of an elaborate RNA silencing machinery, we sought to determine the small RNA landscape of this organism. To this aim, we prepared total RNA from freshly released, filtered parasites. Ethidium bromide staining revealed a relatively abundant class of small (s)RNAs, ∼30 nucleotides (nt) in length (Figure S1A). The 20–40nt sRNA fraction was recovered by gel excision, cloned and subjected to deep sequencing using the Illumina technology. The sRNA library was constructed so as to represent only those sRNAs with a 5′ monophosphate and a 3′ hydroxyl group, the termini expected of miRNAs and siRNAs [28]. About 75% of a total of 5,701,506 reads, represented tRNA and rRNA turnover products, as previously reported for other organisms (Figure S1B) [29]. After filtering low quality reads, 3′, 5′ adapters, and reads shorter than 17 nucleotides, a remaining 1,555,290 reads (∼30% of total reads) matched the Toxoplasma genome (ToxoDB, version 4.3) (Figure S1B). Comparing mRNA and sRNA data for highly expressed genes suggested that the sRNA fraction contained only a very low level of degradation products from longer mRNAs (data not shown). Most sRNA sequences were found to be 25–27nt in length, with 25nt representing the dominant size class (Figure 3A). Of the 1,222,203 total reads, 94,170 corresponded to non-redundant sRNAs, and 92% of these were single reads, thus unraveling a highly complex sRNA population. Plotting Toxoplasma sRNAs (Tg-sRNAs) species with 100% match on the reference genome (in 10-kb sliding windows) showed that non-redundant Tg-sRNA with high read numbers (>1000) originate predominantly from non-coding intergenic regions or are embedded within introns of protein-coding transcriptional units (TUs) (Figure 3B). Other, medium-to-low abundance Tg-sRNA, by contrast, mapped to protein-coding TUs and a variety of DNA repeats and satellites (Figure 3B). These two classes of Tg-sRNA were detailed further, as described in the following sections.
Fig. 3. A repertoire of Toxoplasma endogenous small RNAs. 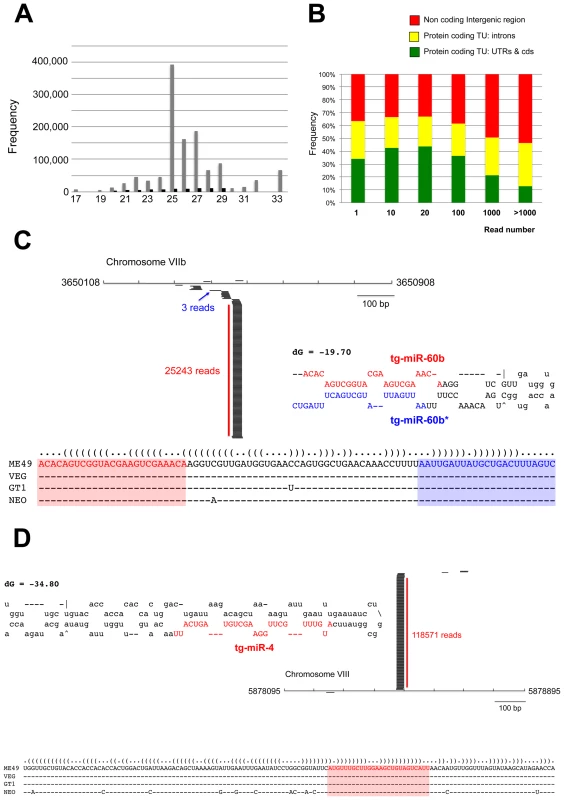
(A) Size distribution of Toxoplasma sRNAs. The sets of redundant (grey) and unique (black) small RNAs were used to generate a histogram quantifying the number of sequences obtained for each size class. nt., nucleotides. (B) Distribution of small RNAs across the Toxoplasma genome. TU: Transcriptional unit, CDS: CoDing Sequence, UTRs: UnTranslated Regions. (C) A miR-60b production hot spot in chromosome VIIb is shown. Small RNAs with perfect matches were plotted within a 800-bp sliding window. Short thin lines above the long bars represent small RNAs derived from the antisense strands, and lines below the bars represent small RNAs from the sense strands. Vertical bars represent the consensus positions of sequencing reads that mapped to the predicted precursors, and the values indicate the total number of these reads. The fold-back structure of the precursor predicted with mfold is provided below. The mature region is shown in red and the passenger strand (microRNA*) in blue. The miR-60b–containing locus for the three canonical strains of Toxoplasma and Neospora caninum is depicted by sequence and structure. (D) A miR-4 production hot spot in chromosome VIII is shown along with the predicted structure and the sequence conservation across parasite species. Toxoplasma miRNAs
In a search for putative Toxoplasma miRNA candidates, we evaluated sRNA reads exhibiting the following features: (1) high abundance of sequence reads sharing the same 5′ terminus; (2) exact match to one or several genomic loci displaying a characteristic fold-back structure typical of MIRNA precursors; and (3), when applicable, low abundant sequence reads corresponding to the labile (miRNA*) passenger strand of miRNA/miRNA* duplexes, as predicted within the fold back structures. Fourteen sRNA families cloned at a high frequency met these MIRNA features and were thus annotated with high confidence as T. gondii miRNAs (Tg-miRNAs) (Figures 3, S2-S13 and Table S1). Genome browser views of some of these Tg-MIRNA indeed indicated the existence of a low frequency, single miRNA* (passenger strand) corresponding to the opposite strand of the duplex within the fold back-structure (e.g. Tg-miR-60b; Figure 3C). Moreover, the reconstituted duplexes sometimes had small 3′ overhangs characteristic of Dicer processing (Figures 3C, S8-S10). Of the 14 annotated Tg-miRNA families, 7 gave detectable hybridization signals in Northern analysis carried out with 5′ end-labeled antisense oligonucleotides (Figures 4A, 4C and S14). No signal was detected with RNA extracted from host cells, confirming that these sRNAs are Toxoplasma-specific. Hybridization often unraveled discrete sRNA species that were heterogeneous in size, reminiscent of the sRNA signals observed in S. pombe [30] and other organisms in which Dicer lacks a PAZ domain, required for the precise sizing of processed sRNAs [31], [32], [33]. In all cases, hybridization with antisense probes from precursor sequences flanking the mature miRNA gave no signal (data not shown), confirming the excision, by Tg-Dicer, of a single sRNA species, a landmark of plant and metazoan miRNA biogenesis. The members of the remaining 7 miRNA families were below detection levels, in agreement with their much lower cloning/sequencing frequencies (Figures S8-S13). In all cases -and as expected from their poor read counts due to their intrinsic instability - cloned miRNA* were also below detection levels of Northern analysis.
Fig. 4. Expression patterns and characteristics of representative microRNAs in Toxoplasma. 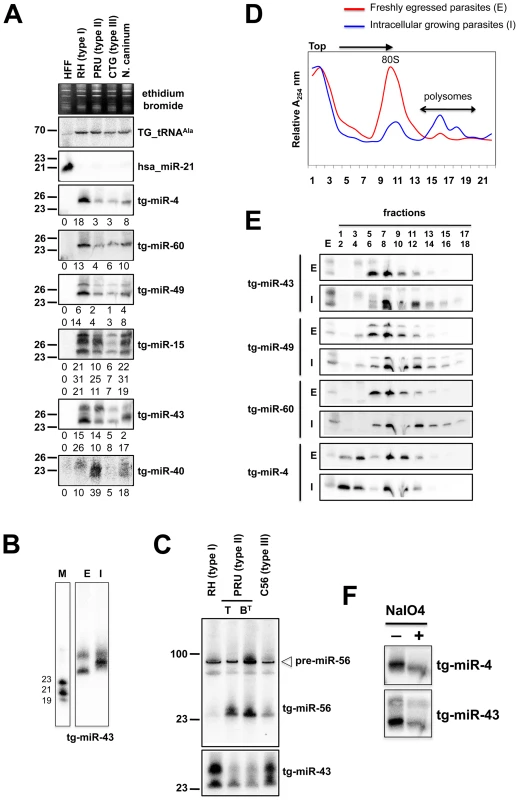
(A) Expression profile analyses of six parasite microRNA candidates by small-RNA Northern hybridization in three canonical strains of Toxoplasma and its close relative, Neospora caninum. To control for loading, blots were stripped and rehybridized with the indicated miRNA and the control probes (tRNAAla and hsa_miR-21). Ethidium bromide staining of rRNA was used as loading control. Hybridization signals were quantified with the ImageJ software, normalized to the amount of Toxoplasma tRNAAla signal present in each sample, and shown as digit below each Northern blot. For miR-15, -43 and -49, each individual signal was separately quantified. The number next to each panel represents the position of RNA markers. Tg, Toxoplasma gondii; hsa, Homo sapiens. (B) Total RNA from freshly egressed (E) and intracellular growing parasites (I) were analyzed by Northern hybridization with complementary probes for Tg-miR-43. RNA markers (left lane) are 19, 21 and 23 nucleotides. (C) Total RNA from types I, II (tachyzoite and pH-converted bradyzoite) and III were analyzed by Northern hybridization with complementary probes for Tg-miR-43 and -miR-56. Pre-miR indicates the foldback RNA precursor form; Tg-miR refers to the mature, predominant form. The number next to each panel represents the position of RNA markers. (D) Total RNA from freshly egressed (E, in red) and intracellular growing parasites (I, in blue) were subjected to sedimentation on 5%–45% sucrose gradients in the presence of cycloheximide (to preserve the association of translating ribosomes with mRNAs). Measurements of the total RNA in the gradient fractions reveal signature patterns of peaks corresponding to messenger-ribonucleoprotein complexes (mRNP), followed by the 80S ribosomal fractions and the polyribosomal fraction. As can be appreciated from the A254 profiles, the rapid proliferation of tachyzoites inside of the host cell (in blue) is diagnosed by the high level of translating polyribosomes. (E) A proportion of Tg-miRNAs cosediment with polyribosomes. Total RNA was extracted from each fraction and analyzed by Northern blots with complementary probes for 6 Tg-miRNAs [miR-43, -49, and 60 (shown) and mir-4, -15, and 40, (not shown)]. All of the Tg-miRNAs tested are predominantly localized to the mRNP with a clear shift to polyribosome fractions in intracellular growing parasites. (F) The 3′ end of Tg-miR-4 and -43 are not modified. Gel-fractionated small RNAs were examined by b-elimination assay (see methods). Sequencing and Northern analyses showed that the miR-60 family largely dominates the Toxoplasma miRNA landscape, accounting for 335,014 reads, of which 61% (280,723 reads) were contributed by miR-60a alone (Figure S2B). MIR-60, together with MIR-4, also constitute the two most diversified Tg-MIRNA gene families (with 8 distinct members in each) among the 14 families identified with high confidence (Figure S2B and S3B). In most cases of Tg-miRNAs with multiple precursors (6 families, Table S1), the mature miRNAs were not located on the same fold-back arms, which, furthermore, were also found to vary in sequence, suggesting that these genes do not share a common ancestor and, thus, have evolved separately. The 14 high-confidence Tg-miRNAs showed no significant homology to any of the known miRNAs of plants and metazoans, as assessed in the central miRBase depositary (release 14). Nearly all Tg-miRNAs and Tg-miRNA* (when available) had, however, directly identifiable orthologs in the genome of the apicomplexan Neospora caninum (dog parasite), when up to 3-nucleotide polymorphism was tolerated (Figures 3, S2-S8 and S13). Moreover, the size and abundance of these orthologous N. caninum (Nc)-miRNAs was confirmed by Northern blot analysis using the same antisense oligonucleotide probes employed for detection of Tg-miRNAs (Figures 4A and S14). The notable exception to sequence conservation in N. caninum was observed with Tg-miR-62, -64, -65 or -66, although this could be attributed to the incomplete N. caninum genome annotation (Figures S9-S12). Taking into account recent observations made in the single cell algae Chlamydomonas, apicomplexans thus provide the second reported example of unicellular organisms that produce miRNAs. Unlike in Chlamydomonas, nonetheless, and despite the relatedness of the Dicer proteins found in both organisms (Figure 1A and 1B), Tg-MIRNA fold-backs have length and thermodynamic features that are much closer to those of mammalian hosts than those of Chlamydomonas or higher plants [10]. Consistent with this idea, most Tg-miRNAs display a clear 5′ - nucleotide bias towards A, as also observed for most mammalian miRNAs [34]. Unlike many mammalian MIRNAs, however, Tg-MIRNAs were not found to form genomic clusters. These findings further emphasize the surprising mosaic nature of the Toxoplasma RNA silencing machinery and small RNA loci.
