-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
The cytoplasmic TRIM5α proteins of certain mammalian lineages efficiently recognize the incoming capsids of particular retroviruses and potently restrict infection in a species-specific manner. Successful retroviruses have evolved capsids that are less efficiently recognized by the TRIM5α proteins of the natural hosts. To address whether TRIM5α contributes to the outcome of retroviral infection in a susceptible host species, we investigated the impact of TRIM5 polymorphisms in rhesus monkeys on the course of a simian immunodeficiency virus (SIV) infection. Full-length TRIM5α cDNAs were derived from each of 79 outbred monkeys and sequenced. Associations were explored between the expression of particular TRIM5 alleles and both the permissiveness of cells to SIV infection in vitro and clinical sequelae of SIV infection in vivo. Natural variation in the TRIM5α B30.2(SPRY) domain influenced the efficiency of SIVmac capsid binding and the in vitro susceptibility of cells from the monkeys to SIVmac infection. We also show the importance in vivo of the interaction of SIVmac with different allelic forms of TRIM5, demonstrating that particular alleles are associated with as much as 1.3 median log difference in set-point viral loads in SIVmac-infected rhesus monkeys. Moreover, these allelic forms of TRIM5 were associated with the extent of loss of central memory (CM) CD4+ T cells and the rate of progression to AIDS in the infected monkeys. These findings demonstrate a central role for TRIM5α in limiting the replication of an immunodeficiency virus infection in a primate host.
Published in the journal: . PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000738
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000738Summary
The cytoplasmic TRIM5α proteins of certain mammalian lineages efficiently recognize the incoming capsids of particular retroviruses and potently restrict infection in a species-specific manner. Successful retroviruses have evolved capsids that are less efficiently recognized by the TRIM5α proteins of the natural hosts. To address whether TRIM5α contributes to the outcome of retroviral infection in a susceptible host species, we investigated the impact of TRIM5 polymorphisms in rhesus monkeys on the course of a simian immunodeficiency virus (SIV) infection. Full-length TRIM5α cDNAs were derived from each of 79 outbred monkeys and sequenced. Associations were explored between the expression of particular TRIM5 alleles and both the permissiveness of cells to SIV infection in vitro and clinical sequelae of SIV infection in vivo. Natural variation in the TRIM5α B30.2(SPRY) domain influenced the efficiency of SIVmac capsid binding and the in vitro susceptibility of cells from the monkeys to SIVmac infection. We also show the importance in vivo of the interaction of SIVmac with different allelic forms of TRIM5, demonstrating that particular alleles are associated with as much as 1.3 median log difference in set-point viral loads in SIVmac-infected rhesus monkeys. Moreover, these allelic forms of TRIM5 were associated with the extent of loss of central memory (CM) CD4+ T cells and the rate of progression to AIDS in the infected monkeys. These findings demonstrate a central role for TRIM5α in limiting the replication of an immunodeficiency virus infection in a primate host.
Introduction
The cytoplasmic tripartite motif protein 5α (TRIM5α) has been shown to restrict the replication of a broad range of retroviruses in a species-specific manner [1]–[5]. For example, TRIM5α of rhesus monkeys mediates an early, post-entry block of HIV-1 replication but only a modest block of SIV replication in vitro [4], whereas human TRIM5α restricts N-MLV potently but HIV-1 only weakly [1]–[3],[5]. Although primate TRIM5α variants share a similar domain organization, TRIM5α sequences are highly polymorphic in different species; this interspecies sequence variation of TRIM5α is associated with differences in the viral specificity of TRIM5α-mediated restriction [3],[5],[6],[7]. Multiple TRIM5 alleles have been recently identified in Old World primates, including rhesus monkeys and sooty mangabeys [8],[9]. While a few of these polymorphic forms of TRIM5α have an effect on the susceptibility of transduced cells to in vitro retrovirus infection (HIV-1 and N-MLV), no significant association has been observed between any of these TRIM5 alleles and either the control of SIV replication in vivo or AIDS pathogenesis.
Although they are resistant to infection with HIV-1, rhesus monkeys support the replication of certain strains of SIV and develop an AIDS-like disease following infection with these isolates. The SIV-infected rhesus monkey has become an invaluable model for studying AIDS pathogenesis and evaluating AIDS vaccine strategies [10]–[12]. Interestingly, high levels of viral replication are achieved following infection with SIV in some rhesus monkeys, whereas other rhesus monkeys appear to be more resistant to infection. Certain MHC class I alleles have been associated with efficient control of SIV replication in vivo on the basis of particularly effective SIV-specific cytotoxic T lymphocyte responses [13]. Factors other than adaptive immune responses also have been implicated in the control of SIV replication, since the relative permissivity of a monkey's peripheral blood mononuclear cells (PBMCs) for SIV replication in vitro predicts the level of SIV replication that occurs in vivo when that monkey is infected with SIV [14]. The present study was initiated to explore the contribution of intraspecies TRIM5 allelic polymorphisms to the control of SIV replication in Indian-origin rhesus monkeys.
Results
Sequencing of rhesus monkey TRIM5α
To characterize the TRIM5α variants of rhesus monkeys, full-length TRIM5 cDNAs were derived and sequenced from 79 unrelated Indian-origin rhesus monkeys. Six to 15 independent cDNA clones from each monkey were sequenced. Genomic DNAs from these same animals were also sequenced to confirm the identity of these alleles. We identified a 2-amino acid deletion and 14 single nucleotide polymorphisms (SNPs), each of which was present in at least two animals in this cohort of rhesus monkeys (Fig. 1). The 2-amino acid deletion and most of the nonsynonymous SNPs (nsSNPs) clustered in the coiled-coil (CC) and the B30.2(SPRY) domains of the molecule. An allele was identified with a nsSNP in the L2 linker, and 4 TRIM5 alleles were also characterized that had synonymous polymorphisms clustered in the CC and B30.2(SPRY) regions of the molecule. Based on the distribution of the 10 nsSNPs, the rhesus monkey TRIM5 coding sequences were grouped into 12 distinct alleles. These alleles and their frequencies in a cohort of 79 monkeys are shown in Table 1, ordered according to increasing numbers of nsSNPs. The predicted amino acid sequence of allele 1 was identical to the published rhesus monkey TRIM5α sequence (AY625001), and the predicted sequence of allele 11 was most divergent from allele 1. TRIMCyp, a molecule in which the TRIM5 RBCC domains are fused to cyclophilin A (CypA) [15]–[18], was cloned from cDNAs prepared from the rhesus monkeys and is designated as allele 12. The coincident expression of two different alleles was observed in many of the monkeys.