The above 14 miRNA families were identified through deep-sequencing of small RNAs isolated from freshly egressed parasites, and so other miRNAs might exist that were simply too low in abundance to be cloned under these specific growth conditions. In addition, several Tg-sRNAs cloned at moderate to low frequency mapped to imperfect fold-backs scattered along the genome, with relatively low free energy (Figure S15). These hairpins are much more heterogeneous in size and structure than cognate Tg-MIRNA precursors, yet their processing produces discrete sRNA species. Although their relatively modest cloning frequencies precludes their detection by Northern analysis, including in N. caninum, the corresponding sRNA might represent recently-evolved miRNAs that may engage into miRNA-like regulatory activities. In plants, a model for MIRNA gene evolution, termed “spontaneous evolution” stems from the high density of small-to-medium sized fold-back sequences scattered throughout the Arabidopsis genome. It has been proposed that following the capture of transcriptional regulatory sequences, some of these random fold-backs could occasionally give rise to new MIR genes. Stabilization through co-evolution with targets initially found by chance could then lead to the fixation of these genes in the genome [35].
Biochemical properties of Toxoplasma miRNA
Unlike metazoan miRNAs, plant miRNAs are methylated at the 2′ hydroxy positions of their 3′-last nucleotides [36]. This modification, mediated by the methyl-transferase HEN1, protects miRNA from 3′ end uridylation and subsequent degradation [37]. However, Tg-miRNA species were found sensitive to b-elimination by periodate, which causes a diagnostic shift in sRNA mobility (Figure 4F). Thus, unlike their plant counterparts, but similar to metazoan miRNAs, Tg-miRNAs do not carry 3′-end modifications, a result also consistent with our failure to identify a HEN1 homolog in the Toxoplasma genome (TOXODB, release v5.2). Nonetheless, the 3′ end of several cloned Tg-miRNAs was often found to contain untemplated adenine residues, which must be added, therefore, after processing by an as yet unidentified terminal adenyl-transferase (Figure S3B). It was shown recently that addition of adenylic acid residues on the 3′-end apparently slows down miRNA turnover in Populus trichocarpa [38].
In plants and animals, miRNA are recruited by AGO proteins to enhance the turnover, or inhibit the translation of cognate mRNA targets. Consequently fractions of most plant and metazoan miRNAs associate with polysomes, the sites of active translation. The existence of miRNAs in Toxoplasma together with the absence of detectable slicer residues in the Tg-AGO predicted that Tg-miRNA would also associate, at least partly, with polysomes. To test this idea, protein extracts from freshly egressed parasites (E) ready to invade, or from fast-growing intracellular parasites (I), were fractionated and resolved on sucrose density gradients (see Methods). For the former (I), the absorbance profiles at 254 nM reflected the ribosome pattern expected from rapidly growing cells: there were few monosomes (80S) and the bulk of the ribosomes sedimented in the polysomal fractions (Figure 4D). By contrast, the amount of polyribosomes in invading parasites (E) was substantially reduced, and this was accompanied by a concomitant increase in 80S monosomes (Figure 4D). As previously observed in plants and metazoans, Tg-miRNAs distribution was found to span a wide range of molecular weights across the gradient (Figure 4E) [39], [40], [41]. Nonetheless, a fraction of several Tg-miRNAs co-sedimented with polysomes (Figure 4E, fractions 13–18). Moreover, the association with translating ribosomes was more pronounced in exponentially growing parasites (I), as expected. Not all Tg-miRNA, however, were found associated to polysomes, and this was notably the case of Tg-miR-4 (Figure 4E). Tg-miR-4 and other non-polysomal miRNA may regulate target mRNAs at later stages of parasite differentiation (e. g. bradyzoite); alternatively, they might not be involved in translation control (data not shown), as has been recently shown for a class of Arabidopsis miRNA [41] that use cleavage-competent and/or cleavage-resistant target sites found in specific non-coding RNAs to initiate the production of trans-acting (tasi)RNAs via the action of RDR6 [42]. Whether tasiRNA exist in Toxoplasma is an interesting question for future experiments. In any case, our findings demonstrate that several Tg-miRNAs are present in the form of miRNPs in polyribosome-containing fractions where they are likely to negatively regulate translation of target transcripts.
Complex regulation of Toxoplasma MIRNA gene expression
miRNAs orchestrate many biological functions and are notably involved in cell fate determination and/or integration of developmental or external stimuli. Plant and metazoan miRNA expression may thus vary greatly depending on growth conditions, changes in developmental stages or, in the case of parasites, changes in virulence. To investigate if, similarly, Tg-miRNA accumulation/processing is regulated differentially in Toxoplasma, we sampled miRNA from freshly egressed (E) or intracellular (I) parasites, as well as from three classical Toxoplasma isotypes. These isotypes are representative of the European and North American parasite population and correspond to three clonal lineages, designated type I, II and III, corresponding to reference strains RH, PRU and CTG, respectively [43]. These genotypes display contrasted virulence in mice: type I strains are lethal, whereas type II and III strains are hypo-virulent and typically establish chronic infections. There are additional phenotypic differences in migration, growth rate, and ability to convert from tachyzoite to the cyst-forming bradyzoite stage, notably [43].
For several of the Tg-miR detected by Northern analysis, we observed differential abundances between the Toxoplasma strains (Figure 4A and 4C). Thus, normalized to the Tg-tRNAAla signal, the miR-4 signal was six fold greater in type I than it was in types II and III (Figure 4A). Likewise Tg-miR-4, -49 and -60 were more abundant in type I strain, whereas Tg-miR-40 and -56 were clearly more abundant in type II. Further investigation of these variations in miR-56 levels showed that they were attributable to differences in miRNA processing rather than transcription, because similar levels of pre-miR-56 were observed among the three isotypes, in Northern analyses (Figure 4C). This result reinforces the growing view that MIRNA genes can undergo extensive post-transcriptional regulation through mechanisms that selectively affect pri-miRNA processing and/or pre-miRNA stabilization [44], as uncovered recently with interactions involving murine pre-Let-7, lin-28 [45], [46] and the RNA-binding protein KSRP [47], a homolog of which was indeed found associated with Tg-AGO (see following sections). These observations thus extend this concept to a single cell parasite; given the overall low genetic diversity among Toxoplasma isotypes [43], they further suggest that differential regulations of pathogen's miRNA repertoires might, indeed, influence virulence. Analyses of Tg-miR accumulation between freshly egressed (E) and intracellular (I) Toxoplasma revealed additional scope for modulation of mature miRNA levels between the two parasitic states. For instance, there was a clear mobility shift with miR-43, which is unlikely explained by changes in pre-miR-43 steady levels, but rather, by alternative Dicer-mediated processing events producing small RNA length variants or with modified termini (Figure 4B). Collectively, these observations unravel highly complex regulations of Tg-MIRNA gene expression, which might be used to refine the amplitude or regulatory outputs of target gene regulation during the parasite's multiple biological states.
Toxoplasma miRNA exhibit perfect or near-perfect matches to many target transcripts
We then attempted to identify putative targets for representative members of the 14 unambiguous Tg-MIRNA families retrieved in this study. A bioinformatics approach was used to scan Toxoplasma transcripts for Tg-miRNA complementarity sites. Despite the resemblance of Tg-MIRNA and mammalian MIRNA genes, and the association of both types of molecules to polysomes, the absence of slicer residues in the Tg-AGO protein strongly suggests that the parasites' miRNA engage into a distinct type of pairing to their targets (Figure 2B). Hence, in the mammalian host, most AGO2-bound miRNAs exhibit only moderate pairing to their targets, notably through a stretch of 6–7 contiguous 5′ nucleotides known as ‘seed’, which is usually followed by several central mismatches that sterically hinder the RNAseH activity of AGO2 [48], [49]. This loose miRNA:target pairing, which is thought to favor translational repression over slicing, makes it difficult to predict mammalian miRNA targets using computer algorithms. These algorithms, moreover, are often biased towards 3′ UTRs, because these regions evolve much more rapidly than coding regions, and are, therefore, more prone to the identification of contiguous, 6–7nt seed-complementary sequences [50].
We found that most of the 14 Tg-miRNA analyzed have readily identifiable target sites in a variety of cellular transcripts (Table S2 and data not shown). Interestingly, these sites exhibit complete to near-complete complementarity to miRNAs -a feature of plant but not of metazoan miRNAs - and they are found in 5′-UTR, coding region and 3′-UTR, although there is a clear bias towards the latter region for most miRNA analyzed (Figure S16A and Table S2). Allowing up to 3 mismatches, more than 80 putative target transcripts were identified for miR-60a alone, the most abundantly sequenced Tg-miRNA. Using the same stringent parameters, an average of 25 cellular targets could be retrieved for each of the 14 Tg-miRNA (Figure S16A and Table S2). GO-term analysis of the putative Tg-miRNA target transcripts showed that they encompass virtually all known biological functions, with a somewhat stronger emphasis on translational control and cell cycle regulation, which might be expected for a single-celled, highly dividing parasite (Figure S16B). These predicted Toxoplasma miRNA:target interactions thus constitute an unprecedented situation in all eukaryotes studied so far, whereby a miRNA-loaded, slicer-deficient Ago (see later in the text) might regulate target gene expression, presumably at the translational level, through perfect or near-perfect binding sites that are predominantly –albeit not exclusively - located in 3′-UTRs. To test the possibility of target cleavage and degradation mediated by Tg-AGO, we examined the levels of mRNA predicted as strong targets of isotype-specific Tg-miRNAs (Table S2). Real time PCR analyses revealed little, if any changes in mRNA levels between type I and II isotypes, contrasting with the differential abundances of the corresponding Tg-miRNAs. This result corroborates partially the suggestion that Tg-AGO acts mainly as a translational regulator, which is also in agreement with the cellular factors found in association with Tg-AGO (see later in the text). Owing to the lack of available antibodies for predicted targets and our current inability to generate Tg-Dicer or Tg-AGO mutants, experimental validation of the above hypothesis will be part of future experiments.