Fig. 1. A schematic illustrating TRIM5α and its functional domains. 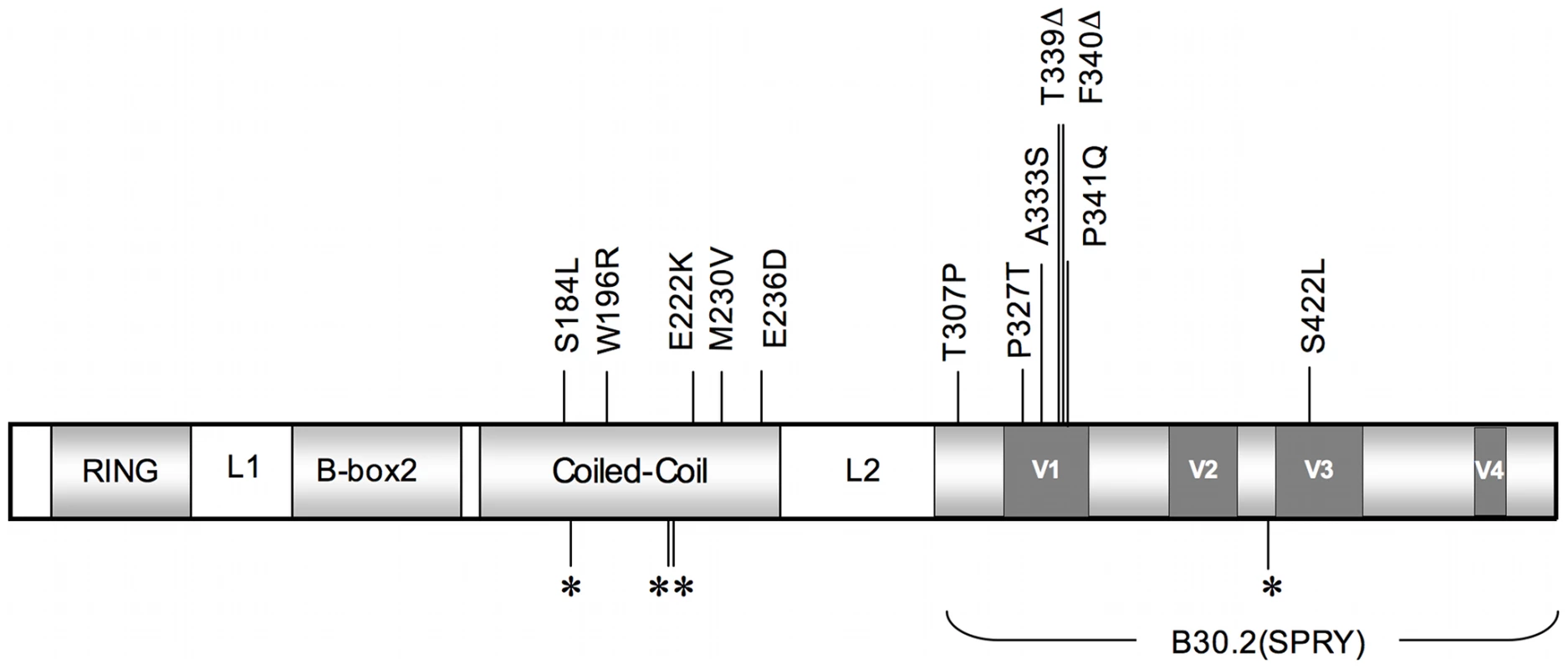
The major domains of TRIM5α are shaded in light grey, and the linker 1 (L1) and linker 2 (L2) regions are designated. The dark grey boxes correspond to variable regions (V1–V4) in the B30.2 (SPRY) domain of the molecule. Locations of coding SNPs are indicated by vertical lines. Nonsynonymous (ns) SNPs with corresponding amino acid changes relative to the major allele are shown above and synonymous SNPs are indicated below by asterisks. These alleles were defined by sequencing full-length cDNA and genomic DNA generated from either B-lymphoblastoid cell lines (B-LCL) or PBMCs of these monkeys. Tab. 1. TRIM5 alleles and their frequencies in Indian-origin rhesus monkeys. 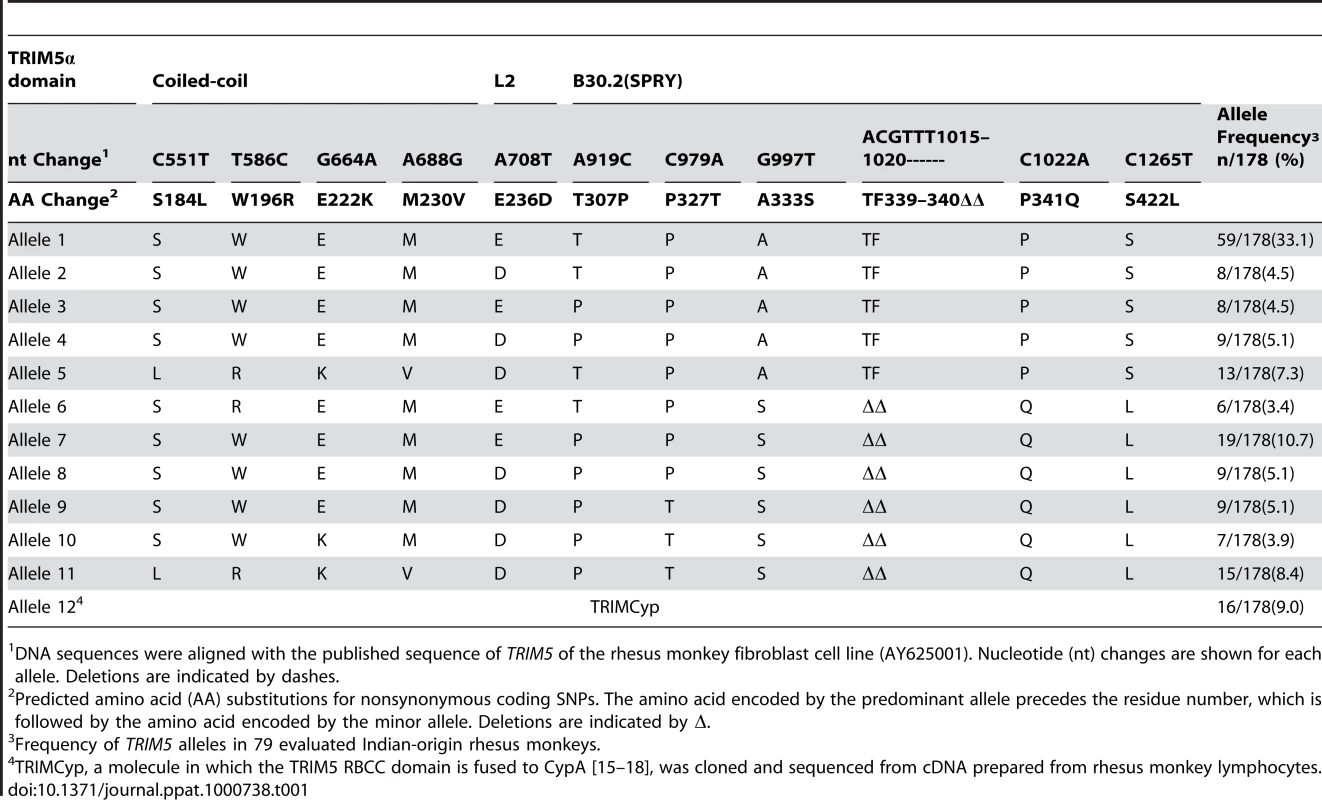
DNA sequences were aligned with the published sequence of TRIM5 of the rhesus monkey fibroblast cell line (AY625001). Nucleotide (nt) changes are shown for each allele. Deletions are indicated by dashes. TRIM5α polymorphisms are associated with susceptibility of B-LCLs to SIV infection
We then sought to assess the impact of this rhesus monkey TRIM5 allelic diversity on the susceptibility of monkey cells to SIV infection. Since TRIM5α restricts the replication of retroviruses by interfering with a post-entry step in their life cycle, we chose first to evaluate the effect of specific TRIM5 allelic products by assessing the in vitro susceptibility of rhesus monkey B-lymphoblastoid cell lines (B-LCLs) expressing defined TRIM5 alleles to infection with a vesicular stomatitis virus G (VSV-G)-pseudotyped SIVmac239-GFP construct. The relative in vitro susceptibility to SIVmac239 infection of rhesus monkey B-LCLs expressing homozygous or heterozygous TRIM5 alleles 1–11 is shown in Fig. 2A and B. A significant variation in susceptibility to SIVmac239 infection was observed among these genetically diverse B-LCLs (P = 0.0164). We then used a Dunn's post test to compare the relative permissiveness for infection of each group of B-LCLs to that of the B-LCLs that expressed only TRIM5 allele 1 (Table 2). Rhesus monkey B-LCLs homozygous for TRIM5 alleles 7, 9 and 10 were significantly more permissive for SIVmac239 infection. The TRIM5α proteins encoded by these alleles share identical B30.2(SPRY) domain residues 328–497. These amino acid residues are also shared by TRIM5α products of alleles 6, 8 and 11. Our inability to demonstrate statistically significant increased permissiveness of alleles 6, 8, and 11 for SIVmac239 infection may be a consequence of the small number of B-LCL populations for evaluation. In fact, when these B-LCLs were assigned to 3 groups on the basis of homozygosity or heterozygosity for expression of alleles 1–5 or 6–11, a statistically significant difference in the permissivity of the cells for SIVmac239 infection was observed (Fig. 2C).