To date, the use of RNAi for specific gene silencing has remained largely inconclusive in Toxoplasma. Many laboratories have attempted to use this tool to down-regulate gene expression but very few reports showed successful double-stranded RNA induced gene silencing and there is currently no evidence for the production of specific siRNA [51]. We note that the use of RNAi is normally expected to result in mRNA turnover, as in metazoans or plants. The nature of the Tg-AGO (slicer deficient) and its possible mode of operation through translational repression (with a usually modest output on gene expression in metazoans) is obviously one parameter that could explain the lack of significant levels of mRNA degradation upon RNAi treatments in this organism.
Toxoplasma repeat-and satellite-associated sRNA
The largest bulk of medium-to-low abundance Tg-sRNAs does not meet the criteria of miRNA annotation and appears to match repetitive elements REP1, REP2 and REP3 (Figure 5A) [52]. REP elements are mitochondrial-like sequences dispersed throughout the nuclear genome of Toxoplasma. They are typically composed of mitochondrial-like genes, including COX1 (cytochrome oxidase subunit 1) and COB (apocytochrome b) that are flanked by a 91 bp short-dispersed repetitive sequence (SDR) organized as a direct or inverted repeat (Figure 5A) that might play roles in generation or dispersal of the REP elements. Nonetheless, there is no sequence similarity between SDRs and other terminal repeats such as those of retroviral LTRs. Moreover, REP elements do not seem to be highly mobile [52].
Fig. 5. Expression patterns and characteristics of repeat-associated Tg-rdsRNA in Toxoplasma. 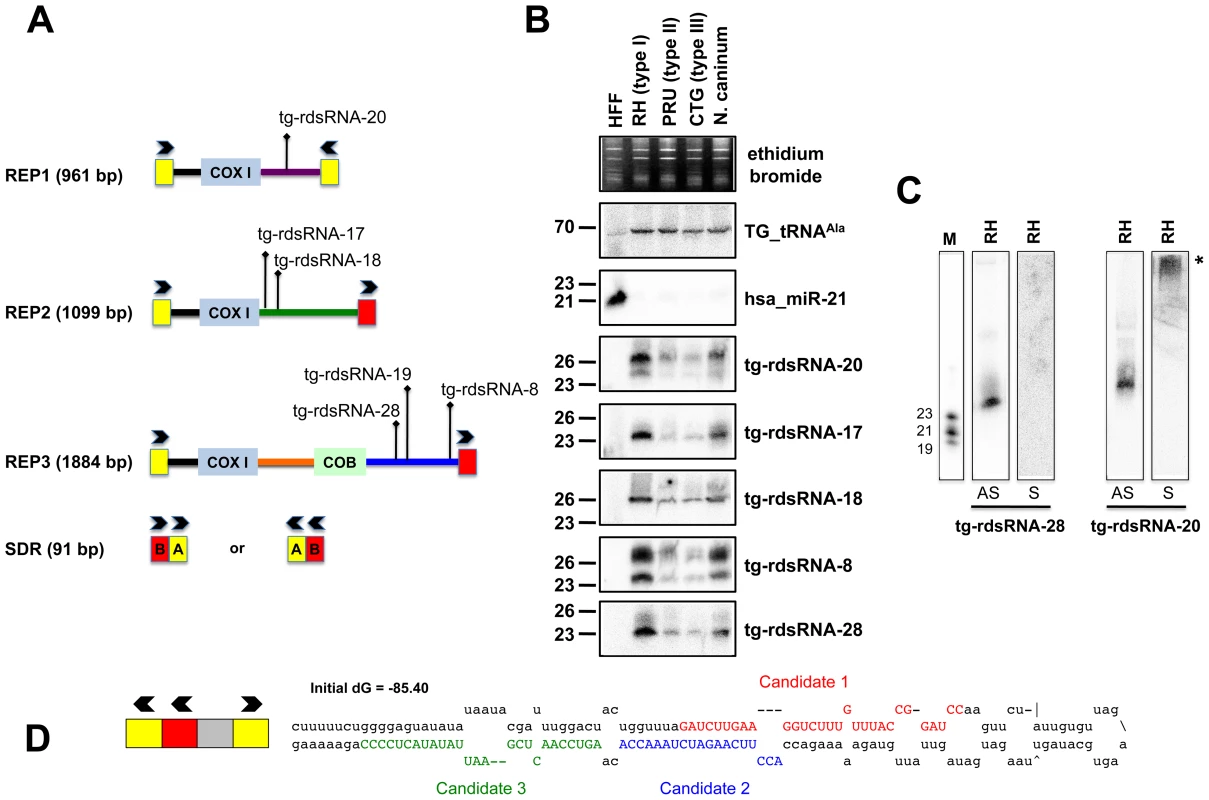
(A) Schematic representation of REP elements. The three REP fragments and the short disperses repeat (SDR) are displayed from 5′ to 3′ with respect to the COX1 (cytochrome oxidase subunit 1) gene fragment. Blocks A and B of the SDR are labeled in yellow and red, respectively. Cloned Tg-rdsRNA are represented above: Tg-rdsRNA-8 (1155 reads), tg-rdsRNA-17 (464 reads), Tg-rdsRNA-18 (1134 reads), Tg-rdsRNA-19 (1326 reads), Tg-rdsRNA-20 (1066 reads) and Tg-rdsRNA-28 (223 reads). (B) Expression profile analyses of five parasite Tg-rdsRNA candidates by small RNA Northern hybridization in three canonical strains of Toxoplasma and its close relative, N. caninum. Controls and quantification method are as in Fig. 2A. The number next to each panel represents the position of RNA markers. (C) Small RNA blots were stripped and re-hybridized with antisense probes of selected Tg-rdsRNA sequence. Antisense and sense oligonucleotides (indicated by AS and S, respectively) were used to confirm the polarity of Tg-rdsRNA-20 and -28. (D) SDR-containing chromosomic region (TGME49_chrX:233424-233609) forms a stereotypic hairpin precursor predicted by mfold that harbor cloned small RNA candidates. Genome mapping showed that Toxoplasma REP-derived sRNAs (rdsRNAs) form discrete species that are exclusively generated from regions located downstream of the COX1 and COB sequences, (Figure 5A), with read counts typically ranging from >10,000 (rdsRNA-17) to a few hundred reads (rdsRNA-28). This fairly high abundance might be explained by the fact that the estimated number of REP elements is >500 copies per genome [52]. While their size range (21–27nt, Table S3) and sensitivity to periodate (not shown) was similar to that of Tg-miRNAs, about half of the rdsRNAs had a 5′ terminal U instead of the prevalent A found in miRNAs. All tested Tg-rdsRNAs were readily detected by Northern blotting using 5′end-labeled antisense oligonucleotides but, unlike Tg-miRNAs, they were consistently much more abundant in the highly virulent Toxoplasma isotype-I (Figure 5B). Interestingly, sense probes generated no signal for the Tg-rdsRNAs tested, consistent with the absence of Illumina reads corresponding to opposite-strand sRNA species (Figure 5C and data not shown). A sense probe for Tg-rdsRNA-20, however, gave a high molecular weight signal potentially resulting from hybridization of a double-stranded RNA precursor, although RNA folding algorithms did not reveal any significant secondary structure at, or in the vicinity of Tg-rdsRNA sequence matches (Figure 5C). Nonetheless, the detection of identical rdsRNA species in the related apicomplexan N. caninum and their isotype-specific accumulation (Figure 5B) together with their abundant loading into Tg-AGO (see below) make it unlikely that these species are simply random degradation products. This rather suggests the existence of a conserved mechanism that accounts for REP-dependent production of precursor molecules required for rdsRNA synthesis. A second class of low abundant Tg-rdsRNAs mapped directly to a long, imperfect stem-loop structure resulting from annealing of an individual ‘solo SDR’ unit. This structure is depicted in Figure 5D, together with the cloned sequences of contiguous or overlapping sRNAs that are likely produced via stepwise processing by the Tg-Dicer. Such imperfect structures might well represent the equivalent of the plant proto-MIRNA genes that arise from DNA-type non-autonomous elements known as miniature inverted-repeat transposable elements (MITEs). MITEs readily fold into imperfect stem-loops typical of miRNA precursors [53], [54] and often generate multiple sRNA species, including heterochromatic siRNA that dampen MITE expression transcriptionally, as well as recently-evolved (or young) miRNAs that may not have yet undergone positive selection for host transcript targeting, and tend to accumulate at low levels, as seen here with the SDR-derived Tg-rdsRNAs.
Sequence analysis also revealed the existence of a third class of repeat-associated sRNAs in Toxoplasma, which map perfectly to high-copy-number (>800 copies per genome) satellite DNA Sat350 (ABGTg/TGR family, Figure 6A) and Sat529a (Figure 6B) [55]. Although these satellite-associated (Tg-sat)RNAs had very low read numbers, they formed near-contiguous stretches of sequence along the corresponding SAT loci. These two features (low read-number, accumulation as populations rather than discrete species) are highly reminiscent of plant heterochromatic siRNAs found at DNA repeats and transposon loci with no intrinsic potential to form fold-back structures. In Arabidopsis, heterochromatic siRNAs are typically synthesized thought the conversion of aberrant RNA molecules into long-dsRNA, via the action of RDR2 [56]. Upon its processing by DCL3, the resulting siRNA population engages into AGO4 or AGO6 to mediate cytosine methylation and histone modifications at the sites of its production, resulting in heterochromatin formation [57]. We speculate that, similarly, Tg-satRNAs originate from the action of Tg-RDR using SAT-derived aberrant transcripts as templates, and contribute to maintain the heterochromatic state found at both SAT350 and SAT529, which, indeed, are enriched in silent chromatin marks including H4K20 and/or H3K9 monomethylations, as assessed by chromatin immunoprecipitation (Figure 6C). Similarly these silencing marks are also poorly but clearly enriched at REP - and MITE-derived sRNA loci (data not shown). We acknowledge that ChIP experiments for histone modifications only provide merely correlative evidence for a functional link between heterochromatin formation/spread and small RNA in Toxoplasma although some of the Tg-AGO-associated factors also support this idea (see later in the text). Assessing the formal contribution of Tg-AGO in DNA-based heterochromatic processes will require further experiments.
Fig. 6. Satellite-associated Tg-satRNA localized to heterochromatin in Toxoplasma. 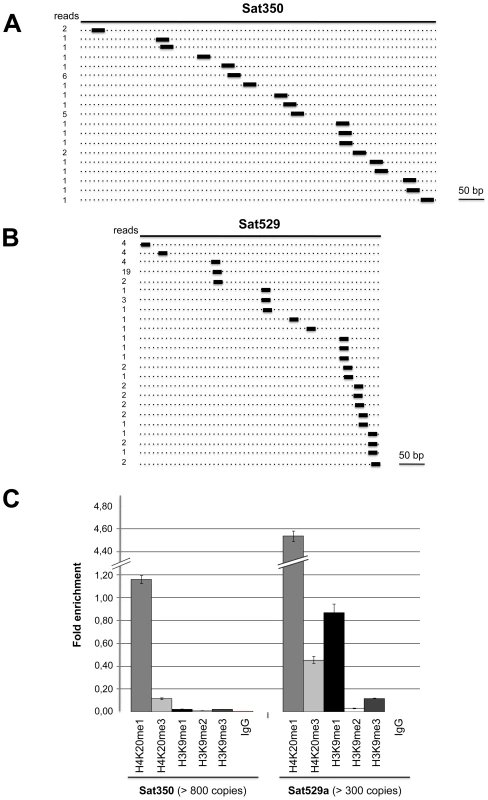
New cloned Tg-satRNA species were assigned to repetitive satellite elements Sat350 and Sat529a, which are embedded in heterochromatin domains. Tg-satRNAs with perfect matches were plotted to satellite elements Sat350 (A) and Sat529a (B). The short thin lines below the long bars represent small RNAs derived from the sense strands with the reads indicated at the left. (C) Analyses of selected modified histones ratio at satellite Sat350 and Sat529a by quantitative chromatin immunoprecipitation PCR. Ratios of DNA precipitated with target modifications over DNA precipitated with core histone H3 were used to calculate relative precipitated fold enrichment shown on the y-axis. Experiments were triplicated and the data sets are concordant. Relative intensity is shown with standard deviations. sRNA loading, sub-cellular localization and polysome association of Tg-AGO
In the absence of obvious, additional Ago-like proteins (including PIWI proteins) in the Toxoplasma genome (TOXODB, release v6.0), both transcriptional and post-transcriptional gene silencing events must, therefore, be operated via the same and unique Tg-AGO. To address this issue, we generated transgenic parasites expressing ectopically HAFlag-tagged, full-length Tg-AGO. RNP complexes were immuno-affinity purified (see next section), co-precipitated RNAs were extracted from the beads and analyzed by Northern using oligonucleotide probes specific to some of the highly abundant Tg-miRNAs and Tg-rdsRNAs studied above. Tg-miR-4 and -43, and as well Tg-rdsRNA-17 and -28 were indeed detected in the HaFlag-Tg-AGO immuno-precipitates but not in control immuno-precipitates (Figure 7A), indicating that Tg-AGO is a common effector of both types of sRNAs. This likely entails both cytoplasmic and nuclear distribution of the protein. Immunofluorescence and confocal microscopy revealed that Tg-AGO accumulates in tachyzoites mostly as granules of unidentified nature, but this labeling was superimposed over a diffuse cytoplasmic signal (Figure 7B and data not shown). Using acetylated histone H4 as a marker, confocal analyses also revealed a faint nuclear staining indicating that a minor portion of Tg-AGO localizes to the nucleus. Nuclear localization of Tg-AGO could be transient or highly dynamic, and under steady-state conditions. Alternatively, nuclear Tg-AGO could be incorporated into large protein complexes that prevent its optimal accessibility to antibodies.
Fig. 7. Small RNA loading, sub-cellular localization and polysome association of Tg-AGO. 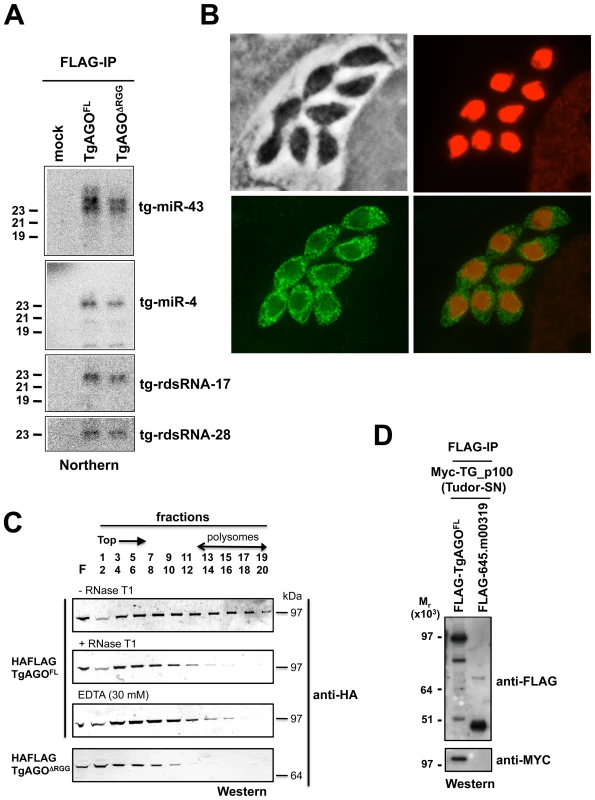
(A) Northern blot analyses of Tg-miRNAs and Tg -rdsRNAs associated with Tg-AGO. Immuno-precipitation using anti-Flag antibody was performed from RH strains expressing ectopically HAFlag-tagged either full-length or delta-RGG truncated Tg-AGO. RNAs isolated from the immunoprecipitates were probed for Tg-miR-4, Tg-miR-43, Tg-rdsRNA-17 and Tg-rdsRNA-28. Mock: non-immune IgG (negative control). (B) Stably expressed recombinant protein HAFlag-TgAGOFL was detected by immunofluorescence assay using an HA antibody (in green) and compared to nuclear localization of acetylated histone H4 (in red). (C) To evaluate the sedimentation characteristics of Tg-AGO complexes, protein extracts containing HAFlag-TgAGOFL or HAFlag-TgAGODRGG were subjected to sedimentation on 5%–45% sucrose gradients in the presence of cycloheximide (to preserve polyribosomes) or 30 mM EDTA (to disrupt polyribosomes). Aliquots of total extracts, either untreated (-RNase T1) or digested with RNase (+ RNase T1) were centrifuged through sucrose gradients and fractionated as described in Methods. Aliquots (equal volume) from indicated fractions were analyzed by Western blots with antibody against HA tag. (D) HAFlag-TgAGOFL and HAFlag-645.m00319 (negative control) were transiently co-expressed with Myc-Tg-p100 (Tudor/SN). Following Flag affinity purification, the bound proteins were analyzed by Western blot using the anti-HA and the anti-myc antibodies. Molecular weight markers are indicated on the left. The fact that a fraction of several Tg-miRNAs co-sediments with polysomes (Figure 4E, fractions 13–18) suggests that a portion of Tg-AGO should also be associated with polysomes, as has been shown for plant and metazoan miRNA-loaded AGOs [41], [58]–[62]. We thus examined the distribution of HaFlag-Tg-AGO using polysome gradients: cytoplasmic extracts from intracellular parasites were prepared and fractionated on sucrose gradients (Figure 7C). The absorbance profiles at 254 nm showed a pattern of ribosomes with ribosomal subunits, monosomes, and polysomes. Consistent with previous findings in metazoans [61], [62], most HAFlag-Tg-AGO was found near the top of the gradient, where soluble material and small ribonucleoprotein particles sediment. Some HAFlag-Tg-AGO was also heterodispersed throughout the gradient fractions, where polyribosomes and Tg-miRNAs co-sediment (Figures 4E and 7C). Treatments of cellular extracts with 30 mM EDTA or RNase T1, known to dissociate polysomes into ribosomal subunits and monosomes, caused a shift in HAFlag-Tg-AGO distribution from the denser fractions to the lighter fractions of the gradient (Figure 7C). This result suggests that a portion of miRNA-loaded Tg-AGO associates with polysomes to regulate translation of Tg-miRNA target mRNAs, perhaps in the cytoplasmic granules observed by immunofluorescence.
As noted previously, a characteristic feature of Tg-AGO is the presence, at the amino terminus, of a repeated RGG-rich region (amino acids 1–68), in which the arginine residues have the potential to undergo methylation (Figure 2B). This post-translational modification is known to influence the stability, activity and/or sub-cellular distribution of some metazoan AGO-like proteins [23]–[25]. Tudor-domain proteins specifically recognize symmetrically dimethylated arginines (sDMA) such as those found in AGO-like proteins [63], [64]. Accordingly, the immunopurified HAFlag-Tg-AGO complex (see below; Tables 1 and S4) was found to contain the Tudor-SN (tudor staphylococcal nuclease)/p100 homolog. Tudor-SN has five staphylococcal/micrococcal nuclease domains as well as Tudor domain, and it was described as a component of RISC in Caenorhabditis elegans, Drosophila and mammals [65]; more recently, the Tudor domain of the fly Tudor-SN was characterized as a specific sDMA-binding protein [64]. Reciprocal immunoprecipitation experiments further confirmed the specific binding of Tg-AGO to Tg-Tudor/SN (Figure 7D). Furthermore, HAFlag-Tg-AGO was also found to co-purify with Tg-PRMT1, which belongs to the family of arginine methyltransferases that use RGG motifs as substrates (Table 1). These results suggest that Tg-AGO is arginine-methylated, and that this modification might be specifically read by Tg-Tudor/SN, possibly to engage Tg-AGO into distinct modes of RNA silencing. In particular, the RGG-rich region of the Trypanosoma brucei Tb-AGO1 was found critical to its association with polysomes [66]. To test if the same was true of Tg-AGO, we engineered HAFlag-Tg-AGODRGG, which carries a deletion of the RGG domain (amino acids 1 to 68). While HAFlag-Tg-AGODRGG was loaded normally with Tg-miRNA and Tg-rdsRNA (Figure 7A), the majority of the mutant protein was found near the top of polysome gradients, and was notably absent in fractions where polyribosomes sediment (Figure 7C). In addition, mass spectrometry analysis of the HAFlag-Tg-AGODRGG complex showed that it was no longer associated to Tg-Tudor/SN (data not shown). Thus, the proposed Tg-AGO arginine-methylation and association with Tg-Tudor/SN might allow post-loading sorting of distinct Tg-AGO-containing RNP complexes towards specific silencing modes. We note that its association to Tg-Tudor/SN through an RGG domain together with its predominant cytoplasmic localization evoke the as yet unexplored possibility that Tg-AGO may serve as a PIWI protein. Thus, in addition to its possible role in heterochromatin formation, Tg-AGO might contribute to post-transcriptional gene silencing of repeats and transposons via Tg-rdsRNAs, as is seen with metazoan PIWI proteins.