Fig. 2. Effect of TRIM5α variants on susceptibility of B-LCLs to SIVmac239 infection. 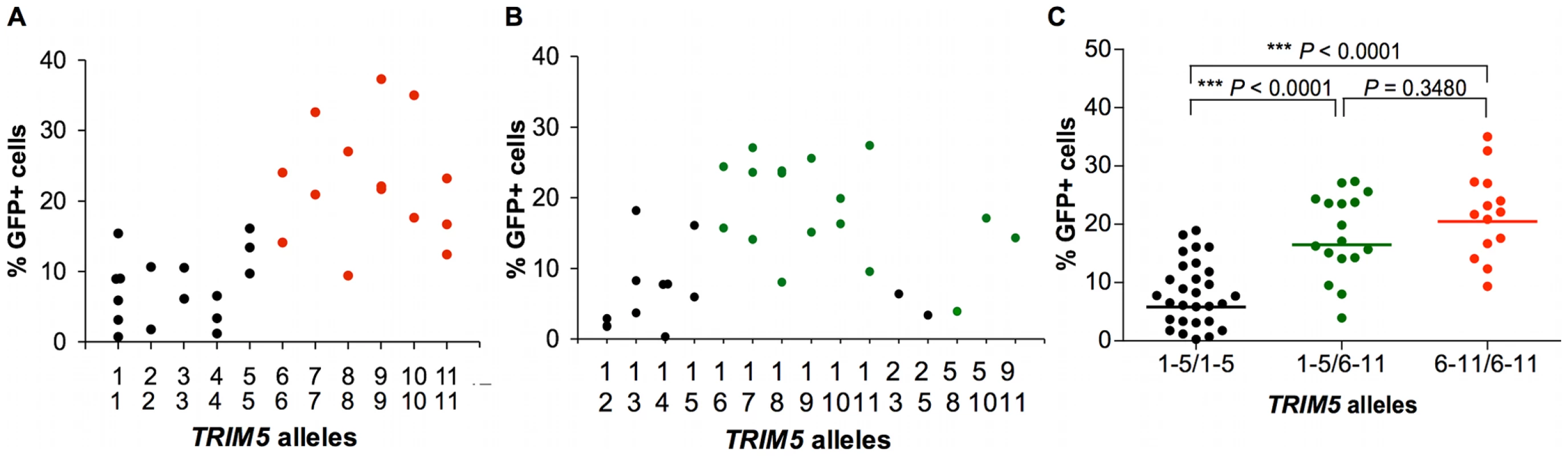
The TRIM5 alleles expressed by selected rhesus monkey B-LCLs were first characterized by cDNA sequencing. These B-LCLs were then evaluated for their relative susceptibility to SIVmac239 replication by incubation for 48 h with a VSV-G-pseudotyped SIVmac239-GFP construct. The percentage of cells expressing GFP was assessed by flow cytometric analysis in B-LCLs homozygous (A) or heterozygous (B) for TRIM5 alleles 1–11. The B-LCLs were then divided into three groups: “1–5/1–5” expressing only TRIM5 alleles 1–5; “1–5/6–11” expressing a TRIM5 allele from the 1–5 groups and another from the 6–11 groups, “6–11/6–11” expressing only TRIM5 alleles 6–11 (C). The sequences of amino acid residues 328–479 of the TRIM5α B30.2 (SPRY) domain encoded by alleles 1–5 are identical to each other and to those previously described as rhesus monkey TRIM5α (AY625001). In contrast, a 2-amino acid deletion (339–340) and 3 nsSNPs (A333S, P341Q and S422L) are present in the B30.2 (SPRY) domain of TRIM5α variants encoded by alleles 6–11. The comparison of the values from the 3 groups of B-LCL was analyzed using a non-parametric one-way ANOVA Kruskal-Wallis test with a Dunn's multiple comparison test. Tab. 2. Comparison of the % GFP values from rhesus monkey B-LCLs expressing various combinations of TRIM5 alleles with a control group of B-LCLs expressing only the TRIM5 allele 1. 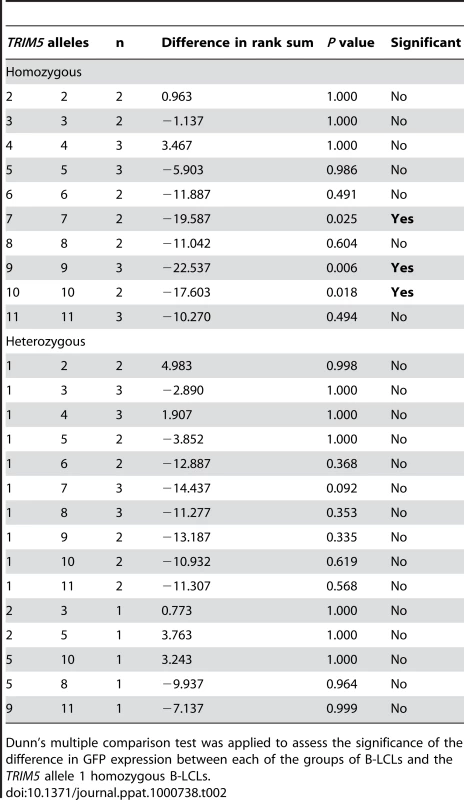
Dunn's multiple comparison test was applied to assess the significance of the difference in GFP expression between each of the groups of B-LCLs and the TRIM5 allele 1 homozygous B-LCLs. The observation above suggested that the permissiveness of rhesus monkey B-LCLs for SIVmac239 replication was associated with amino acid changes clustered in the B30.2(SPRY) domain of TRIM5α. The TRIM5α proteins encoded by the more restricted alleles 1–5 are identical in amino acid residues 328–497 in the B30.2(SPRY) domain. These sequences are also identical to that previously described as rhesus monkey TRIM5α AY625001. Interestingly, alleles 6–11 are all identical in residues 328–497 of the B30.2(SPRY) domain, but differ from alleles 1–5 because of a 2-amino acid deletion (339–340) and 3 nsSNPs (A333S, P341Q and S422L). The results suggest that amino acid changes in the region including residues 328–497 of the TRIM5α B30.2(SPRY) domain determine the efficiency of TRIM5α-mediated restriction for SIVmac239 replication in B-LCLs.
Effects of TRIM5α polymorphisms on retrovirus infections
A number of other complementing observations were made in studying the permissiveness of rhesus monkey B-LCLs assigned to 2 groups on the basis of homozygosity for expression of alleles 1–5 or 6–11 to retrovirus infection. We found that B-LCLs expressing only TRIM5 alleles 1–5 efficiently restricted SIVsmE543 and HIV-1 infection, whereas B-LCLs expressing only TRIM5α molecule from the group of alleles 6–11 were more permissive for infection by these virus constructs (Fig. 3A, B). The overall infectivity of HIV-1 was substantially lower than that of SIVmac239 in the rhesus monkey B-LCLs. This likely reflects the fact that, even in B-LCLs bearing alleles 6–11, rhesus monkey TRIM5α provides a significant barrier to HIV-1 infection. In contrast to this finding, no significant differences in susceptibility to infection by other retroviruses such as equine infectious anemia virus (EIAV), feline immunodeficiency virus (FIV), N-tropic murine leukemia virus (N-MLV) and B-tropic murine leukemia virus (B-MLV) were found between B-LCLs expressing one or the other groups of TRIM5 alleles (Fig. 3C–F). These results suggest the rhesus monkey TRIM5α polymorphisms specifically influence restriction potency for a subset of retroviruses that includes the primate immunodeficiency viruses (PIVs).