Tab. 1. Summary of <i>Tg</i>-AGO-associated proteins identified by mass spectrometry. 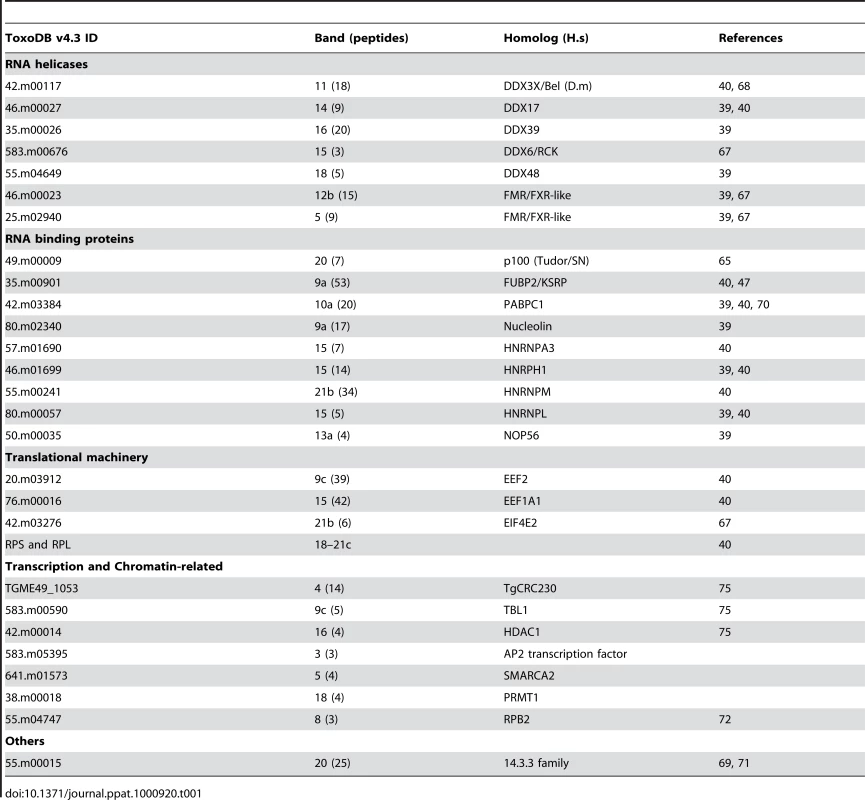
Tg-AGO-associated proteins constitute the near-entire cohort of previously identified human and fly miRNA-RISC components
To characterize the molecular composition of Tg-AGO-containing complexes, HAFlag-Tg-AGO was affinity purified from total cell extracts of intracellular tachyzoite by incubation with anti-FLAG agarose beads. The immunoprecipitated FLAG–protein complexes were eluted using the FLAG peptide. The immunoprecipitated proteins were then separated by SDS-PAGE, excised, and identified by mass spectrometry (Figure 8A). Tables 1 and S4 list the names of the identified proteins, which, remarkably, were direct orthologs of nearly all of the previously identified components of human and Drosophila miRNA-RISC [39], [40], [67]–[69]. These co-purified proteins fell within several functional groups. The largest group encompasses mRNA-binding proteins, in particular the heterologous nuclear ribonucleoproteins: HNRNPA3, HNRNPH1, HNRNPL, and HNRNPM [39], [40]. Several mRNA-binding proteins with putative functions in mRNA transport, stabilization and translation were also identified, including homologs of FUBP2/KSRP, nucleolin and FXR-related proteins, which are well known human and Drosophila Argonaute interactors [39], [40], [68]. Among the DEAD/DEAH box helicases, we found DDX17/DDX5, an ortholog of Drosophila p68, which has been shown to associate with Drosophila Ago2 [9], and DDX3X/Belle or DDX6/p54, which are all required for miRNA function (Table 1) [67], [68]. Consistent with the hypothesis that Tg-AGO associates with mRNPs, a homolog of the polyadenylation binding protein PABPC [70] was identified in the immuno-precipitate, indicating that mRNAs were present in the purifications. Accordingly, treatment of the lysate with RNase T1 prior to immuno-precipitation abolished the integrity of the Tg-AGO1 complex, indicating that the interactions between HAFlag-Tg-AGO and several proteins were RNA-mediated (Figure 8B). Identification of translation initiation and elongation factors, together with various 40S and 60S ribosomal proteins (Table S4) provides further support to the idea that the miRNA-loaded Tg-AGO, which associates with polysomes, might prevent translation of target mRNAs. Another noticeable partner of Tg-AGO was a ortholog of human FUBP2, also known as KHSRP/KSRP, which binds with high affinity to the terminal loop of some miRNA precursors and promotes their maturation [47]. Tg-KSRP might account, at least party, for the post-transcriptional regulation of some Toxoplasma MIRNA genes, as uncovered in this study (Figure 4B and 4C). Consistent with an effect of Tg-KSRP on miRNA maturation rather than activity, Tg-KSRP was found to bind Tg-AGO in an RNA-independent manner (Figure 8C). Accordingly, Tg-KSRP did not associate to polyribosomes (Figure 8D) and was also found in HAFlag-Tg-AGODRGG immuno-precipitates (Figure 8C).
Fig. 8. Proteomic analysis of Tg-AGO complexes. 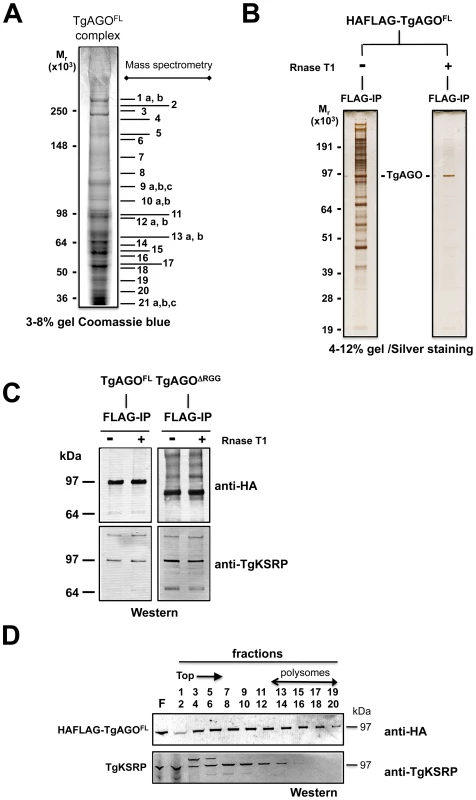
(A) Tg-AGO complexes were immunoprecipitated using Flag antibodies. The co-immunoprecipitated proteins were analysed using mass spectrometry (see Table 1 and Supplemental Table S1). Molecular weight markers are indicated on the left. (B) Transgenic parasites expressing ectopically HAFlag-TgAGOFL were lysed and whole-cell-extracts prepared in the absence or presence (+RNase T1) of ribonuclease T1. HAFlag-TgAGOFL was immunoprecipitated using anti-FLAG beads and flag peptide-eluted fractions were separated by SDS-polyacrylamide gel electrophoresis (4% to 12%), and visualized by silver staining. Molecular weight markers are indicated on the left. (C) Flag-immunopurified fractions from HAFlag-TgAGOFL and HAFlag-TgAGODRGG RNase T1-treated and untreated samples were analyzed by Western blot using a home-made anti-Tg-KSRP antibody. (D) Sedimentation characteristics of Tg-KSRP compared to HAFlag-TgAGOFL. Additional immuno-purified proteins are not obviously related to translational control but have been previously implicated as RISC-associated factors including Tg-Tudor/SN and Tg-PRMT1, already evoked above. Notably, Tg-AGO also co-purified with a conserved 14-3-3 protein (Table 1): 14-3-3 proteins that bind S. pombe Ago1 and human Ago2 are probably required for AGO protein functions in cell cycle and/or gene silencing pathways [71]. 14-3-3 proteins may also act as major regulators for the sorting of AGOs between distinct classes of RNA granules [69], which may include the Tg-AGO foci detected by immunofluorescence in the present study (Figure 7B). Collectively, these results provide compelling evidence that Tg-AGO is part of a functional RISC whose core components are nearly all orthologous to factors required for post-transcriptional gene silencing and its regulation in metazoans.
Consistent with additional, DNA-level silencing functions of Tg-AGO, the second largest subunit of Tg-RNA polymerase II (Rpb2) also co-purified in the HAFlag-Tg-AGOFL immuno-precipitates (Table 1). Interestingly, a mutation of Rpb2 in fission yeast, rpb2-m203, disrupts coupling between transcription and siRNA processing in RNAi-dependent heterochromatin formation [72]. Also reminiscent of the fission yeast heterochromatic RNAi pathway, HAFlag-Tg-AGO was associated with the histone deacetylase TgHDAC3, a protein that may play similar roles to S. pombe Clr3 in the spread of heterochromatin [73], [74]. Remarkably, HAFlag-Tg-AGO was associated with all known components of the major transcriptional co-repressor complex Tg-CRC [75], [76], which contains the two repressor proteins Tg-CRC230 and Tg-TBL1, the catalytic subunit Tg-HDAC3 and a new plant-like AP2-domain transcription factor (Table 1). Moreover, peptide sequencing by tandem mass spectrometry indicated that the subunits of the complex are sub-stoichiometrically represented. This finding is consistent with the as yet unconfirmed idea that Tg-AGO-bound rdsRNA and possibly satRNAs may guide transcriptional gene silencing processes by recruiting histone deacetylases, and subsequently histone methylases (i.e. Tg-SET8 and Tg-SET3, [77]), to heterochromatic regions of the genome.
Conclusions
The present analysis thus uncovers an unsuspected level of complexity in the RNA silencing pathways of the single cell parasite T. gondii. This complexity not only lies in the mere diversity of the sRNAs identified, but also in the apparent mix-and-matched nature of the silencing components found in this organism, both in terms of their evolution and function. In this respect, the T. gondii RNA silencing machinery and its usage by the parasite bewilder many accepted notions in the field. For instance, in no organism studied so far has a single Ago protein evolved to mediate both repeat-associated and miRNA-mediated gene silencing, two pathways usually considered drastically different. Likewise, the metazoan-like Tg-miRNAs have readily identifiable mRNA targets displaying perfect to near-perfect complementarity in both CDS and UTRs, which is unprecedented in animals. Further studies of the Toxoplasma RNA silencing pathways will undoubtedly reveal other surprises and, more importantly, might shed light on the molecular bases of virulence in this important Human parasite.
Materials and Methods
Parasite strains, cell culture, and in vitro bradyzoite differentiation
The parasite strains used in this study are the following: the T. gondii type I RH strain that has lost the ability to complete the two-host life cycle, the T. gondii type II Prugniaud strain that is capable of robust bradyzoite differentiation, and the T. gondii type III CTG and C56 strains. All T. gondii and N. caninum strains were maintained by serial passage in HFF monolayer under tachyzoite conditions in DMEM (Invitrogen) supplemented with 10% (vol/vol) FBS (Invitrogen). T. gondii type II Prugniaud strain was maintained under tachyzoite conditions in DMEM supplemented with 10% (vol/vol) FBS and 25 mM Hepes buffer, pH 7.2. To induce in vitro bradyzoite differentiation, extracellular tachyzoites were allowed to invade HFF cells for 16 hours, and the culture medium was removed and replaced by RPMI-1640 supplemented with 1% FBS and 50 mM Hepes buffer, pH 8.2. After 2–3 days of culture in alkaline medium, bradyzoite induction was assessed for P36 and SUMO expression by IFA as described previously [78]. The RHhxgprt - strain used in these studies contains a deleted or defective HXGPRT gene, which allows for the selection of transfected tachyzoites using mycophenolic acid.
Antibodies
Antisera against Tg-FUBP2/KSRP (35.m00901 gene) were produced by Eurogentec using the ‘Super Speedy immunization’ protocol and the following peptides Tg-FUBP2-1 (H2N-MARKKRGSAATPEEGC-CONH2) and Tg-FUBP2-2 (H2N-GTDKREDRGVTPEE DC-CONH2). Specific antibodies were affinity purified against both peptides. For immunoblot analysis purified antibodies were used at 1∶1000 dilutions. Primary antibodies for IFA, ChIP and Western blot included antibodies against haemagglutinin epitope tag (HA, Roche Diagnostic, dilution at 1∶1000), Polyclonal anti-H4-K20-1me (Abcam ab9051), Polyclonal anti-H4-K20-3me (Abcam ab9053), Polyclonal anti-H4-K20-1me (gift from Rice JC,Sims et al., 2006), Polyclonal anti-H4 Acetylated (K5-K8-K12-K16) (upstate 06-866), Anti-H3-K9-1me (upstate 07-450), Anti-H3-K9-2me (upstate 07-441), Anti-H3-K9-3me (upstate 07-442), Monoclonal anti-Myc (9E10 - sc40X, Santa-Cruz Bio.).
Immunofluorescence microscopy
Infected HFFs grown on coverslips were washed in PBS and fixed/permeabilized for 20 min at room temperature with PBS containing 3% (vol/vol) formaldehyde and 0.2% Triton X-100 (vol/vol). Blocking was performed with PBS containing 5% FBS and 5% goat serum for 1 h at room temperature. Samples were incubated in PBS containing 1% FBS with the primary antibodies, followed by the secondary antibodies goat anti–mouse IgG coupled with Alexa Fluor 488 and goat anti–rabbit IgG coupled with Alexa Fluor 568 (Invitrogen) at a 1∶1,000 dilution each in PBS–1% FBS. Nuclei of host cells and parasites were stained for 10 min at room temperature with Hoechst 33258 at 2 µg/ml in PBS. After four washes in PBS, coverslips were mounted on a glass slide with Mowiol mounting medium (48 mM Tris-HCl [pH 8.5], 4.8% Mowiol 4–88 [wt/vol], 12% glycerol [vol/vol]), and images were acquired with a fluorescence microscope (Axioplan 2; Carl Zeiss, Inc.).
Quantitative chromatin immuno-precipitation (QChIP) assay
QChIP assays were performed based on a modification of previously published methods [77], [79]. Immuno-precipitated DNA were purified through PCR Purification Kit columns (QIAGEN) and used as a template in semiquantitative QPCRs to detect specific targets. Specific primer pairs (melting temperature, 55 to 65°C) amplifying 200 - to 450-bp fragments were used (supplemental Table S1). PCR was performed with 1 µL of DNA and 500 nM primers diluted to a final volume of 20 µL in SYBR Green Reaction Mix (Roche). Accumulation of fluorescent products was monitored by real-time PCR using a LightCycler 2.0 (Roche). Each PCR reaction generated only the expected specific amplicon, as shown by the melting-temperature profiles of final products (dissociation curve, automatically measured by the LightCycler 2.0) and by gel electrophoresis of test PCR reactions. No PCR products were observed in the absence of template. The fold difference of a given target sequence precipitated by a specific antibody was determined by dividing the amount of target sequence in the immunoprecipitate fraction by the amount of target sequence in input DNA (S8, S13). Real-time PCR was carried out in triplicate on 2 ng of DNA at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were collected at 60°C. The concentration of primers and Taqman probes used was determined by following the optimization procedure described in PE Applied Biosystem's protocol. For each experiment, the threshold was set to cross a point at which real-time PCR amplification was linear. For the majority of the experiments, data were analyzed with a threshold of 0.05. Data collected was analyzed and plotted using Microsoft Excel.
Satellite 350B: OL17 (CGACTCGGACGTCAGGCCATGCAGAG) and OL18 (GCGCCTGAACAATACGCCCAACC).
Satellite 529A: OL19 (CTGCAGGGAGGAAGACGAAAGTTG) and OL20 (CTGCAGACACAGTGCATCTGGATT).
Chromatographic purification of Tg-AGO-containing complexes
Whole-cell extract (WCE) from transgenic intracellular tachyzoites expressing ectopically HAFlag-TgAGOFL and HAFlag-TgAGODRGG was incubated with 500 ml of anti-FLAG M2 affinity gel (Sigma) for 1 h at 4°C. Beads were washed with 10 column volumes of BC500 buffer [20 mM Tris (pH 8), 0.5 M KCl, 10% glycerol, 1 mM EDTA, 1 mM DTT, 0.1% NP40, 0.5 mM PMSF, aprotinin, leupeptide, pepstatin, 1 ug ml−1 each]. Bound peptides were eluted stepwise with 250 ug ml−1 FLAG peptide (Sigma) diluted in BC500 buffer. Each preparation was sufficiently clean such that individual peptide bands could be excised and sequenced by mass spectrometry.
Mass spectrometry peptides sequencing
Protein bands were excised from colloidal blue-stained gels (Invitrogen), oxidized with 7% H2O2 and subjected to in-gel tryptic digestion. Peptides were extracted with 5% [v/v] formic acid solution and acetonitrile, and injected into an Ultimate 3000 (Dionex) nanoLC system that was directly coupled to a LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific). MS and MS/MS data were acquired using Xcalibur (Thermo Fischer Scientific) and processed automatically using Mascot Daemon software (Matrix Science). Tandem mass spectra were searched against a compiled T. gondii database using the MASCOT program (Matrix Sciences, London) available via intranet.
Small RNA library preparation
RNA was extracted into TRIzol (Invitrogen), and aliquots of total RNA from RH strain were subjected to small RNA library construction as follows. To avoid any contamination with the host cell small RNAs, freshly released parasites were harvested from the culture supernatant, washed by centrifugation, and filtered through a 3-µm filter before use. For each library, 50 µg of total RNA was size fractionated on a 15% tris-borate-EDTA (TBE) urea polyacrylamide gel (Invitrogen) and a 19–40 base pair fraction was excised. RNA was eluted from the polyacrylamide gel slice in 300 µL of 0.3 M NaCl overnight at 4°C. The resulting gel slurry was passed through a Spin-X cellulose acetate filter column (Corning Inc.) and precipitated by the addition of 750 µL of ethanol and 3 µL of glycogen (5 mg/mL; Ambion). After washing with 75% ethanol, the pellets were allowed to air dry at 25°C and pooled in diethylpyrocarbonate (DEPC)-treated water. The 5′ RNA adapter (5′-GUUCAGAGUUCUACAGUCCGACGAUC-3′) was ligated to the RNA pool with T4 RNA ligase (Promega) in the presence of RNase Out (Invitrogen) 6 hours at 20°C. The ligation reaction was stopped by the addition of 2× Gel Loading Buffer II (Ambion). The ligated RNA was size fractionated on a Novex 15% TBE urea polyacrylamide gel (Invitrogen), and a 40–70 base pair fraction was excised. RNA was eluted from the polyacrylamide gel slice in 300 µL of 0.3 M NaCl overnight at 4°C. The RNA was eluted from the gel and precipitated as described above followed by resuspension in DEPC-treated water. The 3′ RNA adapter (5′-pUCGUAUGCCGUCUUCUGCUUGUidT-3′; p, phosphate; idT, inverted deoxythymidine) was subsequently ligated to the precipitated RNA with T4 RNA ligase (Ambion) in the presence of RNase Out (Invitrogen) 6 hours at 20°C. The ligation reaction was stopped by the addition of 2× Gel Loading Buffer II (Ambion). Ligated RNA was size fractionated on a Novex 10% TBE urea polyacrylamide gel (Invitrogen), and the 70–100 base pair fraction was excised. The RNA was eluted from the polyacrylamide gel and precipitated from the gel as described above and resuspended in 4.5 µL of DEPC-treated water. The RNA was converted to single-stranded cDNA using Superscript II reverse transcriptase (Invitrogen) and Illumina's small RNA RT-Primer (5′-CAAGCAGAAGACGGCATACGA-3′) following the manufacturer's instructions. The resulting cDNA was PCR-amplified with Phusion™ High Fidelity DNA Polymerase (NEB) in 15 cycles using Illumina's small RNA primer set (5′-CAAGCAGAAGACGGCATACGA-3′; 5′ - AATGATACGGCGACCACCGACAGGTTCAGAGTTCTACAGTCCGA-3′). PCR products were purified on a Novex 6% TBE PAGE gel (Invitrogen) and the 100 base pair fraction was excised. The DNA was eluted into 100 µL of 1x NEBuffer 2 at room temperature for 2 hours. The resulting gel slurry was passed through a Spin-X filter (Corning) and precipitated by the addition of 325 µL of ethanol, 10 µL of 3 M sodium acetate, and 3 µL of glycogen (5 mg/mL; Ambion). After washing with 75% ethanol, the pellet was allowed to air dry at 25°C and dissolved in 10 µL of resuspension buffer (10 mM Tris-HCl, pH 8.5). The purified PCR products were quantified on the Agilent DNA 1000 chip and diluted to 10 nM for sequencing on the Illumina 1G (GATC BIOTECH, Konstanz, Germany).
Northerns
RNA from Toxoplasma and Neospora strains was extracted into TRIzol (Invitrogen), deproteinized with phenol chloroform/isoamyl alcohol, and RNA was recovered by ethanol precipitation. For small RNA analyses, 30 ug of purified RNA were separated on a 15% polyacrylamide (w/v) 8 M urea gel and transferred to GeneScreen nylon membranes. DNA oligonucleotides complementary to tg-miRNAs or tg-rasiRNAs were labeled with [g-32P]ATP using T4 PNK (Promega). Hybridizations were performed at 37°C overnight. Hybridized membranes were exposed to imaging plates that were recorded after 5 h (PhosphoImager, FLA-8000, Fuji).