Fig. 3. Effects of TRIM5α variants on susceptibility of rhesus monkey B-LCLs to VSV-G-pseudotyped retrovirus constructs. 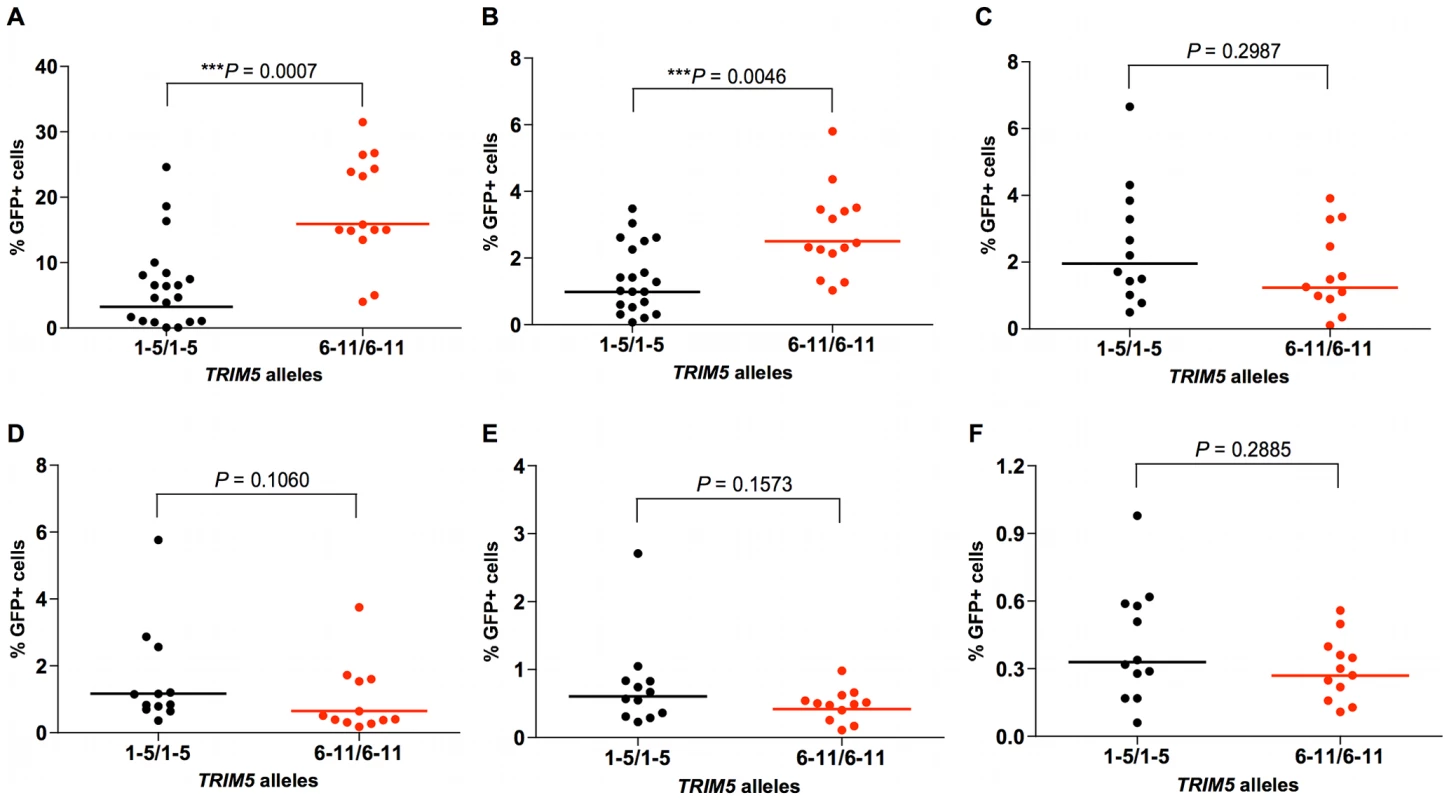
Rhesus monkey B-LCLs expressing selected TRIM5 alleles were divided into two groups, one expressing only TRIM5 alleles 1–5, and the other expressing only TRIM5 alleles 6–11. They were then evaluated for their relative susceptibility to retrovirus infection by incubation for 48 h with VSV-G-pseudotyped recombinant SIVsmE543-GFP (A), HIV-1-GFP (B), EIAV-GFP (C), FIV-GFP (D), N-MLV-GFP (E) or B-MLV-GFP (F) and assessed for % of cells expressing GFP by flow cytometric analysis. The comparison of the values from the 2 groups of B-LCL was analyzed using the Mann-Whitney test. TRIM5 allelic variants differ in their ability to restrict VSV-G-pseudotyped SIVmac239-GFP infection of feline fibroblasts
The contribution of specific rhesus monkey TRIM5 alleles to the inhibition of SIVmac infection was also assessed in TRIM5-transduced cell lines. Feline renal fibroblast (CRFK) cells were stably transduced to express TRIM5α variants encoded by alleles 1, 5, 7 or 11. To produce recombinant viruses, we co-transfected 293T cells with the pLPCX vectors expressing each TRIM5α variant with pVpack-GP and pVPack-VSV-G packaging plasmids. CRFK cells were transduced using the resulting viruses and then selected in G418. These transduced cells were infected with a VSV-G-pseudotyped SIVmac239-GFP construct and subjected to flow cytometric analysis. Consistent with the studies of the B-LCLs, we found that cells transduced with allele 1 or 5 supported a lower level of infection by SIVmac239-GFP, whereas cells transduced with allele 7 or 11 supported a higher level of SIVmac239 infection (Fig. 4A). We also assessed the infectivity of VSV-G-pseudotyped GFP-expressing SIVsmE543, HIV-1, EIAV, FIV, N-MLV and B-MLV viruses these same transduced cells. The restriction of SIVsmE543 and HIV-1 infection was more efficient in cells transduced with allele 1 or 5 than in cells transduced with allele 7 or 11 (Fig. 4A). While transduction of cells with the TRIM5 alleles inhibited infection by EIAV, FIV and N-MLV, all evaluated TRIM5 alleles inhibited the infection with comparable efficiency (Fig. 4B). Expression of these alleles did not restrict B-MLV infection (Fig. 4B). We verified that the HA epitope tag at the C terminus of the TRIM5α protein did not affect the ability of transduced cells to restrict SIVmac239 infection (data not shown). We also confirmed that downregulation of TRIM5α expression by siRNA specifically rescued the infectivity of the restricted SIVmac239 (data not shown). Since the level of TRIM5α protein expression in each of the transduced cells was comparable as determined by Western blotting, differences in TRIM5α protein expression level could not account for the observed differences in the ability of the cells to restrict SIVmac239 infection. The TRIM5α proteins encoded by allele 1 and 7, and by alleles 5 and 11, differ only in the sequences of the B30.2(SPRY) domain (Table 1). Therefore, as in the studies of B-LCLs, the permissiveness for SIVmac replication in the transduced cells was associated with amino acid changes clustered in the B30.2(SPRY) domain of TRIM5α.
Fig. 4. Antiretroviral activity of TRIM5α variants expressed in feline renal fibroblast (CRFK) cells. 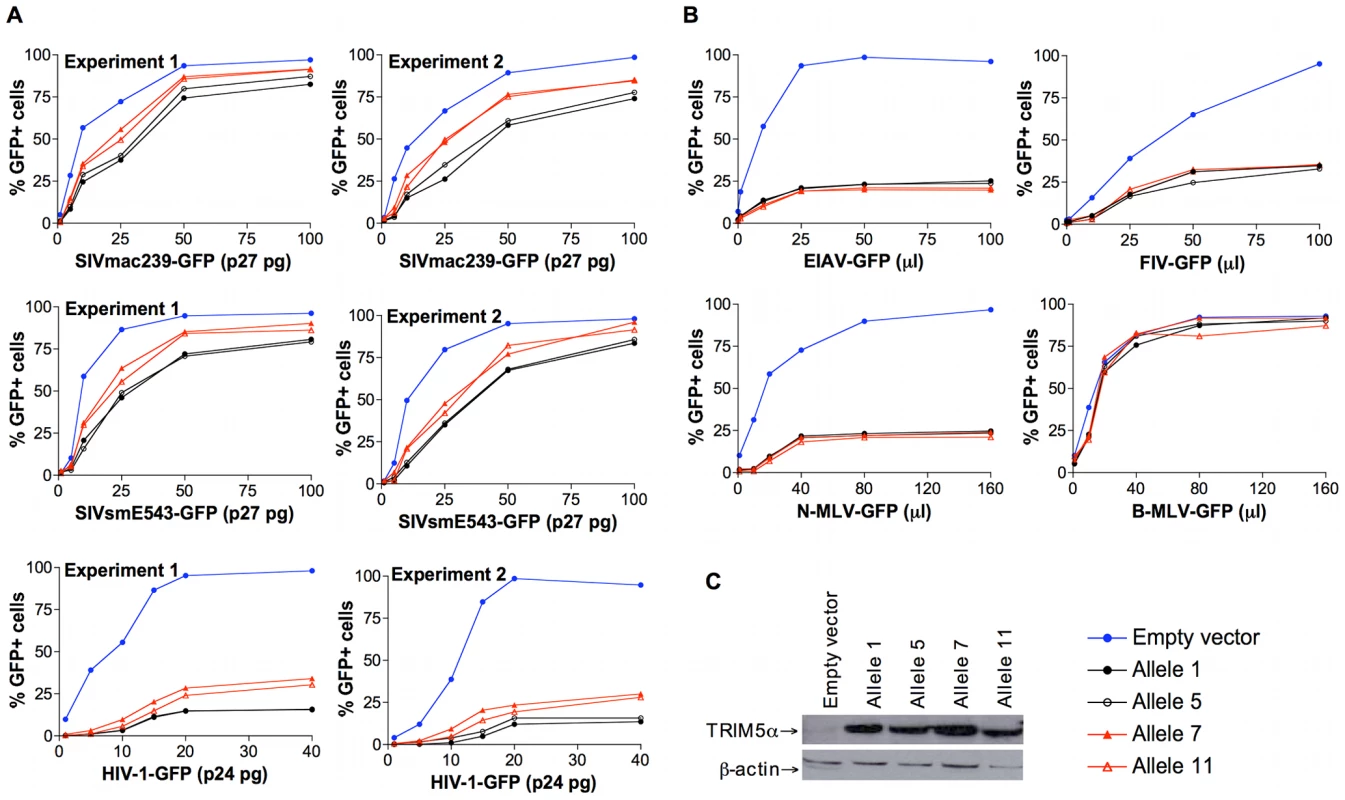
Feline renal fibroblast (CRFK) cells stably expressing TRIM5α variants encoded by allele 1, 5, 7 or 11, or control cells transduced with the empty pLPCX vector were incubated with a VSV-G-pseudotyped recombinant virus construct for 48 h and then subjected to flow cytometric analysis. (A) To assess the effects of TRIM5α variants on the infection of primate immunodeficiency viruses (PIVs), SIVmac239-GFP, SIVsmE543-GFP, and HIV-1-GFP were tested. The results of a repeat experiment are shown. Six dilutions of the stock of SIVmac239-GFP, SIVsmE543-GFP, and HIV-1-GFP were used to infect the transduced cell lines. (B) CRFK cells expressing different TRIM5α variants or control cells transduced with the empty pLPCX vector were incubated with EIAV-GFP, FIV-GFP, N-MLV-GFP or B-MLV-GFP. Infected GFP-positive cells were assessed by flow cytometric analysis. Represent data from repeated experiments are shown. Six dilutions of EIAV-GFP, FIV-GFP, N-MLV-GFP, and B-MLV-GFP were used to achieve 100% transduction of the control cells with the empty pLPCX vector. (C) The expression of TRIM5α protein by the transduced cells was determined by Western blotting using an anti-HA antibody. Levels of β-actin were also assessed as a control for the loading of total protein. Effects of TRIM5α polymorphisms on SIVmac239 capsid binding
We then explored the mechanism accounting for the differences in efficiency of restriction of SIVmac239 infection mediated by these different TRIM5 alleles. A direct interaction of TRIM5α with the retroviral capsid is required for restriction of retrovirus replication [19]–[21]. The recognition of the capsid by TRIM5α is mediated by the B30.2(SPRY) domain. Because the phenotypic differences among rhesus monkey TRIM5α variants grouped according to the sequence of the B30.2(SPRY) domain, we hypothesized that the two groups of TRIM5α variants might exhibit differences in the ability to bind SIVmac capsids. Two TRIM5α proteins encoded by alleles 1 and 7 were studied for capsid-binding ability. TRIM5 allele 1 represents the major allele and encodes a protein identical in sequence to that of the previously reported rhesus monkey TRIM5α [4]. The TRIM5α protein encoded by allele 7 differs from that encoded by allele 1 only in the sequence of the B30.2(SPRY) domain (Table 1). Therefore, TRIM5α variants 1 and 7 were chosen as representatives of the two groups of TRIM5α B30.2(SPRY) domain variants and tested for their ability to bind SIVmac capsid.
For this purpose, we adapted an in vitro capsid-binding assay, which has been used to study TRIM5α binding to the HIV-1 capsid [22]. In our assay, SIVmac239 capsid-nucleocapsid (CA-NC) complexes were generated in vitro, and then incubated with serial dilutions of 293T cell lysates containing TRIM5α allelic variants 1 and 7. After incubation, TRIM5α protein bound to CA-NC complexes was separated from unbound TRIM5α by pelleting through a sucrose cushion. The amount of TRIM5α in both input and pellet was analyzed by Western blotting and densitometry. As expected from previous experience with the HIV-1 capsid-nucleocapsid binding assay [23], both TRIM5α allelic variants bound the SIVmac239 CA-NC complexes in a dose-dependent manner with a sigmoidal pattern. Importantly, however, binding of the variant encoded by TRIM5 allele 7 was less efficient than that of the TRIM5α allele 1 variant (Fig. 5A, B). This finding is consistent with the possibility that differences in the efficiency of binding the SIVmac239 capsid contribute to the observed differences in SIVmac239 restriction potency, with the less efficiently binding allelic variant 7 mediating the less potent restriction of viral replication.
Fig. 5. Effect of TRIM5α variation on SIVmac239 capsid binding. 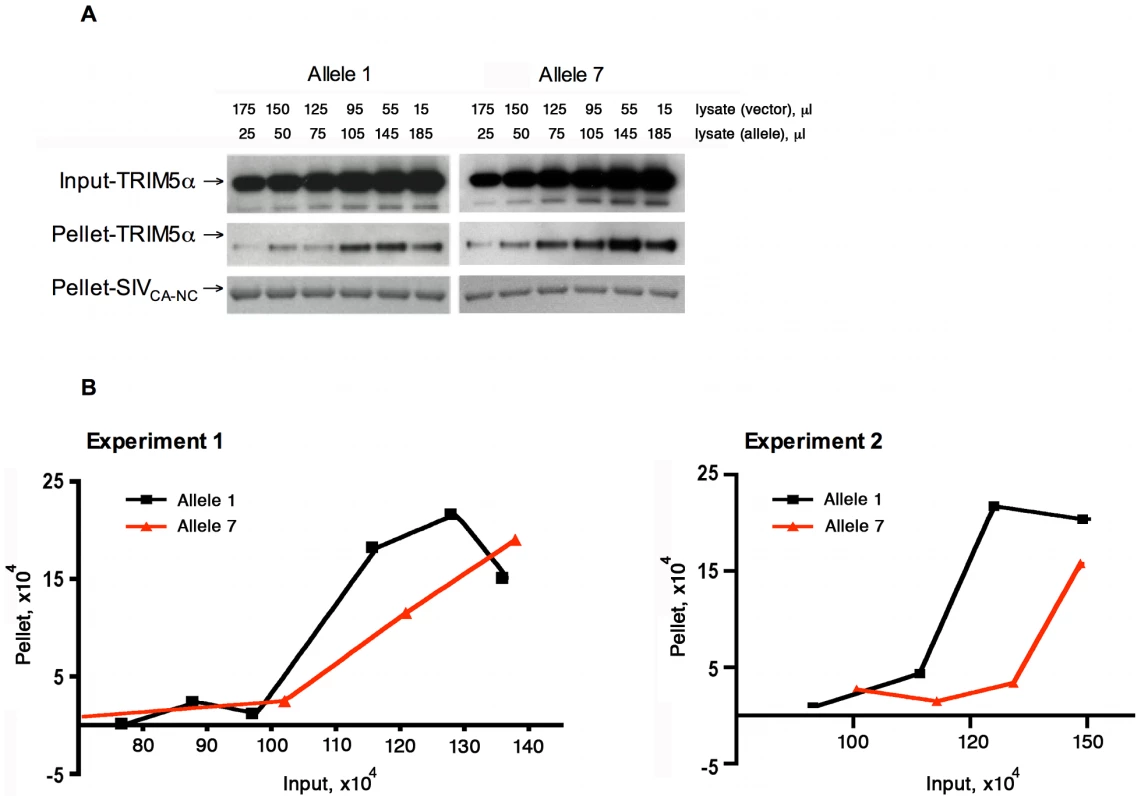
Serial dilutions of 293T cell lysates (input) containing TRIM5α-HA proteins encoded by allele 1 or allele 7 were incubated with equal amounts of SIVmac239 CA-NC complexes assembled in vitro. After incubation, the mixtures were pelleted through a sucrose cushion. The amounts of TRIM5α in both input and pellet were subjected to Western blotting and densitometry. The results of two separate experiments are shown. The Western blot (A) of experiment 1 was quantitatively analyzed by a densitometer with the results shown in (B). The last two samples of the allele 7 variant were beyond the linear range of the densitometry and, therefore, were not included in the final analysis in (B). Both pellet and input amounts are shown in arbitrary densitometric units. TRIM5α polymorphisms are associated with plasma viral RNA levels and clinical sequelae of SIVmac251 infection
To determine the ramifications of these in vitro findings for the control of viral replication in vivo, a study was done to determine whether the expression of particular TRIM5 alleles by Indian-origin rhesus monkeys was associated with the in vivo control of SIVmac251 replication. We tested the specific hypothesis that variation in residues 328–497 of the TRIM5α B30.2(SPRY) domain influences the course of SIVmac251 infection in vivo. The monkeys were divided into 3 groups: in one group, only TRIM5 alleles 1–5 were expressed; in the second group, one copy of alleles 1–5 and one copy of alleles 6–11 were expressed; and in the third group, only alleles 6–11 were expressed. Monkeys were selected for this study that did not express the MHC class I alleles Mamu-A*01, -B*08, or -B*17, as their expression is associated with particularly efficient control of SIV replication [24]–[26]. Eliminating these monkeys from the evaluated animals eliminated an already defined source of variation in virus control.