Polyribosome analysis
Whole cell extracts were prepared as described previously [59] but using a modified polysome buffer containing 100 mM NaCl, 40 mM Tris-Hcl pH 7, 10 mM MgCl2, 1 mM DTT, 1% Triton TX100 and protease inhibitor (Complete). Cycloheximide (100 ug/ml) was added to cells (10 min at 37°C) prior to collecting the cells by centrifugation. The drug was present in all buffers throughout the entire procedure. For polysome fractionation experiment, approximately 1000 OD600 of whole cell extract were layered onto 12 ml 5%–40% sucrose gradients prepared in polysome buffer without Triton, and centrifuged at 4°C for 2h at 36,000 rpm in a Beckman SW41 rotor. After centrifugation, 500 ml fractions were collected from the top of the gradient and the 260-nm absorbance profile was recorded. For Northern-Blot analysis, RNAs from each fraction were precipitated by adding 0,1M NaCl and 3 volumes ethanol and extracted with the TRIzol method. For Western-Blot analysis, 15 ul of each gradient fraction were run on 12% SDS-PAGE gels.
b-Elimination reaction of small RNAs
Total RNAs (30 ug) from RH-infected fibroblasts were incubated in a solution containing 10 mM HEPES (pH 7.0) and 250 mM sodium periodate for 30 min at 22°C. An equal volume of formamide loading dye was added to the samples, followed by incubation for 45 min at 99°C. The reaction mixture was then analysed by Northern-Blot. An equal amount of untreated RNA was also loaded onto the gel for comparison. RNA oligos of known sequence (19, 21 and 23 nt synthetic RNA oligos) were also treated with sodium periodate to check for the completion of b-elimination reaction, and the blots were probed with end-labeled oligos complementary to the synthetic oligos.
Computational analyses
We analysed a pool of 5,701,506 raw reads obtained by the sequencing-by-synthesis (Illumina). Initially, all the sequences fully matching tRNAs or rRNAs were removed. The remaining sequences were used to build a local Mysql database and then trimmed in a 4 steps process: 1) We removed 3′ and 5′ adaptor sequences, using an iterative scheme and updated the database. 2) We removed from each sequence the nucleotides, in the 5′ and 3′ extremities, with a Phred Quality Score (http://www.phrap.com/phred/) below 10 and updated the database. 3) We eliminated all the reads containing more than 6 stretches of C, T or G. 4) We screened the database to keep only reads having a length >19 nucleotides and an average Phred Quality Score >15. The final pool of 1,555,290 reads was used to cluster small RNAs. The sequences without any variation were classified in the same cluster. A total of 275,888 distinct clusters were identified and used for further analysis. All these clusters were compared against the T. gondii genome (http://www.ToxoDB.org, version 4.3) using Blast program with specific parameters (Word size set to 4 and Penalty for a nucleotide mismatch set to -1). This configuration allowed a more refined search of small versus large nucleotide sequences. All the results were saved in the database and used for mapping sRNAs on the chromosomes. Based on the chromosome position, we classify the clusters into families. First, we seeded our classification with results having 100% identity and an alignment length >19 and then recovered the clusters varying with less than three nucleotides. All further analyses were focused on the most abundant families. Images, multi-fastas and alignments against T. gondii genome were automatically generated and manually curated. All these treatments were made using an in-house API (Genobrowser) and functionalities (tools unpublished) written in PHP.
Identification of miRNA candidate loci
Up to 10 sequence windows on both strands, spanning the locus and including variable lengths of flanking regions (5–200 bp on either side), were examined for their potential to form fold-back transcripts by using the RNAfold [80] and Mfold [81] programs. The predicted outcomes, including the minimal folding free energy (MFE), at least 20 kcal/mole (dG = −20 kcal/mole), the length of pre-miRNAs, and the number of nucleotides (A, C, G, or U) in each pre-miRNA were recorded and used for further analysis. Each predicted tg-microRNA was further checked manually to ensure that they were from good quality single-stranded hairpins and that a miRNA/miRNA* pair had 0–2 nt 3′ overhangs.
Construction of T. gondii expression vector
The coding sequence of Tg-AGO (genbank, GU046561) was amplified by RT-PCR to introduce BamHI and HindIII sites at the start and the stop codons respectively. Primers forward (5′-ggatccATGAACGGAGGAGGCAGAGGAAGAG-3′) and reverse (5′ - aagcttCCATCAATGCTGTCTCAACAGAAC-3′) were used for PCR amplification. The PCR product allowed the cloning of Tg-AGOFL in frame with an N-terminal HAFlag tag into the T. gondii expression vector pMAH14 (GRA1 promoter, [75]) digest with BglII-HindIII. Tg-AGODRGG was amplified by PCR with forward (5′ - ggatccCTGTACGATGGAGACCACCTTCTC-3′) and reverse (5′ - aagcttCCATCAATGCTGTCTCAACAGAAC-3′) and cloned subsequently in pMAH14 (BglII-HindIII).
Supporting Information
Zdroje
1. MillerCM
BoulterNR
IkinRJ
SmithNC
2009 The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol 39 23 39
2. BladerIJ
SaeijJP
2009 Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS 117 458 76
3. SullivanWJJr
SmithAT
JoyceBR
2009 Understanding mechanisms and the role of differentiation in pathogenesis of Toxoplasma gondii: a review. Mem Inst Oswaldo Cruz 104 155 161
4. HakimiMA
DeitschKW
2007 Epigenetics in Apicomplexa: control of gene expression during cell cycle progression, differentiation and antigenic variation. Curr Opin Microbiol 10 357 362
5. IyerLM
AnantharamanV
WolfMY
AravindL
2008 Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int J Parasitol 38 1 31
6. SullivanWJJr
HakimiMA
2006 Histone mediated gene activation in Toxoplasma gondii. Mol Biochem Parasitol 148 109 116
7. BougdourA
SautelCF
CannellaD
BraunL
HakimiMA
2008 Toxoplasma gondii gene expression is under the control of regulatory pathways acting through chromatin structure. Parasite 3 206 210
8. HannonGJ
2002 RNA interference. Nature 418 244 251
9. MeisterG
TuschlT
2004 Mechanisms of gene silencing by double-stranded RNA. Nature 431 343 349
10. BartelDP
2004 MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116 281 297
11. LippmanZ
MartienssenR
2004 The role of RNA interference in heterochromatic silencing. Nature 431 364 370
12. MochizukiK
GorovskyMA
2004 Small RNAs in genome rearrangement in Tetrahymena. Curr Opin Genet Dev 14 181 187
13. YaoMC
ChaoJL
2005 RNA-guided DNA deletion in Tetrahymena: an RNAi-based mechanism for programmed genome rearrangements. Annu Rev Genet 39 537 559
14. LeeSR
CollinsK
2006 Two classes of endogenous small RNAs in Tetrahymena thermophila. Genes Dev 20 28 33
15. ZhaoT
LiG
MiS
LiS
HannonGJ
2007 A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev 21 1190 1203
16. MolnárA
SchwachF
StudholmeDJ
ThuenemannEC
BaulcombeDC
2007 miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 447 1126 1129
17. BaumJ
PapenfussAT
MairGR
JanseCJ
VlachouD
2009 Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic Acids Res 37 3788 3798
18. CeruttiH
Casas-MollanoJA
2006 On the origin and functions of RNA-mediated silencing: from protists to man. Curr Genet 50 81 99
19. GajriaB
BahlA
BrestelliJ
DommerJ
FischerS
2008 ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res 36 D553 D556
20. YigitE
BatistaPJ
BeiY
PangKM
ChenCC
2006 Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 127 747 757
21. MaJB
YuanYR
MeisterG
PeiY
TuschlT
2005 Structural basis for 5′-endspecific recognition of guide RNA by the A. fulgidus Piwi protein. Nature 434 666 670
22. ParkerJS
RoeSM
BarfordD
2005 Structural insights into mRNA recognition from a PIWI domain-siRNA guide complex. Nature 434 663 666
23. KirinoY
KimN
de Planell-SaguerM
KhandrosE
ChioreanS
2009 Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol 11 652 658
24. KirinoY
VourekasA
SayedN
de Lima AlvesF
ThomsonT
2010 Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization. RNA 16(1) 70 78
25. VaginVV
WohlschlegelJ
QuJ
JonssonZ
HuangX
2009 Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev 23(15) 1749 1762
26. ZongJ
YaoX
YinJ
ZhangD
MaH
2009 Evolution of the RNA-dependent RNA polymerase (RdRP) genes: Duplications and possible losses before and after the divergence of major eukaryotic groups. Gene 447 29 39
27. KeelingPJ
2009 Chromalveolates and the evolution of plastids by secondary endosymbiosis. J Eukaryot Microbiol 56(1) 1 8
28. LauNC
LimLP
WeinsteinEG
BartelDP
2001 An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294 858 862
29. AravinA
TuschlT
2005 Identification and characterization of small RNAs involved in RNA silencing. FEBS Lett 579 5830 5840
30. DjupedalI
PortosoM
SpåhrH
BonillaC
GustafssonCM
AllshireRC
EkwallK
2005 RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes Dev 19(19) 2301 2306
31. LiuQ
RandTA
KalidasS
DuF
KimHE
2003 R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science 301 1921 1925
32. MacraeIJ
ZhouK
LiF
RepicA
BrooksAN
2006 Structural basis for double-stranded RNA processing by Dicer. Science 311 195 198
33. MacRaeIJ
ZhouK
DoudnaJA
2007 Structural determinants of RNA recognition and cleavage by Dicer. Nat Struct Mol Biol 14 934 940
34. LimLP
LauNC
WeinsteinEG
AbdelhakimA
YektaS
2003 The microRNAs of Caenorhabditis elegans. Genes Dev 17(8) 991 1008
35. FelippesFF
SchneebergerK
DezulianT
HusonDH
WeigelD
2008 Evolution of Arabidopsis thaliana microRNAs from random sequences. RNA 14(12) 2455 2459
36. YuB
YangZ
LiJ
MinakhinaS
YangM
2005 Methylation as a crucial step in plant microRNA biogenesis. Science 307 932 935
37. YangZ
EbrightYW
YuB
ChenX
2006 HEN1 recognizes 21-24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res 34 667 675
38. LuS
SunYH
ChiangVL
2009 Adenylation of plant miRNAs. Nucleic Acids Res 37 1878 1885
39. HöckJ
WeinmannL
EnderC
RüdelS
KremmerE
2007 Proteomic and functional analysis of Argonaute-containing mRNA-protein complexes in human cells. EMBO Rep 8 1052 1060
40. LandthalerM
GaidatzisD
RothballerA
ChenPY
SollSJ
2008 Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA 14 2580 2596
41. LanetE
DelannoyE