Plasma SIV RNA levels were assessed in this cohort of rhesus monkeys through day 178 after SIVmac251 challenge. We measured viral replication for each monkey by doing an area-under-the curve calculation for the plasma SIV RNA levels between days 1 and 70 following infection. A 0.1 log median difference in these values was observed between the allele 1–5 homozygous and the allele 1–5/allele 6–11 heterozygous monkeys, and a 0.6 log median difference in these values was observed between the allele 1–5 homozygous and the allele 6–11 homozygous monkeys (Fig. 6A). Plasma virus RNA levels were also assessed in this cohort of rhesus monkeys on days 14 (peak) and 70 (set-point) following the intravenous inoculation of SIVmac251. Consistent with the decreased in vitro antiviral activity of the products of TRIM5 alleles 6–11, the expression of TRIM5 alleles 6–11 by the rhesus monkeys was associated with higher levels of SIVmac251 replication in vivo. A 0.4 log median difference in plasma virus RNA levels at the time of peak viral replication was observed between the allele 1–5 homozygous and the allele 1–5/allele 6–11 heterozygous monkeys, and a 0.6 log median difference in these values was observed between the allele 1–5 homozygous and the allele 6–11 homozygous monkeys (Fig. 6A). A log median difference in set-point virus RNA levels was 0.7 between the allele 1–5 homozygous and the allele 1–5/allele 6–11 heterozygous monkeys, and 1.3 between the allele 1–5 homozygous and the allele 6–11 homozygous monkeys (Fig. 6A).
Fig. 6. Association of the expression of groups of TRIM5 alleles by rhesus monkeys with viral replication following SIVmac251 infection. 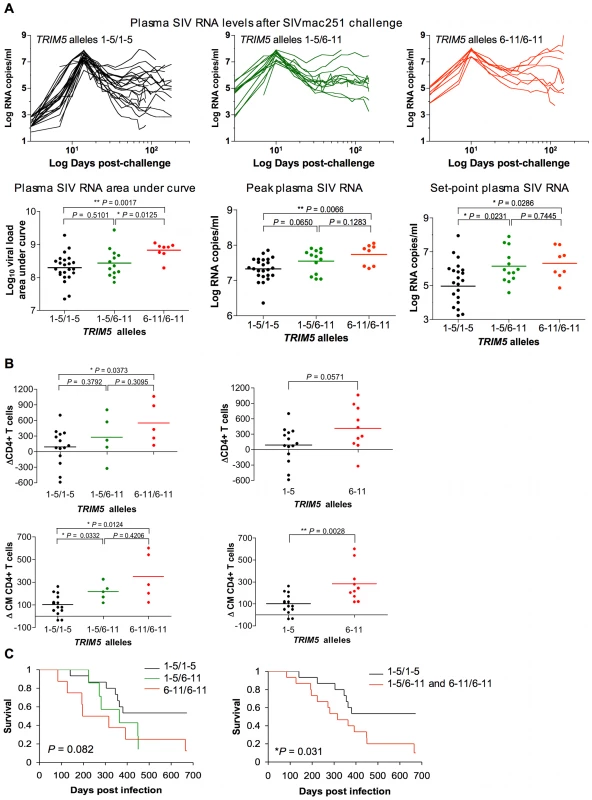
(A) Plasma virus RNA levels were assessed in a cohort of Indian-origin rhesus monkeys between days 1 and 178 after challenge. Area-under-the curve calculations for the plasma SIV RNA levels were assessed in these monkeys. The plasma SIV RNA levels were also assessed on days 14 and 70 following challenge, representing peak and set-point viral load, respectively. (B) The loss of total CD4+ T cells and central memory CD4+ T cells was monitored in the same cohorts of rhesus monkeys following SIVmac251 infection. Data are displayed two ways: dividing the monkeys into 3 cohorts as described in the legend to Fig. 2 or dividing the monkeys into 2 cohorts, one of animlas expressing only alleles 1–5 and the other of animals expressing at least 1 allele of the groups 6–11. (C) The survival of monkeys following SIVmac251 infection is shown in Kaplan-Meier (KM) curves. The P-value corresponds to the log-rank test of equality of the survival curves. The monkeys are divided into either 3 or 2 groups as described above. The comparison of the values from the groups of monkeys was analyzed using a non-parametric one-way ANOVA Kruskal-Wallis test with Dunn's multiple comparison test or the Mann-Whitney test. We then assessed the clinical consequences of infection in these monkeys. First, we evaluated the loss of peripheral blood total CD4+ T cells and CM CD4+ T cells in the monkeys following SIVmac251 inoculation. We sought to determine whether the expression of particular TRIM5 alleles was associated with particular immune sequelae of infection. We displayed the data in two ways: dividing the monkeys into 3 cohorts as described above and dividing the monkeys into 2 cohorts, one group of animals expressing only alleles 1–5 and the other expressing at least 1 allele of the group 6–11. The expression of TRIM5 alleles 6–11 by the rhesus monkeys was associated with a rapid loss of both total CD4+ T cells and CM CD4+ T cells (Fig. 6B). Then we assessed the effect of the expression of TRIM5 alleles 6–11 on the survival of the monkeys following infection (log-rank test of equality, Kaplan-Meyer survival curves). The monkeys expressing only alleles 1–5 maintained a statistically significant survival advantage over the monkeys expressing at least 1 allele of the group 6–11 (Fig. 6C). Therefore, the expression of TRIM5 alleles 6–11 was associated with both less efficient control of SIVmac replication in vivo and an increased rate of disease progression.
Discussion
We demonstrated TRIM5 allele-determined relative resistance to SIV infection in vitro using both B-LCL and transduced fibroblast target cells. However, the variable resistance or permissiveness for infection was more readily apparent in the B-LCL than in the fibroblasts. This difference between target cell populations may reflect differences in TRIM5 expression under normal physiologic control in B-LCLs and in transduced CRFK cells. Thus, overexpression in CRFK cells may partially mask the relative inefficiency of some of the TRIM5 alleles to restrict SIV and HIV replication in vitro.
A number of genes have previously been implicated in the control of HIV-1 replication. These include selected MHC class I alleles and loci, including HLA B*5701, HLA B*27 [27] and HLA C [28], as well as HLA Bw4-8OI in association with KIR3DS1 [29]. Expression of an allelic form of the HIV-1 co-receptor, the delta 32 form of CCR5, is also associated with HIV-1 containment [30]–[32]. These genes and gene loci are thought to contribute to HIV-1 control through classical immune effector mechanisms or viral entry.
The present study shows that naturally occurring TRIM5α B30.2(SPRY) polymorphisms affect immunodeficiency virus infections. These observations demonstrate the importance of SIV/B30.2(SPRY) interactions in vivo. The finding that TRIM5 allelic products are associated with both the permissiveness of cells to HIV-1/SIV infection in vitro and clinical sequelae of SIV infection in vivo expands the known genetic determinants of HIV-1/SIV susceptibility and implicates a novel intracellular mechanism in that virus control.
Our data suggest that the co-expression of restrictive and permissive TRIM5α variants results in a less effective antiretroviral state than the expression of two restrictive TRIM5α variants. Differences in SIVmac restriction potency and capsid binding map to the B30.2(SPRY) domain of the rhesus monkey TRIM5α variants. As the functional TRIM5α moiety is thought to an oligomer [33]–[36], co-expression of TRIM5α variants with different capsid-binding affinities would be expected to result in heterodimers with decreased avidity for the capsid. Dominant-negative effects of less functional TRIM5α variants on more potently restricting TRIM5α variants have been observed [4], [37]–[39].
A number of groups have evaluated the contribution of common TRIM5α polymorphisms to AIDS susceptibility in HIV-1-infected humans [40]–[44]. These various studies have defined associations of nsSNPs in the RING, B-box 2, Coiled-coil domains and Linker 2 region of the molecule with very modest effects on HIV-1 susceptibility and AIDS clinical progression [40]–[43]. The single SPRY domain ns SNP in human TRIM5α, H419Y, was also shown to have only a modest effect on these clinical endpoints [44]. The absence of evidence for a robust association between a SNP in TRIM5α and a substantial HIV-1-associated clinical endpoint in humans is likely a consequence of the fact that the common variant forms of this molecule in humans do not have severely impaired interactions with retroviruses. The human TRIM5 SNPs are for the most part clustered in the B-box 2 and Coiled-coil domains of the molecule, regions of TRIM5α that are not thought to contact the retrovirus capsid. In contrast, as we have shown in the present study, variants of TRIM5α in the rhesus monkey that cluster in the B30.2 (SPRY) domain of the molecule have a significant impact on the interaction of TRIM5α with the capsid of SIV and, as a result, exert a significant effect on SIV replication in vitro and in vivo.