SormaniR
FlorisM
BrodersenP
2009 Biochemical Evidence for Translational Repression by Arabidopsis MicroRNAs. Plant Cell 21 1762 1768
42. VaucheretH
2005 MicroRNA-dependent trans-acting siRNA production. Sci STKE 2005(300) pe43
43. SibleyLD
KhanA
AjiokaJW
RosenthalBM
2009 Genetic diversity of Toxoplasma gondii in animals and humans. Philos Trans R Soc Lond B Biol Sci 364(1530) 2749 2761
44. WinterJ
JungS
KellerS
GregoryRI
DiederichsS
2009 Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3) 228 234
45. HeoI
JooC
ChoJ
HaM
HanJ
KimVN
2008 Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell 32 276 284
46. ViswanathanSR
DaleyGQ
GregoryRI
2008 Selective blockade of microRNA processing by Lin28. Science 320 97 100
47. TrabucchiM
BriataP
Garcia-MayoralM
HaaseAD
FilipowiczW
RamosA
GherziR
RosenfeldMG
2009 The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 459(7249) 1010 1014
48. WangY
JuranekS
LiH
ShengG
TuschlT
PatelDJ
2008 Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456 921 926
49. WangY
JuranekS
LiH
ShengG
WardleGS
TuschlT
PatelDJ
2009 Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461 754 761
50. BrodersenP
VoinnetO
2009 Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol 10(2) 141 148
51. Al RiyahiA
Al-AnoutiF
Al-RayesM
AnanvoranichS
2006 Single argonaute protein from Toxoplasma gondii is involved in the double-stranded RNA induced gene silencing. Int J Parasitol 36(9) 1003 1014
52. OssorioPN
SibleyLD
BoothroydJC
1991 Mitochondrial-like DNA sequences flanked by direct and inverted repeats in the nuclear genome of Toxoplasma gondii. J Mol Biol 222 525 536
53. PiriyapongsaJ
JordanIK
2007 A family of human microRNA genes from miniature inverted-repeat transposable elements. PLoS ONE 2(2) e203
54. PiriyapongsaJ
JordanIK
2008 Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 14(5) 814 821
55. ClementeM
de MiguelN
LiaVV
MatrajtM
AngelSO
2004 Structure analysis of two Toxoplasma gondii and Neospora caninum satellite DNA families and evolution of their common monomeric sequence. J Mol Evol 58 557 567
56. XieZ
JohansenLK
GustafsonAM
KasschauKD
LellisAD
ZilbermanD
JacobsenSE
CarringtonJC
2004 Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2(5) e104
57. MatzkeM
KannoT
DaxingerL
HuettelB
MatzkeAJ
2009 RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21 367 376
58. OlsenPH
AmbrosV
1999 The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol 216 671 680
59. DjikengA
ShiH
TschudiC
ShenS
UlluE
2003 An siRNA ribonucleoprotein is found associated with polyribosomes in Trypanosoma brucei. RNA 9 802 808
60. KimJ
KrichevskyA
GradY
HayesGD
KosikKS
2004 Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci U S A 101 360 365
61. MaroneyPA
YuY
FisherJ
NilsenTW
2006 Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat Struct Mol Biol 13 1102 1107
62. NottrottS
SimardMJ
RichterJD
2006 Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat Struct Mol Biol 13 1108 1114
63. LeeJ
ThompsonJR
BotuyanMV
MerG
2008 Distinct binding modes specify the recognition of methylated histones H3K4 and H4K20 by JMJD2A-tudor. Nat Struct Mol Biol 15 109 111
64. FribergA
CorsiniL
MourãoA
SattlerM
2009 Structure and ligand binding of the extended Tudor domain of D. melanogaster Tudor-SN. J Mol Biol 387(4) 921 934
65. CaudyAA
KettingRF
HammondSM
DenliAM
BathoornAM
2003 A micrococcal nuclease homologue in RNAi effector complexes. Nature 425 411 414
66. ShiH
UlluE
TschudiC
2004 Function of the Trypanosome Argonaute 1 protein in RNA interference requires the N-terminal RGG domain and arginine 735 in the Piwi domain. J Biol Chem 279 49889 49893
67. ChuCY
RanaTM
2006 Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol 4(7) e210
68. ZhouR
HottaI
DenliAM
HongP
PerrimonN
2008 Comparative analysis of argonaute-dependent small RNA pathways in Drosophila. Mol Cell 32 592 599
69. CourchetJ
Buchet-PoyauK
PotemskiA
BrèsA
Jariel-EncontreI
2008 Interaction with 14-3-3 adaptors regulates the sorting of hMex-3B RNA-binding protein to distinct classes of RNA granules. J Biol Chem 283 32131 32142
70. FabianMR
MathonnetG
SundermeierT
MathysH
ZipprichJT
SvitkinYV
RivasF
JinekM
WohlschlegelJ
DoudnaJA
ChenCY
ShyuAB
YatesJR3rd
HannonGJ
FilipowiczW
DuchaineTF
SonenbergN
2009 Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol Cell 35(6) 868 80
71. StoicaC
CarmichaelJB
ParkerH
PareJ
HobmanTC
2006 Interactions between the RNA interference effector protein Ago1 and 14-3-3 proteins: consequences for cell cycle progression. J Biol Chem 281 37646 37651
72. KatoH
GotoDB
MartienssenRA
UranoT
FurukawaK
2005 RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science 309 467 469
73. FischerT
CuiB
DhakshnamoorthyJ
ZhouM
RubinC
ZofallM
VeenstraTD
GrewalSI
2009 Diverse roles of HP1 proteins in heterochromatin assembly and functions in fission yeast. Proc Natl Acad Sci U S A 106 8998 9003
74. WhiteSA
AllshireRC
2008 RNAi-mediated chromatin silencing in fission yeast. Curr Top Microbiol Immunol 320 157 183
75. SaksoukN
BhattiMM
KiefferS
SmithAT
MussetK
2005 Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol Cell Biol 25 10301 10314
76. BougdourA
MaubonD
BaldacciP
OrtetP
BastienO
2009 Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites. J Exp Med 206 953 966
77. SautelCF
CannellaD
BastienO
KiefferS
AldebertD
2007 SET8-mediated methylations of histone H4 lysine 20 mark silent heterochromatic domains in apicomplexan genomes. Mol Cell Biol 27 5711 5724
78. BraunL
CannellaD
PinheiroAM
KiefferS
BelrhaliH
GarinJ
HakimiMA
2009 The small ubiquitin-like modifier (SUMO)-conjugating system of Toxoplasma gondii. Int J Parasitol 39 81 90
79. SautelCF
OrtetP
SaksoukN
KiefferS
GarinJ
2009 The histone methylase KMTox interacts with the redox-sensor peroxiredoxin-1 and targets genes involved in Toxoplasma gondii antioxidant defences. Mol Microbiol 71 212 226
80. HofackerIL
FontanaW
StadlerPF
BonhoefferLS
TackerM
1994 Fast folding and comparison of RNA secondary structures. Monatshefte für Chemie 125 167 188
81. ZukerM
2003 Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31 3406 3415
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing ActivityČlánek The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced MucositisČlánek Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External RegionsČlánek Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The HMW1C Protein Is a Glycosyltransferase That Transfers Hexose Residues to Asparagine Sites in the HMW1 Adhesin
- Analysis of Virion Structural Components Reveals Vestiges of the Ancestral Ichnovirus Genome
- Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing Activity
- Global Migration Dynamics Underlie Evolution and Persistence of Human Influenza A (H3N2)
- The Type III Effectors NleE and NleB from Enteropathogenic and OspZ from Block Nuclear Translocation of NF-κB p65
- VEGF Promotes Malaria-Associated Acute Lung Injury in Mice
- Identification of a Mutant PfCRT-Mediated Chloroquine Tolerance Phenotype in
- The Early Stage of Bacterial Genome-Reductive Evolution in the Host
- Host-Detrimental Role of Esx-1-Mediated Inflammasome Activation in Mycobacterial Infection
- Elevation of Intact and Proteolytic Fragments of Acute Phase Proteins Constitutes the Earliest Systemic Antiviral Response in HIV-1 Infection
- The Pleiotropic CymR Regulator of Plays an Important Role in Virulence and Stress Response
- Alternative Sigma Factor σ Modulates Prophage Integration and Excision in
- Effect of Neuraminidase Inhibitor–Resistant Mutations on Pathogenicity of Clade 2.2 A/Turkey/15/06 (H5N1) Influenza Virus in Ferrets
- Massive APOBEC3 Editing of Hepatitis B Viral DNA in Cirrhosis
- NK Cells and γδ T Cells Mediate Resistance to Polyomavirus–Induced Tumors
- Is Genetically Diverse in Animals and Appears to Have Crossed the Host Barrier to Humans on (At Least) Two Occasions
- Adenylate Cyclase Toxin Mobilizes Its β Integrin Receptor into Lipid Rafts to Accomplish Translocation across Target Cell Membrane in Two Steps
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- HIV-1 Transmitting Couples Have Similar Viral Load Set-Points in Rakai, Uganda
- Few and Far Between: How HIV May Be Evading Antibody Avidity
- Galectin-9/TIM-3 Interaction Regulates Virus-Specific Primary and Memory CD8 T Cell Response
- Perforin Expression Directly by HIV-Specific CD8 T-Cells Is a Correlate of HIV Elite Control
- The Set3/Hos2 Histone Deacetylase Complex Attenuates cAMP/PKA Signaling to Regulate Morphogenesis and Virulence of
- Infidelity of SARS-CoV Nsp14-Exonuclease Mutant Virus Replication Is Revealed by Complete Genome Sequencing
- Combining ChIP-chip and Expression Profiling to Model the MoCRZ1 Mediated Circuit for Ca/Calcineurin Signaling in the Rice Blast Fungus
- Internalin B Activates Junctional Endocytosis to Accelerate Intestinal Invasion
- A Complex Small RNA Repertoire Is Generated by a Plant/Fungal-Like Machinery and Effected by a Metazoan-Like Argonaute in the Single-Cell Human Parasite
- Opc Invasin Binds to the Sulphated Tyrosines of Activated Vitronectin to Attach to and Invade Human Brain Endothelial Cells
- Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa
- PdeH, a High-Affinity cAMP Phosphodiesterase, Is a Key Regulator of Asexual and Pathogenic Differentiation in
- Isolates with Antimony-Resistant but Not -Sensitive Phenotype Inhibit Sodium Antimony Gluconate-Induced Dendritic Cell Activation
- The Microbiota and Allergies/Asthma
- Environmental Factors Determining the Epidemiology and Population Genetic Structure of the Group in the Field
- Prolonged Antigen Presentation Is Required for Optimal CD8+ T Cell Responses against Malaria Liver Stage Parasites
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
- Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
- Effective, Broad Spectrum Control of Virulent Bacterial Infections Using Cationic DNA Liposome Complexes Combined with Bacterial Antigens
- High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men
- The -Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections
- EBV Promotes Human CD8 NKT Cell Development
- Persistent Growth of a Human Plasma-Derived Hepatitis C Virus Genotype 1b Isolate in Cell Culture
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