The findings in the present study indicate that TRIM5α has a function beyond restricting cross-species transmission of retroviruses. We show that TRIM5α variants with an impaired ability to interact with the retrovirus capsid restrict pathogenic retroviruses in a susceptible host species less efficiently both in vitro and in vivo. Therefore, TRIM5α proteins can exert antiretroviral effects ranging from modifications of viral load to complete suppression of infection.
Methods
Cells
HEK293T and feline renal fibroblasts (CRFK) were obtained from American Type Culture Collection and grown in RPMI/10% FBS.
Recombinant viruses
Recombinant SIVmac239-GFP virus was produced by cotransfection of HEK293T cells with pSIVmac239Δenv-GFP, pVSV-G and pRev using the Lipofectamine 2000 (Invitrogen). HIV-1-GFP, SIVsmE543-GFP, EIAV-GFP, FIV-GFP, B - and N-MLV-GFP viruses were prepared as previously described [7]. At 48h after transfection, cell-free supernatant was collected. The titer of virus in the supernatant was determined by infection of HEK293T cells and assessment for % of cells expressing GFP by flow cytometric analysis. An SIV p27 antigen ELISA was performed to measure the p27 gag gene product of SIV.
Establishment of B Lymphoblastoid Cell Lines (B-LCLs)
B-LCLs were generated from the same cohort of 28 uninfected rhesus monkeys and 32 rhesus monkeys previously infected with SIVmac251 as described [45]. Briefly, B cells were transformed by incubating peripheral blood mononuclear cells isolated from rhesus monkeys with Herpesvirus papio. B-LCLs were then propagated in RPMI 1640 supplemented with 20% FBS and penicillin-streptomycin.
Sequencing of TRIM5α cDNA
Total RNA was isolated from H. papio-immortalized rhesus monkey B-LCLs and PBMCs by using the RNeasy Mini kit (Qiagen). Untagged, full-length TRIM5α cDNA clones were generated by RT-PCR with primers TRIM5F1 (5′-CAGACGAATTCCACCATGGCTTCTGGAATCCTG-3′) and TRIM5R1 (5′-GGACGTTCGAAATAGAAAGAAGGGAGACAGC-3′) by using the SuperScript One-Step kit (Invitrogen). PCR products were cloned directly into the TOPO Blunt vector (Invitrogen). Six to 15 independent cDNA clones from individual rhesus monkeys were subjected to automated sequence analysis (GENEWIZ). Genomic DNA was isolated from lymphocytes from the same cohorts of rhesus monkeys by using QIAamp DNA kit (Qiagen), and sequenced. Primer selection was facilitated by the use of the computer program Beacon 3. Primer sequences used to amplify and sequence TRIM5 exons or TRIM5 cDNAs are shown in Table S1. To generate TRIM5α C-terminally tagged with hemagglutinin (HA), genes were amplified with primers TRIM5F1 and TRIM5HA-R (5′-CCACCGGTGGCTCAAGCGTAGTCTGGGACGTCGTATGGGTAGCCGCCAGAGCTTGGTGAGCACAGAG-3′).
Rhesus monkey B-LCL infection
B-LCLs were selected for study that had previously defined TRIM5 alleles. A VSV-G pseudotyped recombinant virus constructs were used for single-round infection. 4×104 cells per well were seeded into 96-well plates and cells were infected with serial dilutions of virus by spinoculation for 2 h at 1,900×g. At 48 h after infection, these B-LCLs were evaluated for their relative susceptibility to retrovirus replication by assessing for the % of cells expressing GFP as determined by flow cytometric analysis.
Restriction in CRFK cells expressing TRIM5 alleles
Feline renal fibroblast (CRFK) cells stably expressing TRIM5α variants encoded by allele 1, 5, 7 or 11 were generated as described by Stremlau et al. [4]. Briefly, 293T cells were co-transfected with the pLPCX vectors containing each TRIM5α cDNA with pVpack-GP and pVPack-VSV-G packaging plasmids (Stratagene) to produce recombinant viruses. CRFK cells were then transduced using the resulting viruses and selected in G418 (0.5mg/ml, Sigma-aldrich). For infection, CRFK cells expressing TRIM5α variants encoded by allele 1, 5, 7 or 11, or control cells transduced with the empty pLPCX vector were harvested and subsequently seeded into 48-well plates (1.2×104 cells per well). Cells were incubated with a VSV-G-pseudotyped recombinant virus construct for 48 h and then subjected to flow cytometric analysis.
Immunoblotting
Feline renal fibroblast (CRFK) cells were transduced as described above and harvested. Cells were then lysed in NP-40 lysis buffer (Boston BioProducts). Total protein was measured by using a BCA assay kit (Pierce) and equal amounts (20 µg) were separated by SDS/PAGE. HA-tagged TRIM5α was detected with either monoclonal anti-HA antibody (Roche) and HRP-conjugated either anti-rat or anti-rabbit IgG secondary antibody, respectively (Sigma-Aldrich). Levels of β-actin were also assessed as a control for the loading of total protein by using anti-β-actin antibody (Sigma-Aldrich).
Capsid-binding assay
The SIVmac239 CA-NC fusion protein was expressed in Escherichia coli and purified with minor modifications from the previously described purification method used for the HIV-1 CA-NC protein [22]. The purified SIVmac CA-NC protein was mixed with (TG)50 DNA oligonucleotides in 50 mM Tris-HCl (pH 7.0) and 500 mM NaCl solution and incubated at 37°C. The resulting SIVmac CA-NC complexes were negatively stained and examined under the electron microscope, confirming that the size and shape of the CA-NC complexes are very similar to those HIV-1 CA-NC complexes.
Approximately 1×107 293T cells were transiently transfected to express the appropriate TRIM5 allele or with a control vector, and then harvested at 24 h post-transfection. The cells were lysed in 1 ml of hypotonic lysis buffer (10 mM Tris-HCl, pH 7.0/10 mM KCl). After brief centrifugation at 4°C to remove cell debris, the supernatant was spun at 110, 000×g for 1 h at 4°C in a Beckman ultracentrifuge. After the pre-cleaning spin, different amounts of the supernatants containing the TRIM5α variants were aliquoted in separate tubes and were complemented with the supernatants from the vector-transformed cells to achieve a final volume of 200 µl. To these mixtures, the same amount of SIVmac CA-NC complexes were added and the final concentration of NaCl was adjusted to 200 mM before incubation at 30°C for 1 h. After incubation, 20 µl of the mixtures were saved for further analysis as an input and the rest of mixtures were layered onto 60% sucrose cushion (prepared in 1× PBS) and centrifuged at 110,000×g for 1 h at 4°C in a Beckman ultracentrifuge. The pellet was resuspended in 1× SDS sample buffer and subjected to SDS/PAGE and Western blotting.
Monoclonal antibody (MAb) staining of cell surface molecules and CD4+ T-lymphocyte counts
Peripheral blood mononuclear cells (PBMCs) were stained with MAbs specific for cell surface molecules, including CD3, CD4, CD28, and CD95, and then subjected to flow cytometric analysis. Peripheral blood CD4+ T lymphocyte and central memory CD4+ T lymphocyte counts were calculated by multiplying the total lymphocytes count by the percentage of CD3+CD4+ T cells, and CD95+CD28+ T cells, respectively, determined by MAb staining and flow cytometric analysis [46].
In vivo infection
Animals
Indian-origin rhesus monkeys used in the analysis were maintained according to the guidelines of the NIH Guide to the Care and Use of Laboratory Animals and the approval of the Institutional Animal Care and Use Committee of Harvard Medical School and the National Institute of Health.
Challenge virus
A stock of uncloned SIVmac251 was expanded on human PBMC and titered in vivo in rhesus monkeys for use in intravenous challenge studies [47]. All monkeys used in the retrospective analysis were received 1 ml of a 1∶3000 dilution of this stock by intravenous route.
Plasma viral RNA assay
Assays were performed using an ultrasensitive branched DNA amplification assay (Bayer Diagnostics).
Statistical analysis
The two-sided nonparametric Mann-Whitney test was used to determine statistical significance. Levels of P <0.05 were considered statistically significant. For multiple comparisons, non-parametric one-way ANOVA Kruskal-Wallis test was used with Dunn's multiple comparison test.
Supporting Information
Zdroje
1. HatziioannouT
Perez-CaballeroD
YangA
CowanS
BieniaszPD
2004 Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5α. Proc Natl Acad Sci U S A 101 10774 10779
2. KeckesovaZ
YlinenLMJ
TowersGJ
2004 The human and African green monkey TRIM5α genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc Natl Acad Sci U S A 101 10780 10785
3. YapM
NisoleS
LynchC
StoyeJ
2004 Trim5α protein restricts both HIV-1 and murine leukemia virus. Proc Natl Acad Sci U S A 101 10786 10791
4. StremlauM
OwensCM
PerronMJ
KiesslingM
AutissierP
2004 The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427 848 853
5. PerronMJ
StremlauM
SongB
UlmW
MulliganRC
2004 TRIM5α mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc Natl Acad Sci U S A 101 11827 11832
6. RichardsonMW
CarrollRG
StremlauM
KorokhovN
HumeauLM
2008 Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. J Virol 82 11117 11128
7. SongB
GoldB
O'HuiginC
JavanbakhtH
LiX
2005 The B30.2(SPRY) domain of the retroviral restriction factor TRIM5alpha exhibits lineage-specific length and sequence variation in primates. J Virol 79 6111 6121
8. NewmanRM
HallL
ConnoleM
ChenGL
SatoS
2006 Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5alpha. Proc Natl Acad Sci U S A 103 19134 19139
9. WilsonSJ
WebbBL
MaplankaC
NewmanRM
VerschoorEJ
2008 Rhesus macaque TRIM5 alleles have divergent antiretroviral specificities. J Virol 82 7243 7247
10. OurmanovI
BilskaM
HirschVM
MontefioriDC
2004 Recombinant modified vaccinia virus ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol 74 2960 2965
11. PolacinoP
StallardV
KlanieckiJE
MontefioriDC
LangloisAJ
1999 Limited breadth of the protective immunity elicited by simian immunodeficiency virus SIVmne gp160 vaccines in a combination immunization regimen. J Virol 73 618 630
12. YasutomiY
ReimannKA
LordCI
MillerMD
LetvinNL
1993 Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol 67 1707 1711
13. PalR
VenzonD
LetvinNL
SantraS
Montefiori
2002 ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol 76 292 302
14. GoldsteinS
BrownCR
DehghaniH
LifsonJD
HirschVM
2000 Intrinsic susceptibility of rhesus macaque peripheral CD4(+) T cells to simian immunodeficiency virus in vitro is predictive of in vivo replication. J Virol 74 9388 9395
15. BrennanG
KozyrevY
HuSL
2009 TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc Natl Acad Sci U S A 105 3569 3574
16. NewmanRM
HallL
KirmaierA
PozziLA
PeryE
2008 Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog 4 e1000003
17. VirgenCA
KratovacZ
BieniaszPD
HatziioannouT
2008 Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc Natl Acad Sci U S A 105 3563 3568
18. WilsonSJ
WebBL
YlinenLM
VerschoorE
HeeneyJL
2008 Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A 105 3557 3562
19. StremlauM
PerronM
LeeM
LiY
SongB
2006 Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci U S A 103 5514 5519
20. PerronMJ
StremlauM
LeeM
JavanbakhtH
SongB
2007 The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J Virol 81 2138 2148
21. JavanbakhtH
YuanW
YeungDF
SongB
Diaz-GrifferoF
2006 Characterization of TRIM5alpha trimerization and its contribution to human immunodeficiency virus capsid binding. Virology 353 234 246
22. GanserBK
LiS
KlishkoVY
FinchJT
SundquistWI
1999 Assembly and analysis of conical models for the HIV-1 core. Science 283 80 83
23. Diaz-GrifferoF
KarA
LeeM
StremlauM
PoeschlaE
2007 Comparative requirements for the restriction of retrovirus infection by TRIM5alpha and TRIMCyp. Virology 369 400 410
24. LoffredoJT
BeanAT
BealDR
LeónEJ
MayGE
2008 Patterns of CD8+ immunodominance may influence the ability of Mamu-B*08-positive macaques to naturally control simian immunodeficiency virus SIVmac239 replication. J Virol 82 1723 1738
25. ManessNJ
YantLJ
ChungC
LoffredoJT
FriedrichTC
2008 Comprehensive immunological evaluation reveals surprisingly few differences between elite controller and progressor Mamu-B*17-positive Simian immunodeficiency virus-infected rhesus macaques. J Virol 82 5245 5254
26. MothéBR
WeinfurterJ
WangC
RehrauerW
WilsonN
2003 Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 77 2736 2740
27. KaslowRA
CarringtonM
AppleR
ParkL
MuñozA
1996 Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nature medicine 2 405 411
28. FellayJ
ShiannaKV
GeD
ColomboS
2007 A whole-genome association study of major determinants for host control of HIV-1. Science 317 944 947
29. MartinMP
GaoX
LeeJH
NelsonGW
DetelsR
2002 Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nature Genetics 31 429 434
30. DeanM
CarringtonM
WinklerC
HuttleyGA
SmithMW
1996 Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273 1856 1862
31. HuangY
PaxtonWA
WolinskySM
NeumannAU
ZhangL
1996 The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nature Medicine 2 1240 1243
32. SmithMW
DeanM
CarringtonM
WinklerC
HuttleyGA
1997 Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science 277 959 965
33. MischeCC
JavanbakhtH
SongB
Diaz-GrifferoF
StremlauM
2005 Retroviral restriction factor TRIM5alpha is a trimer. J Virol 79 14446 14450
34. KarAK
Diaz-GrifferoF
LiY
LiX
SodroskiJ
2008 Biochemical and biophysical characterization of a chimeric TRIM21-TRIM5alpha protein. J Virol 82 11669 11681
35. LangelierCR
SandrinV
EckertDM
ChristensenDE
ChandrasekaranV
2008 Biochemical characterization of a recombinant TRIM5alpha protein that restricts human immunodeficiency virus type 1 replication. J Virol 82 11682 11694
36. LiX
SodroskiJ
2008 The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol 82 11495 11502
37. Perez-CaballeroD
HatziioannouT
YangA
CowanS
BieniaszPD
2005 Human tripartite motif 5alpha domains responsible for retrovirus restriction activity and specificity. J Virol 79 8969
38. JavanbakhtH
Diaz-GrifferoF
StremlauM
SiZ
SodroskiJ
2005 The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5alpha. J Biol Chem 280 26933 26940
39. TorimiroJN
JavanbakhtH
Diaz-GrifferoF
KimJ
CarrJK
2009 A rare null allele potentially encoding a dominant-negative TRIM5alpha protein in Baka pygmies. Virology Epub ahead of print
40. JavanbakhtH
AnP
GoldB
PetersenDC
O'HuiginC
2006 Effects of human TRIM5alpha polymorphisms on antiretroviral function and susceptibility to human immunodeficiency virus infection. Virology 353 234 246
41. SawyerSL
WuLI
AkeyJM
EmermanM
MalikHS
2006 High-frequency persistence of an impaired allele of the retroviral defense gene TRIM5alpha in humans. Curr Biol 16 95 100
42. SpeelmonEC
Livingston-RosanoffD
LiSS
VuQ
BuiJ
GeraghtyDE
2006 Genetic association of the antiviral restriction factor TRIM5alpha with human immunodeficiency virus type 1 infection. J Virol 80 2463 2471
43. van ManenD
RitsMA
BeugelingC
van DortK
SchuitemakerH
2008 The effect of Trim5 polymorphisms on the clinical course of HIV-1 infection. PLoS Pathog 4 e18
44. GoldschmidtV
BleiberG
MayM
MartinezR
OrtizM
2006 Role of common human TRIM5alpha variants in HIV-1 disease progression. Retrovirology 22 54 61
45. KaurA
GrantRM
MeansRE
McClureH
FeinbergM
1998 Diverse Host Responses and Outcomes following Simian Immunodeficiency Virus SIVmac239 Infection in Sooty Mangabeys and Rhesus Macaques. J Virol 72 9597 9611
46. SunY
PermarSR
BuzbyAP
LetvinNL
2007 Memory CD4+ T-lymphocyte loss and dysfunction during primary simian immunodeficiency virus infection. J Immunol 81 8009 8015
47. LetvinNL
MascolaJR
SuY
GorgoneDA
BuzbyAP
2006 Preserved CD4+ Central Memory T Cells and Survival in Vaccinated SIV-Challenged Monkeys. Science 312 1530 1533
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF AirwayČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



